Abstract
Background
Tuberculosis (TB) control has been a challenge in the country and its overall health impact remains significant. COVID-19 has caused significant morbidity and mortality especially among hospitalized patients. TB and COVID-19 co-infection (COVID-TB) may cause more catastrophic consequences and outcomes among afflicted individuals and management may be daunting. There is limited local data on COVID-TB.
Objectives
The clinical profile of COVID-TB patients who were admitted were described. Comparison of the clinical outcomes was also done versus the general admitted COVID-19 patients without concomitant TB in the same institution. Relevant patient outcomes were reported which included admission to an intensive care unit (ICU), length of hospital stay, and mortality rate.
Methods
This is a descriptive study on the demographics and clinical outcomes of patients admitted in the Philippine General Hospital (PGH) for COVID-19 with TB co-infection from March 2020 to September 2020. We aimed to characterize patients with COVID-TB and analyzed their outcomes.
Results
There was a total of 79 patients who were admitted for COVID-19 (confirmed with RT-PCR) with TB co-infection during the study period. Majority of them were males (70.9%) with a median age of 54 (IQR 42 to 64) years. In terms of TB affliction, 75 (94.9%) patients were identified to have pulmonary tuberculosis. Majority of patients had at least one co-morbid illness with hypertension (16.5%), diabetes mellitus (13.9%), and heart failure (11.4%) as the most common. Respiratory symptoms (dyspnea and cough) were the predominant presenting complaint during hospital admission. Majority of the patients were classified as severe (8 or 10.1%) and critical (36 or 45.57%) COVID-19 disease. Fifty-six (70.9%) were bacteriologically confirmed tuberculosis. Radiologic imaging studies revealed findings consistent with pulmonary tuberculosis in 70 (88.61%) through plain radiograph. Forty-seven underwent HRCT and 46 of these (97.8%) had findings suggestive of PTB. Overall, 61 patients (77%) subsequently required oxygen supplementation. The in-hospital mortality within the study population was 36.7% (29/79) in contrast to the general COVID patients admitted in the same period which revealed significantly less fatality at 17.5% (35/200). The length of hospital stay was found to be 21.1 days ± 14.75 days across all study patients, and with median of 20 days for surviving patients. TB treatment outcomes were tracked in the 50 surviving COVID-19 patients where cure was declared in 8/50 (16%) while 22/50 (44%) successfully completed their six-month treatment regimen.
Conclusions
This study of COVID-TB provides an initial evaluation of the potential association between active TB infection and COVID-19 severity and mortality. The data generated from this study may be a starting point to assess the interaction of these two diseases. Furthermore, bidirectional screening may be recommended even at hospitals’ triage areas since both diseases may have similar presentations.
Keywords: tuberculosis, COVID-19, co-infection
INTRODUCTION
In the fourth quarter of 2019, a novel pathogen named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has been reported in hospitalized patients admitted in Wuhan, China.1 Since then, cases have been reported in other cities of China and eventually majority of countries in the world.2 The World Health Organization (WHO) declared this disease as a pandemic in March 12, 2020.3 With the SARS-CoV outbreak in 2002 and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) outbreak in 2012, this coronavirus disease 2019 (COVID-19) has been the third outbreak in the past two decades that has put the WHO and government health institutions on high alert.1,2 In contrast, tuberculosis (TB) is already considered an infectious disease that has been inflicting scourge across the globe with an estimated 10 million cases annually with mortality of 1.2 million in 2018.4 Around one-fourth of world population is estimated to have latent TB infection (LTBI).4 Patients infected with SARS-CoV-2 were usually hospitalized for cough, fever and chest discomfort, which are the same symptoms that can present with patient infected with other related viral infections (e.g., influenza, SARS, Middle East Respiratory Syndrome [MERS]) and TB).5 At this time, experience with concomitant TB and COVID-19 (COVID TB) is still limited.6
Knowledge on the risk factors associated with COVID-19 are still evolving especially on how TB may interact with this disease. A study conducted in China showed the plausible contribution of M. tuberculosis (MTB), either as an infection or disease, as a risk factor for severe pneumonia due to COVID-19.7 Proven causal association between the two diseases has not been rigorously established. Another concern with this co-infection is the mortality associated to it. A published article showed COVID TB has a mortality rate of 12.3%, which is higher than the mortality rate of isolated COVID-19 cases.7 However, it was also documented that majority of those with co-infection have also proven risk factors for mortality (>60 years old, comorbidities like diabetes mellitus, hypertension, heart disease, chronic pulmonary disease).8 Another descriptive study involving 49 cases of COVID-19 with intercurrent and previously treated tuberculosis was published in May 2020.6 No strong conclusion was drawn from this study because of the small sample size. However, there were signals identified that could characterize the interactions of both diseases.
The Philippines has a high disease burden for TB even before the COVID-19 pandemic started. According to recent reports, there are approximately 1 million active cases all over the country and the Philippines ranks fourth in the greatest number of cases globally.4,9 Similar to other countries, there is also scarcity of data regarding COVID-19 and TB co-infection in the country.
The Philippine General Hospital (PGH) is a tertiary government hospital in the heart of Metro Manila which was designated as a COVID referral center last February 2020 by the country’s Department of Health (DOH) as part of the country’s pandemic response. Admitted COVID cases in this facility were mainly moderate to critical in terms of disease severity. The study will describe the cohort of patients admitted with diagnosis of COVID-19 and TB (COVID-TB) during the period of March to September 2020. The data may aid in the analyses of potential interaction of the two diseases and compare the outcomes of patients with concurrent infection and those with isolated COVID-19 disease. Determining the factors affecting the outcomes of patients afflicted with both conditions would provide a possible framework in delivering timely diagnosis, management, and prognostication of these special set of patients.
OBJECTIVES
The clinical profile of patients who were admitted for COVID-19 with concurrent diagnosis of TB infection were described. The patient profiles were based on age, sex, occupation, onset of symptoms, comorbidities, status of TB infection, site of TB infection, TB radiologic findings, TB treatment outcomes, COVID-19 severity, COVID-19 symptoms, COVID-19 radiologic findings, and COVID-19 treatment and outcome. Comparison of the clinical outcomes was also done in patients who were admitted for COVID-19 with concurrent diagnosis of TB infection, versus the general admitted COVID-19 patients in the same institution. Relevant outcomes were reported on the patient which included admission to an intensive care unit (ICU), length of hospital stay, and mortality rate.
METHODS
Study Participants
We included all adult patients admitted for RT-PCR-confirmed COVID-19 infection with deemed concurrent active tuberculosis disease at PGH from March 12, 2020 to September 30, 2020.
Study Design
The study employed a descriptive retrospective cohort design. Patient demographics, clinical presentations, treatment modalities and clinical outcomes were extracted from the PGH electronic medical records [e.g., Registry of Admission and Discharges (RADISH)] and paper medical records. The data gathered were recorded using a standard data collection form and then consolidated in Microsoft Excel worksheet. The results of the imaging studies which included computed tomography scans and plain radiographs were collected from PGH Medical Record System (OpenMRS).
Our study also compared the outcomes to a published study done by Salamat, et al.10 Their study included the first 200 adult patients aged 19 years old and above, with RT-PCR-confirmed COVID-19, admitted at UP-PGH from March 12, 2020, to April 28, 2020. They employed a descriptive retrospective cohort design.
The study was reviewed and approved by the University of the Philippines Manila Research Ethics Board (UPMREB). Since this is a descriptive retrospective study using anonymous patient data, informed consent was not necessary.
Study Variables and Outcomes
The variables that determine the demographics of the patients were collected and included age, sex, employment status, classification of TB diagnosis, site of TB infection, comorbidities, presenting signs and symptoms during hospital admission, COVID severity, diagnostic imaging study, and possible Solidarity trial enrolment. Clinical events and outcomes identified included admission in the ICU, need for mechanical ventilation, length of hospital stay, mortality, and outcome of TB treatment.
TB case definitions and outcomes were based on the National TB Program Manual of Procedures from the Department of Health.11 Whereas the severity of COVID-19 illness was classified based on the Philippine Society for Microbiology and Infectious Diseases (PSMID) guidelines.12 The following operational definitions were used in our study:
Bacteriologically confirmed (BC) TB case: A TB patient from whom a biological specimen is positive by smear microscopy, culture or rapid diagnostic tests (such as Xpert MTB/Rif).
Clinically diagnosed (CD) TB case: A PTB patient who does not fulfill the criteria for bacteriological confirmation but has been diagnosed with active TB by a clinician or other medical practitioner who has decided to give the patient a full course of TB treatment. This definition includes cases diagnosed on the basis of CXR abnormalities or suggestive histology, and extra-pulmonary cases without laboratory confirmation.
Completed (outcome): A patient who completes treatment without evidence of failure but with no record to show that sputum smear or culture results in the last month of treatment and on at least one previous occasion were negative, either because tests were not done or because results are unavailable.
Concurrent active TB: These cases may be bacteriologically confirmed (BC) TB or clinically diagnosed (CD) TB cases. The clinician considers that this case presented with signs and symptoms that can be attributed to TB and not just COVID-19. As such, this case will be managed as a concurrent TB case.
Cured (outcome): A patient with bacteriologically confirmed TB at the beginning of treatment and who was smear- or culture-negative in the last month of treatment and on at least one previous occasion in the continuation phase.
Lost to follow-up (outcome): A patient whose treatment was interrupted for two (2) consecutive months or more. Their outcome cannot be ascertained after this particular period.
Multidrug resistant TB: Multidrug-resistant TB (MDR TB) is caused by TB bacteria that are resistant to at least isoniazid and rifampin, the two most potent TB drugs. It can be readily identified through isolation of bacilli resistant to rifampicin as reported in the Xpert MTBRif report.
Pulmonary TB (PTB): Refers to a case of tuberculosis involving the lung parenchyma.
Severity of COVID-19 illness was classified as follows:
mild - patients with symptoms consistent with COVID-19 but without evidence of pneumonia
moderate - patients with clinical and radiographic evidence of pneumonia, but not requiring oxygen
severe - patients with clinical and radiographic evidence of pneumonia with oxygen saturation <92% on room air, and requiring oxygen support
critical - patients with COVID-19 with ARDS, septic shock, requiring mechanical ventilation, admission to the intensive care unit (ICU) or both.
Solidarity trial: an on-going trial in 2020 from the World Health Organization expert groups that involved the use of four repurposed antiviral drugs — remdesivir, hydroxychloroquine, lopinavir, and interferon beta-1a — in patients hospitalized with coronavirus disease 2019 (COVID-19).
Statistical Analysis
Descriptive statistics was used to summarize the demographic and clinical characteristics of the patients. Frequency and proportion were used for categorical variables, median and inter quartile range for non-normally distributed continuous variables, and mean and SD for normally distributed continuous variables.
RESULTS
A total of 79 patients were included in the analysis out of the 1108 admitted patients who were initially screened. There were 135 patients who were diagnosed with tuberculosis but 56 patients were excluded due to non-active disease status as assessed by the attending clinicians (Figure 1).
Figure 1.
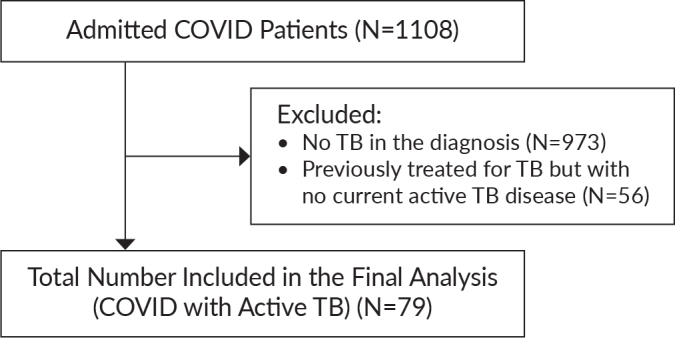
Flow of patient screening and analysis.
Characteristics of the Study Cohort
Table 1 shows the demographics of patients admitted for COVID-19 and TB co-infection. Of the 79 included patients, majority were males (70.9%) with a median age of 54 (IQR 42 to 64) years. Three who were currently employed were identified as healthcare workers (1 midwife, 1 nurse and 1 radiologic technician). In terms of TB, 75 (94.9%) patients were identified to have mainly pulmonary tuberculosis, 3 (3.8%) with disseminated TB, and 1 (1.27%) with gastrointestinal TB. Multidrug resistant TB was identified in three patients while the rest were managed as susceptible type necessitating the use of standard first-line treatment regimen.
Table 1.
General Characteristics of Admitted COVID-19 Patients with TB
| Parameters | Present study (n=79) Number (%) |
|---|---|
| Age | 54 (IQR: 42 to 64) |
| ≥60 years old | 31 (39.24) |
| <60 years old | 48 (60.76) |
|
| |
| Sex | |
| Male | 56 (70.89) |
| Female | 23 (29.11) |
|
| |
| PTB active | |
| Clinically Diagnosed (CD) | 23 (29.11) |
| Bacteriologically Confirmed (BC) | 56 (70.89) |
|
| |
| Diagnostics for BC (n=56) | |
| PCR | 24 (42.86) |
| DSSM | 21 (37.5) |
| MTB Rif | 10 (17.86) |
| Culture | 1 (1.79) |
|
| |
| Employment status | |
| Employed | 28 (35.44) |
| Self employed | 28 (35.44) |
| Unemployed | 23 (29.11) |
|
| |
| Site of TB | |
| Pulmonary TB | 75 (94.9) |
| Disseminated TB | 3 (3.8) |
| Gastrointestinal TB | 1 (1.27) |
|
| |
| Multidrug resistant PTB | 3 (3.8) |
|
| |
| COVID Severity | |
| Mild | 5 (6.33) |
| Moderate | 30 (37.97) |
| Severe | 8 (10.13) |
| Critical | 36 (45.57) |
|
| |
| Comorbidities | |
| HPN | 13 (16.46) |
| DM | 11 (13.92) |
| Metabolic syndrome | 10 (12.66) |
| HF | 9 (11.39) |
| CKD | 7 (8.86) |
| COPD | 5 (6.33) |
| CVD infarct | 5 (6.33) |
| HIV clinical stage 4 | 4 (5.06) |
| Others | 34 (43.04) |
|
| |
| Primary Symptoms | |
| Cough | 52 (65.82) |
| Fever | 46 (58.23) |
| DOB | 40 (50.63) |
| Weight loss | 11 (13.92) |
| Malaise | 8 (10.13) |
| Decreased sensorium | 4 (5.06) |
| Diarrhea | 4 (5.06) |
| Abdominal pain | 3 (3.80) |
| Others | 16 (20.25) |
|
| |
| TB findings on CXR | 70 (88.61) |
|
| |
| TB findings on HRCT (n=47) | 46 (97.87) |
|
| |
| COVID Finding on CXR | 73 (92.41) |
|
| |
| COVID finding on HRCT (n=47) | 45 (95.74) |
Majority of patients had at least one co-morbid illness, with hypertension (13 or 16.5%), diabetes mellitus (11 or 13.9%), and heart failure (9 or 11.4%) as the most common. Metabolic syndrome characterized by a combination of hypertension, impaired fasting glucose or diabetes mellitus, dyslipidemia, and obesity was also seen in 10 (12.66%) patients.
Signs, Symptoms and Severity
Respiratory symptoms were the predominant presenting complaints during hospital admission. Cough and dyspnea were present in 52 (65.8%) and 40 (50.6%) patients, respectively. Constitutional symptoms such as fever 46 (58.2%), anorexia 11 (13.9%), and malaise 8 (10.1%) were also frequently identified. Decreased sensorium though sporadically seen, was noted to be associated with critical illness.
In terms of COVID severity, only 5 (6.3%) were classified as mild infection and 30 (37.9%) as moderate, respectively. Majority of the patients were classified as severe 8 (10.1%) and critical 36 (45.57%).
Diagnostic Tests
All patients were RT-PCR confirmed COVID-19 infection cases and in 56 (70.9%), TB had bacteriologic confirmation. This consisted of the following: Polymerase Chain Reaction or PCR in 24 (42.9%) patients and direct sputum smear microscopy (DSSM or AFB smear) in 21 (37.5%) patients. Radiologic finding consistent with pulmonary tuberculosis was seen in 70 (88.61%) through plain radiograph and 46 (46/47 or 97.87%) through high resolution CT scan among those who underwent this procedure. In terms of COVID-19 pneumonia, findings suggestive of this entity was seen thru plain radiograph and high-resolution CT scan were in 73 (92.4%) and 45 (45/47 or 95.7%) of the patients, respectively. These mainly consisted of reticulonodular infiltrates or ground-glass opacifications especially at the peripheral lung fields.
Interventions
Of the 79 study patients, 40 (50.63%) received standard of care which included the needed oxygen support among hypoxemic patients and antibiotics for pneumonia as well as appropriate management of their co-morbid conditions. During this period, since the Solidarity Trial was on-going, 35 patients who gave consent were given certain investigational medications at the time of hospitalization: remdesivir in 25 (71.43%), hydrochloroquine in 7 (20%), lopinavir/ritonavir in 2 (5.71%), and interferon beta in 1 (2.86%) patient.
Overall, 77.2% (n=61) patients subsequently required oxygen supplementation. This consisted of regular nasal cannula or face mask in 23% (14/61), while 21% (13/61) required high flow nasal cannula. We also observed that in 43% (34/79) of patients, invasive mechanical ventilation was eventually necessary as a form of respiratory support. ICU admission was quite common and was observed in 46.8% (37/79) among patients with COVID-TB.
Outcomes
The in-hospital mortality within the study population was 36.7% (29/79) in contrast to the general COVID patients admitted in our institution during the same study period which revealed less fatality with 17.5% (35/200). Mortality rates were 1.3% (1/79) for mild, 2.5% (2/79) for moderate and 32.9% (26/79) for severe to critical illness. The single mortality in the mild classification succumbed due to sudden fatal arrhythmia. Of the patients with moderate illness, both patients had disease progression. The most common causes of mortality were identified to be Acute Respiratory Distress Syndrome (ARDS), septic and cardiogenic shock, and nosocomial pneumonia and multiorgan failure.
The length of hospital stay was found to be 21.1 days ± 14.75 days across all study patients, and with a median of 20 days for surviving patients.
TB treatment outcomes were able to be tracked in the 50 surviving COVID-19 patients who were all managed under DOTS setting. Cure was declared in eight (16%) of the surviving patients while 22 (44%) completed their six-month treatment regimen. Ten (20%) was lost to follow up and another 10 (20%) expired during the treatment duration. Six of those who died during the treatment were noted to have late-stage malignancy and two had HIV-AIDS.
DISCUSSION
Our present study showed that 79 patients were admitted in our institution with COVID-TB from March 12, 2020 to September 30, 2020. Majority of these were males (70.9%) with a median age of 54 (IQR 42 to 64) years. Majority (94.9%) of them had primary pulmonary TB with bacteriologic confirmation in 70.9% of all cases. Cough and dyspnea were the most common presenting symptoms while hypertension, diabetes, and heart failure were the most common co-morbid conditions. Majority of the patients were classified as COVID severe 8 (10.1%) and critical 36 (45.57%) with more than 77% of cases requiring oxygen support.
The interrelationship between COVID and TB has been a subject of recent studies realizing that these disease entities may have very similar clinical and radiologic presentations.6,8,13 In addition, it has been acknowledged that TB, being the number one infectious disease killer worldwide, was overtaken by COVID-19 in terms of healthcare system attention as the world grappled with this unknown threat.4-7,14
Inoue and colleagues hypothesized that past epidemics of Mycobacterium tuberculosis may confer some protective effect against COVID-19 severity and mortality and this may explain the differences seen among afflicted Asian countries, where TB is a huge burden, and western nations, where TB is relatively well-controlled.15 This concept regarding the mycobacterium’s ability to stimulate a trained immunity at the cellular level, attracted some attention especially after modelling interventions that took into consideration the TB burdens and BCG vaccination coverage at the individual country levels and subsequent negative impacts attributable to COVID-19. An inverse relationship was demonstrated between these factors. A similar concept was studied at the level of hospitals in Turkey where the authors demonstrated that healthcare workers who had continuous exposure in their TB units and had BCG vaccination, when admitted for COVID-19, had lower mortality rates when compared to the general population.16 Again, the potential protective effects of the BCG vaccine as well as the additive stimulus coming from confirmed TB exposure had been implicated to confer a stronger innate immunity that could explain the observed differences in the population studied. However, in both instances, the authors recommended more longitudinal studies with a larger sample size to validate these findings.15,16
These observations on a potential protective effect at the population level, may not be in consonance as to what is being demonstrated by some clinical reviews and studies. In general, there seems to be a poorer outcome among COVID-19 patients who are co-infected with tuberculosis.
In an observational case control study in China, both latent and active TB was associated with more symptom development and a more rapid and severe course for COVID-19.7 In fact, the authors suggested for TB to be considered as an independent risk factor for severe COVID just like diabetes or hypertension. In a propensity score matched sample of COVID-19 patients without and with tuberculosis, using COVID-19 surveillance data in the Philippines, Sy and colleagues conducted a longitudinal cohort analysis of matched COVID-19 patients as of May 17, 2020, and following them until June 15, 2020.17 They were able to demonstrate the following trends: the risk of death in COVID-19 patients with tuberculosis was 2.17 times higher than in those without (95% CI: 1.40-3.37), the risk of recovery in COVID-19 patients with tuberculosis was 25% lower than in those without (RR=0.75, 95% CI 0.63-0.91). Similarly, time-to-death was significantly shorter (p=0.0031) and time-to-recovery was significantly longer in patients with tuberculosis (p=0.0046). In a recent meta-analysis that included a total of 36 studies with a combined total of 89 patients, the pooled ORs of death or severe disease in the COVID-TB group and the non-TB group were shown to be 2.21 (95% CI: 1.80,2.70) and 2.77 (95% CI: 1.33, 5.74) (P<0.01), respectively.18
When a person suffers from a previous or a current active pulmonary illness, the respiratory structure and physiology could be impaired and their resistance to other microbes may be inferior so that this milieu may be conducive to the development of more severe infections.14,19-21 This may account for the poor clinical outcomes including mortality that is generally seen. In addition, the strain on the healthcare system caused by the COVID-19 pandemic, may cause delays in access to care, screening, diagnostic testing, and triage of TB patients (suspected or confirmed) who may already have COVID symptoms.14 As such, they may come already late in their course of illness. The exact mechanisms on how COVID and TB may interact at the individual health level or a country may still require further investigations to be more properly elucidated and characterized.
Our study revealed that affected individuals with COVID-TB were mainly males with a median age of 54 years with co-morbidities present with the lungs as the primary affected organ by TB. This pattern is also like the overview of case reports and case series done by Song and colleagues18 where active TB was isolated in 88.76% of their cases with around 80% of patients being males. In addition, the most common comorbid conditions in their study were diabetes, hypertension, and HIV. A similar trend was also demonstrated from the first-ever global cohort of current or former TB patients (post-TB treatment sequelae) with COVID-19, recruited by the Global Tuberculosis Network (GTN) in eight countries and three continents.7
Cardiovascular, metabolic, and respiratory comorbidities were relatively more common among COVID-19 patients especially among those with a more severe clinical course.7,8,10,13 Diabetes and hypertension were isolated by earlier studies as risk factors in the development of severe to critical forms of COVID-19 and were recommended to be part of the routine screening tests.5,10 Diabetes and any factor which may contribute to a poor immune system of an individual, like HIV, may also pose significant risks in the pathogenesis of TB.4 It is therefore expected that individuals with both COVID and TB may harbor such comorbid conditions.
Our study showed that cough and dyspnea were present in 52 (65.8%) and 40 (50.6%) patients, respectively. Constitutional symptoms such as fever, anorexia, and malaise were also frequently reported. The same observations were described by similar studies.7,18,21 Such a trend may lead to some challenges in terms of the general approach to these patients. With majority of healthcare institutions currently more at heightened alert to address COVID-19, a concomitant TB disease may easily be overlooked in these instances. TB should be considered especially in a high burden setting.14,21 This might have an impact on the diagnostic tests that should be available at points-of-care and access to these examinations, triaging efforts, management of both conditions which should include potential drug-drug interactions, proper interpretation of imaging studies, and monitoring and follow up care of these individuals which might include networking and referral to appropriate institutions like TB-DOTS centers.
COVID-19 has certainly placed a significant strain on various countries’ TB control efforts.14,21-23 Resources may have been diverted from TB control programs to handle the COVID-19 pandemic. The Global Tuberculosis Network (GTN) reported that the COVID-19 pandemic affected TB services in 33 TB centers from 16 countries in the first four months of 2020. TB disease decreased from 32,898 (mean 2,742 ± 177 s.d./month) in 2019 to 16,396 (1,366 ± 308/month; p<0.0001) in 2020 with a sudden decline in March 2020, concomitantly with the commencement of lockdown in majority of the countries.23 TB notification was reduced by approximately 25% in three countries with the highest burden of TB: India, Philippines, and Indonesia during a six month period from January to June 2020 as compared to the same period in the year 2019.14 Quarantine measures and implementation of community lockdowns may have contributed to this worrisome state.
Our study revealed that imaging studies consistent with pulmonary tuberculosis was seen in 70 (88.61%) through plain radiograph and 46 (46/47 or 97.87%) through high resolution CT scan among the 47 who underwent this procedure. Chest CT was done with the clinical consideration of pulmonary embolism or in instances when patient’s clinical condition like persistent dyspnea and/or hypoxemia, warranted further clinical investigation. Chest xray findings mainly consisted of cavitary, fibrotic, and bronchiectatic changes. In terms of COVID-19 pneumonia, findings suggestive of this entity was seen thru plain radiograph and high-resolution CT scan in 73 (92.41%) and 45 (45/47 or 95.7%) of the patients, respectively. These mainly consisted of diffuse reticulonodular infiltrates or ground-glass opacifications especially visualized at the peripheral lung fields.
In the case series done by Song and colleagues, COVID-TB CT findings included bilateral lesions, cavities, infiltrates, ground-glass opacity, nodules, pleural effusion, and fibrosis.16 They also demonstrated that non-survivors were more likely to have bilateral lesions and tree-in-bud pattern. Similar findings of infiltrates and cavitary lesions in 20-33% of cases, were also established by the Global TB Network.6 A bilateral ground-glass opacity picture on HRCT was also documented in 47.7% of cases.
Like those of similar studies, our investigation revealed that no finding on imaging studies may reliably distinguish between the two diseases. In our study, we considered TB among symptomatic patients with the presence of cavitary and bronchiectatic changes localized primarily in the upper lung zones. This prompted bacteriologic examination and confirmation was subsequently established in more than 70% of cases where sputum TB PCR and DSSM were positive in more than 80%. Appropriate treatment was promptly started in these instances and subsequent referrals to TB-DOTS centers was employed among those who were discharged. Cure was declared in 8 (16%) of the surviving patients while 22 (44%) completed their six-month treatment regimen.
Although only 79 patients were included in our COVID-TB research, our study revealed worse outcomes in terms of ICU stay, proportion of patients needing invasive ventilation, and case fatality rate as well as length of hospital stay among non-survivors compared to the study of Salamat and colleagues10 among 200 COVID-19 patients admitted in our institution (Table 2). The same dismal outcomes were also demonstrated by published studies on COVID-TB.7,17,18,20 We attributed this main trend to the resulting worse lung conditions that may be attributed to the dual effect of both diseases as mentioned earlier.
Table 2.
Outcomes of Patients Admitted for COVID-19 and TB Co-infection
| Present study (n=79) Frequency (%); Mean ± SD; Median (IQR) | Salamat MS, et al.10 (n=200) | |
|---|---|---|
| ICU stay | 37 (46.84) | 58 (29) |
| Invasive Ventilation | 34 (43.04) | 38 (19) |
| Case Fatality | 29 (36.71) | 35 (17.5) |
| Hospital Stay (days) | 21.1 ± 14.75 | 16.88 ± 23.59 |
| Survivors | 20 (13 to 28) | 26 (16 to 38) |
| Non survivors | 23.5 (14 to 30) | 7 (3 to 12) |
| Outcome of TB (n=50) | ||
| Completed/Cured | 30 (60) | |
| Expired/Lost to follow up | 20 (40) |
More than three fourths of our patients required oxygen supplementation. We also observed that in 43% (34/79) of patients, invasive mechanical ventilation was eventually necessary as a form of respiratory support. These may imply a sicker cohort of patients compared to those without concomitant TB. This finding is consistent with past observations implying that TB-afflicted lungs are more damaged and this impairment may result in being more prone to subsequent insults from viruses and other organisms and may make them more susceptible to the development of lung injury.7,14,20 In addition, past studies showed that TB was associated with a more severe COVID-19 disease and course.7,17,18,20 In our cohort, the most commonly documented causes of mortality were ARDS, septic and cardiogenic shock, nosocomial pneumonia, and multiorgan failure. There may have been other realities during this period which exacerbated this scenario. These may include delayed health-seeking behavior, inability to access healthcare because of community lockdown conditions, and constrained mobilities of the general population.
COVID-19 and TB share very similar clinical pictures including imaging studies. These may pose a challenge in clinching an accurate diagnosis on screening and subsequent proper management. Since TB is associated with a more rapid and severe COVID-19 course, diagnosis on admission of COVID-TB becomes crucial. Bidirectional screening may be recommended especially in a high prevalent setting like the Philippines. Lessons learned in handling TB may be applied at a wider scale in addressing COVID-19 being cautious however that the efforts gained in TB control will not suffer in the process.22 These may include proper laboratory diagnostic allocation including rapid tests, intensified case finding, patient-centered approaches like home-based testing and utilization of family members as treatment partners and support group, and appropriate and wider use of telehealth facilities and digital adherence technologies.14,22 As our study also illustrated, the continuity of patient care from hospital to discharge back to their community is quite essential in ensuring successful treatment.
Investigational agents, as recommended by the Solidarity Trial, were given in less than 50% of our cases with remdesivir being the most frequent to be administered among those who consented. Also, during the study period, knowledge on the potential benefits of systemic steroids was still evolving. As such, this was not routinely given to all severe-critically ill patients. Almost all our patients received broad spectrum antibiotics consisting mainly of cephalosporins and piperacillin-tazobactam. A similar trend is seen among the COVID-19 patients without TB may be seen.10 This may reflect the changing COVID-19 management recommendations which may be prevalent during this period.
CONCLUSION
Seventy-nine patients were admitted in our institution with COVID-TB from March 12, 2020 to September 30, 2020. Majority of these were males (70.9%) with a median age of 54 (IQR 42 to 64) years. Majority (94.9%) of them had primary pulmonary TB with bacteriologic confirmation in 70.9% of all cases. Cough and dyspnea were the most common presenting symptoms while hypertension, diabetes, and heart failure were the most common co-morbid conditions. Our study revealed worse outcomes in terms of ICU stay, proportion of patients needing invasive ventilation, and case fatality rate as well as length of hospital stay among non-survivors. These findings support published data that TB may be associated with worse outcomes among COVID-19 patients.
This is one of few local studies which focused on the clinical profile and outcomes of patient with COVID-TB in the Philippines. The data generated from this study may be crucial to ensure specific and successful preventive and treatment strategies in the approach to such a patient group.
Recommendations
In the future, it is important that validation studies through larger study population should be made to establish the relationship of COVID-19 and TB that has been uncovered by this study. A multivariate analysis considering TB, comorbidities, and COVID may be performed to further characterize the interaction of these conditions. It is also recommended that studies involving patients who already finished the six-month treatment regimen or those who were cured, with current COVID-19 infection be evaluated and studied further to establish the interaction of both diseases. The potential long-term impacts of TB and COVID-19 on the lungs and the individual’s overall well-being is a fertile area for investigation.
Disclaimer
Views expressed by the authors in the submitted article are their own and not an official position of the institution.
Statement of Authorship
All authors certified fulfillment of ICMJE authorship criteria.
Author Disclosure
All authors declared no conflicts of interest.
REFERENCES
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020. Feb;382(8):727-33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China --- key questions for impact assessment. N Engl J Med. 2020. Feb;382(8):692-4. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 3.Sohrabi C, Alsafi Z, O'neill N, Khan M, Kerwan A, Al-Jabir A, et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020. Apr;76:71-6. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Global Tuberculosis Report 2022 [Internet]. 2022. [cited 2022 Oct]. Available from: https://www.who.int/tb/publications/global_report/en/.
- 5.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Intern Med. 2020. May;35(5):1545-9. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tadolini M, Codecasa LR, García-García JM, Blanc FX, Borisov S, Alffenaar JW, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. 2020. Jul;56(1): 2001398. doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Wang Y, Fleming J, Yu Y, Gu Y, Liu C, et al. Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. MedRxiv. 2020. Mar 16:2020-03. doi: 10.1101/2020.03.10.20033795 [DOI] [Google Scholar]
- 8.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020. Apr;382(18):1708-20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiler G, World Health Organization . It’s Time to End TB in the Philippines. [Internet] 2019. March 19 [cited 2021 Mar]. Available from: https://www.who.int/philippines/news/commentaries/detail/it-s-time-to-end-tb-in-the-philippines
- 10.Salamat MSS, Malundo AFG, Abad CLR, Sandejas JCM, Planta JEG, Poblete JB, et al. Characteristics and factors associated with mortality of 200 COVID-19 patients at a Philippine COVID-19 tertiary referral center. Acta Med Philipp. 2021. Apr;55(2):173-82. doi: 10.47895/amp.v55i2.2845 [DOI] [Google Scholar]
- 11.Department of Health . National Tuberculosis Program. Manual of Procedures 6th edition [Internet]. 2020. [cited 2021 Jun]. Available from: https://ntp.doh.gov.ph
- 12.Philippine Society for Microbiology and Infectious Diseases, Philippine College of Chest Physicians, Philippine College of Physicians, Philippine Rheumatology Association, Philippine College of Hematology and Transfusion Medicine . Interim guidance on the clinical management of adult patients with suspected or confirmed COVID-19 infection [Internet]. [cited 2020 Dec]. Available from: http://philchest.org/xp/wp-content/uploads/2020/07/Final-PCPPSMID-PCCP-COVID-19-Guidelines-20July2020_1595213265.pdf
- 13.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020. Aug;81(2):e16-25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kant S, Tyagi R. The impact of COVID-19 on tuberculosis: challenges and opportunities. Ther Adv Infect Dis. 2021. Jun;8: 20499361211016973. doi: 10.1177/20499361211016973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue K, Kashima S. Association of the past epidemic of Mycobacterium tuberculosis with mortality and incidence of COVID-19. PLoS One. 2021. Jun 18;16(6):e0253169. doi: 10.1371/journal.pone.0253169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torun S, Ozkaya S, Şen N, Kanat F, Karaman I, Yosunkaya S, et al. The relationship between covid-19 severity and Bacillus Calmette-Guérin (BCG)/Mycobacterium tuberculosis exposure history in healthcare workers: a multi-center study. Pathog Glob Health. 2021. Sep;115(6):405-11. doi: 10.1080/20477724.2021.1927605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sy KTL, Haw NJL, Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dis (Lond). 2020. Nov-Dec;52(12):902-7. doi: 10.1080/23744235.2020.1806353. [DOI] [PubMed] [Google Scholar]
- 18.Song WM, Zhao JY, Zhang QY, Liu SQ, Zhu XH, An QQ, et al. COVID-19 and tuberculosis coinfection: an overview of case reports/case series and meta-analysis. Front Med (Lausanne) 2021. Aug;8:657006. doi: 10.3389/fmed.2021.657006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Z, Chen A, Hou W, Graham JM, Li H, Richman PS, et al. Prediction model and risk scores of ICU admission and mortality in COVID-19. PLoS One. 2020. Jul;15(7):e0236618. doi: 10.1371/journal.pone.0236618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Y, Liu M, Chen Y, Shi S, Geng J, Tian J. Association between tuberculosis and COVID‐19 severity and mortality: a rapid systematic review and meta‐analysis. J Med Virol. 2021. Jan;93(1):194-6. doi: 10.1002/jmv.26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Togun T, Kampmann B, Stoker NG, Lipman M. Anticipating the impact of the COVID-19 pandemic on TB patients and TB control programmes. Ann Clin Microbiol Antimicrob. 2020. May;19(1):21. doi: 10.1186/s12941-020-00363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrinivasan R, Rane S, Pai M. India’s syndemic of tuberculosis and COVID-19. BMJ Glob Health. 2020. Nov;5(11):e003979. doi: 10.1136/bmjgh-2020-003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Migliori GB, Thong PM, Alffenaar JW, Denholm J, Tadolini M, Alyaquobi F, et al. Gauging the impact of the COVID-19 pandemic on tuberculosis services: a global study. Eur Respir J. 2021. Nov;58(5):2101786. doi: 10.1183/13993003.01786-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]


