Abstract
Ocular motility has been linked to Sports Concussion Assessment Tool 5 scores. However, the link between ocular motility changes and assessment result changes remains unclear. Hence, we investigated that potential link in patients with sports-related concussions. We retrospectively included participants aged≥18 years who were diagnosed with a sports-related concussion. They underwent smooth pursuit eye movement assessment for allocation to the good improvement (rate of fundamental frequency≥15%) or minor improvement (<15%) groups. Sports Concussion Assessment Tool 5 scores were determined at baseline and two weeks later, and score changes were compared between the groups. Thirteen men (mean±standard deviation age: 20.6±5.0 years) were included: eight (19.0±4.5 years) in the good improvement group and five (20.6±5.7 years) in the minor improvement group. Symptom number (median=2.0 vs. 0.0), symptom severity (median=22.0 vs. 3.0), single-leg stance (median=4.0 vs. 0.5), tandem stance (median=1.0 vs. 0.0), and total errors (median=5.0 vs. 0.5) were worse (all p<0.05) in the minor improvement group. Smooth pursuit eye movement improvements measured using eye-tracking technology was linked to symptom recovery in patients with sports-related concussions. Therefore, ocular motility may be an objective indicator of sports-related concussions. Future studies with more patients are needed to confirm these findings.
Key words: eye tracking, return to play, sports concussion assessment tool, sports-related concussion, ocular motility
Introduction
Sports-related concussions (SRCs) are among one of the most contentious topics in clinical practice owing to their non-specific symptoms and the lack of an established objective diagnostic method 1 . Although computed tomography and magnetic resonance imaging are essential for diagnosing several types of brain injuries, objective diagnoses of SRCs cannot be achieved by imaging techniques, as this condition is often characterized by functional rather than structural anomalies 2 . Therefore, in daily clinical practice, SRC is diagnosed based on various symptoms reported by athletes who suffer from head injuries.
The Sport Concussion Assessment Tool (SCAT) is the standard diagnostic method for diagnosing SRC. It was first introduced at the 2 nd International Conference on Concussion in Sports in 2014 3 . Since its introduction, the tool has undergone multiple revisions, and the 5 th edition (SCAT5) is now the most widely used 4 . Evaluating SRC symptoms using the SCAT5 involves a symptom evaluation (by medical interview), cognitive screening (measuring orientation, immediate memory, and concentration and comprises digits and days of the week backward tasks), neurological screening (a balance test), and delayed recall. However, the SCAT5 scores should not be used as the sole basis for diagnosing concussions, as an indicator of post-SRC recovery, or to determine whether an athlete can return to play. Moreover, since the SCAT5 includes assessments based on subjective symptoms, score improvements may result from inaccurate answers 5 6 7 . Further, although SCAT5 is the recommended standard tool for assessing SRC, its subjective indicators are inconsistent with the neurophysiological recovery process 8 9 10 . In other words, a SCAT5 assessment should be complemented with an objective evaluation of the SRC symptoms.
While the cornerstone of SRC treatment is adequate physical and cognitive rest, the risk of underreporting symptoms in athletes who want to accelerate their recovery may lead to premature return to play. Therefore, the SRC recovery process should be further evaluated using objective indicators.
Ocular motility disorders, one objective SRC symptom, has been reported in 40–90% of patients with an SRC 11 12 13 . Furthermore, ocular motility has been associated with SRC symptoms, making it a potential SRC screening tool 14 . However, ocular motility measurements are not included in the SCAT5, and no studies have investigated the association between ocular motility and symptom recovery in patients with an SRC. If a relationship exists between SRC symptoms and the objective assessment of ocular motility disorders in these patients, then ocular motility evaluations could be valuable for assessing the recovery process for SRC. Furthermore, precise ocular motility evaluation using an eye tracking system is important.
Therefore, the present study aimed to investigate the potential link between ocular motility improvements and symptom recovery using the SCAT5 in patients with SRC.
Materials and Methods
Ethical considerations
The study procedures adhered to the tenets of the Declaration of Helsinki (1964) and its later amendments. The study was approved by our institute’s ethics committee (protocol No. 3628, approved on August 28, 2017). The study was approved by the appropriate Ethics Review Board. The study procedure and objectives were explained to the participants who provided informed consent before the study’s commencement.
Study design and participants
This study involved a retrospective analysis of a case series. Sixty-five participants≥18 years old with an SRC diagnosis at our institution’s Sports Concussion Clinic were recruited between April 2018 and March 2022. Of the 65 participants, 36 consented after hearing the study’s purpose and details. Finally, 13 participants were included in the analysis after excluding 20 patients in early remission, two dropouts, and one patient who did not complete the assessment ( Fig. 1 ).
Fig. 1.

Flowchart of participant recruitment process and group allocation. SCAT5, Sports Concussion Assessment Tool 5; SRC, sports-related concussion.
Data collection
Basic information
The following basic attributes were collected: age, sex, sport, role/position, and the number of days elapsed since injury. SRC symptoms were evaluated using the SCAT5 scores and ocular motility disorder assessment at the initial visit and two weeks later.
Eye tracking
To assess ocular motility disorders 15 , smooth pursuit eye movement (SPM), a type of eye movement in which the eyes remain fixated on a moving object, was measured using an optical device (infrared scleral reflection method). The distance from the target object was fixed at 1 m. In the SPM assessment, the participants were asked to gaze at a visual target rotating at a constant velocity of 3 s/cycle (30°/s), and their eye-movement waveforms in the horizontal and vertical directions were measured. Fourier transform was then applied to the measured waveforms to extract the frequency components. After calculating the rate of fundamental frequency (Rff), an Rff of≥15% was classified as normal ( Fig. 2 ).
Fig. 2.

Ocular motility assessment setup. Patients wore infrared goggles and maintained a fixed head position, and their eye movements following the LED light source were measured. LED, light-emitting diode.
SCAT5
The SCAT5 comprises six steps, but Step 1 (athlete background) and Step 6 (decision) were excluded from the analysis because only Steps 2–5 allowed scoring for SRC symptoms. Scores were calculated for each of the sub-items: Steps 2, 3, 4 and 5.
Group allocation and comparisons
The SCAT5 scores of the participants at the initial visit and two weeks later were compared to check for improvements. Patients with an Rff of≥15% after two weeks were assigned to the good improvement group, whereas those with an Rff of<15% were assigned to the minor improvement group. The scores for each SCAT5 sub-item obtained two weeks later were compared between the groups to examine differences in residual SRC symptoms.
Statistical analyses
The statistical program JMP Pro 15.1.0 (SAS Institute, Cary, NC, USA) was used for the statistical analysis. A Wilcoxon signed-rank test was used for comparisons with a significance level of less than 5%.
Results
Basic characteristics
The median age of the 13 participants was 19.0 years (quartile 18.0–21.0 years), and all participants were men. Moreover, six participants practiced football, two practiced American football, two practiced rugby, one practiced baseball, and one practiced judo. The median number of days elapsed since the injury was 3.8 (quartile 3.0–4.0 days) ( Table 1 ).
Table 1 Study participant characteristics.
| No. | Age | Sex | Sport | Position | Days elapsed since SRC 1 st SPM 2 nd SPM | |
|---|---|---|---|---|---|---|
| 1 | 21 | M | Football | FW | 5 | 16 |
| 2 | 19 | M | Football | FW | 3 | 17 |
| 3 | 18 | M | Rugby | BK | 4 | 17 |
| 4 | 32 | M | Baseball | – | 7 | 17 |
| 5 | 19 | M | American Football | TE | 6 | 13 |
| 6 | 17 | M | Judo | – | 2 | 14 |
| 7 | 18 | M | Football | FW | 3 | 12 |
| 8 | 19 | M | Football | DF | 4 | 14 |
| 9 | 17 | M | Rugby | BK | 3 | 12 |
| 10 | 32 | M | Football | MF | 4 | 14 |
| 11 | 21 | M | American Football | RB | 3 | 13 |
| 12 | 17 | M | Judo | – | 2 | 15 |
| 13 | 18 | M | Football | FW | 3 | 17 |
BK, back; DF, defender; FW, forward; MF, midfielder; TE, tight end; RB, running back; SPM, smooth pursuit eye movement; SRC, sport-related concussion.
Eye tracking and ocular motility changes
Based on the SPM assessment, eight patients were assigned to the good improvement group, and five were assigned to the minor improvement group.
SCAT5 score changes two weeks after the initial assessment
Compared to the minor improvement group, the good improvement group showed significant improvements in symptom number and symptom severity, immediate memory, double-leg stance, single-leg stance, tandem stance, and total errors, and delayed recall after two weeks compared with that at the initial evaluation ( Table 2 ).
Table 2 SCAT5 steps for all participants (N=13).
| SCAT5 | Evaluation items | Scale | Onset | Two weeks after | Calc. | ||
|---|---|---|---|---|---|---|---|
| STEP | Mean (SD) | Median | Mean (SD) | Median | p-value | ||
| 2 | Symptom number | 0–22 | 7.9 (1.44) | 8 | 0.85 (0.94) | 1 | <0.001 |
| Symptom severity | 0–132 | 26.7 (6.80) | 26 | 9.15 (8.40) | 4 | <0.001 | |
| 3 | Orientation | 0–5 | 4.69 (0.46) | 5 | 4.92 (0.27) | 5 | 0.250 |
| Immediate memory | 0–30 | 21.6 (3.79) | 21 | 24.7 (2.61) | 24 | <0.001 | |
| Concentration | 0–5 | 4.31 (0.72) | 4 | 4.69 (0.46) | 5 | 0.188 | |
| 4 | Double-leg | 0–10 | 1.23 (0.70) | 1 | 0.23 (0.42) | 0 | 0.001 |
| Single-leg | 0–10 | 4.38 (0.84) | 4 | 1.54 (1.55) | 1 | <0.001 | |
| Tandem stance | 0–10 | 1.46 (1.15) | 1 | 0.62 (0.74) | 0 | 0.016 | |
| Total errors | 0–30 | 7.08 (2.68) | 6 | 2.38 (2.71) | 1 | <0.001 | |
| 5 | Delayed recall | 0–10 | 6.38 (1.33) | 6 | 8.00 (0.78) | 8 | 0.001 |
SCAT5, Sports Concussion Assessment Tool 5; SD, standard deviation.
SCAT5 score comparisons between the good and minor improvement groups
Initially, all 13 patients had ocular impairment and an Rff<15%; after two weeks of initial evaluation, eight patients reached an Rff of≥15% (i. e., the good improvement group). Thus, the SCAT5 scores after two weeks were compared between the good and minor improvement groups. Scores for symptom number (median=2.0 vs. 0.0; p=0.042) and symptom severity (median=22.0 vs. 3.0; p=0.003) as well as for single-leg stance (median=4.0 vs. 0.5; p=0.006), tandem stance (median=1.0 vs. 0.0; p=0.035), and total errors (median=5.0 vs. 0.5, p=0.014), were significantly worse in the minor improvement group than in the good improvement group ( Fig. 3 ). However, orientation, immediate memory, and concentration as well as double-leg stance and delayed recall did not differ between the two groups ( Table 3 ).
Fig. 3.
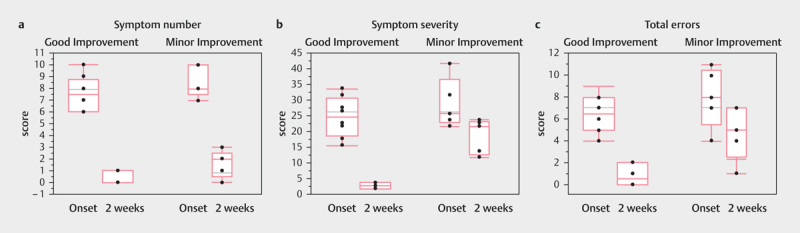
Differences in SCAT5 score; a . Symptom number (Step 2); b . Symptom severity (Step 2;) and c . Total errors (Step 4) at the initial visit and two weeks later.
Table 3 SCAT5 score improvements in participants with minor and good ocular motility recovery.
| SCAT5 | Description | Scale | Minor improvement (N=5) | Good improvement (N=8) | Calc. | ||
|---|---|---|---|---|---|---|---|
| STEP | Median | Quantile | Median | Quantile | p-value | ||
| 2 | Symptom number | 0–22 | 2.0 | 0.5–2.5 | 0.0 | 0.0–1.0 | 0.042 |
| Symptom severity | 0–132 | 22.0 | 13.0–23.5 | 3.0 | 2.0–4.0 | 0.003 | |
| 3 | Orientation | 0–5 | 5.0 | 5.0–5.0 | 5.0 | 5.0–5.0 | 0.429 |
| Immediate memory | 0–30 | 23.0 | 22.0–24.0 | 25.0 | 23.3–28.8 | 0.086 | |
| Concentration | 0–5 | 5.0 | 4.0–5.0 | 5.0 | 4.3–5.0 | 0.584 | |
| 4 | Double-leg | 0–10 | 0.0 | 0.0–1.0 | 0.0 | 0.0–0.0 | 0.271 |
| Single-leg | 0–10 | 4.0 | 2.0–4.0 | 0.5 | 0.0–1.0 | 0.006 | |
| Tandem stance | 0–10 | 1.0 | 0.5–2.0 | 0.0 | 0.0–0.8 | 0.035 | |
| Total errors | 0–30 | 5.0 | 2.5–7.0 | 0.5 | 0.0–2.0 | 0.014 | |
| 5 | Delayed recall | 0–10 | 8.0 | 7.0–8.5 | 8.0 | 7.3–9.0 | 0.485 |
SCAT5, Sports Concussion Assessment Tool 5.
Discussion
In this study we investigated the potential link between the extent of ocular motility improvement and symptom reduction in patients with SRC and found that the group of participants with a higher degree of ocular motility improvement also had better recovery from SRC symptoms.
SCAT5 scores improve over time during the recovery phase 8 9 10 , and our results are consistent with this finding; the scores for most SCAT5 sub-items improved significantly over the two-week recovery period. Notably, differences between the minor and good improvement groups were observed only for subjective symptoms, such as symptom number and symptom severity (Step 2), and balance functions, such as tandem stance and total errors (Step 4). A previous study associated ocular motility disorders with subjective symptoms in patients with mild traumatic brain injury 16 , demonstrating that patients with SRC and residual ocular motility disorder symptoms also reported residual subjective symptoms of SRC; our findings align with the findings of this previous study.
Visual information plays a major role in postural control relative to the vestibular system 17 18 19 20 . Therefore, it is likely that patients with residual ocular motility deficits would also have residual balance disorder symptoms. In contrast, orientation, immediate memory, and concentration (Step 3), as well as double-leg stance (Step 4) and delayed recall (Step 5), did not differ between the two groups. As Step 3 and its five sub-items were used to evaluated cognitive function, it was not considered to be associated with ocular motility. Moreover, the lack of difference in double-leg stance (Step 4), an extremely easy task in which both groups scored 0, was thought to be due to the floor effect.
This study has some limitations, including its small sample size and selection bias regarding sports and sex. To ensure the generalizability of our findings, a study with more patients should be performed. Furthermore, the ocular motility assessment used in this study required specialized equipment, measurements, and specific analytical techniques. Therefore, future studies should consider simpler measurement methods for clinical practice.
In conclusion, precise ocular motility assessments using eye tracking technology could be useful for objectively assessing SRC symptoms during recovery, especially if used with an evaluation tool, such as the SCAT5, for a definitive diagnosis.
Acknowledgement
This work was supported by a KAKEN grant from the Japan Society for the promotion of science (18K17868 and 22K22K11589). Furthermore, we are grateful to Ms. Yuka Sawada and Ms. Shigemi Suzuki, Department of Neurosurgery from St. Marianna University School of Medicine, for their outstanding support and input. We would like to thank Editage (www.editage.com) for English language editing.
Funding Statement
Fundings Japan Society for the Promotion of Science — http://dx.doi.org/10.13039/501100001691; 22K11589 Japan Society for the promotion of science — 18K17868
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Smith A M, Stuart M J, Roberts W O et al. Concussion in ice hockey: current gaps and future directions in an objective diagnosis. Clin J Sport Med. 2017;27:503–509. doi: 10.1097/JSM.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 2.Chamard E, Lichtenstein J D. A systematic review of neuroimaging findings in children and adolescents with sports-related concussion. Brain Inj. 2018;32:816–831. doi: 10.1080/02699052.2018.1463106. [DOI] [PubMed] [Google Scholar]
- 3.McCrory P, Johnston K, Meeuwisse W et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004. Br J Sports Med. 2005;39:196–204. doi: 10.1136/bjsm.2005.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Echemendia R, Meeuwisse W, McCrory P et al. The Sport Concussion Assessment Tool 5 th Edition. Background and rationale . Br J Sports Med. 2017;51:848–850. doi: 10.1136/bjsports-2017-097506. [DOI] [PubMed] [Google Scholar]
- 5.Williamson IJ S, Goodman D.Converging evidence for the under-reporting of concussions in youth ice hockey Br J Sports Med 200640128–132.discussion 128–32; discussion 128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLeod T C, Bay R C, Heil J et al. Identification of sport and recreational activity concussion history through the preparticipation screening and a symptom survey in young athletes. Clin J Sport Med. 2008;8:235–240. doi: 10.1097/JSM.0b013e3181705756. [DOI] [PubMed] [Google Scholar]
- 7.Elliott J, Anderson R, Collins S et al. Sports-related concussion (SRC) assessment in road cycling: a systematic review and call to action. BMJ Open Sport Exerc Med. 2019;5:e000525. doi: 10.1136/bmjsem-2019-000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ntikas M, Hunter A M, Gallagher I J et al. Longer neurophysiological vs. clinical recovery following sport concussion. Front Sports Act Living. 2021;3:737712. doi: 10.3389/fspor.2021.737712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunt S C, Meredith-Duliba T, Didehhani N et al. Resilience and recovery from sports related concussion in adolescents and young adults. J Clin Exp Neuropsychol. 2021;43:677–688. doi: 10.1080/13803395.2021.1990214. [DOI] [PubMed] [Google Scholar]
- 10.Bruce J M, Meeuwisse W, Hutchison M G et al. Determining Sport Concussion Assessment Tool fifth Edition (SCAT5) reliable change in male professional hockey players. Br J Sports Med. 2022;56:1115–1122. doi: 10.1136/bjsports-2021-104851. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor N, Ciuffreda K J, Han Y. Oculomotor rehabilitation in acquired brain injury: a case series. Arch Phys Med Rehabil. 2004;85:1667–1678. doi: 10.1016/j.apmr.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 12.Hunt A W, Mah K, Reed N et al. Oculomotor-based vision assessment in mild traumatic brain injury: a systematic review. J Head Trauma Rehabil. 2016;31:252–261. doi: 10.1097/HTR.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 13.Ciuffreda K J, Kapoor N, Rutner D et al. Occurrence of oculomotor dysfunctions in acquired brain injury: a retrospective analysis. Optometry. 2007;78:155–161. doi: 10.1016/j.optm.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Mucha A, Collins M W, Elbin R J et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42:2479–2486. doi: 10.1177/0363546514543775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hane K. Eye movement measurement using scleral reflection method and an analysis of smooth pursuit eye movement using Fourier transform. Neuro-Ophthalmol Jpn. 2018;35:71–78. [Google Scholar]
- 16.Diwakar M, Harrington D L, Maruta J et al. Filling in the gaps: anticipatory control of eye movements in chronic mild traumatic brain injury. Neuroimage Clin. 2015;8:210–223. doi: 10.1016/j.nicl.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterka R J. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 18.Mucha A, Fedor S, DeMarco D. Vestibular dysfunction and concussion. Handb Clin Neurol. 2018;158:135–144. doi: 10.1016/B978-0-444-63954-7.00014-8. [DOI] [PubMed] [Google Scholar]
- 19.Babicz M A, Woods S P, Cirino P et al. Vestibular/ocular motor screening is independently associated with concussion symptom severity in youths. Clin J Sport Med. 2022;32:40–45. doi: 10.1097/JSM.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 20.Symons G F, Clough M, Mutimer S et al. Cognitive ocular motor deficits and white matter damage chronically after sports-related concussion. Brain Commun. 2021;3:fcab213. doi: 10.1093/braincomms/fcab213. [DOI] [PMC free article] [PubMed] [Google Scholar]


