Magnesium sulfate for preterm fetal neuroprotection reduces cerebral palsy and death or cerebral palsy for children up to 2 years of corrected age.
Abstract
OBJECTIVE:
To systematically review the evidence for the effectiveness and safety of magnesium sulfate as a fetal neuroprotective agent when given to individuals at risk of preterm birth.
DATA SOURCES:
We searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (through March 17, 2023), and reference lists of relevant studies.
METHODS OF STUDY SELECTION:
Randomized controlled trials (RCTs) assessing magnesium sulfate for fetal neuroprotection in pregnant participants at risk of imminent preterm birth were eligible. Two authors assessed RCTs for inclusion, extracted data, and evaluated risk of bias, trustworthiness, and evidence certainty (GRADE [Grading of Recommendations Assessment, Development and Evaluation]).
TABULATION, INTEGRATION, AND RESULTS:
We included six RCTs (5,917 pregnant participants and 6,759 fetuses at less than 34 weeks of gestation at randomization). They were conducted in high-income countries (two in the United States, two across Australia and New Zealand, and one each in Denmark and France) and commenced between 1995 and 2018. Primary outcomes: up to 2 years of corrected age, magnesium sulfate compared with placebo reduced the risk of cerebral palsy (risk ratio [RR] 0.71, 95% CI, 0.57–0.89; six RCTs, 6,107 children) and death or cerebral palsy (RR 0.87, 95% CI, 0.77–0.98; six RCTs, 6,481 children) (high-certainty evidence). Magnesium sulfate had little or no effect on death up to 2 years of corrected age (moderate-certainty evidence) or these outcomes at school age (low-certainty evidence). Although there was little or no effect on death or cardiac or respiratory arrest for pregnant individuals (low-certainty evidence), magnesium sulfate increased adverse effects severe enough to stop treatment (RR 3.21, 95% CI, 1.88–5.48; three RCTs, 4,736 participants; moderate-certainty evidence). Secondary outcome: magnesium sulfate reduced the risk of severe neonatal intraventricular hemorrhage (moderate-certainty evidence).
CONCLUSION:
Magnesium sulfate for preterm fetal neuroprotection reduces cerebral palsy and death or cerebral palsy for children. Further research is required on longer-term benefits and harms for children, effect variation by participant and treatment characteristics, and the generalizability of findings to low- and middle-income countries.
SYSTEMATIC REVIEW REGISTRATION:
The review protocol was based on a standard Cochrane Pregnancy and Childbirth template and our previous Cochrane Systematic Review (doi: 10.1002/14651858.CD004661.pub3; published before the introduction of PROSPERO).
Cerebral palsy remains the most common physical disability in childhood. The birth prevalence of cerebral palsy in high-income countries is 1.6 per 1,000 live births.1 Prevalence is markedly higher in low- and middle-income countries and for children born preterm.1 There is no cure; prevention is crucial.
Magnesium sulfate showed promise for preterm fetal neuroprotection (including cerebral palsy prevention) in observational studies in the 1990s.2–4 In response, several randomized controlled trials (RCTs) were conducted.5–8 These RCTs were included in evidence syntheses,9–11 including a 2009 Cochrane Systematic Review,12 which confirmed the neuroprotective effects of magnesium sulfate. Magnesium sulfate compared with placebo was shown to reduce the risk of cerebral palsy (risk ratio [RR] 0.68, 95% CI, 0.54–0.87; five RCTs, 6,145 children) and death or cerebral palsy (RR 0.85, 95% CI, 0.74–0.98; four RCTs, 4,446 children) up to 2 years of corrected age.12
Over the past decade, adoption of magnesium sulfate for preterm cerebral palsy prevention has ensued across the globe. Across high-income countries, professional bodies, including in the United States,13 have provided committee opinions and clinical practice guidelines.14 In its 2015 guidance on interventions to improve preterm birth outcomes, the World Health Organization (WHO) delivered a strong recommendation for use.15
Despite proven effectiveness, studies, including an individual participant data meta-analysis based on data from RCTs included in the 2009 Cochrane Systematic Review,16 have not established variation in magnesium sulfate's benefits according to participant and treatment characteristics. Recommendations for use therefore vary.14 Local, national, and international translation and uptake are impeded by this variability. Further, in recent years, several new RCTs and school-age follow-up of original RCTs have been completed. Consequently, it is timely to re-evaluate the evidence on prenatal magnesium sulfate for preterm fetal neuroprotection. We aimed to systematically review the evidence for the effectiveness and safety of magnesium sulfate as a fetal neuroprotective agent when administered to individuals at risk of preterm birth in a Cochrane Systematic Review update.
SOURCES
We adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.17 The review protocol was based on a standard Cochrane Pregnancy and Childbirth template and the previous Cochrane Systematic Review12 (published before the introduction of PROSPERO).
For this Cochrane Systematic Review update,18 on March 17, 2023, we searched Cochrane Pregnancy and Childbirth's Trials Register (containing reports from CENTRAL [Cochrane Central Register of Controlled Trials], MEDLINE, EMBASE and CINAHL, hand searched journals and conference proceedings), ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform. Further details of the search strategies can be found in Appendix 1, available online at http://links.lww.com/AOG/D710. We also searched the reference lists of relevant studies and Google Scholar. No language restrictions were applied. Searches were not date restricted (not limited to after the 2009 Cochrane Systematic Review12).
STUDY SELECTION
To minimize potential biases, only RCTs and cluster-RCTs were eligible, in full-text or abstract form. Quasi-RCTs and crossover trials were not eligible. Randomized controlled trials assessing magnesium sulfate given for fetal neuroprotection compared with placebo or no treatment in pregnant participants at risk of imminent preterm birth (at less than 37 weeks of gestation) were eligible. Randomized controlled trials in which magnesium sulfate was used with the primary aim of tocolysis, prevention and treatment of eclampsia and preeclampsia, or as a prenatal dietary supplement were not eligible.
For children, primary outcomes were death (fetal, neonatal, or later), cerebral palsy, death or cerebral palsy, major neurodevelopmental disability, and death or major neurodevelopmental disability. For pregnant participants, primary outcomes were severe outcome potentially related to treatment (death, respiratory arrest, or cardiac arrest) and adverse effects severe enough to stop treatment. Comprehensive secondary outcomes for infants, children, adults, and pregnant individuals were prespecified—the detailed list can be found in Appendix 1 (http://links.lww.com/AOG/D710).
Two reviewers (E.S.S. and a second author not involved in a potentially eligible RCT) independently assessed all potential studies identified for inclusion. Disagreements were resolved through discussion. Randomized controlled trials meeting the inclusion criteria were further evaluated by two reviewers (E.S.S. and S.G., not involved in the eligible RCTs) against predefined criteria for scientific integrity to select studies deemed to be sufficiently trustworthy for analysis (see Appendix 1, http://links.lww.com/AOG/D710, for details of criteria applied).
For included RCTs, data were extracted using a standardized form, including information regarding design, participants, magnesium sulfate regimen, control, outcomes reported, results relevant to the review, and risk of bias. Data were extracted by two reviewers (E.S.S. and S.G.), with differences resolved through discussion. Quality was appraised by two reviewers (E.S.S. and S.G.) using established guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions.19
Data were analyzed using RevMan Web.20 Effect sizes were estimated as RRs for dichotomous outcomes and mean differences for continuous outcomes, with 95% CIs. Where there were sufficient data, pooled estimates were calculated, first using fixed-effects meta-analysis (Mantel-Haenszel method). We performed random-effects meta-analysis to combine effect estimates when there was substantial statistical heterogeneity (I2>30% and either T2>0 or low P-value [<0.10] in the χ2 test).
For the primary outcomes, subgroup analyses were planned based on characteristics of participants (primary reason considered to be at high risk of preterm birth, number of fetuses in utero, gestational age at randomization) and treatment regimens (mode of administration, time treatment intended to be started before birth, loading dose regimen, maintenance dose regimen, repeat treatment permitted). Sensitivity analyses were planned, restricting primary outcome analyses to RCTs with low risk of bias. We planned to investigate reporting biases using funnel plots if 10 or more RCTs were included in meta-analyses.
We planned to assess certainty of the evidence for the primary outcomes and prespecified secondary outcomes (infants: severe intraventricular hemorrhage [grade 3 or 4] and chronic lung disease or bronchopulmonary dysplasia; pregnant individuals: mode of birth (cesarean delivery), postpartum hemorrhage, breastfeeding at hospital discharge, and views of treatment) using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach.21 Evidence was classified as high, moderate, low, or very low certainty considering the following domains: study limitations, consistency, directness, imprecision, and publication bias. Certainty of evidence was assessed by two reviewers (E.S.S. and S.G.); discrepancies were resolved through discussion.
RESULTS
For this updated Cochrane Systematic Review, database searching and other sources identified 215 new records, which we assessed for inclusion, along with 29 records relating to RCTs included in the 2009 Cochrane Systematic Review. After removal of duplicate and irrelevant records, we assessed 137 in full. We included six RCTs (116 records) and excluded six studies (seven records); four studies (four records) are ongoing, and eight studies (10 records) are “awaiting classification” pending further information. A study flow diagram is shown in Figure 1, and Appendix 2, available online at http://links.lww.com/AOG/D710, provides a full list of records by classification.
Fig. 1. Flow diagram of studies identified in the systematic review.

Shepherd. Magnesium Sulfate for Neuroprotection Review. Obstet Gynecol 2024.
The six included RCTs (four of which were included in the 2009 Cochrane Systematic Review5–8) were all individually randomized.5–8,22,23 They were conducted in high-income countries (two in the United States,5,8 two across Australia and New Zealand,6,23 and one each in Denmark22 and France7) and commenced between 19955 and 2018.22 Sample sizes ranged from 575 to 2,241,8 with the RCTs including a total of 5,917 pregnant participants and their 6,759 fetuses alive at randomization.5–8,22,23 Although eligibility criteria varied, all RCTs included pregnant participants in preterm labor or with expected or planned imminent preterm birth at less than 34 weeks of gestation. Lower limits of gestational age varied (no limit,6,7 24 weeks,5,8,22 or 30 weeks23), as did upper limits (less than 30,6 32,8,22 33,7 or 34 weeks5,23). Four RCTs included singletons and twins,5,8,22,23 and two also included higher-order multiple gestations.6,7 All RCTs assessed intravenous magnesium sulfate for fetal neuroprotection compared with a placebo.5–8,22,23 Three administered a 4-g loading dose only,5,7,23 and three included a maintenance dose (a 4–6-g loading dose and 1–2-g/h maintenance dose).6,8,22 Re-treatment was permitted in only two RCTs.8,22 The six RCTs all reported on childhood follow-up outcomes at 18 months to 2 years of corrected age5–8,22,23; two also reported on school-age follow-up.24,25 Further characteristics of the RCTs are provided in Appendix 3 (available online at http://links.lww.com/AOG/D710), including eligibility criteria, magnesium sulfate and control regimens, review outcomes reported, definitions of outcomes, and follow-up assessment methods used in each RCT.
Overall risk of bias for most domains across RCTs was judged to be low. Considering selection bias, five were at low risk6–8,22,23 and one was at unclear risk.5 All RCTs were at low risk of performance and detection bias.5–8,22,23 Five RCTs were at low risk of attrition bias for the main RCT and initial 18-month–2-year corrected age follow-up,6–8,22,23 and one was at unclear risk5; two RCTs were at unclear risk of attrition bias for school-age follow-up.24,25 Four RCTs were at low risk of reporting bias,6,8,22,23 and two were at unclear risk.5,7 Appendix 3 (http://links.lww.com/AOG/D710) provides the detailed quality assessment of each RCT and a summary figure.
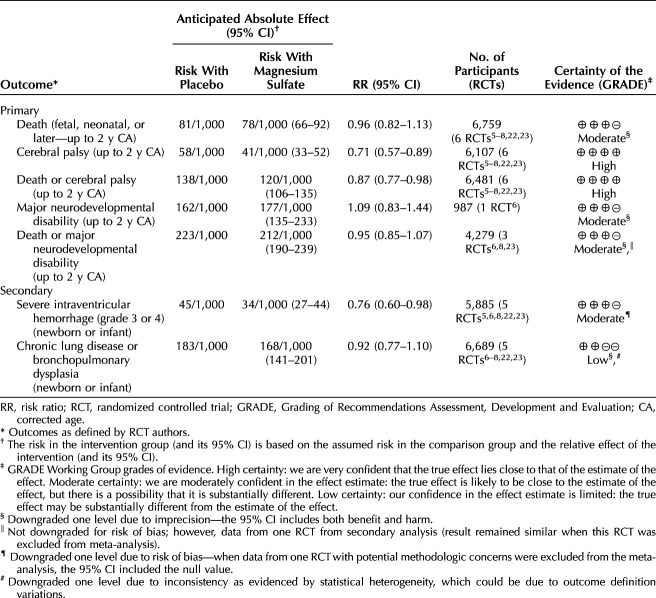
Primary outcomes for infants and children up to 2 years of corrected age: magnesium sulfate compared with placebo reduced cerebral palsy (RR 0.71, 95% CI, 0.57–0.89; six RCTs, 6,107 children; number needed to treat for additional beneficial outcome [NNTB] 60, 95% CI, 41–158) and death or cerebral palsy (RR 0.87, 95% CI, 0.77–0.98; six RCTs, 6,481 children; NNTB 56, 95% CI, 32–363) (both high-certainty evidence). Magnesium sulfate probably resulted in little to no difference in death (fetal, neonatal, or later) (RR 0.96, 95% CI, 0.82–1.13; six RCTs, 6,759 children), major neurodevelopmental disability (RR 1.09, 95% CI, 0.83–1.44; one RCT, 987 children), or death or major neurodevelopmental disability (RR 0.95, 95% CI, 0.85–1.07; three RCTs, 4,279 children) (all moderate-certainty evidence). See Table 1 for a summary of findings and Appendix 4, available online at http://links.lww.com/AOG/D710, for forest plots of meta-analyses.
Table 1.
Summary of Findings for Main Outcomes for Infants and Children Up to 2 Years of Corrected Age
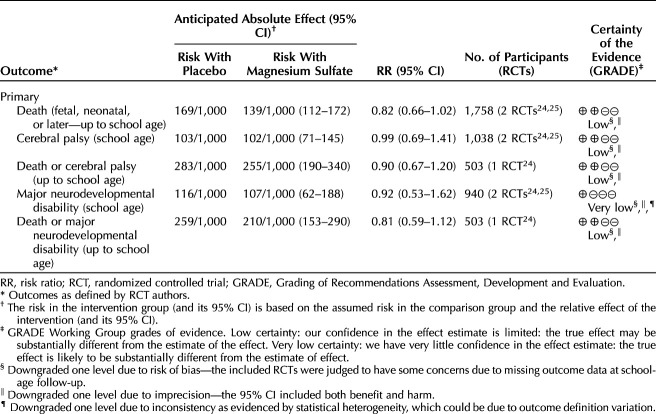
Primary outcomes for infants and children at school age: magnesium sulfate compared with placebo may have resulted in little to no difference in death (fetal, neonatal, or later) (RR 0.82, 95% CI, 0.66–1.02; two RCTs, 1,758 children), cerebral palsy (RR 0.99, 95% CI, 0.69–1.41; two RCTs, 1,038 children), death or cerebral palsy (RR 0.90, 95% CI, 0.67–1.20; one RCT, 503 children), and death or major neurodevelopmental disability (RR 0.81, 95% CI, 0.59–1.12; one RCT, 503 children) (all low-certainty evidence). Magnesium sulfate also may have resulted in little to no difference in major neurodevelopmental disability (RR 0.92, 95% CI, 0.53–1.62; two RCTs, 940 children; very low-certainty evidence). See Table 2 for a summary of findings and Appendix 4 (http://links.lww.com/AOG/D710) for forest plots of meta-analyses.
Table 2.
Summary of Findings for Main Outcomes for Infants and Children Up to School Age
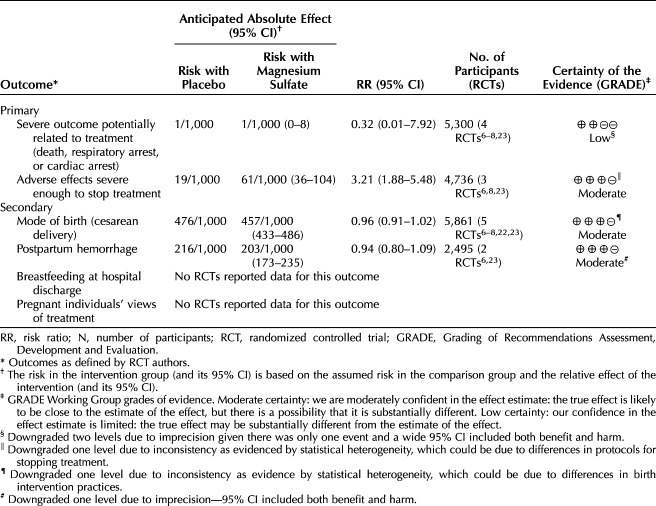
Primary outcomes for pregnant individuals: magnesium sulfate may have resulted in little or no difference in severe outcomes potentially related to treatment (death, cardiac arrest, respiratory arrest) (RR 0.32, 95% CI, 0.01–7.92; four RCTs, 5,300 participants; low-certainty evidence). However, magnesium sulfate probably increased adverse effects severe enough to stop treatment for pregnant individuals compared with placebo (average RR 3.21, 95% CI, 1.88–5.48; three RCTs, 4,736 participants; moderate-certainty evidence). See Table 3 for a summary of findings and Appendix 4 (http://links.lww.com/AOG/D710) for forest plots of meta-analyses.
Table 3.
Summary of Findings for Main Outcomes for Pregnant Individuals
Considering secondary outcomes assessed using GRADE for infants and children, magnesium sulfate probably reduced severe intraventricular hemorrhage (grade 3 or 4) (RR 0.76, 95% CI, 0.60–0.98; five RCTs, 5,885 infants, NNTB 92, 95% CI, 55–1,102; moderate-certainty evidence) and may have resulted in little to no difference in chronic lung disease or bronchopulmonary dysplasia (RR 0.92, 95% CI, 0.77–1.10; five RCTs, 6,689 infants; low-certainty evidence) (Table 1). For pregnant individuals, magnesium sulfate probably resulted in little or no difference in cesarean delivery (five RCTs, 5,861 participants) and postpartum hemorrhage (two RCTs, 2,495 participants) (both moderate-certainty evidence). No data were reported for breastfeeding at discharge or pregnant individuals' views of treatment (Table 3 and Appendix 4 [http://links.lww.com/AOG/D710]).
No evidence of differences was observed for most other secondary outcomes not assessed using GRADE for infants and children. However, we did observe a number of possible benefits with magnesium sulfate compared with placebo: fewer infants required intubation for resuscitation (two RCTs, 3,093 infants), and, up to 2 years of corrected age, fewer children had moderate or severe cerebral palsy (five RCTs, 5,502 children), died or had any neurodevelopmental disability (three RCTs, 3,194 children), had substantial gross motor dysfunction (one RCT, 1,042 children), or died or had substantial gross motor dysfunction (five RCTs, 5,097 children). No harms for infants or children were observed with magnesium sulfate compared with placebo, except for possibly more children up to 2 years of corrected age with behavioral scores (assessed by the Child Behavior Checklist) within the clinical problem range, overall and on the following scales: anxiety, withdrawal, sleeping problems, other (all in one RCT, up to 795 children). See Appendix 5, available online at http://links.lww.com/AOG/D710, for results for all secondary outcomes for infants and children.
For pregnant individuals, largely no evidence of differences in outcomes, including no benefits, were observed. We did, however, observe some possible harms in the magnesium sulfate compared with placebo group: more pregnant individuals experienced any side effects of treatment (four RCTs, 5,300 participants) and the following side effects: hypotension (three RCTs, 3,059 participants), tachycardia (one RCT, 1,062 participants), warmth over body or flushing (four RCTs, 5,300 participants), arm discomfort with infusion (three RCTs, 4,736 participants), mouth dryness (two RCTs, 2,495 participants), nausea or vomiting (four RCTs, 5,300 participants), sleepiness (one RCT, 1,062 participants), sweating (three RCTs, 4,736 participants), dizziness (two RCTs, 2,495 participants), and blurred vision (two RCTs, 2,495 participants). See Appendix 5 (http://links.lww.com/AOG/D710) for results for all secondary outcome for pregnant individuals.
Subgroup analyses for primary outcomes: interaction tests demonstrated no evidence of differences between subgroups with available data (eg, gestational age at randomization, loading dose regimen, maintenance dose regimen, repeat treatment permitted) for infant and child outcomes up to 2 years of corrected age (death, cerebral palsy, death or cerebral palsy, and death or major neurodevelopmental disability), at early school age (death, cerebral palsy, and major neurodevelopmental disability), or for pregnant individuals (severe outcome potentially related to treatment and adverse effects severe enough to stop treatment). See Appendix 5 (http://links.lww.com/AOG/D710) for results from subgroup analyses.
Sensitivity analyses for primary outcomes: where applicable, in sensitivity analyses based on RCT quality (removing one RCT at unclear risk of selection bias5), results for outcomes (up to 2 years of corrected age: death, cerebral palsy, death or cerebral palsy) remained similar to overall analyses. In post hoc sensitivity analyses pooling adjusted effect sizes, when reported by RCTs, results for outcomes (up to 2 years of corrected age: death, cerebral palsy, death or cerebral palsy, and death or major neurodevelopmental disability; at early school age: cerebral palsy) were similar to overall analyses. See Appendix 5 (http://links.lww.com/AOG/D710) for results from sensitivity analyses.
DISCUSSION
This updated Cochrane Systematic Review included six RCTs (enrolling 5,917 pregnant participants at less than 34 weeks of gestation and their 6,759 fetuses alive at randomization) that compared magnesium sulfate with placebo for neuroprotection of the fetus in pregnant individuals at risk of preterm birth.5–8,22,23 Evidence indicates that magnesium sulfate, compared with placebo, reduces cerebral palsy and death or cerebral palsy for children up to 2 years of corrected age and probably reduces severe intraventricular hemorrhage for infants. Current evidence suggests that magnesium sulfate may result in little to no difference in outcomes at school age. Although evidence indicates that magnesium sulfate may result in little to no difference in severe outcomes (death, cardiac arrest, respiratory arrest) for pregnant individuals, it suggests that magnesium sulfate probably increases adverse effects severe enough to stop treatment.
Evidence to assess the effects of magnesium sulfate for preterm fetal neuroprotection is, however, currently incomplete. Although we were able to include data from all six RCTs for the review's primary outcomes of death, cerebral palsy, and death or cerebral palsy up to 2 years of corrected age, for many outcomes, only one to five RCTs contributed data. For several prespecified secondary review outcomes, there were no data reported by the included RCTs. Only two of the six RCTs have reported data up into school age,24,25 and none have reported on follow-up beyond the first decade of life.
This review's findings are further limited by variations in the characteristics of the pregnant participants randomized and treatment regimens used across the included RCTs. Although we attempted to explore variation through subgroup analyses, the ability to do this was limited (eg, inclusion criteria did not enable allocation to one or the other subgroup, or stratified results were not presented, or both).
All included RCTs were conducted in high-income countries and commenced between 1995 and 2018.5–8,22,23 Thus, although there are nearly 6,000 pregnant participants and their children in these RCTs, the applicability to low- and middle-income countries and generalizability to present day clinical context or practice of their findings should be considered.
After screening of potentially eligible studies for trustworthiness, a total of eight were classified as “awaiting classification” (Appendix 2, http://links.lww.com/AOG/D710). These were conducted in various low- and middle-income countries. Their possible future inclusion (along with potential inclusion of “ongoing studies” (Appendix 2, http://links.lww.com/AOG/D710) in a further update to this review may extend the applicability and generalizability of its findings.
Overall risk of bias of the included RCTs was judged to be low. Sensitivity analyses, restricted to the five RCTs at low risk of selection bias,6–8,22,23 supported findings from the main analyses. For primary and secondary review outcomes assessed using GRADE, evidence was determined to be high- to very low-certainty. Evidence was predominantly downgraded due to imprecision, study design limitations, and inconsistency.
We took steps to minimize bias throughout the review process. We conducted a detailed and systematic search, without language, date, or publication status restrictions. Two review authors (E.S.S. and S.G.) not involved in potentially eligible or included RCTs independently assessed RCTs for inclusion; performed data extraction, including risk of bias assessment; and assessed the certainty of the evidence, with disagreements discussed until consensus was reached. Despite independent assessments, these processes are inherently subjective and require a degree of interpretation. In all cases, we sought to be consistent and transparent, documenting our decisions and rationale. We recognize that, with many review outcomes, there is a risk of statistical type 1 error (a “false-positive” result). Results for which there are very few RCTs included, or with moderate or substantial heterogeneity, and “borderline statistical significance” should be treated with caution.
Our review provides the most up-to-date and comprehensive assessment of trustworthy evidence. The results and conclusions of this review are largely consistent with those of prior reviews.9–12,26,27 Compared with the 2009 Cochrane Systematic Review, this review includes two new RCTs22,23 and new school-age follow-up data from two previously included RCTs24,25 and excludes one previously included RCT (magnesium sulfate was administered for preeclampsia not fetal neuroprotection).28 Our review re-confirms that magnesium sulfate reduces cerebral palsy and death or cerebral palsy for children up to 2 years of corrected age. A new finding in this update is that magnesium sulfate probably reduces severe intraventricular hemorrhage for infants. This update also incorporates contemporary methodologies, including GRADE,21 to rate the certainty of the body of evidence.
Our review is also broadly comparable with previous comprehensive reviews of adverse outcomes associated with magnesium sulfate (when administered for the prevention or treatment of eclampsia, for preventing preterm labor and birth [tocolysis], and for fetal neuroprotection).29,30 Magnesium sulfate has not been shown to increase serious adverse effects for pregnant individuals, though an increase in comparatively “minor” adverse treatment effects and treatment cessation has been shown.29 Magnesium sulfate has not been shown to increase neonatal adverse outcomes, including death.30
This review is the first to include data from Crowther et al.23 This RCT was designed to assess the effect of magnesium sulfate at 30–34 weeks of gestation (beyond the gestational age currently recommended in some countries14 based on the previous Cochrane Review).12 Although a reduction in the RCT's composite primary outcome (death or cerebral palsy for children at 2 years of corrected age or the separate components) was not shown, the authors recognize the limited power to detect small between-group differences due to the lower event rates for death and cerebral palsy than predicted and the RCT's sample size.23,31 Despite the absence of benefit observed in Crowther et al 2023,23 the addition of their data to our review's meta-analysis did not negate the overall neuroprotective benefits observed with this treatment.
To date, only one other systematic review has reported on school-age outcomes of antenatal magnesium sulfate for fetal neuroprotection.32 Its findings support ours—an absence of clear benefits or harms and the need for additional follow-up data to determine effects with greater certainty.32
The findings of our review's limited subgroup analyses are consistent with those from a previous individual participant data meta-analysis,16 which similarly demonstrates reductions for children up to 2 years of corrected age in cerebral palsy and death or cerebral palsy—with benefit not clearly affected by characteristics including preterm gestational age and treatment regimen. With the availability of individual participant data, Crowther et al16 were able to assess the influence of characteristics that we were not able to explore in our review (due to the limitations of aggregate data). The important opportunity and need to update this previous individual participant data meta-analysis to include the more recent RCTs and longer-term (school-age) follow-up data has been noted.31
The current international guideline from the WHO on interventions to improve preterm birth outcomes15 used the 2009 Cochrane Systematic Review12 to base their recommendation. Similarly, the clinical practice recommendations provided by professional bodies in many high-income countries (summarized in the systematic review by Jayaram et al14) are based on the previous Cochrane Review.12 Although available clinical practice guidelines all support the use of this treatment for preterm cerebral palsy prevention, given that the systematic reviews have not supported a particular upper gestational age or dosing regimen, recommendations vary. The American College of Obstetricians and Gynecologists' 2010 Committee Opinion (re-affirmed in 2023)13 suggests that physicians should develop specific guidelines regarding inclusion criteria and treatment regimens, in accordance with one of the larger RCTs.6–8 As noted above, the opportunity to further investigate which pregnant individuals to treat (ie, considering primary reason they are at risk of preterm birth, number of fetuses in utero, and gestational age), when to treat (ie, considering how long before anticipated or planned birth), and how to treat (ie, considering loading and maintenance dose regimens) through an updated individual participant data meta-analysis should be explored.
Further research is needed on the longer-term benefits and harms of magnesium sulfate, including follow-up of children into adolescence and adulthood. Any future studies should use robust methodology and aim for consistency in outcome measurement and reporting (using standardized, ideally contemporary assessment methods—particularly for cerebral palsy33) to facilitate pooling of data. This will help to ensure that pregnant individuals whose children are likely to benefit from exposure are not denied treatment and that pregnant individuals whose children will likely not benefit from treatment are not exposed unnecessarily.
In conclusion, high-certainty evidence indicates that magnesium sulfate for preterm fetal neuroprotection reduces cerebral palsy and death or cerebral palsy for children up to 2 years of corrected age. Further research is required on longer-term benefits and harms for children, effect variation by participant and treatment characteristics, and the generalizability of findings to low- and middle-income countries.
Footnotes
Emily Shepherd is funded by an Australian National Health and Medical Research Council (NHMRC) Investigator Grant (ID 2007800) (https://www.nhmrc.gov.au/). This grant also supported Shona Goldsmith's salary in part. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial Disclosure Emily S. Shepherd: former Editor for Cochrane Pregnancy and Childbirth and current Sign-off Editor for Cochrane Central Editorial Service, but had no involvement in the editorial processing of this review. Shona Goldsmith: senior research fellow with Cerebral Palsy Alliance, The University of Sydney. Lex W. Doyle: investigator for an included randomized controlled trial (RCT) (Crowther 2003), and published opinions in medical journals relating to magnesium sulfate use in neuroprotection. Philippa Middleton: investigator for an included RCT (Crowther 2023); former Editor for Cochrane Pregnancy and Childbirth and current Sign-off Editor for Cochrane Central Editorial Service but had no involvement in the editorial processing of this review; and Independent Contractor for National Health and Medical Research Council Stillbirth Centre for Research Excellence. Stéphane Marret: investigator for included RCT (Marret 2006) and works as a health professional in neonatology and neuropaediatrics, Rouen University Hospital, Rouen, France. Dwight J. Rouse: investigator for an included RCT (Rouse 2008). Peter Pryde: investigator for an included RCT (Mittendorf 2002); published opinions in medical journals relating to magnesium sulfate to reduce cerebral palsy; is a retired clinician; and maintains an adjunct faculty position at the University of Wisconsin School of Medicine and Public Health strictly for academic purposes. Hanne W. Wolf: investigator for an included RCT (Wolf 2020), and works as a Gynaecologist and Obstetrician, University Hospital, Denmark. Caroline A. Crowther: investigator for included RCTs (Crowther 2003; Crowther 2023).
Presented at the PSANZ 2024 Congress, April 7–10, 2024, Christchurch, New Zealand; and at EACD 2024, May 29–June 1, 2024, Bruges, Belgium.
Cochrane Pregnancy and Childbirth supported the authors in the development of the Cochrane Systematic Review update. The Cochrane Methods Support Unit (Afroditi Kanellopoulou) also provided statistical advice.
The authors thank Dr. Thomas Sullivan and Dr. Lisa Yelland for statistical advice.
This article is based on a Cochrane Review published in the Cochrane Database of Systematic Reviews (CDSR) 2024, Issue 5, doi: 10.1002/14651858.CD004661. Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and the CDSR should be consulted for the most recent version of the review.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/D711.
REFERENCES
- 1.McIntyre S, Goldsmith S, Webb A, Ehlinger V, Hollung SJ, McConnell K, et al. Global prevalence of cerebral palsy: a systematic analysis. Dev Med Child Neurol 2022;64:1494–506. doi: 10.1111/dmcn.15346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuban KC, Leviton A, Pagano M, Fenton T, Strassfeld R, Wolff M. Maternal toxemia is associated with reduced incidence of germinal matrix hemorrhage in premature babies. J Child Neurol 1992;7:70–6. doi: 10.1177/088307389200700113 [DOI] [PubMed] [Google Scholar]
- 3.Nelson KB, Grether JK. Can magnesium sulfate reduce the risk of cerebral palsy in very low birthweight infants? Pediatrics 1995;95:263–9. doi: 10.1542/peds.95.2.263 [DOI] [PubMed] [Google Scholar]
- 4.Wolf HT, Hegaard HK, Greisen G, Huusom L, Hedegaard M. Treatment with magnesium sulphate in pre-term birth: a systematic review and meta-analysis of observational studies. J Obstet Gynaecol 2012;32:135–40. doi: 10.3109/01443615.2011.638999 [DOI] [PubMed] [Google Scholar]
- 5.Mittendorf R, Dambrosia J, Pryde PG, Lee KS, Gianopoulos JG, Besinger RE, et al. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol 2002;186:1111–8. doi: 10.1067/mob.2002.123544 [DOI] [PubMed] [Google Scholar]
- 6.Crowther CA, Hiller JE, Doyle LW, Haslam RR, Australasian Collaborative Trial of Magnesium Sulphate ACTOMg SO4 Collaborative Group. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA 2003;290:2669–76. doi: 10.1001/jama.290.20.2669 [DOI] [PubMed] [Google Scholar]
- 7.Marret S, Marpeau L, Zupan-Simunek V, Eurin D, Lévêque C, Hellot MF, et al. Magnesium sulphate given before very-preterm birth to protect infant brain: the randomised controlled PREMAG trial. BJOG 2007;114:310–8. doi: 10.1111/j.1471-0528.2006.01162.x [DOI] [PubMed] [Google Scholar]
- 8.Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med 2008;359:895–905. doi: 10.1056/NEJMoa0801187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costantine MM, Weiner SJ, Eunice Kennedy Shriver National Institute of Child Health and Human Development NICHD Maternal–Fetal Medicine Units Network MFMU. Effects of antenatal exposure to magnesium sulfate on neuroprotection and mortality in preterm infants: a meta-analysis. Obstet Gynecol 2009;114:354–64. doi: 10.1097/AOG.0b013e3181ae98c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conde-Agudelo A, Romero R. Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks' gestation: a systematic review and metaanalysis. Am J Obstet Gynecol 2009;200:595–609. doi: 10.1016/j.ajog.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle LW, Crowther CA, Middleton P, Marret S. Antenatal magnesium sulfate and neurologic outcome in preterm infants: a systematic review. Obstet Gynecol 2009;113:1327–33. doi: 10.1097/AOG.0b013e3181a60495 [DOI] [PubMed] [Google Scholar]
- 12.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. The Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No.: CD004661. doi: 10.1002/14651858.CD004661.pub3 [DOI] [PubMed] [Google Scholar]
- 13.Magnesium sulfate before anticipated preterm birth for neuroprotection. Committee Opinion No. 455. American College of Obstetricians and Gynecologists. Obstet Gynecol 2010;115:669–71. doi: 10.1097/AOG.0b013e3181d4ffa5 [DOI] [PubMed] [Google Scholar]
- 14.Jayaram PM, Mohan MK, Farid I, Lindow S. Antenatal magnesium sulfate for fetal neuroprotection: a critical appraisal and systematic review of clinical practice guidelines. J Perinat Med 2019;47:262–9. doi: 10.1515/jpm-2018-0174 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO recommendations on interventions to improve preterm birth outcomes. WHO; 2015. [PubMed] [Google Scholar]
- 16.Crowther CA, Middleton PF, Voysey M, Askie L, Duley L, Pryde PG, et al. Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: an individual participant data meta-analysis. PLoS Med 2017;14:e1002398. doi: 10.1371/journal.pmed.1002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shepherd ES, Goldsmith S, Doyle LW, Middleton P, Marret S, Rouse DJ, et al. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. The Cochrane Database of Systematic Reviews 2024, Issue 5. Art. No.: CD004661. doi: 10.1002/14651858.CD004661.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 6.2. Accessed March 1, 2023. training.cochrane.org/handbook [Google Scholar]
- 20.Cochrane. RevMan Web, version 4.12.0. Accessed March 1, 2023. revman.cochrane.org [Google Scholar]
- 21.Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group; 2013. [Google Scholar]
- 22.Wolf HT, Brok J, Henriksen TB, Greisen G, Salvig JD, Pryds O, et al. Antenatal magnesium sulphate for the prevention of cerebral palsy in infants born preterm: a double-blind, randomised, placebo-controlled, multi-centre trial. BJOG 2020;127:1217–25. doi: 10.1111/1471-0528.16239 [DOI] [PubMed] [Google Scholar]
- 23.Crowther CA, Ashwood P, Middleton PF, McPhee A, Tran T, Harding JE, et al. Prenatal intravenous magnesium at 30-34 weeks' gestation and neurodevelopmental outcomes in offspring: the MAGENTA randomized clinical trial. JAMA 2023;330:603–14. doi: 10.1001/jama.2023.12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chollat C, Enser M, Houivet E, Provost D, Bénichou J, Marpeau L, et al. School-age outcomes following a randomized controlled trial of magnesium sulfate for neuroprotection of preterm infants. J Pediatr 2014;165:398–400.e3. doi: 10.1016/j.jpeds.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 25.Doyle LW, Anderson PJ, Haslam R, Lee KJ, Crowther C, Australasian Collaborative Trial of Magnesium Sulphate ACTOMgSO4 Study Group. School-age outcomes of very preterm infants after antenatal treatment with magnesium sulfate vs placebo. JAMA 2014;312:1105–13. doi: 10.1001/jama.2014.11189 [DOI] [PubMed] [Google Scholar]
- 26.Wolf HT, Huusom LD, Henriksen TB, Hegaard HK, Brok J, Pinborg A. Magnesium sulphate for fetal neuroprotection at imminent risk for preterm delivery: a systematic review with meta-analysis and trial sequential analysis. BJOG 2020;127:1180–8. doi: 10.1111/1471-0528.16238 [DOI] [PubMed] [Google Scholar]
- 27.Zeng X, Xue Y, Tian Q, Sun R, An R. Effects and safety of magnesium sulfate on neuroprotection: a meta-analysis based on PRISMA guidelines. Medicine 2016;95:e2451. doi: 10.1097/md.0000000000002451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magpie Trial Follow-Up Study Collaborative Group. The Magpie Trial: a randomised trial comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for children at 18 months. BJOG 2007;114:289–99. doi: 10.111/j.1471-0528.2006.01165.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bain ES, Middleton PF, Crowther CA. Maternal adverse effects of different antenatal magnesium sulphate regimens for improving maternal and infant outcomes: a systematic review. BMC Pregnancy Childbirth 2013;13:195. doi: 10.1186/1471-2393-13-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shepherd E, Salam RA, Manhas D, Synnes A, Middleton P, Makrides M, et al. Antenatal magnesium sulphate and adverse neonatal outcomes: a systematic review and meta-analysis. PLoS Med 2019;16:e1002988. doi: 10.1371/journal.pmed.1002988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marret S, Chollat C, Sentilhes L. Prenatal intravenous magnesium and neurodevelopmental outcomes in offspring. JAMA 2023;330:2306–7. doi: 10.1001/jama.2023.21723 [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi A, Ito M, Ota E, Namba F. School-age outcomes of antenatal magnesium sulphate in preterm infants. Children 2023;10:1324. doi: 10.3390/children10081324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr 2017;171:897–907. doi: 10.1001/jamapediatrics.2017.1689 [DOI] [PMC free article] [PubMed] [Google Scholar]