Abstract
This systematic review evaluates the efficacy of self-care interventions for atrial fibrillation (AF), focusing on strategies for maintenance, monitoring, and management applied individually or in combination. Adhering to the 2020 PRISMA guidelines, the search strategy spanned literature from 2005 to 2023, utilizing keywords and subject headings for “atrial fibrillation” and “self-care” combined with the Boolean operator AND. The databases searched included Medline, Embase, and CINAHL. The initial search, conducted on February 17, 2021, and updated on May 16, 2023, identified 5160 articles, from which 2864 unique titles and abstracts were screened. After abstract screening, 163 articles were reviewed in full text, resulting in 27 articles being selected for data extraction; these studies comprised both observational and randomized controlled trial designs. A key finding in our analysis reveals that self-care interventions, whether singular, dual, or integrated across all three components, resulted in significant improvements across patient-reported, clinical, and healthcare utilization outcomes compared to usual care. Educational interventions, often supported by in-person sessions or telephone follow-ups, emerged as a crucial element of effective AF self-care. Additionally, the integration of mobile and web-based technologies alongside personalized education showed promise in enhancing outcomes, although their full potential remains underexplored. This review highlights the importance of incorporating comprehensive, theory-informed self-care interventions into routine clinical practice and underscores the need for ongoing innovation and the implementation of evidence-based strategies. The integration of education and technology in AF self-care aligns with the recommendations of leading health organizations, advocating for patient-centered, technology-enhanced approaches to meet the evolving needs of the AF population.
Keywords: Atrial fibrillation, Self-care, Health promotion, Systematic review
Highlights
-
•
Systematic review of atrial fibrillation self-care interventions assessing patient outcomes and healthcare utilization.
-
•
Highlights the efficacy of integrating maintenance, monitoring, and management strategies in AF self-care.
-
•
Emphasizes the emerging role of technology, particularly mobile and web platforms, in enhancing AF self-care.
-
•
Identifies a gap in the use of theoretical frameworks in developing AF self-care interventions.
1. Introduction
Atrial fibrillation (AF), the most prevalent sustained arrhythmia, has seen an increase in morbidity, mortality, stroke, cognitive decline, and reduced quality of life (QOL), particularly amidst aging populations, highlighting the importance of effective AF management strategies [[1], [2], [3], [4]]. Recognizing the critical role of self-care in chronic disease management, as underscored by the American Heart Association's scientific statement, it is particularly vital in preventing and managing cardiovascular disease and stroke [5]. Self-care's efficacy, through key practices such as medication adherence, risk factor modification, and symptom self-monitoring, has been shown to enhance outcomes [[6], [7], [8]], including improved QOL, fewer hospitalizations, more efficient use of healthcare services [[9], [10], [11]] and extended survival [12], for patients with AF [5,12]. However, despite these benefits, self-care is often challenging for patients with AF, who tend to overlook their health regimen, are among the significant portion unaware of their AF condition, or are asymptomatic [[13], [14], [15], [16], [17], [18]]. This underscores the need for patients to acquire the necessary knowledge, confidence, and skills necessary to make informed treatment decisions and manage risk factors contributing to disease progression [17].
Self-care interventions are increasingly being developed for patients with AF, yet compared to other chronic cardiac conditions, the evidence for these interventions are less developed and studied [17]. Riegel's middle range theory of self-care in chronic disease [19] offers a framework for such a synthesis and examination. It conceptualizes self-care as a process of maintenance (e.g., risk reduction, stability), monitoring (e.g., detecting symptom changes), and management (e.g., responding to symptoms), that to be effective must include integrating or combining all three behaviors (see Table 1). Riegel et al. [19] posit that self-care management involves the greatest complexity and should be preceded by the other two self-care components and further that better outcomes result from performing evidence-based than non-evidence-based self-care. The effectiveness of combining these self-care strategies has been demonstrated in other conditions and is hypothesized to be similarly beneficial in AF [20]. Therefore, this study aims to synthesize evidence for the impact of AF self-care interventions on various health outcomes. We hypothesize that self-care activities encompassing maintenance, monitoring, and management will lead to improvements in patient reported outcomes (i.e., QOL), clinical outcomes (i.e., BMI, blood pressure, stroke), and/or healthcare utilization as compared to usual care.
Table 1.
The Middle Range Theory of Self-Care of Chronic Illness, proposed by Riegel et al. (2012), describes the process of self-care with health promotion strategies by individuals diagnosed with chronic illness. Self-care maintenance, self-care monitoring, and self-care management are identified as core elements of patient self-care in chronic illness.
| Self-Care Maintenance in the context of AF | Self-Care Monitoring in the context of AF | Self-Care Management in the context of AF |
|---|---|---|
|
|
|
2. Methods
This systematic review followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) design and reporting guidelines [21].
2.1. Search strategy
A health sciences librarian developed a draft search strategy through review of previous published papers in this area of research [20,22,23] and in consultation with subject matter experts on the research team. Appropriate keywords and subject headings for the concepts of atrial fibrillation and self-care were combined using AND (Appendix A) between the dates of 2005–2023. Databases used in this search included Medline, Embase, and CINAHL. The initial search was conducted on February 17, 2021 and updated to include the most recent literature on May 16, 2023. A total of 5160 articles were retrieved across both timerframes and loaded into Covidence, software designed for the the management of systematic reviews, including the the removal of 2203 duplicates and the screening of 2864 unique titles and abstracts.
2.2. Inclusion/exclusion criteria
Papers selected were evaluated for inclusion based on the following criteria: (1) included adults (≥19 years) with an AF diagnosis; (2) studied self-care, self-management, self-monitoring, and/or symptom management interventions (see Table 1); (3) measured and reported patient-reported outcomes, clinical outcomes, and/or healthcare utilization; and (4) used an observational (retrospective or prospective) or randomized design with usual care as the comparator. Papers were excluded if: (1) they reported preliminary results only; (2) they focused solely on medication adherence without considering other self-care activities; (3) their primary outcome was time in therapeutic range with warfarin therapy or major bleeding; (4) they were not original research (e.g., editorials); (5) were protocols; or (6) were review articles. Exclusion of papers focused solely on medication adherence and time in therapeutic range was intended to ensure the focus of the review was on evidence involving patients' active engagement in self-care.
2.3. Article selection
Seven trained individuals, both experienced and novice, completed article title and abstract screening in pairs with blind oversight to ensure unbiased selection, with conflicts resolved by the lead author. Cohen's Kappa, a measure of interrater reliability, indicated moderate rater agreement of 0.428, as analyzed using R version 4.3.0 [24,25]. The lead author completed the screening of full text papers, finalizing the selection process (see Supplementary Table 1).
2.4. Risk of bias assessment
A quality rating was derived for each paper using the Effective Public Health Practice Project [26] quality assessment tool for quantitative studies. Studies were rated as strong (no weak ratings), moderate (one weak rating), or weak (two or more weak ratings) according to six components: selection bias, study design, confounders, blinding, data collection method, and withdrawals and dropouts. Two reviewers (NM, NA) independently rated all the articles, and discrepancies were discussed with a third team member (LB) until a consensus was reached.
2.5. Data extraction
Data extraction was conducted by four trained Research Assistants and the Primary Investigator, following an intensive training regime on Covidence software and through a collaborative review of sample articles. Also included was detailed categorization of self-care interventions according to Riegel et al.'s [19] model that emphasized assigning interventions into maintenance, monitoring, or management. To ensure the highest levels of accuracy and reliability, the lead author engaged in regular audits and consensus meetings post-extraction. This rigorous methodical process promoted consistency in the collection of inclusive and detailed data across studies, with full extraction details available in Supplementary Table 2.
3. Results
3.1. Study characteristics
Twenty-seven studies were included (Fig. 1, Table 2). Designs included randomized control trials (n = 23), a prospective clinical cohort (n = 1), a pilot cohort from a randomized control trial (n = 1), a cluster-randomized control trial (n = 1), and a retrospective analysis of data from a randomized control trial (n = 1). A total of 8181 participants were represented in the studies. Sample sizes ranged from 46 to 3324 participants, with a median sample size of 114 participants. Approximately 60 % of the participants were male. Studies included participants from thirteen countries: United States (n = 7), China (n = 5), Iran (n = 3), Australia (n = 2), Spain (n = 2), Belgium (n = 1), Canada (n = 1), Denmark (n = 1), Greece (n = 1), South Korea (n = 1), Sweden (n = 1), Taiwan (n = 1), and The Netherlands (n = 1). Study quality was rated ‘strong’ for 13, ‘moderate’ for 11, and ‘weak’ for 3 (see Table 2).
Fig. 1.
PRISMA Flow Chart.
Table 2.
Summary of included studies.
| Author, Year, Country (Quality assessment) | Aim | Intervention | Sample; Male % | Mean Age (SD) | Design | Outcomes |
|---|---|---|---|---|---|---|
| (Abed et al., 2013); Australia (Moderate) [32] | To determine the effect of weight reduction and management of cardiometabolic risk factors on atrial fibrillation burden and cardiac structure. |
Face-to-Face with 24 h Email, Telephone Support: Modified very-low calorie diet (800–1200 kcal daily) to promote weight loss. Very-low-calorie meal replacement sachets (Prima Health Solutions) provided to replace two meals per day. Third meal consisted of calorie-controlled foods with high level of protein and lower GCI. Written exercise plan prescribed low-intensity exercise initially for 20 min 3 times a week then increase to 45 min 3 times a week. Theory: None |
Overweight and obese ambulatory patients (N = 150) with symptomatic AF; 67.33 % male. |
Intervention 59.6 (9.5) Control 60.3 (10.3) |
RCT |
Primary Outcomes AF symptom burden and symptom severity by Atrial Fibrillation Severity Scale; weight; BMI; waist circumference Secondary Outcomes Total AF episodes and duration burden by 7-day Holter; echocardiographic left the atrial area and left ventricular wall thickness. |
| (Caceres et al., 2020); United States (Strong) [45] | To examine the impact of iPhone Helping Evaluate AF Rhythm through Technology (iHEART) intervention on HRQoL in patients with AF. |
Face-to-Application: iPhone equipped with AliveCor Kardia mobile ECG system and unlimited data/text-messages. AliveCor Kardia mobile ECG system captured single‑lead 30 s ECG recordings through 2 electrodes on the mobile device. ECG strips interpreted by staff next day and clinically significant arrhythmias immediately referred to the provider. Text messages from a bank of text messages developed with an expert interdisciplinary panel. Text messages about AF management were sent every Wednesday and about lifestyle factors associated with AF risk on Mondays and Fridays. Theory: None |
Patients (N = 238) with documented AF, undergoing AF treatment with direct cardioversion or radiofrequency ablation to restore normal sinus rhythm; 77 % male. |
Intervention 61.4 (11.9) Control 61.2 (11.8) |
RCT | AF recurrence by AliveCor Mobile system; HRQoL by The Atrial Fibrillation Effect on QOL; Short-Form Health Survey; EuroQol-5D questionnaire; symptom severity by University of Toronto AF Severity Scale. |
| (Cai et al., 2022); China (Strong) [41] | To assess the effectiveness of short-term effectiveness of comprehensive, domiciliary, mobile application-guided and tele-monitored cardiac rehabilitation in patients who have undergone ablation for AF. |
Application: Three-part program: (1) the ShuKang™ application that included video materials with audio instructions focused on aerobic exercise and limb stretching to improve muscle strength, balance and flexibility. (2) Wearable electrocardiogram recording device to monitor heart rhythm and heart rate through application during exercise and at rest. ECG data transmitted via a mobile phone to the monitoring centre. Patients could also transmit electrocardiograms at any time (i.e., in palpitations and chest pain) (3) App Binglijia™ to let patients communicate with physicians and upload and share their daily exercise training records. Theory: None |
AF in-patients (N = 97) who underwent catheter ablation; 64.9 % male. | 57 (10) | RCT | VO2 peak; intervention adherence; minutes spent at target HR; self-reported physical activity by International Physical Activity Questionnaire (IPAQ); Health Beliefs Related to Cardiovascular Disease Scale and Exercise Self-Efficacy Scale; 72-h Holter monitoring. |
| (Chen et al., 2017); United States (Weak) [36] | To examine the impact of PHR-facilitated medication education on medication adherence, knowledge, and patient management. |
Face-to-Face, Web-based: One-on-one PHR training, including logging on, navigating MyChart (PHR), using the messaging system, viewing medication education material. Medication education materials are newsletters prepared by drug information specialists sent to patients' MyChart at 4-, 6-, and 10-weeks. Research coordinates communicated with patients through MyChart regarding importance of anticoagulation treatment. Theory: None |
Predominantly Caucasian (97.8 %) AF patients (N = 90), willing to activate PHR account; 69.6 % male. | 66 | RCT | Medication knowledge level; medication adherence by Medication Possession Ratio (MPR); patient engagement (PAM) |
| (Desteghe et al., 2019); Belgium (Moderate) [15] | To investigate the effect of reinforced targeted in-person education using the Jessa AF Knowledge Questionnaire (JAKQ) |
Face-to-Face: Two allied health professionals reinforced education based on the incorrectly answered questions of the Jessa Atrial fibrillation Knowledge Questionnaire. Healthcare professionals went through the questionnaire together with the patient and indicated whether their response was correct or not. If the answer was correct, the team immediately moved on to the next question. If the answer was wrong, the correct answer was indicated and shortly explained. Consistency achieved between two allied health professionals through training offered by electrophysiologists. Theory: None |
AF patients (N = 67; 26 hospitalized and 41 out-patient) who received AF diagnosis >1 year; 62.7 % male | 72.1 (8.6) | RCT | Jessa AF Knowledge Questionnaire (JAKQ); symptom burden by Leuven ARrhythmia Questionnaire (LARQ); QoL by Short Form Health Survey (SF-12); adherence to NOACs |
| (Ding et al., 2023); China (Moderate) [28] | To investigate the program's effect based on the theory of planned behavior and nudge strategy on adherence to anticoagulant treatment in patients with non-valvular atrial fibrillation. |
Face-to Face with application-based chat and telephone follow-up: With the help of the nurse in the department of cardiology, the intervention was completed during the hospitalization. The aim of intervention during the hospitalization was to shift attitude. The intervention strategy is to improve the behavioral beliefs and salience nudge. After discharge, the communication between participants and the researchers was mainly through the WeChat app and telephone. The intervention was completed with the assistance of the doctors and nurses. The intervention includes the following four parts: shifting attitude, improving perceived behavior control, promoting patients' subjective norms, and enhancing behavior intention changes toward behavior. Theory: theory of planned behavior and nudge strategy |
130 non-valvular AF patients on oral anticoagulants with a CHADS >1; 55 % male | Intervention 65.68 (8.87) | RCT | MMAS-8: Morisky Medication Adherence Scale; Intention; Attitude toward behavior; Perceived behavioral control; Subjective norm; AF-QoL-18 |
| (Fuenzalida et al., 2017); Spain (Moderate) [51] | To determine whether an educational intervention by nurses at discharge from the emergency room (ER) had a long-term effect on decreasing related complications. |
Face-to-Face: Nurse-led education regarding: 1) basic explanation of the arrhythmia; 2) possible AF-related or treatment-related complications that could arise: heart failure, stroke or systemic embolism, brady or tachy arrhythmia, hemorrhage; 3) precautions to consider: take the treatment daily, make follow-up visits to the treating doctor, do the required blood tests, monitor the pulse and perform cardiovascular exercise regularly, avoid alcohol and tobacco, and 4) warning signs and symptoms: palpitations, bradycardia, dyspnoea, chest pain, syncope, hemorrhage. Patients taught how to take pulse manually and encouraged to take pulse weekly. Patients given personalized leaflet about medication prescribed at discharge and summary of the information taught. Theory: None |
Patients (N = 240) with some type of AF (1st episode, paroxysmal, persistent, or permanent) and received consultation; 42.5 % male. | 76.1 (10.9) | RCT | Arrhythmia complication or treatment; number of ED visits; number of hospital admissions. |
| (Gagné et al., 2019); Canada; (Weak) [46] | To assess whether adding a video on atrial fibrillation to a face-to-face educational session improves QoL, knowledge, and health resource utilization among AF patients. |
Video: 8-min educational video called ‘AF’. Video included information about: (1) the conduction system of the heart and (2) normal heartbeat vs AF were described using an orchestra conductor as a metaphor. (3) types of AF, in addition to (4) risk factors for AF, (5) symptoms of AF, and (6) complications from AF, including signs of stroke, (7) pharmacological and non-pharmacological treatments options of AF and anticoagulation therapy. Theory: None |
AF outpatients (N = 60) who had not previously received education on AF at the outpatient AF clinic; 68 % male. |
Intervention 57 (13) Control 56 (13) |
RCT | QoL in AF (AFEQT); Knowledge of AF (KAF); Health Resource Utilization (HRU) HRQoL by SF-12 questionnaire; BP; BMI; smoking status; physical activity by Global Physical Activity Questionnaire; medication adherence by Morisky Medication Adherence Scale; appropriate oral anticoagulation use by CHA2DS2-VASc. |
| (Gallagher et al., 2020); Australia; (Strong) [63] | To determine if a nurse-led education program with tailored advice and goal setting for management of cardiovascular risk factors using a motivational interviewing approach, facilitated by a guideline-based electronic decision support tool to ensure appropriate oral anticoagulation, can improve HRQoL and cardiovascular risk factor status in individuals with AF. |
Face-to-Face with Telephone Follow-up: One-hour nurse-led educational and risk factor management session. Education provided about basic AF pathophysiology, causes, potential complications, treatment options, and appropriate stroke prevention therapies based on individual risk scores. Participants were encouraged to set 3 to 4 realistic risk factors or behavioral goals in line with their priorities and motivation with feedback from nurses. A summary of each participant's goals and strategies to meet their targets was given to them. This also contained written information about each individual's stroke risk score and if current antithrombotic therapy was appropriate. Theory: None |
AF patients (N = 72) referred by cardiologists; 56 % male. |
Intervention 63 (12) Control 66 (10) |
RCT | HRQoL by SF-12 questionnaire; BP; BMI; smoking status; physical activity by Global Physical Activity Questionnaire; medication adherence by Morisky Medication Adherence Scale; appropriate oral anticoagulation use by CHA2DS2-VASc. |
| (Guhl et al., 2020); United States; (Weak) [64] | To measure acceptability and adherence and to assess its effectiveness to improve HRQoL and medication adherence. |
Virtual: Smartphone-based relational agent named Tanya that stimulates face-to-face conversation with a health coach using synthetic speech and accompanying animated behavior. Dialogue content was organized as modules that focused on 3 different domains: AF education, symptoms, and adherence. Tanya can be programmed to refer to prior content areas and obtain repeated assessments to follow resolution of reported problems. Relational agent dialogue referred to the Kardia regularly to reinforce its use, provide instruction on the use of the device, and direct users to check rhythm concomitant with reporting symptoms. Theory: None |
Chronic AF patients (N = 120); 48.3 % male. | 72.1 (9.1) | RCT | HRQoL and AF-HRQoL by AFEQT; medication adherence; duration and frequency of intervention; intervention satisfaction. |
| (Guo et al., 2020); China (Moderate) [52] | To determine whether a mobile health (mHealth) technology-supported AF integrated management strategy would reduce AF-related adverse events. |
Application: Mobile application (Mafa) platform provided to both AF patients and doctors. For patients, application offered dynamic risk-monitoring, time in therapeutic range monitoring if on warfarin, rhythm/heart rare monitoring, educational materials and an interactive game, “What are triggers of AF?”. For doctors, application had clinical decision support tools to facilitate guideline-based treatment recommendations, educational materials and patient involvement strategies with self-care protocols, and structured follow-up, to support the implementation of the ABC pathway for integrated or holistic AF management. Theory: None |
Patients (N = 3324) with AF older than 18 years of age from 40 centers; 62 % male. |
Intervention 67 (15) Control 70 (12) |
Cluster RCT | Questionnaire on the mAFA platform; rates of the composite outcome of ischemic stroke/systemic thromboembolism; all-cause death; rehospitalization. |
| (Hendriks et al., 2014); Netherlands (Strong) [30] | To assess effect of a novel nurse-led integrated chronic care approach on QoL in AF patients. |
Face-to-Face: Nurse-led care consisted of software supported care (Cardio Consult AF), supervised by cardiologists where nurses educated patients about AF symptoms and complications. Nurses discussed diagnostic tests and treatment options. Software based on prevailing ACC/AHA/ESC guidelines for AF to assist professionals to provide comprehensive AF care. 30 min per visit. Theory: Chronic Care Model Framework for quality improvement |
AF patients (N = 534) who participated in previous AF effectiveness trial; 59.0 % male | 66.4 (12.0) | RCT | QoL by Medical Outcomes Study 36-Item Short-Form Survey Instrument (SF-36). |
| (Hickey et al., 2017); United States (Strong) [65] | To investigate the differences between mHealth and usual care over a 6-month follow-up period among patients with a known history of atrial fibrillation. |
Application: AliveCor ECG device given to patients. Device attaches to smartphone with one-time adhesive to capture daily ECG readings. Theory: None |
AF patients (N = 46) whose normal sinus rhythm restored; 69.6 % male. |
Intervention 55 (10) Control 55 (9) |
Pilot cohort from ongoing RCT | QoL by SF-36v2; ECG readings |
| (Hsieh et al., 2021); Taiwan (Strong) [48] | To evaluate the effects of a web-based integrated management program on improving coping strategies, medication adherence, and health-related quality of life (HRQoL) in patients with AF, and to detect its effect on decreasing readmission events. |
Virtual, Web-Based with Telephone Support: Web-based integrated management program included five domains: (1) patient information collection, (2) instructions on AF knowledge (texts and videos about AF), (3) instructions on anticoagulation medicine, (4) self-monitoring of symptoms, and (6) professional consultation from multidisciplinary professionals on any issues related to AF at any time. Research nurse also sent messages every day to monitor the participants' condition through the messaging function of this domain. When participants had an emergency, they could receive textual information or telephonic coaching. Theory: None |
AF patients (N = 231) from cardiovascular outpatient department; 50.2 % male. | 73.08 (11.71) | RCT | Coping strategies (COPE); medication adherence (MARS); HRQoL (EQ-5D & EQ-VAS); readmission events within 2 years. |
| (Joensen et al., 2019); Denmark (Strong) [43] | To investigate whether a rehabilitation programme with group education, physical exercise, optimization of medical treatment and monitoring of lifestyle changes can improve QoL and physical exercise capacity in patients with AF. |
Face-to-face: Rehabilitation exercise program with one-hour group sessions with doctor, nurse, dietician, or psychologist weekly for eight weeks. Education about pathophysiology, risk factors, treatment, diet and coping mechanisms. Exercise programme was conducted as 1-h sessions bi-weekly and supervised by a cardiac rehabilitation physiotherapist. Each training session consisted of at least 30 min of aerobics ≥70 % of maximum exercise capacity estimated from a maximum cycle ergometer test with ECG monitoring and interval training with elements of strengthening exercises. Theory: None |
Inpatients (N = 58) with paroxysmal or persistent, receiving usual care; 69 % male. |
Intervention 62.2 (10.0) Control 60.2 (8.9) |
RCT | 5 QoL questionnaires (AF-QoL-18, AFEQT, GAD-7, PHQ-9, EuroQol 5D); maximum exercise capacity via ergometer cycle test; sub maximum exercise capacity by 6 MW test; strength of lower extremities via 5-repetition-sit-to-stand (5RSS) test; O2 uptake. |
| (Li et al., 2022); Hong Kong (Strong) [31] | To evaluate the feasibility and preliminary effects of a nurse-led empowerment-based care model on HRQoL, AF knowledge, psychological outcomes, medication adherence, and treatment decision-making in patients with AF. |
Face-to-Face with Telephone Follow-up:The nurse-led multi-component behavioral activation programme lasted 13 weeks and comprised four care components to activate self-care behaviors in patients with AF: (i) risk profile assessment and shared decision-making regarding OAC use; (ii) empowerment-based educational module on AF self-care; (iii) nurse-initiated telephone support; and (iv) patient-initiated contact for professional advice Theory: Empowerment-based care model |
Patients documented with AF, not receiving OACs for stroke prevention in HER; 65 % male. | Intervention 71.6 (3.89) Control 72.6 (5.77) |
AFEQT, atrial fibrillation effect on QOL scale; AFKS, atrial fibrillation knowledge scale; HADS, hospital anxiety and depression scale; MGLA, Morisky–Green–Levine adherence scale | |
| (Márquez-Contreras et al., 2018); Spain (Strong) [37] | To assess the efficacy of a mixed intervention, educational, and reminder calendar of the intake, as a strategy to improve therapeutic adherence with dabigatran in patients with non-valvular atrial fibrillation (NVAF). |
Face-to-Face: Education booklet explained to the patient and handed out for at-home use. Booklet highlighted importance of adherence in this disease, the risk of nonadherence, the correct intake of dabigatran according to the SmPC, and what to do in special situations such as hemorrhages. MEMS handed out to track medication adherence. MEMS are electronic monitoring devices that have a digital recording device in the form of a microchip in the lid of the drug container that automatically controls its opening and registers the time and date of the opening. Theory: None |
Patients (N = 625) with chronic non-valvular AF (NVAF) that used dabigatran for stroke and systematic embolism prevention for 2 months continuously; 49.6 % male. | 73.42 (8.4) | RCT | Medication adherence; number of concomitant medications; BP; lipid panel |
| (Mohanty et al., 2022); United States (Moderate) [34] | To evaluate the software's ability to track and stratify patients with AF based on the biometrics; to evaluate the efficacy of the RFMx platform in intervening with health coaching and monitoring compliance for the lifestyle change recommendations. |
Face-to-face with App follow-up: Patients who met the study criteria were given a fitness tracker to track sleep, activity, and heart rate; Bluetooth-enabled weight scale, and blood pressure cuff paired to the RFMx app on their smartphone. They were teamed up with a health coach who created a tailored 12-week diet and exercise program using telehealth- and text-based interventions to help pa- tients achieve at least a 10 % reduction of body weight over the training period that was tracked via the app. Theory: None |
Consecutive AF patients (105) attending clinic; all patients received catheter ablation for AF; 58.5 % male | Intervention; 65.7 (10.8) Control 67.4 (8.6) |
RCT | Compliance; Percentage of group losing weight; Mean reduction in weight |
| (Najafi & Rakhshan, 2018); Iran (Moderate) [44] | To evaluate critical gaps in educational programs and self-management interventions of QoL in patients with AF. |
Face-to-Face with Telephone Follow-up: One-hour education session about AF, symptom control, challenges in psychosocial management of AF, and skills for self-management of chronic diseases and networking collaboration between patients. Educational handbook given out. Telephone follow-up to evaluate quantity of applied educations by patients, answer their questions and motivate them to participate in self-management activities. Theory: None |
Adult patients (N = 72) with resistant or recurrent AF, admitted to CCU, post-CCU and emergency (ED); approx. 59.7 % male. | 59 (13) | RCT | Lifestyle score from Health-Promoting Lifestyle Questionnaire; hospitalization rate of recurrent AF; cerebral embolic complications of AF. |
| (Oh & Hwang, 2021); South Korea (Strong) [49] | To examine effect of an individualized educational intervention on the knowledge, attitudes and self-management ability for outpatients with AF. |
Face-to-Face with Telephone Follow-up: 50–60 min. one-on-one education session, one 5–10 min. telephone counseling and maintenance of self-management diary. Small booklet including the definition, associated risk factors, complications of AF presented in large lettering with colourful pictures to help foster older participants' understanding. Individualized counseling used to identify specific complications, risk factors and management based on the participants' characteristics and comorbidity. Lifestyle risk factors identified, and participants asked to set their own goals for health improvement. Theory: None |
Elderly outpatients (N = 60), aged over 65 years with AF; 58.4 % male. | 71.9 (4.6) | RCT | General & disease-related characteristics (i.e., BMI, CHA₂DS₂-VASc); Knowledge of AF and Stroke Prevention (KAFSP) questionnaire; attitudes toward AF questionnaire |
| (Rakhshan et al., 2019); Iran (Strong) [50] | To investigate the effect of self-management interventions on the lifestyle of patients with AF. |
Face-to-Face with Telephone Follow-up: Two-part self-management intervention: 1) One-hour patient education about AF, and managing psychosocial challenges of living with AF and 2) Telephone follow-up to assess implementation of educated tips by patients, answering questions, and encouraging them to participate actively in self-management activities Theory: None |
AF patients (N = 72) referred to Vali-e-Asr Hospital; 71.67 % male. | 59.18 (13.09) | RCT | Walker's Health-Promoting Lifestyle Profile II (HPLP II) to measure health-promoting lifestyle behaviors. |
| (Schmidt et al., 2021); United States (Moderate) [29] | To determine if perceived self-management and satisfaction with provider communication differed between patients who participated in SMAs compared to patients in standard care. |
Face-to-Face: Shared medical appointments prior to scheduled ablation procedure and post-procedure 3 months. 90-min session with a NP where patients and family members shared experiences, provide education about AF in an interactive manner, and create an individualized care plan with group support. Curriculum with sample scripts and slide presentation was developed for both the pre-procedure SMA appointment and 3-month post-procedure. Short 10-min one-on-one visit offered additional for individual questions and concerns. Theory: Social Cognitive Theory |
Predominantly non-Hispanic White (99 %) patients (N = 123) with AF with an appointment for AF ablation procedure; 66 % male. | 60 | Retrospective analysis of RCT data | Perceived self-management (PAM short-form); perceived quality of healthcare team's chronic disease management (PACIC); AF knowledge (KAF); symptom severity (AFSS part C). |
| (Tang et al., 2017); China (Strong) [38] | To compare and observe the impact of the disease management model of “Treatment-Education-Follow-up” (TEF) on anticoagulant therapy in patients with Stroke with AF (S-AF). |
Face-to-Face with Telephone/Text Follow-up: Patient education on anticoagulant therapy by providing them S-AF prevention and anticoagulant therapy handbooks with regular post-discharge telephone follow-up and outpatient observation. Clinicians provided warfarin anticoagulant therapy to the patients according to their individual risk stratification scores (CHA2DS2-VASc ≥2 points). Patients also given stroke prevention and anticoagulant therapy handbooks at discharge. Post-discharge, patients monitored through outpatient follow-up, telephone or text alert for the monitoring, medication adjustment, health education, and patient need management. Theory: None |
Patients (N = 199) with acute ischemic stroke/TIA combined with AF (paroxysmal, persistent, or permanent); 60.3 % in intervention, 56 % in control male. | Participants intervention and control group majority aged ≥75 (63.8 % in intervention; 60 % in control) | Prospective clinical cohort study | Vascular events; hemorrhagic events; Morisky medication adherence scale; medication compliance; knowledge about warfarin anticoagulant therapy |
| (Toscos et al., 2020); United States (Moderate) [42] | To improve disease knowledge and medication adherence using tailored education and nudges. |
Virtual, Web-based: Educational messages and medication reminders send to participants via MyChart®. 24 educational messages and 14 videos featuring short interviews with four cardiologists and one pharmacist discussing topics surrounding AF and anticoagulation, together with patient testimonials created. Participants sent a medication-specific reminder message upon missing one DOAC dose or two warfarin doses, as recorded by the Smart bottle, with a follow-up message also sent upon missing a second DOAC dose or two additional warfarin doses, including a reminder of importance of OACs in stroke prevention, suggestion to call the doctor about side effects or refills, and a prompt to email the coordinator about bottle issues or routine changes that could cause inaccurate recordings. Theory: None. |
Predominantly white (99 %) adult outpatients (N = 160) with nonvalvular AF and prescribed OAC; 62.5 % male. | 71.1 (8.5) | RCT | Patient engagement by Patient Activation Measure (PAM); health literacy by Newest Vital Sign (NVS) assessment; AF Knowledge Scale; intervention uptake; medication adherence by AdhereTech's Wireless Smart Pill Bottle. |
| (Tzikas et al., 2021); Greece (Moderate) [39] | To assess the impact of an educational, motivational intervention on adherence to oral anticoagulation (OAC) in patients with non-valvular AF. |
Face-to-Face with Telephone Follow-up: 15–20 min education session about AF and rationale for anticoagulation, risks of poor compliance with OAC treatment and value of OAC in AF. Educational leaflet that explained all pertinent information in a simple, direct way given with free telephone number for further questions. Follow-up with three prespecified telephonic interviews for re-education and re-motivation to adhere to OAC. Theory: None |
Patients (N = 1009) with non-valvular atrial fibrillation and received oral anticoagulation for thromboprophylaxis; 53.5 % male. |
Intervention 75.5 Control 76.0 |
RCT | Medication adherence; rate of persistence; treatment gaps in OAC therapy; clinical outcomes. |
| (Wahlström et al., 2020); Sweden (Strong) [33] | To evaluate the effects of MediYoga, in respect of health-related QOL, blood pressure, heart rate, as well as N-terminal pro b-type natriuretic peptide. |
Face-to-Face: MediYoga Group: Therapeutic yoga form based on deep breathing, slow movements and meditation, like from Kundalini yoga. The program aims to stretch the musculature in the chest to achieve a better breathing technique and to balance the autonomous nervous system. Relaxation Group: Listened to relaxing music as a group. Theory: None |
Patients (N = 108) with symptomatic paroxysmal atrial fibrillation; 49.1 % male. |
Intervention Yoga 65 (9) Relaxation 64 (15) Control 63 (10) |
RCT | HRQoL by SF-36, ASTA; BP; HR; N-terminal pro b-type natriuretic peptide (NT-proBN) level. |
| (Zadeh et al., 2019); Iran (Moderate) [40] | To determine effect of treatment regimen on QOL in hospitalized AF patients. |
Face-to-Face with Telephone Follow-up: Adherence to treatment regimen intervention included two educational sessions (45 min), an educational booklet and follow-up after discharge. Educational content derived from the sources and in accordance with the components of the AFEQT questionnaire provided, including information on the type of arrhythmia, the methods of treatment, the drugs used and its complications, the level of activity, the method of coping with mental problems, the importance of performing coagulation tests, the type of nutrition. 10 min telephone follow-up every month. Theory: None |
AF arrhythmia patients (N = 50) hospitalized in Cardiac Care Unit (CCU) and Post CCU Unit and Internal Heart Surgery Unit; 46 % male. | Majority 51–55 years old. | RCT | AF-related QoL by AFEQT. |
3.2. Interventions
A diverse range of self-care interventions for atrial fibrillation were delivered in various settings, including hospitals (n = 7), clinics (n = 12), and through digital platforms (n = 9), which emphasized maintenance (n = 19), monitoring (n = 13), and management (n = 21) strategies (see Table 3). Many interventions featured in-person delivery (n = 19), supplemented by telephone follow-ups (n = 9) and technological aids such as mobile apps, web portals, and a virtual relational agent (n = 1). Only four of the included studies used a theory or model to guide their intervention development [[27], [28], [29], [30]]. Two studies used theories of behavior change [27,28], one used a chronic care framework, specifically designed for quality improvement [29], and one was guided by an empowerment model [30].
Table 3.
Summary of interventions and outcomes.
| Author (setting) | Interventions | Dose Frequency & Duration | Outcomes |
|---|---|---|---|
| Self-Care Maintenance: single focus | |||
| Abed et al., 2013 (AF clinic) |
5 clinic visits quarterly plus daily 24-h email/phone support Target: diet and exercise |
Clinic visits every 3 months over 15 months. | Patient: Reduced symptom burden, severity, episodes Clinical: Reduced weight (14.3 vs. 3.6 kg, respectively), improved cardiac remodeling |
| Mohanty et al., 2022 (clinic) | Tailored 12-week diet and exercise program using telehealth and text-based interventions focused on weight or BMI management | 12 weeks | Patient: Compliance with weight loss program Clinical: Weight loss (p = 0.03) |
| Wahlstrom et al., 2020 (AF clinic) | 12 weekly Medi Yoga and relaxation sessions | Once per week over 12 weeks. | Patient: Improvements in SF health, no change QOL Clinical: Reduced BP: 7 and 5 mmHg systolic and diastolic |
| Self-Care Monitoring: single focus | |||
| Hickey et al., 2017 (clinic) |
Daily ECG monitoring by smartphone | Daily and p.r.n. over 6 months. | Patient: Improved QOL domains Utilization: No change in hospitalizations |
| Self-Care Management: single focus | |||
| Chen et al., 2017 (virtual) |
PHR with in-person training and 3 newsletters re: medications at 4, 6, 10 wks and provider support | Patient-directed use of PHR over 3 months. | Patient: Improved medication adherence (10 %), no change in activation Clinical: Knowledge gain |
| Desteghe et al., 2019 (acute care) | Five (7 min) targeted education sessions by allied health-prof. over 1 year | At first, third, sixth and twelfth month over one year. | Patient: No change QOL, symptom burden, medication adherence Clinical: Knowledge gain |
| Ding et al., 2023 (acute care) | Nurse/Doctor lead education with telephone and text message follow-up over 6 months | One initial session in hospital and then follow-up visits scheduled every month for 20–30 min each time Text messages via app once per week over 6 months. |
Patient: Improved medication adherence, intention, attitude |
| Gallagher et al., 2020 (AF clinic) | Single nurse-led education and 4 follow-up phone calls | One educational and risk factor management session throughout, 3–4 follow-up telephone calls over 3 months. | Patient: No change QOL or medication adherence Clinical: No change BP, BMI, physical activity |
| Hendriks et al., 2014 (clinic) |
Nurse-led education over 4 30-min sessions in 1 year | Follow-ups at 3,6, 12 months over one year. | Patient: No change QOL Clinical: Knowledge gain |
| Marquez Contreras et al., 2018 (acute care) |
Education booklet and reminder calendar at baseline and 6 months | Follow-up at 6 and 12 months over 18 months. | Patient: Improved medication adherence Clinical: Reduced systolic and diastolic BP, weight and LDL in non-adherence intervention group |
| Schmidt et al., 2021 (AF clinic) |
Group medical visit including one-on-one session | Over 3 months | Patient: Improved goal setting, no change symptoms or activation/self-management Clinical: Knowledge gain Utilization: No change hospitalizations or ED visits |
| Tang et al., 2017 (acute care) |
Single education session, handbook and regular post-discharge follow-up | Patient-directed enrollment period, ranges from 6 months – 1.5 years) | Patient: Improved anticoagulant adherence (30 %) Clinical: Knowledge gain, reduced thrombotic events (12 %), no change in bleeding events |
| Tzikas et al., 2021 (acute care) |
One education session using motivational interviewing & handouts at discharge, plus 4 post-discharge phone follow up that involved re-education and motivated to remain motivated to OAC adherence | One session and telephone interviews at 1 wk., 2 months, 6 months over one year. | Patient: Improved 1-year anticoagulant adherence (25 %) Clinical: No change mortality, embolism, bleeding Utilization: No change hospitalizations or ED visits |
| Zedah et al., 2019 (acute care) |
Two 45-min educational sessions, booklet plus 1-mo post-discharge follow up | Over 12 weeks | Patient: Improved QOL at 1 and 3 months |
| Dual component interventions | |||
| Cai et al., 2021 (Maintenance and Monitoring) (clinic) |
12-wk exercise/rehab program (both groups) plus mobile app with ECG data collection | 5–7 days per week of exercise over 3 months. | Patient: Greater exercise self-efficacy and health beliefs related to cardiovascular disease Clinical: Improved V02 peak (4.9 ± 6.6 vs. 9.3 ± 8.0 ml/[min × kg]), no change in arrythmia recurrence |
| Toscos et al., 2020 (Management and Monitoring) (virtual) |
Health professional interview plus education and medication reminders | Over 6 months | Patient: Improved medication adherence Clinical: Knowledge gain |
| Joensen et al., 2019 (Maintenance and Management) (clinic) |
Rehab exercise program plus 8 one-hour, weekly group education sessions | Education sessions once a week over 8 weeks, exercise training sessions twice a week over twelve weeks. | Patient: No change QOL or symptoms Clinical: Improved exercise capacity (6 month) Utilization: No change hospitalizations or ED visits |
| Najafi et al., 2018 (Maintenance and Management) (acute care) |
6 one-hour education sessions over 3 weeks with handbook and 3 phone follow-ups | Follow-up at 4,8, 12 weeks over 3 months. | Patient: Improved lifestyle questionnaire score Clinical: No change stroke, bleeding risk Utilization: Reduced 3-mo hospitalization rate (27.8 % vs. 44.5 %) |
| Caceres et al., 2020 (Monitoring and Management) (AF clinic) |
Daily ECG capture via mobile software and AF messages thrice weekly | Daily ECG & symptom monitoring over 6 months. Text messages 3 times per week over 6 months. |
Patient: No change QOL |
| Gagne et al., 2019 (Monitoring and Management) (clinic) |
Education (both groups) plus 8-min educational video | One session with follow-up at 3 months. | Patient: No change QOL Clinical: No change knowledge Utilization: No change hospitalizations or ED visits |
| Guhl et al., 2020 (Monitoring and Management) (AF clinic) |
1 month smartphone-based, relational health coach | Encouraged daily use over 30 days. | Patient: Improved QOL and medication adherence (16.6 %) |
| Triple component interventions | |||
| Fuenzalida et al., 2017 (clinic) | 8-minute post-ED nurse-led education, handout, pulse monitoring training | One education session at ED discharge with follow-up in one year. | Clinical: Reduced 1-year AF-related complications (31.9 % vs. 48.4 %) Utilization: No change hospitalizations or ED visits |
| Guo et al., 2020 (AF clinic) |
Smartphone monitoring app, interactive game, clinical provider support | Three follow-ups over one year. | Clinical: Reduced composite: ischemic stroke, sys thromboembolism, death, re-hospitalization (1.9 % vs. 6.0 %; HR: 0.39; 95 % CI 0.22 to 0.67) Utilization: Reduced re-hospitalization (1.2 % and 4.5 %; HR: 0.32; 95 % CI: 0.17 to 0.60) |
| Hsieh et al., 2021 (virtual) |
Educational portal, multi-disciplinary care, monitoring, daily messages | Follow-up at 1,3,6 months over 2 years. | Patient: Improved health-related QOL, approach coping, medication adherence Utilization: Reduced 2-yr re-hospitalization (11 vs. 23; OR 0.406; 95 % CI 0.178–0.926) |
| Li et al., 2022 (AF Nurse clinic) | Group-based educational module covered medication management, risk factor management, activities and exercise. Phone follow-up x 4. | Patient: Improved AF QOL (p = 0.03) knowledge QOL (p = 0.01) | |
| Oh et al., 2021 (AF clinic) |
Fifty-min education session with resource and self-management diary, plus single counseling session | Over 12 weeks | Patient: Improved self-management behavior scores for medication, lifestyle, physical activity and symptom monitoring Clinical: Knowledge gain, no change in stroke risk factors |
| Rakhshan et al., 2019 (acute care) | Six one-hour education sessions plus manual and 3 phone follow-up calls | 6 times in 3 weeks over 12 weeks | Patient: Improved lifestyle scores (activity, nutrition, responsibility, stress management) |
3.3. Study outcomes
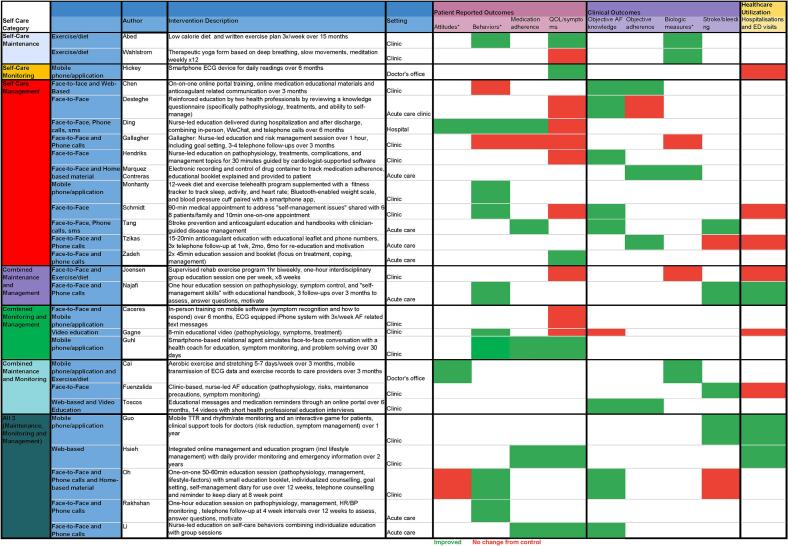
Study outcomes associated with the interventions were classified into patient-reported, clinical, and healthcare utilization outcomes (see Supplementary Table 3 for full list of outcomes). Fifteen studies measured patient-reported outcomes, 16 included clinical outcomes, and 9 studies investigated healthcare utilization or ED visits. Twenty-one of the 34 patient reported outcomes, 21 of 29 clinical outcomes, and 3 of 9 healthcare utilization showed improved outcomes compared to usual care (see Fig. 2). A summary of the effects of interventions (using Riegel's classification) on outcomes are described below.
Fig. 2.
Self-care Category Heat Map.
3.3.1. Single component interventions
Self-care interventions using a single component of Riegel's framework were implemented in 14 studies. Two studies implemented in-person exercise programs resulting in significant improvements in patient reported and clinical outcomes. Abed et al. [31] found a significant decline in patient-reported AF symptom severity in the intervention group compared to the control group (p < 0.001), alongside clinically observed reductions in weight (14.3 and 3.6 kg, respectively; p < 0.001). Compared to the control group, Wahlstrom et al. [32] found a yoga-based intervention decreased both systolic (p = 0.04) and diastolic (p = 0.005) blood pressure.
Among the five studies that implemented nurse-led in-person education programs, the effectiveness varied. Educational interventions using a single, brief and general session were observed to be either ineffective or to result in only a gain in knowledge, without enhancing other significant outcomes like medication adherence [15]. In contrast, an educational intervention, delivered across multiple sessions, was associated with notable improvements in medication adherence (p = 0.001) [27]. Another study using in-person medication adherence education combined with a paper reminder calendar showed improvements in medication adherence (p = 0.05) [33].
An additional four studies supplemented in-person education with telephone follow up. Tzikas et al. [34] found an educational session on AF and anticoagulant medication combined with telephone follow-up achieved an average adherence rate of about 25 % higher than the control group at 6 and 12 months (90.79 % and 89.20 % vs. 64.51 % and 63.22 %; p = 0.001). Similarly, the Tang et al. [35] study demonstrated that multimodal follow-up after in-person anticoagulant education using both telephone and text alerts significantly increased (p = 0.05) the persistence rate of long-term anticoagulant therapy by 30 % (84.5 % vs. 56.0 %) and resulted in a reduced (p = 0.05) recurrence of thrombotic events (4.0 % vs. 16.0 %) [35]. Zadeh et al. [36] found patients demonstrated improved quality of life (p = 0.05), following two focused in-person 45 min education sessions and 2 months of weekly telephone follow-ups. In contrast, an in-person nurse-led education and risk management session followed by 3–4 telephone follow-ups over 3 months found no improvement in patient-reported outcomes or clinical measures compared to the control group [37].
Technology was used exclusively in three studies. Mohanty et al. [38] reported that a 12-week diet and exercise telehealth program supplemented with a smartphone app was associated with higher compliance with weight loss measures (diet (p = 0.025) and exercise (p = 0.042)) compared to the conventional methods. Hickey et al. [39] found a daily smartphone electrocardiogram (ECG) monitoring intervention improved patient reported outcomes including QOL domains for physical functioning (p = 0.008), role physical (0.007), vitality (p = 0.003), and mental health (p = 0.02), but did not impact hospitalizations. Another study using medication-focused educational newsletters delivered through a personal health record (PHR) three times over a 4, 6, and 10-week period increased medication adherence (p = 0.001) over controls by 10 % measured using pharmacy refill data [40], however, information was only available for 48 % of participants.
3.3.2. Dual component interventions
Self-care interventions using two components of Riegel's [19] framework were implemented in seven studies. Three studies used in-person education interventions with mixed outcomes. Fuenzalida [41] implemented nurse-led brief (lasted on average 7.9 ± 2.0 min) in-person educational sessions that covered AF generally (e.g., pathophysiology, risks, maintenance precautions, symptom monitoring) and self-monitoring manual pulse education, demonstration and patient encouragement to perform weekly. Complications from AF were less frequent in the intervention group (p = 0.005) compared to the control group, but there were no significant differences in healthcare utilization. Joensen [42] found that general AF knowledge training followed with a 1-hour, bi-weekly exercise session, over 8 weeks improved the intervention patient group's exercise capacity at six months (p = 0.03) but resulted in no significant difference in patient-reported or healthcare utilization outcomes compared to the control group. Another dual intervention study combined intensive in-person education (six hour-long sessions over three weeks) integrating behavioral health promotion and disease management components (e.g., psychosocial management, problem-solving, decision-making, utilizing resources, and patient networking) with telephone follow-ups [43] and found a significant decrease (p < 0.05) in hospitalization rates for recurrent AF at both one- and three-months post-intervention.
Five studies investigated technology interventions exclusively, yielding significant improvements in various clinical outcomes. Cai et al. [44] reported that participants following a personalized exercise training program delivered through mobile app video and audio combined with transmissible wearable ECG device monitoring for symptomatic episode recordings to a monitoring center had increased AF knowledge (p = 0.02) and improved exercise capacity (VO2 max) (p = 0.003). Similarly, Toscos et al. [45] found increases in knowledge (p = 0.01) and medication adherence (p = 0.001) among patients on oral anticoagulants who received medication reminder messages for missed doses, medication adherence reinforcement, educational messages and videos, and suggestions for addressing potential issues (call Dr). Three studies combined ECG monitoring with other technologies: motivational text messaging [46], an educational video [47], and a smartphone-based, relational health coach simulating in-person conversation for medication and AF-symptom education [48]. Only the combined ECG and health coach intervention resulted in significantly improved QOL (p = 0.03) and self-reported medication adherence at 30 days in the intervention compared to control group (23.2 % vs. 3.5 % control group; adjusted difference 16.6 %, 95 % CI 2.8 %–30.4 %; p = 0.001). Clinical and healthcare utilization outcomes were not reported for these studies.
3.3.3. Triple component interventions
Six studies incorporated interventions encompassing all three self-care categories, resulting in significant improvements across various outcome domains. These six studies combined focused education with ongoing monitoring and support, utilizing both digital tools and personalized follow-up.
Two studies supplemented in-person education with phone follow-up. Li et al. found participants in nurse-led interactive educational sessions over five weeks using role-playing and scenarios to reinforce skills-based learning with six-week follow up telephone calls for support. Significantly increased QOL (p < 0.05) and AF knowledge (p < 0.001) but not medication adherence compared to controls. Similarly, Oh et al. [49] intervention comprised in-person educational and counseling sessions followed by a telephone session at 8 weeks, which focused on AF education and goal-setting. Participants were provided with an educational booklet and a paper self-management diary. Only 22 % (7/31) participants maintained the diary for the duration of the intervention (12 weeks). Intervention participants had significantly higher AF knowledge (p < 0.05) and self-management behaviors (p < 0.05) compared to the control group.
Two studies used technology exclusively to deliver their interventions. Hsieh et al. used an online management program to provide both education (text and video modules) and consultations with health care providers. Participants could also view their personal information. Intervention participants had significantly better medication adherence at 1, 3, and 6 months (p < 0.05) as well as QOL at 3 and 6 months (p < 0.05) compared to control groups. The intervention group also showed significantly fewer readmission events within 2 years (p < 0.05) compared with the control group. In their intervention, Guo [50] used a smartphone application with educational components, provider monitoring and emergency information, as well as provider decision support management following patients over 2 years. Combined rates of ischemic stroke/systemic thromboembolism, death, and rehospitalization’ were lower with the mAFA intervention compared with usual care (1.9 % vs. 6.0 %; hazard ratio [HR]: 0.39; 95 % confidence interval [CI]: 0.22 to 0.67; p < 0.001). Similarly, rehospitalization rates in the intervention group were lower compared to usual care (1.2 % vs. 4.5 %; HR: 0.32; 95 % CI: 0.17 to 0.60; p < 0.001).
4. Discussion
This comprehensive review, covering research from 2005 to 2023, systematically examined the effects of AF self-care interventions – maintenance, monitoring, and management – on patient-reported, clinical, and healthcare utilization outcomes. The key findings of this review were that overall, maintenance, monitoring, and management used singularly or in combination resulted in significant improvements across outcomes. Education emerged as a cornerstone of AF self-care interventions, often delivered in conjunction with in-person sessions or telephone follow-up. Technology integration, particularly through telehealth and virtual provider consultations, showed promise in enhancing outcomes, though its use was limited and its full potential remains underexplored. Further, this review highlights a critical need for theoretically driven AF self-care interventions.
Education emerges as a crucial intervention underlying all AF self-care components in this review. While foundational, education alone is often insufficient for fully engaging patients in long-term self-care and typically does not encompass all components of Riegel's theory [19]. Education was often used in conjunction with in-person and telephone follow-up interventions and with technology only interventions incorporating virtual/telehealth provider consultations over time and with significant patient-reported, clinical and healthcare utlization outcomes. Whether the length of time, delivery modality, or the ongoing contact/support with a provider or a combination of these factors yielded the significant outcomes requires further study. However, evidence suggests patients with AF often need ongoing support with problem-solving and decision-making given the unpredictable nature of the condition and its continually evolving treatment regimes [2].
The combination of mobile and web-based interventions with strategies such as personalized education and continuous support has shown promise in enhancing patient-reported clinical, and healthcare utilization outcomes, a finding that is also echoed in the heart failure literature [51,52]. In the current review, those studies using technology only inclusive of telehealth and virtual provider consultations reduced stroke, mortality, and hospitalizations. However, the majority of studies were in-person only or combined with telephone follow-up. Although these studies demonstrated positive patient-reported and clinical outcomes, they do not reflect the growing recognition of technology's role in managing AF, particularly in the broader context of chronic disease management [53]. To keep pace with the evolving technology landscape, and the preferences of patients with AF to continue virtual and technology integrated care since COVID-19 [54] technology embedded self-care interventions is imperative. Embracing diverse standalone multimedia tools, including smartphones, offers novel opportunities for managing chronic conditions like AF [55]. These developments signal a shift toward more technology-driven, patient-centered approaches in AF self-care, which provide patients with more flexibility and accessibility that may be limited with interventions that emphasize in-person interactions.
Findings from this review align with the theory in showing that interventions integrating all three self-care components achieved consistently significant patient, clinical, and healthcare utilization outcomes. This resonates with other evidence reported in heart failure interventions combining all three self-care components [56,57]. What could not be ascertained from the studies was whether ordering all three self-care components from least (self-maintenance) to most complex (self-management), consistent with Riegel's theory, achieved better outcomes than singular or dual components only. Beginning with foundational knowledge and skills through self-maintenance and self-monitoring activities facilitates key processes such as motivation, reflection, and decision-making needed for patients to self-manage and respond to signs, symptoms and changes in their condition and is expected to improve outcomes [5]. Current findings of improved healthcare utilization with dual and triple component interventions indicate beginning support for patients' enhanced self-care capacity to manage the complexities of AF.
The American Heart Association [5] and the Canadian Cardiovascular Society [58] endorse the integration of self-care interventions into care plans for patients with chronic conditions. Consequently, there is an urgent need for healthcare providers and policymakers to develop and implement evidence-based self-care interventions that align with the recommendations of leading health organizations and effectively address the multifaceted self-care needs of patients with AF. This review highlights that even single and dual component self-care interventions compared to usual care can improve outcomes. As patients AF self-care needs vary over their disease trajectory, single or combined self-care components will be required at different points in time to support symptom recognition (self-maintenance), self-assessment of action effectiveness (self-monitoring), and strategy adjustments (self-management) [59].
4.1. Study limitations
Despite promising evidence for using self-care approaches on outcomes, this review had several limitations. The intent of this review was to examine evidence for the outcomes of interventions using a single or a combination of self-care components. The challenge with use of this approach is that the variation in outcomes across studies made it difficult to compare across the various usages of the self-care components. A further limitation is that none of the studies explicitly used Riegel's [59] theory as a guiding framework, leading to the potential misclassification of maintenance, monitoring, and management-focused interventions. This was mitigated through the clear definition of indicators associated with each of the self-care components and multiple independent extractors for quality assurance. Further, even with our robust and thorough search strategy, there is a chance that we overlooked relevant studies, potentially limiting the scope of our conclusions.
Our conclusions about best approaches to enhancing patient self-care are limited by the paucity of studies targeting self-care maintenance and self-care monitoring approaches, the short-duration (3 months or less) of follow-up for half of the identified studies, and the limited types of outcomes tracked.
5. Conclusion
In conclusion, this systematic review, covering research from 2005 to 2023, offers a thorough analysis of self-care interventions for AF. It highlights the effectiveness of integrating maintenance, monitoring, and management strategies, to enhance patient-reported, clinical and healthcare utilization outcomes. The review also points to the potential of technology, especially mobile and web-based solutions, in aiding AF self-care, though more research is needed to fully understand their impact on healthcare utilization.
A key finding is the current lack of theoretically driven interventions in AF self-care, indicating a vital area for future research. Implementing interventions based on robust frameworks like Riegel's theory could greatly optimize the design of self-care strategies to enhance outcomes and advance self-care science. This review supports the recommendations of leading health organizations for the integration of self-care into chronic disease management plans and underscores the need for, evidence-based self-care strategies tailored to meet the evolving needs of AF patients. This work not only deepens the understanding of AF self-care but also charts a course for future research and clinical practice in this field.
The following are the supplementary data related to this article.
Data extraction process.
Title and abstract screening process.
Results summary.
Source of funding
Michael Smith Health Research BC (C2-2021-2293).
CRediT authorship contribution statement
Ryan E. Wilson: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Lindsay Burton: Writing – review & editing, Writing – original draft, Methodology, Formal analysis. Noah Marini: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Peter Loewen: Writing – review & editing, Writing – original draft, Methodology, Funding acquisition, Conceptualization. Robert Janke: Writing – review & editing, Methodology, Data curation. Noorat Aujla: Writing – review & editing, Writing – original draft, Data curation. Dresya Davis: Writing – review & editing, Writing – original draft, Investigation. Kathy L. Rush: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Medline Search History
Ovid MEDLINE(R) and Epub Ahead of Print, In-Process, In-Data-Review & Other Non-Indexed Citations, Daily and Versions <1946 to May 15, 2023>
1 exp Atrial Fibrillation/ 70437
2 "atrial fibrillation".tw,kf. 91078
3 self care/ or self-management/ 40757
4 exp consumer health information/ or exp health promotion/ or exp patient education as topic/ 181127
5 diagnostic self evaluation/ or self-assessment/ or self efficacy/ 41136
6 (self adj (car* or manag* or monitor* or administrat* or medicat* or efficacy or evaluat* or assess* or test* or exam* or guided or directed or help)).tw,kf. 154691
7 (patient adj1 (educat* or complian* or adher* or noncomplian* or nonadher*)).tw,kf. 41893
8 patient compliance/ 60519
9 ((treatment* or regimen or medication*) adj1 (complian* or adher* or noncomplian* or nonadher*)).tw,kf. 29937
10 (symptom* adj2 manag*).tw,kf. 17146
11 1 or 2 104225
12 or/3-10 462460
13 11 and 12 1294
14 limit 13 to (abstracts and english language) 1066
References
- 1.Åsberg S., Henriksson K.M., Farahmand B., Asplund K., Norrving B., Appelros P., Stegmayr B., Åsberg K.H., Terént A. Ischemic stroke and secondary prevention in clinical practice: a cohort study of 14 529 patients in the Swedish stroke register. Stroke. 2010;41:1338–1342. doi: 10.1161/STROKEAHA.110.580209. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B., Castella M., Diener H.-C., Heidbuchel H., Hendriks J., Hindricks G., Manolis A.S., Oldgren J., Popescu B.A., Schotten U., Van Putte B., Vardas P., Agewall S., Camm J., Baron Esquivias G., Budts W., Carerj S., Casselman F., Coca A., De Caterina R., Deftereos S., Dobrev D., Ferro J.M., Filippatos G., Fitzsimons D., Gorenek B., Guenoun M., Hohnloser S.H., Kolh P., Lip G.Y.H., Manolis A., McMurray J., Ponikowski P., Rosenhek R., Ruschitzka F., Savelieva I., Sharma S., Suwalski P., Tamargo J.L., Taylor C.J., Van Gelder I.C., Voors A.A., Windecker S., Zamorano J.L., Zeppenfeld K. ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. J. Cardiothorac. Surg. 2016;50(2016):e1–e88. doi: 10.1093/ejcts/ezw313. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S., Hart C.L., Hole D.J., McMurray J.J.V. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am. J. Med. 2002;113:359–364. doi: 10.1016/S0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 4.Petryszyn P., Niewinski P., Staniak A., Piotrowski P., Well A., Well M., Jeskowiak I., Lip G., Ponikowski P. Effectiveness of screening for atrial fibrillation and its determinants. A meta-analysis. PLOS ONE. 2019;14 doi: 10.1371/journal.pone.0213198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbara Riegel, Moser Debra K., Buck Harleah G., Vaughan Dickson Victoria, Dunbar Sandra B., Lee Christopher S., Lennie Terry A., JoAnn Lindenfeld, Mitchell Judith E., Treat-Jacobson Diane J., Webber David E., null null Self-care for the prevention and management of cardiovascular disease and stroke. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel D., Mc Conkey N.D., Sohaney R., Mc Neil A., Jedrzejczyk A., Armaganijan L. A systematic review of depression and anxiety in patients with atrial fibrillation: the mind-heart link. Cardiovasc. Psychiatry Neurol. 2013;2013:1–11. doi: 10.1155/2013/159850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCabe P.J. Psychological distress in patients diagnosed with atrial fibrillation: the state of the science. J. Cardiovasc. Nurs. 2010;25:40–51. doi: 10.1097/JCN.0b013e3181b7be36. [DOI] [PubMed] [Google Scholar]
- 8.Rush K.L., Oelke N.D., Shay M., Pedersen C. Seeing the rural healthcare journeys of older adults with atrial fibrillation through a photographic lens. Chronic Illn. 2017;13:204–216. doi: 10.1177/1742395316670462. [DOI] [PubMed] [Google Scholar]
- 9.Ball J., Carrington M.J., McMurray J.J.V., Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int. J. Cardiol. 2013;167:1807–1824. doi: 10.1016/j.ijcard.2012.12.093. [DOI] [PubMed] [Google Scholar]
- 10.Rohrbacker N.J., Kleinman N.L., White S.A., March J.L., Reynolds M.R. The burden of atrial fibrillation and other cardiac arrhythmias in an employed population: associated costs, absences, and objective productivity loss. J. Occup. Environ. Med. 2010;52:383–391. doi: 10.1097/JOM.0b013e3181d967bc. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg B.A., Kim S., Fonarow G.C., Thomas L., Ansell J., Kowey P.R., Mahaffey K.W., Gersh B.J., Hylek E., Naccarelli G., Go A.S., Reiffel J., Chang P., Peterson E.D., Piccini J.P. Drivers of hospitalization for patients with atrial fibrillation: results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF) Am. Heart J. 2014;167:735–742.e2. doi: 10.1016/j.ahj.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riegel B., Jaarsma T., Lee C.S., Strömberg A. Integrating symptoms into the middle-range theory of self-care of chronic illness. Adv. Nurs. Sci. 2019;42:206–215. doi: 10.1097/ANS.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson R.E., Rush K.L., Reid R.C., Laberge C.G. The symptom experience of early and late treatment seekers before an atrial fibrillation diagnosis. Eur. J. Cardiovasc. Nurs. 2020 doi: 10.1177/1474515120952220. [DOI] [PubMed] [Google Scholar]
- 14.Wilson R.E., Rush K.L., Hatt L., Reid R.C., Laberge C.G. The symptom experience of patients with atrial fibrillation before their initial diagnosis. J. Cardiovasc. Nurs. 2020:1. doi: 10.1097/JCN.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 15.Desteghe L., Engelhard L., Vijgen J., Koopman P., Dilling-Boer D., Schurmans J., Delesie M., Dendale P., Heidbuchel H. Effect of reinforced, targeted in-person education using the Jessa atrial fibrillation knowledge questionnaire in patients with atrial fibrillation: a randomized controlled trial. Eur. J. Cardiovasc. Nurs. 2019;18:194–203. doi: 10.1177/1474515118804353. [DOI] [PubMed] [Google Scholar]
- 16.Boriani G., Laroche C., Diemberger I., Fantecchi E., Popescu M.I., Rasmussen L.H., Sinagra G., Petrescu L., Tavazzi L., Maggioni A.P., Lip G.Y.H. Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF pilot general registry. Am. J. Med. 2015;128:509–518.e2. doi: 10.1016/j.amjmed.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 17.McCabe P.J. Moving beyond description to intervention to improve self-management knowledge for patients with atrial fibrillation. Int. J. Cardiol. 2018;272:225–226. doi: 10.1016/j.ijcard.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 18.Rho R.W., Page R.L. Asymptomatic atrial fibrillation. Prog. Cardiovasc. Dis. 2005;48:79–87. doi: 10.1016/j.pcad.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Riegel B., Jaarsma T., Strömberg A. A middle-range theory of self-care of chronic illness. Adv. Nurs. Sci. 2012;35:194–204. doi: 10.1097/ANS.0b013e318261b1ba. [DOI] [PubMed] [Google Scholar]
- 20.Riegel B., Westland H., Iovino P., Barelds I., Bruins Slot J., Stawnychy M.A., Osokpo O., Tarbi E., Trappenburg J.C.A., Vellone E., Strömberg A., Jaarsma T. Characteristics of self-care interventions for patients with a chronic condition: a scoping review. Int. J. Nurs. Stud. 2021;116 doi: 10.1016/j.ijnurstu.2020.103713. [DOI] [PubMed] [Google Scholar]
- 21.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., McKenzie J.E. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruppar T.M., Cooper P.S., Johnson E.D., Riegel B. Self-care interventions for adults with heart failure: a systematic review and meta-analysis protocol. J. Adv. Nurs. 2019;75:676–682. doi: 10.1111/jan.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Q., Chen C., Zhang J., Ye Y., Fan X. Effects of self-management interventions on heart failure: systematic review and meta-analysis of randomized controlled trials. Int. J. Nurs. Stud. 2020;110 doi: 10.1016/j.ijnurstu.2020.103689. [DOI] [PubMed] [Google Scholar]
- 24.McHugh M.L. Interrater reliability: the kappa statistic. Biochem. Medica. 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A language and environment for statistical computing.https://www.R-project.org/ Available online at. [Google Scholar]
- 26.Berghs M., Atkin K., Graham H., Hatton C., Thomas C. Implications for public health research of models and theories of disability: a scoping study and evidence synthesis. Public Health Res. 2016;4:1–166. doi: 10.3310/phr04080. [DOI] [PubMed] [Google Scholar]
- 27.Ding Y., Jiang H., Liu J., Chen D., Yang F. Effects of the theory of planned behavior and nudge strategy-based intervention on the adherence to anticoagulation treatment in patients with non-valvular atrial fibrillation. Geriatr. Nur. (Lond.) 2023;51:17–24. doi: 10.1016/j.gerinurse.2023.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt M.M., Griffin J.M., McCabe P., Stuart-Mullen L., Branda M., OByrne T.J., Bowers M., Trotter K., McLeod C. Shared medical appointments: translating research into practice for patients treated with ablation therapy for atrial fibrillation. PloS One. 2021;16 doi: 10.1371/journal.pone.0246861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendriks J.M.L., Vrijhoef H.J.M., Crijns H.J.G.M., Brunner-La Rocca H.P. The effect of a nurse-led integrated chronic care approach on quality of life in patients with atrial fibrillation. Europace. 2014;16:491–499. doi: 10.1093/europace/eut286. [DOI] [PubMed] [Google Scholar]
- 30.Li P.W.C., Yu D.S.F., Yan B.P. Nurse-led multi-component behavioural activation programme to improve health outcomes in patients with atrial fibrillation: a mixed-methods study and feasibility analysis. Eur. J. Cardiovasc. Nurs. 2022 doi: 10.1093/eurjcn/zvac104. [DOI] [PubMed] [Google Scholar]
- 31.Abed H.S., Wittert G.A., Leong D.P., Shirazi M.G., Bahrami B., Middeldorp M.E., Lorimer M.F., Lau D.H., Antic N.A., Brooks A.G., Abhayaratna W.P., Kalman J.M., Sanders P. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 32.Wahlström M., Rosenqvist M., Medin J., Walfridsson U., Rydell-Karlsson M. MediYoga as a part of a self-management programme among patients with paroxysmal atrial fibrillation – a randomised study. Eur. J. Cardiovasc. Nurs. 2020;19:74–82. doi: 10.1177/1474515119871796. [DOI] [PubMed] [Google Scholar]
- 33.Márquez-Contreras E., Martell-Claros N., Márquez-Rivero S., Hermida-Campa E., Gracia-Diez C., Sanchez-López E., Gil-Guillén V. Compliance and inertia working group, Spanish Society of Hypertension (SEH-LELHA), strategies for improving dabigatran adherence for stroke prevention in patients with non-valvular atrial fibrillation: education and drug intake reminders (FACILITA study) Curr. Med. Res. Opin. 2018;34:1301–1308. doi: 10.1080/03007995.2018.1435519. [DOI] [PubMed] [Google Scholar]
- 34.Tzikas A., Samaras A., Kartas A., Vasdeki D., Fotos G., Dividis G., Paschou E., Forozidou E., Tsoukra P., Kotsi E., Goulas I., Karvounis H., Giannakoulas G. Motivational interviewing to support Oral AntiCoagulation adherence in patients with non-valvular atrial fibrillation (MISOAC-AF): a randomized clinical trial. Eur. Heart J. - Cardiovasc. Pharmacother. 2021;7:f63–f71. doi: 10.1093/ehjcvp/pvaa039. [DOI] [PubMed] [Google Scholar]
- 35.Tang T., Zhang X., Tao L., Sun A., Qiu S., Zhang F., Xie B., Jiao X., Liu X., Tan L. Impact of the disease management model of “treatment-education-follow-up” on anticoagulant therapy in patients with stroke and atrial fibrillation. Biomed. Res. 2017;28:7. [Google Scholar]
- 36.Zadeh F.Y., Moeini M., Shafiee D. Evaluation of the effect of adherence to treatment regimen program on quality of life in atrial fibrillation patients hospitalized in Shahid Chamran Hospital in Isfahan in 2017. Rev. Latinoam. Hipertens. 2019;14:297–304. [Google Scholar]
- 37.Gallagher C., Orchard J., Nyfort-Hansen K., Sanders P., Neubeck L., Hendriks J.M. NursE led atrial fibrillation management: the NEAT study: a randomized controlled trial. J. Cardiovasc. Nurs. 2020;35:456–467. doi: 10.1097/JCN.0000000000000680. [DOI] [PubMed] [Google Scholar]
- 38.Mohanty S., Trivedi C., Della Rocca D.G., Gianni C., MacDonald B., Mayedo A., Shetty S., Natale E., Burkhardt J.D., Bassiouny M., Gallinghouse G.J., Horton R., Al-Ahmad A., Natale A. Impact of digital monitoring on compliance and outcome of lifestyle-change measures in patients with coexistent atrial fibrillation and obesity. Cardiovasc. Digit. Health J. 2022;3:75–79. doi: 10.1016/j.cvdhj.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hickey K.T., Reiffel J., Sciacca R.R., Whang W., Biviano A., Baumeister M., Castillo C., Talathothi J., Garan H. Correlating perceived arrhythmia symptoms and quality of life in an older population with heart failure: a prospective, single centre, urban clinic study. J. Clin. Nurs. 2013;22:434–444. doi: 10.1111/j.1365-2702.2012.04307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y.-C., Roebuck A., Sami A., Ersin Ö., Mirro M. The use of electronic personal health records to improve medication adherence and patient engagement: a randomized study of non-valvular atrial fibrillation patients. J. Innov. Card. Rhythm Manag. 2017;8:2804–2813. doi: 10.19102/icrm.2017.080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuenzalida C., Hernández G., Ferro I., Siches C., Ambrós À., Coll-Vinent B. Long-term benefits of education by emergency care nurses at discharge of patients with atrial fibrillation. Int. Emerg. Nurs. 2017;35:7–12. doi: 10.1016/j.ienj.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Joensen A., Dinesen P., Svendsen L., Hoejbjerg T., Fjerbaek A., Andreasen J., Sottrup M., Lundbye-Christensen S., Vadmann H., Riahi S. Effect of patient education and physical training on quality of life and physical exercise capacity in patients with paroxysmal or persistent atrial fibrillation: a randomized study. J. Rehabil. Med. 2019:0. doi: 10.2340/16501977-2551. [DOI] [PubMed] [Google Scholar]
- 43.Najafi H., Rakhshan M. Effect of self-management interventions on complications of atrial fibrillation: a clinical trial. 2018. https://www.biomedres.info/abstract/effect-of-selfmanagement-interventions-on-complications-of-atrial-fibrillation-a-clinical-trial-10485.html
- 44.Cai C., Bao Z., Wu N., Wu F., Sun G., Yang G., Chen M. A novel model of home-based, patient-tailored and mobile application-guided cardiac telerehabilitation in patients with atrial fibrillation: a randomised controlled trial. Clin. Rehabil. 2022;36:40–50. doi: 10.1177/02692155211032372. [DOI] [PubMed] [Google Scholar]
- 45.Toscos T.R., Coupe A., Wagner S., Drouin M., Roebuck A.E., Daley C.N., Carpenter M.D., Mirro M.J. Can nurses help improve self-care of patients living with atrial fibrillation? A focus group study exploring patients’ disease knowledge gaps. Nurs. Open. 2020;7:998–1010. doi: 10.1002/nop2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caceres B.A., Hickey K.T., Bakken S.B., Biviano A.B., Garan H., Goldenthal I.L., Koleck T.A., Masterson-Creber R., Turchioe M.R., Jia H. Mobile electrocardiogram monitoring and health-related quality of life in patients with atrial fibrillation: findings from the iPhone helping evaluate atrial fibrillation rhythm through technology (iHEART) study. J. Cardiovasc. Nurs. 2020;35:327–336. doi: 10.1097/JCN.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gagné M., Legault C., Boulet L.-P., Charbonneau L., Lemyre M., Giguere A.M.C., Poirier P. Impact of adding a video to patient education on quality of life among adults with atrial fibrillation: a randomized controlled trial. Patient Educ. Couns. 2019;102:1490–1498. doi: 10.1016/j.pec.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Guhl E., Althouse A.D., Pusateri A.M., Kimani E., Paasche-Orlow M.K., Bickmore T.W., Magnani J.W. The atrial fibrillation health literacy information technology trial: pilot trial of a mobile health app for atrial fibrillation. JMIR Cardio. 2020;4 doi: 10.2196/17162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh Y.H., Hwang S.Y. Individualized education focusing on self-management improved the knowledge and self-management behaviour of elderly people with atrial fibrillation: a randomized controlled trial. Int. J. Nurs. Pract. 2021;27 doi: 10.1111/ijn.12902. [DOI] [PubMed] [Google Scholar]
- 50.Guo Y., Lane D.A., Wang L., Zhang H., Wang H., Zhang W., Wen J., Xing Y., Wu F., Xia Y., Liu T., Wu F., Liang Z., Liu F., Zhao Y., Li R., Li X., Zhang L., Guo J., Burnside G., Chen Y., Lip G.Y.H., Guo Y., Lip G.Y.H., Lane D.A., Chen Y., Wang L., Eckstein J., Thomas G.N., Tong L., Mei F., Xuejun L., Xiaoming L., Zhaoliang S., Xiangming S., Wei Z., Yunli X., Jing W., Fan W., Sitong Y., Xiaoqing J., Bo Y., Xiaojuan B., Yuting J., Yangxia L., Yingying S., Zhongju T., Li Y., Tianzhu L., Chunfeng N., Lili Z., Shuyan L., Zulu W., Bing X., Liming L., Yuanzhe J., Yunlong X., Xiaohong C., Fang W., Lina Z., Yihong S., Shujie J., Jing L., Nan L., Shijun L., Huixia L., Rong L., Fan L., Qingfeng G., Tianyun G., Yuan W., Xin L., Yan R., Xiaoping C., Ronghua C., Yun S., Yulan Z., Haili S., Yujie Z., Quanchun W., Weidong S., Lin W., Chan E., Guangliang S., Chen Y., Wei Z., Dandi C., Xiang H., Anding X., Xiaohan F., Ziqiang Y., Xiang G., Fulin G. Mobile health technology to improve care for patients with atrial fibrillation. J. Am. Coll. Cardiol. 2020;75:1523–1534. doi: 10.1016/j.jacc.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 51.Athilingam P., Jenkins B., Johansson M., Labrador M. A Mobile health intervention to improve self-care in patients with heart failure: pilot randomized control trial. JMIR Cardio. 2017;1 doi: 10.2196/cardio.7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seto E., Leonard K.J., Cafazzo J.A., Barnsley J., Masino C., Ross H.J. Mobile phone-based telemonitoring for heart failure management: a randomized controlled trial. J. Med. Internet Res. 2012;14 doi: 10.2196/jmir.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freedman B., Hindricks G., Banerjee A., Baranchuk A., Ching C.K., Du X., Fitzsimons D., Healey J.S., Ikeda T., Lobban T., Mbakwem A., Narasimhan C., Neubeck L., Noseworthy P.A., Philbin D.M., Pinto F.J., Rwebembera J., Schnabel R.B., Svendsen J.H., Aguinaga L., Arbelo E., Böhm M., Farhan H.A., Hobbs F.D.R., Martínez-Rubio A., Militello C., Naik N., Noubiap J.J., Perel P., Piñeiro D., Ribeiro A.L.P., Stępińska J. World heart federation roadmap on atrial fibrillation – a 2020 update. Glob. Heart. 2021;16 doi: 10.5334/gh.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rush K.L., Burton L., Seaton C.L., Loewen P., O’Connor B.P., Moroz L., Corman K., Smith M.A., Andrade J.G. Telehealth satisfaction in patients receiving virtual atrial fibrillation care: quantitative exploratory study. JMIR Hum. Factors. 2023;10 doi: 10.2196/50232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallagher C., Elliott A.D., Wong C.X., Rangnekar G., Middeldorp M.E., Mahajan R., Lau D.H., Sanders P., Hendriks J. Integrated care in atrial fibrillation: a systematic review and meta-analysis. Heart. 2017 doi: 10.1136/heartjnl-2016-310952. [DOI] [PubMed] [Google Scholar]
- 56.Jaarsma T., Hill L., Bayes-Genis A., La Rocca H.-P.B., Castiello T., Čelutkienė J., Marques-Sule E., Plymen C.M., Piper S.E., Riegel B., Rutten F.H., Ben Gal T., Bauersachs J., Coats A.J.S., Chioncel O., Lopatin Y., Lund L.H., Lainscak M., Moura B., Mullens W., Piepoli M.F., Rosano G., Seferovic P., Strömberg A. Self-care of heart failure patients: practical management recommendations from the heart failure Association of the European Society of cardiology. Eur. J. Heart Fail. 2021;23:157–174. doi: 10.1002/ejhf.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riegel B., Lee C.S., Dickson V.V. Self care in patients with chronic heart failure. Nat. Rev. Cardiol. 2011;8:644–654. doi: 10.1038/nrcardio.2011.95. [DOI] [PubMed] [Google Scholar]
- 58.Howlett J.G., Chan M., Ezekowitz J.A., Harkness K., Heckman G.A., Kouz S., Leblanc M.-H., Moe G.W., O’Meara E., Abrams H., Ducharme A., Grzeslo A., Hamilton P., Koshman S.L., Lepage S., McDonald M., McKelvie R.S., Rajda M., Swiggum E., Virani S., Zieroth S. The Canadian cardiovascular society heart failure companion: bridging guidelines to your practice. Can. J. Cardiol. 2016;32 doi: 10.1016/j.cjca.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 59.Moser D.K., Dickson V., Jaarsma T., Lee C., Stromberg A., Riegel B. Role of self-care in the patient with heart failure. Curr. Cardiol. Rep. 2012;14:265–275. doi: 10.1007/s11886-012-0267-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data extraction process.
Title and abstract screening process.
Results summary.