Abstract
Background
Coronary in-stent restenosis (ISR) is a major clinical challenge of contemporary percutaneous revascularization and portends adverse cardiovascular outcomes.
Objectives
We aimed to evaluate gender, race, and ethnicity related outcomes in acute coronary syndromes (ACS) with ISR.
Methods
Primary hospitalizations for ACS and ISR in the National Inpatient Sample database from 2016 to 2019 were included. Patients were stratified by gender, race, and ethnicity. The primary end points were all cause in-hospital mortality and coronary revascularization defined as composite of percutaneous coronary intervention (PCI), balloon angioplasty and/or coronary artery bypass grafting (CABG).
Results
During the study period, a nationally weighted total of 97,680 patients with ACS and ISR were included. There was substantial variation in comorbidities, with greatest burden among Black and Hispanic women. All-cause in-hospital mortality was 2.4 % in the study cohort, but significantly higher in women (2.1 % vs. 2.1 %; aOR: 1.282, 95 % CI: 1.174–1.4; p < 0.001) and revascularization rates were significantly lower in women (77 % vs 80.2 %; aOR: 0.891, 95 % CI: 0.862–0.921; p < 0.001). Compared to White men, all women except Hispanic women, had significantly higher likelihood of in-hospital mortality, while White women, Black men and women, and Hispanic men had lower odds of revascularization.
Conclusions
There are significant gender, racial, and ethnic related differences in revascularization practices and clinical outcomes in patients with ACS and ISR with an adverse impact on women, racial and ethnic minorities in the U.S.
Keywords: In-stent restenosis, Acute coronary syndrome, Race, Gender, Disparities
1. Introduction
Women hospitalized with acute coronary syndromes (ACS) are at a higher risk for adverse outcomes compared to men [[1], [2], [3]]. Additionally, Black and Hispanic patients with ACS are more likely to experience worse outcomes compared to White patients [[4], [5], [6], [7]]. There are various reasons for these disparities despite the significant advances in percutaneous coronary intervention (PCI) and pharmacological treatment of patients with ACS. Coronary in-stent restenosis (ISR) remains one such drawback of PCI and an ongoing clinical challenge in the contemporary era. The mechanism of ISR is multifactorial, including biological, mechanical, operator-related, and patient-related factors. Despite an overlap of risk factors, coronary ISR has a distinctive pathophysiologic process compared to de-novo coronary artery disease with varied clinical manifestations ranging from stable angina to ACS [[8], [9], [10]]. Individuals with coronary ISR are also more likely to have a greater burden of native vessel CAD compared to those without ISR [11]. Moreover, these individuals who present with ACS have poorer cardiovascular outcomes compared with non-ACS presentations, irrespective of stent type [12]. Therefore, ACS patients with ISR constitute a unique high-risk cohort and remain under-recognized.
The rapidly increasing racial and ethnic diversity among the U.S. population has left significant gaps in our understanding of cardiovascular practices and clinical outcomes. Prior cardiovascular research has lumped gender-specific outcomes masking its distinctive interaction with race and ethnicity. To address this knowledge gap, we aimed to assess the combined impact of gender and race-ethnicity on in-hospital mortality and revascularization practices in a diverse cohort of hospitalized ACS patients with coronary ISR.
2. Methods
We performed a 4-year retrospective cohort analysis utilizing data obtained from 2016 to 2019 Healthcare Cost and Utilization Project (HCUP) National Inpatient Sample (NIS) database. NIS registry is the largest publicly available all-payer inpatient care database from the United States. It is developed as part of the Healthcare Cost and Utilization Project (HCUP) and is sponsored by the Agency for Healthcare Research and Quality available at https://www.hcup-us.ahrq.gov/overview.jsp. The NIS includes data from all nonfederal, short-term, general, and other specialty hospitals in the United States (excluding rehabilitation and long-term acute care hospitals) in the form of de-identified patient information containing demographics, discharge diagnoses, comorbidities, procedures, outcomes, and hospitalization costs. All the states that participate in HCUP provide data to the NIS, covering >95 % of the U.S. population. The database was designed to include data from a 20 % sample of discharges from all participating hospitals. This design of the NIS reduces the margin of error for estimates and delivers more stable and precise estimations [13]. The study was exempt from an Institutional Review Board approval because HCUP-NIS is a publicly available database containing only de-identified patient information.
Individuals admitted with acute coronary syndromes (ACS), defined as a composite of hospitalizations with primary discharge diagnosis of either ST- elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI) or unstable angina (UA), were identified using appropriate ICD -10 codes. In this cohort, individuals with newly diagnosed coronary ISR were identified using ICD-10 code - T82.855A and stratified by race-ethnicity and gender using “race” and “female” NIS variables. “White” refers to non-hispanic white individuals, “Black” refers to non-hispanic black individuals, “Hispanic” refers to hispanic individuals of all races and origins, and “Other” refers to asian, pacific-islander and native american individuals. We excluded patients with incomplete data for age, gender, race-ethnicity, length of stay and in-hospital mortality. Furthermore, to reduce the possibility of data duplication, the patients with an indicator for transfer to another acute-care facility were excluded.
Baseline patient characteristics included age, demographics, clinically relevant comorbidities, and Charlson comorbidity index score. Other characteristics, such as teaching status of the hospital, hospital bed size, insurance status, and discharge disposition, were also included. Supplementary Table 1 lists the ICD-10-CM codes used to identify comorbidities.
The primary endpoints were all cause in-hospital mortality and revascularization, defined as composite of percutaneous coronary intervention (PCI) with either drug eluting stent (DES)/ bare metal stent (BMS), balloon angioplasty and/or coronary artery bypass grafting (CABG), during hospitalization for ACS with ISR. Additionally, differences in clinical presentation, procedural outcomes (PCI - DES/BMS, balloon angioplasty, CABG, atherectomy, intra-aortic balloon pump or percutaneous ventricular assist device) and clinical outcomes (cardiogenic shock, stroke, acute kidney injury, major bleeding and length of stay) were compared among the various groups.
We compared the baseline characteristics between patients using the Pearson chi-square test for categorical variables, and Student t-test/Mann-Whitney U test for continuous variables with normal and skewed distribution respectively. National estimates were calculated by applying discharge weights to the data. Multivariate logistic regression was used to compare all-cause in-hospital mortality and revascularization between groups, adjusting for age, Charlson comorbidity index score, hospital bed size, teaching status, insurance, median household income and type of ACS presentation.
Statistical analysis was performed using SPSS Statistics 25.0 (IBM Corp., Armonk, New York) and R statistical software. All p values were 2-sided with a significance threshold of <0.05. Categorical variables were expressed as percentages and continuous variables as mean ± SD for normally distributed data or median with interquartile range for skewed data. Odds Ratio and 95 % confidence intervals (CIs) were used to report the results of logistic regression.
3. Results
We identified a total of 97,680 patients hospitalized with ACS who were diagnosed with coronary ISR lesion(s), of whom 75.9 % were white, 10.8 % were black, 7.5 % were Hispanic, and 6.1 % were of ‘Other’ race/ethnicity. Women constituted 31.5 % (n = 30,815) of the final study sample and were slightly older compared to men (66 years vs. 67 years; p < 0.001). Congestive heart failure, end-stage renal disease (ESRD), prior stroke, carotid artery stenosis, hypertension, diabetes, and obesity were more common in women, whereas prior myocardial infarction, prior coronary artery bypass grafting, alcohol abuse and smoking were more common in men (Table 1). Chronic total occlusion (CTO) was most prevalent in Hispanic men (13.1 %), followed by White men (10.1 %) and Black women (10.1 %). Hispanic women had the highest burden of diabetes (74.1 %) and hypertension (95.9 %). ESRD was most prevalent in Hispanic women (19.4 %), followed by Black women (18.3 %). Smoking was most prevalent in Black men (30.8 %), followed by White men (25.2 %) and White women (25.1 %). Overall, burden of comorbidities (CCI ≥ 5) was greater in women compared to men, particularly black women (44.9 %), followed by Hispanic women (43.6 %).
Table 1.
Baseline characteristics.
| Total, N (%) |
White |
Black |
Hispanic |
Other |
P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | |||
| N (%) | 97,680 (100) | 51,955 (53.2) | 22,150 (22.7) | 5625 (5.8) | 4610 (4.7) | 5070 (5.2) | 2295 (2.3) | 4215 (4.3) | 1760 (1.8) | |
| Age (Median) | 67(58,75) | 67(59,75) | 69(60,77) | 62 (54,69) | 62 (55,71) | 65 (57,73) | 66 (57,74) | 63 (57,72) | 67 (59,76) | <0.01 |
| Comorbidities | ||||||||||
| CTO | 9930 (10.2) | 5635 (10.8) | 1690 (7.6) | 550 (9.8) | 500 (10.8) | 665 (13.1) | 220 (9.6) | 550 (13) | 120 (6.8) | <0.01 |
| CHF | 37,235 (38.1) | 17,780 (34.2) | 9035 (40.8) | 2600 (46.2) | 2260 (49) | 2090 (41.2) | 1030 (44.9) | 1670 (39.6) | 770 (43.7) | <0.01 |
| Prior MI | 42,090 (43.1) | 23,035 (44.3) | 9015 (40.7) | 2560 (45.5) | 1895 (41.1) | 2175 (42.9) | 860 (37.5) | 1810 (42.9) | 740 (42) | <0.01 |
| Prior CABG | 15,455 (15.8) | 8960 (17.2) | 2980 (13.5) | 800 (14.2) | 580 (12.6) | 915 (18) | 350 (15.3) | 625 (14.8) | 245 (13.9) | <0.01 |
| Atrial fibrillation | 17,045 (17.4) | 10,005 (19.3) | 3920 (17.7) | 705 (12.5) | 510 (11.1) | 810 (16) | 275 (12) | 545 (12.9) | 275 (15.6) | <0.01 |
| Hypertension | 89,805 (91.9) | 47,340 (91.1) | 20,305 (91.7) | 5365 (95.4) | 4380 (95) | 4670 (92.1) | 2200 (95.9) | 3880 (92.1) | 1665 (94.6) | <0.01 |
| Diabetes mellitus | 49,025 (50.2) | 23,545 (45.3) | 11,230 (50.7) | 2950 (52.4) | 3065 (66.5) | 2960 (58.4) | 1700 (74.1) | 2425 (57.5) | 1150 (65.3) | <0.01 |
| Dyslipidemia | 81,300 (83.2) | 44,070 (84.8) | 18,255 (82.4) | 4320 (76.8) | 3640 (79) | 4290 (84.6) | 1745 (76) | 3570 (84.7) | 1410 (80.1) | <0.01 |
| PVD | 16,720 (17.1) | 8950 (17.2) | 3900 (17.6) | 1035 (18.4) | 760 (16.5) | 815 (16.1) | 340 (14.8) | 640 (15.2) | 280 (15.9) | <0.01 |
| ESRD | 6395 (6.5) | 2180 (4.2) | 940 (4.2) | 805 (14.3) | 845 (18.3) | 480 (9.5) | 445 (19.4) | 430 (10.2) | 270 (15.3) | <0.01 |
| Prior Stroke | 11,575 (11.8) | 5350 (10.3) | 3030 (13.7) | 810 (14.4) | 855 (18.5) | 530 (10.5) | 320 (13.9) | 425 (10.1) | 255 (14.5) | <0.01 |
| Carotid Artery Stenosis | 3800 (3.9) | 2020 (3.9) | 1085 (4.9) | 90 (1.6) | 135 (2.9) | 175 (3.5) | 115 (5) | 100 (2.5) | 80 (4.5) | <0.01 |
| COPD | 20,005 (20.5) | 10,450 (20.1) | 6060 (27.4) | 885 (15.7) | 945 (20.5) | 665 (13.1) | 255 (11.1) | 515 (12.2) | 230 (13.1) | <0.01 |
| Obesity | 23,385 (23.9) | 12,105 (23.3) | 6030 (27.2) | 1090 (19.4) | 1540 (33.4) | 1020 (20.1) | 580 (25.3) | 630 (14.9) | 390 (22.2) | <0.01 |
| Smoking | 23,795 (24.4) | 13,070 (25.2) | 5550 (25.1) | 1730 (30.8) | 1065 (23.1) | 1035 (20.4) | 310 (13.5) | 765 (18.1) | 270 (15.3) | <0.01 |
| Alcohol Abuse | 1895 (1.9) | 1255 (2.4) | 130 (0.6) | 270 (4.8) | <100 (1.3) | <100 (2) | <100 (0.2) | <100 (1.5) | <100 (0.6) | <0.01 |
| Drug Abuse | 2105 (2.2) | 935 (1.8) | 340 (1.5) | 440 (7.8) | 145 (3.1) | <100 (1.9) | <100 (2.4) | <100 (1.3) | <100 (2.3) | <0.01 |
| Charlson Comorbidity Index (%) | <0.01 | |||||||||
| 0 | 2.9 | 3.4 | 2.6 | 1 | 1.1 | 3.2 | 2.4 | 3.8 | 1.7 | |
| 1–2 | 38.4 | 42.4 | 36 | 33.7 | 24.5 | 36 | 27.5 | 36.8 | 29.3 | |
| 3–4 | 29.3 | 28.7 | 31.3 | 28.8 | 29.5 | 28 | 26.6 | 28.1 | 30.1 | |
| ≥5 | 29.4 | 25.5 | 30.1 | 36.5 | 44.9 | 32.8 | 43.6 | 31.3 | 38.9 | |
| Other characteristics (%) | ||||||||||
| Urban Teaching Hospital | 51.6 | 51 | 50.3 | 54.3 | 55.9 | 52.4 | 53.8 | 54.6 | 56.8 | <0.01 |
| Hospital Bed Size | <0.01 | |||||||||
| Small | 10.6 | 10.4 | 11.8 | 11.3 | 10.5 | 9 | 9.6 | 8.7 | 9.7 | |
| Medium | 20 | 20 | 19.1 | 19.8 | 19.4 | 22.4 | 20.3 | 20.3 | 22.2 | |
| Large | 37.5 | 38 | 37.3 | 36.4 | 37.6 | 31.6 | 39 | 39.3 | 39.5 | |
| Median Household Income | <0.01 | |||||||||
| 0–25 | 30.9 | 26.4 | 30.7 | 52 | 55.3 | 36 | 41.2 | 21.4 | 27.6 | |
| 26–50 | 27.8 | 28.9 | 29.8 | 22.8 | 21.8 | 26.8 | 29.2 | 21 | 22.4 | |
| 51–75 | 23.1 | 24.5 | 23.6 | 15.4 | 14.1 | 22.6 | 19 | 26.2 | 24.4 | |
| 76–100 | 16.4 | 18.3 | 14.5 | 9 | 6.7 | 12.5 | 9.2 | 29.8 | 23.9 | |
| Primary Payer | <0.01 | |||||||||
| Medicare | 61.6 | 60.1 | 69.8 | 53.7 | 61.6 | 57 | 63.6 | 48.9 | 70.2 | |
| Medicaid | 9.2 | 6.9 | 9.0 | 14.8 | 17.2 | 11.5 | 15.9 | 15.2 | 11.1 | |
| Private insurance | 22.3 | 25.6 | 16.5 | 20.5 | 17.4 | 21.2 | 14.4 | 28.9 | 14.8 | |
| Discharge disposition | <0.01 | |||||||||
| Home | 73.2 | 75.7 | 68.2 | 76.6 | 69.4 | 76.2 | 66.2 | 71.8 | 65.3 | |
| Nursing facility | 8.6 | 7.1 | 12.3 | 7.1 | 9.8 | 6.5 | 9.6 | 9.0 | 9.7 | |
NSTEMI was the most common clinical presentation of ACS with a higher prevalence in women (55.3 vs 52.3 %; p < 0.01), followed by unstable angina (31.5 % vs 30.9 %; p = 0.04). Conversely, STEMI was more prevalent in men (16.8 % vs. 13.2 %; p < 0.001). Black men had the highest rates of STEMI (17.4 %) and Black women had the highest rates of NSTEMI (61 %) across all groups. Unstable angina was most common in men of ‘Other’ race/ethnicity (33.5 %) followed by women ‘Other’ race/ethnicity (32.4 %) (Table 2).
Table 2.
Type of ACS presentation stratified by gender and race in patients hospitalized with ACS noted to have coronary ISR.
| Total, N (%) |
White |
Black |
Hispanic |
Other |
P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | |||
| Total, N (%) | 97,680 (100) | 51,955 (53.2) | 22,150 (22.7) | 5625 (5.8) | 4610 (4.7) | 5070 (5.2) | 2295 (2.3) | 4215 (4.3) | 1760 (1.8) | |
| STEMI | 15,685 (16.1) | 8670 (16.7) | 3095 (14) | 980 (17.4) | 485 (10.5) | 865 (17.1) | 245 (10.7) | 730 (17.3) | 240 (13.6) | <0.01 |
| Anterior STEMI | 5155 (5.3) | 2815 (5.4) | 880 (4) | 370 (6.6) | 145 (3.1) | 330 (6.5) | <100 (4.1) | 280 (6.6) | <100 (4.5) | <0.01 |
| Inferior STEMI | 7800 (8) | 4435 (8.5) | 1590 (7.2) | 365 (6.5) | 260 (5.6) | 400 (7.9) | <100 (4.1) | 320 (7.6) | 115 (6.5) | <0.01 |
| Other STEMI | 3010 (3.1) | 1420 (2.7) | 625 (2.8) | 245 (4.4) | <100 (1.7) | 135 (2.7) | <100 (2.4) | 130 (3.1) | <100 (2.6) | <0.01 |
| NSTEMI | 52,775 (54) | 26,840 (51.7) | 11,965 (54) | 3405 (60.5) | 2810 (61) | 2655 (52.4) | 1310 (57.1) | 2075 (49.2) | 950 (54) | <0.01 |
| Unstable Angina | 45,065 (46.1) | 31.7 | 32 | 22 | 28.5 | 30.6 | 32.2 | 33.5 | 32.4 | <0.01 |
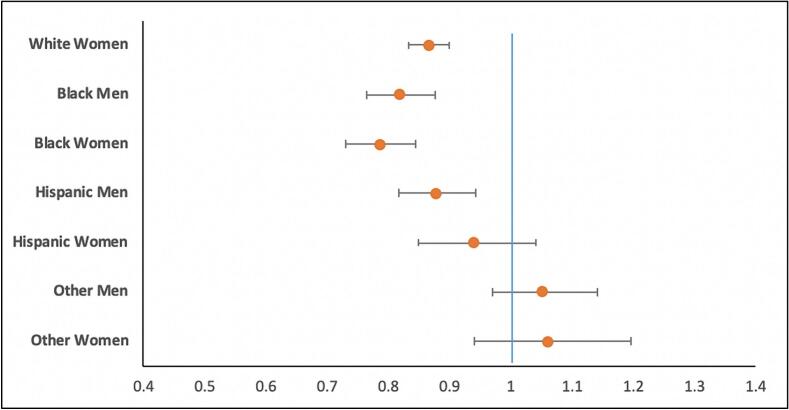
Compared with men, revascularization was significantly lower in women (77 % vs 80.2 %; aOR: 0.891, 95 % CI: 0.862–0.921; p < 0.001). PCI with DES was the most common revascularization strategy but less often utilized in women (52.7 % vs 53.7 %; p = 0.004). Similarly, women had lower rates of CABG compared with men (11.8 % vs 15.2 %; p < 0.001). White women had a revascularization rate of 77.2 %, compared with 80.6 % for White men. Black women had a revascularization rate of 74.5 %, compared with 77.1 % for Black men. Hispanic women had a revascularization rate of 77.6.5 %, compared with 78.3 % for Hispanic men. Furthermore, balloon angioplasty rates were significantly greater in women as compared to men, mostly notable among black individuals (21.6 % vs 18 %, p < 0.01) (Table 3). Black women had lowest rates of PCI with DES (47.8 %), followed by Hispanic women (48.8 %). On multivariate analysis, White women, Black men and women, and Hispanic men had lower overall odds of revascularization compared with White men as the reference (Table 4, Fig. 1).
Table 3.
Procedural and clinical outcomes of ACS and coronary ISR.
| Total, N (%) |
White |
Black |
Hispanic |
Other |
P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | |||
| 97,680 (100) | 51,955 (53.2) | 22,150 (22.7) | 5625 (5.8) | 4610 (4.7) | 5070 (5.2) | 2295 (2.3) | 4215 (4.3) | 1760 (1.8) | ||
| Procedural Outcomes, N (%) | ||||||||||
| Revascularization | 77,310 (79.1) | 41,870 (80.6) | 17,090 (77.2) | 4334 (77.1) | 3435 (74.5) | 3970 (78.3) | 1780 (77.6) | 3420 (81.1) | 1410 (80.1) | <0.01 |
| PCI-DES | 52,110 (53.3) | 28,045 (54.3) | 11,940 (53.9) | 2875 (51.1) | 2205 (47.8) | 2660 (52.5) | 1120 (48.8) | 2300 (54.6) | 965 (54.8) | <0.01 |
| PCI-BMS | 2525 (2.6) | 1370 (2.6) | 655 (3) | 140 (2.5) | 110 (2.4) | 110 (2.2) | <100 (1.7) | <100 (1.5) | <100 (2) | <0.01 |
| Balloon Angioplasty | 16,925 (17.3) | 8695 (16.7) | 3790 (17.1) | 1015 (18) | 995 (21.6) | 920 (18.1) | 420 (18.3) | 715 (17) | 375 (21.3) | <0.01 |
| CABG | 13,895 (14.2) | 8135 (15.7) | 2590 (11.7) | 715 (12.7) | 530 (11.5) | 705 (13.9) | 330 (14.4) | 710 (16.8) | 180 (10.2) | <0.01 |
| IVUS | 5415 (5.6) | 2960 (5.7) | 1085 (4.9) | 290 (5.2) | 315 (6.8) | 265 (5.2) | 135 (5.9) | 295 (7.0) | <100 (4.0) | <0.01 |
| Atherectomy | 6905 (7.1) | 3740 (7.2) | 1350 (6.1) | 400 (7.1) | 305 (6.6) | 350 (6.9) | 115 (5) | 435 (10.3) | 210 (11.9) | <0.01 |
| IABP | 3360 (3.4) | 1615 (3.1) | 635 (2.9) | 260 (4.6) | 150 (3.3) | 245 (4.8) | 120 (5.2) | 200 (4.7) | 135 (7.7) | <0.01 |
| pVAD | 1690 (1.7) | 905 (1.7) | 300 (1.4) | <100 (1.6) | <100 (1.7) | 105 (2.1) | <100 (2) | 115 (2.7) | <100 (2.8) | <0.01 |
| Clinical Outcomes, N (%) | ||||||||||
| Mortality | 2300 (2.4) | 1095 (2.1) | 610 (2.8) | 120 (2.1) | 150 (3.3) | 110 (2.2) | <100 (1.5) | 100 (2.4) | <100 (4.5) | <0.01 |
| Cardiogenic shock | 4625 (4.7) | 2165 (4.2) | 1035 (4.7) | 405 (7.2) | 245 (5.3) | 255 (5) | 125 (5.4) | 235 (5.6) | 160 (9.1) | <0.01 |
| Stroke | 885 (0.9) | 375 (0.7) | 250 (1.1) | <100 (1.1) | <100 (1.7) | <100 (0.9) | <100 (1.3) | <100 (0.9) | <100 (0.3) | <0.01 |
| AKI | 16,640 (17) | 8590 (16.5) | 3460 (15.6) | 110 (19.6) | 940 (20.4) | 985 (19.40 | 425 (18.5) | 765 (18.1) | 375 (21.3) | <0.01 |
| Major Bleeding | 995 (1) | 420 (0.8) | 290 (1.3) | <100 (0.9) | <100 (1.7) | <100 (0.6) | <100 (1.1) | <100 (1.7) | <100 (1.7) | <0.01 |
| LOS | 3(2,6) | 3(2,6) | 3(2,6) | 3(2,6) | 4(2,7) | 3(2,6) | 4(2,8) | 3(2,7) | 4(2,7) | <0.01 |
Table 4.
Adjusted odds ratio in-hospital mortality.
| aOR | 95 % CI | p value | |
|---|---|---|---|
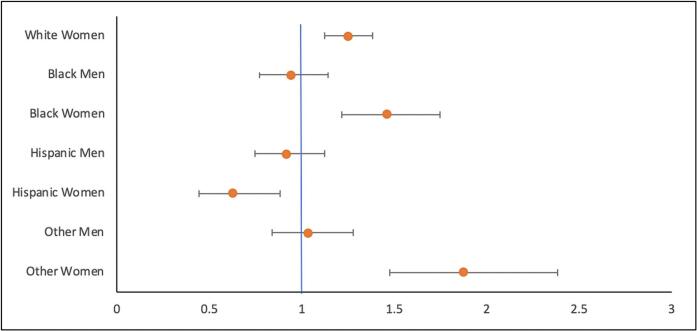
| White women | 1.248 | 1.126–1.383 | <0.001 |
| Black men | 0.939 | 0.771–1.143 | 0.528 |
| Black women | 1.458 | 1.217–1.747 | <0.001 |
| Hispanic men | 0.916 | 0.748–1.121 | 0.394 |
| Hispanic women | 0.628 | 0.445–0.885 | 0.008 |
| Other men | 1.035 | 0.837–1.280 | 0.751 |
| Other women | 1.875 | 1.475–2.382 | <0.001 |
Fig. 1.
Adjusted Odds Ratio In-Hospital mortality highlighting increased odds of mortality in all women except Hispanic women as compared to men.
All-cause in-hospital mortality was 2.4 % in the study cohort, but significantly higher in women (2.8 vs 2.1 %; aOR: 1.282, 95 % CI: 1.174–1.4; p < 0.001). White women had a mortality rate of 2.8 %, compared with 2.1 % for White men. Black women had a mortality rate of 3.3 %, compared with 2.1 % for Black men. Hispanic women had a mortality rate of 1.5 %, compared with 2.2 % for Hispanic men (Table 3). On multivariate analysis using White men as the reference, women from all races/ethnicities had significantly higher odds of in-hospital mortality, in contrast to Hispanic women who had lower odds of in-hospital mortality. Men across all races/ethnicities had no difference in risk of in-hospital mortality compared with White men (Table 5, Fig. 2).
Table 5.
Adjusted odds ratio – revascularization.
| aOR | 95 % CI | p value | |
|---|---|---|---|
| White women | 0.865 | 0.832–0.900 | <0.001 |
| Black men | 0.819 | 0.765–0.877 | <0.001 |
| Black women | 0.786 | 0.731–0.845 | <0.001 |
| Hispanic men | 0.877 | 0.817–0.942 | <0.001 |
| Hispanic women | 0.939 | 0.848–1.040 | 0.231 |
| Other men | 1.051 | 0.969–1.141 | 0.231 |
| Other women | 1.060 | 0.940–1.196 | 0.343 |
Fig. 2.
Adjusted Odds Ratio – Revascularization highlighting disparities in overall rates of revascularization as stratified by gender-race-ethnicity.
4. Discussion
Growing evidence demonstrates heterogeneity in clinical practices in coronary artery disease (CAD) management within the U.S., with variances driven at least in part by a complex interplay of social determinants of health [2,6,14,15]. To our knowledge, this is the first study to report the combined impact of gender and race-ethnicity on procedural and clinical outcomes in ACS with ISR. The main study findings are as follows: (1) clinical characteristics varied significantly according to gender, race, and ethnicity, with Black and Hispanic women demonstrating disproportionately greater burden of comorbidities; (2) NSTEMI was the most common clinical manifestation warranting hospitalization for ACS in patients with ISR, with higher frequency in women, particularly Black women; (3) Revascularization rates, including PCI with DES and CABG were lower in women. After adjusting for potential confounders, White and Black women, had approximately 13 % and 21 % lower odds of revascularization compared with White men. Similarly, Black and Hispanic men were less likely to undergo revascularization; (4) Risk of all cause in-hospital mortality was nearly 28 % higher among women in general. Despite the lower rate of revascularization overall among females, women received more balloon angioplasty (BA) when compared to men and may be attributable to sub-optimal outcomes associated with BA. In reference to White men, mortality was significantly higher in White and Black women, but lower in Hispanic women.
Numerous large-scale studies have demonstrated that Blacks and Hispanics have more modifiable risk factors that have been implicated in the pathophysiology for ACS and coronary ISR, concordant with findings of our study [[16], [17], [18]]. However, studies evaluating the inherent effect of race-ethnicity beyond traditional risk factors on the development of coronary ISR are lacking. Studies assessing the gender-specific impact on risk of coronary ISR have yielded conflicting results. Mehilli et al. reported a lower risk of coronary ISR at a 6-month angiographic follow-up in women who underwent PCI with BMS [19]. However, studies involving DES utilization have not demonstrated such sex differences in the occurrence of ISR despite the higher prevalence of comorbidities and older age in women [20,21]. In one study, younger women (age < 50 years) undergoing PCI were noted to have an increased risk of clinically significant ISR, underscoring the differential impact of premature CAD in women on the risk of ISR [22]. More recently, pooled analysis from 21 randomized PCI trials showed female sex was an independent predictor of ISR [23]. Further exploration of the factors contributing to gender, race, and ethnicity related differences in ISR is warranted.
ISR lesions may demonstrate a constellation of features that may trigger an ACS event, such as inflammatory reaction, thin cap fibroatheroma, lipid-rich neointima, increased macrophage burden, neovascularization, and increased local tissue factor levels [8,9,12]. Prior studies have shown that women and individuals of Black race exhibit greater thrombogenicity, that differentially impacts the risk of ACS [24]. NSTEMI was the predominant ACS manifestation in our study cohort, contrary to unstable angina as reported in a study by Moussa et al. [11] This deviation is likely due to the differences in patient cohort selection as the latter included only patients who underwent ISR- PCI. Our study cohort included patients with newly diagnosed coronary ISR on coronary angiography during hospitalization for ACS, regardless of whether ISR was the culprit lesion.
In contrast to women, men of all race-ethnicities in our study demonstrated no significant increase in in-hospital death despite an observed excess in risk factor burden and lower odds of coronary revascularization among Blacks and Hispanics. Multiple prior studies have independently linked female gender to a higher risk of death in those with ACS, even if they underwent urgent coronary revascularization. The current findings of our study add to the literature of previously reported gender-based differences in outcomes and suggest disparities persist even among women with coronary ISR hospitalized with ACS. Several potential reasons can be postulated to explain these differences. Women present with a higher frequency of prodromal symptoms and varied distribution of cardiac symptoms that are distinct from that of men which might result in delayed evaluation and diagnosis of ACS. Furthermore, traditional risk score tools often underestimate risk in women. Also, gender-based differences in evidence-based therapy of ACS unfavorably affect women. In our cohort of patients, women had higher burden of comorbidities and overall lower rates of coronary revascularization. Thus, women are less likely to have timely and appropriate care that at least in part could have furthered the risk of in-hospital mortality. Additionally, treatment options for ISR with drug-coated balloons is pending approval and may impact outcomes positively in women.
We can only speculate as to the reasons for lower rates of coronary revascularization in women as well as Black and Hispanic men in our study: vessel size, extent and severity of CAD found on coronary angiography, development of complications during hospitalization (bleeding, stroke, AKI, etc.), physician's perception of procedural risk and compliance with post-discharge instructions and other potential confounding socioeconomic factors. Also, non-obstructive coronary artery disease which is more common among women presenting with ACS may not warrant revascularization. Further complicating the clinical course is the wide variability and lack of consensus in treatment of ISR and particularly recurrent ISR. However, despite an increased risk of in-hospital death in White, Black, and ‘Other’ women, an elevated risk was not observed in Hispanic women. On the contrary, Hispanic women had lower mortality compared to white men which could be partially attributed to similar odds of revascularization compared to white men and influence of factors like social support, optimism, and strong familial and social ties that may be potentially protective. This observation remains to be clarified in further prospective studies and the notion of the “Hispanic paradox” could be potentially hazardous for the health of Hispanics given their underlying suboptimal cardiovascular risk profile reported in prior studies as well as in the present study.
5. Limitations
We recognize limitations in our study. First, lack of clinical information such as delays in seeking medical attention, utilization of guideline directed adjunctive medical therapies, laboratory data, access site, timing of angiography/revascularization after diagnosis, door to device time in STEMI, and other residual or unmeasured confounders could have potentially accounted for some of our findings. Second, our study is based on administrative data and is therefore subject to coding bias due to variations in hospital coding practices. Third, lack of angiographic data on culprit lesion: de-novo or ISR, extent and severity of coronary artery disease, and the reasons accounting for lack of revascularization strategies could not be ascertained. Fourth, while we coded for CTO, we were unable to identify which coronary artery was the CTO lesion. Lastly, our study did not account for out-of-hospital deaths and thus we could not determine if the observed differences within in- hospital mortality were potentially exaggerated or offset by post-discharge mortality. Nevertheless, despite these limitations, this study provides a unique disaggregated analysis of cardiovascular care and outcomes in a high-risk patient cohort of ACS with coronary ISR.
6. Conclusion
Patients hospitalized for ACS with coronary ISR were observed to have disproportionate and unfavorable disparities in risk factors, revascularization practices and in-hospital death primarily impacting women, racial and ethnic minorities in the U.S. Our findings underscore the need for targeted research and interventions specific for women, and vulnerable racial and ethnic groups to achieve equitable outcomes.
The following is the supplementary data related to this article.
ICD-10-CM codes used to identify comorbidities.
Ethics statement
This manuscript was performed using data obtained from the Healthcare Cost and Utilization Project (HCUP). The authors attest that we have adhered to the relevant laws and guidelines endorsed with HCUP as well as relevant laws and institutional guidelines.
Funding
None.
CRediT authorship contribution statement
Shivaraj Patil: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Chaitanya Rojulpote: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Conceptualization. William Frick: Writing – review & editing, Conceptualization. Abhijit Bhattaru: Formal analysis. Karanjit Sandhu: Writing – review & editing. Aditya Bakhshi: Writing – review & editing, Writing – original draft. Anum Shahzad: Writing – review & editing, Writing – original draft. Gregg Pressman: Writing – review & editing, Writing – original draft. Antonio Chamoun: Writing – review & editing, Writing – original draft. Div Verma: Writing – review & editing. Chien-Jung Lin: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Vaccarino V., Parsons L., Every N.R., Barron H.V., Krumholz H.M. Sex-based differences in early mortality after myocardial infarction. National Registry of myocardial infarction 2 participants. N. Engl. J. Med. 1999;341(4):217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 2.Wenger N.K. Women and coronary heart disease: a century after Herrick: understudied, underdiagnosed, and undertreated. Circulation. 2012;126(5):604–611. doi: 10.1161/CIRCULATIONAHA.111.086892. [DOI] [PubMed] [Google Scholar]
- 3.Udell J.A., Koh M., Qiu F., et al. Outcomes of women and men with acute coronary syndrome treated with and without percutaneous coronary revascularization. J. Am. Heart Assoc. 2017;6(1) doi: 10.1161/JAHA.116.004319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogunniyi M.O., Mahmoud Z., Commodore-Mensah Y., et al. Eliminating disparities in cardiovascular disease for black women: JACC review topic of the week. J. Am. Coll. Cardiol. 2022;80(18):1762–1771. doi: 10.1016/j.jacc.2022.08.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucholz E.M., Ma S., Normand S.L.T., Krumholz H.M. Race, socioeconomic status, and life expectancy after acute myocardial infarction. Circulation. 2015;132(14):1338. doi: 10.1161/CIRCULATIONAHA.115.017009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham G. Racial and ethnic differences in acute coronary syndrome and myocardial infarction within the United States: from demographics to outcomes. Clin. Cardiol. 2016;39(5):299–306. doi: 10.1002/CLC.22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adam Leigh J., Alvarez M., Rodriguez C.J. Ethnic minorities and coronary heart disease: an update and future directions. Curr. Atheroscler. Rep. 2016;18(2):9. doi: 10.1007/S11883-016-0559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buccheri D., Piraino D., Andolina G., Cortese B. Understanding and managing in-stent restenosis: a review of clinical data, from pathogenesis to treatment. J. Thorac. Dis. 2016;8(10) doi: 10.21037/JTD.2016.10.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shlofmitz E., Iantorno M., Waksman R. Restenosis of drug-eluting stents: a new classification system based on disease mechanism to guide treatment and state-of-the-art review. Circ. Cardiovasc. Interv. 2019;12(8) doi: 10.1161/CIRCINTERVENTIONS.118.007023. [DOI] [PubMed] [Google Scholar]
- 10.Giustino G., Colombo A., Camaj A., et al. Coronary in-stent restenosis: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2022;80(4):348–372. doi: 10.1016/J.JACC.2022.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Moussa I.D., Mohananey D., Saucedo J., et al. Trends and outcomes of restenosis after coronary stent implantation in the United States. J. Am. Coll. Cardiol. 2020;76(13):1521–1531. doi: 10.1016/J.JACC.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Magalhaes M.A., Minha S., Chen F., et al. Clinical presentation and outcomes of coronary in-stent restenosis across 3-stent generations. Circ. Cardiovasc. Interv. 2014;7(6):768–776. doi: 10.1161/CIRCINTERVENTIONS.114.001341. [DOI] [PubMed] [Google Scholar]
- 13.nisoverview @ www.hcup-us.ahrq.govhttps://www.hcup-us.ahrq.gov/nisoverview.jsp
- 14.Tertulien T., Broughton S.T., Swabe G., Essien U.R., Magnani J.W. Association of Race and Ethnicity on the Management of Acute non-ST-segment elevation myocardial infarction. J. Am. Heart Assoc. 2022;11(12) doi: 10.1161/JAHA.121.025758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arora S., Stouffer G.A., Kucharska-Newton A., et al. Fifteen-year trends in management and outcomes of non–ST-segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J. Am. Heart Assoc. 2018;7(19) doi: 10.1161/JAHA.118.010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carnethon M.R., Pu J., Howard G., et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136(21):e393–e423. doi: 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 17.Nadruz W., Claggett B., Henglin M., et al. Widening racial differences in risks for coronary heart disease. Circulation. 2018;137(11):1195–1197. doi: 10.1161/CIRCULATIONAHA.117.030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez C.J., Allison M., Daviglus M.L., et al. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130(7):593–625. doi: 10.1161/CIR.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehilli J., Kastrati A., Bollwein H., et al. Gender and restenosis after coronary artery stenting. Eur. Heart J. 2003;24(16):1523–1530. doi: 10.1016/S0195-668X(03)00320-8. [DOI] [PubMed] [Google Scholar]
- 20.Lansky A.J., Costa R.A., Mooney M., et al. Gender-based outcomes after paclitaxel-eluting stent implantation in patients with coronary artery disease. J. Am. Coll. Cardiol. 2005;45(8):1180–1185. doi: 10.1016/J.JACC.2004.10.076. [DOI] [PubMed] [Google Scholar]
- 21.Stefanini G.G., Kalesan B., Pilgrim T., et al. Impact of sex on clinical and angiographic outcomes among patients undergoing revascularization with drug-eluting stents. JACC Cardiovasc. Interv. 2012;5(3):301–310. doi: 10.1016/j.jcin.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Epps K.C., Holper E.M., Selzer F., et al. Sex differences in outcomes following percutaneous coronary intervention according to age. Circ. Cardiovasc. Qual. Outcomes. 2016;9(2_suppl_1):S16–S25. doi: 10.1161/CIRCOUTCOMES.115.002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosmidou I., Leon M.B., Zhang Y., et al. Long-term outcomes in women and men following percutaneous coronary intervention. J. Am. Coll. Cardiol. 2020;75(14):1631–1640. doi: 10.1016/J.JACC.2020.01.056. [DOI] [PubMed] [Google Scholar]
- 24.Gurbel P.A., Bliden K.P., Cohen E., et al. Race and sex differences in thrombogenicity: risk of ischemic events following coronary stenting. Blood Coagul. Fibrinolysis. 2008;19(4):268–275. doi: 10.1097/MBC.0B013E3282FF76AE. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ICD-10-CM codes used to identify comorbidities.