Abstract
Osilodrostat is an 11β-hydroxylase inhibitor used in the treatment of adult patients with Cushing disease. Prolonged adrenal insufficiency (AI) after osilodrostat use is a rare but significant adverse effect. We present the case of a 41-year-old woman treated with osilodrostat for persistent hypercortisolism following pituitary surgery and Gamma Knife radiosurgery. After 11 months of osilodrostat therapy, she reported AI symptoms, and biochemical testing revealed low serum cortisol following cosyntropin stimulation as well as high plasma adrenocorticotropic hormone (ACTH). The patient was started on physiologic replacement dose of hydrocortisone, which was discontinued 23 months after last osilodrostat exposure when laboratory testing revealed recovery of endogenous cortisol production. The mechanism responsible for the prolonged AI noted with osilodrostat use is unclear and unexpected, given the short half-life of the drug. Although prolonged AI after osilodrostat use is not well understood, providers should be aware of this potential adverse effect and have a low threshold to test for AI in patients reporting AI-related symptoms.
Keywords: Cushing disease, hypercortisolism, adrenal insufficiency, osilodrostat
Introduction
Osilodrostat is a potent, oral 11β-hydroxylase inhibitor used for medical treatment of Cushing disease in patients who either are ineligible for pituitary surgery or have undergone surgery but have persistent disease (1). Given its 4-hour half-life (2), osilodrostat is dosed twice daily and generally titrated to normalize 24-hour urinary free cortisol (UFC) concentrations. Osilodrostat should be uptitrated slowly to reduce the risk of adrenal insufficiency (AI) and glucocorticoid withdrawal symptoms noted in clinical trials (3, 4). Prolonged AI even after osilodrostat discontinuation has been described in 2 recent case series of 5 total patients (5, 6). The mechanism responsible for the persistent AI noted after osilodrostat exposure is unclear and unexpected, given the short half-life of the drug. We report the first documented case of a patient with persistent Cushing disease who developed prolonged primary AI after discontinuation of osilodrostat with subsequent full recovery of cortisol production nearly 2 years later.
Case Presentation
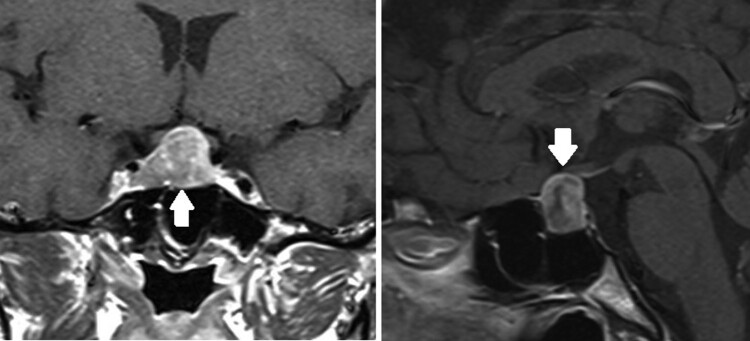
A 41-year-old woman was noted to have an incidental pituitary macroadenoma on magnetic resonance imaging (MRI) of the brain obtained for upper extremity radiculopathy. In retrospect, she noted an 8-kg weight gain in a central distribution over the prior year associated with facial plethora and acne. Biochemical testing revealed elevated 24-hour UFC of 71 µg (195.9 nmol/24 hour) (reference range, 4-50 µg/24 hour, 11-137.9 nmol/24 hour), serum cortisol of 8.1 µg/dL (223.4 nmol/L) after a 1-mg overnight dexamethasone suppression test (DST) (normal, serum cortisol ≤1.8 µg/dL or 49.65 nmol/L), late night salivary cortisol (LNSC) of 0.299 and 0.205 µg/dL (8.24 and 5.65 nmol/L) (reference range, ≤ 0.09 µg/dL, ≤ 2.48 nmol/L), and ACTH of 55 pg/mL (12.1 pmol/L) (reference range, 7.2-63 pg/mL, 1.59-13.9 pmol/L). Dedicated MRI of the pituitary gland revealed a 22 × 11 × 16 mm heterogeneously enhancing suprasellar mass with right lateral sellar and optic chiasm involvement (Fig 1).
Figure 1.
T1-weighted MRI of the pituitary revealed a large, heterogeneously enhancing suprasellar mass (white arrows) with right lateral sellar extension.
The patient underwent transsphenoidal resection of the pituitary macroadenoma. Immunoperoxidase stains demonstrated ACTH expression by neoplastic cells. The patient was treated with hydrocortisone postoperatively but stopped within 2 months of surgery based on basal serum cortisol of 11.1 µg/dL (306.3 nmol/L) with ACTH 38 pg/mL (8.4 pmol/L). Postoperative MRI revealed persistent right cavernous sinus tumor involvement. Subsequently, the patient underwent Gamma Knife radiosurgery 8 months after her pituitary surgery. Her LNSC was elevated at 0.205 and 0.159 mcg/dL (5.7 and 4.4 nmol/L), with 24-hour UFC 23 mcg (63.4 nmol), and ACTH 52 pg/mL (11.5 pmol/L). Because of concern for active, persistent hypercortisolism, the patient was started on osilodrostat 2 mg twice daily and her dose was increased over the course of 3 months to a peak dose of 3 mg twice daily. After 11 months of osilodrostat therapy, the patient presented to our endocrine clinic for a second opinion regarding management.
Diagnostic Assessment
During initial evaluation in our clinic, the patient noted increasing skin pigmentation in her hands. She also reported fatigue, arthralgias, and nausea, concerning for AI. ACTH was checked at this visit and was elevated to 231 pg/mL (50.8 pmol/L). Her 24-hour UFC was undetectable. Osilodrostat was held and 2 weeks later, her serum cortisol was 9.1 µg/dL (251.1 nmol/L) with ACTH 103 pg/mL (22.7 pmol/L) and 24-hour UFC 7.3 µg (20.1 nmol), so osilodrostat was not restarted.
The patient's AI symptoms failed to improve 3 months after discontinuing osilodrostat, and subsequently, a short ACTH (cosyntropin) stimulation test was performed, with peak serum cortisol of 10.4 µg/dL (286.9 nmol/L) (reference range, > 14.5 mcg/dL, > 400 nmol/L) (Table 1). Dehydroepiandrosterone sulfate (DHEA-S) was low at 7.7 mcg/dL (0.21 µmol/L) (reference range, 27-240 mcg/dL, 0.73-6.51 µmol/L). The patient was diagnosed with primary AI. Additional evaluation for etiology of primary AI, consisting of computed tomography (CT) of the abdomen, showed unremarkable adrenal glands bilaterally; 21-hydroxylase antibody and very long chain fatty acid concentrations were not elevated. The patient's mineralocorticoid production was unaffected, with plasma aldosterone concentration of 8 ng/dL (22.2 pmol/L) and plasma renin activity of 2.4 ng/mL/h (30.7 pmol/L/min) (reference range, 0.6-3.0 ng/mL/h, 7.7-38.4 pmol/L/min).
Table 1.
Laboratory values obtained during osilodrostat use (weeks 0-55) and following osilodrostat discontinuation (weeks 67-147)
| Weeks | Basal Serum Cortisol | Peak Serum Cortisol after Cosyntropin Stimulation | Plasma ACTH | Plasma Aldosterone Concentration | Plasma Renin Activity | DHEA-S |
|---|---|---|---|---|---|---|
| 0 (Osilodrostat Started) |
58 pg/mL (12.8 pmol/L) |
|||||
| 24 | 103 pg/mL (22.7 pmol/L) |
|||||
| 55 (Osilodrostat Stopped) |
231 pg/mL (50.8 pmol/L) |
|||||
| 67 | 7.2 μg/dL (198.6 nmol/L) |
10.4 μg/dL (286.9 nmol/L) |
185 pg/mL (40.7 pmol/L) |
|||
| 131 | 9.7 μg/dL (267.6 nmol/L) |
13.3 μg/dL (366.9 nmol/L) |
94 pg/mL (20.7 pmol/L) |
8 ng/dL (22.2 pmol/L) |
2.4 ng/mL/hr (30.7 pmol/L/min) |
7.7 μg/dL (0.21 μmol/L) |
| 147 | 8.4 μg/dL (231.8 nmol/L) |
14.7 μg/dL (405.5 nmol/L) |
61 pg/mL (13.4 pmol/L) |
4 ng/dL (11.1 pmol/L) |
1.7 ng/mL/hr (21.8 pmol/L/min) |
6.5 μg/dL (0.18 μmol/L) |
| Reference Ranges | 5-25 μg/dL (138-690 nmol/L) |
>14.5 μg/dL (>400 nmol/L) |
7.2-63 pg/mL (1.59-13.9 pmol/L) |
< 21 ng/dL (< 58.3 pmol/L) |
0.6-3.0 ng/mL/hr (7.7-38.4 pmol/L/min) |
27-240 μg/dL (0.73-6.51 μmol/L) |
Values in parenthesis are International System of Units (SI).
Treatment
The patient was started on physiologic replacement dose of hydrocortisone 15 mg daily (in 2 divided doses), with modest improvement of AI symptoms. Nineteen months after stopping osilodrostat, ACTH concentration was 94 pg/mL (20.7 pmol/L) with peak serum cortisol of 13.3 µg/dL (366.9 nmol/L) following cosyntropin stimulation testing, so hydrocortisone was tapered to 10 mg daily.
Outcome and Follow-Up
As of the patient's last clinic visit, 23 months off osilodrostat therapy, her ACTH concentration decreased to 61 pg/mL (13.4 pmol/L) with peak serum cortisol 14.7 µg/dL (405.5 nmol/L) after cosyntropin stimulation testing. Hydrocortisone was discontinued without recurrence of AI symptoms and the patient's hyperpigmentation resolved.
Discussion
This case highlights the development of prolonged primary AI after osilodrostat exposure in a patient with Cushing disease with subsequent recovery of adrenal function. The patient was treated with osilodrostat because of persistent postoperative hypercortisolism based on clinical features of glucocorticoid excess and elevated LNSC concentrations. Our case is notable for the prolonged primary AI course following 11 months of osilodrostat, with eventual recovery of adrenal function 99 weeks after last exposure to osilodrostat. This is the first reported case of a patient developing prolonged AI after osilodrostat exposure with eventual full recovery of glucocorticoid production, which occurred nearly 2 years later.
While osilodrostat-mediated AI necessitating temporary glucocorticoid rescue has been described in clinical trials (3, 4, 7), this has generally been attributed to an aggressive uptitration schedule, but prolonged AI from osilodrostat use has not been noted in trials. Mechanistically, osilodrostat is not expected to cause prolonged primary AI after drug discontinuation, as it is a reversible inhibitor of 11β-hydroxylase with a short half-life of 4 hours. Recently published reports have documented 5 patients total with prolonged AI following osilodrostat use (5, 6). In 4 of these cases, the patients had persistent AI after osilodrostat exposure without adrenal recovery. In the fifth case, the patient was able to discontinue hydrocortisone 64 weeks after osilodrostat exposure, but only partial biochemical recovery of adrenal function was noted with peak serum cortisol of 10.5 mcg/dL (289.7 nmol/L) following cosyntropin stimulation test (6). Notably, 3 of the patients were treated with metyrapone prior to starting osilodrostat, which may have also contributed to persistent AI. Interestingly, the authors found that the patients’ AI was associated with low/normal 11-deoxycortisol and low DHEA-S concentrations despite elevated plasma ACTH, suggesting potential inhibition of adrenal steroidogenesis upstream of 11β-hydroxylase. Similarly, our patient was noted to have low DHEA-S concentration and unaffected mineralocorticoid production, indicating additional effects of osilodrostat on steroidogenesis that are not explained by inhibition of 11β-hydroxylase. An inhibitory effect of osilodrostat on 17α-hydroxylase has been noted in vitro (8); however, this effect is short lived and would not account for the prolonged lowering of 11-deoxycortisol and cortisol concentrations documented. Although an adrenolytic effect of osilodrostat has not yet been described, a recent case report found that the adrenal glands reduced in size after treatment with osilodrostat, indicating a possible mechanism by which osilodrostat may induce prolonged AI (9).
While the mechanism of prolonged AI after osilodrostat exposure is not well understood, prescribers should be cognizant of this potential adverse effect due to the severity of a missed diagnosis. We recommend that providers have a low threshold for AI testing in patients recently treated with osilodrostat who develop AI-related symptoms. Our case also suggests that mere discontinuation of osilodrostat is not adequate when AI is suspected. Rather, if AI is diagnosed, patients should be treated with glucocorticoid replacement therapy in addition to stopping osilodrostat. Patients should then undergo periodic reassessment of their adrenal function since delayed recovery, occurring even years after osilodrostat exposure, may be seen. As osilodrostat becomes more commonly used, prolonged AI may become a more frequently identified adverse effect. Future studies will be needed to identify mechanisms for prolonged AI following osilodrostat exposure, patients at highest risk of developing this complication, and strategies to mitigate this adverse effect.
Learning Points
Prolonged primary adrenal insufficiency is emerging as an uncommon but significant adverse effect of osilodrostat use.
Providers should have a low threshold to test for adrenal insufficiency in patients with a history of osilodrostat exposure who develop adrenal insufficiency-related symptoms.
Individuals noted to have persistent, primary adrenal insufficiency following osilodrostat discontinuation should be evaluated for alternate etiologies of adrenal insufficiency, with consideration for adrenal imaging and measurement of 21-hydroxylase antibodies.
Patients who develop prolonged adrenal insufficiency after osilodrostat use require glucocorticoid replacement therapy with periodic reassessment of adrenal function to test for delayed adrenal recovery.
Contributors
All authors made individual contributions to authorship. S.M. and N.T. were involved in the diagnosis and treatment of the patient. S.T. and S.M. were involved in manuscript writing and submission. J.A., N.T., and O.H. were involved in the case discussion. All authors reviewed and approved the manuscript draft.
Abbreviations
- ACTH
adrenocorticotropic hormone
- AI
adrenal insufficiency
- DHEA-S
dehydroepiandrosterone sulfate
- LNSC
late night salivary cortisol
- MRI
magnetic resonance imaging
- UFC
urinary free cortisol
Contributor Information
Sanaa Tejani, Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA.
Jessica Abramowitz, Division of Endocrinology and Metabolism, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA.
Nicholas A Tritos, Neuroendocrine Unit and Neuroendocrine and Pituitary Tumor Clinical Center, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Oksana Hamidi, Division of Endocrinology and Metabolism, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA.
Sasan Mirfakhraee, Division of Endocrinology and Metabolism, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA.
Funding
No public or commercial funding.
Disclosures
O.H. reports advisory board participation with Corcept Therapeutics, Neurocrine Biosciences, Recordati Rare Diseases, Xeris Pharma, and Lantheus. The remaining authors have nothing to disclose.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.
References
- 1. Fleseriu M, Pivonello R, Young J, et al. Osilodrostat, a potent oral 11beta-hydroxylase inhibitor: 22-week, prospective, phase II study in Cushing’s disease. Pituitary. 2016;19(2):138‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Center for Biotechnology Information . PubChem Compound Summary for CID 44139752. Osilodrostat. Accessed January 23, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/Osilodrostat
- 3. Pivonello R, Fleseriu M, Newell-Price J, et al. Efficacy and safety of osilodrostat in patients with Cushing’s disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol. 2020;8(9):748‐761. [DOI] [PubMed] [Google Scholar]
- 4. Fleseriu M, Newell-Price J, Pivonello R, et al. Long-term outcomes of osilodrostat in Cushing's disease: LINC 3 study extension. Eur J Endocrinol. 2022;187(4):531‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poirier J, Bonnet-Serrano F, Thomeret L, Bouys L, Bertherat J. Prolonged adrenocortical blockade following discontinuation of Osilodrostat. Eur J Endocrinol. 2023;188(6):K29‐K32. [DOI] [PubMed] [Google Scholar]
- 6. Ferriere A, Salenave S, Puerto M, Young J, Tabarin A. Prolonged adrenal insufficiency following discontinuation of osilodrostat treatment for intense hypercortisolism. Eur J Endocrinol. 2024;190(1):L1‐L3. [DOI] [PubMed] [Google Scholar]
- 7. Fleseriu M, Biller BMK, Bertherat J, et al. Long-term efficacy and safety of osilodrostat in Cushing's disease: final results from a phase II study with an optional extension phase (LINC 2). Pituitary. 2022;25(6):959‐970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Creemers SG, Feelders RA, de Jong FH, et al. Osilodrostat is a potential novel steroidogenesis inhibitor for the treatment of Cushing syndrome: an in vitro study. J Clin Endocrinol Metab. 2019;104(8):3437‐3449. [DOI] [PubMed] [Google Scholar]
- 9. Sawabe F, Hayafusa R, Kosugi R, Ariyasu H. A case of an ectopic ACTH-producing tumor with adrenal shrinkage during osilodrostat administration. JCEM Case Rep. 2024;2(2):luae008. Published 2024 Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.