Summary
Wingless-related integration site or Wingless and Int-1 or Wingless-Int (WNT) signaling is crucial for embryonic development, and adult tissue homeostasis and regeneration, through its essential roles in cell fate, patterning, and stem cell regulation. The biophysical characteristics of WNT ligands have hindered efforts to interrogate ligand activity in vivo and prevented their development as therapeutics. Recent breakthroughs have enabled the generation of synthetic WNT signaling molecules that possess characteristics of natural ligands and potently activate the pathway, while also providing distinct advantages for therapeutic development and manufacturing. This review provides a detailed discussion of the protein engineering of these molecular platforms for WNT signaling agonism. We discuss the importance of WNT signaling in several organs and share insights from the initial application of these new classes of molecules in vitro and in vivo. These molecules offer a unique opportunity to enhance our understanding of how WNT signaling agonism promotes tissue repair, enabling targeted development of tailored therapeutics.
Subject areas: molecular medicine, cell biology
Graphical abstract
Molecular medicine; Cell biology
Introduction
WNT (“Wingless-related integration site” or “Wingless and Int-1” or “Wingless-Int”) signaling plays key roles in controlling development, tissue homeostasis, and the regeneration of many organs and tissues.1 Although modulation of WNT signaling pathways has the potential to treat many degenerative diseases and tissue injuries, developing research tools and therapeutics that modulate specific aspects of WNT signaling has been challenging. Beyond the complexity of the signaling components and the number of WNT ligands and receptor systems, the biology of the WNT signaling in each adult tissue remains to be fully elucidated. The ability to design appropriate molecules endowed with drug-like properties will be critical to address unanswered research questions and to develop novel therapeutics. In this review, we describe efforts by our group and others in developing novel synthetic WNT agonists. These extensive efforts have enabled researchers to directly modulate the WNT signaling pathway, thereby promoting repair and regeneration in specific tissues.
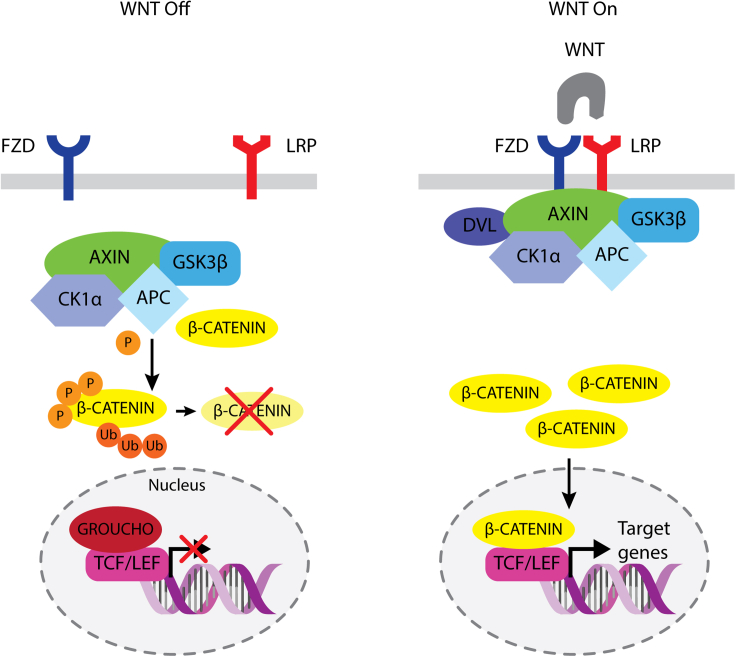
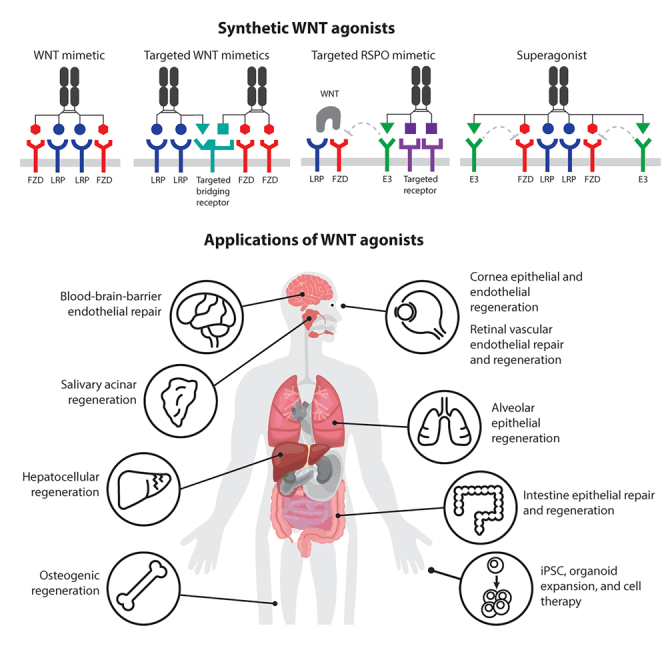
WNT ligands activate diverse downstream signaling cascades. One widely studied WNT signaling pathway involves the Frizzled (FZD) family of receptors, low-density lipoprotein receptor-related protein (LRP) coreceptors, and the transcription factor β-catenin. After WNT binding, FZD and LRP play important roles in recruiting molecules such as disheveled (DVL) and AXIN to the cell membrane.2,3 Subsequently, the β-catenin destruction complex containing AXIN, APC, GSK-3, and CK1 in the cytoplasm disassembles, thereby stabilizing β-catenin. Finally, β-catenin enters the nucleus and with TCF/LEF transcription factors activates downstream target genes of WNT/β-catenin signaling, resulting in cell proliferation and cell fate determination. The complex WNT/β-catenin signaling is regulated by multiple mechanisms including self-limiting negative feedback regulation (Figure 1). A total of 19 WNT ligands have been identified in mammals, and ten FZDs (FZD1–10) and two LRPs (LRP5 and 6) have been found to play roles in the WNT signaling system.4 WNT ligands are promiscuous in that each WNT can bind multiple FZDs and LRPs. WNTs are also post-translationally modified; for example, long-chain fatty acid modification is required for receptor binding and signaling. Consequently, endogenous WNT proteins pose challenges in drug development, and this pathway was long believed to be undruggable. Four FZD subfamilies, FZD1,2,7, FZD5,8, FZD4, and FZD9,10, mediate WNT/β-catenin (canonical) signaling, whereas FZD3,6 is involved primarily in non-canonical signaling independent of β-catenin.5,6 Overlap in FZD expression often exists; however, distinct expression patterns are also observed.7,8 WNT ligands have undesirable biophysical properties and a lack of specificity and consequently are unsuitable for answering specific biological questions or developing therapeutics. On the other hand, directly targeting specific FZD and LRP receptors with molecules that mimic natural ligands has promise in research and therapeutic activation of WNT/β-catenin signaling in specific tissues/cell types. However, how to engage the receptors to mimic the natural ligand actions and induce efficient downstream signaling was determined only recently. Platforms of WNT ligand mimetics have been developed that assemble FZDs and LRPs in proximity, with the desired geometry and valency.
Figure 1.
The canonical WNT signaling pathway
WNT off: in the absence of WNT binding to FZD and LRP, the destruction complex, consisting of AXIN, APC, CK1⍺, and GSK3β, targets cytoplasmic β-catenin for degradation. Consequently, GROUCHO binds TCF/LEF in the nucleus and blocks transcription of target genes. WNT on: after binding of WNT to FZD and LRP, the destruction complex is recruited to, and anchored at, the membrane via DVL. β-catenin is free to accumulate in the cytoplasm and translocates to the nucleus, where it binds TCF/LEF and initiates transcription of target genes.
Cell surface-bound E3 ubiquitin ligases, zinc and ring finger 3 (ZNRF3), and ring finger protein 43 (RNF43) are induced after WNT/β-catenin pathway activation.6,9 ZNRF3 and RNF43 target FZD (and LRP) for degradation and turn off WNT/β-catenin signaling. R-spondins (RSPO1–4) are a family of ligands that bind the receptor complex containing ZNRF3/RNF43 and co-receptor leucine-rich repeat-containing G-protein-coupled receptors (LGR4, 5, or 6). RSPO binding causes clearance or sequestration of the ternary receptor complex, thereby removing ZNRF3 and RNF43 and limiting their effects on FZD, stabilizing WNT receptors, and amplifying WNT/β-catenin signaling. This endogenous WNT/β-catenin signaling enhancing system plays important roles in tissue homeostasis and injury repair, particularly when WNT ligands are abundant, but FZD receptor-mediated signals are limiting. However, LGRs are not tissue or cell type specific, and exogenous RSPO treatment has the potential to amplify WNT/β-catenin signaling in multiple tissues/organs. Therefore, tissue- and cell type-specific RSPO mimetics are desirable. Details of novel targeted RSPO mimetics are described in the following. Molecules with a combination of WNT and RSPO ligand characteristics (“superagonists”) may also be considered in certain contexts.
Cell-to-cell signaling is integral to the complex orchestration of growth, fate choice, and movements of cells that ultimately lead to tissue and organ formation during embryogenesis, homeostasis, and injury-induced repair in adults. WNT/β-catenin signaling is a major developmental pathway that plays crucial roles in many embryonic contexts. In adults, it provides critical niche signals for stem cell renewal and differentiation, and, in some tissues, WNT/β-catenin can imbue stem cells with a self-organizing ability, orchestrating regeneration and restoration of tissue structure and function.10 In this review, we discuss efforts regarding the design principles of various tools for modulating WNT signaling. We additionally describe the biological insights learned from the initial application of these tools, including WNT mimetics, RSPO mimetics, and superagonists with various receptor and cell specificity (collectively called “WNT agonists”), to promote cell expansion in vitro and tissue repair in vivo. These tools will further advance understanding of the mechanistic interactions of WNT signaling with inflammation, fibrosis, tissue regeneration, and other signaling pathways culminating in physiological tissue repair. With this enhanced understanding, WNT signaling agonists can be designed to promote tissue regeneration in disease or after injury, and to tailor therapeutics to benefit patients.
Novel WNT-activating approaches
Discovery and development of WNT mimetics activating WNT/β-catenin signaling
Elucidating the individual ligand or receptor functions of the 19 mammalian WNT ligands, ten FZD receptors (FZD1–10) and two LRP coreceptors (LRP5 and LRP6) in tissues or cell types of interest have been hindered by several challenges. First, WNT ligands undergo extensive posttranslational modifications. In particular, palmitoylation on a conserved serine residue, a modification critical for secretion and FZD binding, makes the ligands insoluble and difficult to produce.11 Second, WNT and receptor interactions are promiscuous, such that each ligand can bind and activate multiple FZD/LRP pairs.12,13 These challenges pose major obstacles in basic research aimed at understanding the roles and functions of an individual ligand or receptor, as well as in the development of potential regenerative therapeutics targeting this pathway. Recent advances in WNT mimetic (or surrogate) development have offered exciting opportunities to overcome these challenges that have hampered the field for decades.
In the seminal work by Janda et al., the first WNT surrogate was constructed by linking an FZD binder and an LRP binder unrelated to WNTs into a single polypeptide chain, thereby creating a bispecific molecule capable of heterodimerizing these two receptors (Janda et al.14; Table 1; Figure 2B). This strategy was favored over re-engineering natural ligands, to avoid the inherent challenges of acylation and the extensive receptor binding site homology.12 Two FZD binders were selected for the initial surrogate construction: one was the single-chain variable fragment (scFv) of an antagonistic antibody, vantictumab (18R5), with FZD1,2,5,7,8 specificity; the other was a de novo-designed four-helix bundle protein, B12, which binds FZD5,8. The LRP binder was the C-terminal domain of the human WNT antagonist DKK1. These two WNT surrogates, 18R5scFv-DKK1c and B12-DKK1c (Table 1), have been found to induce WNT/β-catenin signaling in an FZD-selective fashion in vitro, thus replacing WNT3A in supporting growth of a range of human organoid cultures, and to exhibit WNT activity in vivo in the mouse liver after adenovirus-mediated expression.14 Alternative FZD-binding domains based on ankyrin repeat proteins (designed repeat protein binder, DRPB) were subsequently used to improve the affinity, FZD specificity, and potency of the early WNT surrogates (Miao et al.15; Table 1). Beyond organoid studies, these new molecules, when delivered via adenovirus expression in vivo, revealed FZD subfamily-specific effects in the intestine and liver. These studies demonstrated that WNT/β-catenin signaling can be activated by heterodimerization of receptors, thus laying the groundwork for further development of these tools for research and therapeutics.
Table 1.
Partial list of representative WNT mimetic formats
|
Format |
Molecule specificity summary |
Ref. | Date filed or published | |
|---|---|---|---|---|
| FZD specificity | LRP specificity | |||
 scFv-DKK1c scFv-DKK1cHelix bundle-DKK1c DARPin-DKK1c |
1,2,5,7,8 5,8 1,2,7 4 |
5,6 | WO2016/040895 Janda et al.14 WO2020/018445 Miao et al.15 |
09/2014 05/2017 07/2018 08/2020 |
 VHH-IgG VHH-IgG |
1,2,5,7,8 1,2,7 5,8 4 9,10 Other mono or poly specificities |
5,6 | WO2019/126398 WO2020/010308 Chen et al.16 Fowler et al.19 Xie et al.7 Nguyen et al.24 |
12/2017 07/2018 03/2020 05/2021 05/2022 09/2022 |
 scFvs-Fc scFvs-Fc |
1,2,5,7,8 1,2,7 5,8 7 |
5,6 | WO2019/126398 WO2020/010308 Chen et al.16 Gumber et al.23 |
12/2017 07/2018 03/2020 12/2020 |
 Diabody-Fc-diabody Diabody-Fc-diabody |
1,2,5,7,8 2 4 5 7 |
5,6 | WO2019/126398 WO2019/159084 Tao et al.18 Chidiac et al.25 |
12/2017 02/2018 08/2019 06/2021 |
 VHHs-Fc VHHs-Fc |
8 and various specificities | 5,6 | WO2019/126398 | 12/2017 |
 Fv-IgG Fv-IgG |
1,2,5,7,8 1,2,7 5,8 4 4,9 10 |
5,6 | WO2019/126398 WO2020/010308 WO2021/173726 Post et al.20 Ding et al.87 |
12/2017 07/2018 02/2020 07/2023 06/2023 |
 Fab-IgG Fab-IgG |
1,2,5,7,8 | 5,6 | WO2019/126398 WO2020/010308 WO2021/173726 Post et al.20 |
12/2017 07/2018 02/2020 07/2023 |
Only representative formats and molecules are listed. Additional information on other combinations of different binding domains, linker lengths, orientations, geometries, combinations of different receptor specificities, stoichiometry, and symmetrical vs. nonsymmetrical designs, with or without the Fc domain, etc. can be found in the references.
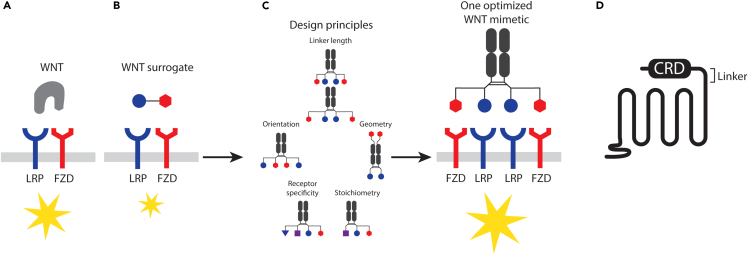
Figure 2.
Discovery and development of WNT mimetics
(A) Endogenous WNT ligand binding FZD and LRP, resulting in downstream signaling events.
(B) WNT surrogates consisting of an FZD binder and an LRP binder elicit similar signaling cascades.
(C) Design principles and one optimized WNT mimetic.
(D) Linker region after the CRD of FZD. Yellow stars denote activation of the WNT signaling pathway.
The development of WNT mimetics has also focused on antibody-based scaffolds. However, in contrast to the WNT mimetics described in the preceding paragraph, in which one FZD binder was linked to one LRP binder (a bivalent bispecific format denoted 1:1 design herein), WNT mimetics using antibody fragments for both FZD and LRP binding in the 1:1 format resulted in little to no activity (WO2019/126398,16,17 WO2019/159084;18). In one such study of bivalent bispecific antibody-based molecules, WNT mimetic activity was present in crudely purified protein preparations but was lost after the final size exclusion chromatography. Analysis of all size exclusion chromatography fractions indicated recovery of activity in fractions containing species with molecular weights of two or more times the expected molecular weights of the monomers (WO2019/126398,16,17). This unexpected finding suggested that WNT mimetic generation based on antibody fragments may require clustering of receptor complexes of more than one FZD and one LRP for efficient signaling. Early examples of such multivalent molecules used a tetravalent bispecific format, or 2:2 design, with broad or subfamily FZD specificity (WO2019/126398). Given that these tetravalent molecules have two FZD binding arms, each of which can bind the same or different FZDs, or can bind one or two copies of FZDs at the same time, and that the same binding variations can occur on the LRP side, many different permutations could potentially occur in the receptor complexes assembled on the cell surface for signaling. For example, the assembled complex could contain two of the same FZDs and two of the same LRPs, two different FZDs and two of the same LRPs, two of the same or different FZDs and one or two LRPs, etc. Therefore, different design parameters, including valency, linker lengths, relative orientation of binders, geometry, stoichiometry, epitope, and receptor specificity, have been systematically evaluated to further understand signaling requirements (WO2019/126398,16 WO2020/010308,19 WO2019/15908418; Figure 2C; Table 1). Although many parameters appear to be binder specific, multivalent binding to receptors with a ratio of two FZDs to two LRP binders (tetravalent or 2:2 design) appeared optimal in all cases tested (WO2019/126398,16 WO2019/15908418,20; Figure 2C; Table 1). A ratio of two FZDs and one LRP binder (trivalent or 2:1 design) has also been found to be highly effective in some reports (WO2019/126398,16 WO2020/010308), although such findings have not been observed in other studies (WO2019/15908418). This stoichiometry requirement with the WNT mimetics is consistent with findings from structural studies revealing FZD dimerization after natural ligand binding as either a 2:121 or 2:2 complex with LRP.22 These systematic analyses have also revealed that receptor complexes consisting of multimers of different FZD or LRP isotypes produce efficient signaling16,18 (Figure 2C). These observations may be biologically relevant. Given the promiscuous binding of WNT/FZD/LRP interactions and the ability of FZD to dimerize after ligand binding, natural ligand/receptor complexes might potentially contain different stoichiometries and various permutations.
Such design concepts have been applied to several antibody-based formats. Different antibody formats may vary in the distances and geometries between various binding arms. In a direct comparison of tandem scFv-Fc, Fv-IgG, and Fab-IgG formats with the same set of FZD and LRP binders, Fv-IgG format has been found to produce the most active molecules20 (Table 1). Further studies are necessary to determine whether this preference is specific to the FZD/LRP binder combination tested in the study or a general preference of this particular format for WNT mimetic construction. A set of tool molecules covering all WNT/β-catenin signaling FZDs with subfamily or monospecificity has been developed20 and may aid in the study of the effects of different FZDs in tissues and cell types. Other formats described to date include tandem scFv-Fc,16,23 VHH-immunoglobulin G (IgG),7,19,24 VHH-VHH-Fc or VHH-Fc-VHH (WO2019/126398), and diabody-Fc-diabody18,25 (Table 1). The WNT mimetics from the Surrozen group have been referred to as Surrozen WNT signal activating proteins (SWAPs),7,16,19,20,24 and the molecules from the Sidhu and Angers groups have been called FZD/LRP agonists (FLAgs).18
Because WNTs bind the cysteine-rich domain (CRD) of FZDs,12 most efforts in antibody binder discovery and WNT mimetic construction have focused on the FZD CRD.14,16,18 Two antibodies specific to the FZD7 linker region after the CRD (1291 and 1791, WO2016/205551 and WO2016/205566, Figure 2D), when assembled in a tetravalent bispecific format (2:2) with either an LRP5 binder in the VHH-IgG format (1291-3 and 1791-3, WO2020/010308) or an LRP6 binder in the tandem scFv-Fc format (F7L6, F7 is 179123), have also shown WNT mimetic activity with monospecificity toward FZD7. These studies have expanded the potential effective epitopes on FZD suitable for WNT mimetic construction, further supporting that WNT/β-catenin signaling can be activated by heteromultimerization of receptors.
Beyond antibody-based modalities, small-molecule WNT mimetics capable of clustering FZD and LRP have also been explored. A small molecule (MFH) targeting site 2 of FZD8 CRD, when fused to a peptide derived from the N-terminal region of human DKK1 (MFH-ND), has been found to weakly induce WNT/β-catenin signaling alone and significantly augment WNT3A activity.26 Other modalities, such as peptides, cyclic peptides, or aptamers, may also be suitable for WNT mimetic construction. Similar principles may be applied to the study of WNT/β-catenin-independent signaling pathways. Overall, the WNT mimetics based on non-WNT scaffolds, particularly antibody-based molecules, offer advantages of improved biophysical properties, such as solubility and stability, ease of production, and ability to target specific FZD/LRP pairs. This approach is expected to advance basic research and regenerative therapeutic development.
Cell-targeted WNT mimetics
Although FZD and LRP specificity can now be achieved with the WNT mimetic agonistic antibodies described earlier, because each of the ten FZDs and two LRP coreceptors is broadly expressed on many cell types in different tissues, tissue/cell targeting cannot be achieved with WNT mimetics alone. Given the important roles of WNT in many tissues during development, and in adult tissue homeostasis and injury repair, often with opposing roles in parenchymal vs. nonparenchymal cells, being able to target WNT or WNT mimetic action to a specific cell type is critical for research and development of therapeutics.
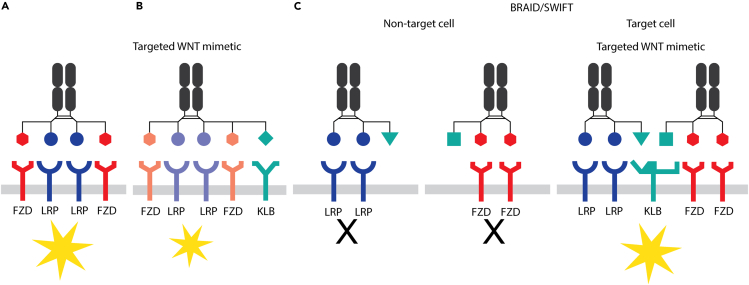
Cell-targeted WNT mimetics can be generated by fusing a binding domain to a cell-targeting receptor to a WNT mimetic (Figure 3B). This approach, sometimes referred to as chimeric activators, has been explored in other growth factor or cytokine systems.27 Another novel cell targeting approach called bridged activation by intra/intermolecular division (BRAID) has been developed, in which an active drug molecule is divided into inactive parts that are assembled via a bridging receptor specific to the target cell.27 The application of this concept to WNT mimetics is called splitting of WNT to induce functional targeting (SWIFT). In a proof-of-concept study, taking advantage of active WNT mimetics requiring multivalent binding to FZD and LRP in the same molecule, an active tetravalent bispecific WNT mimetic (Figure 3A) has been split into two inactive molecules, one consisting of two FZD binding arms and the other having two LRP binding arms. These two inactive components were then linked to two different binders capable of binding to two different epitopes on a common bridging receptor, βKlotho (KLB) (Figure 3C). The mixture of the two tethered inactive components has been found to specifically induce WNT signaling in primary hepatocytes or a hepatoma cell line expressing βKlotho, but not HEK293 or intestinal organoids in which βKlotho is not expressed.27 This novel approach now enables specific targeting of WNT activation in desired cell types, to enhance the responses of target cells in a microenvironment and decrease undesirable off-target effects.
Figure 3.
Cell-targeted WNT mimetics
(A) A bispecific tetravalent WNT mimetic.
(B) A cell targeting receptor fused to a WNT mimetic.
(C) A WNT mimetic split into two inactive molecules: one with two FZD binding arms and the other with two LRP binding arms. Each molecule is connected to a binder that binds a different epitope of the common bridging receptor βKlotho (KLB). Yellow stars denote activation of WNT signaling.
Development of cell/tissue-targeted RSPO mimetics
WNT signaling is fine-tuned by both positive and negative regulators. One such control system involves RSPO proteins, which work synergistically with WNTs to enhance signaling levels but do not activate in the absence of WNTs. This pathway consists of two membrane E3 ubiquitin ligases, secreted RSPO1–4, and their receptors, LGR4–6 or heparin sulfate proteoglycans (HSPGs).28 The two E3 ligases, ZNRF3 and RNF43, are WNT target genes that ubiquitylate FZD and LRP and consequently lead to receptor degradation and downregulation on the cell surface, thereby inhibiting the cellular response to endogenous WNTs (9,29; Figure 4A). RSPOs counter E3 actions by binding both E3 ligases and either LGRs or HSPGs, thus leading to cell membrane clearance of the E3 ligases, increasing FZD and LRP levels, increasing cellular sensitivity to endogenous WNTs, and thereby amplifying WNT signaling30 (Figures 4A and 4B). Therefore, RSPOs also play important roles in stem cell renewal, tissue homeostasis, and injury repair.31 Given that RSPOs do not have an intrinsic ability to activate WNT signaling, RSPO selectively boosts WNT signaling only in cells in which endogenous WNT ligands are present. However, the potential applications of RSPOs in regenerative medicine are limited, because of the broad distribution of their receptors and their pleotropic effects on many tissues.
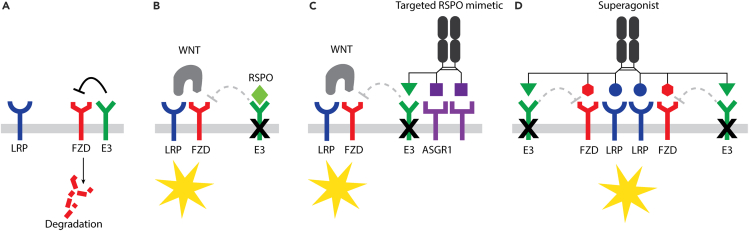
Figure 4.
Cell-targeted RSPO mimetics and WNT superagonists
(A) An E3 ligase targets FZD for receptor degradation, thus inhibiting the cellular response to WNT.
(B) RSPO enhances the cellular response to WNT by interfering with E3 ligase-mediated FZD degradation. RSPO alone does not activate WNT signaling in the absence of endogenous WNTs.
(C) An ASGR1-targeted RSPO molecule specifically enhances WNT signaling in ASGR1-expressing hepatocytes.
(D) A WNT superagonist consisting of a WNT mimetic and an RSPO mimetic can replace both WNTs and RSPOs. Yellow stars denote activation of WNT signaling.
Tissue-selective RSPO mimetics have been developed by redirecting E3 ligase sequestration or degradation away from LGRs and HSPGs, and toward cell-specific cell surface proteins. Two studies have successfully deployed a bispecific molecule consisting of a fusion between specific E3 binders and a cell surface receptor binder of interest. The E3 binders described to date are either specific antibodies that bind RNF43 or ZNRF3,32 or mutant RSPOs that retain the ability to bind E3 but are unable to bind LGRs and HSPGs33 (Figure 4C). The cell surface receptor binders explored include a cytokine interleukin (IL)-2 for immune cell targeting via binding CD25 32 and an anti-ASGR1 antibody for hepatocyte targeting.33 ASGR1 is a receptor localized on the surfaces of hepatocytes. The ASGR1-targeted RSPO mimetic molecules (termed Surrozen WNT signal enhancers engineered for tissue specificity; SWEETS) show specific signaling in hepatocytes and avoid RSPO-sensitive intestinal tissue (Figure 4C). ASGR1 SWEETS works by enhancing local endogenous WNT signaling in the liver. Both in vitro and in vivo, these molecules have been found to lead to increased hepatocyte proliferation and improved liver function in a mouse model of liver injury.33 Additional examples of SWEETS targeting other cell surface receptors, such as the transferrin receptor (TFRC), Ly6/PLAUR domain-containing protein 3 (LYPD3), and Desmoglein-3 (DSG3), have also been reported.33 Interestingly, these RSPO mimetic molecules act as protein degraders. Antibody-based targeting of E3 ligases and ASGR1 have been found to facilitate proteasomal degradation via ubiquitylation or lysosomal pathway degradation.32,34,35,36,37,38,39,40,41 These studies have demonstrated the feasibility of cell-targeted RSPO mimetics and enabled new opportunities for the development of RSPO mimetic WNT modulators for regenerative medicine.
Development of WNT superagonists
For the generation of potent antagonist molecules to inhibit WNT signaling, bispecific molecules that bind the two E3 ligases (ZNRF3 and RNF43) and FZD have been constructed to enhance this natural negative regulatory mechanism in FZD receptor ubiquitination and degradation20 (WO2021/173726). Removal of WNT receptors from the cell surface would be expected to inhibit pathway activation. This concept is similar to that underlying other targeted protein degrader systems, such as PROTAC (proteolysis targeting chimera42). In contrast to the expected results of enhanced FZD degradation, these bispecific E3-FZD engagers have shown the opposite activity, increasing FZD levels and enhancing cellular sensitivity to WNTs, similarly to RSPO mimetic treatment.20 Although the mechanisms leading to these unexpected results remain to be determined, these observations have provided an opportunity to combine an RSPO mimetic and a WNT mimetic into a single molecule, thus creating a WNT “superagonist”20 (Figure 4D). The WNT superagonist platform combines WNT and RSPO mimetics’ effects into one trispecific hexavalent molecule by binding FZD, LRP, and E3 ligase. This platform can be fine-tuned as a set of tool molecules activating all WNT/β-catenin FZD receptors with subfamily or monospecificity replacing both WNTs and RSPOs. For example, in organoid cultures dependent on both WNT and RSPO, a single superagonist can replace both factors in the medium. This set of WNT agonist combination molecules is easily expressed and purified and supports better expansion of various human and mouse organoid systems. These WNT-modulating platforms can also be broadly applied to organoids, pluripotent stem cells (PSCs), and in vivo research and may serve as a basis for future regenerative therapeutic development.
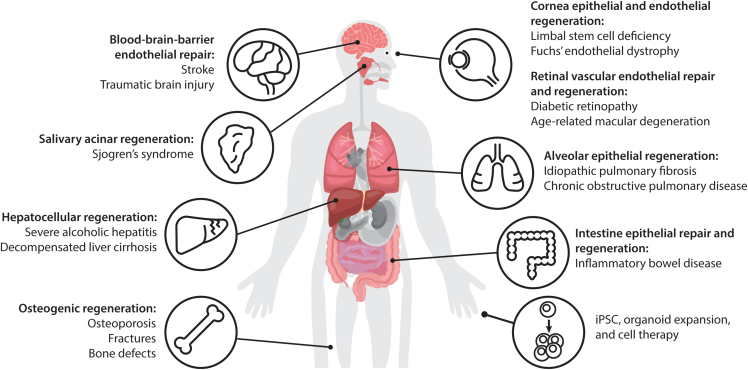
Applications of WNT agonists
Genetic manipulation of the components of the WNT pathway has made enormous progress toward the mechanistic understanding of this pathway in development and adult tissue function. However, mutations in signal transduction components, such as APC, RNF43, or β-catenin, may have fundamentally different effects from endogenous ligands; moreover, genetic manipulations of ligands and receptors do not recapitulate the transient nature of the signaling and are complicated by the promiscuous interactions between WNTs and their receptors, and the functional redundancies in the system. Therefore, additional approaches are needed to elucidate this complex signaling pathway. The development of receptor-specific and cell type-specific modulators, as described in the proceeding section, has offered unprecedented ability to probe this pathway. In this section, we discuss several applications of these tools reported to date. Further refinement of these tools is expected to enable additional biological insights and the expansion of studies into an even broader set of tissues.
Liver
Hepatocytes and non-parenchymal cells of the adult liver are organized as anatomical and functional units termed liver lobules.43 Within each lobule, the expression of metabolic genes and their associated functions display a zonal distribution along the radial axis between the hepatic portal and central veins—a phenomenon described as metabolic zonation. WNT/β-catenin signaling activity near the central vein is a master regulator of liver zonation. Pathway inactivation by liver-specific deletion of receptors, ligands, or β-catenin abolishes pericentral gene expression signatures.44 Pathway overactivation leads to expansion of the pericentral gene profile across the liver lobule.45,46 In addition to its role in patterning the adult liver lobule, WNT/β-catenin activity acts as a critical instructive signal for hepatocyte proliferation and regeneration, inducing the expression of key cell-cycle regulators such as cyclin D1.44,47,48,49 Genetic pathway inactivation in hepatocytes decreases liver size in mice and significantly delays hepatocyte proliferation and liver regeneration after partial hepatectomy (PH).44 Conditional deletion of Apc, in contrast, causes hepatocyte hyperproliferation and increased liver size.45 Hence, given the crucial roles of WNT and RSPO in liver regeneration and metabolic zonation, stimulating WNT/β-catenin activity to treat various liver diseases has great therapeutic promise.
The original WNT surrogate 18R5scFv-DKK1c14 and the next-generation surrogate WNTs15 have both been shown to induce signaling, zonation, and proliferation in the liver. Based on Fzd expression and the comparison of WNT agonists with different FZD specificities, WNT signaling in the liver is induced by different FZD subtypes. When expressed together with RSPO2 in mice, surrogate WNTs specific for FZD1,2,7 or FZD4 expand pericentral WNT target gene expression while repressing expression of periportal genes; in contrast, an FZD5,8-specific surrogate WNT has not demonstrated such effects in the mouse liver.15 In another study using antibody-based WNT mimetics, Post et al. have shown that pan-FZD-specific, FZD1,2,7-, or FZD4-specific agonists induce liver growth.20 Hu et al. have investigated the treatment effects of an FZD1,2,5,7,8 WNT agonist (FL6.13) in liver injury models. Similar to findings from other studies, FL6.13 has shown signaling, zonation, and proliferation effects in naive mouse liver.50 After PH, FL6.13 has been found to rescue metabolic zonation and liver regeneration delay. In a high-dose APAP mouse liver injury model, treatment of FL6.13 at 32 h post-APAP injection has been found to promote liver repair by activating WNT/β-catenin signaling and inducing hepatocyte proliferation, whereas early treatment of FL6.13 increases mortality.50 This work demonstrates the regenerative potential of WNT agonists for treating liver diseases but also highlights potential challenges in developing WNT mimetics for liver regeneration. Of note, FL6.13 not only lacks FZD specificity but also may activate WNT signaling in non-parenchymal liver cells and other non-targeting tissues. Development of hepatocyte-targeted WNT mimetics (Section Cell-targeted WNT mimetics) may facilitate investigation of the roles of specific FZD effects on hepatocytes.
Various studies have shown that the effects of WNT mimetics in the liver are enhanced by simultaneous RSPO treatment15,20; consequently, receptor expression level might be a limiting factor in mediating WNT stimulatory effects. This conclusion is further supported by the WNT superagonist described by Post et al.,20 which has more potent effects on primary hepatocytes than WNT mimetics alone. The presence of endogenous WNT ligands in the liver may render treatment with RSPO alone sufficient to elicit pro-proliferative responses. Treating mice with recombinant RSPO1 alone induces hepatocyte proliferation and accelerates liver regeneration after PH.46 However, the strong proliferation-promoting effect of RSPOs in other tissues, including the intestines, prevents direct application of RSPO for therapeutic purposes. As described in the section “Development of cell/tissue-targeted RSPO mimetics,” Zhang et al.33 first described a hepatocyte-targeted RSPO2 mimetic for liver regeneration. A mutant RSPO2 fragment fused with an antibody to ASGR1 (αASGR1-RSPO2-RA) was found to specifically stimulate WNT signaling in ASGR1-expressing hepatocytes in vitro and in vivo. αASGR1-RSPO2-RA also demonstrated liver-specific proliferation effects but did not affect another RSPO-responsive tissue, the intestine.33 In a TAA liver injury study, αASGR1-RSPO2-RA also improved liver coagulation function, as indicated by accelerated INR recovery.33 Further protein engineering to improve biophysical properties and achieve higher stability and more drug-like properties has yielded the clinical candidate compound SZN-043 (www.surrozen.com; Fisher et al.51).
SZN-043 has shown robust efficacy in preclinical animal models of liver injury, by stimulating hepatocyte proliferation, improving synthetic function, and decreasing fibrosis. SZN-043 is the first cell type-specific RSPO mimetic to enter clinical studies and is currently being evaluated for severe liver diseases that might benefit from rapid hepatocyte regeneration, including severe alcoholic hepatitis (March 2022, ANZCTR registry ACTRN12622000392763).
Intestine
Cells in the intestinal epithelium are continually renewed by intestinal stem cells (ISCs), to maintain a healthy and functional epithelial barrier against mechanical, chemical, and biological insults from the intestinal lumen.52 Stem cell niche signals consist of WNTs expressed by epithelial cells, neighboring mesenchymal cells, and enteric neuronal cells, as well as RSPOs secreted by mesenchymal cells and muscle cells in the muscularis mucosa layer beneath the intestinal crypts; the generated WNT/β-catenin signal gradient is crucial to ISC maintenance, proliferation, and differentiation.53 Severe injuries may compromise or deplete the LGR5+ ISCs located at the crypt base. In these injury scenarios, progenitors and fate-defined epithelial cells of absorptive, enteroendocrine, tuft, and Paneth cell lineages also display plasticity and can de-differentiate and subsequently repopulate the ISCs for epithelial repair.54 WNT is also among the most investigated signaling pathways for damage-induced intestinal epithelial repair mechanisms.55,56,57,58 For instance, in response to DSS-induced colitis damage, WNT-producing stromal cells significantly increase in number and express higher levels of RSPO3. In contrast, when WNT ligand secretion is blocked in mice, radiation-induced intestinal epithelial damage cannot be repaired.59
Treating chemotherapy-induced intestine mucositis and chemically induced colitis mouse models with RSPO1 has further demonstrated the possibility of improving epithelial repair with WNT agonism.31,60 However, although direct application of RSPO is effective in protecting cells against colitis damage or accelerating repair process, its strong mitogenic activity in the intestine, stimulating intestine hyperplasia, has prevented its further development for therapeutic use.60,61 WNT mimetics in various formats have been tested on regeneration and repair of intestinal epithelium in vitro and in vivo. The original WNT surrogate 18R5scFv-DKK1c stimulates intestinal organoid growth in vitro.14 The tetravalent WNT agonizing antibodies engineered by Tao et al. are also active toward intestinal organoids and maintain LGR5+ stem cells when cotreated with a porcupine inhibitor in mice.18 Yan et al. have shown that 18R5scFv-DKK1c alone can maintain LGR5+ ISCs in the absence of endogenous WNT signals in the mouse small intestine and can stimulate epithelium hyperproliferation when combined with RSPO1. These results suggest non-equivalent roles of WNT mimetic and RSPO in maintaining ISC and stimulating epithelial regeneration.61
Fzds display a differential expression pattern along the crypt-villus axis in the intestinal epithelium.16 The identification of the specific FZD subfamily essential for intestinal regeneration continues to be of great interest. Ongoing efforts can be divided into FZD-specific agonists, antagonists, or genetic approaches. Chen et al.16 have shown that WNT mimetics tailored toward FZD5 specificity demonstrate better regenerative potential in intestinal organoids. Both Chen et al.16 and Miao et al.15 have found that WNT mimetics with FZD1,2,7 specificity are not as potent in stimulating organoid growth, or in stimulating mouse intestinal epithelial cell proliferation in vivo, as FZD5,8-specific WNT mimetics in the presence of exogenous RSPO.15,16 Consistently, an inhibitory study has shown that an FZD5,8-specific binder induces catastrophic intestinal crypt and villus loss in intestinal organoids and in mice, whereas binders targeting other FZDs do not.62 Interestingly, other genetic depletion and inhibitory studies appear to suggest that FZD7 is the key WNT receptor in ISCs mediating WNT signaling and epithelial regeneration. Conditionally knocking out FZD7 in the adult intestinal epithelium or inhibiting its function causes ISC loss and impaired epithelial regeneration after damage.63,64 Further investigations may be warranted to comprehensively understand the differential roles of all FZDs expressed in the intestinal epithelium. However, studies to date suggest overlapping roles of FZD5 and FZD7 in ISC regeneration and pathway stimulation, although FZD5 may have greater therapeutic potential for intestinal diseases such as inflammatory bowel disease.
WNT mimetics with FZD5,8 specificity, compared with FZD1,2,7 specificity, have shown superior efficacy in eliciting colon epithelium repair and regeneration in a mouse colitis model, thus achieving better histology and disease activity improvement.7 In agreement with previous reports, WNT mimetics alone effectively repair damaged epithelium but do not cause hyperplasia of the intestinal epithelium.7 Using single-cell RNA sequencing, Xie et al.7 have observed epithelial-specific WNT target gene expression and a transient expansion of progenitor cell proliferation after FZD5,8 mimetic treatment, but no alterations in epithelial cell differentiation potential after WNT mimetic treatment.7 The observed decrease in inflammation and amelioration of animal disease appear to be secondary to healing of the epithelium and barrier. SZN-1326, an optimized version of the FZD5,8-specific mimetic described by Xie et al.,7 is the first tetravalent WNT mimetic molecule to enter clinical studies (March 2022, ANZCTR registry ACTRN12622000392763). SZN-1326’s ability to directly induce epithelial repair and healing differentiates it from all current standard-of-care medicines targeting inflammatory components of the disease. This new mechanism offers great promise in directly improving histological remission and achieving better long-term outcomes in ulcerative colitis.
Vascular blood-retinal and blood-brain barriers
The major roles of WNT/β-catenin signaling in vascular biology, particularly in the retina, and in brain vasculature development and function, have been well documented. Norrin, an atypical WNT ligand, and its receptor complex, FZD4/LRP5/TSPAN12, have been recognized for their importance in the development of retinal vascular structure and the maintenance of the blood-retinal barrier function.65,66,66,67 Genetic perturbations in these four proteins cause vascular defects and visual impairments in humans and rodents.1,68,69 Dysregulation of WNT/β-catenin signaling in the eyes has also been observed in human ocular diseases such as diabetic retinopathy (DR). Abnormally elevated β-catenin levels have been reported in patients with non-proliferative DR70; however, DKK1 levels are also significantly elevated in the vitreous in patients with DR.71
Given its essential roles in the retinal vessels, WNT/β-catenin signaling modulation was explored as a therapeutic approach for retinopathies. SZN-413, a tetravalent bispecific WNT mimetic antibody targeting FZD4 and LRP5, is highly potent in inducing WNT/β-catenin signaling in vascular endothelial cells in vitro, as compared with the natural ligands Norrin or WNT3A.24 In an oxygen-induced retinopathy (OIR) mouse model, SZN-413 significantly decreased pathological neovascular tuft formation to a similar extent as the current standard-of-care anti-VEGF drug aflibercept, while unexpectedly achieving superior regeneration of normal vessels in this model. SZN-413 also upregulated the tight junction proteins CLDN5 and ZO1 in retinal vascular cells and inhibited VEGF-driven retinal vascular leakage in a rabbit retinopathy model.24 It is remarkable that this novel FZD4/LRP5-targeting WNT mimetic molecule can simultaneously correct both vascular non-perfusion and leakage, thus addressing the underlying pathophysiology of retinopathies. Another tetravalent FZD4/LRP5-targeting WNT mimetic molecule, F4L5.13, has been independently developed.25 Similarly to SZN-413, F4L5.13 has been demonstrated to decrease neovascular tufts in OIR mice and restore blood-retinal barrier function in Tspan12 knockout (KO) mice.25,72 However, F4L5.13 does not restore the vessels in the ischemic area in the developing eyes of OIR mice, in contrast to SZN-413.25 Further experiments are necessary to clarify the reasons for the differences between F4L5.13 and SZN-413 in the vascular regeneration observed in the OIR model. Nevertheless, these results suggest that FZD4-targeting WNT mimetics have great promise for treating patients with retinal vascular diseases, such as DR.69 An optimized version of F4L5.13, called Restoret (EYE103), is the second tetravalent WNT mimetic molecule to achieve clinical applications; this therapy is currently being evaluated for diabetic macular edema and advanced neovascular age-related macular degeneration (June 2023,73 clinicaltrials.gov NCT05919693).
FZD4-targeting WNT mimetics may also modulate blood-brain barrier (BBB) function. Mice with genetic mutations in any components of the Norrin/FZD4/LRP5/TSPAN12 axis show phenotypically similar defects in the retina and the brain vasculature.65,67,74,75 The BBB defects in the KO mice are observed in the cerebellum and are caused by downregulation of tight junction proteins and/or upregulation of plasmalemma vesicle-associated protein in vascular endothelial cells.76,77,78,79 BBB defects have been suggested as a cause of the cognitive/psychosocial impairments observed in a subpopulation of patients with Norrie disease.80
Beyond the Norrin/FZD4/LRP5/TSPAN12 signaling axis, the WNT7/FZD/GPR124/RECK signaling axis is another critical WNT/β-catenin-dependent signaling pathway for angiogenesis in the central nervous system (CNS) and BBB formation/maintenance.81,82,83,84 Genetic ablation of Wnt7, Gpr124, or Reck in vascular endothelial cells impairs CNS angiogenesis and BBB formation.79,82,85 The Norrin/FZD4/LRP5/TSPAN12 and WNT7/FZD/GPR124/RECK signaling axes both regulate CNS angiogenesis and maintain BBB function, thus demonstrating the critical function of WNT/β-catenin signaling in CNS vascular development and function, though exerting their effects in different regions of the CNS.77 A BBB-specific WNT agonist targeting GPR124/RECK has been shown to protect against ischemic stroke infarction and glioblastoma expansion.86 Moreover, a bioengineered FZD4-selective WNT mimetic, L6-F4-2, also significantly restores the function of BBB in aberrant brain vessels under ischemic stroke conditions and rescues stroke phenotypes.87 Therefore, the collective scientific evidence suggests that the agonistic approaches to FZD4 regulation have therapeutic potential not only for retinal vascular diseases but also for brain diseases caused by BBB dysfunction such as stroke, traumatic brain injury, epilepsy, and neurodegenerative diseases.
Bone
WNT/β-catenin signaling is critical for skeletal development, bone mass regulation in adults, and injury repair.88,89,90,91,92 WNT/β-catenin signaling activation promotes osteogenic differentiation, induces bone formation,89,90,91,92 and decreases bone resorption.93 Human mutations in multiple WNT pathway genes result in bone disorders. For instance, gain-of-function or loss-of-function mutations in the human LRP5 gene induce an autosomal dominant high-bone-mass trait or diminished bone mass with osteoporosis-pseudoglioma syndrome, respectively.94,95 Mutations in the WNT16 gene influence bone strength and increase osteoporotic fracture risk,96 and mutations in WNT1 induce osteogenesis imperfecta.97 Sclerostin, a glycoprotein produced primarily by osteocytes, is a key WNT pathway inhibitor that competes with endogenous ligands for binding LRP5/6.98,99 Genetic abnormalities in the SOST gene, encoding sclerostin protein, cause high bone mass in human diseases such as sclerosteosis and Van Buchem’s disease.100,101 A neutralizing monoclonal antibody against sclerostin, romosozumab, activates WNT/β-catenin signaling, increases bone formation, and protects patients with osteoporosis against high fracture risk.100 Thus, activation of WNT signaling has promise in enhancing bone regeneration and healing in conditions such as osteoporosis, fractures, and bone defects.
The importance of WNT/β-catenin signaling in bone regeneration has led to the evaluation of WNT mimetics in various bone studies. The original WNT surrogate, 18R5scFv-DKK1c, described in the section “Discovery and development of WNT mimetics activating WNT/β-catenin signaling,” has been shown to upregulate the early osteogenic marker alkaline phosphatase in mouse mesenchymal stem cells in vitro.14 FZDs are broadly expressed in osteoblasts and osteoblast precursors; notably, expression of FZD1, FZD2, and FZD7 has been observed in both mouse and human bones.19 The new generation of bispecific antibody-based WNT mimetics targeting FZD1,2,7 or FZD1,2,5,7,8 shows rapid and potent osteogenic effects in various bone disease models, including osteoporosis, aging, and bone fractures, thus demonstrating the remarkable potential of WNT mimetics in achieving effective bone regeneration,19 and serving as therapeutic agents for bone diseases.
In addition to FZD1,2,7 subtypes, other FZDs have also been reported to regulate bone homeostasis. For example, loss of Fzd8 induces osteoclastogenesis, thereby leading to osteopenia.102 Fzd9 is induced during osteoblast differentiation, and Fzd9 KO mice exhibit diminished bone mass due to impaired bone formation.103 Additionally, Fzd4 is expressed in osteoblasts and is crucial for normal bone acquisition.104 Interestingly, loss of Fzd4 is compensated for by the upregulation of Fzd8; consequently, these two FZDs may have redundant function in osteoblasts.104 The availability of FZD-selective WNT mimetics and the potential to develop cell-specific modulators described in the section “Novel WNT-activating approaches” are expected to advance understanding of the roles of various FZDs in different bone cell types and facilitate the development of WNT mimetics as therapeutics for bone diseases.
Cornea
The corneal epithelium, the outermost layer of the eye, functions to refract light and forms the primary barrier of the eye against external insults.105 Once injured, the corneal epithelium must be quickly and properly repaired to restore optical clarity and to maintain vision. WNT/β-catenin signaling is active under normal physiology and is necessary to maintain the homeostasis of the corneal epithelium.106,107 In the corneal limbus, where limbal stem cells reside, the WNT/β-catenin pathway is an important niche factor in the control of proliferation and cell fate.108 Exogenous activation of WNT/β-catenin signaling by small molecules or recombinant WNT proteins induces the proliferation and colony-forming efficiency of human corneal epithelial stem cells in vitro.109,110,111,112 The small molecule WNT mimetic MFH-ND (described in the section “Discovery and development of WNT mimetics activating WNT/β-catenin signaling”) also enhances limbal epithelial cell stemness and proliferation in culture.26 In some corneal disorders, such as diabetic cornea, WNT/β-catenin signaling-associated factors are downregulated. In a mouse model of diabetic corneal alterations, treatment with the pathway activator lithium chloride promotes accelerated healing.113 Together, these results suggest that WNT mimetics may be used to stimulate corneal epithelial healing in disease or injury. Among the ten FZDs, Fzd7 is differentially expressed in the limbal stem cell population113,114; therefore, testing an FZD7-specific WNT mimetic for treatment of corneal epithelial diseases such as limbal stem cell deficiency and neurotrophic keratopathy would be of particular interest.
The corneal endothelium is a single layer of specialized cells on the inner surface of the cornea. This layer is a critical structure for the cornea, which maintains corneal clarity through its pump function. Endothelial cells are lost over time with aging but can also be damaged through chemical and mechanical exposures or genetic dystrophies.115 Although human corneal endothelial cells have limited proliferation capability, WNT/β-catenin signaling activation is of interest in tissue-regeneration approaches.116 Corneal endothelial cell density has been found to significantly increase in vitro after RSPO1 treatment in rabbit and human cornea organ cultures.117,118 In addition, WNT10B promotes corneal endothelial cell proliferation.119 The latest generation of WNT mimetics will be of interest in further exploration of therapeutic approaches to corneal endothelial cell dysfunction. Future investigations may include deciphering specific WNT receptor expression profiles in endothelial cells and the proliferative ability of human cells in vivo.
Although both cell types of the cornea respond to WNT/β-catenin signaling, an approach tailored to each distinct cell layer is likely to be necessary to explore the full therapeutic potential of pathway activation.
Salivary glands
Salivary glands are exocrine glands that produce saliva through a system of acini and ducts. The three major glands produce serous, mucinous, or seromucous secretions, which facilitate digestion and lubrication. WNT signaling has been implicated in salivary gland development and adult stem cell maintenance and is activated after injury. Components of the WNT pathway, such as WNT protein and receptors, are expressed in adult salivary glands in both mice and humans.120,121 Using a transgenic WNT reporter, Hai and colleagues have demonstrated that WNT signaling is active in developing mice and is reactivated in adult submandibular glands during regeneration.122 In another study, transient Wnt1 overexpression has been found to protect tissue against radiation-induced chronic salivary gland dysfunction.123 Furthermore, in vitro, WNT3A is a key driver of salivary gland stem cell expansion as organoids.124 More recently a novel platform of FZD-specific WNT mimetics has elucidated the role of FZD1,2,7 in this pro-proliferative salivary gland phenotype.20 In a comparison of subfamily-specific WNT mimetics targeting FZD1,2,5,7,8, FZD1,2,7, FZD5,8, FZD4, FZD4,9, or FZD10, FZD1,2,7-targeting WNT activators had the most potent effects in vitro and in vivo. With systemic treatment, WNT activation through FZD1,2,7 resulted in salivary gland tissue expansion, as measured by acinar cell proliferation and organ weight increase. In addition, murine organoid expansion was enhanced in a dose-dependent manner.20 The salivary gland is one of the first studied tissues with a strong biased response to a single FZD subfamily. Many other tissue cell types respond to both FZD1,2,7 and FZD5,8 activation, despite having differential receptor mRNA expression levels. The exact mechanisms underlying salivary gland FZD1,2,7 receptor preference, and whether these rules apply to other exocrine glandular tissues such as the lacrimal gland or pancreas, remain unknown. In addition, the FZD-specific effects on human salivary gland cells remain to be determined. Thus, further exploration of WNT signaling in salivary gland regeneration is encouraged. The tissues’ easy accessibility is expected to open avenues for local administration of WNT mimetics or cell therapy approaches.
Lungs
The lungs are a complex organ with myriad epithelial, stromal, and immune cell types125 and a highly regionalized architecture. The proximal and distal airways and the alveoli have regenerative ability after injury.126 In the alveoli, a subset of AT2 cells are stem cells that can self-renew and differentiate into AT1 cells.127,128,129,130 WNT/β-catenin signaling in AT2 cells is critical for their renewal and alveolar regeneration.129,130 During normal homeostasis and after injury, PDGFRA+ fibroblasts secrete WNT ligands, which signal to AT2 cells129,131,132; AT2 cells also use autocrine WNT signals to regulate expansion after injury.129 In contrast, WNT/β-catenin signaling must be diminished for AT2 cells to differentiate into AT1 cells.129,130 The association of active WNT/β-catenin signaling with epithelial stem/progenitor cell renewal and expansion, and downregulation of the pathway to differentiation are shared features among the proximal and distal airway compartments, and other adult stem cell-maintained tissues such as the intestinal epithelium.
A fine balance of WNT signaling in stromal and epithelial cells in the lungs underlies normal renewal and repair after injury, and changes in the levels of WNT/β-catenin signaling are associated with lung diseases such as chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF). The loss of alveoli (emphysema) in COPD is associated with decreased WNT/β-catenin signaling,133,134,135 whereas activation of WNT/β-catenin signaling leads to less airspace expansion in elastase and smoking models of COPD.133,134,136,137 In hypercapnia (elevated blood CO2 level) injury models, similarly to COPD, loss of alveoli is associated with decreased WNT/β-catenin signaling, and pathway activation rescues AT2 cell proliferation.132 On the basis of these studies, further studies may investigate whether targeting WNT and RSPO mimetics to AT2 cells and/or disease-specific distal epithelial cells facilitates alveolar regeneration.
IPF is characterized by myofibroblast-mediated deposition of extracellular matrix.138,139 In addition to the loss of alveoli, aberrant disease-associated epithelial cells contribute to driving fibrosis.140,141 Critically, in the bleomycin model, loss of WNT/β-catenin signaling in AT2 cells leads to enhanced pulmonary fibrosis142; therefore, defects in AT2 cell renewal and alveolar regeneration may enhance fibrotic damage, and alveolar regeneration might have anti-fibrotic benefits. A WNT mimetic that targets FZD5, which is enriched in alveolar cells, has been shown to expand AT2 organoids; however, in the bleomycin model, treatment with the FZD5-specific WNT mimetic leads to AT2 cell expansion into the airways but does not restore alveoli or decrease fibrosis.143 Although the dosing in this experiment limited the relevance of the findings to repairing established fibrosis, one interpretation may be that activating WNT/β-catenin signaling only on AT2 cells may be insufficient to repair the lungs after severe injury such as fibrosis.
An additional complication in developing WNT/β-catenin signaling modulators to treat IPF is that many studies have reported a connection between active WNT/β-catenin signaling and the formation of pulmonary fibrosis.136,144,145 These results suggest that a WNT/β-catenin signaling antagonist targeted to fibroblasts might limit the formation of fibrosis, whereas a pathway activator should avoid this cell type. However, perhaps surprisingly, a broad-spectrum FZD-binding WNT mimetic that engages FZD1,2,5,7,8 and LRP6 has been found to activate the pathway in multiple lung cell types, decrease inflammation and fibrosis in the lungs, and restore lung function in an acute bleomycin model.146 In line with these results, broader, non-targeted activation of the pathway via an antibody to DKK1, an LRP antagonist, has been found to decrease fibrosis in the acute bleomycin model.147 Potentially relevant to these findings, WNT/β-catenin signaling may also play key roles in other cells including immune cells relevant in IPF. For example, in several pulmonary injury models, macrophages have been reported to respond to RSPO3 by shifting from a pro- to an anti-inflammatory phenotype.148
Because WNT/β-catenin signaling plays multiple roles in lung injury, disease, and repair, and in light of the recent finding that pathway activation in multiple cell types is beneficial in an animal model of pulmonary fibrosis, several questions must be answered to engineer targeted WNT/β-catenin signaling modulators for repairing damaged/diseased lung tissue. Will focused activation of WNT/β-catenin signaling (via WNT/RSPO mimetics) in one cell type be sufficient to promote alveolar regeneration and limit or decrease fibrosis, or must multiple cell types be targeted to orchestrate regeneration? Can targeted WNT/β-catenin signaling agonists revert aberrant, disease-specific epithelial cells to a more normal phenotype in vivo? Is there a time and place where pathway antagonism could be beneficial? Even though there are many unresolved questions, the strong rationale and recent data make it an exciting time to explore the development of regenerative therapeutics for lung disease.
Ex vivo stem cell expansion
The discovery of WNT signaling as an essential cellular signaling pathway for stem cell maintenance in vivo has further enabled researchers to expand and maintain stem cells in culture. Three-dimensional grown structures derived from PSCs or adult stem cells are known as organoids. By recapitulating the stem cell niche, with factors such as WNT, organoids self-organize into organ-specific structures with differentiated cell types. In most organoid culture systems, WNT signaling activation is essential to maintain stemness and proliferation. One earlier well-known example is the derivation of small intestinal epithelial organoids from single LGR5+ stem cells.149 In the presence of RSPO1 and other growth factors, crypt stem cells proliferate and form crypt-like budding structures. In murine intestinal organoids, WNT3A is secreted from Paneth cells, and the addition of RSPO1 to the culture medium importantly further enhances WNT signaling pathway activity. However, most organoid cultures described to date have insufficient endogenous secretion of WNT protein and must be supplemented with exogenous ligands. The establishment of other WNT-dependent organoid cultures, such as human colon cultures, has built on the addition of recombinant WNT3A protein or WNT3A-conditioned medium.150 Other examples include human liver organoids,151 human pancreatic organoids,152 human stomach organoids,153 and human salivary gland organoids.124 In addition, embryonic stem cells and induced PSCs (iPSCs) are responsive to WNT signaling activation. Supplementation with WNT3A protein has been found to reprogram differentiated cells to pluripotent state and promote the generation of iPSCs.154 Moreover, activation of WNT signaling through inhibition of GSK-3 has been reported to maintain pluripotency in human and mouse embryonic stem cells.155 The GSK-3 inhibitor CHIR99021 has also been used to expand adult stem cell-derived organoids of liver hepatocytes and lung alveolar type 2 cells.156,157,158
The discovery and increased availability of WNT mimetics have also transformed the field of organoid research. Antibody-based WNT mimetics enable potent induction of proliferation and stemness, as well as deciphering FZD/LRP-specific biology in vitro. Surrogate WNT molecules are superior, or comparable, to recombinant WNT3A protein or WNT3A-conditioned medium in promoting continuous growth and single-cell outgrowth efficiency in several organoid types.14,15,16,20 The newest generation of WNT mimetics is tailored to activate the pathway through a subfamily or a single FZD receptor. Application of these new tools is beginning to elucidate some of the FZD subtype-specific functions in stem cell biology. Activation through FZD1,2,7 or FZD5,8, for example, supports mouse intestinal organoid growth,16 whereas WNT activation through FZD1,2,7 appears to be the most potent stimulant in murine salivary gland organoid expansion.20 An FZD7- and LRP6-selective WNT activator has demonstrated that activation through FZD7 alone is sufficient to promote mesendodermal differentiation of hPSCs.23 Using lung alveolar epithelial cell cultures, Nabhan et al. have shown that FZD5- and FZD6-specific agonists activate canonical WNT signaling in stem cells.143 These WNT mimetics can replace CHIR99021 and have been found to outperform recombinant WNT3A in alveolar cell expansion in vitro.143 More recently, cell-specific WNT mimetics or WNT superagonists that combine both WNT and RSPO activity have shown ongoing improvement in WNT mimetics and ready applicability in organoid research.20,27
The ability of WNT mimetics to improve the expansion of stem cells and progenitor cells will be of interest in cell therapy. Epithelial cell cultures such as organoids retain the ability to rebuild their tissue of origin after in vivo transplantation. Successful transplantation of organoids for regenerative purposes has been demonstrated in mouse models of intestinal and salivary gland injury.159,160,161 Transplantation of organoids from these two tissues is currently in human clinical trials to test for functional organ recovery after cell therapy. WNT mimetics may potentially provide an abundant and sustainable source of cells for therapeutic purposes.
Discussion, conclusion, and future directions
More than four decades after the discovery of WNT and many seminal studies on this fundamental developmental signaling pathway, we are in the dawn of an exciting era of understanding WNT biology in adult tissue repair. Through the efforts of many researchers, new tools including receptor-specific WNT mimetics, enhancers of WNT signaling including cell-specific RSPO mimetics, superagonists, and other tissue-specific engineered tools have been created. In this review, we highlighted the recent advances in these molecules and tools, collectively referred to as WNT agonists. We then summarized WNT agonists’ early applications and the rationale for their use in liver, intestine, retina vessel, BBB, cornea, salivary gland, lung, and ex vivo stem cells. We also discussed functional studies applying WNT mimetics, and the effects of specific FZDs and FZD subfamilies on tissues of interest. Beyond the list of tissues described earlier, we and others in the field have investigated the effects of WNT agonists on many more tissues/organs and certain specific cell types within those tissues. These ongoing efforts are likely to improve understanding of the similarities and distinct features of regenerative effects of WNT signaling in various tissues, thus culminating in a pipeline of knowledge, and supporting a rationale for therapeutic design for specific tissues and diseases.
Among WNT agonists, the ideal context for engineered WNT mimetics might be diseases involving endogenous WNT ligand deficiency or a loss of connection between WNT producer cells and target stem or precursor cells because of tissue damage. If abundant WNT ligands are present in diseased tissues, but the signaling toward critical parenchymal cells is insufficient, RSPO mimetics, tissue/cell-specific WNT enhancers, might be desirable to channel WNT signaling toward parenchymal cells and away from other cells, such as inflammatory cells and fibroblasts. In situations in which both WNT ligands and receptors are deficient, a superagonist approach combining WNT and RSPO mimetics might be considered. These theoretical assumptions could be tested in disease models of different tissues, and the effects of WNT or RSPO mimetics or superagonists could be compared.
Several lines of evidence suggest that WNT or RSPO mimetics, in the context of acute treatment, benefit tissue healing in a self-limited manner: (1) these molecules are designed to retain key negative feedback inhibitors of WNT signaling, such as AXIN2 and NOTUM; (2) from liver, intestine, and retina vascular studies, for example, WNT or RSPO mimetics have been found to have effective but transient effects on cell proliferation; (3) tissue functions are properly restored by newly regenerated healthy parenchymal cells; (4) tissue structures are restored with normal organization and restoration of all key cell types (from liver, intestine, and retina vessel disease models). These results further suggest the tremendous therapeutic potential of WNT and RSPO mimetics and indicate that the effects of these molecules fundamentally differ from those caused by mutations in genes such as APC, RNF43, or CTNNB1, which result in constitutive activation of the pathway.
The effects of these new WNT agonist tools appear to mimic the phenotype of endogenous WNT or RSPO ligands on the basis of comparison of these new studies and previous genetic studies. Furthermore, these new tools—such as FZD subfamily- or individual FZD-specific WNT mimetics or superagonists, and tissue-specific RSPO mimetics—may provide critical insights into specific receptor and tissue biology. In contrast, when feasible, antagonists inhibiting specific FZD receptors may be invaluable tools to complement agonist studies, and provide validation, contrast, or additional insights into the WNT signaling biology of specific tissues.
The WNT mimetic agonists described herein are in therapeutically developable molecular formats, and many demonstrate excellent drug-like properties. These molecules work effectively in various in vitro and in vivo preclinical models and may confer potential benefits in multiple therapeutic areas, such as those detailed in this review and beyond. Moreover, a wealth of literature in many disease areas has implicated WNT signaling in tissue repair and regeneration (Figure 5). For example, strong evidence from human genetics indicates that WNT signaling is required for healthy bone density, and the anti-sclerostin antibody romosozumab has been approved by the Food and Drug Administration (FDA) for the treatment of osteoporosis. Together, these findings validate the potential benefits of activating WNT signaling in adult tissues and suggest the feasibility of achieving safe and effective treatment with WNT signaling activation to promote adult tissue repair. Future directions will include incorporating knowledge of disease-specific characteristics, such as WNT receptor expression, expression of other targeting receptors, and dysregulation of WNT signaling, to enable the design of targeted, fit-for-purpose therapeutics.
Figure 5.
Applications of WNT mimetics
Different WNT mimetics targeting different FZDs and cell types have been applied and have shown regenerative potential in bone, liver, cornea, intestine, lung, retina vessel, BBB, and salivary gland. WNT mimetics can also be applied to enhance patient-derived or allogeneic iPSC or organoid expansion ex vivo that can be used as cell therapy.
Acknowledgments
The authors thank all staff at Surrozen, Inc., for their dedication and contributions to the development and study of various WNT modulating platforms. We thank our scientific founders Christopher Garcia, Roeland Nusse, Clavin Kuo, and Hans Clevers and our current and former Scientific Advisory Board members and Board of Directors for their guidance and support. We thank Craig Parker for helpful discussions and critical reading of this manuscript. This work was funded by Surrozen, Inc.
Author contributions
Y.P., C.L., R.B.F., W.-C.Y., H.N., and S.-J.L., conceptualization, formal analysis, resources, investigation, visualization, methodology, writing – original draft, writing – review and editing; Y.L., conceptualization, formal analysis, resources, investigation, visualization, supervision, funding acquisition, methodology, writing – original draft, writing – review and editing.
Declaration of interests
All authors are current or former full-time employees and shareholders of Surrozen, Inc. Y.L. is Executive Vice President of Research at Surrozen, Inc. Patent applications are pending for the work described in this manuscript.
References
- 1.Nusse R., Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Tauriello D.V.F., Jordens I., Kirchner K., Slootstra J.W., Kruitwagen T., Bouwman B.A.M., Noutsou M., Rüdiger S.G.D., Schwamborn K., Schambony A., Maurice M.M. Wnt/beta-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc. Natl. Acad. Sci. USA. 2012;109:E812–E820. doi: 10.1073/pnas.1114802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao J., Wang J., Liu B., Pan W., Farr G.H., 3rd, Flynn C., Yuan H., Takada S., Kimelman D., Li L., Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 4.Clevers H., Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi A., Yamamoto H., Sato A., Matsumoto S. New insights into the mechanism of Wnt signaling pathway activation. Int. Rev. Cell Mol. Biol. 2011;291:21–71. doi: 10.1016/B978-0-12-386035-4.00002-1. [DOI] [PubMed] [Google Scholar]
- 6.Niehrs C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 7.Xie L., Fletcher R.B., Bhatia D., Shah D., Phipps J., Deshmukh S., Zhang H., Ye J., Lee S., Le L., et al. Robust Colonic Epithelial Regeneration and Amelioration of Colitis via FZD-Specific Activation of Wnt Signaling. Cell. Mol. Gastroenterol. Hepatol. 2022;14:435–464. doi: 10.1016/j.jcmgh.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gayden J., Hu S., Joseph P.N., Delgado E., Liu S., Bell A., Puig S., Monga S.P., Freyberg Z. A Spatial Atlas of Wnt Receptors in Adult Mouse Liver. Am. J. Pathol. 2023;193:558–566. doi: 10.1016/j.ajpath.2023.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao H.X., Xie Y., Zhang Y., Charlat O., Oster E., Avello M., Lei H., Mickanin C., Liu D., Ruffner H., et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 10.Clevers H., Loh K.M., Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 11.Janda C.Y., Garcia K.C. Wnt acylation and its functional implication in Wnt signalling regulation. Biochem. Soc. Trans. 2015;43:211–216. doi: 10.1042/BST20140249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janda C.Y., Waghray D., Levin A.M., Thomas C., Garcia K.C. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dijksterhuis J.P., Baljinnyam B., Stanger K., Sercan H.O., Ji Y., Andres O., Rubin J.S., Hannoush R.N., Schulte G. Systematic mapping of WNT-FZD protein interactions reveals functional selectivity by distinct WNT-FZD pairs. J. Biol. Chem. 2015;290:6789–6798. doi: 10.1074/jbc.M114.612648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janda C.Y., Dang L.T., You C., Chang J., de Lau W., Zhong Z.A., Yan K.S., Marecic O., Siepe D., Li X., et al. Surrogate Wnt agonists that phenocopy canonical Wnt and beta-catenin signalling. Nature. 2017;545:234–237. doi: 10.1038/nature22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao Y., Ha A., de Lau W., Yuki K., Santos A.J.M., You C., Geurts M.H., Puschhof J., Pleguezuelos-Manzano C., Peng W.C., et al. Next-Generation Surrogate Wnts Support Organoid Growth and Deconvolute Frizzled Pleiotropy In Vivo. Cell Stem Cell. 2020;27:840–851.e6. doi: 10.1016/j.stem.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H., Lu C., Ouyang B., Zhang H., Huang Z., Bhatia D., Lee S.J., Shah D., Sura A., Yeh W.C., Li Y. Development of Potent, Selective Surrogate WNT Molecules and Their Application in Defining Frizzled Requirements. Cell Chem. Biol. 2020;27:598–609.e4. doi: 10.1016/j.chembiol.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Chen H., Lu C., Lee S.J., Li Y. Protocol to Generate and Characterize Potent and Selective WNT Mimetic Molecules. STAR Protoc. 2020;1:100043. doi: 10.1016/j.xpro.2020.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao Y., Mis M., Blazer L., Ustav M., Jnr, Steinhart Z., Chidiac R., Kubarakos E., O'Brien S., Wang X., Jarvik N., et al. Tailored tetravalent antibodies potently and specifically activate Wnt/Frizzled pathways in cells, organoids and mice. Elife. 2019;8:e46134. doi: 10.7554/eLife.46134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowler T.W., Mitchell T.L., Janda C.Y., Xie L., Tu S., Chen H., Zhang H., Ye J., Ouyang B., Yuan T.Z., et al. Development of selective bispecific Wnt mimetics for bone loss and repair. Nat. Commun. 2021;12:3247. doi: 10.1038/s41467-021-23374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Post Y., Dilip A., Xie L., Sura A., Suen N., Ye J., Mutha D., Liu A.T., Nguyen H., Whisler E., et al. Novel Frizzled-specific antibody-based Wnt mimetics and Wnt superagonists selectively activate WNT/beta-catenin signaling in target tissues. Cell Chem. Biol. 2023;30:976–986.e5. doi: 10.1016/j.chembiol.2023.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Nile A.H., Mukund S., Stanger K., Wang W., Hannoush R.N. Unsaturated fatty acyl recognition by Frizzled receptors mediates dimerization upon Wnt ligand binding. Proc. Natl. Acad. Sci. USA. 2017;114:4147–4152. doi: 10.1073/pnas.1618293114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirai H., Matoba K., Mihara E., Arimori T., Takagi J. Crystal structure of a mammalian Wnt-frizzled complex. Nat. Struct. Mol. Biol. 2019;26:372–379. doi: 10.1038/s41594-019-0216-z. [DOI] [PubMed] [Google Scholar]
- 23.Gumber D., Do M., Suresh Kumar N., Sonavane P.R., Wu C.C.N., Cruz L.S., Grainger S., Carson D., Gaasterland T., Willert K. Selective activation of FZD7 promotes mesendodermal differentiation of human pluripotent stem cells. Elife. 2020;9:63060. doi: 10.7554/eLife.63060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen H., Chen H., Vuppalapaty M., Whisler E., Logas K.R., Sampathkumar P., Fletcher R.B., Sura A., Suen N., Gupta S., et al. SZN-413, a FZD4 Agonist, as a Potential Novel Therapeutic for the Treatment of Diabetic Retinopathy. Transl. Vis. Sci. Technol. 2022;11:19. doi: 10.1167/tvst.11.9.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chidiac R., Abedin M., Macleod G., Yang A., Thibeault P.E., Blazer L.L., Adams J.J., Zhang L., Roehrich H., Jo H.N., et al. A Norrin/Wnt surrogate antibody stimulates endothelial cell barrier function and rescues retinopathy. EMBO Mol. Med. 2021;13:e13977. doi: 10.15252/emmm.202113977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C., Mei H., Robertson S.Y.T., Lee H.J., Deng S.X., Zheng J.J. A Small-Molecule Wnt Mimic Improves Human Limbal Stem Cell Ex Vivo Expansion. iScience. 2020;23:101075. doi: 10.1016/j.isci.2020.101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H., Lee S.-J., Li R., Sura A., Suen N., Dilip A., Pomogov Y., Vuppalapaty M., Suen T.T., Lu C., et al. BRAIDing receptors for cell specific targeting. Elife. 2024;12:RP90221. doi: 10.7554/eLife.90221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hao H.X., Jiang X., Cong F. Control of Wnt Receptor Turnover by R-spondin-ZNRF3/RNF43 Signaling Module and Its Dysregulation in Cancer. Cancers. 2016;8:54. doi: 10.3390/cancers8060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo B.K., Spit M., Jordens I., Low T.Y., Stange D.E., van de Wetering M., van Es J.H., Mohammed S., Heck A.J.R., Maurice M.M., Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 30.de Lau W., Peng W.C., Gros P., Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 2014;28:305–316. doi: 10.1101/gad.235473.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J., de Vera J., Narushima S., Beck E.X., Palencia S., Shinkawa P., Kim K.A., Liu Y., Levy M.D., Berg D.J., et al. R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology. 2007;132:1331–1343. doi: 10.1053/j.gastro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Luca V.C., Miao Y., Li X., Hollander M.J., Kuo C.J., Garcia K.C. Surrogate R-spondins for tissue-specific potentiation of Wnt Signaling. PLoS One. 2020;15:e0226928. doi: 10.1371/journal.pone.0226928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z., Broderick C., Nishimoto M., Yamaguchi T., Lee S.J., Zhang H., Chen H., Patel M., Ye J., Ponce A., et al. Tissue-targeted R-spondin mimetics for liver regeneration. Sci. Rep. 2020;10:13951. doi: 10.1038/s41598-020-70912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampathkumar P., Jung H., Chen H., Zhang Z., Suen N., Yang Y., Huang Z., Lopez T., Benisch R., Lee S.-J., et al. Targeted protein degradation systems to enhance Wnt signaling. Elife. 2023;13:RP93908. doi: 10.7554/eLife.93908.1. [DOI] [Google Scholar]
- 35.Pance K., Gramespacher J.A., Byrnes J.R., Salangsang F., Serrano J.A.C., Cotton A.D., Steri V., Wells J.A. Modular cytokine receptor-targeting chimeras for targeted degradation of cell surface and extracellular proteins. Nat. Biotechnol. 2023;41:273–281. doi: 10.1038/s41587-022-01456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn G., Banik S.M., Miller C.L., Riley N.M., Cochran J.R., Bertozzi C.R. LYTACs that engage the asialoglycoprotein receptor for targeted protein degradation. Nat. Chem. Biol. 2021;17:937–946. doi: 10.1038/s41589-021-00770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marei H., Tsai W.T.K., Kee Y.S., Ruiz K., He J., Cox C., Sun T., Penikalapati S., Dwivedi P., Choi M., et al. Antibody targeting of E3 ubiquitin ligases for receptor degradation. Nature. 2022;610:182–189. doi: 10.1038/s41586-022-05235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cotton A.D., Nguyen D.P., Gramespacher J.A., Seiple I.B., Wells J.A. Development of Antibody-Based PROTACs for the Degradation of the Cell-Surface Immune Checkpoint Protein PD-L1. J. Am. Chem. Soc. 2021;143:593–598. doi: 10.1021/jacs.0c10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siepe D.H., Picton L.K., Garcia K.C. Receptor Elimination by E3 Ubiquitin Ligase Recruitment (REULR): A Targeted Protein Degradation Toolbox. ACS Synth. Biol. 2023;12:1081–1093. doi: 10.1021/acssynbio.2c00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D., Duque-Jimenez J., Brixi G., Facchinetti F., Rhee K., Feng W.W., Jänne P.A., Zhou X. Transferrin Receptor Targeting Chimeras (TransTACs) for Membrane Protein Degradation. bioRxiv. 2023 doi: 10.1101/2023.08.10.552782. Preprint at. [DOI] [Google Scholar]
- 41.Sun R., Meng Z., Lee H., Offringa R., Niehrs C. ROTACs leverage signaling-incompetent R-spondin for targeted protein degradation. Cell Chem. Biol. 2023;30:739–752.e8. doi: 10.1016/j.chembiol.2023.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Zhao L., Zhao J., Zhong K., Tong A., Jia D. Targeted protein degradation: mechanisms, strategies and application. Signal Transduct. Targeted Ther. 2022;7:113. doi: 10.1038/s41392-022-00966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben-Moshe S., Itzkovitz S. Spatial heterogeneity in the mammalian liver. Nat. Rev. Gastroenterol. Hepatol. 2019;16:395–410. doi: 10.1038/s41575-019-0134-x. [DOI] [PubMed] [Google Scholar]
- 44.Nejak-Bowen K., Monga S.P. Wnt-beta-catenin in hepatobiliary homeostasis, injury, and repair. Hepatology. 2023;78:1907–1921. doi: 10.1097/HEP.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benhamouche S., Decaens T., Godard C., Chambrey R., Rickman D.S., Moinard C., Vasseur-Cognet M., Kuo C.J., Kahn A., Perret C., Colnot S. Apc tumor suppressor gene is the "zonation-keeper" of mouse liver. Dev. Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Planas-Paz L., Orsini V., Boulter L., Calabrese D., Pikiolek M., Nigsch F., Xie Y., Roma G., Donovan A., Marti P., et al. The RSPO-LGR4/5-ZNRF3/RNF43 module controls liver zonation and size. Nat. Cell Biol. 2016;18:467–479. doi: 10.1038/ncb3337. [DOI] [PubMed] [Google Scholar]
- 47.Jin Y., Anbarchian T., Wu P., Sarkar A., Fish M., Peng W.C., Nusse R. Wnt signaling regulates hepatocyte cell division by a transcriptional repressor cascade. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2203849119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelsen C.J., Rickheim D.G., Timchenko N.A., Stanley M.W., Albrecht J.H. Transient expression of cyclin D1 is sufficient to promote hepatocyte replication and liver growth in vivo. Cancer Res. 2001;61:8564–8568. [PubMed] [Google Scholar]
- 49.Tan X., Behari J., Cieply B., Michalopoulos G.K., Monga S.P.S. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 50.Hu S., Liu S., Bian Y., Poddar M., Singh S., Cao C., McGaughey J., Bell A., Blazer L.L., Adams J.J., et al. Single-cell spatial transcriptomics reveals a dynamic control of metabolic zonation and liver regeneration by endothelial cell Wnt2 and Wnt9b. Cell Rep. Med. 2022;3:100754. doi: 10.1016/j.xcrm.2022.100754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher T., Le T., Patel M., Newman M., Yeh W.C., Vanhove G.F., Tibbitts J., Baribault H. SZN-043, A Hepatocyte-Targeted R-Spondin Mimetic, Stimulates Hepatocyte Proliferation in an Acute Alcoholic Hepatitis Model. Gastroenterology. 2021;160 S-794. [Google Scholar]
- 52.Gehart H., Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 2019;16:19–34. doi: 10.1038/s41575-018-0081-y. [DOI] [PubMed] [Google Scholar]
- 53.Hageman J.H., Heinz M.C., Kretzschmar K., van der Vaart J., Clevers H., Snippert H.J.G. Intestinal Regeneration: Regulation by the Microenvironment. Dev. Cell. 2020;54:435–446. doi: 10.1016/j.devcel.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 54.Meyer A.R., Brown M.E., McGrath P.S., Dempsey P.J. Injury-Induced Cellular Plasticity Drives Intestinal Regeneration. Cell. Mol. Gastroenterol. Hepatol. 2022;13:843–856. doi: 10.1016/j.jcmgh.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Degirmenci B., Valenta T., Dimitrieva S., Hausmann G., Basler K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature. 2018;558:449–453. doi: 10.1038/s41586-018-0190-3. [DOI] [PubMed] [Google Scholar]
- 56.Murata K., Jadhav U., Madha S., van Es J., Dean J., Cavazza A., Wucherpfennig K., Michor F., Clevers H., Shivdasani R.A. Ascl2-Dependent Cell Dedifferentiation Drives Regeneration of Ablated Intestinal Stem Cells. Cell Stem Cell. 2020;26:377–390.e6. doi: 10.1016/j.stem.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papapietro O., Teatero S., Thanabalasuriar A., Yuki K.E., Diez E., Zhu L., Kang E., Dhillon S., Muise A.M., Durocher Y., et al. R-spondin 2 signalling mediates susceptibility to fatal infectious diarrhoea. Nat. Commun. 2013;4:1898. doi: 10.1038/ncomms2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saha S., Aranda E., Hayakawa Y., Bhanja P., Atay S., Brodin N.P., Li J., Asfaha S., Liu L., Tailor Y., et al. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat. Commun. 2016;7:13096. doi: 10.1038/ncomms13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kabiri Z., Greicius G., Madan B., Biechele S., Zhong Z., Zaribafzadeh H., Bunte R., Edison, Aliyev J., Aliyev J., Wu Y., et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 60.Kim K.A., Kakitani M., Zhao J., Oshima T., Tang T., Binnerts M., Liu Y., Boyle B., Park E., Emtage P., et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 61.Yan K.S., Janda C.Y., Chang J., Zheng G.X.Y., Larkin K.A., Luca V.C., Chia L.A., Mah A.T., Han A., Terry J.M., et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5(+) intestinal stem-cell self-renewal. Nature. 2017;545:238–242. doi: 10.1038/nature22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dang L.T., Miao Y., Ha A., Yuki K., Park K., Janda C.Y., Jude K.M., Mohan K., Ha N., Vallon M., et al. Receptor subtype discrimination using extensive shape complementary designed interfaces. Nat. Struct. Mol. Biol. 2019;26:407–414. doi: 10.1038/s41594-019-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flanagan D.J., Phesse T.J., Barker N., Schwab R.H.M., Amin N., Malaterre J., Stange D.E., Nowell C.J., Currie S.A., Saw J.T.S., et al. Frizzled7 functions as a Wnt receptor in intestinal epithelial Lgr5(+) stem cells. Stem Cell Rep. 2015;4:759–767. doi: 10.1016/j.stemcr.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nile A.H., de Sousa E Melo F., Mukund S., Piskol R., Hansen S., Zhou L., Zhang Y., Fu Y., Gogol E.B., Kömüves L.G., et al. A selective peptide inhibitor of Frizzled 7 receptors disrupts intestinal stem cells. Nat. Chem. Biol. 2018;14:582–590. doi: 10.1038/s41589-018-0035-2. [DOI] [PubMed] [Google Scholar]
- 65.Richter M., Gottanka J., May C.A., Welge-Lüssen U., Berger W., Lütjen-Drecoll E. Retinal vasculature changes in Norrie disease mice. Invest. Ophthalmol. Vis. Sci. 1998;39:2450–2457. [PubMed] [Google Scholar]
- 66.Ye X., Wang Y., Cahill H., Yu M., Badea T.C., Smallwood P.M., Peachey N.S., Nathans J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Junge H.J., Yang S., Burton J.B., Paes K., Shu X., French D.M., Costa M., Rice D.S., Ye W. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell. 2009;139:299–311. doi: 10.1016/j.cell.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 68.Rattner A., Wang Y., Nathans J. Signaling Pathways in Neurovascular Development. Annu. Rev. Neurosci. 2022;45:87–108. doi: 10.1146/annurev-neuro-111020-102127. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen H., Lee S.J., Li Y. Selective Activation of the Wnt-Signaling Pathway as a Novel Therapy for the Treatment of Diabetic Retinopathy and Other Retinal Vascular Diseases. Pharmaceutics. 2022;14:2476. doi: 10.3390/pharmaceutics14112476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y., Hu Y., Zhou T., Zhou K.K., Mott R., Wu M., Boulton M., Lyons T.J., Gao G., Ma J.X. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am. J. Pathol. 2009;175:2676–2685. doi: 10.2353/ajpath.2009.080945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiu F., He J., Zhou Y., Bai X., Wu G., Wang X., Liu Z., Chen Y., Ma J.X., Liu Z. Plasma and vitreous fluid levels of Dickkopf-1 in patients with diabetic retinopathy. Eye (Lond) 2014;28:402–409. doi: 10.1038/eye.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang L., Abedin M., Jo H.N., Levey J., Dinh Q.C., Chen Z., Angers S., Junge H.J. A Frizzled4-LRP5 agonist promotes blood-retina barrier function by inducing a Norrin-like transcriptional response. iScience. 2023;26:107415. doi: 10.1016/j.isci.2023.107415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O'Brien S., Chidiac R., Angers S. Modulation of Wnt-beta-catenin signaling with antibodies: therapeutic opportunities and challenges. Trends Pharmacol. Sci. 2023;44:354–365. doi: 10.1016/j.tips.2023.03.008. [DOI] [PubMed] [Google Scholar]
- 74.Kato M., Patel M.S., Levasseur R., Lobov I., Chang B.H.J., Glass D.A., 2nd, Hartmann C., Li L., Hwang T.H., Brayton C.F., et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Q., Wang Y., Dabdoub A., Smallwood P.M., Williams J., Woods C., Kelley M.W., Jiang L., Tasman W., Zhang K., Nathans J. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y., Rattner A., Zhou Y., Williams J., Smallwood P.M., Nathans J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151:1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y., Cho C., Williams J., Smallwood P.M., Zhang C., Junge H.J., Nathans J. Interplay of the Norrin and Wnt7a/Wnt7b signaling systems in blood-brain barrier and blood-retina barrier development and maintenance. Proc. Natl. Acad. Sci. USA. 2018;115:E11827–E11836. doi: 10.1073/pnas.1813217115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Z., Liu C.H., Huang S., Fu Z., Tomita Y., Britton W.R., Cho S.S., Chen C.T., Sun Y., Ma J.X., et al. Wnt signaling activates MFSD2A to suppress vascular endothelial transcytosis and maintain blood-retinal barrier. Sci. Adv. 2020;6:eaba7457. doi: 10.1126/sciadv.aba7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou Y., Nathans J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev. Cell. 2014;31:248–256. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jokela M., Karhu J., Nurminen J., Martikainen M.H. Multiple Cranial Nerve Gadolinium Enhancement in Norrie Disease. Ann. Neurol. 2022;91:158–159. doi: 10.1002/ana.26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang J., Mancuso M.R., Maier C., Liang X., Yuki K., Yang L., Kwong J.W., Wang J., Rao V., Vallon M., et al. Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat. Med. 2017;23:450–460. doi: 10.1038/nm.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cho C., Smallwood P.M., Nathans J. Reck and Gpr124 Are Essential Receptor Cofactors for Wnt7a/Wnt7b-Specific Signaling in Mammalian CNS Angiogenesis and Blood-Brain Barrier Regulation. Neuron. 2017;95:1221–1225. doi: 10.1016/j.neuron.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 83.Cullen M., Elzarrad M.K., Seaman S., Zudaire E., Stevens J., Yang M.Y., Li X., Chaudhary A., Xu L., Hilton M.B., et al. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc. Natl. Acad. Sci. USA. 2011;108:5759–5764. doi: 10.1073/pnas.1017192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuhnert F., Mancuso M.R., Shamloo A., Wang H.T., Choksi V., Florek M., Su H., Fruttiger M., Young W.L., Heilshorn S.C., Kuo C.J. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stenman J.M., Rajagopal J., Carroll T.J., Ishibashi M., McMahon J., McMahon A.P. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 86.Martin M., Vermeiren S., Bostaille N., Eubelen M., Spitzer D., Vermeersch M., Profaci C.P., Pozuelo E., Toussay X., Raman-Nair J., et al. Engineered Wnt ligands enable blood-brain barrier repair in neurological disorders. Science. 2022;375:eabm4459. doi: 10.1126/science.abm4459. [DOI] [PubMed] [Google Scholar]
- 87.Ding J., Lee S.J., Vlahos L., Yuki K., Rada C.C., van Unen V., Vuppalapaty M., Chen H., Sura A., McCormick A.K., et al. Therapeutic blood-brain barrier modulation and stroke treatment by a bioengineered FZD(4)-selective WNT surrogate in mice. Nat. Commun. 2023;14:2947. doi: 10.1038/s41467-023-37689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krishnan V., Bryant H.U., Macdougald O.A. Regulation of bone mass by Wnt signaling. J. Clin. Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cawthorn W.P., Bree A.J., Yao Y., Du B., Hemati N., Martinez-Santibañez G., MacDougald O.A. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone. 2012;50:477–489. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bennett C.N., Longo K.A., Wright W.S., Suva L.J., Lane T.F., Hankenson K.D., MacDougald O.A. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. USA. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kang S., Bennett C.N., Gerin I., Rapp L.A., Hankenson K.D., Macdougald O.A. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 93.Canalis E. Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat. Rev. Endocrinol. 2013;9:575–583. doi: 10.1038/nrendo.2013.154. [DOI] [PubMed] [Google Scholar]
- 94.Gong Y., Slee R.B., Fukai N., Rawadi G., Roman-Roman S., Reginato A.M., Wang H., Cundy T., Glorieux F.H., Lev D., et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 95.Little R.D., Carulli J.P., Del Mastro R.G., Dupuis J., Osborne M., Folz C., Manning S.P., Swain P.M., Zhao S.C., Eustace B., et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng H.F., Tobias J.H., Duncan E., Evans D.M., Eriksson J., Paternoster L., Yerges-Armstrong L.M., Lehtimäki T., Bergström U., Kähönen M., et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012;8:e1002745. doi: 10.1371/journal.pgen.1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pyott S.M., Tran T.T., Leistritz D.F., Pepin M.G., Mendelsohn N.J., Temme R.T., Fernandez B.A., Elsayed S.M., Elsobky E., Verma I., et al. WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta. Am. J. Hum. Genet. 2013;92:590–597. doi: 10.1016/j.ajhg.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim J., Han W., Park T., Kim E.J., Bang I., Lee H.S., Jeong Y., Roh K., Kim J., Kim J.S., et al. Sclerostin inhibits Wnt signaling through tandem interaction with two LRP6 ectodomains. Nat. Commun. 2020;11:5357. doi: 10.1038/s41467-020-19155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li X., Zhang Y., Kang H., Liu W., Liu P., Zhang J., Harris S.E., Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 100.Lim S.Y. Romosozumab for the treatment of osteoporosis in women: Efficacy, safety, and cardiovascular risk. Womens Health (Lond) 2022;18 doi: 10.1177/17455057221125577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhong Z., Ethen N.J., Williams B.O. WNT signaling in bone development and homeostasis. Wiley Interdiscip. Rev. Dev. Biol. 2014;3:489–500. doi: 10.1002/wdev.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Albers J., Keller J., Baranowsky A., Beil F.T., Catala-Lehnen P., Schulze J., Amling M., Schinke T. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J. Cell Biol. 2013;200:537–549. doi: 10.1083/jcb.201207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Albers J., Schulze J., Beil F.T., Gebauer M., Baranowsky A., Keller J., Marshall R.P., Wintges K., Friedrich F.W., Priemel M., et al. Control of bone formation by the serpentine receptor Frizzled-9. J. Cell Biol. 2011;192:1057–1072. doi: 10.1083/jcb.201008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kushwaha P., Kim S., Foxa G.E., Michalski M.N., Williams B.O., Tomlinson R.E., Riddle R.C. Frizzled-4 is required for normal bone acquisition despite compensation by Frizzled-8. J. Cell. Physiol. 2020;235:6673–6683. doi: 10.1002/jcp.29563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bashir H., Seykora J.T., Lee V. Invisible Shield: Review of the Corneal Epithelium as a Barrier to UV Radiation, Pathogens, and Other Environmental Stimuli. J. Ophthalmic Vis. Res. 2017;12:305–311. doi: 10.4103/jovr.jovr_114_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ma J., Lwigale P. Transformation of the Transcriptomic Profile of Mouse Periocular Mesenchyme During Formation of the Embryonic Cornea. Invest. Ophthalmol. Vis. Sci. 2019;60:661–676. doi: 10.1167/iovs.18-26018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang L., Wang Y.C., Okada Y., Zhang S., Anderson M., Liu C.Y., Zhang Y. Aberrant expression of a stabilized beta-catenin mutant in keratocytes inhibits mouse corneal epithelial stratification. Sci. Rep. 2019;9:1919. doi: 10.1038/s41598-018-36392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li W., Hayashida Y., Chen Y.T., Tseng S.C.G. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17:26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bonnet C., Oh D., Mei H., Robertson S., Chang D., Bourges J.L., Behar-Cohen F., Zheng J.J., Deng S.X. Wnt6 plays a complex role in maintaining human limbal stem/progenitor cells. Sci. Rep. 2021;11:20948. doi: 10.1038/s41598-021-00273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gonzalez S., Oh D., Baclagon E.R., Zheng J.J., Deng S.X. Wnt Signaling Is Required for the Maintenance of Human Limbal Stem/Progenitor Cells In Vitro. Invest. Ophthalmol. Vis. Sci. 2019;60:107–112. doi: 10.1167/iovs.18-25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lyu J., Joo C.K. Wnt-7a up-regulates matrix metalloproteinase-12 expression and promotes cell proliferation in corneal epithelial cells during wound healing. J. Biol. Chem. 2005;280:21653–21660. doi: 10.1074/jbc.M500374200. [DOI] [PubMed] [Google Scholar]
- 112.Nakatsu M.N., Ding Z., Ng M.Y., Truong T.T., Yu F., Deng S.X. Wnt/beta-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Invest. Ophthalmol. Vis. Sci. 2011;52:4734–4741. doi: 10.1167/iovs.10-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dan J., Tan T., Wu M., Gong J., Yang Q., Wang L., Wang P. Lithium chloride promotes diabetic corneal epithelial wound healing by activating the Wnt/beta-catenin signaling pathway. Exp. Ther. Med. 2023;26:373. doi: 10.3892/etm.2023.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mei H., Nakatsu M.N., Baclagon E.R., Deng S.X. Frizzled 7 maintains the undifferentiated state of human limbal stem/progenitor cells. Stem Cell. 2014;32:938–945. doi: 10.1002/stem.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gupta P.K., Berdahl J.P., Chan C.C., Rocha K.M., Yeu E., Ayres B., Farid M., Lee W.B., Beckman K.A., Kim T., et al. The corneal endothelium: clinical review of endothelial cell health and function. J. Cataract Refract. Surg. 2021;47:1218–1226. doi: 10.1097/j.jcrs.0000000000000650. [DOI] [PubMed] [Google Scholar]
- 116.Park S., Leonard B.C., Raghunathan V.K., Kim S., Li J.Y., Mannis M.J., Murphy C.J., Thomasy S.M. Animal models of corneal endothelial dysfunction to facilitate development of novel therapies. Ann. Transl. Med. 2021;9:1271. doi: 10.21037/atm-20-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hirata-Tominaga K., Nakamura T., Okumura N., Kawasaki S., Kay E.P., Barrandon Y., Koizumi N., Kinoshita S. Corneal endothelial cell fate is maintained by LGR5 through the regulation of hedgehog and Wnt pathway. Stem Cell. 2013;31:1396–1407. doi: 10.1002/stem.1390. [DOI] [PubMed] [Google Scholar]
- 118.Okumura N., Nakamura T., Kay E.P., Nakahara M., Kinoshita S., Koizumi N. R-spondin1 regulates cell proliferation of corneal endothelial cells via the Wnt3a/beta-catenin pathway. Invest. Ophthalmol. Vis. Sci. 2014;55:6861–6869. doi: 10.1167/iovs.14-14091. [DOI] [PubMed] [Google Scholar]
- 119.Lee J.G., Heur M. WNT10B enhances proliferation through beta-catenin and RAC1 GTPase in human corneal endothelial cells. J. Biol. Chem. 2015;290:26752–26764. doi: 10.1074/jbc.M115.677245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoffman M.P., Kidder B.L., Steinberg Z.L., Lakhani S., Ho S., Kleinman H.K., Larsen M. Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development. 2002;129:5767–5778. doi: 10.1242/dev.00172. [DOI] [PubMed] [Google Scholar]
- 121.Sun Q.F., Sun Q.H., Du J., Wang S. Differential gene expression profiles of normal human parotid and submandibular glands. Oral Dis. 2008;14:500–509. doi: 10.1111/j.1601-0825.2007.01408.x. [DOI] [PubMed] [Google Scholar]
- 122.Hai B., Yang Z., Millar S.E., Choi Y.S., Taketo M.M., Nagy A., Liu F. Wnt/beta-catenin signaling regulates postnatal development and regeneration of the salivary gland. Stem Cells Dev. 2010;19:1793–1801. doi: 10.1089/scd.2009.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hai B., Yang Z., Shangguan L., Zhao Y., Boyer A., Liu F. Concurrent transient activation of Wnt/beta-catenin pathway prevents radiation damage to salivary glands. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:e109–e116. doi: 10.1016/j.ijrobp.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maimets M., Rocchi C., Bron R., Pringle S., Kuipers J., Giepmans B.N.G., Vries R.G.J., Clevers H., de Haan G., van Os R., Coppes R.P. Long-Term In Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals. Stem Cell Rep. 2016;6:150–162. doi: 10.1016/j.stemcr.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun X., Perl A.K., Li R., Bell S.M., Sajti E., Kalinichenko V.V., Kalin T.V., Misra R.S., Deshmukh H., Clair G., et al. A census of the lung: CellCards from LungMAP. Dev. Cell. 2022;57:112–145.e2. doi: 10.1016/j.devcel.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aros C.J., Pantoja C.J., Gomperts B.N. Wnt signaling in lung development, regeneration, and disease progression. Commun. Biol. 2021;4:601. doi: 10.1038/s42003-021-02118-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barkauskas C.E., Cronce M.J., Rackley C.R., Bowie E.J., Keene D.R., Stripp B.R., Randell S.H., Noble P.W., Hogan B.L.M. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Flozak A.S., Lam A.P., Russell S., Jain M., Peled O.N., Sheppard K.A., Beri R., Mutlu G.M., Budinger G.R.S., Gottardi C.J. Beta-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J. Biol. Chem. 2010;285:3157–3167. doi: 10.1074/jbc.M109.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nabhan A.N., Brownfield D.G., Harbury P.B., Krasnow M.A., Desai T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359:1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zacharias W.J., Frank D.B., Zepp J.A., Morley M.P., Alkhaleel F.A., Kong J., Zhou S., Cantu E., Morrisey E.E. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zepp J.A., Zacharias W.J., Frank D.B., Cavanaugh C.A., Zhou S., Morley M.P., Morrisey E.E. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell. 2017;170:1134–1148.e10. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dada L.A., Welch L.C., Magnani N.D., Ren Z., Han H., Brazee P.L., Celli D., Flozak A.S., Weng A., Herrerias M.M., et al. Hypercapnia alters stroma-derived Wnt production to limit beta-catenin signaling and proliferation in AT2 cells. JCI Insight. 2023;8:e159331. doi: 10.1172/jci.insight.159331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiang Z., Lao T., Qiu W., Polverino F., Gupta K., Guo F., Mancini J.D., Naing Z.Z.C., Cho M.H., Castaldi P.J., et al. A Chronic Obstructive Pulmonary Disease Susceptibility Gene, FAM13A , Regulates Protein Stability of β-Catenin. Am. J. Respir. Crit. Care Med. 2016;194:185–197. doi: 10.1164/rccm.201505-0999OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kneidinger N., Yildirim A.Ö., Callegari J., Takenaka S., Stein M.M., Dumitrascu R., Bohla A., Bracke K.R., Morty R.E., Brusselle G.G., et al. Activation of the WNT/β-Catenin Pathway Attenuates Experimental Emphysema. Am. J. Respir. Crit. Care Med. 2011;183:723–733. doi: 10.1164/rccm.200910-1560OC. [DOI] [PubMed] [Google Scholar]
- 135.Uhl F.E., Vierkotten S., Wagner D.E., Burgstaller G., Costa R., Koch I., Lindner M., Meiners S., Eickelberg O., Königshoff M. Preclinical validation and imaging of Wnt-induced repair in human 3D lung tissue cultures. Eur. Respir. J. 2015;46:1150–1166. doi: 10.1183/09031936.00183214. [DOI] [PubMed] [Google Scholar]
- 136.Baarsma H.A., Königshoff M. ‘WNT-er is coming’ : WNT signalling in chronic lung diseases. Thorax. 2017;72:746–759. doi: 10.1136/thoraxjnl-2016-209753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Conlon T.M., John-Schuster G., Heide D., Pfister D., Lehmann M., Hu Y., Ertüz Z., Lopez M.A., Ansari M., Strunz M., et al. Inhibition of LTβR signalling activates WNT-induced regeneration in lung. Nature. 2020;588:151–156. doi: 10.1038/s41586-020-2882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Glass D.S., Grossfeld D., Renna H.A., Agarwala P., Spiegler P., DeLeon J., Reiss A.B. Idiopathic pulmonary fibrosis: Current and future treatment. Clin. Respir. J. 2022;16:84–96. doi: 10.1111/crj.13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pardo A., Selman M. Lung Fibroblasts, Aging, and Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2016;13:S417–S421. doi: 10.1513/AnnalsATS.201605-341AW. [DOI] [PubMed] [Google Scholar]
- 140.Chakraborty A., Mastalerz M., Ansari M., Schiller H.B., Staab-Weijnitz C.A. Emerging Roles of Airway Epithelial Cells in Idiopathic Pulmonary Fibrosis. Cells. 2022;11:1050. doi: 10.3390/cells11061050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moss B.J., Ryter S.W., Rosas I.O. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. 2022;17:515–546. doi: 10.1146/annurev-pathol-042320-030240. [DOI] [PubMed] [Google Scholar]
- 142.Tanjore H., Degryse A.L., Crossno P.F., Xu X.C., McConaha M.E., Jones B.R., Polosukhin V.V., Bryant A.J., Cheng D.-S., Newcomb D.C., et al. β-Catenin in the Alveolar Epithelium Protects from Lung Fibrosis after Intratracheal Bleomycin. Am. J. Respir. Crit. Care Med. 2013;187:630–639. doi: 10.1164/rccm.201205-0972OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nabhan A.N., Webster J.D., Adams J.J., Blazer L., Everrett C., Eidenschenk C., Arlantico A., Fleming I., Brightbill H.D., Wolters P.J., et al. Targeted alveolar regeneration with Frizzled-specific agonists. Cell. 2023;186:2995–3012.e15. doi: 10.1016/j.cell.2023.05.022. [DOI] [PubMed] [Google Scholar]
- 144.Aros C.J., Vijayaraj P., Pantoja C.J., Bisht B., Meneses L.K., Sandlin J.M., Tse J.A., Chen M.W., Purkayastha A., Shia D.W., et al. Distinct Spatiotemporally Dynamic Wnt-Secreting Niches Regulate Proximal Airway Regeneration and Aging. Cell Stem Cell. 2020;27:413–429.e4. doi: 10.1016/j.stem.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Skronska-Wasek W., Gosens R., Königshoff M., Baarsma H.A. WNT receptor signalling in lung physiology and pathology. Pharmacol. Ther. 2018;187:150–166. doi: 10.1016/j.pharmthera.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 146.Patel M., Post Y., Hill N., Sura A., Ye J., Fisher T., Suen N., Zhang M., Cheng L., Pribluda A., et al. A WNT mimetic with broad spectrum FZD-specificity decreases fibrosis and improves function in a pulmonary damage model. Respir. Res. 2024;25:153. doi: 10.1186/s12931-024-02786-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sung E.A., Park M.H., Henegariu O., Sime P.J., Chae W.J. Dickkopf1 Promotes Pulmonary Fibrosis upon Bleomycin-Induced Lung Injury. Am. J. Pathol. 2023;193:1130–1142. doi: 10.1016/j.ajpath.2023.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou B., Magana L., Hong Z., Huang L.S., Chakraborty S., Tsukasaki Y., Huang C., Wang L., Di A., Ganesh B., et al. The angiocrine Rspondin3 instructs interstitial macrophage transition via metabolic–epigenetic reprogramming and resolves inflammatory injury. Nat. Immunol. 2020;21:1430–1443. doi: 10.1038/s41590-020-0764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 150.Sato T., Stange D.E., Ferrante M., Vries R.G.J., Van Es J.H., Van den Brink S., Van Houdt W.J., Pronk A., Van Gorp J., Siersema P.D., Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 151.Huch M., Gehart H., van Boxtel R., Hamer K., Blokzijl F., Verstegen M.M.A., Ellis E., van Wenum M., Fuchs S.A., de Ligt J., et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Boj S.F., Hwang C.I., Baker L.A., Engle D.D., Tuveson D.A., Clevers H. Model organoids provide new research opportunities for ductal pancreatic cancer. Mol. Cell. Oncol. 2016;3:e1014757. doi: 10.1080/23723556.2015.1014757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bartfeld S. Modeling infectious diseases and host-microbe interactions in gastrointestinal organoids. Dev. Biol. 2016;420:262–270. doi: 10.1016/j.ydbio.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 154.Marson A., Foreman R., Chevalier B., Bilodeau S., Kahn M., Young R.A., Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sato N., Meijer L., Skaltsounis L., Greengard P., Brivanlou A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 156.Hu H., Gehart H., Artegiani B., LÖpez-Iglesias C., Dekkers F., Basak O., van Es J., Chuva de Sousa Lopes S.M., Begthel H., Korving J., et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell. 2018;175:1591–1606.e19. doi: 10.1016/j.cell.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 157.Peng W.C., Logan C.Y., Fish M., Anbarchian T., Aguisanda F., Alvarez-Varela A., Wu P., Jin Y., Zhu J., Li B., et al. Inflammatory Cytokine TNFalpha Promotes the Long-Term Expansion of Primary Hepatocytes in 3D Culture. Cell. 2018;175:1607–1619.e15. doi: 10.1016/j.cell.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Katsura H., Sontake V., Tata A., Kobayashi Y., Edwards C.E., Heaton B.E., Konkimalla A., Asakura T., Mikami Y., Fritch E.J., et al. Human Lung Stem Cell-Based Alveolospheres Provide Insights into SARS-CoV-2-Mediated Interferon Responses and Pneumocyte Dysfunction. Cell Stem Cell. 2020;27:890–904.e8. doi: 10.1016/j.stem.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yui S., Nakamura T., Sato T., Nemoto Y., Mizutani T., Zheng X., Ichinose S., Nagaishi T., Okamoto R., Tsuchiya K., et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat. Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 160.Fordham R.P., Yui S., Hannan N.R.F., Soendergaard C., Madgwick A., Schweiger P.J., Nielsen O.H., Vallier L., Pedersen R.A., Nakamura T., et al. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell. 2013;13:734–744. doi: 10.1016/j.stem.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pringle S., Maimets M., van der Zwaag M., Stokman M.A., van Gosliga D., Zwart E., Witjes M.J.H., de Haan G., van Os R., Coppes R.P. Human Salivary Gland Stem Cells Functionally Restore Radiation Damaged Salivary Glands. Stem Cell. 2016;34:640–652. doi: 10.1002/stem.2278. [DOI] [PubMed] [Google Scholar]