Abstract
In this part 1 of a 2-part continuing medical education series, the epidemiology, clinical features, and diagnostic methods for fungal skin neglected tropical diseases (NTDs), which include eumycetoma, chromoblastomycosis, paracoccidioidomycosis, sporotrichosis, emergomycosis, talaromycosis, and lobomycosis, are reviewed. These infections, several of which are officially designated as NTDs by the World Health Organization (WHO), cause substantial morbidity and stigma worldwide and are receiving increased attention due to the potential for climate change-related geographic expansion. Domestic incidence may be increasing in the setting of global travel and immunosuppression. United States dermatologists may play a central role in early detection and initiation of appropriate treatment, leading to decreased morbidity and mortality.
Keywords: neglected tropical diseases, fungal infections, endemic mycoses, implantation mycoses, systemic mycoses, eumycetoma, chromoblastomycosis, paracoccidioidomycosis, sporotrichosis, emergomycosis, talaromycosis, lobomycosis, epidemiology, diagnostics
Introduction
Key points
Fungal skin neglected tropical diseases (NTDs) primarily affect socioeconomically disadvantaged populations globally.
Familiarity with fungal skin NTDs is important given associations with severe morbidity.
We provide an overview of fungal skin NTDs, focusing on epidemiology, clinical features, and diagnosis.
Fungal skin NTDs are a diverse group of environmental fungal infections (Table 1) which cause substantial disability and receive little public health attention. Consequently, the World Health Organization (WHO) designated mycetoma, chromoblastomycosis, and “other deep mycoses” as NTDs.1 NTDs disproportionately affect low-income countries, but due to travel to endemic regions and immunosuppression, NTDs are also reported in high income countries. NTD geographic expansion may occur with climate change, globalization, and increased host susceptibility with use of immunosuppressive therapies.2–5 The majority of fungal skin NTDs are not reportable diseases,6–12 resulting in a large unknown disease burden. Diagnosis is challenging, as clinical presentation frequently resembles other infectious and non-infectious conditions (Table 2). In the United States (US), dermatologists have limited fungal skin NTD patient encounters, precluding clinical pattern recognition and affecting long term outcomes.13 This review addresses this practice gap by outlining epidemiology, clinical features, and diagnosis of fungal skin NTDs to improve patient care by dermatologists.
Table 1.
Summary of fungal skin NTDs—epidemiology and clinical features
| Fungal skin NTD | Endemic region | Mode of transmission | Patient population | Dermatologic clinical features | Cutaneous areas of involvement | Other organ involvement |
|---|---|---|---|---|---|---|
| Eumycetoma | Tropical and subtropical regions between latitude 15°S and 30°N (‘mycetoma belt’) | Traumatic transcutaneous inoculation | Barefoot-walking immunocompetent populations, male (3:1 male/female), third to fourth decade | Painless, firm subcutaneous masses, formation of multiple sinuses within the mass, and a purulent or seropurulent discharge containing sand-like particles called “grains, | Lower extremities are most common, but other anatomical sites can be involved | Muscle, bone |
| Chromoblastomycosis | Tropical and subtropical regions | Traumatic transcutaneous inoculation | Immunocompetent male agricultural workers | Verrucous papule (early stage), which progressively enlarges into variable morphologies (nodular, verrucous, tumorous, cicatricial, plaque). Black dots (sclerotic lesions) may be present on plaques | Lower extremities are most common, but other anatomical sites can be involved | Internal organ involvement very rare |
| Paracoccidioidomycosis | Tropical and subtropical regions of South and Central America | Inhalation of conidia, possible rare direct transcutaneous inoculation | Male agricultural workers between third and fifth decades, smokers | Ulcers or vegetations with fine granulations or hemorrhagic spots | Upper respiratory tract mucosa, face, extremities, trunk | Lungs, adrenal glands |
| Sporotrichosis | Global distribution | Traumatic transcutaneous inoculation or contact with exudate from infected cat | Patients in contact with vegetation, soil, or cats; veterinarians or those that work with animals | Lymphocutaneous (nodular lymphangitis) or cutaneous (polymorphic skin lesions including papules, nodules, ulcers, verrucous lesions, or plaques following trauma) | Extremities | Pulmonary, osteoarticular, ophthalmic, central nervous system |
| Emergomycosis | North America, Europe, Africa, Asia | Inhalation of conidia | HIV-infected patients or patients with other immunosuppressive conditions, such as diabetes mellitus, hematologic malignancy, or organ transplant. | Umbilicated papules, hyperkeratotic plaques, nodules, verrucous lesions, ulcerations. Lesions may be polymorphic in one patient or in one lesion over time | Widespread | Lungs, gastrointestinal tract, liver, spleen, bone marrow, lymph nodes, and cervix |
| Talaromycosis | Tropical and subtropical regions of Asia | Inhalation of spores | HIV-infected patients or patients with other immunosuppressive conditions, such as cancer, organ transplant, or auto-immune diseases. | Painless papules with central umbilication | Face, neck, extremities | Upper and lower respiratory tract, bone, gastrointestinal tract |
| Lobomycosis | Central and South America | Traumatic transcutaneous inoculation | Forest workers, farmers, hunters, and fishermen | Slowly developing (months, years, decades), keloid-like nodular lesions | Lower extremities, ears, upper extremities, head | No internal organ involvement; testicular involvement reported in one case |
Table 2.
Common pathogens and differential diagnoses of fungal skin NTDs
| Fungal skin NTD | Common pathogens | Infectious diseases differential diagnoses | Non-infectious diseases differential diagnoses |
|---|---|---|---|
| Eumycetoma |
Madurella mycetomatis
Madurella pseudomycetomatis Madurella fahalii Madurella tropicana Trematosphaeria grisea Medicopsis romeroi Scedosporium boydii Falciformispora senegalensis |
Cutaneous tuberculosis Coccidioidomycosis Chromoblastomycosis Hyalohyphomycosis Sporotrichosis Blastomycosis Dermatophyte pseudomycetomas Botryomycosis |
Fibroma Rheumatoid nodule Keloid Fibrolipoma Dermatofibroma Dermatofibroma protuberans Kaposi’s sarcoma Malignant melanoma Verrucous carcinoma |
| Chromoblastomycosis |
Fonsecaea pedrosoi
Fonsecaea monophora Fonsecaea nubica Cladophialophora carrionii Phialophora verrucosa Rhinocladiella aquaspersa Exophiala spp. |
Lobomycosis Sporotrichosis Phaeohyphomycosis Protothecosis Tuberculosis verrucose Leishmaniasis Hanseniasis Eumycetoma Botryomycosis |
Keratoacanthoma Lupus erythematous Cutaneous sarcoidosis Basal Cell Carcinoma Psoriasis |
| Paracoccidioidomycosis |
Paracoccidioides brasiliensis
Paracoccidioides lutzii |
Tuberculosis Coccidioidomycosis Histoplasmosis Leishmaniasis Chromoblastomycosis Leprosy Syphilis |
Sarcoidosis Pneumoconiosis Interstitial pneumonitis Neoplasm |
| Sporotrichosis |
Sporothrix schenckii
Sporothrix globosa Sporothrix luriei Sporothrix brasiliensis |
Mycobacteriosis Actinomycosis American tegumentary leishmaniasis Blastomycosis Cryptococcosis Paracoccidioidomycosis |
Pyoderma gangrenosum |
| Emergomycosis |
Emergomyces pasteurianus
Emergomyces africanus Emergomyces canadensis Emergomyces orientalis Emergomyces europaeus |
Histoplasmosis Cryptococcosis Sporotrichosis Blastomycosis Tuberculosis Non-tuberculous mycobacterial infection Bacillary angiomatosis Varicella Papular pruritic eruption of HIV Secondary syphilis |
Drug reactions Kaposi’s sarcoma Guttate psoriasis Pyoderma gangrenosum |
| Talaromycosis | Talaromyces marneffei | Histoplasmosis Molloscum contagiosum Mycetoma Viral wart Cutaneous tuberculosis Sporotrichosis Chromoblastomycosis Melioidosis |
Epidermoid cyst Lipoma |
| Lobomycosis | Lacazia loboi | Lepromatous leprosy Diffuse cutaneous leishmaniasis Sporotrichosis Chromoblastomycosis Paracoccidioidomycosis |
Keloids Dermatofibrosarcoma protuberans Kaposi’s sarcoma Squamous cell carcinoma |
In addition to NTDs recognized by the WHO, this review also includes mycoses on the WHO NTD 2021–2030 roadmap, mycoses recognized as NTDs by the Public Library of Science Neglected Tropical Diseases, and mycoses for which there are global calls for NTD recognition.
Eumycetoma
Key points
Eumycetoma occurs most commonly in the tropical and subtropical mycetoma belt, but cases have been reported worldwide.
Immunocompetent men in their 30s-40s in the mycetoma belt without sufficient foot coverage are most often affected.
Physical examination is commonly notable for subcutaneous swelling, multiple discharging sinuses, and presence of macroscopic granules.
Overview.
Mycetoma is caused by fungi (eumycetoma), bacteria (actinomycetoma), or combinations. Eumycetoma is most frequently caused by Madurella mycetomatis,14 which is present in soil and gains cutaneous entry after minor trauma, which has led to the term “Madura foot.” However, metagenomic studies demonstrate diversity of implicated pathogens.15
Epidemiology.
Populations of tropical/subtropical regions in the mycetoma belt (latitudes 15° S -30° N) are primarily affected (Figure 1).16 Sudan has the largest reported mycetoma burden;17 other endemic regions include Senegal,18 India,19 Mexico,20 Brazil, and Argentina.21 Men in their 30s-40s are most frequently affected, likely related to agriculture occupations.22 Risk factors include walking barefoot or wearing footwear without sufficient coverage.
Figure 1.
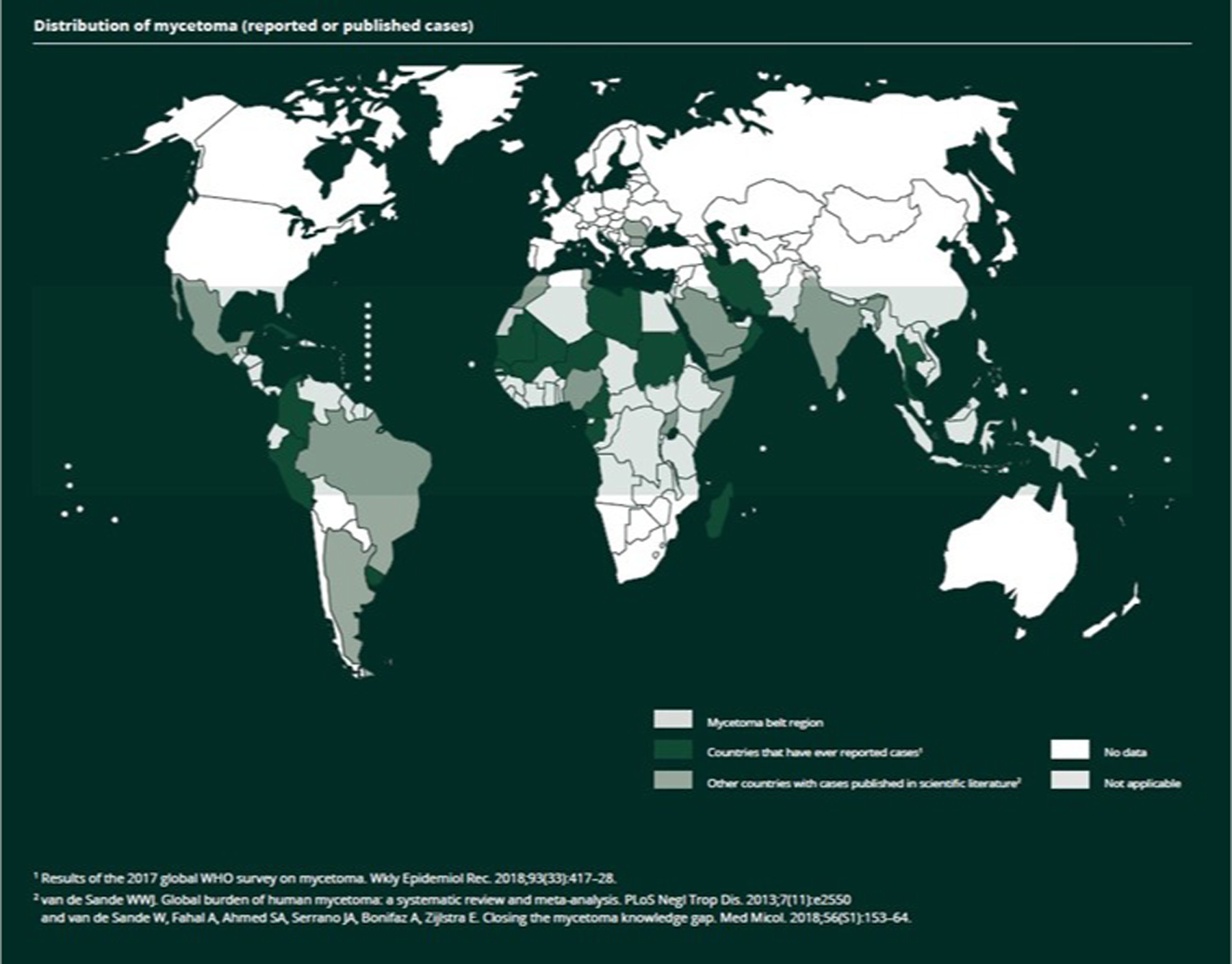
Distribution of mycetoma (reported or published cases). Reproduced from the World Health Organization (published under Creative Commons CC BY-NC-SA 4.0 DEED license).
Epidemiologic trends.
In an epidemiologic study (2020),23 eumycetoma and actinomycetoma were reported beyond tropical endemic areas of the mycetoma belt, to the US (57 cases), Italy (8), China (9), and Australia (5). There are eumycetoma cases in Mexican patients who migrate to the US.24–26 In a US epidemiologic study13 of implantation mycoses (2017–2021), there were 238 eumycetoma cases (5.2 per 1,000,000), with highest prevalence in the Midwest and more commonly among immunosuppressed patients. There was a female predominance for eumycetoma in the US in contrast to international settings, possibly due to different care-seeking behaviors.21
Clinical features.
The classic triad of disease includes slow, progressive subcutaneous swelling, multiple discharging sinuses, and macroscopic granules.27 Lower extremities are most frequently affected, with painless plaques demonstrating woody swelling and communicating sinuses, with the latter draining purulent material and granular grains (black-fungal, red-bacterial, white/yellow-fungal or bacterial) (Figure 2).28 Clinical variants include nodules without sinuses, cysts,29 and verrucous plaques.30,31 If left untreated, infection may spread via fascial planes to involve muscle or bone.28,32
Figure 2.
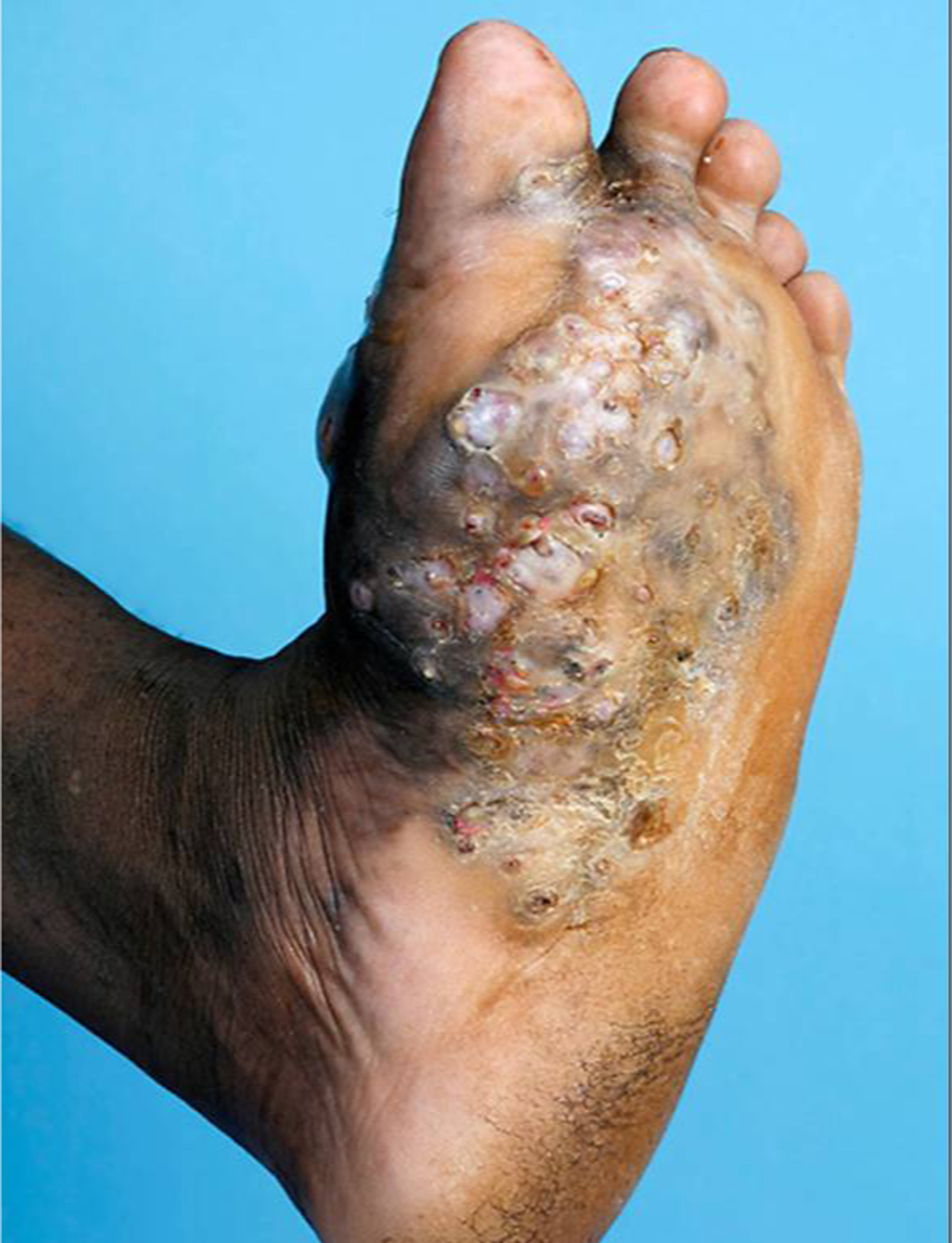
A massive eumycetoma lesion of the plantar foot with multiple discharging sinuses and black grains, Mexico.
Diagnosis and evaluation.
Ultrasound may distinguish eumycetoma from other subcutaneous masses, and between eumycetoma (black grains produce sharp hyperechoic foci) and actinomycetoma (grains less distinct and seen at bottom of lesions).33,34 Sensitivity and specificity for ultrasound examination of eumycetoma were 94.3% and 50%, respectively, in a cross-sectional study35 of 222 patients suspected of fungal mycetoma caused by Madurella mycetomatis. Sinus material may be obtained via ultrasound-guided fine needle aspiration for histopathologic examination, which is required for definitive diagnosis (Figure 3).36 Tissue fungal culture identifies the causative organism. Magnetic resonance imaging (MRI) may assess for extent of soft tissue involvement.37,38 The “dot-in-circle” sign on MRI refers to small, round-shaped hyperintense lesions with small central focus correlating to granulomata-containing grains (Figure 4).39 X-ray and computed tomography (CT) may assess for bone involvement.
Figure 3.
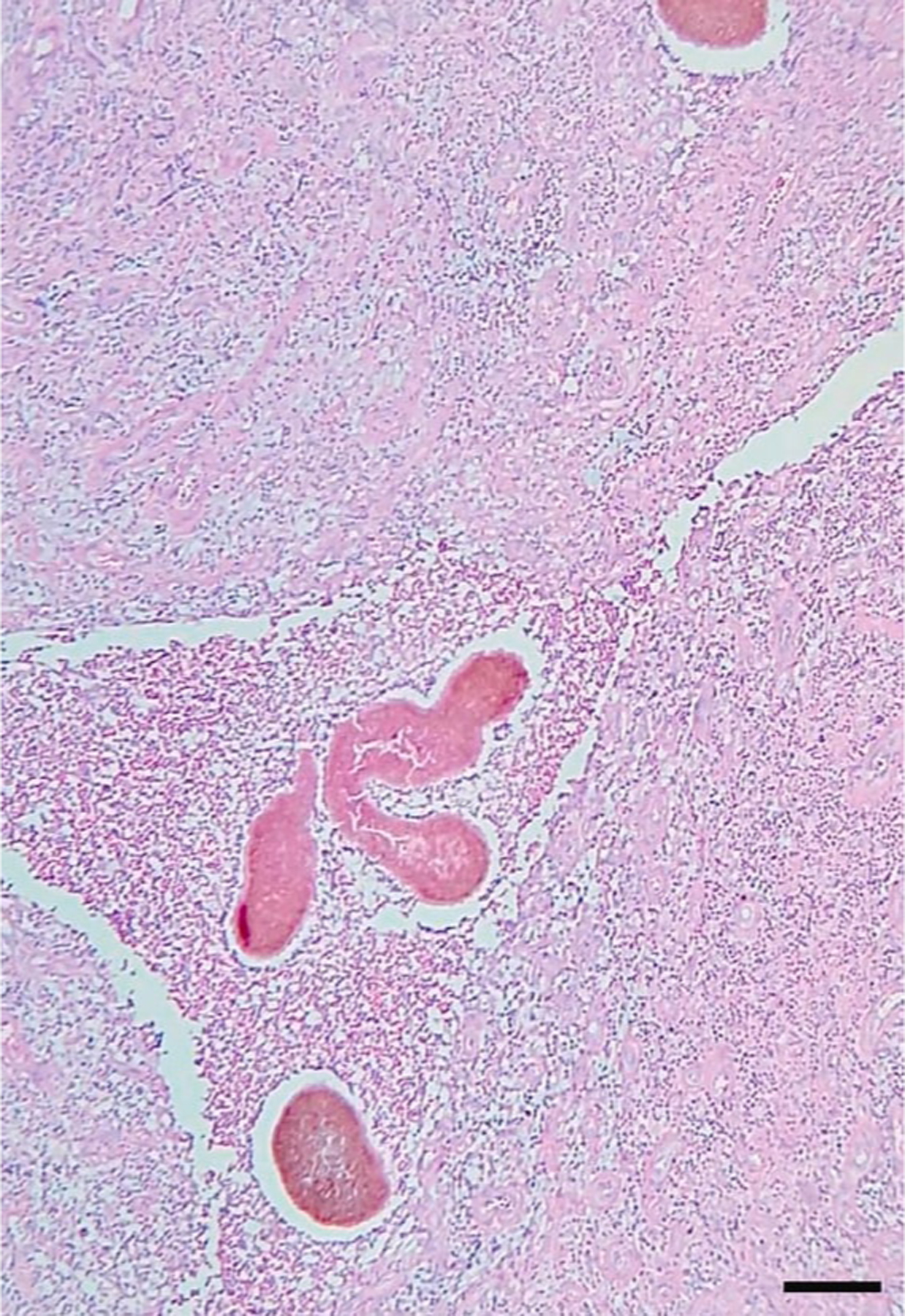
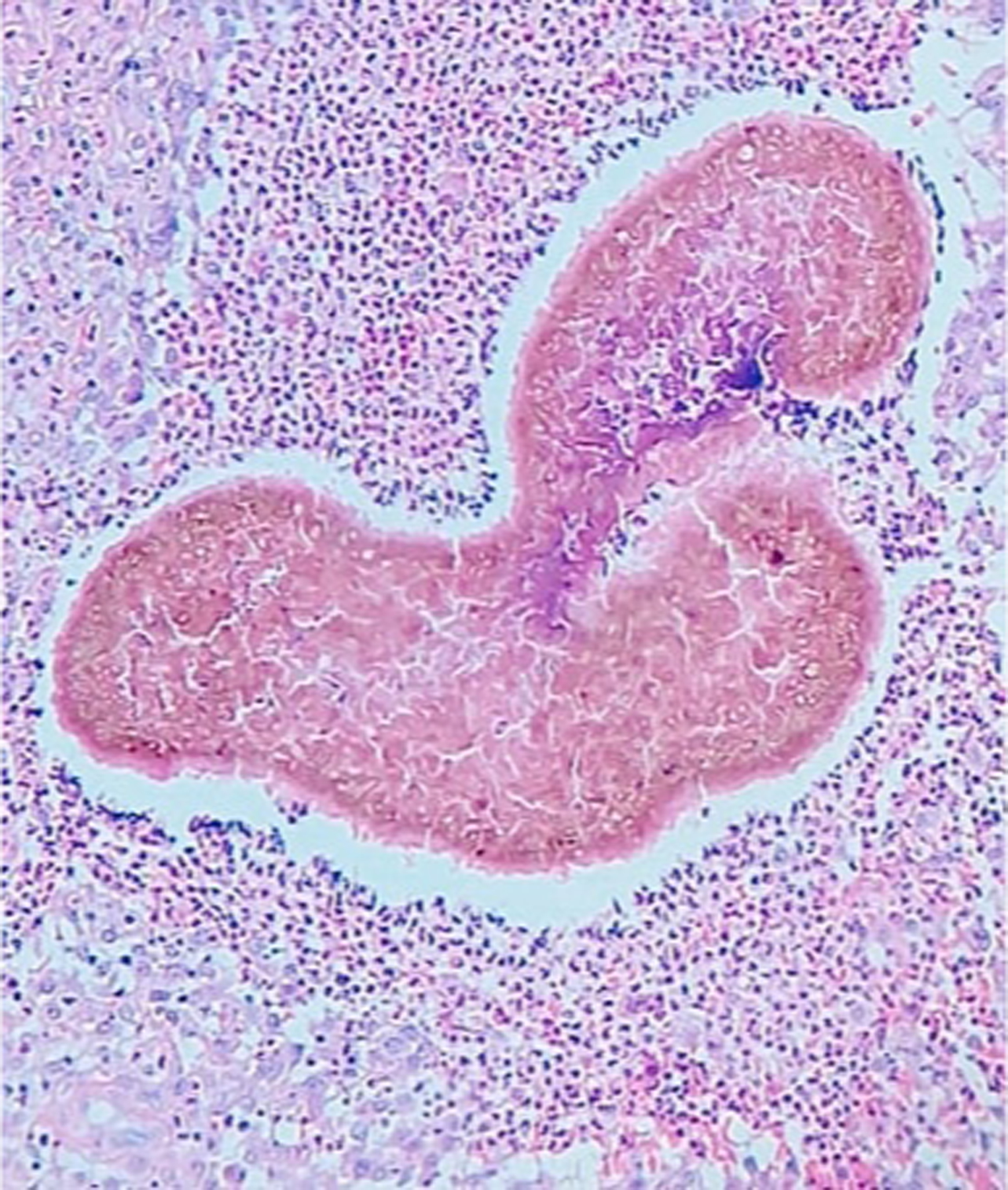
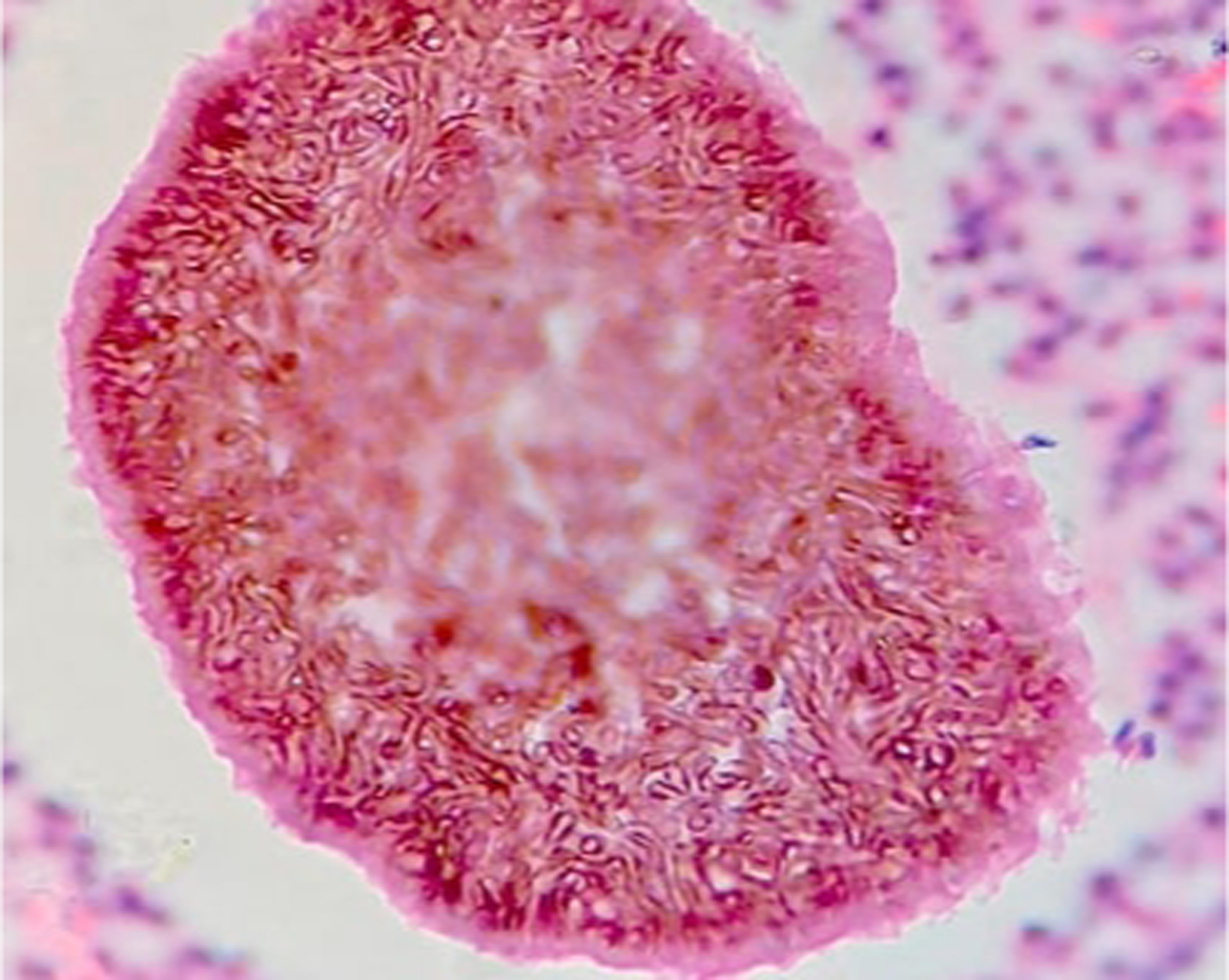
Eumycetoma histopathologic examination (skin biopsy) demonstrating A. suppurative granulomatous inflammatory infiltrates with neutrophils, lymphocytes, and histiocytes; B. large multilobed grains with Splendore-Hoeppli phenomenon; C. clusters of radially branched brown hyphae (Hematoxylin and eosin stain, 100x). Reproduced from Arteaga et al.141 (published under Creative Commons CC-BY-NC-ND license).
Figure 4.
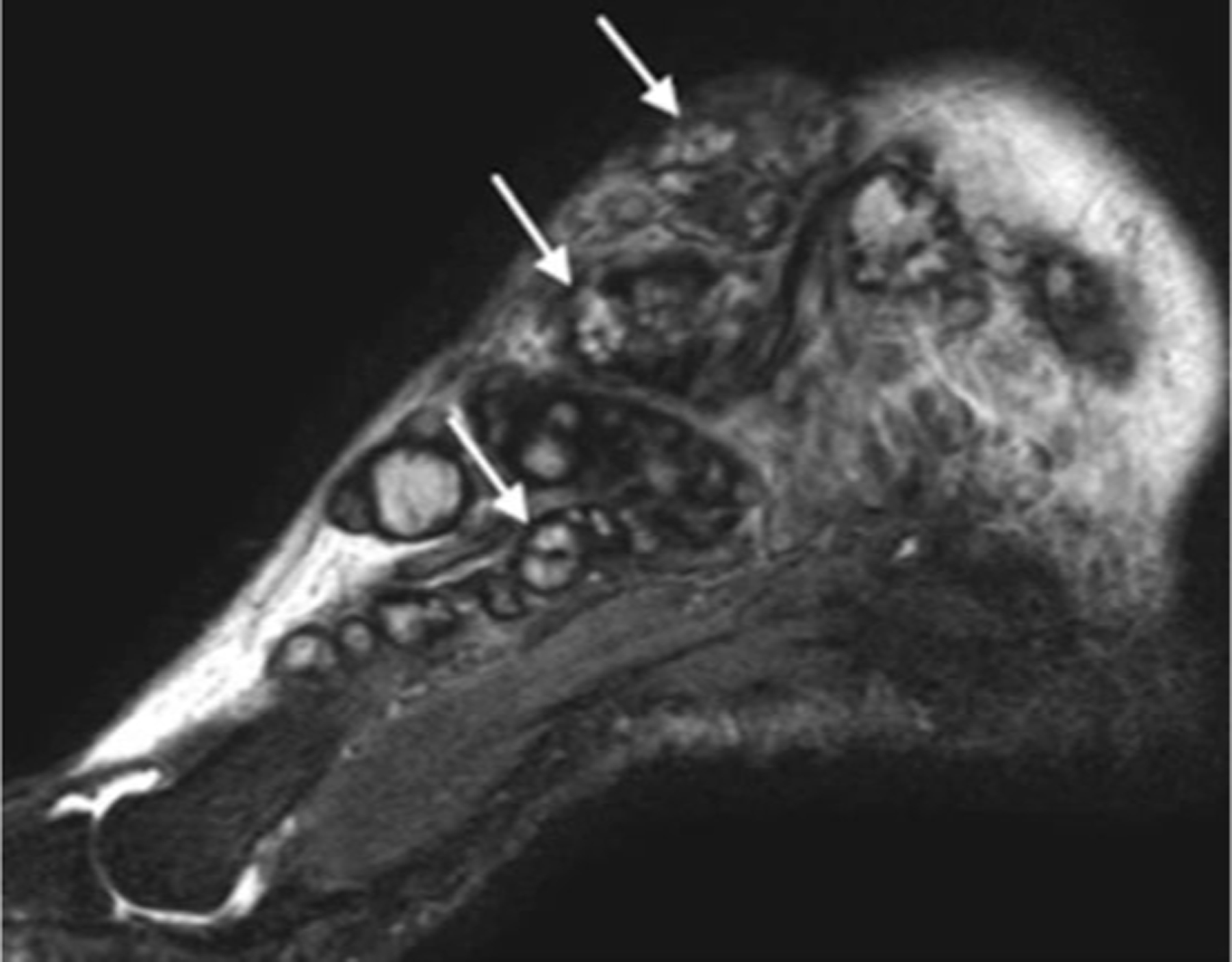
Sagittal MRI show multiple, small, round-to-spherical hyperintense mycetoma lesions separated by peripheral hypointense tissue. Some of the lesions contain a central hypointense dot, resulting in the ‘dot-in-circle’ sign (arrows). Reproduced from Laohawiriyakamol et al.39 (published under Creative Commons CC-BY license).
Chromoblastomycosis
Key points
Chromoblastomycosis most commonly occurs in tropical and subtropical regions.
Immunocompetent male agricultural workers are most frequently affected, but anyone may be affected.
Physical examination is notable for diverse clinical findings, including macules, papules, plaques, nodules, and verruciform or scar-like lesions. Cayenne pepper black dots may be present.
Overview.
Chromoblastomycosis is a chronic granulomatous infection most frequently caused by Fonsecaea and Cladophialophora spp. and acquired through traumatic inoculation.
Epidemiology.
Chromoblastomycosis most commonly occurs in tropical and subtropical regions, and most frequently affects agricultural workers, though not limited to this population (Figure 5). In a review7 of 7,740 chromoblastomycosis cases (1914–2020), cases were most common in South America, Africa, Central America and Mexico, and Asia. Men (81.7%) were frequently affected with mean age 57.1 years.
Figure 5.
Distribution of deep mycoses. Reproduced from the World Health Organization (published under Creative Commons CC BY-NC-SA 4.0 DEED license).
Epidemiologic trends.
In a US epidemiologic study13 of implantation mycoses (2017–2021), there were 667 chromoblastomycosis and phaeohyphomycotic abscess cases (14.7 per 1,000,000), with highest prevalence in the Northeast and more commonly among immunosuppressed patients.
Clinical features.
Chromoblastomycosis most frequently affects lower extremities, followed by upper extremities, and head/neck. In early disease stages, chromoblastomycosis may present as a small verrucous papule following trauma (Figure 6). Lesions enlarge over time, forming hypertrophic and verrucous plaques, sometimes associated with pruritus and pain. Black dots with “cayenne pepper” appearance may be present on the surface (Figure 7).40 In the aforementioned study of 7,740 chromoblastomycosis patients, the most common morphologies were verruciform, tumorous, and plaque-like.7 Only one lesion type was present in 63.2% of patients, while combinations were observed in 36.8%. In the US, chromoblastomycosis typically presents as small to moderate sized plaques with heavy scale-crust, unlike the tumoral masses and large verrucous plaques more typical of long-neglected lesions in endemic regions (Figure 8).41 Chromoblastomycosis lesions may clinically and histopathologically mimic squamous cell carcinoma.42
Figure 6.
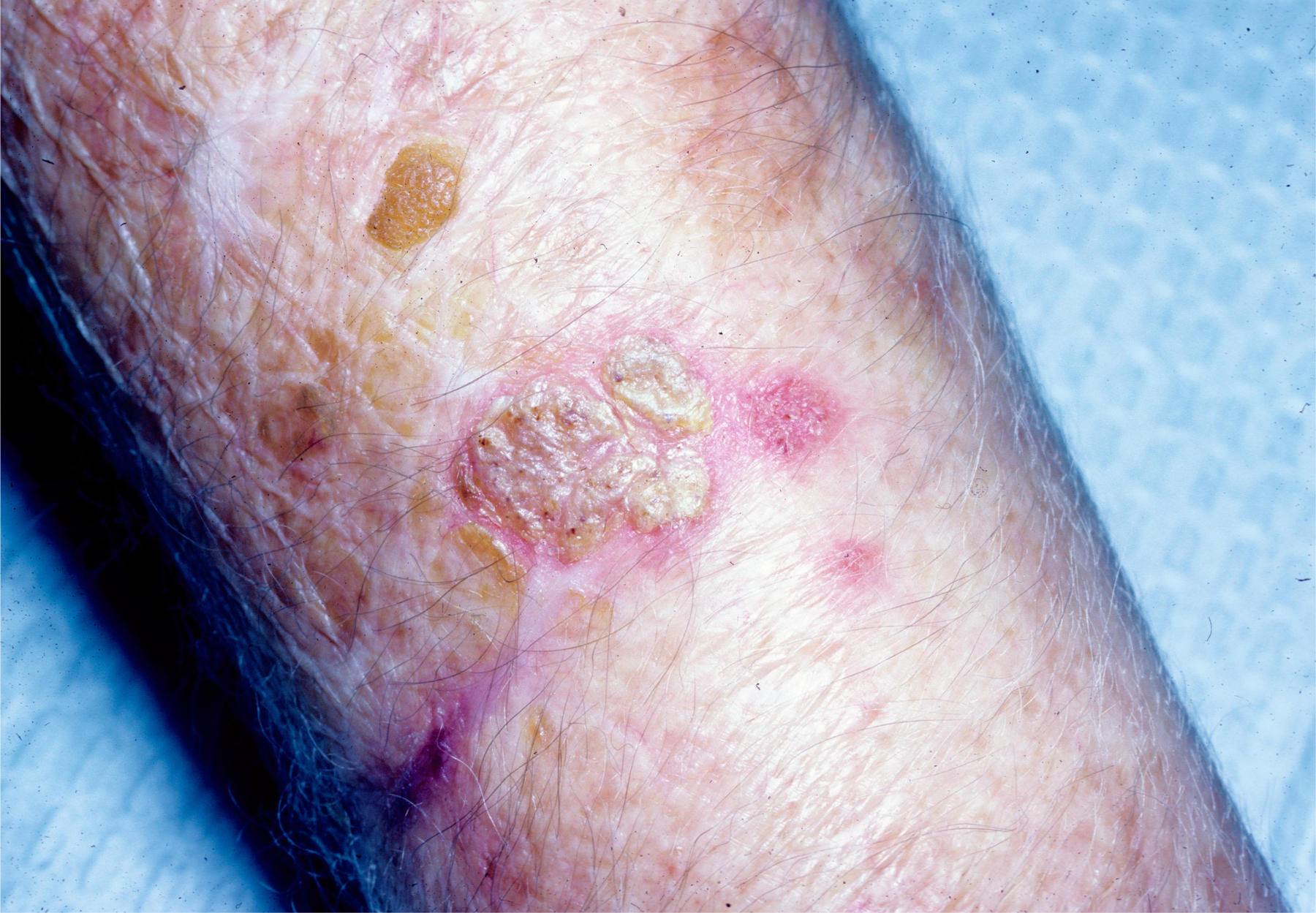
Small, scale-crust covered lesion on the forearm found to be chromoblastomycosis; submitted for biopsy as Bowen’s disease. Patient from Houston, Texas.
Figure 7.
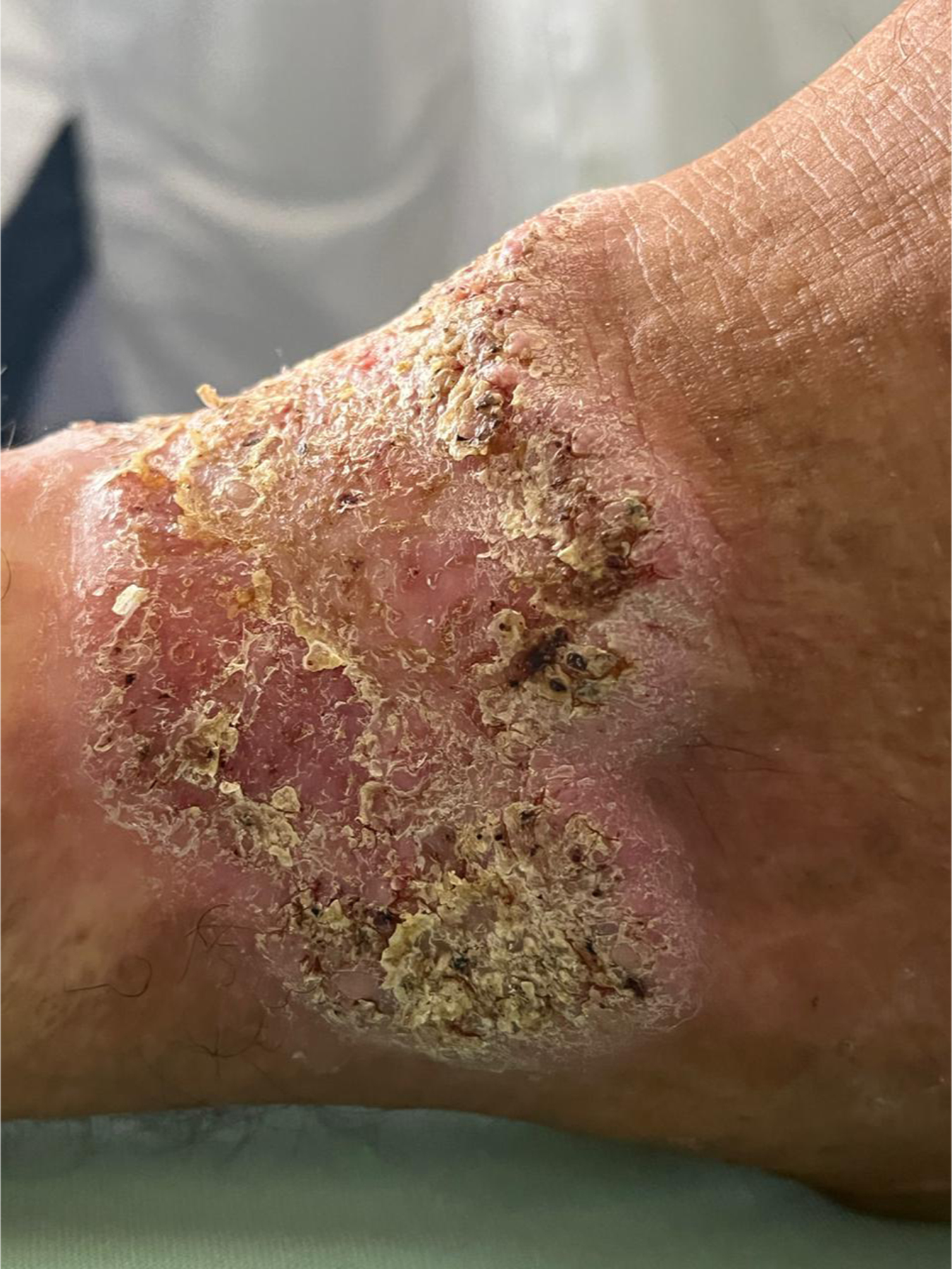
Chromoblastomycosis, hyperkeratotic plaque, with warty dry lesions, with moderate severity disease localized to the distal third of the leg. Lesions with small black dots (cayenne pepper appearance).
Figure 8.
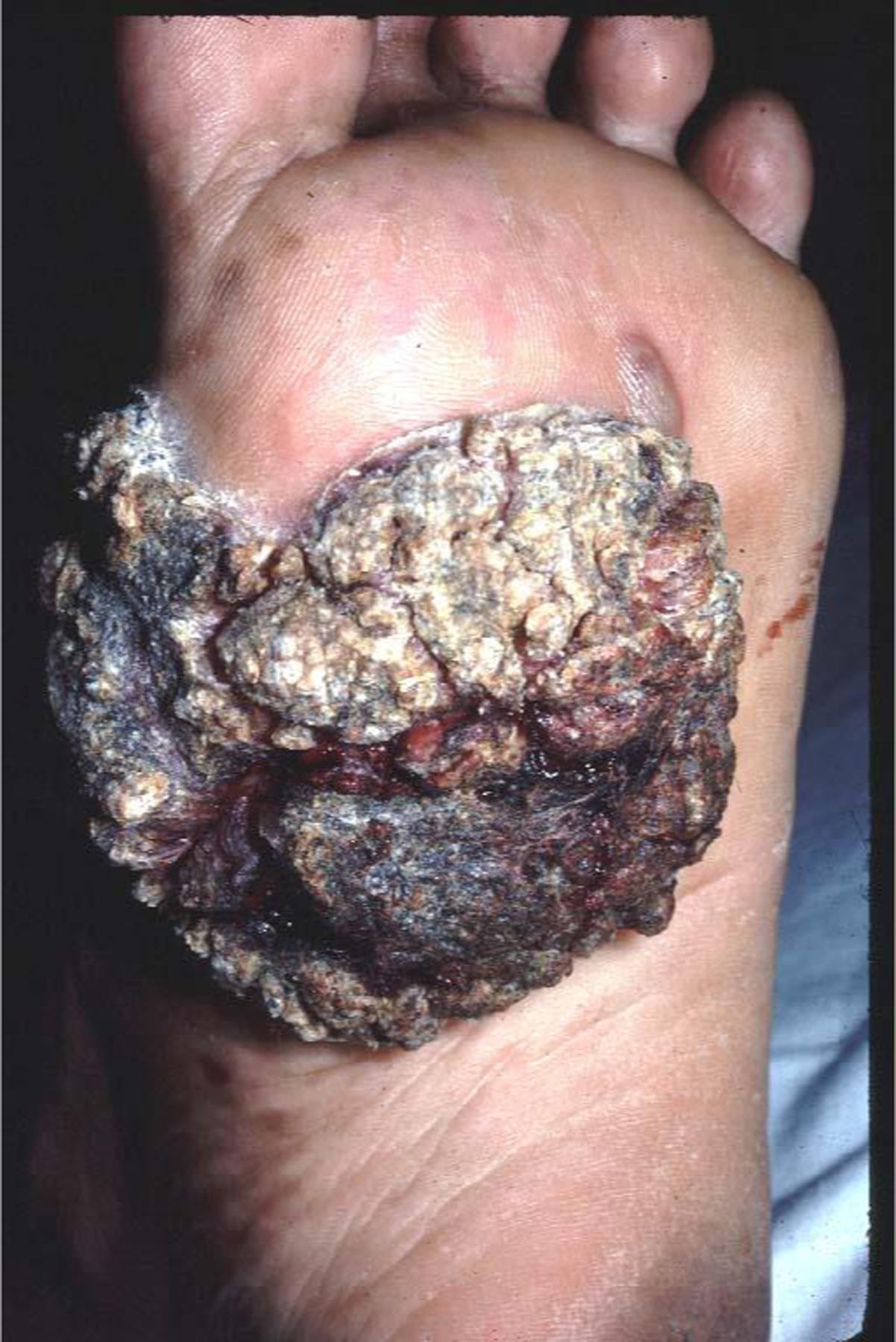
Tumoral mass clinically diagnosed as verrucous squamous cell carcinoma and diagnosed with biopsy as chromoblastomycosis. Patient from Panama.
Diagnosis and evaluation.
Diagnosis is confirmed through identification of sclerotic bodies (thick-walled cells [4–12μm in diameter] resembling copper pennies), also known as muriform cells or medlar bodies, seen on potassium hydroxide (KOH) preparation with microscopy or skin biopsy with histopathology (Figures 9, 10).43 Skin biopsy is preferentially performed in an affected area with visible black dots.44 Skin scraping is typically sufficient due to the robust nature of medlar bodies.45 Skin scrapings may be used for early and long-standing lesions.45 Tissue fungal culture identifies the causative organism.44 In a retrospective review of chromoblastomycosis cases (1989–2018) in Brazil, average time to diagnosis was nine years.46
Figure 9.
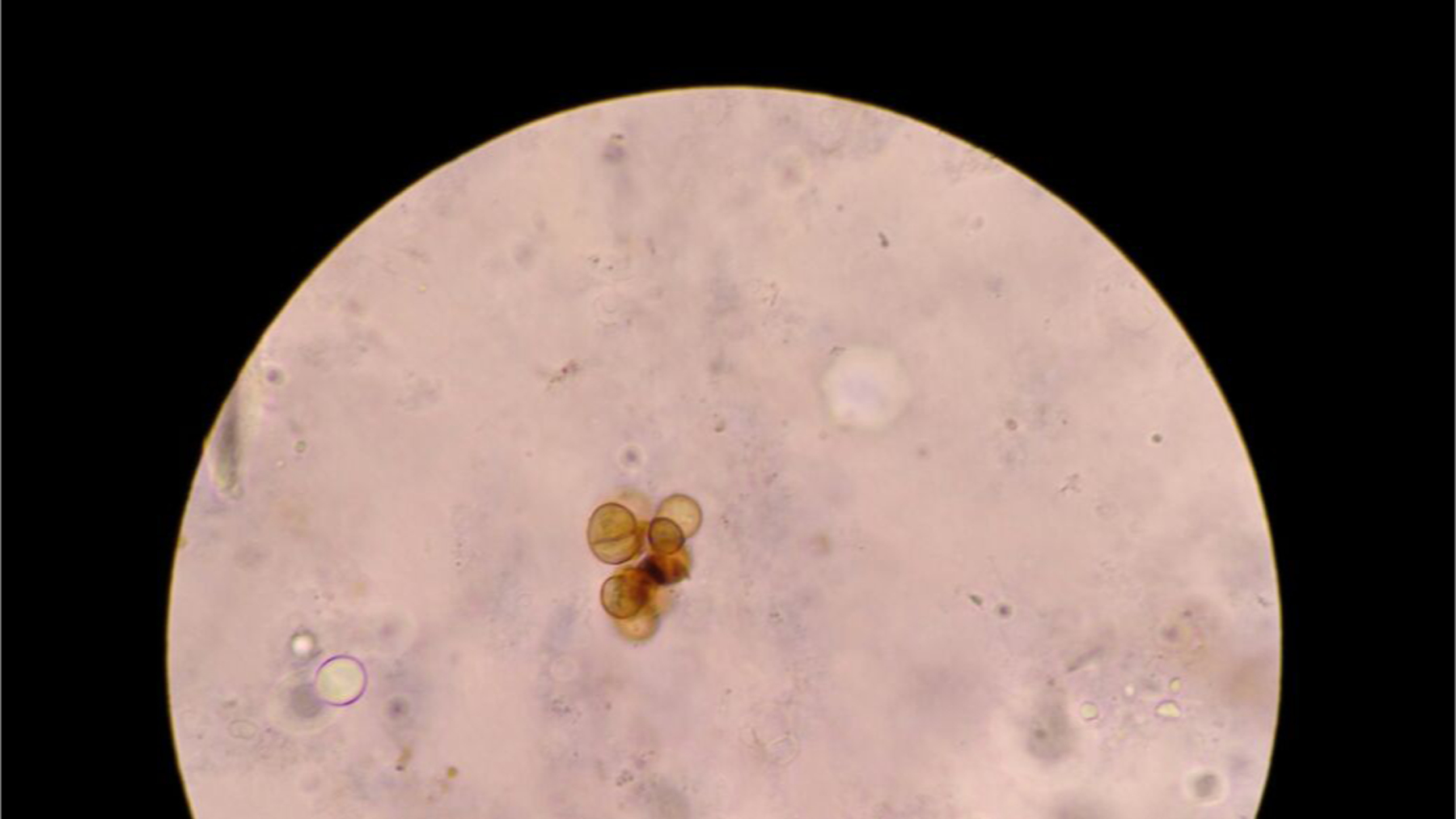
Chromoblastomycosis, clustered muriform cells depicted as round to polyhedral (chestnut-like) cells, visualized on direct microscopy potassium hydroxide exam.
Figure 10.
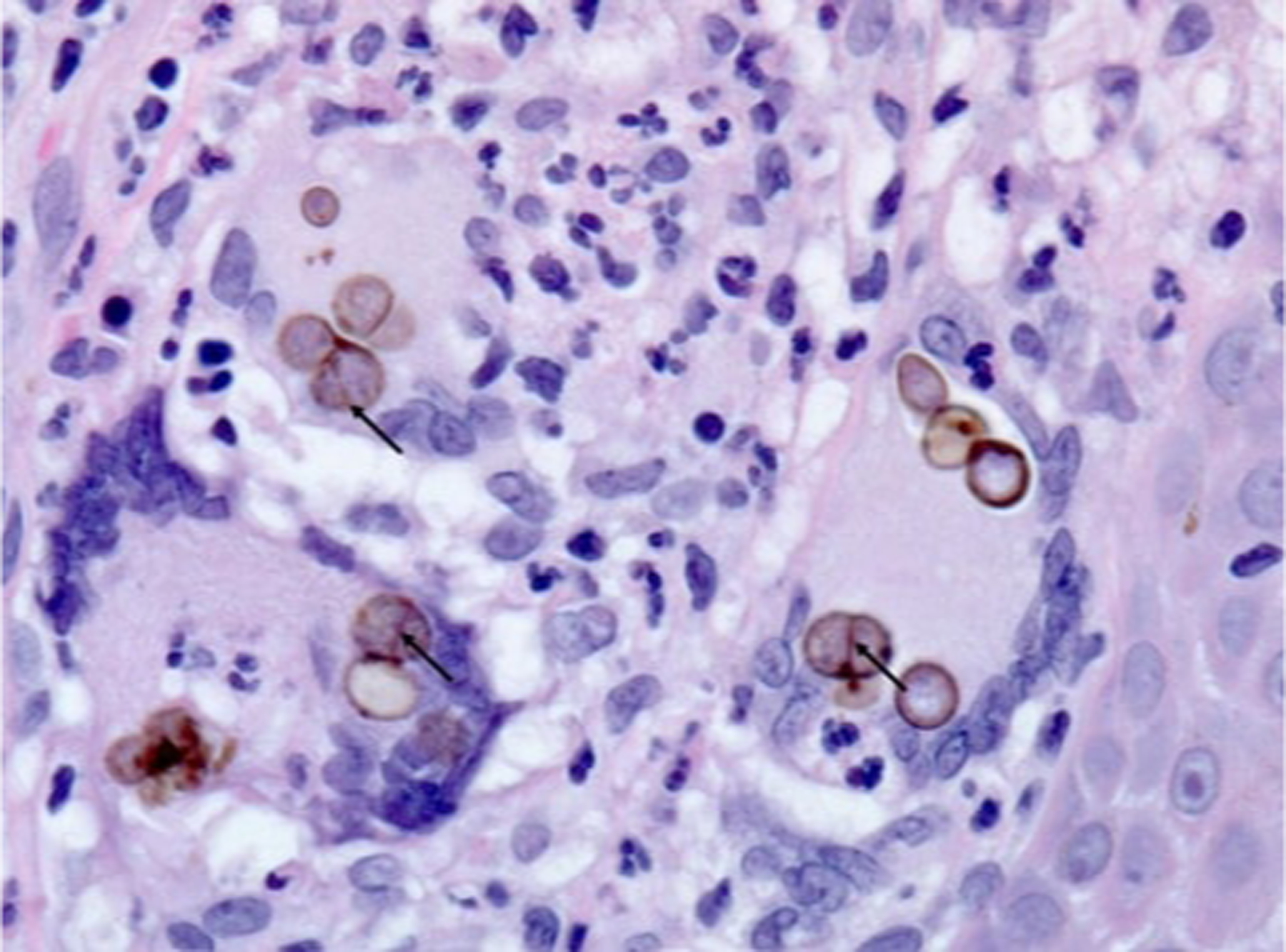
Chromoblastomycosis, skin, H&E stain: Clusters of round, thick-walled, brown-pigmented fungal spores (“sclerotic bodies,” “Medlar bodies” or “copper bodies”) within multinucleated giant cells in a focus of subepidermal granulomatous inflammation. Sclerotic bodies are fungal spores that are usually 5–12 microns and divide by internal septation (arrows). Original magnification: x630.
Paracoccidioidomycosis
Key points
Paracoccidioidomycosis is endemic in tropical and subtropical regions of Latin America.
Male agricultural workers in their 30s-50s are primarily affected.
Dermatological examination is notable for mucosal and cutaneous ulcers with hemorrhagic spots, often accompanied by severe local adenopathy.
Overview.
Paracoccidioidomycosis is caused by dimorphic fungi of the genus Paracoccidioides, which are soil saprophytes.47 Infection occurs via inhalation of conidia, with hematogenous spread to other organs, including the skin.48 Direct cutaneous or mucosal inoculation is rare.49,50 Paracoccidioidomycosis is classified into acute-subacute (juvenile) and chronic (adult) forms. Most cases (74–96%) are chronic,51 and are classified into unifocal form (single organ involvement—typically lungs), and multiple form, (multiple organ involvement—typically skin, mucosa, and lungs).49
Epidemiology.
Paracoccidioidomycosis is endemic to tropical and subtropical regions of South and Central America (Figure 5). Infection is associated with exposure to rural environments and agricultural activity, with higher risk in people working in coffee, sugar cane, corn, and tobacco plantations.52,53 Men in their 30s-50s are most frequently affected in the chronic form,51 and 90% are smokers, with disease risk 14 times higher among smokers vs. non-smokers.54,55 Adolescents of both sexes are most frequently affected in the acute-subacute form.51
Epidemiologic trends.
Brazil has the highest proportion of cases (80%),56 with incidence rising in the northern region (Amazon) due to new settlements and concurrent deforestation.51,57–59 Urban cases of paracoccidioidomycosis may be increasing.60
Clinical features of chronic form.
The lungs are most frequently involved (90%), followed by upper respiratory tract mucosa and skin.51 Mucosal lesions are painful, erythematous ulcers with ragged borders and hemorrhagic spots.61 In a retrospective study of 161 paracoccidioidomycosis patients, 60.2% presented with oral lesions, most commonly mulberry-like ulcers and gingival hemorrhagic spots (Figure 11),62 and 27.8% presented with skin involvement. Cutaneous lesions usually present as erythematous papules or ulcers/vegetations with fine granulations and hemorrhagic spots, most commonly involving the face, extremities, and trunk (Figure 12).63 There is a rare sarcoidosis-like form (Figure 13).64
Figure 11.
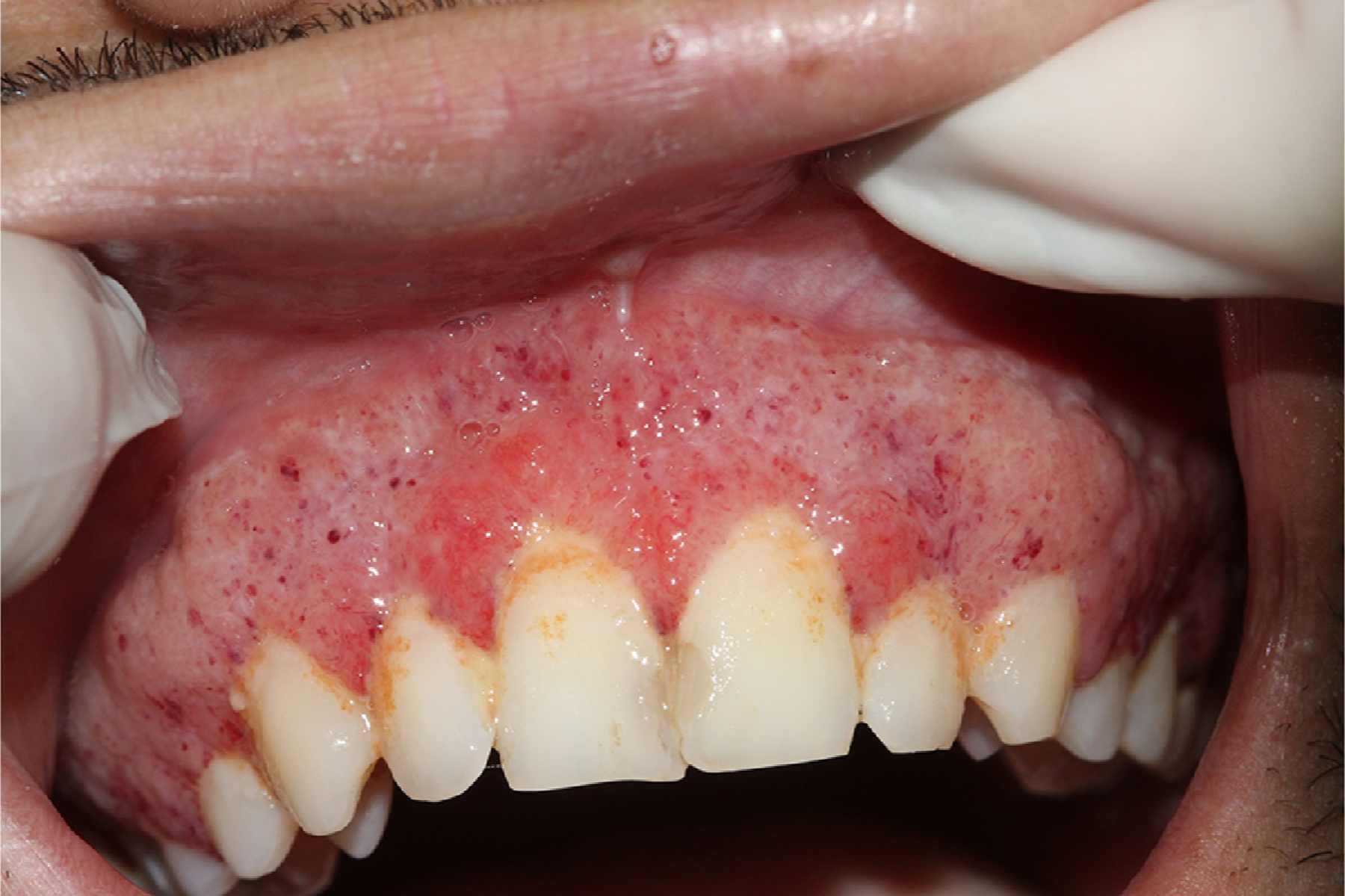
Oral lesions of paracoccidioidomycosis in the gingiva and mulberry-like ulcers with hemorrhagic dots. Photograph from a patient treated at University Hospital Cassiano Antonio Moraes, Federal University of Espirito Santo. Reproduced from Dutra et al.142 (published under Creative Commons CC-BY-NC-ND license).
Figure 12.
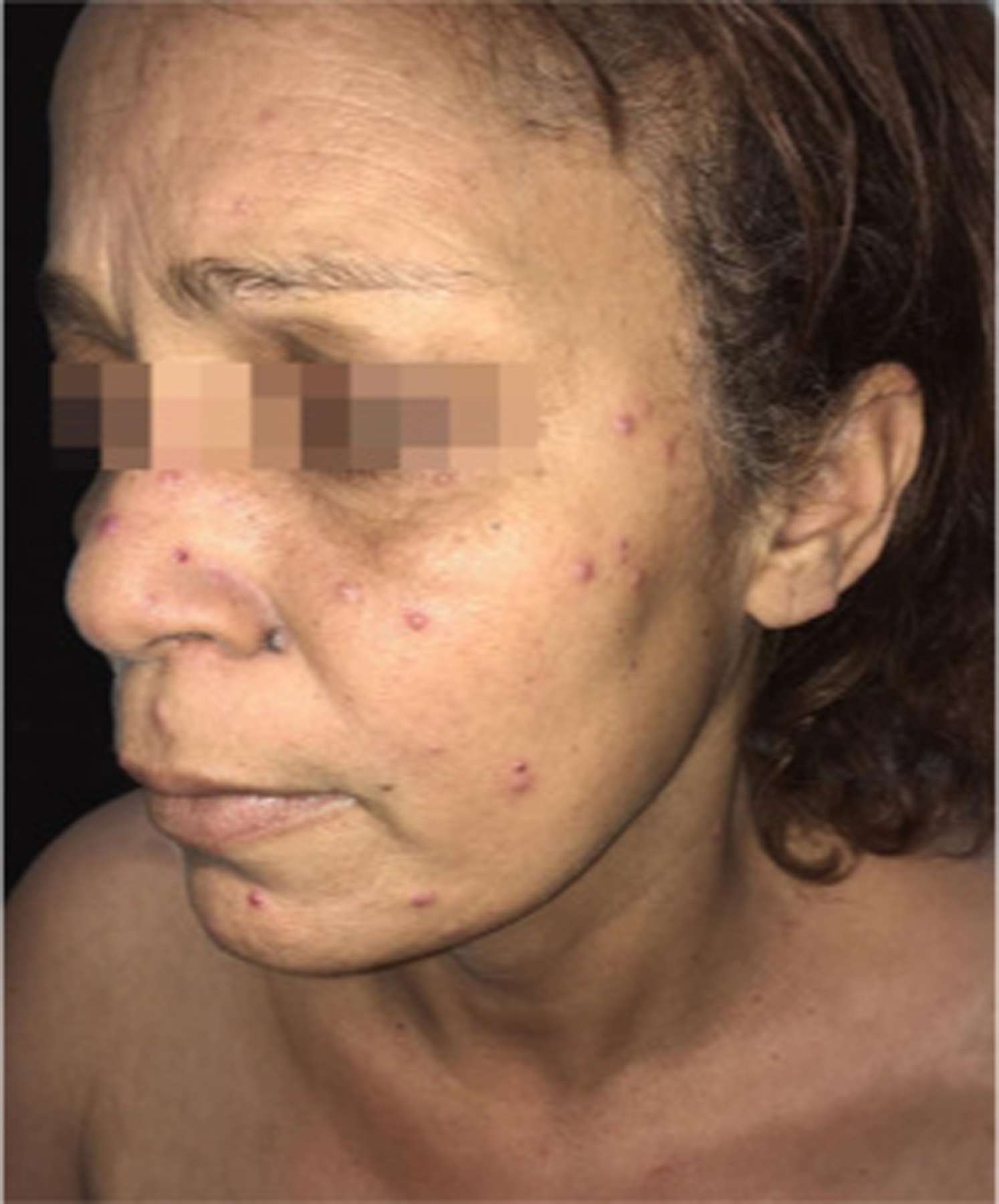
Paracoccidioidomycosis and liver transplantation. Acneiform lesions and erythematous papules, some with an ulcerated center, disseminated on the face. Reproduced from Valentim et al.143 (published under Creative Commons CC-BY license).
Figure 13.
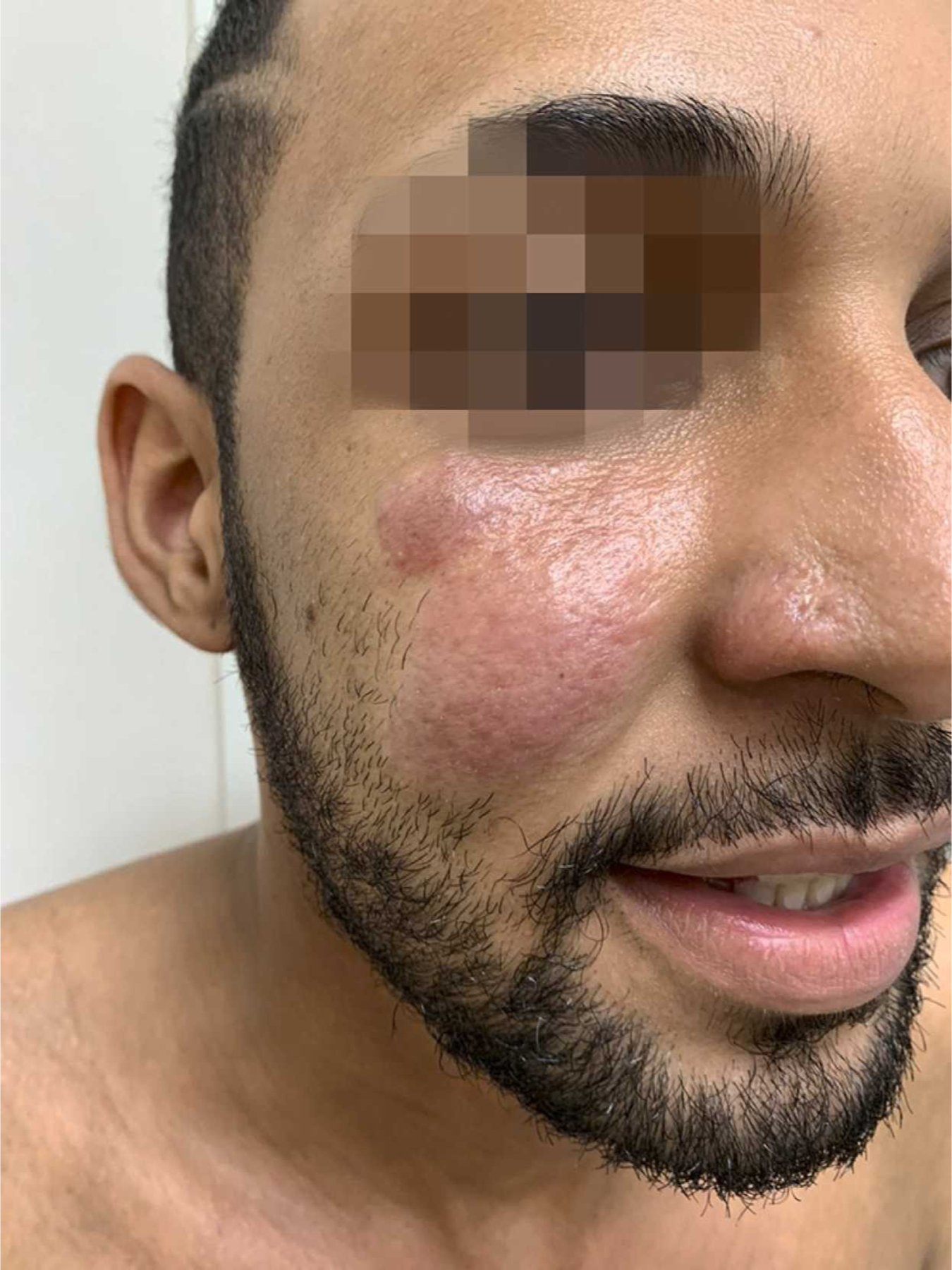
Paracoccidioidomycosis, infiltrated, sarcoid-like cutaneous lesions in the right malar region. Reproduced from Cárcano et al.64 (published under Creative Commons CC-BY-NC-ND license).
Clinical features of acute-subacute form.
Most symptoms involve the phagocytic-mononuclear system and frequently include generalized lymphadenopathy and hepatosplenomegaly.51 Cutaneous lesions are present in 16.3% of cases.65
Diagnosis and evaluation.
Direct microscopy with KOH of tissue samples or skin scrapings demonstrates large yeasts (10–30μm) with a thick, birefringent cell wall with multiple buds resembling a “mariner’s wheel” or “Mickey Mouse Ears.”8,66,67 Histopathologic examination with Gomori’s methenamine silver (GMS) stain aids in organism identification (Figure 14). Tissue fungal culture identifies the causative organism. Serologic testing is used to monitor response to therapy.66 Chest CT is recommended to evaluate for pulmonary involvement.47
Figure 14.
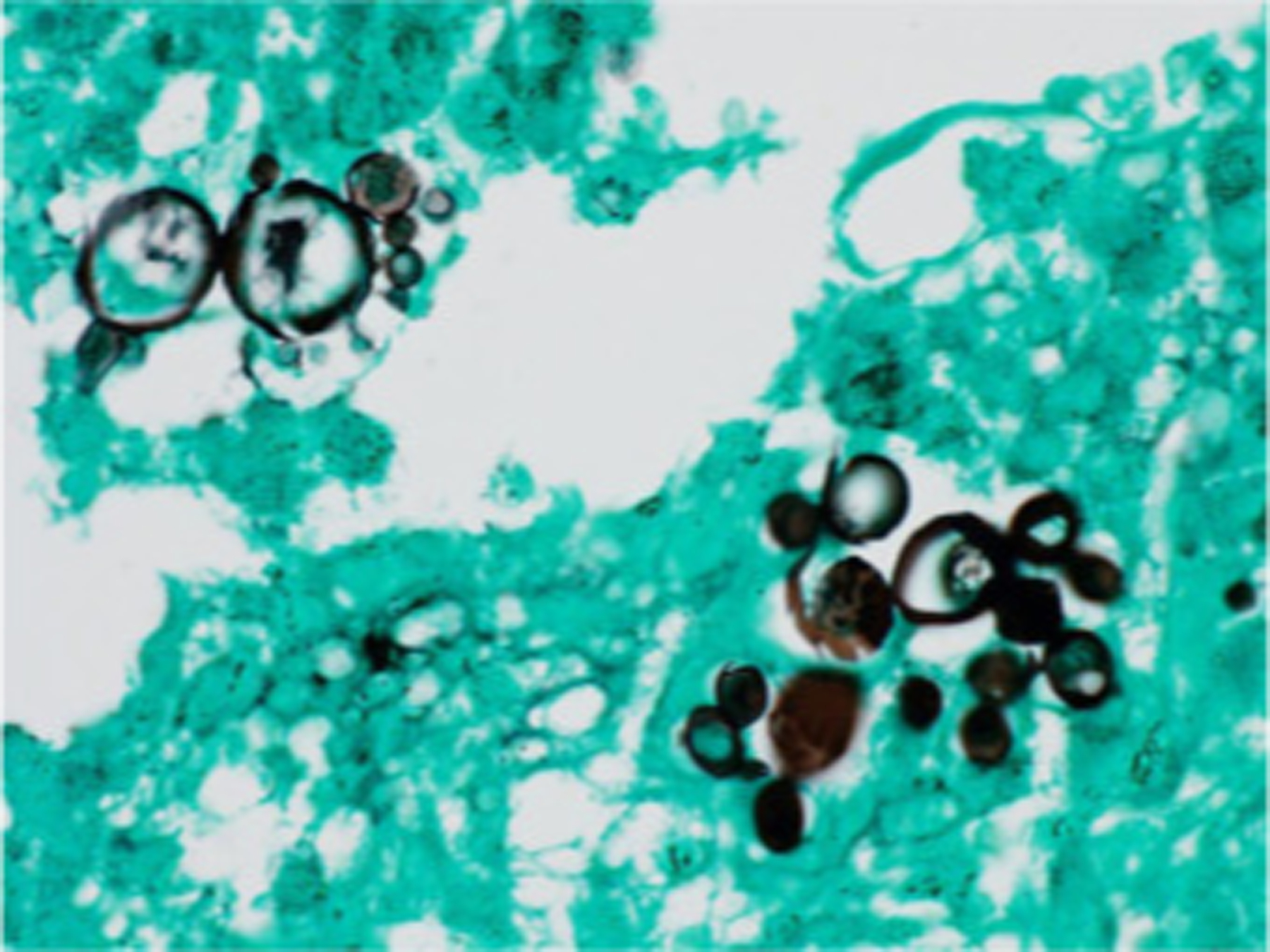
Paracoccidioidomycosis and liver transplantation, histopathologic examination (skin biopsy) demonstrating multi-budding fungal cells, “ship’s wheel” finding pathognomonic for disease (Grocott-Gomori. Immersion). Reproduced from Valentim et al.143 (published under Creative Commons CC-BY license).
Sporotrichosis
Key points
Sporotrichosis is thought to be the most prevalent implantation mycosis worldwide.
Patients in contact with vegetation, soil, and cats are most frequently affected.
Physical examination classically shows ascending nodular lymphangitis.
Overview.
Sporotrichosis is caused by the dimorphic fungal genus Sporothrix spp. The most common species vary by region, including Sporothrix schenckii (North America, Australia, South Africa),68 Sporothrix globosa (Asia),68,69 and Sporothrix brasiliensis (Brazil).70,71 Transmission varies by species, with the classical route defined as traumatic inoculation of soil or plant material for Sporothrix schenckii, and the emerging route defined as inoculation through a bite, scratch or contact with respiratory droplets (sneezing, licking) from infected cat for Sporothrix brasiliensis.72 In feline sporotrichosis, transmission to humans may occur via contact with infected animal secretions without trauma.
Epidemiology.
Sporotrichosis has a global distribution, with endemic areas being Latin America (especially Brazil, Mexico, Colombia), Asia (especially China, India, Japan), and to a lesser extent, US and Australia (Figure 5).73 It is often considered the most prevalent and widespread implantation mycosis worldwide.74 Veterinarians and those who work with animals may have increased infection risk.72,75
Epidemiologic trends.
An ongoing sporotrichosis epidemic in Rio de Janeiro, Brazil, primarily from feline transmission of Sporothrix brasiliensis, caused an increase in human cases from 759 in 1998–2004 to >4,000 in 2014.74 In a case series of 122 sporotrichosis patients infected with Sporothrix brasiliensis, 70.5% were female, which may be related to the high proportion of women working as housewives or cleaners in Brazil with close contact to domesticated cats.76
Clinical features.
The cutaneous form is most common, including lymphocutaneous (55%) and fixed cutaneous (25%) involvement (Figures 15, 16).77 In the lymphocutaneous form, a papulonodular lesion develops days to weeks after inoculation and subsequently ulcerates, often resembling the initial lesion of syphilis and therefore referred to as chancriform (Figure 17). Similar lesions thereafter occur along regional lymphatic channels proximal to the original lesion (nodular lymphangitis). In the cutaneous form, polymorphic skin lesions, including papules, nodules, ulcers, verrucous lesions, and plaques develop following trauma.74 Extracutaneous involvement is associated with chronic obstructive pulmonary disease, alcoholism, steroids, AIDS, solid organ transplantation, and TNF-alpha inhibitors, and may include pulmonary, osteoarticular, ophthalmic, and central nervous system infection.74,78
Figure 15.
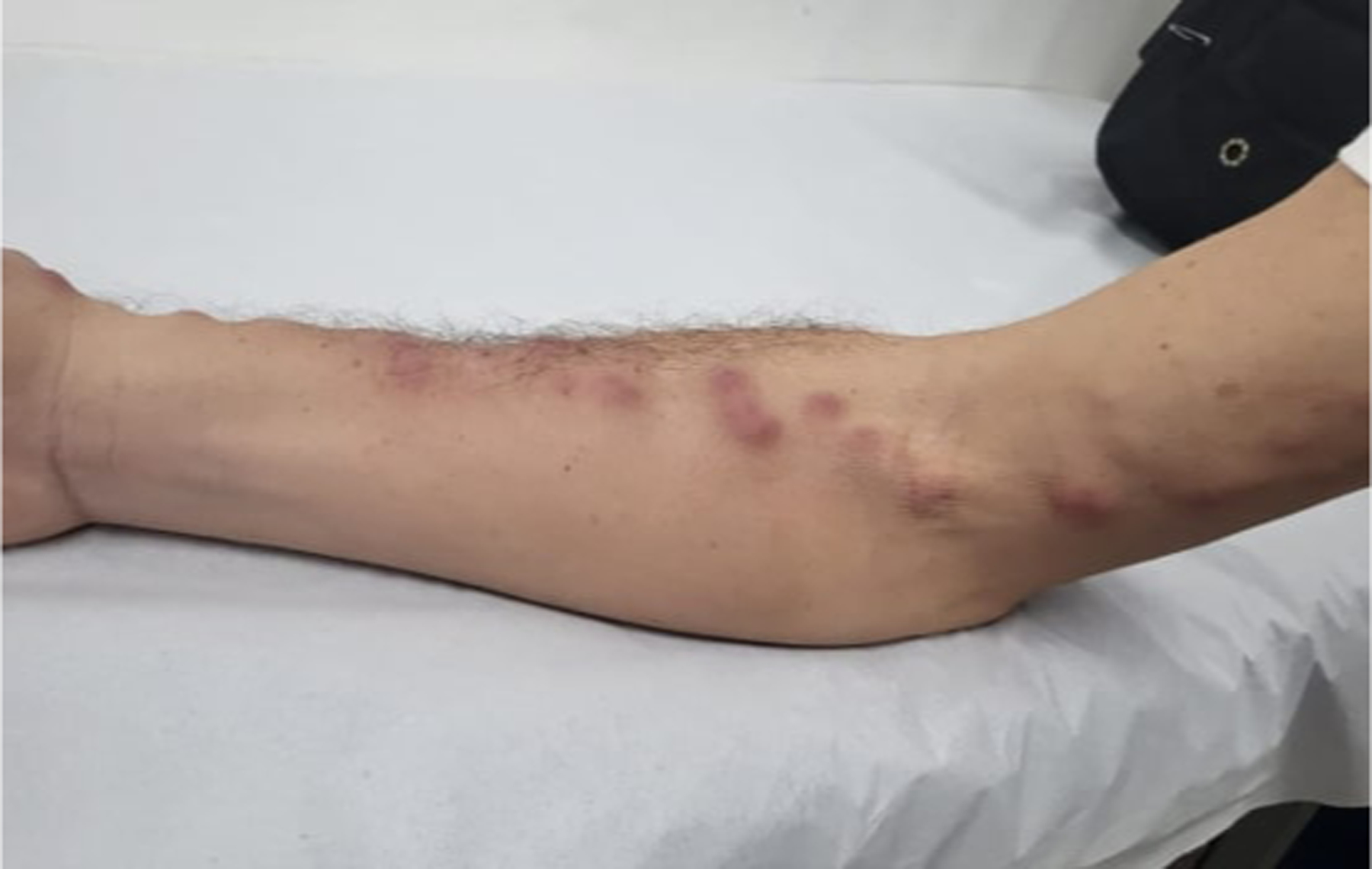
Sporotrichosis, feline dissemination. Erythematous lesions, with subcutaneous nodules, painful on palpation, with lymphatic distribution involving the right arm.
Figure 16.
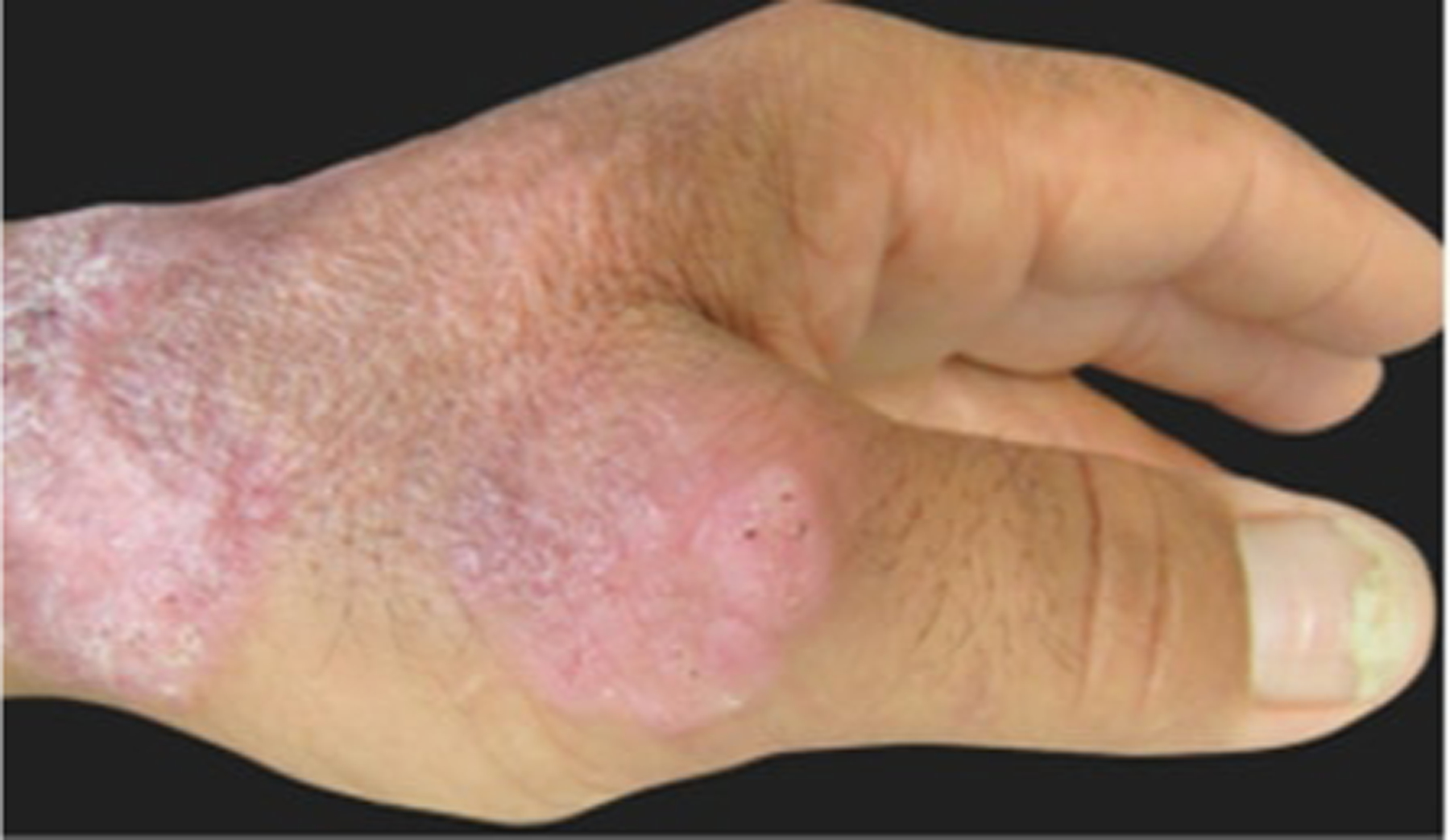
Sporotrichosis fixed cutaneous form, verrucous lesion on the dorsum of the hand. Reproduced from Orofino-Costa et al.144 (published under Creative Commons CC-BY license).
Figure 17.
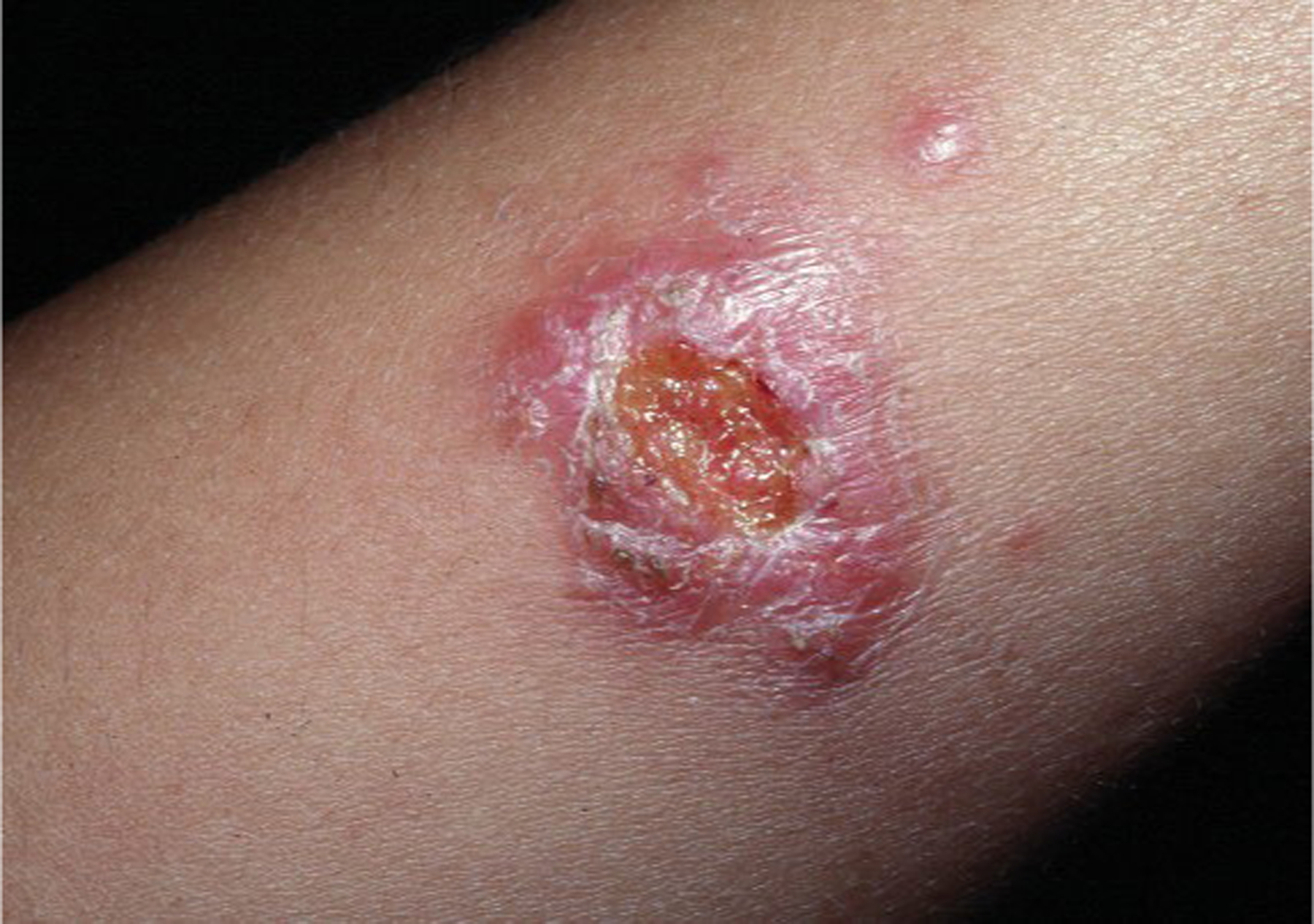
Primary chancriform lesion of sporotrichosis after trauma with rose thorn.
Diagnosis and evaluation.
Tissue fungal culture is the primary diagnostic test.79–83 Histopathology has low sensitivity, showing only an inflammatory or granulomatous reaction, and rarely oval or spherical yeasts (2–6μm in diameter) sometimes elongated in a cigar shape (Figure 18).79 Because organisms may be sparse, immunohistochemistry may enhance sensitivity. Polymerase chain reaction (PCR) is a fast, accurate, and highly sensitive diagnostic method. 84 However, immunohistochemistry and PCR are not readily available for sporotrichosis diagnosis in the US.
Figure 18.
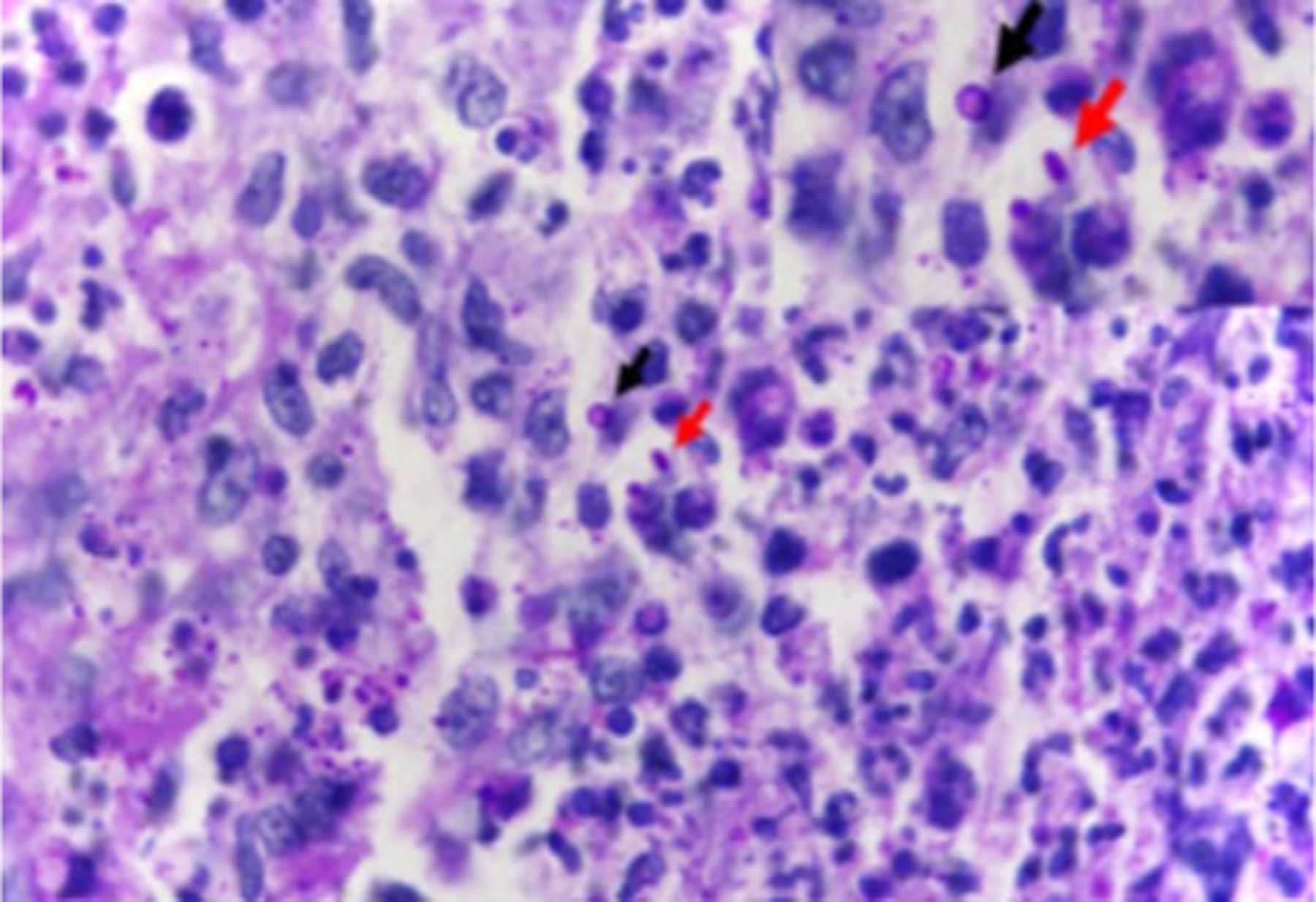
Sporotrichosis histopathologic examination (skin) demonstrating suppurative granuloma and parasitic fungus. Epithelioid cells on the left, neutrophils and myocytes on the right, round yeast-like (black arrow) and elongated or navicular fungal cells (red arrow) (Periodic acid-Schiff stain, 1000x). Reproduced from Orofino-Costa et al.144 (published under Creative Commons CC-BY license).
Emergomycosis
Key points
Emergomycosis is a recently recognized dimorphic fungal infection with known distribution in North America, Africa, Asia, and Europe.
Immunosuppressed patients are most frequently affected.
Physical examination shows widespread polymorphic cutaneous lesions.
Overview.
Emergomycosis is caused by a group of previously unknown dimorphic fungal species that have recently been reclassified to the genus Emergomyces. Emergomyces spp. are found in soil and are likely spread by inhalation of conidia.85 It is unknown whether Emergomyces spp. have truly emerged or whether they are now recognized due to increased immunosuppressed, susceptible hosts, and the adoption of molecular identification techniques.86
Epidemiology.
Emergomycosis cases have been reported in North America, Africa, Asia, and Europe.87 The most cosmopolitan and widespread species is Es. pasteurianus,88 diagnosed in the Netherlands,89 Italy,90 Spain,91 France,92 India,93 China,94,95 Uganda,96 and South Africa.97 Other species include Es. canadensis (North America), Es. orientalis (Asia), Es. europaeus (Europe) and Es. africanus (Southern Africa).85,87,98,99 Emergomycosis most commonly affects immunosuppressed people, including those with advanced HIV, solid organ transplantation, diabetes mellitus, hematologic malignancy, and those taking immunosuppressants.88,100,101,102 The highest burden of cases is among HIV-infected patients in South Africa.103
Epidemiologic trends.
Emergomycosis is now recognized as the most commonly diagnosed dimorphic fungal infection in South Africa.86 Recent cases of emergomycosis have been reported in North America, including four cases in immunocompromised patients in the US (Colorado, New Mexico) and Canada (Saskatchewan).104
Clinical Features.
Emergomycosis may involve the skin, mucous membranes, lungs, gastrointestinal tract, liver, spleen, bone marrow, lymph nodes, and cervix.85,86,88,105–107 In a retrospective study of 55 emergomycosis patients in South Africa, 96% had skin lesions at presentation, and 88% had pulmonary involvement.108 Cutaneous lesions are typically widespread and polymorphic,105 and may include umbilicated papules, hyperkeratotic plaques, nodules, verrucous lesions, and ulcerations (Figures 19, Figure 20).85,105,106,108,109
Figure 19.

Mucocutaneous manifestations of HIV-associated disseminated emergomycosis in a South African patient who presented with a 1-year history of nodular lesions on the face, limbs, and trunk. Papules and plaques with central necrosis. Reproduced from Reddy et al.145 with permission. Figure courtesy of Dr. Matilda Mphahlele (Division of Dermatology, Chris Hani Baragwanath Academic Hospital, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa).
Figure 20.
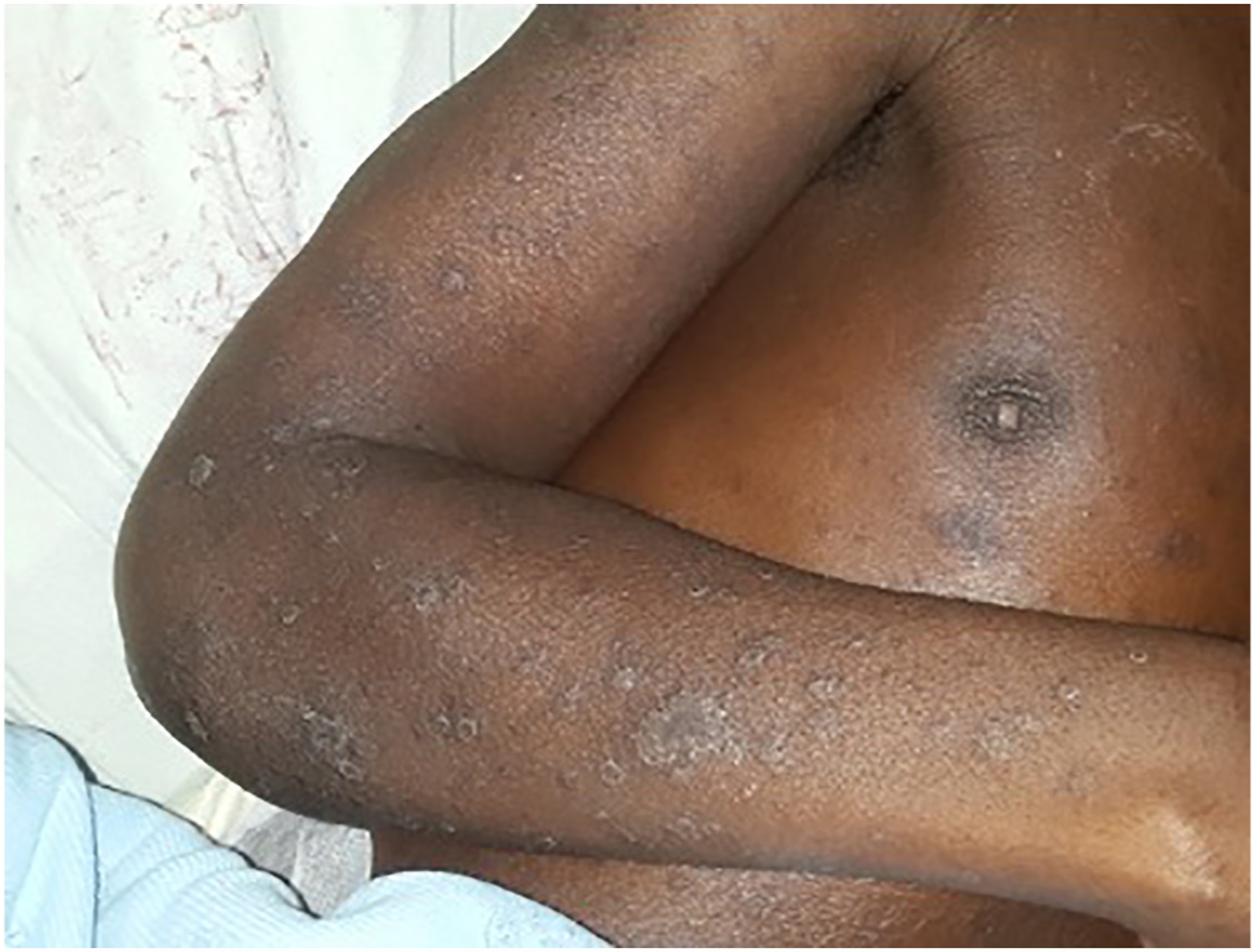
Clinical image of a South African patient with HIV-associated disseminated emergomycosis, showing hyperpigmented and violaceous plaques with surface scaling. Reproduced from Reddy et al.145 with permission.
Diagnosis and evaluation.
Diagnosis is via tissue fungal culture in conjunction with histologic evidence of tissue invasion.100 Punch or incisional skin biopsies may be used for tissue culture and histopathology.100 Histopathologic sections demonstrate 2–5μm intracellular (in histiocytes) and extracellular oval yeasts with narrow-based budding and inflammatory change, best visualized with hematoxylin-eosin, periodic acid Schiff (PAS), GMS, and Wright-Giemsa stains (Figure 21).85,88,101 Histopathology alone is not sufficient for diagnosis, as yeast cells of Es. africanus and Es. orientalis resemble those of Histoplasma capsulatum and Blastomyces dermatitidis, respectively.85,88,100
Figure 21.
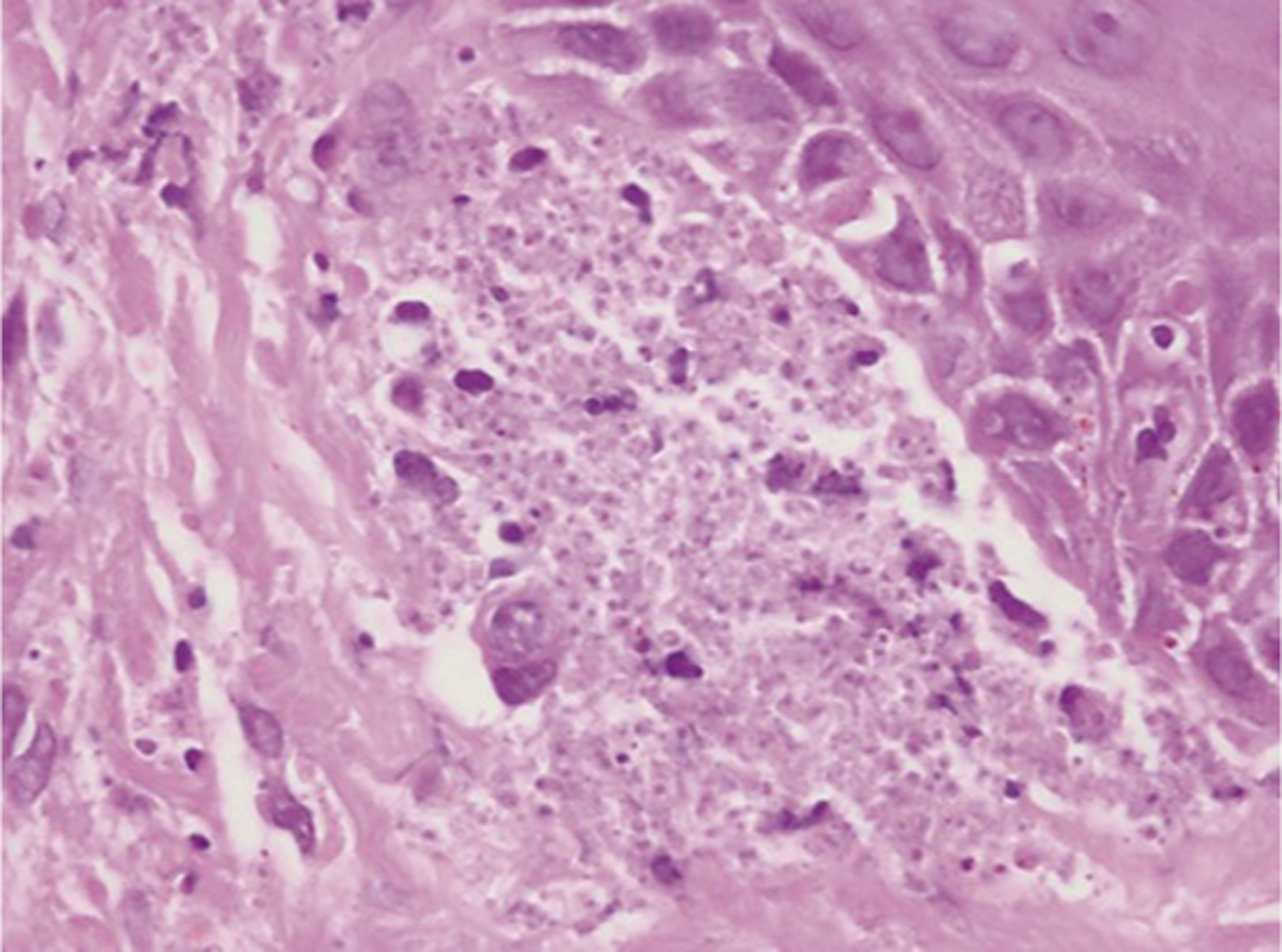
Histopathology of skin biopsies from South African patients with HIV-associated disseminated emergomycosis caused by Es. Africanus. Es. africanus organisms occupying the papillary dermis, as seen under oil emersion (haematoxylin and eosin, original magnification x 1,000). Reproduced from Govender et al.106 with permission.
Talaromycosis
Key points
Talaromycosis is endemic to tropical and subtropical regions of Asia.
Patients living with HIV are most frequently affected.
Physical examination findings are papules with central umbilication involving the face, neck, and extremities.
Overview.
Talaromycosis is caused by the fungus Talaromyces marneffei, which is spread via inhalation of spores.110
Epidemiology.
Talaromycosis is endemic to tropical and subtropical regions of Asia and primarily affects HIV patients.111 Patients with other immunosuppressive conditions, including cancer and organ transplantation, may also be affected.112 A total of 24,622 talaromycosis cases were reported in 33 countries by late 2018, with an estimated 288,000 talaromycosis cases globally 1964–2018, and approximately 17,300 cases diagnosed annually.113
Epidemiologic trends.
It is estimated that talaromycosis incidence will increase by 35% 2020–2025,113 due to rising incidence of advanced HIV among newly diagnosed HIV patients in Indonesia, Thailand, Vietnam, and the Philippines.111
Clinical features.
The main clinical manifestations are painless papules involving the face, neck, and extremities.114–117 In a retrospective study of 80 HIV patients with disseminated talaromycosis, 71% had cutaneous findings, and 87% of papules had central umbilication (Figure 22). Non-HIV patients less often had skin lesions (44% vs. 71%),118,119 and had longer time to diagnosis (180 vs. 45 days)120 and higher mortality (29% vs. 21%)121 compared to HIV-coinfected patients. Papules less often have central umbilication in non-HIV-coinfected vs. HIV-coinfected patients.122
Figure 22.
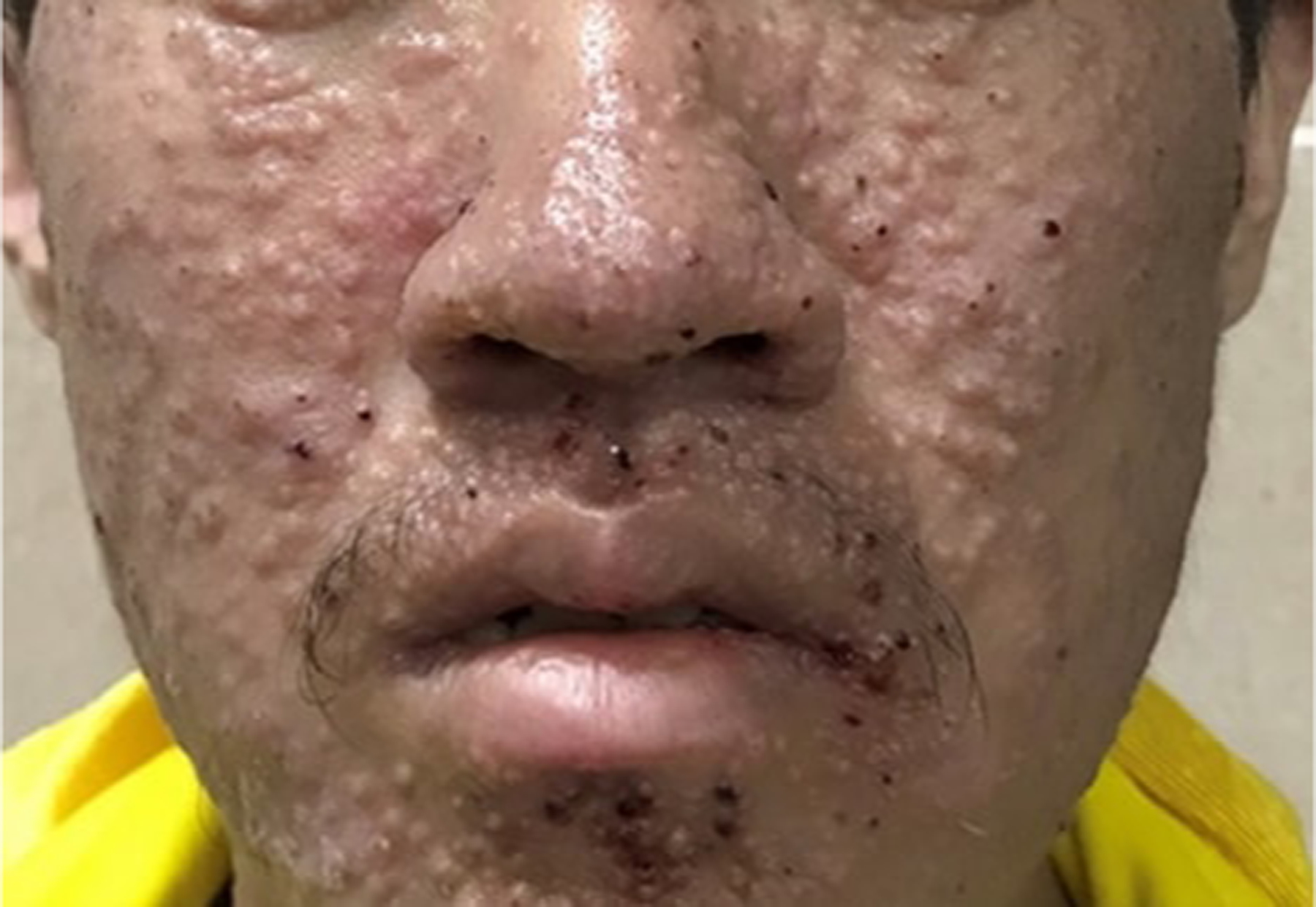
Talaromycosis physical examination. Multiple cuticolor follicular papules with or without central umbilication, nodules, and acne-like lesions were developed densely on his face, neck, and upper anterior chest. Some of them were covered with reddish brown crusts. Reproduced from Xian et al.146 (published under Creative Commons CC-BY license).
Diagnosis and evaluation.
Tissue fungal culture is the primary diagnostic test.123 Presumptive diagnosis may be made via microscopy of Wright-stained touch smears from a skin biopsy, showing clear basophilic, oval yeasts with central septation.124–126 Histopathology with GMS or PAS staining demonstrate round or oval yeast cells within macrophages (Figure 23),126 but may resemble that of other fungal diseases associated with parasitized histiocytes, including emergomycosis and histoplasmosis. Antigen detection is an accurate and inexpensive diagnostic option for advanced HIV patients with high fungal burden.100 An inexpensive, point-of-care immunochromatographic strip test with high sensitivity/specificity using clinical urine specimens was recently developed, which is not yet readily available in the US.127
Figure 23.

Talaromycosis histopathologic examination (skin biopsy) demonstrating abundant yeast-like organisms in the cytoplasm of histocytes. The organisms were spherical to oval, about 3-μm in diameter and occasional contained septum (Periodic acid-Schiff stain, 40x). Reproduced from Xian et al.146 (published under Creative Commons CC-BY license).
Lobomycosis
Key points
Lobomycosis primarily occurs in tropical regions of Latin America.
Individuals with trauma in the setting of environmental exposure are most frequently affected.
Physical examination is notable for keloid-like nodules involving the lower extremities or ears.
Overview.
Lobomycosis, also known as lacaziosis or Jorge Lobo’s disease, is caused by the fungus Lacazia loboi, which presumably gains cutaneous entry after minor trauma.128 This pathogen is non-cultivable, and taxonomy is therefore debated.129,130 Dolphins are the only other known host. Recent increase in dolphin varieties infected with Lacazia loboi131 raises concern for increased geographical distribution of disease in humans.
Epidemiology.
Lobomycosis primarily occurs in Central and South America, and most commonly affects forest workers, farmers, hunters, manual laborers, and fishermen. There were 907 reported cases globally as of 2022, which is likely an underestimate.130
Epidemiologic trends.
Incidence has increased in Acre, Brazil.132,133 Cases have been reported in the US and Canada, likely caused by preceding exposure in the Amazon Basin.134,135
Clinical features.
Lobomycosis is characterized by long incubation period (sometimes years) followed by slow, progressive development of painless keloid-like nodules, which may ulcerate or become verrucous (Figure 24).10 In the localized form, lower extremities are most frequently affected, followed by ears, upper extremities, and head (Figure 25).136,137 In a retrospective study of 249 lobomycosis cases in Brazil, localized, multifocal, and disseminated cutaneous lesions were observed in 61%, 22%, and 16% of patients, respectively.138
Figure 24.
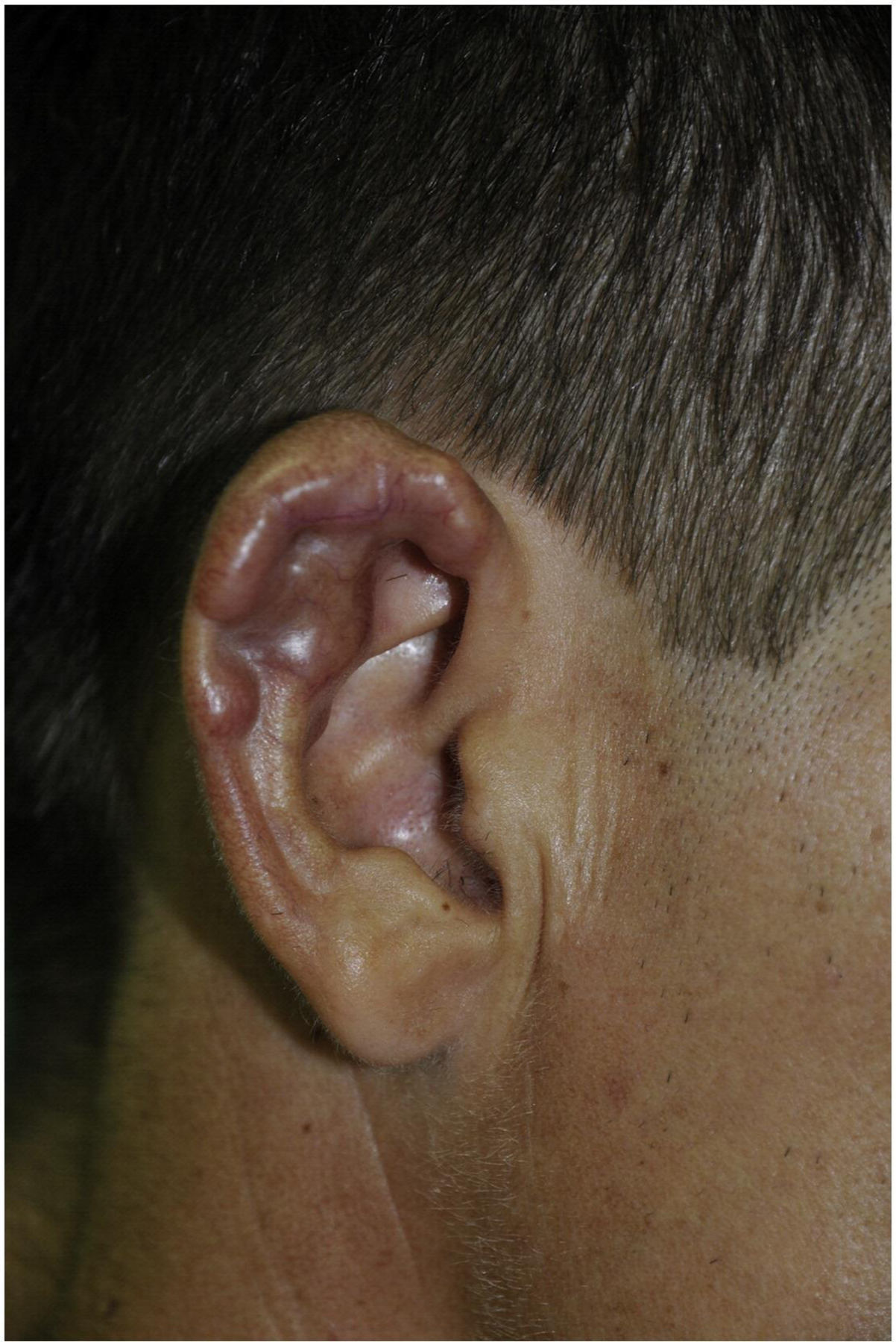
Lobomycosis, classic infiltrative nodules on the right outer ear. Reproduced from Talhari et al. 139 Permission granted for figure reproduction.
Figure 25.
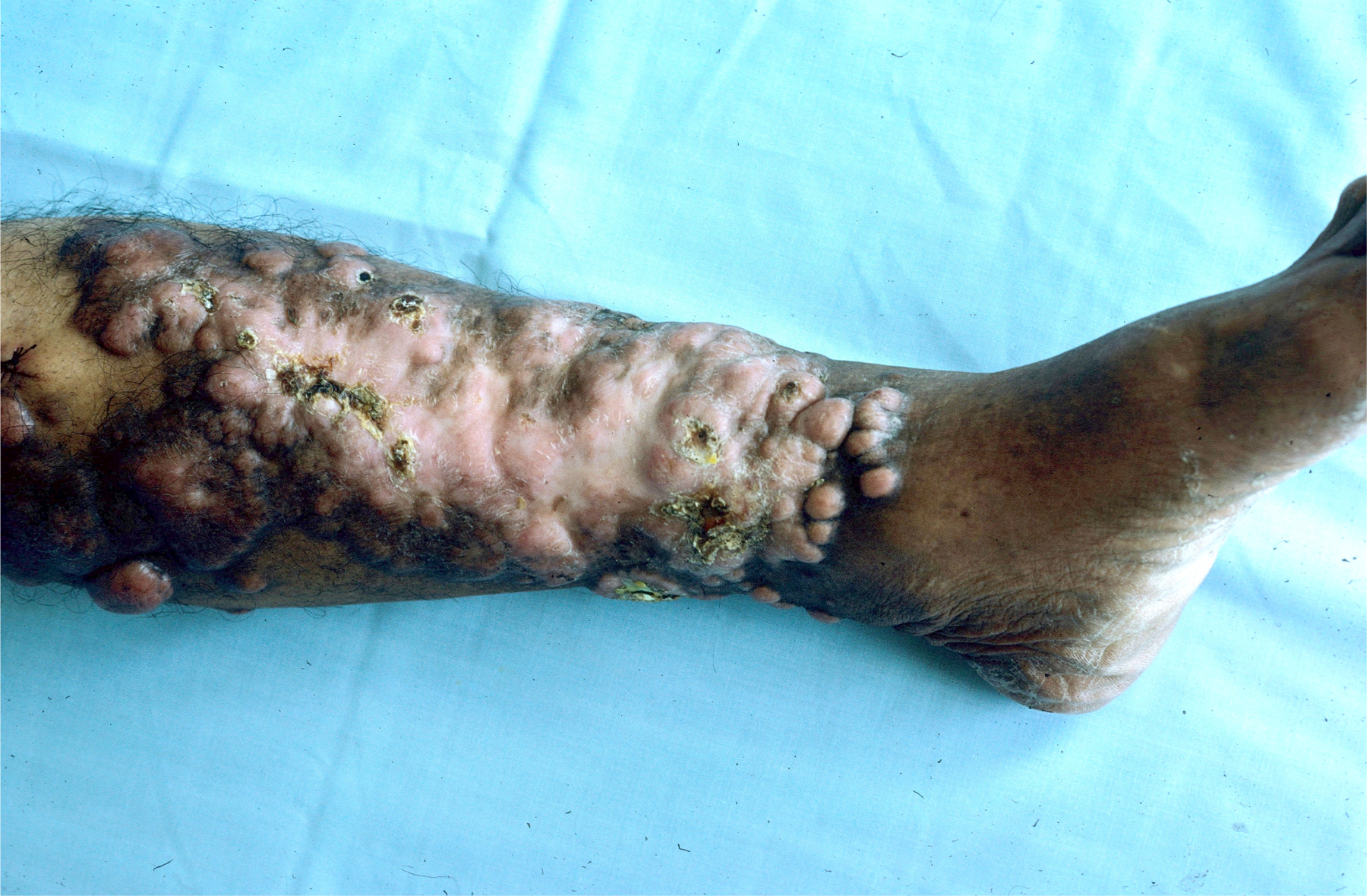
Large aggregate of keloid-like nodules admixed with sclerotic areas diagnosed as lobomycosis on biopsy. Patient from Panama.
Diagnosis and evaluation.
Diagnosis is made via skin biopsy with histopathology,139 showing isolated round yeast-like cells with a thick wall containing melanin, or in 2–10 cell chains connected via tubular projections (Figure 26).139 In scaly lesions, fungal visualization using direct microscopy on lesional scrapings is useful. 10,140
Figure 26.
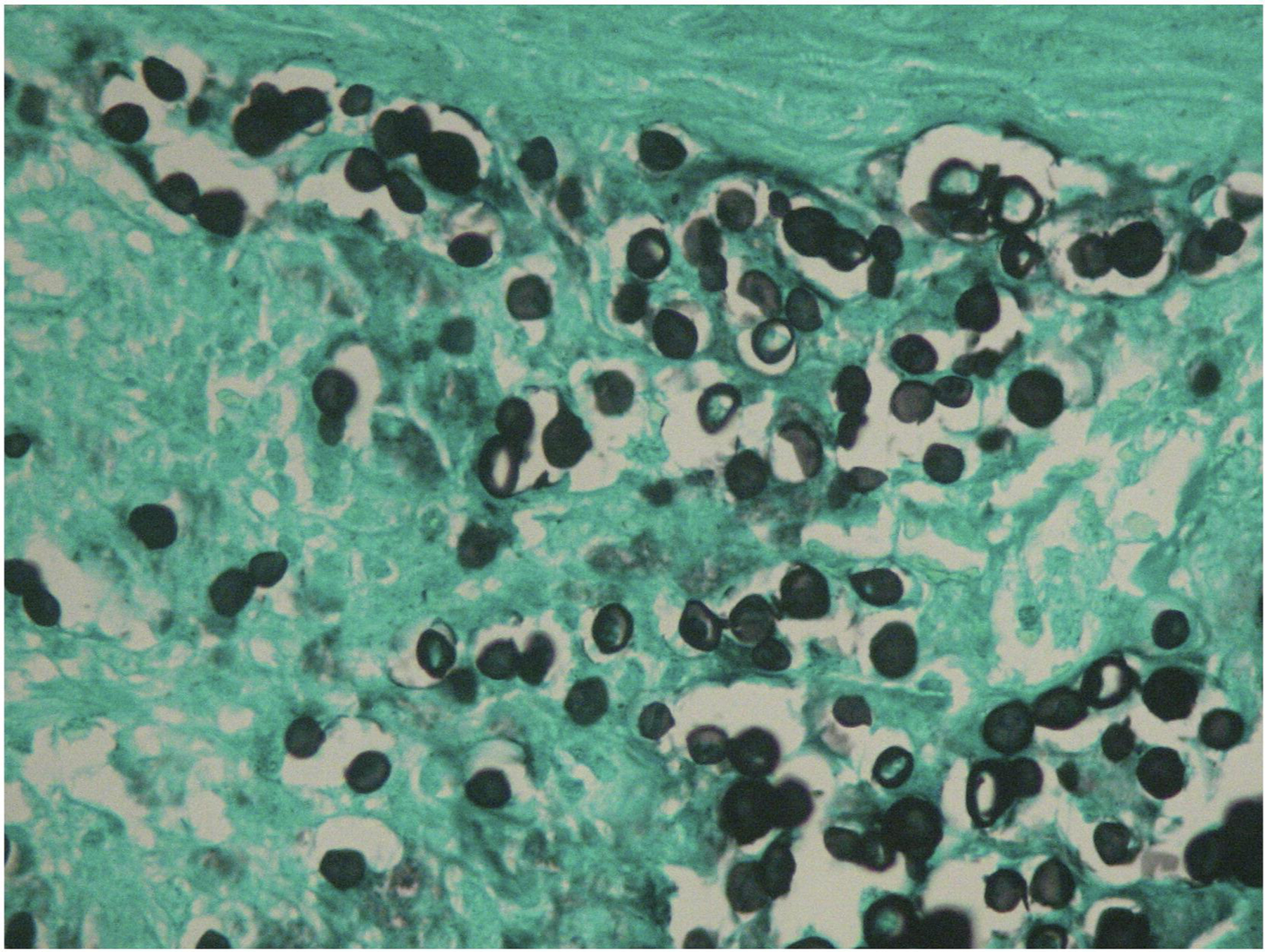
Lobomycosis histopathology examination demonstrating multiple isolated and chained parasites (Grocott stain, 40x). Reproduced from Talhari et al.139 Permission granted for figure reproduction.
Conclusion.
Fungal skin NTDs have been and continue to be neglected, despite association with substantial morbidity and stigma. Public health efforts are needed to improve surveillance and to increase access to diagnostic tools in endemic regions, since diagnosis is challenging. A high index of suspicion for fungal NTDs based on demographics and clinical presentation is necessary, with adequate tissue biopsy for histological examination and fungal culture. Recognition of cutaneous manifestations and prompt diagnosis is imperative for improved outcomes.
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- CT
Computed tomography
- HIV
Human immunodeficiency virus
- GMS
Gomori’s methenamine silver
- KOH
Potassium hydroxide
- MRI
Magnetic resonance imaing
- NTD
Neglected tropical disease
- PAS
Periodic acid Schiff
- PCR
Polymerase chain reaction
- TNF
Tumor necrosis factor
- US
United States
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Attachments: CME proposal form, CME clinical vignette quiz section
Conflicts of Interest: Ms. Curtis, Dr. Gold, Dr. Ritter, Dr. Rosen, Dr. Santos, Dr. Smith, and Dr. Lipner have no conflicts of interest relevant to the content of the submission. Financial disclosures: Dr. Shari Lipner has served as a consultant for Ortho-Dermatologics, Eli Lilly, BelleTorus Corporation, and Moberg Pharmaceuticals.
Patient Consent: Not applicable
IRB approval status: Not applicable
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Note: This work is not under consideration at any other journal and has not been previously presented.
References
- 1.Hotez PJ, Alvarado M, Basanez MG et al. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis 2014; 8:e2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gadre A, Enbiale W, Andersen LK, Coates SJ. The effects of climate change on fungal diseases with cutaneous manifestations: a report from the International Society of Dermatology Climate Change Committee. J Clim Change Health. 2022;6: 100156. [Google Scholar]
- 3.Lafferty KD. The Ecology of Climate Change and Infectious Diseases. Vol. 90, Source: Ecology. 2009. [DOI] [PubMed] [Google Scholar]
- 4.Thompson GR 3rd, Le T, Chindamporn A, Kauffman CA, Alastruey-Izquierdo A, Ampel NM, Andes DR, Armstrong-James D, Ayanlowo O, Baddley JW, Barker BM, Lopes Bezerra L, Buitrago MJ, Chamani-Tabriz L, Chan JFW, Chayakulkeeree M, Cornely OA, Cunwei C, Gangneux JP, Govender NP, Hagen F, Hedayati MT, Hohl TM, Jouvion G, Kenyon C, Kibbler CC, Klimko N, Kong DCM, Krause R, Lee Lee L, Meintjes G, Miceli MH, Rath PM, Spec A, Queiroz-Telles F, Variava E, Verweij PE, Schwartz IS, Pasqualotto AC. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis 2021. Dec;21(12):e364–e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonifaz A, Robles-Tenorio A, Tirado-Sánchez A (2022). Climate Change Impact on Chromoblastomycosis. In: Frías-De-León MG, Brunner-Mendoza C, Reyes-Montes M.d.R., Duarte-Escalante E (eds) The Impact of Climate Change on Fungal Diseases. Fungal Biology. Springer, Cham. [Google Scholar]
- 6.Fahal AH, Ahmed KO, Saeed AA, Elkhawad AO, Bakhiet SM. Why the mycetoma patients are still neglected. PLoS Negl Trop Dis 2022. Dec 29;16(12):e0010945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos DWCL, de Azevedo CMPES, Vicente VA, Queiroz-Telles F, Rodrigues AM, de Hoog GS, Denning DW, Colombo AL. The global burden of chromoblastomycosis. PLoS Negl Trop Dis 2021. Aug 12;15(8):e0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn RC, Hagen F, Mendes RP, Burger E, Nery AF, Siqueira NP, Guevara A, Rodrigues AM, de Camargo ZP. Paracoccidioidomycosis: Current Status and Future Trends. Clin Microbiol Rev 2022. Dec 21;35(4):e0023321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold JA, Derado G, Mody RK, Benedict K. Sporotrichosis-Associated Hospitalizations, United States, 2000–2013. Emerg Infect Dis 2016. Oct;22(10):1817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonçalves FG, Rosa PS, Belone AFF, Carneiro LB, de Barros VLQ, Bispo RF, Sbardelott YADS, Neves SAVM, Vittor AY, Woods WJ, Laporta GZ. Lobomycosis Epidemiology and Management: The Quest for a Cure for the Most Neglected of Neglected Tropical Diseases. J Fungi (Basel). 2022. May 10;8(5):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith DJ, Gold JAW, Benedict K, Wu K, Lyman M, Jordan A, Medina N, Lockhart SR, Sexton DJ, Chow NA, et al. Public Health Research Priorities for Fungal Diseases: A Multidisciplinary Approach to Save Lives. Journal of Fungi. 2023; 9(8):820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J Assessing global fungal threats to humans. mLife. 2022; 1: 223–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold JAW, Smith DJ, Benedict K, Lockhart SR, Lipner SR. Epidemiology of implantation mycoses in the United States: An analysis of commercial insurance claims data, 2017 to 2021. J Am Acad Dermatol 2023. Aug;89(2):427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed AO, van Leeuwen W, Fahal A, van de Sande W, Verbrugh H, van Belkum A. Mycetoma caused by Madurella mycetomatis: a neglected infectious burden. Lancet Infect Dis 2004. Sep;4(9):566–74. [DOI] [PubMed] [Google Scholar]
- 15.Santona A, Mhmoud NA, Siddig EE, Deligios M, Fiamma M, Paglietti B, Bakhiet SM, Rubino S, Fahal AH. Metagenomic detection of eumycetoma causative agents from households of patients residing in two Sudanese endemic villages in White Nile State. PLoS Negl Trop Dis 2022. Aug 30;16(8):e0010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hay R, Denning DW, Bonifaz A, Queiroz-Telles F, Beer K, Bustamante B, Chakrabarti A, Chavez-Lopez MG, Chiller T, Cornet M, Estrada R, Estrada-Chavez G, Fahal A, Gomez BL, Li R, Mahabeer Y, Mosam A, Soavina Ramarozatovo L, Rakoto Andrianarivelo M, Rapelanoro Rabenja F, van de Sande W, Zijlstra EE. The Diagnosis of Fungal Neglected Tropical Diseases (Fungal NTDs) and the Role of Investigation and Laboratory Tests: An Expert Consensus Report. Trop Med Infect Dis 2019. Sep 24;4(4):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hay RJ, Fahal AH. Mycetoma: an old and still neglected tropical disease. Trans R Soc Trop Med Hyg 2015. Mar;109(3):169–70. [DOI] [PubMed] [Google Scholar]
- 18.Sow D, Ndiaye M, Sarr L, Kanté MD, Ly F, Dioussé P, et al. Mycetoma epidemiology, diagnosis management, and outcome in three hospital centres in Senegal from 2008 to 2018. PLoS One. 2020;15(4):e0231871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubey N, Capoor MR, Hasan AS, Gupta A, Ramesh V, Sharma S, et al. Epidemiological profile and spectrum of neglected tropical disease eumycetoma from Delhi, North India. Epidemiol Infect 2019;147:e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López-Martínez R, Méndez-Tovar LJ, Bonifaz A, Arenas R, Mayorga J, Welsh O, et al. Actualización de la epidemiología del micetoma en México. Revisión de 3,933 casos. Gac Med Mex 2013;149(5):586–92. [PubMed] [Google Scholar]
- 21.Emery D, Denning DW. The global distribution of actinomycetoma and eumycetoma. PLoS Negl Trop Dis 2020;14(9):e0008397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reis CMS, Reis-Filho EGM. Mycetomas: An epidemiological, etiological, clinical, laboratory and therapeutic review. An Bras Dermatol 2018;93:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emery D, Denning DW. The global distribution of actinomycetoma and eumycetoma. PLoS Negl Trop Dis 2020. Sep 24;14(9):e0008397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Southern PM Jr. Mycetoma due to Madurella grisea acquired in Mexico. Trop Doct 1996. Oct;26(4):187–8. [DOI] [PubMed] [Google Scholar]
- 25.Montes LF, Freeman RG, McClarin W. Maduromycosis due to Madurella grisea. Report of the fifth North American case. Arch Dermatol 1969. Jan;99(1):74–9. [PubMed] [Google Scholar]
- 26.Standish SN, Goldstein W, Stuart CR. Pedal Fungal Mass in the Midwest. J Am Podiatr Med Assoc 2018. Jul;108(4):334–339. [DOI] [PubMed] [Google Scholar]
- 27.Husain U, Verma P, Suvirya S, Priyadarshi K, Gupta P. An overview of mycetoma and its diagnostic dilemma: Time to move on to advanced techniques. Indian J Dermatol Venereol Leprol 2023. Jan-Frebuary;89(1):12–17. [DOI] [PubMed] [Google Scholar]
- 28.Nenoff P, van de Sande WW, Fahal AH, Reinel D, Schöfer H. Eumycetoma and actinomycetoma--an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol 2015. Oct;29(10):1873–83. [DOI] [PubMed] [Google Scholar]
- 29.Fahal AH, el Hassan AM, Abdelalla AO, Sheik HE Cystic mycetoma: An unusual clinical presentation of Madurella mycetomatis infection. Trans. R. Soc. Trop. Med. Hyg 1998;92:66–67. [DOI] [PubMed] [Google Scholar]
- 30.Fahal AH, Suliman SH, Hay R Mycetoma: The Spectrum of Clinical Presentation. Trop. Med. Infect. Dis 2018;3:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao X, Cognetti M, Burch-Smith R, Mejia EO, Mirkin G. Mycetoma: Development of Diagnosis and Treatment. J Fungi (Basel). 2022. Jul 19;8(7):743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verma P, Jha A. Mycetoma: reviewing a neglected disease. Clin Exp Dermatol 2019. Mar;44(2):123–129. [DOI] [PubMed] [Google Scholar]
- 33.Yadav T, Meena VK, Shaikh M, Khera S, Sureka B, Garg P, Khera PS. Clinico-radiological-pathological correlation in eumycetoma spectrum: Case series. North Clin Istanb 2019. Jul 12;7(4):400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fahal AH, Sheik HE, Homeida MM, Arabi YE, Mahgoub ES. Ultrasonographic imaging of mycetoma. Br J Surg 1997. Aug;84(8):1120–2. [PubMed] [Google Scholar]
- 35.Siddig EE, Nyuykonge B, Mhmoud NA, Abdallah OB, Bahar MEN, Ahmed ES, Nyaoke B, Zijlstra EE, Verbon A, Bakhiet SM, Fahal AH, van de Sande WWJ. Comparing the performance of the common used eumycetoma diagnostic tests. Mycoses. 2023. May;66(5):420–429. [DOI] [PubMed] [Google Scholar]
- 36.Siddig EE, Mhmoud NA, Bakhiet SM, Abdallah OB, Mekki SO, El Dawi NI, Van de Sande W, Fahal AH. The Accuracy of Histopathological and Cytopathological Techniques in the Identification of the Mycetoma Causative Agents. PLoS Negl Trop Dis 2019. Aug 29;13(8):e0007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bahar ME, Bakheet OELH, Fahal AH. Mycetoma imaging: the best practice. Trans R Soc Trop Med Hyg 2021. Apr 14;115(4):387–396. [DOI] [PubMed] [Google Scholar]
- 38.El Shamy ME, Fahal AH, Shakir MY, Homeida MM. New MRI grading system for the diagnosis and management of mycetoma. Trans R Soc Trop Med Hyg 2012. Dec;106(12):738–42. [DOI] [PubMed] [Google Scholar]
- 39.Laohawiriyakamol T, Tanutit P, Kanjanapradit K, Hongsakul K, Ehara S. The “dot-in-circle” sign in musculoskeletal mycetoma on magnetic resonance imaging and ultrasonography. Springerplus. 2014. Nov 13;3:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Queiróz AJR, Pereira Domingos F, Antônio JR. Chromoblastomycosis: clinical experience and review of literature. Int J Dermatol 2018. Nov;57(11):1351–1355. [DOI] [PubMed] [Google Scholar]
- 41.Rosen T, Joseph LM. Chromoblastomycosis. Int J Dermatol 1979. Apr;18(3):226–7. [DOI] [PubMed] [Google Scholar]
- 42.Rolon AM, Tolaymat LM, Sokumbi O, Bodiford K. The Role of Excision for Treatment of Chromoblastomycosis: A Cutaneous Fungal Infection Frequently Mistaken for Squamous Cell Carcinoma. Dermatol Surg 2023. Jul 1;49(7):649–653. [DOI] [PubMed] [Google Scholar]
- 43.Gajurel K, Ahrens WA. Medlar bodies of chromoblastomycosis. Transpl Infect Dis 2023. Jun;25(3):e14047. [DOI] [PubMed] [Google Scholar]
- 44.Passero LFD, Cavallone IN, Belda W Jr. Reviewing the Etiologic Agents, Microbe-Host Relationship, Immune Response, Diagnosis, and Treatment in Chromoblastomycosis. J Immunol Res 2021. Nov 1;2021:9742832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosen T, Overholt M. Persistent viability of the Medlar body. Int J Dermatol 1996. Feb;35(2):96–8. [DOI] [PubMed] [Google Scholar]
- 46.Santos DWCL, Vicente VA, Weiss VA, de Hoog GS, Gomes RR, Batista EMM, Marques SG, Queiroz-Telles F, Colombo AL, Azevedo CMPES. Chromoblastomycosis in an Endemic Area of Brazil: A Clinical-Epidemiological Analysis and a Worldwide Haplotype Network. J Fungi (Basel). 2020. Oct 3;6(4):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peçanha PM, Peçanha-Pietrobom PM, Grão-Velloso TR, Rosa Júnior M, Falqueto A, Gonçalves SS. Paracoccidioidomycosis: What We Know and What Is New in Epidemiology, Diagnosis, and Treatment. J Fungi (Basel). 2022. Oct 18;8(10):1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivitti EA, Aoki V: Deep fungal infections in tropical countries. Clin Dermatol 1999; 17: pp. 171–190. [DOI] [PubMed] [Google Scholar]
- 49.Pacheco FB, Venier NAB, Bueno AL, Almeida AL, Milman L, Koehler A, Pagani DM, Scroferneker ML. Isolated cutaneous lesions in paracoccidioidomycosis: a suggestive case of acquisition through cutaneous inoculation. Rev Inst Med Trop Sao Paulo 2021. Mar 24;63:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendes RP, Cavalcante RS, Marques SA, Marques MEA, Venturini J, Sylvestre TF, Paniago AMM, Pereira AC, da Silva JF, Fabro AT, Bosco SMG, Bagagli E, Hahn RC, Levorato AD. Paracoccidioidomycosis: Current Perspectives from Brazil. Open Microbiol J 2017. Oct 31;11:224–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shikanai-Yasuda MA, Mendes RP, Colombo AL, Queiroz-Telles F, Kono ASG, Paniago AMM, Nathan A, Valle ACFD, Bagagli E, Benard G, Ferreira MS, Teixeira MM, Silva-Vergara ML, Pereira RM, Cavalcante RS, Hahn R, Durlacher RR, Khoury Z, Camargo ZP, Moretti ML, Martinez R. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev Soc Bras Med Trop 2017. Sep-Oct;50(5):715–740. [DOI] [PubMed] [Google Scholar]
- 52.Bellíssimo-Rodrigues F, Machado AA, Martinez R Paracoccidioidomycosis epidemiological features of a 1000-cases series from a hyperendemic area on the Southeast of Brazil. Am. J. Trop. Med. Hyg 2011;85:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coimbra Junior CF, Wanke B, Santos RV, do Valle AC, Costa RL, Zancopé-Oliveira RM Paracoccidioidin and histoplasmin sensitivy in Tupi-Mondé Ameridian populations from Brazilian Amazonia. Ann. Trop. Med. Parasitol 1994;88:197–207. [DOI] [PubMed] [Google Scholar]
- 54.Rodrigues Gda S, Severo CB, Oliveira Fde M, Moreira Jda S, Prolla JC, Severo LC Association between paracoccidioidomycosis and cancer. J. Bras. Pneumol 2010;36:356–362. [DOI] [PubMed] [Google Scholar]
- 55.Dos Santos WA, da Silva BM, Passos ED, Zandonade E, Falquete A Associação entre tabagismo e paracoccidioidomicose: Um estudo de caso-controle no Estado do Espírito Santo, Brasil. Cad. Saude Publica 2003;19:245–253. [DOI] [PubMed] [Google Scholar]
- 56.Martinez R New Trends in Paracoccidioidomycosis Epidemiology. J Fungi (Basel). 2017. Jan 3;3(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vieira Gde D, Alves Tda C, Lima SM, Camargo LM, Sousa CM Paracoccidioidomycosis in a western Brazilian Amazon State: Clinical-epidemiologic profile and spatial distribution of the disease. Rev. Soc. Bras. Med. Trop 2014;47:63–68. [DOI] [PubMed] [Google Scholar]
- 58.Barrozo LV, Benard G, Silva ME, Bagagli E, Marques SA, Mendes RP First description of a cluster of acute/subacute paracoccidioidomycosis cases and its association with a climatic anomaly. PLoS Negl. Trop. Dis 2010;4:e643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arantes TD, Theodoro RC, Teixeira Mde M, Bosco Sde M, Bagagli E Environmental Mapping of Paracoccidioides spp. in Brazil Reveals New Clues into Genetic Diversity, Biogeography and Wild Host Association. PLoS Negl. Trop. Dis 2016;10:e0004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.do Valle ACF, Marques de Macedo P, Almeida-Paes R, Romão AR, Lazéra MDS, Wanke B. Paracoccidioidomycosis after Highway Construction, Rio de Janeiro, Brazil. Emerg Infect Dis 2017. Nov;23(11):1917–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramos-e-Silva M: Facial and oral aspects of some venereal and tropical diseases. Acta Dermatovenereol Croat 2004; 12: pp. 173–180. [PubMed] [Google Scholar]
- 62.Dutra LM; Silva THM; Falqueto A; Pecanha PM; Souza LRM; Goncalves SS; Velloso TRG Oral paracoccidioidomycosis in a single-center retrospective analysis from a Brazilian southeastern population. J. Infect. Public Health 2018, 11, 530–533. [DOI] [PubMed] [Google Scholar]
- 63.Ramos-E-Silva M, Saraiva Ldo E. Paracoccidioidomycosis. Dermatol Clin 2008. Apr;26(2):257–69, vii. [DOI] [PubMed] [Google Scholar]
- 64.Cárcano CBM, Tanaka VD’, Alessi C, Reis MT, Ferreira MS. Paracoccidioidomycosis with sarcoid-like cutaneous lesion: A clinicopathological challenge. IDCases. 2022. Jul 18;29:e01574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romaneli MTDN, Tardelli NR, Tresoldi AT, Morcillo AM, Pereira RM. Acute-subacute paracoccidioidomycosis: A paediatric cohort of 141 patients, exploring clinical characteristics, laboratorial analysis and developing a non-survival predictor. Mycoses. 2019. Nov;62(11):999–1005. [DOI] [PubMed] [Google Scholar]
- 66.Pinheiro BG, Hahn RC, Camargo ZP, Rodrigues AM. Molecular Tools for Detection and Identification of Paracoccidioides Species: Current Status and Future Perspectives. J Fungi (Basel). 2020. Nov 18;6(4):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Queiroz-Telles F; Escuissato DL Pulmonary paracoccidioidomycosis. Semin. Respir. Crit. Care Med 2011, 32, 764–774. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Hagen F, Stielow B, Rodrigues AM, Samerpitak K, Zhou X, et al. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14,000 human and animal case reports. Persoonia. 2015;35:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moussa TAA, Kadasa NMS, Al Zahrani HS, Ahmed SA, Feng P, Gerrits van den Ende AHG, et al. Origin and distribution of Sporothrix globosa causing sapronoses in Asia. J Med Microbiol 2017;66:560–569. [DOI] [PubMed] [Google Scholar]
- 70.Rodrigues AM, de Melo Teixeira M, de Hoog GS, Schubach TM, Pereira SA, Fernandes GF, et al. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl Trop Dis 2013;7:e2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodrigues AM, de Hoog G, Zhang Y, de Camargo ZP. Emerging sporotrichosis is driven by clonal and recombinant Sporothrix species. Emerg Microbes Infect 2014;3:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Andrade Galliano Daros Bastos F, Raimundo Cognialli RC, Rodrigues de Farias M, Dos Santos Monti F, Wu K, Queiroz-Telles F. Spread of Sporothrix spp. through respiratory droplets from infected cats: A potential route of transmission. Med Mycol 2022. Nov 12;60(11):myac079. [DOI] [PubMed] [Google Scholar]
- 73.Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S Global epidemiology of sporotrichosis. Med Mycol 2015;53:3–14. [DOI] [PubMed] [Google Scholar]
- 74.Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto AC. Neglected endemic mycoses. Lancet Infect Dis 2017. Nov;17(11):e367–e377. [DOI] [PubMed] [Google Scholar]
- 75.Xavier JRB, Waller SB, Osório LDG, Vives PS, Albano APN, Aguiar ESV, Ferreira MRA, Conceição FRD, Faria RO, Meireles MCA, Gomes ADR. Human sporotrichosis outbreak caused by Sporothrix brasiliensis in a veterinary hospital in Southern Brazil. J Mycol Med 2021. Sep;31(3):101163. [DOI] [PubMed] [Google Scholar]
- 76.de Oliveira Bento A, de Sena Costa AS, Lima SL, do Monte Alves M, de Azevedo Melo AS, Rodrigues AM, da Silva-Rocha WP, Milan EP, Chaves GM. The spread of cat-transmitted sporotrichosis due to Sporothrix brasiliensis in Brazil towards the Northeast region. PLoS Negl Trop Dis 2021. Aug 30;15(8):e0009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Queiroz-Telles F, McGinnis MR, Salkin I, Graybill JR. Subcutaneous mycoses. Infect Dis Clin North Am 2003; 17: 59–85 [DOI] [PubMed] [Google Scholar]
- 78.Rojas FD, Fernández MS, Lucchelli JM, Lombardi D, Malet J, Vetrisano ME, Cattana ME, Sosa MLÁ, Giusiano G. Cavitary Pulmonary Sporotrichosis: Case Report and Literature Review. Mycopathologia. 2017. Dec;182(11–12):1119–1123. [DOI] [PubMed] [Google Scholar]
- 79.Rodrigues AM, Della Terra PP, Gremião ID, Pereira SA, Orofino-Costa R, de Camargo ZP. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 2020. Oct;185(5):813–842. [DOI] [PubMed] [Google Scholar]
- 80.Rodrigues AM; Orofino-Costa R; de Camargo ZP Sporothrix spp. In Pocket Guide to Mycological Diagnosis, 1st ed.; Cordeiro Rde A, Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 99–113. [Google Scholar]
- 81.Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and Sporotrichosis. Clin Microbiol Rev 2011. Oct;24(4):633–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodrigues AM; de Hoog GS; de Camargo ZP Feline Sporotrichosis. In Emerging and Epizootic Fungal Infections in Animals; Seyedmousavi S, de Hoog GS, Guillot J, Verweij PE, Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 1, pp. 199–231. [Google Scholar]
- 83.Kauffman CA, Bustamante B, Chapman SW, Pappas PG; Infectious Diseases Society of America. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007. Nov 15;45(10):1255–65. [DOI] [PubMed] [Google Scholar]
- 84.Zhang M, Li F, Li R, Gong J, Zhao F. Fast diagnosis of sporotrichosis caused by Sporothrix globosa, Sporothrix schenckii, and Sporothrix brasiliensis based on multiplex real-time PCR. PLoS Negl Trop Dis 2019. Feb 28;13(2):e0007219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Samaddar A, Sharma A. Emergomycosis, an emerging systemic mycosis in immunocompromised patients: current trends and future prospects. Front. Med 2021;8:670731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwartz IS, Govender NP, Sigler L, Jiang Y, Maphanga TG, Toplis B, Botha A, Dukik K, Hoving JC, Muñoz JF, de Hoog S, Cuomo CA, Colebunders R, Kenyon C. Emergomyces: The global rise of new dimorphic fungal pathogens. PLoS Pathog 2019. Sep 19;15(9):e1007977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spallone A, Schwartz IS. Emerging Fungal Infections. Infect Dis Clin N Am 2021;35:261–77. [DOI] [PubMed] [Google Scholar]
- 88.Schwartz IS, Maphanga TG & Govender NP Emergomyces: a New Genus of Dimorphic Fungal Pathogens Causing Disseminated Disease among Immunocompromised Persons Globally. Curr Fungal Infect Rep 12, 44–50 (2018). [Google Scholar]
- 89.Gast KB, van der Hoeven A, de Boer MGJ, van Esser JWJ, Kuijper EJ, Verweij JJ, et al. Two cases of Emergomyces pasteurianus infection in immunocompromised patients in the Netherlands. Med Mycol Case Rep 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gori S, Drouhet E. Cutaneous disseminated mycosis in a patient with AIDS due to a new dimorphic fungus. J Mycol Med 1998;8: 57–63 [Google Scholar]
- 91.Pelegrín I, Alastruey-Izquierdo A, Ayats J, Cuenca-Estrella M, Cabellos C. A second look at Emmonsia infection can make the difference. Transpl Infect Dis 2014;0:1–2. [DOI] [PubMed] [Google Scholar]
- 92.Lavergne R-A, Kandel-Aznar C, Khatchatourian L, Garcia-Hermoso D, Jeddi F, Boutoille D, et al. Emmonsia pasteuriana: une cause rare d’infection fongique chez l’immunodéprimé. J Mycol Med 2017;27(3):e7–8. [Google Scholar]
- 93.Malik R, Capoor MR, Vanidassane I, Gogna A, Singh A, Sen B, et al. Disseminated Emmonsia pasteuriana infection in India: a case report and a review. Mycoses. 2016;59(2):127–32. [DOI] [PubMed] [Google Scholar]
- 94.Feng P, Yin S, Zhu G, Li M, Wu B, Xie Y, et al. Disseminated infection caused by Emmonsia pasteuriana in a renal transplant recipient. J Dermatol 2015;42(12):1179–82. [DOI] [PubMed] [Google Scholar]
- 95.Tang XH, Zhou H, Zhang XQ, De Han J, Gao Q. Cutaneous disseminated emmonsiosis due to Emmonsia pasteuriana in a patient with cytomegalovirus enteritis. JAMA Dermatology. 2015;151(11):1263–4. [DOI] [PubMed] [Google Scholar]
- 96.Wilmes D, Rooms I, Heidemarie L, McCormick Smith I, Haase G, Rickerts V. Emergomycosis: Case report of a disseminated Emergomyces pasteurianus infection and review of an emerging fungal infection. 52nd Scientific Conference of the German speaking Mycological Society (DMykG) together with the Austrian Society for Medical Mycology (ÖGMM), 6 September– 8 September 2018; 6–8 September, 2018; Innsbruck, Austria: Mycoses; 2018. [Google Scholar]
- 97.Maphanga TG, Britz E, Zulu TG, MR S, Naicker SD, Schwartz IS, et al. In vitro antifungal susceptibility of the yeast- and mould-phases of the dimorphic fungal pathogen, Emergomyces africanus (formerly Emmonsia species), from HIV-infected south African patients. J Clin Microbiol 2017;55(6):1812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wellinghausen N, Kern WV, Haase G, Rozdzinski E, Kern P, Marre R, et al. Chronic granulomatous lung infection caused by the dimorphic fungus Emmonsia sp. Int J Med Microbiol 2003;293(6):441–5. [DOI] [PubMed] [Google Scholar]
- 99.Wang P, Kenyon C, de Hoog S, Guo L, Fan H, Liu H, et al. A novel dimorphic pathogen, Emergomyces orientalis (Onygenales), agent of disseminated infection. Mycoses. 2017;60(5):310–9. [DOI] [PubMed] [Google Scholar]
- 100.Thompson GR, Le T, Chindamporn A, Kauffman CA, Alastruey-Izquierdo A, Ampel NM, et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwartz IS, Govender NP, Sigler L, Jiang Y, Maphanga TG, Toplis B, et al. Emergomyces: the global rise of new dimorphic fungal pathogens. PLoS Pathog 2019;15 (9):e1007977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gunaydin SD, Arikan-Akdagli S, Akova M. Fungal infections of the skin and soft tissue. Curr Opin Infect Dis 2020;33:130–6 [DOI] [PubMed] [Google Scholar]
- 103.Ibe C, Mnyambwa NP, Mfinanga SG. Emergomycosis in Africa: Time to Pay Attention to This Emerging Deadly Fungal Infection. Int J Gen Med 2023. Jun 7;16:2313–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schwartz IS, Sanche S, Wiederhold NP, Patterson TF, Sigler L. Emergomyces canadensis, a dimorphic fungus causing fatal systemic human disease in North America. Emerg Infect Dis 2018;24(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwartz IS, Govender NP, Corcoran C, Dlamini S, Prozesky H, Burton R, et al. Clinical characteristics, diagnosis, management, and outcomes of disseminated emmonsiosis: a retrospective case series. Clin Infect Dis 2015;61(6):1004–12. [DOI] [PubMed] [Google Scholar]
- 106.Govender NP, Grayson W. Emergomycosis (Emergomyces africanus) in advanced HIV disease. Dermatopathology 2019;6:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rooms I, Mugisha P, Gambichler T, Hadaschik E, Esser S, Rath PM, et al. Disseminated emergomycosis in a person with HIV infection, Uganda. Emerg Infect Dis 2019;25(9):1750–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schwartz IS, Kenyon C, Lehloenya R, Claasens S, Spengane Z, Prozesky H, Burton R, Parker A, Wasserman S, Meintjes G, Mendelson M, Taljaard J, Schneider JW, Beylis N, Maloba B, Govender NP, Colebunders R, Dlamini S. AIDS-Related Endemic Mycoses in Western Cape, South Africa, and Clinical Mimics: A Cross-Sectional Study of Adults With Advanced HIV and Recent-Onset, Widespread Skin Lesions. Open Forum Infect Dis 2017. Aug 25;4(4):ofx186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kenyon C, Bonorchis K, Corcoran C, Meintjes G, Locketz M, Lehloenya R, et al. A dimorphic fungus causing disseminated infection in South Africa. N Engl J Med 2013;369:1416–24. [DOI] [PubMed] [Google Scholar]
- 110.Vanittanakom N; Cooper CR Jr.; Fisher MC; Sirisanthana T Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin. Microbiol. Rev 2006, 19, 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Narayanasamy S, Dat VQ, Thanh NT, Ly VT, Chan JF, Yuen KY, Ning C, Liang H, Li L, Chowdhary A, Youngchim S, Supparatpinyo K, Aung NM, Hanson J, Andrianopoulos A, Dougherty J, Govender NP, Denning DW, Chiller T, Thwaites G, van Doorn HR, Perfect J, Le T. A global call for talaromycosis to be recognised as a neglected tropical disease. Lancet Glob Health. 2021. Nov;9(11):e1618–e1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Centers for Diseases Control and Prevention. Talaromycosis (Formerly Penicilliosis). Available online: https://www.cdc.gov/fungal/diseases/other/talaromycosis.html (accessed on 12 August 2023).
- 113.Ning CWWXB, Thanh NT, Ye Li, Liang H, Le T. The global distribution, drivers, and burden of talaromycosis 1964–2018. Conference of Retrovirus and Opportunistic Infections; 2020; Boston, MA, USA; 2020. [Google Scholar]
- 114.Chan JF; Lau SK; Yuen KY; Woo PC Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg. Microbes Infect 2016, 5, e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Supparatpinyo K; Khamwan C; Baosoung V; Nelson KE; Sirisanthana T Disseminated Penicillium marneffei infection in southeast Asia. Lancet 1994, 344, 110–113. [DOI] [PubMed] [Google Scholar]
- 116.Vanittanakom N; Cooper CR Jr.; Fisher MC; Sirisanthana T Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin. Microbiol. Rev 2006, 19, 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Narayanasamy S; Dat VQ; Thanh NT; Ly VT; Chan JF; Yuen KY; Ning C; Liang H; Li L; Chowdhary A; et al. A global call for talaromycosis to be recognised as a neglected tropical disease. Lancet Glob. Health 2021, 9, e1618–e1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Le T, Wolbers M, Chi NH, et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis 2011; 52(7): 945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect 2016; 5(3): e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang JQ, Yang ML, Zhong XN, et al. A comparative analysis of the clinical and laboratory characteristics in disseminated penicilliosis marneffei in patients with and without human immunodeficiency virus infection. Chinese Journal of Tuberculosis and Respiratory Diseases 2008; 31(10): 740–6. [PubMed] [Google Scholar]
- 121.Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis 2013; 13: 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis 2013. Oct 5;13:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pruksaphon K, Intaramat A, Ratanabanangkoon K, Nosanchuk JD, Vanittanakom N, Youngchim S Diagnostic laboratory immunology for talaromycosis (penicilliosis): Review from the bench-top techniques to the point-of-care testing. Diagn. Microbiol. Infect. Dis 2020;96:114959. [DOI] [PubMed] [Google Scholar]
- 124.Qin L, Zhao L, Tan C, Chen XU, Yang Z, Mo W A novel method of combining Periodic Acid Schiff staining with Wright-Giemsa staining to identify the pathogens Penicillium marneffei, Histoplasma capsulatum, Mucor and Leishmania donovani in bone marrow smears. Exp. Ther. Med 2015;9:1950–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vanittanakom N, Cooper CR Jr., Fisher MC, Sirisanthana T Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin. Microbiol. Rev 2006;19:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zaongo SD, Zhang F, Chen Y. An Overview of Diagnostic and Management Strategies for Talaromycosis, an Underrated Disease. J Fungi (Basel). 2023. Jun 6;9(6):647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pruksaphon K, Intaramat A, Simsiriwong P, Mongkolsuk S, Ratanabanangkoon K, Nosanchuk JD, Kaltsas A, Youngchim S. An inexpensive point-of-care immunochromatographic test for Talaromyces marneffei infection based on the yeast phase specific monoclonal antibody 4D1 and Galanthus nivalis agglutinin. PLoS Negl Trop Dis 2021. May 4;15(5):e0009058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Brito AC; Quaresma JAS Lacaziose (doença de Jorge Lobo): Revisão e atualização. An. Bras. Dermatol 2007, 82, 461–474. [Google Scholar]
- 129.Vilela R, Huebner M, Vilela C, Vilela G, Pettersen B, Oliveira C, Mendoza L. The taxonomy of two uncultivated fungal mammalian pathogens is revealed through phylogeny and population genetic analyses. Sci Rep 2021. Sep 13;11(1):18119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gonçalves FG (Serviço Estadual de Dermatologia do Acre, Programa Estadual de Dermatologia do Acre, Programa Estadual de Controle da Hanseníase, SESACRE/FUNDHACRE, Rio Branco, Acre, Brazil). Personal communication, 2022. [Google Scholar]
- 131.Francesconi VA, Klein AP, Santos AP, Ramasawmy R, Francesconi F. Lobomycosis: epidemiology, clinical presentation, and management options. Ther Clin Risk Manag 2014. Oct 9;10:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de Brito AC; Quaresma JAS Lacaziose (doença de Jorge Lobo): Revisão e atualização. An. Bras. Dermatol 2007, 82, 461–474. [Google Scholar]
- 133.Silva AR; Pinheiro GS; Matos WB; Couto MJA; Gonçalves EGR Doença de Jorge Lobo: Primeiro caso registrado no Estado do Maranhão. Cad. Pesqui 2013, 20, 64–67. [Google Scholar]
- 134.Burns RA; Roy JS; Woods C; Padhye AA; Warnock DW Report of the first human case of Lobomycosis in United States. J. Clin. Microb 2000, 38, 1283–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Elsayed S, Kuhn SM, Barber D, Church DL, Adams S, Kasper R. Human case of lobomycosis. Emerg Infect Dis 2004. Apr;10(4):715–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pradinaud R Entre le Yucatan, la Floride et la Guyane Française, la lobomycose existe-t-elle aux Antilles. Bull. Société Pathol. Exot 1984, 77, 392–400. [PubMed] [Google Scholar]
- 137.Vilela R; Mendoza L; Rosa PS; Belone AF; Madeira S; Opromolla DV; Resende MA Molecular model for studying the uncultivated fungal pathogen Lacazia loboi. J. Clin. Microbiol 2005, 43, 3657–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Woods WJ, Belone Ade F, Carneiro LB, Rosa PS. Ten years experience with Jorge Lobo’s disease in the state of Acre, Amazon region, Brazil. Rev Inst Med Trop Sao Paulo 2010. Sep-Oct;52(5):273–8. [DOI] [PubMed] [Google Scholar]
- 139.Talhari S, Talhari C. Lobomycosis. Clin Dermatol 2012. Jul-Aug;30(4):420–4. [DOI] [PubMed] [Google Scholar]
- 140.Opromolla DVA, Belone AFF, Taborda PRO, Taborda VBA Correlação clínico-patológica em 40 casos novos de lobomicose. An. Bras. Dermatol 2000;75:425–434. [Google Scholar]
- 141.Arteaga D, Tirado-Sánchez A, Vázquez-González D, Moreno LM, van de Sande W, Bonifaz A. Encapsulated eumycetoma caused by Biatriosporamackinnonii. Med Mycol Case Rep 2022. Sep 13;38:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dutra LM, Silva THM, Falqueto A, Peçanha PM, Souza LRM, Gonçalves SS, Velloso TRG. Oral paracoccidioidomycosis in a single-center retrospective analysis from a Brazilian southeastern population. J Infect Public Health. 2018. Jul-Aug;11(4):530–533. [DOI] [PubMed] [Google Scholar]
- 143.Valentín FO, Tsutsui GM, Abbade LPF, Marques SA. Disseminated paracoccidioidomycosis in a liver transplant patient. An Bras Dermatol 2021. May-Jun;96(3):346–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Orofino-Costa R, Freitas DFS, Bernardes-Engemann AR, Rodrigues AM, Talhari C, Ferraz CE, Veasey JV, Quintella L, Sousa MSLA, Vettorato R, Almeida-Paes R, de Macedo PM. Human sporotrichosis: recommendations from the Brazilian Society of Dermatology for the clinical, diagnostic and therapeutic management. An Bras Dermatol 2022. Nov-Dec;97(6):757–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reddy DL, Nel J, Govender NP. Review: Emergomycosis. J Mycol Med 2023. Mar;33(1):101313. Figure courtesy of Dr. Matilda Mphahlele (Division of Dermatology, Chris Hani Baragwanath Academic Hospital, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa). [DOI] [PubMed] [Google Scholar]
- 146.Xian J, Huang X, Li Q, Peng X, Peng X. Dermatoscopy for the rapid diagnosis of Talaromyces marneffei infection: a case report. BMC Infect Dis 2019. Aug 9;19(1):707. [DOI] [PMC free article] [PubMed] [Google Scholar]


