Abstract

Narcissus tazetta var. chinensis is a perennial monocot plant that is well known for its pharmaceutical and ornamental uses. This study aimed to understand the changes in the primary and secondary metabolites in different in vitro tissues of N. tazetta (callus, adventitious root, and shoot) using high-performance liquid chromatography and gas chromatography time-of-flight mass spectrometry. In addition, to optimize the most efficient in vitro culture methods for primary and secondary metabolite production, N. tazetta bulbs were used as explants and cultivated in Murashige and Skoog (MS) medium containing different hormones at various concentrations. In addition, the present study found suitable hormonal concentrations for callus, adventitious root, and shoot induction and analyzed the primary and secondary metabolites. The MS medium supplemented with 1.0 mg L–1 dicamba, 3.0 mg L–1 indole-3-butyric acid (IBA), and 3.0 mg L–1 6-benzylaminopurine (BAP) was the most efficient media for callus, adventitious root, and shoot induction in N. tazetta. The tissue induced in this medium was subjected to primary (amines, amino acids, organic acids, sugars, and sugar alcohols) and secondary metabolite (galantamine and phenolic acids) analysis. The shoots and roots showed the highest amounts of metabolites. This study showed that bulb in vitro culture can be an efficient micropropagation method for N. tazetta and the production of primary and secondary metabolites, offering implications for the mass production of primary and secondary metabolite compounds from N. tazetta tissues generated in vitro.
Introduction
Narcissus tazetta var. chinensis, a perennial monocot plant in the Amaryllidaceae family, is well-known for its pharmaceutical and ornamental uses.1,2 Amaryllidaceae alkaloids are produced by species of Amaryllidaceae, including Narcissus spp. (daffodil). Most of the alkaloids in Amaryllidaceae are known to have biological activity. All major structural classes of alkaloids found in Amaryllidaceae have biological activities.3,4 As a result, many alkaloids from Amaryllidaceae are used as medicines, and other alkaloids in the family have the potential to be used in pharmaceuticals.5
Secondary metabolites in plants include various compounds that are synthesized in numerous ways through precursors and in response to stimuli.6 The secondary metabolites in plants have various functions, such as attracting pollinators, repelling herbivores, and responding to environmental stress.7,8 Plants are an essential source for discovering novel bioactive compounds, and the metabolites found in plants are used as essential substances to make products for use in the pharmaceutical, cosmetic, and food industries.9,10 Plant cultivation is mainly used to harvest secondary metabolites from plants; however, the innovation of tissue culture in plant systems has opened another path for the collection of secondary metabolites.11
Galantamine is an alkaloid from the Amaryllidaceae family. It is a dibenzofuran-type base that has been found in several species of this family.12,13 Acetylcholinesterase inhibitor galantamine is a powerful drug used worldwide to treat the symptoms of Alzheimer-type senile dementia.14,15 The galantamine used in the medical field can be produced in two ways, total synthesis and extraction, using Amaryllidaceae plants.16,17 Phenylpropanoids comprise a broad group of organic compounds that are synthesized by a combination of the amino acids tyrosine and phenylalanine in plants.18,19 Previous studies have reported that phenylpropanoids have various positive benefits for human health (helps to prevent diabetes, cancer, and heart disease) and pharmaceutical sectors.20−22
According to Fazili et al.,23 tissue culture is a sustainable technique that is unaffected by a variety of environmental factors and adaptable to different growth parameters. Additionally, various culture systems, such as callus, shoot, root, hairy roots, and suspension cell culture, can be used to selectively produce bioactive compounds in plants on a laboratory scale.24,25 Thus, the identification of in vitro culture systems to produce bioactive substances suitable for commercial scale is crucial.26 Chen et al.27 optimized conditions for callus induction and plant regeneration from N. tazetta L. var. chinensis Roem anthers. In another study, callus cultures from infected commercial bulbs were obtained, although callus induction and bulblet regeneration in Narcissus remained difficult.28 To date, there has not been much study conducted on callus induction and plant regeneration in N. tazetta L. var. chinensis. In addition, to the best of our knowledge, no studies have analyzed the primary and secondary metabolites from different N. tazetta tissues generated in vitro.
N. tazetta bulb has various medicinal properties;29 however, the natural vegetative proliferation of Narcissus bulb is very slow, with an average yearly rate of daughter bulb production of 1.6.30 Thus, in this study, suitable protocols for callus, adventitious root, and shoot induction were optimized. In addition, we analyzed the primary and secondary metabolites from different N. tazetta tissues using gas chromatography-time-of-flight mass spectrometry (GC-TOFMS) and high-performance liquid chromatography (HPLC) to analyze the metabolites in different organs.
Results
N. tazetta Callus Establishment
In the first part of this study, various tissue inductions were conducted to investigate phenolic compounds, including galantamine. As a result, the optimal conditions for inducing calli, adventitious roots, and shoots in N. tazetta were identified (Figure 1). No callus was induced in the absence of auxin (control). Increasing concentrations of 2,4-D led to an increase in both induction efficiency and callus weight up to a certain point. At a 4.0 mg L–1 concentration, the induction efficiency reached 92%, and the callus weight was 0.34 g per callus. This indicates that 2,4-D is effective in promoting callus induction and growth in N. tazetta meristems. Dicamba also showed a trend similar to that of 2,4-D, with increasing concentrations leading to higher induction efficiency and initial callus weight. The highest induction efficiency (92%) and callus weight (0.39 g per callus) were observed with 1.0 mg L–1 dicamba. Comparing the two auxins, dicamba generally led to higher induction efficiency and callus weight compared with 2,4-D, especially at lower concentrations. However, at higher concentrations (4.0 mg L–1), the efficiency decreased for dicamba, indicating a possible inhibitory effect at this concentration (Table 1 and Figure 1).
Figure 1.
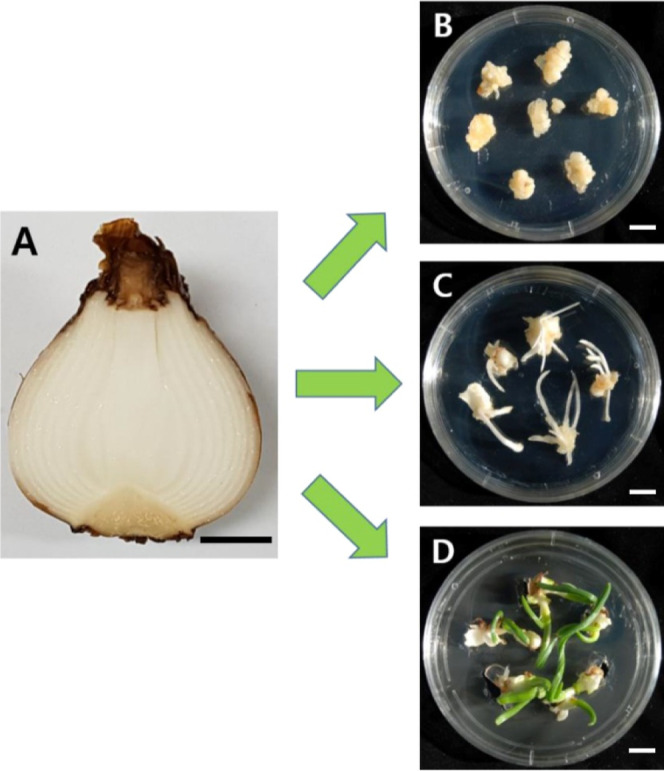
Establishment of callus, adventitious root, and shoot culture of N. tazetta using meristems in disinfected bulbs. (A) N. tazetta bulb, (B) N. tazetta callus induced on MS agar containing auxin (dicamba 1.0 mg L–1), (C) N. tazetta adventitious root induced on MS agar containing auxin (IBA 3.0 mg L–1), and (D) N. tazetta shoot induced on MS agar containing cytokinin (BAP 3.0 mg L–1). The scale bar indicates 1 cm.
Table 1. Effect of Different Auxin Concentrations on Callus Induction from N. tazetta Meristems after 6 Weeks on MS Mediuma.
| auxin (mg L–1) | induction efficiency (%) | callus weight (FW g/callus) |
|---|---|---|
| control 0 | 0 | 0 |
| 2,4-D 0.5 | 71 | 0.27 ± 0.01e |
| 2,4-D 1.0 | 85 | 0.32 ± 0.01d |
| 2,4-D 2.0 | 88 | 0.35 ± 0.01bc |
| 2,4-D 4.0 | 92 | 0.34 ± 0.01c |
| dicamba 0.5 | 79 | 0.34 ± 0.01c |
| dicamba 1.0 | 92 | 0.39 ± 0.01a |
| dicamba 2.0 | 90 | 0.36 ± 0.01b |
| dicamba 4.0 | 77 | 0.28 ± 0.01e |
Different alphabetical letters a–e denote significant differences (p < 0.05).
Adventitious Root Establishment in N. tazetta
Auxin was not present in the control, which resulted in a 0% induction efficiency and no root production. Increasing the IAA concentrations led to an increase in both the induction efficiency and the number of roots per explant. Additionally, root length generally increased with increasing IAA concentrations, reaching a maximum number of roots per explant at 5.0 mg L–1 of IAA. Similar to IAA, higher IBA concentrations resulted in higher induction efficiency and an increased number of roots per explant. However, the trend in root length varied, with the maximum length observed at 3.0 mg L–1 rather than 5.0 mg L–1 after IBA treatment. NAA showed a different trend compared to IAA and IBA. While 1.0 and 3.0 mg L–1 concentrations led to a relatively high induction efficiency and root number, the highest concentration (5.0 mg L–1) resulted in no adventitious root induction. IAA generally showed a positive effect on adventitious root induction, with the highest efficiency observed at 5.0 mg L–1. IBA also demonstrated a positive effect, with the highest induction efficiency at 3.0 mg L–1. NAA showed a varied response, with efficient induction observed at lower concentrations but inhibitory effects at higher concentrations (Figure 1 and Table 2).
Table 2. Effect of Different Auxin Concentrations on Adventitious Root Induction from N. tazetta Callus after 6 Weeks on MS Mediuma.
| auxin (mg L–1) | induction efficiency (%) | number of root/explants | root length(cm) |
|---|---|---|---|
| control 0 | 0 | 0 | 0 |
| IAA 1.0 | 65 | 1.10 ± 0.06f | 1.45 ± 0.02f |
| IAA 3.0 | 73 | 2.60 ± 0.08e | 2.11 ± 0.03c |
| IAA 5.0 | 76 | 2.90 ± 0.07d | 2.05 ± 0.03d |
| IBA 1.0 | 71 | 3.10 ± 0.07c | 2.18 ± 0.03b |
| IBA 3.0 | 84 | 3.70 ± 0.09a | 2.53 ± 0.05a |
| IBA 5.0 | 82 | 3.50 ± 0.11b | 1.74 ± 0.02e |
| NAA 1.0 | 80 | 2.80 ± 0.08d | 1.14 ± 0.02g |
| NAA 3.0 | 62 | 1.00 ± 0.08f | 0.56 ± 0.02g |
| NAA 5.0 | 0 | 0 | 0 |
Different alphabetical letters a–g denote significant differences (p < 0.05).
Shoot Culture Establishment in N. tazetta
In the absence of cytokinin (control), some shoot induction occurred, with a 69% induction efficiency, an average of 2.30 shoots per explant, and an average length of 1.12 cm. Increasing the BAP concentrations led to an increase in both induction efficiency and the number of shoots per explant. Additionally, shoot length generally increased with increasing BAP concentration, reaching a maximum at 3 mg L–1. Similar to BAP, higher kinetin concentrations resulted in a higher induction efficiency and an increased number of shoots per explant. The trend in shoot length varied, with the maximum length observed at 3 mg L–1 rather than at 5 mg L–1. Both BAP and kinetin showed a positive effect on shoot induction, with higher concentrations leading to an increased induction efficiency, number of shoots per explant, and shoot length. Overall, 3 3 mg L–1 BAP and 3 3 mg L–1 kinetin showed the highest induction efficiency and produced shoots with the most extended average lengths (Figure 1 and Table 3).
Table 3. Effect of Different Cytokinin Concentrations on Shoot Induction from N. tazetta Meristems after 6 Weeks on MS Mediuma.
| cytokinin (mg L–1) | induction efficiency (%) | number of shoot/explants | shoot length(cm) |
|---|---|---|---|
| control 0 | 69 | 2.30 ± 0.05d | 1.12 ± 0.01g |
| BAP 1 | 75 | 2.80 ± 0.06c | 1.29 ± 0.01e |
| BAP 3 | 86 | 3.60 ± 0.07a | 1.52 ± 0.01a |
| BAP 5 | 84 | 3.10 ± 0.07b | 1.38 ± 0.01b |
| kinetin 1 | 72 | 2.40 ± 0.05d | 1.24 ± 0.01f |
| kinetin 3 | 82 | 3.10 ± 0.06b | 1.36 ± 0.01c |
| kinetin 5 | 85 | 2.90 ± 0.06c | 1.33 ± 0.01d |
Different alphabetical letters a–g denote significant differences (p < 0.05).
Galantamine Content in the Callus, Adventitious Root, and Shoot Culture of N. tazetta
The galantamine content of the different in vitro generated tissues (callus, shoot, and adventitious root) was analyzed using HPLC. The hormonal concentration that produced the highest induction efficiency and weight from the different in vitro generated tissues was determined by further analysis of primary and secondary metabolites. The shoot (0.158 mg/g dry weight), followed by the roots (0.109 mg/g dry weight) and callus (0.026 mg/g dry weight), had the highest galantamine content (Figure 2).
Figure 2.
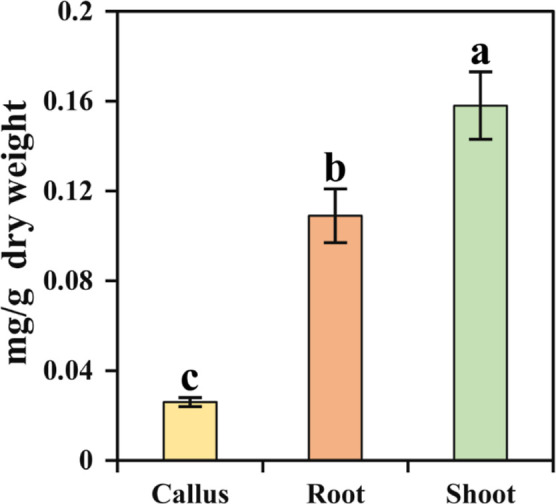
Galantamine content in the callus, roots, and shoot of N. tazetta. The error bar represents the standard deviation of 10 biological replicates.
Phenolic Compound Content in N. tazetta Calli, Roots, and Shoots
Phenolic compounds were detected in three in vitro-generated N. tazetta tissues (callus, root, and shoot). Each compound showed a distinct distribution pattern among the tissues, with some being more abundant in specific tissues than others. For example, rutin and quercetin were more abundant in shoot tissue than in callus and root tissues. These compounds were not detected (ND) in callus and shoot tissues but were found in adventitious root tissue. This indicates the tissue-specific accumulation of certain phenolic compounds. The shoot tissue had the highest total phenolic compound content compared to those of the adventitious root and callus tissues. This suggests that shoot tissue may have a higher accumulation of phenolic compounds compared to the other tissues analyzed (Table 4).
Table 4. Phenolic Compound Content (μg/g of Dry Weight) in the Callus, Roots, and Shoot of N. tazettaa,b.
| compound names | callus | root | shoot |
|---|---|---|---|
| gallic acid | 13.5 ± 0.8b | 12.4 ± 0.6b | 24.2 ± 1.1a |
| catechin | 64.6 ± 2.5a | 64.4 ± 3.2a | 63.8 ± 2.8a |
| 4-hydroxybenzoic acid | 8.7 ± 1.0a | ND | ND |
| chlorogenic acid | 63.6 ± 2.9b | 63.7 ± 3.6b | 94.11 ± 4.5a |
| caffeic acid | 21.0 ± 1.4a | 22.7 ± 1.2a | 20.6 ± 0.9a |
| (−)-epicatechin | 57.4 ± 2.5b | 87.6 ± 3.3a | 51.2 ± 2.1c |
| epicatechin gallate | 61.4 ± 6.2a | 61.7 ± 4.6a | 61.4 ± 3.7a |
| ferulic acid | ND | 36.5 ± 1.1a | ND |
| sinapic acid | ND | 11.6 ± 0.8a | ND |
| benzoic acid | 62.2 ± 4.2a | 62.2 ± 3.8a | 62.8 ± 5.1a |
| rutin | 204.3 ± 14.2c | 310.4 ± 19.5b | 509.7 ± 26.7a |
| quercetin | 137.1 ± 9.5a | 135.2 ± 7.9a | 134.1 ± 7.1a |
| kaempferol | 48.9 ± 2.3a | 53.1 ± 6.8a | 48.8 ± 4.3a |
| total | 742.7 ± 12.27c | 921.5 ± 6.84b | 1070.71 ± 10.22a |
ND: not detected.
Different alphabetical letters a–c denote significant differences (p < 0.05).
Metabolic Profiling of Metabolites Identified from Callus, Adventitious Root, and Shoot Cultures of N. tazetta
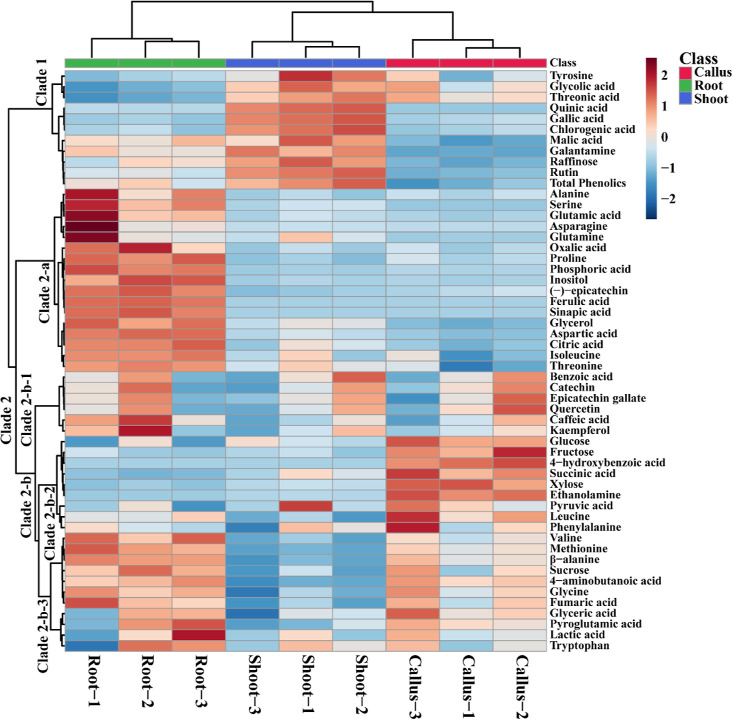
Hydrophilic metabolites extracted from N. tazetta were analyzed by HPLC and GC-TOFMS. The analysis identified 54 metabolites (amines, galantamine, amino acids, phenolic acids, organic acids, sugar alcohols, and sugars), and a heat map was generated based on hierarchical clustering (Figure 3). The heat map showed that most of the metabolites were significantly higher in adventitious root tissue. The heat map cluster was classified into two clades: clade 1 and clade 2. Clade 1 was composed of 11 metabolites, which were most abundant in the shoot, although some of them were also slightly present in the callus and roots. Clade 2 formed a mixed cluster with metabolites rich in all organs. Clade 2 was divided into clades 2-a and 2-b. Clade 2-a consisted of rich metabolites only in the adventitious root tissue, whereas clade 2-b formed a cluster in which the metabolites were present in all of the tissues. Clade 2-b was further split into three groups: clade 2-b-1, clade 2-b-2, and clade 2-b-3. Clade 2-b-1 consisted of metabolites present in all tissues, whereas clade 2-b-2 consisted of compounds containing various monosaccharides and a few amino acids that were relatively unique to the callus. In the case of clade 2-b-3, the compounds that were highly present in both the callus and adventitious roots were separated. The heat map showed that most of the metabolites were rich in adventitious root tissue.
Figure 3.
Heat map of the relative concentrations of hydrophilic metabolites identified from three N. tazetta tissues using HPLC and GC-TOFMS. Three biological replicates were used.
In detail, the tricarboxylic acid (TCA) intermediate showed a differential accumulation in different tissues. Pyruvic acid showed the highest accumulation in the callus and shoot, whereas fumaric acid was rich in the callus and adventitious roots. The other three TCA cycle intermediates, namely, citric acid, malic acid, and succinic acid, were significantly highest in the adventitious roots, shoot, and callus, respectively. The sugars, such as glucose and fructose, were significantly high only in the callus tissue, whereas the shoot had the highest raffinose content. The sucrose content was significantly higher in both the root and the callus tissues. The sugar alcohols, such as glycerol and inositol, were considerably rich in adventitious roots, whereas xylose showed a significantly high accumulation in adventitious root tissue. The organic acid levels, such as threonic acid and quinic acid content, were abundant in the shoot, whereas in the roots, the oxalic acid content was highest (Figure 3). In addition, the glyceric and lactic acid contents were highest in the roots and callus, whereas the pyruvic acid content was highest in the callus and shoot tissues. Most of the amino acid content (alanine, serine, asparagine, glutamic acid, glutamine, proline, aspartic acid, isoleucine, and threonine) was highest only in the roots. Amino acids, such as phenylalanine, leucine, lactic acid, and tryptophan, were present in root, shoot, and callus tissues. Valine, methionine, β-alanine, glycine, and pyroglutamic acid were significantly present in the roots, and these amino acids were also slightly present in the callus. These results showed that most metabolites were highest in the root tissue compared with other organs.
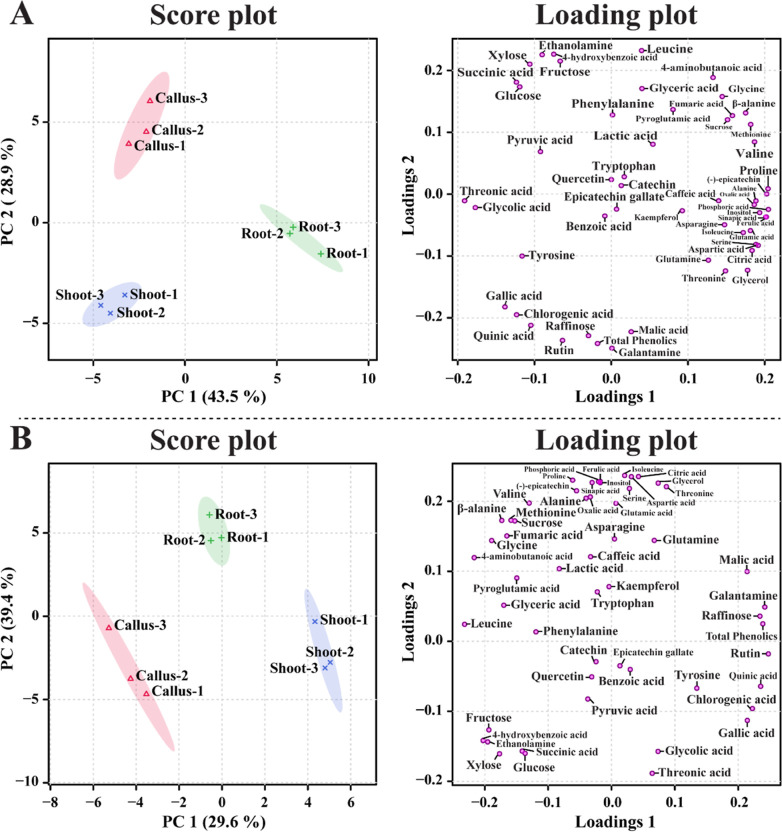
Principal component analysis (PCA) was performed to understand the differences in the identified metabolites among the callus, root, and shoot tissues. PCA showed a clear separation between tissue types, which resulted in a 43.5% (principal component 1) and 28.9% (PC2) variance with a two-component analysis (Figure 4). This clear separation might be due to amino acids, phenolic compounds, sugars, and sugar alcohols. In the PCA, phosphoric acid, proline, (−)-epicatechin, ferulic acid, sinapic acid, inositol, aspartic acid, serine, alanine, and valine had positive eigenvector values of 0.20481, 0.20435, 0.20251, 0.20204, 0.20138, 0.19337, 0.19137, 0.18861, 0.18858, and 0.18672, respectively, and threonic acid, glycolic acid, gallic acid, succinic acid, chlorogenic acid, glucose, tyrosine, xylose, quinic acid, and pyruvic acid had negative eigenvector values of −0.19111, −0.17711, −0.1385, −0.1236, −0.12333, −0.11928, −0.11614, −0.10611, −0.10475, and −0.090047, respectively. The partial least-squares discriminant analysis (PLS-DA) results also showed separation between the callus, root, and shoot tissues, with 29.6% and 39.4% of the variance in the two-component analysis. This clear separation could be due to the following metabolites: rutin, galantamine, total phenolics, quinic acid, raffinose, chlorogenic acid, gallic acid, malic acid, tyrosine, and threonine, which had positive eigenvector values of 0.2475, 024,186, 0.23881, 0.23532, 0.23427, 0.22223, 0.21433, 0.21397, 0.1347, and 0.086601, respectively, and leucine, 4-aminobutanoic acid, 4-hydroxybenzoic acid, ethanolamine, fructose, glycine, xylose, β-alanine, glyceric acid, and fumaric acid, which showed negative eigenvector values of −0.23173, −0.21661, −0.2026, −0.19538, −0.19338, −0.18908, −0.17668, −0.17308, −0.16995, and −0.16504, respectively. A clear separation was detected between the callus, root, and shoot tissues in PCA and PLS-DA, which exhibited significant VIP values (>1) for rutin, galantamine, raffinose, total phenolics, quinic acid, malic acid, leucine, 4-hydroxybenzoic acid, chlorogenic acid, ethanolamine, 4-aminobutanoic acid, fructose, xylose, and glycine (Figure 5). This supports the heat map results, indicating that the considerable difference between the identified primary and secondary metabolites in the callus, root, and shoot tissues might be due to TCA cycle intermediates, amino acids, organic acids, sugars, sugar alcohols, and phenolic compounds.
Figure 4.
PCA (A) and PLS-DA (B) score and loading plots of the identified metabolites from the three N. tazetta tissues.
Figure 5.
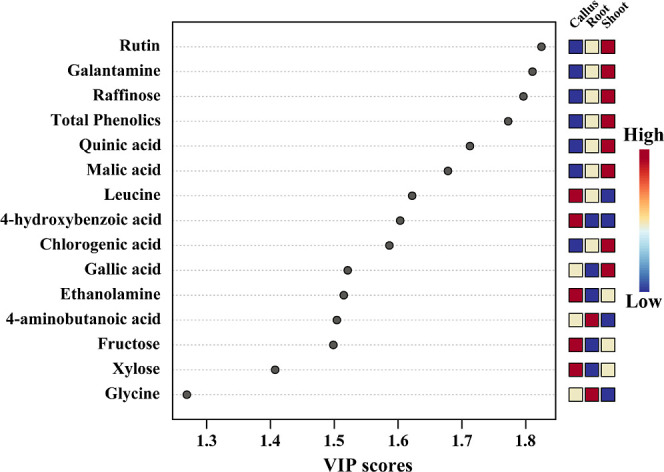
Main metabolites that differentiate the three N. tazetta tissues are based on the VIP scores obtained using the PLS-DA model. Colored squares on the right denote the relative concentrations of the three corresponding N. tazetta tissues.
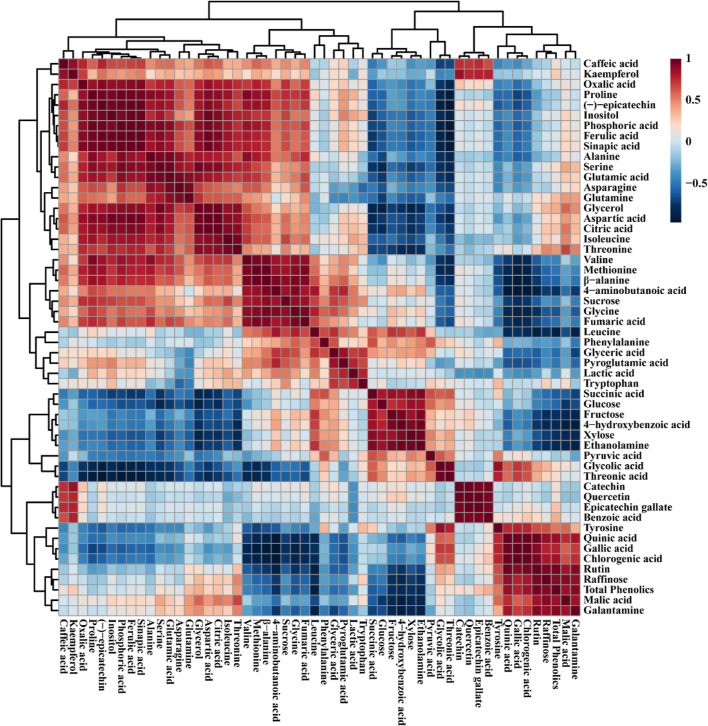
The correlations between the metabolites identified from the callus, root, and shoot cultures were investigated using Pearson’s correlation (Figure 6). The shikimate pathway is a well-known pathway for the biosynthesis of aromatic amino acids (tryptophan, phenylalanine, and tyrosine). Phenylalanine is a primary amino acid used for the synthesis of various phenolic acids, such as gallic acid, catechin, chlorogenic acid, (−) epicatechin, caffeic acid, epicatechin gallate, benzoic acid, rutin, kaempferol, and quercetin. Among these identified compounds, phenylalanine showed a positive correlation with a few phenolic compounds, including kaempferol, catechin, quercetin, and benzoic acid, whereas compounds including ferulic acid, caffeic acid, epicatechin gallate, gallic acid, rutin, and chlorogenic acid showed a negative correlation. In addition, phenylalanine showed a positive correlation with all TCA cycle intermediates, including oxalic, fumaric, citric, malic, and succinic acid. Citric acid showed a positive correlation with glutamic acid (r = 0.80704, p = 0.008556), and its byproducts, including pyroglutamic acid and glutamine, showed a positive correlation with citric acid. Sucrose showed a positive correlation with the TCA cycle intermediates, including oxalic, citric, and fumaric acids, whereas it showed a negative correlation with succinic and malic acid. Galantamine, which is also synthesized from phenylalanine, showed a negative correlation with phenylalanine (r = −0.47978, p = 0.19122), whereas it showed a strong correlation with total phenolics (r = 0.92002, p = 0.32987). In this study, among the phenolic compounds, rutin showed the highest accumulation in all organs and a strong correlation with galantamine content (r = 0.91747, and p = 0.00049). Galantamine showed a positive correlation with most of the TCA cycle intermediates, such as oxalic, citric, and malic acids, whereas it showed a negative correlation with fumaric acid (r = −0.47533, p = 0.19596) and succinic acid (r = −7009, p = 0.03543). Galantamine showed a positive correlation with sugar (raffinose) and sugar alcohols (glycerol and inositol).
Figure 6.
Relationships between the metabolites identified from the three N. tazetta tissues are shown in a correlation matrix; each colored square shows the Pearson’s correlation coefficient for a pair of metabolites; the strength of the red or blue color, as shown on the color scale, represents the correlation coefficient value for each colored box.
Fifty-six pathways were identified in the different organs of N. tazetta. For metabolic pathway analysis, the Kyoto Encyclopedia of Genes and Genomes was selected based on the Arabidopsis thaliana plant database (Table S1). In this study, 31 pathways were impacted. The list of pathways impacting different organs included amino acid biosynthesis, metabolic, and carbohydrate metabolism pathways. The most impacted pathways were as follows: flavone and flavonol biosynthesis, isoquinoline alkaloid biosynthesis, glycine, serine, and threonine metabolism, alanine, aspartate, and glutamate metabolism, and phenylalanine metabolism (Figure 7). In addition, pathways such as valine, leucine, and isoleucine degradation, ubiquinone and other terpenoid–quinone biosynthesis, arginine biosynthesis, amino sugar and nucleotide sugar metabolism, and lysine biosynthesis were not impacted (Table S1).
Figure 7.
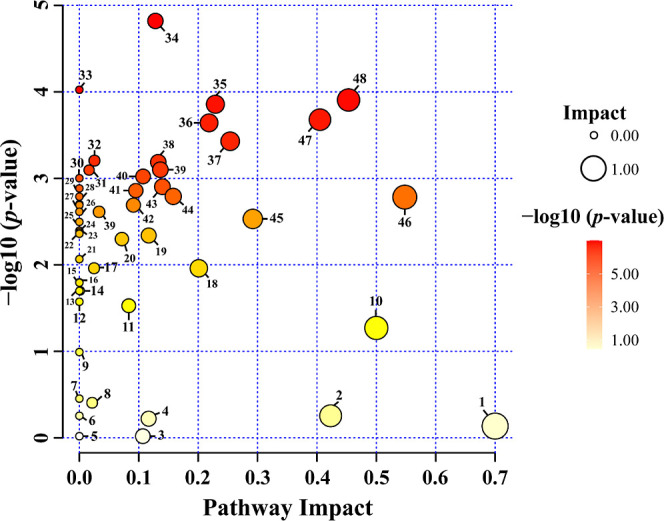
Identified metabolites and their pathway impact on three different tissues of N. tazetta. (1) Flavone and flavonol biosynthesis, (2) phenylalanine metabolism, (3) tryptophan metabolism, (4) glycolysis/gluconeogenesis, (5) indole alkaloid metabolism, (6) tropane, piperidine, and pyridine alkaloid biosynthesis, (7) terpenoid backbone biosynthesis, (8) phenylalanine, tyrosine, and tryptophan biosynthesis, (9) nitrogen metabolism, (10) isoquinoline alkaloid biosynthesis, (11) glutathione metabolism, (12) d-amino acid metabolism, (13) thiamine metabolism, (14) lipoic acid metabolism, (15) fatty acid degradation, (16) monobactam biosynthesis, (17) flavonoid biosynthesis, (18) tyrosine metabolism, (19) pantothenate and CoA biosynthesis, (20) fructose and mannose metabolism, (21) propanoate metabolism, (22) biosynthesis of various plant secondary metabolites, (23) sphingolipid metabolism, (24) glucosinolate biosynthesis, (25) pyrimidine metabolism, (26) cyanoamino acid metabolism, (27) ascorbate and aldarate metabolism, (28) amino sugar and nucleotide sugar metabolism, (29) arginine biosynthesis, (30) valine, leucine, and isoleucine degradation, (31) arginine and proline metabolism, (32) glycerophospholipid metabolism, (33) ubiquinone and other terpenoid–quinone biosynthesis, (34) galactose metabolism, (35) glyoxylate and dicarboxylate metabolism, (36) citrate cycle (TCA cycle), (37) beta-alanine metabolism, (38) phenylpropanoid biosynthesis, (39) butanoate metabolism, (40) valine, leucine, and isoleucine biosynthesis, (41) carbon fixation in photosynthetic organisms, (42) inositol phosphate metabolism, (43) cysteine and methionine metabolism, (44) glycerolipid metabolism, (45) pyruvate metabolism, (46) glycine, serine, and threonine metabolism, (47) starch and sucrose metabolism, and (48) alanine, aspartate, and glutamate metabolism.
Discussion
In our study, we found that the induction efficiency, number of root/explants, and root length were increased with increasing IAA concentration. However, at the lowest IBA concentrations (1 and 3 mg L–1), the adventitious root induction was increased, whereas it was decreased at the highest concentration. A similar result was obtained in Plumbago zeylanica, where the percentage and root number gradually increased with increasing auxin (IAA and IBA) concentration.31 In other studies, it has been reported that the adventitious root number and root length were increased at the lowest auxin concentrations, whereas at the highest concentration, the root induction was significantly decreased in Echinacea purpurea,32Fagopyrum tataricum,33 and Psoralea corylifolia.34 In this study, the NAA treatment showed a different tendency, where the concentration increased and adventitious root induction was significantly decreased. A similar trend was achieved in F. tataricum; at the lowest NAA concentration, the greatest number of adventitious roots was achieved, whereas it was decreased at the highest concentration of NAA.33 In Boerhaavia diffusa, in vitro, induction of adventitious roots showed that 1.0 mg L–1 NAA produced the highest number of roots, whereas high concentrations (2 and 4 mg L–1 NAA) produced the most profuse rooting.35 Similarly, the NAA produced the greatest number of adventitious roots in Robinia pseudoacacia, while the IBA treatment exhibited the highest number of adventitious root formations in Grewia optiva.36 The medium supplemented with 1.0 mg L–1 of IBA and 0.5 mg L–1 of NAA showed the highest number of adventitious roots in P. zeylanica.31 From these results, it is clearly stated that specific types of auxins must be selected for efficient root induction in a specific species.
This study identified the ideal conditions for producing primary and secondary metabolites in N. tazetta using different in vitro tissue cultures. This suggests an efficient and high-yielding method for the commercial-scale production of galantamine. Similar to our study, research on galantamine and metabolites using Narcissus pseudo narcissus, which is closely related to Narcissus gazzetta, also showed that the galantamine content is lowest in the callus.37 However, unlike our data, this study was not able to detect a significant amount of galantamine in the root, which may be due to the different conditions for root induction. For example, unlike our tissue culture conditions, for root induction, we used cytokinin, which can act as a NAA antagonist. Another reason might be the time duration for the induction of the root tissue. For example, Ferdausi et al.37 analyzed the galantamine content in 14 week old root cultures, whereas in our study, we analyzed 6 week old cultures, which might explain the highest accumulation of galantamine in N. tazetta roots. Additionally, another study found that the timing of tissue harvest depends on the developmental stage, such as before and after flowering, which alters the galantamine content in a tissue-specific manner.38 More specifically, the galantamine content was higher in the roots and stems before bloom but significantly reduced after flowering. Thus, the slight difference in the galantamine content between this study and the others could be caused by various factors.
Amino acids are significantly detected in wounded Lycoris radiata callus.39 Similar amino acids were also detected in this study of the N. tazetta callus. However, amino acids, such as methionine, have not been identified in the L. radiata callus. Amino acids, such as alanine, asparagine, glutamic acid, glutamine, and serine, are present in L. radiata, whereas they are ND in the N. tazetta callus. In addition, Park et al.39 reported that wounding of the callus enhances galantamine biosynthesis and its pathway genes. However, the N. tazetta callus produced a more significant amount of galantamine than the wounded L. radiata callus. These results show that N. tazetta might accumulate a significant amount of galantamine compared to other Amaryllidaceae families.
The analysis of metabolites in L. radiata root showed a significant number of metabolites.40 Similar results were obtained in our study, in which most of the metabolites were present in the adventitious roots of N. tazetta. Specifically, amino acids, such as alanine, glutamine, glutamic acid, asparagine, and aspartic acid, have been identified in L. radiata and N. tazetta root tissues. In addition to amino acids, other metabolites, such as phosphoric acid, citric acid, lactic acid, and glycerol, have also been identified in both tissues. The metabolites identified in the leaf tissues of L. radiata were also present in the in vitro shoot tissues of N. tazetta. For example, quinic acid, malic acid, raffinose, and glycolic acid are present in the leaf tissues of L. radiata and in vitro-generated shoot tissues of N. tazetta. Although this study identified tissue culture conditions that produce large amounts of galantamine, other molecular mechanisms behind the production have yet to be elucidated. This is due to the need for genetic studies of phenolic and galantamine compound biosynthesis pathways and their complexity. However, based on several similar studies previously conducted involving molecular works, it is possible to infer the mechanism behind these pathways. For example, ferulic acid is known as a precursor of chlorogenic acid. In our data, the chlorogenic acid level in the shoots was significantly higher than those in the callus and roots. This may indicate that the gene converting ferulic acid to chlorogenic acid is positively regulated in the shoot. Surprisingly, the amount of ferulic acid was significant in the root tissue, while it was not present in the other tissues. This indicates that either ferulic acid synthase catechol-O-methyltransferase (COMT) is positively regulated in the roots or UDP-glucosyltransferase 84 (UGT84) and hydroxycinnamoyl-d-glucose: quinate hydroxycinnamoyl transferase (HCGQT), which convert ferulic acid to chlorogenic acid, are negatively regulated in this tissue.41 Galantamine biosynthesis is also known to be induced by various stressors. For example, previous research confirmed that the physical wounding of L. radiata callus causes galantamine accumulation by inducing the gene expression of seven genes responsible for galantamine biosynthesis.42 This indicates that galantamine may be regulated by various wounding-related signaling pathways, such as salicylic acid, jasmonic acid, and ethylene signaling. Transcriptomic analysis by Li et al.43 indicated that JA and other hormones may regulate galantamine biosynthesis. However, further molecular genetic and biochemical studies are needed to elucidate the profound mechanism of the hormone-mediated galantamine biosynthesis pathway.
Conclusions
In conclusion, this study identified optimal conditions for N. tazetta tissue culture for calli, shoots, and roots. In addition, primary and secondary metabolites were analyzed in three N. tazetta tissues. These results will provide a clear understanding of the presence of various primary and secondary metabolites in each tissue of N. tazetta. In addition, this research may be helpful to researchers for the mass production of primary and secondary metabolites in N. tazetta in vitro tissues. In the future, scaling up of specific pharmaceutical compounds from the particular N. tazetta in vitro tissues can be used for the commercial production of medicines, cosmetics, and skincare products. In addition, this can be done in small areas, which will avoid damage or depletion of large areas of grown plants. Furthermore, it is important to fill the gaps in knowledge about the antioxidant activities, antiaging, and antiwrinkling activities of each N. tazetta in vitro tissue.
Materials and Methods
Plant Materials
A 1 year-old bulb of N. tazetta var. chinensis cv. ‘Geumjanogdae’ was purchased from Xplant Co., Songpa-gu, Seoul, Korea (37°29′05.9″N 127°07′16.6″E). The bulbs were grown on a greenhouse experimental farm at the Chungnam National University in Daejeon, Korea. After one year, the bulbs were used as resources for inducing callus or shoot growth. For each in vitro tissue (callus, adventitious root, and shoot), we have grown 30 plates/tissues. In each Petri plate, we keep ∼5–7 explants.
Callus Induction
The bulbs were first collected and then cleansed using flowing tap water, followed by immersion in 70% ethanol for 1 min. N. tazetta var. chinensis bulbs were immersed in a 2% sodium hypochlorite (NaOCl) solution for 12 min and then rinsed with sterilized water 4–5 times. The bulbs were placed in a laminar air flow chamber, and the water on the bulb was dried by using sterile tissue paper. The efficiency of the disinfectant is ∼60%. The meristems were excised from the center of the bulbs, dissected into fragments of about 0.5 × 0.5 × 0.2 cm, and then placed in a 90 mm Petri dish containing ∼20 mL of Murashige and Skoog (MS) medium44 containing salts and vitamins, 30 g/L of sucrose, and 0.8% of Phytoagar (Duchefa, Netherlands). Additionally, media with varying concentrations (0, 0.5, 1, 2, and 4 mg L–1) of 2,4-D (2,4-dichlorophenoxyacetic acid) or dicamba (3,6-dichloro-2-methoxybenzoic acid) were added. All plant hormones used in this study were acquired from Sigma (St. Louis, MO, USA). The pH of the media was adjusted to 5.8 before the addition of the agar. Subsequently, the medium was sterilized by autoclaving at 121 °C for 20 min. The cultures were incubated in a growth room devoid of light at a constant temperature of 25 ± 1 °C. To induce callus formation from the meristem, 5–7 explants per Petri dish were transferred to new callus induction media every 2 weeks. Compact calli developed after 6 weeks.
Adventitious Root Induction
To induce adventitious roots, the callus was sliced into sections of roughly 0.5 × 0.5 × 0.2 cm. The sliced callus was placed on a sterile solidified MS medium (pH 5.8) containing 0.8% (w/v) Phytoagar (Sigma, St. Louis, MO., USA), with varying concentrations (0, 1, 3, and 5 mg L–1) of IAA (indole-3-acetic acid), IBA (indole-3-butyric acid), or NAA (naphthalene acetic acid). The cultures were incubated at 25 ± 1 °C in a growth chamber without light for 6 weeks.
Shoot Regeneration
To induce shoot regeneration, the meristems were excised from disinfected N. tazetta bulbs and cut into pieces (0.5 × 0.5 × 0.2 cm). To optimize the suitable shoot regeneration conditions, the meristems were kept on a sterilized solidified MS medium (pH 5.8) enriched with 0, 1, 3, and 5 mg L–1 of BAP (6-benzylaminopurine) or kinetin (N6-furfuryladenine). The cultures were maintained at 25 ± 1 °C in a growth chamber with a 16 h photoperiod, using a conventional cool white fluorescent lamp (35 μmol s–1 m–2), for 6 weeks.
Analysis of Galantamine by HPLC
Galantamine extraction was performed using a protocol previously described by Park et al.39 with minor adjustments. Specifically, a 2 mL solution of 0.1% trifluoroacetic acid was added to a mixture of 100 mg of powders derived from different types (callus, adventitious root, and stem) of N. tazetta. Then, 2 mL of 0.1% trifluoroacetic acid was added, and the mixture was sonicated for 30 min. The mixture was then stored at 4 °C overnight. The incubated mixture was centrifuged at 13,000 rpm for 10 min. The liquid supernatant was passed through a 0.45 μm Acrodisc syringe filter (Pall Corporation, Port Washington, NY, USA) and collected in a screw cap vial. An HPLC analysis of galantamine was conducted using an NS-4000 HPLC system connected to an NS-6000 autosampler and a UV–vis detector (Futecs Corporation, Daejeon, Korea). A column of OptimaPak C18 (250 × 4.6 mm, 5 μm, RStech Corp., Daejeon, Korea) was used to separate the galantamine. The mobile phase consisted of solvent A, which was a 50 mM aqueous buffer of ammonium formate, and solvent B, which was acetonitrile. The separation process was carried out at a flow rate of 1 mL/min, and the oven column was maintained at 30 °C. The detection wavelength was 285 nm, and 20 μL of the sample was injected for each run. The gradient program and identification and quantification of galantamine content were performed according to the protocol described by Park et al.39 The data were expressed as the means of three replicates.
Analysis of Hydrophilic Compounds by GC-TOFMS
The hydrophilic metabolites were extracted using the methodology outlined in a recent work by Yeo et al.45 with minor modifications. Briefly, 1 mL of water, CHCl3, and MeOH in a ratio of 1:1:2.5 (v/v/v) was added to 10 mg of N. tazetta tissues (callus, adventitious root, and stem) generated in vitro, followed by the addition of 60 μL of 0.2 g/L adonitol as an internal standard. Extraction was conducted in a small thermomixer with a mixing frequency of 1200 rpm and 37 °C for 30 min. The polar phase of the samples was separated by centrifugation at 10,000 rpm for 5 min. After centrifugation, 800 μL of the polar phase was transferred to a sterile Eppendorf tube, and then 400 μL of deionized water was added. Finally, the mixture was centrifuged at 10,000 rpm for 5 min to separate the methanol–water phase, which contained the polar metabolites. The resulting mixture was then evaporated by using a centrifugal concentrator (CVE-2000, Daejeon, Korea) for 3 h. The residues were dehydrated for 15 h using a freeze dryer. The lyophilized materials were processed in two steps: methoxide derivatization and trimethylsilyl etherification. In a vial, 80 μL of methoxyamine hydrochloride and pyridine (20 g/L) were agitated for 90 min at 30 °C. Then, 80 μL of N-methyl-N-(trimethylsilyl) trifluoroacetamide was added to the vial. The mixture was heated at 37 °C for 30 min. The products obtained were examined using an Agilent 7890 GC system (Agilent, Atlanta, GA, USA). The operating conditions, flow rate, and gradient program adhered to the procedure outlined in the study conducted by Yeo et al.45 The metabolites were identified by using an internal library. Quantification was determined by comparing the peak areas of the metabolites to the peak areas of the internal standard.
Statistical Analysis
The data were analyzed using Duncan’s multiple range test at a significance threshold of p < 0.05. The analysis was performed using SAS 9.4 software from the SAS Institute, Inc., located in Cary, NC, USA. Heat map, PCA, PLS-DA, variable importance in projection (VIP), Pearson correlation, and pathway impact analyses of the 54 identified metabolites from different in vitro tissues of N. tazetta were performed using Metabo Analyst 6.0 (http://www.metaboanalyst.ca/) with autoscaling.
Acknowledgments
This work was supported by a grant from the Nakdonggang National Institute of Biological Resources (NNIBR), funded by the Ministry of Environment (MOE) of the Republic of Korea (NNIBR20243113).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c01735.
Metabolic pathways where the identified metabolites and their pathway impact on three different tissues of N. tazetta (PDF)
Author Contributions
# C.P. and R.S. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Abdel-Rahman H.; Al-Ansary A.; Rashed K.; Rizkalla A. Micropropagation of Narcissus tazetta ‘Chinensis’ and its relation to secondary metabolites content. J. Appl. Life Sci. Int. 2017, 14 (1), 1–11. 10.9734/JALSI/2017/36410. [DOI] [Google Scholar]
- Fan Y.; Peng J.; Wu J.; Zhou P.; He R.; Allan A. C.; Zeng L. NtbHLH1, a JAF13-like bHLH, interacts with NtMYB6 to enhance proanthocyanidin accumulation in Chinese Narcissus. BMC Plant Biol. 2021, 21, 275. 10.1186/s12870-021-03050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M.-P.; Karimzadegan V.; Héneault M.; Cloutier F.; Bérubé G.; Berthoux L.; Mérindol N.; Desgagné-Penix I. Chemical synthesis and biological activities of Amaryllidaceae alkaloid norbelladine derivatives and precursors. Molecules 2022, 27 (17), 5621. 10.3390/molecules27175621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J. J.; van Staden J. Antiviral alkaloid principles of the plant family Amaryllidaceae. Phytomedicine 2023, 108, 154480. 10.1016/j.phymed.2022.154480. [DOI] [PubMed] [Google Scholar]
- Kilgore M. B.; Augustin M. M.; May G. D.; Crow J. A.; Kutchan T. M. CYP96T1 of Narcissus sp. aff. Pseudonarcissus catalyzes formation of the Para-Para’CC phenol couple in the Amaryllidaceae alkaloids. Front. Plant Sci. 2016, 7, 225. 10.3389/fpls.2016.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecka A.; Jedrzejczak-Rey N.; Bednarek P. Secondary metabolites in plant innate immunity: conserved function of divergent chemicals. New Phytol. 2015, 206 (3), 948–964. 10.1111/nph.13325. [DOI] [PubMed] [Google Scholar]
- Pang Z.; Chen J.; Wang T.; Gao C.; Li Z.; Guo L.; Xu J.; Cheng Y. Linking plant secondary metabolites and plant microbiomes: A review. Front. Plant Sci. 2021, 12, 621276. 10.3389/fpls.2021.621276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E.; Gang D. R. Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective. Trends Plant Sci. 2000, 5 (10), 439–445. 10.1016/S1360-1385(00)01741-6. [DOI] [PubMed] [Google Scholar]
- Chandran H.; Meena M.; Barupal T.; Sharma K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450 10.1016/j.btre.2020.e00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero G.; Berni R.; Muñoz-Sanchez J.; Apone F.; Abdel-Salam E. M.; Qahtan A. A.; Alatar A. A.; Cantini C.; Cai G.; Hausman J.-F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9 (6), 309. 10.3390/genes9060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCosmo F.; Misawa M. Plant cell and tissue culture: alternatives for metabolite production. Biotechnol. Adv. 1995, 13 (3), 425–453. 10.1016/0734-9750(95)02005-N. [DOI] [PubMed] [Google Scholar]
- Ayaz M.; Nawaz A.; Naz F.; Ullah F.; Sadiq A.; Islam Z. U. Phytochemicals-based therapeutics against Alzheimer’s disease: an update. Curr. Top. Med. Chem. 2022, 22 (22), 1811–1820. 10.2174/1568026622666220815104305. [DOI] [PubMed] [Google Scholar]
- Bastida J.; Lavilla R.; Viladomat F. Chapter 3 Chemical and Biological Aspects of Narcissus Alkaloids. Alkaloids Chem. Biol. 2006, 63, 87–179. 10.1016/S1099-4831(06)63003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ago Y.; Koda K.; Takuma K.; Matsuda T. Pharmacological aspects of the acetylcholinesterase inhibitor galantamine. J. Pharmacol. Sci. 2011, 116 (1), 6–17. 10.1254/jphs.11R01CR. [DOI] [PubMed] [Google Scholar]
- Kavanagh S.; Van Baelen B.; Schäuble B. Long-term effects of galantamine on cognitive function in Alzheimer’s disease: a large-scale international retrospective study. J. Alzheimer’s Dis. 2011, 27 (3), 521–530. 10.3233/JAD-2011-110417. [DOI] [PubMed] [Google Scholar]
- Keglevich P.; Szántay C.; Hazai L. The chemistry of galanthamine. classical synthetic methods and comprehensive study on its analogues. Mini-Rev. Med. Chem. 2016, 16 (18), 1450–1461. 10.2174/1389557516666160321114556. [DOI] [PubMed] [Google Scholar]
- Küenburg B.; Czollner L.; Fröhlich J.; Jordis U. Development of a pilot scale process for the anti-Alzheimer drug (−)-galanthamine using large-scale phenolic oxidative coupling and crystallisation-induced chiral conversion. Org. Process Res. Dev. 1999, 3 (6), 425–431. 10.1021/op990019q. [DOI] [Google Scholar]
- Sathasivam R.; Choi M.; Radhakrishnan R.; Kwon H.; Yoon J.; Yang S. H.; Kim J. K.; Chung Y. S.; Park S. U. Effects of various Agrobacterium rhizogenes strains on hairy root induction and analyses of primary and secondary metabolites in Ocimum basilicum. Front. Plant Sci. 2022, 13, 983776. 10.3389/fpls.2022.983776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathasivam R.; Park S. U.; Kim J. K.; Park Y. J.; Kim M. C.; Nguyen B. V.; Lee S. Y. Metabolic profiling of primary and secondary metabolites in kohlrabi (Brassica oleracea var. gongylodes) sprouts exposed to different light-emitting diodes. Plants 2023, 12 (6), 1296. 10.3390/plants12061296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do T. M. H.; Choi M.; Kim J. K.; Kim Y. J.; Park C.; Park C. H.; Park N. I.; Kim C.; Sathasivam R.; Park S. U. Impact of light and dark treatment on phenylpropanoid pathway genes, primary and secondary metabolites in Agastache rugosa transgenic hairy root cultures by overexpressing Arabidopsis transcription factor AtMYB12. Life 2023, 13 (4), 1042. 10.3390/life13041042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y.; Kwon H.; Kim J. K.; Park C. H.; Sathasivam R.; Park S. U. Comparative analysis of glucosinolate and phenolic compounds in green and red kimchi cabbage (Brassica rapa L. ssp. pekinensis) hairy roots after exposure to light and dark conditions. Horticulturae 2023, 9 (4), 466. 10.3390/horticulturae9040466. [DOI] [Google Scholar]
- Park C. H.; Sathasivam R.; Kim T. J.; Park B. B.; Kim J. K.; Park S. U. Metabolic profiling and secondary metabolite accumulation during fruit development of Cornus officinalis Sieb. et Zucc. Ind. Crops Prod. 2022, 189, 115779. 10.1016/j.indcrop.2022.115779. [DOI] [Google Scholar]
- Fazili M. A.; Bashir I.; Ahmad M.; Yaqoob U.; Geelani S. N. In vitro strategies for the enhancement of secondary metabolite production in plants: A review. Bull. Natl. Res. Cent. 2022, 46 (1), 35. 10.1186/s42269-022-00717-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudheer W.; Thiruvengadam M.; Nagella P. A. comprehensive review on tissue culture studies and secondary metabolite production in Bacopa monnieri L. Pennell: A nootropic plant. Crit. Rev. Biotechnol. 2023, 43 (6), 956–970. 10.1080/07388551.2022.2085544. [DOI] [PubMed] [Google Scholar]
- Wawrosch C.; Zotchev S. B. Production of bioactive plant secondary metabolites through in vitro technologies—status and outlook. Appl. Microbiol. Biotechnol. 2021, 105 (18), 6649–6668. 10.1007/s00253-021-11539-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazian M.; Sabbatini P. Traditional in vitro strategies for sustainable production of bioactive compounds and manipulation of metabolomic profile in medicinal, aromatic and ornamental plants. Planta 2021, 254 (6), 111. 10.1007/s00425-021-03771-5. [DOI] [PubMed] [Google Scholar]
- Chen L.; Zhu X.; Gu L.; Wu J. Efficient callus induction and plant regeneration from anther of Chinese narcissus (Narcissus tazetta L. var. Chinensis Roem). Plant ell Rep. 2005, 24 (7), 401–407. 10.1007/s00299-005-0958-4. [DOI] [PubMed] [Google Scholar]
- Xu P. S.; Niimi Y.; Araki H. Production of virus-free bulblets from callus induced from scale culture of Lilium longiflorum ’Georgia’. J. JPN Soc. Hortic. Sci. 2000, 69 (1), 97–102. 10.2503/jjshs.69.97. [DOI] [Google Scholar]
- Rameshk M.; Sharififar F.; Mehrabani M.; Pardakhty A.; Farsinejad A.; Mehrabani M. Proliferation and in vitro wound healing effects of the microniosomes containing Narcissus tazetta L. bulb extract on primary human fibroblasts (HDFs). DARU J. Pharm. Sci. 2018, 26 (1), 31–42. 10.1007/s40199-018-0211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J.; Santos I.; Salema R. In vitro production of bulbs of Narcissus bulbocodium flowering in the first season of growth. Sci. Hortic. 1998, 76 (3–4), 205–217. 10.1016/S0304-4238(98)00141-1. [DOI] [Google Scholar]
- Sivanesan I.; Jeong B. R. Induction and establishment of adventitious and hairy root cultures of Plumbago zeylanica L. Afr. J. Biotechnol. 2009, 8 (20), 5294–5300. [Google Scholar]
- Choffe K. L.; Murch S. J.; Saxena P. K. Regeneration of Echinacea purpurea: induction of root organogenesis from hypocotyl and cotyledon explants. Plant Cell Tissue Organ Cult. 2000, 62, 227–234. 10.1023/A:1006444821769. [DOI] [Google Scholar]
- Choi M.; Sathasivam R.; Nguyen B. V.; Park N. I.; Woo S.-H.; Park S. U. Expression analysis of phenylpropanoid pathway genes and metabolomic analysis of phenylpropanoid compounds in adventitious, hairy, and seedling roots of Tartary buckwheat. Plants 2021, 11 (1), 90. 10.3390/plants11010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran P.; Jayabalan N. Psoralen production in hairy roots and adventitious roots cultures of Psoralea coryfolia. Biotechnol. Lett. 2009, 31, 1073–1077. 10.1007/s10529-009-9957-9. [DOI] [PubMed] [Google Scholar]
- Jenifer U.; Francina Cecilia K.; Ravindhran R. In vitro adventitious root and hairy root cultures in Boerhaavia diffusa L. Int. J. Curr. Res. 2012, 4 (1), 65–67. [Google Scholar]
- Swamy S.; Puri S.; Singh A. Effect of auxins (IBA and NAA) and season on rooting of juvenile and mature hardwood cuttings of Robinia pseudoacacia and Grewia optiva. New Forests 2002, 23, 143–157. 10.1023/A:1015653131706. [DOI] [Google Scholar]
- Ferdausi A.; Chang X.; Jones M. Enhancement of galanthamine production through elicitation and NMR-based metabolite profiling in Narcissus pseudonarcissus cv. Carlton in vitro callus cultures. In Vitro Cell. Dev. Biol.Plant 2021, 57, 435–446. 10.1007/s11627-020-10139-z. [DOI] [Google Scholar]
- Ferri D.; Ubaldi C.; Marcozzi G.; Fasciani P.; Bacchetta L.; Pace L. Chemical characterization of Narcissus poeticus from Sirente-Velino (Apennines-Italy): Galantamine accumulation and distribution of allergenic compounds in the flower. Nat. Prod. Commun. 2017, 12, 15–18. 10.1177/1934578X1701200105. [DOI] [PubMed] [Google Scholar]
- Park C. H.; Sathasivam R.; Nguyen B. V.; Baek S.-A.; Yeo H. J.; Park Y. E.; Kim H. H.; Kim J. K.; Park S. U. Metabolic profiling of primary metabolites and galantamine biosynthesis in wounded Lycoris radiata callus. Plants 2020, 9 (11), 1616. 10.3390/plants9111616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak A.; Simlat M.; Morańska E.; Skrzypek E.; Warchoł M.; Tarakemeh A.; Laurain-Mattar D. Exogenous melatonin stimulated Amaryllidaceae alkaloid biosynthesis in in vitro cultures of Leucojum aestivum L. Ind. Crops Prod. 2019, 138, 111458. 10.1016/j.indcrop.2019.06.021. [DOI] [Google Scholar]
- Volpi e Silva N.; Mazzafera P.; Cesarino I. Should I stay or should I go: are chlorogenic acids mobilized towards lignin biosynthesis?. Phytochem. 2019, 166, 112063. 10.1016/j.phytochem.2019.112063. [DOI] [PubMed] [Google Scholar]
- Ptak A.; Morańska E.; Saliba S.; Zieliński A.; Simlat M.; Laurain-Mattar D. Elicitation of galanthamine and lycorine biosynthesis by Leucojum aestivum L. and L. aestivum ‘Gravety Giant’plants cultured in bioreactor RITA®. Plant Cell Tissue Organ Cult. 2017, 128, 335–345. 10.1007/s11240-016-1113-3. [DOI] [Google Scholar]
- Li Q.; Xu J.; Zheng Y.; Zhang Y.; Cai Y. Transcriptomic and metabolomic analyses reveals that exogenous methyl jasmonate regulates galanthamine biosynthesis in Lycoris longituba seedlings. Front. Plant Sci. 2021, 12, 713795. 10.3389/fpls.2021.713795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T.; Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15 (3), 473–497. 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Yeo H. J.; Lim S.-Y.; Park C. H.; Kim C. Y.; Sathasivam R.; Kim J. K.; Park S. U. Metabolic analyses and evaluation of antioxidant activity in purple kohlrabi sprouts after exposed to UVB radiation. Antioxidants 2022, 11 (8), 1443. 10.3390/antiox11081443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.