This cross-sectional study investigates the prevalence of up-to-date lung cancer screening across the 50 US states and the District of Columbia in 2022.
Key Points
Question
What was the prevalence of eligible US individuals being up to date with recommended lung cancer screening (LCS) in 2022?
Findings
In this nationwide cross-sectional study of 25 958 individuals eligible for LCS, 18% reported being up to date with LCS overall, but prevalence varied across states (range, 10%-31%). Rates were lower in southern states that also had high LC mortality burden, but state Medicaid expansion and higher LCS capacity levels were associated with higher prevalence of up-to-date LCS.
Meaning
Recommended LCS uptake was low in 2022, and prevalence did not correspond with LC burden across states; however, improving health care access and LCS capacity may be associated with higher rates.
Abstract
Importance
The US Preventive Services Task Force (USPSTF) recommends annual lung cancer screening (LCS) with low-dose computed tomography in high-risk individuals (age 50-80 years, ≥20 pack-years currently smoking or formerly smoked, and quit <15 years ago) for early detection of LC. However, representative state-level LCS data are unavailable nationwide.
Objective
To estimate the contemporary prevalence of up-to-date (UTD) LCS in the US nationwide and across the 50 states and the District of Columbia.
Design, Setting, and Participants
This cross-sectional study used data from the 2022 Behavioral Risk Factor Surveillance System (BRFSS) population-based, nationwide, state-representative survey for respondents aged 50 to 79 years who were eligible for LCS according to the 2021 USPSTF eligibility criteria. Data analysis was performed from October 1, 2023, to March 20, 2024.
Main Outcomes and Measures
The main outcome was self-reported UTD-LCS (defined as past-year) prevalence according to the 2021 USPSTF eligibility criteria in respondents aged 50 to 79 years. Adjusted prevalence ratios (APRs) and 95% CIs compared differences.
Results
Among 25 958 sample respondents eligible for LCS (median [IQR] age, 62 [11] years), 61.5% reported currently smoking, 54.4% were male, 64.4% were aged 60 years or older, and 53.0% had a high school education or less. The UTD-LCS prevalence was 18.1% overall, but varied across states (range, 9.7%-31.0%), with relatively lower levels in southern states characterized by high LC mortality burden. The UTD-LCS prevalence increased with age (50-54 years: 6.7%; 70-79 years: 27.1%) and number of comorbidities (≥3: 24.6%; none: 8.7%). A total of 3.7% of those without insurance and 5.1% of those without a usual source of care were UTD with LCS, but state-level Medicaid expansions (APR, 2.68; 95% CI, 1.30-5.53) and higher screening capacity levels (high vs low: APR, 1.93; 95% CI, 1.36-2.75) were associated with higher UTD-LCS prevalence.
Conclusions and Relevance
This study of data from the 2022 BRFSS found that the overall prevalence of UTD-LCS was low. Disparities were largest according to health care access and geographically across US states, with low prevalence in southern states with high LC burden. The findings suggest that state-based initiatives to expand access to health care and screening facilities may be associated with improved LCS rates and reduced disparities.
Introduction
Lung cancer (LC) continues to be the leading cause of death in the US, with 125 070 estimated deaths in 2024.1 Earlier-stage diagnosis is associated with improved survival. Yet, more than 40% of LCs are still diagnosed at a distant stage,2 for which 5-year relative survival is just 8%.1 In 2013, the US Preventive Services Task Force (USPSTF) recommended annual LC screening (LCS) with low-dose computed tomography (CT) in high-risk individuals (age 55-80 years with a ≥30 pack-year smoking history).3,4 This recommendation was based on results from the National Lung Cancer Screening Trial showing a 20% reduction in LC mortality associated with annual low-dose CT compared with chest radiography.3,4 In 2021, the USPSTF LCS eligibility criteria were updated,5 lowering the age to start screening from 55 to 50 years and pack-year history from 30 to 20 pack-years. This update was based on evidence of reduced LC deaths associated with LCS at ages 50 to 55 years and lower pack-year histories.6 The USPSTF also concluded that there was adequate evidence of moderate LCS-related harms (eg, invasive procedures and patient anxiety from false-positive results, overdiagnosis, and radiation exposure).5 This prompted the panel to recommend implementation of shared decision-making between patient and clinician, which includes discussion of potential benefits, limitations, and harms about screening.5
While additional trial evidence has accumulated about the mortality benefits of LCS,7 its uptake remains low in clinical settings. Only about 6.5% of eligible individuals were up to date (UTD) with LCS nationally in 2020 according to American College of Radiology (ACR) Lung Cancer Screening Registry data, with wide state-level variation from less than 4% in southern and western states to 14% to 15% in northeastern states.8,9 Major national and state-representative surveillance systems have measured LCS less regularly. The UTD-LCS prevalence was less than 4% according to the 2015 National Health Interview Survey (NHIS), but there is no planned update to LCS measurement in the NHIS until 2024.10 The LCS estimates from the state-based Behavioral Risk Factor Surveillance System (BRFSS), which until 2021, included LCS measurement as an optional state module, are generally higher: 7% to 21% across 19 states in 2019 to 21.2% across 4 states in 2021.11,12
In 2022, the BRFSS measured LCS across all states, allowing for the first time, an assessment of representative state-level LCS prevalence nationwide.13 Using these contemporary data, we estimated UTD-LCS prevalence across US states and nationally according to major demographic, socioeconomic, health care access, and health-related factors. Additionally, we studied the association of state-level LC mortality burden, health care access, and LCS capacity with UTD-LCS prevalence to inform policy and implementation efforts.
Methods
This cross-sectional study included data from the 2022 BRFSS survey, which is a state-based landline and cellular telephone survey that collected data in all 50 states, the District of Columbia, Guam, Puerto Rico, and the US Virgin Islands on health-related risk behaviors, chronic health conditions, health care access, and use of preventive services from the noninstitutionalized adult population (age ≥18 years).13 The response rates of analyzed states ranged from 33.9% to 66.8%, with a median rate of 45.1%. Because the BRFSS does not provide specific ages for respondents aged 80 years or older, we restricted our sample to those aged 50 to 79 years. State-level LC mortality rates from the National Center for Health Statistics,14 state Medicaid expansion status under the Patient Protection and Affordable Care Act as of December 2021,15 and number of screening facilities from the ACR Lung Cancer Screening Registry were also included.16 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies. The study was deemed exempt by the Morehouse School of Medicine institutional review board, with a waiver of informed consent, as it used deidentified public-use data.
Measures
The outcome measure was prevalence of UTD-LCS according to USPSTF 2021 eligibility criteria in participants aged 50 to 79 years (LCS eligible: ≥20 pack-years, currently smoking or formerly smoked with <15 years since quitting; LCS ineligible: <20 pack-years currently smoking or formerly smoked with ≥15 years since quitting). Those who responded “yes” to whether they “ever had a CT or CAT [computed axial tomography] scan of your chest area” and whether “any of the CT or CAT scans of your chest area were done mainly to check or screen for LC” were asked about the recency of the test. Those who responded “within the past year (anytime <12 months ago)” were considered UTD for LCS.
Covariates were identified from prior literature10,17 and included individual demographic variables (age group, self-identified sex and race and ethnicity, and urban vs rural county residence), socioeconomic status (educational level, annual household income), health care access variables (primary insurance type, usual source of care), and health status variables (number of comorbidities, diagnoses of cancer, chronic obstructive pulmonary disease, and cardiovascular disease). Race and ethnicity were analyzed to assess contemporary LCS differences across racial and ethnic groups, given previously noted inequities in LCS eligibility and LC mortality burden across these groups. Categories included American Indian or Alaska Native, non-Hispanic; Asian or Pacific Islander, non-Hispanic; Black, non-Hispanic; Hispanic; White, non-Hispanic; and multiracial. State-level variables included LCS capacity (defined as number of screening facilities in 2021 divided by the number of LCS-eligible individuals in the population estimated from the 2022 BRFSS survey), health care access (Medicaid expansion status: expansion vs nonexpansion), and LC burden (age-adjusted mortality rate per 100 000 persons among those aged 50-79 years from 2016 to 2020) and were ranked as high, medium, and low tertiles.
Statistical Analysis
Data were analyzed from October 1, 2023, to March 20, 2024. The UTD-LCS prevalence was estimated nationally, by state (50 states and the District of Columbia), and by selected covariates. To understand geographic state-level differences in UTD-LCS prevalence, state-specific estimates were compared with the unadjusted prevalence in the state with the lowest prevalence and with the national mean in logistic regression models with UTD-LCS as the outcome and state indicator variable as the main exposure while adjusting for age, sex, race and ethnicity, and educational level. State-level differences were estimated as adjusted prevalence ratios (APRs) and 95% CIs from contrasts of predicted marginals. Similar regression models were used to estimate differences in national UTD-LCS prevalence across respondent characteristics. Finally, the associations of state-level Medicaid expansion status and LCS capacity with UTD-LCS prevalence were estimated as APRs and 95% CIs from adjusted logistic regression models that included state fixed effects to account for unmeasured time-invariant state-level confounders. Pearson correlation coefficient was used to examine the correlation of state-level UTD-LCS prevalence with LC burden. All estimates were weighted using the BRFSS weighting variable to be population representative and accounted for complex survey sampling. Analyses were conducted in SAS and SAS-callable SUDAAN, version 9.4 (SAS Institute Inc) and RStudio, version 2022.06.1 + 524 (R Foundation for Statistical Computing) packages tidyverse, tigris, sf, ggplot2, and patchwork. All statistical tests were 2 sided, with significance set at P < .05.
Results
Among the 99 730 respondents to the 2022 BRFFS survey who were aged 50 to 79 years, 28 483 (corresponding to 13.17 million with population weighting) were eligible for screening according to USPSTF 2021 criteria after excluding incomplete eligibility and current or former smoking status data (n = 14 087). After excluding respondents with missing LCS data, the analytic sample to estimate UTD-LCS prevalence included 25 958 screening-eligible (missing, n = 2525) and 52 511 (missing, n = 4649) screening-ineligible respondents (eFigure 1 in Supplement 1). Of these individuals, 45.6% were female and 54.4% were male; median (IQR) age was 62 (11) years. A total of 1.7% were American Indian or Alaska Native; 1.6%, Asian or Pacific Islander; 8.1%, Black; 6.7%, Hispanic; 78.4%, White; and 3.5%, multiracial. Most were older (≥60 years: 64.4%), had lower socioeconomic status (high school education or less: 53.0%), lived in urban counties (89.5%), and reported currently smoking (61.5%), having private (36.3%) or Medicare (32.4%) plans as primary health insurance, and having a usual source of care (89.4%). A total of 81.2% reported 1 or more comorbidities, of whom 35.6% reported 3 or more comorbidities (Table 1). In addition to the screening-eligible sample, there were an additional 57 160 participants (representing 25.49 million persons) who had ever smoked but were ineligible for LCS. Of those with complete LCS test data (n = 52 511), 82.8% had formerly smoked (72.3% had ≥15 years since quitting; 10.5% had <15 years since quitting and <20 pack-years), and 17.1% currently smoked with less than a 20 pack-year history.
Table 1. Prevalence of UTD-LCS by Respondent Characteristics Among Screening-Eligible and Screening-Ineligible US Adults, 2022a.
| Variable | Sample, No. (weighted %) | Unadjusted UTD-LCS prevalence, weighted % (95% CI)b | APR (95% CI)c |
|---|---|---|---|
| Screening-eligible persons who ever smoked | |||
| Total, No. | 28 483 | NA | NA |
| Weighted No., millions | 13.17 | NA | NA |
| With complete LCS data, No. | 25 958 | 18.1 (17.2-19.1) | NA |
| Smoking history | |||
| Currently smoking, 20 pack-years | 15 961 (61.5) | 16.9 (15.8-18.1) | 0.91 (0.81-1.01) |
| Formerly smoked, <15 y since quitting, 20 pack-years | 9997 (38.5) | 20.0 (18.5-21.7) | 1.00 [Reference] |
| Sex | |||
| Female | 12 461 (45.6) | 17.9 (16.6-19.3) | 0.95 (0.85-1.05) |
| Male | 13 497 (54.4) | 18.4 (17.1-19.7) | 1.00 [Reference] |
| Age, yd | |||
| 50-54 | 3513 (15.8) | 6.7 (5.4-8.2) | 0.25 (0.19-0.31) |
| 55-59 | 4636 (19.8) | 12.7 (11.0-14.5) | 0.46 (0.39-0.54) |
| 60-69 | 11 338 (42.8) | 20.4 (19-21.9) | 0.74 (0.65-0.83) |
| 70-79 | 6471 (21.6) | 27.1 (24.6-29.7) | 1.00 [Reference] |
| Race and ethnicity | |||
| American Indian or Alaska Native, non-Hispanic | 587 (1.7) | 14.2 (9.7-20.3) | 0.83 (0.58-1.18) |
| Asian or Pacific Islander, non-Hispanic | 348 (1.6) | NAe | NAe |
| Black, non-Hispanic | 1296 (8.1) | 20.0 (16.4-24.2) | 1.04 (0.85-1.27) |
| Hispanic | 954 (6.7) | 16.6 (12.5-21.8) | 1.04 (0.77-1.39) |
| White, non-Hispanic | 21 447 (78.4) | 18.4 (17.4-19.4) | 1.00 [Reference] |
| Multiracial | 640 (3.5) | 12.8 (9.4-17.2) | 0.74 (0.55-1.01) |
| Annual household income, $ | |||
| <25 000 | 6664 (30.7) | 19.5 (17.6-21.4) | 1.08 (0.91-1.28) |
| 25 000 to <75 000 | 10 552 (45.3) | 18.9 (17.5-20.5) | 0.99 (0.85-1.15) |
| ≥75 000 | 4756 (24) | 17.1 (14.9-19.6) | 1.00 [Reference] |
| Educational level | |||
| Less than high school | 2590 (17.6) | 15.7 (13.7-18.0) | 0.96 (0.80-1.16) |
| High school or GED | 9282 (35.4) | 18.6 (17.1-20.2) | 1.07 (0.92-1.24) |
| Some college | 8737 (34.1) | 18.9 (17.2-20.6) | 1.06 (0.91-1.24) |
| College graduate | 5283 (12.9) | 18.1 (15.8-20.7) | 1.00 [Reference] |
| Residence location | |||
| Urban county | 21 100 (89.5) | 18.4 (17.4-19.4) | 1.03 (0.90-1.18) |
| Rural county | 4491 (10.5) | 16.9 (14.9-19.1) | 1.00 [Reference] |
| Health insurance type | |||
| Private | 7496 (36.3) | 14.4 (12.8-16.0) | 1.00 [Reference] |
| Medicare (age ≥65 y) | 8631 (32.4) | 26.3 (24.3-28.5) | 1.29 (1.10-1.51) |
| Medicaid | 3149 (14.5) | 16.2 (14.0-18.7) | 1.13 (0.94-1.36) |
| Other | 2248 (10.4) | 19.2 (16.4-22.4) | 1.14 (0.94-1.38) |
| Uninsured | 1240 (6.4) | 3.7 (2.4-5.6) | 0.31 (0.20-0.47) |
| Usual source of care | |||
| Yes | 23 267 (89.4) | 19.7 (18.7-20.8) | 1.00 [Reference] |
| No | 2524 (10.6) | 5.1 (3.4-7.5) | 0.30 (0.20-0.45) |
| Comorbidities, No.f | |||
| 0 | 4670 (18.9) | 8.7 (7.4-10.2) | 1.00 [Reference] |
| 1 | 6351 (23.8) | 14.3 (12.5-16.2) | 1.56 (1.27-1.93) |
| 2 | 5652 (21.8) | 20.1 (17.9-22.4) | 2.13 (1.74-2.61) |
| ≥3 | 9284 (35.6) | 24.6 (22.9-26.3) | 2.63 (2.19-3.15) |
| Cancer diagnosis | |||
| No | 21 516 (83.6) | 15.6 (14.6-16.6) | 1.00 [Reference] |
| Yes | 4239 (16.4) | 30.7 (27.9-33.6) | 1.78 (1.59-1.99) |
| COPD | |||
| No | 17 118 (67.1) | 12.1 (11.1-13.1) | 1.00 [Reference] |
| Yes | 8656 (32.9) | 30.6 (28.7-32.7) | 2.45 (2.21-2.71) |
| Asthma | |||
| No | 21 646 (82.8) | 17.5 (16.5-18.6) | 1.00 [Reference] |
| Yes | 4219 (17.2) | 20.7 (18.4-23.3) | 1.24 (1.09-1.42) |
| CVDg | |||
| No | 19 262 (76.1) | 17.1 (16.1-18.2) | 1.00 [Reference] |
| Yes | 5993 (23.9) | 21.1 (19.1-23.2) | 1.14 (1.01-1.28) |
| Screening-ineligible persons who ever smoked | |||
| Total, No. | 57 160 | NA | NA |
| Weighed No., millions | 25.49 | NA | NA |
| With complete LCS data, No. | 52 511 | 6.3 (5.8-6.8) | |
| Currently smoking, <20 pack-years | 7913 (17.1) | 9.5 (8.4-10.7) | 1.00 [Reference] |
| Formerly smoked, ≥15 y since quitting, ≥20 pack-years | 11 898 (20.7) | 8.7 (7.7-9.9) | 0.66 (0.53-0.82) |
| Formerly smoked, <15 y since quitting, <20 pack-years | 4348 (10.5) | 9.0 (6.6-12.2) | 1.01 (0.72-1.42) |
| Formerly smoked, ≥15 y since quitting, <20 pack-years | 28 352 (51.6) | 3.7 (3.2-4.2) | 0.34 (0.27-0.42) |
Abbreviations: APR, adjusted prevalence ratio; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; GED, General Education Diploma; LCS, lung cancer screening; NA, not applicable; UTD, up-to-date.
Among people aged 50 to 79 years according to US Preventive Services Task Force 2021 LCS criteria. Eligible: 20 or more pack-years, currently smoking or formerly smoked with less than 15 years since quitting; ineligible: less than 20 pack-years currently smoking or formerly smoked with 15 or more years since quitting.
Estimate includes 50 states, the District of Columbia, Puerto Rico, US Virgin Islands, and Guam.
Models were adjusted for age, sex, race and ethnicity, educational level, and region.
The 80-year-old respondents were not included as Behavioral Risk Factor Surveillance System collapses data for respondents aged 80 years or older.
Suppressed due to statistical instability defined as a relative SE greater than 30.
Comorbidity number based on report of ever diagnosis of myocardial infarction, coronary heart disease, stroke, asthma, COPD, arthritis, depression, kidney disease, diabetes, or cancer.
Ever diagnosed with myocardial infarction, coronary heart disease, or stroke.
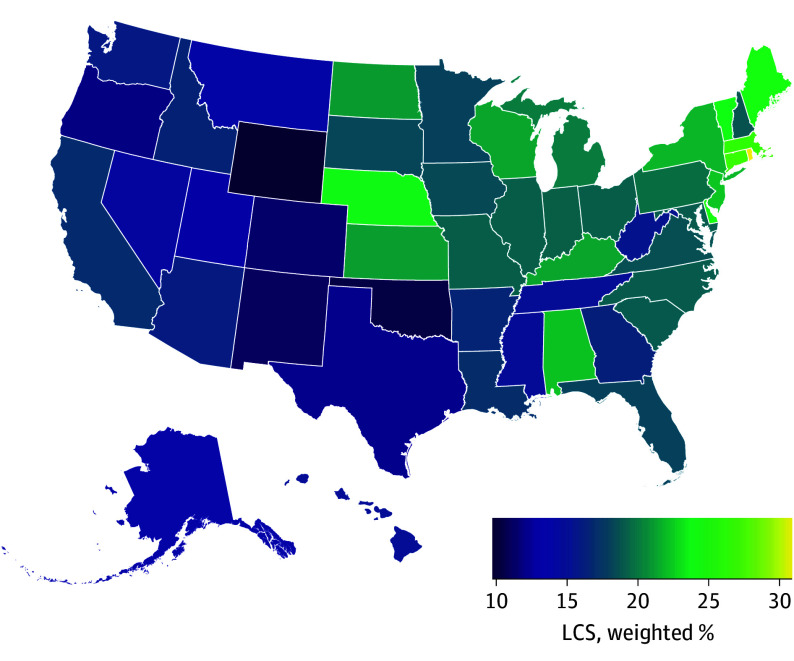
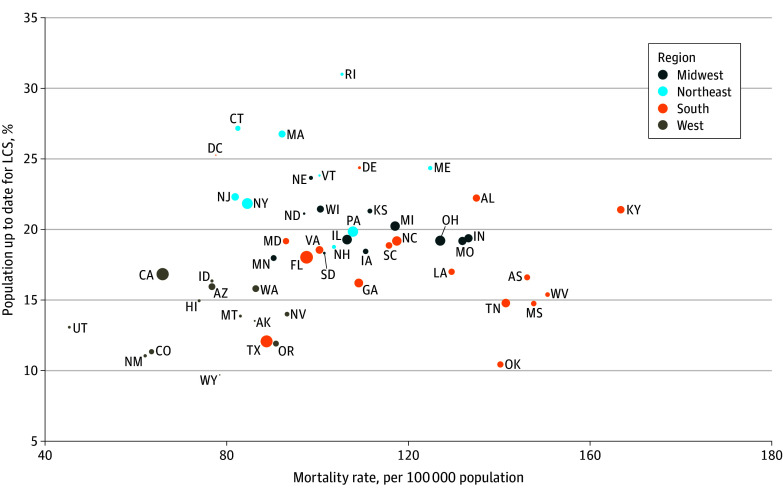
Nationally, among LCS-eligible adults in 2022, 18.1% (95% CI, 17.2%-19.1%), or 2.17 million (95% CI, 2.10 million-2.24 million), reported being UTD with LCS (Table 1). The UTD-LCS prevalence was 18.6% (95% CI, 17.2%-20.0%) when restricted to eligible persons without a prior LC diagnosis in 29 states with these data. Across the US states, there was wide variation in UTD-LCS prevalence (highest in Rhode Island: 31.0%; lowest in Wyoming: 9.7%; APR, 2.95; 95% CI, 1.93-4.49) (Figure 1 and Table 2). The UTD-LCS prevalence was not correlated with state-level LC mortality burden (r = 0.16; P = .26) (Table 2 and Figure 2). The UTD-LCS tended to be lower in southern states despite a higher LC burden in this region. For example, 15 of 17 southern states had high or medium LC burden, but only 2 of these states had UTD-LCS prevalence significantly above the national mean. Conversely, all 9 northeastern states had low or medium LC burden, but UTD-LCS prevalence was significantly above the national mean in 7 of these states. The UTD-LCS prevalence was correlated with LC burden to a larger degree in the midwestern region, where there was high or medium burden and above-mean UTD-LCS prevalence, and in the western region, where there was low burden and below-mean UTD-LCS prevalence.
Figure 1. State-Level Prevalence of Up-to-Date Lung Cancer Screening (LCS) in the US, 2022.
Data are for people aged 50 to 79 years who were eligible for LCS according to 2021 US Preventive Services Task Force eligibility criteria (≥20 pack-years, currently smoking or formerly smoked with <15 years since quitting).
Table 2. Regional and State-Level Prevalence of UTD-LCS and LC Burden in the US, 2022.
| Variable | UTD-LCSa | LC burden | ||||
|---|---|---|---|---|---|---|
| Weighted unadjusted % (95% CI) | APR (95% CI)b | Rankc | Age-adjusted mortality rate, per 100 000 persons aged 50-79 y | Rankc | ||
| Reference: state with lowest prevalence | Reference: national mean | |||||
| Northeast | ||||||
| Mean | 22.4 (19.8-25.1) | 1.51 (1.25-1.82)d | 1.22 (1.10-1.35) | NA | 98.1 | NA |
| Rhode Island | 31.0 (25.0-37.8) | 2.95 (1.93-4.49) | 1.71 (1.39-2.10) | High | 105.4 | Medium |
| Massachusetts | 26.8 (20.9-33.6) | 2.46 (1.58-3.82) | 1.42 (1.12-1.80) | High | 92.2 | Medium |
| Connecticut | 27.2 (21.2-34.0) | 2.54 (1.64-3.94) | 1.47 (1.16-1.87) | High | 82.4 | Low |
| Vermont | 23.8 (17.8-31.1) | 2.32 (1.46-3.69) | 1.34 (1.01-1.78) | High | 100.4 | Medium |
| Maine | 24.4 (20.4-28.7) | 2.40 (1.60-3.59) | 1.38 (1.16-1.64) | High | 124.9 | High |
| New Jersey | 22.3 (14.6-32.5) | 2.01 (1.17-3.45) | 1.17 (0.79-1.73) | High | 81.9 | Low |
| New York | 21.8 (18.1-26.0) | 2.16 (1.43-3.26) | 1.25 (1.05-1.50) | High | 84.5 | Low |
| Pennsylvania | 19.9 (14.5-26.5) | 1.82 (1.14-2.92) | 1.05 (0.79-1.39) | High | 107.8 | Medium |
| New Hampshire | 18.8 (14.5-24.1) | 1.83 (1.18-2.86) | 1.06 (0.82-1.36) | Medium | 103.7 | Medium |
| Midwest | ||||||
| Mean | 19.7 (18.1-21.3) | 1.36 (1.15-1.61)d | 1.09 (1.02-1.18) | NA | 110.6 | NA |
| Nebraska | 23.7 (18.9-29.1) | 2.29 (1.50-3.51) | 1.32 (1.06-1.65) | High | 98.6 | Medium |
| Wisconsin | 21.4 (17.9-25.5) | 2.03 (1.35-3.05) | 1.17 (0.98-1.40) | High | 100.7 | Medium |
| Kansas | 21.3 (17.3-25.9) | 2.14 (1.41-3.24) | 1.23 (1.01-1.51) | High | 111.5 | High |
| North Dakota | 21.1 (15.9-27.5) | 1.98 (1.25-3.14) | 1.14 (0.86-1.51) | High | 97.1 | Medium |
| Michigan | 20.2 (16.9-24.1) | 1.88 (1.25-2.83) | 1.08 (0.91-1.30) | High | 117.2 | High |
| Indiana | 19.4 (16.3-22.9) | 1.96 (1.30-2.93) | 1.13 (0.95-1.34) | Medium | 133.3 | High |
| Illinois | 19.3 (12.7-28.1) | 1.79 (1.04-3.07) | 1.03 (0.70-1.52) | Medium | 106.6 | Medium |
| Missouri | 19.2 (15.2-23.9) | 1.77 (1.15-2.73) | 1.02 (0.82-1.28) | Medium | 132 | High |
| Ohio | 19.2 (16.2-22.6) | 1.98 (1.33-2.95) | 1.14 (0.98-1.33) | Medium | 127.1 | High |
| Iowa | 18.4 (14.8-22.6) | 1.71 (1.12-2.63) | 0.99 (0.80-1.23) | Medium | 110.6 | High |
| South Dakota | 18.3 (10.4-30.4) | 1.69 (0.88-3.24) | 0.97 (0.57-1.67) | Medium | 101.6 | Medium |
| Minnesota | 18.0 (14.8-21.6) | 1.71 (1.13-2.58) | 0.99 (0.81-1.19) | Medium | 90.3 | Low |
| South | ||||||
| Mean | 16.8 (15.4-18.4) | 1.16 (0.97-1.39)d | 0.94 (0.88-1.00) | NA | 121.6 | NA |
| District of Columbia | 25.3 (14.2-40.8) | 2.36 (1.20-4.63) | 1.37 (0.79-2.39) | High | 77.6 | Low |
| Delaware | 24.4 (18.0-32.2) | 2.38 (1.49-3.79) | 1.37 (1.03-1.83) | High | 109.3 | Medium |
| Alabama | 22.2 (17.0-28.5) | 2.22 (1.42-3.48) | 1.28 (1.00-1.65) | High | 135 | High |
| North Carolina | 19.2 (14.0-25.7) | 1.82 (1.12-2.94) | 1.05 (0.78-1.42) | Medium | 117.4 | High |
| Kentucky | 21.4 (16.1-27.8) | 1.97 (1.25-3.12) | 1.14 (0.87-1.50) | High | 166.9 | High |
| Virginia | 18.6 (14.8-23.0) | 1.80 (1.17-2.78) | 1.04 (0.83-1.30) | Medium | 100.4 | Medium |
| Maryland | 19.2 (15.4-23.7) | 1.80 (1.17-2.76) | 1.04 (0.83-1.29) | Medium | 93.1 | Medium |
| South Carolina | 18.9 (14.9-23.6) | 1.93 (1.25-2.96) | 1.11 (0.89-1.39) | Medium | 115.7 | High |
| Florida | 18.0 (13.9-23.0) | 1.69 (1.08-2.62) | 0.98 (0.77-1.24) | Medium | 97.6 | Medium |
| Louisiana | 17.0 (12.9-22.1) | 1.68 (1.07-2.66) | 0.97 (0.75-1.27) | Medium | 129.6 | High |
| Arkansas | 16.6 (12.9-21.2) | 1.61 (1.03-2.51) | 0.93 (0.72-1.20) | Medium | 146.2 | High |
| Georgia | 16.2 (12.3-21.1) | 1.44 (0.91-2.27) | 0.83 (0.63-1.09) | Low | 109.0 | Medium |
| West Virginia | 15.4 (12.2-19.3) | 1.59 (1.03-2.45) | 0.91 (0.72-1.15) | Low | 150.7 | High |
| Tennessee | 14.8 (10.5-20.4) | 1.56 (0.96-2.56) | 0.90 (0.65-1.24) | Low | 141.5 | High |
| Mississippi | 14.8 (10.4-20.6) | 1.45 (0.87-2.43) | 0.84 (0.59-1.20) | Low | 147.6 | High |
| Texas | 12.1 (8.2-17.4) | 1.15 (0.68-1.94) | 0.67 (0.47-0.95) | Low | 88.8 | Low |
| Oklahoma | 10.5 (7.7-14.0) | 1.06 (0.66-1.7) | 0.61 (0.45-0.82) | Low | 140.3 | High |
| West | ||||||
| Mean | 15.1 (12.7-17.8) | 1 [Reference] | 0.81 (0.71-0.93) | NA | 75.6 | NA |
| California | 16.8 (11.6-23.9) | 1.52 (0.94-2.48) | 0.89 (0.66-1.20) | Medium | 65.9 | Low |
| Idaho | 16.4 (11.1-23.4) | 1.41 (0.84-2.37) | 0.81 (0.56-1.18) | Medium | 76.8 | Low |
| Arizona | 15.9 (12.0-20.8) | 1.45 (0.91-2.31) | 0.84 (0.63-1.12) | Low | 76.8 | Low |
| Washington | 15.8 (13.5-18.5) | 1.48 (0.99-2.2) | 0.85 (0.72-1.00) | Low | 86.4 | Low |
| Hawaii | 15.0 (9.5-22.7) | 1.31 (0.67-2.57) | 0.77 (0.45-1.34) | Low | 73.9 | Low |
| Montana | 13.9 (10.0-18.9) | 1.40 (0.86-2.26) | 0.81 (0.59-1.10) | Low | 83.0 | Low |
| Nevada | 14.0 (8.9-21.4) | 1.25 (0.70-2.23) | 0.73 (0.47-1.13) | Low | 93.3 | Medium |
| Alaska | 13.5 (9.6-18.8) | 1.40 (0.85-2.31) | 0.81 (0.58-1.14) | Low | 86.2 | Low |
| Utah | 13.1 (8.6-19.5) | 1.34 (0.77-2.33) | 0.78 (0.51-1.17) | Low | 45.3 | Low |
| Oregon | 11.9 (8.6-16.4) | 1.16 (0.70-1.91) | 0.67 (0.48-0.94) | Low | 90.8 | Medium |
| Colorado | 11.4 (7.7-16.4) | 1.11 (0.65-1.88) | 0.64 (0.44-0.94) | Low | 63.4 | Low |
| New Mexico | 11.1 (6.3-18.7) | 1.04 (0.52-2.08) | 0.61 (0.34-1.09) | Low | 62.0 | Low |
| Wyoming | 9.7 (6.7-14.0) | 1.00 [Reference] | 0.58 (0.40-0.84) | Low | 78.4 | Low |
Abbreviations: APR, adjusted prevalence ratio; LC, lung cancer; LCS, lung cancer screening; NA, not applicable; UTD, up-to-date.
Data are for people aged 50 to 79 years who were eligible for LCS according to 2021 US Preventive Services Task Force eligibility criteria (≥20 pack-years, currently smoking or formerly smoked with <15 years since quitting).
Models included 50 states and the District of Columbia only and were adjusted for age, sex, race and ethnicity, and educational level.
Rank is based on tertile categorization.
Comparison is with the western region.
Figure 2. Lung Cancer (LC) Mortality Rate and to Up-to-Date LC Screening (LCS) Prevalence in the 50 US States and District of Columbia, 2022.
Data are for people aged 50 to 79 years who were eligible for LCS according to 2021 US Preventive Services Task Force eligibility criteria (≥20 pack-years, currently smoking or formerly smoked with <15 years since quitting). Circle size is proportional to state-specific LC death counts.
State-level Medicaid expansion and LCS capacity were positively associated with UTD-LCS prevalence. The UTD-LCS prevalence was higher among the 39 Medicaid expansion states than among 12 nonexpansion states (8 southern, 3 midwestern, and 1 western) (eTable in Supplement 1) among adults aged 50 to 64 years (APR, 2.68; 95% CI, 1.30-5.53) and among adults aged 50 to 64 years (APR, 2.92; 95% CI, 1.04-8.19) with lower income (Table 3). Compared with lower-level LCS capacity states, UTD-LCS prevalence was higher in states with high-level (APR, 1.93; 95% CI, 1.36-2.75) and medium-level (APR, 1.44; 95% CI, 1.00-2.07) capacity (Table 3 and eFigure 2 and the eTable in Supplement 1).
Table 3. Association of UTD-LCS Prevalence With State-Level Health Care Access and Screening Capacity in the US, 2022a.
| State-level factor | No. | UTD-LCS, % (95% CI)b | APR (95% CI)b | ||
|---|---|---|---|---|---|
| States | Sample | Weighted unadjusted | Adjusted | ||
| Health care access c | |||||
| Age 50-64 y, all income levels | |||||
| Expansion state | 39 | 10 328 | 14.0 (12.8-15.3) | 19.4 (13.8-26.7) | 2.68 (1.30-5.53) |
| Nonexpansion state | 12 | 3493 | 12.3 (10.4-14.5) | 7.3 (4.8-10.9) | 1.00 [Reference] |
| Age 50-64 y, income<$25 000 | |||||
| Expansion state | 39 | 2660 | 18.9 (16.2-21.9) | 24.9 (5.9-15.2) | 2.92 (1.04-8.19) |
| Nonexpansion state | 12 | 902 | 13.2 (9.5-18) | 8.5 (2.7-4.5) | 1.00 [Reference] |
| LC screening capacity d | |||||
| Low | 17 | 8105 | 16.2 (14.6-17.8) | 13.9 (11.3-16.9) | 1.00 [Reference] |
| Medium | 17 | 8942 | 19.5 (18.0-21.1) | 20.0 (16.5-23.9) | 1.44 (1.00-2.07) |
| High | 17 | 8550 | 20.0 (18.5-21.6) | 26.8 (21.8-32.4) | 1.93 (1.36-2.75) |
Abbreviations: APR, adjusted prevalence ratio; LC, lung cancer; LCS, lung cancer screening; UTD, up-to-date.
Data are for people aged 50 to 79 years who were eligible for LCS according to 2021 US Preventive Services Task Force eligibility criteria (≥20 pack-years, currently smoking or formerly smoked with <15 years since quitting).
Models include 50 states and District of Columbia only and adjusted for age, sex, race and ethnicity, educational level, region, and state fixed effects.
Medicaid expansion status.
Number of LCS facilities per LCS-eligible adults. Rank is based on tertile categorization.
At the national level, UTD-LCS prevalence was statistically similar between persons who currently smoked (≥20 pack-years) at 16.9% compared with 20.0% among persons who formerly smoked (<15 years since quitting) (P = .07) (Table 1). The UTD-LCS prevalence increased with increasing age (6.7% [95% CI, 5.4%-8.2%] among those aged 50-54 years vs 27.1% [95% CI, 24.6%-29.7%] among those aged 70-79 years; APR, 0.25; 95% CI, 0.19-0.31; P < .001) and comorbidity burden (≥3 conditions: 24.6% [95% CI, 22.9%-26.3%]; no conditions: 8.7% [95% CI, 7.4%-10.2%]; P < .001), but there was no difference between females and males (17.9% [95% CI, 16.6%-19.3%] vs 18.4% [95% CI, 17.1%-19.7%]; P = .30) or between residents of urban and rural counties (18.4% [17.4%-19.4%] vs 16.9% [95% CI, 14.9%-19.1%]; P = .69). The UTD-LCS prevalence was similar across income and educational levels (less than high school: 15.7% [95% CI, 13.7%-18.0%]; college: 18.1% [95% CI, 15.8%-20.7%]; P = .69) but varied widely by health care access; it was lowest among uninsured persons (3.7% [95% CI, 2.4%-5.6%]; APR vs private, 0.31; 95% CI, 0.20-0.47; P < .001), similar among privately insured (14.4%; 95% CI, 12.8%-16.0%) and Medicaid-insured persons (16.2%; 95% CI, 14.0%-18.7%; P = .20), and highest among Medicare-insured individuals (26.3% [95% CI, 24.3%-28.5%]; APR vs private, 1.29; 95% CI, 1.10-1.51; P = .001). Respondents with a usual source of care were significantly more likely to be UTD for LCS (19.7%; 95% CI, 18.7%-20.8%) than were those without a usual source of care (5.1% [95% CI, 3.4%-7.5%]) (APR, 0.30; 95% CI, 0.20-0.45; P < .001).
In the sample of participants who had a smoking history but were ineligible for LCS, UTD-LCS prevalence was 9.5% (95% CI, 8.4%-10.7%) among those currently smoking with less than a 20 pack-year history (Table 1). Among LCS-ineligible persons with former smoking status, UTD-LCS prevalence was 8.7% (95% CI, 7.7%-9.9%) among those with 15 or more years since quitting and 20 or more pack-years, 9.0% (95% CI, 6.6%-12.2%) among those with less than 15 years since quitting and less than 20 pack-years, and 3.7% (95% CI, 3.2%-4.2%) among those with 15 or more years since quitting and less than 20 pack-years.
Discussion
This study found that a decade after the USPSTF initially recommended annual LCS, an estimated 18.1% of eligible US adults were UTD with LCS in 2022, with prevalence varying nearly 3-fold across US states. Notably, UTD-LCS prevalence was discordant with LC burden; for example, southern states had higher LC burden but lower UTD-LCS prevalence. Nationally, 3.7% of LCS-eligible persons without insurance and 5.1% without a usual source of care reported being UTD with LCS, but state-level Medicaid expansion and higher screening capacity were associated with higher prevalence, pointing to potential policy and implementation levers to improve LCS levels.
Comparing UTD-LCS prevalence from this study with previously published estimates is challenging because of evolving LCS guideline criteria and differences in data collection methods. This study’s UTD-LCS prevalence estimate of 18.1% is arguably low and may reflect several barriers, including patient and physician concerns regarding LCS-related harms vs benefits18,19; low acceptance, awareness, and knowledge of LCS19,20; and health care coverage and infrastructure inadequacies.21,22 Yet, 18.1% is substantially higher than previously published population-based UTD-LCS estimates,8,9,10,11,12,17 reflecting in part improvements in policy-, systems-, practitioner-, and patient-level factors over time.22,23,24 For example, the National Coverage Determination covering LCS without co-pay in Medicare-eligible populations has been associated with significant LCS uptake23 and was possibly reflected in the significantly higher UTD-LCS prevalence among Medicare-insured persons compared with persons with other insurance types in this study. Similarly, screening capacity measured using ACR-registered facility density has increased over time,22 with 26.5 facilities per 100 000 screening-eligible persons in 2022 compared with 15.9 in 2016.16
Yet, variation in some of these same factors may have also contributed to the observed 3-fold difference in UTD-LCS prevalence across states. For example, we showed that state Medicaid expansions were cross-sectionally associated with higher UTD-LCS prevalence. This finding may potentially explain the comparable UTD-LCS prevalence that we also found among Medicaid-insured and privately insured persons nationally, likely reflecting the Patient Protection and Affordable Care Act requirement for plans to cover USPSTF-recommended preventive services without co-pay. This finding is also consistent with a large body of evidence supporting expansions of public insurance to improve access to critical cancer preventive services among persons with health care access barriers.25,26,27 Additionally, higher state LCS capacity was associated with higher UTD-LCS prevalence, consistent with prior registry-based studies.8,22 With few exceptions, high LC burden states with lower-than-mean UTD-LCS prevalence were southern or midwestern states that had not expanded Medicaid and/or had low screening capacity. Improving health care access, including through Medicaid expansions and removal of cost barriers to follow-up testing and treatment, will be particularly useful among underserved and minoritized racial and ethnic groups who are disproportionately affected by access barriers.21,28 Also needed are institutional and societal efforts to address LCS capacity, including screening infrastructure investments and quality benchmarking to incentivize implementation.21,29 Ultimately, multilevel initiatives that span patient, practitioner, system, and societal levels may be necessary to match LCS need with delivery.21
Published studies indicate that eligibility disparities between Black and White persons noted with previous guidelines will likely be reduced under the expanded 2021 criteria.5,17,30,31,32,33 Our results show that UTD-LCS prevalence according to the expanded criteria was statistically similar among racial and ethnic groups. Yet, newly eligible Black individuals and other high-risk racial and ethnic minority individuals are more likely to be younger and uninsured compared with White persons,17 highlighting the need to address insurance and cost barriers in these groups. Additionally, efforts should ensure that inequities do not arise because of barriers elsewhere in the screening continuum (eg, follow-up, cessation and oncologic treatment, and screening and treatment quality), which may further exacerbate noted racial and ethnic inequities in LC mortality burden.28,32,34
Among screening-eligible persons nationally, we found that that UTD-LCS prevalence was higher among older persons than among younger persons, a finding consistent with other studies.10,17 As evidence emerges of shifting late-stage diagnosis burden to younger ages,35 this finding suggests the need for targeted demand (eg, education campaigns) and delivery (eg, addressing lower health care coverage, leveraging allied health professionals) interventions among younger LCS-eligible persons. Conversely, after adjusting for age, we observed higher UTD-LCS prevalence with increasing number of comorbidities. This finding may indicate that recommendations to discontinue screening in the presence of marked comorbidities that limit life expectancy are not being strictly followed.36 Patient-practitioner communication weighing patient values and preferences with information regarding risks and benefits from the practitioner may be central to guide decision-making in such contexts.
A total of 72.3% of persons who were ineligible for LCS according to the 2021 USPSTF criteria had quit smoking 15 or more years earlier. However, evidence suggests that cancer risk associated with never smoking remains elevated even 30 or more years after quitting.37,38 Additionally, screening-ineligible persons with lower-risk smoking histories are overrepresented among racial and ethnic minority groups, who have higher LC risk even at lower smoking intensities.31,32,39 The UTD-LCS prevalence among screening-ineligible persons, while lower than among eligible persons, still ranged from 4% to 10%. This finding highlights the challenge of implementing eligibility criteria based on age, smoking intensity, and years since quitting that were originally derived from the National Lung Cancer Screening Trial in clinical practices. The American Cancer Society’s 2023 LCS guideline update eliminated years since quitting as an eligibility criterion based on modeling showing increased relative mortality benefit with no loss in screening efficiency.36,40,41 As LCS implementation ramps up, studies are needed to understand the benefits vs harms of modifying eligibility within clinical population-based settings and to ensure equal treatment for equal risk. Additionally, the potential for integrating risk calculators, which have been shown to improve screening outcomes while also reducing disparities,42,43 should be explored.
Limitations
This study has limitations. Measurement bias from LCS self-report (eg, inaccurate recall of screening specifically for LC, unawareness of the difference between screening and surveillance testing) and survey-related biases (nonresponse, social desirability) could be present in the BRFSS. However, self-reported data for other screening measures are generally accurate,44 and our overall findings regarding state patterns of LCS prevalence and its association with LCS capacity align with prior registry-based studies.8 We could also not exclude those who had a prior LC diagnosis because states did not uniformly assess this measure. However, UTD-LCS prevalence among eligible adults without an LC diagnosis in 29 states with these data was similar to the overall study estimate. Weighted population estimates of eligible and ineligible individuals may be underestimates because we excluded incomplete data on pack-years and years since quitting. The UTD-LCS prevalence may be overestimated as 80-year-old LCS-eligible respondents could not be included. We expect the potential overestimation to be minimal because 80-year-old respondents constituted approximately 0.81% of the USPSTF 2021 LCS-eligible sample in NHIS 2015 data. The association demonstrated between state-level factors and UTD-LCS prevalence is cross-sectional and precludes causal conclusions. Future longitudinal and quasi-experimental studies are needed to evaluate these and other intervenable state-level factors in relation to LCS uptake. Finally, LCS capacity, as measured in this study, does not consider facility characteristics (eg, size, volume) or account for transportation distances to screening facilities, which is an important barrier in states with large rural areas.45 Despite these limitations, to our knowledge, this study is the first representative state-level assessment of LCS nationwide based on a large population sample that allowed a comprehensive assessment across several relevant factors, including policy and implementation factors.
Conclusions
This cross-sectional study found that a decade after the LCS was initially recommended, in 2022, UTD-LCS prevalence remained low; 18.1% of eligible adults were UTD with recommended LCS nationally. The largest disparities in UTD-LCS were according to health care access factors and across US states, with state-level LCS discordant with LC burden, particularly in high-burden southern states. Therefore, improving health care access for persons with low income through Medicaid expansion and increasing screening capacity may be associated with increased uptake and reduced disparities.
eFigure 1. Sample Selection
eFigure 2. Lung Cancer Screening Capacity in Relation to UTD-LCS Prevalence, US States, 2022
eTable. State-Level Screening Capacity, and Healthcare Access, United States, 2022
Data Sharing Statement
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi: 10.3322/caac.21820 [DOI] [PubMed] [Google Scholar]
- 2.Kratzer TB, Bandi P, Freedman ND, et al. Lung cancer statistics, 2023. Cancer. 2024;130(8):1330-1348. doi: 10.1002/cncr.35128 [DOI] [PubMed] [Google Scholar]
- 3.Reduced lung-cancer mortality with low-dose computed tomographic screening . N Engl J Med. 2011;365(5):395-409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moyer VA; U.S. Preventive Services Task Force . Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi: 10.7326/M13-2771 [DOI] [PubMed] [Google Scholar]
- 5.Krist AH, Davidson KW, Mangione CM, et al. ; US Preventive Services Task Force . Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi: 10.1001/jama.2021.1117 [DOI] [PubMed] [Google Scholar]
- 6.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi: 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 7.Becker N, Motsch E, Trotter A, et al. Lung cancer mortality reduction by LDCT screening—results from the randomized German LUSI trial. Int J Cancer. 2020;146(6):1503-1513. doi: 10.1002/ijc.32486 [DOI] [PubMed] [Google Scholar]
- 8.Fedewa SA, Kazerooni EA, Studts JL, et al. State variation in low-dose computed tomography scanning for lung cancer screening in the United States. J Natl Cancer Inst. 2021;113(8):1044-1052. doi: 10.1093/jnci/djaa170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fedewa SA, Bandi P, Smith RA, Silvestri GA, Jemal A. Lung cancer screening rates during the COVID-19 pandemic. Chest. 2022;161(2):586-589. doi: 10.1016/j.chest.2021.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jemal A, Fedewa SA. Lung cancer screening with low-dose computed tomography in the United States—2010 to 2015. JAMA Oncol. 2017;3(9):1278-1281. doi: 10.1001/jamaoncol.2016.6416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Pan IE, Tak HJ, Vlahos I, Volk R, Shih YT. Assessment of uptake appropriateness of computed tomography for lung cancer screening according to patients meeting eligibility criteria of the US Preventive Services Task Force. JAMA Netw Open. 2022;5(11):e2243163. doi: 10.1001/jamanetworkopen.2022.43163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maki KG, Tan NQP, Toumazis I, Volk RJ. Prevalence of lung cancer screening among eligible adults in 4 US states in 2021. JAMA Netw Open. 2023;6(6):e2319172. doi: 10.1001/jamanetworkopen.2023.19172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention . Behavioral Risk Factor Surveillance System survey data, 2022. Accessed January 19, 2024. https://www.cdc.gov/brfss/annual_data/annual_2022.html
- 14.National Center for Health Statistics, Centers for Disease Control and Prevention . Detailed mortality—all counties, 2003-2021, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. 2023. Accessed November 14, 2023. https://www.cdc.gov/nchs/data_access/vitalstatsonline.htm#Mortality_Multiple/
- 15.Kaiser Family Foundation . Status of state Medicaid expansion decisions: interactive map. 2023. Accessed December 5, 2023. https://www.kff.org/medicaid/issue-brief/status-of-state-medicaid-expansion-decisions-interactive-map/
- 16.American College of Radiology . LCSR state reports. 2023. Accessed December 5, 2023. https://nrdrsupport.acr.org/support/solutions/articles/11000093991
- 17.Lozier JW, Fedewa SA, Smith RA, Silvestri GA. Lung cancer screening eligibility and screening patterns among Black and White adults in the United States. JAMA Netw Open. 2021;4(10):e2130350. doi: 10.1001/jamanetworkopen.2021.30350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seehusen DA. Should family physicians routinely screen for lung cancer in high-risk populations? no: the USPSTF’s recommendation for lung cancer screening is overreaching. Am Fam Physician. 2014;90(2):73D-74D. [PubMed] [Google Scholar]
- 19.Kota KJ, Ji S, Bover-Manderski MT, Delnevo CD, Steinberg MB. Lung cancer screening knowledge and perceived barriers among physicians in the United States. JTO Clin Res Rep. 2022;3(7):100331. doi: 10.1016/j.jtocrr.2022.100331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavers D, Nelson M, Rostron J, et al. Understanding patient barriers and facilitators to uptake of lung screening using low dose computed tomography: a mixed methods scoping review of the current literature. Respir Res. 2022;23(1):374. doi: 10.1186/s12931-022-02255-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osarogiagbon RU, Yang PC, Sequist LV. Expanding the reach and grasp of lung cancer screening. Am Soc Clin Oncol Educ Book. 2023;43:e389958. doi: 10.1200/EDBK_389958 [DOI] [PubMed] [Google Scholar]
- 22.Kale MS, Wisnivesky J, Taioli E, Liu B. The landscape of US lung cancer screening services. Chest. 2019;155(5):900-907. doi: 10.1016/j.chest.2018.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson LM, Benefield TS, Bearden SC, et al. Changes in physician knowledge, attitudes, beliefs, and practices regarding lung cancer screening. Ann Am Thorac Soc. 2019;16(8):1065-1069. doi: 10.1513/AnnalsATS.201812-867RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun J, Perraillon MC, Myerson R. The impact of Medicare health insurance coverage on lung cancer screening. Med Care. 2022;60(1):29-36. doi: 10.1097/MLR.0000000000001655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao J, Mao Z, Fedewa SA, et al. The Affordable Care Act and access to care across the cancer control continuum: a review at 10 years. CA Cancer J Clin. 2020;70(3):165-181. doi: 10.3322/caac.21604 [DOI] [PubMed] [Google Scholar]
- 26.Moss HA, Wu J, Kaplan SJ, Zafar SY. The Affordable Care Act’s Medicaid expansion and impact along the cancer-care continuum: a systematic review. J Natl Cancer Inst. 2020;112(8):779-791. doi: 10.1093/jnci/djaa043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fedewa SA, Yabroff KR, Smith RA, Goding Sauer A, Han X, Jemal A. Changes in breast and colorectal cancer screening after Medicaid expansion under the Affordable Care Act. Am J Prev Med. 2019;57(1):3-12. doi: 10.1016/j.amepre.2019.02.015 [DOI] [PubMed] [Google Scholar]
- 28.Dwyer LL, Vadagam P, Vanderpoel J, Cohen C, Lewing B, Tkacz J. Disparities in lung cancer: a targeted literature review examining lung cancer screening, diagnosis, treatment, and survival outcomes in the United States. J Racial Ethn Health Disparities. Published online May 19, 2023. doi: 10.1007/s40615-023-01625-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Committee for Quality Assurance . New measure coming for lung cancer screening. 2022. Accessed March 12, 2024. https://www.ncqa.org/blog/new-measure-coming-for-lung-cancer-screening/
- 30.Pu CY, Lusk CM, Neslund-Dudas C, Gadgeel S, Soubani AO, Schwartz AG. Comparison between the 2021 USPSTF lung cancer screening criteria and other lung cancer screening criteria for racial disparity in eligibility. JAMA Oncol. 2022;8(3):374-382. doi: 10.1001/jamaoncol.2021.6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF lung cancer screening guidelines among African American adult smokers. JAMA Oncol. 2019;5(9):1318-1324. doi: 10.1001/jamaoncol.2019.1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haddad DN, Sandler KL, Henderson LM, Rivera MP, Aldrich MC. Disparities in lung cancer screening: a review. Ann Am Thorac Soc. 2020;17(4):399-405. doi: 10.1513/AnnalsATS.201907-556CME [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinsky PF, Lau YK, Doubeni CA. Potential disparities by sex and race or ethnicity in lung cancer screening eligibility rates. Chest. 2021;160(1):341-350. doi: 10.1016/j.chest.2021.01.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kearney L, Nguyen T, Steiling K. Disparities across the continuum of lung cancer care: a review of recent literature. Curr Opin Pulm Med. Published online February 26, 2024. doi: 10.1097/MCP.0000000000001064 [DOI] [PubMed] [Google Scholar]
- 35.Potter A, Senthil P, Mansur A, Mathey-Andrews C, Auchincloss H, Yang CFJ. Early diagnosis of lung cancer among younger vs. older adults: widening disparities in the era of lung cancer screening. Paper presented at: International Association for the Study of Lung Cancer World Conference on Lung Cancer; August 7, 2022; Vienna, Austria. [Google Scholar]
- 36.Wolf AMD, Oeffinger KC, Shih TY, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2024;74(1):50-81. doi: 10.3322/caac.21811 [DOI] [PubMed] [Google Scholar]
- 37.Thomson B, Islami F. Association of smoking cessation and cardiovascular, cancer, and respiratory mortality. JAMA Intern Med. 2024;184(1):110-112. doi: 10.1001/jamainternmed.2023.6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kondo KK, Rahman B, Ayers CK, Relevo R, Griffin JC, Halpern MT. Lung cancer diagnosis and mortality beyond 15 years since quit in individuals with a 20+ pack-year history: a systematic review. CA Cancer J Clin. 2024;74(1):84-114. doi: 10.3322/caac.21808 [DOI] [PubMed] [Google Scholar]
- 39.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333-342. doi: 10.1056/NEJMoa033250 [DOI] [PubMed] [Google Scholar]
- 40.Landy R, Cheung LC, Young CD, Chaturvedi AK, Katki HA. Absolute lung cancer risk increases among individuals with >15 quit-years: analyses to inform the update of the American Cancer Society lung cancer screening guidelines. Cancer. 2024;130(2):201-215. doi: 10.1002/cncr.34758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meza R, Cao P, de Nijs K, et al. Assessing the impact of increasing lung screening eligibility by relaxing the maximum years-since-quit threshold: a simulation modeling study. Cancer. 2024;130(2):244-255. doi: 10.1002/cncr.34925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landy R, Young CD, Skarzynski M, et al. Using prediction models to reduce persistent racial and ethnic disparities in the draft 2020 USPSTF lung cancer screening guidelines. J Natl Cancer Inst. 2021;113(11):1590-1594. doi: 10.1093/jnci/djaa211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landy R, Gomez I, Caverly TJ, et al. Methods for using race and ethnicity in prediction models for lung cancer screening eligibility. JAMA Netw Open. 2023;6(9):e2331155. doi: 10.1001/jamanetworkopen.2023.31155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17(4):748-757. doi: 10.1158/1055-9965.EPI-07-2629 [DOI] [PubMed] [Google Scholar]
- 45.Sahar L, Douangchai Wills VL, Liu KKA, et al. Geographic access to lung cancer screening among eligible adults living in rural and urban environments in the United States. Cancer. 2022;128(8):1584-1594. doi: 10.1002/cncr.33996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Sample Selection
eFigure 2. Lung Cancer Screening Capacity in Relation to UTD-LCS Prevalence, US States, 2022
eTable. State-Level Screening Capacity, and Healthcare Access, United States, 2022
Data Sharing Statement