Abstract
BACKGROUND
Essential tremor (ET) is one of the most common movement disorders worldwide. In medically refractory ET, deep brain stimulation (DBS) of the ventral intermediate nucleus of the thalamus is the current standard of care. However, DBS carries an inherent 2% to 3% risk of hemorrhage, a risk that can be much higher in patients with concomitant coagulopathy. Magnetic resonance imaging–guided focused ultrasound (MRgFUS) thalamotomy is a surgical alternative that is highly effective in treating ET, with no reports of intracranial hemorrhage to date.
OBSERVATIONS
This is the first documented case of successful MRgFUS thalamotomy in a patient with von Willebrand disease (VWD). A 60-year-old left-handed male had medically refractory ET, VWD type 2B, and a family history of clinically significant hemorrhage after DBS. He underwent right-sided MRgFUS thalamotomy and received a perioperative course of VONVENDI (recombinant von Willebrand factor) to ensure appropriate hemostasis. Postprocedure imaging confirmed a focal lesion in the right thalamus without evidence of hemorrhage. The patient reported 90% improvement of his left-hand tremor and significant improvement in his quality of life without obvious side effects.
LESSONS
MRgFUS thalamotomy with peri- and postoperative hematological management is a promising alternative to DBS for patients with underlying coagulopathies.
KEYWORDS: focused ultrasound, essential tremor, von Willebrand disease, coagulation, case report
ABBREVIATIONS: CRST = Clinical Rating Scale for Tremor, CT = computed tomography, DBS = deep brain stimulation, DRTT = dentato-rubro-thalamic tract, ET = essential tremor, FGATIR = fast gray matter acquisition T1 inversion recovery, FLAIR = fluid-attenuated inversion recovery, FVII = coagulation factor VIII, MRgFUS = magnetic resonance imaging–guided focused ultrasound, MRI = magnetic resonance imaging, rVWF = recombinant von Willebrand factor, VIM = ventral intermediate, VWD = von Willebrand disease, VWF = von Willebrand factor
Essential tremor (ET) is one of the most common movement disorders worldwide.1 Classically, it presents as a 4- to 12-Hz kinetic tremor involving one or both upper extremities; however, it can also involve the head, face, voice, and lower extremities.2–4 Although some consider it a benign condition, ET can prove quite debilitating and often has a significant impact on one’s quality of life and function.5–7
Medical management of ET consists of pharmacological options including propranolol, primidone, and gabapentin.8,9 Although medical therapies can prove helpful in some cases, 30% to 50% prove refractory.10,11 For these cases, surgical options can be considered, including deep brain stimulation (DBS), magnetic resonance imaging–guided focused ultrasound (MRgFUS) thalamotomy, and Gamma Knife radiosurgery.1,12,13 Of the surgical options, DBS of the ventral intermediate (VIM) nucleus of the thalamus is the current standard of care.14,15 Although highly efficacious, with a 70% to 80% tremor reduction, DBS involves the placement of electrodes deep into the brain and thus carries an inherent risk of hemorrhage at a rate of approximately 2% to 3%.16,17 This risk is even higher in patients with acquired or inherited coagulopathies.18–20
MRgFUS thalamotomy, approved by the Food and Drug Administration in 2016, is a relatively new surgical therapy for ET.21 MRgFUS uses ultrasound focused to a single point to create a small (6–8 mm) lesion in the thalamus without having to make a skin incision or burr hole.22 Similar to DBS, it has proven highly effective in treating tremor, with a reported tremor reduction of 60% to 70%.23 Additionally, its safety profile has been quite favorable with no reports of intracranial hemorrhage to date.1,13,24,25 Thus, some have suggested that for patients with coagulopathies, MRgFUS may be a good alternative to DBS.
In this case report, we share the first documented case of MRgFUS in a patient with von Willebrand disease (VWD), the most common autosomal bleeding disorder.26 Affecting approximately 1% of the population, VWD is caused by a deficient or defective von Willebrand factor (VWF), a large glycoprotein instrumental to the mediation of platelet hemostasis and stabilization of coagulation factor VIII (FVIII).27 Because of this deficient protein, these patients are prone to bleeding following minor trauma and thus high-risk surgical candidates requiring close collaboration with hematology.28,29 In this report, we detail the surgical decision-making, perioperative and postoperative management, and procedural outcomes of a patient with medically refractory ET as well as VWD. On the basis of our experience, we subsequently propose a framework through which to approach the surgical management of ET in patients with VWD and other coagulopathies.
Illustrative Case
A 60-year-old, left-handed male with a history of hypertension and VWD type 2B had been diagnosed with ET in his early 40s. He initially noted tremor in both of his hands; over time, however, tremor in his left hand proved much more severe than that in his right. As his tremor progressed, he tried multiple medications including propranolol, primidone, and gabapentin, but none provided satisfactory tremor control. At the time of presentation to our team, he described his tremor as “disabling,” as he was unable to use utensils to eat, unable to write, and unable to drink from a glass without using two hands. An assessment with the Clinical Rating Scale for Tremor (CRST) was completed, and the score was calculated as 34. Aside from the patient’s tremor, the remainder of his neurological examination was unremarkable.
Based on the patient’s tremor severity, bilaterality of symptoms, impact on his quality of life, failure of medical therapies, and age, our team proposed placement of bilateral DBS electrodes into the ventral intermediate (VIM) nucleus to address his tremor. However, the patient declined, because his father, who also had ET and VWD type 2B, had undergone DBS surgery 10 years earlier and encountered significant hemorrhagic complications, requiring the surgery to be aborted. Given this, we subsequently recommended right-sided MRgFUS thalamotomy, to which the patient agreed.
Although MRgFUS thalamotomy has an excellent safety profile with respect to bleeding complications, there are no published cases of procedural outcomes in patients with VWD. Thus, our patient was evaluated by the Benign Hematology Service with the goal of developing a plan to execute MRgFUS safely. Notably, the patient had successfully undergone an uncomplicated thymoma resection approximately 9 years earlier. At that time, he was given perioperative Humate-P supplementation, an intermediate-purity plasma-derived VWF/FVIII concentrate successful in treating nearly all surgery-related bleeding events in patients with VWD.30 After the Humate-P administration, he had an excellent response in his VWF activity and FVIII activity. Interestingly, he did have brief thrombocytopenia that self-resolved. He was continued on Humate-P for 9 days after surgery and did not have any hemorrhagic complications.
In preparation for MRgFUS, the hematology team obtained baseline VWF activity and antigen levels and FVIII activity levels. VWF activity was low (28.0%), but VWF antigen was normal (69%), consistent with the diagnosis of type 2B VWD. FVIII activity was normal (82%). Therefore, VWF supplementation was recommended. Because his FVIII activity level was normal, he did not need treatment with a replacement product that included both VWF and FVIII, such as Humate-P. Instead, hematology recommended the use of VONVENDI (a recombinant VWF [rVWF] that includes no FVIII) in the peri- and postoperative periods.
We calculated the patient’s skull density ratio as 0.50 and obtained a magnetic resonance imaging (MRI) scan of his brain with diffusion tensor imaging sequences. As previously described, we utilized these MRI scans in conjunction with BrainLab to perform fiber tracking and subsequently identify the ipsilateral corticospinal tract, medial lemniscus, nondecussating dentato-rubro-thalamic tract (DRTT), and decussating DRTT.31,32 These fiber tracts along with image-based and atlas-based consensus coordinates were used to identify two targets for the MRgFUS thalamotomy (Table 1).
TABLE 1.
Coordinates of target lesions from anatomical landmarks
| Target | Anatomical Distances (mm) |
||
|---|---|---|---|
| Lat to Midline | Ant to PC | Superior to AC-PC Line | |
| 1 | 12.5 | 6.0 | 1.5 |
| 2 | 14.0 | 6.4 | 3.0 |
AC = anterior commissure; ant = anterior; PC = posterior commissure.
On the day of the procedure, the patient’s hematological workup was consistent with his baseline, that is, low VWF activity levels and normal FVIII activity levels. As recommended by hematology, the patient was given 50 U/kg of VONVENDI. Thirty minutes later, VWF activity was rechecked and was 147% (reference range 45%–150%). Thus, we proceeded with the procedure. Two sonications were performed at target 1 to confirm ultrasound alignment and proper tissue heating. For these sonications, the average temperature did not exceed 50°C. Satisfied with alignment, a verification sonication was performed, during which the average temperature reached 52°C. After this sonication, the patient endorsed a 50% improvement in his left-hand tremor. Importantly, he denied any issues with numbness, tingling, weakness, dysarthria, or dysmetria. Therefore, a treatment sonication was performed at target 1 with a peak temperature of 59°C reached. We once again confirmed satisfactory tremor improvement in the absence of significant side effects. The ultrasound was then refocused to target 2, which was anterior, superior, and lateral to the first target. Target 2 was selected based on patient-specific tractography to follow the demonstrated trajectory of the DRTT while staying safely away from the medial lemniscus and internal capsule. A final sonication was delivered at target 2, reaching an average temperature of 57°C. After the final sonication, the patient endorsed a 70% reduction in tremor without any side effects. The only bleeding issue encountered was prolonged oozing from a frame pin site; however, after 15 minutes of pressure, this stopped and did not require any further intervention.
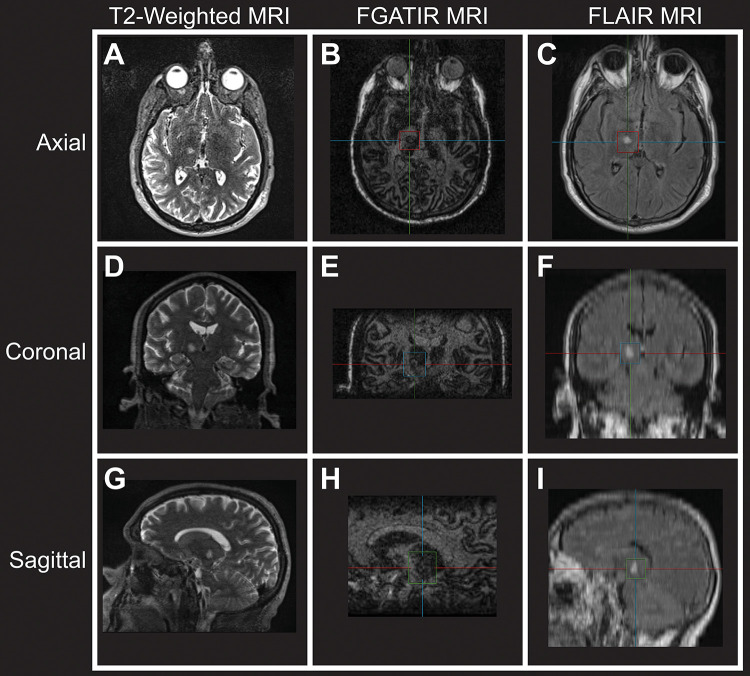
Postprocedure, fast gray matter acquisition T1 inversion recovery (FGATIR), fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted imaging sequences confirmed a focal lesion targeted to the right thalamus with expected perilesional edema. Importantly, no evidence of hemorrhage was appreciated (Fig. 1). The patient was then admitted to the Neurosurgery Service at Duke University Hospital. Eight hours after the procedure, VWF activity was reassessed and found to be 104%. A head computed tomography (CT) scan was obtained at the same time, revealing a focal hypodensity in the right thalamus consistent with interval thalamotomy but no evidence of intracranial hemorrhage (Fig. 2).
FIG. 1.
Postprocedure axial T2-weighted (A), FGATIR (B), and FLAIR (C) MRI sequences at the level of the ablation showing a right thalamic lesion. The same sequences in the coronal (D–F) and sagittal (G–I) planes.
FIG. 2.
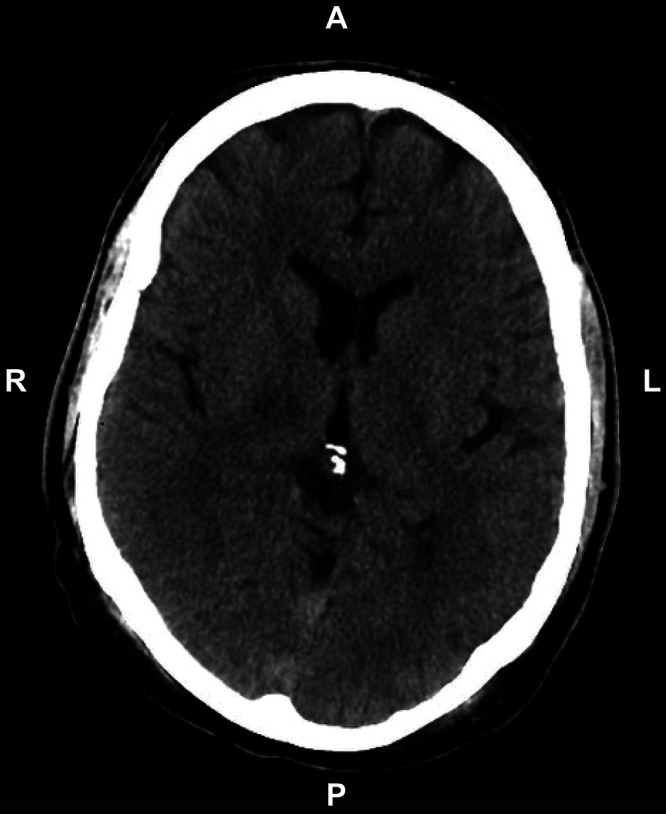
Postprocedure CT scan showing no evidence of hemorrhage.
For the remainder of the patient’s hospitalization, he continued daily VONVENDI per hematology, and VWF activity levels remained consistently within his goal range of 80% to 100%. Therefore, he was discharged home on postprocedure day 3. He was continued on daily VONVENDI dosing to complete a total of 10 days of therapy (Fig. 3).
FIG. 3.
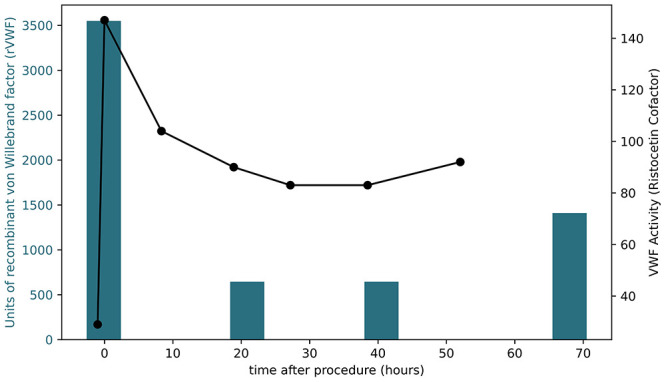
VWF and VWF activity levels before and after MRgFUS. The left axis shows the units of rVWF given, corresponding to the blue bars, whereas the right axis shows the VWF activity, corresponding to the black line and data points.
The patient returned to the clinic 12 days after the procedure. At that time, he reported a subjective 90% improvement of his left-hand tremor and marked (“100%” per his description) improvement in his quality of life without any obvious side effects (Fig. 4). His tremor score for his treated hand went from 13 to 3 on parts A and B of the CRST, whereas his level of dysfunction went from 12 to 1 on part C. He noted that on postprocedure days 1 through 4, he experienced mild dysarthria, minor gait imbalance, and slight numbness/tingling of the tip of the tongue. However, these symptoms self-resolved within 3 days of onset. Additionally, he denied any significant issues with bleeding or bruising around his pin sites. He was very satisfied with the results of his procedure.
FIG. 4.
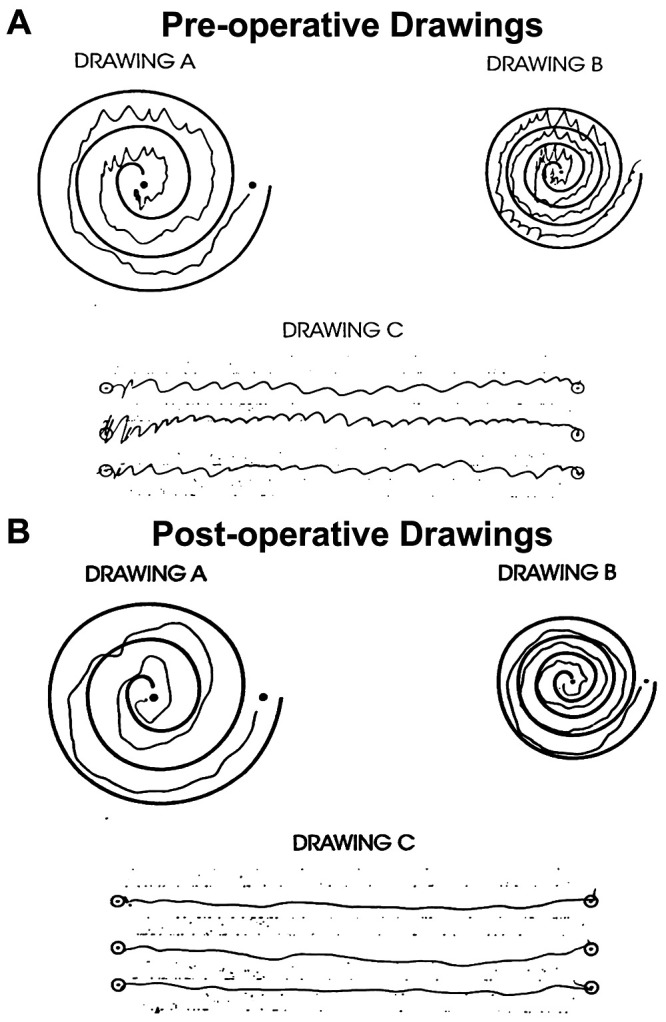
CRST drawings with the left hand before (A) and after (B) MRgFUS.
Patient Informed Consent
The necessary patient informed consent was obtained in this study. Furthermore, the CARE (CAse REport) guidelines were followed during the writing of this case report.33
Discussion
Observations
In this report, we describe the first documented case of a patient with an inherited coagulopathy, VWD type 2B, to undergo MRgFUS thalamotomy for medically refractory ET. Our experience highlights three key results: 1) MRgFUS thalamotomy can produce excellent tremor control in a patient with an inherited coagulopathy (90% reduction), 2) the procedure can be successfully performed without causing significant neurological or hemorrhagic complications, and 3) achieving these results is best accomplished in close collaboration with hematology. Our experience establishes a proof of safety, as well as a protocol for successfully and safely treating patients with inherited coagulopathies such as VWD using MRgFUS thalamotomy and thus establishing it as a good alternative to DBS in this patient population.
DBS has proven to be a highly effective approach for treating medically refractory ET, Parkinson’s disease, dystonia, epilepsy, and obsessive-compulsive disorder. Despite such efficacy, DBS does have a 1% to 2% risk of intracranial hemorrhage, a risk that has proven much higher in patients with coagulopathies, either acquired or inherited. Despite efforts to address these coagulopathies—holding medications that induce acquired coagulopathies or providing factor supplementation to address inherited coagulopathies—intracranial hemorrhage does still occur, as highlighted by the experience of our patient’s father. Although hemorrhagic complications are often temporary and manageable, in rare cases they can prove devastating and life-threatening, something that must be carefully assessed considering the elective nature of DBS.
MRgFUS thalamotomy is an incisionless ablative therapy currently used to treat medically refractory ET and tremor-dominant Parkinson’s disease. To date, its hemorrhagic safety profile has been far superior to DBS. In fact, no reported cases of hemorrhage during or after MRgFUS thalamotomy exist in the literature. Thus, some have proposed that this procedure could be an excellent alternative to DBS in patients with coagulopathies. In support of this, recent work has highlighted the safety of MRgFUS thalamotomy with respect to acquired coagulopathies secondary to anticoagulation or antiplatelet use. As detailed by Caston et al.,25 patients on long-term anticoagulation or with antiplatelet complications did not have any hemorrhagic complications after MRgFUS thalamotomy.
Although incredibly encouraging, these results cannot be directly translated to inherited coagulopathies because of the distinct biology underlying these conditions. In some acquired coagulopathies, bleeding dysfunction results from the administration of medications such as warfarin, factor Xa inhibitors, or aspirin, which have strong but transient effects on coagulation factors and platelets. Because these medications have half-lives measured in hours to days, their biological effect diminishes over time, 2 to 7 days depending on the agent. In contrast, inherited coagulopathies such as VWD have an inherent deficiency in coagulation factors that typically remains unchanged over time. Therefore, a distinct therapeutic approach for treating inherited coagulopathies is warranted in situations associated with an increased risk of bleeding, such as surgery.
Our experience detailed here provides novel evidence that patients with both VWD and medically refractory ET can be successfully treated with MRgFUS thalamotomy combined with appropriate factor supplementation. Moving forward, several key questions remain. First, although our experience in this patient with VWD should be applicable to patients with other inherited coagulopathies, such as hemophilia, this will need to be confirmed in additional case reports and series. Second, for patients with milder bleeding defects, it remains unclear whether factor supplementation is also needed. Because MRgFUS acts through coagulative necrosis of the brain tissue being targeted, some have proposed that such a mechanism already limits hemorrhagic complications and thus may eliminate the need for factor supplementation. In support of this, a recent investigation highlighted how MRgFUS can safely be performed without stopping or reversing a patient’s antiplatelet and anticoagulant medications.25 However, despite these results and in an abundance of caution, we advocate for close collaboration with hematology to make this decision on a case-by-case basis.
In summary, our case report demonstrates that MRgFUS thalamotomy can be a safe and efficacious treatment modality for medically refractory ET in a patient with VWD. MRgFUS thalamotomy is a relatively new surgical option for ET with tremor reduction rates comparable to those for DBS and a more favorable safety profile with respect to hemorrhagic complications. We detail the surgical decision-making, perioperative and postoperative management, and medical optimization for ET in the setting of a coagulopathy to decrease the risk of hemorrhagic complications.
Although our experience is based on a single patient, it can serve as a guide for developing a practice guideline that can be validated or perhaps further optimized by results from future studies. Thus, we propose the following framework for the safe implementation of MRgFUS in patients with VWD. 1) Prior to surgery, consult with your local hematology team to confirm the patient’s hematological diagnosis, assess the patient’s coagulation status, and jointly develop a plan for safely performing MRgFUS. 2) On the day of surgery, reassess the patient’s coagulation profile, and based on these results, administer rVWF. Thirty minutes postinfusion, recheck the serum VWF activity. If it is between 45% and 150%, proceed with the procedure. 3) Perform MRgFUS with serial neurological assessments, as one would normally do. 4) Postprocedure, admit the patient to the inpatient neurosurgical ward for overnight observation. Within 6 hours of the completed surgery, obtain a postoperative MRI or CT scan to evaluate for hemorrhage. 5) After surgery, redose rVWF to maintain daily VWF activity levels between 80% and 100%. 6) Continue to follow the patient’s VWF activity levels with appropriate rVWF dosing for 10 days. This can be done as an inpatient or outpatient, depending on local resource availability.
Lessons
Patients with coagulopathies have an inherent risk for hemorrhagic complications after intracranial surgery such as DBS. MRgFUS thalamotomy is a promising surgical alternative to DBS in the treatment of medically refractory ET. Published studies to date have demonstrated a favorable safety profile for MRgFUS thalamotomy with no reported cases of intracranial hemorrhage; however, there are currently no literature cases of the successful and safe application of MRgFUS thalamotomy in patients with VWD. Our case is the first documented instance of MRgFUS thalamotomy in a patient with ET and concomitant VWD. Our experience demonstrates that with proper perioperative and postoperative management of coagulopathies, the risk of intracranial hemorrhage can be minimized and MRgFUS thalamotomy can be safely and effectively used to treat ET in the setting of coagulopathies. While our protocol involved postprocedural hospitalization for VONVENDI administration and close monitoring, we hope that future work will investigate whether such measures are necessary. If patients with coagulopathies can also be treated on an outpatient basis, this would be a positive advance forward from the patient perspective.
Author Contributions
Conception and design: Harward, Folz, Shah, Lad. Acquisition of data: Harward, Folz, Seas, Chinyengetere, Harris, Oyedeji, Lad. Analysis and interpretation of data: Harward, Folz, Seas, Ortel, Shah, Lad. Drafting the article: Harward, Folz, Seas, Oyedeji, Shah. Critically revising the article: Harward, Folz, Seas, Chinyengetere, Oyedeji, Ortel, Shah, Lad. Reviewed submitted version of manuscript: Harward, Folz, Seas, Chinyengetere, Oyedeji, Ortel. Approved the final version of the manuscript on behalf of all authors: Harward. Statistical analysis: Harward. Administrative/technical/material support: Harward, Folz, Beasley, Harris, Lad. Study supervision: Harward, Lad. Clinical evaluation: Chinyengetere. Patient management plan: Ortel.
Supplemental Information
Previous Presentations
This paper was presented as an eposter at the 2024 American Association of Neurological Surgeons Meeting in Chicago, Illinois, May 3-6, 2024.
References
- 1. Hopfner F, Deuschl G. Managing essential tremor. Neurotherapeutics. 2020;17(4):1603–1621. doi: 10.1007/s13311-020-00899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis ED, Gerbin M, Galecki M. Essential tremor 10, 20, 30, 40: clinical snapshots of the disease by decade of duration. Eur J Neurol. 2013;20(6):949–954. doi: 10.1111/ene.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reich SG. Essential tremor. Med Clin North Am. 2019;103(2):351–356. doi: 10.1016/j.mcna.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 4. Louis ED. Essential tremor. Lancet Neurol. 2005;4(2):100–110. doi: 10.1016/S1474-4422(05)00991-9. [DOI] [PubMed] [Google Scholar]
- 5. Louis ED, Barnes L, Albert SM, et al. Correlates of functional disability in essential tremor. Mov Disord. 2001;16(5):914–920. doi: 10.1002/mds.1184. [DOI] [PubMed] [Google Scholar]
- 6. Louis ED, Okun MS. It is time to remove the ‘benign’ from the essential tremor label. Parkinsonism Relat Disord. 2011;17(7):516–520. doi: 10.1016/j.parkreldis.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen JJ, Swope DM. Essential tremor. J Pharm Pract. 2007;20(6):458–468. [Google Scholar]
- 8. Shanker V. Essential tremor: diagnosis and management. BMJ. 2019;366:l4485. doi: 10.1136/bmj.l4485. [DOI] [PubMed] [Google Scholar]
- 9. Pal PK. Guidelines for management of essential tremor. Ann Indian Acad Neurol. 2011;14(1) suppl 1:S25–S28. doi: 10.4103/0972-2327.83097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kosmowska B, Wardas J. The pathophysiology and treatment of essential tremor: the role of adenosine and dopamine receptors in animal models. Biomolecules. 2021;11(12):1813. doi: 10.3390/biom11121813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langford BE, Ridley CJA, Beale RC, Caseby SCL, Marsh WJ, Richard L. Focused ultrasound thalamotomy and other interventions for medication-refractory essential tremor: an indirect comparison of short-term impact on health-related quality of life. Value Health. 2018;21(10):1168–1175. doi: 10.1016/j.jval.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 12. Giammalva GR, Maugeri R, Umana GE, et al. DBS, tcMRgFUS, and gamma knife radiosurgery for the treatment of essential tremor: a systematic review on techniques, indications, and current applications. J Neurosurg Sci. 2022;66(6):476–484. doi: 10.23736/S0390-5616.22.05524-2. [DOI] [PubMed] [Google Scholar]
- 13. Rohani M, Fasano A. Focused ultrasound for essential tremor: review of the evidence and discussion of current hurdles. Tremor Other Hyperkinet Mov (N Y). 2017;7:462. doi: 10.7916/D8Z89JN1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giordano M, Caccavella VM, Zaed I, et al. Comparison between deep brain stimulation and magnetic resonance-guided focused ultrasound in the treatment of essential tremor: a systematic review and pooled analysis of functional outcomes. J Neurol Neurosurg Psychiatry. 2020;91(12):1270–1278. doi: 10.1136/jnnp-2020-323216. [DOI] [PubMed] [Google Scholar]
- 15. Iorio-Morin C, Fomenko A, Kalia SK. Deep-brain stimulation for essential tremor and other tremor syndromes: a narrative review of current targets and clinical outcomes. Brain Sci. 2020;10(12):925. doi: 10.3390/brainsci10120925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beric A, Kelly PJ, Rezai A, et al. Complications of deep brain stimulation surgery. Stereotact Funct Neurosurg. 2001;77(1–4):73–78. doi: 10.1159/000064600. [DOI] [PubMed] [Google Scholar]
- 17. Shin HK, Kim MS, Yoon HH, Chung SJ, Jeon SR. The risk factors of intracerebral hemorrhage in deep brain stimulation: does target matter? Acta Neurochir (Wien). 2022;164(2):587–598. doi: 10.1007/s00701-021-04977-y. [DOI] [PubMed] [Google Scholar]
- 18. Runge J, Cassini Ascencao L, Blahak C, et al. Deep brain stimulation in patients on chronic antiplatelet or anticoagulation treatment. Acta Neurochir (Wien). 2021;163(10):2825–2831. doi: 10.1007/s00701-021-04931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jung IH, Chang KW, Park SH, Chang WS, Jung HH, Chang JW. Complications after deep brain stimulation: a 21-year experience in 426 patients. Front Aging Neurosci. 2022;14:819730. doi: 10.3389/fnagi.2022.819730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Topp G, Ghulam-Jelani Z, Chockalingam A, et al. Safety of deep brain stimulation lead placement on patients requiring anticlotting therapies. World Neurosurg. 2021;145:e320–e325. doi: 10.1016/j.wneu.2020.10.047. [DOI] [PubMed] [Google Scholar]
- 21. Jung NY, Chang JW. Magnetic resonance-guided focused ultrasound in neurosurgery: taking lessons from the past to inform the future. J Korean Med Sci. 2018;33(44):e279. doi: 10.3346/jkms.2018.33.e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bachu VS, Kedda J, Suk I, Green JJ, Tyler B. High-intensity focused ultrasound: a review of mechanisms and clinical applications. Ann Biomed Eng. 2021;49(9):1975–1991. doi: 10.1007/s10439-021-02833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375(8):730–739. doi: 10.1056/NEJMoa1600159. [DOI] [PubMed] [Google Scholar]
- 24. Fishman PS, Elias WJ, Ghanouni P, et al. Neurological adverse event profile of magnetic resonance imaging-guided focused ultrasound thalamotomy for essential tremor. Mov Disord. 2018;33(5):843–847. doi: 10.1002/mds.27401. [DOI] [PubMed] [Google Scholar]
- 25. Caston RM, Campbell JM, Rahimpour S, Moretti P, Alexander MD, Rolston JD. Hemorrhagic safety of magnetic resonance-guided focused ultrasound thalamotomy for tremor without interruption of antiplatelet or anticoagulant therapy. Stereotact Funct Neurosurg. 2023;101(5):314–318. doi: 10.1159/000533590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. James PD, Connell NT, Ameer B, et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021;5(1):280–300. doi: 10.1182/bloodadvances.2020003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA) Haemophilia. 2008;14(2):171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 28. O’Donnell JS, Lavin M. Perioperative management of patients with von Willebrand disease. Hematology (Am Soc Hematol Educ Program). 2019;2019(1):604–609. doi: 10.1182/hematology.2019000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miesbach W. Perioperative management for patients with von Willebrand disease: defining the optimal approach. Eur J Haematol. 2020;105(4):365–377. doi: 10.1111/ejh.13462. [DOI] [PubMed] [Google Scholar]
- 30. Auerswald G, Kreuz W. Haemate P/Humate-P for the treatment of von Willebrand disease: considerations for use and clinical experience. Haemophilia. 2008;14(suppl 5):39–46. doi: 10.1111/j.1365-2516.2008.01850.x. [DOI] [PubMed] [Google Scholar]
- 31. Feltrin FS, Chopra R, Pouratian N, et al. Focused ultrasound using a novel targeting method four-tract tractography for magnetic resonance-guided high-intensity focused ultrasound targeting. Brain Commun. 2022;4(6):fcac273. doi: 10.1093/braincomms/fcac273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shah BR, Lehman VT, Kaufmann TJ, et al. Advanced MRI techniques for transcranial high intensity focused ultrasound targeting. Brain. 2020;143(9):2664–2672. doi: 10.1093/brain/awaa107. [DOI] [PubMed] [Google Scholar]
- 33. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013;2013:bcr2013201554. doi: 10.1186/1752-1947-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]