Highlights
-
•
Application of ultrasound technology to freeze storage.
-
•
UIF-175 W can effectively minimized the changes in MP structure.
-
•
UIF-175 W can protect the lipid during the freeze process.
-
•
A schematic illustration of protein and lipid oxidations was proposed.
Keywords: Large yellow croaker, Multi-frequency ultrasound, Myofibrillar protein, Lipid oxidation, Frozen storage
Abstract
In this study, large yellow croaker (Larimichthys crocea) was frozen using multi-frequency ultrasound-assisted freezing (MUIF) with different powers (160 W, 175 W, and 190 W, respectively) and stored at −18 °C for ten months. The effect of different ultrasound powers on the myofibrillar protein (MP) structures and lipid oxidation of large yellow croaker was investigated. The results showed that MUIF significantly slowed down the oxidation of MP by inhibiting carbonyl formation and maintaining high sulfhydryl contents. These treatments also held a high activity of Ca2+-ATPase in the MP. MUIF maintained a higher ratio of α-helix to β-sheet during frozen storage, thereby protecting the secondary structure of the tissue and stabilizing the tertiary structure. In addition, MUIF inhibited the production of thiobarbituric acid reactive substances value and the loss of unsaturated fatty acid content, indicating that MUIF could better inhibit lipid oxidation of large yellow croaker during long-time frozen storage.
1. Introduction
Large yellow croaker (Larimichthys crocea) has high economic value, tender meat, rich in protein, and is loved by consumers [1]. Nevertheless, large yellow croaker is vulnerable to oxidation of proteins and lipids when kept in cold storage, resulting in reduced food quality [2]. The majority of muscle protein in fish is myofibrillar protein [3], which is a salt-soluble protein, and its content is closely related to the storage quality of fish [4]. The oxidation and denaturation of MP have a certain effect on the quality, color and texture of fish [5]. However, the growth of ice crystals during freezing disrupts the MP structure, which exacerbates MP oxidation, further leading to sulfhydryl destruction, MP aggregation, carbonyl formation, and conformational changes [6], [7]. The use of some freezing techniques in the freezing process can effectively slow down the growth of ice crystals through rapid freezing and reduce the damage of ice crystals to fish tissue. Therefore, freezing methods are critical to inhibit protein and lipid oxidation during fish storage.
Common frozen foods are obtained by air freezing (AF) or plate freezing. However, the slow freezing speed of these methods results in the formation of large and irregular ice crystals [8], [9]. The location and size of the ice crystals as well as the speed at which the fish freezes are affected by the freezing techniques [10]. In recent years, novel freezing techniques such as ultrasonic-assisted immersion freezing (UIF) have been developed to address these problems. UIF is a method of freezing aquatic products developed in recent years [11]. The application of UIF in the food freezing process has been studied, including the nucleation, inhibiting the growth of ice crystals [12], and the acceleration of the freezing speed [13]. The rupture and collapse of cavitation bubbles generate strong physical forces that break down the original ice crystals into smaller fragments, which also act as new nuclei, thus promoting secondary nucleation to form small and uniform ice crystals.
Cavitation bubble movement creates microcurrent, which could improve heat transfer efficiency [14]. Sun et al. [10] indicated that UIF at 175 W significantly maintained the protein structure during freezing. Zhang et al. [15] found that UIF with specific power could significantly increase the freezing rate of samples. As a result, UIF can prevent the destruction of the food microstructure during freezing while also reducing freezing time, thus improving quality. In one of our previous studies, MUIF-treated large yellow croakers were evaluated by freezing rate, quality and structural characteristics. MUIF could speed up the freezing speed [16], which improved the frozen quality of large yellow croaker. The research shows that compared with single frequency ultrasound, multi-frequency ultrasound increases the range of ultrasonic cavitation effect, avoids the problem of uneven sound field when using single frequency ultrasound, expands the cavitation region, has more cavitation bubble growth rate and collapse pressure, and significantly improves the cavitation effect and freezing efficiency [17]. However, it remains unclear how different MUIF power levels affect the structure of MP and lipid oxidation. As consequently, the present research intended to investigate the influence of different MUIF power levels on MP and lipids during the frozen storage of large yellow croaker samples.
2. Materials and methods
2.1. Sample and freezing process
Fresh large yellow croaker samples were obtained from Nanhui New Town (Shanghai, China). Sixty-three fresh large yellow croaker (550 ± 20 g, 30 ± 2 cm) were purchased, placed in a foam box with ice, and delivered to the laboratory within 30 min. The samples were flushed and individually wrapped in polyethylene bags. Each group of samples was treated differently: Immersed freezing (IF) and MUIF at different ultrasonic powers (UIF-160, UIF-175, and UIF-190 W, respectively). Three fresh yellow croakers were used for 0-day samples before freezing. The remaining sixty fish were separated randomly into 4 groups of 15 fish each.
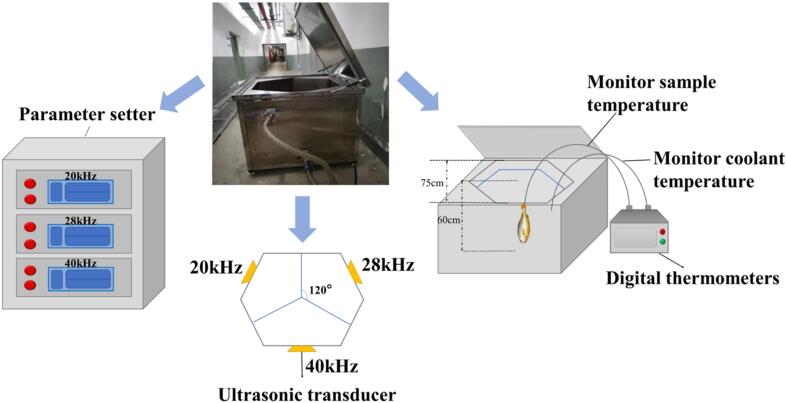
Ultrasonic freezing equipment is divided into two parts: the hexahedral tank and the control cabinet. The hexahedral tank is equipped with ultrasonic generators, and the ultrasonic generators facing the surface of the sink have the same frequency, which are set to 20, 28 and 40 kHz, respectively. Set the ultrasonic frequency to 20/28/40 kHz. Large yellow croaker samples were placed at −25 °C into an ultrasonic device containing a coolant consisting of 29.3 % calcium chloride (Fig. 1). Transfer the samples and store in the −18 °C refrigerator as soon as the temperature in the center of the samples reaches −18 °C. The samples were frozen for up to 300 days. Every 60 days, three fish at random from each group were chosen for a quality assessment, and the ultimate outcome was based on the average value. Prior to testing, frozen samples were thawed under running water until they reached a core temperature of 4 °C. Then cut the large yellow croaker into fillets by removing the head and slicing the fish in the direction of the bones. Each analysis (described below) was triplicated for each fish.
Fig. 1.
Schematic of the ultrasound-assisted immersion freezing apparatus.
2.2. Extraction of MP
The description of Liu et al. [18] was used to extract MP. Proteins were extracted from the muscles found on the backs of the fish fillets. 20 mL of Tris-Maleate (0.05 mol/L KCl) was combined with 2 g of fish flesh, and the mixture was centrifuged at 10,614 × g for 15 min at 4 °C. The precipitate from centrifugation was repeated as previously described. After 3 h of standing at 4 °C, 20 mL of Tri-Maleate (0.06 mol/L KCl, pH 7.0) was blended evenly with the sediment. The supernatant obtained by centrifugation with the given circumstances was MP. MP solution was prepared for the determination of total sulfhydryl content, carbonyl content, Ca2+-ATPase, MP secondary and tertiary structure.
2.3. Determination of total sulfhydryl contents
The Zhao et al. [19] method was followed to determine the total sulfhydryl content. The extinction coefficient of 2-nitro-5-mercaptobenzoate (NTB) was measured to determine the total sulfhydryl content (μmol/g) of the protein.
2.4. Carbonyl contents
The carbonyl contents were measured as described by Sharafodin & Soltanizadeh [20]. Derivatization of a protein hydrazone using 2,4-dinitrophenylhydrazine determines the carbonyl contents.
2.5. Ca2+-ATPase activity
The corresponding assay kits were obtained from Nanjing Jiancheng Institute of Bioengineering, China, and used a microscopic method to determine the activity of Ca2+-ATPase in actin according to the instructions.
2.6. MP secondary structure
Potassium bromide powder and dried myofibril samples were ground and mixed well. The mixture was pressed into thin sheets. The secondary structure was identified using FTIR (Spectrum 3; PerkinElmer, USA). The following are the test parameters: the resolution is 4 cm−1, and the scan wavelength range is 4500 to 5000 cm−1 [21].
2.7. MP tertiary structure
Utilizing a fluorescence spectrophotometer (F-7100), the intrinsic fluorescence of the MP solution at 300–400 nm was scanned. A slit width of 5 nm and an excitation wavelength of 295 nm were used. There was a 1200 nm/min scanning rate.
2.8. Determination of thiobarbituric acid reactive substances (TBARS)
The same methodology as Yang et al. [22] was used to determine TBARS with slight modifications. The fish flesh from the abdomen (5 g) was evenly mixed with 20 mL of a 20 % trichloroacetic acid (TCA) solution. The mixture was left standing for 1 h and then centrifuged at 11,960 × g for 10 min at 4 °C. Once filtered, dilute the supernatant to 50 ml. After mixing 5 mL of diluent with 5 mL of thiobarbituric acid (TBA) at 0.02 M, the mixture reacted in water at 100 °C for 20 min. Allow it to cool and measure the absorbance of the solution at 532 nm using a UV spectrophotometer (Evolution 220, Thermo Fisher Scientific, MA, USA). Using the following formula, the TBARS value was calculated and expressed as mg MDA/kg:
where ω0 is the mass fraction of the standard, ω1 is the mass fraction of the sample, A0 is the absorbance of the standard, and A1 is the absorbance of the sample.
2.9. Fatty acids determination
Lipid extraction was obtained according to Abd El-Ghafour et al. [23]. A 300 μL mixture of methanol, acetonitrile, and water (volume ratio 2:1:2) was used to grind 50 mg of fish flesh from the abdomen for 2 min. The mixture should be centrifuged for 15 min at 4 °C at 12,000 × g. After centrifugation, the supernatant was collected in a clean centrifuge tube. A mixture of 200 μL extraction and the precipitate was centrifuged for 5 min at 4 °C at 12,000 × g. The above experimental procedure was repeated after collecting the supernatant into the centrifuge tube. Chloroform (chloroform:supernatant = 4:5) was equally mixed with the combined supernatant and centrifuged for 5 min at 4 °C at 12,000 × g. After moving the chloroform layer to a glass bottle, it evaporated until it was completely dry. The dried sample was evenly mixed with 100 μL solvent (dichloromethane: isopropanol: methanol = 1:1:2), and then centrifuged for 5 min at 4 °C at 12,000 × g. Liquid chromatography-mass spectrometry was utilized to ascertain the fatty acid content of the supernatant. (ACQUITY UPLC I-Class system and VION IMS QTOF Mass spectrometer). The content of fatty acid was quantitatively determined by an external standard method. Qualitative of fatty acids was performed using retention time of standard.
2.10. Statistical analysis
Independent repetition of each experimental sample three times. Data analysis was done using one-way analysis of variance (ANOVA). IBM SPSS 22.0 software was used to perform Duncan's test, and mean ± standard deviation was used to report the experimental results.
3. Results and discussion
3.1. Total sulfhydryl contents
When sulfhydryl is oxidized, it forms covalent disulfide bonds (—S—S—) that result in MP cross-linking [24]. Therefore, changes in MP structure can be reflected by changes in sulfhydryl contents [25]. The total sulfhydryl contents in each sample varied similarly and showed a declining trend as storage time increased (Fig. 2A). The total sulfhydryl contents in IF, UIF-160 W, UIF-175 W, and UIF-190 W decreased rapidly to 49.27 %, 38.09 %, 34.54 % and 39.41 % of the initial value after 300 days storage. During frozen storage, some active sulfhydryl groups oxidized, resulting in a drop in total sulfhydryl concentration [26]. Compared with IF samples, the total sulfhydryl group contents of MUIF samples were higher. The total sulfhydryl contents of the UIF-175 W samples were 11.06 %, 7.02 %, and 4.46 % higher than that in the IF, UIF-160 W, and UIF-190 W samples on the 180th day, respectively. The rapid freezing rate and ultrasonic cavitation effect formed the regular ice crystals, thus inhibiting the destruction of spatial protein structure [27], reducing the degree of oxidation of sulfhydryl groups. In contrast, the IF sample freezes more slowly, which leads to the formation of irregular ice crystals that exacerbate the denaturation of MP (Fig. 3) [28]. Qiu et al. [11] also mentioned the effect of UIF on MP structure and sample quality. They demonstrated that compared to IF samples, UIF samples had higher total sulfhydryl contents, similar to the current study. The total sulfhydryl contents of the UIF-190 W sample decreased faster compared to the UIF-175 W sample, indicating that the sulfhydryl groups were oxidized to disulfide bonds at the higher ultrasonic power, which promoted the denaturation of MP. However, Sun et al. [10] indicated that the total sulfhydryl contents of UIF samples with different power levels were not significantly different, which may be related to the short freezing time.
Fig. 2.
Average (±standard deviation) of total sulfhydryl contents (A), carbonyl content (B) and Ca2+-ATPase activity (C) of large yellow croaker samples during frozen storage (IF: immersion freezing; UIF: ultrasound-assisted immersion freezing at different ultrasound powers). Letters are used “a” to “e” significance differences between the samples (p < 0.05, n = 3). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Freezing curves of large yellow croaker with different freezing treatments (Ma et al., 2022). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Carbonyl contents
Utilizing the carbonyl contents, one can assess the extent of oxidative denaturation of MPs throughout the process and storage [29]. As shown in Fig. 2B, the variation in carbonyl contents of the samples was proportional to the increased storage time. Proteins developed oxidative denaturation, resulting in increased carbonyl contents [29]. The carbonyl contents of the UIF-160 W, UIF-175 W, and UIF-190 W samples were dramatically lower than those of IF samples during frozen storage. The mechanical effect of cavitation could be responsible for this result. During the freezing process, the cavitation effect improves the mass and heat transfer, resulting in more uniform ice crystal formation and reducing enzymatic activity [30]. Therefore, UIF could inhibit MP oxidation and reduce structural integrity damage [31]. The carbonyl contents of the UIF-190 W samples increased faster, indicating that the high ultrasonic power could induce MP oxidation. According to the study by Kang et al. [32], high ultrasonic intensity increases free radical production, resulting in increased carbonyl content in beef proteins. Shi et al. [29] pointed out that the carbonyl contents in the grass carp samples did not change significantly (p > 0.05). The short storage time caused different results from the present study.
3.3. Ca2+-ATPase activity
Microstructural alterations occur in MP integrity, resulting in a reduction in Ca2+-ATPase activity [33]. Following frozen storage, all samples showed a significant decrease in Ca2+-ATPase activity (Fig. 2C), indicating that the freezing process compromised myosin integrity. Ca2+-ATPase activities of the IF, UIF-160 W, UIF-175 W and UIF-190 W samples decreased by 74.07 %, 70.54 %, 66.50 % and 69.36 % compared to the fresh samples taken toward completion of frozen storage, respectively. The structural alterations in the myosin head were the cause of the reduced Ca2+-ATPase activity during the formation and storage of ice crystals. When frozen storage came to an end, the UIF-175 W sample had more Ca2+-ATPase activity than the other samples. The ice crystals formed during freezing had less damage to muscle cells and reduced the denaturation and inactivation of antioxidant enzymes, which was beneficial to maintaining the activity of Ca2+-ATPase [34]. The UIF-190 W sample exhibited lower Ca2+-ATPase activity than the UIF-175 W sample. The cavitation bubble rupture produced by high ultrasound power caused mechanical vibrations, which led to myosin changes [35]. M. Zhang et al. [15] and Sun et al. [10] also remarked that mechanical vibration could also cause some damage to the MP.
3.4. MP secondary structure
The secondary structure of MP consists mainly of α-helices, β-sheets, β-turns, and random coils, which is a structure consisting of the backbone of certain peptide segments in the peptide chain (Fig. 4A). The stable secondary structure has a large number of α helices and β-sheets, while the increased content of β turns and random coils reflects the instability of the protein structure. The α-helix structure is related to the formation of intramolecular hydrogen bonds and is the main secondary structure, and its content can reflect the changes in the molecular structure of the protein. The proportion of α helices decreased during frozen storage compared to fresh samples, whereas the content of β sheets, β rings and random coils increased. The decrease in α-helix contents was mainly related to the irreversible denaturation of proteins [36]. The UIF samples had the highest α-helix contents and UIF-175 W samples had the lowest random coil contents during frozen storage (Fig. 4B). The reason for this phenomenon is that the collapse of the cavitation bubbles generated by the UIF generates physical forces that break the ice crystals into smaller fragments, inducing secondary nucleation of the ice crystals, while also promoting heat transfer efficiency [10]. Therefore, UIF may accelerate the freezing speed and form small, regular ice crystals, resulting in less ice crystal damage to the muscles of the fish [28]. The higher ultrasound power (UIF-190 W) promoted the degradation of the MP structure, which was due to the mechanical action of ultrasonic waves, resulting in the unfolding of the MP structure [27]. IF samples had the lowest α-helix contents. Furthermore, the α-helix content of IF on the 300th day decreased by 22.86 % compared to the fresh samples, which was higher than the MUIF samples during frozen storage. The increase in β-turn and random coil contents usually reflects the increased looseness of the MP structure [37]. The IF samples exhibited a comparatively higher concentration of β-turns and random coils, suggesting a transition from a stable to an unstable secondary structure for the MP, which was usually manifested by the MP structure becoming loose. The FTIR results from this investigation revealed that IF and MUIF influenced both the secondary structure of MP during frozen storage, and that MUIF (especially UIF-175 W) could reduce freezing-induced alterations in the secondary structure of MP. This is similar to the study of Bian et al. [38], they point out that MUAF better to maintain the stability of the large yellow croaker MP secondary structure.
Fig. 4.
Secondary structure (A, B), fluorescence intensity (FI, C, D) of large yellow croaker samples during frozen storage (IF: immersion freezing; UIF: ultrasound-assisted immersion freezing at different ultrasound powers). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. MP tertiary structure
Endogenous fluorescence spectroscopy is a method for monitoring tertiary structural changes in MPs by measuring tryptophan residues. The change of fluorescence intensity can reflect the aggregation and folding of proteins. The decrease of fluorescence intensity indicates that the MP structure is destroyed and the protein oxidation leads to the unstable tertiary structure. The value of λmax for fresh samples in this study was 337 nm (Fig. 4(C and D)). As freezing time increased, the intrinsic fluorescence of MP decreased, suggesting that freezing exposed partially buried tryptophan residues to a polar environment [39]. In comparison to IF samples, MUIF samples had a higher fluorescence intensity. This result may be due to the synergistic effect of MUIF on the unfolding of MP structures and the accelerated freezing process to reduce muscle tissue damage. Sun et al. [10] indicated that the high ultrasound power affected the unfolding of MP structures. They found that the UIF-175 W sample had a higher fluorescence intensity than other samples. Therefore, MUIF, at the appropriate power, may induce nucleation, which can dramatically increase the number of ice nuclei in food during frozen storage, thus reducing the damage of ice crystals to muscle structure [15]. Bian et al. [38] also pointed out that the MUAF resulted in less damage to the tertiary structures of MP. Therefore, the UIF-175 W could reduce damage to frozen fish MP structure, which could effectively reduce the degree of oxidation of MPs and keep the tertiary structure of MP stable.
3.6. TBARS
The fresh large yellow croaker is rich in polyunsaturated fatty acids, which are prone to oxidative rancidity during long-term frozen storage, which leads to a decline in the freshness and quality of the fish [40]. Frozen samples had higher TBARS values compared to fresh samples, suggesting that lipid oxidation levels increased over frozen time (Fig. 5). The TBARS value of the fresh sample was about 0.1200 mg MDA/kg. It increased rapidly to 0.7306, 0.6162, 0.6786, and 0.7100 mg MDA/kg on the 180th day for the IF, UIF-160 W, UIF-175 W and UIF-190 W samples, respectively. Compared with the MUIF sample, the TBARS of the IF sample were significantly higher, indicating that MUIF could effectively reduce the lipid oxidation rate. Some reports indicated that ice crystals could puncture cells and cause the release of oxidants, leading to accelerated oxidation [34], [41]. MUIF has a faster freezing rate, causing uniform, tiny ice crystals to form within the tissue. which slows the fat oxidation rate of the sample during frozen storage [42]. In addition, MUIF can reduce the activity of lipase, phospholipase and lipoxygenase, thereby inhibiting lipid oxidation. Sun et al. [42] also found that UIF samples had a low degree of lipid oxidation during frozen storage. Therefore, MUIF can reduce the degradation of fatty acids and quality deterioration during frozen storage.
Fig. 5.
Average TBARS of myofibrillar proteins of large yellow croaker muscles during frozen storage (IF: immersion freezing; UIF: ultrasound-assisted immersion freezing at different ultrasound powers). Letters are used “a” to “c” significance differences between the samples (p < 0.05, n = 3). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.7. Fatty acids
The degree of unsaturated fatty acids, oxygen exposure, storage duration, and temperature all affect the rate and degree of lipid autoxidation [43]. The fatty acids composition (μg/g) and changes in the fresh and MUIF samples during frozen storage are summarized in Table 1. A total of 29 fatty acids were identified and quantified, among which 11 were unsaturated fatty acids (UFAs). The fresh large yellow croaker contained higher amounts of UFAs and lower amounts of saturated fatty acids (SFAs). Docosahexaenoic acid (DHA) was the highest at the beginning and followed by eicosapentaenoic acid (EPA). These two UFAs have special effects on preventing cardiovascular and cerebrovascular diseases, antitumor, and anti-inflammatory activities [44], [45]. The contents of DHA and EPA in the IF samples gradually decreased with the extension of frozen time. In particular near the end of frozen storage, these two UFAs could be better maintained in the MUIF samples. MUIF treatment reduces tissue damage and iron release from hemoglobin and myoglobin, thus inhibiting lipid autoxidation [27]. However, high ultrasound powers generated cavitation and microstreaming, generating highly reactive free radicals that promoted lipid oxidation in frozen fish. The contents of SFAs increased during frozen storage, and the increase in C16: 0 was particularly obvious, indicating that the SFAs in the flesh degraded and some UFAs converted to SFAs during frozen storage.
Table 1.
Average (±standard deviation) for fatty acid composition of large yellow croaker samples during frozen storage.
| Fatty acid (µg/mL) | 0 day |
180th day |
300th day |
||||||
|---|---|---|---|---|---|---|---|---|---|
| IF | UIF-160 | UIF-175 | UIF-190 | IF | UIF-160 | UIF-175 | UIF-190 | ||
| C12:0 | 0.594 ± 0.031f | 0.991 ± 0.092f | 0.930 ± 0.024f | 5.144 ± 0.062a | 3.17 ± 0.23c | 1.600 ± 0.058e | 1.055 ± 0.047e | 1.379 ± 0.090e | 0.732 ± 0.016f |
| C13:0 | 0.793 ± 0.030d | 0.675 ± 0.021d | 0.419 ± 0.046e | 2.31 ± 0.14a | 1.546 ± 0.036b | 1.241 ± 0.021c | 0.908 ± 0.034d | 0.340 ± 0.020e | ND |
| C14:0 | 1.10 ± 0.13d | 1.339 ± 0.031c | 0.198 ± 0.012d | 1.25 ± 0.20c | 3.59 ± 0.15a | 3.5909 ± 0.0080a | ND | ND | 0.540 ± 0.029d |
| C14:1 | 6.580 ± 0.067b | 0.996 ± 0.019e | 1.163 ± 0.022e | 8.17 ± 0.24a | 4.414 ± 0.071c | 1.87 ± 0.14e | 1.630 ± 0.031e | 1.039 ± 0.022e | 0.366 ± 0.017e |
| C15:0 | 4.4806 ± 0.0062d | 2.86 ± 0.11f | 3.011 ± 0.038e | 3.156 ± 0.085e | 4.811 ± 0.087d | 6.43 ± 0.11b | 7.824 ± 0.066a | 4.16 ± 0.22d | 2.791 ± 0.019f |
| C15:1 | 0.468 ± 0.026d | 0.450 ± 0.044d | 0.756 ± 0.034d | 3.3950 ± 0.0082a | 1.968 ± 0.030c | 0.461 ± 0.024d | 0.667 ± 0.032d | 0.386 ± 0.011d | 0.443 ± 0.021d |
| C16:0 | 114.8 ± 1.7e | 258.39 ± 0.13d | 182.94 ± 0.24e | 144.86 ± 0.15e | 169.34 ± 0.24e | 583.7 ± 4.1a | 397.2 ± 3.0c | 324.4 ± 3.5c | 367.0 ± 4.7c |
| C16:1 | 34.99 ± 0.30e | 30.75 ± 0.40e | 32.98 ± 0.25e | 32.8 ± 2.9e | 36.9 ± 4.6e | 110.0000 ± 0.0055a | 74.74 ± 0.98c | 34.24 ± 0.24e | 44.64 ± 0.54d |
| C17:0 | 7.05 ± 0.24d | 2.91 ± 0.12d | 5.51 ± 0.15d | 37.53 ± 0.19a | 21.06 ± 0.94b | 6.651 ± 0.092d | 11.402 ± 0.093c | 4.676 ± 0.081d | 3.10 ± 0.16d |
| C17:1 | 34.12 ± 0.42a | 3.343 ± 0.072d | 5.72 ± 0.13d | 34.748 ± 0.077a | 28.51 ± 0.40b | 12.615 ± 0.083c | 14.78 ± 0.21c | 9.65 ± 0.13d | 5.071 ± 0.088d |
| C18:0 | 196.4 ± 2.5f | 236.7 ± 2.2d | 234.36 ± 1.52d | 285.7 ± 1.2a | 248.21 ± 0.59c | 251.4 ± 1.6c | 250.6 ± 1.7c | 265.8 ± 1.4b | 281.5 ± 2.5a |
| C18:1n-9 | 58.61 ± 0.42d | 48.20 ± 0.13e | 68.20 ± 0.73d | 56.5 ± 2.9d | 57 ± 11 d | 245.7 ± 9.9a | 176.3 ± 1.8b | 86.71 ± 0.25d | 85.4 ± 1.7d |
| C18:2t | 44.865 ± 0.055c | 36.31 ± 0.25d | 53.80 ± 0.26b | 56.78 ± 0.45b | 58.4 ± 1.0b | 56.70 ± 0.18b | 52.19 ± 0.39b | 61.0 ± 1.0a | 31.11 ± 0.19d |
| C18:3n-3 | 33.66 ± 0.10b | 2.701 ± 0.028e | 4.348 ± 0.098e | 42.23 ± 0.27a | 27.04 ± 0.40c | 10.73 ± 0.12d | 8.55 ± 0.13e | 6.71 ± 0.14e | 2.08 ± 0.11e |
| C20:0 | 3.31 ± 0.25d | 0.225 ± 0.022e | 1.558 ± 0.045e | 4.957 ± 0.060c | 3.325 ± 0.050d | 9.368 ± 0.098a | 5.68 ± 0.33c | 4.70 ± 0.11c | 4.67 ± 0.12c |
| C20:1n-9 | 94.42 ± 0.15a | 3.32 ± 0.12e | 5.825 ± 0.018e | 66.569 ± 0.077b | 59.23 ± 0.58c | 16.44 ± 0.12e | 12.8588 ± 0.059e | 3.9669 ± 0.0060e | 5.26 ± 0.13e |
| C20:2n-6 | 13.96 ± 0.12a | 1.40 ± 0.13e | 4.74 ± 0.23e | 13.322 ± 0.049e | 11.81 ± 0.67f | 3.96 ± 0.11f | 4.105 ± 0.073e | 4.79 ± 0.16a | 2.14 ± 0.20b |
| C20:3n-6 | 10.675 ± 0.080a | 0.608 ± 0.044f | 1.616 ± 0.037f | 6.35 ± 0.13c | 3.85 ± 0.17e | 2.631 ± 0.029e | 2.362 ± 0.039e | 3.30 ± 0.13e | 2.464 ± 0.080e |
| C20:4n-6 | 82.31 ± 0.80a | 27.6 ± 2.3d | 52.39 ± 0.95c | 86.73 ± 0.34a | 91.52 ± 0.51a | 44.96 ± 0.38c | 66.8 ± 2.5b | 49.18 ± 0.93c | 24.253 ± 0.040d |
| C20:5n-3 (EPA) | 182.53 ± 0.58a | 80.13 ± 0.18f | 98.5 ± 1.1f | 171.93 ± 0.79b | 164.85 ± 0.76b | 56.0 ± 1.5 h | 71.34 ± 0.22 g | 142.30 ± 0.96c | 146.065 ± 0.020c |
| C21:0 | 1.600 ± 0.044a | 0.360 ± 0.022d | 0.628 ± 0.023c | 1.539 ± 0.010a | 1.6123 ± 0.0085a | 0.305 ± 0.024d | 0.603 ± 0.011c | 0.661 ± 0.017c | 0.1739 ± 0.0087d |
| C22:0 | 0.505 ± 0.021e | ND | ND | 1.307 ± 0.012a | 0.478 ± 0.010e | ND | ND | 0.689 ± 0.054d | ND |
| C22:1n-9 | 7.0131 ± 0.0014d | 1.126 ± 0.025d | 1.918 ± 0.037d | 32.37 ± 0.34a | 14.31 ± 0.23c | 1.823 ± 0.041d | 6.33 ± 0.58d | 9.165 ± 0.043d | 3.121 ± 0.037d |
| C22:2 | 3.201 ± 0.087c | 0.530 ± 0.034d | 0.581 ± 0.024d | 6.98 ± 0.11a | 3.736 ± 0.060c | 0.509 ± 0.022d | 1.072 ± 0.045d | 1.561 ± 0.090d | 0.26 ± 0.26d |
| C22:4n-6 | 25.278 ± 0.091a | 2.23 ± 0.22f | 5.813 ± 0.030e | 29.94 ± 0.15a | 13.30 ± 0.71d | 6.690 ± 0.087e | 12.60 ± 0.31d | 6.574 ± 0.035e | 2.665 ± 0.022f |
| C22:5n-3 | 41.7 ± 1.0b | 5.65 ± 0.21f | 18.99 ± 0.15e | 59.83 ± 0.28a | 37.52 ± 0.50c | 14.22 ± 0.13e | 30.34 ± 0.45c | 27.05 ± 0.65c | 6.388 ± 0.018f |
| C22:6n-3 (DHA) | 186.01 ± 0.40a | 106.3 ± 1.7e | 158.0 ± 1.1b | 179.86 ± 0.80b | 171.2 ± 1.2b | 91.6 ± 1.6f | 94.71 ± 0.54f | 149.4 ± 1.4c | 141.7 ± 1.1c |
| C24:0 | 0.168 ± 0.021e | ND | ND | 0.724 ± 0.022d | 0.167 ± 0.011e | 2.16 ± 0.11a | ND | ND | ND |
| C24:1n-9 | 7.496 ± 0.098c | ND | ND | 10.091 ± 0.025a | 5.4595 ± 0.0023d | 0.179 ± 0.028f | 1.313 ± 0.024f | 1.515 ± 0.050f | 0.065 ± 0.010f |
Different letters represent significant differences among samples for the same fatty acid (p < 0.05, n = 3).
3.8. Mechanism schematic
A schematic of the effect of MUIF on lipid and protein oxidation during freezing storage is shown in Fig. 6. IF samples induced the formation of large ice crystals, which continued to grow during freezing and severely damaged the sample MP structure. MUIF decomposed large ice crystals into smaller fragments through the cavitation effect, promoted secondary nucleation of ice crystals, accelerated freezing rate, reduced the destruction of MP structure by ice crystals, reduced protein unfolding, and slowed down the degree of lipid oxidation.
Fig. 6.
Schematic mechanism of the effects of the MUIF on lipid and protein of large yellow croaker during frozen storage. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Conclusions
The effects of MUIF (frequency of 20/28/40 kHz) with different powers treatments on MP structure and lipid oxidation were studied during 300 days of frozen storage of large yellow croaker. MUIF could reduce the rate of carbonyl group formation, slow the decrease in total sulfhydryl contents and Ca2+-ATPase activities, and better maintain the secondary and tertiary structures of MP. At the same time, the results of TBARS and fatty acids showed that appropriate ultrasound power (UIF-175 W) delayed the degradation of fatty acids and reduced muscle protein damage of the large yellow croaker. This study indicated that MUIF with proper power (175 W) solved the problem of muscle protein damage caused by freezing and effectively delayed the extent of lipid oxidation. However, the damage to the chemical structure and oxidative stability of meat products caused by the increase in ultrasonic power needs to be considered and solved. Therefore, the optimization of ultrasonic parameters should be considered in future research to better meet commercial requirements.
CRediT authorship contribution statement
Weihao Yang: Writing – original draft, Software, Formal analysis, Data curation, Conceptualization. Yixuan Dong: Software, Resources, Formal analysis, Data curation. Xuan Ma: Methodology, Formal analysis, Conceptualization. Jing Xie: Writing – review & editing, Supervision, Funding acquisition. Jun Mei: Writing – review & editing, Validation, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the earmarked fund for CARS-47.
Contributor Information
Jing Xie, Email: jxie@shou.edu.cn.
Jun Mei, Email: jmei@shou.edu.cn.
Data availability
Data will be made available on request.
References
- 1.Ahn J.J., Kim Y., Hong J.Y., Kim G.W., Kim S.Y., Hwang S.Y. Probe-based fluorescence melting curve analysis for differentiating larimichthys polyactis and larimichthys crocea. Food Anal. Methods. 2016;9:2036–2041. doi: 10.1007/s12161-015-0381-6. [DOI] [Google Scholar]
- 2.Kim T.-K., Lee M.H., Yong H.I., Jung S., Paik H.-D., Jang H.W., Choi Y.-S. Effect of interaction between mealworm protein and myofibrillar protein on the rheological properties and thermal stability of the prepared emulsion systems. Foods. 2020;9:1443. doi: 10.3390/foods9101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z., Zhao X., Yang W., Mei J. Jing Xie, Effect of magnetic nano-particles combined with multi-frequency ultrasound-assisted thawing on the quality and myofibrillar protein-related properties of salmon (Salmo salar) Food Chem. 2024;445 doi: 10.1016/j.foodchem.2024.138701. [DOI] [PubMed] [Google Scholar]
- 4.Xu Z., Pei J., Mei J., Yu H., Chu S., Xie J. Effect of gum tragacanth–sodium alginate coatings incorporated with epigallocatechin gallate on the quality and shelf life of large yellow croaker (Larimichthys crocea) during superchilling storage. Food Qual. Saf. 2024;8 doi: 10.1093/fqsafe/fyad039. [DOI] [Google Scholar]
- 5.Liu Y., Tan Y., Luo Y., Li X., Hong H. Evidence of myofibrillar protein oxidation and degradation induced by exudates during the thawing process of bighead carp fillets. Food Chem. 2024;434 doi: 10.1016/j.foodchem.2023.137396. [DOI] [PubMed] [Google Scholar]
- 6.Domínguez R., Pateiro M., Munekata P.E.S., Zhang W., Garcia-Oliveira P., Carpena M., Prieto M.A., Bohrer B., Lorenzo J.M. Protein oxidation in muscle foods: a comprehensive review. Antioxidants. 2021;11:60. doi: 10.3390/antiox11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y., Fu Z., Tan Y., Luo Y., Li X., Hong H. Protein oxidation-mediated changes in digestion profile and nutritional properties of myofibrillar proteins from bighead carp (Hypophthalmichthys nobilis) Food Res. Int. 2023;174 doi: 10.1016/j.foodres.2023.113546. [DOI] [PubMed] [Google Scholar]
- 8.Mahato S., Zhu Z., Sun D.-W. Glass transitions as affected by food compositions and by conventional and novel freezing technologies: a review. Trends Food Sci. Technol. 2019;94:1–11. doi: 10.1016/j.tifs.2019.09.010. [DOI] [Google Scholar]
- 9.Astráin-Redín L., Abad J., Rieder A., Kirkhus B., Raso J., Cebrián G., Álvarez I. Direct contact ultrasound assisted freezing of chicken breast samples. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Q., Chen Q., Xia X., Kong B., Diao X. Effects of ultrasound-assisted freezing at different power levels on the structure and thermal stability of common carp (Cyprinus carpio) proteins. Ultrason. Sonochem. 2019;54:311–320. doi: 10.1016/j.ultsonch.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Qiu S., Cui F., Wang J., Zhu W., Xu Y., Yi S., Li X., Li J. Effects of ultrasound-assisted immersion freezing on the muscle quality and myofibrillar protein oxidation and denaturation in Sciaenops ocellatus. Food Chem. 2022;377 doi: 10.1016/j.foodchem.2021.131949. [DOI] [PubMed] [Google Scholar]
- 12.Das K., Zhang M., Bhandari B., Chen H., Bai B., Roy M.C. Ultrasound generation and ultrasonic application on fresh food freezing: effects on freezing parameters, physicochemical properties and final quality of frozen foods. Food Rev. Int. 2023;39:4465–4495. doi: 10.1080/87559129.2022.2027436. [DOI] [Google Scholar]
- 13.Farshbaf Aghajani P., Soltani Firouz M., Alikhani Chamgordani P. The improvement of freezing time and functional quality of frozen mushrooms by application of probe-type power ultrasound. Ultrason. Sonochem. 2023;100 doi: 10.1016/j.ultsonch.2023.106637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.N. Mehta, R.A. Kantale, O.P. Malav, P. Kumar, A.K. Verma, P. Umaraw, S.K. Khatkar, K. Kaur, Application of Ultrasound Techniques for Meat, Fish, and Poultry Processing Industries, in: Non-Thermal Process. Technol. Meat, Fish, Poult. Ind., CRC Press, Boca Raton, 2023: pp. 145–180. 10.1201/9781003251958-8.
- 15.Zhang M., Haili N., Chen Q., Xia X., Kong B. Influence of ultrasound-assisted immersion freezing on the freezing rate and quality of porcine longissimus muscles. Meat Sci. 2018;136:1–8. doi: 10.1016/j.meatsci.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Ma X., Mei J., Xie J. Effects of multi-frequency ultrasound on the freezing rates, quality properties and structural characteristics of cultured large yellow croaker (Larimichthys crocea) Ultrason. Sonochem. 2021;76 doi: 10.1016/j.ultsonch.2021.105657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H., Mei J., Xie J. New ultrasonic assisted technology of freezing, cooling and thawing in solid food processing: a review. Ultrason. Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C., Wang J., Su W., Chen G., Zhang J. Effects of mango and partridge tea extracts on microbial, physical, and chemical properties of tilapia fillets treated with in-package cold plasma during refrigerator storage. Food Qual. Saf. 2024;8:fyae001. doi: 10.1093/fqsafe/fyae001. [DOI] [Google Scholar]
- 19.Zhao J., Dong F., Li Y., Kong B., Liu Q. Effect of freeze–thaw cycles on the emulsion activity and structural characteristics of soy protein isolate. Process Biochem. 2015;50:1607–1613. doi: 10.1016/j.procbio.2015.06.021. [DOI] [Google Scholar]
- 20.Sharafodin H., Soltanizadeh N. Potential application of DBD Plasma Technique for modifying structural and physicochemical properties of Soy Protein Isolate. Food Hydrocoll. 2022;122 doi: 10.1016/j.foodhyd.2021.107077. [DOI] [Google Scholar]
- 21.Duppeti H., Manjabhatta S.N., Kempaiah B.B. Physicochemical, structural, functional and flavor adsorption properties of white shrimp (Penaeus vannamei) proteins as affected by processing methods. Food Res. Int. 2023;163 doi: 10.1016/j.foodres.2022.112296. [DOI] [PubMed] [Google Scholar]
- 22.Yang X., Fang S., Xie Y., Mei J., Xie J. Preservative effects of flaxseed gum-sodium alginate active coatings containing carvacrol on quality of turbot (Scophthalmus maximus) during cold storage. Coatings. 2024;14:338. doi: 10.3390/coatings14030338. [DOI] [Google Scholar]
- 23.Abd El-Ghafour S., Zakar A.H., Mohamad A.S. Changes of fatty acid profile of mullet fish (Mugil cephalus) fillets as influenced by gamma irradiation. Egypt. J. Aquat. Res. 2018;44:241–244. doi: 10.1016/j.ejar.2018.07.001. [DOI] [Google Scholar]
- 24.Sompongse W., Itoh Y., Obatake A. Effect of cryoprotectants and a reducing reagent on the stability of actomyosin during ice storage. Fish. Sci. 1996;62:73–79. doi: 10.2331/fishsci.62.73. [DOI] [Google Scholar]
- 25.Higuera-Barraza O.A., Torres-Arreola W., Ezquerra-Brauer J.M., Cinco-Moroyoqui F.J., Rodríguez Figueroa J.C., Marquez-Ríos E. Effect of pulsed ultrasound on the physicochemical characteristics and emulsifying properties of squid (Dosidicus gigas) mantle proteins. Ultrason. Sonochem. 2017;38:829–834. doi: 10.1016/j.ultsonch.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Nikoo M., Benjakul S., Xu X. Antioxidant and cryoprotective effects of Amur sturgeon skin gelatin hydrolysate in unwashed fish mince. Food Chem. 2015;181:295–303. doi: 10.1016/j.foodchem.2015.02.095. [DOI] [PubMed] [Google Scholar]
- 27.Lu N., Ma J., Sun D.-W. Enhancing physical and chemical quality attributes of frozen meat and meat products: mechanisms, techniques and applications. Trends Food Sci. Technol. 2022;124:63–85. doi: 10.1016/j.tifs.2022.04.004. [DOI] [Google Scholar]
- 28.Ma X., Mei J., Qiu W., Xie J. Influence of multi-frequency ultrasound-assisted freezing on the freezing rate, physicochemical quality and microstructure of cultured large yellow croaker (larimichthys crocea) Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.906911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Z., Zhong S., Yan W., Liu M., Yang Z., Qiao X. The effects of ultrasonic treatment on the freezing rate, physicochemical quality, and microstructure of the back muscle of grass carp (Ctenopharyngodon idella) LWT. 2019;111:301–308. doi: 10.1016/j.lwt.2019.04.071. [DOI] [Google Scholar]
- 30.Chemat F., Zill-e-Huma M.K. Khan, Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason. Sonochem. 2011;18:813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Qiu L., Zhang M., Chitrakar B., Bhandari B. Application of power ultrasound in freezing and thawing Processes: effect on process efficiency and product quality. Ultrason. Sonochem. 2020;68 doi: 10.1016/j.ultsonch.2020.105230. [DOI] [PubMed] [Google Scholar]
- 32.Kang D., Zou Y., Cheng Y., Xing L., Zhou G., Zhang W. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrason. Sonochem. 2016;33:47–53. doi: 10.1016/j.ultsonch.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Mahato S., Sun D.-W., Zhu Z. Ca2+ATPase enzyme activities and lipid and protein oxidations of grass carp (Ctenopharyngodon idellus) stored at 4 °C for 30 min under electromagnetic fields. Food Chem. 2023;399 doi: 10.1016/j.foodchem.2022.133914. [DOI] [PubMed] [Google Scholar]
- 34.Benjakul S., Bauer F. Biochemical and physicochemical changes in catfish (Silurus glanis Linne) muscle as influenced by different freeze–thaw cycles. Food Chem. 2001;72:207–217. doi: 10.1016/S0308-8146(00)00222-3. [DOI] [Google Scholar]
- 35.Jayasooriya S.D., Torley P.J., D’Arcy B.R., Bhandari B.R. Effect of high power ultrasound and ageing on the physical properties of bovine Semitendinosus and Longissimus muscles. Meat Sci. 2007;75:628–639. doi: 10.1016/j.meatsci.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Jia G., Nirasawa S., Ji X., Luo Y., Liu H. Physicochemical changes in myofibrillar proteins extracted from pork tenderloin thawed by a high-voltage electrostatic field. Food Chem. 2018;240:910–916. doi: 10.1016/j.foodchem.2017.07.138. [DOI] [PubMed] [Google Scholar]
- 37.Cai L., Zhang W., Cao A., Cao M., Li J. Effects of ultrasonics combined with far infrared or microwave thawing on protein denaturation and moisture migration of Sciaenops ocellatus (red drum) Ultrason. Sonochem. 2019;55:96–104. doi: 10.1016/j.ultsonch.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Bian C., Yu H., Yang K., Mei J., Xie J. Effects of single-, dual-, and multi-frequency ultrasound-assisted freezing on the muscle quality and myofibrillar protein structure in large yellow croaker (Larimichthys crocea) Food Chem. X. 2022;15 doi: 10.1016/j.fochx.2022.100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang C.-H., Sun X., Foegeding E.A. Modulation of physicochemical and conformational properties of kidney bean vicilin (phaseolin) by glycation with glucose: implications for structure-function relationships of legume vicilins. J. Agric. Food Chem. 2011;59:10114–10123. doi: 10.1021/jf202517f. [DOI] [PubMed] [Google Scholar]
- 40.Fernández-Segovia I., Fuentes A., Aliño M., Masot R., Alcañiz M., Barat J.M. Detection of frozen-thawed salmon (Salmo salar) by a rapid low-cost method. J. Food Eng. 2012;113:210–216. doi: 10.1016/j.jfoodeng.2012.06.003. [DOI] [Google Scholar]
- 41.Xia X., Kong B., Liu Q., Liu J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze–thaw cycles. Meat Sci. 2009;83:239–245. doi: 10.1016/j.meatsci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Sun Q., Sun F., Xia X., Xu H., Kong B. The comparison of ultrasound-assisted immersion freezing, air freezing and immersion freezing on the muscle quality and physicochemical properties of common carp (Cyprinus carpio) during freezing storage. Ultrason. Sonochem. 2019;51:281–291. doi: 10.1016/j.ultsonch.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Tomás M.C., Anón M.C. Study on the influence of freezing rate on lipid oxidation in fish (salmon) and chicken breast muscles. Int. J. Food Sci. Technol. 1990;25:718–721. doi: 10.1111/j.1365-2621.1990.tb01134.x. [DOI] [Google Scholar]
- 44.Chen J., Garssen J., Redegeld F. The efficacy of bortezomib in human multiple myeloma cells is enhanced by combination with omega-3 fatty acids DHA and EPA: timing is essential. Clin. Nutr. 2021;40:1942–1953. doi: 10.1016/j.clnu.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 45.So J., Wu D., Lichtenstein A.H., Tai A.K., Matthan N.R., Maddipati K.R., Lamon-Fava S. EPA and DHA differentially modulate monocyte inflammatory response in subjects with chronic inflammation in part via plasma specialized pro-resolving lipid mediators: a randomized, double-blind, crossover study. Atherosclerosis. 2021;316:90–98. doi: 10.1016/j.atherosclerosis.2020.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.