Abstract
Introduction
Spontaneous preterm birth complicates ∼7% of pregnancies and causes morbidity and mortality. Although infection is a common etiology, our understanding of the fetal immune system in vivo is limited. This study aimed to utilize T2-weighted imaging and T2* relaxometry (which is a proxy of tissue oxygenation) of the fetal spleen in uncomplicated pregnancies and in fetuses that were subsequently delivered spontaneously prior to 32 weeks.
Methods
Women underwent imaging including T2-weighted fetal body images and multi-eco gradient echo single-shot echo planar sequences on a Phillips Achieva 3T system. Previously described postprocessing techniques were applied to obtain T2- and T2*-weighted imaging of the fetal spleen and T2-weighted fetal body volumes.
Results
Among 55 women with uncomplicated pregnancies, an increase in fetal splenic volume, splenic:body volume, and a decrease in splenic T2* signal intensity was demonstrated across gestation. Compared to controls, fetuses who were subsequently delivered prior to 32 weeks’ gestation (n = 19) had a larger spleen when controlled for the overall size of the fetus (p = 0.027), but T2* was consistent (p = 0.76).
Conclusion
These findings provide evidence of a replicable method of studying the fetal immune system and give novel results on the impact of impending preterm birth on the spleen. While T2* decreases prior to preterm birth in other organs, preservation demonstrated here suggests preferential sparing of the spleen.
Keywords: Chorioamnionitis, Fetal immunity, Infection, Magnetic resonance imaging, Preterm rupture of membranes, Preterm delivery
Introduction
Preterm birth (delivery prior to 37 completed weeks’ gestation) remains the leading cause of neonatal mortality and morbidity worldwide accounting for around 10% of deliveries globally, with the incidence of poor outcomes inversely proportional to gestation at delivery [1]. Accordingly, although extreme preterm birth (prior to 32 completed weeks’ gestation) accounts for only 1.2% of deliveries, it is disproportionately responsible for mortality and morbidity, including cerebral palsy, developmental delay, chronic lung disease, and necrotizing enterocolitis [2, 3]. Between 40 and 70% of cases of delivery prior to 32 weeks’ are associated with chorioamnionitis, rising as high as 94% when delivery occurs prior to 24 weeks of which around 50% are additionally associated with a fetal inflammatory response, evidencing a fetal immune response [4].
Sitting in the upper left abdomen, the spleen is embryologically distinct from the gastrointestinal tract and serves as a secondary lymphoid organ, hosting hematopoetic progenitor cells from 15 weeks [5], and is responsible for lymphoid immune responses via the reticuloendothelial system, although it also has a purpose in filtering red blood cells [6, 7]. Animal studies have demonstrated histopathological changes in the spleen in cases of chorioamnionitis, with rhesus macaques demonstrated to develop diffuse reticuloendothelial hyperplasia secondary to chorioamnionitis following inoculation with Ureaplasma parvum [8]. Conversely, both perinatal and adult postmortem in cases of sepsis have demonstrated severe depletion in T and B cells in the spleen [9–11], although no study has compared splenic volumes in postmortem in fetuses with and without chorioamnionitis.
While the spleen is not routinely assessed in fetal ultrasound, it can be identified in the axial view of the upper abdomen. Normograms of splenic circumference suggest quadratic growth with gestation [12]. While there is evidence of increased splenic size in fetal anemia [13], no studies have assessed ultrasonographical changes in size in the presence of the fetal inflammatory response. Measurement of splenic size has not been adopted into clinical practice, likely because of the good predictive value of middle cerebral artery peak systolic velocity in fetal anemia [14], obviating the need for splenic assessment for this reason, but also because of the difficulties in accurately measuring the diameter of irregularly shaped organs on ultrasound. However, abnormal splenic vein Dopplers (pulsatile rather than continuous wave flow) have been observed in fetuses born preterm with chorioamnionitis, although these findings have never been validated [15, 16].
Magnetic resonance imaging (MRI) could improve our understanding of volumetric and functional changes in the fetal spleen during the inflammatory response associated with preterm birth. It has previously been utilized to study anatomical variations of the spleen in specific pathologies, such as situs anomalies and cardiac isomerism [17, 18], and one study has demonstrated feasibility of obtaining T2* relaxometry values [19]. However, normal ranges for anatomical or functional data have not been established and no attempts have been made to correlate with fetuses who go on to be delivered extremely preterm. Both techniques have the potential to provide additional in vivo insights into the fetal immune system prior to preterm birth: the application of deformable slice to volume reconstruction (DSVR) [20], which corrects for the impact of fetal motion, allows for accurate assessment of splenic volume; and the application of T2* relaxometry, which utilizes the differing magnetic properties of oxy- and deoxyhemoglobin may give insight indirectly into tissue oxygenation. This study aimed to utilize both anatomical T2-weighted imaging and T2* relaxometry of the fetal spleen in uncomplicated pregnancies throughout gestation and in a cohort of fetuses that were subsequently delivered very preterm to determine alterations in volume and tissue oxygenation owing to the inflammatory changes that precede preterm birth.
Methods
Ethical Approval
Ethical approval was obtained under 16/LO/1573 (the placental imaging project), 19/SS/0032 (antenatal assessment of fetal infection using advanced MRI protocols), 21/YH/0210 (the use of advanced MRI techniques to evaluate antenatal lung development), and 21/SS/0082 (individual risk prediction of adverse neonatal outcomes in pregnancies that deliver preterm using advance MRI techniques and machine learning). All regulatory approvals were obtained prior to commencing research. All women provided informed written consent prior to participation.
Recruitment
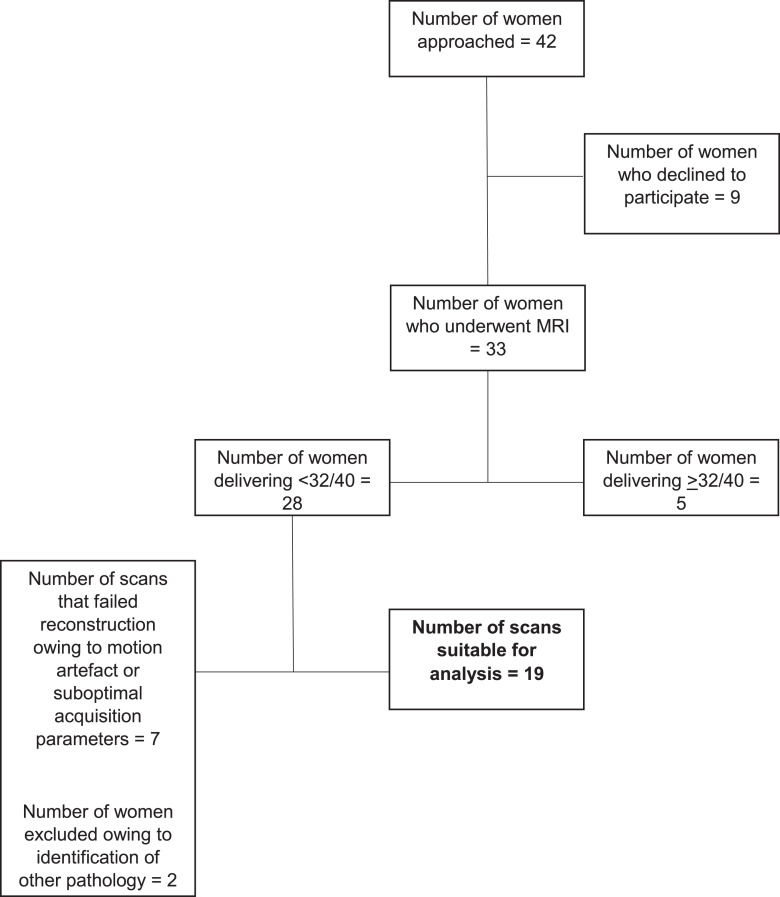
Pregnant women between 18 and 31+6 weeks’ gestation who were considered to be at high risk of preterm birth were prospectively recruited from St Thomas’ Hospital between December 2015 and August 2023. Inclusion criteria were preterm prelabor rupture of the membranes, or exposed membranes, or a >75% risk of delivery before 32 weeks’ gestation based on a validated algorithm utilizing cervical length, quantitative fetal fibronectin and preexisting risk factors for spontaneous preterm birth (QUiPP App) [21]. Women were retrospectively excluded if they subsequently delivered after 32 weeks’ gestation or acquired other obstetric complications.
The control group was retrospectively obtained from low-risk pregnancies that delivered at term where woman had volunteered to have an MRI as part of the above-mentioned studies. For cases and controls, exclusion criteria were contraindication to MRI, twin pregnancy, chromosomal or structural abnormalities, active labor, and inability to give informed consent.
Image Acquisition
All women underwent a fetal MRI on a Phillips Achieva 3T clinical MR scanner using a 32-channel cardiac coil. During the recruitment period, no significant changes were made to the MRI hard- or software. Imaging was obtained in approximately 1 h, with a break offered around half-way through. Scanning was undertaken in the supine position, with continuous maternal heart rate and oxygen saturation and intermittent blood pressure monitoring.
Anatomical imaging of the fetal body was acquired using 2D T2-weighted single-shot turbo spin echo in at least three orthogonal planes reoriented with respect to maternal habitus or the fetal body and brain. A B0 map was acquired using an in-house tool and manual image-based shimming performed to improve homogeneity of the magnetic field [22]. A multi-echo gradient echo single-shot echo planar sequence was performed with the following parameters: 3 mm3 resolution, five echo times (13.8 ms/70.4 ms/127.0 ms/183.6 ms/270.2 ms), repetition time 3 s, parallel imaging SENSE factor = 3, flip angle 90°. The field of view was set to 360 mm × (320–400) mm × (60–120) mm using scanner co-ordinates in order to encompass the whole body and minimize artefact from parallel imaging reconstruction techniques.
Image Analysis
A perinatal radiologist reviewed all images for structural pathology. All other image analysis was undertaken after completion of recruitment. Fetal body volume was calculated following segmentation of the least motion corrupted T2 stack of the whole fetus using a semiautomated pipeline developed in-house followed by manual editing using ITK-SNAP [23]. We have previously confirmed good inter and interobserver variability in assessment of fetal volume [24]. The DSVR image was then used to generate an anatomically oriented, aligned T2* map (Fig. 1).
Fig. 1.
MR images of the spleen acquired in a control fetus at 31+2 weeks’ gestation. Top row: axial images; bottom row: coronal images. a T2-weighted images following reconstruction using deformable slice to volume registration (spleen in red). b T2 multi-echo prior to reconstruction (second echo time shown). c T2* map generated from T2ME images and reconstructed in T2 space (spleen in red).
Semiautomated segmentations of the fetal spleen generated via an in-house pipeline [25] onto the T2-weighted DSVR images were manually refined by MH on ITK-SNAP and volumes calculated from this [23] (3 years of MRI experience, interclass correlation confirmed with LS, 15 years MRI experience, to be 0.98) (Fig. 1). A comparison of manual segmentation of the oriented T2* map as compared to application of the T2-segmented spleen was undertaken on five sets of images and no difference was noted (intraclass correlation coefficient 0.95), and so the refined T2 segmentations were also applied to the T2* images. T2* maps were generated using a monoexponential decay model via an in-house python script to obtain proton density and T2* maps: 10 random initializations were performed and the voxel-wise median value was used [26].
Outcome Data
Data were collected on maternal demographics and obstetric outcomes, including maternal antenatal corticosteroid (ACS) administration. Neonatal outcomes including gestation at delivery, sex of infant, birthweight, and birthweight centile (calculated using INTERGROWTH-21 charts), neonatal unit admission, and survival to discharge were collected. A composite outcome consisting of persistent periventricular hyperechoic foci, grade 3–4 intraventricular hemorrhage, retinopathy of prematurity, chronic lung disease (defined as supplementary oxygen requirement at 36 weeks’ corrected gestation), and necrotizing enterocolitis was calculated for all neonates who survived to discharge [27]. Placental histopathology was reported by a specialist perinatal histopathologist in accordance with the Amsterdam Criteria for women delivering prior to 32 weeks’ gestation [28].
Statistical Analysis
Statistical analysis was undertaken in SPSS version 29 and graphs made in Excel 16.75. No formal power calculation was performed as this was a pilot study that explored a novel hypothesis. Demographic and neonatal outcome data were analyzed using a Student’s t test for continuous data and a χ2 test for categorical data. After testing for normality, differences between groups were investigated accounting for the effect of gestation using linear regression analysis, where data were not normally distributed, a log transformation was first performed. The Royston-Wright method was used for development of normal ranges [29].
Results
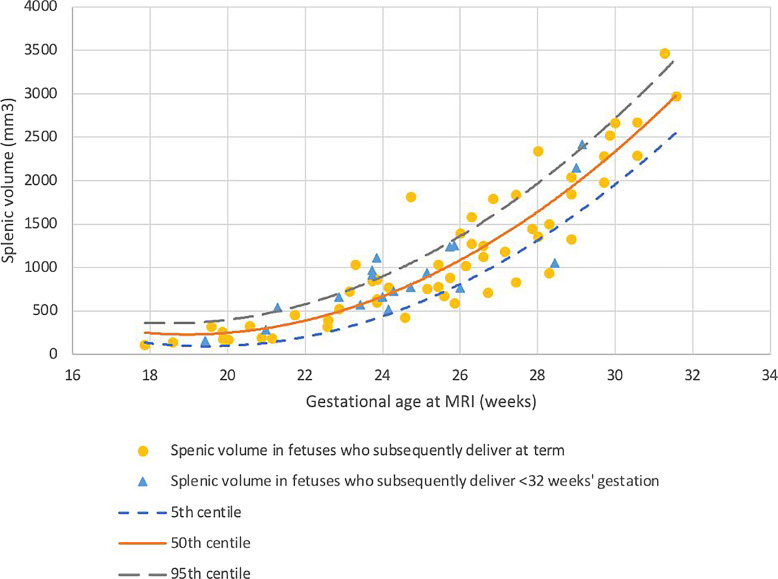
Nineteen women who subsequently delivered prior to 32 weeks’ gestation and who had complete imaging of the fetal abdomen were included: 11 had PPROM prior to recruitment. Fifteen cases had placental histopathology available, of which nine had chorioamnionitis and funisitis, 11 had chorioamnionitis alone, and four had no pathological anomalies, no other placental anomalies were identified in any case. High-risk recruitment is summarized in Figure 2. Fifty-five controls were identified, all of whom delivered at term without obstetric or neonatal complication. Clinical characteristics for both cohorts are summarized in Table 1. No overt pathology was identified in any cases. Splenic volume increased quadratically with gestation (p < 0.001) and was not significantly different in fetuses who were subsequently delivered extremely preterm (p = 0.140) (Fig. 3).
Fig. 2.
Summary of recruitment of women at high risk of spontaneous preterm birth prior to 32 gestational weeks.
Table 1.
Clinical characteristics of cases and controls
| Characteristic | Term cohort (n = 55) | Preterm cohort (n = 19) | p value | 95% CI |
|---|---|---|---|---|
| Maternal age, years | ||||
| Mean (SD) | 33.20 (6.39) | 28.91 (11.87) | 0.148 | −1.64–10.22 |
| BMI, kg/m2 | ||||
| Mean (SD) | 22.91 (4.42) | 21.65 (8.73) | 0.557 | −3.09–5.59 |
| Ethnicity, n (%) | ||||
| White | 39 (71) | 11 (58) | ||
| Black | 6 (11) | 3 (16) | ||
| South Asian | 5 (9) | 4 (21) | ||
| Mixed race | 3 (5) | 0 | ||
| Other | 2 (4) | 1 (5) | ||
| Ethnicity grouping, n (%) | ||||
| White | 39 (71) | 11 (58) | 0.413 | |
| Non-white | 16 (29) | 8 (42) | ||
| Parity, n (%) | ||||
| Primiparous | 43 (78) | 14 (74) | 0.688 | |
| Multiparous | 12 (22) | 5 (26) | ||
| Gestational age at MRI, weeks | ||||
| Mean (SD) | 25.45 (3.46) | 24.51 (2.56) | 0.214 | −0.57–2.446 |
| Gestational age at birth, weeks | ||||
| Mean (SD) | 39.73 (1.26) | 26.24 (3.43) | ||
| Birth weight, g | ||||
| Mean (SD) | 3,357.64 (378.10) | 984.74 (583.29) | ||
| Birthweight centiles of live births, n (%)* | ||||
| 0–3 | 0 | 0 | 0.023 | |
| 3–10 | 1 (2) | 2 (13) | ||
| 10–25 | 2 (4) | 0 | ||
| 25–50 | 21 (38) | 1 (6) | ||
| 50–75 | 8 (15) | 7 (44) | ||
| 75–90 | 15 (27) | 4 (25) | ||
| 90–97 | 8 (15) | 2 (13) | ||
| 97–100 | 0 | 0 | ||
| Sex of infant, n (%) | ||||
| Female | 28 (51) | 10 (53) | 0.897 | |
| Male | 27 (49) | 9 (47) | ||
| Undetermined | 0 | |||
| Outcome, n (%) | ||||
| Live at discharge | 55 (100) | 12 (63) | ||
| Death prior to discharge | ||||
| Second trimester pregnancy loss | 4 (21) | |||
| Neonatal death | 3 (16) | |||
Continuous data analyzed using Student’s t test; categorical data using χ2.
BMI, body mass index; MRI, magnetic resonance imaging.
*Calculated using the INTERGROWTH-21 formula. Centiles not available for deliveries <23 weeks’ gestation.
Fig. 3.
Fetal splenic volume in control fetuses and fetuses who were subsequently delivered prior to 32 gestational weeks. Yellow dots: control fetuses (n = 55); blue triangles: fetuses who were subsequently delivered prior to 32 weeks (n = 19) (p = 0.146). Small dashed blue line: 5th centile; solid orange line: 50th centile; long dashed gray line: 95th centile. Formulas for generating centiles where GA = gestational age at MRI: 50th centile: y = (6438.557745157574) + (−655.102252428557 × GA) + (17.27958002820324 × GA × GA); 5th and 95th centiles: y = (6438.557745157574) + (−655.102252428557 × GA) + (17.27958002820324 × GA × GA) ± (1.645 × [0 + −1.673780165632131 × GA] + [0.5140457330570599 × GA × GA]).
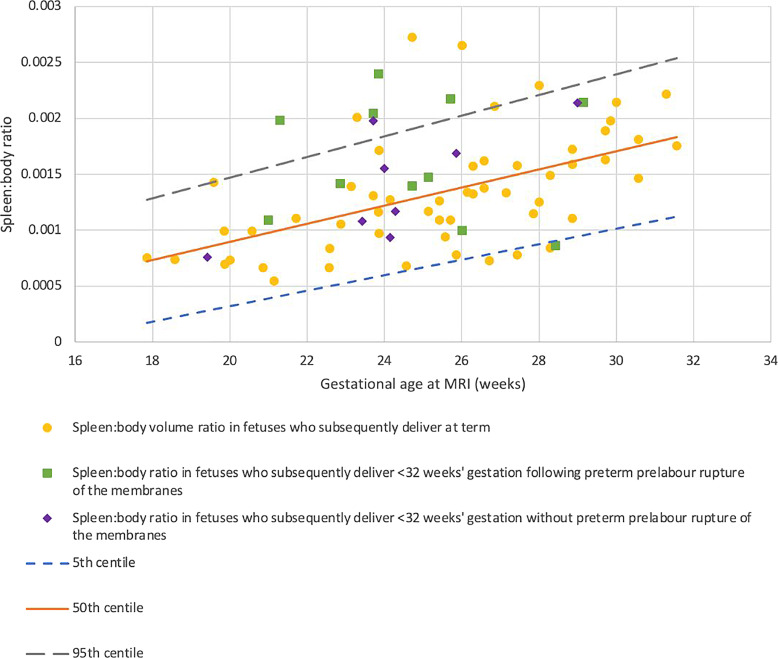
Body volumes were significantly lower in fetuses who went on to be delivered extremely preterm (p = 0.001). Spleen:body volume increases with gestation (p < 0.001) and was increased in fetuses who were subsequently delivered extremely preterm (p = 0.028) (Fig. 4); this effect seems to have been driven by cases of PPROM (n = 11, p = 0.026) rather than those with membranes intact (n = 9, p = 0.283) (Fig. 5).
Fig. 4.
Fetal spleen volume:body volume ratio in control fetuses and fetuses who were subsequently delivered prior to 32 gestational weeks. Yellow dots: control fetuses (n = 55); blue triangles: fetuses who were subsequently delivered prior to 32 weeks (n = 19) (p = 0.027). Small dashed blue line: 5th centile; solid orange line: 50th centile; long dashed gray line: 95th centile. Formulas for generating centiles where GA = gestational age at MRI: 50th centile: y = (8.080781009961062e−05 × GA) + (−0.000720353421025719); 5th and 95th centiles: y = (8.080781009961062e−05 × GA) + (−0.000720353421025719) ± (1.645 × [7.041853238979974e−06 × GA + 0.0002079991357377875]).
Fig. 5.
Fetal spleen volume:body volume ratio demonstrating the impact of preterm prelabor rupture of the membranes. Control fetuses: yellow dots (n = 55); green squares: fetuses who were subsequently delivered prior to 32 gestational weeks following preterm prelabor rupture of the membranes (n = 11) (p = 0.026); blue diamonds: fetuses who were subsequently delivered prior to 32 gestational weeks without preterm prelabor rupture of the membranes (n = 9) (p = 0.283). Small dashed blue line: 5th centile; solid orange line: 50th centile; long dashed gray line: 95th centile.
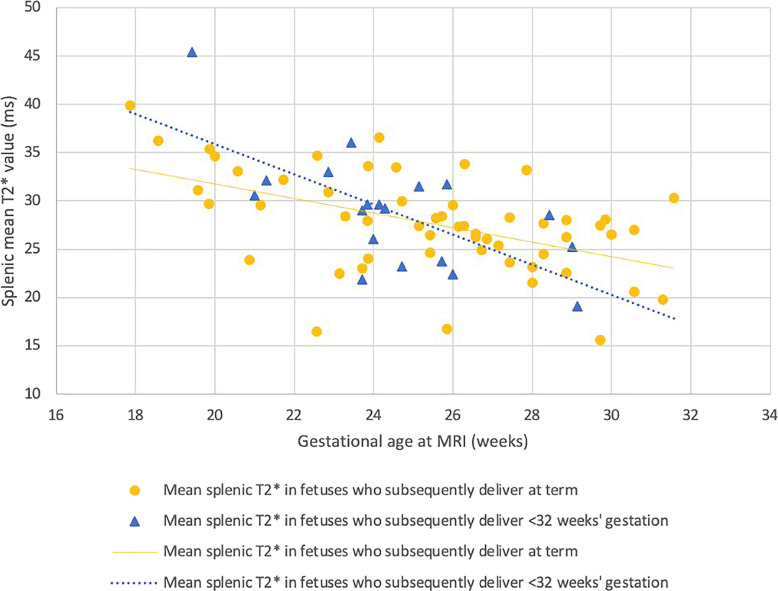
Mean spleen T2* decreased across gestation in low-risk fetuses (p < 0.001). This relationship was maintained in fetuses who were subsequently born extremely preterm (p = 0.755) (Fig. 6).
Fig. 6.
Fetal splenic mean T2* in control fetuses and fetuses who were subsequently delivered prior to 32 gestational weeks. Yellow dots: control fetuses (n = 55); blue triangles: fetuses who were subsequently delivered prior to 32 weeks (n = 19) (p = 0.755).
Frequency of neonatal morbidity is too small to correlate with MR findings; however, a summary of outcomes in all babies born after viability is given in Table 2. Of the 14 infants who survived to discharge, four had a neonatal composite morbidity score of one, and all others scored zero.
Table 2.
Severe neonatal morbidity in preterm cohort
| Morbidity | Frequency |
|---|---|
| Intraventricular hemorrhage grade 3–4 diagnosed on cranial ultrasound, n (%) | 1 (7) |
| Persistent periventricular echo densities diagnosed on cranial ultrasound, n (%) | 0 |
| Retinopathy of prematurity, n (%) | 0 |
| Chronic lung disease, n (%) | 4 (27) |
| Necrotizing enterocolitis, n (%) | 3 (20) |
| Early-onset sepsis confirmed on culture, n (%) | 5 (33) |
Discussion
Splenic volume can be reproducibly calculated on fetal MRI and increases linearly with gestation between 16 and 32 weeks’ gestation. Fetuses who are subsequently born spontaneously prior to 32 weeks’ gestation have a reduced body volume as compared to their term-born counterparts, and a higher spleen:body ratio. Splenic mean T2* decreases across gestation in the control group, and this is maintained in the cases.
Fetal splenic size across gestation has been calculated on ultrasound using either 2D or 3D measurements. The largest study of normally progressing pregnancies (n = 684) measured splenic circumference and demonstrated quadratic growth between 14 and 40 gestational weeks [12], while another large study of 405 fetuses noted circumference to grow linearly, it did note a quadratic relationship with volume [30], as has been shown here. The consistency between these studies and our best model of growth with gestational age adds further support to the use of MRI as an accurate means of determining fetal splenic volume.
Our finding of reduced body volumes in fetuses who go on to be delivered very preterm is consistent with our previous work [24], and highlights the need for organ:body volume ratios, rather than reliance on absolute size of the organ. Of note, the preterm group are in higher birthweight centiles than the term-born infants, this is likely to reflect a combination of issues: first, differing body composition between term and preterm cohorts so driving a difference between volume and weight [31]; and the reliance on a relatively small number of infants born preterm to create centiles for this cohort as compared to the term-born group [32, 33]. The increase in splenic volume relative to fetal size in the cohort who subsequently delivers preterm is a novel finding: to our knowledge, there are no human or animal studies comparing splenic volume following chorioamnionitis. This finding could reflect increased function of the organ owing to inflammation prior to preterm birth. While data on changes in size of the fetal spleen following chorioamnionitis (for example, on postmortem) are lacking, splenic enlargement is a recognized finding in adults who have died with sepsis [34], and it conceivable that a similar mechanism is at play here.
A small study of nine fetuses has demonstrated feasibility of assessing mean splenic T2* in the fetus and suggests a decrease in mean T2* across gestation. Fetal spleen mean T2* has been assessed in one other study that was conducted at 0.55 T with the aim of defining an automated pipeline for organ segmentation, rather than establishing normal ranges across gestation, and inaccuracies in the final pipeline were not corrected [35]. This study did not show a statistically significant decrease in T2* with gestational age, although this finding appears skewed by a group of outliers at term – which could reflect physiological changes at term or may relate to the pipeline described. Our finding of reduced mean T2* across gestation could reflect a true decrease in relative oxygenation as gestation progresses or may reflect changes in erythropoiesis across gestation.
Imaging-based assessment of the fetal spleen has been utilized in order to understand nonstructural-based pathologies in the perinatal period, in particular fetal anemia (using ultrasound) [13, 36] and neonatal hemochromatosis (using T2*) [37]. However, in both these conditions, pathological changes are associated with erythrocyte filtration, iron storage, and erythropoiesis, and not the immune functions of the spleen. One ultrasound study by Musilova et al. [38] has used splenic venous Dopplers to interrogate the function of the spleen in fetuses following PPROM (n = 79) and has demonstrated pulsatile flow in cases where funisitis is subsequently diagnosed. The cause of this is hypothesized to be a consequence of abnormal flow in the hepatic system, rather than directly related to splenic pathology itself, although this was not demonstrated in their work. Our finding of a preserved T2* in the spleen of fetuses who were subsequently delivered extremely preterm is unlike that noted in the placenta [39] where a decrease is seen: these findings are likely to represent varying responses to a single pathology, and in the case of the spleen could reflect preservation of immune function in response to an inflammatory event.
Postmortem studies following severe chorioamnionitis do show a critical role for the spleen with severe T- and B-cell depletion demonstrated [9]. A similar T-cell depletion is seen in the thymus at postmortem [40], and ovine models suggest that, in this case, this is driven by movement of cells into the circulation [41, 42]. It is plausible that a similar event happens in relation to the spleen, although the increased size of the spleen is in contrast to the reduction seen in the thymus [43]. A likely hypothesis comes from analysis of splenic-derived cytokines in a preterm lamb model following lipopolysaccharide exposure in utero which demonstrates a more protracted response from the spleen than has been demonstrated in the thymus: the study characterized a CD3 cell response from 5 h postexposure and lasting up to 15 days, suggesting that intrauterine inflammation causes both a rapid and prolonged change in fetal splenic function [44]. They further hypothesize that this response could be biphasic in response to the initial insult and a secondary response to inflammatory processes in other organs, such as the gut.
However, in the fetal spleen at postmortem increased areas of granulopoesis have also been described which may explain the need for preserved metabolic function suggested by the unchanged mean T2* value demonstrated in this study [10]. In a rhesus macaques model, reticuloendothelial hyperplasia has also been described which would also support this theory [8]. However, in the case of both postmortem and animal studies, only very severe disease is represented, and whether these findings are directly applicable to in vivo assessment of the spleen is not clear. Nonetheless, the apparent impact on organ size of PPROM, which is more strongly associated with chorioamnionitis and funisitis than preterm birth with intact membranes [45], does support a theory of inflammation-driven change.
Strengths and Limitations
To our knowledge, this is the first study to describe normal volumetry of the fetal spleen across gestation, determine relative size of the spleen in fetuses who go on to be delivered extremely preterm, and utilize a functional imaging technique to interrogate the impact of preterm birth on this part of the fetal immune system, which to date has only been studied minimally, particularly in human fetuses. As well as being novel, we have demonstrated that these techniques are reproducible. We utilize advanced imaging techniques that can be utilized elsewhere as pipelines are accessible [46]. While the rate of data loss appears high, we have included studies that did not formally image the fetal body; in studies that have formally imaged this rate of data loss is 20%, including cases of suboptimal imaging owing to shortened scan protocols for patient discomfort. We anticipate that this will continue to fall. Furthermore, MRI has overcome several issues associated with ultrasound, in particular difficulties obtaining images because of maternal tissues or because of oligohydramnios (a particular concern in women at high risk of spontaneous preterm birth) and provided reproducible means of functional assessment.
There are several limitations to this work. As this is a pilot study, the sample size is small, which hinders our ability to study the direct impact of chorioamnionitis and funisitis. While related research is lacking, there is potential that antenatal corticosteroid administration may impact the spleen. While Musilova’s work did consider the effect of steroids and suggests more women with fetal Doppler abnormalities had received steroids; however, this finding related to the higher rates of funisitis in this group, and so steroid use could reflect a more severe clinical picture rather than being in any way causative. It was not feasible to further investigate this given national protocols on steroid administration dictated that participants above 23 weeks’ gestation had all received steroids, and those below had not.
T2* relaxometry is an indirect measurement of tissue oxygenation and can be influenced by several factors. Inadvertent inclusion of other tissues could confound results, although conservative segmentation on T2 images as well as applying thresholding reduced the impact of this. Most particular to the spleen, increased iron deposition influences T2* results. However, we have found no evidence of alterations in splenic iron deposition prior to spontaneous preterm birth, limiting concern surrounding this.
Future Research
These pilot data provide support for the use of MR based imaging methodologies in the assessment of the fetal spleen in vivo and provide further insight into the role of the spleen, a relatively understudied immune organ as compared to the fetal thymus or placenta. While building on this dataset may achieve greater understanding of the implications of preterm birth and chorioamnionitis and funisitis on splenic volume and the impact on T2* relaxometry, other related avenues are also of value, especially given the limited role of isolated splenic assessment in the prediction of preterm birth. Longitudinal assessment of women at high risk of preterm birth could give greater understanding of the immune changes that occur as chorioamnionitis develops, particularly given animal model-derived hypothesis of a biphasic response; if assessment of the spleen were to be combined with that of other relevant structures such as the thymus, adrenal glands, or placenta, this would add greater granularity still to our understanding of fetal immunity prior to preterm birth. The histopathological changes described in both animal and human spleens following severe chorioamnionitis lend themselves to assessment via diffusion imaging which is better suited for analyzing microstructural changes in the organ which could add further insights still. While there is extensive neonatal T-cell work, the potential to understand the origins of cell lineages in the circulation immediately following preterm birth could add greater insight, although this may be confounded by the process of active labor itself. Furthermore, the imaging techniques described have translation to other conditions where improved understanding of the anatomical and functional properties of the fetal spleen is of interest such as isomerism but also to better understand the antenatal antecedence of pediatric complications of conditions such as hemochromatosis and hemoglobinopathies.
This study demonstrates feasibility of T2 volumetry and T2* relaxometry as applied to the fetal spleen. Among fetuses who go on to be delivered extremely preterm, splenic volume is increased as compared to fetal size; T2* is maintained, suggesting equivalence in oxygenation in both groups. This work provides convincing preliminary data on the value of these techniques in the assessment of the fetal spleen prior to spontaneous preterm birth and gives foundations for future use of MR as a methodology to improve in vivo understanding of the fetal immune system.
Acknowledgments
The authors are grateful to the women who participated in these research studies.
Statement of Ethics
The placental imaging project was reviewed by the London Central Research Ethics Committee, approval ID 16/LO/1573. Antenatal assessment of fetal infection using advanced MRI protocols and individual risk prediction of adverse neonatal outcomes in pregnancies that deliver preterm using advanced MRI techniques and machine learning were both reviewed by the South East Scotland Research Ethics Committee with approval IDs 19/SS/0032 and 21/SS/0082, respectively. The use of advanced MRI techniques to evaluate antenatal lung development was reviewed by the Yorkshire and Humber – South Yorkshire Research Ethics Committee, Approval No. 21/YH/0210. All participants gave informed, written consent prior to participation in these studies.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
L.S. is funded by the National Institute for Health Research (NIHR) (NIHR Advanced Fellowship [301664]). This work was supported by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z], by the NIH Human Placenta Project grant 1U01HD087202-01 (Placenta Imaging Project [PIP]), by the Wellcome Trust, Sir Henry Wellcome Fellowship to J.H., [201374/Z/16/Z], by the UKRI, FLF to JH [MR/T018119/1], and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Author Contributions
M.H.: conceptualization, investigation, formal analysis, data curation, writing – original draft, and visualization; A.U.: methodology, software, and writing – review and editing; M.P.: investigation and writing – review and editing; N.S.: funding acquisition, writing – review and editing, and supervision; D.G. and M.R.: writing – review and editing; A.S.: supervision and writing – review and editing; J.H.: methodology, software, formal analysis, and writing – review and editing. L.S.: funding acquisition, conceptualization, investigation, data curation, supervision, and writing – review and editing.
Funding Statement
L.S. is funded by the National Institute for Health Research (NIHR) (NIHR Advanced Fellowship [301664]). This work was supported by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z], by the NIH Human Placenta Project grant 1U01HD087202-01 (Placenta Imaging Project [PIP]), by the Wellcome Trust, Sir Henry Wellcome Fellowship to J.H., [201374/Z/16/Z], by the UKRI, FLF to JH [MR/T018119/1], and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Data Availability Statement
The data that support the finding of the study are not publicly available due to privacy reasons but are available from the corresponding author (megan.hall@kcl.ac.uk) upon reasonable request.
References
- 1. World Health Organisation . Born too soon: decade of action on preterm birth. Available from: https://www.who.int/publications/i/item/9789240073890 (accessed May 16th, 2023).
- 2. Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russell P. Inflammatory lesions of the human placenta: clinical significance of acute chorioamnionitis. Am J Diagn Gynecol Obstet. 1979;1. [Google Scholar]
- 5. Golub R, Cumano A. Embryonic hematopoiesis. Blood Cells Mol Dis. 2013;51(4):226–31. [DOI] [PubMed] [Google Scholar]
- 6. Varga I, Babala J, Kachlik D. Anatomic variations of the spleen: current state of terminology, classification, and embryological background. Surg Radiol Anat. 2018;40(1):21–9. [DOI] [PubMed] [Google Scholar]
- 7. Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5(8):606–16. [DOI] [PubMed] [Google Scholar]
- 8. Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16(1):56–70. [DOI] [PubMed] [Google Scholar]
- 9. Toti P, De Felice C, Occhini R, Schuerfeld K, Stumpo M, Epistolato MC, et al. Spleen depletion in neonatal sepsis and chorioamnionitis. Am J Clin Pathol. 2004;122(5):765–71. [DOI] [PubMed] [Google Scholar]
- 10. Stallmach T, Karolyi L. Augmentation of fetal granulopoiesis with chorioamnionitis during the second trimester of gestation. Hum Pathol. 1994;25(3):244–7. [DOI] [PubMed] [Google Scholar]
- 11. Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE Jr, Hui JJ, Chang KC, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166(11):6952–63. [DOI] [PubMed] [Google Scholar]
- 12. Srisupundit K, Piyamongkol W, Tongprasert F, Luewan S, Tongsong T. Reference range of fetal splenic circumference from 14 to 40 weeks of gestation. Arch Gynecol Obstet. 2011;283(3):449–53. [DOI] [PubMed] [Google Scholar]
- 13. Oepkes D, Meerman RH, Vandenbussche FP, van Kamp IL, Kok FG, Kanhai HH. Ultrasonographic fetal spleen measurements in red blood cell-alloimmunized pregnancies. Am J Obstet Gynecol. 1993;169(1):121–8. [DOI] [PubMed] [Google Scholar]
- 14. Martinez-Portilla RJ, Lopez-Felix J, Hawkins-Villareal A, Villafan-Bernal JR, Paz Y Miño F, Figueras F, et al. Performance of fetal middle cerebral artery peak systolic velocity for prediction of anemia in untransfused and transfused fetuses: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2019;54(6):722–31. [DOI] [PubMed] [Google Scholar]
- 15. Musilova I, Spacek R, Stranik J, Jacobsson B, Kacerovsky M. Fetal portal system flowmetry and intra-amniotic inflammation in preterm prelabor rupture of membranes. Fetal Diagn Ther. 2019;46(5):323–32. [DOI] [PubMed] [Google Scholar]
- 16. Musilova I, Kacerovsky M, Andrys C, Kostal M, Slaba K, Jacobsson B. The fetal splenic vein flow pattern and fetal inflammatory response in the preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2014;27(8):770–4. [DOI] [PubMed] [Google Scholar]
- 17. Liu YP, Huang YL, Tsai PS, Lin DC, Chen CP. Prenatal diagnosis of abdominal lymphatic malformations. Taiwan J Obstet Gynecol. 2021;60(1):13–9. [DOI] [PubMed] [Google Scholar]
- 18. Nemec SF, Brugger PC, Nemec U, Bettelheim D, Kasprian G, Amann G, et al. Situs anomalies on prenatal MRI. Eur J Radiol. 2012;81(4):e495–501. [DOI] [PubMed] [Google Scholar]
- 19. Sethi S, Giza SA, Goldberg E, Empey MEET, de Ribaupierre S, Eastabrook GDM, et al. Quantification of 1.5 T T1 and T2* relaxation times of fetal tissues in uncomplicated pregnancies. J Magn Reson Imaging. 2021;54(1):113–21. [DOI] [PubMed] [Google Scholar]
- 20. Uus A, Zhang T, Jackson LH, Roberts TA, Rutherford MA, Hajnal JV, et al. Deformable slice-to-volume registration for motion correction of fetal body and placenta MRI. IEEE Trans Med Imaging. 2020;39(9):2750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuhrt K, Smout E, Hezelgrave N, Seed PT, Carter J, Shennan AH. Development and validation of a tool incorporating cervical length and quantitative fetal fibronectin to predict spontaneous preterm birth in asymptomatic high-risk women. Ultrasound Obstet Gynecol. 2016;47(1):104–9. [DOI] [PubMed] [Google Scholar]
- 22. Gaspar AS, Nunes RG, Ferrazzi G, Hughes EJ, Hutter J, Malik SJ, et al. Optimizing maternal fat suppression with constrained image-based shimming in fetal MR. Magn Reson Med. 2019;81(1):477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–28. [DOI] [PubMed] [Google Scholar]
- 24. Story L, Zhang T, Steinweg JK, Hutter J, Matthew J, Dassios T, et al. Foetal lung volumes in pregnant women who deliver very preterm: a pilot study. Pediatr Res. 2020;87(6):1066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uus A, Hall M, Grigorescu I, Zampieri CA, Collado AE, Payette K, et al. 3D T2w fetal body MRI: automated organ volumetry, growth charts and population-averaged atlas. medRxiv. 2023:2023.05. [Google Scholar]
- 26. Hutter J, Slator PJ, Jackson L, Gomes ADS, Ho A, Story L, et al. Multi-modal functional MRI to explore placental function over gestation. Magn Reson Med. 02 2019;81(2):1191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shah PS, Lui K, Sjörs G, Mirea L, Reichman B, Adams M, et al. Neonatal outcomes of very low birth weight and very preterm neonates: an international comparison. J Pediatr. 2016;177:144–52.e6. [DOI] [PubMed] [Google Scholar]
- 28. Khong TY, Mooney EE, Ariel I, Balmus NCM, Boyd TK, Brundler MA, et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med. 2016;140(7):698–713. [DOI] [PubMed] [Google Scholar]
- 29. Royston P, Wright EM. How to construct “normal ranges” for fetal variables. Ultrasound Obstet Gynecol. 1998;11(1):30–8. [DOI] [PubMed] [Google Scholar]
- 30. You JH, Lv GR, Liu XL, He SZ. Reference ranges of fetal spleen biometric parameters and volume assessed by three-dimensional ultrasound and their applicability in spleen malformations. Prenat Diagn. 2014;34(12):1189–97. [DOI] [PubMed] [Google Scholar]
- 31. Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics. 2012;130(3):e640–9. [DOI] [PubMed] [Google Scholar]
- 32. Villar J, Giuliani F, Fenton TR, Ohuma EO, Ismail LC, Kennedy SH, et al. INTERGROWTH-21st very preterm size at birth reference charts. Lancet. 2016;387(10021):844–5. [DOI] [PubMed] [Google Scholar]
- 33. Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–68. [DOI] [PubMed] [Google Scholar]
- 34. Torgersen C, Moser P, Luckner G, Mayr V, Jochberger S, Hasibeder WR, et al. Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesth Analg. 2009;108(6):1841–7. [DOI] [PubMed] [Google Scholar]
- 35. Payette K, Uus, A, Aviles Verdera, J, Avena Zampieri, C, Hall, M, Story, L, et al. An automated pipeline for quantitative T2* fetal body MRI and segmentation at low field. ArXiv. 2023:358–67. [Google Scholar]
- 36. Haugen G, Husby H, Helbig AE, Schmidt-Melbye AC. Ultrasonographic monitoring of pregnancies complicated by red blood cell alloimmunization in a cohort with mild to moderate risk according to previous obstetric outcome. Acta Obstet Gynecol Scand. 2002;81(3):227–33. [DOI] [PubMed] [Google Scholar]
- 37. Schoennagel BP, Remus CC, Wedegaertner U, Salzmann I, Grabhorn E, Adam G, et al. Quantification of prenatal liver and spleen iron in a sheep model and assessment of iron stores in a human neonate with neonatal hemochromatosis using R2* mapping. Magn Reson Med Sci. 2014;13(3):167–73. [DOI] [PubMed] [Google Scholar]
- 38. Musilova I, Kacerovsky M, Hornychova H, Kostal M, Jacobsson B. Pulsation of the fetal splenic vein: a potential ultrasound marker of histological chorioamnionitis and funisitis in women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2012;91(9):1119–23. [DOI] [PubMed] [Google Scholar]
- 39. Hutter J, Slator PJ, Avena Zampieri C, Hall M, Rutherford M, Story L. Multi-modal MRI reveals changes in placental function following preterm premature rupture of membranes. Magn Reson Med. 2023;89(3):1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toti P, De Felice C, Stumpo M, Schürfeld K, Di Leo L, Vatti R, et al. Acute thymic involution in fetuses and neonates with chorioamnionitis. Hum Pathol. 2000;31(9):1121–8. [DOI] [PubMed] [Google Scholar]
- 41. Kunzmann S, Glogger K, Been JV, Kallapur SG, Nitsos I, Moss TJ, et al. Thymic changes after chorioamnionitis induced by intraamniotic lipopolysaccharide in fetal sheep. Am J Obstet Gynecol. 2010;202(5):476.e1–476.e4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuypers E, Collins JJP, Jellema RK, Wolfs TGAM, Kemp MW, Nitsos I, et al. Ovine fetal thymus response to lipopolysaccharide-induced chorioamnionitis and antenatal corticosteroids. PLoS One. 2012;7(5):e38257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Story L, Zhang T, Uus A, Hutter J, Egloff A, Gibbons D, et al. Antenatal thymus volumes in fetuses that delivered <32 weeks’ gestation: an MRI pilot study. Acta Obstet Gynecol Scand. 2021;100(6):1040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuypers E, Willems MGM, Jellema RK, Kemp MW, Newnham JP, Delhaas T, et al. Responses of the spleen to intraamniotic lipopolysaccharide exposure in fetal sheep. Pediatr Res. 2015;77(1–1):29–35. [DOI] [PubMed] [Google Scholar]
- 45. Gomez-Lopez N, Galaz J, Miller D, Farias-Jofre M, Liu Z, Arenas-Hernandez M, et al. The immunobiology of preterm labor and birth: intra-amniotic inflammation or breakdown of maternal-fetal homeostasis. Reproduction. 2022;164(2):R11–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uus A. Available from: https://github.com/SVRTK/SVRTK (accessed October 17th, 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the finding of the study are not publicly available due to privacy reasons but are available from the corresponding author (megan.hall@kcl.ac.uk) upon reasonable request.