Abstract
Objectives
To evaluate asthma characteristics and treatment patterns, including short-acting β2-agonist (SABA) prescriptions, in primary and specialist care in the Singapore cohort of the SABA use IN Asthma (SABINA III) study.
Design
Cross-sectional, observational study.
Setting
Multicentre study conducted at five sites across Singapore.
Methods
In patients with asthma (aged ≥12 years), data on demographics, disease characteristics and asthma treatment prescriptions were collected using electronic case report forms. Patients were classified by investigator-defined asthma severity (guided by 2017 Global Initiative for Asthma recommendations) and practice type (primary/specialist care).
Results
Of the 205 patients analysed (mean (SD) age, 53.6 (16.8) years; female, 62%), 55.9% were enrolled by specialists and 44.1% by primary care physicians. Most study patients (80.5%) had moderate-to-severe asthma (86.0% in specialist care and 74.4% in primary care). In the 12 months before study enrolment, 18.0% of patients experienced ≥1 severe exacerbation. Asthma was well or partly controlled in 78.0% of patients. Overall, 17.1% of all patients were overprescribed SABA (≥3 SABA canisters/year) in the preceding 12 months, and overprescription was greater in specialist versus primary care (26.3% vs 5.6%). Only 2.9% of patients were prescribed SABA monotherapy, while 41.0% received SABA in addition to maintenance therapy. Among the latter, 40.5% were overprescribed SABA. Overall, a higher percentage of patients prescribed ≥3 SABA canisters (vs 0–2 SABA canisters) were assessed as having uncontrolled asthma during the study visit (42.9% vs 17.6%). Maintenance therapy in the form of inhaled corticosteroids (ICS) or ICS/long-acting β2 agonist fixed-dose combinations were prescribed to 14.1% and 84.9% of patients, respectively, in the 12 months before enrolment.
Conclusions
In this Singapore cohort, ~17% of all patients and more than 40% of patients prescribed SABA in addition to maintenance therapy were overprescribed SABA. These findings emphasise the need to align clinical practices with the latest evidence-based treatment recommendations.
Trial registration
Keywords: Asthma, Health policy, RESPIRATORY MEDICINE (see Thoracic Medicine)
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This observational study highlights real-world asthma-management practices and the prevalence of short-acting β2-agonist overprescription in both primary and specialist care settings in Singapore.
Study sites were selected using purposive sampling with the aim of obtaining a sample representative of asthma management within Singapore, leading to a relatively balanced representation of patients from primary and specialist care.
Prescription data were collected retrospectively and may not be reflective of actual medication use or treatment adherence.
Introduction
The prevalence of asthma in the Asia-Pacific region, although lower than that in Western countries and Australia, is rapidly increasing due to economic development and urbanisation.1 2 In 2010, the prevalence of asthma in Singapore was 10.5% among adults aged 18–69 years.3 Between 1990 and 2019, asthma mortality rates significantly decreased across Asia-Pacific countries, with the greatest reduction observed in Singapore (80.8%), followed by Malaysia (64.9%), Japan (64.3%) and Korea (62.6%) (source: own calculations based on Institute for Health Metrics and Evaluation data4). Since 2010, asthma mortality rates in Singapore have been approximately 0.8 per 100 000 total population,4 which is on par with, if not lower, than that observed in many other developed countries, including the USA, Australia and the UK (1.2%, 1.9% and 2.2%, respectively). Nonetheless, despite having an efficient and well-established healthcare system,5 the burden of asthma is high in Singapore, in part, due to recurrent exacerbations in some individuals, the allergen-sensitive environment, poor medication adherence and limited health literacy.6
Results from the Asthma Insight and Management (AIM) survey conducted in eight Asia-Pacific countries and Hong Kong indicated a discrepancy between self-reported and Global Initiative for Asthma (GINA)-assigned asthma control.7 In Singapore, 65% of patients self-reported having completely controlled/well-controlled asthma; however, when assessed objectively using the GINA assessment of asthma control, only 14% of patients had controlled/well-controlled asthma.7 This discordance between patients’ perceptions and guideline-defined levels of control affects how patients manage their asthma, including adherence to maintenance treatment.8 Uncontrolled asthma has also been associated with significantly high asthma costs and low quality of life.9 10
The key to improving clinical outcomes in patients with asthma is improving adherence to inhaled corticosteroids (ICS), which treat the underlying inflammation of asthma, and minimising reliance on short-acting β2-agonists (SABAs).11 Although SABAs are prescribed for immediate symptom relief,11 reliance on SABAs is associated with an increased risk of preventable asthma attacks in patients of all severities.12–15 Consequently, since 2019, GINA no longer recommends SABA monotherapy.16 Low-dose ICS-formoterol, a fast-acting reliever,17 18 is now recommended as the preferred reliever for adults and adolescents at GINA treatment steps 1 and 2 and for patients prescribed ICS-formoterol maintenance therapy at steps 3–5.11 This landmark change represents a fundamental shift in asthma management, ensuring that anti-inflammatory treatment is now at the forefront of asthma treatment for all patients.
An overview of prescription patterns for asthma medications in Singapore, particularly SABA, will help clinicians understand the potential burden of SABA overuse, potentially allowing for timely identification of at-risk patient groups across primary and specialist care settings. Therefore, as part of the SABA use IN Asthma (SABINA) programme,19 SABINA III, a real-world observational study, was conducted globally to describe SABA prescription patterns across 24 countries outside of Europe and North America.20 Here, we report the results from the Singapore cohort of SABINA III.
Methods
Study design
The methodology of SABINA III has been described previously.20 In brief, this was a cross-sectional, multicentre, observational study conducted at five sites across Singapore (two private general physician clinics, two polyclinics (ie, public primary healthcare centres) and one respiratory specialist clinic in an academic university hospital), with patients recruited from October 2019 to January 2020. A national coordinator selected potential sites using purposive sampling with the aim of obtaining a sample cohort representative of current treatment practices within each participating site. The national coordinator also provided advice on the different types of centres (primary care centres, different types of hospitals and geographical distribution), and facilitated the selection of the investigators. The study objectives were to describe the demographic and clinical features of the asthma population under primary and specialist care by asthma severity and to assess both SABA and ICS prescriptions in patients.
Retrospective data were obtained from existing medical records, and patient data, including an assessment of current asthma symptom control, were collected during a study visit and entered on an electronic case report form (eCRF). Physicians entered data on exacerbation history, comorbidities and information on medication prescriptions for asthma in the eCRF based on patient medical records. At the study visit, physicians also enquired as to whether patients had experienced exacerbations that were not captured in their medical records. Data for SABA over-the-counter (OTC) purchase was based on patient recall and obtained directly from patients at the study visit, which was subsequently entered in the eCRF by the investigator.
Study population
At each site, patients aged ≥12 years with a documented physician diagnosis of asthma in their medical records, ≥3 documented consultations for asthma with the same healthcare practitioner over their lifetime to exclude those patients seen by a number of different physicians, and medical records containing data for ≥12 months before the study visit were eligible for enrolment. To minimise selection bias, eligible patients were enrolled consecutively at each study site. Patients with a diagnosis of other chronic respiratory diseases, such as chronic obstructive pulmonary disease, were excluded.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Data collection
Patients were categorised by practice type (primary or specialist care) and investigator-classified asthma severity (guided by GINA 2017 recommendations).21 Data were collected on demographics; disease characteristics, including severe exacerbation history22; and both prescribed asthma treatments (including SABA, ICS, ICS/long-acting β2-agonist (LABA) fixed-dose combinations, oral corticosteroid (OCS) burst treatment (defined as a short course of intravenous corticosteroids or OCS administered for 3–10 days or a single dose of an intramuscular corticosteroid to treat an exacerbation) and antibiotics for asthma) and OTC SABA purchases without a prescription in the 12 months before study initiation. SABA prescriptions were categorised as 0, 1–2, 3–5, 6–9, 10–12 and ≥13 canisters per year, with prescription of ≥3 SABA canisters considered as overprescription.12 14 23 ICS-containing prescriptions were expressed according to the prescribed average daily dose—low, medium or high (online supplemental table 1).21 Asthma symptom control was assessed using the GINA 2017 assessment for asthma control and categorised as well controlled, partly controlled or uncontrolled.21 Severe exacerbations in the preceding 12 months were based on the American Thoracic Society/European Respiratory Society recommendations22 and defined as a deterioration in asthma resulting in hospitalisation or emergency room treatment or the need for intravenous corticosteroids or OCS for ≥3 days or a single dose of an intramuscular corticosteroid.
bmjopen-2022-064245supp001.pdf (81.7KB, pdf)
Statistical analysis
Continuous variables are summarised by the number of non-missing values, mean (SD) and median (range). Categorical variables are summarised by frequency counts and percentages. A post hoc analysis using the Chi-square test was conducted to compare sociodemographic and disease characteristics of patients prescribed 0–2 and ≥3 SABA canisters in the preceding 12 months.
Results
Study population
Of the 212 patients enrolled, 7 were excluded because their asthma duration was less than 12 months, and 205 were included in the final analysis (figure 1). Overall, 55.9% of patients were treated by specialists and 44.1% by primary care physicians. In primary care, 25.6% and 74.4% of patients had investigator-classified mild (GINA steps 1/2) and moderate-to-severe (GINA steps 3–5) asthma, respectively. In specialist care, 14.0% and 86.0% of patients had mild and moderate-to-severe asthma, respectively.
Figure 1.
Patient disposition and study population by practice type and investigator-classified asthma severity in the SABINA III Singapore cohort. *All patients under specialist care were treated by a pulmonologist. SABA, short-acting β2-agonist; SABINA, SABA use IN Asthma.
Demographics and disease characteristics
The mean (SD) age of patients was 53.6 (16.8) years. Patients under specialist care were, on average, older than those under primary care (mean (SD) age, 57.3 (15.7) vs 49.1 (17.2) years) (table 1). Overall, most patients were female (62.0%) and non-smokers (80.5%). The mean (SD) body mass index (BMI) was 26.8 (6.7) kg/m2, and more than half of all patients (55.1%) had a BMI ≥25 kg/m2, indicating obesity (Asia-Pacific classification).24 More patients under specialist care vs primary care had a BMI ≥25 kg/m2 (58.8% vs 50.0%). A total of 20.5% of patients had high school education, while 24.9% had university and/or post-university education. Only 5.9% of patients had fully reimbursed healthcare, with the majority (64.4%) receiving partial reimbursement. A greater proportion of patients treated in specialist care had partially reimbursed healthcare compared with those in primary care (81.6% vs 42.2%).
Table 1.
Demographics and disease characteristics according to asthma severity and practice type in the SABINA III Singapore cohort
| Demographics and disease characteristics | All (n=205) |
Primary care (n=90) |
Specialist care (n=114) |
||||
| Mild asthma (n=23) | Moderate-to-severe asthma (n=67) | All (n=90) | Mild asthma (n=16) | Moderate-to-severe asthma (n=98) | All (n=114) | ||
| Demographics | |||||||
| Age (years) | |||||||
| Mean±SD | 53.6±16.8 | 50.0±15.3 | 48.8±18.0 | 49.1±17.2 | 54.3±14.8 | 57.8±15.9 | 57.3±15.7 |
| Median (min, max) | 56.0 (15.0, 91.0) |
55.0 (20.0, 72.0) |
51.0 (15.0, 91.0) |
52.0 (15.0, 91.0) |
55.5 (34.0, 79.0) |
62.0 (21.0, 86.0) |
59.5 (21.0, 86.0) |
| Gender | |||||||
| Female | 127 (62.0) | 11 (47.8) | 45 (67.2) | 56 (62.2) | 9 (56.2) | 61 (62.2) | 70 (61.4) |
| BMI* (kg/m2) | |||||||
| Mean±SD | 26.8±6.7 | 28.0±7.3 | 25.1±5.0 | 25.8±5.7 | 26.6±5.8 | 27.6±7.5 | 27.4±7.3 |
| Median (min, max) | 25.8 (14.2, 53.8) |
26.5 (19.4, 49.7) |
24.7 (16.6, 40.5) |
24.9 (16.6, 49.7) |
27.0 (18.2, 37.1) |
26.4 (14.2, 53.8) |
26.4 (14.2, 53.8) |
| BMI group* (kg/m2) | |||||||
| ≥25 | 113 (55.1) | 13 (56.5) | 32 (47.8) | 45 (50.0) | 8 (50.0) | 59 (60.2) | 67 (58.8) |
| Smoking status history | |||||||
| Current smoker | 11 (5.4) | 2 (8.7) | 4 (6.0) | 6 (6.7) | 2 (12.5) | 3 (3.1) | 5 (4.4) |
| Ex-smoker | 29 (14.1) | 4 (17.4) | 4 (6.0) | 8 (8.9) | 2 (12.5) | 19 (19.4) | 21 (18.4) |
| Non-smoker | 165 (80.5) | 17 (73.9) | 59 (88.1) | 76 (84.4) | 12 (75.0) | 76 (77.6) | 88 (77.2) |
| Number of comorbidities | |||||||
| 0 | 89 (43.4) | 12 (52.2) | 38 (56.7) | 50 (55.6) | 7 (43.8) | 32 (32.7) | 39 (34.2) |
| 1–2 | 84 (41.0) | 9 (39.1) | 18 (26.9) | 27 (30.0) | 6 (37.5) | 50 (51.0) | 56 (49.1) |
| 3–4 | 26 (12.7) | 2 (8.7) | 9 (13.4) | 11 (12.2) | 3 (18.8) | 12 (12.2) | 15 (13.2) |
| ≥5 | 6 (2.9) | 0 (0.0) | 2 (3.0) | 2 (2.2) | 0 (0.0) | 4 (4.1) | 4 (3.5) |
| Education level | |||||||
| Primary and/or secondary school | 104 (50.7) | 11 (47.8) | 31 (46.3) | 42 (46.7) | 6 (37.5) | 55 (56.1) | 61 (53.5) |
| High school | 42 (20.5) | 2 (8.7) | 12 (17.9) | 14 (15.6) | 6 (37.5) | 22 (22.4) | 28 (24.6) |
| University and/or post-university education | 51 (24.9) | 8 (34.8) | 20 (29.9) | 28 (31.1) | 4 (25.0) | 19 (19.4) | 23 (20.2) |
| Healthcare insurance/medication funding | |||||||
| Not reimbursed | 43 (21.0) | 7 (30.4) | 17 (25.4) | 24 (26.7) | 2 (12.5) | 17 (17.3) | 19 (16.7) |
| Partially reimbursed | 132 (64.4) | 9 (39.1) | 29 (43.3) | 38 (42.2) | 13 (81.2) | 80 (81.6) | 93 (81.6) |
| Fully reimbursed | 12 (5.9) | 1 (4.3) | 9 (13.4) | 10 (11.1) | 1 (6.2) | 1 (1.0) | 2 (1.8) |
| Unknown | 18 (8.8) | 6 (26.1) | 12 (17.9) | 18 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Disease characteristics | |||||||
| Asthma duration (years) | |||||||
| Mean±SD | 18.1±16.5 | 18.8±14.8 | 13.3±12.5 | 14.7±13.3 | 18.9±17.1 | 20.9±18.4 | 20.6±18.2 |
| Median (min, max) | 10.0 (1.0, 67.0) |
17.0 (3.0, 58.0) |
9.0 (1.0, 56.0) |
9.5 (1.0, 58.0) |
13.5 (1.0, 50.0) |
10.5 (1.0, 67.0) |
10.5 (1.0, 67.0) |
| Number of severe asthma exacerbations in the 12 months before study initiation | |||||||
| Mean±SD | 0.4±1.2 | 0.2±0.5 | 0.4±0.9 | 0.3±0.8 | 0.1±0.5 | 0.4±1.4 | 0.4±1.4 |
| Median (min, max) | 0.0 (0.0, 11.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 5.0) |
0.0 (0.0, 5.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 11.0) | 0.0 (0.0, 11.0) |
| Number of severe asthma exacerbations in the 12 months before study initiation | |||||||
| 0 | 168 (82.0) | 20 (87.0) | 55 (82.1) | 75 (83.3) | 15 (93.8) | 78 (79.6) | 93 (81.6) |
| 1 | 20 (9.8) | 2 (8.7) | 4 (6.0) | 6 (6.7) | 0 (0.0) | 14 (14.3) | 14 (12.3) |
| 2 | 11 (5.4) | 1 (4.3) | 5 (7.5) | 6 (6.7) | 1 (6.2) | 3 (3.1) | 4 (3.5) |
| ≥3 | 6 (2.9) | 0 (0.0) | 3 (4.5) | 3 (3.3) | 0 (0.0) | 3 (3.1) | 3 (2.6) |
| Level of asthma symptom control | |||||||
| Well controlled | 100 (48.8) | 12 (52.2) | 29 (43.3) | 41 (45.6) | 12 (75.0) | 47 (48.0) | 59 (51.8) |
| Partly controlled | 60 (29.3) | 10 (43.5) | 28 (41.8) | 38 (42.2) | 2 (12.5) | 19 (19.4) | 21 (18.4) |
| Uncontrolled | 45 (22.0) | 1 (4.3) | 10 (14.9) | 11 (12.2) | 2 (12.5) | 32 (32.7) | 34 (29.8) |
All data are described as n (%) unless otherwise specified.
*Defined as per Asia-Pacific classification.24
BMI, body mass index; SABA, short-acting β2-agonist; SABINA, SABA use IN Asthma.
Patients had a mean (SD) asthma duration of 18.1 (16.5) years, higher in specialist care versus primary care (20.6 (18.2) vs 14.7 (13.3)) (table 1). Patients reported a mean (SD) of 0.4 (1.2) severe asthma exacerbations in the 12 months before the study visit, with 18% experiencing ≥1 severe exacerbation. The mean (SD) number of severe exacerbations among patients in primary and specialist care was comparable (0.3 (0.8) vs 0.4 (1.4), respectively). Most patients (84.4%) had ≤2 comorbidities; however, a larger proportion of patients in primary care had no comorbidities compared with those in specialist care (55.6% vs 34.2%). Asthma symptom control was assessed as well controlled in 48.8% of all patients, partly controlled in 29.3% and uncontrolled in 22.0%. The proportion of patients with well-controlled or partly controlled asthma was greater in primary care versus specialist care (87.8% vs 70.2%).
SABA treatment trends
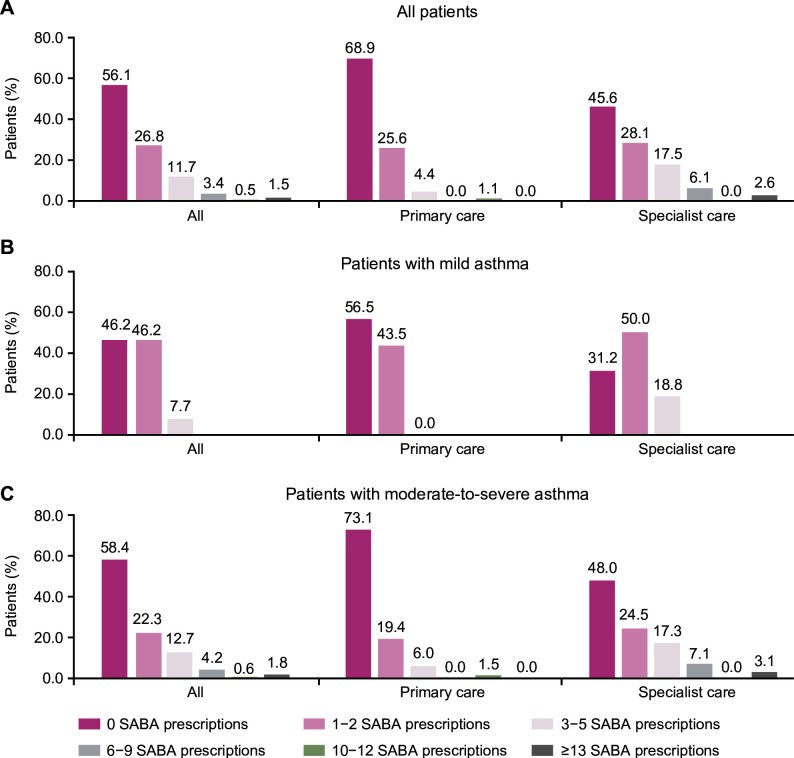
Overall, 17.1% of patients were prescribed ≥3 SABA canisters in the 12 months preceding the study visit (figure 2; table 2). Fewer patients with mild versus moderate-to-severe asthma were overprescribed SABA (7.7% vs 19.3%), while a higher proportion of patients in specialist care were prescribed ≥3 SABA canisters compared with those in primary care (26.3% vs 5.6%). Further, 2.0% of all patients, 1.1% of those treated in primary care, and 2.4% of those treated in specialist care were prescribed ≥10 SABA canisters.
Figure 2.
SABA prescriptions for (A) all patients, (B) patients with mild asthma and (C) patients with moderate-to-severe asthma, according to practice type in the SABINA III Singapore cohort. SABA, short-acting β2-agonist; SABINA, SABA use IN Asthma.
Table 2.
Asthma treatments in the preceding 12 months in the SABINA III Singapore cohort
| Asthma treatments in the 12 months before study initiation | All (n=205) | Primary care (n=90) | Specialist care (n=114) | ||||
| Mild asthma (n=23) | Moderate-to-severe asthma (n=67) | All (n=90) | Mild asthma (n=16) | Moderate-to-severe asthma (n=98) | All (n=114) | ||
| Type of SABA prescription | |||||||
| SABA as monotherapy | 6 (2.9) | 4 (17.4) | 0 (0.0) | 4 (4.4) | 2 (12.5) | 0 (0.0) | 2 (1.8) |
| SABA in addition to maintenance therapy | 84 (41.0) | 6 (26.1) | 18 (26.9) | 24 (26.7) | 9 (56.2) | 51 (52.0) | 60 (52.6) |
| SABA overprescription* | 35 (17.1) | 0 (0.0) | 5 (7.5) | 5 (5.6) | 3 (18.8) | 27 (27.6) | 30 (26.3) |
| Number of SABA canisters/inhalers prescribed per patient in the 12 months before study initiation | |||||||
| SABA as monotherapy | |||||||
| Mean±SD | 1.8±1.6 | 1.2±0.5 | NA | 1.2±0.5 | 3.0±2.8 | NA | 3.0±2.8 |
| Median (min, max) | 1.0 (1.0, 5.0) | 1.0 (1.0, 2.0) | NA | 1.0 (1.0, 2.0) | 3.0 (1.0, 5.0) | NA | 3.0 (1.0, 5.0) |
| SABA in addition to maintenance therapy | |||||||
| Mean±SD | 3.0±3.2 | 1.5±0.5 | 2.3±2.6 | 2.1±2.3 | 2.1±0.6 | 3.6±3.6 | 3.4±3.4 |
| Median (min, max) | 2.0 (1.0, 16.0) | 1.5 (1.0, 2.0) | 1.0 (1.0, 12.0) | 1.0 (1.0, 12.0) | 2.0 (1.0, 3.0) | 3.0 (1.0, 16.0) | 2.0 (1.0, 16.0) |
| Other prescriptions† | |||||||
| ICS | 29 (14.1) | 8 (34.8) | 1 (1.5) | 9 (10.0) | 14 (87.5) | 6 (6.1) | 20 (17.5) |
| ICS-LABA | 174 (84.9) | 8 (34.8) | 67 (100) | 75 (83.3) | 0 (0.0) | 98 (100) | 98 (86) |
| LABA-LAMA | 2 (1.0) | 0 (0) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 1 (1.0) | 2 (1.8) |
| LAMA | 16 (7.8) | 0 (0) | 0 (0.0) | 0 (0.0) | 2 (12.5) | 14 (14.3) | 16 (14.0) |
| LTRA | 24 (11.7) | 2 (8.7) | 21 (31.3) | 23 (25.6) | 0 (0) | 1 (1.0) | 1 (0.9) |
| Xanthines | 1 (0.5) | 0 (0.0) | 1 (1.5) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Monoclonal antibodies | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 1 (0.9) |
| OCS burst | 60 (29.3) | 6 (26.1) | 23 (34.3) | 29 (32.2) | 2 (12.5) | 29 (29.6) | 31 (27.2) |
| Antibiotic | 4 (2) | 3 (13) | 1 (1.5) | 4 (4.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
All data are described as n (%), unless otherwise specified.
*Defined as ≥3 canisters or inhalers prescribed per patient in the preceding 12 months.
†Percentage values do not add up to 100% since patients may have been prescribed more than one treatment.
ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; NA, not applicable; OCS, oral corticosteroid; SABA, short-acting β2-agonist; SABINA, SABA use IN Asthma.
Only 2.9% of patients, all of whom had mild asthma, were prescribed SABA monotherapy (table 2). Among patients who were prescribed SABA in addition to maintenance therapy (41.0%), SABA overprescription was common in 40.5%. Of these patients, a higher proportion in specialist care were prescribed ≥3 SABA canisters compared with those in primary care (48.3% vs 27.8%; online supplemental table 2).
SABA overprescription: patient profile
Sociodemographic characteristics, except for BMI, were not significantly different between patients prescribed 0–2 and ≥3 SABA canisters (table 3). However, significant between-group differences were observed in disease characteristics and outcomes. Compared with those prescribed 0–2 SABA canisters, a higher percentage of patients prescribed ≥3 SABA canisters had uncontrolled asthma (17.6% vs 42.9%) and were at GINA step 4/5 (50.0% vs 80.0%).
Table 3.
Sociodemographic and asthma characteristics of patients prescribed 0–2 and ≥3 SABA canisters in the preceding 12 months in the SABINA III Singapore cohort
| 0–2 SABA canisters (n=170) |
≥3 SABA canisters (n=35) |
|
| Sociodemographic characteristics | ||
| Age groups | ||
| 12–17 years | 3 (1.8) | 0 (0) |
| ≥18–54 years | 78 (45.9) | 17 (48.6) |
| ≥55 years | 89 (52.4) | 18 (51.4) |
| Missing values | 0 | 0 |
| Total | 170 | 35 |
| Gender | ||
| Female | 103 (60.6) | 24 (68.6) |
| Male | 67 (39.4) | 11 (31.4) |
| Missing values | 0 | 0 |
| Total | 170 | 35 |
| BMI groups*,†(kg/m2 ) | ||
| <18.5 | 7 (4.2) | 6 (17.1) |
| ≥18.5–22.9 | 43 (25.6) | 5 (14.3) |
| ≥23–24.9 | 24 (14.3) | 5 (14.3) |
| ≥25 | 94 (56) | 19 (54.3) |
| Missing values | 2 | 0 |
| Total | 168 | 35 |
| Education level | ||
| Unknown | 6 (3.5) | 2 (5.7) |
| Primary and/or secondary school | 86 (50.6) | 18 (51.4) |
| High school | 31 (18.2) | 11 (31.4) |
| University and/or post-university education | 47 (27.6) | 4 (11.4) |
| Missing values | 0 | 0 |
| Total | 170 | 35 |
| Healthcare insurance/medication funding | ||
| Unknown | 17 (10) | 1 (2.9) |
| Not reimbursed | 36 (21.2) | 7 (20) |
| Partially reimbursed | 105 (61.8) | 27 (77.1) |
| Fully reimbursed | 12 (7.1) | 0 (0) |
| Missing values | 0 | 0 |
| Total | 170 | 35 |
| Smoking status history | ||
| Active smoker | 9 (5.3) | 2 (5.7) |
| Former smoker | 23 (13.5) | 6 (17.1) |
| Nonsmoker | 138 (81.2) | 27 (77.1) |
| Missing values | 0 | 0 |
| Total | 170 | 35 |
| Number of comorbidities | ||
| 0 | 78 (45.9) | 11 (31.4) |
| 1–2 | 66 (38.8) | 18 (51.4) |
| 3–4 | 22 (12.9) | 4 (11.4) |
| ≥5 | 4 (2.4) | 2 (5.7) |
| Missing values | 0 | 0 |
| Total | 170 | 35 |
| Asthma characteristics | ||
| Number of severe asthma exacerbations in the 12 months prior to study initiation | ||
| 0 | 142 (83.5) | 26 (74.3) |
| 1 | 16 (9.4) | 4 (11.4) |
| 2 | 9 (5.3) | 2 (5.7) |
| ≥3 | 2 (1.2) | 1 (2.9) |
| Missing values | 1 | 2 |
| Total | 169 | 33 |
| Level of asthma symptom control† | ||
| Well controlled | 88 (51.8) | 12 (34.3) |
| Partly controlled | 52 (30.6) | 8 (22.9) |
| Uncontrolled | 30 (17.6) | 15 (42.9) |
| Missing values | 0 | 0 |
| Total | 170 | 35 |
| GINA 2017 classification† ‡ | ||
| Step 1 | 8 (4.7) | 1 (2.9) |
| Step 2 | 28 (16.5) | 2 (5.7) |
| Step 3 | 49 (28.8) | 4 (11.4) |
| Step 4 | 66 (38.8) | 18 (51.4) |
| Step 5 | 19 (11.2) | 10 (28.6) |
| Missing values | 0 | 0 |
| Total | 170 | 35 |
All data are described as n (%) unless otherwise specified. Comparisons were non-significant unless otherwise specified.
*Defined as per Asia-Pacific classification.24
†P value <0.05 obtained using the χ2 test.
‡According to GINA 2017 classification, step 1=as needed SABA, step 2=regular low-dose ICS±SABA, step 3=low-dose ICS/LABA±SABA, step 4=medium dose ICS/LABA+add-on maintenance medication±SABA, step 5=high-dose ICS/LABA+add-on maintenance medication±SABA.
BMI, body mass index; GINA, Global Initiative for Asthma; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; SABA, short-acting β2-agonist; SABINA, SABA use IN Asthma.
SABA purchase OTC
Only 4.4% of patients, the majority of whom had moderate-to-severe asthma, purchased SABA OTC. Of these patients, 77.8% purchased 1–2 SABA canisters and 22.2% purchased ≥3 SABA canisters in the 12 months before study enrolment (online supplemental table 3).
Other prescribed asthma treatments in the 12 months prior to study enrolment
Maintenance medication prescriptions
Overall, 14.1% of patients were prescribed ICS, with a mean (SD) of 5.1 (3.8) ICS canisters in the preceding 12 months (table 2, online supplemental table 4). The majority of these patients were prescribed low-dose ICS (53.6%), while 32.1% and 14.3% were prescribed medium-dose and high-dose ICS, respectively (online supplemental table 4). In contrast, most patients (84.9%), the majority of whom had moderate-to-severe asthma, were prescribed ICS/LABA fixed-dose combinations (table 2); 35.1%, 47.7% and 17.2% received low-dose, medium-dose and high-dose ICS, respectively. A comparable proportion of patients treated in primary and specialist care were prescribed ICS/LABA fixed-dose combinations (83.3% vs 86%, respectively, online supplemental table 4).
In the preceding 12 months, 11.7% and 7.8% of patients were prescribed leukotriene receptor antagonists and long-acting muscarinic antagonists (LAMAs), respectively (table 2, online supplemental table 4). Additionally, LABA/LAMA fixed-dose combinations were prescribed to 1.0% of patients, while a comparable proportion of patients were prescribed monoclonal antibodies (0.5%) or xanthines (0.5%).
Other medications
Overall, 29.3% of patients (32.2% in primary care and 27.2% in specialist care) were prescribed short-course OCSs (table 2, online supplemental table 4). Only four (2%) patients, all of whom were treated in primary care, were prescribed antibiotics for asthma.
Discussion
This cross-sectional study is the first to compare the asthma medication prescription patterns under both primary (private and public) and specialist care settings in Singapore. Our findings showed that SABA overprescription was observed in 17% of all study patients and was lower in primary care (6%) compared with specialist care (26%). While almost all patients were prescribed ICS or ICS/LABA, among those prescribed SABA relievers on a background of maintenance therapy, SABA overprescription was widespread, occurring in over 40% of patients, with a similar pattern of greater overprescription in specialist versus primary care.
The Pan International SABINA III study showed that SABA overprescription was associated with an increased risk of severe exacerbations and increased odds of poorly controlled asthma.20 Several other studies have demonstrated that overreliance on SABA is associated with an increased risk of preventable exacerbations even in mild asthma.12–15 An investigation led by the National Review of Asthma Deaths found that among patients who died due to asthma, 56% and 39% were prescribed 6 or more and 12 or more SABA canisters, respectively, in the year before their death.25 Thus, patients being overprescribed SABA in Singapore may be at risk of poor clinical outcomes. Taken together, these data reveal that while the primary healthcare system fares better than specialist care in SABA prescription practices, there is a need for improvement across prescriber types in the country.
Compared with the pooled data from all SABINA III countries,20 Singapore fared relatively better in some respects. For instance, SABA overprescription and the percentage of patients with ≥1 severe exacerbation in the 12 months before the study initiation were lower in Singapore versus the country-aggregated SABINA III cohort (17% vs 38% and 18% vs 45%, respectively).20 These findings may be attributed to the large proportion of patients in Singapore receiving anti-inflammatory treatment in the form of ICS-LABA (85%), including the relatively high proportion of patients receiving low-dose ICS-LABA (35%), since the addition of LABA to ICS has been shown to improve asthma control faster and at a lower ICS dose compared with ICS alone.26 This shift toward increased ICS/LABA prescription in primary care has been previously reported by a 10-year longitudinal cost-analysis study by Tan et al.27 In Singapore, efforts to improve asthma-related health outcomes were facilitated by the Singapore National Asthma Programme (SNAP), launched in 2001 and funded by the Ministry of Health (MoH), through sustainable integrated asthma programmes, effective use of written asthma action plans28 and the use of objective process indicators (eg, preventer–reliever ratio to increase ICS adherence).29 Additionally, relatively low healthcare costs due to a copayment structure involving payment by both the patient and government as well as national savings and healthcare insurance schemes reduce out-of-pocket costs and increase ownership.30 However, despite the availability of subsidised healthcare in Singapore,30 only 5.9% of patients reported full healthcare reimbursement in this study. This is of particular importance since insurance status may impact the quality of care received31 32 and the subsequent need for urgent/emergency care.33 Factors such as healthcare coverage gaps, lack of insurance, or having partial or public insurance are associated with cost barriers to asthma care,34 unmet healthcare needs35 and poorer asthma control.36 Indeed, a comparable proportion of patients in our study had no healthcare reimbursement (21%) and uncontrolled asthma (22%). Although our results are consistent with those from a 2021 cross-sectional online survey involving Singapore residents, which reported uncontrolled asthma in a comparable proportion of patients (29%), this survey did not assess healthcare insurance coverage of the participants.37 Of note, despite representing less than one-third of survey participants, patients with uncontrolled disease accounted for 46% of the total costs associated with asthma.37 Thus, our findings, combined with this previous report from Singapore,37 underscore the need for ensuring continuity of healthcare coverage to improve asthma control and reduce avoidable healthcare expenditure.
In many Asia-Pacific countries, including India,38 Malaysia39 and Australia,40 SABAs may be obtained without a prescription, thereby increasing the possibility of reliance on SABAs without physician oversight. Compared with 18% (n=1503) of patients in the overall SABINA III population,20 only 4% (n=9) of patients in Singapore purchased SABA OTC. It is unclear how these nine patients may have bought SABA OTC as SABA purchase is strictly regulated in Singapore and SABAs usually cannot be bought without a prescription.41 However, some clinics may provide SABA to their long-term associated patients without a prescription,41 and patients may have also purchased SABA overseas or erroneously answered the question on the eCRF. Of note, following the GINA update in 2019,16 pharmacy associations in Singapore were informed of these updated recommendations and are aware that SABA monotherapy is no longer encouraged.
Overall, no significant differences in sociodemographic characteristics were observed between patients prescribed 0–2 and ≥3 SABA canisters. This could be attributed to the small sample size in Singapore, as other studies have reported that certain patient populations defined by age, gender and smoking history are more susceptible to SABA over-reliance.42–44 However, consistent with previous research,43 patients prescribed ≥3 SABA canisters (vs 0–2 SABA canisters) in the prior year had poorer asthma control, with 43% and 18% of patients, respectively, having uncontrolled asthma.
Altogether, 29.3% of patients were prescribed an OCS burst. This finding was comparable to that reported in the AIM survey, where 26% of patients from Singapore reported OCS use, which was the lowest rate observed in all countries in the Asia-Pacific region.7 Nevertheless, our findings show that a substantial proportion of patients from this Singapore cohort were prescribed an OCS burst; while typically this treatment is reserved for the management of acute asthma exacerbations, only 18% of patients in this study experienced ≥1 severe exacerbation in the 12 months before the study visit. This discordance may be attributed to the prescription of OCS burst treatment for the management of other comorbid conditions requiring anti-inflammatory treatment, such as eczema or arthritis. Reassuringly, only 2% of patients in this Singapore cohort were prescribed antibiotics for asthma; this indicates familiarity with GINA recommendations, which do not support the routine use of antibiotics without strong evidence of lung infection.11 This may also be explained by the work undertaken by the MoH that appointed the National Antimicrobial Taskforce in 2009 to tackle the problem of antimicrobial resistance, and recommended mandatory surveillance of drug-resistant bacteria and antibiotic prescriptions.45 Moreover, antimicrobial stewardship programmes were implemented in all public acute hospitals, leading to greater engagement with private hospitals and physicians.45
Further improvements in asthma outcomes in the country will require sustained attempts at reducing SABA prescriptions and, in parallel, increasing maintenance medication prescriptions and ensuring adherence. Our findings showed that more specialists overprescribed SABA compared with primary care physicians. Compared with primary care physicians, specialists treated more patients with moderate-to-severe asthma in our study. The greater overprescription observed might be related to difficult-to-treat asthma traits in cases referred to specialists, which may make patients more susceptible to SABA over-reliant behaviour. Alternatively, this may, in part, be the result of better management of asthma at the primary care level, spearheaded by SNAP,28 emphasising the important role that primary care providers play in asthma care.46 We also observed that 80% of patients prescribed ≥3 SABA canisters were at GINA step 4/5 (moderate-to-severe asthma), suggesting the need for careful physician review of SABA prescriptions in such patients. Interestingly, a recent systematic literature review (SLR) and meta-analysis of 24 randomised controlled trials documented that treatment with SABA monotherapy, if used within prescribed limits for symptom relief, did not result in increased mortality or excess serious adverse events in adult and adolescent patients with asthma.47 However, this SLR and meta-analysis did not include data from observational studies,47 thereby limiting the real-world clinical relevance of findings. In contrast, results from the SABINA studies,12 14 23 including the SABINA III Singapore cohort, are representative of real-world asthma management practices and highlight the need for close monitoring of reliever use and patients at risk of poor outcomes.
Although the sample size of this study was small, a relatively similar representation from primary and specialist care (44% and 56%, respectively) allowed for a reasonable comparison between the two practice types. In this study, patients treated in specialist care were older and more likely to be obese than those treated in primary care. Furthermore, the proportion of patients with uncontrolled asthma was higher in specialist care compared with primary care. For this difficult-to-treat group, integrated asthma care processes between the primary and specialist care settings need to be coordinated to minimise cost and care fragmentation.
This study has some limitations. First, the results could have been confounded by responder and selection bias, that is, patients with milder asthma or non-adherent high-risk patients might have been excluded. Thus, firm epidemiological conclusions related to prescriber type and asthma severity are best answered by a large database study. Nonetheless, patient inclusion aimed to be representative of patient and physician practices in both primary and specialist care. Second, owing to its observational nature, therapies may be differently prescribed depending on disease severity.48 Indeed, 1.5% and 6.1% of patients classified with moderate-to-severe asthma in primary and specialist care, respectively, were prescribed ICS, suggesting differences in local treatment practices compared with GINA recommendations. Third, the ethnicity of patients, which has been shown to impact asthma severity and treatment response,49 was unknown. Fourth, prescription data were used to estimate inhaler use; however, information on dispensed inhalers, prescription adherence, correct inhaler use or stockpiling was not available. Fifth, due to the low patient numbers, it was not feasible to examine the association between SABA prescriptions and asthma-related clinical outcomes. Sixth, our study was not designed to examine the association between healthcare insurance coverage and level of asthma control, or SABA overprescription and reimbursement; however, a previous analysis of the SABINA III study in 8351 patients with asthma revealed that a lack of reimbursed healthcare lowered the odds of symptom control, even in patients with mild asthma.50 Finally, this study only recorded the number of comorbidities (categorised as 0, 1–2, 3–4 and ≥5) in the eCRF, while data on the type and rate of comorbidities were not captured. Despite these limitations, this is the first such analysis in Singapore to provide an insight into asthma severity, maintenance treatment and SABA prescriptions in a multiethnic Asian population representative of both primary and specialist care-treated patients. Moreover, these findings provide an overview of real-world asthma care and may aid policymakers and clinicians in initiating targeted changes in clinical practices to further improve outcomes in the country.
Conclusions
Results from the Singapore cohort of SABINA III demonstrated that nearly one-fifth of all patients were overprescribed SABA (≥3 canisters in the preceding 12 months), with greater overprescription in specialist care versus primary care. While nearly all patients were prescribed maintenance medication in the form of either ICS or ICS/LABA fixed-dose combinations, more than 40% of these patients receiving SABA relievers were overprescribed SABA. Continued efforts are thus required to ensure that clinical practices in the country are aligned with the latest evidence-based treatment recommendations.
Supplementary Material
Acknowledgments
Writing and editorial support was provided by Riva Verma of Cactus Life Sciences (part of Cactus Communications), Mumbai, India in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3) and fully funded by AstraZeneca.
Footnotes
Contributors: MJHIB designed the study. DHYT, TLT, WHT, MJHIB, JHK, SSK, GRW and HFL contributed to data collection. DHYT, TLT, WHT, MJHIB, JHK, SSK, GRW and HFL contributed to data analysis, data interpretation, and drafting and reviewing the manuscript. All authors take full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: AstraZeneca funded (grant number: N/A) all the SABINA studies, was involved in the study design, developed the study protocol, conducted the studies and performed the analyses. AstraZeneca reviewed the publication, without influencing the opinions of the authors, to ensure medical and scientific accuracy, and the protection of intellectual property. AstraZeneca also funded medical writing support.
Competing interests: DHYT has received honorarium from AstraZeneca as well as consultation fees from GSK in the past. TLT has received honorarium from AstraZeneca in the past. CC was an employee of AstraZeneca at the time of manuscript development. MJHIB was an employee of AstraZeneca at the time of the study conduct. WHT, JHK, SSK, GRW and HFL have no conflicts of interest to declare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request. Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available, outlining further details: https://vivli.org/ourmember/astrazeneca/.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and the study was conducted in compliance with the study protocol, the Declaration of Helsinki, and local ethics approval from National Healthcare Group Domain Specific Review Board (NHG DSRB; reference number 2019/00876) and Parkway Independent Ethics Committee (reference number PIEC/2019/029). Signed informed consent was collected from patients or their legal guardians. Participants gave informed consent to participate in the study before taking part.
References
- 1. Song W-J, Kang M-G, Chang Y-S, et al. Epidemiology of adult asthma in Asia: toward a better understanding. Asia Pac Allergy 2014;4:75–85. 10.5415/apallergy.2014.4.2.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong GWK, Leung TF, Ko FWS. Changing prevalence of allergic diseases in the Asia-Pacific region. Allergy Asthma Immunol Res 2013;5:251–7. 10.4168/aair.2013.5.5.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Health Survey . Ministry of Health. 2010. Available: https://www.moh.gov.sg/docs/librariesprovider5/resources-statistics/reports/nhs2010---low-res.pdf [Accessed 10 Dec 2022].
- 4. Global Burden of Disease . Institute for health Metrics and evaluation. 2019. Available: https://vizhub.healthdata.org/gbd-compare/ [Accessed 10 Dec 2022].
- 5. The Economist Intelligence Unit Healthcare . Health outcomes and cost: a 166-country comparison. 2014. Available: www.eiu.com/public/topical_report.aspx?campaignid=Healthoutcome2014 [Accessed 10 Dec 2022].
- 6. Koh MS, Yii AC, Ong YY. Asthma in Singapore: past, present and future. Ann Acad Med Singap 2017;46:81–3. [PubMed] [Google Scholar]
- 7. Thompson PJ, Salvi S, Lin J, et al. Insights, attitudes and perceptions about asthma and its treatment: findings from a multinational survey of patients from 8 Asia-Pacific countries and Hong Kong. Respirology 2013;18:957–67. 10.1111/resp.12137 [DOI] [PubMed] [Google Scholar]
- 8. Price D, David-Wang A, Cho S-H, et al. Time for a new language for asthma control: results from REALISE Asia. J Asthma Allergy 2015;8:93–103. 10.2147/JAA.S82633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Accordini S, Corsico AG, Braggion M, et al. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol 2013;160:93–101. 10.1159/000338998 [DOI] [PubMed] [Google Scholar]
- 10. Doz M, Chouaid C, Com-Ruelle L, et al. The association between asthma control, health care costs, and quality of life in France and Spain. BMC Pulm Med 2013;13:15. 10.1186/1471-2466-13-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Global Initiative for Asthma (GINA) . Global strategy for asthma management and prevention. 2022, Available: https://ginasthma.org/ [Accessed 10 Dec 2022].
- 12. Bloom CI, Cabrera C, Arnetorp S, et al. Asthma-related health outcomes associated with short-acting β2-agonist inhaler use: an observational UK study as part of the SABINA global program. Adv Ther 2020;37:4190–208. 10.1007/s12325-020-01444-5 [DOI] [PubMed] [Google Scholar]
- 13. FitzGerald JM, Tavakoli H, Lynd LD, et al. The impact of inappropriate use of short acting beta agonists in asthma. Respir Med 2017;131:135–40. 10.1016/j.rmed.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 14. Nwaru BI, Ekström M, Hasvold P, et al. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J 2020;55:1901872. 10.1183/13993003.01872-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stanford RH, Shah MB, D’Souza AO, et al. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol 2012;109:403–7. 10.1016/j.anai.2012.08.014 [DOI] [PubMed] [Google Scholar]
- 16. Global Initiative for Asthma (GINA) . Global strategy for asthma management and prevention. 2019. Available: https://ginasthma.org/ [Accessed 10 Dec 2022].
- 17. Beasley R, Holliday M, Reddel HK, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med 2019;380:2020–30. 10.1056/NEJMoa1901963 [DOI] [PubMed] [Google Scholar]
- 18. O’Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med 2018;378:1865–76. 10.1056/NEJMoa1715274 [DOI] [PubMed] [Google Scholar]
- 19. Cabrera CS, Nan C, Lindarck N, et al. SABINA: global programme to evaluate prescriptions and clinical outcomes related to short-acting β2-agonist use in asthma. Eur Respir J 2020;55:1901858. 10.1183/13993003.01858-2019 [DOI] [PubMed] [Google Scholar]
- 20. Bateman ED, Price DB, Wang H-C, et al. Short-acting β2-agonist prescriptions are associated with poor clinical outcomes of asthma: the multi-country, cross-sectional SABINA III study. Eur Respir J 2022;59:2101402. 10.1183/13993003.01402-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Global Initiative for Asthma (GINA) . Global strategy for asthma management and prevention. 2017. Available: https://ginasthma.org/ [Accessed 10 Dec 2022].
- 22. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009;180:59–99. 10.1164/rccm.200801-060ST [DOI] [PubMed] [Google Scholar]
- 23. Janson C, Menzies-Gow A, Nan C, et al. SABINA: an overview of short-acting Beta(2)-Agonist use in asthma in European countries. Adv Ther 2020;37:1124–35. 10.1007/s12325-020-01233-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization . The Asia-Pacific perspective: redefining obesity and its treatment. 2000. Available: https://apps.who.int/iris/handle/10665/206936 [Accessed 10 Dec 2022].
- 25. Royal College of Physicians . National review of asthma deaths (NRAD). Why asthma still kills. 2015. Available: https://www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills [Accessed 10 Dec 2022].
- 26. Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med 2004;170:836–44. 10.1164/rccm.200401-033OC [DOI] [PubMed] [Google Scholar]
- 27. Tan NC, Nguyen HV, Lye WK, et al. Trends and predictors of asthma costs: results from a 10-year longitudinal study. Eur Respir J 2016;47:801–9. 10.1183/13993003.00188-2015 [DOI] [PubMed] [Google Scholar]
- 28. Singapore National Asthma Program . Primary care pages. Available: https://www.primarycarepages.sg/patient-care/chronic-disease-management/singapore-national-asthma-programme-(snap) [Accessed 10 Dec 2022].
- 29. Chong PN, Tan NC, Lim TK. Impact of the Singapore National Ssthma Program (SNAP) on preventor-reliever prescription ratio in polyclinics. Ann Acad Med Singap 2008;37:114–7. [PubMed] [Google Scholar]
- 30. Healthcare schemes and subsidies. Ministry of health Singapore. Available: https://www.moh.gov.sg/cost-financing/healthcare-schemes-subsidies [Accessed 10 Dec 2022].
- 31. Apter AJ, Van Hoof TJ, Sherwin TE, et al. Assessing the quality of asthma care provided to Medicaid patients enrolled in managed care organizations in Connecticut. Ann Allergy Asthma Immunol 2001;86:211–8. 10.1016/S1081-1206(10)62693-2 [DOI] [PubMed] [Google Scholar]
- 32. Shireman TI, Heaton PC, Gay WE, et al. Relationship between asthma drug therapy patterns and Healthcare utilization. Ann Pharmacother 2002;36:557–64. 10.1345/aph.1A067 [DOI] [PubMed] [Google Scholar]
- 33. Markovitz BP, Andresen EM. Lack of insurance coverage and urgent care use for asthma: a retrospective cohort study. BMC Public Health 2006;6:14. 10.1186/1471-2458-6-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pate CA, Qin X, Bailey CM, et al. Cost barriers to asthma care by health insurance type among children with asthma. J Asthma 2020;57:1103–9. 10.1080/02770903.2019.1640730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gushue C, Miller R, Sheikh S, et al. Gaps in health insurance coverage and emergency department use among children with asthma. J Asthma 2019;56:1070–8. 10.1080/02770903.2018.1523929 [DOI] [PubMed] [Google Scholar]
- 36. Peters AT, Klemens JC, Haselkorn T, et al. Insurance status and asthma-related health care utilization in patients with severe asthma. Ann Allergy Asthma Immunol 2008;100:301–7. 10.1016/S1081-1206(10)60590-X [DOI] [PubMed] [Google Scholar]
- 37. Finkelstein EA, Lau E, Doble B, et al. Economic burden of asthma in Singapore. BMJ Open Respir Res 2021;8:e000654. 10.1136/bmjresp-2020-000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Sickle D. Management of asthma at private pharmacies in India. Int J Tuberc Lung Dis 2006;10:1386–92. [PubMed] [Google Scholar]
- 39. Raja Gopal V, Abdullah Thani NSI, Tan W, et al. Cost-effectiveness analysis of Budesonide/Formoterol (Symbicort®) as needed for mild asthma in Malaysia. Drugs Ther Perspect 2021;37:439–51. 10.1007/s40267-021-00855-w [DOI] [Google Scholar]
- 40. Azzi EA, Kritikos V, Peters MJ, et al. Understanding Reliever Overuse in patients purchasing over-the-counter short-acting Beta(2) agonists: an Australian community Pharmacy-based survey. BMJ Open 2019;9:e028995. 10.1136/bmjopen-2019-028995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tan NC, Tay IH, Ngoh A, et al. Factors influencing family physicians' drug prescribing behaviour in asthma management in primary care. Singapore Med J 2009;50:312–9. [PubMed] [Google Scholar]
- 42. Amin S, Soliman M, McIvor A, et al. Usage patterns of short-acting Β2-Agonists and inhaled corticosteroids in asthma: A targeted literature review. J Allergy Clin Immunol Pract 2020;8:2556–64. 10.1016/j.jaip.2020.03.013 [DOI] [PubMed] [Google Scholar]
- 43. Azzi E, Kritikos V, Price D, et al. Reliever overuse when treatable traits go untreated. ERS International Congress 2019 abstracts; September 28, 2019:suppl 10.1183/13993003.congress-2019.OA5151 [DOI] [Google Scholar]
- 44. Wang C-Y, Lai C-C, Wang Y-H, et al. The prevalence and outcome of short-acting β2-agonists overuse in asthma patients in Taiwan. NPJ Prim Care Respir Med 2021;31:19. 10.1038/s41533-021-00231-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. National strategic action plan on antimicrobial resistance. Singapore. 2017. Available: https://rr-asia.woah.org/wp-content/uploads/2020/03/singapore_singapore-national-strategic-action-plan-on-amr.pdf [Accessed 10 Dec 2022].
- 46. Koh GC, Lim JF. Bridging the gap between primary and specialist care: formidable challenges ahead. Ann Acad Med Singap 2008;37:89–2. [PubMed] [Google Scholar]
- 47. Sriprasart T, Waterer G, Garcia G, et al. Safety of SABA monotherapy in asthma management: a systematic review and meta-analysis. Adv Ther 2023;40:133–58. 10.1007/s12325-022-02356-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roche N, Reddel H, Martin R, et al. Quality standards for real-world research. focus on observational database studies of comparative effectiveness. Ann Am Thorac Soc 2014;11 Suppl 2:S99–104. 10.1513/AnnalsATS.201309-300RM [DOI] [PubMed] [Google Scholar]
- 49. Forno E, Celedon JC. Asthma and ethnic minorities: socioeconomic status and beyond. Curr Opin Allergy Clin Immunol 2009;9:154–60. 10.1097/aci.0b013e3283292207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bateman ED, Price DB, Wang H-C, et al. Late breaking abstract - reimbursement for asthma care is a universal barrier to achieving asthma symptom control: the SABINA III study. ERS International Congress 2020 abstracts; September 7, 2020:suppl 10.1183/13993003.congress-2020.2665 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-064245supp001.pdf (81.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are available upon reasonable request. Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available, outlining further details: https://vivli.org/ourmember/astrazeneca/.