Abstract
While mortality caused by sepsis remains an unsolved problem, studies showed conflicting results about effectiveness of monoclonal and polyclonal antibodies in patients suffering sepsis. For this reason, this current study provides an update of review clinical randomized trial studies until March 2024. The main object of this study is to determine effects of monoclonal and polyclonal antibodies on mortality rate and hospitalization of patients suffering sepsis. Search of Scopus, Web of science, EMBASE, PubMed and Cochrane were performed and randomized controlled trials which conducted in patients with septic shock or bacterial sepsis were included. Two reviewers assessed all searched trials for eligibility according to already defined criteria and did data collection and analyses afterwards. Present study showed monoclonal and polyclonal antibodies are a safe strategy with mild-to-moderate adverse effects. However, most studies indicate no significant change among inter-and intra-group comparison (p > 0.05) and further studies are needed, results showed an increase in survival rate, ventilator-and ICU-free days, resolve organ dysfunction, mediating inflammation related cytokines.
Keywords: Monoclonal antibody, Sepsis, Septic shock, Septicemia, Randomized clinical trial
1. Introduction
Sepsis is a serious condition in which patients may experience multiple organ failure. Every year in the world, about 50 million people get sepsis which about 11 million patients die [1]. This disorder is due to the excessive reaction of the immune system to infectious (and non-infectious) agents [2]. Based on pathophysiology, sepsis begins following the identification of Pathogen-associated molecular patterns (PAMPs) and Damage-associated molecular patterns (DAMPs) on the surface of the host cells by pattern-recognition receptors (PRR) such as toll-like receptors (TLR), nucleotide-binding oligomerization domain (Nod), retinoic-inducible gene I (RIG-I) and C-type lectin receptors (CLR's), on antigen-presenting cells (APCs) [3,4]. This reaction leads to the activation of TLR types 2, 3, 4, 7 and 9, increased transcription of genes related to inflammation, activation of transcription factors mitogen-activated protein kinase (MAPK) and nuclear factor- κB (NF-κB) and then the production of pro-inflammatory cytokines such as IL-8, 12, 18, tumor necrosis factor alpha (TNF-α) and interferons (INFs) [5,6]. The imbalance between inflammatory and anti-inflammatory responses and the unregulated release of them are described as the main cause of organ failure [2].
According to the CDC, a person with one or more symptoms including high heart rate or low blood pressure, fever, shivering, or feeling very cold, confusion or disorientation, shortness of breath, extreme pain or discomfort, and clammy or sweaty skin is said to have sepsis [7]. The early diagnosis of sepsis and the beginning of treatment will play an important role in saving the lives of patients. Although supportive treatments such as fluid replacement along with antibiotic therapy are the only methods that are commonly prescribed for sepsis [8], immunotherapy have also been introduced [6]. Improper administration of broad-spectrums antibiotics and antibiotic resistance increased the need for effective antibiotic [9]. The function of intravenous immunoglobulins (IVIg) were described for their anti-inflammatory properties and neutralization of invading agents and their toxins [10]. Although immunoglobulins were effective in reducing symptoms and organ failure, they had no effect on the mortality rate of sepsis in animal model study [11]. Therefore, other agents including monoclonal antibodies (m-Ab) was designed to selectively prevent the disease by targeting inflammatory factors in inflammatory signaling pathways and pathogens [6]. Studies investigated the effect of monoclonal antibodies on diseases including viral infections [12] and cancer [13], neuromyelitis optica spectrum disorder [14], rheumatoid arthritis [15], psoriasis [16], and even on the recent COVID-19 pandemic [17]. m-Abs have been less studied in the bacteriology and until today only three FDA-approved m-Abs have been described for bacterial infections [18]. The present study reviewed the effects of monoclonal and polyclonal (m-Ab and p-Ab) antibodies on sepsis, their advantages and disadvantages, and effectiveness until March 2024.
2. Method
2.1. Search strategy
A comprehensive search was conducted in the following electronic databases: Web of science, Scopus, EMBASE, PubMed and Cochrane. The search will cover articles published up to the date of March 2024. The protocol follows the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines [19]. A combination of Medical Subject Headings (MeSH) terms and keywords were employed in the search strategy. The syntax will encompass the following terms: ("Antibody" OR "Antibody*" OR "Ab" OR "Immunoglobulin*" OR "Immune Globulins*") AND ("Sepsis" OR "Septic shock" OR "Septicemia") AND ("Randomized Controlled Trial*" OR "Randomized Clinical Trial" OR "RCT").
2.2. Inclusion and exclusion criteria
Studies eligible for inclusion is full-text articles published in English, randomized controlled trials (RCTs) involving human subjects diagnosed with sepsis, and reporting the use of monoclonal and polyclonal antibodies as an intervention will considered. Studies that do not meet the inclusion criteria, involve animals, observational studies, and systematic reviews/meta-analyses or unpublished were excluded. The exclusion of non-RCT studies is consistent with the study’s emphasis on the highest quality of clinical evidence.
2.3. Data management
For efficient organization and retrieval, data and records extracted from studies will be managed using both EndNote and Rayyan [20] software. This dual approach ensures comprehensive data management and facilitates systematic review processes.
2.4. Study selection
Two independent reviewers will screen studies based on these criteria. Discrepancies will be resolved through discussion or a third-party consultation, reflecting a rigorous and unbiased selection process.
2.5. Data extraction
Two independent reviewers will systematically extract data from selected studies, ensuring comprehensive and accurate data collection crucial for the integrity of the systematic review. In instances of disagreement or discrepancy, a structured discussion will be initiated to reach a consensus, and if necessary, a third-party expert will be consulted to provide an unbiased resolution. This process is designed to maintain the rigor and objectivity of the extraction process. Detailed data will be extracted on several key aspects, including study characteristics (author, publication year, study design), participant characteristics (sample size, age, sex, comorbidities), specifics of the antibodies used (type, dosage, administration routes, and duration of treatment), and study outcomes (mortality rates, adverse events). Additionally, the methodological quality of each study will be assessed, examining aspects like blinding, allocation concealment, and handling of dropouts, to evaluate the robustness of the findings and ensure a thorough understanding of each study's reliability and validity.
2.6. Quality assessment
The Joanna Briggs Institute (JBI) criteria will be used for assessing the methodological quality of RCTs, reflecting a focused and standardized approach to quality evaluation.
2.7. Consideration of study findings
The systematic review will reflect on the varied efficacy of antibodies in different patient populations and bacterial infections, as observed in the results. This includes the consideration of factors such as antibody type (monoclonal vs. polyclonal), target (e.g., TNF-α, endotoxin), administration route, and dosage, as these have shown to influence treatment outcomes significantly.
3. Results
3.1. Results of the search and characteristics of the included RCT studies
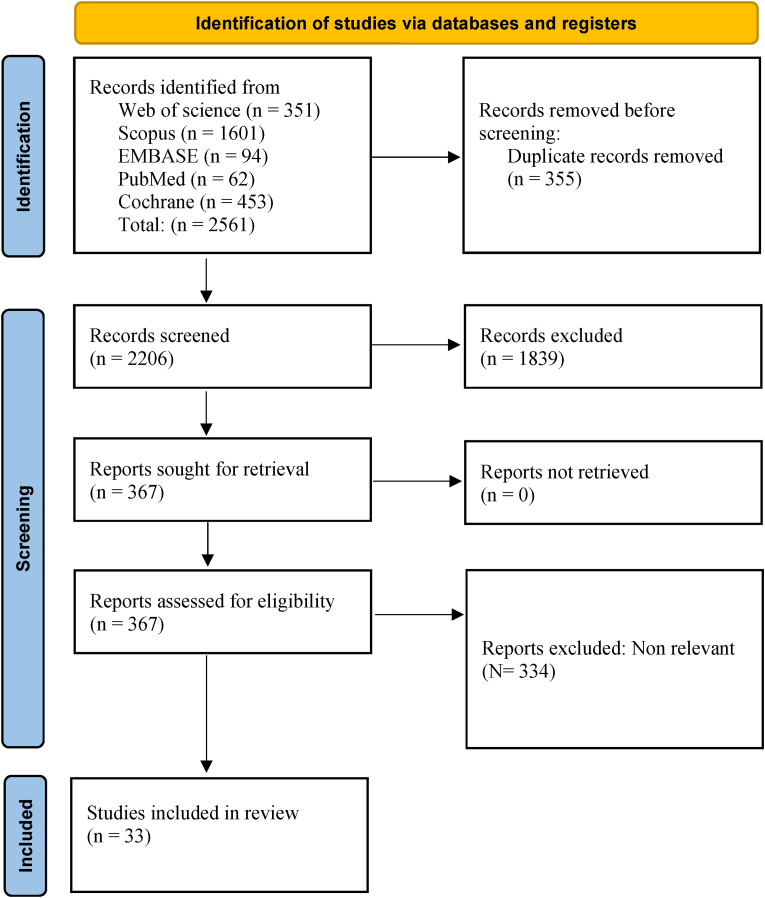
Thirty-three eligible trials were retrieved based on inclusion criteria (Fig. 1). Among thirty-three trials, thirteen (39.9 %) and twenty (60 %) studies used m-Ab and p-Ab respectively. Currently there are eight candidates under investigation in phase Ⅱ which belongs to m-Abs. By reviewing RCTs, different m-Ab and p-Ab were used including as HA-1A (anti-endotoxin), AZD9773 (anti-TNF-α), Afelimomab (anti-TNF-α), Adrecizumab (or HAM8101; anti-Adrenomedullin), Pentaglobin (polyvalent immunoglobulin M (IgM) against LPS and outer membrane proteins), pagibaximab (anti-lipoteichoic acid), Pentoxifylline (anti-TNF-α), CytoFab (anti-TNF-α), LG-1, MAB-T88 (anti-lipopolysaccharide), E5 (anti-endotoxin) and IG preparation containing IgG, IgM and IgA. Among RCTs, Pentaglobin, HA-1A, and AZD9773 were the most frequently used hybridoma antibodies. Also, endotoxin and TNF-α are the main selected targets for reducing symptoms and complications in patients with sepsis. Almost all studies administration route was performed intravenously (IV). Moreover, duration of receiving antibodies varied from 1 week to 90 days which among included RCTs, 45 % lasted less than 10 days (15 RCTs). Most studies were conducted in 1991, 2014 and 2021 (each year three RCT). Most trials were conducted in the USA (10 RCTs) followed by Germany (6 RCTs), Netherlands, France and Belgium (each country 4 RCTs). In addition to found all patients had history of sepsis, total participants of included studies were recorded as 13,633 volunteers, which 52.65 % and 43.69 % were male and female (7179 females VS 5957 male), respectively. In order to avoid further dispersion, the age groups examined in this review were divided into three main groups; early childhood (4Y), child (7–12 Y) and adult (>18 years). Among 33 trials, 9 early childhood trials, 4 child trials and 18 adult trials investigated the effect of m-Ab and p-Ab. Ten out of thirty-three assessed anti- LPS components m-Ab and p-Ab, while six out of thirty-three assessed anti-inflammatory m-Ab and p-Ab, and few studies evaluated an anti-Adrenomedullin (ADM) and anti-Lipoteichoic acid. Table-1 shows details of included RCTs which evaluated effect of m-Ab and p-Ab on patients suffering bacterial sepsis or septic shock. In the majority of studies, human serum albumin was administered to the placebo group as a control because it is safe for patients.
Fig. 1.
Flow chart of study selection for inclusion in the systematic review. Most articles related to animals, observational studies, and systematic reviews/meta-analyses or unpublished were excluded. Finally, nineteen articles were excluded from the study due to the non-availability of the full text and also the lack of their English version.
Table-1.
The details of included RCTs which evaluated effect of m-Ab and p-Ab on patients suffering bacterial sepsis or septic shock.
| Author Country Year |
Study design & Phase |
Participants (T/C) Age group Gender (F:M) History |
Drug name Type Target |
Doses (n) Concentration Duration |
Parameter measure | Outcomes |
|---|---|---|---|---|---|---|
| Aitchison et al. South Africa (1985) |
DB-RCT NA |
34 (17/17) Adult 7:10, 8:9 Sepsis |
LG-1 m-Ab LPS |
NA | Serum endotoxin, anti-LPS levels at the time of admission to the study and 24 h later. | Hospital mortality did not changed. |
| Greenman et al. USA (1991) |
DB-RCT NA |
316 (164/152) Adult 108:208 Sepsis |
E5 m-Ab Endotoxin |
IV doses 2 mg/kg 1 h |
Physical examinations and laboratory tests. | Great significant survival and resolution of individual organ failures. |
| Schedel et al. Germany (1991) |
RCT NA |
55 (28/27) Adult 26:29 Sepsis |
P-Ab LPS |
IV doses NA NA |
Mortality rate and IgG serum titers. | Reduction in death related to the septic reduced in treatment group. |
| Ziegler et al. USA,canada, Europe (1991) |
DB-RCT NA |
200 (95/105) Adult 83:117 Sepsis |
HA-1 A m-Ab LPS |
IV doses Single 100 mg NA |
Mortality rate,severity of illness index, vital signs, Hematologic and clinical chemistry tests, and antibody titers. | Improved survival in the treatment group. |
| McCloskey et al. USA (1994) |
DB-RCT NA |
621 (293/328) Adult NA Sepsis |
HA-1 A m-Ab LPS |
IV doses Single 100 mg NA |
Mortality rate | No significant effect in reducing the mortality rate between the two groups. |
| Linden et al. USA (1995) |
DB-RCT NA |
393 (131/262) Adult 165:228 Sepsis |
HA-1 A m-Ab LPS |
IV doses 100 mg of HA-1A diluted with 50 mL normal saline Multiple doses at 24 h. |
Severity of illness and mortality rate | Great severity of illness and higher mortality in treatment group. |
| Derkx et al. Nine centers/USA (1999) |
DB-RCT NA |
269 (130/137) Child 112:155 Sepsis |
HA-1 A m-Ab LPS |
IV doses 6 mg/kg NA |
Mortality rate | Reduction in mortality rate. |
| Angus et al. USA (2000) |
PDB-RCT Phase Ⅲ |
1102 (1090/12) Adult 490:600 Sepsis |
E5 m-Ab LPS |
IV doses 2 mg/kg per 1 h as 1 ml/kg per day 24 h |
Mortality rate | No significant differences in symptoms of sepsis, laboratory data and rates of morbidities and mortality between the two groups. |
| Gallagher et al. USA (2001) |
Open-label RCT Phase I/II |
36 (9/27) Adult 18:18 Sepsis |
Afelimomab m-Ab TNF-α |
IV doses 0.3, 1.0, or 3.0 mg/kg over 20 min Every 8 h for 72 h |
Serum concentrations of TNF-α, serum IL-6. | Improvement in TNF-α and IL-6 concentrations. |
| Reinhart et al. Europe and Israel (2001) |
DB-RCT NA |
446 (C:222/224) Adult 168:278 Sepsis |
Afelimomab m-Ab TNF-a |
IV doses 1 mg/kg per 8 h For 3D |
Mortality rate, and IL-6 concentration. | Significant reduction in mortality rate. |
| Albertson et al. France (2003) |
DB-RCT NA |
16 (8/8) Adult NA sepsis |
m-Ab LPS |
IV doses 300 mg/kg 1 M |
Compliance, laboratory tests, vital signs, and organ dysfunction. | Significant increase in the survival rate and reduction of LOS. |
| Rice et al. USA and Canada (2006) |
DB-RCT Phase II |
81 (38/43) Adult 30:51 Sepsis |
CytoFab p-Ab TNF-a |
IV doses 250-U/kg loading dose, followed by nine doses of 50 U/kg every 12 h, or 5 mg/kg human albumin as placebo Every 12 h |
TNF-α, IL-6, IL-8 concentrations, hematology and biochemistry measurements. | Improvement in TNF-α and IL-6 concentrations. No significant differences in adverse events, laboratory and vital sign abnormalities between the two groups. |
| Weisman et al. USA (2011) |
DB-RCT Phase II |
88 (42/46) Early childhood 42:46 Staphylococcal Sepsis |
Pagibaximab m-Ab LPS |
IV doses 90 or 60 mg/kg 1 W |
Serum levels of anti-LTA, anti-monoclonal/chimeric antibody levels. | No significant differences in demography, mortality, or morbidity rate between the two groups. |
| Morris et al. USA (2012) |
DB-RCT Phase IIa |
70 (23/47) Adult 38:32 Sepsis |
AZD9773 p-Ab TNF-a |
IV doses Single doses of 50 or 250 U/kg; multiple doses of 250 U/kg loading and 50 U/kg maintenance. Every 12h for 5D |
Serum levels of TNF-ɑ, laboratory tests, ECG measurements, and mortality rate. | Reduction of TNF-ɑ in treatment group. |
| Aikawa et al. Japan (2013) |
DB-RCT Phase II |
Chohort1:(7/3) chohort2: (7/3) Adult 3:4 & 4:3 Sepsis |
AZD9773 p-Ab TNF a |
IV doses Cohort 1: 250/50 U/kg; Cohort 2: 500/100 U/kg For 5D |
Serum levels of TNF-ɑ. | Reduction of TNF-ɑ in treatment group. |
| Australia, Belgium, Canada, Czech Republic, Finland, France, and Spain (2014) | DB-RCT Phase II |
493 (394/99) Adult 72:128 Sepsis |
AZD9773 p-Ab TNF-α |
IV doses Single dose of AZD9773 250 U/kg followed by 50 U/kg every 12 h (low dose), a single loading infusion of AZD9773 500 U/kg followed by 100 U/kg every 12 h (high dose) For 5D |
Serum levels of TNF-α, IL-6, and IL-8 concentrations from days 1–6. | No significant differences in adverse events, laboratory and vital sign abnormalities between the two groups. |
| Domizi et al. Italy (2019) |
DB-RCT Phase II |
20 (10/10) Adult 5:15 Sepsis |
p-Ab NA |
IV doses 250 mg/kg per day For 72h |
Mean arterial pressure,heart rate, laboratory tests, IL-1 β, IL-6, IL-8, IL-10 and TNF-α. | Increase in PVD and microvascular flow index in the treatment group. No significant changes in cytokines and NIRS-derived parameters between the two groups. Significant reduction of IL-6 and IL-10 in the treatment group. |
| Geven et al. Belgium, France, Germany, the Netherlands and Italy (2019) |
DB-RCT Phase II |
300 (150/150) Adult NA Sepsis |
Adrecizumab m-Ab Adrenomedullin (ADM) |
IV doses Two dosages of adrecizumab, 2 and 4 mg/kg, administered per hrs. Primary: for 90 D. Secondary: for 14D. |
Mortality rate | Unacceptable high morbidity and mortality rate. |
| Biagioni et al. Italy (2021) |
PDB-RCT NA |
356 NA NA Sepsis |
Pentaglobin p-Ab NA |
IV doses Daily dose achieving serum titers above 100 mg/dL For 7D |
Mortality rate | The mortality rate reduced in treatment group in compare to control goup. |
| Laterre et al. Belgium, France, Germany and the Netherlands (2021) |
DB-RCT Phase IIa |
301 (149/152) Adult 168:133 Sepsis |
Adrecizumab m-Ab Adrenomedullin (ADM) |
IV doses Treatment arm A: 2 mg/kg. Treatment arm B: adrecizumab 4 mg/kg NA |
TEAEs | No differences in mortality rate, frequency and severity in TEAEs between the two groups. |
| Acunas et al. London (1994) |
DB-RCT NA |
97 (67/30) Early childhood 35:62 Sepsis |
p-Ab NA |
IV doses Lyophilized human normal immunoglobulin (3 g/100 ml vial with 5 % sucrose) Fresh frozen plasma (15 ml/kg body weight) or Sandoglobulin (500 mg/kg body weight) NA |
Adverse effects, immune markers and C reactive protein. | Increase in IgG subclasses, IgA and complement component C4, and decrease in C reactive protein in treatment group. |
| Akdag et al. Ankara, Turkey (2014) |
PDB-RCT NA |
204 (equal number of 51 in four group) Early childhood 79:129 Sepsis |
Pentoxifylline (PTX) p-Ab NA |
IV doses Group 1: Standard treatment plus placebo. Daily for 3 consecutive D Group2: Standard treatment plus 6 mg/kg/hIV over 4 h. Daily for 3 consecutive D Group3: Standard treatment plus 250 mg/kgIV over daily IgM-enriched IVIG. Daily for 3 consecutive D Group 4: Standard treatment plus 6 mg/kg/h IV PTX over 4 h (first) plus 250mg/kgIV over4 hours IgM-enriched IVIG Daily for 3 consecutive D |
laboratory tests, CRP, IL-6, neutrophil CD64 expression and TNF-α levels. | No significant differences for laboratory data, symptoms of sepsis among groups, and mortality rate. |
| Bancalari et al. Chile (2020) |
P-RCT NA |
61 Early childhood 40:21 Sepsis |
Pentoxifylline p-Ab NA |
IV doses 500 mg/kg/day 7D |
Mortality rate | No AE were observed in groups. |
| Brocklehurst et al. Nine countries (2008) |
PDB-RCT NA |
3493 (1759/1734) Early childhood 1493:2000 Sepsis |
Pentoxifylline p-Ab NA |
IV doses 10 ml/kg of immunoglobulin over 4–6 h repeated 48 h later |
Mortality rate or major disability at two years. | Potential in reduction of mortality in severe neonatal sepsis. |
| Brocklehurst et al. Nine countries (2011) |
DB-RCT NA |
3493 (1759/1734) Early childhood 1493:2000 Sepsis |
p-Ab NA |
IV doses 500 mg (10 ml) per kg of body and repeated after 48 h. |
Mortality rate or major disability at two years. | No significant difference in the sepsis episodes and rates of major or non-major disability or of adverse events between-the two groups. |
| Goto et al. Japan (2022) |
R-RCT NA |
80 (40/40) Child 31:49 Sepsis |
p-Ab NA |
IV doses Dose of 5 g/D For 3 D |
Serum IgG levels | No significant difference in lengths of artificial ventilation and ICU stays between the two groups. |
| Haque et al. Saudi Arabia (1995) |
PDB-RCT NA |
130 (65/65) Early childhood NA Sepsis |
p-Ab NA |
IV doses 250 mg/kg per day For 4 D |
Mortality rate | Improvement in the outcome of neonatal sepsis when used as an adjunct to supportive and antibiotic therapy. |
| Hentrich et al. Germany (2006) |
P-RCT NA |
211(106/105) Adult 84:121 Neutropenic patients with hematologic malignancies and sepsis |
p-Ab NA |
IV doses 1300 mL of iviGMA (7.8 g IgM, 7.8 g IgA, and 49.4 g IgG) 72 h. |
Mortality rate | No significant differences in mortality rate between the two groups. |
| Jaspers et al. NA (1987) |
DB-RCT NA |
17 NA NA Sepsis |
p-Ab LPS |
IV doses Day 1: anti-lipoid A antibodies were infused at a dose of 8 ml (400 mg) per kg body weight at a rate of 1–2 ml/min. Day 2 (24 h after the first infusion): half the dose NA |
Clinical symptoms and laboratory tests | No significant differences in Clinical symptoms and laboratory parameters between the two groups. |
| Kola et al. Albania (2014) |
DB-RCT NA |
78 (39/39) Child 24:54 Sepsis |
Pentaglobin p-Ab NA |
IV doses 20–40 ml/kg 1 h. |
Survival rate | The survival rate was higher in the treatment group. |
| Nassir et al. Iraq (2021) |
RCT NA |
272 (136/136) Early childhood 138:134 Sepsis |
p-Ab NA |
IV doses 5 ml/kg daily 3 consecutive D |
laboratory tests, liver and kidney function test, Mortality rate and duration of hospitalization. | Significant higher mortality risk for mortality in patient treated by standard antibiotic protocol than patients received p-Ab adjuvant therapy. |
| Shenoi et al. Bangalore (1999) |
RCT NA |
50 (25/25) Early childhood 12:38 Sepsis |
p-Ab NA |
IV doses 1 g/kg of IVIG 3 consecutive D |
Mortality rate | Significant higher number of babies with positive blood culture in placebo group. No significant reduction in death rate. |
| Takahashi et al. Japan (2020) |
RCT NA |
80 (33/47) Child 40:40 Septic DIC |
p-Ab NA |
IV doses 5000 mg/D For 3 D |
Increased platelet counts and mortality rate | Significant and non-significant increase in platelet count and mortality rates, respectively. |
RCT: randomized controlled trial, DB: double blind, PDB-RCT: prospective RCT, R-RCT: retrospective RCT, T/C: treatment/control, F:M: female: male, m-Ab: monoclonal antibody, p-Ab: polyclonal antibody, NA: not available, IV: intravenous, mg/kg: milligram/kilogram, hrs.: hours, M: month, D: day, AE: adverse effect, IL: interleukin, TNF: Tumor necrosis factor, LOS: lipooligosaccharide, LPS: lipopolysaccharide, PVD; perfused vessel density, ECG: electrocardiogram.
3.2. Outcomes
Primary outcomes are considered as mortality and adverse effects (treatment emergent adverse events (TEAEs)) after taking m-Ab and p-Ab. At the same time, laboratory measurement like hematology, biochemistry, immunological, and microbiological analysis were considered secondary outcomes. This includes as examination of blood cells count (platelet, white and red blood cells), anti-endotoxin, anti-LTA, anti-antibody levels (IgG serum titers), TNF-α, interleukin (IL)-6, IL-8 and determining bacterial species. Tolerability of m-Ab and p-Ab, efficacy endpoints including health service utilization like duration of stay in hospital, ventilator-, intensive care unit (ICU)- and shock-free days, change in sequential-related organ failure assessment (SOFA) like sepsis multi organ dysfunction and severity of illness index are also secondary outcomes. All parameters measured at the time of admission to the study until the second measurement which were differ as 24 h later, a week and 14 and 28 days’ assessment.
3.3. Effects of monoclonal antibody and polyclonal antibody on mortality rates of patients with sepsis
Mortality rates were evaluated in twenty-five studies [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]]. Significant increase in survival of patients and reduction of mortality rate were observed (p < 0.05) [22,26,31,32,36,39,42], but 16 trials showed no significantly change in survival or mortality rates in inter-and intra-group comparison [[21], [22], [23], [24],[26], [27], [28],30,34,35,37,38,41,43,44,46], and unacceptable increase in mortality [25,29,38] (p > 0.05). Patients who received antibiotics plus antibody as adjuvant therapy showed less risk for death in compare to patients who give antibiotics only [47]. Mortality were also studied in 4 trial which studied death rate in gram negative bacteremia patients [22,30,33,37], and showed death rate significantly was decreased than sepsis induced by gram positive bacteria (GPB) patients (p < 0.05) [33,37], or no change in survival of confirmed gram negative bacteremia group’s patients [22,30]. Also, 1 trial studied mortality rate in IL-6 positive patients which reported mortality rate was not different from randomized IL-6 positive placebo and non-randomized patients with a negative IL-6 test [31].
3.4. Microbiological analysis following monoclonal antibody and polyclonal antibody
Nine trials analyzed bacterial species in sepsis patients who were under treatment by antibodies [[37], [38], [39],[41], [42], [43], [44], [45],47], which get different results. Gram negative bacteria (GNB) like Escherichia coli, Klebsiella spp. and Enterobacter was mostly isolated in patients with sepsis than most found GPBs like Methicillin-resistant Staphylococcus aureus (MRSA) and Enterococcus spp. in patients with sepsis [37,43,47]. In contrast, GPB like Coagulase negative staphylococcus (CONS) [38,41,44], Staphylococcus epidermidis [39,42] and Group B Streptococcus [42] were most found GPB in proven sepsis patients and responsible for death. Most frequent cites which bacterial agent of sepsis was isolated were wounds, sputum, urine [37], and blood [[37], [38], [39],42,44,47]. In addition, the opsonophagocytic (bacterial killing) [44] and bactericidal [46] activity of antibody was analyzed, which showed increase serum opsonophagocytic activity in antibody treated group in compare to control.
3.5. Effects of monoclonal antibody and polyclonal antibody on levels of serum components
As one of laboratory’s measurement, endotoxin (LPS or LOS) level in serum were evaluated by three studies [21,22,32], which 1 trials showed significant reduction in levels of endotoxins in serum of antibody treated patients (p < 0.05) [22,32]. Cytokines including TNF- α, IL-1, -6, -8 and -10 levels in serum were measured by 8 study [24,31,38,[48], [49], [50], [51], [52]], which 3 out of 8 trials for TNF-α in serum [48,49,51,52] and 1 trial for TNF-α in BAL [52], 2 out of 8 trials for IL-6 [24,48,52] and 1 out of 8 trial for IL-8 [48] reports were observed as significantly decrease in levels and 1 out of 8 trial increase in IL-10 [50] levels (p < 0.05). However, few trials showed increasing in TNF-α [50] and IL-6 [31]. Increased of anti-LTA [44], anti-lipid A [46] and TNF-α-antibody complexes levels [24] in serum also were observed.
By analyzing other laboratory markers, 1 trial showed improvement of DIC scores and significant increasing platelet counts as coagulation marker [37,47], 1 trial showed significant reduction of C-reactive protein (CRP) (p < 0.05) [53] and white blood cell (WBC) [47], and 2 trial showed no differences in change of plasma CRP level, WBC count [38], and neutrophil CD64 (MFI) as sepsis biomarker in in critically ill patients [38,42,53], between antibody treated group in compare to other examined group.
3.6. Effects of monoclonal antibody and polyclonal antibody on tolerability and TEAEs
Safety and tolerability of antibody administration in patients with sepsis syndrome were considered as primary objective in 3 trials [25,26,28,52], which showed there’s no safety concern about antibody and well-tolerate by patients [25,28,33,34,51]. In various studies, the severity of side effects as undesired harmful effect is classified into several categories: mild, moderate, severe, life-threatening, and disabling. Adverse events (AE) following administration of m-Ab and p-Ab were analyzed in 16 study [[22], [23], [24], [25], [26],28,30,31,40,41,[43], [44], [45],48,49,52] which most of them showed similar or no significant change AE between treatment and placebo [23,31,41,52], and described as mild-to-moderate [26,51]. Other results were increase frequency of serious adverse effects (SAE) [22,41], and m-Ab/p-Ab independent AE [24] or not seen any AE/SAE [43,44].
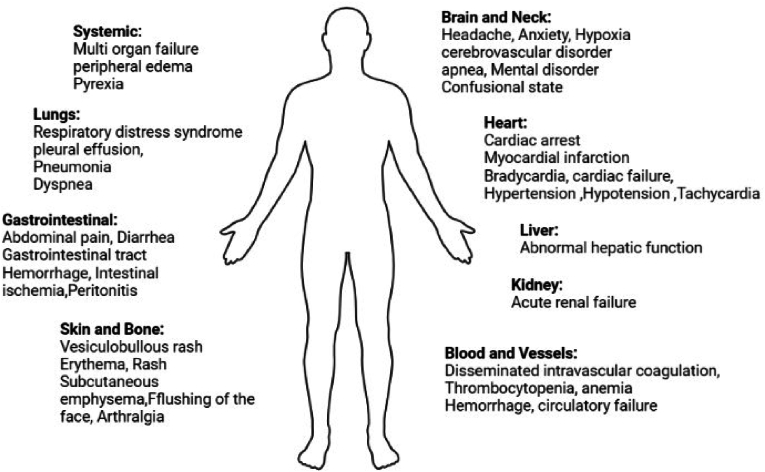
Among nine early childhood trials, one trial related to using m-Ab and the remaining eight used p-Ab. Except for one trial [23], other trials examined the adverse effects during the study. The only m-Ab related trial [24] and four p-Ab trial were not associated with any other side effects [25–28]. The most important complication reported in early Childhood included Necrotizing enterocolitis [29] and pneumonia [30, 31] which was not considered as SAE. One m-Ab trial and three p-Ab trials performed on children's sepsis. Only one trial reported hypersensitivity and allergic reactions which became life-threatening [32]. In adult studies, pleural effusion and peripheral edema [23] following p-Ab and reversible allergic reactions [24] following m-Ab were the most common side effects. Summary of side effect gathered in Fig. 2.
Fig. 2.
Adverse effects induced by m-AB and p-Ab in patients with bacterial sepsis or septic shock.
3.7. Effects of monoclonal antibody and polyclonal antibody on organ dysfunction and severity in illness
Organ dysfunction and severity sepsis support Index (SSI) were considered as the primary efficacy of the secondary outcomes. Organ dysfunction were studied in 7 study [22,26,28,31,37,38,45], that 1 study indicated significantly resolve in organ failure (p < 0.05) [26,37]. While there were no differences between treatment and placebo [31,38], one study reported increase in failure in kidney [22]. Moreover, tissue oxygenation and microvascular reactivity were evaluated which increase in perfused vessel density during sepsis was reported [50].
Studying of severity in illness showed no significant change between treatment and placebo groups (p > 0.05) [22,25,28,29], while one trial reported an increase in severity of sepsis [29]. Apnea and respiratory distress were recorded as most sepsis severity in patients [44], and necrotizing enterocolitis, disseminated intravascular coagulation (DIC) and Pulmonary hemorrhage introduce as co-morbidities following combination using of m-Ab and p-Ab [38].
3.8. Effects of monoclonal antibody and polyclonal antibody on hospitalization and critical care
The number of ventilator, shock and ICU free days (or need for ventilation and ICU) [28,35,43,45,49,52], adequacy of using antibiotics (or no use of antibiotics) [22,45,47], need for surgical treatment [22], length of stay in hospital [36,40,43,45], and need for granulocyte monocyte colony stimulating factor (GM-CSF) [43] in patients were studied. Trials showed there was no changes in number of shock-free days [52] or using ventilator [35,43], and hospitalization [43] in treated and placebo groups. Also, significant increase in ventilator-free days and ICU-free days [35], shorter stay in hospital (hospitalization) [36,47], and reduction of disease duration in antibody treated sepsis patients [47] were reported in compare to longer illness duration of patients who give antibiotics (p < 0.05).
4. Discussion
Despite the availability of antibiotics as common therapy for patient with sepsis, sepsis is still leading cause of death and organ failure in patients. Antibiotics lack the ability to prevent the toxic effects caused by endotoxin, as in some cases they may stimulate the release of bacterial endotoxin [32]. Undeveloped immune system of babies and children as well as the slowness of the antibody production pathway reinforces the need for immunotherapy as adjuvant treatment for sepsis [54] and another treatment strategy for sepsis caused by GNBs. A number of studies have shown that the serum of patients immunized with E. coli J5 is effective in preventing sepsis and septic shock [32]. Studies emphasized on the effectiveness of immunoglobulin in the treatment of sepsis which prevent infant mortality by inducing a better immune response [55,56]. Immunoglobulin are design based on their affinity to the specific targets which after opsonization and fixing complement, result in clearance of pathogens harboring that surface antigen [22]. TNF-α was one of the most common targets for treatment of sepsis using antibody, due to its stability and conserved structure, as well as less side effects. Although endotoxin was another common target against sepsis but variability and lipid structure become challenging, although further studies were needed to prove. Targeting cytokines like TNF-α are considered as one of main markers of microbial sepsis, is steadily increased in most patients with sepsis and is related to the death rate in these patients [57]. Studies indicate as the level of TNF-α is higher in patients with more severe disease, the clinical effects of anti-TNF-α drugs such as CytoFab were also observed in this group of patients [58,59]. Present study supports GNB as the main bacterial species responsible for sepsis syndrome which mostly derived from bloodstream infection. Also, antibodies are well tolerating with no safety concern strategy which in some cases induced reversible mild-moderate AE. They resolved the organ failure and rarely exacerbate the organ dysfunction in patients with sepsis. Also, m-Ab/p-Ab mediate patient’s need for critical care and stay in hospital. By increasing of m-Ab/p-Ab in patient’s serum, they have ability to reduce endotoxins, TNF-α, IL-1, 6 and 8, and increase IL-10. Despite of an improvement of DIC, increasing platelet, decrease of CRP and WBC, we found that antibodies in most studies weren’t effective in reducing mortality rates in patients with sepsis, and they were not effective enough as expected for normalizing CRP, WBC and CD64 marker in patients who suffering sepsis syndrome. The outcome affecting mortality depends on, first, which GNBs or GPBs caused the sepsis [37], and second, whether other drugs such as antibiotics are prescribed along with antibodies or not. Studies indicate significant effectiveness of co-administration of antibody as an adjuvant along the common and conventional treatment of sepsis, antibiotics on mortality and its improvement in the level of laboratory markers [42,47]. The finding of these studies have made these antibodies to be suggested as a complementary and supportive strategy for infants with sepsis.
In the past, it was thought that sepsis was mostly caused by GNB like klebsiella spp., Escherichia coli (E. coli), and Pseudomonas aeruginosa (P. auroginosa). while recently the role of gram-positive bacteria (GPB) such as Staphylococcus aureus (S. aureus), Streptococcus pyogenes (S. pyogenes) has also been mentioned [60,61]. Endotoxin in GNBs which is composed of lipid A, an oligosaccharide core, and the O-antigen polysaccharide, is the main cause of shock in sepsis. When Lipid A is recognized by the innate immune system, Toll-like receptor 4 (TLR4) as the key factor, enhances the production of pro-inflammatory cytokines by macrophage stimulation. Briefly, LPS is recognized by LPS-binding protein (LBP) in the serum and transferred to CD14. This molecule transfers LPS to the MD2, then binds to TLR4 and forms a TLR4-MD2 receptor complex. Through activation of the TLR domain, the subsequent processes of LPS recognition are started. The important point of this process is the MyD88-independent pathway that leads to the activation of Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and stimulation of the release of pro-inflammatory cytokines such as TNF-α [62]. On the other hand, LTA present in the cell wall of GPBs such as staphylococcus are important in stimulating the production of pro-inflammatory cytokines in addition to controlling bacterial cell division [63]. Because they are ligands for TLR2 and together with peptidoglycan, they contribute to the exacerbating inflammation [64]. Designing hybrid antibody against LTA such as pagibaximab showed that it can be successful in neutralizing LTA in GPBs and reducing pro-inflammatory cytokines. Based on Weisman et al. finding, despite of level of anti-LTA in the serum of volunteer patients can be high, it may not show a great opsonization effect which is probably because not all binding activities are limited to opsonization activities [44]. Probably the survival of the patient with sepsis caused by GNB is higher following the use of IVIG, so the mortality in this group of people is greatly reduced [65]. Although the reason is not exactly known, it is attributed to mechanisms such as reducing the levels of endotoxin in the serum [66] and neutralizing the GNBs toxin [33], such as neutralizing the toxin type 3 secretion system by P. aeruginosa and preventing the action of this toxin following the administration of antibodies [67].
The importance of organ failure associate with prognosis of sepsis [26]. The failure in organs such as the kidney, and the serious condition of the patients are the reasons for the increase in the death rate in some studies [22]. Endotoxin is involved in failure, especially in GNB induced sepsis. Since circulating endotoxin level is a good predictor of anti-endotoxin antibody response, in such cases antibodies against endotoxin and its other components may provide greater improvement in organ failure. E5 and HA-1A, produced as anti-lipid A antibody from IgM with half-life span [26]. In contrast to antibodies in other trials, they have not impressive in the survival of sepsis patients as expected. There are several reasons in this regard, the antigenicity of the antibodies is different from each other, the health and care background of the patients like risk factors and underlying disease including surgeries, trauma, cancer and diabetes has a direct impact on the reducing cytokines, side effects and the mortality in the group receiving hybrid antibodies [48]. Also, there is direct relationship between increasing age and IL-8 with the death rate which as the age increases, the level of IL-8 tending to increase and elderly patients are susceptible to severe complications of the sepsis [49]. Dosage can be also important in the mortality risk in patients receiving hybrid antibodies. For example, in response to a low dose of AZD [49] and IVIG [35], decrease in the need of patients to use a ventilator and an increase in the survival of patients were observed which indicates significant decrease in the death rate among these patients. Low dose of antibody has a complementary effect in which it leaves positive effects in patients with sepsis without over-stimulating the immune system [35]. In contrast to cytokines like TNF-α, intact and usual antibodies are not able to penetrate tissues such as the lung due to their larger size. When TNF-α reaches to the lung, it triggers an inflammatory response which leads to permeabilization of the lung tissue and entry of other cytokines into the alveolar space [68]. Designing novel antibodies with shorter fragment antigen-binding (Fab) region such as polyclonal CytoFab that can penetrate the alveolar space and effectively neutralize TNF-α [69]. In order to confirm this statement, when the amount of cytokines present in bronchoalveolar lavage (BAL) in mechanically ventilated patient with sepsis syndrome and lung injuries who receiving antibody were compared with placebo group, it is found that the amount of pro-inflammatory cytokines is clearly decreased in the receiving antibodies group while placebo showed an increase of these cytokines [52].
The lack of clinical and protective effect of antibodies on the outcome of sepsis may depend on special group of patients. It is clear that anti-endotoxin antibody has more protective effect on sepsis patients, but it is difficult to diagnose sepsis patients in the initial stages. Since sepsis is a complex disease, it is unreasonable to expect that an antibody alone can provide protective effects. Therefore, the goal of preventing sepsis in people infected with Gram-negatives needs trials with more than seven hundred volunteers [70]. It was thought that TNF-α has a direct effect on the production of other inflammatory cytokines, including IL-8 and IL-6, while now different results obtained in the trials showed despite the reduction of TNF-α, decreasing in IL-8 and IL-6 levels were not observed. This is probably due to the fact that TNF-α is no longer the dominant factor in the cytokine cascade and does not induce a biological function in patients with sepsis. The lack of effect of hybrid antibodies in reducing inflammatory cytokines is another reason for the lack of effective treatment of this type of antibodies and no significant report in reduction of death rate [49]. Rapid diagnosis of sepsis patients helps in their treatment process. Screening patients for high levels of IL-6 helps to identify patients in hyperinflammatory response during severe sepsis and prioritize them in treatments. Although Reinhart et al.'s study using Afelimomab had no significant effect on mortality in IL-6-positive subjects, it showed that IL-6 was highly predictive of disease outcome [31]. Improvement of serum platelet level is associated with improvement in coagulation function and better prognosis of DIC in sepsis patients [37].
Researchers consider the effects of polyvalent immunoglobulins to be more effective than monoclonal antibodies in neonatal and adult patients suffering sepsis, for instance antibodies with base of IgM preparation like Pentaglobin has shown a better effect on reducing mortality and side effects [42,71]. Compared to monoclonal antibodies, polyclonal antibodies can show better clinical effects by targeting several domains of TNF-α and establishing more interactions [72]. Small design of a trial leads to insufficient evidence, which makes it impossible to reach a correct decision about the result of using an antibody. By the highest titers of antibodies against the pathogens associated with sepsis and their toxins, IgM preparation have a better effect compared to the conventional antibodies that are usually prepared. As we know, the first reaction of the immune system against pathogens is the production of IgM, which is induced earlier than IgG and is faster and more effective in activating the immune system. Therefore, they speed up the final goal which is the production of specific antibodies [73,74]. Another reason for the effectiveness of polyvalent IgM is the lack of IgMs following their early clearance from the baby's body, which compensates the low levels of IgM during the fetal period [36]. Current study highlights that monoclonal antibody of Pagibaximab [23], HA-1A [24], adrenomedullin antibody adrecizumab [25, 26], Afelimomab [27, 28], ovine anti-TNF fragment antigen binding (Fab) fragments (CytoFab) [29] and polyclonal antibody of Pentaglobin [24, 30, 31], AZD9773 [32–34], E5 [26], IVIG and immunoglobulin containing IgM, IgA and IgG [35–37] were well-tolerate, effective and safe for treatment of patients with sepsis by reduction of mortality. Although the effective mechanism of immunoglobulins has not been well defined and confirmed, but studies using Pentaglobin have shown that they do this by changing the profile of cytokine production towards anti-inflammatory response, changing the activation of the complement system and neutralizing toxins [75]. While IVIG acts through antibody-dependent killing following c3b deposition and potentiating opsonization, studies showed Pentaglobin as IGM-enriched IVIG have more opsonization activity than IVIG or IgG based antibodies, studies support the claim that IgM-enriched IVIG works better in the treatment of sepsis for some reason [47,76]. First, IgM preferably reacts to bacterial toxins and antigens by pentameric structure. Due to their size, IgM are able to neutralize LPS more effectively and have a significant ability to activate the complement system as well as greater opsonization power in neutralizing bacteria [42]. In contrast, there are controversial results from number of E5 [41], HA-1A [42, 43], Pentaglobin [38], and others including LG-l [40], and MAB-T88 [39] as m-Ab and pentoxifylline (PTX) [38], IVIG [15, 44–47], IgmA-enriched immunoglobulin [48] as p-Ab in some studies which were not recommend and need further study in future on increasing survival of patients. The reason that a similar antibody showed a different effect in another study can be explained by the difference in the preparation of the antibody, the dose of the antibody, and the route of administration which obtain different results. Also, the conditions of the patients in each study are not the same [39].
5. Conclusion
The therapeutically effect of an antibody against sepsis different depends on the patient and type of bacteria. Even adequate sample size and high enrolled patients, antibodies did not change the death rate and improvement in survival. The use of polyvalent IgM preparation in the treatment of pediatric sepsis patients suggested for an increase in the survival rate, a reduction in the LOS and an improvement in infection severity.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
All authors agreed to publish this manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by the deputy for research and technology of the Iran University of Medical Sciences [grant number 1401-4-73-24841].
CRediT authorship contribution statement
Marzie Mahdizade Ari: Writing – original draft, Investigation, Formal analysis, Data curation. Mohammad Esmaeil Amini: Resources, Methodology, Data curation. Mohammad Sholeh: Methodology, Investigation, Data curation. Abed Zahedi Bialvaei: Writing – review & editing, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Patricia Schlagenhauf
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nmni.2024.101435.
Contributor Information
Marzie Mahdizade Ari, Email: marziemahdizadeari@gmail.com.
Mohammad Esmaeil Amini, Email: moh.es.amini@gmail.com.
Mohammad Sholeh, Email: mohammad.sholeh.mail@gmail.com.
Abed Zahedi Bialvaei, Email: zahedi.ab@iums.ac.ir.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet (London, England) 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39(5):517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 3.van der Poll T., Shankar-Hari M., Wiersinga W.J. The immunology of sepsis. Immunity. 2021;54(11):2450–2464. doi: 10.1016/j.immuni.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Gong T., Liu L., Jiang W., Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 5.Font M.D., Thyagarajan B., Khanna A.K. Sepsis and Septic Shock – basics of diagnosis, pathophysiology and clinical decision making. Med Clin. 2020;104(4):573–585. doi: 10.1016/j.mcna.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Kharga K., Kumar L., Patel S.K.S. Recent advances in monoclonal antibody-based approaches in the management of bacterial sepsis. Biomedicines. 2023;11(3) doi: 10.3390/biomedicines11030765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.https://www.cdc.gov/sepsis/diagnosis/index.html.
- 8.Martínez M.L., Plata-Menchaca E.P., Ruiz-Rodríguez J.C., Ferrer R. An approach to antibiotic treatment in patients with sepsis. J Thorac Dis. 2020;12(3):1007–1021. doi: 10.21037/jtd.2020.01.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadgir C.A., Biswas D.A. Antibiotic resistance and its impact on disease management. Cureus. 2023;15(4) doi: 10.7759/cureus.38251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarczak D., Kluge S., Nierhaus A. Use of intravenous immunoglobulins in sepsis therapy-A clinical view. Int J Mol Sci. 2020;21(15) doi: 10.3390/ijms21155543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagiwara J., Yamada M., Motoda N., Yokota H. Intravenous immunoglobulin attenuates cecum ligation and puncture-induced acute lung injury by inhibiting apoptosis of alveolar epithelial cells. J Nippon Med School = Nippon Ika Daigaku zasshi. 2020;87(3):129–137. doi: 10.1272/jnms.JNMS.2020_87-303. [DOI] [PubMed] [Google Scholar]
- 12.Mokhtary P., Pourhashem Z., Mehrizi A.A., Sala C., Rappuoli R. Recent progress in the discovery and development of monoclonal antibodies against viral infections. Biomedicines. 2022;10(8) doi: 10.3390/biomedicines10081861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahavi D., Weiner L. Monoclonal antibodies in cancer therapy. Antibodies. 2020;9(3) doi: 10.3390/antib9030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aungsumart S., Youngkong S., Dejthevaporn C., Chaikledkaew U., Thadanipon K., Tansawet A., et al. Efficacy and safety of monoclonal antibody therapy in patients with neuromyelitis optica spectrum disorder: a systematic review and network meta-analysis. Front Neurol. 2023;14 doi: 10.3389/fneur.2023.1166490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka T., Hishitani Y., Ogata A. Monoclonal antibodies in rheumatoid arthritis: comparative effectiveness of tocilizumab with tumor necrosis factor inhibitors. Biol Targets & Ther. 2014;8:141–153. doi: 10.2147/BTT.S37509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Fernández K., Mangas-Sanjuán V., Merino-Sanjuán M., Martorell-Calatayud A., Mateu-Puchades A., Climente-Martí M., et al. Impact of pharmacokinetic and pharmacodynamic properties of monoclonal antibodies in the management of psoriasis. Pharmaceutics. 2022;14(3) doi: 10.3390/pharmaceutics14030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez A.V., Piscoya A., Pasupuleti V., Phan M.T., Julakanti S., Khen P., et al. Beneficial and harmful effects of monoclonal antibodies for the treatment and prophylaxis of COVID-19: systematic review and meta-analysis. Am J Med. 2022;135(11) doi: 10.1016/j.amjmed.2022.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavaco M., Castanho M., Neves V. The use of antibody-antibiotic conjugates to fight bacterial infections. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.835677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:1–10. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aitchison J.M., Arbuckle D.D. Anti-endotoxin in the treatment of severe surgical septic shock. Results of a randomized double-blind trial. South Afr Med J = Suid-Afrikaanse tydskrif vir geneeskunde. 1985;68(11):787–789. [PubMed] [Google Scholar]
- 22.Albertson T.E., Panacek E.A., MacArthur R.D., Johnson S.B., Benjamin E., Matuschak G.M., et al. Multicenter evaluation of a human monoclonal antibody to Enterobacteriaceae common antigen in patients with Gram-negative sepsis. Crit Care Med. 2003;31(2):419–427. doi: 10.1097/01.CCM.0000045564.51812.3F. [DOI] [PubMed] [Google Scholar]
- 23.Angus D.C., Birmingham M.C., Balk R.A., Scannon P.J., Collins D., Kruse J.A., et al. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Stud Investigat Jama. 2000;283(13):1723–1730. doi: 10.1001/jama.283.13.1723. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher J., Fisher C., Sherman B., Munger M., Meyers B., Ellison T., et al. A multicenter, open-label, prospective, randomized, dose-ranging pharmacokinetic study of the anti-TNF-alpha antibody afelimomab in patients with sepsis syndrome. Intensive Care Med. 2001;27(7):1169–1178. doi: 10.1007/s001340100973. [DOI] [PubMed] [Google Scholar]
- 25.Geven C., Blet A., Kox M., Hartmann O., Scigalla P., Zimmermann J., et al. A double-blind, placebo-controlled, randomised, multicentre, proof-of-concept and dose-finding phase II clinical trial to investigate the safety, tolerability and efficacy of adrecizumab in patients with septic shock and elevated adrenomedullin concentration (AdrenOSS-2) BMJ Open. 2019;9(2) doi: 10.1136/bmjopen-2018-024475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenman R.L., Schein R.M., Martin M.A., Wenzel R.P., MacIntyre N.R., Emmanuel G., et al. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. The XOMA Sepsis Study Group. JAMA. 1991;266(8):1097–1102. [PubMed] [Google Scholar]
- 27.Hentrich M., Fehnle K., Ostermann H., Kienast J., Cornely O., Salat C., et al. IgMA-enriched immunoglobulin in neutropenic patients with sepsis syndrome and septic shock: a randomized, controlled, multiple-center trial. Crit Care Med. 2006;34(5):1319–1325. doi: 10.1097/01.CCM.0000215452.84291.C6. [DOI] [PubMed] [Google Scholar]
- 28.Laterre P.F., Pickkers P., Marx G., Wittebole X., Meziani F., Dugernier T., et al. Safety and tolerability of non-neutralizing adrenomedullin antibody adrecizumab (HAM8101) in septic shock patients: the AdrenOSS-2 phase 2a biomarker-guided trial. Intensive Care Med. 2021;47(11):1284–1294. doi: 10.1007/s00134-021-06537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linden P.K., Angus D.C., Chelluri L., Branch R.A. The influence of clinical study design on cost-effectiveness projections for the treatment of gram-negative sepsis with human anti-endotoxin antibody. J Crit Care. 1995;10(4):154–164. doi: 10.1016/0883-9441(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 30.McCloskey R.V., Straube R.C., Sanders C., Smith S.M., Smith C.R. Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. CHESS Trial Study Group. Ann Intern Med. 1994;121(1):1–5. doi: 10.7326/0003-4819-121-1-199407010-00001. [DOI] [PubMed] [Google Scholar]
- 31.Reinhart K., Menges T., Gardlund B., Harm Zwaveling J., Smithes M., Vincent J.L., et al. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: the RAMSES Study. Crit Care Med. 2001;29(4):765–769. doi: 10.1097/00003246-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Schedel I., Dreikhausen U., Nentwig B., Höckenschnieder M., Rauthmann D., Balikcioglu S., et al. Treatment of gram-negative septic shock with an immunoglobulin preparation: a prospective, randomized clinical trial. Crit Care Med. 1991;19(9):1104–1113. doi: 10.1097/00003246-199109000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Ziegler E.J., Fisher C.J., Jr., Sprung C.L., Straube R.C., Sadoff J.C., Foulke G.E., et al. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991;324(7):429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- 34.Derkx B., Wittes J., McCloskey R. Trial EPMSS. Randomized, placebo-controlled trial of HA-1A, a human monoclonal antibody to endotoxin, in children with meningococcal septic shock. Clin Infect Dis. 1999;28(4):770–777. doi: 10.1086/515184. [DOI] [PubMed] [Google Scholar]
- 35.Goto K., Yasuda N., Sato Y. Effects of low-dose intravenous immunoglobulin as the adjunctive therapy in septic shock patients with and without hypogammaglobulinemia: a retrospective cohort study. Ann Palliat Med. 2022;11(8):2600–2608. doi: 10.21037/apm-21-3694. [DOI] [PubMed] [Google Scholar]
- 36.Elmira K., Celaj E., Bakalli I., Lluka R., Kuli-Lito G., Sallabanda S. Efficacy of an IgM preparation in the treatment of patients with sepsis: a double-blind randomized clinical trial in a pediatric intensive care unit. South Eastern Eur J Publ Health. 2015;1 [Google Scholar]
- 37.Takahashi G., Shibata S. Study of usefulness of low-dose IgG for patients with septic disseminated intravascular coagulation. Biomarkers Med. 2020;14(13):1189–1196. doi: 10.2217/bmm-2020-0204. [DOI] [PubMed] [Google Scholar]
- 38.Akdag A., Dilmen U., Haque K., Dilli D., Erdeve O., Goekmen T. Role of pentoxifylline and/or IgM-enriched intravenous immunoglobulin in the management of neonatal sepsis. Am J Perinatol. 2014;31(10):905–912. doi: 10.1055/s-0033-1363771. [DOI] [PubMed] [Google Scholar]
- 39.Bancalari A., Muñoz T., Martínez P. Prolonged intravenous immunoglobulin treatment in very low birth weight infants with late onset sepsis. J Neonatal Perinat Med. 2020;13(3):381–386. doi: 10.3233/NPM-190259. [DOI] [PubMed] [Google Scholar]
- 40.The INIS Study. International Neonatal Immunotherapy Study: non-specific intravenous immunoglobulin therapy for suspected or proven neonatal sepsis: an international, placebo controlled, multicentre randomised trial. BMC Pregnancy Childbirth. 2008;8:52. doi: 10.1186/1471-2393-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brocklehurst P., Farrell B., King A., Juszczak E., Darlow B., Haque K., et al. Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med. 2011;365(13):1201–1211. doi: 10.1056/NEJMoa1100441. [DOI] [PubMed] [Google Scholar]
- 42.Haque K.N., Remo C., Bahakim H. Comparison of two types of intravenous immunoglobulins in the treatment of neonatal sepsis. Clin Exp Immunol. 1995;101(2):328–333. doi: 10.1111/j.1365-2249.1995.tb08359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shenoi A., Nagesh N.K., Maiya P.P., Bhat S.R., Subba Rao S.D. Multicenter randomized placebo controlled trial of therapy with intravenous immunoglobulin in decreasing mortality due to neonatal sepsis. Indian Pediatr. 1999;36(11):1113–1118. [PubMed] [Google Scholar]
- 44.Weisman L.E., Thackray H.M., Steinhorn R.H., Walsh W.F., Lassiter H.A., Dhanireddy R., et al. A randomized study of a monoclonal antibody (pagibaximab) to prevent staphylococcal sepsis. Pediatrics. 2011;128(2):271–279. doi: 10.1542/peds.2010-3081. [DOI] [PubMed] [Google Scholar]
- 45.Biagioni E., Tosi M., Berlot G., Castiglione G., Corona A., De Cristofaro M.G., et al. Adjunctive IgM-enriched immunoglobulin therapy with a personalised dose based on serum IgM-titres versus standard dose in the treatment of septic shock: a randomised controlled trial (IgM-fat trial) BMJ Open. 2021;11(2) doi: 10.1136/bmjopen-2019-036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaspers L., Marget W., Mar P.J., Hoffmann K., Langecker P., Ruckdeschel G., et al. [Antibody to lipoid A in the treatment of septic shock] Infection. 1987;15(Suppl 2):S89–S95. doi: 10.1007/BF01644200. [DOI] [PubMed] [Google Scholar]
- 47.Nassir K.F., Al-Saddi Y.I., Abbas H.M., Al Khames Aga Q.A., Al Khames Aga L.A., Oudah A.A. Pentaglobin (immunoglobulin M-enriched immunoglobulin) as adjuvant therapy for premature and very low-birth-weight neonates with sepsis. Indian J Pharmacol. 2021;53(5):364–370. doi: 10.4103/ijp.ijp_881_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aikawa N., Takahashi T., Fujimi S., Yokoyama T., Yoshihara K., Ikeda T., et al. A Phase II study of polyclonal anti-TNF-α (AZD9773) in Japanese patients with severe sepsis and/or septic shock. J Infect Chemother : Off J Japan Soc Chemother. 2013;19(5):931–940. doi: 10.1007/s10156-013-0612-y. [DOI] [PubMed] [Google Scholar]
- 49.Bernard G.R., Francois B., Mira J.P., Vincent J.L., Dellinger R.P., Russell J.A., et al. Evaluating the efficacy and safety of two doses of the polyclonal anti-tumor necrosis factor-α fragment antibody AZD9773 in adult patients with severe sepsis and/or septic shock: randomized, double-blind, placebo-controlled phase IIb study. Crit Care Med. 2014;42(3):504–511. doi: 10.1097/CCM.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 50.Domizi R., Adrario E., Damiani E., Scorcella C., Carsetti A., Giaccaglia P., et al. IgM-enriched immunoglobulins (Pentaglobin) may improve the microcirculation in sepsis: a pilot randomized trial. Ann Intensive Care. 2019;9(1):135. doi: 10.1186/s13613-019-0609-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris P.E., Zeno B., Bernard A.C., Huang X., Das S., Edeki T., et al. A placebo-controlled, double-blind, dose-escalation study to assess the safety, tolerability and pharmacokinetics/pharmacodynamics of single and multiple intravenous infusions of AZD9773 in patients with severe sepsis and septic shock. Crit Care. 2012;16(1):R31. doi: 10.1186/cc11203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rice T.W., Wheeler A.P., Morris P.E., Paz H.L., Russell J.A., Edens T.R., et al. Safety and efficacy of affinity-purified, anti-tumor necrosis factor-alpha, ovine fab for injection (CytoFab) in severe sepsis. Crit Care Med. 2006;34(9):2271–2281. doi: 10.1097/01.CCM.0000230385.82679.34. [DOI] [PubMed] [Google Scholar]
- 53.Acunas B.A., Peakman M., Liossis G., Davies E.T., Bakoleas B., Costalos C., et al. Effect of fresh frozen plasma and gammaglobulin on humoral immunity in neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 1994;70(3):F182–F187. doi: 10.1136/fn.70.3.f182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen J., Carlet J. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International Sepsis Trial Study Group. Crit Care Med. 1996;24(9):1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Capasso L., Borrelli A., Cerullo J., Pisanti R., Figliuolo C., Izzo F., et al. Role of immunoglobulins in neonatal sepsis. Translation Med @ UniSa. 2015;11:28–33. [PMC free article] [PubMed] [Google Scholar]
- 56.Haque K.N., Zaidi M.H., Bahakim H. IgM-enriched intravenous immunoglobulin therapy in neonatal sepsis. Am J Dis Child. 1960;142(12):1293–1296. doi: 10.1001/archpedi.1988.02150120047038. [DOI] [PubMed] [Google Scholar]
- 57.Gogos C.A., Drosou E., Bassaris H.P., Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181(1):176–180. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 58.Függer R., Zadrobilek E., Götzinger P., Klimann S., Rogy M., Winkler S., et al. Perioperative TNF alpha and IL-6 concentrations correlate with septic state, organ function, and Apache II scores in intra-abdominal infection. Eur J Surg= Acta Chirurgica. 1993;159(10):525–529. [PubMed] [Google Scholar]
- 59.Eichacker P.Q., Parent C., Kalil A., Esposito C., Cui X., Banks S.M., et al. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med. 2002;166(9):1197–1205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]
- 60.Parrillo J.E., Parker M.M., Natanson C., Suffredini A.F., Danner R.L., Cunnion R.E., et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113(3):227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 61.Opal S.M., Garber G.E., LaRosa S.P., Maki D.G., Freebairn R.C., Kinasewitz G.T., et al. Systemic host responses in severe sepsis analyzed by causative microorganism and treatment effects of drotrecogin alfa (activated) Clin Infect Dis : Off Publ Infect Dis Soc Am. 2003;37(1):50–58. doi: 10.1086/375593. [DOI] [PubMed] [Google Scholar]
- 62.Ramachandran G. Gram-positive and gram-negative bacterial toxins in sepsis: a brief review. Virulence. 2014;5(1):213–218. doi: 10.4161/viru.27024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gründling A., Schneewind O. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proc Natl Acad Sci USA. 2007;104(20):8478–8483. doi: 10.1073/pnas.0701821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kimbrell M.R., Warshakoon H., Cromer J.R., Malladi S., Hood J.D., Balakrishna R., et al. Comparison of the immunostimulatory and proinflammatory activities of candidate Gram-positive endotoxins, lipoteichoic acid, peptidoglycan, and lipopeptides, in murine and human cells. Immunol Lett. 2008;118(2):132–141. doi: 10.1016/j.imlet.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziegler E.J., McCutchan J.A., Fierer J., Glauser M.P., Sadoff J.C., Douglas H., et al. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- 66.Endo S., Inada K., Yamada Y., Takakuwa T., Nakae H., Kasai T., et al. The effects of immune globulin on endotoxemia. Clin Therapeut. 1992;14(6):781–784. [PubMed] [Google Scholar]
- 67.Frank D.W. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol. 1997;26(4):621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- 68.Haitsma J.J., Uhlig S., Göggel R., Verbrugge S.J., Lachmann U., Lachmann B. Ventilator-induced lung injury leads to loss of alveolar and systemic compartmentalization of tumor necrosis factor-alpha. Intensive Care Med. 2000;26(10):1515–1522. doi: 10.1007/s001340000648. [DOI] [PubMed] [Google Scholar]
- 69.Schaumann W., Kaufmann B., Neubert P., Smolarz A. Kinetics of the Fab fragments of digoxin antibodies and of bound digoxin in patients with severe digoxin intoxication. Eur J Clin Pharmacol. 1986;30(5):527–533. doi: 10.1007/BF00542410. [DOI] [PubMed] [Google Scholar]
- 70.Derkx B., Wittes J., McCloskey R. Randomized, placebo-controlled trial of HA-1A, a human monoclonal antibody to endotoxin, in children with meningococcal septic shock. Eur Pediatr Meningococ Septic Shock Trial Stud Group. Clin Infect Dis : Off Publ Infect Dis Soc Am. 1999;28(4):770–777. doi: 10.1086/515184. [DOI] [PubMed] [Google Scholar]
- 71.Goldstein B., Giroir B., Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med : J Soc Critic Care Med World Federat Pediatr Intens Critic Care Soc. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 72.Nelson P.N., Reynolds G.M., Waldron E.E., Ward E., Giannopoulos K., Murray P.G. Monoclonal antibodies. Mol Pathol : Materialprüfung. 2000;53(3):111–117. doi: 10.1136/mp.53.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norrby-Teglund A., Haque K.N., Hammarström L. Intravenous polyclonal IgM-enriched immunoglobulin therapy in sepsis: a review of clinical efficacy in relation to microbiological aetiology and severity of sepsis. J Intern Med. 2006;260(6):509–516. doi: 10.1111/j.1365-2796.2006.01726.x. [DOI] [PubMed] [Google Scholar]
- 74.Olas K., Butterweck H., Teschner W., Schwarz H.P., Reipert B. Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin Exp Immunol. 2005;140(3):478–490. doi: 10.1111/j.1365-2249.2005.02779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitrevski M., Marrapodi R., Camponeschi A., Cavaliere F.M., Lazzeri C., Todi L., et al. Intravenous immunoglobulin and immunomodulation of B-cell - in vitro and in vivo effects. Front Immunol. 2015;6:4. doi: 10.3389/fimmu.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boonsopa C. Comparison of adjunctive treatment with IgMEnriched IVIG and antibiotics alone in treatment of neonatal sepsis. Siriraj Med J. 2021;72:84–91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.