Abstract
The recalcitrant, fibrous protein keratin is found in the outermost layer of vertebrate skin, feathers, hair, horn, and hooves. Approximately, 10 million tons of keratin wastes are produced annually worldwide, of which around 8.5 million tons are from feather wastes. The biodegradation of keratin has been a challenge due to the lack of understanding of biological parameters that modulate the process. Few soil-borne microbes are capable of producing keratinase enzyme which has the potential to degrade the hard keratin. However, various pesticides are abundantly used for the management of poultry farms and reports suggest the presence of the pesticide residues in feather. Hence, it was hypothesized that pesticides would interact with the substrate-binding or allosteric sites of the keratinase enzyme and interferes with the keratin-degradation process. In the present study, molecular interactions of 20 selected pesticides with the keratinase enzyme were analyzed by performing molecular docking. In blind docking, 14 out of 20 pesticides showed higher inhibitory potential than the known inhibitor phenylmethylsulfonyl flouride, all of which exhibited higher inhibitory potential in site-specific docking. The stability and strength of the protein complexes formed by the top best potential pesticides namely fluralaner, teflubenzuron, cyhalothrin, and cyfluthrin has been further validated by molecular dynamic simulation studies. The present study is the first report for the preliminary investigation of the keratinase-inhibitory potential of pesticides and highlights the plausible role of these pesticides in hindering the biological process of keratin degradation and thereby their contribution in environmental pollution.
Graphical abstract
Illustration depicting the hypothesis, experimental procedure, and the resultant keratinase-inhibitory potential of selected pesticides.
Keywords: Keratinase, Pesticide, Blind docking, Site-specific docking, Molecular dynamic simulation, Hydrogen bond
Introduction
Keratin is an insoluble structural protein found in the epidermis of skin, feathers, nail, hair, wool, horn and hooves of vertebrate animals. It is considered to be the third most abundant polymer after cellulose and chitin (Lange et al. 2016; Li 2021; McKittrick et al. 2012). Owing to the presence of hydrophobic interactions, hydrogen bonds, and intra/intermolecular disulfide cross-linkages, keratin is mechanically stable, recalcitrant and resistant to degradation by common proteases such as trypsin, pepsin, and papain (Lai et al. 2023). Feather waste contributes to approximately 8.5 million tons out of 10 million tons of keratin wastes generated annually around the globe, thereby leading to environmental pollution (Anbesaw 2022; Lai et al. 2023). Microorganisms are abundantly found in the soil and are capable of producing various proteases such as keratinase, which can hydrolyze the recalcitrant keratin (Yadav et al. 2022). Both bacteria and fungi have been identified as effective decomposers of keratin substrates (Nnolim et al. 2020b). Some of the microorganisms which secrete keratinase include Pseudomonas stutzeri, Bacillus licheniformis ALW1, Bacillus sp. FPF-1, Bacillus cereus HD1, Aspergillus flavus, Aphanoascus keratinophilus and Chrysosporium tropicum, Trichoderma harzianum isolate HZN12, Meiothermus taiwanensis WR-220 (Abdel-fattah et al. 2018; Bagewadi et al. 2018; Bohacz et al. 2020; Chaturvedi et al. 2014; Masood et al. 2021; Nnolim et al. 2020a; Wu et al. 2017; Yahaya et al. 2022). Most microbial keratinases are released into the extracellular matrix when keratin or keratin-containing substrates like feathers are present in the culture medium indicating that keratinase production is an inducible process (Li 2021).
In the poultry industry, various pesticides are used to control pests such as lice, ticks, and mites and protect the animals from the pest attacks (Cui et al. 2019; Kyakuwaire et al. 2019; Mahmoud and Abdel-Mohsein 2019). Traces of some of the known pesticides like fipronil, bifenthrin, cyfluthrin and cypermethrin are reported to accumulate in the poultry wastes (Corrias et al. 2021; da Silva Soares et al. 2021), and their excessive use may hinder biological processes taking place in the soil. Also, one study suggested the interaction of fungicide (pesticide), fluconazole with keratinase protein (Nwofor et al. 2022). Thus, in the present study, it was hypothesized that the occurrence of the pesticides in poultry wastes could affect the biodegradation of keratin by inhibiting/enhancing keratinase activity. Pesticides that are largely used in the poultry farms were selected to see their molecular interactions with keratinase and their role in the inhibition of keratin (feather) degradation. The 20 considered pesticides were acetamiprid, amitraz, bifenthrin, chlorpyrifos, cyfluthrin, cyhalothrin, cypermethrin, dichlorvos, fenitrothion, fluconazole, fluralaner, fipronil, imidacloprid, permethrin, pirimiphosmethyl, pyriproxyfen, phoxim, tetrachlorvinphos, teflubenzuron and thiram (da Silva Soares et al. 2021; Petersen et al. 2021; Sammarco et al. 2023; Townsend 2015; Weng et al. 2020). The study (i) performs blind and site-specific docking of these pesticides with keratinase, (ii) analyzes their molecular interactions with the enzyme and evaluates their keratinase-inhibitory potential, (iii) performs molecular dynamic simulation, and (iv) puts forward the most potential pesticide(s) with the ability to inhibit keratinase activity and hinder the biodegradation of keratin.
Methodology
Protein structure preparation
The 3D structure of keratinase from Meiothermus taiwanensisWR-220 (PDB id: 5wsl) was obtained from https://www.rcsb.org/in the.pdb format. For blind docking study, whole protein was taken into account and was loaded in PyRx and converted to.pdbqt format as a step of protein preparation (Dallakyan and Olson 2015). For site-specific docking study, the substrate binding sites i.e., the S1 site (most preferable site) of the enzyme containing the amino acid residues Ser130, Leu131, Gly132, Ala156, Ala157, Gly158, and Tyr282 including the conserved catalytic triad (Asp39, His72, and Ser224) and Ser104 and Ser105 was considered and the protein was prepared in PyMol with the above mentioned residues (Wu et al. 2017).
Ligand preparation
20 pesticides (ligands) that are mostly used in the poultry farms were selected. The 3-dimensional structures of the ligands were downloaded from NCBI PubChem compounds database (https://pubchem.ncbi.nlm.nih.gov/) in SDF (Structure-Data File) format and their SMILES notation was also obtained (Table 1). The ligand preparation was performed using the Open Babel inbuilt in PyRx interface and their SDF formats were converted to.pdbqt format. Considering previous studies, phenylmethylsulfonylflouride (PMSF) was selected as the known inhibitor of keratinase and retrieved by the above mentioned process (Banerjee et al. 2014; Kandasamy et al. 2018).
Table 1.
Chemical structure, molecular weight and PubChem CID of the ligands (pesticides) used in the study
| Sl. No. | Ligands | Chemical structure | Mol. Wt. (g/mol) | PubChem CID |
|---|---|---|---|---|
| 1. | Phenylmethylsufonlyflouride-control |  |
174.19 | 4784 |
| 2. | Acetamiprid |  |
222.67 | 213,021 |
| 3. | Amitraz | 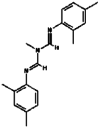 |
293.4 | 36,324 |
| 4. | Bifenthrin |  |
422.9 | 6,442,842 |
| 5. | Chlorpyrifos | 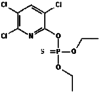 |
350.6 | 2730 |
| 6. | Cyfluthrin |  |
434.3 | 104,926 |
| 7. | Cyhalothrin |  |
449.8 | 5,281,873 |
| 8. | Cypermethrin | 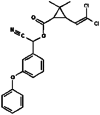 |
416.3 | 2912 |
| 9. | Dichlorvos |  |
220.97 | 3039 |
| 10. | Fenitrothion | 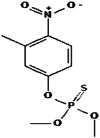 |
277.24 | 31,200 |
| 11. | Fluconazole |  |
306.27 | 3365 |
| 12. | Fluralaner | 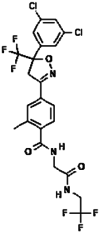 |
556.3 | 25,144,319 |
| 13. | Fipronil |  |
437.1 | 3352 |
| 14. | Imidacloprid | 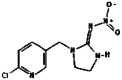 |
255.66 | 86,287,518 |
| 15. | Permethrin | 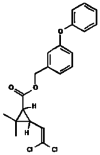 |
391.3 | 40,326 |
| 16. | Pirimiphos-methyl | 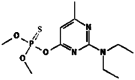 |
305.34 | 34,526 |
| 17. | Pyriproxyfen | 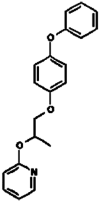 |
321.4 | 91,753 |
| 18. | Phoxim | 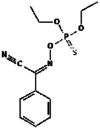 |
298.30 | 9,570,290 |
| 19. | Tetrachlorvinphos | 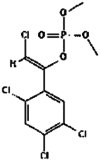 |
366.0 | 5,284,462 |
| 20. | Teflubenzuron | 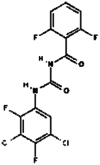 |
381.1 | 91,734 |
| 21. | Thiram | 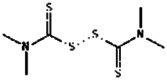 |
240.4 | 5455 |
Molecular docking
Molecular docking was performed using the AutoDockVina interface inbuilt in the PyRx platform. In blind docking, the multi-ligand–protein interactions were carried out inside a grid box with a dimension of 88.14 Å, 45.45Å and 100Å along the co-ordinates X, Y, and Z-axis respectively. Similarly, for site-specific docking, the multi-ligand–protein interactions were carried out inside the grid box having dimensions of 36.69 Å, 32.52 Å, and 52.25 Å along the co-ordinates X, Y, and Z-axis respectively (Nwofor et al. 2022; Saha et al. 2021; Trott and Olson 2012).
Visualization of ligand-receptor molecular interactions
The protein–ligand interactions were visualized using BIOVIA Discovery studio visualizer which analyzes the interactions of the ligands with keratinase showing various molecular interactions including conventional hydrogen bonds, carbon hydrogen bonds, Van der Waals interactions, Amide-pi stacked, pi–pi T shaped, alkyl, pi-alkyl, pi-sigma, pi-sulpur bonds, halogen bonds, along with visualization of the unfavorable bonds.
Molecular dynamics simulation (MD)
Molecular dynamic (MD) simulation of the top four docked pesticide-keratinase complexes, known inhibitor-keratinase complex, and free keratinase was performed on Gromacs version 2016.3. The protein was prepared by the AMBER99SB-ILDN force field and the ligands were prepared by the General Amber Force Field (GAFF) (Lindorff-Larsen et al. 2010). The system was solvated by the Transferable Intermolecular Potential with 3 Points (TIP3P) water model, centering the free protein and ligand-bound protein complexes within a 3-dimensional 2 nm side’s cubic box. Ions were added to neutralize the system and maintaining biological conditions. Energy minimization of the system was carried out by employing steepest descent algorithm with convergence threshold of force < 1000 kJ/mol (Rahimnahal et al. 2023). Electrostatic forces were computed using Partial Mesh Ewald (PME) summation, while the Van der Waals interactions were computed with a shift cut off ranging from 10.0 to 12.0 Å. The hydrogen bonds were constrained using Linear Constraint Solver (LINCS). The whole system was equilibrated to 300 K temperature using 100 ps NVT and NPT ensembles. After equilibration, MD simulation was performed for 50 nanoseconds (ns) with a resolution of 100 ps and the analysis was performed over the whole trajectory. The output of the simulation was analyzed from the Root Mean Square Deviations (RMSD) in the backbone atoms of protein, Root Mean Square Fluctuations (RMSF) in the individual amino acid residues of protein, Radius of Gyration (RG), Solvent Accessible Solvent Area (SASA), number of H-bonds formed by the ligands, and binding free energies of the complexes. The binding free energy of ligand–protein complexes was calculated by Molecular Mechanisc/Poisson-Boltzmann Surface Area (MMPBSA). The binding energy (∆G) is the summation of energies of Van der Waals forces (∆E VDW), electrostatic forces (∆E EL), contribution of polar component (∆EPB), and contribution of non-polar component (∆ESASA) (Genheden and Ryde 2015).
Results
Interaction of pesticides with keratinase
Blind docking studies
The blind docking study showed that out of 20 pesticides investigated, 14 pesticides exhibited higher binding affinities for keratinase as compared to the common protease inhibitor PMSF. The higher negative value indicates a stronger interaction, showing that ligands with higher negative values inhibited the protein with greater efficacy (Mazumder et al. 2019). PMSF showed a binding efficiency of − 5.4 kcal/mol and formed a total of 10 molecular bonds with keratinase. Amongst the pesticides, fluralaner exhibited the highest binding efficiency of − 8.3 kcal/mol and formed a total of 27 bonds with keratinase. The second highest binding affinity was shown by Teflubenzuron with a docking score of − 7.8 kcal/mol and formed total 14 molecular interactions with the enzyme. Fipronil showed the third highest binding efficiency of − 7.3 kcal/mol and formed a total of 12 interactions with keratinase, followed by cyfluthrin which showed a binding efficiency of − 6.9 kcal/mol and formed 13 interactions with keratinase. The docking scores and details of molecular interactions formed by all 14 pesticides are given in Table 2 and the 3D and 2D pictures of molecular interactions of four best docked pesticide-keratinase complexes are shown in Fig. 1.
Table 2.
Various interactions found in keratinase pesticides molecular docking studies (blind docking)
| Ligands | Conventional hydrogen bond | Carbon hydrogen bond | Vander waals interactions | Pi interactions | Halogen (flourine) | Others | Binding affinity (kcal/mol) |
|---|---|---|---|---|---|---|---|
| 1. PMSF |
B chain Ser160 Arg162 C chain Ala1 |
– |
B chain Gly133, Gly134 C chain Tyr26, Ser27 and Tyr282 |
B chain Phe167 (pi-sulpur) Phe167 (pi–pi) |
– | – | − 5.4 |
| 2. Acetamiprid |
C chain Thr2 |
B chain Ala135 |
B chain Ser137, Arg171 and Ala135 C chain Thr2, Asp14, Gly15, Ser21, Thr23, Thr25 |
– | – |
C chain Asp14 |
− 4.9 |
| 3. Amitraz | – |
B chain Asn159 Ser160 |
B chain Ser102, Gly133, Gly134 C chain Ala1, Thr25, Ser27, Asn28, Tyr282 |
B chain Phe167 (pi–pi) C chain Leu281 (pi-alkyl) |
– | C chain Asn280 (unfavourable donor–donor) | − 6.7 |
| 4. Bifenthrin | – |
A chain Ser108 B chain Thr258 |
A chain Gly109, Ile111, Ala112, Thr141 B chain Arg16, Gly255, Ala257, Ser259 |
Ala138 (pi-alkyl) | – | – | − 6.3 |
| 5. Chlorpyrifos |
C chain Gly132 |
– |
C chain Gly103, Gly133, Ala156, Ala157, Gly158, Asn159, Thr223, Ser224 |
C chain His72, Ala170 Phe167, Leu99, Leu131 (pi-alkyl) Gly132 (Pi-amide) |
– | – | − 5.4 |
| 6. Cyfluthrin |
A chain Arg-171 B chain Thr23 |
– |
A chain Gly134, Ser137, Gly166 B chain Thr2, Asp14, Gly15, Pro19, Ser21, Thr25 |
A chain Phe167 (pi-alkyl) A chain Ala135 (pi-sigma) |
B chain Asp14 |
– | − 6.9 |
| 7. Cyhalothrin |
C chain Asp159, Ser224 |
Gly158 |
C chain Leu99, Thr40, Gly103, Asn70, Leu131, Ala156, Leu139, Asn159, Gly132, Gly133, Asn192, Phe167, Ser160 |
C chain His72 (Pi- cation) C chain Phe167 (pi-sigma) |
– | – | − 6.6 |
| 8. Cypermethrin |
C chain Asn159, Ser224 |
C chain Gly158, |
C chain Thr40, Asn70, Leu99, Gly103, Leu131, Gly132, Gly133, Ser160 |
C chain His72 (Pi- cation) Phe167 (pi-sigma) |
– | – | − 6.8 |
| 9. Dichlorvos |
A chain Cys101 Ser102 |
– |
A chain- Ile60, Asn100, Asp62 B chain- Tyr237, Thr248, Ala244, Ser243, Asn241 |
– | – | – | − 4.1 |
| 10. Fenitrothion |
B chain Asn100 C chain Tyr237, Asn241, Ser245, Thr248 |
– |
B chain Ile60, Asp62, Cys101, Ser102 C chain Ser243, |
C chain Ala244 (pi-alkyl) |
– | – | − 5.6 |
| 11. Fluconazole |
B chain Ser160 C chain Ala1, Ser27 |
B chain Gly134 |
B chain Gly133, Gly134, Asn159 C chain Thr25, Tyr26, Asn280, Tyr282, |
B chain Phe167 (pi–pi) |
– | – | − 6.7 |
| 12. Fluralaner |
B chain Ala59 Cys101 Asp62 C chain Asn241, Asn251 |
– |
B chain Gly61, Arg97, Ser102 Ser104, Ser105, Ser106 C chain Tyr237, Ser243, Ala244, Ser245, Thr248, Ala252, Gly255 |
B chain Cys101 (pi-sulpur and alkyl) |
B chain Ile 60, Asn100, Ala59 |
– | − 8.3 |
| 13. Fipronil |
A chain Arg171 B chain Ser21 |
B chain Ser21 |
A chain Thr4, Ser137, Gly166 B chain Thr23, Thr25, |
A chain Ala135 (pi-sigma) B chain Thr2 (pi-sigma) |
B chain Asp14, Gly15 |
– | − 7.3 |
| 14. Imidacloprid |
C chain Ser130, Asp159, Thr223, Ser224 |
C chain Thr40, Leu99, Gly103, Leu131, Gly132, Ala156, Gly158 |
His72 (pi-alkyl) | – | His72 (unfavourable positive-positive) | − 6.2 | |
| 15. Permethrin |
A chain Arg171 |
A chain Ser137 B chain Thr2, Asp14, Gly15, Leu20, Ser21, Thr23, Thr25 |
A chain Ala135 (pi-alkyl) B chain Pro19 (pi-alkyl) |
– | – | − 6.3 | |
| 16. Pirimiphos-methyl | – | – |
B chain Gly134, Gly133, Phe167, Ser160 C chain Tyr25, Try26, Ser27, Asn159, Asn180, Tyr282 |
– | – |
C chain Ala1 |
− 5.1 |
| 17. Pyriproxyfen | – | – |
A chain Ser137, Gly166, Arg171 B chain Asp14, Gly15, Pro19, Ser21, Thr23, Thr25 |
A chain Ala135 B chain (pi-alkyl) Pro19 (alkyl) |
– |
B chain Thr2 (pi-donor hydrogen bond) |
− 5.6 |
| 18. Phoxim |
C chain His72, Asp159, Ser224 |
C chain Ser130 |
C chain Thr40, Leu99, Gly103, Ser130, Phe167, Ala170, Gly158, Thr223 |
C chain Leu131, Ala157 (amide-pi stacked) and Ala156 (pi-alkyl) |
– |
C chain Gly132 (Unfavourable donor-donor) |
− 6.7 |
| 19. Tetrachlorvinphos | – |
B chain Arg16, Thr258 and Ser259, |
A chain Ala138, Ser108, Ile111 B chain Thr17, Ala257 |
B chain Val261 (alkyl) |
– | – | − 4.9 |
| 20. Teflubenzuron |
B chain Ser160 C chain Ala1, Ser2 |
– |
B chain Gly133, Arg162 C chain Asn28, Asn280, Tyr282 |
B chain Phe167 (pi–pi T shaped) C chain Thr29 (pi-sigma) Ala1 (pi-alkyl) and Tyr26 (alkyl and pi-alkyl) |
– | – | − 7.8 |
| 21. Thiram | – |
A chain Asp62 |
A chain Ile60, Asp100, Ser102 B chainTyr237, Asn241, Ser243, Ala244, Thr248 |
A chain Cys101 (pi-alkyl) |
– | – | − 3.3 |
The bold signifies those ligands which showed highest binding affinities with the target protein
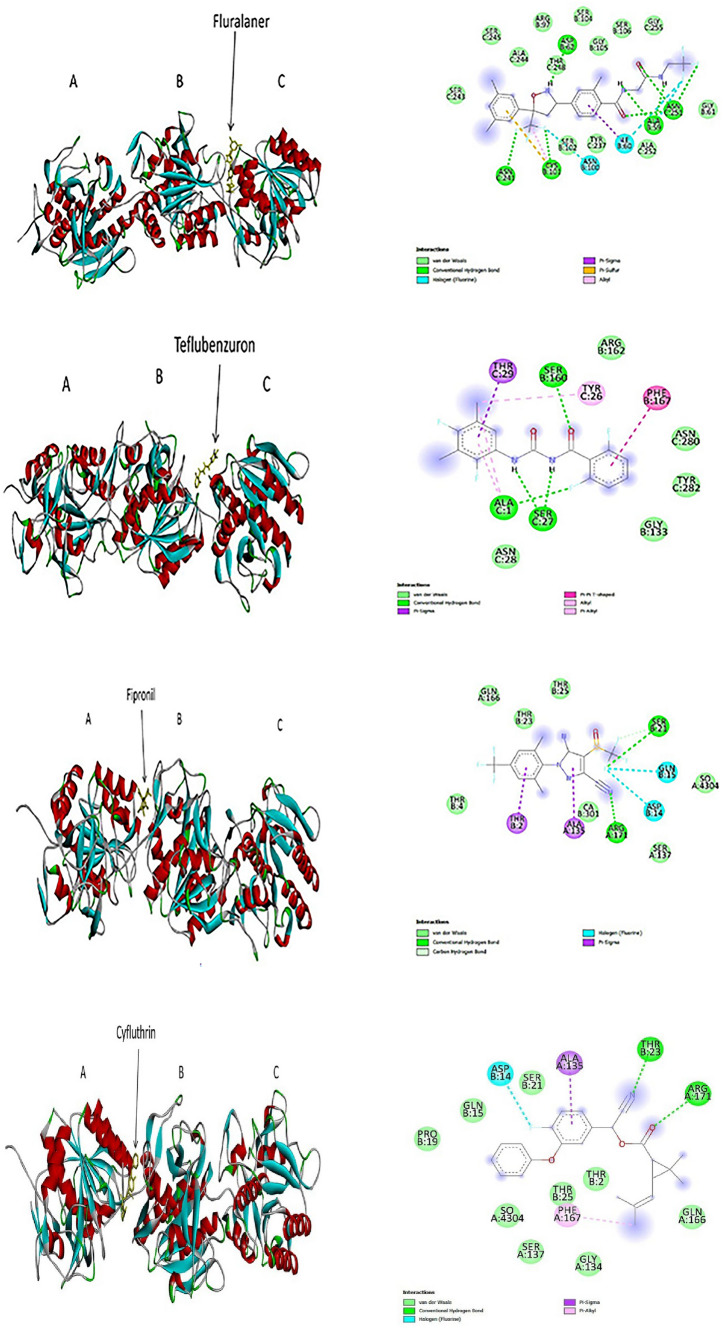
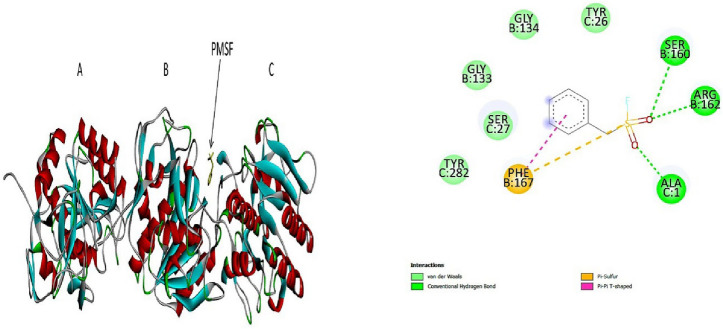
Fig. 1.
3D (left) and 2D (right) photographs of 4 best docked pesticides (fluralaner, teflubenzuron, fipronil, cyfluthrin) and the control PMSFwith keratinase enzyme in blind docking study
Site-specific docking
Out of the 20 pesticides studied in blind docking experiment, those 14 pesticides which showed binding efficiencies higher than that of the control PMSF were further proceeded for site-specific docking. The active site binding pocket was taken from literature. In this experiment, PMSF showed the binding efficiency of − 3.8 kcal/mol and formed a total of 8 molecular interactions with keratinase. Interestingly, 3 pesticides Fluralaner, cyhalothrin, and teflubenzuron showed the highest binding efficiency of − 5.2 kcal/mol and the number of bonds formed by them with the active site of keratinase were 18, 8, and 13 respectively. Cyfluthrin showed the next highest binding energy of − 5.1 kcal/mol and formed a total of 8 molecular interactions with the enzyme (Fig. 2). The docking scores and details of the molecular interactions formed by all 14 pesticides with the active site of keratinase are provided in Table 3 and the 3D and 2D pictures of the 4 best docked complexes are illustrated in Fig. 2.
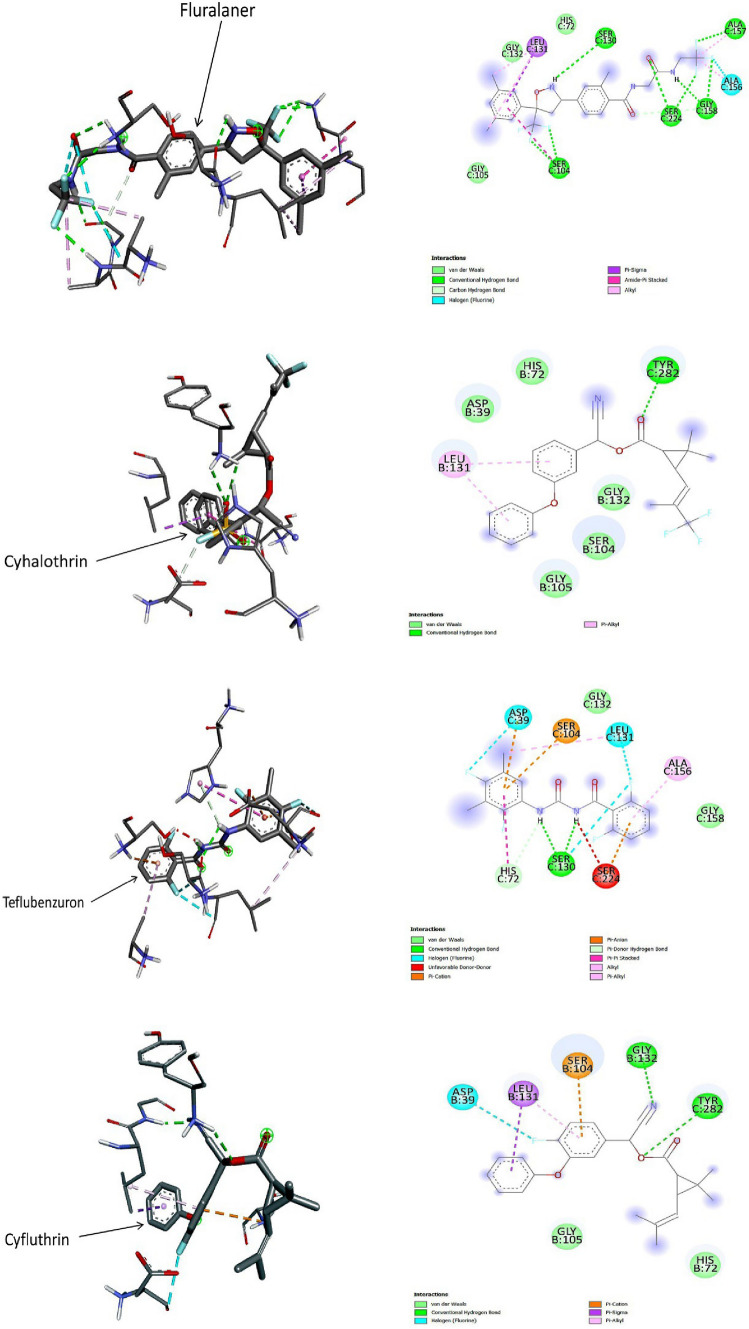
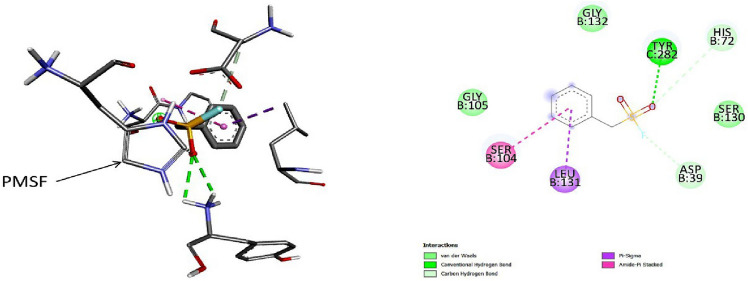
Fig. 2.
3D (left) and 2D (right) photographs of 4 best docked pesticides (fluralaner, cyhalothrin, teflubenzuron, cyfluthrin) and the control PMSF with keratinase in site-specific docking study
Table 3.
Various interactions found in keratinase pesticides molecular docking studies (site specific molecular docking)
| Ligands | Conventional hydrogen bond | Carbon hydrogen bond | vander Waals interactions | Pi-interactions | Halogen (flourine) | others | Binding energy (Kcal/mol) |
|---|---|---|---|---|---|---|---|
| 1. PMSF |
C chain Tyr282 |
B chain Asp39, His72 |
B chain Gly105, Ser130, Gly132 |
B chain Ser104 (amide pi stacked) Leu131 (pi-sigma) |
– | – | − 3.8 |
| 2. Amitraz | – | – |
B chain Gly105, Ser130, Gly132 |
B chain Ser104 (pi-cation) and Leu131 (pi-sigma and pi-alkyl) C chain Tyr282 (pi-cation) |
– |
B chain His72, Ser104 C chain Tyr282 (unfavourable positive positive bond) |
− 4.5 |
| 3. Bifenthrin |
B chain: Ser104, Gly105 |
B chain: Ser104 and Gly132 |
B chain Asp39, Leu131 |
C chain Tyr282 (pi–pi T shaped) |
– | – | − 4.7 |
| 4. Cyfluthrin |
B chain Gly132 C chain Tyr282 |
– |
B chain His72, Gly105 |
B chain Leu131 (pi-sigma and pi-alkyl) Ser104 (pi-cation) |
B chain Asp39 (flourine) |
– | − 5.1 |
| 5. Cyhalothrin |
C chain Tyr282 |
– |
B chain Asp39, His72, Ser104, Gly105, Gly132 |
B chain Leu131 (pi-alkyl) |
– | – | − 5.2 |
| 6. Cypermethrin |
B chain Gly132 C chain Tyr282 |
– |
B chain Asp39, His72, Gly105 |
B chain Ser104 (pi-cation) Leu131 (pi-alkyl and pi-sigma) |
– | – | − 4.9 |
| 7. Fenitrothion |
B chain Gly132 C chain Tyr282 |
– | B chain His72, Gly105, Ser130, Gly132 | Leu131 (alkyl and pi-alkyl) | – | – | − 3.9 |
| 8. Fluconazole |
B chain Ser104 |
– |
B chain Asp39, His72 C chain Tyr282 |
B chain Leu131 (pi-alkyl) |
B Chain Gly132 |
– | − 4.4 |
| 9. Fluralaner |
C chain Ser104, Ser130, Ser224, Ala157, Gly158 |
C chain Gly158 |
C chain His72, Gly105, Gly132 |
C chain Leu131 (pi-amide and pi-alkyl) Ser104 (amide pi-stacked) |
C chain Ala156 (flourine) |
– | − 5.2 |
| 10. Fipronil |
C chain His72, Ser224, Ser130, Gly132 |
C chain Gly105 |
– | – |
C chain Asp39, Ser104, Leu131 |
− 4.5 | |
| 11. Imidacloprid |
B chain Gly105 C chain Tyr282 |
– |
B chain Ser130, Leu131, Gly132, Ser224 |
B chain Asp39, His72 (pi-cation) |
– |
B chain Ser104 (Unfavourable positive positive) |
− 4.1 |
| 12. Permethrin |
C chain Tyr282 |
– |
B chain Asp39, His72, Ser104, Gly105, Gly132 |
B chain Leu131 (pi-alkyl |
– | – | − 4.9 |
| 13. Pyriproxyfen | – | – |
B chain His72, Ser104, Gly105, Ser130, Gly132 C chain Tyr282 |
B chain Leu131 (pi-sigma and pi-alkyl) Asp39 (pi-alkyl) |
– | – | − 4.3 |
| 14. Phoxim |
C chain Tyr282 |
– |
B chain His72, Ser104, Gly105, Gly132 |
B chain Leu131 (pi-alkyl) |
– | – | − 4.7 |
| 15. Teflubenzuron |
C chain Ser130 |
– |
C chain Gly132, Gly158 |
C chain Asp39 (pi-anion) His72 (pi–pi T shaped) Ser104, Ser224 (pi-cation) Leu131 (alkyl and pi-alkyl) |
C Chain Asp39, Ser130, Leu131 |
C chain Ser224 (unfavourable donor-donor) His72 (pi-donor hydrogen bond) |
− 5.2 |
The bold signifies those ligands which showed highest binding affinities with the target protein
Molecular weight and binding energy
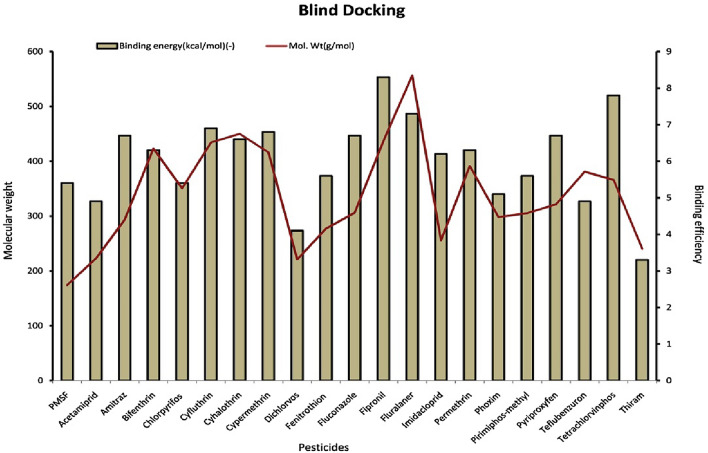
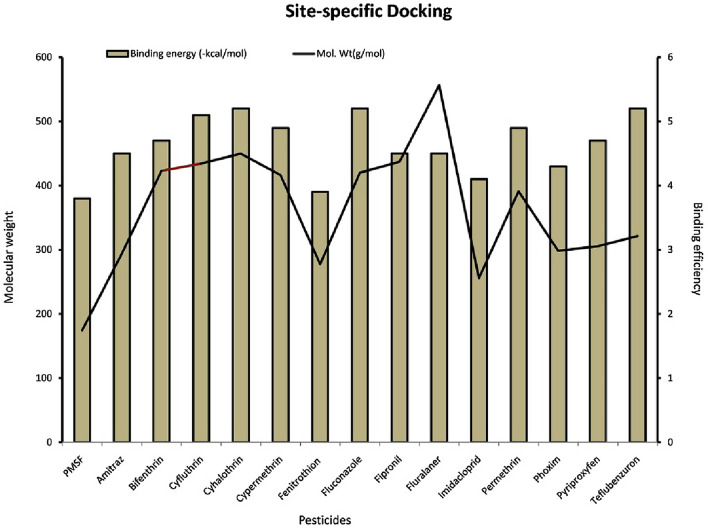
In the blind docking studies, it was found that the binding energy of the pesticides doesn’t correlate with the molecular weight of the compound except fluralaner. Other pesticides (fipronil, permethrin, cypermethrin, cyhalothrin, cyfluthrin and bifenthrin) with molecular weight ranging from (400–500 g/mol) showed lower binding energy than teflubenzuron (381.1 g/mol) which showed higher binding energy after fluralaner. The pesticide (thiram, mol. Wt-240.4 g/mol) has lowest binding energy (− 3.3 kcal/mol) while having higher molecular weight than more than PMSF (174.19 g/mol). In both blind and site specific docking, it was found that that there is no correlation between the molecular weight of pesticides and their binding energies (Figs. 3 and 4).
Fig. 3.
Graph showing the relationship between molecular weight of the pesticides and their binding effficiencies to keratinase in blind docking study
Fig. 4.
Graph showing the relationship between molecular weight of the pesticides and their binding effficiencies to keratinase in site-specific docking study
Molecular dynamic studies
Through molecular docking, four pesticides, fluralaner, teflubenzuron, cyfluthrin and cyhalothrin, are identified as having the most favourable binding interaction with the keratinase protein. In order to validate their interactions and their structural satbility with the protein, molecular dynamic simulation was performed using GroMacs software (Pawara et al. 2021).
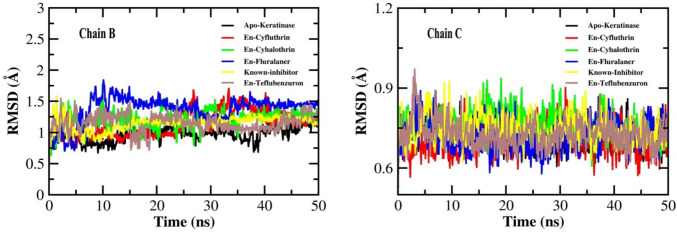
Analysis of root mean square deviation (RMSD)
The RMSD refers to the deviations in the backbone atoms (Cα) of protein from its initial (free/bound) conformation to the final (free/bound) conformation. Smaller deviations between the two states indicate more stability of the protein (free/bound). Throuhgout the 50 ns simulation, fluralaner-keratinase complex and teflubenzuron-keratinase complex showed small deviations during the initial 10 ns and 15 ns respectively, at chain B of the protein. A few deviations were also seen in cyfluthrin-keratinase complex at chain B between 25 and 50 ns, before which it maintained a stable comformation. While, no major deviations were observed in the complexes formed by cyhalotrhin, and PMSF individually, as well as in the unbound protein (Fig. 5). Also, all the ligand–protein complexes (pesticides and PMSF) and the unbound protein remained mostly stable at chain C throughout the simulation time (Fig. 5). This result indicates considerale stability of the pesticide-protein complexes mainly cyfluthrin and cyhalothrin.
Fig. 5.
The root-mean-square deviation (RMSD) plots of keratinase and the docked complexes with ligands, En- known inhibitor, En-Cyfluthrin, En-Cyhalothrin, En-Fluralaner and En-Teflubenzuron during the simulation of 50 ns
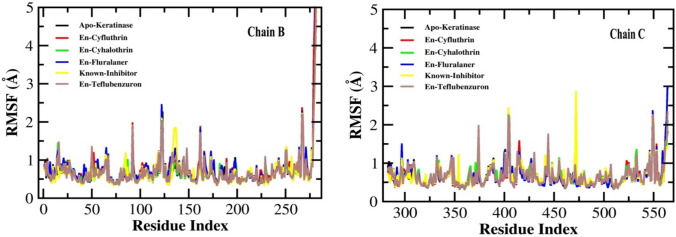
Analysis of root mean square fluctuation (RMSF)
Analysis of root mean square fluctuation (RMSF)
The RMSF value represents the flexibility and mobility of the amino acid residues of the protein when bound with the ligand during simulation. The minimal fluctuation in the RMSF value designates that the compound is packed with the binding sites of the protein (Nath et al. 2024). The Fig. 6 depicts the RMSF value of the B and C chain of the protein during the formation of protein–ligand complex. Teflubenzuron-keratinase complex showed four minor fluctuations near 95th, 125th, 160th, and 270th residues and a major fluctuation near 275th residue of chain B. While in chain C, it showed five minor fluctutions near 375th, 400th, 445th, 550th, and 555th residues. Fluralaner-keratinase complex showed three minor fluctuations near 125th, 160th, 270th residues of chain B and two minor fluctuations near 550th and 555th residues of chain C. Cyflutrhin-protein complex showed three minor fluctuations near 99th, 160th, and 270th residues and a major fluctuation near 275th residue of chain B, while it showed a smaller fluctuation near 415th residue of chain C. No considerable fluctuations were observed in cyhalothrin-protein complex. The known inhibitor PMSF however showed only one minor fluctuation near 130th residue of chain B and two such fluctuations near 400th and 475th residues of chain C. These results indicate relatively more stability of the cyhalothrin-protein and cyfluthrin-protein complexes than the other pesticides as well as PMSF.
Fig. 6.
The root mean square fluctuation (RMSF) plots of keratinase (Apo-Keratinase) and the docked complexes with ligands, En-known inhibitor complex, En-Cyfluthrin, En-Cyhalothrin, En-Fluralaner and En-Teflubenzuron during the simulation of 50 ns
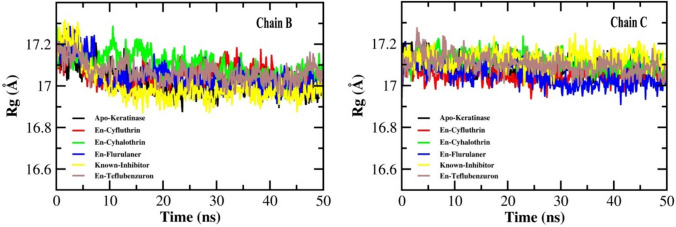
Analysis of radius of gyration (Rg)
Radius of gyration was evaluated to measure the structural compactness and robustness of the protein during the binding of the protein in a simulated condition for a duration of 50 ns. A relatively steady value of RG over the course of simulation indicates that the protein remains stably folded (Ghasemi et al. 2016). The RG values of all the pesticide-protein complexes, PMSF-protein complex, and free protein showed a slight decline from 17.2 to 17 Å for both chain B and C, and remained stable within this range for the entire simulation time (Fig. 7). This slight fluctuation in RG indicates that the designed systems did not lose their compactness over the course of simulation.
Fig. 7.
Radius of gyration (RG) plot for keratinase protein (Apo-keratinase) and the docked complexes with ligands, En-known inhibitor, En-cyfluthrin, En-fluralaner, En-teflubenzuron, and En-cyhalothrin during the simulation of 50 ns
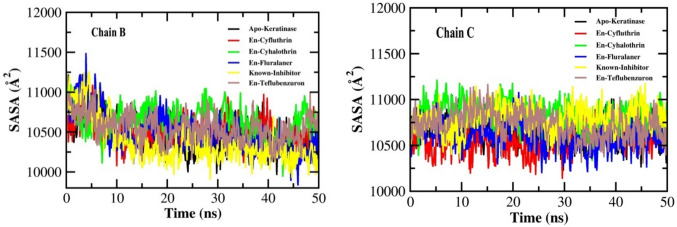
Analysis of the solvent-accessible surface area (SASA)
Another parameter to determine the structural stability and folding of a protein in the presence of solvent is the solvent accessible surface area (SASA). It refers to the surface area of protein which is available for its interaction with water molecules. The SASA was calculated during a 50 ns MD simulation to measure the protein–ligand complex's interaction with the solvent and anticipate the extent of conformational changes that occurred during the interaction (Joshi et al. 2021). During the entire course of simulation, The SASA values of all the four ligand–protein complexes (cyfluthrin, cyhalothrin, teflubenzuron, PMSF) as well as the free protein were found to range between 10,500 and 11,000 Å2 for both the chains (Fig. 8). However, the fluralaner-protein complex showed a SASA value about 115,000 Å2 at chain B for the initial 5 ns. No major change in the SASA values was observed in the protein due to ligand binding, indicating the maintenance of structural stability in the complexes.
Fig. 8.
Solvent-accessible surface area (SASA) plots for chains B and C for keratinase (Apo-Keratinase) and docked complexes with ligands En-known inhibitor, En-Cyfluthrin, En-Cyhalothrin, En-Fluralaner and En-Teflubenzuron for 50 ns simulation
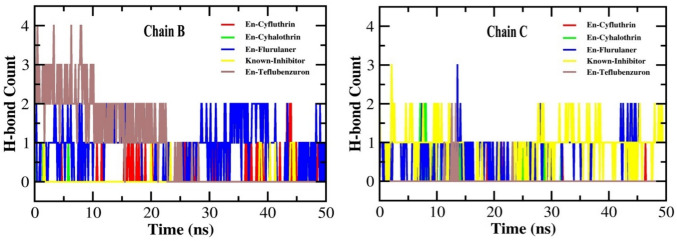
Hydrogen bond count
H-bond interaction is crucial for maintaining the structural integrity of protein and for ensuring the stable localization of the ligands around the active site of the protein (Nath et al. 2024). The analysis of H-bond formation by the pesticide-protein complexes revealed that fluralaner formed two H-bonds with both chain B throughout the course of simulation, while it formed three H-bonds with chain C during the initial 15 ns which reduced to two bonds for the rest of simulation. Teflubenzuron formed four H-bonds with chain B during the initial 10 ns which reduced to 2 and 1 for the next 20 ns, and showed no bond formation during the remaining 20 ns. With chain C, it formed only two H-bonds during the initial 15 ns. Cyfluthrin formed one H-bond with chain B for the initial 40 ns which increased to two for the remaining 10 ns, while it formed only one H-bond with chain C throughout the simulation. Cyhalothrin formed only one H-bond with chain B during the first 6 ns, while it formed two H-bonds with chain C for the initial 10 ns which reduced to one bond for the next 20 ns. The known inhibitor PMSF formed only one H-bond with chain B for the entire simulation, while it formed three bonds with chain C for the initial 2 ns which reduced to two bonds for the remaining simulation period (Fig. 9). These results indicate that the H-bonds formed by fluralaner and cyfluthrin individually with the protein remained relatively more stable than those formed by other pesticides.
Fig. 9.
Number of H-bond formation in keratinase complex with ligands En-known inhibitor, En-Cyfluthrin, En-Cyhalothrin, En-Fluralaner and En-Teflubenzuron for a simulation period of 50 ns
Binding free energy
Like molecular docking, in MD simulation also a higher negative value of binding energy denotes greater affinity of the ligands towards the protein. The MM/PBSA analysis of binding free energies of the complexes revealed that the known inhibitor PMSF showed a binding energy of − 17.66 kcal/mol, while amongst pesticides, the highest binding energy was shown by cyfluthrin (− 41.57 kcal/mol), followed by fluralaner (− 36.63 kcal/mol) and teflubenzuron (− 25.02 kcal/mol). Cyhalothrin however showed a very negligible binding energy of − 0.05 kcal/mol. This results indicate a greater bidning affinity of cyfluthrin, and fluralaner, teflubenzuron for the protein during the entire simulation course (Table 4).
Table 4.
Details of binding free energies of pesticide-keratinase docked complexes
| Ligand | ∆E VDW (kcal/mol) | ∆E EL (kcal/mol) | ∆EPB (kcal/mol) | ∆ESASA (kcal/mol) | ∆G (kcal/mol) |
|---|---|---|---|---|---|
| Cyfluthrin | − 43.18 | − 2.77 | 8.73 | − 4.35 | − 41.57 |
| Teflubenzuron | − 24.99 | − 2.23 | 4.91 | − 2.70 | − 25.02 |
| Fluralaner | − 37.10 | − 5.27 | 9.57 | − 3.83 | − 36.63 |
| Cyhalothrin | − 0.02 | 0.00 | − 0.02 | 0.00 | − 0.05 |
| PMSF | − 17.92 | − 3.40 | 5.88 | − 2.22 | − 17.66 |
Discussion
In the present work, the molecular interactions between pesticides and keratinase were studied in order to comprehend their impact on the biodegradation of keratin. The blind docking study demonstrated that 14 out of the 20 selected pesticides have favorable binding affinities for keratinase, while all the above 14 pesticides showed good binding efficiency for keratinase in the site-specific docking also, with fluralaner, cyhalothrin, cyfluthrin, and teflubenzuron considered as the best hits. The molecular dynamic simulation study of these four best hits further validated the inhibitory potential of these pesticides.
Molecular docking is considered to be a reliable computational tool to predict the binding efficiency of substrate with a receptor. The higher negative binding energy and number of molecular interactions formed during the substrate-receptor complex formation is positively correlated with the strength the formed complex (Mazumder et al. 2018). Various types of molecular interactions contribute to the ligand-receptor docking which include hydrogen bonds, Van der Waals interactions, pi–pi interactions, pi-sulphur interactions, pi-alkyl interactions, as well as fluorine bonds. These interactions participate in intercalating the ligand to the binding site of receptor, increasing the binding affinity, strengthening and stabilizing the ligand-receptor complex formed (Khan et al. 2017; Owoloye et al. 2022; Pantsar and Poso 2018). In the present study, 20 pesticides were tested for blind docking with keratinase enzyme out of which 14 pesticides (namely, amitraz, bifenthrin, chlorpyrifos, cyfluthrin, cyhalothrin, cypermethrin, fenitrothion, fluconazole, fluralaner, fipronil, imidacloprid, pyriproxyfen, phoxim, and teflubenzuron) showed binding efficiency to keratinase higher than that of the control PMSF. In blind docking, the pesticides were found to bind mostly in the B and C chains of the keratinase and interact with various amino acids not particular to the active sites or substrate binding site residues. PMSF is an irreversible inhibitor of keratinase enzyme whose binding efficiency to the enzyme isolated from Bacillus sp. was found to be − 4.41 kcal/mol (Banerjee et al. 2014). In our study, the binding efficiency of control PMSF was found to be-5.4 kcal/mol and it formed 2 conventional H-bonds (Ser160 and Arg162) at chain B and 1 conventional H-bond (Ala1) at chain C, 2 Van der Waals interactions (Gly133 and Gly134) at chain and 3 Van der Waals forces (Tyr26, Ser27, Tyr282) at chain C, 1 pi-sulphur bond (Phe167) at chain B, and 1 pi–pi interaction (Phe167) at chain B of keratinase. Fluralaner exhibited the best binding with keratinase with a binding efficiency of − 8.3 kcal/mol and formed 4 conventional H-bonds (Ala59, Cys101, Asp62) at chain B and 3 conventional H-bonds (Asn251 and Asn241) of chain C, 6 Van der Waals interactions (Gly61, Arg97, Ser102, Ser104, Ser105, Ser106) at chain B and 7 such bonds (Tyr237, Ser243, Ala244, Ser245, Thr248, Ala252, Gly255) at chain C, 1 pi-sulphur bond (Cys101) at B chain, 1 alkyl bond (Cys101) at B chain, 1 pi-sigma bond (Ile60) at B chain, and 3 fluorine bonds (Ile60, Asn100, Asn100) at B chain of with the enzyme. The second best binding efficiency was shown by teflubenzuron which exhibited a binding energy of − 7.8 kcal/mol and formed 1 conventional H-bond (Ser160) at B chain and 3 such bonds (Ser27, Ala1) at chain C, 2 Van der Waals interactions (Gly133, Arg162) at B chain and 3 such bonds (Asn28, Tyr282, Asn280) at C chain, 1 alkyl (Tyr26) at C chain, 2 pi-alkyl bonds (Ala1, Tyr26) at C chain, 1 pi–pi T shaped bond (Phe167) at B chain and 1 pi-sigma bond (Thr29) at chain C of the enzyme. Following teflubenzuron, fipronil showed a binding efficiency of − 7.3 kcal/mol and formed 2 conventional H-bonds (one each) with Arg171 and Ser21 of A and B chains respectively, 1 carbon-hydrogen bond (Ser21) of B chain, 2 Van der Waals interactions (Ser137, Gln166) at chain A and 3 such bonds (Thr4, Thr27, Thr25) at B chain, 2 fluorine bonds (Asp14 at A chain and Gln15 at B chain), and 2 pi-sigma bonds (Ala135 at A chain and Thr2 at B chain) with keratinase. Cyfluthrin showed a bonding efficiency of − 6.9 kcal/mol and formed 2 conventional H-bonds (1 each) with the Arg171 and Thr23 of A and B chains respectively, 8 Van der Waals interactions (Ser137, Gly134, Gln166 at A chain, and Gln15, Thr2, Thr25, Ser21, Pro19 at B chain), 1 pi-alkyl bond (Phe167 at A chain), 1 fluorine bond (Asp14 of B chain), and 1 pi-sigma bond (Ala135 of A chain) with keratinase. A previous study reported that the pesticide fluconazole showed a relatively lower binding efficiency of − 6.7 kcal/mol to the fungal keratinase (Nwofor et al. 2022). Similarly, in our study also flucanazole was found to exhibit relatively lower binding energy (− 6.7 kcal/mol) and lesser number of molecular interactions with the protein as shown in Table 2. The details of the blind docking molecular interactions of the pesticides along with their docking scores are provided in Fig. 1 and Table 2.
One study reported that the preferred active site of keratinase enzyme isolated from M. taiwanensis lies at the S1 site which includes the amino acid residues Ser130, Leu131, Gly132, Ala156, Ala157, Gly158, and Tyr282 which reside between the B and C chains of the enzyme. Notably, the study also demonstrated that the catalytic triad of the enzyme lies nearby the S1 active site and consists of Asp39, His72 and Ser224 amino acid residues (Wu et al. 2017). The study further suggested that the amino acid residues Ser104 and Ser105 also participate in the substrate-binding activity. 14 pesticides that showed highest docking score in blind docking were further tested for site-specific docking considering the above mentioned residues, out of which fluralaner, teflubenzuron, cyhalothrin and cyfluthrin showed highest binding affinities towards keratinase (Table 3). In site specific docking, the PMSF showed binding energy − 3.8 kcal/mol and formed 1 conventional H-bond (Tyr282 of C chain), 2 carbon-hydrogen bonds (Asp39 and His72 of B chain), 1 pi-sigma bond with Leu131 of B chain, 1 pi-amide stacked bond with Ser104 of B chain, and 3 Van der Waals interactions (Gly105, Ser130 and Gly132 of B chain). Fluralaner, teflubenzuron and cyhalothrin showed second highest binding energy of − 5.2 kcal/mol. Fluralaner formed 8 conventional H-bonds (Ser104, Ser130, Ala157, Gly158, Ser224 of C chain), 1 carbon-hydrogen bond (Ala158), 3 Van der Waals interactions (His72, Gly105, Gly132) at chain C, 1 amide-pi stacked bond (Ser104 of C chain), 1 pi-sigma bond (Leu131), 3 alkyl bonds (Leu131, Ala156, Ala157), and 1 halogen bond (Ala156) of C chain. Teflubenzuron formed 2 conventional H-bond with Ser130, 2 Van der Waals forces (Gly132, Gly158), 1 pi-anion bond (Asp39), 1 pi–pi stacked bond (His72), 2 pi-cation bonds (Ser104, Ser224), 2 alkyl bonds (Leu131, Ala156) and 3 halogen bonds (Leu131, Ser130, Asp39) of C chain. Cyhalothrin formed 2 pi-alkyl bonds with Leu131 of B chain, 5 Van der Waals interactions with Asp39, His72, Ser104, Gly105, Gly132 of B chain along with 1 conventional H-bond with Tyr282 of C chain. Cyfluthrin formed 2 Van der Waals interaction with His72 and Gly105 of B chain, 1 halogen bond with Asp39 of B chain, and 2 conventional H-bonds with Gly132 of B chain, and 1 conventional H-bondTyr282 of C chain, 1 pi-cation bond with Ser104 of B chain, and 1 pi-sigma and 1 pi-alkyl bond with Leu131 of B chain. The details of the site-specific molecular interactions of the pesticides along with their docking scores are provided in Fig. 2 and Table 3.
A previous study showed a negative correlation between the molecular weight of the ligands and their corresponding docking score (Mazumder et al. 2019), however, in our study no such correlation was found except for fluralaner whose molecular weight and docking score was found to be positively correlated. Such contradicting results warrant further extensive studies in this domain to unveil the mechanism behind any relation between the molecular weight and binding affinity of ligands.
Interestingly, from the above discussion it can be traced out that fluralaner, teflubenzuron, and cyfluthrin which exhibited highest binding efficiency and higher number of molecular interactions with keratinase in the blind docking experiment also showed similar result in the site-specific docking. However, fipronil though showed a highly favourable binding efficiency in blind docking study, did not appear to be a potential substrate for keratinase in the site-specific docking. On the other hand, cyhalothrin which did not show a much higher binding score in blind docking, was one of the top 3 pesticides with highest binding affinity in site-specific docking. The 3D and 2D docked (blind and site-specific) pictures of these pesticides are illustrated in Figs. 1 and 2. Higher binding efficiencies of these pesticides to or within the active site of keratinase as compared to the known inhibitor of keratinase PMSF indicate their plausible effect in modulating the structural conformation of the enzyme, thereby blocking the keratin-keratinase binding phenomenon. Further, to validate the strength and stability of these docked complexes, the four best hit complexes were exposed to MD simulation.
MD simulation is an advanced approach to determine the binding, interaction, stability, and strength of docked ligand–protein complexes in a simulated environment which is more or less similar to a biological system. The RG and SASA values of all the protein complexes formed by pesticides and PMSF, as well as for the unbound protein suggest their considerable stability throughout the simulation. The RMSD and RMSF values revealed the higher stability of cyfluthrin-keratinase and cyhalothrin-keratinase complexes, while more number of stable H-bonds were also formed by cyfluthrin and fluralaner. Interestingly, cyfluthrin and fluralaner showed the lowest binding free energy and thus highest binding affinity towards the protein.
Together with the findings of molecular docking and the molecular dynamic simulation, the pesticides fluralaner and cyfluthrin exhibited best binding and interaction with the active site of keratinase with higher stability and strength. Our study thus presents fluralaner and cyfluthrin as two most potential keratinase, however, the potential of the other two pesticides teflubenzuron and cyhalothrin should not be neglected.
Conclusion
Environmental pollution is a great havoc to human health which can lead to various disease and ailments. Huge amount of poultry feathers get dumped to the soil nearby poultry farms and the biological degradation of the primary constituent of feather i.e. keratin is extremely necessary to avoid feather accumulation, soil pollution, and the consequent health issues. Soil microbes are capable of producing keratinase enzyme which responsible for the degradation of keratin. However, excessive use of pesticides in the poultry farms in order to protect the poultry from pest attacks may hinder with the keratin-degradation activity of keratinase enzyme. Proceeding with this hypothesis, the present study investigated the keratinase-inhibitory potential of 20 selected pesticides that are largely used in poultry farms through computational approach. Screened through both blind and site-specific docking analysis, Fluralaner, teflubenzuron, cyhalothrin, and cyfluthrin appeared to be the most potential inhibitors of keratinase, while fluralaner and cyfluthrin emerged as the two most potential inhibitors from MD simulation study. Therefore, the present study puts forward a preliminary investigation of numerous pesticides and their demonstration as potent inhibitors of keratinase enzyme, thereby elucidating their role in hindering the keratin-degradation process in the soil and hence their contribution to environmental pollution. The study is first of its kind to identify the most potential keratinase inhibitors and directs towards their minimal use as pesticides in farms.
Acknowledgements
We acknowledged late Prof. Sanjeev Kumar for his valuable guidance and conceptualization of the present work.
Author contributions
I.G.—Conceptualization, experimentation, data analysis, data interpretation, data validation and writing the original draft, R.R.—Writing and editing, figure preparation, A.S.—Molecular Dynamic Simulation, B.C.P.—Writing and editing, S.K.—Conceptualization, D.K.—Review and editing, P.P.—Review and editing, P.B.—Reviewing and editing, and A.B.—Conceptualization, review and editing.
Funding
No financial support received.
Data availability
All dataset generated or analyzed during this study are included in the manuscript and are available with the corresponding author, Indira Gahatraj for any further correspondence.
Declarations
Conflict of interest
Authors disclosed no conflict of interest.
Consent to publish
The manuscript is original and has not submitted in any other journals. The copyright of the manuscript is authorized by the authors to the journal upon acceptance for publication.
Ethical approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Indira Gahatraj, Email: indiragahatraj55@gmail.com.
Anupom Borah, Email: anupomborahh@gmail.com.
References
- Abdel-Fattah AM, El-Gamal MS, Ismail SA, Emran MA, Hashem AM. Journal of Genetic Engineering and Biotechnology Biodegradation of feather waste by keratinase produced from newly isolated Bacillus licheniformis ALW1. J Genet Eng Biotechnol. 2018;16(2):311–318. doi: 10.1016/j.jgeb.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anbesaw MS. Bioconversion of keratin wastes using keratinolytic microorganisms to generate value-added products. Int J Biomater. 2022;2022:1–24. doi: 10.1155/2022/2048031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagewadi ZK, Mulla SI, Ninnekar HZ. Response surface methodology based optimization of keratinase production from Trichoderma harzianum isolate HZN12 using chicken feather waste and its application in dehairing of hide. J Environ Chem Eng. 2018;6(4):4828–4839. doi: 10.1016/j.jece.2018.07.007. [DOI] [Google Scholar]
- Banerjee A, Sahoo DK, Thatoi H, Pati BR, Mondal KC, Sen A, Mohapatra PK, Das. Structural characterization and active site prediction of bacterial keratinase through molecular docking. J Bioinform. 2014;1(November):67–82. [Google Scholar]
- Bohacz J, Korni T, Kitowski I, Ciesielska A (2020) International Biodeterioration & Biodegradation Degradation of chicken feathers by Aphanoascus keratinophilus and Chrysosporium tropicum strains from pellets of predatory birds and its practical aspect, 151(April). 10.1016/j.ibiod.2020.104968
- Chaturvedi V, Bhange K, Bhatt R, Verma P. Production of kertinases using chicken feathers as substrate by a novel multifunctional strain of Pseudomonas stutzeri and its dehairing application. Biocatal Agric Biotechnol. 2014;3(2):167–174. doi: 10.1016/j.bcab.2013.08.005. [DOI] [Google Scholar]
- Corrias F, Atzei A, Taddeo R, Arru N, Casula M, Salghi R, Russo M, Angioni A. Fipronil and fipronil sulfone distribution in chicken feathers and eggs after oral and dermal exposure. Foods. 2021;10(12):1–15. doi: 10.3390/foods10123077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B, Wang F, Wang LDL, Pan C, Ke J, Tian Y. A comparative analysis of risk perception and coping behaviors among chinese poultry farmers regarding human and poultry infection with avian influenza. Int J Environ Res Public Health. 2019;16(20):1–13. doi: 10.3390/ijerph16203832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Soares CEC, Scussel VMV, Dahlke FF (2021) Pyrethroid and residues in chickens and poultry litter, July, 145–166. 10.1007/978-3-030-54712-7_4
- Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Methods Mol Biol. 2015;1263(January 2015):243–250. doi: 10.1007/978-1-4939-2269-7_19. [DOI] [PubMed] [Google Scholar]
- Genheden S, Ryde U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov. 2015;10(5):449–461. doi: 10.1517/17460441.2015.1032936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi F, Zomorodipour A, Karkhane AA, Khorramizadeh MR. In silico designing of hyper-glycosylated analogs for the human coagulation factor IX. J Mol Graph Model. 2016;68:39–47. doi: 10.1016/j.jmgm.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Joshi T, Joshi T, Sharma P, Chandra S, Pande V. Molecular docking and molecular dynamics simulation approach to screen natural compounds for inhibition of Xanthomonas oryzae pv. Oryzae by targeting peptide deformylase. J Biomolecul Struct Dyn. 2021;39(3):823–840. doi: 10.1080/07391102.2020.1719200. [DOI] [PubMed] [Google Scholar]
- Kandasamy S, Duraisamy S, Chinnappan S, Balakrishnan S, Thangasamy S, Muthusamy G, Arumugam S, Palanisamy S. Molecular modeling and docking of protease from Bacillus sp. for the keratin degradation. Biocatal Agric Biotechnol. 2018;13(December 2017):95–104. doi: 10.1016/j.bcab.2017.11.016. [DOI] [Google Scholar]
- Khan AM, Shawon J, Halim MA. Multiple receptor conformers based molecular docking study of fluorine enhanced ethionamide with mycobacterium enoyl ACP reductase (InhA) graphical abstract N1-InhA. J Mol Graph Model. 2017 doi: 10.1016/j.jmgm.2017.09.010. [DOI] [PubMed] [Google Scholar]
- Kyakuwaire M, Olupot G, Amoding A, Nkedi-Kizza P, Basamba TA. How safe is chicken litter for land application as an organic fertilizer? A review. Int J Environ Res Public Health. 2019 doi: 10.3390/ijerph16193521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Wu X, Zheng X, Li W, Wang L. Insights into the keratin efficient degradation mechanism mediated by Bacillus sp. CN2 based on integrating functional degradomics. Biotechnol Biofuels Bioprod. 2023;16(1):1–14. doi: 10.1186/s13068-023-02308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange L, Huang Y, Busk PK. Microbial decomposition of keratin in nature—a new hypothesis of industrial relevance. Appl Microbiol Biotechnol. 2016;100(5):2083–2096. doi: 10.1007/s00253-015-7262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. Structure, application, and biochemistry of microbial keratinases. Front Microbiol. 2021;12(June):1–14. doi: 10.3389/fmicb.2021.674345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindorff-Larsen K, Piana S, Palmo K, Maragakis P, Klepeis JL, Dror RO, Shaw DE. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Prot Struct Funct Bioinform. 2010;78(8):1950–1958. doi: 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud MAM, Abdel-Mohsein HS. Hysterical tetracycline in intensive poultry farms accountable for substantial gene resistance, health and ecological risk in Egypt-manure and fish. Environ Pollut. 2019;255:113039. doi: 10.1016/j.envpol.2019.113039. [DOI] [PubMed] [Google Scholar]
- Masood S, Hussain A, Javid A, Bukahri SM, Ali W, Ali S, Ghaffar I, Imtiaz A, Amin HMA, Salahuddin H, Inayat M, Razzaq S, Kafayat F, Rafiq H, Yasmeen M, Muneeb M, Sattar S. Fungal decomposition of chicken-feather waste in submerged and solid-state fermentation. Braz J Biol. 2021;83:1–8. doi: 10.1590/1519-6984.246389. [DOI] [PubMed] [Google Scholar]
- Mazumder MK, Paul R, Phukan BC, Dutta A, Chakrabarty J, Bhattacharya P, Borah A. Garcinol, an effective monoamine oxidase-B inhibitor for the treatment of Parkinson’s disease. Med Hypoth. 2018;117(April):54–58. doi: 10.1016/j.mehy.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Mazumder MK, Choudhury S, Borah A. An in silico investigation on the inhibitory potential of the constituents of Pomegranate juice on antioxidant defense mechanism: relevance to neurodegenerative diseases. IBRO Rep. 2019;6(December 2018):153–159. doi: 10.1016/j.ibror.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKittrick J, Chen PY, Bodde SG, Yang W, Novitskaya EE, Meyers MA. The structure, functions, and mechanical properties of keratin. Jom. 2012;64(4):449–468. doi: 10.1007/s11837-012-0302-8. [DOI] [Google Scholar]
- Nath M, Bhowmik D, Saha S, Nandi R, Kumar D. Identification of potential inhibitor against Leishmania donovani mitochondrial DNA primase through in-silico and in vitro drug repurposing approaches. Sci Rep. 2024;14(1):1–20. doi: 10.1038/s41598-024-53316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnolim NE, Okoh AI, Nwodo UU. Bacillus sp. FPF-1 produced keratinase with high potential for chicken feather degradation. Molecules. 2020;25(7):1–16. doi: 10.3390/molecules25071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnolim NE, Udenigwe CC, Okoh AI, Nwodo UU (2020b) Microbial keratinase: next generation green catalyst and prospective applications, 11(December). 10.3389/fmicb.2020.580164 [DOI] [PMC free article] [PubMed]
- Nwofor CN, Duru CE, Onyenwe NE. Computational identification of small molecules in Mitracarpus scaber ethanolic leaf extract with fungal keratinase inhibitory potentials. J Nat Pesticide Res. 2022;2(May):100010. doi: 10.1016/j.napere.2022.100010. [DOI] [Google Scholar]
- Owoloye AJ, Ligali FC, Enejoh OA, Musa AZ, Aina O, Idowu ET, Oyebola KM. Molecular docking, simulation and binding free energy analysis of small molecules as Pf HT1 inhibitors. PLoS ONE. 2022;17(8 July):1–18. doi: 10.1371/journal.pone.0268269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantsar T, Poso A (2018) Binding affinity via docking : fact and fiction, 1–11. 10.3390/molecules23081899 [DOI] [PMC free article] [PubMed]
- Pawara R, Ahmad I, Surana S, Patel H. Computational identification of 2,4-disubstituted amino-pyrimidines as L858R/T790M-EGFR double mutant inhibitors using pharmacophore mapping, molecular docking, binding free energy calculation, DFT study and molecular dynamic simulation. In Silico Pharmacol. 2021 doi: 10.1007/s40203-021-00113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen I, Johannhörster K, Pagot E, Escribano D, Zschiesche E, Temple D, Thomas E. Assessment of fluralaner as a treatment in controlling Dermanyssus gallinae infestation on commercial layer farms and the potential for resulting benefits of improved bird welfare and productivity. Parasit Vectors. 2021;14(1):1–10. doi: 10.1186/s13071-021-04685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimnahal S, Meimandipour A, Fayazi J, Asghar Karkhane A, Shamsara M, Beigi Nassiri M, Mirzaei H, Hamblin MR, Tarrahimofrad H, Bakherad H, Zamani J, Mohammadi Y. Biochemical and molecular characterization of novel keratinolytic protease from Bacillus licheniformis (KRLr1) Front Microbiol. 2023;14(May):1–24. doi: 10.3389/fmicb.2023.1132760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Nandi R, Vishwakarma P, Prakash A, Kumar D. Discovering potential RNA dependent RNA polymerase inhibitors as prospective drugs against COVID-19: an in silico approach. Front Pharmacol. 2021;12(February):1–13. doi: 10.3389/fphar.2021.634047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammarco BC, Hinkle NC, Crossley MS. Biology and management of lesser mealworm alphitobius diaperinus (coleoptera: tenebrionidae) in broiler houses. J Integr Pest Manag. 2023;1:1. doi: 10.1093/jipm/pmad003. [DOI] [Google Scholar]
- Townsend L (2015) Insecticide control on poultry. 26 February 2015, 1–4. http://www.uky.edu/Ag/PAT/recs/livestk/recpou/poufly.htm
- Trott O, Olson JA. Software news and updates gabedit—a graphical user interface for computational chemistry softwares. J Comput Chem. 2012;32:174–182. doi: 10.1002/jcc. [DOI] [PubMed] [Google Scholar]
- Weng R, Lou S, Pang X, Song Y, Su X, Xiao Z, Qiu J. Multi-residue analysis of 126 pesticides in chicken muscle by ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Chem. 2020;309(April 2019):125503. doi: 10.1016/j.foodchem.2019.125503. [DOI] [PubMed] [Google Scholar]
- Wu WL, Chen MY, Tu IF, Lin YC, Eswarkumar N, Chen MY, Ho MC, Wu SH. The discovery of novel heat-stable keratinases from Meiothermus taiwanensis WR-220 and other extremophiles. Sci Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-04723-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Bumbra P, Laura JS, Khosla B. Bioresource Technology Reports Optimization of nutritional and physical parameters for enhancing the keratinase activity of Bacillus cereus isolated from soil of poultry dump site in Gurugram, Haryana. Bioresour Technol Rep. 2022;18(June):101108. doi: 10.1016/j.biteb.2022.101108. [DOI] [Google Scholar]
- Yahaya RSR, Phang LY, Normi YM, Abdullah JO, Ahmad SA, Sabri S. Feather-degrading Bacillus cereus HD1: genomic analysis and its optimization for keratinase production and feather degradation. Curr Microbiol. 2022;79(6):1–15. doi: 10.1007/s00284-022-02861-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All dataset generated or analyzed during this study are included in the manuscript and are available with the corresponding author, Indira Gahatraj for any further correspondence.