Abstract
Plants provide compounds that can be used to treat diseases, and in silico methods help to expedite drug discovery while reducing costs. This study explored the phytochemical profile of methanol extract of O. alismoides using GC-MS to identify potential bioactive compounds. Autodock 4.2.6. was employed for molecular docking evaluation of the efficacy of these identified compounds against Estrogen Receptor Alpha (ERα), Human Epidermal Growth Factor Receptor 2 (HER2), and Epidermal Growth Factor Receptor (EGFR), proteins. Additionally, the ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) properties of the compounds were predicted using the SwissADME online tool. The preliminary phytochemical analysis revealed the presence of alkaloids, carbohydrates, glycosides, and steroids. During the GC-MS analysis, seven compounds were identified, and drug-likeness prediction of these compounds showed good pharmacokinetic properties having high gastrointestinal absorption, and orally bioavailable. The molecular docking studies exhibited promising binding affinities of bioactive compounds against all target proteins. Specifically, the compounds Tricyclo[5.2.1.0(2,6)]decan-10-ol and 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide demonstrated the highest binding affinities with the ERα (-6.3 and − 6.0 k/cal), HER2 (-5.6 and − 6.1 k/cal), and EGFR (-5.4 and − 5.4 k/cal), respectively. These findings suggest the potential of O. alismoides as a source for developing new cancer therapeutics. The study highlights the effectiveness of in silico approaches for accelerating drug discovery from natural sources and paves the way for further exploration of these promising compounds.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40203-024-00227-y.
Keywords: Molecular Docking, Ottelia alismoides, Palmitic acid, Ethyl palmitate and drug-likeness properties
Introduction
Cancer is defined by the unchecked growth and division of cells, disrupting the usual cell division process (Ur Rashid et al. 2019). It’s among the most significant health challenges we confront. Moreover, cancer-related fatalities are increasing, expected to surpass cardiovascular disease deaths soon (Al-Warhi et al. 2020). The WHO reported 19.3 million new cancer cases and 10 million deaths in 2020. It is expected to reach 30.2 million new cases and 16.3 million fatalities by 2040 (Globocan, 2020). Cancer cells can be invasive, aggressive, and metastatic, spreading to various organs. Breast cancer is a highly heterogeneous disease that disrupts the normal function of mammary epithelial cells. It is the most common non-skin cancer and a leading cause of cancer-related death in women worldwide (Yedjou et al. 2019). Globally, it affects more than one in ten women (Torre et al., 2012). Excessive estrogen production is considered a major contributor to breast cancer. The estrogen receptor (ER), a nuclear receptor activated by binding to estrogen (17β-estradiol), plays a crucial role. Humans naturally express ER-α and ER-β, which regulate various physiological processes like cell growth and differentiation. Notably, ER-α is highly expressed in the mammary gland and uterus (Rafeeq 2022). HER2, on the other hand, belongs to a superfamily of human peptide ligands called epidermal growth factor receptors (EGFR) that includes HER1, HER2, HER3, and HER4. Notably, EGFR and HER2 are considered some of the most promising therapeutic targets for cancer treatment (Sait et al. 2020). Worldwide, intense research aims to create drugs that accurately target and eradicate cancer cells. Often, non-toxic plant extracts are combined with chemotherapy treatments to reduce the dose and side effects (Al-Zahrani et al. 2022). Medicinal plant compounds can be used to treat various illnesses and support chemotherapy side effect reduction (Hosseinzadeh et al. 2015; Mintah et al. 2019; Sathish et al. 2024). Plants have multifaceted roles. They’re key sources of essential nutrients and abound with health-promoting bioactive components that fight numerous diseases (Liu, 2003). These components, including lipids, phytochemicals, and pharmaceuticals, are utilized in the food, medicine, and beauty sectors. In places like India, incredibly under-resourced areas, plant-based traditional medicines are integral to healthcare due to their precise phytochemical components (Velmurugan and Anand 2017; Sathish et al. 2024). Particularly in India’s rural northeast, diverse plants and local socio-economic conditions have fostered reliance on traditional medicine. Plants from this region, abundant in bioactive compounds, exhibit antimicrobial, anticancer, anti-inflammatory, and antioxidant properties. Traditional remedies often use extracts rich in phytochemicals for chronic and infectious conditions (Sahoo and Manchikant, 2013).
Ottelia alismoides, or Duck lettuce, is adapted to saline and freshwater aquatic environments. These plants have evolved traits for submerged or floating life (Hutchinson GE, 1975; Cook CDK, 1974). Many countries spanning tropical and subtropical Asia and Australia have reported its presence, encompassing a broad geographical expanse. Within India, the plant can be located in diverse aquatic environments like tanks, ponds, streams, and ditches (Pullaiah et al. 2003). This plant stands out prominently in various locations, such as the aquatic herb situated in the rear waters of Madhuban Dam near Dudhani, the Silvassa region, Kamrup district in Assam, Pondicherry, Ariyalur, Kunnathur tank in Madurai district, Tamil Nadu (Sumithira et al. 2017), and even along the Vellaru River in Pudukkottai District. Traditionally, O. alismoides has been a remedy for diseases like cancer, tuberculosis, and diabetes. Yet, treating certain cancers, especially lung and skin, is challenging due to rapid growth and drug resistance (Das et al. 2023). While many plants have bioactive secondary metabolites, few have been intensely researched as key bioactive sources. Hence, creating effective screening techniques for new compounds is crucial (Keskes et al. 2017). Advances in extraction processes have produced potent medicines (Yadav et al. 2017). Gas chromatography-mass spectrometry (GC-MS) is typical for pinpointing plants’ bioactive elements (Satapute et al. 2019; Fan et al. 2018). Razack et al. (2015) vouched for GC-MS’s efficacy in detecting compounds in plant extracts. With technological progress, state-of-the-art tools aid drug discovery, screening medicinal plants for potential drugs (Sliwoski et al. 2014). Computational prediction is vital in drug development to comprehend drug features, promoting pharmaceutical advancements (Loza-Mejia et al. 2018). Among cost-effective strategies, molecular docking provides crucial insights into drug-protein interactions (Lee and Kim, 2019; Bharathi et al. 2014). This research examines the limited studies on O. alismoides and its bioactive components, focusing on identifying these compounds and conducting in silico molecular docking studies for anticancer potential. These in silico results can guide lab experiments in vitro, in vivo, or ex vivo.
Materials and methods
Collection and preparation of plant extract
The plant material chosen for this study was whole plant parts of Ottelia alismoides (L.) Pers., a member of the Hydrocharitaceae family. It was gathered from the Vellaru River in Sivapuram, Pudukkottai district, Tamil Nadu, India, in December 2022. The identification and confirmation of the plant were conducted by the PG and Research Department of Botany at J.J. College of Arts and Science (Autonomous), Pudukkottai, Tamil Nadu, India. The collected plant materials underwent a thorough washing with tap water to remove sediment particles. To prepare the methanolic extract, the plant materials were again washed meticulously and then spread out on blotting paper in the shade at room temperature for drying. Subsequently, the shade-dried samples were finely powdered using a tissue blender. A total of 30 g of powdered samples were placed in a Soxhlet apparatus and separately extracted with methanol for a duration of 8 h (Iniya et al. 2017).
Preliminary phytochemical analysis of O. alismoides
The methanolic extract of O. alismoides underwent qualitative phytochemical prescreening using established protocols (Tiwari et al. 2011). To prepare the standard solution, 100 mg of plant extract was dissolved in 10 mL of methanol. Subsequently, these solutions were screened to detect the presence of various phytochemicals, namely Tannins, Alkaloids, Flavonoids, Proteins, Carbohydrates, Glycosides, Saponins, Triterpenoids, Steroids, and Starch.
Gas chromatography and mass spectrometry (GC–MS) analysis of O. alismoides methanolic extract
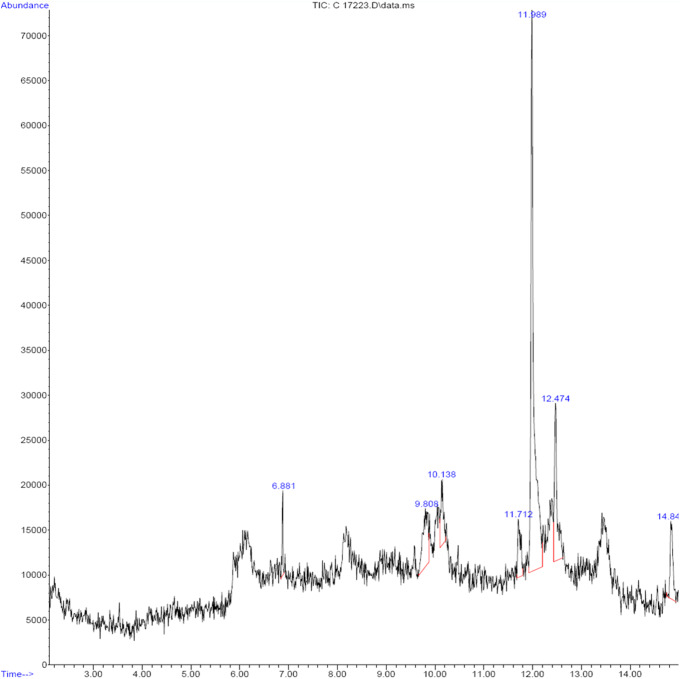
Using a Thermo Scientific Co. GC-MS system (model Thermo GC-TRACE ultra, version 5.0, Thermo MsDsq II), the phytoconstituent of O. alismoides extract was identified. A 100 µl methanolic extract of O. alismoides was dissolved in 1 ml of solvent, mixed, and filtered using a membrane filter. Chromatography was conducted on a DB 35-MS capillary standard non-polar column with dimensions of 15 m length, 0.25 μm film thickness, and 0.25 mm inner diameter. Helium was the carrier gas at a 1.0 ml/min flow rate. The sample injection volume was 1 µl in the split mode at a ratio of 1:10. The injector temperature was set at 240 ºC, while the transfer line temperature was maintained at 280°C. The instrument’s column temperature started at 50ºC for 2 min and then increased at 5ºC per minute until it reached 260ºC, where it was held for 10 min. The instrument operated at 70 eV, and the sample underwent scanning within a mass spectral range of 42–350 (m/z). Subsequently, the obtained GC-MS compound peaks were analyzed using NIST (Fig. 1).
Fig. 1.
GC–MS chromatogram of the methanol extract of O. alismoides
ADMET analysis
Identified bioactive compounds from GC-MS analysis of O. alismoides were evaluated the Absorption, Distribution, Metabolites, Excretion and Toxicity (ADMET) properties using a free web tool of SwissADME (http://www.swissadme.ch) (Hodgson 2001; Daina et al. 2017).
Molecular Docking studies
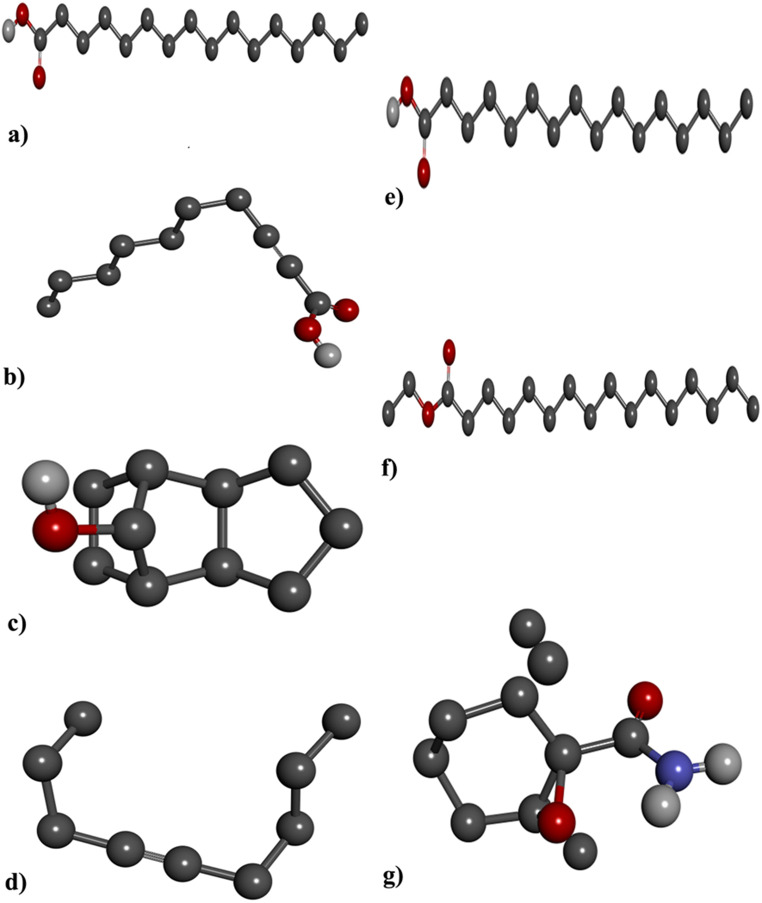
The three-dimensional (3D) structure of identified bioactive compounds (Fig. 2) in O. alismoides were retrieved from the PubChem database and then converted into Mol, PDBQT, and PDB formats utilizing the Open Babel software (Neema et al. 2015). Structure-based molecular docking was performed with various cancer proteins (Fig. 3), such as the X-ray crystal structure of the three molecular targets Estrogen Receptor Alpha (PDB ID: 3ERT) (Raju et al. 2021), Epidermal Growth Factor Receptor (PDB ID: 2J6M) (Acharya et al. 2019), and Human Epidermal Growth Factor Receptor 2(PDB ID: 1N8Z) (Megantara et al. 2022). These proteins were sourced from the Protein Data Bank of the Research Collaboratory for Structural Bioinformatics and were converted to pdbqt coordinates using Auto Dock Tools. Before transforming the protein for auto docking, the protein was purged of water molecules and inhibitors. Polar hydrogen atoms were added using Auto Dock Tools, Kollman charges were designated, and the altered enzyme structure was supplied as an input for the Autogrid program. Each docking system underwent 150 Lamarckian genetic algorithm iterations during the Autodock search. The optimal pose for each ligand was selected for further investigation of its interactions with target proteins. The results were visualized and interpreted using Discovery Studio 4.0 Client (Neema et al. 2015).
Fig. 2.
3D Structure of Bioactive compounds in O. alismoides (a) 3,5-Di-tert-butylphenol; (b) 2-Decynoic acid; (c) Tricyclo[5.2.1.0 (2,6)]decan-10-ol; (d) 4-Nonyne; (e) Palmitic Acid; (f) Ethyl palmitate and (g) 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide
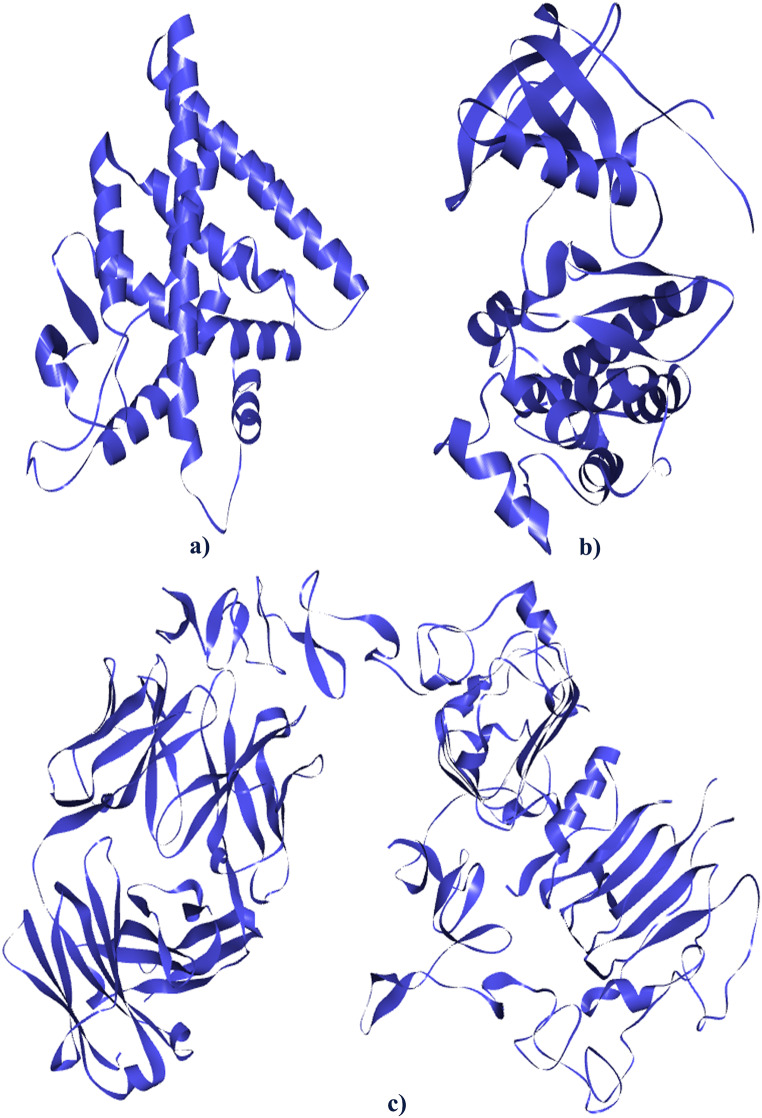
Fig. 3.
3D Structure of Target Proteins (a) Estrogen Receptor Alpha (PDB ID: 3ERT), (b) Epidermal Growth Factor, (b) Human Epidermal Growth Factor Receptor 2
Results
Preliminary phytochemical analysis of O. alismoides
The preliminary phytochemical analysis of the methanol extract from O. alismoides aerial plant parts revealed the presence of multiple bioactive compounds such as, tannins, alkaloids, carbohydrates, glycosides, and steroids (Table 1).
Table 1.
Preliminary Phytochemical Screening of Methanol Extract of O. alismoides
| S.No | Phytochemical | Methanol Extract |
|---|---|---|
| 1. | Tannins | + |
| 2. | Alkaloids | + |
| 3. | Flavonoids | - |
| 4. | Proteins | - |
| 5. | Carbohydrates | - |
| 6. | Glycosides | - |
| 7. | Saponins | - |
| 8. | Triterpenoids | + |
| 9. | Steroids | - |
| 10. | Starch | - |
Present (+) Absent (-)
GC-MS analysis of O. alismoides
The methanol extract of O. alismoides underwent further analysis via GC-MS to discern its phytochemical constituents. The chromatograph produced seven prominent peaks, indicating the presence of seven compounds such as 3,5-Di-tert-butylphenol, 2-Decynoic acid, Tricyclo[5.2.1.0(2,6)]decan-10-ol, 4-Nonyne, Palmitic Acid, Ethyl palmitate, and 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide Acid. Table 2; Fig. 1 represent the chromatogram of the extract, demonstrating various compounds, retention times (RT), molecular formula (MF), molecular weights (MW), and respective concentrations (%).
Table 2.
GC-MS Analysis of Methanol extract of O. alismoides
| P. No | Peak Name | Compound Name | M.W | M. F | Area % | RT |
|---|---|---|---|---|---|---|
| 1. | Phenol, 3,5-bis(1,1-dimethylethyl)- | 3,5-Di-tert-butylphenol | 206.32 | C14H22O | 2.53 | 6.881 |
| 2. | 2-Decanynoic acid | 2-Decynoic acid | 168.23 | C10H16O2 | 9.79 | 9.812 |
| 3. | Tricyclo[5.2.1.0 (2,6)]decan-10-ol | Tricyclo[5.2.1.0 (2,6)]decan-10-ol | 152.23 | C10H16O | 5.55 | 10.133 |
| 4. | 4-Nonyne | 4-Nonyne | 124.22 | C9H16 | 5.43 | 11.712 |
| 5. | n-Hexadecanoic acid | Palmitic Acid | 256.42 | C16H32O2 | 54.98 | 11.987 |
| 6. | Hexadecanoic acid, ethyl ester | Ethyl palmitate | 284.5 | C18H36O2 | 14.53 | 12.478 |
| 7. | 7-Oxabicyclo[4.1.0]heptane, 1,5- | 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide | 244.5 | C7H8Cl3NO2 | 7.19 | 14.842 |
Peak No (P. No); Molecular Weight (M.W) Molecular Formula (M.F); Retention Time (R.T)
Physicochemical and pharmacokinetic properties of Phytocompounds from O. alismoides
The evaluation encompassed the ADMET attributes of the interacting bioactive compounds from the methanolic extract of O. alismoides. These identified compounds possessed a molecular weight that did not exceed 500 g/mol. Seven compounds displayed X Log P3 values from 0.90 to 4.67, while their TPSA values were confined to 0.00 to 55.62. A variation between − 2.06 and − 5.51 was observed regarding Log S values. Fraction Csp3 values, on the other hand, showcased a spectrum from 0.57 to 1.00. Rotatable bonds, ranging from 1 to 16, were characteristic of all compounds (Table 3). Prominent among the interacting phytochemicals were those that conformed to Lipinski’s rule of five, indicating favourable interactions. Notably, a substantial portion of these compounds exhibited the capacity to traverse the blood-brain barrier (BBB) effectively and displayed high levels of intestinal absorption. Within the analyzed phytochemicals, a bioavailability score of 0.55 was generally noted. However, 2-Decynoic acid and Palmitic Acid stood out with a higher score of 0.85 (Table 4). Certain compounds, such as 2-Decynoic acid, Tricyclo[5.2.1.0(2,6)]decan-10-ol, 4-Nonyne, and 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptanecarboxamide, exhibited no discernible inhibition of CYP450 enzymes. Conversely, distinct compounds, including 3,5-Di-tert-butylphenol and Ethyl palmitate, were found to inhibit specific CYP450 enzymes, specifically CYP2D6 and CYP1A2, respectively. Notably, Palmitic Acid demonstrated inhibition of two CYP450 enzymes, specifically CYP1A2 and CYP2C9. Furthermore, the potential for skin permeation was assessed using log Kp values, revealing that the compound 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide exhibited remarkably low skin permeation ability with a value of -6.92. For comprehensive insights into cytochrome and skin permeation attributes (Table 5).
Table 3.
Physicochemical properties of bioactive compounds in O. alismoides
| S. No | Compound Name |
Molecular Weight | Log P | TPSA (Å) |
Log S (ESOL) |
Fraction Csp3 |
HBR |
|---|---|---|---|---|---|---|---|
| 1. | 3,5-Di-tert-butylphenol | 206.32 | 3.87 | 20.23 | -4.38 | 0.57 | 2 |
| 2. | 2-Decynoic acid | 168.23 | 2.47 | 37.30 | -3.02 | 0.70 | 5 |
| 3. | Tricyclo[5.2.1.0 (2,6)]decan-10-ol | 152.23 | 2.45 | 20.23 | -2.23 | 1.00 | 0 |
| 4. | 4-Nonyne | 124.22 | 4.38 | 0.00 | -2.88 | 0.78 | 3 |
| 5. | Palmitic Acid | 256.42 | 4.19 | 37.30 | -5.02 | 0.94 | 14 |
| 6. | Ethyl palmitate | 284.5 | 4.67 | 26.30 | -5.51 | 0.94 | 16 |
| 7. | 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide | 244.5 | 0.90 | 55.62 | -2.06 | 0.86 | 1 |
Table 4.
Pharmacokinetic properties of bioactive compounds in O. alismoides
| S. No | Compounds Name | Lipinski Role |
BBB | HIA | PGP- | Bioavailability Score |
|---|---|---|---|---|---|---|
| 1. | 3,5-Di-tert-butylphenol | Yes,0 | Yes | High | No | 0.55 |
| 2. | 2-Decynoic acid | Yes,0 | Yes | High | No | 0.85 |
| 3. | Tricyclo[5.2.1.0 (2,6)]decan-10-ol | Yes,0 | Yes | High | No | 0.55 |
| 4. | 4-Nonyne | Yes,1 | Yes | Low | No | 0.55 |
| 5. | Palmitic Acid | Yes,1 | Yes | High | No | 0.85 |
| 6. | Ethyl palmitate | Yes,1 | No | High | No | 0.55 |
| 7. | 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide | Yes,0 | Yes | High | No | 0.55 |
Table 5.
Cytochrome properties and skin permeation of bioactive compounds in O. alismoides
| S. No | Compounds Name | CYP1A2 inhibitor |
CYP2C19 inhibitor |
CYP2C9 inhibitor |
CYP2D6 inhibitor |
CYP3A4 inhibitor |
Log Kp (cm/s) |
|---|---|---|---|---|---|---|---|
| 1. | 3,5-Di-tert-butylphenol | No | No | No | Yes | No | -4.07 |
| 2. | 2-Decynoic acid | No | No | No | No | No | -4.54 |
| 3. | Tricyclo[5.2.1.0 (2,6)]decan-10-ol | No | No | No | No | No | -5.60 |
| 4. | 4-Nonyne | No | No | No | No | No | -4.28 |
| 5. | Palmitic Acid | Yes | No | Yes | No | No | -2.77 |
| 6. | Ethyl palmitate | Yes | No | No | No | No | -2.44 |
| 7. | 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide | No | No | No | No | No | -6.92 |
Molecular docking
The study examined the three-dimensional structures of different cancer-related proteins, such as Human Epidermal Growth Factor Receptor 2 (HER2), Epidermal Growth Factor Receptor (EGFR), and Estrogen Receptor Alpha (ERα), as shown in Fig. 2. These proteins were analyzed alongside seven phytocompounds from the methanol extract of O. alismoides using GC–MS analysis. The docking of these compounds with the target proteins was conducted, and the resulting data, including binding energy and hydrogen bonding, were compiled in Table 6. Notably, when tested against the HER2 protein, the bioactive compounds − 3,5-Di-tert-butylphenol, 2-Decynoic acid, Tricyclo[5.2.1.0 (2,6)]decan-10-ol, 4-Nonyne, Palmitic Acid, Ethyl palmitate, and 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide, exhibited binding affinities of -4.1, -4.7, -5.6, -4.6, -4.7, -4.4, and − 6.1 kcal/mol, respectively. Noteworthy interactions were observed: 2-Decynoic acid forming hydrogen bonds with THR5 and TYR281, Palmitic Acid with THR5, and 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide with ASN280 and SER441 residues. Some compounds showed no hydrogen bond interactions (Fig. 4 and Table S1).
Table 6.
Molecular Docking Value of bioactive compounds in O. alismoides against Target Proteins
| Target Protein (PDB ID) |
Compound Name | Binding Affinity (kcal/mol) | Number of Hydrogen Bond | Name of Amino acids residues |
|---|---|---|---|---|
| 1N8Z | 3,5-Di-tert-butylphenol | -4.1 | 0 | - |
| 2-Decynoic acid | -4.7 | 2 |
THR5 TYP281 |
|
| Tricyclo[5.2.1.0 (2,6)]decan-10-ol | -5.6 | 0 | - | |
| 4-Nonyne | -4.6 | 0 | - | |
| Palmitic Acid | -4.7 | 1 | THR5 | |
| Ethyl palmitate | -4.4 | 0 | ||
| 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide | -6.1 | 2 |
ASN280 SER441 |
|
| 2J6M | 3,5-Di-tert-butylphenol | -3.6 | 2 |
ILE878 LYS879 |
| 2-Decynoic acid | -4.7 | 2 |
MET793 GLY796 |
|
| Tricyclo[5.2.1.0 (2,6)]decan-10-ol | -5.4 | 1 | THR854 | |
| 4-Nonyne | -4.3 | 0 | - | |
| Palmitic Acid | -3.8 | 1 | ASP1012 | |
| Ethyl palmitate | -4.9 | 1 | THR854 | |
| 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide | -5.4 | 3 |
LYS745 THR854 ASP855 |
|
| 3ERT | 3,5-Di-tert-butylphenol | -4.1 | 2 |
MET522 GLU523 |
| 2-Decynoic acid | -4.4 | 0 | ||
| Tricyclo[5.2.1.0 (2,6)]decan-10-ol | -6.3 | 1 | GLU353 | |
| 4-Nonyne | -4.8 | 0 | - | |
| Palmitic Acid | -5.2 | 0 | - | |
| Ethyl palmitate | -4.3 | 0 | - | |
| 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide | -6.0 | 0 | - |
Fig. 4.
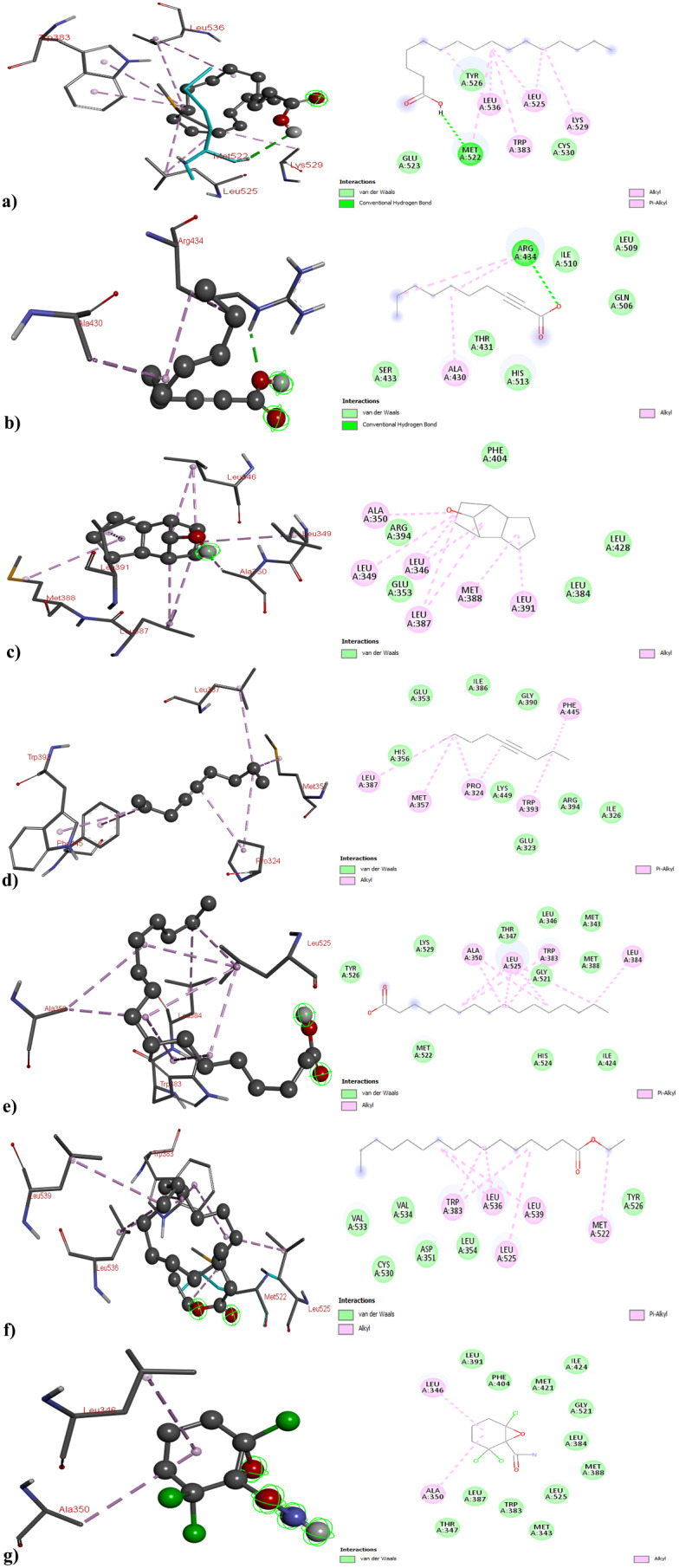
3D and 2D Structure of Estrogen Receptor Alpha with (a) 3,5-Di-tert-butylphenol; (b) 2-Decynoic acid; (c)Tricyclo[5.2.1.0 (2,6)]decan-10-ol; (d) 4-Nonyne (e) Palmitic Acid; (f) Ethyl palmitate; and (g) 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide
Similarly, against the EGFR, the binding affinities of 3,5-Di-tert-butylphenol, 2-Decynoic acid, Tricyclo[5.2.1.0 (2,6)]decan-10-ol, 4-Nonyne, Palmitic Acid, Ethyl palmitate, and 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide were determined as -3.6, -4.7, -5.4, -4.3, -3.8, -4.9, and − 5.41 kcal/mol, respectively. Particularly strong interactions were observed with 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide, forming hydrogen bonds with LYS745, THR854, and ASP855. 3,5-Di-tert-butylphenol established hydrogen bonds with ILE878 and LYS879, while 2-Decynoic acid formed interactions with MET793 and GLY796. Tricyclo[5.2.1.0 (2,6)]decan-10-ol, Palmitic Acid, and Ethyl palmitate exhibited a hydrogen bond with THR854, ASP1012, and THR854, respectively. Some compounds did not show hydrogen bond interactions (Fig. 5 and Table SS2).
Fig. 5.
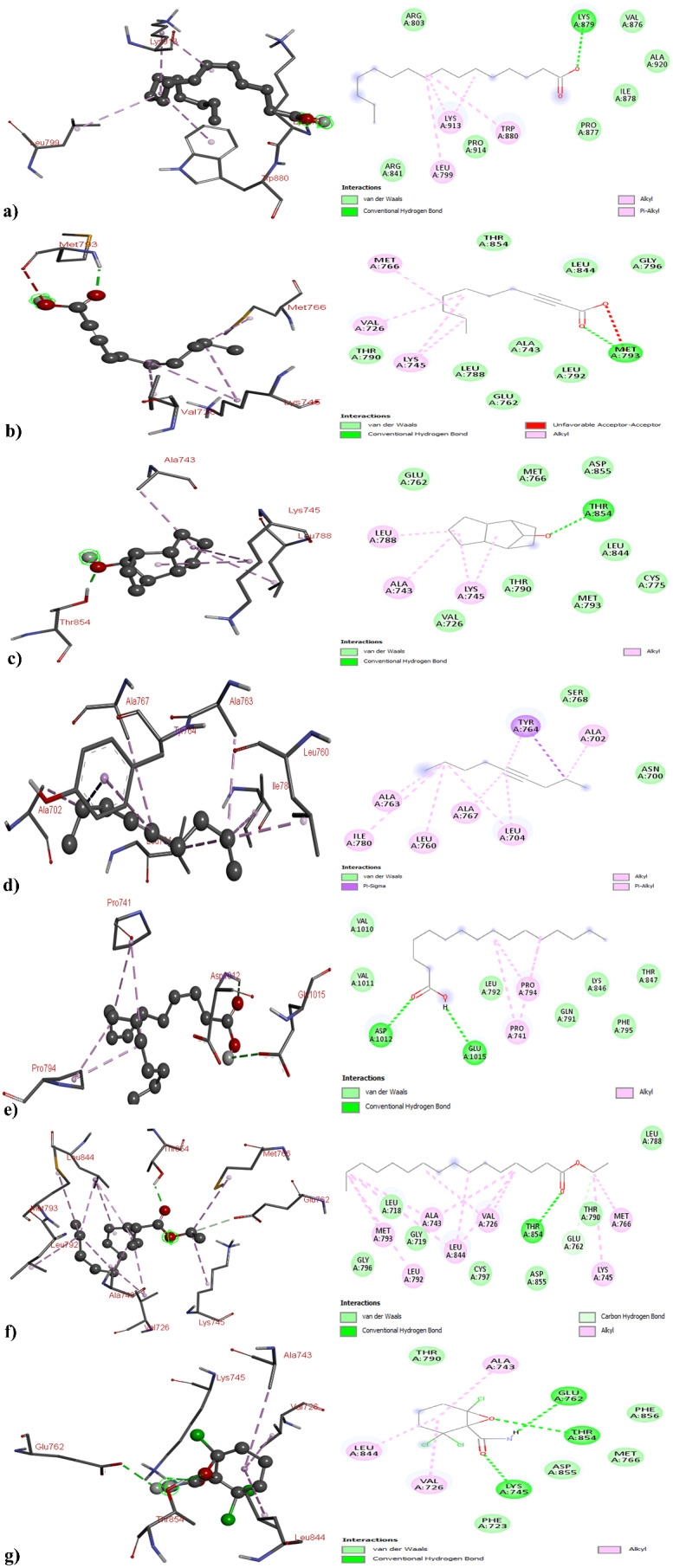
3D and 2D Structure of Epidermal Growth Factor Receptor with (a) 3,5-Di-tert-butylphenol; (b) 2-Decynoic acid; (c)Tricyclo[5.2.1.0 (2,6)]decan-10-ol; (d) 4-Nonyne; (e) Palmitic Acid; (f) Ethyl palmitate; and (g) 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide
Concerning ERα, the binding affinities of the bioactive compounds − 3,5-Di-tert-butylphenol, 2-Decynoic acid, Tricyclo[5.2.1.0 (2,6)]decan-10-ol, 4-Nonyne, Palmitic Acid, Ethyl palmitate, and 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide - were found to be -4.1, -4.4, -6.3, -4.8, -5.2, -4.3, and − 6.0 kcal/mol, respectively. Significant interactions were observed, with 3,5-Di-tert-butylphenol establishing hydrogen bonds with MET522 and GLU523 and Tricyclo[5.2.1.0 (2,6)]decan-10-ol forming an interaction with GLU353. Some compounds did not exhibit hydrogen bond interactions (Fig. 6 and Table S3). This study highlighted the promising performance of the identified bioactive compounds, with particular emphasis on 4-Nonyne and 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide. These compounds demonstrated noteworthy effects across all the cancer protein targets.
Fig. 6.
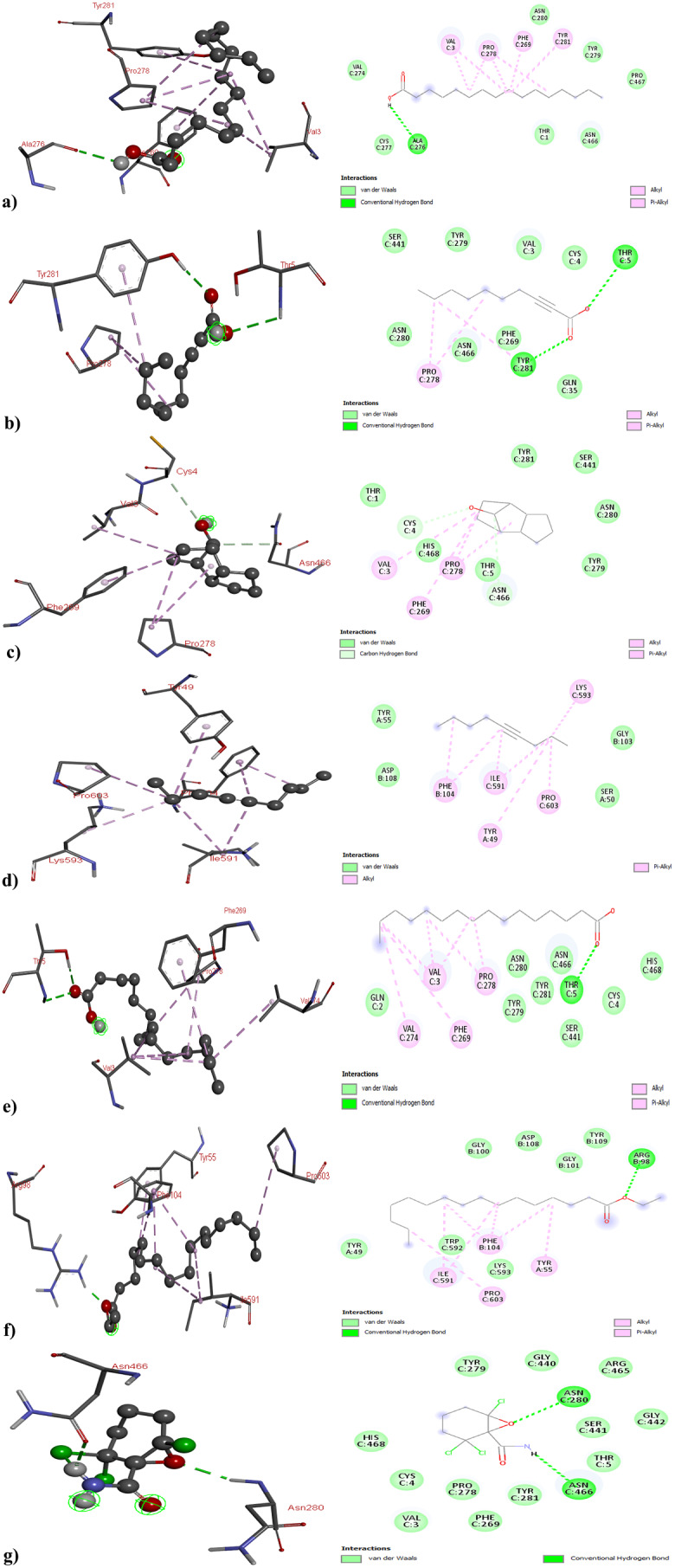
3D and 2D Structure of Human Epidermal Growth Factor Receptor 2 with (a) 3,5-Di-tert-butylphenol; (b) 2-Decynoic acid; (c)Tricyclo[5.2.1.0 (2,6)]decan-10-ol; (d) 4-Nonyne (e) Palmitic Acid; (f) Ethyl palmitate; and (g) 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide
Discussion
Natural compounds are gaining prominence in searching for lead molecules with anticancer and antioxidant properties for cancer treatment (Shapira, 2017). Although computational algorithms are extensively studied in medicinal synthetic chemistry, their application in the domain of natural phytochemicals still needs to be explored. The primary goal of molecular docking is to predict the structure of ligand-receptor complexes using computational methods (Muthusamy et al. 2011; Sathish et al. 2024). This docking process involves a virtual screening of compound libraries, with the results ranked based on scores. Theoretical models are then formulated to explain how ligands inhibit the target receptor’s activity, which is crucial for lead optimization (Feinstein and Brylinski 2015). Docking outcomes are influenced by various factors, including intramolecular forces like bond characteristics, angles, and dihedral angles, as well as intermolecular forces such as electrostatic interactions, dipolar forces, hydrogen bonds, and hydrophobic interactions (Kerns and Di 2003; Duran-Iturbide et al. 2020). In this study, the plant of O. alismoides was extracted using methanol and analyzed through phytochemical screening and in silico molecular docking. This study presents a pioneering achievement by reporting the molecular docking of O. alismoides secondary metabolites with various cancer proteins. The results highlight the solid binding capabilities of the chosen bioactive compounds to their designated receptors, suggesting molecular docking as a valuable method for identifying inhibitors. O. alismoides is known for its potential antitubercular activity, cytotoxicity and anticancer activity (TsaiYuan et al. 2012; Hoye et al. 2013).
The preliminary phytochemical analysis showed the presence of tannins, alkaloids, carbohydrates, glycosides, and steroid compounds. SahiraBanu and Cathrine 2015 reported phytochemicals of glycosides, alkaloids, flavonoids, flavones, terpenoids, tannins and phenolic compounds (SahiraBanu and Cathrine 2015). The previous report showed Alkaloids exhibit anti-inflammatory, anticancer, antimicrobial, antifungal, analgesic, anaesthetic, and neuropharmacological effects and act as pain relievers (Vijayakumar et al. 2019). Glycosides contribute antioxidant, antitumor, anticancer, antidiabetic, and hepatoprotective benefits (Kumar et al. 2017; Vijayakumar et al. 2019).
The methanol extract from O. alismoides was analyzed using GC-MS, revealing seven prominent compounds such as 3,5-Di-tert-butylphenol, 2-Decynoic acid, Tricyclo[5.2.1.0(2,6)]decan-10-ol, 4-Nonyne, Palmitic Acid, Ethyl palmitate, and 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide Acid. Similarly, Seif-Eldin et al. 1998 and Thomas et al. 2013 isolated two diastereomeric 4-methylene-2-cyclohexenones, Otteliones A and B and 3a-hydroxyottelione in O. alismoides.
The compounds’ drug-likeness is contingent upon several critical physicochemical properties (Hodgson 2001). Compounds with a molecular weight of 500 g/mol or lower hold promise for easy absorption, diffusion, and transport. The compound’s lipophilic nature affects its solubility, selectivity, and permeability, all pivotal factors in determining its potential as a drug candidate (Muegge et al. 2001). Lead compounds exhibiting (XlogP3) values within the range of -0.7 to + 5.0 have demonstrated their worth. All compounds in this study adhere to this range. Overly high lipophilicity in a compound can lead to rapid metabolic turnover, reduced solubility, limited intestinal absorption, and the risk of toxicity in vital organs (Arnott and Planey 2012).
Compounds displaying polarity within the range of 20 to 130 Å and a solubility not exceeding 6 meet the acceptable benchmarks for drug-likeness. The compound’s aqueous solubility can be estimated directly from its molecular structure and weight. A fraction of carbons with sp3 hybridization no less than 0.25 serves as an efficient marker, a criterion satisfied by all compounds in this study, each surpassing the specified threshold. Flexibility, characterized by the presence of rotatable bonds (limited to 9 or fewer), plays a role in a compound’s versatility. This investigation found that Palmitic Acid and Ethyl palmitate compounds exceeded the 9-rotatable-bond limit, while the others had fewer than 9. Lipinski established the “Rule of 5” (Ro5) to define molecular characteristics of compounds for drug design assistance. The research verifies that the investigated phytocompounds fall within Lipinski’s parameters. Ro5 was applied as a filter to recognize compounds with high potential as drug candidates (Lipinski et al. 2012). The compounds found in the methanol extract of O. alismoides exhibit a high likelihood of being efficiently absorbed through the gastrointestinal tract. This implies that when taken orally, these compounds can readily cross the gastrointestinal barrier (Pires et al. 2018). In contrast, the blood-brain barrier (BBB) is a physiological partition formed by the brain’s microvascular endothelial cell layer, effectively isolating the brain from the bloodstream.
The evaluation focused on the phytocompounds’ capacity to penetrate the blood-brain barrier (BBB). Notably, 85% of the compounds could breach the BBB effectively. This ability to cross the BBB is a pivotal criterion for compounds that target the central nervous system. In contrast, ethyl palmitate exhibited an inability to traverse the BBB, which suggests a potential for minimizing adverse effects within the central nervous system. In Lin’s 2003 study, all other compounds demonstrated probabilities of BBB penetration (Lin et al. 2003). P-glycoproteins (P-gp) function as transporters located in the cell membrane’s intracellular and extracellular regions. The role of P-glycoprotein is of notable importance in drug absorption and excretion (El-Kattan and Varma 2012). The potential resistance of various compounds at distinct target sites is a significant consideration. This membrane transporter protein seems to primarily affect the limitation of cellular drug uptake from the bloodstream into the brain and from the intestinal lumen into epithelial cells, as opposed to mainly promoting the elimination of drugs from hepatic cells and renal tubules (Hunter and Hirst, 1997; Suzuki and Sugiyama 2000). The compounds 2-Decynoic acid and Palmitic Acid exhibit a higher bioavailability value of 0.85, in contrast to the remaining compounds, which possess a bioavailability value 0.55. This observation aligns with the adherence of these compounds to the Lipinski rule of five, implying a 55% probability of bioavailability. The vital aspect of drug design lies in the oral bioavailability of a drug, which denotes the portion of the administered dose that reaches the bloodstream. The process is mainly influenced by gastrointestinal absorption. A drug’s aqueous solubility should be favourable for optimal oral bioavailability and absorption (Levin 2012; Veber et al. 2002).
The enzyme Cytochrome P450 monooxygenase is crucial to drug metabolism and elimination in biological systems. Around 90% of the molecules under investigation in this study serve as substrates for five distinct isoforms of the enzyme: CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP3A4 (Stegemann et al. 2007). These compounds exhibit no inhibitory effects on the activities of these enzymes, implying a strong likelihood of undergoing transformation and subsequent bioavailability following oral administration. Notably, these compounds do not hinder the functioning of CYP450 enzymes and do not provoke any adverse reactions. Nevertheless, it’s essential to recognize that if these compounds were to inhibit the CYP isoenzymes, it could disrupt the metabolism process, potentially leading to decreased bioavailability and the risk of accumulating in the body, which could have toxic effects (Srimai et al. 2013). Within the range of compounds studied, a subset does display inhibitory actions on CYP450 enzymes, which could result in unforeseen adverse reactions. This inhibition of specific enzyme variants raises significant concerns regarding potential interactions between different drugs, particularly in relation to the movement and processing of drugs within the body. Such inhibition, combined with the possibility of accumulation, has the potential to impact critical aspects of drug behaviour, including absorption, distribution, metabolism, and excretion (ADME), which could ultimately lead to unfavourable consequences (Wang et al. 2015). The skin serves as a discerning barrier, allowing the entry of diverse compounds for penetration. Skin permeability holds significant importance in appraising compounds suitable for transdermal administration. A molecule’s skin permeability, indicated by its negative logarithmic Kp value, is inversely proportional to its permeation potential—lower values correspond to reduced permeability. In this current research, all compounds exhibit impermeability, as reflected by their negative logarithmic Kp values (Raies and Bajic 2016).
Our investigation encompassed molecular docking studies involving phytocompounds from O. alismoides and their interactions with three distinct cancer proteins. The primary focus was on how these phytochemicals interacted with the active sites of the target proteins, potentially enhancing their functional roles. Notably, the phytocompounds identified in our study adhered to the Lipinski rule of five, indicating their probable safety for human consumption. The study extensively covered various aspects, including binding affinity, specific bonding patterns like hydrogen bonds, the interactions between ligand amino acid residues and the protein, and the bond lengths between ligand atoms and target proteins.
Valeriote et al. (2002) assessed the target protein binding strengths in kcal/mol for all phytocompounds (ligands). The study accurately pinpointed the locations where the ligands attached to specific amino acids on the protein structure. Encouragingly, all compounds exhibited favourable binding affinities. For instance, in the case of the HER2 protein, compounds including 3,5-Di-tert-butylphenol, 2-Decynoic acid, Tricyclo[5.2.1.0(2,6)]decan-10-ol, 4-Nonyne, Palmitic Acid, Ethyl palmitate, and 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide showcased binding affinities of -4.1, -4.7, -5.6, -4.6, -4.7, -4.4, and − 6.1 kcal/mol, respectively. Similarly, concerning the EGFR protein, the binding affinities were − 3.6, -4.7, -5.4, -4.3, -3.8, -4.9, and − 5.4 kcal/mol for the mentioned compounds. As for the ERα protein, binding affinities were measured at -4.1, -4.4, -6.3, -4.8, -5.2, -4.3, and − 6.0 kcal/mol, respectively. These binding affinities are comparable to those reported for standard drugs against the same target proteins. For instance, Mendie and Hemalatha (2022) documented binding energies of -6.03 kcal/mol and − 6.59 kcal/mol for Dovitinib and Gefitinib against HER2, respectively. Similarly, Yusuf et al. (2023) studied molecular docking of the standard drug Erlotinib against EGFR protein, which has a binding energy of -8.539 kcal/mol. Another study showed a binding energy of -8.2 kcal/mol for Gemcitabine against the ERα protein (Munshi et al. 2022). Therefore, it can be inferred from the molecular docking studies that Tricyclo[5.2.1.0(2,6)]decan-10-ol and 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide have good affinity for the target proteins.
Conclusion
The present study investigated the methanol extract of O. alismoides. GC-MS analysis revealed seven bioactive compounds with favorable physicochemical properties according to SwissADME, falling within recommended ranges for molecular weight, Log P, and TPSA. Additionally, most ADMET properties, including bioavailability, logS, human intestinal absorption, and BBB permeation, were within acceptable ranges, suggesting their potential as drug candidates. Furthermore, a majority of the phytoconstituents exhibited promising bioactivity scores, cytochrome properties, and skin permeation, indicative of good drug-like characteristics. Molecular docking studies revealed promising binding affinities of these compounds against selected target proteins, particularly Tricyclo[5.2.1.0(2,6)]decan-10-ol and 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide, which showed the highest binding affinities with ERα, HER2, and EGFR. Notably, no prior in silico studies have investigated this plant’s effects on cancer proteins. Further in vitro and in vivo investigations are warranted to validate these findings comprehensively.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge the SR Academy and Management of J.J. College of Arts and Science (A), Pudukkottai, for providing lab facilities, to carry out this research work.
Abbreviations
- GC-MS
Gas Chromatography and Mass Spectrum
- WHO
World Health Organization
- ADMET
Absorption, Distribution, Metabolites, Excretion and Toxicity
- PDB
Protein Data Bank
- PDBQT
Protein Data Bank, Partial Charge (Q), & Atom Type (T)
- TPSA
Topological Polar Surface Area
- BBB
Blood-Brain Barrier
- Ro5
Rule of 5
- CYP
Cytochrome P450
- HER2
Human Epidermal Growth Factor Receptor 2
- EGFR
Epidermal Growth Factor Receptor
- ERα
Estrogen Receptor Alpha
- THR
Threonine
- TYR
Tyrosine
- ASN
Asparagine
- SER
Serine
- LYS
Lysine
- ASP
Aspartic Acid
- ILE
Isoleucine
- MET
Methionine
- GLY
Glycine
- GLU
Glutamic acid
Author contributions
SM., SS and CR., wrote the main manuscript text and JP.,JP., and SM., prepared all figures and tables. All authors reviewed the manuscript.
Declarations
Supporting information
This manuscript contains supplementary file.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acharya R, Chacko S, Bose P, Lapenna A, Pattanayak SP. Structure based Multitargeted Molecular Docking analysis of selected furanocoumarins against breast Cancer. Sci Rep. 2019;9(1):15743. doi: 10.1038/s41598-019-52162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Warhi T, Sabt A, Elkaeed EB, Eldehna WM. Recent advancements of coumarin-based anticancer agents: an up-to-date review. Bioorg Chem. 2020;103:104163. doi: 10.1016/j.bioorg.2020.104163. [DOI] [PubMed] [Google Scholar]
- Al-Zahrani A, Ibraheem F, El-Senduny FF (2022) Anticancer activities of Saudi Juniperus procera extracts and their effects on the regulatory mechanisms in controlling growth of human cancerous cells. Res Sq 1–25. 10.21203/rs.3.rs-1666736/v1
- Arnott JA, Planey SL. The influence of lipophilicity in drug discovery and design. Expert Opin Drug Discov. 2012;7:863–875. doi: 10.1517/17460441.2012.714363. [DOI] [PubMed] [Google Scholar]
- Banu KS, Cathrine L. General techniques involved in phytochemical analysis. Int j adv res chem sci. 2015;2(4):25–32. [Google Scholar]
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Rahaman A, Nath R, Das Talukdar A, Nath D, Bhattacharjee S, Mandal DP, Choudhury MD, Das D, Das G, Patra JK. Effect of acetone fraction of Ottelia alismoides on the G2/M cell cycle arrest and apoptosis in the human carcinoma cell lines. J Ethnopharmacol. 2023;10:115729. doi: 10.1016/j.jep.2022.115729. [DOI] [PubMed] [Google Scholar]
- Duran-Iturbide NA, Diaz-Eufracio BI, Jose LF. In silico ADME/Tox profiling of natural products: a focus on Biofacquimacs. ACS Omega. 2020;5:16076–16084. doi: 10.1021/acsomega.0c01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kattan A, Varma M. Oral absorption, intestinal metabolism and human oral bioavailability. Top Drug Metab. 2012;10:31087–31097. [Google Scholar]
- Fan S, Chang J, Zong Y, Hu G, Jia J. GC-MS analysis of the composition of the essential oil from Dendranthema indicum Var. Aromaticum using three extraction methods and two columns. Molecules. 2018;23:576. doi: 10.3390/molecules23030576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein WP, Brylinski M. Calculating an optimal box size for ligand docking and virtual screening against experimental and predicted binding pockets. J Cheminform. 2015;7:18–25. doi: 10.1186/s13321-015-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson J. ADMET—Turning chemicals into drugs. Nat Biotechnol. 2001;19:722–726. doi: 10.1038/90761. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh S, Jafarikukhdan A, Hosseini A, Armand R. The application of medicinal plants in traditional and modern medicine: a review of Thymus vulgaris. Int J Clin Med. 2015;6:635. doi: 10.4236/ijcm.2015.69084. [DOI] [Google Scholar]
- Hoye TR, Ayyad SEN, Beckord H, Brown SG. New diarylheptanoids and a hydroxylated ottelione from Ottelia alismoides. Nat Prod Commun. 2013;8(3):1934578X1300800320. doi: 10.1177/1934578X130080032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J, Hirst BH (1997) Intestinal secretion of drugs: The role of P-glycoprotein and related drug efflux systems in limiting oral drug absorption. Adv Drug Deliv Rev 25:129–157. 10.1016/S0169-409X(97)00497-3
- Iniya Udhaya C, John Peter Paul J. Screening of anti-oxidant activity of methanolic extract of Gracilaria Fergusonii J.Ag. (Red seaweed) in Hare Island, Thoothukudi, Tamil Nadu, India. Indo Am j Pharm sci. 2017;4(9):2724–2727. [Google Scholar]
- Kerns EH, Di L. Pharmaceutical profiling in drug discovery. Drug Discov Today. 2003;8:316–323. doi: 10.1016/S1359-6446(03)02649-7. [DOI] [PubMed] [Google Scholar]
- Keskes H, Belhadj S, Jlail L, El Feki A, Damak M, Sayadi S, Allouche N. LC-MS–MS and GC-MS analyses of biologically active extracts and fractions from Tunisian Juniperus phoenice leaves. Pharm Biol. 2017;55(1):88–95. doi: 10.1080/13880209.2016.1230139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin GM. P-glycoprotein: why this drug transporter may be clinically important. Curr Psychiatr. 2012;11:38–40. doi: 10.1007/s11418-021-01575-1. [DOI] [Google Scholar]
- Lin J, Sahakian DC, Morais SM, Xu JJ, Polzer RJ, Winter SM. The role of absorption, distribution, metabolism, excretion and toxicity in drug discovery. Curr Top Med Chem. 2003;3:1125–1154. doi: 10.2174/1568026033452096. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2012;64:4–17. doi: 10.1016/j.addr.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Loza-Mejía MA, Salazar JR, Sánchez-Tejeda JF (2018) In Silico studies on compounds derived from Calceolaria: Phenylethanoid glycosides as potential multitarget inhibitors for the development of pesticides. Biomolecules 8(4):121. 10.3390/biom8040121 [DOI] [PMC free article] [PubMed]
- Megantara S, Wathoni N, Mohammed AFA, Suhandi C, Ishmatullah MH, Putri MFFD. Silico Study: combination of α-Mangostin and Chitosan Conjugated with trastuzumab against Human Epidermal Growth Factor Receptor 2. Polym (Basel) 2022;14(13):2747. doi: 10.3390/polym14132747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta G, Islam K. Total synthesis of (±)-Otteliones a and B. Angew Chem. 2002;114(13):2502–2504. doi: 10.1002/1521-3757(20020703)114:13<2502::AID-ANGE2502>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Mendie LE, Hemalatha S. Molecular docking of phytochemicals targeting GFRs as therapeutic sites for cancer: an in silico study. Appl Biochem Biotechnol. 2022;194(1):215–231. doi: 10.1007/s12010-021-03791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintah SO, Asafo-Agyei T, Archer MA, Junior PAA, Boamah D, Kumadoh D, Appiah A, Ocloo A, Boakye YD, Agyare C. Medicinal plants for treatment of prevalent diseases. Pharmacogn Med Plants. 2019;9:1–19. [Google Scholar]
- Munshi M, Zilani MNH, Islam MA, Biswas P, Das A, Afroz F, Hasan MN. Novel compounds from endophytic fungi of Ceriops decandra inhibit breast cancer cell growth through estrogen receptor alpha in in-silico study. IMU. 2022;32:101046. doi: 10.1016/j.imu.2022.101046. [DOI] [Google Scholar]
- Muegge I, Heald SL, Brittelli D (2001) Simple selection criteria for drug-like chemical matter. J Med Chem 44:1841–1846. 10.1021/jm015507e [DOI] [PubMed]
- Muthusamy K, Kirubakaran P, Singh D, Nagamani S, Sindhu S (2011) Molecular docking studies of bitter melon compounds against BRCA1 protein. J Pharm Res 4(2):388–390.
- Neema T, Kumud P, Bhasker P. Docking studies of grayanotoxin as potential inhibitor for major virulent proteins of encephalitis virus. Int J Pharmtechres. 2015;7:156–164. [Google Scholar]
- Pires DE, Kaminskas LM, Ascher DB. Prediction and optimization of pharmacokinetic and toxicity properties of the ligand. Methods mol biol. 2018;1762:271–284. doi: 10.1007/978-1-4939-7756-7_14. [DOI] [PubMed] [Google Scholar]
- Rafeeq MM. Molecular docking analysis of phytochemicals with estrogen receptor alpha. Bioinformation. 2022;18(8):697. doi: 10.6026/97320630018697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raies AB, Bajic VB. In silico toxicology: computational methods for the prediction of chemical toxicity. Wiley Interdiscip Rev Comput Mol Sci. 2016;6:147–172. doi: 10.1002/wcms.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju L, Lipin R, Eswaran R. Identification, ADMET evaluation and molecular docking analysis of phytosterols from Banaba (Lagerstroemia speciosa (L)Pers) seed extract against breast cancer. Silico Pharmacol. 2021;9(1):43. doi: 10.1007/s40203-021-00104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razack S, Kumar KH, Nallamuthu I, Naika M, Khanum F Antioxidant, biomolecule oxidation protective activities of Nardostachys jatamansi DC and its phytochemical analysis by RP-HPLC and GC-MS. Antioxidants 4:185–203. 10.3390/antiox4010185 [DOI] [PMC free article] [PubMed]
- Sahoo N, Manchikanti P. Herbal drug regulation and commercialization: an Indian industry perspective. J Altern Complement Med. 2013;19(12):957–963. doi: 10.1089/acm.2012.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sait KHW, Mashraqi M, Khogeer AA, Alzahrani O, Anfinan NM, Sait HK, Almutairi A, Alam Q. Molecular docking analysis of HER-2 inhibitor from the ZINC database as anticancer agents. Bioinformation. 2020;16(11):882. doi: 10.6026/97320630016882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satapute P, Murali KP, Kurjogi M, Jogaiah S. Physiological adaptation and spectral annotation of Arsenic and Cadmium heavy metal-resistant and susceptible strain Pseudomonas taiwanensis. Environ Pollut. 2019;251:555–563. doi: 10.1016/j.envpol.2019.05.054. [DOI] [PubMed] [Google Scholar]
- Sathish M, Manickam A, Prakasham R, Kannaiah S (2024) In silico studies of Byttneria herbacea Roxb. Bioactive compounds against anti-inflammatory (COX-1) protein, JMBFS, 13(5):e10249. 10.55251/jmbfs.10249
- Shapira N (2017) The potential contribution of dietary factors to breast cancer prevention. Eur J Cancer Prev 26(5): 385–395. 10.1097/CEJ.0000000000000406 [DOI] [PMC free article] [PubMed]
- Sliwoski G, Kothiwale S, Meiler J, Lowe EW. Computational methods in drug discovery. Pharmacol Res. 2014;66(1):334–395. doi: 10.1124/pr.112.007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srimai V, Ramesh M, Parameshwar KS, Parthasarathy T. Computer-aided design of selective cytochrome P450 inhibitors and docking studies of alkyl resorcinol derivatives. Med Chem. 2013;22:5314–5323. doi: 10.1007/s00044-013-0532-5. [DOI] [Google Scholar]
- Stegemann S, Leveiller F, Franchi D, De Jong H, Linden H. When poor solubility becomes an issue: from early stage to proof of concept. Eur J Pharm Sci. 2007;31:249–261. doi: 10.1016/j.ejps.2007.05.110. [DOI] [PubMed] [Google Scholar]
- Sumithira G, Kavya V, Ashma A, Kavinkumar MC (2017) A Review of Ethanobotanical and Phytopharmacology of Ottelia alismoides (L.) PERS. IJRPP 6(3);302–311
- Suzuki H, Sugiyama Y. Role of metabolic enzymes and efflux transporters in the absorption of drugs from the small intestine. Eur J Pharm Sci. 2000;12:3–12. doi: 10.1016/S0928-0987(00)00178-0. [DOI] [PubMed] [Google Scholar]
- Tiwari G, Wang S, Tang J, Birla SL. Analysis of radio frequency (RF) power distribution in dry food materials. J Food Eng. 2011;104(4):548–556. doi: 10.1016/j.jfoodeng.2011.01.015. [DOI] [Google Scholar]
- TsaiYuan C, Tu YP, Wei WY, Chen HY, Chen CS, Lee YSE, Huang JJ, Sha CK (2012) Synthesis and antiproliferative activities of ottelione a analogues. ACS Med Chem Lett 3(12):1075–1080. [DOI] [PMC free article] [PubMed]
- Ur Rashid H, Xu Y, Muhammad Y, Wang L, Jiang J. Research advances on anticancer activities of matrine and its derivatives: an updated overview. Eur J Med Chem. 2019;161:205–238. doi: 10.1016/j.ejmech.2018.10.037. [DOI] [PubMed] [Google Scholar]
- Valeriote F, Grieshaber CK, Media J, Pietraszkewics H, Hoftmann J. Discovery and development of anticancer agents from plants. J Exp Ther Oncol. 2002;2:228–236. doi: 10.1046/j.1359-4117.2002.01038.x. [DOI] [PubMed] [Google Scholar]
- Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 45:2615–2623. 10.1021/jm020017n [DOI] [PubMed]
- Velmurugan G, Anand SP. Biosciences and Plant Biology. Int J Curr Res Biosci Plant Biol. 2017;4(8):67–72. doi: 10.20546/ijcrbp.2017.408.009. [DOI] [Google Scholar]
- Vijayakumar AS, Jeyaraj M, Selvakumar M, Abirami E. Pharmacological activity of silver nanoparticles, ethanolic extract from Justicia gendarussa (burm) f plant leaves. Res j life sci Bioinform. 2019;5(2):462–475. doi: 10.26479/2019.0502.33. [DOI] [Google Scholar]
- Wang Y, Xing J, Xu Y, Zhou N, Peng J, Xiong Z, Liu X, Luo X, Luo C, Chen K, Zheng M. Silico ADMET modelling for rational drug design. Q Rev Biophys. 2015;48:488–515. doi: 10.1017/S0033583515000190. [DOI] [PubMed] [Google Scholar]
- Yadav MK, Upadhyay P, Purohit S, Pandey BL, Shah H. Phytochemistry and pharmacological activity of Mucuna pruriens: a review. Int J Green Pharm. 2017;11(2):69–73. [Google Scholar]
- Yedjou CG, Sims JN, Miele L, Noubissi F, Lowe L, Fonseca DD, Alo RA, Payton M, Tchounwou PB (2019) Health and racial disparity in breast cancer. Breast cancer Metastasis drug Resistance: Challenges Progress 31–49 [DOI] [PMC free article] [PubMed]
- Yusuf AJ, Adegboyega AE, Yakubu AH, Johnson GI, Asomadu RO, Adeduro MN, Chukwuma IF, Ugwah-Oguejiofor CJ, Okoh OS, Johnson TO. Exploring Scutellaria baicalensis bioactives as EGFR tyrosine kinase inhibitors: Cheminformatics and molecular docking studies. IMU. 2023;43:101406. doi: 10.1016/j.imu.2023.101406. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.