Abstract
Primary graft dysfunction (PGD) is a severe form of acute lung injury resulting from lung ischemia/reperfusion injury (I/R) in lung transplantation (LTx), associated with elevated post-transplant morbidity and mortality rates. Neutrophils infiltrating during reperfusion are identified as pivotal contributors to lung I/R injury by releasing excessive neutrophil extracellular traps (NETs) via NETosis. While alveolar macrophages (AMs) are involved in regulating neutrophil chemotaxis and infiltration, their role in NETosis during lung I/R remains inadequately elucidated. Extracellular histones constitute the main structure of NETs and can activate AMs. In this study, we confirmed the significant involvement of extracellular histone-induced M1 phenotype of AMs (M1-AMs) in driving NETosis during lung I/R. Using secretome analysis, public protein databases, and transwell co-culture models of AMs and neutrophils, we identified Cathepsin C (CTSC) derived from AMs as a major mediator in NETosis. Further elucidating the molecular mechanisms, we found that CTSC induced NETosis through a pathway dependent on NADPH oxidase-mediated production of reactive oxygen species (ROS). CTSC could significantly activate p38 MAPK, resulting in the phosphorylation of the NADPH oxidase subunit p47phox, thereby facilitating the trafficking of cytoplasmic subunits to the cell membrane and activating NADPH oxidase. Moreover, CTSC up-regulated and activated its substrate membrane proteinase 3 (mPR3), resulting in an increased release of NETosis-related inflammatory factors. Inhibiting CTSC revealed great potential in mitigating NETosis-related injury during lung I/R. These findings suggests that CTSC from AMs may be a crucial factor in mediating NETosis during lung I/R, and targeting CTSC inhition may represent a novel intervention for PGD in LTx.
Keywords: Lung ischemia-reperfusion injury, NETosis, Alveolar macrophages, Cathepsin C, NADPH oxidase
Graphical abstract
1. Introduction
Lung transplantation (LTx) has emerged as a life-saving option for thousands of patients with end-stage lung disease (ESLD) [1]. While significant progress has been achieved in organ preservation, surgical techniques, and transplant rejection treatment in recent years, the persistent challenge of primary graft dysfunction (PGD) continues to impact post-LTx morbidity and mortality [2,3]. PGD typically presents within the first 72 h after LTx, and lung ischemia/reperfusion (I/R) injury is widely recognized as a primary trigger of PGD [4]. The influx of neutrophils into the alveolar space is a prominent feature upon reperfusion, and they function as pivotal executive cells in the inflammatory cascade during lung I/R [5]. Neutrophil extracellular traps (NETs) released from activated neutrophils play a key role in trapping and eliminating pathogenic microorganisms [6]. However, the cell death resulting from exaggerated NETs formation can have detrimental effects, which has been termed NETosis since 2004 and has been demonstrated to exacerbate lung I/R injury and contribute to PGD [[7], [8], [9], [10], [11]].
Importantly, the occurrence of NETosis is not an independent event of neutrophils but is regulated by a variety of activated immune cells, such as platelets, non-classical monocytes, and NK cells [8,12,13]. These cells facilitate neutrophil recruitment and trigger NETosis during lung I/R. Alveolar macrophages (AMs) are essential tissue-resident cells located within the alveolar cavity, and they represent among the earliest responders to injury stimuli [14]. The crucial role of cross-talk between macrophages and neutrophils in regulating the innate immune response has been explored [[15], [16], [17]]. In murine models of acute lung injury (ALI) following hemorrhagic shock, AMs were demonstrated to induce neutrophil necroptosis by secreting exosomes [18]. Additionally, neutrophil-released NETs could drive the M1 phenotype of AMs (M1-AMs), leading to pyroptosis occurrence and ALI exacerbation in mice [19]. However, the impact of AMs on NETosis remains less explored.
In 2009, Xu et al. identified extracellular histones as novel damage-associated molecular patterns (DAMPs) [20]. Remarkably, these histones are integral components of NETs and have been acknowledged as markers for NETosis [21]. Our previous research revealed a notable elevation in plasma histone levels in patients after LTx, with higher levels detected in patients with PGD compared to those without PGD [22]. We further observed that extracellular histones activated AMs through NLRP3 inflammasome activation [23]. Considering the involvement of AMs in the initial inflammatory response and the prolonged inflammation orchestrated by neutrophils during the later reperfusion phase, we hypothesize that AMs activated by extracellular histones could release inflammatory mediators, leading to neutrophil recruitment and subsequent NETosis occurrence. This process, in turn, results in the release of more extracellular histones, establishing a vicious cycle that worsens lung I/R injury.
Cathepsin C (CTSC) is a cysteine exopeptidase primarily located within lysosomes. By cleaving the N-terminal dipeptide from substrates, CTSC selectively activates neutrophil serine proteases (NSPs) [24]. Significantly, activated NSPs participate in chromatin decondensation and subsequent NET release [25,26]. Numerous investigations have suggested CTSC as a promising target for autoimmune or inflammatory diseases [27,28]. Pharmacological inhibition of CTSC is recommended for the treatment of SARS-CoV-2 infection and acute respiratory distress syndrome (ARDS) [29]. Genetic knockout of CTSC has shown significant reductions in inflammatory cytokine levels and their infiltration into target organs in murine models of asthma, sepsis, and abdominal aortic aneurysm [[30], [31], [32]]. Additionally, CTSC secretion by tumor cells and gastric epithelial cells fostered tumor metastasis and activated neutrophils [33,34]. However, research on CTSC in organ I/R injury has been limited.
The leakage of soluble lysosomal enzymes into the cytoplasm occurs as a consequence of changes in lysosomal membrane stability and permeability, known as lysosomal membrane permeability (LMP) [35]. Previous studies have demonstrated that extracellular histones can increase plasma membrane permeability due to their direct cytotoxic effects [[36], [37], [38], [39]]. Moreover, histones within the cytoplasm contribute to the instability of mitochondrial membranes [[40], [41], [42]]. However, the impact of histones on LMP remains uncertain. In this study, we demonstrated that extracellular histones provoked LMP in AMs, resulting in the secretion of CTSC. Additionally, we identified that CTSC derived from AMs promoted NETosis through a pathway dependent on reduced nicotinamide adenine dinuc-leotide phosphate (NADPH) oxidase-mediated reactive oxygen species (ROS) burst. These findings suggest a previously unidentified pathway of crosstalk between AMs and neutrophils, potentially amplifying AM activation and NETosis, thus exacerbating lung I/R injury. Targeting CTSC inhibition may offer an optimized therapeutic approach for the prevention and treatment of lung I/R and PGD during LTx.
2. Materials and methods
2.1. Patients
We enrolled 70 ESLD patients who were admitted to the Department of Thoracic Surgery at Shanghai Pulmonary Hospital, Tongji University, China, and underwent allogeneic single or double LTx surgery from June 2022 to July 2023 (Supplementary Material, Table 1). The exclusion criteria included patients under 18 years old, those lacking preoperative basic information, or those who died during surgery. Venous blood samples (10 ml) were collected from the patients through a central venous catheter at the following time points: before surgery, immediately after surgery, and at 24, 48, and 72 h post-surgery. The samples were processed within 2 h. Additionally, 10 ml of peripheral blood from 20 healthy volunteers was collected to serve as controls. This study received approval from the Institutional Medical Ethics Committee of Shanghai Pulmonary Hospital (No. K22-267Z), and informed consent forms were signed with the patients' representatives.
2.2. Animals and treatments
Male C56BL/6 strain mice, aged 6–8 weeks, were housed in the Experimental Animal Center of Shanghai Pulmonary Hospital. The breeding environment was maintained under specific pathogen-free conditions at a suitable temperature (22–25 °C) and humidity (50–60 %). We established a murine model of lung I/R by clamping the left pulmonary hilum for 2 h followed by 4 h of reperfusion, as described by Sayah et al. [8]. In brief, the mice underwent anesthesia, orotracheal intubation, and left-side thoracotomy. Upon exposure of the left pulmonary hilum, a microvascular clip were used to clamp the pulmonary artery, pulmonary vein, and left bronchus. The successful induction of ischemia was indicated by the left lung exhibiting a dark purple color and lack of expansion. Following 2 h of ischemia, the microvascular clip was removed, and the left lung underwent 4 h of reperfusion. The Sham group involved a left-side thoracotomy and exposure of the pulmonary hilum without clamping. After reperfusion, the mice were euthanized by dislocation, and samples of the left lung, blood, and bronchoalveolar lavage fluid (BALF) were collected. For depletion of AMs in mice, Clodronate liposomes (Clo-lip) (Yeasen, China) were administered at a dose of 150 μl (5 μg/μl) via tail vein injection. Subsequently, the mouse was maintained in an upright position, and 50 μl of Clo-lip was delivered via micropipette for inhalation during spontaneous breathing. After 24 h, the lung I/R model was established. For neutralization of extracellular histones in mice, N-acetylated heparin (Sigma, USA) was intravenously injected at a dosage of 250 U/kg immediately before preparing the lung I/R model. For inhibition of CTSC in mice, AZD7986 (MCE, USA) was intragastrically administered at a dose 1.25 mg/kg twice with a 12-h interval. The lung I/R model was established 12 h after the injection. All experimental procedures were conducted in accordance with the Guidelines for the Management and Use of Laboratory Animals of National Institutes of Health and had received approval from the Institutional Medical Ethics Committee of Shanghai Pulmonary Hospital (K23-199).
2.3. Neutrophil isolation
In this study, human polymorphonuclear neutrophils (PMNs) and bone marrow-derived neutrophils (BMDNs) from mice were isolated. Briefly, human blood samples were centrifuged, and the plasma was separately collected and stored. The blood pellets, diluted with an equal volume of 1 × PBS, were layered on top of Histopaque 1119 (Sigma, USA) and Ficoll-Paque (Cytiva, USA) at a volume ratio of 2:1:1. Following density gradient centrifugation, the PMN layer was collected. These cells underwent washing and treatment with red blood cell lysate (BD Pharming, USA). For the isolation of BMDNs from mice, a mouse bone marrow neutrophil isolation kit (TBD Sciences, China) was used. The femurs and tibias of the mice were isolated, and the bone marrow was flushed repeatedly until it appeared white. Then the cell suspension underwent density gradient centrifugation following the manufacturer's instructions. The BMDN layer was collected, washed, and lysed. Hoechst 33342 staining (Sigma, USA) was used to observe the nucleus morphology, while flow cytometry analysis was conducted to assess the purity of PMNs and BMDNs (Supplementary Material, Figs. S1a–h).
2.4. Transwell co-culture model of AMs and neutrophils and siRNA transfection
The murine AMs (MH-S cell line) were cultured in RPMI 1640 complete culture medium (supplemented with 10 % fetal bovine serum and 1 % penicillin-streptomycin) under conditions of 5 % CO2 at 37 °C. In the co-culture system, MH-S cells were placed in the upper Transwell chamber (Corning, USA), while BMDNs were placed in the lower chamber (Supplementary Material, Figs. S1i and j). Additionally, MH-S were primed with calf thymus histones (Sigma, USA) at a concentration of 50 μg/ml for 3 h before co-culturing with BMDNs. For CTSC inhibition, MH-S cells were transfected with siRNA targeting CTSC mRNA and negative control siRNA at a concentration of 100 nM using Lipofectamine 2000 (Invitrogen, USA) transfection reagent prior to the treatment of calf thymus histones. The different siRNA sequences (GenePharma, China) were provided in Supplementary Material, Table 2.
2.5. Extraction and mass spectrometric-based secretome analysis of primary AMs
To analyze changes in the secretome of AMs stimulated by extracellular histones, we used label-free mass spectrometry (MS). We firstly harvested primary AMs from normal mouse BALF through lavaging the lungs with fresh PBS multiple times. After lavaging, we centrifuged the samples and treated the sedimental cells with red blood cell lysate. Then, we washed and resuspended the cells in complete PRMI 1640 medium. They were then incubated, and non-adherent cells were removed, and adherent cells were exposed to 50 μg/ml of extracellular histones. After 3 h of incubation, we collected the cells for flow cytometry and the supernatant for MS analysis, conducted by Biomarker Technologies Co., Ltd.(China). The processes included protein extraction, quality inspection, enzymatic desalting, liquid-phase fractionation, MS analysis, database comparison, and bioinformatics analysis.
2.6. Flow cytometry analysis
For flow cytometry analysis, cells were centrifuged and resuspended in cold Stain Buffer (BD Pharming, USA). They were then incubated with Fc-block (BD Pharming, USA) to inhibit nonspecific binding mediated by Fc-receptors. For detection of the M1 phenotype of AMs, the MH-S cells were incubated with anti-CD80 (#104705; BioLegend, USA) and anti-CD86 (#159203; BioLegend, USA), and the primary AMs were incubated with anti-F4/80 (#123115; BioLegend, USA), anti-CD80, and anti-CD86 (#159203; BioLegend, USA). For assessing the purity of neutrophils, PMNs were incubated with anti-CD15 (#301903; BioLegend, USA), and BMDNs were incubated with anti-Ly6G (#127613; BioLegend, USA) and anti-CD11b (#101205; BioLegend, USA). Additionally, PMNs were incubated with anti-PR3 (#ab91181; Abcam, UK) to detect the expression of membrane proteinase 3 (mPR3). The data were collected using a BD FACS flow cytometer.
2.7. Immunofuorescence staining
To analyze the components of in vitro NETs, PMNs or BMDNs at a concentration of 5 × 105 cells were seeded on the sterile cell slides pre-coated with poly-l-lysine (Sigma, USA) in 24-well plates. Then these cells were immobilized for 10 min with 4 % paraformaldehyde and blocked for 30 min with immunostaining blocking solution. They were then incubated overnight at 4 °C with anti-histone H3 (dilution at 1:1000; #ab5103; Abcam, UK) and anti-myeloperoxidase (MPO) (dilution at 1:200 or 1:1000; #GB14115 or #GB12224; Servicebio, China). The following day, the cells were incubated with the respective secondary antibodies (dilution at 1:200; Proteintech, China). To visually observe the morphology of NETs, PMNs were incubated with 1 μM of SYTOX Green dye (Thermo Fisher Scientific, USA) for 15 min. Additionally, to evaluate the impact of extracellular histones on LMP, MH-S cells were incubated with Anti-CTSC (dilution at 1:100; #sc-74590; Santa Cruz, USA), anti-LAMP1 (dilution at 1:100; #sc-20011; Santa Cruz, USA), and Anti-Galectin-3 (dilution at 1:50; #14979-1-AP; Proteintech, China) along with their corresponding secondary antibodies.
For immunofluorescence staining of paraffin-embedded lung tissues, the tissues were sectioned, de-waxed, subjected to antigen retrieval, and then blocked with an immunostaining blocking solution. To assess neutrophil infiltration, slides were incubated overnight at 4 °C with anti-Ly6G antibody (diluted at 1:100; #sc-53515; Santa Cruz, USA). For the analysis of NET components in lung tissue, the slides were incubated with anti-histone H3 (dilution at 1:1000; #ab5103; Abcam, UK) and anti-MPO (dilution at 1:1000; #GB12224; Servicebio, China). Moreover, to elucidate the distribution of CTSC in murine lung tissue, the slides were incubated with Anti-CTSC, anti-podoplanin (diluted at 1:500; #ab256559; Abcam, UK), anti-prosurfactant (ProSPC) (diluted at 1:500; #ab211326; Abcam, UK), and anti-F4/80 (diluted at 1:200; #28463-1-AP; Proteintech, China) along with their respective secondary antibodies. Finally, the cells or sections were stained with Hoechst 33342 for nucleus staining, and then observed and photographed using an Olympus microscope.
2.8. Detection of circulating NETs
To analyze circulating NETs, double stranded DNA (dsDNA) and citrullinated histone3 (CiH3) in plasma, BALF and cell supernatant were detected using the PicoGreen dsDNA Quantification Kit (Solarbio, China) and the Citrullinated Histone H3 ELISA Kit (Cayman Chemical, USA), respectively. All experimental manipulations were conducted following the manufacturer's instructions.
2.9. Western blot analysis
For protein extraction, cells were collected and lysed with RIPA lysis buffer containing protease inhibitors on ice for 15 min. Frozen tissues (20 mg) were mixed with RIPA lysis buffer and homogenized using a high-speed cryogenic tissue grinder, followed by further lysis on ice for 15 min. The lysate of cells or tissues was centrifuged at 12,000 g for 15 min at 4 °C. After centrifugation, the supernatant was collected. The protein concentration was measured using a BCA Protein Assay Kit (Beyotime, China). These proteins were separated on SDS-PAGE and transferred onto PVDF membranes. After blocking, primary antibodies were added and incubated overnight at 4 °C. Proteins in MH-S cells were incubated with anti-inducible nitric oxide synthase (iNOS) (diluted at 1:1000; #EPR16630; Abcam, UK) to detect the M1 phenotype. Proteins in PMNs, BMDNs, and lung tissues were incubated with anti-histone H3 (diluted at 1:1000; #ab5103; Abcam, UK) and anti-protein arginine deiminase type 4 (PAD4) (diluted at 1:1000; #ab96758; Abcam, UK). Proteins in PMNs were incubated with anti-p38 mitogen-activated protein kinase (MAPK) (diluted at 1:1000; #8690T; CST, USA), anti-phospho-p38 MAPK (diluted at 1:1000; #4511T; CST, USA), anti-p67phox (diluted at 1:5000; ab109523; Abcam, UK), anti-p47phox (diluted at 1:500; A5143; ABclonal, China) and anti-phospho-p47phox (diluted at 1:500; ab63554; Abcam, UK).
Furthermore, to evaluate the protein expression of CTSC in lysosomes or cytoplasm, the Lysosome Isolation Kit (Sigma; USA) was used based on centrifugation density gradient centrifugation, following the manufacturer's instructions. After obtaining the lysosome and cytoplasmic fractions, they underwent the aforementioned protein extraction process and were incubated with anti-CTSC (diluted at 1:250; #sc-74590; Santa Cruz, USA). For detection the protein expression of NADPH oxidase subunits and mPR3, the Mem-PER Plus Membrane Protein Extraction Kit (Thermo Fisher Scientific, USA) was used. Plasma membrane and cytoplasmic proteins were extracted according to the manufacturer's instructions. They were then incubated with anti-p67phox, anti-p47phox, and anti-PR3 (diluted at 1:1000; #ab91181; Abcam, UK). As internal controls, anti-GAPDH (total), anti-β-actin (total), anti-LAMP1 (lysosome), and anti-Na+/K+ATPase (plasma membrane) were used.
2.10. AO staining and PI staining
Acridine Orange (AO) is a weakly alkaline fluorescent dye capable of permeating both cell and lysosomal membranes. When the lysosomal membrane remains intact, AO emits orange-red fluorescence when excited by a 488 nm light source within the lysosome. Conversely, if the lysosomal membrane is compromised and LMP occurs, AO displays green fluorescence when excited by a 488 nm light source in the cytoplasm [43,44]. Additionally, we used the Calcein-AM/PI Double Stain Kit (Yeasen, China) to evaluate the membrane permeability of MH-S cells. Propidium Iodide (PI) is unable to enter cells when the cell membrane is intact. Calcein AM characterized by strong hydrophobicity can permeate cell membrane and bind to DNA, generating green fluorescence. When the cell membrane's permeability increases, PI can infiltrate the cell and bind to DNA, generating red fluorescence [45]. In the experiment, MH-S cells seeded in a 6-well culture plate were washed with PBS, resuspended in 1 ml of AO working solution (5 μg/ml) (Sigma, USA) or 1 ml of calcein-AM/PI working solution (1 μM PI and 1 μM Calcein-AM), and then incubated at 37 °C for 30 min. The fluorescence intensity of AO or PI staining was observed under an Olympus microscope and images were captured.
2.11. Detection of intracellular ROS and mitochondrial superoxide levels
The Reactive Oxygen Species Assay Kit (Solarbio, China) and MitoSOX Red Mitochondrial Superoxide Indicator (Yeasen, China) were used to detect intracellular ROS and mitochondrial superoxide levels, respectively. Briefly, PMNs seeded in a 24-well culture plate pre-coated with poly-l-lysine were washed with PBS, resuspended in 200 μl of DCFH-DA working solution (10 mM) or 200 μl of MitoSOX Red working solution (5 μM), and then incubated at 37 °C for 30 min. The fluorescence intensity of DCFH-DA or MitoSOX Red was observed under an Olympus microscope and images were captured.
2.12. Detection of NADPH oxidase activity
The NADP+/NADPH detection kit (Beyotime, China) based on the WST-8 method was used to detect the activity of NADPH oxidase in PMNs. According to the manufacturer's instructions, PMNs in each sample were divided into two aliquots. One aliquot containing both NADP+ and NADPH was lysed on ice for 15 min, while the other was incubated at 60 °C for 30 min to deplete NADP+ and retain only NADPH. After centrifugation, 100 μl of G6PDH working solution was added to the sample wells and incubated in the dark for 10 min at 37 °C. The samples were then measured at 450 nm using a fluorescent microplate reader to determine the optical density (OD) value. The ratio of NADP+/NADPH was calculated.
2.13. Detection of PR3 activity
The detection of proteinase 3 (PR3) activity was based on the cleavage of a fluorescent substrate. Each PMN sample was resuspended in 200 μl of assay buffer containing 50 mM HEPES, 150 mM NaCl, and 0.05 % Triton X-100. Then, 2 μl of substrate solution with 1 mM of Abz-Val-Ala-Asp-Nva-Arg-Asp-Arg-Gln-EDDnp (GlpBio, USA) was added to each sample. The reaction mixture was transferred to a fluorescent microplate reader, where the fluorescence signal was monitored using an excitation wavelength of 360 nm and emission wavelength of 460 nm, with intensity data recorded every minute for 30 min.
2.14. Assessment of lung histology
The morphological changes in murine lung tissue were evaluated using hematoxylin and eosin (HE) staining. Briefly, the paraffin-embedded lung tissues were sectioned, de-waxed, stained with hematoxylin and eosin, dehydration, and then cleared. Then they were observed and captured under an Olympus microscope. The lung injury scores were determined based on specific histological elements, including alveolar congestion, infiltration of red blood cells, neutrophil infiltration or aggregation in the alveoli or vessel walls, and thickness of the alveolar wall or hyaline membrane formation. Each element was graded from 0 (indicating minimal damage) to 4 (indicating maximal damage), and the scores were summed to derive the overall lung injury scores.
2.15. ELISA analysis
To detect the levels of tumor necrosis factor (TNF-α) and interleukin-1β (IL-1β) in murine BALF and plasma, the Mouse TNF-α ELISA Kit (Cusabio, China) and Mouse IL-1β ELISA Kit (Cusabio, China) were used. Additionally, the Mouse CTSC ELISA Kit (ABclonal, China) was used to measure the levels of CTSC in murine BALF and cell supernatant. Human TNF-α ELISA Kit (Cusabio, China) and Human IL-1β ELISA Kit (Cusabio, China) were used to detect TNF-α and IL-1β levels in PMN supernatant. The experiments were conducted according to the manufacturer's instructions.
2.16. Statistical analysis
Data analysis was conducted using SPSS statistics 20 (IBM, USA). Following Shapiro-Wilk test, normally distributed continuous variables were presented as mean ± standard deviations. For data meeting the assumptions of normality and homogeneity of variance, Student's t-test was used for comparisons between two groups, while one-way analysis of variance (ANOVA) with the LSD or Bonferroni post hoc test was used for comparisons involving more than two groups. If the data did not meet these criteria, the Mann-Whitney U test was used for two-group comparisons, and the Kruskal-Wallis test for comparisons involving more than two groups. Statistical significance was set at P < 0.05. GraphPad Prism 9.0 (San Diego, USA) was used for generating the figures.
3. Results
3.1. NETosis is increased following clinical LTx and lung I/R in mice
Firstly, we confirmed the occurrence of NETosis following clinical lung I/R in LTx. As depicted in Fig. 1a, the immunofluorescence staining in PMNs post-LTx revealed heightened fiber NET components of MPO and CiH3 compared to both healthy controls and pre-LTx PMNs. Given that the PAD4-dependent histone citrullination event is pivotal for NETosis, and PAD4 and CiH3 expression are considered relevant markers of NETosis [46,47], we detected their protein expression in PMNs. Our western blot analysis revealed a significant increase in PAD4 and CiH3 expression in PMNs post-LTx compared to both pre-LTx PMNs and healthy controls (Fig. 1b). Moreover, since dsDNA and CiH3 within NETs can be released into circulation, we further measured their concentration in plasma. The CiH3 concentration in plasma significantly increased 24 h after LTx compared to pre-LTx and healthy controls (Fig. 1c). The dsDNA concentration within 3 days post-LTx exhibited a significant increase compared to pre-LTx levels (Fig. 1d). The average level of dsDNA within three days post-LTx was significantly higher than that in healthy controls (Fig. 1e). Additionally, LTx patients experiencing PGD displayed higher dsDNA levels in plasma compared to those who did not develop PGD (Fig. 1f). These findings indicate a significant increase in PMN NETosis following clinical lung I/R in LTx.
Fig. 1.
Elevated NETosis is observed after clinical LTx and lung I/R in mice.
a. Representative immunofluorescence images of CiH3 and MPO in PMNs from healthy controls and LTx patients. Scale Bar = 50 μm. b. Protein levels of PAD4 and CiH3 in PMNs from healthy controls and LTx patients were detected using western blot (n = 3 per group). c. The levels of plasma CiH3 of healthy controls and LTx patients were detected using ELISA (n = 8 per group). d. The levels of plasma dsDNA of LTx patients at different time points were detected using the Picogreen method (n = 28 per group). e. The levels of plasma dsDNA were compared between LTx patients and healthy control individuals (n ≥ 20 per group). f. The levels of plasma dsDNA were compared between PGD patients and those without PGD (n ≥ 10 per group). g. Representative immunofluorescence images of Ly6G in murine lung subjected to I/R are present, accompanied by the mean optical density of Ly6G (n = 6 per group). Scale Bar = 20 μm. h. The percentage of neutrophils stained with Ly6G and CD11b in the BALF of mice subjected to I/R was detected by flow cytometry (n = 6 per group). i. Representative images demonstrating NET presence through co-expression of CiH3 and MPO in the lung subjected to I/R, marked by white arrows. Scale Bar = 20 μm. j. Protein levels of PAD4 and CiH3 in murine lung subjected to I/R were detected using western blot (n = 3 per group). k. & l. The levels of CiH3 and dsDNA in the plasma and BALF of mice subjected to I/R were detected using ELISA and Picogreen method (n = 6 per group). Graphs represent means ± standard deviations. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ns signifies P > 0.05 when comparing data between two groups.
Next, we established a murine model of lung I/R. HE staining revealed disrupted alveolar structural, notable thickening of alveolar septa, infiltration of numerous inflammatory cells, and accumulation of exudate within the alveolar cavity in the I/R group (Fig. S2a). The lung injury score was significantly higher in the I/R group compared to the Sham group (Fig. S2a). Additionally, mice in the I/R group exhibited reduced oxygenation index (Fig. S2b), increased total protein content (Fig. S2c) in BALF, and elevated levels of TNF-α and IL-1β in both BALF and plasma (Figs. S2d and e). These findings confirm the successful establishment of the murine model of lung I/R injury. The immunohistochemical analysis of Ly6G in I/R group, a specific marker for murine neutrophils, revealed significant neutrophil infiltration in the lung tissue (Fig. 1g), accompanied by an elevated count of CD11b+Ly6G+ cells in BALF (Fig. 1h). Furthermore, the I/R group exhibited increased NET formation in lung tissues (Fig. 1i), along with increased expression of PAD4 and CiH3 compared to the Sham group (Fig. 1j). Levels of CiH3 and dsDNA in the blood and BALF were also significantly elevated in the I/R group compared to the Sham group (Fig. 1k and l). These findings collectively suggest that neutrophil infiltration and enhanced NETosis occurrence are prominent features of lung I/R injury in mice.
3.2. NETosis is closely associated with extracellular histone-induced M1-AMs during lung I/R
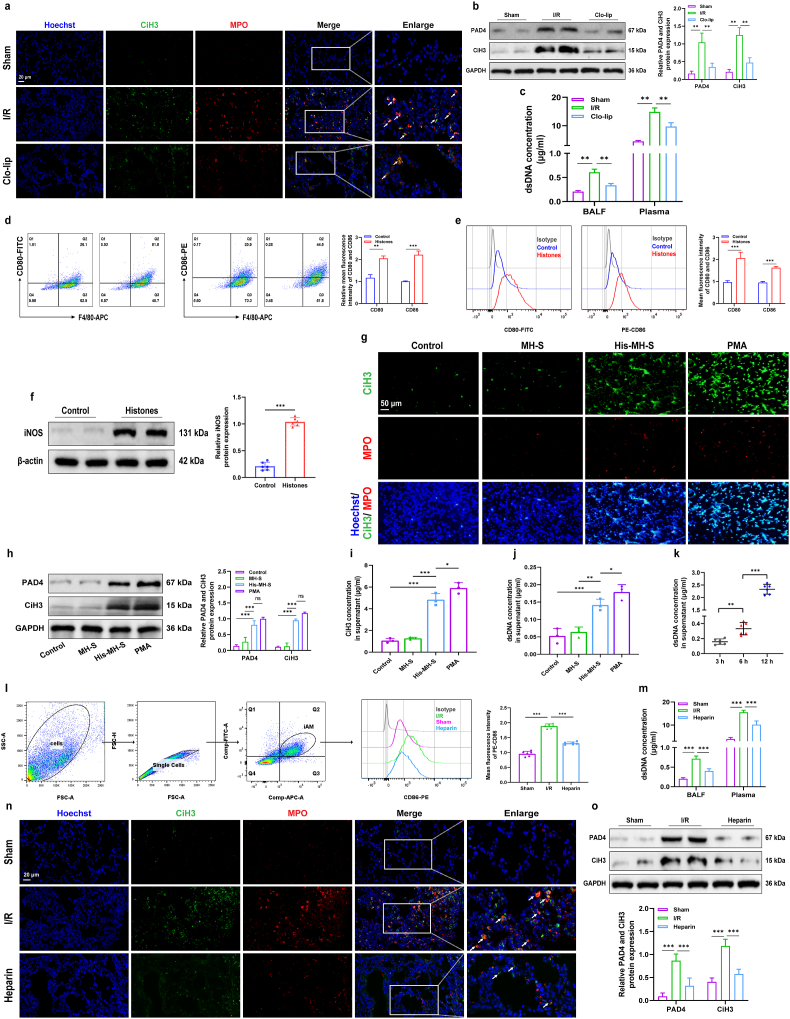
NETosis is regulated by various immune cells [48]. To explore the involvement of AMs in NETosis during lung I/R, we administered Clo-lip via tail vein and nasal injection to deplete AMs before establishing the lung I/R model. Flow cytometry and immunofluorescence staining results confirmed successful AM depletion (Figs. S2f and g). Immunofluorescence staining of NET components was significantly diminished in the Clo-lip group compared to the I/R group (Fig. 2a). The protein expression of PAD4 and CiH3 in lung tissue of the Clo-lip group was markedly down-regulated compared to the I/R group (Fig. 2b), along with a decreased level of dsDNA in both blood and BALF (Fig. 2c). These results suggest a potential pivotal role of AMs in NETosis during lung I/R. However, depleting AMs or neutrophils is not feasible in clinical settings because macrophages play a crucial role in fighting infections, tissue repair, and maintaining immune homeostasis [14]. Neutrophils are essential for the initial immune response, contributing to the engulfment and elimination of invading pathogens [6]. Considering that histones can activate AMs and are the primary component of NETs, we hypothesized the presence of cross-talk between AMs and neutrophils during lung I/R. Subsequently, we investigated the impact of extracellular histone-stimulated AMs on NETosis.
Fig. 2.
Extracellular histone-induced M1-AMs play a pivotal role in NETosis during lung I/R.
a. Representative images demonstrating NET presence through co-expression of CiH3 and MPO in the lung subjected to I/R and Clo-lip treatment, marked by white arrows. Scale Bar = 20 μm. b. Protein levels of PAD4 and CiH3 in lung tissues of mice subjected to I/R and pretreated with Clo-lip were detected using western blot (n = 3 per group). c. The levels of dsDNA in the plasma and BALF of mice subjected to I/R and pretreated with Clo-lip were detected using the Picogreen method (n = 6 per group). d. & e. The M1 polarization in primary AMs and MH-S cell lines treated with extracellular histones was assessed based on CD80 or CD86 expression using flow cytometry (n = 3 per group). f. iNOS expression in MH-S cells treated with extracellular histones was assessed by western blot (n = 3 per group). g. Representative immunofluorescence images of CiH3 and MPO in BMDNs co-cultured with extracellular histone-treated MH-S cells are present and PMA serves as a positive control. Scale Bar = 50 μm. h. Protein levels of PAD4 and CiH3 in BMDNs co-cultured with extracellular histone-treated MH-S cells were detected using western blot (n = 3 per group). i. & j. The levels of CiH3 and dsDNA in the supernatant of BMDNs co-cultured with extracellular histone-treated MH-S cells were detected using ELISA and Picogreen method, with PMA serving as a positive control (n = 3 per group). k. The levels of dsDNA in the supernatant of BMDNs co-cultured with extracellular histone-treated MH-S cells at different time points were detected using the Picogreen method (n = 6 per group). l. The percentage of M1-AMs stained with F4/80, CD11b, and CD86 in the BALF of mice subjected to I/R and pretreated with heparin was detected using flow cytometry (n = 6 per group), and the F4/80 and CD11b positive population represents activated AMs (iAMs). m. The levels of dsDNA in murine plasma and BALF were detected using the Picogreen method (n = 6 per group). n. Representative images demonstrating NET presence through co-expression of CiH3 and MPO in the lung subjected to I/R and heparin treatment, marked by white arrows. Scale Bar = 20 μm. o. Protein levels of PAD4 and CiH3 in the lung of mice subjected to I/R and pretreated with heparin were detected using western blot (n = 3 per group). Graphs represent means ± standard deviations. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ns signifies P > 0.05 when comparing data between two groups.
The induction of M1-AMs by extracellular histones was verified. We extracted primary AMs from mice BALF and stimulated them with 50 μg/ml of extracellular histones for 3 h. As depicted in Fig. 2d, a significant increase in CD80 and CD86 membrane expression of AMs was observed after histone stimulation, suggesting M1 polarization of AMs. This finding was further corroborated in MH-S cell lines (Fig. 2e). Additionally, we observed elevated protein expression of iNOS in MH-S cells, another marker of M1 polarization, demonstrating a significant upregulation in the histone-treated group (Fig. 2f). Next, we established a Transwell co-culture model involving extracellular histone-stimulated MH-S cells and BMDNs. Unstimulated MH-S cells were used as negative controls, and PMA (50 nM) treatment as positive controls. Immunofluorescence and western blot analysis revealed that histone-stimulated MH-S cells significantly promoted NETosis, evidenced by an increase in fiber NET components of MPO and CiH3 (Fig. 2g), along with elevated protein expression of PAD4 and CiH3 (Fig. 2h). Furthermore, co-culturing BMDNs with histone-stimulated MH-S cells resulted in a significant increase in CiH3 and dsDNA concentration in the BMDN supernatant (Fig. 2i and j). Importantly, the increase in dsDNA levels showed a time-dependent trend, reaching its peak after 12 h of co-culture (Fig. 2k). These findings collectively suggest that extracellular histone-induced M1-AMs notably enhance NETosis.
Finally, we administered the histone neutralizer, heparin, intravenously before establishing the lung I/R model to ascertain the role of extracellular histones in lung I/R injury. Flow cytometry analysis revealed a significant reduction in the proportion of M1-AMs in the BALF of the heparin-treated group (Fig. 2l). Both BALF and blood dsDNA concentrations significantly decreased after heparin injection (Fig. 2m). Immunofluorescence staining and western blot analysis of NETosis makers in lung tissues indicated a notable reduction in NETosis occurrence following heparin administration (Fig. 2n and o). These findings underscore the pivotal role of histone-induced M1-AMs in triggering neutrophil NETosis during lung I/R injury.
3.3. Extracellular histones promote the secretion of CTSC from AMs
Based on the above results, we hypothesize that AMs may secrete specific substances that mediate NETosis under extracellular histone stimulation. We first analyzed the secretion profile of AMs stimulated by extracellular histoneion using label-free mass spectrometry. The score plot of principal component analysis (PCA) revealed complete separation between the histone-treated group and the control group (Fig. 3a). The levels of secreted proteins in each group were depicted in the heatmap (Fig. 3b). Using criteria of fold change (FC) ≥ 1.2 and P value ≤ 0.05, 490 up-regulated proteins were identified in the histone-treated group. Notably, KEGG enrichment analysis indicated significant enrichment of up-regulated proteins from the histone-treated group in the Lysosome pathway (Fig. 3c), with CTSC being one of the prominently up-regulated proteins in the Lysosome pathway (Fig. 3d).
Fig. 3.
CTSC is enriched in AMs and extracellular histones trigger CTSC secretion through LMP.
a. Analyses of PCA in different replicated protein samples. b. Heatmap showing the expression landscape of all of the quantified proteins. c. Bubble diagram illustrating the KEGG pathway enrichment of up-regulated secreted proteins in the histone-treated group. d. Heatmap showing expression of lysosomal proteins involved in KEGG-enriched pathways. e. Relative expression levels of CTSC mRNA in different human tissues within the Consensus dataset. f. Relative expression levels of CTSC mRNA in different cell types within human lung tissue. g. A general overview of CTSC mRNA enrichment in different cell types and tissues. h. Representative immunofluorescence images depicting makers for AMs (F4/80) and alveolar epithelial cells (Podoplanin and ProSPC) co-localized with CTSC in murine lung tissues are present. Scale Bar = 50 μm. i. Protein levels of CTSC in various murine tissues were detected using western blot (n = 3 per group). j. & k. Concentrations of CTSC in the supernatant of MH-S cells and murine BALF were measured using ELISA (n = 6 per group). l. Protein levels of CTSC in the cytoplasm and lysosome of MH-S cells treated with extracellular histones were detected using western blot (n = 3 per group). m. Representative AO staining images of MH-S cells treated with extracellular histones or LPS. Scale Bar = 20 μm. n. Representative immunofluorescence images of CTSC in MH-S cells treated with extracellular histones or LPS. Scale Bar = 20 μm. o. Representative immunofluorescence images depicting makers for lysosome (LMP1) co-localized with Galectin-3 in MH-S cells treated with extracellular histones or LPS. Scale Bar = 20 μm. p. Representative images and quantitative analysis of staining for Calcein-AM and PI in MH-S cells treated with extracellular histones (n = 6 per group). Scale Bar = 50 μm. Graphs represent means ± standard deviations. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ns signifies P > 0.05 when comparing data between two groups.
Further exploration of the Human Protein Atlas database revealed high expression of CTSC mRNA in human lung tissue (Fig. 3e, Fig. S3a). Analysis of CTSC mRNA expression in various peripheral blood cell types revealed a notably elevated level in monocytes compared to other cell types (Figs. S3b and c). Single-cell type specificity and tissue cell specificity analysis of mRNA underscored the pronounced enrichment of CTSC mRNA in the mononuclear phagocyte system and alveolar macrophages (Fig. 3f and g, Fig. S3d). A comparative analysis of mRNA levels for various Cathepsins (CTSA, CTSB, CTSC, CTSD, CTSE, CTSK, CTSL, CTSS, CTSV, and CTSX) in THP1 (a human monocytic leukemia cell line) and A549 (a human lung cancer alveolar basal epithelial cell line) was conducted. Strikingly, the relative mRNA levels of CTSC in THP1 were much higher than those in A549 (Fig. S3e). Moreover, our immunofluorescence analysis of murine lung demonstrated significant co-localization of CTSC with the macrophage marker F4/80, while co-localization with the alveolar type I epithelial cell marker podoplanin and alveolar type II epithelial cell marker ProSPC was minimal (Fig. 3h). Our western blot results further confirmed the high expression of CTSC protein in murine lung tissues (Fig. 3i). Based on both database findings and our experimental results, we concluded that CTSC is highly enriched in AMs. ELISA results indicated significantly elevated levels of CTSC in the supernatant of MH-S cells treated with extracellular histones compared to both the control and LPS-treated groups (Fig. 3j). Moreover, CTSC levels in the BALF from the I/R group were significantly elevated compared to the Sham group. However, following heparin administration, CTSC levels notably decreased (Fig. 3k). These findings suggest that extracellular histones may play a role in mediating the secretion of CTSC from AMs during lung I/R.
Next, we investigated the mechanism by which extracellular histones promote the secretion of CTSC from AMs. Since CTSC is located in the lysosome, we conducted experiments to separate lysosomal and cytoplasmic proteins, confirming that histone treatment induced an elevation in CTSC protein levels in the cytoplasm (Fig. 3l). Then we explored the effect of histones on LMP using several staining methods. The AO staining results revealed an increased green fluorescence intensity in the histone-treated group, whereas both the Control and LPS groups exhibited orange fluorescence (Fig. 3m). Immunofluorescence analysis of CTSC within MH-S cells demonstrated diffuse staining in the histone-treated group, while the Control and LPS groups displayed speckled staining patterns (Fig. 3n). Furthermore, Galectin-3, a marker indicating lysosomal membrane damage, was used to assess LMP [49]. Upon LMP occurrence, the N-acetylglucosamine subunit on the lysosomal membrane becomes exposed, facilitating the recruitment of Galectin-3 and resulting in enhanced co-localization with the lysosomal membrane marker LAMP1 under fluorescence microscopy. Our findings indicated increased co-localization of Galectin-3 and LAMP1 in histone-treated cells (Fig. 3o), confirming the occurrence of LMP. Additionally, PI staining was conducted to assess cell membrane permeability following histone stimulation. The results revealed a significant increase in PI-positive cells after extracellular histone treatment (Fig. 3p). These findings collectively demonstrate that extracellular histones induce LMP in AMs and increase cell membrane permeability, consequently leading to elevated secretion of CTSC.
3.4. NETosis is induced by CTSC derived from AMs
To investigate the role of AM-derived CTSC in mediating NETosis, we used small interfering RNA (siRNA) to silence CTSC expression in MH-S cells. The transfection efficiency was determined by western blot analysis (Fig. 4a). Subsequently, MH-S cells transfected with siCTSC-2 were subjected to 3 h of extracellular histone stimulation. Western blot and ELISA analysis revealed that the levels of CTSC in the cytoplasm and supernatant did not increase following extracellular histone stimulation (Fig. 4b and c). Additionally, NETosis was inhibited in BMDNs when co-cultured with siRNA-treated and histone-stimulated MH-S cells. This inhibition was evidenced by a decrease in the network structure of NETs (Fig. 4d), as well as reduced protein expression of PAD4 and CiH3 (Fig. 4e) and the concentration of dsDNA in the BMDN supernatant (Fig. 4f). These results underscore the significant role of CTSC derived from AMs in promoting NETosis.
Fig. 4.
CTSC derived from AMs leads to NETosis.
a. Protein levels of CTSC in MH-S cells treated with various siRNA sequences were detected using western blot (n = 3 per group). b. Protein levels of CTSC in the cytoplasm and lysosome of MH-S cells treated with extracellular histones and siCTSC were detected using western blot (n = 3 per group). c. Concentrations of CTSC in the supernatant of MH-S cells treated with extracellular histones and siCTSC were measured using ELISA (n = 3 per group). d. Representative images of NET formation stained with SYTOX Green were presented and quantifed (Scale Bar = 50 μm). e. & f. Protein levels of PAD4 and CiH3 (n = 3 per group) and concentrations of dsDNA in the supernatant (n = 3 per group) of BMDNs co-cultured with siRNA-treated and histone-stimulated MH-S cells were presented. g. & h. Concentrations of dsDNA in the supernatant (n = 3 per group) and protein levels of PAD4 and CiH3 (n = 3 per group) in PMNs treated with CTSC were presented. i. Representative images of NET formation were presented and quantifed in PMNs treated with CTSC (Scale Bar = 20 μm). Graphs represent means ± standard deviations. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ns signifies P > 0.05 when comparing data between two groups. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Subsequently, we isolated healthy adult PMNs and subjected them to in vitro stimulation with activated human recombinant CTSC protein (100 μg/ml). The CTSC-stimulatd PMNs exhibited a significant increase in NETosis compared to the control group, characterized by a notable elevation of dsDNA levels in the PMN supernatant (Fig. 4g), up-regulation of PAD4 and CiH3 protein expression (Fig. 4h), and an augmentation in the network structure of NETs (Fig. 4i). These findings indicate that CTSC promotes NETosis, and CTSC derived from AMs may play a crucial role in mediating the crosstalk between AMs and neutrophils.
3.5. CTSC triggers a substantial ROS burst originating from NADPH oxidase
We then focused on the molecular mechanism by which CTSC regulates NETosis. It is well established that the occurrence of NETosis is triggered by bursts of ROS, leading to the release of NETs [6]. Elevated ROS levels activate PAD4, causing the conversion of arginine to citrulline on histone 3 and resulting in chromatin depolymerization. To elucidate the effects of CTSC on ROS levels, we performed DCFH-DA staining and the results revealed a notable increase in ROS levels induced by CTSC, as indicated by a rise in DCFH-DA-positive cells. (Fig. 5a). To ascertain whether CTSC-induced NETosis is dependent on ROS, we used ROS scavengers including N-acetylcysteine (NAC; 5 mM), glutathione ethyl ester (GEE; 2 mM), and Trolox (200 μM). Following ROS clearance, the expression of PAD4 and CiH3 in PMNs exhibited a marked decrease (Fig. 5b), along with a notable reduction in dsDNA levels in PMN supernatant (Fig. 5c) and the network structure of NETs (Fig. 5d). These findings strongly suggest the dependency of CTSC-induced NETosis on ROS.
Fig. 5.
CTSC induces NETosis through a pathway dependent on NADPH oxidase-mediated ROS production.
a. Representative images and quantitative analysis of staining for DCFH-DA in PMNs (n = 6 per group). Scale Bar = 50 μm. b. & c. Protein levels of PAD4 and CiH3 (n = 3 per group) and concentrations of dsDNA in the supernatant (n = 3 per group) of PMNs treated with CTSC and ROS scavengers were presented. d. Representative images of NET formation of PMNs treated with CTSC and ROS scavengers were presented and quantifed (Scale Bar = 20 μm). e. Representative images of MitoSOX Red staining in PMNs treated with CTSC were presented, with Rotenone (10 μM) serving as a positive control (Scale Bar = 20 μm) f-h. Representative images and quantitative analysis of NET formation (Scale Bar = 20 μm), protein levels of PAD4 and CiH3 (n = 3 per group), and concentrations of dsDNA in the supernatant (n = 3 per group) of PMNs treated with CTSC or various antioxidant inhibitors were presented. Graphs represent means ± standard deviations. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ns signifies P > 0.05 when comparing data between two groups. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The main sources of intracellular ROS are NADPH oxidase and mitochondria. To elucidate the source of CTSC-induced ROS, we used the MitoSox Red indicator to assess the levels of mitochondrial superoxide (MitoSox) in PMNs. Notably, CTSC did not increase MitoSox levels, as indicated by weakened MitoSox Red staining (Fig. 5e). We further detected NETosis subsequent to treatment with the mitochondria-targeting antioxidant Mito-TEMPO (200 μM) and the inhibitors of NADPH oxidase DPI (10 μM), Apocynin (30 μM), and VAS2870 (10 μM). Our findings demonstrated that Mito-TEMPO treatment failed to attenuate CTSC-induced NETosis, whereas NADPH oxidase inhibitors significantly suppressed this process. This suppression was evidenced by the reduction in the network structure of NETs (Fig. 5f), the down-regulation of PAD4 and CiH3 expression in PMNs (Fig. 5g), and the decrease in dsDNA levels in PMN supernatant (Fig. 5h). Therefore, it is reasonable to conclude that NADPH oxidase acts as the primary source of CTSC-induced ROS, mediating CTSC-triggered NETosis.
3.6. CTSC induces the phosphorylation of p47phox and activates NADPH oxidase via p38 MAPK
Next, we explored the mechanisms by which CTSC induced ROS production. The regulatory relationship between the mitogen-activated protein kinase (MAPK) subfamily p38 and CTSC has been well established in previous studies. CTSC has been demonstrated to enhance the metastasis of hepatocellular carcinoma by activating p38 MAPK in liver cancer cell lines SK-HEP-1 and SMMC-7721 [50]. Furthermore, CTSC has been found to promote M1 polarization of microglia and macrophages in neuroinflammation and atherosclerotic plaques through a p38 MAPK-dependent pathway [[51], [52], [53]]. We further examined the expression of p38 MAPK in PMNs following CTSC treatment. Western blot analysis revealed a significant activation of the p38 MAPK pathway by CTSC, as indicated by a notable increase in phosphorylated p38 (p-p38) protein expression. (Fig. 6a). Thus, we hypothesize that a close relationship exists between p38 MAPK and ROS. Activation of p38 MAPK may potentially influence ROS production. We pretreated with the p38 MAPK inhibitor SB203580 (10 μM) before administering CTSC, and then measured NADPH oxidase activity, ROS levels, and NETosis. Consequently, SB203580 inhibited NADPH oxidase activity (Fig. 6b), resulting in a reduction in CTSC-induced ROS production (Fig. 6c) and NETosis, as indicated by a decrease in the network structure of NETs (Fig. 6d), lowered PAD4 and CiH3 protein expression in PMNs (Fig. 6e), and decreased dsDNA levels in the supernatant (Fig. 6f).
Fig. 6.
CTSC activates p38 MAPK and results in p47phox phosphorylation and trafficking of intracellular NADPH oxidase subunits to the cell membrane.
a. Protein levels of p-p38 and p38 in PMNs treated with CTSC were detected using western blot (n = 3 per group). b. The activity of NADPH oxidase was estimated using the WST-8 method, indicated by the NADP+/NADPH ratio (n = 3 per group). c. Representative images and quantitative analysis of staining for DCFH-DA in PMNs treated with CTSC and SB203580 (n = 3 per group). Scale Bar = 50 μm. d-f. Representative images and quantitative analysis of NET formation (Scale Bar = 20 μm), protein levels of PAD4 and CiH3 (n = 3 per group), and concentrations of dsDNA in the supernatant (n = 3 per group) of PMNs pretreated with CTSC and SB203580 were presented. g. Protein levels of p-p38 and p38 in PMNs after treated with CTSC and inhibitors of ROS or NADPH oxidase were detected (n = 3 per group). h. Schematic illustrations depicting the structure of NADPH oxidase in both resting and activated states. i. Protein levels of p67phox and p47phox in both the plasma membrane and the whole cell in PMNs treated with CTSC were detected using western blot. Relative intensities were determined and normalized to the band intensity of GAPDH and Na+/K+-ATPase (n = 3 per group). j. Protein levels of p-p47phox and p47phox in the whole cell, as well as p67phox and p47phox in the plasma membrane of PMNs treated with CTSC and SB203580 were detected using western blot. Relative intensities were determined and normalized to the band intensity of GAPDH and Na+/K+-ATPase (n = 3 per group). Graphs represent means ± standard deviations. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ns signifies P > 0.05 when comparing data between two groups.
To further investigate the regulatory relationship between p38 MAPK and ROS, we pretreated PMNs with the NADPH oxidase inhibitor DPI (10 μM) and the ROS scavenger NAC (5 mM) prior to CTSC stimulation. Subsequently, we examined the alterations in the p38 MAPK pathway. Our results revealed that inhibiting NADPH oxidase and ROS had minimal inhibitory effects on p38 phosphorylation (Fig. 6g), suggesting that CTSC likely activates NADPH oxidase via the p38 MAPK pathway, driving a ROS burst-dependent NETosis.
NADPH oxidase is a multi-subunit complex comprising membrane-bound subunits, gp91phox/NOX2, and p22phox, along with cytosolic subunits, including p47phox, p67phox, p40phox, and Rac1/2 [54]. The phosphorylation of p47phox initiates the trafficking of cytoplasmic subunits toward the cell membrane, facilitating the assembly and activation of NADPH oxidase [55] (Fig. 6h). Through membrane protein isolation and western blot analysis, we found that CTSC treatment significantly up-regulated the expression of p47phox and p67phox in the membrane fraction of PMNs, while their expression in the whole cell remained unchanged (Fig. 6i). This suggests that CTSC promotes the trafficking of intracellular NADPH oxidase subunits to the membrane, thereby enhancing the assembly and activation of NADPH oxidase in PMNs. Previous studies have demonstrated the crucial role of p38 MAPK in regulating the phosphorylation of p47phox [[56], [57], [58]]. Given the significance of CTSC in activating p38 MAPK, we pretreated PMNs with SB203580 (10 μM) to inhibit p38 MAPK and subsequently examined the expression of phosphorylated p47phox (p-p47phox) following CTSC treatment. Our results indicated that CTSC induced elevated expression of p-p47phox in PMNs (Fig. 6j). However, inhibition of p38 MAPK resulted in decreased p47phox phosphorylation, accompanied by significant reductions in the expression of p47phox and p67phox on the cell membrane (Fig. 6j). These findings suggest CTSC may facilitate the assembly and activation of NADPH oxidase through p38 MAPK-dependent phosphorylation of p47phox.
3.7. CTSC promotes inflammatory response via up-regulating and activating mPR3
PR3 is one of NSPs and can be activated by CTSC [25,26]. The activation of mPR3 in PMNs has been proven to efficiently cleave precursors of anti-inflammatory proteins and cytokines [59,60]. Previous studies have demonstrated that membrane-bound TNF-α and IL-1β are direct substrates of mPR3 [[61], [62], [63], [64]]. Considering that NETosis is accompanied by a release of inflammatory cytokines, we subsequently investigated the potential involvement of CTSC-activated mPR3 in the inflammatory response during NETosis. Previous studies have shown that cytoplasmic PR3 can be trafficked to the cell membrane under TNF-α stimulation through the p38 MAPK pathway [65]. Consequently, we hypothesized that CTSC may similarly upregulate mPR3 expression through the p38 MAPK pathway. Our western blot and flow cytometry analysis revealed a notable increase in mPR3 expression after CTSC stimulation (Fig. 7a and b). Consistent with previous findings, the flow cytometry data for mPR3 exhibited a characteristic bimodal distribution [65] (Fig. 7b). The activity of PR3 in PMNs significantly increased following treatment with CTSC (Fig. 7c). Remarkably, inhibition of p38 MAPK did not attenuate this increase. Therefore, we proposed that CTSC not only up-regulated the expression of mPR3 but also activated these constitutively expressed mPR3. Next, we detected the levels of TNF-α and IL-1β in PMN supernatant. CTSC notably elevated these inflammatory cytokine levels. However, their levels decreased upon treatment with the PR3 inhibitor Sivelestat, suggesting the involvement of mPR3 in NETosis-related inflammation and indicating that CTSC enhances cytokine release through up-regulating and activating mPR3.
Fig. 7.
CTSC enhances inflammatory cytokine release through an mPR3-dependent pathway.
a. Protein levels of PR3 in both the plasma membrane and the whole cell in PMNs treated with CTSC and SB203580 were detected using western blot. Relative intensities were determined and normalized to the band intensity of β-actin and Na+/K+-ATPase (n = 3 per group). b. Expression of mPR3 in PMNs treated with CTSC and SB203580 was assessed by flow cytometry (n = 6 per group). c. Activity of PR3 in PMNs treated with CTSC and SB203580 was detected (n = 3 per group). d. & e. Concentrations of TNF-α and IL-1β in the supernatant of PMNs treated with CTSC and PR3 inhibitor were measured by ELISA (n = 6 per group). Graphs represent means ± standard deviations. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ns signifies P > 0.05 when comparing data between two groups.
3.8. CTSC inhibition attenuates NETosis and lung I/R injury in mice
Finally, our aim was to explore the role of CTSC inhibition in alleviating lung I/R injury in mice. Prior to establishing the lung I/R model, we administered AZD7986, a specific CTSC inhibitor, intragastrically to the mice. Our findings demonstrated that AZD7986 significantly decreased NETosis in the lungs during I/R in mice, as evidenced by decreased protein expression of PAD4 and CiH3 in lung tissues (Fig. 8a), lower levels of dsDNA in the blood and BALF (Fig. 8b), and a decrease in NET formation (Fig. 8c). These results strongly suggest that CTSC inhibition can effectively reduce NETosis in lung I/R.
Fig. 8.
The NETosis and lung injury are alleviated after CTSC inhibition during lung I/R in mice.
a. Protein levels of PAD4 and CiH3 in the lung of mice subjected to I/R and AZD7986 treatment were detected using western blot (n = 3 per group). b. The levels of dsDNA in the plasma and BALF were detected using the Picogreen method (n = 6 per group). c. Representative images demonstrating NET presence through co-expression of CiH3 and MPO in the lung subjected to I/R and AZD7986 treatment, marked by white arrows. Scale Bar = 20 μm. d. Histopathological changes in the lung of mice subjected to I/R and AZD7986 treatment were evaluated using HE staining and lung injury scores (n = 6 per group). Scale Bar = 50 μm. Inflammatory cell infiltration or aggregation in the alveoli or vessel walls is indicated by ♦ annotations. Alveolar congestion or infiltration of red blood cells is denoted by ▲ annotations, while protein fragments in the alveolar space is denoted by ★ annotations. Thickening of the alveolar walls is highlighted with ↑ annotations. e. The oxygenation index was assessed by blood gas analysis (n = 6 per group). f-h. Quantitative analysis of total protein concentration, TNF-α and IL-1β levels in the BALF and plasma of mice subjected to I/R and AZD7986 treatment were detected (n = 6 per group). Graphs represent means ± standard deviations. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ns signifies P > 0.05 when comparing data between two groups. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Histological staining of lung tissue revealed substantial inflammatory cell infiltration in the I/R group, accompanied by thickened alveolar septa and accumulation of inflammatory exudates within the alveolar cavities (Fig. 8d). Conversely, in the AZD7986 group, the lung injury score was notably attenuated, marked by reduced inflammatory cell infiltration, thinner alveolar septa, and preserved alveolar structure (Fig. 8d). The oxygenation index was higher than that observed in the I/R group (Fig. 8e). Furthermore, the protein concentration in BALF of the AZD7986 group markedly decreased compared to that of the I/R group, indicating improved lung barrier function (Fig. 8f). Levels of TNF-α and IL-1β in both BALF and plasma of AZD7986-treated mice were significantly reduced compared to those in the I/R group (Fig. 8g and h). These observations underscore the potential of CTSC inhibition in ameliorating NETosis and lung I/R injury.
4. Discussion
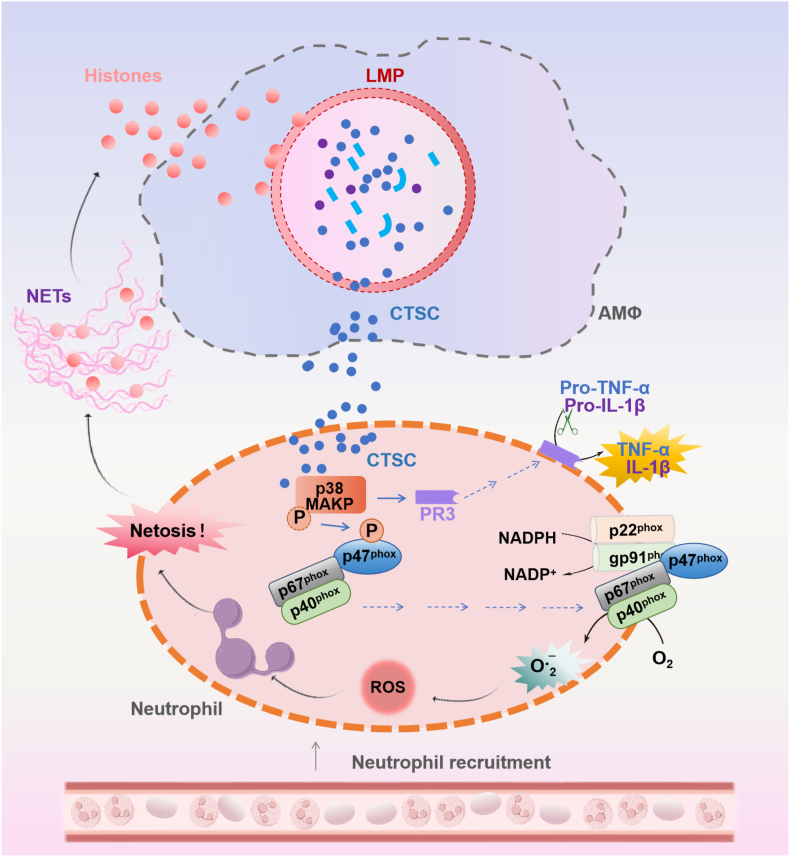
Herein, we elucidated that extracellular histone-induced M1-AMs promoted NETosis during lung I/R. AMs-derived CTSC played a pivotal role in NETosis by activating the p38 MAPK pathway. Mechanistically, p38 MAPK activation leaded to the phosphorylation of the NADPH oxidase subunit p47phox, which in turn promoted NADPH oxidase activation, ROS production, and NETosis. Moreover, CTSC up-regulated and activated mPR3 through p38 MAPK, resulting in an increased release of NETosis-related inflammatory cytokines (Fig. 9). This novel molecular mechanism potentially underlies lung I/R and underscores the crucial involvement of CTSC in mediating the positive feedback loop between NETosis and extracellular histones during lung I/R.
Fig. 9.
Schematic illustration of the mechanism of CTSC from extracellular histone-induced M1 AMs in promoting NETosis during lung I/R injury. Extracellular histones induce M1-AMs and promote CTSC secretion through LMP. AM-derived CTSC induces NETosis through a pathway dependent on NADPH oxidase-mediated ROS production. Specifically, CTSC activates p38 MAPK in neutrophils and facilitated the phosphorylation of the NADPH oxidase subunit p47phox, thus promoting NADPH oxidase activation. On the other hand, CTSC up-regulates and activates mPR3 through p38 MAPK, resulting in an increased release of NETosis-related inflammatory cytokine release. Targeting CTSC inhibition revealed great potential in mitigating lung I/R injury.
The high incidence of PGD (estimated at approximately 20–30 %) poses a formidable obstacle in the advancement of LTx [2,3]. Lung I/R injury predominantly underlies the development of PGD [4]. Upon the restoration of blood flow to the transplanted lung, neutrophils traverse the endothelial barrier into the alveolar space, where they undergo activation and intensified the sterile inflammatory response in lung I/R injury [5]. Li et al. documented a dynamically progressive increase in neutrophil infiltration within lung tissue post-reperfusion using intravital imaging. Even 24 h after reperfusion, a continued influx of neutrophils persisted, indicating the pivotal role of neutrophils in the inflammatory cascade during later phases of lung I/R [66]. Consistent with existing literature, our study revealed a marked elevation in neutrophil infiltration within both lung tissue and BALF following 4 h of reperfusion.
The influx of numerous neutrophils during reperfusion can initiate enduring and extensive tissue damage, with the release of NETs being a significant contributing factor to lung transplant injury [[8], [9], [10], [11]]. The clinical investigation in our study revealed a considerable rise in NETosis following LTx. This increase was also observed in our murine models of lung I/R post-reperfusion. Studies have elucidated several strategies for mitigating NETs-induced lung injury in mice, such as depleting neutrophils using anti-Ly6G antibodies, disrupting the scaffold of NETs with deoxyribonuclease I (DNase I), inhibiting NET formation through PAD4 inhibition, and neutralizing histones [8,9,67]. However, despite these promising strategies, directly targeting neutrophils or NETs may present challenges in clinical LTx due to the essential roles of NETs in immobilizing, capturing, and eliminating pathogens. Neutrophil depletion or NET clearance could facilitate the dissemination of numerous pathogens, thereby increasing the risk of post-LTx infections. Therefore, targeting the upstream signaling pathways responsible for NETosis may provide a means to achieve an optimal balance in immune function.
The early stages of lung I/R injury involve the release of a amount of DAMPs by damaged cells, rendering resident cells activated [68,69]. The activation of resident AMs constitutes a critical initial step in the inflammatory cascade during lung I/R [70]. While previous studies have emphasized the role of macrophages in neutrophil migration and chemotactic function [[71], [72], [73]], there has been limited focus on the effects of NETosis in activted AMs. Our findings revealed that extracellular histones induced M1-AMs, which in turn significantly enhanced NETosis when co-cultured with neutrophils. Additionally, we administered mice with Clo-lip or heparin to deplete AMs or neutralize extracellular histones, both of which effectively alleviated NETosis. However, given the critical role of macrophages in tissue repair and homeostasis maintenance, AM depletion is not a feasible standard treatment [14]. Heparin is a negatively charged molecule and has shown therapeutic potential in various injury models by neutralizing positively charged histones [[74], [75], [76]]. Nonetheless, its clinical utility is limited by its tendency to induce bleeding, necessitating the clinical translation of non-anticoagulant forms of heparin [77].
CTSC is a lysosomal cysteine protease and is implicated in the progression of both acute and chronic inflammation [24,[27], [28], [29]]. However, its involvement in organ I/R injury remains unexplored. Through protein database analysis and experimental validation in mice, our investigation revealed high enrichment of CTSC in AMs within lung tissue. Notably, CTSC is also a secreted protein due to the presence of a signal peptide within its structure. Previous research has identified mast cells in the trachea and AMs in the alveoli as primary sources of CTSC [78]. Moreover, studies have indicated that several DAMPs including cholesterol crystals and ATP, could induce LMP in macrophages and lead to the release of lysosomal proteases [79,80]. In our study, we confirmed that extracellular histones, among these same DAMPs, induced LMP in AMs using various fluorescence staining methods. Through secretome and ELISA analysis, we further validated that extracellular histones triggered the secretion of CTSC from AMs. Additionally, neutralization of extracellular histones resulted in a significant reduction in CTSC levels in murine BALF after lung I/R.
By silencing CTSC expression in AMs using siRNA, our findings demonstrated that AM-derived CTSC notably triggered NETosis. Moreover, our results indicated a ROS-dependent mechanism underlying CTSC-induced NETosis. While intracellular ROS are primarily produced by NADPH oxidase, previous research has identified mitochondria-derived ROS as responsible for NETosis induced by ribonucleoprotein immune complexes [81]. In our study, through the use of inhibitors targeting mitochondria or NADPH oxidase, we validated the essential role of NADPH oxidase in CTSC-induced ROS generation and subsequent NETosis. The trafficking of NADPH oxidase subunits from the cytoplasm to the cell membrane marks the initial step in NADPH oxidase activation [55,82]. Our study demonstrated that CTSC promoted the membrane translocation of p47phox and p67phox and activated NADPH oxidase. However, the specific interaction mechanism between these membrane subunits remains unexplored. A recent study illustrated that this translocation prompted a restructuring of the subunit configuration on the membrane, ultimately facilitating NADPH oxidase activation [83].
The p38 MAPK is a crucial member of the MAPK subfamily that play important roles in phosphorylating downstream effector proteins [84]. Previous studies have elucidated a close regulatory relationship between CTSC and p38 MAPK in disease models of hepatocellular carcinoma, neuroinflammation, and atherosclerosis [[51], [52], [53]]. In our study, we discovered that CTSC stimulation induced a marked activation of p38 MAPK. Moreover, while previous studies have indicated the involvement of p38 MAPK downstream of ROS signaling [85], our results showed that CTSC-induced p38 MAPK activation preceded ROS signaling, corroborating the observations of Awasthi et al. and Han et al. [86,87]. This underscores a nuanced mechanism underlying NETosis in response to diverse stimuli. Furthermore, we found that activated p38 MAPK facilitated the phosphorylation of p47phox, thereby promoting the translocation of NADPH oxidase subunits from the cytoplasm to the cell membrane, ultimately resulting in NADPH oxidase activation.
NSPs act as direct substrate of CTSC, whose activation not only contributes to NETosis but also inflicts damage on other immune cells upon extracellular release [25,26]. Importantly, NSP activation predominantly occurs during the maturation stage of bone marrow neutrophils [24]. Consequently, the infiltrating neutrophils during reperfusion possess active NSP forms, making it improbable for CTSC to reactivate NSPs. However, PR3 is constitutively expressed on neutrophil membranes in addition to its cytoplasmic granular form [88,89]. These membrane-expressed forms of PR3 can cleave precursors of inflammatory cytokines and facilitate their release upon activation [[61], [62], [63], [64]]. Studies have demonstrated heightened expression of mPR3 in activated neutrophils, and CTSC inhibition could result in decreased mPR3 expression [64,[90], [91], [92]]. Our study revealed that CTSC directly up-regulated and activated mPR3, promoting NETosis-related inflammatory responses.
Given the pivotal pro-inflammatory role of CTSC in inflammatory and autoimmune diseases, various CTSC inhibitors have been developed [24]. Among these, AZD7986, a nitrile compound, has emerged as a notable candidate and is currently in initial clinical trials [93].The results of a phase 2 global multicenter clinical trial investigating the effects of AZD7986 (Brensocatib) in bronchiectasis patients were reported in 2020, known as the WILLOW trial [94]. This trial demonstrated Brensocatib's efficacy in treating bronchiectasis, indicated by a reduction in the time to lung function deterioration. Additionally, Brensocatib has received breakthrough drug designation from the FDA, underscoring its potential as an innovative first-in-class therapy for bronchiectasis. Recognizing the significant role of CTSC-induced NETosis during lung I/R, we administered AZD7986 to mice intragastrically before establishing a murine lung I/R model. The results revealed a substantial reduction in NETosis and lung I/R injury following CTSC inhibition. While the precise mechanism of CTSC inhibition in reducing NETosis during lung I/R in mice remains unexplored, it is plausible that the inhibitor not only targets AM-derived CTSC but may also directly affect neutrophils, given the crucial role of endogenous CTSC in these cells. The observed beneficial effect of CTSC inhibition in reducing lung I/R injury may signify an additional advantage of systemic CTSC inhibition in NSP activity.
There are several limitations that warrant discussion. Firstly, in the context of clinical LTx, the donor lung undergoes cold ischemic injury during long-time low-temperature preservation, whereas the I/R model in our study induces thermal ischemic injury. As a result, the inflammatory response profiles and severity of I/R injury may vary between these scenarios [95], necessitating validation using a murine LTx model in future study. Secondly, our investigation revealed that extracellular histones can activate AMs, leading to the promotion of NETosis and subsequent heightened histone release. While we detected the release of NETs and CiH3 within this vicious feedback loop, the histones we used was exogenous, and NETs were not extracted to stimulate AMs. Future studies will involve intervening with AMs by using extracted NETs to gain deeper insights. Moreover, regarding the co-culture model of macrophages and neutrophils, we exclusively used MH-S cells and BMDNs. Our future plane involves isolating human PBMCs and inducing their differentiation into macrophages to establish a co-culture model with human PMNs, thus enhancing the translational relevance of our findings. Lastly, in our study, mice were euthanized immediately after 4 h of reperfusion without assessing their short-term survival rate. Future research will incorporate data on the impact of AZD7986 on the post-I/R survival rate of mice.
5. Conclusion
Our study suggests the pivotal role of AM-derived CTSC in mediating the vicious feedback loop between histones and NETosis during lung I/R injury. We discovered that extracellular histones induced M1-AMs and promoted CTSC secretion through LMP. AM-derived CTSC induced NETosis through a ROS-dependent pathway, which activated p38 MAPK in neutrophils and facilitated the phosphorylation of the NADPH oxidase subunit p47phox, thus promoting NADPH oxidase activation. Inhibiting CTSC could alleviate NETosis-related injury during lung I/R. Our findings underscore the significance of CTSC as a central mediator of NETosis in lung I/R injury, positioning CTSC as a potential novel target for the prevention and treatment of both lung I/R injury and PGD in LTx.
Funding
This study received support from the Foundation of the Science and Technology Commission of Shanghai Municipality (grant number 23ZR1453300), the National Natural Science Foundation of China (grant number 82170107), the Excellent Subject Leader Program of Shanghai Municipal Health Commission (grant number 2022XD007), and the Program of Shanghai Municipal Health Commission (grant number 202140406).
CRediT authorship contribution statement
Jing Yu: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Yu Fu: Software, Methodology, Investigation, Data curation, Conceptualization. Jiameng Gao: Software, Methodology, Investigation, Data curation, Conceptualization. Qingqing Zhang: Methodology, Investigation. Nan Zhang: Methodology, Investigation. Zhiyuan Zhang: Methodology, Investigation. Xuemei Jiang: Methodology, Investigation. Chang Chen: Writing – review & editing, Supervision. Zongmei Wen: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103231.
Contributor Information
Chang Chen, Email: chenthoracic@163.com.
Zongmei Wen, Email: wzm1103@126.com.
Abbreviations
- ALI
acute lung injury
- AMs
alveolar macrophages
- AO
Acridine Orange
- ARDS
acute respiratory distress syndrome
- BALF
bronchoalveolar lavage fluid
- BMDNs
bone marrow-derived neutrophils
- CiH3
citrullinated histone3
- Clo-lip
Clodronate Liposomes
- CTSC
Cathepsin C
- DAMPs
damage-associated molecular patterns
- dsDNA
double stranded DNA
- ESLD
end-stage lung disease
- HE
hematoxylin and eosin
- I/R
ischemia/reperfusion
- IL-1β
interleukin-1β
- iNOS
inducible nitric oxide synthase
- LMP
lysosomal membrane permeability
- LTx
lung transplantation
- M1-AMs
M1 phenotype of alveolar macrophages
- MAPK
mitogen-activated protein kinase
- MPO
myeloperoxidase
- mPR3
membrane proteinase 3
- NADPH
nicotinamide adenine dinuc-leotide phosphate
- NETs
neutrophil extracellular traps
- NSPs
neutrophil serine proteases
- PAD4
protein arginine deiminase type 4
- PGD
primary graft dysfunction
- PI
Propidium Iodide
- PMNs
polymorphonuclear neutrophils
- p-p38
phosphorylated p38
- p-p47phox
phosphorylated p47phox
- PR3
proteinase 3
- ProSPC
prosurfactant
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor α
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Swaminathan A.C., Todd J.L., Palmer S.M. Advances in human lung transplantation. Annu. Rev. Med. 2021;72:135–149. doi: 10.1146/annurev-med-080119-103200. [DOI] [PubMed] [Google Scholar]
- 2.Cantu E., Diamond J.M., Cevasco M., Suzuki Y., Crespo M., Clausen E., et al. Contemporary trends in PGD incidence, outcomes, and therapies. J. Heart Lung Transplant. 2022;41(12):1839–1849. doi: 10.1016/j.healun.2022.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chacon-Alberty L., Fernandez R., Jindra P., King M., Rosas I., Hochman-Mendez C., et al. Primary graft dysfunction in lung transplantation: a review of mechanisms and future applications. Transplantation. 2023;107(8):1687–1697. doi: 10.1097/TP.0000000000004503. [DOI] [PubMed] [Google Scholar]
- 4.Capuzzimati M., Hough O., Liu M. Cell death and ischemia-reperfusion injury in lung transplantation. J. Heart Lung Transplant. 2022;41(8):1003–1013. doi: 10.1016/j.healun.2022.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd H.M., Gauthier J.M., Terada Y., Li W., Krupnick A.S., Gelman A.E., et al. Updated views on neutrophil responses in ischemia-reperfusion injury. Transplantation. 2022;106(12):2314–2324. doi: 10.1097/TP.0000000000004221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18(2):134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 8.Sayah D.M., Mallavia B., Liu F., Ortiz-Muñoz G., Caudrillier A., DerHovanessian A., et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 2015;191(4):455–463. doi: 10.1164/rccm.201406-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallavia B., Liu F., Lefrançais E., Cleary S.J., Kwaan N., Tian J.J., et al. Mitochondrial DNA stimulates TLR9-dependent neutrophil extracellular trap formation in primary graft dysfunction. Am. J. Respir. Cell Mol. Biol. 2020;62(3):364–372. doi: 10.1165/rcmb.2019-0140OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonneau S., Landry C., Bégin S., Adam D., Villeneuve L., Clavet-Lanthier M.É., et al. Correlation between neutrophil extracellular traps (NETs) expression and primary graft dysfunction following human lung transplantation. Cells. 2022;11(21):3420. doi: 10.3390/cells11213420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindstedt S., Niroomand A., Mittendorfer M., Hirdman G., Hyllén S., Pierre L., et al. Nothing but NETs: cytokine adsorption correlates with lower circulating nucleosomes and is associated with decreased primary graft dysfunction. J. Heart Lung Transplant. 2023;42(10):1358–1362. doi: 10.1016/j.healun.2023.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Z., Chiu S., Akbarpour M., Sun H., Reyfman P.A., Anekalla K.R., et al. Donor pulmonary intravascular nonclassical monocytes recruit recipient neutrophils and mediate primary lung allograft dysfunction. Sci. Transl. Med. 2017;9(394) doi: 10.1126/scitranslmed.aal4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabrese D.R., Tsao T., Magnen M., Valet C., Gao Y., Mallavia B., et al. NKG2D receptor activation drives primary graft dysfunction severity and poor lung transplantation outcomes. JCI. Insight. 2022;7(24) doi: 10.1172/jci.insight.164603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malainou C., Abdin S.M., Lachmann N., Matt U., Herold S. Alveolar macrophages in tissue homeostasis, inflammation, and infection: evolving concepts of therapeutic targeting. J. Clin. Invest. 2023;133(19) doi: 10.1172/JCI170501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakazawa D., Shida H., Kusunoki Y., Miyoshi A., Nishio S., Tomaru U., et al. The responses of macrophages in interaction with neutrophils that undergo NETosis. J. Autoimmun. 2016;67:19–28. doi: 10.1016/j.jaut.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Yalcinkaya M., Fotakis P., Liu W., Endo-Umeda K., Dou H., Abramowicz S., et al. Cholesterol accumulation in macrophages drives NETosis in atherosclerotic plaques via IL-1β secretion. Cardiovasc. Res. 2023;119(4):969–981. doi: 10.1093/cvr/cvac189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warnatsch A., Ioannou M., Wang Q., Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349(6245):316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiao Y., Li Z., Loughran P.A., Fan E.K., Scott M.J., Li Y., et al. Frontline Science: macrophage-derived exosomes promote neutrophil necroptosis following hemorrhagic shock. J. Leukoc. Biol. 2018;103(2):175–183. doi: 10.1189/jlb.3HI0517-173R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H., Li Y., Song C., Hu Y., Dai M., Liu B., et al. Neutrophil extracellular traps augmented alveolar macrophage pyroptosis via AIM2 inflammasome activation in LPS-induced ALI/ARDS. J. Inflamm. Res. 2021;14:4839–4858. doi: 10.2147/JIR.S321513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J., Zhang X., Pelayo R., Monestier M., Ammollo C.T., Semeraro F., et al. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009;15(11):1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda S., Nakazawa D., Shida H., Miyoshi A., Kusunoki Y., Tomaru U., et al. NETosis markers: quest for specific, objective, and quantitative markers. Clin. Chim. Acta. 2016;459:89–93. doi: 10.1016/j.cca.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Jin Y., Sun M., Lv X., Wang X., Jiang G., Chen C., et al. Extracellular histones play a pathogenic role in primary graft dysfunction after human lung transplantation. RSC Adv. 2020;10(21):12485–12491. doi: 10.1039/d0ra00127a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang P., Jin Y., Sun M., Jiang X., Yang J., Lv X., et al. Extracellular histones aggravate inflammation in ARDS by promoting alveolar macrophage pyroptosis. Mol. Immunol. 2021;135:53–61. doi: 10.1016/j.molimm.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Korkmaz B., Caughey G.H., Chapple I., Gauthier F., Hirschfeld J., Jenne D.E., et al. Therapeutic targeting of cathepsin C: from pathophysiology to treatment. Pharmacol. Ther. 2018;190:202–236. doi: 10.1016/j.pharmthera.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Othman A., Sekheri M., Filep J.G. Roles of neutrophil granule proteins in orchestrating inflammation and immunity. FEBS J. 2022;289(14):3932–3953. doi: 10.1111/febs.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pham C.T. Neutrophil serine proteases: specific regulators of inflammation. Nat. Rev. Immunol. 2006;6(7):541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 27.Aghdassi A.A., Pham C., Zierke L., Mariaule V., Korkmaz B., Rhimi M. Cathepsin C role in inflammatory gastroenterological, renal, rheumatic, and pulmonary disorders. Biochimie. 2024;216:175–180. doi: 10.1016/j.biochi.2023.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Korkmaz B., Lamort A.S., Domain R., Beauvillain C., Gieldon A., Yildirim A.Ö., et al. Cathepsin C inhibition as a potential treatment strategy in cancer. Biochem. Pharmacol. 2021;194 doi: 10.1016/j.bcp.2021.114803. [DOI] [PubMed] [Google Scholar]
- 29.Korkmaz B., Lesner A., Marchand-Adam S., Moss C., Jenne D.E. Lung protection by Cathepsin C inhibition: a new hope for COVID-19 and ARDS? J. Med. Chem. 2020;63(22):13258–13265. doi: 10.1021/acs.jmedchem.0c00776. [DOI] [PubMed] [Google Scholar]
- 30.Akk A.M., Simmons P.M., Chan H.W., Agapov E., Holtzman M.J., Grayson M.H., et al. Dipeptidyl peptidase I-dependent neutrophil recruitment modulates the inflammatory response to Sendai virus infection. J. Immunol. 2008;180(5):3535–3542. doi: 10.4049/jimmunol.180.5.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mallen-St Clair J., Pham C.T., Villalta S.A., Caughey G.H., Wolters P.J. Mast cell dipeptidyl peptidase I mediates survival from sepsis. J. Clin. Invest. 2004;113(4):628–634. doi: 10.1172/JCI19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagano M.B., Bartoli M.A., Ennis T.L., Mao D., Simmons P.M., Thompson R.W., et al. Critical role of dipeptidyl peptidase I in neutrophil recruitment during the development of experimental abdominal aortic aneurysms. Proc. Natl. Acad. Sci. U. S. A. 2007;104(8):2855–2860. doi: 10.1073/pnas.0606091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Y., Cong M., Li J., He D., Wu Q., Tian P., et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39(3):423–437. doi: 10.1016/j.ccell.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y.G., Teng Y.S., Cheng P., Kong H., Lv Y.P., Mao F.Y., et al. Abrogation of cathepsin C by Helicobacter pylori impairs neutrophil activation to promote gastric infection. Faseb. J. 2019;33(4):5018–5033. doi: 10.1096/fj.201802016RR. [DOI] [PubMed] [Google Scholar]
- 35.Cao M., Luo X., Wu K., He X. Targeting lysosomes in human disease: from basic research to clinical applications. Signal Transduct. Targeted Ther. 2021;6(1):379. doi: 10.1038/s41392-021-00778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abrams S.T., Zhang N., Manson J., Liu T., Dart C., Baluwa F., et al. Circulating histones are mediators of trauma-associated lung injury. Am. J. Respir. Crit. Care Med. 2013;187(2):160–169. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh I.N., Deluna X., White M.R., Hartshorn K.L. Histone H4 directly stimulates neutrophil activation through membrane permeabilization. J. Leukoc. Biol. 2021;109(4):763–775. doi: 10.1002/JLB.3A0620-342R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silvestre-Roig C., Braster Q., Wichapong K., Lee E.Y., Teulon J.M., Berrebeh N., et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature. 2019;569(7755):236–240. doi: 10.1038/s41586-019-1167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung J., Lee L.E., Kim H., Kim J.E., Jang S.H., Roh J.S., et al. Extracellular histones aggravate autoimmune arthritis by lytic cell death. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.961197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Major L.A., Sauterer R. Demonstration of exogenous nuclear histone H3 binding to mitochondria and subsequent cytochrome C release in cauliflower. Cell Biol. Int. 2019;43(11):1323–1329. doi: 10.1002/cbin.11223. [DOI] [PubMed] [Google Scholar]
- 41.Cascone A., Bruelle C., Lindholm D., Bernardi P., Eriksson O. Destabilization of the outer and inner mitochondrial membranes by core and linker histones. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh A., Verma S., Modak S.B., Chaturvedi M.M., Purohit J.S. Extra-nuclear histones: origin, significance and perspectives. Mol. Cell. Biochem. 2022;477(2):507–524. doi: 10.1007/s11010-021-04300-4. [DOI] [PubMed] [Google Scholar]
- 43.Eriksson I., Vainikka L., Persson H.L., Öllinger K. Real-time monitoring of lysosomal membrane permeabilization using acridine orange. Method Protoc. 2023;6(4):72. doi: 10.3390/mps6040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomé M.P., Filippi-Chiela E.C., Villodre E.S., Migliavaca C.B., Onzi G.R., Felipe K.B., et al. Ratiometric analysis of Acridine Orange staining in the study of acidic organelles and autophagy. J. Cell Sci. 2016;129(24):4622–4632. doi: 10.1242/jcs.195057. [DOI] [PubMed] [Google Scholar]
- 45.Evavold C.L., Hafner-Bratkovič I., Devant P., D'Andrea J.M., Ngwa E.M., Boršić E., et al. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell. 2021;184(17):4495–4511. doi: 10.1016/j.cell.2021.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Li M., Stadler S., Correll S., Li P., Wang D., et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009;184(2):205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewis H.D., Liddle J., Coote J.E., Atkinson S.J., Barker M.D., Bax B.D., et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 2015;11(3):189–191. doi: 10.1038/nchembio.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poli V., Zanoni I. Neutrophil intrinsic and extrinsic regulation of NETosis in health and disease. Trends Microbiol. 2023;31(3):280–293. doi: 10.1016/j.tim.2022.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aits S., Kricker J., Liu B., Ellegaard A.M., Hämälistö S., Tvingsholm S., et al. Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy. 2015;11(8):1408–1424. doi: 10.1080/15548627.2015.1063871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang G.P., Yue X., Li S.Q. Cathepsin C interacts with TNF-α/p38 MAPK signaling pathway to promote proliferation and metastasis in hepatocellular carcinoma. Cancer. Res. Treat. 2020;52(1):10–23. doi: 10.4143/crt.2019.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Q., Zhang Y., Liu S., Liu Y., Yang X., Liu G., et al. Cathepsin C promotes microglia M1 polarization and aggravates neuroinflammation via activation of Ca2+-dependent PKC/p38MAPK/NF-κB pathway. J. Neuroinflammation. 2019;16(1):10. doi: 10.1186/s12974-019-1398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai J., Liu J., Zhang Q., An Y., Xia B., Wan C., et al. Cathepsin C is involved in macrophage M1 polarization via p38/MAPK pathway in sudden cardiac death. Cardiovasc. Ther. 2021;2021 doi: 10.1155/2021/6139732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alam S., Liu Q., Liu S., Liu Y., Zhang Y., Yang X., et al. Up-regulated Cathepsin C induces macrophage M1 polarization through FAK-triggered p38 MAPK/NF-κB pathway. Exp. Cell Res. 2019;382(2) doi: 10.1016/j.yexcr.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 54.Begum R., Thota S., Abdulkadir A., Kaur G., Bagam P., Batra S. NADPH oxidase family proteins: signaling dynamics to disease management. Cell. Mol. Immunol. 2022;19(6):660–686. doi: 10.1038/s41423-022-00858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El-Benna J., Dang P.M., Gougerot-Pocidalo M.A. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin. Immunopathol. 2008;30(3):279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 56.El Benna J., Han J., Park J.W., Schmid E., Ulevitch R.J., Babior B.M. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch. Biochem. Biophys. 1996;334(2):395–400. doi: 10.1006/abbi.1996.0470. [DOI] [PubMed] [Google Scholar]
- 57.Ricci E., Ronchetti S., Gabrielli E., Pericolini E., Gentili M., Roselletti E., et al. GILZ restrains neutrophil activation by inhibiting the MAPK pathway. J. Leukoc. Biol. 2019;105(1):187–194. doi: 10.1002/JLB.3AB0718-255R. [DOI] [PubMed] [Google Scholar]
- 58.Brown G.E., Stewart M.Q., Bissonnette S.A., Elia A.E., Wilker E., Yaffe M.B. Distinct ligand-dependent roles for p38 MAPK in priming and activation of the neutrophil NADPH oxidase. J. Biol. Chem. 2004;279(26):27059–27068. doi: 10.1074/jbc.M314258200. [DOI] [PubMed] [Google Scholar]
- 59.Chu T.Y., Zheng-Gérard C., Huang K.Y., Chang Y.C., Chen Y.W., I K.Y., et al. GPR97 triggers inflammatory processes in human neutrophils via a macromolecular complex upstream of PAR2 activation. Nat. Commun. 2022;13(1):6385. doi: 10.1038/s41467-022-34083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Richthofen H.J., Westerlaken G.H.A., Gollnast D., Besteman S., Delemarre E.M., Rodenburg K., et al. Soluble signal inhibitory receptor on leukocytes-1 is released from activated neutrophils by proteinase 3 cleavage. J. Immunol. 2023;210(4):389–397. doi: 10.4049/jimmunol.2200169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coeshott C., Ohnemus C., Pilyavskaya A., Ross S., Wieczorek M., Kroona H., et al. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc. Natl. Acad. Sci. U. S. A. 1999;96(11):6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armstrong L., Godinho S.I., Uppington K.M., Whittington H.A., Millar A.B. Tumour necrosis factor-alpha processing in interstitial lung disease: a potential role for exogenous proteinase-3. Clin. Exp. Immunol. 2009;156(2):336–343. doi: 10.1111/j.1365-2249.2009.03906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schreiber A., Pham C.T., Hu Y., Schneider W., Luft F.C., Kettritz R. Neutrophil serine proteases promote IL-1β generation and injury in necrotizing crescentic glomerulonephritis. J. Am. Soc. Nephrol. 2012;23(3):470–482. doi: 10.1681/ASN.2010080892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y., Liu Z., Pan T., Chen E., Mao E., Chen Y., et al. JMJD3 is involved in neutrophil membrane proteinase 3 overexpression during the hyperinflammatory response in early sepsis. Int. Immunopharm. 2018;59:40–46. doi: 10.1016/j.intimp.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 65.Kettritz R., Schreiber A., Luft F.C., Haller H. Role of mitogen-activated protein kinases in activation of human neutrophils by antineutrophil cytoplasmic antibodies. J. Am. Soc. Nephrol. 2001;12(1):37–46. doi: 10.1681/ASN.V12137. [DOI] [PubMed] [Google Scholar]
- 66.Li W., Terada Y., Tyurina Y.Y., Tyurin V.A., Bery A.I., Gauthier J.M., et al. Necroptosis triggers spatially restricted neutrophil-mediated vascular damage during lung ischemia reperfusion injury. Proc. Natl. Acad. Sci. U. S. A. 2022;119(10) doi: 10.1073/pnas.2111537119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caudrillier A., Kessenbrock K., Gilliss B.M., Nguyen J.X., Marques M.B., Monestier M., et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Invest. 2012;122(7):2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shepherd H.M., Gauthier J.M., Li W., Krupnick A.S., Gelman A.E., Kreisel D. Innate immunity in lung transplantation. J. Heart Lung Transplant. 2021;40(7):562–568. doi: 10.1016/j.healun.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Q., Lan P. Activation of immune signals during organ transplantation. Signal Transduct. Targeted Ther. 2023;8(1):110. doi: 10.1038/s41392-023-01377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao M., Fernandez L.G., Doctor A., Sharma A.K., Zarbock A., Tribble C.G., et al. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291(5):1018–1026. doi: 10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- 71.Akbarpour M., Lecuona E., Chiu S.F., Wu Q., Querrey M., Fernandez R., et al. Residual endotoxin induces primary graft dysfunction through ischemia/reperfusion-primed alveolar macrophages. J. Clin. Invest. 2020;130(8):4456–4469. doi: 10.1172/JCI135838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spahn J.H., Li W., Bribriesco A.C., Liu J., Shen H., Ibricevic A., et al. DAP12 expression in lung macrophages mediates ischemia/reperfusion injury by promoting neutrophil extravasation. J. Immunol. 2015;194(8):4039–4048. doi: 10.4049/jimmunol.1401415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prakash A., Mesa K.R., Wilhelmsen K., Xu F., Dodd-o J.M., Hellman J. Alveolar macrophages and Toll-like receptor 4 mediate ventilated lung ischemia reperfusion injury in mice. Anesthesiology. 2012;117(4):822–835. doi: 10.1097/ALN.0b013e31826a4ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu C., Liang Y., Liu Y., Shu W., Luan Z., Ma X. Unfractionated heparin protects microcirculation in endotoxemic rats by antagonizing histones. J. Surg. Res. 2023;282:84–92. doi: 10.1016/j.jss.2022.09.019. [DOI] [PubMed] [Google Scholar]
- 75.Zhu C., Liang Y., Li X., Chen N., Ma X. Unfractionated heparin attenuates histone-mediated cytotoxicity in vitro and prevents intestinal microcirculatory dysfunction in histone-infused rats. J. Trauma Acute Care Surg. 2019;87(3):614–622. doi: 10.1097/TA.0000000000002387. [DOI] [PubMed] [Google Scholar]
- 76.Iba T., Hashiguchi N., Nagaoka I., Tabe Y., Kadota K., Sato K. Heparins attenuated histone-mediated cytotoxicity in vitro and improved the survival in a rat model of histone-induced organ dysfunction. Intens. Care Med. Exp. 2015;3(1):36. doi: 10.1186/s40635-015-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jaimes F., De La Rosa G., Morales C., Fortich F., Arango C., Aguirre D., et al. Unfractioned heparin for treatment of sepsis: a randomized clinical trial (The HETRASE Study) Crit. Care Med. 2009;37(4):1185–1196. doi: 10.1097/CCM.0b013e31819c06bc. [DOI] [PubMed] [Google Scholar]
- 78.Wolters P.J., Laig-Webster M., Caughey G.H. Dipeptidyl peptidase I cleaves matrix-associated proteins and is expressed mainly by mast cells in normal dog airways. Am. J. Respir. Cell Mol. Biol. 2000;22(2):183–190. doi: 10.1165/ajrcmb.22.2.3767. [DOI] [PubMed] [Google Scholar]
- 79.Duewell P., Kono H., Rayner K.J., Sirois C.M., Vladimer G., Bauernfeind F.G., et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santos S.A.C.S., Persechini P.M., Henriques-Santos B.M., Bello-Santos V.G., Castro N.G., Costa de Sousa J., et al. P2X7 receptor triggers lysosomal leakage through calcium mobilization in a mechanism dependent on pannexin-1 hemichannels. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.752105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lood C., Blanco L.P., Purmalek M.M., Carmona-Rivera C., De Ravin S.S., Smith C.K., et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016;22(2):146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belambri S.A., Rolas L., Raad H., Hurtado-Nedelec M., Dang P.M., El-Benna J. NADPH oxidase activation in neutrophils: role of the phosphorylation of its subunits. Eur. J. Clin. Invest. Suppl. 2018;2 doi: 10.1111/eci.12951. [DOI] [PubMed] [Google Scholar]
- 83.Liu X., Shi Y., Liu R., Song K., Chen L. Structure of human phagocyte NADPH oxidase in the activated state. Nature. 2024;627(8002):189–195. doi: 10.1038/s41586-024-07056-1. [DOI] [PubMed] [Google Scholar]
- 84.Johnson G.L., Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 85.Keshari R.S., Verma A., Barthwal M.K., Dikshit M. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J. Cell. Biochem. 2013;114(3):532–540. doi: 10.1002/jcb.24391. [DOI] [PubMed] [Google Scholar]
- 86.Awasthi D., Nagarkoti S., Kumar A., Dubey M., Singh A.K., Pathak P., et al. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radic. Biol. Med. 2016;93:190–203. doi: 10.1016/j.freeradbiomed.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Han F., Ding Z.F., Shi X.L., Zhu Q.T., Shen Q.H., Xu X.M., et al. Irisin inhibits neutrophil extracellular traps formation and protects against acute pancreatitis in mice. Redox Biol. 2023;64 doi: 10.1016/j.redox.2023.102787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guarino C., Seren S., Lemoine R., Hummel A.M., Margotin J.E., El-Benna J., et al. Constitutive and induced forms of membrane-bound proteinase 3 interact with antineutrophil cytoplasmic antibodies and promote immune activation of neutrophils. J. Biol. Chem. 2023;299(4) doi: 10.1016/j.jbc.2023.103072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu N., Westra J., Kallenberg C.G. Membrane-bound proteinase 3 and its receptors: relevance for the pathogenesis of Wegener's Granulomatosis. Autoimmun. Rev. 2009;8(6):510–514. doi: 10.1016/j.autrev.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 90.Matsumoto T., Kaneko T., Wada H., Kobayashi T., Abe Y., Nobori T., et al. Proteinase 3 expression on neutrophil membranes from patients with infectious disease. Shock. 2006;26(2):128–133. doi: 10.1097/01.shk.0000223122.11147.5a. [DOI] [PubMed] [Google Scholar]
- 91.Seren S., Rashed Abouzaid M., Eulenberg-Gustavus C., Hirschfeld J., Nasr Soliman H., Jerke U., et al. Consequences of Cathepsin C inactivation for membrane exposure of proteinase 3, the target antigen in autoimmune vasculitis. J. Biol. Chem. 2018;293(32):12415–12428. doi: 10.1074/jbc.RA118.001922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jerke U., Eulenberg-Gustavus C., Rousselle A., Nicklin P., Kreideweiss S., Grundl M.A., et al. Targeting cathepsin C in PR3-ANCA vasculitis. J. Am. Soc. Nephrol. 2022;33(5):936–947. doi: 10.1681/ASN.2021081112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doyle K., Lönn H., Käck H., Van de Poël A., Swallow S., Gardiner P., et al. Discovery of second generation reversible covalent DPP1 inhibitors leading to an oxazepane amidoacetonitrile based clinical candidate (AZD7986) J. Med. Chem. 2016;59(20):9457–9472. doi: 10.1021/acs.jmedchem.6b01127. [DOI] [PubMed] [Google Scholar]
- 94.Chalmers J.D., Haworth C.S., Metersky M.L., Loebinger M.R., Blasi F., Sibila O., et al. Phase 2 trial of the DPP-1 inhibitor Brensocatib in bronchiectasis. N. Engl. J. Med. 2020;383(22):2127–2137. doi: 10.1056/NEJMoa2021713. [DOI] [PubMed] [Google Scholar]
- 95.Iskender I., Cypel M., Martinu T., Chen M., Sakamoto J., Kim H., et al. Effects of warm versus cold ischemic donor lung preservation on the underlying mechanisms of injuries during ischemia and reperfusion. Transplantation. 2018;102(5):760–768. doi: 10.1097/TP.0000000000002140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.