Abstract

The SARS-CoV-2 (COVID-19) pandemic outbreak led to enormous social and economic repercussions worldwide, felt even to this date, making the design of new therapies to combat fast-spreading viruses an imperative task. In the face of this, diverse cutting-edge nanotechnologies have risen as promising tools to treat infectious diseases such as COVID-19, as well as challenging illnesses such as cancer and diabetes. Aside from these applications, nanoscale metal–organic frameworks (nanoMOFs) have attracted much attention as novel efficient drug delivery systems for diverse pathologies. However, their potential as anti-COVID-19 therapeutic agents has not been investigated. Herein, we propose a pioneering anti-COVID MOF approach by studying their potential as safe and intrinsically antiviral agents through screening various nanoMOF. The iron(III)-trimesate MIL-100 showed a noteworthy antiviral effect against SARS-CoV-2 at the micromolar range, ensuring a high biocompatibility profile (90% of viability) in a real infected human cellular scenario. This research effectively paves the way toward novel antiviral therapies based on nanoMOFs, not only against SARS-CoV-2 but also against other challenging infectious and/or pulmonary diseases.
Keywords: COVID-19, metal−organic frameworks, nanomedicines, antiviral, therapy
1. Introduction
The global pandemic COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had an enormous social and economic effect worldwide: since 2019,1 > 770 million infections with ∼7 million deaths have been reported, resulting in > €8 trillion economic burden for health services.2 It highlights the urgent need for rapid and preventative responses to combat the fastest-spreading virus in the history of the modern world. Among the diverse recovery approaches, the European Union has steered the transition toward a sustainable and resilient Europe through the NextGenerationEU program, with a budget of €806 billion (sustaining €338 billion only for funded grants). One of the attractive, rapid, and economically viable strategies is drug repurposing,3 providing novel treatment options based on a time- and cost-effective discovery of new therapeutic directions arising from current clinical or ongoing investigational drug trials. For instance, the immunosuppressant monoclonal antibody Tocilizumab and the antimalarial Chloroquine (both used in rheumatoid arthritis) along with the antiviral Remdesivir (candidate for the treatment of Ebola) are examples of treatments for SARS-CoV-2 infection, the latter being a unique FDA (United States Food and Drug Administration)-approved emergency medicine for hospitalized COVID-19 infected patients.4 In parallel, the scientific community has advanced toward alternative antiviral prophylaxes, focusing on cutting-edge nanovaccines such as mRNA (mRNA) vaccines using lipid nanoparticles (NPs; BNT162b2 by Pfizer-BioNTech and mRNA-1273 by Moderna). These are some of the few commercialized FDA nanotechnologies, with their reliability demonstrated through more than 13 million administered doses since 2019.5 Furthermore, the greatest anti-COVID-19 activities have been achieved by distinct metal-based complexes and/or nanoparticles (NPs). For instance, potent inhibition of SARS-CoV-2 replication and other members of the coronavirus family has been realized using Au(I) complexes (e.g., auranofin),6 Ag(0) NPs,7,8 or other types of cations, salts, or oxides of biological interests (e.g., Bi(III)-metal salts),9,10 iron oxide NPs (IONs: Fe3O4/γ-Fe2O3),11 Zn(II) and Zn/Ru-ionophores,12 and ZnO-based biocomposites13).
Among emerging metal-based nanomedicines studied so far, nanoscale Metal–Organic Frameworks (MOFs) have attracted great attention as effective drug delivery systems (DDS).14−16 Compared to classic DDS, this new class of crystalline hybrid nanomaterials (composed of inorganic nodes linked by polydentate organic ligands, assembled into multidimensional periodic structures) offers several advantages such as (i) their chemical and structural versatility, which permits suitable biocompatibility and control of their in vivo fate, (ii) an ideal amphiphilic internal microenvironment that can be conveniently adapted to host a variety of active pharmaceutical ingredients (APIs; e.g., biological gases, drugs, enzymes, and nucleic acids), (iii) be used to release them in a controlled manner under physiological conditions, (iv) easy and scalable synthesis, following green methods with high yields, (v) proven in vivo safety (e.g., mice, nematodes, and fish), and (vi) their external surface engineering, endowing further multifunctional abilities such as furtivity, targeting, imaging, or enhancing their stability (chemical/structural/colloidal) under biorelevant conditions.17−20 However, despite all these favorable features and previous therapeutic outcomes achieved with other pathologies (e.g., cancer, diabetes, wound healing, or even as antimicrobial agents, mainly as antibacterial),17,21,22 MOFs have not been deeply investigated for antiviral purposes, and even less, for fighting COVID-19.
The scarce existing reports so far (∼70 articles since 2020, 22 publications in 2023; Web of Science, March 2024) have been mostly dealing with COVID-19 detection10,23−25 and viral spreading control (as surface coatings of filtration systems26 and as active micrometric MOFs for decontamination27). For the specific purpose of viral inhibition,28 only two recent works have reported the activity of drug-loaded MOFs (i.e., topical antiseptic-acriflavine29 and the antimalarial drug-hydroxychloroquine28), one of them exclusively as a passive nanocarrier while the other studied as well the intrinsic metal activity of copper in two Cu-based MOFs (Cu-BDC, HKUST-1). However, their novelty aside, the authors employed artificial pseudovirus-based entry assays for their performance evaluation, estimating the presence of the recombinant luciferase-labeled virus.28 Considering this, to the best of our knowledge, nanoMOFs have never been systematically explored as stand-alone antiviral agents or as DDS using real SARS-CoV-2 pathogenesis.
Given these precedents, we propose here an anti-COVID-19 nanoMOF-based platform for revealing, for the first time, their potential intrinsic antiviral activity. Hence, a preliminary screening of six nanoscaled MOFs with diverse structures and compositions has been performed to identify the most efficient nanoMOF against SARS-CoV-2-infected human cells. Considering the COVID transmission route, nanoMOF biosafety and infection efficacy (replication) were evaluated using a pulmonary human cell line, selecting the prototypes based on their porosities and biostabilities as well as a priori biocompatibility and potentially active composition (antimicrobial Fe11 and Bi9,10 sites; biocompatible Zr,29,30 trimesate and aminoterephthalate;31 antioxidant ellagate):32,33 (i) the heavily investigated mesoporous Fe(III)-trimesate structure, denoted MIL-100(Fe)34 (MIL stands for Material from Institute Lavoisier; Brunauer–Emmett–Teller surface SBET = 1800 m2·g–1; pore diameter Dp = 25 and 29 Å, accessible via 5 and 8.6 Å windows), with proven in vitro and in vivo biosafety and high efficacy as a DDS;17,35,36 (ii) the highly porous and biocompatible Fe(III)–aminoterephthalate MIL-101-NH2(Fe) (SBET = 1840 m2·g–1; Dp = 29 and 34 Å, accessible via 12 and 16 Å windows);37 (iii) its polymorph MIL-88B-NH2(Fe),31,37−39 with a highly flexible framework (open form SBET(theo.) = 2815 m2·g–1; Dp = 9 Å);31,39 (iv) the microporous Zr(IV)-aminoterephthalate UiO-66-NH2(Zr)40,41 (UiO stand for Universitetet i Oslo) (SBET = 1000 m2·g–1; Dp = 6 and 12.5 Å, accessible via 6 Å windows), whose with in vivo biosafety has already been proven as well as its pulmonary drug delivery;42−44 (v) the bioinspired and highly porous Zr(IV)-ellagate structure, SU-102(Zr) (SBET = 570 m2·g–1; Dp = 12 Å);45,46 and (vi) the biocompatible Bi(III)-ellagate phase, SU-101(Bi) (SBET = 410 m2·g–1; Dp = 6–7 Å),32 recently investigated as a potential DDS, showing an outstanding biostability profile (e.g., ciprofloxacin and dopamine).
Furthermore, in an attempt to have a better understanding of the nanoMOF inhibition performance, their structural, chemical, and colloidal biostability was investigated under relevant physiological media, paying special attention to the nature of their external surface physicochemical properties.
2. Results and Discussion
2.1. NanoMOF Anti-COVID-19 Activity
The nanoMOF in vitro anti-COVID-19 activity was verified using infected human lung adenocarcinoma A549-ACE2 cells. These were selected due to their accuracy in representing human airway and lung epithelia (primary route of COVID-19 transmission) as well as their susceptibility to SARS-CoV-2 infection since they express the human angiotensin-converting enzyme type-2 receptor [angiotensin-converting enzyme 2 (ACE2)], which is the main target of the viral spike glycoprotein S, used for the virus entry (i.e., cell-virus membrane fusion).47 A preliminary screening of six chosen nanoMOFs was undertaken in a dose–response assay, focusing on potential self-immunoactive MOFs (potential tolerogenic or immuno-active carriers)19 or cations with reported antiviral activity (e.g., Fe, Zr, and Bi).48−50 The infection efficacy was indirectly evaluated by assessing the level of viral antigen expression (levels of SARS-CoV-2 nucleoprotein-N) in the absence or presence of nanoMOFs through automatized immunofluorescence microscopy in SARS-CoV-2 infected cells. We aimed to seek a lower viral replication and, in turn, a reduction of viral-induced cell death when a nanoMOF is present. For this purpose, the number of cells during this viral prevention was reliably assessed through a DAPI (4′,6-diamidino-2-fenilindol) nuclei staining method, determining the nanoMOF concentration able to reduce 50 or 90% of viral antigen accumulation (EC50 and EC90; more details in Section S4). Simultaneously, the cellular viability was evaluated on equal terms by the metabolic MTT-based assay [stands for (3-[4,5-dimethilthyazol-2-yl]-2,5-diphenyl tetrazolium bromide)], estimating the CC50 value (nanoMOF concentration able to produce 50% of cytotoxicity), contributing to discard the highly toxic nanosystems.
After 48 h of contact with SARS-CoV-2 infected A549-ACE2 cells, a moderate but significant reductive effect (average of ∼43%; Figure 1) was observed on the viral infection induced by all the tested nanoMOFs at the highest tested concentration (100 μg·mL–1). However, to consider a new antiviral treatment as a relevant active compound in clinical trials, its therapeutic window (i.e., drug dosage range with a minimum effective therapeutic concentration giving a minimum toxic effect) needs to be investigated.51,52 In other words, 90% of viral inhibition must be triggered to provide a pharmacological-active NPs dose.
Figure 1.
NanoMOF antiviral activity in SARS-CoV-2 infected A549-ACE2 cells. Infection efficacy (%, green bars) and biocompatibility profiles (MTT assays, purple bars; DAPI-dyed cells, blue bars) were plotted after 48 h in the presence of the indicated doses (μg·mL–1) of MIL-100(Fe), MIL-88B-NH2(Fe), MIL-101-NH2(Fe), UiO-66-NH2(Zr), SU-102(Zr), and SU-101(Bi). Data are shown as mean ± standard deviation (SD) of three different experiments in triplicates (n = 9). Statistical significance was determined using ANOVA and Dunnetts post hoc method.
Taking this into account, it should be highlighted that a substantial inhibition of the viral propagation is observed with the addition of MIL-100(Fe) NPs, showing a decrease in the infection efficiency of up to ∼92% at moderate concentrations (20 μg·mL–1; Figures 1 and S3) with little to no increase of cytotoxicity (viability values ∼98%). Despite these outcomes, the activities are far from those of a strong antiviral agent: the clinically approved Remdesivir—taken here as positive quality control of the assay—has shown an EC50 of 60 nM with >99% of viral infection reduction at 3 μg·mL–1; see Supporting Information: Section S3, Figure S3. Even so, these results highlight the potential of using nanoMOFs in anti-COVID-19 therapies, evidenced here through the use of real COVID-infected cells. Even if the native nanoMOF activity is significantly smaller than that of Remdesivir (2 vs 0.5 μM for a total of 99% viral inhibition, respectively), considerable possibility for improvement is expected due to the nature of nanoMOF materials, including the potential of varied compositions, high API loadings, progressive release, and external coatings, making nanoMOFs promising as dual-action antiviral drug-delivery platforms.18,53,54
In contrast to MIL-100(Fe), high viral inhibition (with an average of ∼87%) was only obtained at the maximum tested dose for the rest of the nanoMOFs (∼91 > 90 > 88 > 85 > 81% of MIL-88B-NH2(Fe), SU-102(Zr), MIL-101-NH2(Fe), SU-101(Bi), UiO-66-NH2(Zr), respectively), which was associated with certain cytotoxicity, as denoted by both DAPI-dyed cells and MTT-based assay. This diverse performance profile (infection, biocompatibility) could be a consequence of the versatile nanoMOF composition and/or structures, which would impact not only their external viral interaction and/or cell internalization but also the cellular homeostasis such as redox processes (e.g., oxidative stress-ROS) or the initial activation of the immune response through, for example, complement activation. Notably, the highest inhibition effect is observed for the Fe-based MOFs (the highest inhibition effect: ∼ ≥90%) than Bi- or Zr-based materials (∼85%), showing a moderate prevention of the SARS-CoV-2 propagation upon decomposition. In the case of the Zr-based MOFs (UiO-66-NH2, SU-102), it is more evident that despite being compromised of the same metal cation, other factors such as structure, stability, size, and external surface, could be playing a key role in their therapeutic performance (antiviral performance: 90 vs 81%; cell viability: 89 vs 68% for SU-102 and UiO-66-NH2, respectively). Considering the possible activity of the individual nanoMOF components, we have also investigated their antiviral effect (Figure 2).
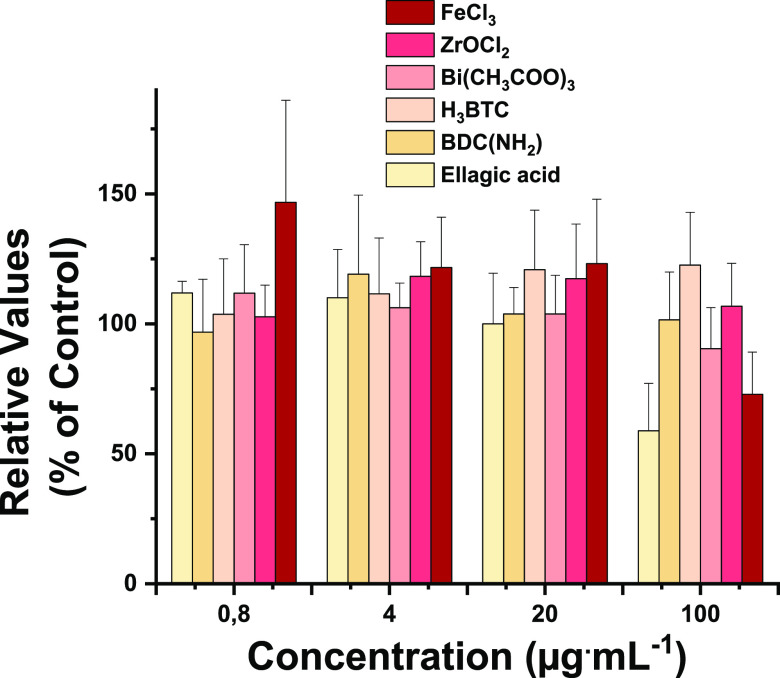
Figure 2.
Precursor antiviral activity in SARS-CoV-2 infected A549-ACE2 cells.
Infection efficacy (%) is determined after 48 h in the presence of chosen doses (μg·mL–1) of each precursor (the applied corresponding amount in each MOF prototype: specific metallic salts and linkers). Data are shown as mean ± SD of three different experiments in triplicates (n = 9). Statistical significance was determined using ANOVA and Dunnett’s posthoc method.
Overall, the presence of a metallic salt (FeCl3, ZrOCl2, Bi(CH3COO)3) or linker precursor (H3BTC, NH2–BDC) was not able to significantly hamper the virus infection (tested at the corresponding MOF maximum dose; more details in Sections 4.2–4.5), excepting the case of ellagic acid (EA), the common linker of SU-101(Bi) and SU-102(Zr) (constituting 42.9 and 64.6% of the total composition, respectively; Tables S1–S2) and the Fe salt, with just a slight decrease of the viral infection. This behavior would agree with its renowned antioxidant properties,55,56 which seem to have a protective role against SARS-CoV-2. Thus, one could suggest that the whole nanoMOF structure (associated with steady nanoparticle stability) rather than the individual components is required for successful viral interference.
In this context, the nanoMOF stability in terms of crystalline structure preservation (X-ray diffraction–XRD patterns–structural) and the potential release of their constituents to the cell culture media (HPLC-chemical; more details in Section 4.5) were evaluated. Except for the highly stable SU-101(Bi) and SU-102(Zr), after 48 h in contact with supplemented culture media [Dulbecco’s modified Eagle medium (DMEM)], the rest of the nanoMOFs showed a partial degradation associated with an initial ligand leaching up to 8 h, reaching then a stabilization phase at 48 h (Figure 3A). MIL-101-NH2(Fe) showed a degradation of 60%, followed by MIL-100(Fe), UiO-66-NH2, and MIL-88B–NH2(Fe) with values of ∼47, 25, and 6%, respectively. During this incubation, a crystalline structure loss was observed except for MIL-100(Fe) showing a decrease in their crystallinity already after 24 h (Figure S4). From one side, the slow but apparent degradation of the crystalline structure could have contributed to the largest observed antiviral effect, since the NP can prolong its exposure time, and thus its action as an intact structure against SARS-CoV-2. On the other hand, its partial degradation throughout the experiment (∼53% of iron leaching to the media) agrees with a previously reported docking study evidencing that iron species were able to interact with the viral spike protein receptor binding domain (S-RBD), blocking or reducing the viral entrance into the cell, and consequently, to the infection efficacy.
Figure 3.
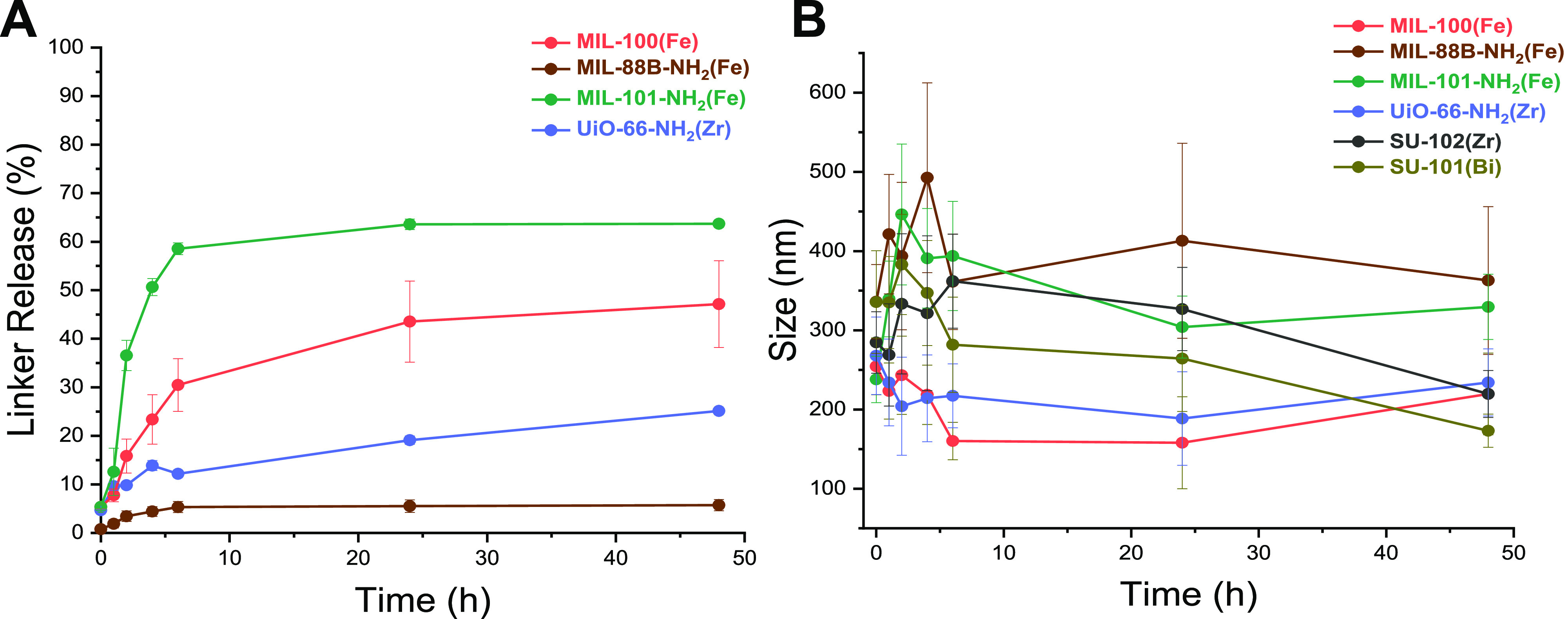
NanoMOF stability. (A) Chemical: Linker release [H3BTC, NH2BDC; % by high-performance liquid chromatography (HPLC)] in supplemented DMEM at 37 °C after 48 h. Data are shown as mean ± SD with three replicates per experiment. SU-102(Zr) and SU-101(Bi) did not show linker release; (B) Colloidal: Size (nm) variation in supplemented DMEM at 37 °C after 48 h.
In the case of ellagate-based MOFs, SU-102(Zr) and SU-101(Bi), it should be emphasized that both materials showed the highest chemical and structural stability with no linker release and unaltered crystallinities, contributing to their observed proactive antiviral effect (Figures 1, 3A, and S4). The exceptional robustness of these MOFs is mainly associated with the strong chelating phenolates, as previously reported.32,46 This behavior evidenced again that the intact MOF structure is playing a crucial role in their antiviral performance. Likewise, the presence of diverse surface charges of individual compounds or alternative surface chemistries (external moieties, functional groups) accessible to easily interact with the surroundings will directly affect their potential aggregation in the media (shifting the particle size) and, subsequently, their biological performance.
To shed light on this attractive approach, the nanoMOF–SARS-CoV-2 interaction in an infected cellular environment was further investigated in depth. The physicochemical properties of nanocarriers (e.g., surface chemistry, charge, stability, and particle size) highly affect their affinity to diverse biological structures (e.g., proteins, cells, tissues, and nucleic acids), which in turn governs their efficacy and biodistribution.57,58 To such ends, the nanoMOF colloidal stability and surface chemistry were monitored by dynamic light scattering (DLS, expressed as the average of the hydrodynamic particle size) and ζ -potential measurements, respectively, using diverse biorelevant media such as aqueous solution and supplemented DMEM (see Section 4.5.2 and Supporting Information, Table S3).
Aqueous suspensions of the diverse nanoMOFs exhibited a nanometric particle size (Table S3), in agreement with their crystal dimensions in a dry state (as observed by TEM, Figure S2; ∼250 nm; Figure 3B and Table S3). In contrast, when suspended in cell culture media, the overall nanoMOF size slightly increased to ∼280 nm, being stabilized in shorter times up to 48 h (Figure 3B). Note here that the smallest hydrodynamic sizes are displayed for both MIL-100(Fe) and UiO-66-NH2(Zr) (∼200 nm), in contrast with the rest of the nanoMOFs (≥250 nm). Hence, although one could imply that the smaller the crystal dimension, the higher the antiviral activity (surface-to-volume ratio, reactivity, and/or viral contact), this factor might not be the unique parameter since although MIL-100(Fe) showed the most efficient antiviral performance the least were UiO-66-NH2(Zr) NPs.
On the other hand, the presence of biochemical compounds in the cell culture medium may be able to directly interplay with the external surface charge of the nanoMOFs, modifying their interactions with their surroundings and, potentially leading to aggregation phenomena (as initially observed in all the nanoMOFs; Figure 3B) and/or different interactions with other biological entities, such as the virus. Initially, the nanoMOF surface charge in simpler aqueous solution shows significantly different values depending on their composition. If the positively charged surfaces of MIL-101-NH2(Fe) and MIL-88B–NH2(Fe) NPs could be explained by the presence of –NH3+ of the ligand (pKa ∼ 9.5, pH = 7.4),59,60 the negative charge in the other nanoMOFs might be associated with partially coordinated ligands (carboxylate, pKa ∼ 3.5 and 4.4 or hydroxyl groups pKa ∼ 5.5, 6.2, 11.8 and 12.4)59−61 and/or metals (coordination sphere completed with =O, –OH2, or–OH; Table S3). In contrast, the ζ-potential values turn to a more neutral surface charge in DMEM than in aqueous solutions (from −5 to −17 mV, Figure S5 and Table S3). This shift could be a result of diverse protein–nanoMOF surface combinations, exhibiting the previously mentioned reactive groups. However, note here that despite the interaction with the medium proteins, a higher affinity toward the viral surface might shift the adsorption equilibrium,61,62 leading to the exposure of the initial MOF surface to the virus. Indeed, viral adsorption is highly affected by the pH of the media (i.e., protonation/deprotonation of the virion surface-active moieties). At pH values lower than the isoelectric point of surface glycoproteins (pI = 6.24) of surface glycoproteins, the overall charge of SARS-CoV-2 would be positive. Further, the tendency of the iron carboxylate MOFs to decrease the pH (pH ∼ 6 in cell culture)61 could also influence the viral surface, affecting its cellular entry. The resulting external surface of the nanoMOFs exposed toward the SARS-CoV-2 targets (i.e., S-RBD protein) suggests a direct consequence in the viral cellular entry, which in turn, may provide insight into, the potential mechanism of action.
Although it has not been properly evaluated for these selected nanoMOFs, it cannot be excluded that a mix of both prophylactic and/or therapeutic mechanisms is present. On one hand, a therapeutic mechanism could be expected as the nanoMOFs may act as carriers, affecting the metabolism of the infected cell through their potential uptake of substances. For instance, it is known that higher iron intake by Fe-based NPs has demonstrated their repercussion on the cellular iron metabolism by decreasing the SARS-CoV-2 induced ferroptosis63 as well as on the oxidative stress,11 hampering the virus replication and exocytosis of the new virions. On the other hand, a prophylactic mechanism may present through the hindering of viral internalization into the host cell due to a greater affinity to the nanoMOF surface. However, a better understanding of the chemical nature of the outer nanoMOF surfaces and their interaction with the biological surroundings (e.g., proteins, ions, and pH)64 will be required to shed light on the effective interaction between the nanoMOF-SARS-CoV-2 interfaces, which compromise the hijacking of the host cell machinery and the viral multiplication.
In the case of the most promising inhibitor, MIL-100(Fe), a combination of effects may give rise to its exceptional antiviral activity. First, as a consequence of its relatively high structural biostability, and consequentially intact surface, as well as its capacity to acidify the medium (more positively charged virus), the interactions between the carboxylate groups present on the surface and the virus would be highly favored. Additionally, the progressive and moderate degradation of MIL-100(Fe) could promote an antiviral action, releasing gradually the proven active iron species. Finally, the reported effective internalization of MIL-100(Fe) in diverse cell lines (e.g., cervical-HeLa, macrophagic-J774A.1, lung-A549 and Calu-3, hepatic-HepG2, and Hep3B cells)31,65 might also reinforce its therapeutic efficacy. In other words, this Fe-based MOF seems to be able to trigger a hostile environment for the SARS-CoV-2 infection, not only during the binding process to the host cells but also by inducing intracellular processes such as oxidative stress without relevant repercussions on the whole cellular viability. However, further experimental assays should be performed to reveal the particular nanoMOF trafficking mechanism involved in the interference of the SARS-CoV-2 propagation.
3. Conclusions
In a nutshell, to the best of our knowledge, this is the first article evidencing the intrinsic role of nanoMOFs against a real SARS-CoV-2 infected scenario, emphasizing the imperative of the integrity of the nanoparticulate MOF for triggering their antiviral effect. It is also worth pointing out that, among diverse tested MOF families, MIL-100(Fe) has emerged as the most promising anti-COVID-19 candidate, exhibiting a significant viricide effect (EC50 < 2 μM) associated with an elevated biosafe profile (>99%) in a real infected human cellular scenario. It seems that this effective antiviral performance might be related to a combination of prophylactic/therapeutic effects. These promising outcomes along with their attractive features as DDS (e.g., exceptional drug loadings, biosafety) pave the way toward future preclinical and clinical research for the COVID treatment or other challenging infectious diseases related to pulmonary pathologies.
4. Experimental Section
4.1. Materials and Methods
FeCl3·6H2O (Sigma-Aldrich, 97%), trimesic acid (benzene-1,3,5-tricarboxylic acid, 97% Sigma-Aldrich), 2-aminoterephthalic acid (2-aminobenzene-1,4-dicarboxylic acid, 99% Acros Organics), Ti(OiPr)4 (Sigma-Aldrich, 97%), ZrOCl2·8H2O (Sigma-Aldrich), Bi(CH3COO)3 (Alfa Aesar, 99%), EA (Acros Organics, 97%), N,N-dimethylformamide(DMF, Chemlab, 99.5%), methanol (MeOH, JT. Baker, analytical grade), ethanol (EtOH, 96%), glacial acetic acid (Merck, 100%), DMEM (Sigma-Aldrich), fetal bovine serum (FBS, Sigma-Aldrich), penicillin/streptomycin (P/S, Sigma-Aldrich), nonessential amino acids (Sigma-Aldrich), NaH2PO4 (Sigma-Aldrich), H3PO4 (Sigma-Aldrich), MTT (3-[4,5-dimethilthyazol-2-yl]-2,5-diphenyl tetrazolium bromide, Across Organics), DMSO (dimethyl sulfoxide), Triton-X100, Remdesivir (Santa Cruz), 4% paraformaldehyde (PFA), bovine serum albumin (BSA; Sigma-Aldrich), tetramethylbenzidine (TBM) substrate (Thermo Scientific ref: 34024), DAPI (Life Technologies), Recombinant 1.26 nanobody against S protein (PMID—PubMed ref: 35547740), rabbit monoclonal primary antibody against N protein (GeneTex HL344), goat antirabbit secondary antibody conjugated to Alexa 488 (Invitrogen-Carlsbad, CA, USA), and goat antihuman secondary antibody conjugated to horseradish peroxidase (HRP, Invitrogen ref: 31410) were used as received without further purification.
Phosphate-buffered solution (PBS, 0.04 M, pH = 2.5) for HPLC determination was prepared by solving 2.4 g (0.02 mol) of NaH2PO4 and 2.84 g (0.02 mol) of Na2HPO4 in 1 L of deionized water. The pH was then adjusted to 2.5 with H3PO4 (≥85%).
Supplemented cell culture media solution for the stability and in vivo studies was prepared by adding to the DMEM, 2% FBS, 1% P/S, and 1% nonessential amino acids.
4.2. NanoMOF Synthesis
The synthesis of the selected MOFs: MIL-100(Fe),66 MIL-88B–NH2(Fe),39 MIL-101-NH2(Fe)67 UiO-66-NH2(Zr),68 SU-102(Zr),45 and SU-101(Bi)32 was performed following the published synthetic methods, adapting the synthesis conditions to obtain nanoparticles (NPs) as described in the Supporting Information (more details in Table S1).
4.3. Physicochemical Characterization
X-ray powder diffraction (XRPD) patterns were collected in a D8 Advance Bruker diffractometer with Cu Kα1 radiation (λ = 1.54056 Å). Profiles were generally collected in the 3–35° 2θ range with a typical step size of 0.02° and a scanning speed of 0.1°·s–1 in continuous mode. Fourier transform infrared (FTIR) spectra were collected using a Nicolet 6700 instrument (Thermo Scientific USA) from 4000 to 400 cm–1. Thermogravimetric analyses (TGA; 5–10 mg) were collected on a PerkinElmer Diamond TGA/DTA STA 6000 (25–600 °C at 5 °C·min–1 with an airflow of 100 mL·min–1). The particle size and colloidal stabilities were monitored by DLS, and zeta potential (ζ) was registered via Electrophoretic Light Scattering using a Zetasizer Nano series Nano-ZS (Malvern Instruments, Worcestershire, UK) with a concentration of 0.1 mg·mL–1 at room temperature or 37 °C in the desired media by the use of an ultrasound tip (10% amplitude for 1 min; Digital Sonifer 450, Branson). Similarly, for biocompatibility assays, the NP solutions were prepared at a 10-fold higher concentration (due to a 1:10 direct dilution in each), yielding a diverse concentration range (more details in Section 4.5). The chemical stability was monitored with a reverse-phase HPLC in a Jasco LC-4000 series model, with a photodiode array (PDA) detector MD-4015, as well as a multisampler AS-4150 operated by a ChromNav software (Jasco Inc., Japan) with a Purple ODS reverse-phase column (5 μm, 4.6 × 150 mm, Análisis Vínicos, Spain). For the quantification of the analyzed ligands, isocratic conditions were used, with a flow rate of 1 mL·min–1, a fixed column temperature of 298 K, and an injection volume of 30 μL. The mobile phase was a mixture of PBS (0.04 M, pH: 2.5) and MeOH (60:40) for trimesic acid (H3BTC) and 2-aminoterephthalic acid (NH2–BDC) with retention times (rt) and absorption maximums of 4.17 min and 228 nm, and 4.72 min and 225 nm, respectively. Enzyme-linked immunoassays (ELISAs) were performed following the manufacturer’s protocols and analyzed by an automated multimode plate reader (TECAN Spark Cyto, Grödig, Austria).
4.4. In Vitro Studies
4.4.1. Cell Lines and Culture
A549, a pulmonary epithelial cell line derived from human lung adenocarcinoma, was kindly provided by Dr. Pablo Gastaminza (CNB-CSIC, Madrid, Spain). A549-ACE2 cells stably expressing the ACE2 type receptor were obtained by retroviral vector transduction and selected under blasticidine (2.5 μg·mL–1) restricted conditions.69 Cell cultures were maintained in complete high glucose DMEM supplemented with 10% FBS and 1% of PS (100 U·mL–1) with nonessential amino acids at 37 °C in a 5% CO2 humidified atmosphere. For the infection experiments, 2% FBS and the absence of selection antibiotics were used.
4.4.2. SARS-CoV-2 Infection Assays
All the infection experiments were performed by inoculating A549-ACE2 cells seeded onto 96-well plates (2 × 104 cells per well) with the SARS-CoV-2 virus (Orthocoronavirinae; Alphacoronavirus; Sarbecovirus; strain NL/2020) kindly provided by Dr. R. Molenkamp (Erasmus University Medical Center Rotterdam) at low multiplicity of infection (MOI) of 0.005, as indicated below. All treatments were diluted in the completed media from the aqueous stock solutions (at 1 mg·mL–1, see below) to achieve the selected compound concentrations. All infection experiments were conducted in an authorized BSL3 facility at the CNB-CSIC following international regulations and under the CSIC Ethics Committee supervision.
4.4.3. NanoMOF Antiviral Activity
Automatized immunofluorescence microscopy was used to evaluate the potential antiviral effect. A549-ACE2 cells were seeded onto 96-well plates as indicated above and infected (MOI = 0.005) in the presence of the indicated compound dose. After 48 h postinfection, cells were fixed with 4% paraformaldehyde in PBS for 20 min at RT, washed twice with PBS, and kept for 1 h in the incubation buffer (3% BSA; 0.3% Triton-X100 in PBS). Cells were then incubated for 1 h with a rabbit monoclonal primary antibody against N protein (dilution 1:2000), washed with PBS, and subsequently incubated with a goat antirabbit secondary antibody conjugated to Alexa 488 (dilution 1:500). The nuclei were labeled with DAPI during the secondary antibody incubation following the manufactureŕs instructions. Cells were then washed with PBS and visualized using an automated multimode plate reader. As controls, uninfected cells were used to determine the assay background (0% of infection, C−) along with the maximum virus infection (100%) as vehicle-treated cells (water, C+). Additionally, an internal control, Remdesivir,69 was also tested for the validation/reliability of the experiment. The antiviral activity of the selected prototypes was indirectly calculated from the experimental fluorescence measurements (%) related to the negative controls (vehicle-treated infected cells; Figure S3).
Alternatively, an additional colorimetric assay (ELISA) was used to evaluate the potential nanoMOF antiviral effect considering the MOF intrinsic fluorescence, which could hamper the immunofluorescence microscopy readout. In this case, the recombinant 1.26 nanobody against S protein ([0.1 μg·mL–1]) together with a goat antihuman secondary antibody conjugated to HRP ([0.04 μg·mL–1]) were here replaced instead of the previous antibodies. Tetramethylbenzidine (TBM) substrate was used following the manufacturer’s instructions. The colorimetric signal was acquired at λ = 450 nm in a multimode TEKAN plate reader.
4.4.4. Cytotoxicity Assays
NanoMOF biocompatibility profiles were evaluated by the MTT colorimetric assay following the manufacturer’s instructions.70 Briefly, adherent A549-ACE2 cells were seeded 24 h prior to the assay in 96-well plates at a density of 2 × 104 cells per well in a complete medium. A wide range of nanoMOF and/or precursor solutions (stock 1 mg·mL–1) were prepared, yielding diverse concentrations (from 0.8 to 100 μg·mL–1) to achieve the desired dilutions. Cells treated with the vehicle or with 1% Triton-X100 were considered positive and negative controls, respectively. The nanoMOF cytotoxicity profile was evaluated after 48 h by adding the MTT reactant (0.5 mg·mL–1 in PBS, incubated at 37 °C for 2 h), dissolving the formazan crystals with DMSO (100 μL per well). Absorbance was determined at λ = 570 nm using a microplate reader (Omega, BMG Labtech). Cell viability was calculated as the percentage of the experimental absorbance measurements of control growth, subtracting the absorbances derived from NPs &/or the media.
4.5. NanoMOF Stabilities
MOF physicochemical properties (chemical stability-HPLC, structural-XRD, and colloidal-DLS) assays were carried out in supplemented cell culture media (DMEM, see Sections 4.1, S4) under shaking conditions (37 °C, up to 48 h) to mimic the potential in vitro viral interaction (cells + virus + nanoMOFs). Every experiment was done at least three times using three replicates each (n = 9).
4.5.1. Chemical and Structural Stability
A nanoMOF concentration of 5 mg·mL–1 (weight considering dry powder) was suspended in DMEM, being sonicated with an ultrasound bath (10% amplitude, 20s). The resulting suspension was kept in an orbital incubator shaker at 37 °C using bidimensional stirring. At each given time (0, 1, 2, 4, 6, 24, and 48 h), the samples were centrifuged (13400 rpm, 3 min), collecting a 0.5 mL aliquot of the supernatant which was replaced with the same volume of “fresh” supplemented DMEM to continue the kinetic experiment. This aliquot was used for the quantification of the linker release by HPLC, determining its chemical stability profile (Figure 3A). After diverse incubation times (0 > 24 > 48 h), the MOF structural stability was checked by XRPD (Figure S4).
4.5.2. Colloidal Stability
One mg of nanoMOF (weight considering dry powder) was dispersed in 10 mL of supplemented DMEM (0.1 μg·mL–1), monitoring the particle size and surface charge over time (up to 48 h, 37 °C). The resulting measurements are depicted in Figure 3B and figures in the Supporting Information (Figure S5).
4.6. Ethics Statement
All the experiments involving virus infection of cell cultures were performed by the Antiviral Screening Platform in the biosafety level 3 (BSL-3) facilities of the Centro Nacional de Biotecnología (CNB) (Madrid, Spain).
4.7. Statistics
The results of the different assays are represented as mean ± SD. Ordinary Two-way ANOVA analysis of variance followed by Dunnett’s multiple comparison tests were carried out to determine significant differences using GraphPad Prism 9.2 software (GraphPad Software, Inc., La Jolla, CA, USA). Each experiment was performed at least three times (n ≥ 3). In the graphs, the results are indicated as P > 0.05, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001.
Acknowledgments
“Comunidad de Madrid” and European Regional Development Fund—FEDER 2014-2020-OE REACT-UE 1—financial support to the VIRMOF-CM project associated with R&D projects in response to COVID-19. T.H., C.B., and P.H.: European Union’s Horizon Europe Research and Innovation Programme under the Marie Sklodowska-Curie grant agreement (no. 897678-NeuroMOF and no. 101061833-BioPolyMOF). Also, this work has been supported by other MSCA action, ITN—Marie Skłodowska-Curie Innovative Training Networks (ITN; no.: 860942-HeatNMof). The authors would like to thank Dr. Urtzi Garaigorta and Dr. Pablo Gastaminza from the Centro Nacional de Biotecnología (CNB-CSIC) for the fruitful discussion and also highlight the TOC design to our Communication Manager, Lorena Martínez.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.4c06174.
Synthesis, physicochemical characterization, stabilities, and in vitro cellular studies (PDF)
Author Present Address
§ Department of Chemistry and Biochemistry, Materials Science Institute, University of Oregon, Eugene, Oregon 97403, United States
Author Contributions
∥ I.Á.-M. and B.F. are co-first authors and contributed equally to this work. Inés Álvarez-Miguel: Methodology, Investigation, Writing–original draft; Beatrice Fodor: Methodology, Investigation, Writing–original draft. Guillermo Gutiérrez: Methodology, Investigation; Catalina Biglione: Investigation, Supervision; Erik Svensson Grape: Visualization, Writing–review; A. Ken Inge: Visualization, Writing–review. Tania Hidalgo: Conceptualization, Methodology, Investigation, Supervision, Writing–original draft, review and editing, Funding acquisition; Patricia Horcajada: Conceptualization, Supervision, Writing–review and editing, Funding acquisition.
The authors declare no competing financial interest.
Supplementary Material
References
- Agarwal R.; Gopinath G.; Farrar J.; Hatchett R.; Sands P. A Global Strategy to Manage the Long-Term Risks of COVID-19. IMF Work. Pap. 2022, 2022 (068), 1. 10.5089/9798400205996.001. [DOI] [Google Scholar]
- World Health Organization(WHO) . World Health Organization. [Internet]. https://data.who.int/dashboards/covid19/deaths. Accessed date (April 2024).
- Elkashlan M.; Ahmad R. M.; Hajar M.; Al Jasmi F.; Corchado J. M.; Nasarudin N. A.; Mohamad M. S. A Review of SARS-CoV-2 Drug Repurposing: Databases and Machine Learning Models. Front. Pharmacol 2023, 14 (August), 1182465. 10.3389/fphar.2023.1182465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration (FDA) . Remdesivir (Veklury) [package insert]: Highlights of prescribing information. Remdesivir (Veklury) [package insert]: Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/214787s019lbl.pdf. Accessd date (March 2024).
- Huang X.; Kon E.; Han X.; Zhang X.; Kong N.; Mitchell M. J.; Peer D.; Tao W. Nanotechnology-Based Strategies against SARS-CoV-2 Variants. Nat. Nanotechnol. 2022, 17 (10), 1027–1037. 10.1038/s41565-022-01174-5. [DOI] [PubMed] [Google Scholar]
- Laplantine E.; Chable-Bessia C.; Oudin A.; Swain J.; Soria A.; Merida P.; Gourdelier M.; Mestiri S.; Besseghe I.; Bremaud E.; Neyret A.; Lyonnais S.; Favard C.; Benaroch P.; Hubert M.; Schwartz O.; Guerin M.; Danckaert A.; Del Nery E.; Muriaux D.; Weil R. The FDA-Approved Drug Auranofin Has a Dual Inhibitory Effect on SARS-CoV-2 Entry and NF-ΚB Signaling. iScience 2022, 25 (10), 105066. 10.1016/j.isci.2022.105066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilaquinga F.; Morey J.; Torres M.; Seqqat R.; Piña M. d. l. N. Silver Nanoparticles as a Potential Treatment against SARS-CoV-2: A Review. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2021, 13 (5), 1–19. 10.1002/wnan.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q.; Lu J.; Liu N.; Lu W.; Li Y.; Shang C.; Li X.; Hu L.; Jiang G. Antiviral Properties of Silver Nanoparticles against SARS-CoV-2: Effects of Surface Coating and Particle Size. Nanomater 2022, 12 (6), 990. 10.3390/nano12060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.; Chan J. F.-W.; Wang S.; Li H.; Zhao J.; Ip T. K.-Y.; Zuo Z.; Yuen K.-Y.; Yuan S.; Sun H. Orally Administered Bismuth Drug Together with N-Acetyl Cysteine as a Broad-Spectrum Anti-Coronavirus Cocktail Therapy. Chem. Sci. 2022, 13 (8), 2238–2248. 10.1039/D1SC04515F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X.; Zhang L.; Du L.; Liao R.; Cai H.; Lu K.; Zhao Z.; Xie Y.; Wang P.-H.; Pan J.-A.; Zhang Y.; Li G.; Dai J.; Mao Z.-W.; Xia W. Allosteric Inhibition of SARS-CoV-2 3CL Protease by Colloidal Bismuth Subcitrate. Chem. Sci. 2021, 12 (42), 14098–14102. 10.1039/D1SC03526F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeDiego M. L.; Portilla Y.; Daviu N.; López-García D.; Villamayor L.; Mulens-Arias V.; Ovejero J. G.; Gallo-Cordova Á.; Veintemillas-Verdaguer S.; Morales M. P.; Barber D. F. Iron Oxide and Iron Oxyhydroxide Nanoparticles Impair SARS-CoV-2 Infection of Cultured Cells. J. Nanobiotechnol. 2022, 20 (1), 352. 10.1186/s12951-022-01542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kladnik J.; Dolinar A.; Kljun J.; Perea D.; Grau-Expósito J.; Genescà M.; Novinec M.; Buzon M. J.; Turel I. Zinc Pyrithione Is a Potent Inhibitor of PLPro and Cathepsin L Enzymes with Ex Vivo Inhibition of SARS-CoV-2 Entry and Replication. J. Enzyme Inhib. Med. Chem. 2022, 37 (1), 2158–2168. 10.1080/14756366.2022.2108417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AbouAitah K.; Allayh A. K.; Wojnarowicz J.; Shaker Y.; Swiderska-Sroda A.; Lojkowski W. Nanoformulation Composed of Ellagic Acid and Functionalized Zinc Oxide Nanoparticles Inactivates DNA and RNA Viruses. Pharmaceutics 2021, 13, 2174. 10.3390/pharmaceutics13122174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing F.; Xu J.; Zhou Y.; Yu P.; Zhe M.; Xiang Z.; Duan X.; Ritz U. Recent Advances in Metal-Organic Frameworks for Stimuli-Responsive Drug Delivery. Nanoscale 2024, 16 (9), 4434–4483. 10.1039/D3NR05776C. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Gao N.; Li X.; Ling G.; Zhang P. Metal Organic Framework-Based Variable-Size Nanoparticles for Tumor Microenvironment-Responsive Drug Delivery. Drug Delivery Transl. Res. 2024, 14, 1737–1755. 10.1007/s13346-023-01500-x. [DOI] [PubMed] [Google Scholar]
- Karimi Alavijeh R.; Akhbari K. Cancer Therapy by Nano MIL-n Series of Metal-Organic Frameworks. Coord. Chem. Rev. 2024, 503, 215643. 10.1016/j.ccr.2023.215643. [DOI] [Google Scholar]
- Horcajada P.; Chalati T.; Serre C.; Gillet B.; Sebrie C.; Baati T.; Eubank J. F.; Heurtaux D.; Clayette P.; Kreuz C.; Chang J.-S.; Hwang Y. K.; Marsaud V.; Bories P.-N.; Cynober L.; Gil S.; Férey G.; Couvreur P.; Gref R. Porous Metal-Organic-Framework Nanoscale Carriers as a Potential Platform for Drug Delivery and Imaging. Nat. Mater. 2010, 9 (2), 172–178. 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]
- Rojas S.; Arenas-Vivo A.; Horcajada P. Metal-Organic Frameworks: A Novel Platform for Combined Advanced Therapies. Coord. Chem. Rev. 2019, 388, 202–226. 10.1016/j.ccr.2019.02.032. [DOI] [Google Scholar]
- Hidalgo T.; Simón-Vázquez R.; González-Fernández A.; Horcajada P. Cracking the Immune Fingerprint of Metal-Organic Frameworks. Chem. Sci. 2022, 13 (4), 934–944. 10.1039/D1SC04112F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.; Walden M.; Ettlinger R.; Kiessling F.; Gassensmith J. J.; Lammers T.; Wuttke S.; Peña Q. Biomedical Metal-Organic Framework Materials: Perspectives and Challenges. Adv. Funct. Mater. 2023, 2308589. 10.1002/adfm.202308589. [DOI] [Google Scholar]
- Al Sharabati M.; Sabouni R.; Husseini G. A. Biomedical Applications of Metal-Organic Frameworks for Disease Diagnosis and Drug Delivery: A Review. Nanomaterials 2022, 12, 277. 10.3390/nano12020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin L.; Harvey P. D. Virus Management Using Metal-Organic Framework-Based Technologies. ACS Appl. Mater. Interfaces 2023, 15 (11), 13844–13859. 10.1021/acsami.3c00922. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Wang Z.; Gupta P.; Morrissey J. J.; Naik R. R.; Singamaneni S. Enhancing the Stability of COVID-19 Serological Assay through Metal-Organic Framework Encapsulation. Adv. Healthcare Mater. 2021, 10 (18), 2100410. 10.1002/adhm.202100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Batra K.; Clavier G.; Maurin G.; Ding B.; Tissot A.; Serre C. Ln-MOF Based Ratiometric Luminescent Sensor for the Detection of Potential COVID-19 Drugs. Chem.—Eur. J. 2023, 29 (12), e202203136 10.1002/chem.202203136. [DOI] [PubMed] [Google Scholar]
- Wang X.; Clavier G.; Zhang Y.; Batra K.; Xiao N.; Maurin G.; Ding B.; Tissot A.; Serre C. A MOF/DNA Luminescent Sensing Platform for Detection of Potential COVID-19 Biomarkers and Drugs. Chem. Sci. 2023, 14 (20), 5386–5395. 10.1039/D3SC00106G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A. V.; Vornholt S. M.; Major L. L.; Ettlinger R.; Jansen C.; Rainer D. N.; de Rome R.; So V.; Wheatley P. S.; Edward A. K.; Elliott C. G.; Pramanik A.; Karmakar A.; Armstrong A. R.; Janiak C.; Smith T. K.; Morris R. E. Surface-Functionalized Metal-Organic Frameworks for Binding Coronavirus Proteins. ACS Appl. Mater. Interfaces 2023, 15 (7), 9058–9065. 10.1021/acsami.2c21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plastiras O.-E.; Bouquet P.; Lecœur C.; Dhainaut J.; Dacquin J.-P.; Royer S.; Loiseau T.; Goffard A.; Volkringer C. Cytotoxicity and Effectiveness of Archetypal Metal-Organic Frameworks (HKUST-1, UiO-66, MIL-53, MIL-125) against Coronaviruses (HCoV-229E and SARS-CoV-2). Microporous Mesoporous Mater. 2024, 367, 112975. 10.1016/j.micromeso.2023.112975. [DOI] [Google Scholar]
- Ejsmont A.; Warowicka A.; Broniarczyk J.; Goscianska J. The Synergistic Effect of Cu-MOF Nanoparticles and Immunomodulatory Agent on SARS-CoV-2 Inhibition. Chem. Commun. 2023, 59 (33), 4907–4910. 10.1039/D3CC00894K. [DOI] [PubMed] [Google Scholar]
- Jodłowski P. J.; Dymek K.; Kurowski G.; Jaśkowska J.; Bury W.; Pander M.; Wnorowska S.; Targowska-Duda K.; Piskorz W.; Wnorowski A.; Boguszewska-Czubara A. Zirconium-Based Metal-Organic Frameworks as Acriflavine Cargos in the Battle against Coronaviruses–A Theoretical and Experimental Approach. ACS Appl. Mater. Interfaces 2022, 14 (25), 28615–28627. 10.1021/acsami.2c06420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo C.; Xiao J.; Xiao K.; Zou S.; Wang M.; Qi P.; Liu T.; Hu Y. Pre-Treatment with Zirconia Nanoparticles Reduces Inflammation Induced by the Pathogenic H5N1 Influenza Virus. Int. J. Nanomed. 2020, 15, 661–674. 10.2147/IJN.S221667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamames-Tabar C.; Cunha D.; Imbuluzqueta E.; Ragon F.; Serre C.; Blanco-Prieto M. J.; Horcajada P. Cytotoxicity of Nanoscaled Metal-Organic Frameworks. J. Mater. Chem. B 2014, 2 (3), 262–271. 10.1039/C3TB20832J. [DOI] [PubMed] [Google Scholar]
- Grape E. S.; Flores J. G.; Hidalgo T.; Martínez-Ahumada E.; Gutiérrez-Alejandre A.; Hautier A.; Williams D. R.; O’Keeffe M.; Öhrström L.; Willhammar T.; Horcajada P.; Ibarra I. A.; Inge A. K. A Robust and Biocompatible Bismuth Ellagate MOF Synthesized Under Green Ambient Conditions. J. Am. Chem. Soc. 2020, 142 (39), 16795–16804. 10.1021/jacs.0c07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceci C.; Lacal P. M.; Tentori L.; De Martino M. G.; Miano R.; Graziani G. Experimental Evidence of the Antitumor, Antimetastatic and Antiangiogenic Activity of Ellagic Acid. Nutrients 2018, 10, 1756. 10.3390/nu10111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horcajada P.; Surblé S.; Serre C.; Hong D.-Y.; Seo Y.-K.; Chang J.-S.; Grenèche J. M.; Margiolaki I.; Férey G. Synthesis and Catalytic Properties of MIL-100(Fe), an Iron(Iii) Carboxylate with Large Pores. Chem. Commun. 2007, (27), 2820–2822. 10.1039/B704325B. [DOI] [PubMed] [Google Scholar]
- Fernández-Paz C.; Rojas S.; Salcedo-Abraira P.; Simón-Yarza T.; Remuñán-López C.; Horcajada P. Metal-Organic Framework Microsphere Formulation for Pulmonary Administration. ACS Appl. Mater. Interfaces 2020, 12 (23), 25676–25682. 10.1021/acsami.0c07356. [DOI] [PubMed] [Google Scholar]
- Hidalgo T.; Alonso-Nocelo M.; Bouzo B. L.; Reimondez-Troitiño S.; Abuin-Redondo C.; de la Fuente M.; Horcajada P. Biocompatible Iron(Iii) Carboxylate Metal-Organic Frameworks as Promising RNA Nanocarriers. Nanoscale 2020, 12 (8), 4839–4845. 10.1039/C9NR08127E. [DOI] [PubMed] [Google Scholar]
- Bauer S.; Serre C.; Devic T.; Horcajada P.; Marrot J.; Férey G.; Stock N. High-Throughput Assisted Rationalization of the Formation of Metal Organic Frameworks in the Iron(III) Aminoterephthalate Solvothermal System. Inorg. Chem. 2008, 47 (17), 7568–7576. 10.1021/ic800538r. [DOI] [PubMed] [Google Scholar]
- Serre C.; Mellot-Draznieks C.; Surblé S.; Audebrand N.; Filinchuk Y.; Férey G. Role of Solvent-Host Interactions That Lead to Very Large Swelling of Hybrid Frameworks. Science 2007, 315 (5820), 1828–1831. 10.1126/science.1137975. [DOI] [PubMed] [Google Scholar]
- Horcajada P.; Salles F.; Wuttke S.; Devic T.; Heurtaux D.; Maurin G.; Vimont A.; Daturi M.; David O.; Magnier E.; Stock N.; Filinchuk Y.; Popov D.; Riekel C.; Férey G.; Serre C. How Linker’s Modification Controls Swelling Properties of Highly Flexible Iron(III) Dicarboxylates MIL-88. J. Am. Chem. Soc. 2011, 133 (44), 17839–17847. 10.1021/ja206936e. [DOI] [PubMed] [Google Scholar]
- Cavka J. H.; Jakobsen S.; Olsbye U.; Guillou N.; Lamberti C.; Bordiga S.; Lillerud K. P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130 (42), 13850–13851. 10.1021/ja8057953. [DOI] [PubMed] [Google Scholar]
- DeStefano M. R.; Islamoglu T.; Garibay S. J.; Hupp J. T.; Farha O. K. Room-Temperature Synthesis of UiO-66 and Thermal Modulation of Densities of Defect Sites. Chem. Mater. 2017, 29 (3), 1357–1361. 10.1021/acs.chemmater.6b05115. [DOI] [Google Scholar]
- He C.; Lu K.; Liu D.; Lin W. Nanoscale Metal-Organic Frameworks for the Co-Delivery of Cisplatin and Pooled SiRNAs to Enhance Therapeutic Efficacy in Drug-Resistant Ovarian Cancer Cells. J. Am. Chem. Soc. 2014, 136 (14), 5181–5184. 10.1021/ja4098862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyra À.; Yazdi A.; Espín J.; Carné-Sánchez A.; Roher N.; Lorenzo J.; Imaz I.; Maspoch D. Synthesis, Culture Medium Stability, and In Vitro and In Vivo Zebrafish Embryo Toxicity of Metal-Organic Framework Nanoparticles. Chem.—Eur. J. 2015, 21 (6), 2508–2518. 10.1002/chem.201405380. [DOI] [PubMed] [Google Scholar]
- Jarai B. M.; Stillman Z.; Attia L.; Decker G. E.; Bloch E. D.; Fromen C. A. Evaluating UiO-66 Metal-Organic Framework Nanoparticles as Acid-Sensitive Carriers for Pulmonary Drug Delivery Applications. ACS Appl. Mater. Interfaces 2020, 12 (35), 38989–39004. 10.1021/acsami.0c10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson Grape E.; Chacón A. J.; Rojas S.; Pérez Y.; Jaworski A.; Nero M.; Åhlén M.; Martínez-Ahumada E.; Narongin-Fujikawa M.; Ibarra I.; Cheung O.; Baresel C.; Willhammar T.; Horcajada P.; Inge A. K. Efficient Removal of Aqueous Pharmaceutical Pollutants by a Robust Anionic Zirconium Ellagate Framework. ChemRxiv 2022, 10.26434/chemrxiv-2022-h3530. [DOI] [Google Scholar]
- Svensson Grape E.; Chacón-García A. J.; Rojas S.; Pérez Y.; Jaworski A.; Nero M.; Åhlén M.; Martínez-Ahumada E.; Galetsa Feindt A. E.; Pepillo M.; Narongin-Fujikawa M.; Ibarra I. A.; Cheung O.; Baresel C.; Willhammar T.; Horcajada P.; Inge A. K. Removal of Pharmaceutical Pollutants from Effluent by a Plant-Based Metal-Organic Framework. Nat. Water. 2023, 1 (5), 433–442. 10.1038/s44221-023-00070-z. [DOI] [Google Scholar]
- Hoffmann M.; Kleine-Weber H.; Schroeder S.; Krüger N.; Herrler T.; Erichsen S.; Schiergens T. S.; Herrler G.; Wu N. H.; Nitsche A.; Müller M. A.; Drosten C.; Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181 (2), 271. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhi R.; Srinoi P.; Ngo N.; Tran H.-V.; Lee T. R. Nanoparticle-Based Strategies to Combat COVID-19. ACS Appl. Nano Mater. 2020, 3 (9), 8557–8580. 10.1021/acsanm.0c01978. [DOI] [PubMed] [Google Scholar]
- Ibrahim Fouad G. A Proposed Insight into the Anti-Viral Potential of Metallic Nanoparticles against Novel Coronavirus Disease-19 (COVID-19). Bull. Natl. Res. Cent. 2021, 45 (1), 36. 10.1186/s42269-021-00487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karges J.; Cohen S. M. Metal Complexes as Antiviral Agents for SARS-CoV-2. ChemBioChem 2021, 22 (16), 2600–2607. 10.1002/cbic.202100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce R. L. Translational Nanomedicine - through the Therapeutic Window. Nanomedicine 2015, 10 (21), 3249–3260. 10.2217/nnm.15.168. [DOI] [PubMed] [Google Scholar]
- Habet S. Narrow Therapeutic Index Drugs: Clinical Pharmacology Perspective. J. Pharm. Pharmacol. 2021, 73 (10), 1285–1291. 10.1093/jpp/rgab102. [DOI] [PubMed] [Google Scholar]
- Agostoni V.; Chalati T.; Horcajada P.; Willaime H.; Anand R.; Semiramoth N.; Baati T.; Hall S.; Maurin G.; Chacun H.; Bouchemal K.; Martineau C.; Taulelle F.; Couvreur P.; Rogez-Kreuz C.; Clayette P.; Monti S.; Serre C.; Gref R. Towards an Improved Anti-HIV Activity of NRTI via Metal-Organic Frameworks Nanoparticles. Adv. Healthcare Mater. 2013, 2 (12), 1630–1637. 10.1002/adhm.201200454. [DOI] [PubMed] [Google Scholar]
- Marcos-Almaraz M. T.; Gref R.; Agostoni V.; Kreuz C.; Clayette P.; Serre C.; Couvreur P.; Horcajada P. Towards Improved HIV-Microbicide Activity through the Co-Encapsulation of NRTI Drugs in Biocompatible Metal Organic Framework Nanocarriers. J. Mater. Chem. B 2017, 5 (43), 8563–8569. 10.1039/C7TB01933E. [DOI] [PubMed] [Google Scholar]
- Alexova R.; Alexandrova S.; Dragomanova S.; Kalfin R.; Solak A.; Mehan S.; Petralia M. C.; Fagone P.; Mangano K.; Nicoletti F.; Tancheva L. Anti-COVID-19 Potential of Ellagic Acid and Polyphenols of Punica Granatum L. Molecules 2023, 28 (9), 3772. 10.3390/molecules28093772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G.; Wang X.; Cheng M.; Wang S.; Ma K. The Multifaceted Mechanisms of Ellagic Acid in the Treatment of Tumors: State-of-the-Art. Biomed. Pharmacother. 2023, 165 (July), 115132. 10.1016/j.biopha.2023.115132. [DOI] [PubMed] [Google Scholar]
- Mitragotri S.; Burke P. A.; Langer R. Overcoming the Challenges in Administering Biopharmaceuticals: Formulation and Delivery Strategies. Nat. Rev. Drug Discovery 2014, 13 (9), 655–672. 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattal E.; Tsapis N. Nanomedicine Technology: Current Achievements and New Trends. Clin. Transl. Imaging 2014, 2 (1), 77–87. 10.1007/s40336-014-0053-3. [DOI] [Google Scholar]
- National Center for Biotechnology Information . PubChem. Compound Summary for CID 7489, Terephthalic acid. https://pubchem.ncbi.nlm.nih.gov/compound/Terephthalic-acid. Accessed date (February 2024).
- Park C.; Sheehan R. J.. Phthalic Acids and Other Benzenepolycarboxylic Acids. Kirk-Othmer Encycl. Chem. Technol., Wiley; 2000. [Google Scholar]
- Bellido E.; Guillevic M.; Hidalgo T.; Santander-Ortega M. J.; Serre C.; Horcajada P. Understanding the Colloidal Stability of the Mesoporous MIL-100(Fe) Nanoparticles in Physiological Media. Langmuir 2014, 30 (20), 5911–5920. 10.1021/la5012555. [DOI] [PubMed] [Google Scholar]
- Giménez-Marqués M.; Hidalgo Crespo T.; Serre C.; Horcajada P. Nanostructured Metal-Organic Frameworks and Their Bio-Related Applications. Coord. Chem. Rev. 2015, 307, 342. 10.1016/j.ccr.2015.08.008. [DOI] [Google Scholar]
- Wang Y.; Huang J.; Sun Y.; Stubbs D.; He J.; Li W.; Wang F.; Liu Z.; Ruzicka J. A.; Will E.; Rayman M. P.; Wan X.; Zhang J.. Since January 2020 Elsevier Has Created a COVID-19 Resource Centre with Free Information in English and Mandarin on the Novel Coronavirus COVID- 19. The COVID-19 Resource Centre Is Hosted on Elsevier Connect, the Company ’ S Public News and Information, 2020.
- Joonaki E.; Hassanpouryouzband A.; Heldt C. L.; Areo O. Surface Chemistry Can Unlock Drivers of Surface Stability of SARS-CoV-2 in a Variety of Environmental Conditions. Chem 2020, 6 (9), 2135–2146. 10.1016/j.chempr.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grall R.; Hidalgo T.; Delic J.; Garcia-Marquez A.; Chevillard S.; Horcajada P. In Vitro Biocompatibility of Mesoporous Metal (III; Fe, Al, Cr) Trimesate MOF Nanocarriers. J. Mater. Chem. B 2015, 3 (42), 8279–8292. 10.1039/C5TB01223F. [DOI] [PubMed] [Google Scholar]
- García Márquez A.; Demessence A.; Platero-Prats A. E.; Heurtaux D.; Horcajada P.; Serre C.; Chang J.-S.; Férey G.; de la Peña-O’Shea V. A.; Boissière C.; Grosso D.; Sanchez C. Green Microwave Synthesis of MIL-100(Al, Cr, Fe) Nanoparticles for Thin-Film Elaboration. Eur. J. Inorg. Chem. 2012, 2012 (32), 5165–5174. 10.1002/ejic.201200710. [DOI] [Google Scholar]
- Arenas-Vivo A.; Avila D.; Horcajada P. Phase-Selective Microwave Assisted Synthesis of Iron(III) Aminoterephthalate MOFs. Materials 2020, 13 (6), 1469. 10.3390/ma13061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A.; Wan L.; Caro J. Microwave-Assisted Synthesis of Well-Shaped UiO-66-NH2 with High CO2 Adsorption Capacity. Mater. Res. Bull. 2018, 98 (October 2017), 308–313. 10.1016/j.materresbull.2017.10.038. [DOI] [Google Scholar]
- Ginex T.; Garaigorta U.; Ramírez D.; Castro V.; Nozal V.; Maestro I.; García-Cárceles J.; Campillo N. E.; Martinez A.; Gastaminza P.; Gil C. Host-Directed FDA-Approved Drugs with Antiviral Activity against SARS-CoV-2 Identified by Hierarchical In Silico/In Vitro Screening Methods. Pharmaceuticals 2021, 14, 332. 10.3390/ph14040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration (FDA) . Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed date (April 2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.