Abstract
Octopamine, the functional analog of noradrenaline, modulates many different behaviors and physiological processes in invertebrates. In the central nervous system, a few octopaminergic neurons project throughout the brain and innervate almost all neuropils. The center of memory formation in insects, the mushroom bodies, receive octopaminergic innervations in all insects investigated so far. Different octopamine receptors, either increasing or decreasing cAMP or calcium levels in the cell, are localized in Kenyon cells, further supporting the release of octopamine in the mushroom bodies. In addition, different mushroom body (MB) output neurons, projection neurons, and dopaminergic PAM cells are targets of octopaminergic neurons, enabling the modulation of learning circuits at different neural sites. For some years, the theory persisted that octopamine mediates rewarding stimuli, whereas dopamine (DA) represents aversive stimuli. This simple picture has been challenged by the finding that DA is required for both appetitive and aversive learning. Furthermore, octopamine is also involved in aversive learning and a rather complex interaction between these biogenic amines seems to modulate learning and memory. This review summarizes the role of octopamine in MB function, focusing on the anatomical principles and the role of the biogenic amine in learning and memory.
The biogenic amine octopamine (OA) is a neurohormone, neurotransmitter, and neuromodulator in invertebrates (for review, see Roeder 1999, 2005; Farooqui 2007). OA is synthetized by tyramine (TA), a neuromodulator by itself, which is produced from the amino acid tyrosine (Barker et al. 1972; Livingstone and Tempel 1983; Monastirioti et al. 1996; Cole et al. 2005). OA and TA have been shown to modulate a range of behaviors, including feeding, locomotion, sleep, aggression, courtship, and learning and memory (Hammer 1993; Roeder 2005, 2020; Crocker et al. 2010; Aonuma and Watanabe 2012; Kim et al. 2013; Stevenson and Rillich 2016; Iliadi et al. 2017; Youn et al. 2018; Sabandal et al. 2020). By activating or inhibiting neuronal circuits, they are thought to orchestrate behavior (Sombati and Hoyle 1984; Claßen and Scholz 2018). OA especially seems to bias the decision between behavioral outcomes, like courtship versus aggression or feeding versus locomotion, but also functions as an independent transmitter (Sombati and Hoyle 1984; Certel et al. 2010; Andrews et al. 2014; Sayin et al. 2019; Selcho and Pauls 2019). The mushroom body (MB) has been demonstrated to be involved in behaviors such as locomotion, sleep, courtship, and, most notably, learning and memory, all of which are affected by OA (Martin et al. 1998; McBride et al. 1999; Heisenberg 2003; Popov et al. 2004; Davis 2005; Joiner et al. 2006; Pitman et al. 2006; Fiala 2007; Busto et al. 2010; Bushey and Cirelli 2011; Bushey et al. 2011; Kaun et al. 2011). Furthermore, OA neurons (OANs) innervate the MB, and OA receptor expression has been identified in Kenyon cells in insects (Han et al. 1998; Cayre et al. 1999; Grohmann et al. 2003; Busch et al. 2009; Sinakevitch et al. 2011; Kim et al. 2013; Wu et al. 2013; Shih et al. 2019). The role of OA in the MB is predominantly investigated in the genetically tractable fruit fly; thus, this review focuses on the current knowledge in Drosophila. However, studies in other invertebrates suggest a conserved role of biogenic amines in MB-driven behaviors like learning and memory (for review, see Roeder 2005; Verlinden et al. 2010; Perry and Barron 2013; Van Damme et al. 2021).
Octopaminergic neurons involved in learning and memory
The first hints that OA in the MB is involved in learning and memory came from experiments performed in honeybees (Mercer and Menzel 1982). OA injection in the peduncle next to the calyx enhanced memory formation and recall of the rewarded stimulus and injection of OA into the calyces could substitute the sugar reward in olfactory conditioning (Bicker and Menzel 1989; Hammer and Menzel 1998). The VUMmx1 neuron of the honeybee was suggested to mediate the sugar reward for olfactory learning, as depolarization substitute for the sugar reward in olfactory learning (Hammer 1993; Hammer and Menzel 1998). Indeed, optogenetic and thermogenetic activation, respectively, of almost all OA/TA neurons in Drosophila was sufficient to substitute the sugar reward during olfactory learning (Schroll et al. 2006; Burke et al. 2012). OA was supposed to signal rewarding stimuli, whereas dopamine (DA) was suggested to mediate aversive stimuli to the MB where coincidence detection of an odor leads to olfactory learning and memory (Farooqui et al. 2003; Schwaerzel et al. 2003; Mizunami et al. 2009; Tomchik and Davis 2009). This simple model was challenged by the finding that DA neurons mediate the appetitive and aversive stimuli in olfactory learning and the observation that OA represents sweet taste rather than the nutrient value of the sugar reward in olfactory learning, potentially signaling the perception/presence of reward (Kim et al. 2007; Selcho et al. 2009, 2014; Burke et al. 2012; Mizunami et al. 2015). Different sets of DA cells modulate aversive or appetitive learning via the DA receptor dDA1R in the MB (Kim et al. 2007; Claridge-Chang et al. 2009; Aso et al. 2010; Liu et al. 2012). In addition, thermogenetic activation of OANs paired with an odor introduced an appetitive memory in flies, only if DA signaling was intact (Burke et al. 2012). This further suggested that OA plays a minor role in learning and memory.
On the other hand, recent publications demonstrate the role of OA in appetitive as well as aversive learning and indicate a rather complex action of both biogenic amines in learning and memory. Tyramine β hydroxylase (TβH) mutants, lacking OA, showed impaired appetitive and aversive learning that could be rescued by TβH expression in OA/TA neurons (Iliadi et al. 2017). A specific knockdown of TβH, and thereby OA, in OANs leads to impaired aversive learning (Wu et al. 2013; Sabandal et al. 2020). Appetitive learning depends on intact OA signaling to α/β and γ KCs, and OA signaling to the MB also mediates aversive stimuli (Zhou et al. 2012; Kim et al. 2013). Blocking the transmitter release from OANs in the training phase leads to impaired courtship learning, whereas thermogenetic activation of these cells introduced artificial courtship behavior (Zhou et al. 2012). Therefore, octopamine signaling in the MB is clearly involved in learning and memory. The following sections will discuss the anatomical basis for the functional role of OA in the MB circuitry, focusing on the morphology of the OANs and localization of the receptors.
Morphological description of OANs innervating the mushroom body
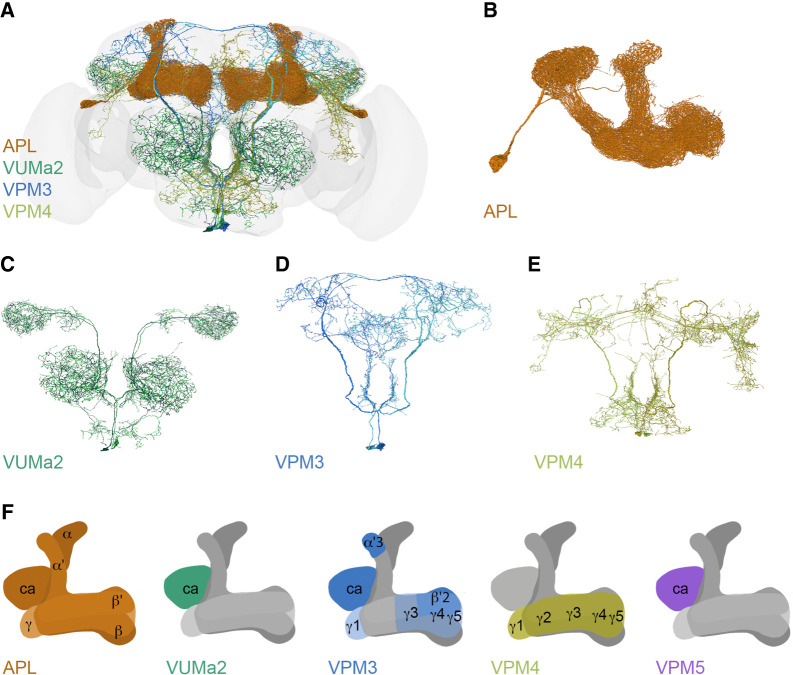
The morphology of the OANs has been studied in great detail in a diverse group of insects, with a focus on dorsal unpaired median (DUM) and ventral unpaired median (VUM) neurons, a group of symmetrically projecting cells located at the dorsal or ventral midline in the subesophageal, thoracic, and abdominal ganglia (Stevenson and Spörhase-Eichmann 1995; Monastirioti 1999; Stern 1999). The broad innervation of central brain neuropils arises from an overall small number of VUM/DUM neurons in the subesophageal ganglion and a few cells located in the brain itself. Interestingly, all insects analyzed for OA immunoreactivity showed staining in the MBs. OA labeling was observed in the MBs of fruit flies and blowflies (Monastirioti et al. 1995; Sinakevitch and Strausfeld 2006), honeybees (Kreissl et al. 1994; Sinakevitch et al. 2005), locusts (Konings et al. 1988; Bräunig 1991; Stevenson et al. 1992; Kononenko et al. 2009), crickets (Spörhase-Eichmann et al. 1992), cockroaches (Sinakevitch et al. 2005), and hawkmoths (Dacks et al. 2005). More specifically, all of these insects possess OA-positive neurites in the calyces. The peduncles (locusts, moths), vertical lobes (bees, cockroaches, blowflies), and medial lobes (Drosophila, blowflies, bees, moths) contain restrictive octopaminergic fibers (Konings et al. 1988; Kreissl et al. 1994; Dacks et al. 2005; Sinakevitch et al. 2005; Sinakevitch and Strausfeld 2006). Single OANs with a similar innervation pattern have been described in locusts, bees, and fruit flies, allowing several groups to speculate that there is a conserved function of OA in insects (Bräunig 1991; Hammer 1993; Bräunig and Burrows 2004; Schröter et al. 2007; Busch et al. 2009; Wu et al. 2013; Selcho et al. 2014). In the following, I will concentrate on the description of the single cells identified in Drosophila melanogaster. Here, five different octopaminergic cell types have been reported to innervate the MB: VUMa2, VPM3, VPM4, VPM5, and the anterior paired lateral (APL) neuron (Fig. 1A–F; Table 1; Busch et al. 2009; Wu et al. 2013). The cell bodies of the VM cluster neurons are distributed along the midline in the subesophageal ganglion and have been divided into three different subclusters depending on the location of the cell bodies in the mandibular (md), maxillary (mx), or labial (lb) neuromere. The VUM cells of the md and mx clusters are duplicated (Busch and Tanimoto 2010). One VUM cell type (VUMa2) projecting into the MB located in the md and mx neuromere has been identified. In addition, three different paired VM neurons (VPM3, VPM4, and VPM5) innervate the MB (Fig. 1C–F; Busch et al. 2009). The two VUMa2 neurons ramify within the calyces (Fig. 1C,F; Busch et al. 2009), while the VPM5 cell type mimics the arborization of the VUMa2 but projects only unilaterally. The two VPM3 cells innervate the γ-lobe, α′β′-lobes, and the calyx (Fig. 1D,F; Busch et al. 2009; Aso et al. 2014). The γ-lobe is also innervated by the two VPM4 neurons (Fig. 1E,F; Busch et al. 2009; Aso et al. 2014). Beside the ascending cells of the VM cluster, another paired OAN with its cell body in the anterior lateral brain arborizes extensively in the MB. The APL neuron projects to the calyx, accessory calyx, peduncle, and all lobes with few arborizations outside the MB (Fig. 1B,F; Tanaka et al. 2008; Liu and Davis 2009).
Figure 1.
Octopaminergic neurons innervate the mushroom body. (A–E) Four octopaminergic cell types that arborize in the MB identified on an electron microscopic level using FlyWire neuroglancer. The VPM5 cell type has not yet been identified in the FlyWire data set. (B) The APL neuron innervates all parts of the MB. (C) Two VUMa2 neurons project from the antennal lobe via the iACT to both calyces and the lateral horn. (D) The paired VPM3 cells innervate the contralateral calyx and lobes, the fan-shaped body, parts of the dorsolateral protocerebrum, and the SEZ. (E) The VPM4 cell type arborizes in the contralateral γ-lobe and innervates the dorsomedial and -lateral protocerebrum as well as the SEZ. (F) Overview of the MB (gray) and the appropriate OA release sites (colored) of the four different octopaminergic cell types shown in B–E and the VPM5 cell type. Based on the data from Busch et al. (2009), Aso et al. (2014), and the analysis of the whole brain EM volume using FlyWire neuroglancer (Zheng et al. 2018; Dorkenwald et al. 2022, 2023). (APL) Anterior paired lateral, (Ca) calyx, (iACT) inner antennocerebral tract, (SEZ) subesophageal zone, (VPM) ventral paired median, (VUM) ventral unpaired median.
Table 1.
Anatomical information on MB-innervating OANs
| OA cell typea | MB compartment innervatedb | Vesiclesc | Co-transmitterd |
|---|---|---|---|
| OA-VUMa2 | ca (bs) | DCV, SV | TA, Glut |
| OA-VPM3 | ca, γ1,3–5, α′3, β′2 (co) | DCV, SV | TA, Glut, (NO) |
| OA-VPM4 | γ1–5 (co) | DCV, SV | TA, Glut, (NO) |
| OA-VPM5 | ca (ip) | ? | ? |
| APL | ca, ped, α1–3, α′1–3, β1–2, β′1–2, γ1–5 (ip) | DCV, SV | TA, GABA |
(α,β,α′,β′,γ) Appropriate MB lobe, (ca) calyx, (ped) peduncle, (bs) both sites, (co) contralateral, (ip) ipsilateral, (DCV) dense-core vesicle, (SV) synaptic vesicle, (TA) tyramine, (Glut) glutamate, (NO) nitric oxide, (?) not identified.
aData from Busch et al. (2009) and Wu et al. (2013).
bData from Busch et al. (2009), Chen et al. (2012), and Aso et al. (2014).
cObserved in FlyWire neuroglancer (Fig. 2).
dData from Liu and Davis (2009), Aso et al. (2019), and Sherer et al. (2020).
The arborization pattern of the VM cells has been identified using the Tdc2-GAL4 line (Cole et al. 2005). Tyrosine decarboxylase 2 (Tdc2) is the enzyme necessary for TA production and therefore all GAL4-positive cells should contain TA and potentially also OA. As all Tdc2-GAL4-positive VM cells were stained by an OA antibody and an antibody against TβH, the enzyme for OA synthesis, these cells are indeed octopaminergic (Busch et al. 2009; Schneider et al. 2012). The APL neuron, which is not included in the Tdc2-GAL4 line, is also OA- and TβH-positive (Wu et al. 2013). So far, 10 out of approximately 100 octopaminergic cells in the Drosophila brain have been shown to innervate the MB.
One other paired neuron was also marked by OA antibody labeling. The DAL (dorsal–anterior–lateral) neuron contacts α/β KCs in the calyx and was shown to be involved in long-term memory (LTM) formation but independent of OA production within the cell (Chen et al. 2012). A detailed anatomical description is not available and the DAL neuron has not yet been identified at the EM level. Another OA cell cluster located in the anterior superior medial protocerebrum (ASM) might contain additional MB innervating cells (FlyCircuit) (Chiang et al. 2011; Zhou et al. 2012). One neuron innervating the αβ-lobes was identified, but in contrast to the VM cluster, not all Tdc2-GAL4-positive cells of the ASM cluster are OA-immunoreactive (Busch et al. 2009). Therefore, this MB-innervating cell could be tyraminergic. Further investigations need to show if other so far uncharacterized OANs exist that contribute to OA release into the MB circuitry.
Octopaminergic neurons contain a diverse set of transmitters
OA is synthetized from TA; therefore, all OANs contain TA, which can act as a neuromodulator by itself (Nagaya et al. 2002; Schützler et al. 2019). In addition, OANs are reported to express at least one more transmitter (many glutamate, some GABA, or acetylcholine) and in many cases additional peptides (Schneider et al. 2012; Wu et al. 2013; Croset et al. 2018; Sherer et al. 2020). Certel and colleagues (Sherer et al. 2020) have shown that nearly all OANs of the VM cluster show colocalization of the Drosophila vesicular glutamate transporter (dVGlut) and therefore contain glutamate as a classical co-transmitter. In contrast, the APL neuron has been demonstrated to contain GABA as a co-transmitter (Liu and Davis 2009). Even though co-transmission is a common principle of many neurons across species, the anatomical principles and behavioral consequences of these combinations of transmitters are poorly understood (Hnasko and Edwards 2012; Vaaga et al. 2014; Nusbaum et al. 2017; Nässel 2018). The Drosophila vesicular monoamine transporter mediates the transport of biogenic amines into a large dense-core vesicle (DCV) and small synaptic vesicle (SV) (Greer et al. 2005; Deshpande et al. 2020). Therefore, OA can be released extrasynaptically by fusion of DCVs to the membrane as shown for type II motor neuron endings and potentially also from SVs at presynaptic active zones like classical transmitters, which are only packed into SVs (Grygoruk et al. 2014; Stocker et al. 2018).
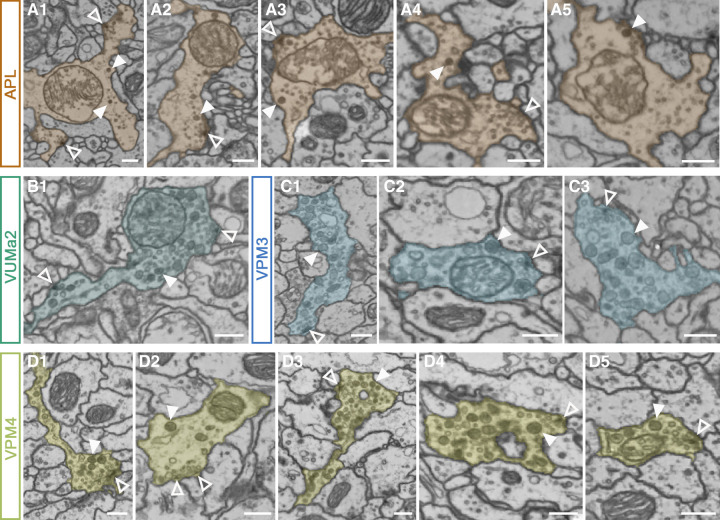
Indeed, using FlyWire neuroglancer (Dorkenwald et al. 2022) to analyze the morphology of the OANs in the whole brain EM volume (Zheng et al. 2018), t-bars (the structural correlate of SV release sites), SVs, and DCVs can be identified in MB-innervating OA cell types (Fig. 2A–D). DCVs are visible in the arborizations of OANs in the MB calyces and lobes, whereas all cell types contain additional classical synapses. This suggests that OANs exert their function in the MB through locally restricted synaptic connections to specific postsynaptic partners and through volume transmission targeting a broader set of neurons within the MB. The identification of SVs opens in addition the possibility that VM-OANs corelease glutamate in the MBs. In contrast, the APL neuron was discovered as a GABAergic neuron and corelease of OA was identified a few years later (Liu and Davis 2009; Wu et al. 2013). The observation that the APL cell contains DCVs supports the octopaminergic identity of these neurons (Fig. 2A; Takemura et al. 2017). Further investigations are needed to show if the transmitters are released together at the same synapse or if release can occur at distinct sites. Indeed, the first experiments analyzing the distribution of the vesicular monoamine transporter and dVGlut in VPM4 neurons showed potential colocalization of both transporters and, in addition, areas of distinct localization (Sherer et al. 2020). Interestingly, co-transmission has recently been shown in the MB for a subset of DA neurons (Aso et al. 2019). Here, the co-transmitter nitric oxide (NO) seems to activate the soluble guanylate cyclase in KCs to form an inverse memory, acting antagonistically to DA. The effect of NO is slower than the DA effect and seems to shorten memory retention to enable rapid updating of memories (Aso et al. 2019). In addition, Aso and colleagues proposed that OA-VPM3 or -VPM4 neurons also contain NO as they seem to express NO synthase. Whether this observation holds true, and whether a similar function can be achieved by the release of this co-transmitter from OANs, remains to be tested in further experiments.
Figure 2.
Ultrastructure of octopaminergic neurons in the mushroom body. (A–D) Example images of the ultrastructure of the MB-innervating OA cell types show large dense-core vesicles (DCVs, arrowheads), synaptic vesicles (SVs, open arrowheads), and potential SV release sites (t-bars, open arrowheads). FlyWire neuroglancer was used to investigate the morphology of OANs in the whole brain EM volume (Zheng et al. 2018; Dorkenwald et al. 2022). (A) The APL neuron contains mostly SVs and only a few DCVs in all areas. Example boutons from the calyx (A1), α- (A2), β- (A3), α′- (A4), and γ-lobes (A5). (B) DCVs and SV release sites in a VUMa2 cell bouton in the calyx. (C) Ultrastructure of the VPM3 neuron in the γ- (C1) and α′-lobe (C2) and the calyx (C3). (D) The VPM4 cell innervates the complete γ-lobe. DCVs, as well as SVs and their release sites, are visible in the γ1 (D1), γ2 (D2), γ3 (D3), γ4 (D4), and γ5 (D5) compartments. Scale bars, 300 nm.
OA receptors expressed in the mushroom bodies
OA receptors have been studied intensively in order to decipher the function of OA in the MB circuitry. OA receptors are G-protein-coupled and can be classified as α-adrenergic-like OA receptors (Octα1-R and Octα2-R) and β-adrenergic-like OA receptors (Octβ1-R, Octβ2-R, Octβ3-R) according to their structural and functional similarities to vertebrate α- and β-adrenergic receptor subtypes (Balfanz et al. 2005; Evans and Maqueira 2005; Maqueira et al. 2005; Wu et al. 2014; Qi et al. 2017). Octα-R activation leads to an increase in intracellular calcium levels and influences intracellular cAMP concentration (up-regulation in Octα1-R and down-regulation in Octα2-R (in vitro studies: Han et al. 1998; Balfanz et al. 2005; Beggs et al. 2011; Wu et al. 2014; Qi et al. 2017; Nakagawa et al. 2022; in vivo: Lee et al. 2009). In contrast, Octβ-R activation elicits an increase in intracellular cAMP but not in calcium levels (Balfanz et al. 2005; Maqueira et al. 2005; ex vivo: Sabandal et al. 2020; in vivo: Lim et al. 2014). In addition to the OA-affiliated receptors, three TA receptor types have been identified (Tyr1-R, Tyr2-R, and Tyr3-R). Tyr1-R and Tyr3-R respond to TA and OA, whereas Tyr2-Rs are specifically activated by TA. Activation of these receptors leads to either an increase or decrease of intracellular cAMP levels, or to changes in intracellular calcium levels (for review, see Finetti et al. 2021).
The first OA receptor identified in the MBs was the Drosophila Octα1-R named OAMB (Han et al. 1998). Using antibody staining, the MBs were highlighted because of the high amount of receptor expression in this densely packed neuropil. El-Kholy et al. (2015) used promoter GAL4 lines to visualize OA and TA receptor expression. All known Drosophila OA and TA receptors seem to be expressed in the MBs. Single-cell type RNA-seq data of α/β and γ KCs revealed expression of Octα1-R (OAMB), Octβ1-R (OA2), Octβ2-R, and TyrR in α/β KCs and OAMB and OA2 in γ KCs (Crocker et al. 2016). RNA-seq data from all KC types revealed expression of OAMB and Octα2-R in α/β and γ KCs and less strong in α′/β′ KCs. Octβ1-R, Octβ2-R, and Octβ3-R are detected in α/β, α′/β′, and γ KCs, respectively, with Octβ3-R exclusively expressed in γd subtypes (Shih et al. 2019). Octopamine receptors (OARs) appear to be broadly expressed within the MB (Table 2), but such a large-scale distribution seems rather unrealistic considering the innervation pattern of the OANs. Therefore, it is necessary to investigate the exact localization of the receptors within a KC.
Table 2.
Octopamine receptors in the MB
| OA receptor | Expression in KCsa | IR in MB regionsb | Functional role for learning and memory (OAR localization) |
|---|---|---|---|
| Octα1-R (OAMB) | α/β, α′/β′, γ KCs | α, α′, β, β′, γ, ped, ca | Appetitive olfactory learning (α/β, γ KCs)c; sweet taste reinforcement (PAM DAN)d; courtship learning (αβ lobes)e |
| Octα2-R | α/β, α′/β′, γ KCs | ? | ? |
| Octβ1-R | α/β, α′/β′, γ KCs | ? | Aversive olfactory learning (α/β KCs); appetitive olfactory learning (PN)f |
| Octβ2-R | α/β, α′/β′, γ KCs | α′, β′ | sweet taste reinforcement (PPL1 DAN)d; ARM (α′/β′ KCs)g |
| Octβ3-R | α/β, α′/β′, γ KCs | ? | ? |
(α,β,α′,β′,γ) Appropriate MB lobe, (ARM) anesthesia-resistant memory, (ca) calyx, (DAN) dopaminergic neuron, (IR) immunoreactivity, (KCs) Kenyon cells, (OAR) octopamine receptor, (ped) peduncle, (?) unknown.
aData from Crocker et al. (2016) and Shih et al. (2019).
bData from Han et al. (1998), Crittenden et al. (1998), Lee et al. (2003), Wu et al. (2013), and Kim et al. (2013).
cData from Kim et al. (2013).
dData from Burke et al. (2012) and Huetteroth et al. (2015).
eData from Zhou et al. (2012).
fData from Sabandal et al. (2020).
gData from Wu et al. (2013).
Behavioral experiments in Drosophila revealed that OA signaling via OAMB in the α/β and γ KCs is sufficient to rescue learning (Kim et al. 2013). Additionally, courtship learning depends on OAMB expression in the MB (Zhou et al. 2012). The sweet taste information in sugar reward learning is mediated by OAMB expressing dopaminergic PAM neurons activating β′2 and γ4 compartments of the MB (Burke et al. 2012; Huetteroth et al. 2015). OA signaling via Octβ1-R in α/β KCs is necessary for aversive olfactory learning, while signaling via Octβ1-R in projection neurons is involved in appetitive olfactory learning (Sabandal et al. 2020). In addition, Octβ2-R in the dopaminergic MB-MP1 PPL1 neurons is needed to modulate negative DA signals for sweet taste learning (Burke et al. 2012). Octβ2-R RNAi in α′/β′ KCs impaired anesthesia-resistant memory (ARM) (Wu et al. 2013). Analyzing receptor function in specific sets of KCs, MB output neurons (MBONs), or MB input neurons (MBINs) is an effective way to demonstrate the role of OA in MB function (Table 2).
Function of OANs signaling to the mushroom body circuitry
Currently, there is limited information available regarding the function of the individual MB-innervating OANs. Only two cell types have been linked to behavioral function: The VPM4 cell was found to play a role in feeding-related behaviors, whereas APL neurons are involved in learning and memory (Liu and Davis 2009; Pitman et al. 2011; Ren et al. 2012; Wu et al. 2012, 2013; Youn et al. 2018; Sayin et al. 2019; Zhou et al. 2019). The VPM4 cells promote proboscis extension to sugar, the first step in feeding behavior. The modulation of the proboscis extension reflex depends on functional OA synthesis within the VPM4 cells and on OAMB activity in sugar-sensing gustatory receptor neurons, increasing sensory responses to sugar (Youn et al. 2018). Additionally, VPM4 neurons reduce hunger-driven odor tracking by signaling to MBON-γ1pedc > αβ (MBON11) in the γ1 compartment (Sayin et al. 2019). Together with the findings that VPM4 neurons reinforce proboscis extension, it seems that these OANs promote the behavioral switch from food search to feeding (Youn et al. 2018; Sayin et al. 2019). Manjila et al. (2019) demonstrated that VPM4 neurons modulate flight duration through OAMB activation in PAM dopaminergic neurons. Octopaminergic input to PAM neurons innervating the MB β′1 compartment reduces the inhibitory output of MBON-β′1 leading to prolonged flight bouts. This OA–PAM–MBON circuit might influence flight in response to food availability, feeding state, or other sensory input (Manjila et al. 2019).
The APL neurons play a role in learning and memory and enable sparse odor coding by KCs through feedback inhibition in a compartment-specific manner (Liu and Davis 2009; Pitman et al. 2011; Ren et al. 2012; Wu et al. 2012, 2013; Lei et al. 2013; Lin et al. 2014; Zhou et al. 2019; Amin et al. 2020; Prisco et al. 2021). APL neurons suppress aversive olfactory learning, and their activity is reduced by olfactory learning via dopaminergic PPL1 neurons in the vertical lobes (Liu and Davis 2009; Zhou et al. 2019). Furthermore, both forms of intermediate-term memory, ARM and anesthesia-sensitive memory, are modulated by the APL neurons (Wu et al. 2011, 2013). However, only ARM is regulated by OA. Memory formation is modulated by APL cells via Octβ2-R signaling in α′/β′ KCs (Wu et al. 2013). APL neurons corelease OA and GABA. The decrease in OA levels does not affect aversive learning, but impairs ARM formation. Conversely, the decrease in GABA levels within the cells affects learning, but not intermediate-term memory formation (Liu and Davis 2009; Wu et al. 2013). The APL neuron exhibits a separation between co-transmitter functions, possibly due to the different localization of the appropriate receptor.
Outlook
MB function is modulated by OA. Ten OANs that innervate the MB have been described, and all cells have multiple transmitters. To holistically decipher the role of OANs in the MB circuitry, it is essential to gain further insights into the set of co-transmitters released from these neurons. The first step to a functional connectome has been taken by the precise morphological analysis within the openly accessible connectome of the complete fly brain (FlyWire.ai). However, the synaptic connections within the MB are insufficient as they do not cover DCV release. Therefore, to obtain a comprehensive morphological overview of all release sites, it is necessary to identify a marker for DCV “synapses.” Furthermore, fundamental questions remain to be answered: Is OA packed in SVs and released from synapses? Are co-transmitters spatially separated? Is the release of co-transmitters regulated? In addition, it is essential to study the localization of the OA receptors in more detail in order to identify postsynaptic partners. Knockdown of receptor expression in specific sets of KCs, MBONs, or MBINs is an effective way to demonstrate the role of OA in MB function.
Acknowledgments
I thank Dennis Pauls for comments on the manuscript and the Princeton FlyWire team and members of the Murthy and Seung laboratories, as well as members of the Allen Institute for Brain Science, for the development and maintenance of FlyWire (supported by BRAIN Initiative grants MH117815 and NS126935 to Murthy and Seung). I also acknowledge members of the Princeton FlyWire team and the FlyWire consortium for neuron proofreading and annotation.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053839.123.
Freely available online through the Learning & Memory Open Access option.
References
- Amin H, Apostolopoulou AA, Suárez-Grimalt R, Vrontou E, Lin AC. 2020. Localized inhibition in the Drosophila mushroom body. Elife 9: e56954. 10.7554/eLife.56954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews JC, Fernández MP, Yu Q, Leary GP, Leung AKW, Kavanaugh MP, Kravitz EA, Certel SJ. 2014. Octopamine neuromodulation regulates Gr32a-linked aggression and courtship pathways in Drosophila males. PLoS Genet 10: e1004356. 10.1371/journal.pgen.1004356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aonuma H, Watanabe T. 2012. Octopaminergic system in the brain controls aggressive motivation in the ant, Formica japonica. Acta Biol Hung 63: 63–68. 10.1556/ABiol.63.2012.Suppl.2.6 [DOI] [PubMed] [Google Scholar]
- Aso Y, Siwanowicz I, Bräcker L, Ito K, Kitamoto T, Tanimoto H. 2010. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol 20: 1445–1451. 10.1016/j.cub.2010.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo T-T, Dionne H, Abbott L, Axel R, Tanimoto H, et al. 2014. The neuronal architecture of the mushroom body provides a logic for associative learning. Elife 3: e04577. 10.7554/eLife.04577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Ray RP, Long X, Bushey D, Cichewicz K, Ngo T-T, Sharp B, Christoforou C, Hu A, Lemire AL, et al. 2019. Nitric oxide acts as a cotransmitter in a subset of dopaminergic neurons to diversify memory dynamics. Elife 8: e49257. 10.7554/eLife.49257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfanz S, Strünker T, Frings S, Baumann A. 2005. A family of octapamine receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster: Drosophila octopamine receptors. J Neurochem 93: 440–451. 10.1111/j.1471-4159.2005.03034.x [DOI] [PubMed] [Google Scholar]
- Barker DL, Molinoff PB, Kravitz EA. 1972. Octopamine in the lobster nervous system. Nat New Biol 236: 61–63. 10.1038/newbio236061a0 [DOI] [PubMed] [Google Scholar]
- Beggs KT, Tyndall JDA, Mercer AR. 2011. Honey bee dopamine and octopamine receptors linked to intracellular calcium signaling have a close phylogenetic and pharmacological relationship. PLoS ONE 6: e26809. 10.1371/journal.pone.0026809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicker G, Menzel R. 1989. Chemical codes for the control of behaviour in arthropods. Nature 337: 33–39. 10.1038/337033a0 [DOI] [PubMed] [Google Scholar]
- Bräunig P. 1991. Suboesophageal DUM neurons innervate the principal neuropiles of the locust brain. Phil Trans R Soc Lond B 332: 221–240. 10.1098/rstb.1991.0051 [DOI] [Google Scholar]
- Bräunig P, Burrows M. 2004. Projection patterns of posterior dorsal unpaired median neurons of the locust subesophageal ganglion: locust descending DUM neurons. J Comp Neurol 478: 164–175. 10.1002/cne.20287 [DOI] [PubMed] [Google Scholar]
- Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, Waddell S. 2012. Layered reward signalling through octopamine and dopamine in Drosophila. Nature 492: 433–437. 10.1038/nature11614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch S, Tanimoto H. 2010. Cellular configuration of single octopamine neurons in Drosophila. J Comp Neurol 518: 2355–2364. 10.1002/cne.22337 [DOI] [PubMed] [Google Scholar]
- Busch S, Selcho M, Ito K, Tanimoto H. 2009. A map of octopaminergic neurons in the Drosophila brain. J Comp Neurol 513: 643–667. 10.1002/cne.21966 [DOI] [PubMed] [Google Scholar]
- Bushey D, Cirelli C. 2011. From genetics to structure to function: exploring sleep in Drosophila. Int Rev Neurobiol 99: 213–244. 10.1016/B978-0-12-387003-2.00009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D, Tononi G, Cirelli C. 2011. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science 332: 1576–1581. 10.1126/science.1202839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busto GU, Cervantes-Sandoval I, Davis RL. 2010. Olfactory learning in Drosophila. Physiology 25: 338–346. 10.1152/physiol.00026.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayre M, Buckingham SD, Yagodin S, Sattelle DB. 1999. Cultured insect mushroom body neurons express functional receptors for acetylcholine, GABA, glutamate, octopamine, and dopamine. J Neurophysiol 81: 1–14. 10.1152/jn.1999.81.1.1 [DOI] [PubMed] [Google Scholar]
- Certel SJ, Leung A, Lin C-Y, Perez P, Chiang A-S, Kravitz EA. 2010. Octopamine neuromodulatory effects on a social behavior decision-making network in Drosophila males. PLoS ONE 5: e13248. 10.1371/journal.pone.0013248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-C, Wu J-K, Lin H-W, Pai T-P, Fu T-F, Wu C-L, Tully T, Chiang A-S. 2012. Visualizing long-term memory formation in two neurons of the Drosophila brain. Science 335: 678–685. 10.1126/science.1212735 [DOI] [PubMed] [Google Scholar]
- Chiang A-S, Lin C-Y, Chuang C-C, Chang H-M, Hsieh C-H, Yeh C-W, Shih C-T, Wu J-J, Wang G-T, Chen Y-C, et al. 2011. Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Curr Biol 21: 1–11. 10.1016/j.cub.2010.11.056 [DOI] [PubMed] [Google Scholar]
- Claßen G, Scholz H. 2018. Octopamine shifts the behavioral response from indecision to approach or aversion in Drosophila melanogaster. Front Behav Neurosci 12: 131. 10.3389/fnbeh.2018.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenböck G. 2009. Writing memories with light-addressable reinforcement circuitry. Cell 139: 405–415. 10.1016/j.cell.2009.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. 2005. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem 280: 14948–14955. 10.1074/jbc.M414197200 [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EM, Han KA, Kalderon D, Davis RL. 1998. Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem 5: 38–51. 10.1101/lm.5.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A, Shahidullah M, Levitan IB, Sehgal A. 2010. Identification of a neural circuit that underlies the effects of octopamine on sleep: wake behavior. Neuron 65: 670–681. 10.1016/j.neuron.2010.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A, Guan X-J, Murphy CT, Murthy M. 2016. Cell-type-specific transcriptome analysis in the Drosophila mushroom body reveals memory-related changes in gene expression. Cell Rep 15: 1580–1596. 10.1016/j.celrep.2016.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croset V, Treiber CD, Waddell S. 2018. Cellular diversity in the Drosophila midbrain revealed by single-cell transcriptomics. Elife 7: e34550. 10.7554/eLife.34550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks AM, Christensen TA, Agricola H-J, Wollweber L, Hildebrand JG. 2005. Octopamine-immunoreactive neurons in the brain and subesophageal ganglion of the hawkmoth Manduca sexta. J Comp Neurol 488: 255–268. 10.1002/cne.20556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. 2005. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci 28: 275–302. 10.1146/annurev.neuro.28.061604.135651 [DOI] [PubMed] [Google Scholar]
- Deshpande SA, Freyberg Z, Lawal HO, Krantz DE. 2020. Vesicular neurotransmitter transporters in Drosophila melanogaster. Biochim Biophys Acta 1862: 183308. 10.1016/j.bbamem.2020.183308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorkenwald S, McKellar CE, Macrina T, Kemnitz N, Lee K, Lu R, Wu J, Popovych S, Mitchell E, Nehoran B, et al. 2022. FlyWire: online community for whole-brain connectomics. Nat Methods 19: 119–128. 10.1038/s41592-021-01330-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorkenwald S, Matsliah A, Sterling AR, Schlegel P, Yu S, McKellar CE, Lin A, Costa M, Eichler K, Yin Y, et al. 2023. Neuronal wiring diagram of an adult brain. bioRxiv 10.1101/2023.06.27.546656 [DOI]
- El-Kholy S, Stephano F, Li Y, Bhandari A, Fink C, Roeder T. 2015. Expression analysis of octopamine and tyramine receptors in Drosophila. Cell Tissue Res 361: 669–684. 10.1007/s00441-015-2137-4 [DOI] [PubMed] [Google Scholar]
- Evans PD, Maqueira B. 2005. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci 5: 111–118. 10.1007/s10158-005-0001-z [DOI] [PubMed] [Google Scholar]
- Farooqui T. 2007. Octopamine-mediated neuromodulation of insect senses. Neurochem Res 32: 1511–1529. 10.1007/s11064-007-9344-7 [DOI] [PubMed] [Google Scholar]
- Farooqui T, Robinson K, Vaessin H, Smith BH. 2003. Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honeybee. J Neurosci 23: 5370–5380. 10.1523/JNEUROSCI.23-12-05370.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala A. 2007. Olfaction and olfactory learning in Drosophila: recent progress. Curr Opin Neurobiol 17: 720–726. 10.1016/j.conb.2007.11.009 [DOI] [PubMed] [Google Scholar]
- Finetti L, Roeder T, Calò G, Bernacchia G. 2021. The insect type 1 tyramine receptors: from structure to behavior. Insects 12: 315. 10.3390/insects12040315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer CL, Grygoruk A, Patton DE, Ley B, Romero-Calderon R, Chang H-Y, Houshyar R, Bainton RJ, DiAntonio A, Krantz DE. 2005. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine. J Neurobiol 64: 239–258. 10.1002/neu.20146 [DOI] [PubMed] [Google Scholar]
- Grohmann L, Blenau W, Erber J, Ebert PR, Strünker T, Baumann A. 2003. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain: octopamine receptor from Apis mellifera. J Neurochem 86: 725–735. 10.1046/j.1471-4159.2003.01876.x [DOI] [PubMed] [Google Scholar]
- Grygoruk A, Chen A, Martin CA, Lawal HO, Fei H, Gutierrez G, Biedermann T, Najibi R, Hadi R, Chouhan AK, et al. 2014. The redistribution of Drosophila vesicular monoamine transporter mutants from synaptic vesicles to large dense-core vesicles impairs amine-dependent behaviors. J Neurosci 34: 6924–6937. 10.1523/JNEUROSCI.0694-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer M. 1993. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature 366: 59–63. 10.1038/366059a0 [DOI] [PubMed] [Google Scholar]
- Hammer M, Menzel R. 1998. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn Mem 5: 146–156. 10.1101/lm.5.1.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K-A, Millar NS, Davis RL. 1998. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci 18: 3650–3658. 10.1523/JNEUROSCI.18-10-03650.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M. 2003. Mushroom body memoir: from maps to models. Nat Rev Neurosci 4: 266–275. 10.1038/nrn1074 [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Edwards RH. 2012. Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol 74: 225–243. 10.1146/annurev-physiol-020911-153315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huetteroth W, Perisse E, Lin S, Klappenbach M, Burke C, Waddell S. 2015. Sweet taste and nutrient value subdivide rewarding dopaminergic neurons in Drosophila. Curr Biol 25: 751–758. 10.1016/j.cub.2015.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliadi KG, Iliadi N, Boulianne GL. 2017. Drosophila mutants lacking octopamine exhibit impairment in aversive olfactory associative learning. Eur J Neurosci 46: 2080–2087. 10.1111/ejn.13654 [DOI] [PubMed] [Google Scholar]
- Joiner WJ, Crocker A, White BH, Sehgal A. 2006. Sleep in Drosophila is regulated by adult mushroom bodies. Nature 441: 757–760. 10.1038/nature04811 [DOI] [PubMed] [Google Scholar]
- Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. 2011. A Drosophila model for alcohol reward. Nat Neurosci 14: 612–619. 10.1038/nn.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-C, Lee H-G, Han K-A. 2007. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci 27: 7640–7647. 10.1523/JNEUROSCI.1167-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-C, Lee H-G, Lim J, Han K-A. 2013. Appetitive learning requires the alpha1-like octopamine receptor OAMB in the Drosophila mushroom body neurons. J Neurosci 33: 1672–1677. 10.1523/JNEUROSCI.3042-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings PNM, Vullings HGB, Geffard M, Buijs RM, Diederen JHB, Jansen WF. 1988. Immunocytochemical demonstration of octopamine-immunoreactive cells in the nervous system of Locusta migratoria and Schistocerca gregaria. Cell Tissue Res 251: 371–379. 10.1007/BF00215846 [DOI] [PubMed] [Google Scholar]
- Kononenko NL, Wolfenberg H, Pflüger H-J. 2009. Tyramine as an independent transmitter and a precursor of octopamine in the locust central nervous system: an immunocytochemical study. J Comp Neurol 512: 433–452. 10.1002/cne.21911 [DOI] [PubMed] [Google Scholar]
- Kreissl S, Eichmüller S, Bicker G, Rapus J, Eckert M. 1994. Octopamine-like immunoreactivity in the brain and subesophageal ganglion of the honeybee: octopamine-IR in the brain of the honeybee. J Comp Neurol 348: 583–595. 10.1002/cne.903480408 [DOI] [PubMed] [Google Scholar]
- Lee H-G, Seong C-S, Kim Y-C, Davis RL, Han K-A. 2003. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev Biol 264: 179–190. 10.1016/j.ydbio.2003.07.018 [DOI] [PubMed] [Google Scholar]
- Lee H-G, Rohila S, Han K-A. 2009. The octopamine receptor OAMB mediates ovulation via Ca2+/calmodulin-dependent protein kinase II in the Drosophila oviduct epithelium. PLoS ONE 4: e4716. 10.1371/journal.pone.0004716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z, Chen K, Li H, Liu H, Guo A. 2013. The GABA system regulates the sparse coding of odors in the mushroom bodies of Drosophila. Biochem Biophys Res Commun 436: 35–40. 10.1016/j.bbrc.2013.05.036 [DOI] [PubMed] [Google Scholar]
- Lim J, Sabandal PR, Fernandez A, Sabandal JM, Lee H-G, Evans P, Han K-A. 2014. The octopamine receptor Octβ2R regulates ovulation in Drosophila melanogaster. PLoS ONE 9: e104441. 10.1371/journal.pone.0104441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AC, Bygrave AM, De Calignon A, Lee T, Miesenböck G. 2014. Sparse, decorrelated odor coding in the mushroom body enhances learned odor discrimination. Nat Neurosci 17: 559–568. 10.1038/nn.3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Davis RL. 2009. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat Neurosci 12: 53–59. 10.1038/nn.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Plaçais P-Y, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. 2012. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 488: 512–516. 10.1038/nature11304 [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Tempel BL. 1983. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature 303: 67–70. 10.1038/303067a0 [DOI] [PubMed] [Google Scholar]
- Manjila SB, Kuruvilla M, Ferveur J-F, Sane SP, Hasan G. 2019. Extended flight bouts require disinhibition from GABAergic mushroom body neurons. Curr Biol 29: 283–293.e5. 10.1016/j.cub.2018.11.070 [DOI] [PubMed] [Google Scholar]
- Maqueira B, Chatwin H, Evans PD. 2005. Identification and characterization of a novel family of Drosophila β-adrenergic-like octopamine G-protein coupled receptors. J Neurochem 94: 547–560. 10.1111/j.1471-4159.2005.03251.x [DOI] [PubMed] [Google Scholar]
- Martin JR, Ernst R, Heisenberg M. 1998. Mushroom bodies suppress locomotor activity in Drosophila melanogaster. Learn Mem 5: 179–191. 10.1101/lm.5.1.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SMJ, Giuliani G, Choi C, Krause P, Correale D, Watson K, Baker G, Siwicki KK. 1999. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron 24: 967–977. 10.1016/S0896-6273(00)81043-0 [DOI] [PubMed] [Google Scholar]
- Mercer AR, Menzel R. 1982. The effects of biogenic amines on conditioned and unconditioned responses to olfactory stimuli in the honeybee Apis meilifera. J Comp Physiol A 145: 363–368. 10.1007/BF00619340 [DOI] [Google Scholar]
- Mizunami M, Unoki S, Mori Y, Hirashima D, Hatano A, Matsumoto Y. 2009. Roles of octopaminergic and dopaminergic neurons in appetitive and aversive memory recall in an insect. BMC Biol 7: 46. 10.1186/1741-7007-7-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizunami M, Hamanaka Y, Nishino H. 2015. Toward elucidating diversity of neural mechanisms underlying insect learning. Zool Lett 1: 8. 10.1186/s40851-014-0008-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M. 1999. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc Res Tech 45: 106–121. [DOI] [PubMed] [Google Scholar]
- Monastirioti M, Gorczyca M, Rapus J, Eckert M, White K, Budnik V. 1995. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J Comp Neurol 356: 275–287. 10.1002/cne.903560210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M, Linn CE Jr, White K. 1996. Characterization of Drosophila tyramine β-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci 16: 3900–3911. 10.1523/JNEUROSCI.16-12-03900.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya Y, Kutsukake M, Chigusa SI, Komatsu A. 2002. A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster. Neurosci Lett 329: 324–328. 10.1016/S0304-3940(02)00596-7 [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maehara S, Kume K, Ohta H, Tomita J. 2022. Biological functions of α2-adrenergic-like octopamine receptor in Drosophila melanogaster. Genes Brain Behav 21: e12807. 10.1111/gbb.12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nässel DR. 2018. Substrates for neuronal cotransmission with neuropeptides and small molecule neurotransmitters in Drosophila. Front Cell Neurosci 12: 83. 10.3389/fncel.2018.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Marder E. 2017. Functional consequences of neuropeptide and small-molecule co-transmission. Nat Rev Neurosci 18: 389–403. 10.1038/nrn.2017.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CJ, Barron AB. 2013. Neural mechanisms of reward in insects. Annu Rev Entomol 58: 543–562. 10.1146/annurev-ento-120811-153631 [DOI] [PubMed] [Google Scholar]
- Pitman JL, McGill JJ, Keegan KP, Allada R. 2006. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature 441: 753–756. 10.1038/nature04739 [DOI] [PubMed] [Google Scholar]
- Pitman JL, Huetteroth W, Burke CJ, Krashes MJ, Lai S-L, Lee T, Waddell S. 2011. A pair of inhibitory neurons are required to sustain labile memory in the Drosophila mushroom body. Curr Biol 21: 855–861. 10.1016/j.cub.2011.03.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov AV, Peresleni AI, Popova EVS, Wolf R, Heisenberg M. 2004. The role of the mushroom bodies and of the central complex of Drosophila melanogaster brain in the organization of courtship behavior and communicative sound production. J Evol Biochem Physiol 40: 641–652. [Google Scholar]
- Prisco L, Deimel SH, Yeliseyeva H, Fiala A, Tavosanis G. 2021. The anterior paired lateral neuron normalizes odour-evoked activity in the Drosophila mushroom body calyx. Elife 10: e74172. 10.7554/eLife.74172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y-X, Xu G, Gu G-X, Mao F, Ye G-Y, Liu W, Huang J. 2017. A new Drosophila octopamine receptor responds to serotonin. Insect Biochem Mol Biol 90: 61–70. 10.1016/j.ibmb.2017.09.010 [DOI] [PubMed] [Google Scholar]
- Ren Q, Li H, Wu Y, Ren J, Guo A. 2012. A GABAergic inhibitory neural circuit regulates visual reversal learning in Drosophila. J Neurosci 32: 11524–11538. 10.1523/JNEUROSCI.0827-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder T. 1999. Octopamine in invertebrates. Prog Neurobiol 59: 533–561. 10.1016/S0301-0082(99)00016-7 [DOI] [PubMed] [Google Scholar]
- Roeder T. 2005. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol 50: 447–477. 10.1146/annurev.ento.50.071803.130404 [DOI] [PubMed] [Google Scholar]
- Roeder T. 2020. The control of metabolic traits by octopamine and tyramine in invertebrates. J Exp Biol 223: jeb194282. 10.1242/jeb.194282 [DOI] [PubMed] [Google Scholar]
- Sabandal JM, Sabandal PR, Kim Y-C, Han K-A. 2020. Concerted actions of octopamine and dopamine receptors drive olfactory learning. J Neurosci 40: 4240–4250. 10.1523/JNEUROSCI.1756-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin S, De Backer J-F, Siju KP, Wosniack ME, Lewis LP, Frisch L-M, Gansen B, Schlegel P, Edmondson-Stait A, Sharifi N, et al. 2019. A neural circuit arbitrates between persistence and withdrawal in hungry Drosophila. Neuron 104: 544–558.e6. 10.1016/j.neuron.2019.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Ruppert M, Hendrich O, Giang T, Ogueta M, Hampel S, Vollbach M, Büschges A, Scholz H. 2012. Neuronal basis of innate olfactory attraction to ethanol in Drosophila. PLoS ONE 7: e52007. 10.1371/journal.pone.0052007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Völler T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, et al. 2006. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol 16: 1741–1747. 10.1016/j.cub.2006.07.023 [DOI] [PubMed] [Google Scholar]
- Schröter U, Malun D, Menzel R. 2007. Innervation pattern of suboesophageal ventral unpaired median neurones in the honeybee brain. Cell Tissue Res 327: 647–667. 10.1007/s00441-006-0197-1 [DOI] [PubMed] [Google Scholar]
- Schützler N, Girwert C, Hügli I, Mohana G, Roignant J-Y, Ryglewski S, Duch C. 2019. Tyramine action on motoneuron excitability and adaptable tyramine/octopamine ratios adjust Drosophila locomotion to nutritional state. Proc Natl Acad Sci 116: 3805–3810. 10.1073/pnas.1813554116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. 2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci 23: 10495–10502. 10.1523/JNEUROSCI.23-33-10495.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcho M, Pauls D. 2019. Linking physiological processes and feeding behaviors by octopamine. Curr Opin Insect Sci 36: 125–130. 10.1016/j.cois.2019.09.002 [DOI] [PubMed] [Google Scholar]
- Selcho M, Pauls D, Han K-A, Stocker RF, Thum AS. 2009. The role of dopamine in Drosophila larval classical olfactory conditioning. PLoS ONE 4: e5897. 10.1371/journal.pone.0005897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcho M, Pauls D, Huser A, Stocker RF, Thum AS. 2014. Characterization of the octopaminergic and tyraminergic neurons in the central brain of Drosophila larvae. J Comp Neurol 522: 3485–3500. 10.1002/cne.23616 [DOI] [PubMed] [Google Scholar]
- Sherer LM, Catudio Garrett E, Morgan HR, Brewer ED, Sirrs LA, Shearin HK, Williams JL, McCabe BD, Stowers RS, Certel SJ. 2020. Octopamine neuron dependent aggression requires dVGLUT from dual-transmitting neurons. PLoS Genet 16: e1008609. 10.1371/journal.pgen.1008609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih M-FM, Davis FP, Henry GL, Dubnau J. 2019. Nuclear transcriptomes of the seven neuronal cell types that constitute the Drosophila mushroom bodies. Adv Genet 9: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinakevitch I, Strausfeld NJ. 2006. Comparison of octopamine-like immunoreactivity in the brains of the fruit fly and blow fly. J Comp Neurol 494: 460–475. 10.1002/cne.20799 [DOI] [PubMed] [Google Scholar]
- Sinakevitch I, Niwa M, Strausfeld NJ. 2005. Octopamine-like immunoreactivity in the honey bee and cockroach: comparable organization in the brain and subesophageal ganglion. J Comp Neurol 488: 233–254. 10.1002/cne.20572 [DOI] [PubMed] [Google Scholar]
- Sinakevitch I, Mustard JA, Smith BH. 2011. Distribution of the octopamine receptor AmOA1 in the honey bee brain. PLoS ONE 6: e14536. 10.1371/journal.pone.0014536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombati S, Hoyle G. 1984. Generation of specific behaviors in a locust by local release into neuropil of the natural neuromodulator octopamine. J Neurobiol 15: 481–506. 10.1002/neu.480150607 [DOI] [PubMed] [Google Scholar]
- Spörhase-Eichmann U, Vullings HGB, Buijs RM, Hörner M, Schürmann F-W. 1992. Octopamine-immunoreactive neurons in the central nervous system of the cricket, Gryllus bimaculatus. Cell Tissue Res 268: 287–304. 10.1007/BF00318798 [DOI] [PubMed] [Google Scholar]
- Stern M. 1999. Octopamine in the locust brain: cellular distribution and functional significance in an arousal mechanism. Microsc Res Tech 45: 135–141. [DOI] [PubMed] [Google Scholar]
- Stevenson PA, Rillich J. 2016. Controlling the decision to fight or flee: the roles of biogenic amines and nitric oxide in the cricket. Curr Zool 62: 265–275. 10.1093/cz/zow028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson PA, Pflüger H-J, Eckert M, Rapus J. 1992. Octopamine immunoreactive cell populations in the locust thoracic-abdominal nervous system: octopamine immunoreactive neurones. J Comp Neurol 315: 382–397. 10.1002/cne.903150403 [DOI] [PubMed] [Google Scholar]
- Stevenson PA, Spörhase-Eichmann U. 1995. Localization of octopaminergic neurones in insects. Comp Biochem Physiol A Physiol 110: 203–215. 10.1016/0300-9629(94)00152-J [DOI] [PubMed] [Google Scholar]
- Stocker B, Bochow C, Damrau C, Mathejczyk T, Wolfenberg H, Colomb J, Weber C, Ramesh N, Duch C, Biserova NM, et al. 2018. Structural and molecular properties of insect type II motor axon terminals. Front Syst Neurosci 12: 5. 10.3389/fnsys.2018.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura S, Aso Y, Hige T, Wong A, Lu Z, Xu CS, Rivlin PK, Hess H, Zhao T, Parag T, et al. 2017. A connectome of a learning and memory center in the adult Drosophila brain. Elife 6: e26975. 10.7554/eLife.26975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka NK, Tanimoto H, Ito K. 2008. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol 508: 711–755. 10.1002/cne.21692 [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Davis RL. 2009. Dynamics of learning-related cAMP signaling and stimulus integration in the Drosophila olfactory pathway. Neuron 64: 510–521. 10.1016/j.neuron.2009.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaaga CE, Borisovska M, Westbrook GL. 2014. Dual-transmitter neurons: functional implications of co-release and co-transmission. Curr Opin Neurobiol 29: 25–32. 10.1016/j.conb.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme S, De Fruyt N, Watteyne J, Kenis S, Peymen K, Schoofs L, Beets I. 2021. Neuromodulatory pathways in learning and memory: lessons from invertebrates. J Neuroendocrinol 33: e12911. 10.1111/jne.12911 [DOI] [PubMed] [Google Scholar]
- Verlinden H, Vleugels R, Marchal E, Badisco L, Pflüger H-J, Blenau W, Broeck JV. 2010. The role of octopamine in locusts and other arthropods. J Insect Physiol 56: 854–867. 10.1016/j.jinsphys.2010.05.018 [DOI] [PubMed] [Google Scholar]
- Wu C-L, Shih M-FM, Lai JS-Y, Yang H-T, Turner GC, Chen L, Chiang A-S. 2011. Heterotypic gap junctions between two neurons in the Drosophila brain are critical for memory. Curr Biol 21: 848–854. 10.1016/j.cub.2011.02.041 [DOI] [PubMed] [Google Scholar]
- Wu Y, Ren Q, Li H, Guo A. 2012. The GABAergic anterior paired lateral neurons facilitate olfactory reversal learning in Drosophila. Learn Mem 19: 478–486. 10.1101/lm.025726.112 [DOI] [PubMed] [Google Scholar]
- Wu C-L, Shih M-FM, Lee P-T, Chiang A-S. 2013. An octopamine-mushroom body circuit modulates the formation of anesthesia-resistant memory in Drosophila. Curr Biol 23: 2346–2354. 10.1016/j.cub.2013.09.056 [DOI] [PubMed] [Google Scholar]
- Wu S-F, Xu G, Qi Y-X, Xia R-Y, Huang J, Ye G-Y. 2014. Two splicing variants of a novel family of octopamine receptors with different signaling properties. J Neurochem 129: 37–47. 10.1111/jnc.12526 [DOI] [PubMed] [Google Scholar]
- Youn H, Kirkhart C, Chia J, Scott K. 2018. A subset of octopaminergic neurons that promotes feeding initiation in Drosophila melanogaster. PLoS ONE 13: e0198362. 10.1371/journal.pone.0198362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Lauritzen JS, Perlman E, Robinson CG, Nichols M, Milkie D, Torrens O, Price J, Fisher CB, Sharifi N, et al. 2018. A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell 174: 730–743.e22. 10.1016/j.cell.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Huang H, Kim SM, Lin H, Meng X, Han K-A, Chiang A-S, Wang JW, Jiao R, Rao Y. 2012. Molecular genetic analysis of sexual rejection: roles of octopamine and its receptor OAMB in Drosophila courtship conditioning. J Neurosci 32: 14281–14287. 10.1523/JNEUROSCI.0517-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Chen N, Tian J, Zeng J, Zhang Y, Zhang X, Guo J, Sun J, Li Y, Guo A, et al. 2019. Suppression of GABAergic neurons through D2-like receptor secures efficient conditioning in Drosophila aversive olfactory learning. Proc Natl Acad Sci 116: 5118–5125. 10.1073/pnas.1812342116 [DOI] [PMC free article] [PubMed] [Google Scholar]