Abstract
Across animal species, dopamine-operated memory systems comprise anatomically segregated, functionally diverse subsystems. Although individual subsystems could operate independently to support distinct types of memory, the logical interplay between subsystems is expected to enable more complex memory processing by allowing existing memory to influence future learning. Recent comprehensive ultrastructural analysis of the Drosophila mushroom body revealed intricate networks interconnecting the dopamine subsystems—the mushroom body compartments. Here, we review the functions of some of these connections that are beginning to be understood. Memory consolidation is mediated by two different forms of network: A recurrent feedback loop within a compartment maintains sustained dopamine activity required for consolidation, whereas feed-forward connections across compartments allow short-term memory formation in one compartment to open the gate for long-term memory formation in another compartment. Extinction and reversal of aversive memory rely on a similar feed-forward circuit motif that signals omission of punishment as a reward, which triggers plasticity that counteracts the original aversive memory trace. Finally, indirect feed-forward connections from a long-term memory compartment to short-term memory compartments mediate higher-order conditioning. Collectively, these emerging studies indicate that feedback control and hierarchical connectivity allow the dopamine subsystems to work cooperatively to support diverse and complex forms of learning.
Dopamine systems are ubiquitously important for learning in both vertebrates and invertebrates. Although mammalian dopaminergic neurons (DANs) were once regarded as a relatively uniform population that broadcasts reward signals, it has become clear that they consist of functionally diverse groups, with some of them mediating aversive stimuli (Matsumoto and Hikosaka 2009; Lerner et al. 2015; Menegas et al. 2018; de Jong et al. 2019; Yuan et al. 2019). The heterogeneity of DANs is not limited to the signs of valence they convey. In the primate caudate nucleus, two subregions receiving projections from distinct populations of DANs (Kim et al. 2014) contribute to flexible short-lasting memory and stable long-lasting memory in reward conditioning tasks (Kim and Hikosaka 2013). Thus, anatomically distinct groups of DANs mediate distinct types of memory. Furthermore, the benefits of having multiple dopamine subsystems bearing diverse properties in reward predictions and learning rates have been theoretically predicted and are supported by physiological and behavioral experiments (Iigaya et al. 2019; Dabney et al. 2020). However, major outstanding questions include what circuit mechanisms underlie the distinct properties of dopamine subsystems and how those subsystems interact with each other to enable diverse forms of learning and memory.
The Drosophila mushroom body (MB) has been an important model to study the mechanisms of dopamine-dependent learning. The orderly arrangement of the dopaminergic input and genetic tractability of individual circuit elements allowed detailed functional characterization of each dopamine subsystem. Importantly, like mammalian systems, DANs in the Drosophila MB exhibit considerable heterogeneity in their contribution to olfactory learning. Moreover, advanced anatomical studies in this system revealed that DANs receive numerous direct and indirect inputs from the output neurons of the dopamine subsystems. In this review, we spotlight these feedback and feed-forward networks within and across dopamine subsystems.
Basic circuit principles of the Drosophila MB
The adult Drosophila MB consists of ∼2600 neurons, of which ∼2000 are Kenyon cells (KCs) that predominantly receive inputs from the second-order olfactory neurons in the antennal lobes (Li et al. 2020). Each KC is narrowly tuned to odors, and as a population, KCs accurately represent odor identity in a sparse format (Turner et al. 2008; Honegger et al. 2011). KCs can be divided into three major anatomical classes (α/β, α′/β′, and γ), each forming separate axon bundles that collectively make up five MB lobes (α, β, α′, β′, and γ) (Crittenden et al. 1998; Lee et al. 1999). Those MB lobes can be further divided into 15 anatomical compartments (α1–3, β1–3, α′1–2, β′1–2, and γ1–5) defined by matching innervation patterns of the dendrites of the MB output neurons (MBONs), the postsynaptic neurons of the KCs, and the axons of DANs (Fig. 1A; Tanaka et al. 2008; Aso et al. 2014a). Thus, the overall structure of the MB can be described as repeated anatomical units formed by pairs of MBONs and DANs arranged along the axis of KC axon bundles.
Figure 1.
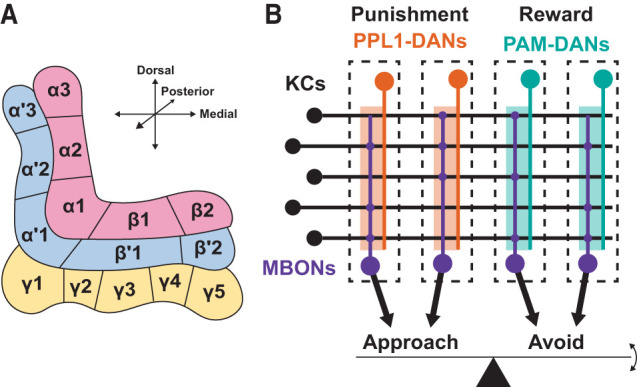
Basic anatomy and function of the Drosophila MB. (A) Arrangement of the MB lobes and 15 MB compartments. (Red) α/β Lobes, (blue) α′/β′ lobes, (yellow) γ lobe. (B) Schematic illustrating the anatomy underlying the valence balance model, which proposes that odor-directed behavior is executed based on the balance of valence-related output from the MBON population. In general, punishment-encoding PPL1-DANs (orange) innervate compartments that activate approach-promoting MBONs, and reward-encoding PAM-DANs (teal) innervate compartments that activate avoidance-promoting MBONs. The depression of KC–MBON synapses by dopamine therefore promotes the appropriate behavior by biasing behavior toward avoidance (in the case of modulation by DANs activated by punishment) or approach (in the case of modulation by DANs activated by reward).
Just like anatomically segregated dopamine subsystems in mammals mediate distinct valences and types of memory (as introduced above), Drosophila MB compartments represent functionally diverse memory units. Distinct populations of DANs are involved in appetitive and aversive learning. The compartments innervated by DANs belonging to the protocerebral anterior medial (PAM) cluster are generally appetitive memory compartments, while those innervated by the protocerebral posterior lateral 1 (PPL1) cluster DANs are aversive memory compartments (Fig. 1B; Schwaerzel et al. 2003; Burke et al. 2012; Liu et al. 2012; Aso and Rubin 2016). Moreover, memory dynamics are also distinct across compartments. Memories in some compartments develop slowly but are resistant to extinction, while those in other compartments are quickly formed but easily overwritten by subsequent conditioning (Yamagata et al. 2015; Aso and Rubin 2016). The key step of memory formation is cAMP-dependent long-term depression (LTD) at KC–MBON synapses (Yamada and Hige 2023) induced by coactivation of KCs and DANs (Cohn et al. 2015; Hige et al. 2015; Berry et al. 2018), which is thought to occur during simultaneous presentation of odor and punishment or reward. Since optogenetic activation of each MBON can promote either approach or avoidance behavior, they are considered to signal either positive or negative valence (Aso et al. 2014b; Owald et al. 2015; Perisse et al. 2016). Thus, compartment-specific induction of LTD should tip the balance of the overall valence signaled by the MB and bias the behavioral choice (Fig. 1B). In support of this valence balance model (Owald and Waddell 2015; Hige 2018), a recent study identified a cluster of neurons that integrate synaptic inputs from multiple MBONs, whose odor-evoked activity is unmasked upon LTD induction in one of the presynaptic MBONs (Aso et al. 2023).
As expected from the nonoverlapping tiling pattern of the MB compartments, recent studies using dopamine sensors revealed compartment-specific spatial patterns of dopamine signals (Sun et al. 2020; Noyes and Davis 2023; Yamada et al. 2023), which explains compartment-specific modulation of MBON activity (Cohn et al. 2015; Hige et al. 2015) and transmitter release from KCs (Davidson et al. 2023). However, MB compartments are not entirely functionally independent from each other or from their own output. Anatomical analysis of the total population of MBONs identified five neuropils where their axon terminals converge (Aso et al. 2014a). Four of those neuropils collectively contain 90% of the dendritic arbors of DANs, suggesting the presence of MBON–DAN connections. The EM connectome indeed confirmed that there are extensive monosynaptic and disynaptic connections from MBONs to DANs, constituting feedback and feed-forward networks (Li et al. 2020). Those connections imply that MB compartments can dynamically tune their function based on ongoing activity of themselves, history of learning, and the hierarchical relationships between compartments. This interplay enables the influence of existing memories on future learning. This dense MBON–DAN network was originally reported in the connectome of the Drosophila larval MB (Eschbach et al. 2020). The connectome-based computational modeling predicted that both direct and indirect MBON–DAN connections are important for multiple types of learning, including classical conditioning, higher-order conditioning, context-dependent conditioning, and extinction (Eschbach et al. 2020). Simulated neuronal activity showed altered firing rates of modulatory neurons after conditioning, which may underlie at least some forms of learning. However, some of the conditioning paradigms have been experimentally demonstrated so far to be effective only in adult flies. Below, we introduce recent advances in understanding the functions of MBON–DAN networks in the adult brain by classifying them into three categories: memory consolidation, memory update, and higher-order conditioning.
Memory consolidation—feedback mechanism
Perhaps the simplest form of the circuit mediated by MBON–DAN connections is a feedback loop, where MBONs of a given MB compartment synapse onto the DANs projecting back to the same compartment. Among all MB compartments, α1 is the only compartment whose resident MBONs and DANs do not directly communicate with DANs or MBONs in other compartments (Li et al. 2020). However, they do form a feedback loop from α/β KCs to glutamatergic MBON-α1 to a subpopulation of PAM-α1 (DANs in α1) (Fig. 2A), and the activity of this recurrent circuit is implicated in the consolidation of appetitive memory. While there are several appetitive memory compartments (Burke et al. 2012; Liu et al. 2012; Huetteroth et al. 2015), α1 is particularly essential for long-term memory (LTM) formed by nutritious sugar rather than for short-term memory (STM) formed by nonnutritious sweetness (Yamagata et al. 2015; Aso and Rubin 2016). Transient blockade of any one of the three components of the feedback loop (i.e., α/β KCs, MBON-α1, and PAM-α1) during or shortly after sugar conditioning impairs memory measured after 24 h, representing LTM (Ichinose et al. 2015). However, synaptic outputs from those neurons are no longer needed between 22 h posttraining and the test period. These results led to the model that self-sustained activity of the feedback loop after learning contributes to the consolidation of memory. However, the postulated reverberant circuit activity during the consolidation period is yet to be demonstrated. The memory consolidation function of this circuit depends on both Dop1R1 dopamine receptors on α/β KCs and N-methyl-D-aspartate (NMDA)-type glutamate receptors on PAM-α1 (Ichinose et al. 2015). While dopamine can depolarize the postsynaptic neurons (Cohn et al. 2015; Takemura et al. 2017), glutamatergic transmission in the central brain of Drosophila has been generally considered inhibitory (Liu and Wilson 2013). In particular, transmission from glutamatergic MBON-α1 to another group of postsynaptic neurons is also inhibitory (Aso et al. 2023). Cell type-specific transcriptome analysis suggests that PAM-α1 abundantly expresses glutamate-gated chloride channels in addition to NMDA receptors (Aso et al. 2019). Thus, it is entirely possible that an additional indirect pathway from MBON-α1 to PAM-α1 is responsible for the function of this feedback loop. Interestingly, a systematic study on the behavioral contribution of each MBON revealed that all MBONs making major contributions to appetitive memory (namely, MBON-α1, MBON-β1, MBON-γ5β′2a, and MBON-β′2mp) are glutamatergic and form recurrent circuits at least anatomically (Ichinose et al. 2021). Thus, it is possible that this glutamatergic feedback motif plays a common role in reward memory beyond memory consolidation.
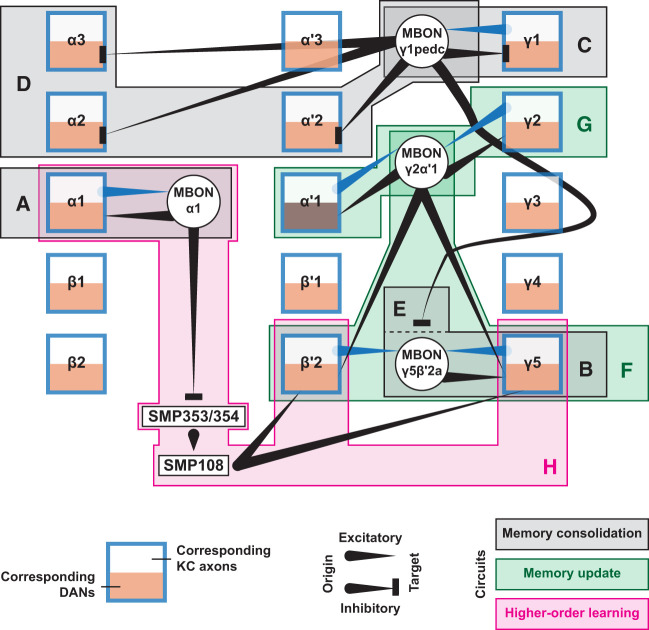
Figure 2.
The interconnected nature of feedback and feed-forward networks underlying memory consolidation, memory update, and higher-order conditioning. Each square with thick blue borders represents a compartment of the MB. Connecting lines emerging from the top half of a square (white) represent output from KC axons, and connecting lines ending in the bottom half of a square (orange) represent input to the DANs innervating this compartment. Connecting lines ending in a point denote excitatory connections, while those ending in a perpendicular bar denote inhibitory connections. Each circle represents an MBON. For simplicity, only connections that are directly mentioned in this review are drawn, although some of the connections are not physiologically confirmed. Note that this integrated illustration highlights the role of MBON-γ1pedc and MBON-γ2α′1 as hub neurons interconnecting different compartments and involvement of the circuits around the γ5 compartment in multitudes of functions. (A) Feedback loop from α/β KCs to MBON-α1 to PAM-α1; involved in the consolidation of appetitive memory (Ichinose et al. 2015). (B) Excitatory feedback loop from γ KCs to MBON-γ5β′2a to PAM-γ5; involved in the consolidation of courtship memory (Krüttner et al. 2015; Zhao et al. 2018). Together with the connection illustrated in E, this circuit is also implicated in the extinction of aversive memory (Felsenberg et al. 2018). (C) Inhibitory feedback loop from MBON-γ1pedc to PPL1-γ1pedc; involved in the consolidation of appetitive memory (Pavlowsky et al. 2018). (D) Depression of MBON-γ1pedc olfactory activity is considered to disinhibit PPL1-α′2α2 and PPL1-α3; involved in the consolidation of aversive memory (Awata et al. 2019; Schnitzer et al. 2022). (E) Depression of MBON-γ1pedc olfactory activity is also considered to disinhibit MBON-γ5β′2a, leading to excitatory feedback from MBON-γ5β′2a to PAM-γ5 via the circuit illustrated in B; involved in the extinction of aversive memory (Felsenberg et al. 2018). (F) Feed-forward excitation from MBON-γ2α′1 to PAM-β′2a and PAM-γ5; involved in the reversal of aversive memory (McCurdy et al. 2021) and in learning relative aversive value (Villar et al. 2022). (G) Excitatory feedback loop from MBON-γ2α′1 to PPL1-γ2α′1; involved in the reconsolidation of appetitive memory (Felsenberg et al. 2017). (H) Depression of MBON-α1 disinhibits SMP353/354, activating SMP108, which excites PAM-β′2a and PAM-γ5 among other PAM-DANs; involved in second-order conditioning (Aso et al. 2023; Yamada et al. 2023).
Similar reverberant activity of a feedback loop is considered to underlie the consolidation of courtship memory. When a naive male is placed with a recently mated female, repetitive courtship rejections by the female suppress the male's subsequent courtship toward mated females (Siegel and Hall 1979). PAM-γ5 DANs, originally annotated as fruitless-positive aSP13 DANs, play a crucial role in this learning (Keleman et al. 2012). Transient blockade of these DANs during the consolidation period can prevent the formation of LTM after long conditioning, whereas artificial activation can convert STM, formed by short conditioning, to LTM (Krüttner et al. 2015). Like in α1, the three sets of neurons (γ KCs, glutamatergic MBON-γ5β′2a, and PAM-γ5) form a recurrent loop (Fig. 2B), but in this case, the excitatory nature of connections is demonstrated by calcium imaging; γ KCs can excite MBON-γ5β′2a only in the presence of dopamine, and a transient activation of MBON-γ5β′2a induces a prolonged activation of PAM-γ5 (Zhao et al. 2018). The time scale of the prolonged activity of PAM-γ5 (i.e., a few minutes after termination of MBON-γ5β′2a activation) (Zhao et al. 2018) does not explain the time window in which PAM-γ5 activity is required for consolidation (i.e., 1–3 h after the end of training) (Krüttner et al. 2015). This mismatch may suggest the involvement of additional circuits to reactivate or maintain the activity of PAM-γ5. Interestingly, PAM-γ5 is activated during daytime sleep, the duration of which increases after the long conditioning required for LTM (Dag et al. 2019). A key difference from the appetitive olfactory memory formed in α1 is that the recurrent circuit is also required for STM formation; blockade of MBON-γ5β′2a during training impairs courtship STM (Zhao et al. 2018), whereas that of MBON-α1 does not affect appetitive olfactory STM (Ichinose et al. 2015). This difference may be related to the difference in the typical duration of conditioning: 1 h for short-term courtship memory versus 1 min for appetitive olfactory memory.
The above two examples propose the importance of sustained excitatory activity of the feedback loop. Another case shows that inhibitory feedback loops could also contribute to memory consolidation. Immediately after conditioning with nutritious sugar, PPL1-γ1pedc, the DAN projecting to the γ1 compartment, shows an increased rhythmic activity, slowly oscillating at ∼0.04 Hz for 0.5–1 h (Musso et al. 2015). The transiency of this activity is key for memory consolidation because artificial activation of PPL1-γ1pedc after this time window impairs LTM (Pavlowsky et al. 2018). The elevated activity of PPL1-γ1pedc initially suppresses MBON-γ1pedc but later excites it by recruiting different types of dopamine receptors. The activated MBON-γ1pedc, which is GABAergic, in turn suppresses PPL1-γ1pedc via GABAB receptors (Fig. 2C). Thus, the complex reciprocal interplay between PPL1-γ1pedc and MBON-γ1pedc shapes the temporal dynamics of the dopamine activity beneficial for consolidation. However, it remains to be studied how the transient activity of dopamine in γ1pedc, which is known as one of the aversive memory compartments (Claridge-Chang et al. 2009; Aso et al. 2010), helps consolidate the appetitive memory and how DAN activity outside the narrow time window disrupts the consolidation process.
One potential problem shared by the consolidation models involving sustained recurrent activity is the maintenance of the stimulus specificity of memory. That is, during the consolidation period, there is no longer an olfactory input that evokes odor-specific activity in KCs; meanwhile, DANs keep releasing dopamine to all KCs in the compartment. This may result in nonspecific modulation of KC outputs and therefore a decrease in stimulus specificity. Such a decline in stimulus specificity of LTM as compared with STM has been shown in appetitive memory (Ichinose et al. 2015). In the case of courtship learning, this trade-off may be less significant because the role of accurate sensory coding in KCs is not well defined in this behavioral paradigm. Another related issue that remains to be solved is how sustained input from DANs modulates KC outputs. At this point, bidirectional modulation of KC–MBON synapses is only demonstrated as dependent on the relative timing of the activity of KCs and DANs (Handler et al. 2019), the size of odor-evoked calcium transient in the KC presynaptic terminal (Davidson et al. 2023), or the second-messenger pathway implicated in a cotransmitter of DANs (Aso et al. 2019; Yamada and Hige 2023), none which can easily explain the action of dopamine during consolidation. Aversive LTM formation also depends on protein synthesis in a small subset of MBONs at specific time points during the consolidation period (Wu et al. 2017), suggesting the involvement of plasticity on the postsynaptic neurons. The presence of transcriptional memory trace has been also suggested in at least one of the MBONs in the context of appetitive LTM (Widmer et al. 2018).
Memory consolidation—feed-forward mechanism
Multiple lines of evidence indicate that different classes of KCs preferentially contribute to different phases of memory. In general, γ KCs serve as an important “gateway” of memory or primary association site for olfactory and punishment/reward signals, whereas output from α/β KCs is critical for LTM (Isabel et al. 2004; Yu et al. 2006; Krashes et al. 2007; Krashes and Waddell 2008; Blum et al. 2009; Trannoy et al. 2011; Qin et al. 2012; Huang et al. 2013). These findings are in line with MB compartment-specific contributions to STM and LTM. For appetitive learning, a series of studies using artificial activation and blockade of DANs or MBONs identified α1 as one of the LTM compartments and γ5β′2a as an STM compartment (Huetteroth et al. 2015; Yamagata et al. 2015; Aso and Rubin 2016). Similarly, for aversive learning, γ1pedc was identified as one of the STM compartments, and α3 as an LTM compartment (Pai et al. 2013; Aso and Rubin 2016; Jacob and Waddell 2020), although α3 is also implicated in appetitive LTM (Plaçais et al. 2013). The observations that memories with different retention times depend on distinct neuronal substrates of the MB invited active debates over the origin of LTM in relation to STM. In an extreme scenario, STM and LTM could form and develop in parallel in completely independent, segregated circuits. The LTM induction mechanisms that we reviewed so far do not contradict this view because they reside within a single MB compartment, even though they may involve additional neurons outside the MB. However, the mechanisms that we introduce next, which are mediated by MBON–DAN feed-forward circuits, are in support of another view: Formation of STM sequentially induces LTM, involving multiple KC classes and MB compartments.
Aversive olfactory LTM is typically induced by so-called spaced training, in which odor–shock conditioning is repeated multiple times with certain intervals (Tully et al. 1994; Beck et al. 2000), and the optimum interval is at least in part determined by the level of protein phosphatase activity (Pagani et al. 2009). During later cycles of the spaced training, GABAergic MBON-γ1pedc and a subset of the presynaptic γ KCs decrease their odor-evoked activity (measured by the level of phosphorylation of extracellular signal-related kinase [pERK]) in a Dop1R1-dependent manner (Awata et al. 2019). In contrast, PPL1-α′2α2, one of the DANs postsynaptic to MBON-γ1pedc, shows a concurrent increase in the pERK reported activity, suggesting disinhibition from MBON-γ1pedc (Fig. 2D). This suggests that in the later cycles of spaced training, both KCs and DANs are strongly activated in the α′2α2 compartment. Simultaneous thermogenetic activation of PPL1-α′2α2 and α/βs KCs, which are the subset of α/β KCs identified as particularly essential for LTM retrieval (Huang et al. 2013), induces Arc2 mRNA expression in α/βs KCs, which is implicated in LTM formation. Thus, these observations suggest that LTM induction involves the coordinated action of multiple MB compartments.
Although care must be taken when interpreting these results because of the use of indirect measurement of neuronal activity and plasticity (Awata et al. 2019), a similar circuit mechanism was independently proposed by another study using elegant voltage imaging tools (Schnitzer et al. 2022). This work also points to the importance of MBON-γ1pedc plasticity as a trigger to open the gate for plasticity in other MB compartments in the α lobe. Repetitive odor–shock pairing induces an odor-specific decline in spike responses in MBON-γ1pedc in later cycles, as expected from the above-mentioned pERK reported activity (Awata et al. 2019) as well as other studies (Hige et al. 2015; Perisse et al. 2016; Felsenberg et al. 2018; Cervantes-Sandoval et al. 2020; McCurdy et al. 2021; Hancock et al. 2022; Davidson et al. 2023; Zeng et al. 2023). This activity decline is accompanied by the odor-specific activity increase in PPL1-α3, which is also postsynaptic to MBON-γ1pedc (Scheffer et al. 2020), as well as in PPL1-α′2α2 (Fig. 2D; Schnitzer et al. 2022). Importantly, MBON-α3 shows odor-specific depression for up to 48 h after conditioning (Schnitzer et al. 2022), whereas MBON-γ1pedc recovers from depression after 1 h (Cervantes-Sandoval et al. 2020; Schnitzer et al. 2022; but see Perisse et al. 2016; Felsenberg et al. 2018). Thus, MBON–DAN feed-forward circuits assign hierarchical relationships between MB compartments, enabling STM formation in one compartment to gate the plasticity in another compartment for LTM induction. Since bypassing this circuit mechanism by direct optogenetic activation of DANs in γ1pedc and α3 results in the formation of STM and LTM, respectively, with different learning rates (Aso and Rubin 2016), intrinsic properties of the MB compartments, such as compartment-specific expression of a DAN cotransmitter (Aso et al. 2019), are also an important determinant of the induction of LTM.
Memory update
While forming, stabilizing, and maintaining a memory are certainly important aspects of learning, memories also require sufficient flexibility to be updated; for example, in response to more recent experience that does not align with the previously learned association. We next introduce the circuit mechanisms of various types of memory update involving MBON–DAN connections.
After forming an association between an odor (conditioned stimulus+ [CS+]) and shock, the aversive olfactory memory can be extinguished by subsequent presentation of the CS+ alone (Tempel et al. 1983; Schwaerzel et al. 2002), and this extinction involves an excitatory MBON–DAN connection (Felsenberg et al. 2018) that also plays a role in the aforementioned consolidation process of courtship memory (Zhao et al. 2018). As repeatedly mentioned, aversive conditioning induces CS+-specific depression in MBON-γ1pedc. This GABAergic MBON sends axon collaterals to the MB lobes to make contacts with multiple MBONs, including MBON-γ5β′2a (Fig. 2E), which belongs to an appetitive memory compartment. After aversive training, the CS+ response of the axon of MBON-γ5β′2a is potentiated (as compared with control odors), presumably due to disinhibition from MBON-γ1pedc (Owald et al. 2015; Perisse et al. 2016; Felsenberg et al. 2018). This relative potentiation of the CS+ response is expected to contribute to conditioned avoidance of CS+ because direct activation of a cluster of MBONs, including MBON-γ5β′2a, drives avoidance behavior (Aso et al. 2014b; Owald et al. 2015). During re-exposure to CS+ or extinction training, this potentiated CS+ response drives excitation of a subset of the PAM-γ5 DANs via direct synaptic contacts (Otto et al. 2020), which in turn induces depression in the γ5 branch of the MBON-γ5β′2a dendrites (Felsenberg et al. 2018). Although MBON-γ1pedc remains depressed after extinction training, the dendritic depression of MBON-γ5β′2a neutralizes the potentiation effect in its axon. Thus, this excitatory feedback connection, perhaps representing the “reward” of the absence of shock or prediction error, induces plasticity in the appetitive memory compartment, which counteracts that induced by the original aversive training. A similar antagonism between coexisting opposing memories is considered to underlie the extinction of appetitive memory (Felsenberg et al. 2017). In this case, activation of punishment-encoding PPL1-DANs via corresponding MBONs during extinction training is crucial, although a less detailed process compared with aversive memory extinction is known.
Reversal learning is somewhat similar to extinction but requires more flexible updates of memory, as it completely flips the stimulus contingency between trainings rather than simply omitting the reinforcement. In this paradigm, after normal associative learning, the cue that was presented without reinforcement during original training (conditioned stimulus− [CS−]) is paired with reinforcement, while the original CS+ is presented alone without reinforcement (Shuai et al. 2010; Cervantes-Sandoval et al. 2016; Berry et al. 2018; McCurdy et al. 2021). Unsurprisingly, the reversal of aversive learning induces neuronal activity changes that overlap with those observed after the extinction of aversive memory (McCurdy et al. 2021). As mentioned above, aversive conditioning potentiates the CS+ response of MBON-γ5β′2a presumably via disinhibition from MBON-γ1pedc. Just like in extinction, the central mechanism of attenuation of CS+ avoidance after reversal learning is also neutralization of the plasticity (or depotentiation) of MBON-γ5β′2a. However, in reversal learning, the depression that counteracts the ongoing potentiation is mediated by PAM-β′2a DANs, not PAM-γ5 as seen in the case of extinction (Felsenberg et al. 2018; Otto et al. 2020). The CS+ response of PAM-β′2a, previously depressed by aversive conditioning, regrows during reversal training to depotentiate MBON-γ5β′2a (McCurdy et al. 2021). This activity pattern of PAM-β′2a mirrors that of cholinergic MBON-γ2α′1 (Berry et al. 2018; McCurdy et al. 2021), which is presynaptic to PAM-β′2a (Fig. 2F). Thus, although slightly different in the routes leading to plasticity, both reversal learning and extinction induce parallel opposing memory traces via MBON–DAN pathways to attenuate the conditioned behavior. Interestingly, a closely overlapping mechanism is used for learning of relative aversive value (Villar et al. 2022). In this learning, flies experience strong electric shocks with odor A and then milder shocks with odor B, after which they develop a relative preference of B over A (Yin et al. 2009; Perisse et al. 2013). During pairing with odor B, a subset of reward-encoding PAM-DANs (namely, PAM-β′2a and PAM-γ5) shows an increased activity, likely driven by elevated activity of MBON-γ2α′1 (Fig. 2F; Villar et al. 2022). Thus, the circuitry around PAM-β′2a and PAM-γ5 may have a specialized role of representing “relative reward” based on the animal's past experience.
Memory update does not always mean that memory is extinguished or reversed; some memory just needs to be refreshed and re-established, especially since memory appears to become temporarily labile after retrieval (Nader et al. 2000; Schafe and LeDoux 2000), often triggering reconsolidation to maintain the originally learned association. In flies, moderate reactivation of aversive memory by re-exposure to CS+ alone triggers reconsolidation, whereas intense reactivation results in extinction (Lagasse et al. 2009). Reconsolidation of appetitive memory can be initiated by postlearning re-exposure to CS− alone, and this process relies on the excitation of multiple types of DANs via both feedback and feed-forward MBON–DAN networks (Felsenberg et al. 2017). After appetitive learning, MBON-γ2α′1 shows an increased response to CS−, which is counterintuitive because MBON-γ2α′1 promotes approach behavior (Aso et al. 2014b); the elevated response to CS− could counteract the conditioned approach to CS+. However, this cholinergic MBON feeds a critical excitatory drive to its partner DAN PPL1-γ2α′1 (Fig. 2G) via a feedback loop and also to PAM-DANs via feed-forward connections, both of whose activity is required for reconsolidation with different timing; PPL1-γ2α′1 is required during CS− re-exposure, and PAM-DANs are required right after it (Felsenberg et al. 2017). Although the detailed process is still elusive, sequential action of those DANs driven by MBON–DAN connections is expected to induce destabilization and restabilization of memory.
Higher-order conditioning
The examples so far show that the hierarchical interconnection between dopamine subsystems enables existing memory to gate LTM or update it based on new experiences. In general, this circuit architecture allows previous learning to influence future learning. This feature also enables a complex form of learning, in which animals use a previously learned, rather than an innate, valence of stimuli to learn a new association. Second-order conditioning is one such type of indirect learning that has been observed in many different species, including Drosophila (Rizley and Rescorla 1972; Rashotte et al. 1977; Bitterman et al. 1983; Hawkins et al. 1998; Brembs and Heisenberg 2001; Mizunami et al. 2009; Tabone and de Belle 2011; Yamada et al. 2023). In appetitive unimodal olfactory second-order conditioning, starved flies first undergo odor (S1)–sugar conditioning. This S1 serves as a reward in the following round of training, in which flies are presented with another odor (S2) followed by S1 but not by sugar. After this training, flies develop a robust preference toward S2 even though it was never presented with the primary reward (Yamada et al. 2023). Optogenetic imprinting of S1 memory in targeted reward memory compartments followed by S2–S1 conditioning identified α1, the LTM compartment, as the most efficient “teacher” compartment capable of instructing second-order memory. Paradoxically, as mentioned above, α1 is the most “isolated” compartment in terms of direct connectivity with other compartments, lacking monosynaptic connection between MBON-α1 and non-α1 DANs (Li et al. 2020). It turned out, however, that there is a robust network indirectly connecting MBON-α1 to multiple PAM-DANs innervating reward memory compartments. A cluster of cholinergic neurons (SMP353/354) receiving inhibitory inputs from MBON-α1 acquires an excitatory S1 response upon induction of depression in α1, mimicking S1 memory formation (Aso et al. 2023). SMP353/354 converge on a single cholinergic neuron named SMP108, which also acquires an excitatory S1 response (Yamada et al. 2023). SMP108 in turn synapses on PAM-DANs and, upon activation, can induce dopamine release in multiple “student” compartments, including the STM compartments γ5 and β′2a (Fig. 2H). S2–S1 conditioning after S1 memory formation in α1 indeed induces S2-specific depression in MBON-γ5β′2a. Successful second-order conditioning generally involves repetitive S2–S1 pairing. This requires S1 memory to be resistant to extinction because no reward is presented during S2–S1 pairing. This requirement may explain why α1, which can hold stable and long-lasting memory, is most effective as a teacher compartment. On the other hand, the retention time of S2 memory is known to be short across species (Gewirtz and Davis 2000). This feature could be explained by the memory properties of the student compartments. Thus, the identified circuit mechanisms of second-order conditioning may account for distinct properties of first- and second-order memory. A somewhat similar mechanism may underlie aversive second-order learning because suppression of MBON-γ1pedc, which mimics the plasticity following first-order conditioning, paired with an odor presentation induces aversive memory (Ueoka et al. 2017; König et al. 2019), although the exact mechanisms are yet to be studied.
Current limitations and future prospects
Apart from a notable exception of the second-order learning study (Yamada et al. 2023), the majority, if not all, of the studies mentioned in this review focused on, or attributed the identified functions to, direct MBON–DAN connections. However, experimental evidence to support the specific role of the direct connections is typically circumstantial and does not formally exclude the potential involvement of indirect connections. For example, even the presence of an EM-confirmed monosynaptic connection from an MBON to DANs and corresponding behavioral evidence of the requirement of DAN expression of receptors for the transmitter released by the MBON do not rule out the possibility that other neurons that use the same transmitter and synapse on the DANs play an important role. Additionally, although the relationship between the ultrastructural arrangement of synapses and physiologically measured synaptic strength has been studied in certain cell types (Liu et al. 2022), it is unclear whether we can extrapolate the results to other cells. In general, we need more information about the subcellular localization of transmitter receptors to validate the connectome-identified synapses. We also need tools to manipulate specific synapses or axon branches rather than switching the entire output of a given neuron on and off to understand the function of the specific connections of interest. For example, a recent study in T4/T5 neurons of the fly visual system used epitope-tagged endogenous transmitter receptors together with expansion microscopy to demonstrate subtype-specific localization of receptors to dendritic domains within a neuron (Sanfilippo et al. 2023). Subtype compositions of the receptors are also distinct among synapses within the same dendritic domains. Such high-resolution information may enable input-specific manipulation of synapses by, for example, cell type-specific knockout of a specific receptor subtype. To explore the presence of indirect connections, the combination of EM connectome data and the neurotransmitter prediction from the EM images (Eckstein et al. 2023) would be a powerful tool. However, to test the functions of the identified connections, genetic drivers for the interneurons connecting MBONs and DANs are required. Although such an approach has proven to be successful in identifying the circuit mediated by two interneurons (Yamada et al. 2023), the creation of specific genetic drivers for interneurons can be a rate-limiting step.
Another complicating factor is the heterogeneity of DANs projecting to the same MB compartment. While there is only a single cell per cell type of PPL1-DANs, which occupies the entire MB compartment, there are a few up to dozens of neurons per cell type of PAM-DANs (Li et al. 2020). A given PAM-DAN cell type, as a population, occupies the entire MB compartment, but individual cells project to a subregion of the compartment. In the γ5 compartment, which is innervated by ∼20 PAM-DANs, such anatomical heterogeneity has been successfully linked to their functional segregation (Otto et al. 2020). However, the ability to interrogate the different roles across subclasses of PAM-DANs is again limited by the availability of specific genetic drivers.
Finally, to monitor the dynamic sequential changes of neuronal activity across multiple MB compartments, simultaneous recording of neurons and behavioral changes would be ideal. The recent development of optical probes represented by genetically encoded voltage sensors made it possible to report the spiking activity of neurons in head-fixed-behaving flies (Schnitzer et al. 2022). However, monitoring the activity of neurons while flies show learning-induced behavioral changes is still challenging, presumably due to the impact of surgery and head fixation on their internal state. One avenue to mitigate the problem might be to record from free-moving flies using less invasive imaging with bioluminescence probes (Mercier et al. 2018). Given the dense network of MBONs and DANs, however, the flip side of monitoring actual learning-induced changes is that it may make it difficult to discriminate the primary location of plasticity from the regions undergoing plasticity induced by the primary plasticity. To probe these relationships, artificial learning induced by site-directed memory imprinting using methods such as optogenetic activation of a specific DAN population should be useful.
Conclusions
In a simplified view, the Drosophila MB can be regarded as repeating memory modules controlled in parallel by dopamine and operating with different intrinsic parameters for plasticity induction and maintenance. However, the intricate network interconnecting the MB compartments revealed by the EM connectome suggests that these memory modules are far from independent from each other. Recent studies confirm that DANs in a given module are under the effect of its own or other modules’ output, which enables previous learning to influence future learning. Computational modeling studies have also begun to account for such connectivity, illustrating the importance of these connections in processes like extinction learning and testing reward prediction capabilities of DANs (Bennett et al. 2021; Jiang and Litwin-Kumar 2021; Springer and Nawrot 2021; Zhao et al. 2021; Gkanias et al. 2022). It should be noted that, as evident from the circuit underlying second-order conditioning, even MBONs and DANs that are three synapses away can mediate a robust function. Thus, the potential impact of MBON–DAN connections should be even more significant than what one would expect from direct connectivity, which already appears dense. Indeed, there are more functions of the MB that are likely to involve this MBON–DAN network (Hattori et al. 2017; Jacob and Waddell 2020). Moreover, DAN activity is influenced not only by previous learning but also by a complex combination of the innate value of stimuli, the internal state of the animal, ongoing locomotion, and microcircuits inside the MB compartments (Krashes et al. 2009; Cohn et al. 2015; Cervantes-Sandoval et al. 2017; Tsao et al. 2018; Lyutova et al. 2019; Siju et al. 2020; Zolin et al. 2021; Schnitzer et al. 2022; Kato et al. 2023). Thus, the MB offers ample opportunities to explore the functions of higher-order connections between dopamine subsystems.
Acknowledgments
We thank Yoshinori Aso for helpful comments on an earlier version of the manuscript. A.M.D. is supported by a Postdoctoral Fellowship provided by the BRAIN (Brain Research through Advancing Innovative Neurotechnologies) Initiative of the National Institutes of Health (F32MH125582). T.H. is supported by grants from the National Institutes of Health (R01DC018874), the National Science Foundation (2034783), and the United States–Israel Binational Science Foundation (2019026).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053807.123.
Freely available online through the Learning & Memory Open Access option.
References
- Aso Y, Rubin GM. 2016. Dopaminergic neurons write and update memories with cell-type-specific rules. Elife 5: e16135. 10.7554/eLife.16135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Siwanowicz I, Bräcker L, Ito K, Kitamoto T, Tanimoto H. 2010. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol 20: 1445–1451. 10.1016/j.cub.2010.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo T-TB, Dionne H, Abbott LF, Axel R, Tanimoto H, et al. 2014a. The neuronal architecture of the mushroom body provides a logic for associative learning. Elife 3: e04577. 10.7554/eLife.04577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Sitaraman D, Ichinose T, Kaun KR, Vogt K, Belliart-Guérin G, Plaçais P-Y, Robie AA, Yamagata N, Schnaitmann C, et al. 2014b. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. Elife 3: e04580. 10.7554/eLife.04580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Ray RP, Long X, Bushey D, Cichewicz K, Ngo T-T, Sharp B, Christoforou C, Hu A, Lemire AL, et al. 2019. Nitric oxide acts as a cotransmitter in a subset of dopaminergic neurons to diversify memory dynamics. Elife 8: e49257. 10.7554/eLife.49257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Yamada D, Bushey D, Hibbard KL, Sammons M, Otsuna H, Shuai Y, Hige T. 2023. Neural circuit mechanisms for transforming learned olfactory valences into wind-oriented movement. Elife 12: e85756. 10.7554/eLife.85756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awata H, Takakura M, Kimura Y, Iwata I, Masuda T, Hirano Y. 2019. The neural circuit linking mushroom body parallel circuits induces memory consolidation in Drosophila. Proc Natl Acad Sci 116: 16080–16085. 10.1073/pnas.1901292116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CD, Schroeder B, Davis RL. 2000. Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. J Neurosci 20: 2944–2953. 10.1523/JNEUROSCI.20-08-02944.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JEM, Philippides A, Nowotny T. 2021. Learning with reinforcement prediction errors in a model of the Drosophila mushroom body. Nat Commun 12: 2569. 10.1038/s41467-021-22592-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JA, Phan A, Davis RL. 2018. Dopamine neurons mediate learning and forgetting through bidirectional modulation of a memory trace. Cell Rep 25: 651–662.e5. 10.1016/j.celrep.2018.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman ME, Menzel R, Fietz A, Schäfer S. 1983. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol 97: 107–119. 10.1037/0735-7036.97.2.107 [DOI] [PubMed] [Google Scholar]
- Blum AL, Li W, Cressy M, Dubnau J. 2009. Short- and long-term memory in Drosophila require cAMP signaling in distinct neuron types. Curr Biol 19: 1341–1350. 10.1016/j.cub.2009.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembs B, Heisenberg M. 2001. Conditioning with compound stimuli in Drosophila melanogaster in the flight simulator. J Exp Biol 204: 2849–2859. 10.1242/jeb.204.16.2849 [DOI] [PubMed] [Google Scholar]
- Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl DM, Silies M, Certel S, Waddell S. 2012. Layered reward signalling through octopamine and dopamine in Drosophila. Nature 492: 433–437. 10.1038/nature11614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Sandoval I, Chakraborty M, MacMullen C, Davis RL. 2016. Scribble scaffolds a signalosome for active forgetting. Neuron 90: 1230–1242. 10.1016/j.neuron.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Sandoval I, Phan A, Chakraborty M, Davis RL. 2017. Reciprocal synapses between mushroom body and dopamine neurons form a positive feedback loop required for learning. Elife 6: e04577. 10.7554/eLife.23789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Sandoval I, Davis RL, Berry JA. 2020. Rac1 impairs forgetting-induced cellular plasticity in mushroom body output neurons. Front Cell Neurosci 14: 258. 10.3389/fncel.2020.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, MiesenbOck G. 2009. Writing memories with light-addressable reinforcement circuitry. Cell 139: 405–415. 10.1016/j.cell.2009.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn R, Morantte I, Ruta V. 2015. Coordinated and compartmentalized neuromodulation shapes sensory processing in Drosophila. Cell 163: 1742–1755. 10.1016/j.cell.2015.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EM, Han K-AA, Kalderon D, Davis RL. 1998. Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem 5: 38–51. 10.1101/lm.5.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabney W, Kurth-Nelson Z, Uchida N, Starkweather CK, Hassabis D, Munos R, Botvinick M. 2020. A distributional code for value in dopamine-based reinforcement learning. Nature 577: 671–675. 10.1038/s41586-019-1924-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dag U, Lei Z, Le JQ, Wong A, Bushey D, Keleman K. 2019. Neuronal reactivation during post-learning sleep consolidates long-term memory in Drosophila. Elife 8: e42786. 10.7554/eLife.42786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AM, Kaushik S, Hige T. 2023. Dopamine-dependent plasticity is heterogeneously expressed by presynaptic calcium activity across individual boutons of the Drosophila mushroom body. eNeuro 10: ENEURO.0275-23.2023. 10.1523/ENEURO.0275-23.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim CK, Tian L, Deisseroth K, Lammel S. 2019. A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron 101: 133–151.e7. 10.1016/j.neuron.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein N, Bates AS, Champion A, Du M, Yin Y, Schlegel P, Lu AK-Y, Rymer T, Finley-May S, Paterson T, et al. 2023. Neurotransmitter classification from electron microscopy images at synaptic sites in Drosophila melanogaster. bioRxiv 10.1101/2020.06.12.148775v3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschbach C, Fushiki A, Winding M, Schneider-Mizell CM, Shao M, Arruda R, Eichler K, Valdes-Aleman J, Ohyama T, Thum AS, et al. 2020. Recurrent architecture for adaptive regulation of learning in the insect brain. Nat Neurosci 23: 544–555. 10.1038/s41593-020-0607-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenberg J, Barnstedt O, Cognigni P, Lin S, Waddell S. 2017. Re-evaluation of learned information in Drosophila. Nature 544: 240–244. 10.1038/nature21716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenberg J, Jacob PF, Walker T, Barnstedt O, Edmondson-Stait AJ, Pleijzier MW, Otto N, Schlegel P, Sharifi N, Perisse E, et al. 2018. Integration of parallel opposing memories underlies memory extinction. Cell 175: 709–722.e15. 10.1016/j.cell.2018.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M. 2000. Using Pavlovian higher-order conditioning paradigms to investigate the neural substrates of emotional learning and memory. Learn Mem 7: 257–266. 10.1101/lm.35200 [DOI] [PubMed] [Google Scholar]
- Gkanias E, McCurdy LY, Nitabach MN, Webb B. 2022. An incentive circuit for memory dynamics in the mushroom body of Drosophila melanogaster. Elife 11: e75611. 10.7554/eLife.75611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock CE, Rostami V, Rachad EY, Deimel SH, Nawrot MP, Fiala A. 2022. Visualization of learning-induced synaptic plasticity in output neurons of the Drosophila mushroom body γ-lobe. Sci Rep 12: 10421. 10.1038/s41598-022-14413-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler A, Graham TGW, Cohn R, Morantte I, Siliciano AF, Zeng J, Li Y, Ruta V. 2019. Distinct dopamine receptor pathways underlie the temporal sensitivity of associative learning. Cell 178: 60–75.e19. 10.1016/j.cell.2019.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori D, Aso Y, Swartz KJ, Rubin GM, Abbott LF, Axel R. 2017. Representations of novelty and familiarity in a mushroom body compartment. Cell 169: 956–969.e17. 10.1016/j.cell.2017.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Greene W, Kandel ER. 1998. Classical conditioning, differential conditioning, and second-order conditioning of the Aplysia gill-withdrawal reflex in a simplified mantle organ preparation. Behav Neurosci 112: 636–645. 10.1037/0735-7044.112.3.636 [DOI] [PubMed] [Google Scholar]
- Hige T. 2018. What can tiny mushrooms in fruit flies tell us about learning and memory? Neurosci Res 129: 8–16. 10.1016/j.neures.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Hige T, Aso Y, Modi MN, Rubin GM, Turner GC. 2015. Heterosynaptic plasticity underlies aversive olfactory learning in Drosophila. Neuron 88: 985–998. 10.1016/j.neuron.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger KS, Campbell RAA, Turner GC. 2011. Cellular-resolution population imaging reveals robust sparse coding in the Drosophila mushroom body. J Neurosci 31: 11772–11785. 10.1523/JNEUROSCI.1099-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang P, Xie Z, Wang L, Zhong Y. 2013. The differential requirement of mushroom body α/β subdivisions in long-term memory retrieval in Drosophila. Protein Cell 4: 512–519. 10.1007/s13238-013-3035-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huetteroth W, Perisse E, Lin S, Klappenbach M, Burke C, Waddell S. 2015. Sweet taste and nutrient value subdivide rewarding dopaminergic neurons in Drosophila. Curr Biol 25: 751–758. 10.1016/j.cub.2015.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Aso Y, Yamagata N, Abe A, Rubin GM, Tanimoto H. 2015. Reward signal in a recurrent circuit drives appetitive long-term memory formation. Elife 4: e10719. 10.7554/eLife.10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Kanno M, Wu H, Yamagata N, Sun H, Abe A, Tanimoto H. 2021. Mushroom body output differentiates memory processes and distinct memory-guided behaviors. Curr Biol 31: 1294–1302.e4. 10.1016/j.cub.2020.12.032 [DOI] [PubMed] [Google Scholar]
- Iigaya K, Ahmadian Y, Sugrue LP, Corrado GS, Loewenstein Y, Newsome WT, Fusi S. 2019. Deviation from the matching law reflects an optimal strategy involving learning over multiple timescales. Nat Commun 10: 1466. 10.1038/s41467-019-09388-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabel G, Pascual A, Preat T. 2004. Exclusive consolidated memory phases in Drosophila. Science 304: 1024–1027. 10.1126/science.1094932 [DOI] [PubMed] [Google Scholar]
- Jacob PF, Waddell S. 2020. Spaced training forms complementary long-term memories of opposite valence in Drosophila. Neuron 106: 977–991.e4. 10.1016/j.neuron.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Litwin-Kumar A. 2021. Models of heterogeneous dopamine signaling in an insect learning and memory center. PLoS Comput Biol 17: e1009205. 10.1371/journal.pcbi.1009205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Ohta K, Okanoya K, Kazama H. 2023. Dopaminergic neurons dynamically update sensory values during olfactory maneuver. Cell Rep 42: 113122. 10.1016/j.celrep.2023.113122 [DOI] [PubMed] [Google Scholar]
- Keleman K, Vrontou E, Krüttner S, Yu JY, Kurtovic-Kozaric A, Dickson BJ. 2012. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature 489: 145–149. 10.1038/nature11345 [DOI] [PubMed] [Google Scholar]
- Kim HF, Hikosaka O. 2013. Distinct basal ganglia circuits controlling behaviors guided by flexible and stable values. Neuron 79: 1001–1010. 10.1016/j.neuron.2013.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HF, Ghazizadeh A, Hikosaka O. 2014. Separate groups of dopamine neurons innervate caudate head and tail encoding flexible and stable value memories. Front Neuroanat 8: 120. 10.3389/fnana.2014.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König C, Khalili A, Niewalda T, Gao S, Gerber B. 2019. An optogenetic analogue of second-order reinforcement in Drosophila. Biol Lett 15: 20190084. 10.1098/rsbl.2019.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Waddell S. 2008. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci 28: 3103–3113. 10.1523/JNEUROSCI.5333-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. 2007. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron 53: 103–115. 10.1016/j.neuron.2006.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. 2009. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139: 416–427. 10.1016/j.cell.2009.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüttner S, Traunmüller L, Dag U, Jandrasits K, Stepien B, Iyer N, Fradkin LG, Noordermeer JN, Mensh BD, Keleman K. 2015. Synaptic Orb2A bridges memory acquisition and late memory consolidation in Drosophila. Cell Rep 11: 1953–1965. 10.1016/j.celrep.2015.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagasse F, Devaud J-M, Mery F. 2009. A switch from cycloheximide-resistant consolidated memory to cycloheximide-sensitive reconsolidation and extinction in Drosophila. J Neurosci 29: 2225–2230. 10.1523/JNEUROSCI.3789-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Lee A, Luo L. 1999. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development 126: 4065–4076. 10.1242/dev.126.18.4065 [DOI] [PubMed] [Google Scholar]
- Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, Zalocusky KA, Crow AK, Malenka RC, Luo L, Tomer R, et al. 2015. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162: 635–647. 10.1016/j.cell.2015.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Lindsey JW, Marin EC, Otto N, Dreher M, Dempsey G, Stark I, Bates AS, Pleijzier MW, Schlegel P, et al. 2020. The connectome of the adult Drosophila mushroom body provides insights into function. Elife 9: e62576. 10.7554/eLife.62576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WW, Wilson RI. 2013. Glutamate is an inhibitory neurotransmitter in the Drosophila olfactory system. Proc Natl Acad Sci 110: 10294–10299. 10.1073/pnas.1220560110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Plaçais P-Y, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. 2012. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 488: 512–516. 10.1038/nature11304 [DOI] [PubMed] [Google Scholar]
- Liu TX, Davoudian PA, Lizbinski KM, Jeanne JM. 2022. Connectomic features underlying diverse synaptic connection strengths and subcellular computation. Curr Biol 32: 559–569.e5. 10.1016/j.cub.2021.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyutova R, Selcho M, Pfeuffer M, Segebarth D, Habenstein J, Rohwedder A, Frantzmann F, Wegener C, Thum AS, Pauls D. 2019. Reward signaling in a recurrent circuit of dopaminergic neurons and peptidergic Kenyon cells. Nat Commun 10: 3097. 10.1038/s41467-019-11092-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. 2009. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459: 837–841. 10.1038/nature08028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy LY, Sareen P, Davoudian PA, Nitabach MN. 2021. Dopaminergic mechanism underlying reward-encoding of punishment omission during reversal learning in Drosophila. Nat Commun 12: 1115. 10.1038/s41467-021-21388-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegas W, Akiti K, Amo R, Uchida N, Watabe-Uchida M. 2018. Dopamine neurons projecting to the posterior striatum reinforce avoidance of threatening stimuli. Nat Neurosci 21: 1421–1430. 10.1038/s41593-018-0222-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier D, Tsuchimoto Y, Ohta K, Kazama H. 2018. Olfactory landmark-based communication in interacting Drosophila. Curr Biol 28: 2624–2631.e5. 10.1016/j.cub.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Mizunami M, Unoki S, Mori Y, Hirashima D, Hatano A, Matsumoto Y. 2009. Roles of octopaminergic and dopaminergic neurons in appetitive and aversive memory recall in an insect. BMC Biol 7: 46. 10.1186/1741-7007-7-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso P-Y, TchEnio P, Preat T. 2015. Delayed dopamine signaling of energy level builds appetitive long-term memory in Drosophila. Cell Rep 10: 1023–1031. 10.1016/j.celrep.2015.01.036 [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. 2000. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406: 722–726. 10.1038/35021052 [DOI] [PubMed] [Google Scholar]
- Noyes NC, Davis RL. 2023. Innate and learned odor-guided behaviors utilize distinct molecular signaling pathways in a shared dopaminergic circuit. Cell Rep 42: 112026. 10.1016/j.celrep.2023.112026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto N, Pleijzier MW, Morgan IC, Edmondson-Stait AJ, Heinz KJ, Stark I, Dempsey G, Ito M, Kapoor I, Hsu J, et al. 2020. Input connectivity reveals additional heterogeneity of dopaminergic reinforcement in Drosophila. Curr Biol 30: 3200–3211.e8. 10.1016/j.cub.2020.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Waddell S. 2015. Olfactory learning skews mushroom body output pathways to steer behavioral choice in Drosophila. Curr Opin Neurobiol 35: 178–184. 10.1016/j.conb.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Felsenberg J, Talbot CB, Das G, Perisse E, Huetteroth W, Waddell S. 2015. Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron 86: 417–427. 10.1016/j.neuron.2015.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani MR, Oishi K, Gelb BD, Zhong Y. 2009. The phosphatase SHP2 regulates the spacing effect for long-term memory induction. Cell 139: 186–198. 10.1016/j.cell.2009.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai T-P, Chen C-C, Lin H-H, Chin A-L, Lai JS-Y, Lee P-T, Tully T, Chiang A-S. 2013. Drosophila ORB protein in two mushroom body output neurons is necessary for long-term memory formation. Proc Natl Acad Sci 110: 7898–7903. 10.1073/pnas.1216336110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlowsky A, Schor J, Plaçais P-Y, Preat T. 2018. A GABAergic feedback shapes dopaminergic input on the Drosophila mushroom body to promote appetitive long-term memory. Curr Biol 28: 1783–1793.e4. 10.1016/j.cub.2018.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisse E, Yin Y, Lin AC, Lin S, Huetteroth W, Waddell S. 2013. Different Kenyon cell populations drive learned approach and avoidance in Drosophila. Neuron 79: 945–956. 10.1016/j.neuron.2013.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisse E, Owald D, Barnstedt O, Talbot CB, Huetteroth W, Waddell S. 2016. Aversive learning and appetitive motivation toggle feed-forward inhibition in the Drosophila mushroom body. Neuron 90: 1086–1099. 10.1016/j.neuron.2016.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaçais P-Y, Trannoy S, Friedrich AB, Tanimoto H, Preat T. 2013. Two pairs of mushroom body efferent neurons are required for appetitive long-term memory retrieval in Drosophila. Cell Rep 5: 769–780. 10.1016/j.celrep.2013.09.032 [DOI] [PubMed] [Google Scholar]
- Qin H, Cressy M, Li W, Coravos JS, Izzi SA, Dubnau J. 2012. γ Neurons mediate dopaminergic input during aversive olfactory memory formation in Drosophila. Curr Biol 22: 608–614. 10.1016/j.cub.2012.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte ME, Griffin RW, Sisk CL. 1977. Second-order conditioning of the pigeon's keypeck. Anim Learn Behav 5: 25–38. 10.3758/BF03209127 [DOI] [Google Scholar]
- Rizley RC, Rescorla RA. 1972. Associations in second-order conditioning and sensory preconditioning. J Comp Physiol Psychol 81: 1–11. 10.1037/h0033333 [DOI] [PubMed] [Google Scholar]
- Sanfilippo P, Kim AJ, Bhukel A, Yoo J, Mirshahidi PS, Pandey V, Bevir H, Yuen A, Mirshahidi PS, Guo P, et al. 2023. Mapping of multiple neurotransmitter receptor subtypes and distinct protein complexes to the connectome. bioRxiv 10.1101/2023.10.02.560011v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. 2000. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci 20: RC96. 10.1523/JNEUROSCI.20-18-j0003.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer LK, Xu CS, Januszewski M, Lu Z, Takemura S, Hayworth KJ, Huang GB, Shinomiya K, Maitlin-Shepard J, Berg S, et al. 2020. A connectome and analysis of the adult Drosophila central brain. Elife 9: e57443. 10.7554/eLife.57443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer M, Huang C, Luo J, Woo SJ, Roitman L, Li J, Pieribone V, Kannan M, Vasan G. 2022. Dopamine signals integrate innate and learnt valences to regulate memory dynamics. Res Square 10.21203/rs.3.rs-1915648/v1 [DOI] [Google Scholar]
- Schwaerzel M, Heisenberg M, Zars T. 2002. Extinction antagonizes olfactory memory at the subcellular level. Neuron 35: 951–960. 10.1016/S0896-6273(02)00832-2 [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. 2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci 23: 10495–10502. 10.1523/JNEUROSCI.23-33-10495.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai Y, Lu B, Hu Y, Wang L, Sun K, Zhong Y. 2010. Forgetting is regulated through Rac activity in Drosophila. Cell 140: 579–589. 10.1016/j.cell.2009.12.044 [DOI] [PubMed] [Google Scholar]
- Siegel RW, Hall JC. 1979. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci 76: 3430–3434. 10.1073/pnas.76.7.3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siju KP, Stih V, Aimon S, Gjorgjieva J, Portugues R, Grunwald Kadow IC. 2020. Valence and state-dependent population coding in dopaminergic neurons in the fly mushroom body. Curr Biol 30: 2104–2115.e4. 10.1016/j.cub.2020.04.037 [DOI] [PubMed] [Google Scholar]
- Springer M, Nawrot MP. 2021. A mechanistic model for reward prediction and extinction learning in the fruit fly. eNeuro 8: ENEURO.0549-20.2021. 10.1523/ENEURO.0549-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Zhou J, Dai B, Qian T, Zeng J, Li X, Zhuo Y, Zhang Y, Wang Y, Qian C, et al. 2020. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat Methods 17: 1156–1166. 10.1038/s41592-020-00981-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabone CJ, de Belle JS. 2011. Second-order conditioning in Drosophila. Learn Mem 18: 250–253. 10.1101/lm.2035411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura S-Y, Aso Y, Hige T, Wong A, Lu Z, Xu CS, Rivlin PK, Hess H, Zhao T, Parag T, et al. 2017. A connectome of a learning and memory center in the adult Drosophila brain. Elife 6: 5643. 10.7554/eLife.26975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka NK, Tanimoto H, Ito K. 2008. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol 508: 711–755. 10.1002/cne.21692 [DOI] [PubMed] [Google Scholar]
- Tempel BL, Bonini N, Dawson DR, Quinn WG. 1983. Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci 80: 1482–1486. 10.1073/pnas.80.5.1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trannoy S, Redt-Clouet C, Dura J-M, Preat T. 2011. Parallel processing of appetitive short- and long-term memories in Drosophila. Curr Biol 21: 1647–1653. 10.1016/j.cub.2011.08.032 [DOI] [PubMed] [Google Scholar]
- Tsao C-H, Chen C-C, Lin C-H, Yang H-Y, Lin S. 2018. Drosophila mushroom bodies integrate hunger and satiety signals to control innate food-seeking behavior. Elife 7: e35264. 10.7554/eLife.35264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. 1994. Genetic dissection of consolidated memory in Drosophila. Cell 79: 35–47. 10.1016/0092-8674(94)90398-0 [DOI] [PubMed] [Google Scholar]
- Turner GC, Bazhenov M, Laurent G. 2008. Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol 99: 734–746. 10.1152/jn.01283.2007 [DOI] [PubMed] [Google Scholar]
- Ueoka Y, Hiroi M, Abe T, Tabata T. 2017. Suppression of a single pair of mushroom body output neurons in Drosophila triggers aversive associations. FEBS Open Bio 7: 562–576. 10.1002/2211-5463.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar ME, Pavão-Delgado M, Amigo M, Jacob PF, Merabet N, Pinot A, Perry SA, Waddell S, Perisse E. 2022. Differential coding of absolute and relative aversive value in the Drosophila brain. Curr Biol 32: 4576–4592.e5. 10.1016/j.cub.2022.08.058 [DOI] [PubMed] [Google Scholar]
- Widmer YF, Fritsch C, Jungo MM, Almeida S, Egger B, Sprecher SG. 2018. Multiple neurons encode CrebB dependent appetitive long-term memory in the mushroom body circuit. Elife 7: 16699. 10.7554/eLife.39196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J-K, Tai C-Y, Feng K-L, Chen S-L, Chen C-C, Chiang A-S. 2017. Long-term memory requires sequential protein synthesis in three subsets of mushroom body output neurons in Drosophila. Sci Rep 7: 7112. 10.1038/s41598-017-07600-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada D, Hige T. 2023. Cyclic nucleotide-induced bidirectional long-term synaptic plasticity in Drosophila mushroom body. bioRxiv 10.1101/2023.09.28.560058v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada D, Bushey D, Li F, Hibbard KL, Sammons M, Funke J, Litwin-Kumar A, Hige T, Aso Y. 2023. Hierarchical architecture of dopaminergic circuits enables second-order conditioning in Drosophila. Elife 12: e79042. 10.7554/eLife.79042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata N, Ichinose T, Aso Y, Plaçais P-Y, Friedrich AB, Sima RJ, Preat T, Rubin GM, Tanimoto H. 2015. Distinct dopamine neurons mediate reward signals for short- and long-term memories. Proc Natl Acad Sci 112: 578–583. 10.1073/pnas.1421930112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Chen N, Zhang S, Guo A. 2009. Choice strategies in Drosophila are based on competition between olfactory memories. Eur J Neurosci 30: 279–288. 10.1111/j.1460-9568.2009.06821.x [DOI] [PubMed] [Google Scholar]
- Yu D, Akalal D-BG, Davis RL. 2006. Drosophila α/β mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron 52: 845–855. 10.1016/j.neuron.2006.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Dou Y-N, Sun Y-G. 2019. Topography of reward and aversion encoding in the mesolimbic dopaminergic system. J Neurosci 39: 6472–6481. 10.1523/JNEUROSCI.0271-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Li X, Zhang R, Lv M, Wang Y, Tan K, Xia X, Wan J, Jing M, Zhang X, et al. 2023. Local 5-HT signaling bi-directionally regulates the coincidence time window for associative learning. Neuron 111: 1118–1135.e5. 10.1016/j.neuron.2022.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Lenek D, Dag U, Dickson BJ, Keleman K. 2018. Persistent activity in a recurrent circuit underlies courtship memory in Drosophila. Elife 7: e04577. 10.7554/eLife.31425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Widmer YF, Diegelmann S, Petrovici MA, Sprecher SG, Senn W. 2021. Predictive olfactory learning in Drosophila. Sci Rep 11: 6795. 10.1038/s41598-021-85841-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolin A, Cohn R, Pang R, Siliciano AF, Fairhall AL, Ruta V. 2021. Context-dependent representations of movement in Drosophila dopaminergic reinforcement pathways. Nat Neurosci 24: 1555–1566. 10.1038/s41593-021-00929-y [DOI] [PMC free article] [PubMed] [Google Scholar]