Abstract
Objective
To assess the effect of different antiplatelet strategies on clinical outcomes after coronary artery bypass grafting.
Design
Five year follow-up of randomised Different Antiplatelet Therapy Strategy After Coronary Artery Bypass Grafting (DACAB) trial.
Setting
Six tertiary hospitals in China; enrolment between July 2014 and November 2015; completion of five year follow-up from August 2019 to June 2021.
Participants
500 patients aged 18-80 years (including 91 (18.2%) women) who had elective coronary artery bypass grafting surgery and completed the DACAB trial.
Interventions
Patients were randomised 1:1:1 to ticagrelor 90 mg twice daily plus aspirin 100 mg once daily (dual antiplatelet therapy; n=168), ticagrelor monotherapy 90 mg twice daily (n=166), or aspirin monotherapy 100 mg once daily (n=166) for one year after surgery. After the first year, antiplatelet therapy was prescribed according to standard of care by treating physicians.
Main outcome measures
The primary outcome was major adverse cardiovascular events (a composite of all cause death, myocardial infarction, stroke, and coronary revascularisation), analysed using the intention-to-treat principle. Time-to-event analysis was used to compare the risk between treatment groups. Multiple post hoc sensitivity analyses examined the robustness of the findings.
Results
Follow-up at five years for major adverse cardiovascular events was completed for 477 (95.4%) of 500 patients; 148 patients had major adverse cardiovascular events, including 39 in the dual antiplatelet therapy group, 54 in the ticagrelor monotherapy group, and 55 in the aspirin monotherapy group. Risk of major adverse cardiovascular events at five years was significantly lower with dual antiplatelet therapy versus aspirin monotherapy (22.6% v 29.9%; hazard ratio 0.65, 95% confidence interval 0.43 to 0.99; P=0.04) and versus ticagrelor monotherapy (22.6% v 32.9%; 0.66, 0.44 to 1.00; P=0.05). Results were consistent in all sensitivity analyses.
Conclusions
Treatment with ticagrelor dual antiplatelet therapy for one year after surgery reduced the risk of major adverse cardiovascular events at five years after coronary artery bypass grafting compared with aspirin monotherapy or ticagrelor monotherapy.
Trial registration
NCT03987373ClinicalTrials.gov NCT03987373.
Introduction
Aspirin monotherapy is recommended after coronary artery bypass graft surgery to improve graft patency and reduce major adverse cardiovascular events.1 2 3 4 Findings from randomised and observational studies suggest that dual antiplatelet therapy is more effective than aspirin monotherapy in preventing saphenous vein graft failure.5 6 7 8 9 However, the evidence is inconclusive with regard to the effect of dual antiplatelet therapy on clinical outcomes,4 7 10 11 12 13 14 15 16 17 18 and current clinical guidelines recommend dual antiplatelet therapy only in selected patients at high ischaemic risk after coronary artery bypass grafting.3 19 20 21 22
The Different Antiplatelet Therapy Strategy After Coronary Artery Bypass Graft Surgery (DACAB)23 trial was a multicentre, randomised, open label trial comparing ticagrelor dual antiplatelet therapy, ticagrelor monotherapy, and aspirin monotherapy for one year after coronary artery bypass grafting. Vein graft patency (primary outcome) at one year was significantly higher in patients randomised to dual antiplatelet therapy compared with patients randomised to aspirin monotherapy (88.7% v 76.5%; P<0.001) and numerically higher compared with patients randomised to ticagrelor monotherapy (88.7% v 82.8%; P=0.068).
The DACAB trial was not powered for clinical outcomes. At one year after coronary artery bypass grafting, the rate of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) was 1.8% in the dual antiplatelet therapy group, 2.4% in the ticagrelor monotherapy group, and 5.4% in the aspirin monotherapy group. The rate of major bleeding was 1.8% in the dual antiplatelet therapy group and 1.2% in the ticagrelor monotherapy group; no major bleeding event was reported in the aspirin monotherapy group.
We have now extended the clinical follow-up of patients included in the DACAB trial to five years (DACAB-Follow-up Extension (DACAB-FE) study), with the aim of investigating the effect of different antiplatelet strategies on clinical outcomes at five years after coronary artery bypass grafting.
Methods
Study design
The DACAB trial was a prospective, multicentre, open label, evaluator blind, randomised, controlled trial that compared the effect of dual antiplatelet therapy with ticagrelor plus aspirin, ticagrelor monotherapy, and aspirin monotherapy on saphenous vein graft patency in patients aged 18 to 80 years who had elective coronary artery bypass graft surgery in six Chinese tertiary hospitals.23 The study design and full inclusion/exclusion criteria have been described previously.23 Briefly, eligible patients were enrolled from 31 July 2014 through 3 November 2015 and randomised 1:1:1 to ticagrelor 90 mg twice daily plus aspirin 100 mg once daily (dual antiplatelet therapy), ticagrelor monotherapy 90 mg twice daily, or aspirin monotherapy 100 mg once daily for one year after coronary artery bypass graft surgery.23 After the first year, antiplatelet therapy was based on the recommendation of the individual treating physician (including aspirin monotherapy, adenosine diphosphate receptor inhibitor monotherapy (ticagrelor or clopidogrel), and dual antiplatelet therapy).
All patients in the DACAB trial were included in the DACAB-FE study. This study was conducted in accordance with the Declaration of Helsinki and Guidelines for Good Clinical Practice.
Data collection
Annual clinical follow-up at local sites was recommended according to clinical status. A face-to-face or telephone visit with the central clinical trial unit was scheduled for all surviving patients at five years (within three months either side) after coronary artery bypass grafting, at which the original medical records of clinical events, related laboratory test results, electrocardiograms, and ultrasonic cardiography results and the use of concomitant medications were collected. Electrocardiograms were read centrally by the Clinical Endpoint Committee, which independently adjudicated the outcome. For deceased patients, the cause of death was adjudicated using the national China Centre for Disease Control database.
Outcomes and endpoints
The primary outcome was major adverse cardiovascular events (a composite of all cause death, myocardial infarction, stroke, and coronary revascularisation). Secondary outcomes included an extended major adverse cardiovascular events outcome (a composite of all cause death, myocardial infarction, stroke, coronary revascularisation, and hospital admission for unstable angina) and a restricted major adverse cardiovascular events outcome (a composite of cardiovascular death, myocardial infarction, and stroke), as well as their individual components. All outcomes were measured at the time to first occurrence from randomisation to last visit. Detailed outcome definitions are provided in the supplementary appendix.
The safety outcome, analysed post hoc, was major bleedings events based on the Thrombolysis in Myocardial Infarction (TIMI) risk criteria (combination of coronary artery bypass grafting related bleeding and non-coronary artery bypass grafting related major bleeding, such as intracranial bleeding, clinically overt signs of haemorrhage with haemoglobin drop ≥5 g/dL, and fatal bleeding). The secondary safety outcome was serious adverse events resulting in hospital admission or emergency department visits. We added net adverse clinical events, a composite of major adverse cardiovascular events and major bleeding events, as a post hoc outcome.
Statistical analysis
The sample size for the DACAB trial was based on a calculated statistical power of 80% to detect a significant difference in the primary outcome of vein graft patency.23 For the DACAB-FE study, we did no formal power calculation. In the DACAB-FE primary analysis, patients were analysed according to randomised treatment groups in the DACAB trial (intention to treat).
We summarised baseline characteristics by using counts and percentages for discrete variables and mean with standard deviation and median with interquartile range for continuous variables. We reported outcomes as frequencies, incidence per 1000 patient months, and cumulative incidence.
We used Kaplan-Meier curves to describe the event-free survival in the groups and univariate Cox regression models to compare the risk of outcomes between treatment groups. We tested the Cox proportionality assumption by means of scaled Schoenfeld residuals. We reported estimates as hazard ratios and 95% confidence intervals. We used competing risk analysis with a Fine-Gray framework for all outcomes except major adverse cardiovascular events, extended major adverse cardiovascular events, and all cause death.24 Patients with no events were right censored at their last visit. For patients who were lost to follow-up, we used the date of the last visit or the most recent known clinical outcome in the primary analysis.
We used a univariate Cox regression model to analyse treatment effects in key patient subgroups (age group; sex; coronary syndrome status; history of myocardial infarction, hypertension, diabetes, hyperlipidaemia, and peripheral arterial disease; pump use; and bleeding risk based on the CRUSADE score25). Additional subgroup analyses were based on history of stroke, chronic pulmonary disease, chronic kidney disease, smoking, SYNTAX score, EuroSCORE, Lp(a) lipoprotein concentrations, left main disease, internal mammary artery use, and completeness of revascularisation. We tested treatment effect modification by using interaction terms.
Sensitivity analyses
We did multiple post hoc sensitivity analyses. To evaluate the effect of one year of dual antiplatelet therapy on late events, we did landmark analysis starting at one year after randomisation by using univariate Cox proportional hazard models. We did this analysis in participants who remained in the trial and had not had a primary endpoint event during the first year. We repeated the main analysis including only patients who received the planned dose of study drug without interruption for more than 60 days during the first year (per protocol).
In addition, we did two as-treated analyses. In the first analysis, we compared patients on the basis of the antiplatelet treatment that they received in year 1 and in years 2-5 by using an adjusted multivariable Cox regression model. We identified the covariates included in the model by using a causal directed acylic graph (supplementary figure S1). Considering the limitations of conditional models and the likelihood of reverse causation bias, we fitted a marginal structural model through stable inverse probability weighting in the second analysis.26 We divided the entire follow-up period into seven time segments (0-2 months, 2-4 months, 4-6 months, 6-8 months, 8-10 months, 10-12 months, and 12-60 months) and regarded antiplatelet treatment at different time segments as time dependent exposure. The covariates included in the effect estimation were the same as in the first as-treated analysis (see as-treated analysis methods section of supplementary appendix for more details).
In another post hoc analysis, we compared major adverse cardiovascular events, all cause death, cardiovascular death, and myocardial infarction between patients with all patent grafts and patients with at least one failed graft at one year imaging. To investigate a potential dependency of the treatment effect by the enrolling centres, we used a multivariable Cox regression model with an interaction term between treatment and study centre.
We applied a two sided significance level of 0.05 to all statistical analyses without multiplicity adjustment. We used SAS version 9.4 for statistical analyses.
Patient and public involvement
Although patients and the public were not directly involved in this paper owing to the lack of funding and covid-19, we spoke to patients about the study and asked one representative of the patients to read the manuscript and give us advice before submission.
Results
Patient characteristics
The primary analysis included 500 patients whose demographics and baseline characteristics have been previously published.23 Among these patients, 168 received dual antiplatelet therapy during the first year after coronary artery bypass grafting, 166 received ticagrelor monotherapy, and 166 received aspirin monotherapy. Most of the patients enrolled were men (81.8%), and the mean age was 63.1 years. Table 1 summarises the baseline characteristics of the included patients.
Table 1.
Demographic and baseline characteristics of included patients. Values are numbers (percentages) unless stated otherwise
| Characteristic | Overall (n=500) | Ticagrelor plus aspirin (n=168) | Ticagrelor monotherapy (n=166) | Aspirin monotherapy (n=166) |
|---|---|---|---|---|
| Mean (SD) age, years | 63.1 (8.2) | 63.0 (8.1) | 62.9 (8) | 63.5 (8.1) |
| Sex: | ||||
| Male | 409 (81.8) | 134 (80) | 134 (81) | 141 (85) |
| Female | 91 (18.2) | 34 (20) | 32 (19) | 25 (15) |
| Mean (SD) body mass index | 25.2 (3.3) | 25.3 (3.2) | 25.2 (3.0) | 25.2 (3.6) |
| Coronary syndrome status: | ||||
| Chronic coronary syndrome | 168 (33.6) | 55 (33) | 63 (38) | 50 (30) |
| Acute coronary syndrome | 332 (66.4) | 113 (67) | 103 (62) | 116 (70) |
| Medical history: | ||||
| Myocardial infarction | 156 (31.2) | 53 (32) | 60 (36) | 43 (26) |
| Stroke | 61 (12.2) | 26 (15) | 13 (8) | 22 (13) |
| Hypertension* | 369 (73.8) | 127 (76) | 122 (73) | 120 (72) |
| Diabetes† | 217 (43.4) | 75 (45) | 75 (45) | 67 (40) |
| Hyperlipidaemia‡ | 364 (72.8) | 121 (72) | 124 (75) | 119 (72) |
| Smoking | 246 (49.2) | 85 (51) | 74 (45) | 87 (52) |
| Peripheral artery disease | 82 (16.4) | 26 (15) | 27 (16) | 29 (17) |
| Chronic kidney disease§ | 89 (17.8) | 31 (18) | 25 (15) | 33 (20) |
| Median (IQR) LVEF, % | 62.0 (57.0-67.0) | 61.0 (56.0-67.0) | 62.0 (58.0-66.0) | 63.0 (56.0-68.0) |
| Pump use | 121 (24.2) | 39 (23) | 36 (22) | 46 (28) |
| IMA graft use | 418 (83.6) | 141 (84) | 144 (87) | 133 (80) |
IMA=internal mammary artery; IQR=interquartile range; LVEF=left ventricular ejection fraction; SD=standard deviation.
Defined as systolic/diastolic blood pressure ≥140/90 mm Hg.
Defined as glycated haemoglobin (HbA1c) >6.5%.
Defined as baseline low density lipoprotein cholesterol >1.8 mmol/L with or without statin therapy.
Defined as baseline serum creatinine >100 μmol/L.
Follow-up
The first five year visit occurred in August 2019 and the last in June 2021. Completeness of follow-up for the primary outcome of major adverse cardiovascular events at five years was 95.4% (477/500 patients; fig 1). Median follow-up time was 61.1 months (supplementary table S1). For the five year assessment, 197 (39.4%) patients were followed up in person and 280 (56.0%) by telephone interview. Electrocardiographic or echocardiographic assessment was obtained in 428 (85.6%) patients at one year and in 402 (80.4%) patients at five years.
Fig 1.
Flow of patients through Different Antiplatelet Therapy Strategy After Coronary Artery Bypass Grafting (DACAB) trial and DACAB follow-up extension study
Antiplatelet and other concomitant medications
Within the first year from randomisation, 17 (3.4%) patients discontinued the allocated study drug for longer than 60 days, of whom five (3%) had been randomised to dual antiplatelet therapy, eight (5%) to ticagrelor monotherapy, and four (2%) to aspirin monotherapy. At the five year follow-up, 324 (64.8%) of 500 patients were receiving aspirin monotherapy: 103 (61%) in the dual antiplatelet therapy group, 102 (61%) in the ticagrelor monotherapy group, and 119 (72%) in the aspirin monotherapy group. Full details of concomitant medications at one year and at five years are shown in supplementary figure S2 and supplementary tables S2 and S3.
Primary outcome
Major adverse cardiovascular events occurred in 148 patients: 39 in the dual antiplatelet therapy group, 54 in the ticagrelor monotherapy group, and 55 in the aspirin monotherapy group. At the five year follow-up, the rate of major adverse cardiovascular events was 22.6% in the dual antiplatelet therapy group, 32.9% in the ticagrelor monotherapy group, and 29.9% in the aspirin monotherapy group (table 2). Patients in the dual antiplatelet therapy group had a significantly lower risk of major adverse cardiovascular events compared with patients in the aspirin monotherapy group (hazard ratio 0.65, 95% confidence interval 0.43 to 0.99; P=0.04) and in the ticagrelor monotherapy group (0.66, 0.44 to 1.00; P=0.05); we found no significant difference between patients in the ticagrelor monotherapy group and those in the aspirin monotherapy group (0.99, 0.68 to 1.44; P=0.97) (table 3 and fig 2). The global Schoenfeld residuals test verified the Cox proportionality assumption (P=0.63).
Table 2.
Incidence of primary and secondary outcomes in randomised treatment groups
| Outcome | Ticagrelor plus aspirin (n=168) | Ticagrelor monotherapy (n=166) | Aspirin monotherapy (n=166) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No (%) with events | Events per 1000 patient months | Cumulative incidence at 5 years—% (95% CI) | No (%) with events | Events per 1000 patient months | Cumulative incidence at 5 years—% (95% CI) | No (%) with events | Events per 1000 patient months | Cumulative incidence at 5 years—% (95% CI) | |||
| MACE* | 39 (23) | 4.5 | 22.6 (16.9 to 29.8) | 54 (33) | 7.0 | 32.9 (26.2 to 40.7) | 55 (33) | 7.0 | 29.9 (23.5 to 37.6) | ||
| 0-1 year | 17 (10) | 9.2 | 10.1 (6.4 to 15.8) | 28 (17) | 16.5 | 16.9 (12.0 to 23.6) | 29 (17) | 17.2 | 17.5 (12.5 to 24.2) | ||
| 2-5 years† | 22 (15) | 3.2 | 17.1 (11.2 to 25.5) | 26 (20) | 4.3 | 21.7 (14.8 to 31.1) | 26 (19) | 4.2 | 32.9 (20.1 to 50.8) | ||
| Extended MACE‡ | 39 (23) | 4.5 | 22.6 (16.9 to 29.8) | 55 (33) | 7.1 | 32.9 (26.2 to 40.7) | 56 (34) | 7.2 | 30.5 (24.1 to 38.2) | ||
| Restricted MACE§ | 27 (16) | 3.0 | 15.8 (11.1 to 22.4) | 44 (27) | 5.5 | 26.2 (20.2 to 33.8) | 45 (27) | 5.5 | 26.6 (20.6 to 34.3) | ||
| All cause mortality | 16 (10) | 1.7 | 9.3 (5.7 to 15.0) | 11 (7) | 1.1 | 6.3 (3.5 to 11.4) | 12 (7) | 1.2 | 6.2 (3.4 to 11.1) | ||
| CV mortality | 9 (5) | 0.9 | 5.1 (2.7 to 9.8) | 6 (4) | 0.6 | 3.4 (1.5 to 7.5) | 7 (4) | 0.7 | 4.0 (1.9 to 8.3) | ||
| MI | 18 (11) | 1.9 | 10.9 (7.0 to 16.9) | 36 (22) | 4.4 | 21.8 (16.3 to 29.2) | 32 (19) | 3.7 | 19.6 (14.3 to 26.7) | ||
| Non-silent MI | 10 (6) | 1.0 | 6.0 (3.3 to 11.1) | 14 (8) | 1.5 | 8.5 (5.1 to 14.1) | 16 (10) | 1.7 | 9.7 (6.1 to 15.5) | ||
| Silent MI | 9 (5) | 0.9 | 5.4 (2.9 to 10.2) | 22 (13) | 2.5 | 13.5 (9.1 to 19.9) | 18 (11) | 1.9 | 10.5 (6.7 to 16.5) | ||
| Stroke | 6 (4) | 0.6 | 3.5 (1.6 to 7.6) | 6 (4) | 0.6 | 3.4 (1.6 to 7.6) | 15 (9) | 1.5 | 8.7 (5.3 to 14.2) | ||
| Ischaemic | 1 (1) | 0.1 | 0.6 (0.1 to 3.9) | 5 (3) | 0.5 | 2.7 (1.1 to 6.6) | 12 (7) | 1.2 | 6.6 (3.8 to 11.7) | ||
| Haemorrhagic | 5 (3) | 0.5 | 3.1 (1.3 to 7.3) | 1 (1) | 0.1 | 0.6 (0.1 to 4.4) | 3 (2) | 0.3 | 1.9 (0.6 to 5.7) | ||
| Coronary revascularisation | 7 (4) | 0.7 | 3.7 (1.7 to 7.7) | 8 (5) | 0.8 | 4.1 (2.0 to 8.3) | 10 (6) | 1.0 | 5.2 (2.8 to 9.8) | ||
| Hospital admission for unstable angina | 4 (2) | 0.4 | 2.2 (0.8 to 6.0) | 8 (5) | 0.8 | 4.5 (2.2 to 8.9) | 8 (5) | 0.8 | 4.5 (2.3 to 9.0) | ||
| Major bleeding | 8 (5) | 0.8 | 4.9 (2.5 to 9.6) | 4 (2) | 0.4 | 2.5 (0.9 to 6.5) | 7 (4) | 0.7 | 4.3 (2.1 to 8.9) | ||
| NACE ¶ | 42 (25) | 5.0 | 24.4 (18.5 to 31.8) | 55 (33) | 7.2 | 33.5 (26.8 to 41.4) | 58 (35) | 7.6 | 31.7 (25.2 to 39.5) | ||
CI=confidence interval; CV=cardiovascular; MACE=major adverse cardiac events; MI=myocardial infarction; NACE=net adverse clinical events.
Composite of all cause mortality, myocardial infarction, stroke, and coronary revascularisation.
Analysis population is risk population at beginning of second year: 151 in ticagrelor plus aspirin group, 133 in ticagrelor monotherapy group, and 134 in aspirin monotherapy group.
Composite of all cause mortality, myocardial infarction, stroke, coronary revascularisation, and hospital admission for unstable angina.
Composite of cardiovascular mortality, myocardial infarction, and stroke.
Composite of all cause mortality, myocardial infarction, stroke, coronary revascularisation, and major bleeding.
Table 3.
Risk comparison of primary and secondary outcomes between randomised treatment groups
| Outcome | Ticagrelor plus aspirin v aspirin monotherapy | Ticagrelor plus aspirin v ticagrelor monotherapy | Ticagrelor monotherapy v aspirin monotherapy | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| MACE* | 0.65 (0.43 to 0.99) | 0.04 | 0.66 (0.44 to 1.00) | 0.05 | 0.99 (0.68 to 1.44) | 0.97 | ||
| 0-1 year | 0.56 (0.31 to 1.01) | 0.05 | 0.58 (0.32 to 1.06) | 0.07 | 0.96 (0.57 to 1.61) | 0.88 | ||
| 2-5 years | 0.76 (0.43 to 1.34) | 0.34 | 0.74 (0.42 to 1.30) | 0.31 | 1.03 (0.60 to 1.77) | 0.91 | ||
| Extended MACE† | 0.64 (0.43 to 0.96) | 0.03 | 0.65 (0.43 to 0.98) | 0.04 | 0.99 (0.68 to 1.44) | 0.96 | ||
| Restricted MACE‡ | 0.56 (0.35 to 0.90) | 0.02 | 0.57 (0.35 to 0.91) | 0.02 | 0.98 (0.65 to 1.48) | 0.94 | ||
| All cause mortality | 1.35 (0.64 to 2.86) | 0.44 | 1.49 (0.69 to 3.21) | 0.30 | 0.91 (0.40 to 2.06) | 0.82 | ||
| CV mortality | 1.30 (0.49 to 3.49) | 0.60 | 1.52 (0.54 to 4.23) | 0.43 | 0.86 (0.29 to 2.53) | 0.78 | ||
| MI | 0.54 (0.30 to 0.95) | 0.03 | 0.47 (0.27 to 0.82) | 0.01 | 1.14 (0.71 to 1.82) | 0.58 | ||
| Non-silent MI | 0.62 (0.28 to 1.34) | 0.22 | 0.70 (0.32 to 1.56) | 0.39 | 0.88 (0.44 to 1.77) | 0.72 | ||
| Silent MI | 0.51 (0.23 to 1.13) | 0.10 | 0.38 (0.18 to 0.83) | 0.01 | 1.32 (0.70 to 2.49) | 0.39 | ||
| Stroke | 0.39 (0.15 to 1.00) | 0.05 | 0.99 (0.32 to 3.06) | 0.99 | 0.39 (0.15 to 1.00) | 0.05 | ||
| Ischaemic | 0.08 (0.01 to 0.62) | 0.02 | 0.20 (0.02 to 1.67) | 0.14 | 0.41 (0.15 to 1.16) | 0.09 | ||
| Haemorrhagic | 1.67 (0.40 to 6.98) | 0.48 | 4.99 (0.59 to 42.42) | 0.14 | 0.34 (0.04 to 3.22) | 0.34 | ||
| Coronary revascularisation | 0.69 (0.26 to 1.81) | 0.45 | 0.87 (0.32 to 2.39) | 0.78 | 0.80 (0.32 to 2.01) | 0.63 | ||
| Hospital admission for unstable angina | 0.49 (0.15 to 1.63) | 0.25 | 0.49 (0.15 to 1.61) | 0.24 | 1.01 (0.38 to 2.69) | 0.98 | ||
| Major bleeding | 1.14 (0.42 to 3.14) | 0.80 | 1.99 (0.60 to 6.61) | 0.26 | 0.57 (0.17 to 1.96) | 0.38 | ||
| NACE§ | 0.67 (0.45 to 1.00) | 0.05 | 0.70 (0.47 to 1.05) | 0.09 | 0.95 (0.66 to 1.38) | 0.80 | ||
CI=confidence interval; CV=cardiovascular; HR=hazard ratio; MACE=major adverse cardiac events; MI=myocardial infarction; NACE=net adverse clinical events
Composite of all cause mortality, myocardial infarction, stroke, and coronary revascularisation.
Composite of all cause mortality, myocardial infarction, stroke, coronary revascularisation, and hospital admission for unstable angina.
Composite of cardiovascular mortality, myocardial infarction, and stroke.
Composite of all cause mortality, myocardial infarction, stroke, coronary revascularisation, and major bleeding.
Fig 2.
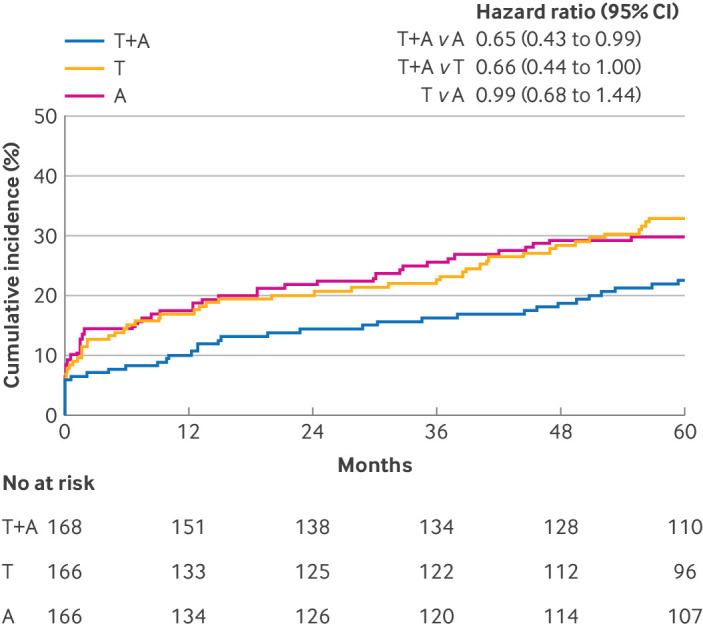
Kaplan-Meier estimate of time to primary major adverse cardiovascular events outcome. A=aspirin monotherapy; CI=confidence interval; HR=hazard ratio; T=ticagrelor monotherapy; T+A=ticagrelor plus aspirin
Sensitivity analyses
Results were consistent in all the sensitivity analyses. In the landmark analysis restricted to patients who were event-free at one year, patients in the dual antiplatelet therapy group had a numerically lower risk of late (2-5 years) major adverse cardiovascular events compared with patients in the aspirin monotherapy group (hazard ratio 0.76, 0.43 to 1.34; P=0.34) and patients in the ticagrelor monotherapy group (0.74, 0.42 to 1.30; P=0.31) (supplementary figure S3). In the per protocol analyses, patients in the dual antiplatelet therapy group had a significantly lower risk of major adverse cardiovascular events compared with patients in the aspirin monotherapy group (hazard ratio 0.63, 0.42 to 0.96; P=0.03) and patients in the ticagrelor monotherapy group (0.62, 0.41 to 0.94; P=0.03).
The baseline characteristics and the incidence of primary and secondary outcomes in the first as-treated analysis are summarised in supplementary tables S4 and S5. Patients who received dual antiplatelet therapy in the first year and aspirin monotherapy in years 2-5 had a significantly lower risk of major adverse cardiovascular events compared with those who received aspirin monotherapy during the first year and in years 2-5 (hazard ratio 0.54, 0.33 to 0.88; P=0.01) and those who received adenosine diphosphate receptor inhibitor monotherapy in the first year and aspirin monotherapy in years 2-5 (0.56, 0.34 to 0.93; P=0.03) (supplementary table S6). In the marginal structural model with stable inverse probability weighting (mean weight was 1.006, minimum weight was 0.2489, and maximum weight was 1.3255), use of dual antiplatelet therapy in the first year and aspirin monotherapy in years 2-5 was associated with a significantly lower risk of major adverse cardiovascular events compared with the use of aspirin monotherapy for the whole study period (hazard ratio 0.45, 0.28 to 0.72; P<0.001) and the use of adenosine diphosphate receptor inhibitor monotherapy for the first year followed by aspirin monotherapy in years 2-5 (0.65, 0.44 to 0.95; P=0.04).
The rate of major adverse cardiovascular events and myocardial infarction was higher in patients with at least one failed graft compared with patients with all grafts patent at one year (44.1% v 24.7% (P<0.001) and 31.5% v 14.0% (P<0.001), respectively; supplementary table S7). We found no significant differences in the rates of major adverse cardiovascular events between participating centres (supplementary table S8).
Secondary outcomes
The extended major adverse cardiovascular events outcome occurred in 39 patients in the dual antiplatelet therapy group, 55 patients in the ticagrelor monotherapy group, and 56 patients in the aspirin monotherapy group; the rates at five years were 22.6%, 32.9%, and 30.5%, respectively (table 2). The risk of extended major adverse cardiovascular events was lower in patients in the dual antiplatelet therapy group compared with patients in the aspirin monotherapy group (hazard ratio 0.64, 0.43 to 0.96; P=0.03) and in the ticagrelor monotherapy group (0.65, 0.43 to 0.98; P=0.04) (table 3 and fig 3).
Fig 3.

Kaplan-Meier estimate of time to extended major adverse cardiovascular events. A=aspirin monotherapy; CI=confidence interval; HR=hazard ratio; T=ticagrelor monotherapy; T+A=ticagrelor plus aspirin
The restricted major adverse cardiovascular events outcome occurred in 27 patients in the dual antiplatelet therapy group, 44 patients in the ticagrelor monotherapy group, and 45 patients in the aspirin monotherapy group; the rates at five years were 15.8%, 26.2%, and 26.6%, respectively (table 2). The risk of restricted major adverse cardiovascular events was lower in patients in the dual antiplatelet therapy group compared with patients in the aspirin monotherapy group (hazard ratio 0.56, 0.35 to 0.90; P=0.02) and in the ticagrelor monotherapy group (0.57, 0.35 to 0.91; P=0.02) (table 3 and fig 4).
Fig 4.

Kaplan-Meier estimate of time to restricted major adverse cardiovascular events. A=aspirin monotherapy; CI=confidence interval; HR=hazard ratio; T=ticagrelor monotherapy; T+A=ticagrelor plus aspirin
The rates of all cause mortality and cardiovascular mortality at five year were numerically higher in the patients in dual antiplatelet therapy group compared with patients in the aspirin monotherapy group (9.3% v 6.2% and 5.1% v 4.0%, respectively) and in the ticagrelor monotherapy group (9.3% v 6.3% and 5.1% v 3.4%, respectively) (table 2). The individual clinical outcomes are summarised in table 2 and supplementary tables S9 to S13.
Safety outcomes
Nineteen patients experienced major bleeding events, including eight in the dual antiplatelet therapy group, four in the ticagrelor monotherapy group, and seven in the aspirin monotherapy group; the rates at five years were 4.9%, 2.5%, and 4.3%, respectively (table 2). The risk of major bleeding was not significantly different between patients in the dual antiplatelet therapy group and patients in the aspirin monotherapy group (hazard ratio 1.14, 0.42 to 3.14; P=0.80) and in the ticagrelor monotherapy group (1.99, 0.60 to 6.61; P=0.26) (table 3). The other adverse events were similar between groups (supplementary table S14).
Net adverse clinical outcomes (post hoc analysis)
The risk of net adverse clinical events was significantly lower in patients in the dual antiplatelet therapy group compared with patients in the aspirin monotherapy group (24.4% v 31.7% at five years; hazard ratio 0.67, 0.45 to 1.00; P=0.05) (table 2 and table 3). We found no significant difference in net adverse clinical events between patients in the dual antiplatelet therapy and ticagrelor monotherapy groups (24.4% v 33.5% at five years; hazard ratio 0.70, 0.47 to 1.05; P=0.09) (tables 2 and table 3).
Subgroup analysis
Subgroup treatment effect interactions are reported in table 4 and supplementary table S15.
Table 4.
Subgroup analyses of primary major adverse cardiac events outcome between randomised treatment groups using univariate Cox regression
| Subgroup | Patients with events/total patients (%) | Ticagrelor plus aspirin v aspirin monotherapy | Ticagrelor plus aspirin v ticagrelor monotherapy | Ticagrelor monotherapy v aspirin monotherapy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ticagrelor plus aspirin | Ticagrelor monotherapy | Aspirin monotherapy | HR (95% CI) | P value* | HR (95% CI) | P value* | HR (95% CI) | P value* | ||||
| All patients | 39/168 (23) | 54/166 (33) | 55/166 (33) | 0.65 (0.43 to 0.99) | 0.66 (0.44 to 1.00) | 0.99 (0.68 to 1.44) | ||||||
| Age: | 0.71 | 0.58 | 0.85 | |||||||||
| ≤70 years | 33/140 (24) | 41/133 (31) | 42/130 (32) | 0.68 (0.43 to 1.08) | 0.70 (0.45 to 1.11) | 0.98 (0.63 to 1.50) | ||||||
| >70 years | 6/28 (21) | 13/33 (39) | 13/36 (36) | 0.56 (0.21 to 1.47) | 0.52 (0.20 to 1.36) | 1.08 (0.50 to 2.32) | ||||||
| Sex: | 0.72 | 0.11 | 0.05 | |||||||||
| Male | 29/134 (22) | 46/134 (34) | 43/141 (30) | 0.66 (0.41 to 1.06) | 0.56 (0.35 to 0.89) | 1.19 (0.78 to 1.80) | ||||||
| Female | 10/34 (29) | 8/32 (25) | 12/25 (48) | 0.55 (0.24 to 1.28) | 1.27 (0.50 to 3.23) | 0.44 (0.18 to 1.07) | ||||||
| Coronary syndrome status: | 0.29 | 0.64 | 0.53 | |||||||||
| CCS | 9/55 (16) | 17/63 (27) | 16/50 (32) | 0.44 (0.19 to 0.99) | 0.55 (0.24 to 1.23) | 0.84 (0.42 to 1.66) | ||||||
| ACS | 30/113 (27) | 37/103 (36) | 39/116 (34) | 0.76 (0.47 to 1.22) | 0.69 (0.43 to 1.11) | 1.10 (0.70 to 1.72) | ||||||
| History of MI: | 0.49 | 0.93 | 0.51 | |||||||||
| Yes | 13/53 (25) | 20/60 (33) | 12/43 (28) | 0.83 (0.38 to 1.81) | 0.68 (0.34 to 1.37) | 1.23 (0.60 to 2.52) | ||||||
| No | 26/115 (23) | 34/106 (32) | 43/123 (35) | 0.60 (0.37 to 0.98) | 0.65 (0.39 to 1.09) | 0.92 (0.59 to 1.44) | ||||||
| History of hypertension: | 0.19 | 0.25 | 0.90 | |||||||||
| Yes | 32/127 (25) | 38/122 (31) | 38/120 (32) | 0.77 (0.48 to 1.23) | 0.75 (0.47 to 1.21) | 1.01 (0.64 to 1.58) | ||||||
| No | 7/41 (17) | 16/44 (36) | 17/46 (37) | 0.39 (0.16 to 0.95) | 0.44 (0.18 to 1.08) | 0.94 (0.48 to 1.87) | ||||||
| History of diabetes: | 0.86 | 1.00 | 0.86 | |||||||||
| Yes | 17/75 (23) | 24/75 (32) | 22/67 (33) | 0.62 (0.33 to 1.17) | 0.66 (0.35 to 1.22) | 0.97 (0.54 to 1.72) | ||||||
| No | 22/93 (24) | 30/91 (33) | 33/99 (33) | 0.68 (0.40 to 1.17) | 0.67 (0.39 to 1.16) | 1.02 (0.62 to 1.67) | ||||||
| History of hyperlipidaemia: | 0.43 | 0.40 | 0.10 | |||||||||
| Yes | 27/121 (22) | 35/124 (28) | 41/119 (34) | 0.59 (0.36 to 0.96) | 0.74 (0.45 to 1.22) | 0.81 (0.52 to 1.27) | ||||||
| No | 12/47 (26) | 19/42 (45) | 14/47 (30) | 0.90 (0.41 to 1.97) | 0.51 (0.25 to 1.06) | 1.65 (0.82 to 3.31) | ||||||
| History of PAD: | 0.27 | 0.87 | 0.14 | |||||||||
| Yes | 7/26 (27) | 10/27 (37) | 15/29 (52) | 0.42 (0.17 to 1.03) | 0.70 (0.27 to 1.85) | 0.55 (0.25 to 1.23) | ||||||
| No | 32/142 (23) | 44/139 (32) | 40/137 (29) | 0.74 (0.47 to 1.18) | 0.66 (0.42 to 1.04) | 1.14 (0.74 to 1.75) | ||||||
| Pump use: | 0.97 | 0.51 | 0.50 | |||||||||
| Yes | 11/39 (28) | 11/36 (31) | 17/46 (37) | 0.67 (0.31 to 1.44) | 0.85 (0.37 to 1.97) | 0.80 (0.37 to 1.71) | ||||||
| No | 28/129 (22) | 43/130 (33) | 38/120 (32) | 0.65 (0.40 to 1.07) | 0.61 (0.38 to 0.98) | 1.08 (0.70 to 1.68) | ||||||
| CRUSADE score: | 0.53 | 0.40 | 0.84 | |||||||||
| Low risk | 34/154 (22) | 48/149 (32) | 50/152 (33) | 0.63 (0.41 to 0.97) | 0.63 (0.41 to 0.98) | 1.00 (0.68 to 1.49) | ||||||
| High risk | 5/14 (36) | 6/17 (35) | 5/14 (36) | 0.92 (0.27 to 3.17) | 1.16 (0.35 to 3.79) | 0.85 (0.26 to 2.78) | ||||||
ACS=acute coronary syndrome; CCS=chronic coronary syndrome; CI=confidence interval; HR=hazard ratio; MI=myocardial infarction; PAD=peripheral artery disease.
P value for interaction..
Discussion
In this extended follow-up study of the DACAB trial, the risk of major adverse cardiovascular events up to five years after coronary artery bypass grafting was lower with dual antiplatelet therapy with ticagrelor plus aspirin for one year compared with aspirin monotherapy or ticagrelor monotherapy. The beneficial effect of ticagrelor dual antiplatelet therapy seemed to continue after the first year and was consistent across key clinical subgroups, including different baseline bleeding risks.
Strengths and limitations of study
This study provides evidence of clinical benefit with the use of ticagrelor dual antiplatelet therapy for one year after coronary artery bypass grafting. Given the substantial rate of cardiovascular events after coronary artery bypass grafting despite standard aspirin therapy, evidence for the clinical effect of ticagrelor dual antiplatelet therapy is of critical importance to inform clinical practice. Strengths of this study include the evaluation of three different antiplatelet strategies, the high adherence to the randomised treatment during the first year, the high rate of complete five year follow-up, and the combined evaluation of clinical events and imaging.
The main limitation is that the randomised allocation to antiplatelet therapy was for the first year only. However, coronary artery bypass graft thrombosis typically occurs within the first year after surgery,27 28 and the prevention of graft occlusion in the first postoperative year could conceivably have long term effects on reducing the risk of major adverse cardiovascular events29; in addition, the long term benefit of one year of ticagrelor dual antiplatelet therapy was confirmed in all the sensitivity analyses.
In clinical practice, patients with coronary artery bypass grafts are generally followed by a specialist early after surgery and then referred to their general physician, and changes in medical therapy are frequent during this transition and also driven by the patient’s health status. The change in antiplatelet treatment could be seen as an intercurrent event, and we presented the intention-to-treat analysis and two as-treated analyses to describe the effect of the interventions both from a treatment policy estimand perspective and from a hypothetical strategy perspective. However, the intention-to-treat analysis is of principal interest for inference as it describes the practical consequences of the specific antiplatelet treatment over the first year (and collider stratification, confounding by indication, and unmeasured bias may be present in the as-treated analyses).
Other limitations include the fact that owing to restrictions associated with the covid-19 pandemic, more than half of the patients completed the five year follow-up remotely, rather than attending an in-person visit, and this may have affected in particular the ascertainment of silent myocardial infarctions. Moreover, given the small number of events, the analysis of the individual outcomes may be underpowered. Finally, the use of the internal mammary artery for coronary artery bypass grafting was lower compared with that in Western registries, which may affect the generalisability of results, but this is consistent with contemporary Chinese cardiac surgical practice.30
Comparison with other studies
Previous studies on the clinical effect of dual antiplatelet therapy, aspirin monotherapy, and ticagrelor monotherapy after coronary artery bypass graft surgery reported mixed results.7 12 13 14 31 32 In the CASCADE study, at a median follow-up of 7.6 years, treatment with clopidogrel plus aspirin for one year was associated with a numerical reduction in major adverse cardiovascular events compared with aspirin monotherapy.13 In the prematurely terminated TICAB trial, no significant differences in the incidence of major adverse cardiovascular events one year after coronary artery bypass grafting were observed in patients who were treated with ticagrelor monotherapy and those treated with aspirin monotherapy, although the study may have been underpowered to detect even moderate treatment effects.32 In a subgroup analysis of the PLATO trial, among patients with acute coronary syndromes who had coronary artery bypass grafting, ticagrelor plus aspirin for one year was superior to clopidogrel plus aspirin in reducing the risk of major adverse cardiovascular events at 12 months after surgery.31 However, this was an observational analysis in a subgroup of patients enrolled in a prospective randomised trial.31
Clinical implications
In the one year imaging analysis of the DACAB trial, ticagrelor dual antiplatelet therapy was associated with a significantly higher saphenous vein graft patency rate compared with aspirin monotherapy, but too few events occurred to allow meaningful intergroup comparisons of clinical outcomes, although the absolute rate of major adverse cardiovascular events was lower in the ticagrelor dual antiplatelet therapy group than in the aspirin monotherapy group.23 The attrition rate of coronary bypass grafts is known to be higher in the first year after surgery (mostly owing to early thrombosis and reactive intimal hyperplasia) and relatively lower during postoperative years 2-5.28 33 Our analysis suggests that ticagrelor dual antiplatelet therapy during the first postoperative year may protect coronary bypass grafts from early occlusion, leading to sustained postoperative clinical benefit. We hypothesise that this benefit is due to the protective effect of patent surgical grafts against coronary artery disease progression and acute coronary events.34
We observed an absolute increase in mortality in patients who received dual antiplatelet therapy. This increased incidence of mortality was based on a low number of events and was not accompanied by an increase in bleeding events; further investigation is needed. An increase in the risk of major bleeding events was noted in a recent meta-analysis comparing ticagrelor dual antiplatelet therapy with aspirin monotherapy in coronary artery bypass grafting patients,9 but this was not seen in our study, in which a significant net clinical benefit was seen with the use of ticagrelor dual antiplatelet therapy for one year after surgery; however, our sample size may have been underpowered for safety outcomes. Treatment decisions must be based on clinical and surgical characteristics of the individual patient.
Future directions
Future studies should investigate the effect of short term ticagrelor dual antiplatelet therapy after coronary artery bypass grafting, as well as its optimal duration. Furthermore, research comparing the efficacy and safety of ticagrelor dual antiplatelet therapy with aspirin monotherapy or ticagrelor monotherapy in specific patient populations, such as people with diabetes or older people, or in patients taking concomitant drugs, would provide additional guidance to clinicians treating patients after coronary artery bypass graft surgery.
Conclusions
The DACAB-FE study provides evidence of a significant five year clinical benefit of one year of ticagrelor dual antiplatelet therapy after coronary artery bypass grafting compared with aspirin monotherapy or ticagrelor monotherapy.
What is already known on this topic
Dual antiplatelet therapy with ticagrelor is more effective than aspirin monotherapy in preventing saphenous vein graft failure after coronary artery bypass graft surgery (CABG)
However, the effect of dual antiplatelet therapy on clinical outcomes, including major adverse cardiovascular events (MACE), after CABG remains unclear
In the DACAB trial, dual antiplatelet therapy with ticagrelor plus aspirin significantly improved vein graft patency rate at one year compared with aspirin monotherapy
What this study adds
Ticagrelor dual antiplatelet therapy for one year significantly reduced the five year risk of MACE after CABG, compared with aspirin monotherapy or ticagrelor monotherapy
This five year follow-up extension study of the DACAB trial suggests that one year of dual antiplatelet therapy improves clinical outcomes after CABG
Acknowledgments
We thank Jing Ji for her wise advice as the representative of the participants, DAP Software (Beijing) Co Ltd for data management, and Shanghai Bestudy Medical Technology Co Ltd for clinical research coordinator services. We also thank Kaicheng Huang, Zhaoxi Qi, Aleksandra Erac-Zganec, Alice Carruthers, and Qing Yun Chong of Nucleus Global for providing medical writing assistance funded by Ruijin Hospital, Shanghai, China. This study was presented as a featured science abstracts oral presentation at the 2021 American Heart Association’s Scientific Sessions and as a late-breaking trials oral presentation at the 37th European Association for Cardio-Thoracic Surgery’s annual meeting on 6 October 2023.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Web appendix: Protocol
Contributors: YZhu and WZ contributed equally to this work and are joint first authors. QZ and MG contributed equally to this work and are joint last/corresponding authors. YZhu, MG, and QZ conceived and designed the study. YZhu, YZhou, QX, JH, MT, RW, YS, LK, and BR acquired, analysed, and interpreted the data. YZhu, WZ, AD, MG, and QZ drafted the manuscript. All authors critically revised the manuscript for important intellectual content. YZhu WZ, and AD did the statistical analyses. QZ obtained funding. LH, ZC, JM, XC, XW, and QZ provided administrative, technical, or material support. MG and QZ provided supervision and are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The DACAB and DACAB-FE studies were funded as an investigator initiated trial by AstraZeneca. AstraZeneca provided the study medication free of charge and had no role in considering the study design or in the collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication. The DACAB-FE study was also funded by the Shanghai Jiao Tong University School of Medicine (project No DLY201802) and Science and Technology Commission of Shanghai Municipality (project No 21Y21901300), which had no role in considering the study design or in the collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from AstraZeneca for the submitted work; YZhu has received personal fees and non-financial support from AstraZeneca, Johnson & Johnson, Medtronic, Novartis, Sanofi, and Chugai and grants from the Science and Technology Commission of Shanghai Municipality and the Shanghai Shenkang Hospital Development Centre; WZ has received personal fees and non-financial support from MSD, Pfizer, Boston Scientific, Cordis, and Becton, Dickinson and Company; LH has received grants from the Science and Technology Commission of Shanghai Municipality. ZC has received personal fees and non-financial support from AstraZeneca, Medtronic, Johnson & Johnson, and Edwards life science and grants from the Science and Technology Commission of Henan Municipality, Henan Provincial Health Commission, Henan Provincial People's Hospital, and Zhengzhou University; JM has received grants from the National Natural Science Foundation of China and the Science and Technology Commission of Shanghai Municipality; XC has received grants from the National Natural Science Foundation of China; XW has received personal fees and non-financial support from AstraZeneca, Johnson & Johnson, and Medtronic and grants from the National Natural Science Foundation of China; QX has received personal fees and non-financial support from Medtronic and Novartis; JH has received personal fees and non-financial support from Medtronic and Johnson & Johnson; MT has received personal fees from Pfizer and grants from the National Natural Science Foundation of China; RW has received personal fees from Novartis. MG has received grants from the National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research; QZ has received grants, personal fees, and non-financial support from AstraZeneca, personal fees and non-financial support from Medtronic and Johnson & Johnson, and grants from the National Science Foundation of China, Science and Technology Commission of Shanghai Municipality, Shanghai Jiao Tong University, Ruijin Hospital Shanghai Jiao Tong University School of Medicine, Shanghai Shenkang Hospital Development Centre, and Chugai; no other relationships or activities that could appear to have influenced the submitted work.
Transparency: The lead authors (the manuscript’s guarantors) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The results of this study were presented at the 2021 American Heart Association annual meeting and the 2023 annual meeting of the European Association for Cardio-Thoracic Surgery and will be disseminated through scientific publications in peer reviewed journals and presentations at national meetings in China. The results will also be shared with the clinicians at participating sites via an investigator meeting.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The protocol for DACAB-FE (available as an online supplement) was reviewed and approved by the independent institutional review board of Ruijin Hospital Shanghai Jiao Tong University School of Medicine (KY-2019-49). Written informed consent was obtained from all patients.
Data availability statement
All data that underlie the results reported in this article will be provided on reasonable request.
References
- 1. Ferraris VA, Saha SP, Oestreich JH, et al. Society of Thoracic Surgeons . 2012 update to the Society of Thoracic Surgeons guideline on use of antiplatelet drugs in patients having cardiac and noncardiac operations. Ann Thorac Surg 2012;94:1761-81. 10.1016/j.athoracsur.2012.07.086 [DOI] [PubMed] [Google Scholar]
- 2. Kulik A. Secondary prevention after coronary artery bypass graft surgery: a primer. Curr Opin Cardiol 2016;31:635-43. 10.1097/HCO.0000000000000331 [DOI] [PubMed] [Google Scholar]
- 3. Kulik A, Ruel M, Jneid H, et al. American Heart Association Council on Cardiovascular Surgery and Anesthesia . Secondary prevention after coronary artery bypass graft surgery: a scientific statement from the American Heart Association. Circulation 2015;131:927-64. 10.1161/CIR.0000000000000182 [DOI] [PubMed] [Google Scholar]
- 4. Solo K, Lavi S, Kabali C, et al. Antithrombotic treatment after coronary artery bypass graft surgery: systematic review and network meta-analysis. BMJ 2019;367:l5476. 10.1136/bmj.l5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deo SV, Dunlay SM, Shah IK, et al. Dual anti-platelet therapy after coronary artery bypass grafting: is there any benefit? A systematic review and meta-analysis. J Card Surg 2013;28:109-16. 10.1111/jocs.12074 [DOI] [PubMed] [Google Scholar]
- 6. Saw J, Wong GC, Mayo J, et al. Ticagrelor and aspirin for the prevention of cardiovascular events after coronary artery bypass graft surgery. Heart 2016;102:763-9. 10.1136/heartjnl-2015-308691 [DOI] [PubMed] [Google Scholar]
- 7. Agarwal N, Mahmoud AN, Patel NK, et al. Meta-Analysis of Aspirin Versus Dual Antiplatelet Therapy Following Coronary Artery Bypass Grafting. Am J Cardiol 2018;121:32-40. 10.1016/j.amjcard.2017.09.022 [DOI] [PubMed] [Google Scholar]
- 8. Gao G, Zheng Z, Pi Y, Lu B, Lu J, Hu S. Aspirin plus clopidogrel therapy increases early venous graft patency after coronary artery bypass surgery a single-center, randomized, controlled trial. J Am Coll Cardiol 2010;56:1639-43. 10.1016/j.jacc.2010.03.104 [DOI] [PubMed] [Google Scholar]
- 9. Sandner S, Redfors B, Angiolillo DJ, et al. Association of Dual Antiplatelet Therapy With Ticagrelor With Vein Graft Failure After Coronary Artery Bypass Graft Surgery: A Systematic Review and Meta-analysis. JAMA 2022;328:554-62. 10.1001/jama.2022.11966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benedetto U, Altman DG, Gerry S, et al. ART Investigators . Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the Arterial Revascularization Trial. Eur J Cardiothorac Surg 2017;52:456-61. 10.1093/ejcts/ezx075 [DOI] [PubMed] [Google Scholar]
- 11. Cardoso R, Knijnik L, Whelton SP, et al. Dual versus single antiplatelet therapy after coronary artery bypass graft surgery: An updated meta-analysis. Int J Cardiol 2018;269:80-8. 10.1016/j.ijcard.2018.07.083 [DOI] [PubMed] [Google Scholar]
- 12. Fox KAA, Mehta SR, Peters R, et al. Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial . Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial [B]. Circulation 2004;110:1202-8. 10.1161/01.CIR.0000140675.85342.1B [DOI] [PubMed] [Google Scholar]
- 13. Hage A, Voisine P, Erthal F, et al. Eight-year follow-up of the Clopidogrel After Surgery for Coronary Artery Disease (CASCADE) trial. J Thorac Cardiovasc Surg 2018;155:212-222.e2. 10.1016/j.jtcvs.2017.06.039 [DOI] [PubMed] [Google Scholar]
- 14. Qu J, Zhang H, Rao C, et al. Dual Antiplatelet Therapy with Clopidogrel and Aspirin Versus Aspirin Monotherapy in Patients Undergoing Coronary Artery Bypass Graft Surgery. J Am Heart Assoc 2021;10:e020413. 10.1161/JAHA.120.020413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Diepen S, Fuster V, Verma S, et al. Dual Antiplatelet Therapy Versus Aspirin Monotherapy in Diabetics With Multivessel Disease Undergoing CABG: FREEDOM Insights. J Am Coll Cardiol 2017;69:119-27. 10.1016/j.jacc.2016.10.043 [DOI] [PubMed] [Google Scholar]
- 16. von Scheidt M, Bongiovanni D, Tebbe U, et al. Ticagrelor-based antiplatelet regimens in patients treated with coronary artery bypass grafting: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2020;57:520-8. [DOI] [PubMed] [Google Scholar]
- 17. Willemsen LM, Janssen PWA, Peper J, et al. Effect of Adding Ticagrelor to Standard Aspirin on Saphenous Vein Graft Patency in Patients Undergoing Coronary Artery Bypass Grafting (POPular CABG): A Randomized, Double-Blind, Placebo-Controlled Trial. Circulation 2020;142:1799-807. 10.1161/CIRCULATIONAHA.120.050749 [DOI] [PubMed] [Google Scholar]
- 18. Kulik A, Le May MR, Voisine P, et al. Aspirin plus clopidogrel versus aspirin alone after coronary artery bypass grafting: the clopidogrel after surgery for coronary artery disease (CASCADE) Trial. Circulation 2010;122:2680-7. 10.1161/CIRCULATIONAHA.110.978007 [DOI] [PubMed] [Google Scholar]
- 19. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016;134:e123-55. [DOI] [PubMed] [Google Scholar]
- 20. Sousa-Uva M, Storey R, Huber K, et al. ESC Working Group on Cardiovascular Surgery and ESC Working Group on Thrombosis . Expert position paper on the management of antiplatelet therapy in patients undergoing coronary artery bypass graft surgery. Eur Heart J 2014;35:1510-4. 10.1093/eurheartj/ehu158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Valgimigli M, Bueno H, Byrne RA, et al. ESC Scientific Document Group. ESC Committee for Practice Guidelines (CPG) ESC National Cardiac Societies . 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213-60. 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 22. Lawton JS, Tamis-Holland JE, Bangalore S, et al. Writing Committee Members . 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e21-129. 10.1016/j.jacc.2021.09.006 [DOI] [PubMed] [Google Scholar]
- 23. Zhao Q, Zhu Y, Xu Z, et al. Effect of Ticagrelor Plus Aspirin, Ticagrelor Alone, or Aspirin Alone on Saphenous Vein Graft Patency 1 Year After Coronary Artery Bypass Grafting: A Randomized Clinical Trial. JAMA 2018;319:1677-86. 10.1001/jama.2018.3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 1999;94:496-509 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 25. Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009;119:1873-82. 10.1161/CIRCULATIONAHA.108.828541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561-70. 10.1097/00001648-200009000-00012 [DOI] [PubMed] [Google Scholar]
- 27. Antonopoulos AS, Odutayo A, Oikonomou EK, et al. SAFINOUS-CABG (Saphenous Vein Graft Failure—An Outcomes Study in Coronary Artery Bypass Grafting) group . Development of a risk score for early saphenous vein graft failure: An individual patient data meta-analysis. J Thorac Cardiovasc Surg 2020;160:116-127.e4. 10.1016/j.jtcvs.2019.07.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xenogiannis I, Zenati M, Bhatt DL, et al. Saphenous Vein Graft Failure: From Pathophysiology to Prevention and Treatment Strategies. Circulation 2021;144:728-45. 10.1161/CIRCULATIONAHA.120.052163 [DOI] [PubMed] [Google Scholar]
- 29. Gaudino M, Sandner S, An KR, et al. Graft Failure After Coronary Artery Bypass Grafting and Its Association With Patient Characteristics and Clinical Events: A Pooled Individual Patient Data Analysis of Clinical Trials With Imaging Follow-Up. Circulation 2023;148:1305-15. 10.1161/CIRCULATIONAHA.123.064090 [DOI] [PubMed] [Google Scholar]
- 30. Li X, Gu D, Wang X, et al. Trends of Coronary Artery Bypass Grafting Performance in a Cohort of Hospitals in China Between 2013 and 2018. Circ Cardiovasc Qual Outcomes 2021;14:e007025. 10.1161/CIRCOUTCOMES.120.007025 [DOI] [PubMed] [Google Scholar]
- 31. Held C, Asenblad N, Bassand JP, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol 2011;57:672-84. 10.1016/j.jacc.2010.10.029 [DOI] [PubMed] [Google Scholar]
- 32. Schunkert H, Boening A, von Scheidt M, et al. Randomized trial of ticagrelor vs. aspirin in patients after coronary artery bypass grafting: the TiCAB trial. Eur Heart J 2019;40:2432-40. 10.1093/eurheartj/ehz185 [DOI] [PubMed] [Google Scholar]
- 33. Gaudino M, Antoniades C, Benedetto U, et al. ATLANTIC (Arterial Grafting International Consortium) Alliance . Mechanisms, Consequences, and Prevention of Coronary Graft Failure. Circulation 2017;136:1749-64. 10.1161/CIRCULATIONAHA.117.027597 [DOI] [PubMed] [Google Scholar]
- 34. Doenst T, Haverich A, Serruys P, et al. PCI and CABG for Treating Stable Coronary Artery Disease: JACC Review Topic of the Week. J Am Coll Cardiol 2019;73:964-76. 10.1016/j.jacc.2018.11.053 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials
Web appendix: Protocol
Data Availability Statement
All data that underlie the results reported in this article will be provided on reasonable request.



