Abstract
Suppression of neuroinflammation using small molecule compounds targeting the key pathways in microglial inflammation has attracted great interest. Recently, increasing attention has been gained to the role of the second bromodomain (BD2) of the bromodomain and extra-terminal (BET) proteins, while its effect and molecular mechanism on microglial inflammation has not yet been explored. In this study, we evaluated the therapeutic effects of ABBV-744, a BD2 high selective BET inhibitor, on lipopolysaccharide (LPS)-induced microglial inflammation in vitro and in vivo, and explored the key pathways by which ABBV-744 regulated microglia-mediated neuroinflammation. We found that pretreatment of ABBV-744 concentration-dependently inhibited the expression of LPS-induced inflammatory mediators/enzymes including NO, TNF-α, IL-1β, IL-6, iNOS, and COX-2 in BV-2 microglial cells. These effects were validated in LPS-treated primary microglial cells. Furthermore, we observed that administration of ABBV-744 significantly alleviated LPS-induced activation of microglia and transcriptional levels of pro-inflammatory factors TNF-α and IL-1β in mouse hippocampus and cortex. RNA-Sequencing (RNA-seq) analysis revealed that ABBV-744 induced 508 differentially expressed genes (DEGs) in LPS-stimulated BV-2 cells, and gene enrichment and gene expression network analysis verified its regulation on activated microglial genes and inflammatory pathways. We demonstrated that pretreatment of ABBV-744 significantly reduced the expression levels of basic leucine zipper ATF-like transcription factor 2 (BATF2) and interferon regulatory factor 4 (IRF4), and suppressed JAK-STAT signaling pathway in LPS-stimulated BV-2 cells and mice, suggesting that the anti-neuroinflammatory effect of ABBV-744 might be associated with regulation of BATF2-IRF4-STAT1/3/5 pathway, which was confirmed by gene knockdown experiments. This study demonstrates the effect of a BD2 high selective BET inhibitor, ABBV-744, against microglial inflammation, and reveals a BATF2-IRF4-STAT1/3/5 pathway in regulation of microglial inflammation, which might provide new clues for discovery of effective therapeutic strategy against neuroinflammation.
Keywords: microglial inflammation, BD2, ABBV-744, BATF2, IRF4, JAK/STAT
Introduction
Microglia, the resident macrophages of the central nervous system (CNS), act as the first line of defense in the brain by rapidly responding to abnormal substances in the microenvironment [1]. Normally, microglia directly phagocytose cellular debris and invading pathogens, and release neurotrophic factors to maintain CNS homeostasis [2]. Neurotoxic factors, however, are induced by the over-activation of microglia such as nitric oxide (NO), tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) which mediate complex signaling and further worsen the pathological process [2–5]. Although the precise mechanisms of microglial activation remain to be elucidated, increasing evidence suggests that complex reciprocal interactions exist during microglial pathophysiological cascade, which elicit or retract the progress of microglia-mediated neuroinflammation [6, 7]. Recently, an impressive amount of research has been accumulated regarding discovery of novel key signaling pathways that successfully reduce the production of multiple pro-inflammatory factors and hamper sequential pathological events [8–12], highlighting that discovery of novel potent anti-microglial inflammation small molecules and elucidating their key signaling pathways have great potential for future drug development.
As epigenetic “reader”, the bromodomain and extra-terminal (BET) family mediates gene transcription by recognizing and binding to histone and non-histone acetylated lysines through its bromodomains [13, 14], which acts at the initiation and elongation stages of transcription [15–17]. In recent years, targeting the second bromodomain (BD2) of BET protein, the key to the rapid response of BET proteins, has shown great promise in countering various types of diseases, shown by potent therapeutic effects in preclinical animal models of solid tumor [18], arthritis [19], psoriasis and inflammatory bowel disease [20], periodontitis [21], etc., and such beneficial roles are believed to be attributed to the regulatory role of selective BD2 inhibitors in inflammatory responses happened in peripheral tissues or cells. For example, RVX-297, a BET inhibitor with more than 40-fold selectivity towards the BD2 domains [22], reduced the expression of pro-inflammatory mediators in mouse spleen and serum of arthritis and autoimmune disease animal models [19]; GSK620, a selective BET-BD2 inhibitor with more than 200-fold selectivity over all other bromodomains [23], significantly reduced disease-associated pro-inflammatory gene expression in joint, skin and liver of arthritis, psoriasis and hepatitis animal models [18]; RVX-208, a BET inhibitor with more than 20-fold selectivity towards the BD2 domains [24], inhibited inflammatory gene expression and cytokine production in macrophages and gingival epithelial cells of periodontitis cell models [21]. Nevertheless, the regulatory effect and molecular mechanism of BD2 high selective BET inhibitor on microglia-mediated neuroinflammation have not been explored.
Hence, in this study, we evaluated the effect of ABBV-744, a highly potent and selective inhibitor of the BD2 domain of BET family proteins with drug-like properties [25], on lipopolysaccharide (LPS)-induced microglial inflammation both in vitro and in vivo, and further explored the potential key pathways by which ABBV-744 regulate microglia-mediated neuroinflammation, employing RNA-sequencing technology.
Materials and methods
Preparation of compounds
Compounds ABBV-744 and AG490, a JAK-STAT signaling pathway inhibitor [26, 27] (MedChemExpress, Shanghai, China), were dissolved in 100% dimethyl sulfoxide as a stock solution at 10 mM and diluted to specific concentrations in cell culture medium for in vitro studies. For in vivo experiments, compound ABBV-744 was dissolved in DMSO (5%, v/v), PEG300 (40%, v/v) and saline (55%, v/v) in sequence to the desired concentration and was orally administered to experimental mice. DMSO, PEG300 and saline were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Cell culture
The BV-2 cell line, a type of microglial cell derived from C57BL/6J mice, is widely used for discovery and mechanistic studies of candidate compounds against microglial inflammation [28–30]. The BV-2 cell used in the article was a gift from Prof Lin-yin Feng (Shanghai Institute of Materia Medica, Chinese Academy of Sciences). BV-2 cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA) containing 10% heat-inactivated fetal bovine serum (Gibco, Penrose, Auckland, NZ), 60 mg/L ampicillin (Sigma-Aldrich, St Louis, MO, USA) and 50 mg/L streptomycin (Sangon Biotech, Shanghai, China). All cells were cultured in a humidified incubator at 37 °C with 5% carbon dioxide.
Primary mouse microglia culture
The cortex of one-day-old C57B/L mice (Charles River, Beijing, China) was separated under a microscope and mechanically disrupted, transferred into a centrifuge tube containing warm Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 (D-MEM/F-12) culture medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum. The centrifuged cell pellet was resuspended in D-MEM/F-12 culture medium, and the cell suspension was passed through a disposable mesh and seeded in a cell culture flask pre-coated with 50 μg/mL poly-lysine (Sangon Biotech, Shanghai, China). The entire culture medium was refreshed after 24 h and then half of the medium was refreshed every three days. On day 11, primary microglia were harvested by shaking at 200 rpm for 2 h and seeded on culture plates pre-coated with poly-lysine. Thirty minutes after seeding, the cell culture medium was completely refreshed to remove nonadherent cells. Associated animal experimental procedures were in accordance with relevant guidelines and approved by the Institutional Animal and Use Committee of Shanghai Institute of Materia Medica, Chinese Academy of Sciences (IACUC: 2021-04-ZHY-162, 2022-02-ZHY-185).
Nitrite detection
BV-2 cells were seeded in 96-well plates at a density of 2 × 105 cells/mL for 24 h. The cells were then preincubated with compounds of specified concentrations for 2 h followed by lipopolysaccharide (LPS, Sigma-Aldrich, St Louis, MO, USA) challenge at a final concentration of 100 ng/mL for 24 h. The nitrite oxide content of the cell supernatant was measured according to the instructions of the nitric oxide detection kit (Beyotime, Shanghai, China).
Cell viability detection
The MTT method was used to evaluate the effects of different concentrations of compounds on the cell survival rate of BV-2 cells. In brief, BV-2 cells were seeded in 96-well culture plates at a density of 1.25 × 104 cells/mL for 24 h. The culture media were refreshed and the cells were incubated with corresponding concentrations of compounds for another 24 h. Each well of the plates was added with 10 μL of 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sangon Biotech, Shanghai, China) (5 mg/mL) and the plates were continued to be cultured for another 3 h. After discarding all the culture medium, 100 μL/well 100% DMSO was added and the absorbance at 490 nm was measured by MD Flex Station 3 (Molecular Devices, Sunnyvale, CA, USA).
Enzyme-linked immunosorbent assay
BV-2 cells were seeded in 12-well plate at a density of 2 × 105 cells/mL for 24 h. The culture medium was then refreshed and the cells were preincubated with compounds of corresponding concentrations for 2 h. After stimulation with LPS (100 ng/mL) for 24 h, the cell supernatant was collected and measured for IL-6 and TNF-α contents according to the instructions of the ELISA detection kit (MULTI SCIENCES, Hangzhou, China).
RNA isolation, RNA reverse transcription, and quantitative real-time PCR
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. cDNA was prepared using HiScript III RT Super Mix for qPCR (+gDNA wiper) (Vazyme, Nanjing, China) with 1000 ng total RNA. Quantitative real-time PCR was performed using cDNA and Taq Pro Universal SYBR qPCR Master (Vazyme, Nanjing, China) on Bio-Rad CFX96 (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. qPCR primers were synthesized by Sangon Biotech (Shanghai, China) and the sequences were provided in Supplementary Table S1. Relative gene expression was calculated using the ΔΔCT method and was normalized to GAPDH mRNA expression in the corresponding samples.
Western blotting
Cells or tissues were lysed with RIPA lysis buffer (50 mM Tris-base, 150 mM NaCl, 2 mM EDTA, 0.5% sodium deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 1 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 1% protease inhibitor cocktail (Invitrogen, Carlsbad, CA, USA)) for 30 min on ice. After centrifugation at 12,000 × g for 15 min at 4 °C, the supernatant was collected and the protein concentration was determined according to the instructions of the BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). After leveling the concentration, the samples were mixed with loading buffer and boiled at 95 °C for 10 min. According to the experimental needs, the protein samples were separated by 7.5% or 10% SDS-polyacrylamide gel electrophoresis. Next, proteins were transferred to 0.2 μm nitrocellulose membrane (Whatman, Maidstone, Kent, UK) using the Turbo semi-dry machine (Bio-Rad, Hercules, CA, USA) and blocked with 5% skim milk for 1 h at 25 °C. Membranes were then incubated with the corresponding primary antibodies overnight at 4 °C: iNOS (610328, 1:500) from BD Biosciences (San Jose, CA, USA); COX2 (12282, 1:1000), IRF4 (15106, 1:1000), p-JAK2 (3776, 1:1000), JAK2 (3230, 1:1000), p-STAT1 (9167, 1:1000), STAT1 (14994, 1:5000), p-STAT3 (9145, 1:1000), STAT3 (30835, 1:5000), p-STAT5 (4322, 1:1000), STAT5 (94205, 1:5000), GAPDH (2118, 1:20,000), α-tubulin (3873, 1:20,000) from Cell Signaling Technology (Beverly, MA, USA); BATF2 (PA537138, 1:1000) from Invitrogen (Rockford, IL, USA), β-actin (A5441, 1:10,000) from Sigma-Aldrich (St Louis, MO, USA). Membranes were then washed with TBST solution (50 mM Tris-base, 150 mM NaCl, 0.05% v/v Tween 20, pH 7.4) for three times and incubated with the secondary antibody for 2 h at room temperature: goat anti-rabbit IgG(H + L) (KC-RB-035, 1:5000), goat anti-mouse IgG(H + L) (KC-MM-035, 1:5000) from Kangcheng Biotech (Shanghai, China); IRDye 800CW goat anti-mouse (926-32210, 1:5000) from LI-COR (Lincoln, NE, USA). Finally, the membranes were imaged with Immobilon Western Chemiluminescent HRP Substrate (Millipore, Bedford, MA, USA) through a fully automated chemiluminescence image analysis system (Tanon, Shanghai, China) or a comprehensive imaging system (Bio-Rad, Hercules, CA, USA). ImageJ software was used to analyze the gray value of the protein bands.
RNA-sequencing
For transcriptomic analysis, BV-2 cells were preincubated with or without 0.03 μM ABBV-744 for 2 h and then stimulated with 100 ng/mL LPS for 3 h. BV-2 cell samples (n = 4) were collected with TRIzol and sent to Majorbio Company (Majorbio, Shanghai, China) on dry ice for RNA sequencing with the Illumina HiSeq™ 2000 platform. The sequencing data were analyzed on Majorbio Cloud Platform (https://www.majorbio.com/) according to the instructions. Differentially expressed genes (DEGs) at a 5% false discovery rate (FDR) (adjusted P value ≤ 0.05) were identified. Kyoto Encyclopedia of Genes and Genomes (KEGG) database was used for the classification and pathway enrichment of the DEGs. Gene Ontology (GO) biological process enrichment analysis of the DEGs was implemented with Goatools software. GO terms with P values < 0.05 were considered significantly enriched in DEGs.
Small interfering RNA transfection
BATF2 and IRF4 knockdown in BV-2 cells were achieved by small interfering RNA (siRNA) synthesized by OBiO Tech (Shanghai, China). Initially, three siRNAs targeting the different regions of BATF2 and IRF4 mRNA were tested and the one showing the highest knockdown efficacy was chosen for further assays. In brief, BV-2 cells were seeded in a 24-well plate at a density of 1 × 105 cells/mL. After 24 h of culture, the cells were replaced with antibiotic-free DMEM (HG) culture medium. siRNAs and Lipofectamine RNAiMAX Transfection Reagent (Invitrogen, Carlsbad, CA, USA) were diluted with Opti-MEM culture medium (Gibco, Grand Island, NY, USA) and transfected to BV-2 cells according to the instructions of the transfection kit. siBATF2: 5′-GGACCAGAGUGUUCACAGAACTT-3′, 5′-GUUCUGUGAACACUCUGGUCCTT-3′; siIRF4: 5′-GACCAGUCACACCCAGAAAUCTT-3′, 5′-GAUUUCUGGGUGUGACUGGUCTT-3′ and non-targeting siRNA containing scrambled sequences as a negative control (NC). The siRNAs were removed after 24 h and BV-2 cells were harvested another 3 h later for further assays.
Animals and in vivo experimental design
Male C57BL/6J mice (20–22 g, Charles River, Beijing, China) were housed in a specific pathogen-free facility under the following conditions: temperature, 24–26 °C; humidity, 50%–60%; ad libitum access to food/water; 12-h light/dark cycle. All animal experimental procedures were approved by the Animal Care and Use Committee of Shanghai Institute of Materia Medica, Chinese Academy of Sciences (IACUC: 2022-02-ZHY-186, 2024-02-ZHY-226).
In the in vivo efficacy evaluation experiments, the mice were randomly assigned to five groups (eight mice in each group): vehicle group, LPS group, 0.05 mg/kg ABBV-744 treated group, 0.1 mg/kg ABBV-744 treated group, 0.2 mg/kg ABBV-744 treated group. Mice in the vehicle group and LPS group were given normal solvent (5% DMSO + 40% PEG300 + 55% saline) daily by gavage for two days. Mice in ABBV-744 treated group were administered orally with ABBV-744 in corresponding concentrations daily for two days. On the second day, mice in the LPS group and ABBV-744 treated groups were injected intraperitoneally with LPS at the dose of 5 mg/kg one hour after administration by gavage while mice in the vehicle group were injected intraperitoneally with normal saline. After 24 h, mice were subjected to cardiac perfusion, then half of the brain was fixed with 4% PFA for immunofluorescence staining experiments, and the hippocampus and cortex of the other half of the brain were isolated for biochemical indexes.
In the in vivo mechanism study experiments, the mice were randomly assigned to five groups (twelve mice in each group): Vehicle group, LPS group, 0.05 mg/kg ABBV-744 treated group, 0.1 mg/kg ABBV-744 treated group, 0.2 mg/kg ABBV-744 treated group. Mice were treated the same as the above experiments on the first two days while on the second day, the brain of mice was sacrificed 3 h after LPS injection for further mechanism study.
Immunofluorescence staining
Half of the mouse brain was fixed in 4% paraformaldehyde, dehydrated in gradients of 20% and 30% sucrose solutions, and then embedded in OCT embedding agent. Then, the mouse brain was frozen and cut into 30 μm-thick sections. Brain sections were blocked in blocking solution containing 1% bovine serum albumin, 5% goat serum, and 0.2% Triton X-100 for 2 h at 25 °C. Sections were then incubated with rabbit anti-IBA1 (1:200, 019-19741, Wako, Japan) overnight at 4 °C. Next, sections were incubated with Alexa FluorTM 564-conjugated anti-rabbit IgG (1:200, 1887149, Invitrogen) for 2 h at room temperature and mounted on lysine-coated slides. Images of stained sections were captured using a pathology slide scanner (NanoZoomer HT, Hamamatsu, Japan) and analyzed using ImageJ software.
Statistical analysis
All data are presented as the mean ± SEM and statistical analyses were performed with GraphPad Prism 9.4.1 software. Normality tests were first done based on the Shapiro-Wilk method using Prism software before assessing statistical significance. Outliers were determined and removed by ROUT method in Prism software. Differences between normally distributed values of two experimental groups were analyzed by an unpaired Student’s t-test. Differences among normally distributed values of three or more experimental groups were analyzed by One-way ANOVA followed by a Dunnett’s multiple comparisons test. Differences at P < 0.05 were considered statistically significant.
Results
ABBV-744 inhibited LPS-induced expression of pro-inflammatory mediators and genes in microglial cells
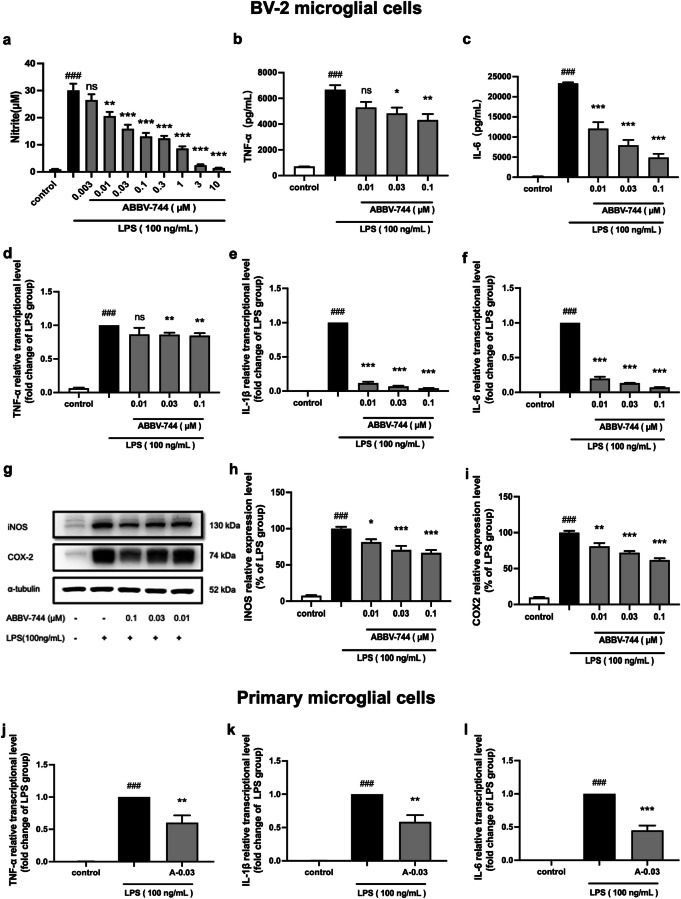
To investigate the effect of ABBV-744 on LPS-induced neuroinflammation, BV-2 microglial cells were pretreated with ABBV-744 for 2 h and then exposed to LPS (100 ng/mL) for 24 h. First, a nitric oxide detection kit was used to detect nitric oxide (NO) production level in the cell supernatant [31]. Results showed that ABBV-744 at 0.01 μM to 10 μM significantly inhibited the production of NO in LPS-exposed BV-2 cells in a concentration-dependent manner (Fig. 1a). ABBV-744 did not affect the viability of BV-2 cells with/without LPS exposure, at concentrations up to 30 nM (Supplementary Fig. S1). In order to further assess the concentration-dependent anti-inflammatory effects of ABBV-744 under no/low cytotoxicity, three sequential concentrations, 0.01, 0.03, and 0.1 μM were chosen in the following studies. Similar to the results on NO production, ELISA test indicated that ABBV-744 concentration-dependently inhibited the expression of two pro-inflammatory cytokines, TNF-α and IL-6 (Fig. 1b, c). Next, quantitative real-time PCR technology was used to detect pro-inflammatory gene expression in BV-2 cells induced by LPS stimulation. Experimental results showed that after 3 h of exposure to LPS, ABBV-744 significantly inhibited the expression of three pro-inflammatory genes, TNF-α, IL-1β, and IL-6 in BV-2 cells (Fig. 1d–f). To further determine the inhibitory activity of ABBV-744 against LPS-stimulated inflammatory responses in BV-2 cells, upstream pro-inflammatory mediators inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) expression levels were examined by Western blot analysis. Consistent with the result on NO production and pro-inflammatory cytokines, ABBV-744 treatment also downregulated LPS-induced overproduction of iNOS and COX2 in the LPS-stimulated BV-2 cells (Fig. 1g–i).
Fig. 1. Effect of ABBV-744 on LPS-induced inflammatory responses in microglial cells.
a–c BV-2 cells were pretreated with ABBV-744 (0.003, 0.01, 0.03, 0.1, 0.3, 1, 3, 10 μM) for 2 h and then exposed to LPS (100 ng/mL) for 24 h (n = 5). The production of NO was measured using nitric oxide detection kit (a). The levels of pro-inflammatory factors TNF-α (b) and IL-6 (c) were measured using ELISA. d-f BV-2 cells were pretreated with ABBV-744 (0.01, 0.03, 0.1 μM) for 2 h and then exposed to LPS (100 ng/mL) for 3 h (n = 5). The mRNA levels of TNF-α (d), IL-1β (e) and IL-6 (f) were measured by quantitative real-time PCR. g–i BV-2 cells were pretreated with ABBV-744 (0.01, 0.03, 0.1 μM) for 2 h and then exposed to LPS (100 ng/mL) for 24 h (n = 5). The protein levels of iNOS, COX2 and α-tubulin were measured using immunoblot analysis (g). The quantification of the intensities of iNOS (h) and COX2 (i) relative to α-tubulin are shown. j–l Primary microglial cells were pretreated with ABBV-744 (0.03 μM) for 2 h and then exposed to LPS (100 ng/mL) for 3 h (n = 5). The mRNA levels of TNF-α (j), IL-1β (k), and IL-6 (l) were measured by quantitative real-time PCR. Comparisons between control group and LPS group were generated with unpaired two-tailed Student’s t-test. Comparisons between LPS group and ABBV-744-treated groups were generated with One-way ANOVA followed by Dunnett’s multiple comparison test. Data are shown as the mean ± SEM, ###P < 0.001 vs. the control group, *P < 0.05, **P < 0.01, ***P < 0.001, ns (no significant difference) vs. the LPS group. A-0.03: 0.03 μM ABBV-744.
To further validate the anti-inflammatory effects in microglial cells, we also evaluated whether ABBV-744 diminished the LPS-induced mRNA levels of pro-inflammatory genes in primary microglial cells. Similarly, ABBV-744 at 0.03 μM significantly reduced the transcriptional levels of pro-inflammatory genes TNF-α, IL-1β and IL-6 in the LPS-stimulated primary microglial cells, to approximately 50% of those in the LPS alone group (Fig. 1j–l). Similar to that in BV-2 cells, ABBV-744 treatment also downregulated LPS-induced overexpression of iNOS and COX2 genes in the LPS-exposed primary microglial cells (Supplementary Fig. S2).
ABBV-744 alleviated LPS-induced activation of microglia and expression of pro-inflammatory genes in mouse hippocampus and cortex
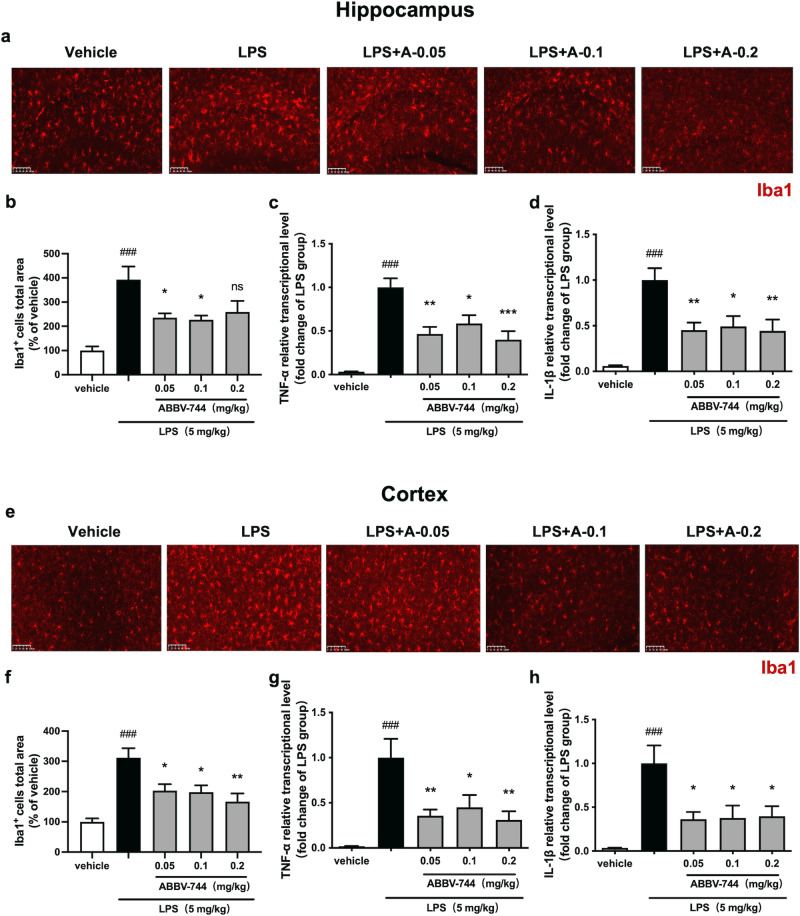
In order to further evaluate the anti-neuroinflammatory effect of ABBV-744 in vivo, C57BL/6 J mice were treated with vehicle (5% DMSO + 40% PEG300 + 55% saline; p.o.) or ABBV-744 (0.05, 0.1, 0.2 mg/kg; p.o.) daily for 2 days, followed by injection with saline or LPS (5 mg/kg; i.p.) for 1 day. Mice were sacrificed 24 h after LPS treatment. Then hippocampus and cortex, two brain regions widely chosen for assessing neuroinflammatory responses [32, 33] or pharmacological effects [34, 35] of active compounds against neuroinflammation, were dissected for studying anti-inflammatory responses of ABBV-744. Subsequently, immunofluorescence staining was performed with anti-Iba1 antibody to detect activation of microglia (Fig. 2a, e). The results showed that intraperitoneal injection of LPS exhibited a significant increase in Iba1-positive microglia levels in the hippocampus of mice, while ABBV-744 at doses of 0.05 and 0.1 mg/kg were able to significantly reduce the levels of Iba1 in the hippocampus of LPS-injected mice (Fig. 2a, b, Supplementary Fig. S3a). Activated microglia was associated with pro-inflammatory responses and neuroinflammation, therefore real-time PCR was performed to detect the mRNA levels of pro-inflammatory genes TNF-α and IL-1β. The results showed that ABBV-744 (0.05, 0.1, and 0.2 mg/kg) significantly down-regulated the mRNA levels of TNF-α and IL-1β in the hippocampus of mice to about 50% of those in the LPS group (Fig. 2c, d).
Fig. 2. Effect of ABBV-744 on LPS-induced activation of microglia and transcription of pro-inflammatory genes in the hippocampus and cortex of mice.
a–h C57BL/6J mice were treated with vehicle (5% DMSO + 40% PEG300 + 55% saline; p.o.) or ABBV-744 (0.05, 0.1, 0.2 mg/kg; p.o.) daily for 2 days, followed by injection with saline or LPS (5 mg/kg; i.p.) for 1 day. Mice were sacrificed 24 h after LPS treatment (n = 7–8). a Activation of microglia in the CA1 region of mice hippocampus was detected by immunofluorescence staining using an anti-Iba1 antibody. Scale bar = 100 μm. b Quantification of Iba1 positive cells in the hippocampus of mice (n = 7–8). The mRNA levels of TNF-α (c) and IL-1β (d) in the hippocampus were measured by quantitative real-time PCR (n = 7–8). e Activation of microglia in the prefrontal cortex of mice was detected by immunofluorescence staining using an anti-Iba1 antibody. Scale bar = 100 μm. f Quantification of Iba1 positive cells in the cortex of mice (n = 7–8). The mRNA levels of TNF-α (g) and IL-1β (h) in the cortex were measured by quantitative real-time PCR (n = 7-8). Comparisons between control group and LPS group were generated with unpaired two-tailed Student’s t-test. Comparisons between the LPS group and ABBV-744-treated groups were generated with One-way ANOVA followed by Dunnett’s multiple comparison test. Data are shown as the mean ± SEM, ###P < 0.001 vs. the vehicle group, *P < 0.05, **P < 0.01, ***P < 0.001, ns (no significant difference) vs. the LPS group. For all immunofluorescence and qPCR experiments: vehicle group, LPS group and 0.1 mg/kg ABBV-744 treatment group, n = 8; 0.05 mg/kg and 0.2 mg/kg ABBV-744 treatment groups, n = 7.
We also examined activation of microglia and expression of pro-inflammatory genes in the cortex of mice. Similar to the results in hippocampus, LPS injection significantly increased the levels of Iba1 positive microglia in the mouse cortical area and the mRNA levels of TNF-α and IL-1β in mouse cortical tissue, while ABBV-744 at doses of 0.05, 0.1, and 0.2 mg/kg could significantly inhibit these inflammatory effects (Fig. 2f–h, Supplementary Fig. S3b). Above results indicated that ABBV-744 can inhibit the neuroinflammatory responses in the brain of mice.
RNA-seq analysis revealed key differential genes and signaling pathway in LPS-exposed BV-2 cells by ABBV-744 regulation
Despite the apparently alleviative effects of ABBV-744 observed on LPS-induced microglial inflammation, understanding of its mechanism of action remains limited. Therefore, we performed RNA sequencing (RNA-seq) to explore the potential molecular mechanism underlying the anti-neuroinflammatory effects of ABBV-744. The volcano plot showed that a total of 508 DEGs were identified between the LPS group and LPS + ABBV-744 group, of which 72 genes were significantly upregulated and 436 genes were significantly down-regulated in the LPS + ABBV-744 group (Fig. 3a). As shown in the Supplementary Table S2, we listed all 436 downregulated DEGs which contained a large number of inflammation-related genes, such as chemokines, interleukins, tumor necrosis factors and matrix metalloproteinases. Upon comparison, IL-1β and IL-6 which were demonstrated to be significantly downregulated by ABBV-744 pretreatment, were also included (Supplementary Table S2). Then, GO functional annotation was performed and the enrichment chord diagram showed top 5 enrichment degrees and related DEGs. Of note, the gene with the largest downregulation fold difference after ABBV-744 treatment compared with the LPS model group was BATF2, a member of the basic leucine zipper ATF-like transcription factor [36], suggesting that this gene might be critical for the anti-inflammatory effects of ABBV-744.
Fig. 3. Analysis of RNA-seq experiment. a-d BV-2 cells were stimulated with LPS (100 ng/mL) for 3 h following pretreatment with ABBV-744 (0.03 μM) for 2 h.
RNA sequencing and analysis were performed (P < 0.05 and two-fold difference, n = 4). a Volcano plot of DEGs between LPS and LPS + ABBV-744 groups. The blue dot and red dot represent that the expression level of genes has difference, the gray dot represents no difference. b The enrichment chord diagram displays top 5 GO terms and corresponding genes among 508 DEGs between the LPS group versus the LPS + ABBV-744 group. c The KEGG functional annotation bar chart shows four major categories of KEGG metabolic pathways. d The KEGG functional enrichment analysis line chart shows top 20 pathways enriched by DEGs.
In addition, the chemokine family such as Ccr5 and Ccl20, the interleukin family such as Il1f6 and Il2b (Fig. 3b), all of which are well reported to be involved in regulation of microglial inflammation [37–40], again suggesting the antagonistic effect of ABBV-744 on microglial inflammation. Moreover, the KEGG functional annotation results showed that 508 DEGs were annotated into four major categories of KEGG metabolic pathways, namely “Human Diseases”, “Organismal Systems”, “Cellular Process” and “Environmental Information Processing”. Among them, the number of DEGs annotated to “Immune system” was the largest, with 55; followed by “Signaling molecules and interaction” and “Signal transduction” with 48 and 44, respectively (Fig. 3c), suggesting that ABBV-744 may exert its anti-inflammatory effects mainly by modulating signal transduction in the immune response of microglia.
Considering that BET proteins, as epigenetic “readers”, mediate the transcriptional regulation of target genes by recruiting transcription factors, we performed transcription factor prediction analysis based on these 508 DEGs. The bar chart displayed the predicted transcription factor families and corresponding members (Supplementary Fig. S4). Interestingly, IRF4, a member of the Interferon Regulatory Factor (IRF) family of transcription factors [41], which regulates macrophage polarity to mediate the regulation of inflammation, was the only core transcription factors predicted in the IRF family (Supplementary Fig. S4). In addition, IRF4 also appeared on the enriched chord plot as one of the DEGs with a significantly higher degree of downregulation (Fig. 3b), suggesting that IRF4 might play an important role in the anti-inflammatory effects mediated by ABBV-744. Finally, we performed KEGG functional annotation and enrichment analysis to find key regulatory pathways. Among the inflammation-related signaling pathways illustrated in the KEGG functional enrichment analysis line chart, we prioritized the JAK/STAT signaling pathway that mediates inflammatory signal transduction and transcriptional activation of inflammatory genes (Fig. 3d).
ABBV-744 down-regulated LPS-induced expression of BATF2 and IRF4 and inhibited the activation of JAK/STAT signaling pathway in BV-2 cells
Combining the results of RNA-seq analysis and literature survey, we further verified the regulative effects of ABBV-744 on potential key regulatory genes BATF2, IRF4, and JAK/STAT signaling pathway in the LPS-stimulated BV-2 cells. BV-2 cells were preincubated with ABBV-744 (0.03 μM) for 2 h and then exposed to LPS (100 ng/mL) for 3 h. The results of real-time PCR experiments showed that after exposure to LPS for 3 h, ABBV-744 treatment could significantly downregulated the LPS-induced overexpression of BATF2 (Fig. 4a) and IRF4 in BV-2 cells (Fig. 4b) and primary microglial cells (Supplementary Fig. S5). Similarly, Western blot experiments showed that ABBV-744 treatment significantly attenuated the LPS-induced upregulation of BATF2 (Fig. 4c) and IRF4 in BV-2 cells (Fig. 4d). As for LPS-induced JAK/STAT signaling pathway, we tested the phosphorylation levels of JAK and STAT using Western blot analysis. As shown in Fig. 4e–h, ABBV-744 markedly reversed the increased phosphorylation levels of JAK2, STAT1, STAT3 and STAT5 in the LPS-exposed BV-2 cells. Above results further support aforementioned RNA-seq analysis results.
Fig. 4. Effect of ABBV-744 on LPS-induced expression levels of BATF2, IRF4, and JAK/STAT signaling pathway in BV-2 cells.
a, b BV-2 cells were pretreated with ABBV-744 (0.03 μM) for 2 h and then exposed to LPS (100 ng/mL) for 3 h (n = 4–5). The mRNA levels of BATF2 (a) and IRF4 (b) were measured by quantitative real-time PCR. c BV-2 cells were pretreated with ABBV-744 (0.03 μM) for 2 h and then exposed to LPS (100 ng/mL) for 15 min (n = 5). The protein level of BATF2 was measured by Western blot. The quantification of the intensity of BATF2 relative to β-actin is shown. d–h BV-2 cells were pretreated with ABBV-744 (0.03 μM) for 2 h and then exposed to LPS (100 ng/mL) for 3 h (n = 6–7). Protein levels of IRF4 (d), p-JAK2 and JAK2 (e), p-STAT1 and STAT1 (f), p-STAT3 and STAT3 (g), p-STAT5 and STAT5 (h) were measured by Western blot and β-actin or GAPDH were used as a loading control. Comparisons of groups were generated with unpaired two-tailed Student’s t-test. Data are shown as the mean ± SEM, ##P < 0.01, ###P < 0.001 vs. the control group, **P < 0.01, ***P < 0.001 vs. the LPS group. A-0.03: 0.03 μM ABBV-744.
Knocking down BATF2 and IRF4 or suppressing JAK/STAT signaling pathway significantly inhibited LPS-induced pro-inflammatory gene expression in BV-2 cells
In order to clarify whether downregulation of BATF2 and IRF4, and inhibition of the JAK/STAT signaling pathway by ABBV-744 are associated with its anti-microglial inflammation effect, we used small interfering RNAs to knockdown BATF2 or IRF4 and used AG490 to suppress JAK/STAT signaling pathway in BV-2 cells. siRNA targeting the BATF2 or IRF4 sequence significantly reduced the mRNA level of BATF2 or IRF4 (Supplementary Fig. S6). After exposed to LPS for 3 h, three common pro-inflammatory genes (TNF-α, IL-1β, and IL-6) were detected by real-time PCR. As shown in Fig. 5a–c, transfection with BATF2-targeted siRNA significantly decreased the elevated mRNA levels of TNF-α, IL-1β, and IL-6 in LPS-stimulated BV-2 cells. Similarly, transfection with IRF4-targeted siRNA markedly decreased the elevated expression of TNF-α, IL-1β, and IL-6 (Fig. 5d–f). Also, AG490 (10 μM) treatment significantly inhibited the transcriptional levels of the above three pro-inflammatory genes induced by LPS (Fig. 5g–i). The above results demonstrate that down-regulating of BATF2 and IRF4 and inhibiting JAK/STAT signaling pathway suppresses the inflammatory responses in LPS-exposed BV-2 cells, suggesting that the anti-microglial inflammation effect of ABBV-744 might be attributed to its regulation on BATF2, IRF4, and JAK/STAT signaling.
Fig. 5. Effect of knocking down of BATF2 or IRF4 and suppressing JAK-STAT signaling pathway on LPS-induced expression levels of pro-inflammatory genes in BV-2 cells.
a–f BV-2 cells were transfected with small interfering RNAs for 24 h and then exposed to LPS (100 ng/mL) for 3 h (n = 7). The mRNA levels of TNF-α (a), IL-1β (b) and IL-6 (c) were measured by quantitative real-time PCR after knocking down of BATF2. The mRNA levels of TNF-α (d), IL-1β (e) and IL-6 (f) were measured by quantitative real-time PCR after knocking down of IRF4. g–i BV-2 cells were pretreated with AG490 (10 μM) for 2 h and then exposed to LPS (100 ng/mL) for 3 h (n = 7). The mRNA levels of TNF-α (g), IL-1β (h) and IL-6 (i) were measured by quantitative real-time PCR. Comparisons of groups were generated with unpaired two-tailed Student’s t-test. Data are shown as the mean ± SEM, ###P < 0.001 vs. the control group, *P < 0.05, ***P < 0.001 vs. the LPS group. AG-10: 10 μM AG490.
Knocking down of BATF2 downregulated IRF4 and inhibited JAK-STAT signaling pathway in LPS-stimulated BV-2 cells
Aforementioned results suggested that ABBV-744 might regulate LPS-induced microglial inflammation by downregulating BATF2 and IRF4 and inhibiting the JAK/STAT signaling pathway. However, the relationships among BATF2, IRF4, and JAK/STAT remain unclear. We further evaluated the influence of BATF2 on IRF4 and JAK/STAT signaling pathway in LPS-exposed BV-2 cells using Western blot analysis. As shown in Fig. 6a, knocking down of BATF2 significantly downregulated LPS-induced protein levels of IRF4 (Fig. 6a). At the same time, we detected the JAK/STAT signaling pathway in the LPS-exposed BV-2 cells. By contrast, knocking down of BATF2 did not affect the level of phosphorylated JAK2, but significantly downregulated the phosphorylation levels of STAT1, STAT3 and STAT5 in the BV-2 cells stimulated with LPS (Fig. 6b–e), suggesting that IRF4 and STAT signaling may be located downstream of BATF2 to regulate microglial inflammation. In order to further clarify the relationship between IRF4 and JAK-STAT signaling, we used small interfering RNA to knockdown IRF4 in BV-2 cells and detected changes in key proteins of the JAK/STAT signaling pathway. As shown in Fig. 6f–i, knocking down of IRF4 gene markedly reversed the increased phosphorylation levels of JAK2, STAT1, STAT3, and STAT5 in the LPS-exposed BV-2 cells. It is speculated that knocking down the BATF2 gene can downregulate the expression of IRF4 induced by LPS, thereby inhibiting the activation of the JAK/STAT signaling pathway and exerting an inflammatory regulatory effect in microglial cells.
Fig. 6. Effect of knocking down of BATF2 on IRF4 and JAK-STAT signaling pathway in LPS-stimulated BV-2 cells.
a–e BV-2 cells were transfected with BATF2 small interfering RNA for 24 h and then exposed to LPS (100 ng/mL) for 3 h (n = 6). Protein levels of IRF4 (a), p-JAK2 and JAK2 (b), p-STAT1 and STAT1 (c), p-STAT3 and STAT3 (d), p-STAT5 and STAT5 (e) were measured by Western blot and β-actin was used as a loading control. f–i BV-2 cells were transfected with IRF4 small interfering RNA for 24 h and then exposed to LPS (100 ng/mL) for 3 h (n = 5–7). Protein levels of p-JAK2 and JAK2 (f), p-STAT1 and STAT1 (g), p-STAT3 and STAT3 (h), p-STAT5 and STAT5 (i) were measured by Western blot and β-actin was used as a loading control. Comparisons of groups were generated with unpaired two-tailed Student’s t-test. Data are shown as the mean ± SEM, ###P < 0.001 vs. the NC (negative control) group, *P < 0.05, **P < 0.01, ***P < 0.001, ns (no significant difference) vs. the NC + LPS group.
ABBV-744 downregulated BATF2 and IRF4 and inhibited the activation of JAK/STAT signaling pathway in LPS-injected mice
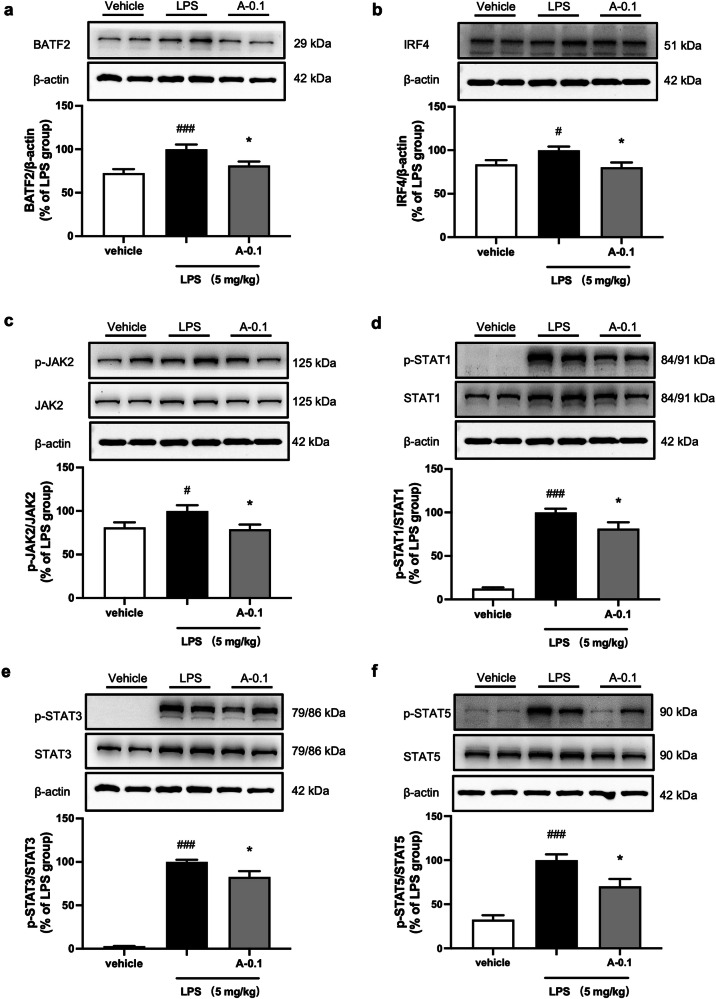
In Fig. 3, we have demonstrated that ABBV-744 could significantly downregulate the protein levels of BATF2 and IRF4 and inhibit the activation of the JAK/STAT signaling pathway in the LPS-induced microglial cell inflammation model. To further verify in vivo regulative effects of ABBV-744 on BATF2, IRF4, and JAK/STAT signaling, we first detected the protein changes of the above pathways in the mouse hippocampus of LPS-injected mice. Western blot experiments showed that intraperitoneal injection of 5 mg/kg LPS significantly upregulated the protein levels of BATF2 and IRF4 in mouse hippocampus tissue, while 0.1 mg/kg ABBV-744 obviously reduced the protein levels of BATF2 and IRF4 (Fig. 7a, b). Consistent with the results of in vitro experiments, ABBV-744 at 0.1 mg/kg significantly inhibited the activation of JAK/STAT signaling pathway, showing by reduced protein levels of p-JAK2, p-STAT1, p-STAT3, and p-STAT5 in the hippocampus of LPS-injected mice (Fig. 7c–f). Similar to the results in the hippocampus, ABBV-744 treatment showed a down-regulatory effect on the expression levels of BATF2, IRF4 and phosphorylation of STAT1, STAT3 and STAT5 in the cortex of LPS-injected mice (Supplementary Fig. S7). Above results indicate that in vivo anti-neuroinflammatory effects of ABBV-744 might be associated with regulation of BATF2-IRF4-STAT1/3/5 signaling pathway.
Fig. 7. Effect of ABBV-744 on LPS-induced expression levels of BATF2, IRF4, and JAK-STAT signaling pathway in LPS-injected mice.
a–f C57BL/6J mice were treated with vehicle (5% DMSO + 40% PEG300 + 55% saline; p.o.) or ABBV-744 (0.1 mg/kg; p.o.) daily for 2 days, followed by injection with saline or LPS (5 mg/kg; i.p.) for 1 day. Mice were sacrificed 3 h after LPS treatment (n = 12). Protein levels of BATF2 (a), IRF4 (b), p-JAK2 and JAK2 (c), p-STAT1 and STAT1 (d), p-STAT3 and STAT3 (e), p-STAT5 and STAT5 (f) were measured by Western blot and β-actin was used as a loading control. Comparisons of groups were generated with unpaired two-tailed Student’s t-test. Data are shown as the mean ± SEM, #P < 0.05, ###P < 0.001 vs. the vehicle group, *P < 0.05 vs. the LPS group. A-0.1: 0.1 mg/kg ABBV-744.
Discussion
In order to successfully fight even halt neuroinflammation and eventually delay the progress of neuroinflammatory disorders, discovery of novel small molecules and key therapeutic target/signaling pathways against microglial inflammation continuing attracting attentions of researchers. In the current study, we demonstrated for the first time that: 1) A BD2 high selective BET inhibitor, ABBV-744, exhibits potent inhibitory effects against LPS-induced microglial inflammation both in vivo and in vitro; 2) ABBV-744 suppresses BATF2-IRF4-STAT1/3/5 signaling in LPS-stimulated neuroinflammation models both in vitro and in vivo; 3) BATF2 exists as the upstream of IRF4 and STAT signaling in the inflammatory microglia.
Although previous studies have demonstrated the preclinical value of the significant inhibitory effects of ABBV-744 in tumor models [42–46], to our knowledge, no reports were found about the anti-inflammatory effect of this small molecule. In the current study, we observed that ABBV-744 exhibited significant inhibitory effects on LPS-induced upregulation of pro-inflammatory mediator, inflammatory cytokines and enzymes in microglial cells (Fig. 1). Similar to the in vitro results, we further verified that ABBV-744 reduced microglial activation and overexpression of pro-inflammatory genes in the brain of LPS-stimulated mice (Fig. 2). In the present study, transcription level of IL-6 was not measured in the brain of LPS-injected mice, since our previous studies have found that relative weak and unstable response of IL-6 transcription to LPS stimulation in the brain of mice, either comparing to the LPS-stimulated robust changes of TNF-α and IL-1β mRNA levels in vivo, or comparing to the LPS-stimulated robust changes of IL-6 mRNA level in microglial cells in vitro, suggesting the changes of pro-inflammatory gene IL-6 might not be a good indicator of inflammatory responses in this animal model. As a matter of fact, the intraperitoneal injection of LPS (5 mg/kg) could cause weight loss of mice (Supplementary Fig. S8) and was reported to induce other periphery responses [47, 48], by contrast, a significant amelioration in body weight reduction was found in the LPS-injected mice treated with 0.2 mg/kg ABBV-744 (Supplementary Fig. S8), indicating the anti-neuroinflammatory effects of ABBV-744 might be interfered by peripheral factors. Our preliminary study employing UPLC-MS/MS analysis found that ABBV-744 is able to deliver across the blood-brain barrier of mice, with the exposure of ABBV-744 in brain tissue homogenates remained sustained levels during 0.5–4 h after drug administration. However, the exposure level of ABBV-744 in the brain of mice is quite low, as compared to that in the plasma or its in vitro effective concentration. Taken together the aberrant influence of LPS and the ameliorative effects of ABBV-744 in body weights (Supplementary Fig. S8), it is possible that the suppressive effect of ABBV-744 in inflammatory responses in the brain could be a synergistic role of ABBV-744 on peripheral system, and/or on complex brain system composed of various cell types. Therefore, further studies will be needed to understand the pharmacokinetic properties of ABBV-744, and the relationship between brain exposure and in vivo anti-neuroinflammatory efficacy of ABBV-744. Together with the RNA sequencing results regarding the effective regulation of ABBV-744 on multiple activated microglial genes and inflammatory pathways in LPS-stimulated BV-2 cells (Fig. 3b, c), the present study demonstrates the potent activities of ABBV-744, a BD2 high selective BET inhibitor, against microglial inflammation.
In order to further elucidate the regulatory molecular mechanisms of ABBV-744, we firstly focused on BATF2, the most significantly down-regulated DEG by ABBV-744, in LPS-stimulated microglial BV-2 cells (Fig. 3b). BATF2, a member of ATF-like basic leucine zipper (bZIP) transcription factor family [49], is predominantly expressed in monocytes/macrophages [50]. Although many studies indicate that BATF2 plays an important regulatory role in peripheral inflammatory processes [51–55], only one study reported the upregulation of BATF2 in TNFα/IFNγ-stimulated retinal microglia [56]. Nevertheless, the intervention role and molecular mechanisms by which BATF2 regulates microglial neuroinflammation have not been reported. In the present study, we found that the significant downregulation of BATF2 by ABBV-744 (Figs. 4, 7) is closely associated with its potent suppressive effects against microglial inflammation (Figs. 1 and 2), and further confirmed that knocking down of BATF2 significantly suppressed the expression of pro-inflammatory genes in LPS-stimulated microglial cells (Fig. 5), suggesting that potent anti-microglial inflammatory effect could be achieved through effective regulation of BATF2 signaling.
Besides BATF2, IRF4 was another important DEG regulated by ABBV-744 in the LPS-stimulated neuroinflammatory responses. IRF4, a member of the interferon regulatory factor (IRF) family, is closely associated with macrophage alternative activation status [57–61], while in the CNS, IRF4 has been characterized as a central regulator of neuroinflammation in ischemic stroke [62–64]. Though presumably the IRF4-ralated regulatory axis is a potential therapeutic target for neuroinflammation, small molecules that efficiently modulate IRF4 have not been reported. In this study, we found that ABBV-744 significantly downregulated LPS-induced IRF4 overexpression both in vivo and in vitro, and knockdown experiments confirmed that IRF4 had a significant inhibitory effect on pro-inflammatory gene expression in LPS-exposed BV-2 cells.
Following the results of KEGG functional annotation and enrichment analysis, we further verified the potent suppressive effect of ABBV-744 on the Janus kinase/signal transducer and activator of transcription (JAK-STAT) signaling pathway, a well-explored signaling pathway involved in periphery and central inflammatory responses [65–67], in the LPS-stimulated neuroinflammation. Currently, small molecules targeting JAKs are emerging therapies for the treatment of autoimmune and inflammatory diseases [68, 69] and increasing evidence showed that compounds with potent inhibitory effects on the JAK/STAT signaling pathway can effectively suppressed microglial inflammation [70–73]. Consistent with these previous findings, we further confirmed that downregulation of JAK-STAT signaling, using JAK inhibitor AG490, reduced the expression of pro-inflammatory genes in LPS-stimulated BV-2 cells (Fig. 5). Taken together the promising role of JAK/STAT signaling in regulating multiple gene transcription [65, 66] and the fact that no study yet reports the relationship between BET inhibition and JAK/STAT regulation in microglial inflammation, exploring the involvement of JAK-STAT signaling pathway on anti-neuroinflammatory effects of ABBV-744 become the priority choice of the present study. Nevertheless, other signaling pathways such as TNFα signaling could also contribute to the anti-inflammatory effects of ABBV-744, therefore, further study will be needed for making comprehensive conclusion.
As far as we know, the molecular mechanisms by which BATF2 mediates the regulation of neuroinflammation have not been reported. Although BATF2 has been sporadically reported to regulate macrophage-mediated immune responses through the JAK/STAT pathway [74, 75] and the Batf2/Irf1 pathway [76], the upstream and downstream regulators of BATF2 in microglial inflammation remain unknown. In this study, we surprisingly found that knocking down of BATF2 in BV-2 cells not only downregulated LPS-induced overexpression of IRF4, but also inhibited the activation of the JAK/STAT signaling pathway (Fig. 6). This indicates that BATF2 is likely to be upstream of IRF4 and JAK/STAT in the regulation of microglial inflammation and the results of IRF4 knockdown experiments further suggest that the JAK/STAT signaling pathway may be regulated by IRF4. Therefore, we speculate that BATF2 exists as the upstream of IRF4 and JAK-STAT signaling in the inflammatory microglia, and ABBV-744 regulates microglial neuroinflammation through the BATF2-IRF4-STAT1/3/5 pathway. To better illustrate the relationship between ABBV-744 and BATF2/IRF4/JAK-STAT regulation in LPS-exposed BV-2 cells, strategy with knocking down of BET, the known target of ABBV-744, was further performed. As shown in Supplementary Fig. S9, knocking down of BRD2, BRD3, and BRD4 genes all significantly inhibited the expression levels of LPS-stimulated overexpression of pro-inflammatory cytokines and mediators in LPS-exposed BV-2 cells. At the gene transcription level, knocking down of BRD2 in BV-2 cells significantly inhibited LPS-induced expression of BATF2 and IRF4, while knocking down of BRD4 in BV-2 cells significantly inhibited LPS-induced expression of IRF4 (Supplementary Fig. S10). At the protein expression level, knocking down of BRD2 in BV-2 cells significantly inhibited LPS-induced overexpression of BATF2 and over-phosphorylation STAT3 and STAT5, while knocking down of BRD4 in BV-2 cells significantly inhibited LPS-induced expression of BATF2 and over-phosphorylation of JAK2, STAT1, STAT3 and STAT5 (Supplementary Fig. S11). Taken together, it is likely that the regulative effects of ABBV-744 on BATF2, IRF4 and JAK-STAT in microglial inflammation is attributed to its BET inhibition profile, although further studies will be needed for understanding the precise mechanisms.
Τo our knowledge, no literature has reported that inhibition of BET/or ABBV-744 treatment could inhibit either BATF2 or IRF4 expression in microglial inflammation, our study may provide important clues for better understanding the molecular mechanism between BET inhibition and microglial inflammation, as well as providing potent active agents for potently suppression of BATF2 or IRF4 signaling pathways. Beside BATF2 and IRF4, many other differentially expressed genes by ABBV-744 were reported to be involved in regulation of microglial inflammation, therefore a high probability exists that other key molecular mechanisms may be involved in the anti-inflammatory effects of ABBV-744, and further studies will be necessary to understand the full picture of regulative mechanisms of ABBV-744.
In conclusion, we first present a potential role of ABBV-744 in suppressing microglial neuroinflammation both in vitro and in vivo, which are closely associated with its regulation on BATF2-IRF4-STAT1/3/5 pathway. Thus, our study provides a candidate compound, namely ABBV-744, for efficiently regulation of neuroinflammation, and we also illustrate potential novel BATF2-related targets/pathways for regulating microglial inflammation.
Supplementary information
Acknowledgements
This work was supported by the Innovation of Science and Technology 2030-Major Projects (Grant No. 2021ZD0200900).
Author contributions
HYZ, LLW, and HW designed the study. LLW, XYX, and WJH performed the animal and cell experiments. HW and JL provided methodological guidance. LLW and SJL conducted the data analysis. LLW drafted and revised the manuscript. HYZ reviewed and revised the manuscript.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-024-01318-4.
References
- 1.Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179:292–311. [DOI] [PubMed] [Google Scholar]
- 2.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borst K, Dumas AA, Prinz M. Microglia: Immune and non-immune functions. Immunity. 2021;54:2194–208. [DOI] [PubMed] [Google Scholar]
- 4.Wright-Jin EC, Gutmann DH. Microglia as dynamic cellular mediators of brain function. Trends Mol Med. 2019;25:967–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lecca D, Jung YJ, Scerba MT, Hwang I, Kim YK, Kim S, et al. Role of chronic neuroinflammation in neuroplasticity and cognitive function: a hypothesis. Alzheimers Dement. 2022;18:2327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21:1359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan X, Wu J, Geng J, Ji D, Wei D, Ling Y, et al. A novel hybrid of telmisartan and borneol ameliorates neuroinflammation and white matter injury in ischemic stroke through ATF3/CH25H axis. Transl Stroke Res. 2024;15:195–218. [DOI] [PubMed] [Google Scholar]
- 9.Hui Y, Xu Z, Li J, Kuang L, Zhong Y, Tang Y, et al. Nonenzymatic function of DPP4 promotes diabetes-associated cognitive dysfunction through IGF-2R/PKA/SP1/ERp29/IP3R2 pathway-mediated impairment of Treg function and M1 microglia polarization. Metabolism. 2023;138:155340. [DOI] [PubMed] [Google Scholar]
- 10.Sun HY, Wu J, Wang R, Zhang S, Xu H, Kaznacheyeva capital Ie C, et al. Pazopanib alleviates neuroinflammation and protects dopaminergic neurons in LPS-stimulated mouse model by inhibiting MEK4-JNK-AP-1 pathway. Acta Pharmacol Sin. 2023;44:1135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi G, Liu L, Cao Y, Ma G, Zhu Y, Xu J, et al. Inhibition of neutrophil extracellular trap formation ameliorates neuroinflammation and neuronal apoptosis via STING-dependent IRE1alpha/ASK1/JNK signaling pathway in mice with traumatic brain injury. J Neuroinflammation. 2023;20:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Ye C, Zhao X, Tong Y, Lin W, Huang Q, et al. TRIM45 aggravates microglia pyroptosis via Atg5/NLRP3 axis in septic encephalopathy. J Neuroinflammation. 2023;20:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert JP, Barsyte-Lovejoy D, et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149:214–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi J, Vakoc CR. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol Cell. 2014;54:728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donati B, Lorenzini E, Ciarrocchi A. BRD4 and cancer: going beyond transcriptional regulation. Mol Cancer. 2018;17:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borck PC, Guo LW, Plutzky J. BET epigenetic reader proteins in cardiovascular transcriptional programs. Circ Res. 2020;126:1190–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo J, Zheng Q, Peng Y. BET proteins: biological functions and therapeutic interventions. Pharmacol Ther. 2023;243:108354. [DOI] [PubMed] [Google Scholar]
- 18.Gilan O, Rioja I, Knezevic K, Bell MJ, Yeung MM, Harker NR, et al. Selective targeting of BD1 and BD2 of the BET proteins in cancer and immunoinflammation. Science. 2020;368:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jahagirdar R, Attwell S, Marusic S, Bendele A, Shenoy N, McLure KG, et al. RVX-297, a BET bromodomain inhibitor, has therapeutic effects in preclinical models of acute inflammation and autoimmune disease. Mol Pharmacol. 2017;92:694–706. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Yin L, Xiong Z, Huang F, Yang N, Jiang F, et al. Discovery of a bromodomain and extra terminal domain (BET) Inhibitor with the selectivity for the second bromodomain (BD2) and the capacity for the treatment of inflammatory diseases. J Med Chem. 2023;66:10824–48. [DOI] [PubMed] [Google Scholar]
- 21.Sun M, Clayton N, Alam S, Asmussen N, Wong A, Kim JH, et al. Selective BET inhibitor RVX-208 ameliorates periodontal inflammation and bone loss. J Clin Periodontol. 2023;50:1658–69. [DOI] [PubMed] [Google Scholar]
- 22.Kharenko OA, Gesner EM, Patel RG, Norek K, White A, Fontano E, et al. RVX-297- a novel BD2 selective inhibitor of BET bromodomains. Biochem Biophys Res Commun. 2016;477:62–7. [DOI] [PubMed] [Google Scholar]
- 23.Seal JT, Atkinson SJ, Aylott H, Bamborough P, Chung CW, Copley RCB, et al. The optimization of a novel, weak bromo and extra terminal domain (BET) bromodomain fragment ligand to a potent and selective second bromodomain (BD2) inhibitor. J Med Chem. 2020;63:9093–126. [DOI] [PubMed] [Google Scholar]
- 24.Picaud S, Wells C, Felletar I, Brotherton D, Martin S, Savitsky P, et al. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc Natl Acad Sci USA. 2013;110:19754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheppard GS, Wang L, Fidanze SD, Hasvold LA, Liu D, Pratt JK, et al. Discovery of N-Ethyl-4-[2-(4-fluoro-2,6-dimethyl-phenoxy)-5-(1-hydroxy-1-methyl-ethyl)phenyl]-6-methyl-7-oxo-1H-pyrrolo[2,3-c]pyridine-2-carboxamide (ABBV-744), a BET bromodomain inhibitor with selectivity for the second bromodomain. J Med Chem. 2020;63:5585–623. [DOI] [PubMed]
- 26.Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–8. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen M, Kaltoft K, Nordahl M, Ropke C, Geisler C, Mustelin T, et al. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proc Natl Acad Sci USA. 1997;94:6764–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Hao T, Yao X, Che Y, Liu Y, Fang M, et al. Crebanine ameliorates ischemia-reperfusion brain damage by inhibiting oxidative stress and neuroinflammation mediated by NADPH oxidase 2 in microglia. Phytomedicine. 2023;120:155044. [DOI] [PubMed] [Google Scholar]
- 29.Hsu CH, Pan YJ, Zheng YT, Lo RY, Yang FY. Ultrasound reduces inflammation by modulating M1/M2 polarization of microglia through STAT1/STAT6/PPARgamma signaling pathways. CNS Neurosci Ther. 2023;29:4113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li HR, Liu Q, Zhu CL, Sun XY, Sun CY, Yu CM, et al. beta-Nicotinamide mononucleotide activates NAD+/SIRT1 pathway and attenuates inflammatory and oxidative responses in the hippocampus regions of septic mice. Redox Biol. 2023;63:102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng Y, Wang Y, Jia F, Liu W, Zhou D, Jin Q, et al. Tailoring supramolecular prodrug nanoassemblies for reactive nitrogen species-potentiated chemotherapy of liver cancer. ACS Nano. 2021;15:8663–75. [DOI] [PubMed] [Google Scholar]
- 32.Kim H, Seo JS, Lee SY, Ha KT, Choi BT, Shin YI, et al. AIM2 inflammasome contributes to brain injury and chronic post-stroke cognitive impairment in mice. Brain Behav Immun. 2020;87:765–76. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M, Chen H, Zhang W, Liu Y, Ding L, Gong J, et al. Biomimetic remodeling of microglial riboflavin metabolism ameliorates cognitive impairment by modulating neuroinflammation. Adv Sci. 2023;10:e2300180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin Z, Shi DD, Li W, Cheng D, Zhang YD, Zhang S, et al. Berberine ameliorates depression-like behaviors in mice via inhibiting NLRP3 inflammasome-mediated neuroinflammation and preventing neuroplasticity disruption. J Neuroinflammation. 2023;20:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Ali T, He K, Liu Z, Shah FA, Ren Q, et al. Ibrutinib alleviates LPS-induced neuroinflammation and synaptic defects in a mouse model of depression. Brain Behav Immun. 2021;92:10–24. [DOI] [PubMed] [Google Scholar]
- 36.Murphy TL, Tussiwand R, Murphy KM. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat Rev Immunol. 2013;13:499–509. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Li Y, Jiang S, Shi FD, Shi K, Jin WN. Targeting CCL5 signaling attenuates neuroinflammation after seizure. CNS Neurosci Ther. 2023;29:317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao N, Li Y, Han J, Wu S, Liu X, Wang Q, et al. Microglia-derived CCL20 deteriorates neurogenesis following intraventricular hemorrhage. Exp Neurol. 2023;370:114561. [DOI] [PubMed] [Google Scholar]
- 39.Zhu L, Liu X, Nemeth DP, DiSabato DJ, Witcher KG, McKim DB, et al. Interleukin-1 causes CNS inflammatory cytokine expression via endothelia-microglia bi-cellular signaling. Brain Behav Immun. 2019;81:292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider P, Weber-Fahr W, Schweinfurth N, Ho YJ, Sartorius A, Spanagel R, et al. Central metabolite changes and activation of microglia after peripheral interleukin-2 challenge. Brain Behav Immun. 2012;26:277–83. [DOI] [PubMed] [Google Scholar]
- 41.Trujillo-Ochoa JL, Kazemian M, Afzali B. The role of transcription factors in shaping regulatory T cell identity. Nat Rev Immunol. 2023;23:842–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elshazly AM, Sinanian MM, Neely V, Chakraborty E, Alshehri MA, McGrath MK, et al. BRD4 Inhibition as a strategy to prolong the response to standard of care in estrogen receptor-positive breast cancer. Cancers. 2023;15:4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K, Tang J, Fan S, Su H, Yu R, Zhang Y, et al. ABBV-744 induces autophagy in gastric cancer cells by regulating PI3K/AKT/mTOR/p70S6k and MAPK signaling pathways. Neoplasia. 2023;45:100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Cai T, Lin X, Huang X, Bui MH, Plotnik JP, et al. Selective inhibition of the second bromodomain of BET family proteins results in robust antitumor activity in preclinical models of acute myeloid leukemia. Mol Cancer Ther. 2021;20:1809–19. [DOI] [PubMed] [Google Scholar]
- 45.Wright S, Hu J, Wang H, Hyle J, Zhang Y, Du G, et al. Interrogating bromodomain inhibitor resistance in KMT2A-rearranged leukemia through combinatorial CRISPR screens. Proc Natl Acad Sci USA. 2023;120:e2220134120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faivre EJ, McDaniel KF, Albert DH, Mantena SR, Plotnik JP, Wilcox D, et al. Selective inhibition of the BD2 bromodomain of BET proteins in prostate cancer. Nature. 2020;578:306–10. [DOI] [PubMed] [Google Scholar]
- 47.Chang CC, Tien CH, Lee EJ, Juan WS, Chen YH, Hung YC, et al. Melatonin inhibits matrix metalloproteinase-9 (MMP-9) activation in the lipopolysaccharide (LPS)-stimulated RAW 264.7 and BV2 cells and a mouse model of meningitis. J Pineal Res. 2012;53:188–97. [DOI] [PubMed] [Google Scholar]
- 48.Simpson BW, Trent MS. Pushing the envelope: LPS modifications and their consequences. Nat Rev Microbiol. 2019;17:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su ZZ, Lee SG, Emdad L, Lebdeva IV, Gupta P, Valerie K, et al. Cloning and characterization of SARI (suppressor of AP-1, regulated by IFN). Proc Natl Acad Sci USA. 2008;105:20906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guler R, Roy S, Suzuki H, Brombacher F. Targeting Batf2 for infectious diseases and cancer. Oncotarget. 2015;6:26575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitada S, Kayama H, Okuzaki D, Koga R, Kobayashi M, Arima Y, et al. BATF2 inhibits immunopathological Th17 responses by suppressing Il23a expression during Trypanosoma cruzi infection. J Exp Med. 2017;214:1313–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guler R, Mpotje T, Ozturk M, Nono JK, Parihar SP, Chia JE, et al. Batf2 differentially regulates tissue immunopathology in type 1 and type 2 diseases. Mucosal Immunol. 2019;12:390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang L, Shen WW, Shao W, Zhao Q, Pang GZ, Yang Y, et al. MANF ameliorates DSS-induced mouse colitis via restricting Ly6C(hi)CX3CR1(int) macrophage transformation and suppressing CHOP-BATF2 signaling pathway. Acta Pharmacol Sin. 2023;44:1175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dai L, Liu Y, Cheng L, Wang H, Lin Y, Shi G, et al. SARI attenuates colon inflammation by promoting STAT1 degradation in intestinal epithelial cells. Mucosal Immunol. 2019;12:1130–40. [DOI] [PubMed] [Google Scholar]
- 55.Dai L, Liu Y, Yin Y, Li J, Dong Z, Chen N, et al. SARI suppresses colitis-associated cancer development by maintaining MCP-1-mediated tumour-associated macrophage recruitment. J Cell Mol Med. 2020;24:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saddala MS, Yang X, Tang S, Huang H. Transcriptome-wide analysis reveals core sets of transcriptional regulators of sensome and inflammation genes in retinal microglia. Genomics. 2021;113:3058–71. [DOI] [PubMed] [Google Scholar]
- 57.Huang SC, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD, et al. Metabolic reprogramming mediated by the mTORC2-IRF4 signaling axis is essential for macrophage alternative activation. Immunity. 2016;45:817–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sasaki K, Terker AS, Pan Y, Li Z, Cao S, Wang Y, et al. Deletion of myeloid interferon regulatory factor 4 (Irf4) in mouse model protects against kidney fibrosis after ischemic injury by decreased macrophage recruitment and activation. J Am Soc Nephrol. 2021;32:1037–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang H, Liu B, Gao Y, Nie J, Feng S, Yu W, et al. Jmjd3/IRF4 axis aggravates myeloid fibroblast activation and m2 macrophage to myofibroblast transition in renal fibrosis. Front Immunol. 2022;13:978262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ming-Chin Lee K, Achuthan AA, De Souza DP, Lupancu TJ, Binger KJ, Lee MKS, et al. Type I interferon antagonism of the JMJD3-IRF4 pathway modulates macrophage activation and polarization. Cell Rep. 2022;39:110719. [DOI] [PubMed] [Google Scholar]
- 61.Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–44. [DOI] [PubMed] [Google Scholar]
- 62.Ngwa C, Al Mamun A, Qi S, Sharmeen R, Conesa MPB, Ganesh BP, et al. Central IRF4/5 signaling are critical for microglial activation and impact on stroke outcomes. Transl Stroke Res. 2023. 10.1007/s12975-023-01172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al Mamun A, Chauhan A, Qi S, Ngwa C, Xu Y, Sharmeen R, et al. Microglial IRF5-IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes. Proc Natl Acad Sci USA. 2020;117:1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ngwa C, Mamun AA, Xu Y, Sharmeen R, Liu F. Phosphorylation of microglial IRF5 and IRF4 by IRAK4 regulates inflammatory responses to ischemia. Cells. 2021;10:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for Inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McLornan DP, Pope JE, Gotlib J, Harrison CN. Current and future status of JAK inhibitors. Lancet. 2021;398:803–16. [DOI] [PubMed] [Google Scholar]
- 69.Benucci M, Bernardini P, Coccia C, De Luca R, Levani J, Economou A, et al. JAK inhibitors and autoimmune rheumatic diseases. Autoimmun Rev. 2023;22:103276. [DOI] [PubMed] [Google Scholar]
- 70.Long Y, Wang Y, Shen Y, Huang J, Li Y, Wu R, et al. Minocycline and antipsychotics inhibit inflammatory responses in BV-2 microglia activated by LPS via regulating the MAPKs/JAK-STAT signaling pathway. BMC Psychiatry. 2023;23:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma H, Wang C, Han L, Kong F, Liu Z, Zhang B, et al. Tofacitinib promotes functional recovery after spinal cord injury by regulating microglial polarization via JAK/STAT signaling pathway. Int J Biol Sci. 2023;19:4865–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L, Zhao D, Wang H, Wang L, Liu X, Zhang H. FPS-ZM1 inhibits LPS-induced microglial inflammation by suppressing JAK/STAT signaling pathway. Int Immunopharmacol. 2021;100:108117. [DOI] [PubMed] [Google Scholar]
- 73.Ri MH, Xing Y, Zuo HX, Li MY, Jin HL, Ma J, et al. Regulatory mechanisms of natural compounds from traditional Chinese herbal medicines on the microglial response in ischemic stroke. Phytomedicine. 2023;116:154889. [DOI] [PubMed] [Google Scholar]
- 74.Kanemaru H, Yamane F, Tanaka H, Maeda K, Satoh T, Akira S. BATF2 activates DUSP2 gene expression and up-regulates NF-kappaB activity via phospho-STAT3 dephosphorylation. Int Immunol. 2018;30:255–65. [DOI] [PubMed] [Google Scholar]
- 75.Hu Y, Gu J, Wang Y, Lin J, Yu H, Yang F, et al. Promotion effect of EGCG on the raised expression of IL-23 through the signaling of STAT3-BATF2-c-JUN/ATF2. J Agric Food Chem. 2021;69:7898–909. [DOI] [PubMed] [Google Scholar]
- 76.Roy S, Guler R, Parihar SP, Schmeier S, Kaczkowski B, Nishimura H, et al. Batf2/Irf1 induces inflammatory responses in classically activated macrophages, lipopolysaccharides, and mycobacterial infection. J Immunol. 2015;194:6035–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.