Abstract
Non-alcoholic fatty liver disease (NAFLD) is one of the most prevalent causes of chronic liver disease worldwide which is often seen in patients with metabolic abnormalities such as those with obesity and insulin resistance. On the other hand, sarcopenia is a generalized and progressive skeletal muscle disorder characterized by low muscle strength, low muscle quality, low physical performance, or a combination of the three. Both disease entities share several underlying risk factors and pathophysiologic mechanisms. These include: (1) cardiometabolic overlaps such as insulin resistance, chronic systemic inflammation, decreased vitamin D levels, sex hormone modifications; (2) muscle-related factors such as those mitigated by myostatin signaling, and myokines (i.e., irisin); and (3) liver-dysfunction related factors such as those associated with growth hormone/insulin-like growth factor 1 Axis, hepatokines (i.e., selenoprotein P and leukocyte cell-derived chemotaxin-2), fibroblast growth factors 21 and 19 (FGF21 and FGF19), and hyperammonemia. This narrative review will examine the pathophysiologic overlaps that can explain the links between NAFLD and sarcopenia. Furthermore, this review will explore the emerging roles of nonpharmacologic (e.g., weight reduction, diet, alcohol, and smoking cessation, and physical activity) and pharmacologic management (e.g., roles of β-hydroxy-β-methylbutyrate, branched-chain amino acid supplements, and testosterone therapy) to improve care, intervention sustainability, and acceptability for patients with sarcopenia-associated NAFLD.
Keywords: NAFLD, sarcopenia, fatty liver disease
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a chronic liver disease often seen in individuals with metabolic abnormalities (e.g., obesity and insulin resistance). Sarcopenia is a condition characterized by the loss of muscle mass and strength that typically occurs with aging and obesity. Together, sarcopenia and NAFLD share many underlying risk factors and pathophysiologic mechanisms, which are primarily addressed by lifestyle interventions such as alcohol cessation, physical activity, and a hypocaloric diet. Through this descriptive study, we aim to further discuss the clinical associations and pathophysiologic mechanisms that link sarcopenia and NAFLD, then focus on the role of nonpharmacologic management and emerging pharmacologic options in NAFLD-associated sarcopenia.
Non-alcoholic fatty liver disease (NAFLD)
Non-alcoholic fatty liver disease is one of the most important causes of liver diseases worldwide and is likely to emerge as the leading cause of end-stage liver disease in the future.1 In the United States of America (USA), an estimated 80-100 million individuals are affected by NAFLD, and with the spread of obesity worldwide, there has also been a concurrent rise in the prevalence of NAFLD in both developed and developing countries.1,2
According to the American Association for the Study of Liver Diseases (AASLD), NAFLD is defined by (1) evidence of hepatic steatosis [i.e., by imaging or histology], and (2) the lack of secondary causes of hepatic fat accumulation [e.g., significant alcohol consumption, the use of steatogenic medication, or monogenic hereditary disorders].3
Metabolic comorbidities such as type 2 diabetes mellitus (T2DM), dyslipidemia, and obesity are strongly linked with the development of NAFLD.4 Studies have shown that NAFLD is increasingly recognized as the hepatic manifestation of metabolic syndrome.1,2 Furthermore, NAFLD itself is a risk factor for cardiovascular disease (CVD), chronic kidney disease (CKD), and T2DM.4
Sarcopenia
Sarcopenia is defined by the European Working Group on Sarcopenia in Older People (EWGSOP) as a generalized and progressive skeletal muscle disorder characterized by low muscle strength, low muscle quality, low physical performance, or a combination of the three.5 A wide variety of tests and tools are available to diagnose sarcopenia, which utilize the above-mentioned EWGSOP criteria to identify the condition. Muscle mass can be measured with magnetic resonance imaging (MRI) and CT scanning, dual x-ray absorptiometry (DXA), ultrasound, bioimpedance analysis, calf circumference measurement, and D3-creatine dilution.6 Muscle strength is assessed with handgrip strength using a handheld hydraulic dynamometer or knee extensor force, while physical performance can be measured with gait speed, the Short Physical Performance Battery (SPPB) test, and the Timed Up and Go (TUG) test.7-9 Sarcopenia has varying definitions and consequently varying approaches to diagnosis, which inevitably affects its estimated prevalence.10 A recent meta-analysis by Petermann-Rocha et al. found that the prevalence of sarcopenia ranges from 10% to 27% in individuals over 60 years old, noting that the values vary considerably according to the classification and cut-off point used.11 Other definitions for sarcopenia include those developed by the International Working Group on Sarcopenia (IWGS) and the Asian Working Group on Sarcopenia (AWGS).12,13 The EWGSOP2, IWGS, and AWGS 2019 adopt similar approaches in defining sarcopenia based on low muscle mass in combination with poor muscle function. Cut-off points for Asian populations were lower compared to non-Asian populations in the previous AWGS 2014 definition but have since been revised in the AWGS 2019 definition of sarcopenia (Table 1).13,14
Table 1.
Definition of Sarcopenia developed by the European, International, and Asian Working Groups on Sarcopenia
| Low muscle mass | Low muscle strength (grip strength) | Low muscle performance (gait speed, short performance physical battery) | |
|---|---|---|---|
| EWGSOP2 | Males: <7.0 kg/m2 Females: <5.5 kg/m2 |
Males: <27 kg Females: <16 kg |
Gait speed ≤0.8 m/s SPPB ≤8-point score |
| IWGS | Males: <7.23 kg/m2 Females: <5.67 kg/m2 |
- | Gait speed ≤1.0 m/s |
| AWGS 2019 | Males: <7.0 kg/m2 Females: <5.4 kg/m2 |
Males: <28 kg Females: <18 kg |
Gait speed ≤1.0 m/s SPPB ≤9-point score |
Adapted from Cruz-Jentoft et al., 2019; Fielding et al., 2011; Chen et al., 2020
Abbreviations: AWGS = Asian Working Group on Sarcopenia; EWGSOP = European Working Group on Sarcopenia in Older People; IWGS = International Working Group on Sarcopenia
Link between NAFLD and sarcopenia
Sarcopenia and NAFLD share many common underlying pathophysiologic mechanisms, such as insulin resistance, chronic inflammation, nutritional deficiencies, and physical inactivity.15,16 Both are associated with obesity, metabolic syndrome, type 2 diabetes, dyslipidemia, and age.17,18 In a recent population study by Hong et al., low levels of serum vitamin D (i.e. serum 25(OH)D level of 4.85–15.26 ng/mL) were found to be associated with sarcopenia (OR 2.65; 95% CI 1.64–4.27 in males and OR 1.80; 95% CI 1.29–2.51 in females], NAFLD (OR 1.82; 95% CI 1.19–2.96 in males), and sarcopenia in NAFLD (OR 2.25; 95% CI 1.26–4.03 in males).19 Low muscle mass and low muscle strength are also positively associated with NAFLD, and concurrent sarcopenia and obesity are associated with a significantly higher risk of NAFLD.20,21 A prospective study of UK Biobank participants identifies low muscle mass and grip strength as risk factors associated with a higher risk of severe NAFLD.11 The prevalence of sarcopenia increases with age and is closely associated with obesity, especially for older adults.22 In American populations, the prevalence of sarcopenia varies across race and ethnicity, with Hispanics having the highest prevalence, followed by Asians, non-Hispanic Whites, and lastly non-Hispanic Blacks.22,23
Moreover, multiple population studies have demonstrated the association between NAFLD and sarcopenia.24-27 Several of these recent studies are summarized in Table 2. It must be noted that most of these studies were conducted in Asian populations, and the methods of assessing and defining NAFLD and sarcopenia differ as well.
Table 2.
Population studies that link sarcopenia and NAFLD
| Study | Population (N) | Key Findings |
|---|---|---|
| Roh et al. (2022) | Korea (1595) | Those with NAFLD have 1.6x risk of developing low muscle mass (LMM) and 2.29x risk of developing low muscle strength (LMS) |
| Wang et al. (2021) | China (578) | LMM independently associated with NAFLD in both males and females; LMS independently associated with NAFLD in males only |
| Wijarnpreecha et al. (2021) | United States (1925) | Individuals with sarcopenic obesity have significantly increased odds of having NAFLD |
| Song et al. (2022) | Korea (2191) | Prevalence of NAFLD and fibrosis increased significantly according to sarcopenic status |
| Chung et al. (2019) | Korea (5989) | The prevalence of NAFLD was significantly higher in subjects with sarcopenia than in those without |
METHODOLOGY
This narrative review focused on the latest literature on the interrelationship between non-alcoholic fatty liver disease and sarcopenia, the majority dating from 2015-2023. Studies included in this review were done using SCOPUS, MEDLINE, and CINAHL databases. Search strings included ‘Non-Alcoholic Fatty Liver Disease’ OR ‘NAFLD and ‘Sarcopenia.’ Additionally, reference lists of primary articles identified through initial searches and manual searches for further pertinent journals were carried out by the researchers.
PATHOPHYSIOLOGIC MECHANISMS EXPLAINING THE LINK BETWEEN NAFLD AND SARCOPENIA
Cardiometabolic overlaps between NAFLD and sarcopenia
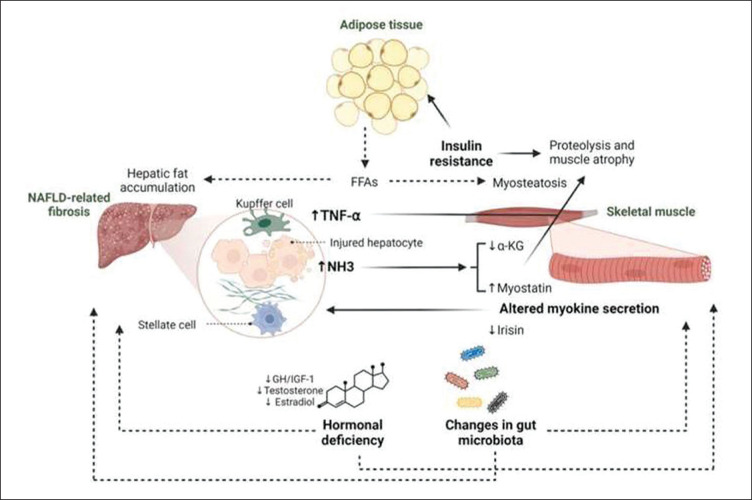
The pathophysiologic processes behind sarcopenia and NAFLD are multifactorial. Recent studies have shown that sarcopenia and NAFLD share several common pathophysiologic mechanisms. Figure 1 illustrates the key mechanisms linking sarcopenia and NAFLD.
Figure 1.
Pathophysiologic mechanisms involved in the relationship between NAFLD and sarcopenia.
Adapted from “Non-alcoholic fatty liver disease-related fibrosis and sarcopenia: An altered liver-muscle crosstalk leading to increased mortality risk,” by Kuchay, M. et al., 2022, Ageing Research Reviews, 80. Copyright [2022] by Elsevier B.V.
Insulin resistance
Skeletal muscles account for nearly 50% of lean body mass. Glucose uptake in the skeletal muscle is essentially insulin-dependent, occurring via glucose transporter 4. Thus, skeletal muscles play an integral role in glucose and energy homeostasis.
Insulin plays an important role in protein synthesis, proteolysis inhibition, amino acid transport in skeletal muscle, and muscle proliferation and hypertrophy.28 Insulin normally increases the activity of phosphatidylinositol 3-kinase (PI3K)/Akt signaling which phosphorylates Forkhead box O (FoxO) transcription factors inhibiting its activation and preventing the induction of atrophy-related muscle-specific ubiquitin ligases: atrogin-1 and muscle ring finger 1 protein (Murf1), that promote skeletal muscle atrophy. Hence, insulin resistance (IR) is an established risk factor for both NAFLD and sarcopenia.29,30
In a state of IR, the insulin-mediated degradation of FoxO transcription factors via the PI3K/Akt pathway is ineffective resulting in its accumulation in the nucleus and induction of the atrophy-related ligases causing skeletal muscle atrophy.29,30 Furthermore, cells fail to respond normally to insulin becoming less effective in taking up glucose from the blood, thereby worsening sarcopenia. Hence, insulin resistance can lead to 1) a reduction of protein synthesis; 2) the promotion of proteolysis and muscle wasting, and 3) the promotion of lipolysis and increased circulating fatty acids (FA) that can be taken up by both muscle and liver. Excess FA stored within the muscle results in myosteatosis (i.e., further reducing muscle protein synthesis which contributes to sarcopenia), while increased delivery of free fatty acids to the liver promotes lipotoxicity that can worsen NAFLD.30,31 Sarcopenia, on the other hand, can enhance IR by reducing cellular targets for insulin action.
Chronic systemic inflammation
NAFLD has been recognized as a subclinical inflammatory state, characterized by the activation of Kupffer cells and subsequent production of tumor necrosis factor-alpha (TNF-α) as well as other pro-inflammatory cytokines that influence disease progression. In an advanced disease state, TNF-α produced by a fibrotic liver can be transported to the skeletal muscle and induce muscle atrophy through the activation of nuclear factor kappa B (NF-kB) resulting in the upregulation of Muscle ring finger 1 (Murf1) protein which mediates sarcomeric protein degradation causing skeletal muscle atrophy.30 Thus, targeting TNF-α inhibition in skeletal muscles may be a potential preventive strategy for liver-fibrosis-induced muscle atrophy. Other proinflammatory cytokines (e.g., Interleukin (IL)-6 and IL-β) can also promote apoptosis in skeletal muscles. This is seen in chronic inflammatory states such as in NAFLD wherein IL-6 inhibits IGF-1 activity, reducing myogenesis.28
Decreased vitamin D levels
Vitamin D is integral in myoblast proliferation and differentiation, skeletal muscle growth, and reduction of muscle inflammation. Furthermore, it regulates the expression of insulin receptors in pancreatic β-cells and peripheral target tissues. Receptors of vitamin D are expressed in cells including the liver and skeletal muscles. Hence, patients with NAFLD have been found to have lower levels of vitamin D leading to worsening inflammation and the promotion of liver fibrosis.28
Sex hormones
Testosterone is an anabolic hormone that promotes muscle protein synthesis, and insulin sensitivity, and decreases fat mass. In men, testosterone deficiency has been associated with an increased accumulation of visceral adipose tissue, insulin resistance, and subsequently increased transport of free fatty acids to the liver, all of which contribute to the development and progression of NAFLD.32 Age-related decrease in sex hormone levels (i.e., testosterone and estrogen deficiency in males and females, respectively) has been associated with sarcopenia, sarcopenic obesity, and its related complications, such as NAFLD. These may explain the higher rates of sarcopenia and NAFLD in elderly patients.30
Muscle-related factors that influence NAFLD progression
Myostatin signaling
Myostatin is a myokine synthesized and secreted in skeletal muscle which plays an integral role in the inhibition of skeletal muscle growth and mass. It works by binding to the activin receptor type IIB (ActRIIB) resulting in the formation and activation of the Smad complex that inhibits muscle hypertrophy and hyperplasia as well as suppresses protein synthesis. Elevated levels of myostatin have been reported in patients with liver fibrosis and cirrhosis.28,30 Myostatin worsens liver fibrosis through the activation of ActRIIB on hepatic stellate cells reducing stellate cell proliferation, inducing cell migration, and increasing procollagen type 1, a biomarker associated with liver injury, inflammation, and fibrosis.30
Irisin
Decreased skeletal muscle as in sarcopenia reduces the secretion of various beneficial myokines such as irisin, which is another myokine that acts on the skeletal muscle, influences glucose homeostasis, increases adipocyte energy expenditure, and improves insulin sensitivity and lipid metabolism. Sarcopenia has also been associated with a reduction of pro-inflammatory cytokines and an elevation of anti-inflammatory cytokines in adipose tissue. Irisin’s ability to regulate lipid metabolism which may help prevent lipid accumulation in liver cells is another potential mechanism that may explain the link between sarcopenia in NAFLD.28,33 Irisin secretion is induced by exercise which may support the link between physical inactivity and NAFLD.34
Liver dysfunction-related factors that promote sarcopenia
Growth hormone/insulin-like growth factor 1 axis
Growth hormone (GH) acts on insulin-sensitive organs (e.g. liver, adipose tissue, and skeletal muscle). Its effects are mediated by insulin-like growth factor-1 (IGF-1) synthesized in the liver. Together, they are key regulators of metabolic homeostasis. It has been demonstrated that patients with NAFLD have reduced IGF-1 gene expression.35 With suppression of the GH/IGF-1 axis activity, Akt is dephosphorylated resulting in the loss of inhibition of FoxO transcription factors and subsequent increased expression of muscle-specific ubiquitin ligases, atrogin-1, and Murf, thus, promoting skeletal muscle atrophy.30 Decreased levels of GH and IGF-1 have also been observed in obesity and in aging which may explain their relationship with NAFLD and sarcopenia.
Hepatokines (Selenoprotein P and leukocyte cellderived chemotaxin-2)
Non-alcoholic fatty liver disease induces the production of oxygen free radicals which results in lipid peroxidation and the production of proinflammatory cytokines, such as TNF-α and TGF-β. Several hepatokines act in an auto-, para-, and endocrine manner to regulate a broad range of metabolic processes. Selenoprotein P (SeP), a hepatokine that works as a selenium transport protein, is increased in NAFLD as well as in type 2 diabetes and pre-diabetes.29,31 SeP inhibits 5’AMP-activated protein kinase (AMPK) in the liver promoting the synthesis of leukocyte cell-derived chemotaxin-2 (LECT2), which induces IR in skeletal muscle. Both SeP and LECT2 provide a direct association between NAFLD and muscle dysfunction.29
Fibroblast growth factors 21 and 19 (FGF21 and FGF19)
FGF21 is synthesized in the liver, pancreas, adipose tissue, and muscle and plays a role in several mechanisms. In the liver, it plays a protective role by inhibiting liver lipogenesis, stimulating hepatic fatty acid oxidation, and reducing lipid transport into the liver by increasing peripheral lipoprotein catabolism and reducing adipocyte lipolysis.36 FGF21 enhances insulin sensitivity in both adipose tissue and skeletal muscle which further enhances glucose utilization. Elevated levels of FGF21 have been observed in patients with NAFLD and other disease processes such as non-alcoholic steatohepatitis, obesity, type 2 diabetes, and chronic kidney disease, among others. However, despite this elevation, FGF21 resistance is hypothesized to occur in these disease states.37 FGF19 also exerts its action in skeletal muscles by inducing muscle hypertrophy and increasing muscle strength. Dysregulation of endocrine FGF signaling, particularly FGF21, and FGF19, has been observed in NAFLD and may help explain its association with sarcopenia.29,37
Hyperammonemia
The liver is primarily involved in physiologic ammonia disposal. However, ureagenesis becomes ineffective in diseases that impair liver function. NAFLD is characterized by urea-cycle dysregulation resulting in hyperammonemia. Two mechanisms have been proposed to explain how hyperammonemia promotes sarcopenia. The first one is due to the increased expression of NF-kB and myostatin. The second mechanism is due to the shift into non-hepatocyte ammonia disposal in skeletal muscles in patients with impaired liver function. Ammonia disposal in skeletal muscles results in the loss of α-keto- glutarate (α-KG), a critical tricarboxylic acid (TCA) cycle intermediate, that promotes skeletal muscle protein synthesis and muscle hypertrophy, as well as inhibits protein degradation.30,38 Hyperammonemia can also promote disease progression by promoting liver fibrosis mainly through the activation of hepatic stellate cells.
DIAGNOSIS
The pioneer in community screening for sarcopenia is the SARC-F questionnaire (Table 3), which is composed of the following domains: Strength, Assistance with walking, Rising from a chair, Climbing stairs, and Falls. Out of 10, a score ≥4 is predictive of sarcopenia. This tool was validated against 3 international consensus panel sarcopenia criteria: European Working Group on Sarcopenia in Older People (EWGSOP), International Working Group on Sarcopenia (IWGS), and AWGS, resulting in a specificity of 94–99% but poor sensitivity of 4–10%.39 Similarly, in a meta-analysis composed of 12,800 eligible subjects, the pooled results of SARC-F specificity and sensitivity were 90% and 21%, respectively using the EWGSOP criteria.40 However, in a recent study on the geriatric population, the ideal SARC-F cut-off was adjusted to 3 when the Youden index was applied, resulting in a specificity of 77% and a sensitivity of 52%.41 In addition, the prognostic utility of SARC-F was studied in primary six racial/ethnic groups (African Americans, Latinos, Japanese, Native Hawaiians, Whites, and other Asian Americans including Filipinos, Chinese, and Koreans), showing statistically significant association with risk of all-cause mortality in males and females, with adjusted HR for overall mortality of 3.73 and 3.10, respectively.40 Due to the low sensitivity demonstrated by SARC-F, Rossi et al. developed the Mini Sarcopenia Risk Assessment (MSRA) questionnaire in two forms, the 5- and 7-items (shown in Table 3), wherein scores of ≤45 and ≤30 are indicative of sarcopenia risk, respectively.42 Against the AWGS criteria, the specificity and sensitivity of MSRA-7 and MRSA-5 were 40%, 87%, 71%, and 90%, respectively.43 Meanwhile, the combination of SARC-F and 7-item MSRA resulted in a specificity of 100% and sensitivity of 63%; hence, this is recommended as a first-line tool for high-risk patients (Table 3).41 In a multicenter, retrospective study involving 23,889 NAFLD patients, a high-risk sarcopenic obesity (SO) screening model was developed to identify high-risk SO subjects. Based on the model showing older age, male, sarcopenia index, and metabolic syndrome as significant risk factors, high-risk SO subjects had significantly higher odds of significant liver fibrosis (fibrosis-4 index >2.67) or atherosclerotic CVD risk score >20% compared to subjects without SO.44 Early identification of sarcopenia before the onset of physical disability is crucial to prevent the progression of sarcopenia.
Table 3.
Screening for Sarcopenia: SARC-F Scoring, 5- and 7-item MSRA
| SARC-F | MRSA | 7 | 5 | |
|---|---|---|---|---|
|
Strength: How much difficulty do you have in lifting and carrying 10 lb? |
None = 0 Some = 1 A lot or unable = 2 |
1. How old are you?
|
0 5 |
0 5 |
|
Assitance in walking: How much difficulty do you have walking across a room? |
None = 0 Some = 1 A lot, use aids, or unable = 2 |
2. Were you hospitalized in the last year?
|
0 5 10 |
0 10 15 |
|
Rise from a chair: How much difficulty do you have transferring from a chair or bed? |
None = 0 Some = 1 A lot or unable without help = 2 |
3. What is your activity level?
|
0 5 |
0 15 |
|
Climb stairs: How much difficulty do you have climbing a flight of 10 stairs? |
None = 0 Some = 1 A lot or unable = 2 |
4. Do you eat 3 meals per day regularly?
|
0 5 |
|
| 0 15 | ||||
|
Falls: How many times have you fallen in the past year? |
None = 0 1-3 falls = 1 > 4 falls = 2 |
5. Do you consume any of the following? Milk or dairy products (yogurt, cheese)
|
0 5 |
- - |
| 6. Do you consume any of the following? Poultry, meat, fish, eggs, legumes, ragout, or ham
|
0 5 |
- - |
||
7. Did you lose weight in the past year?
|
0 5 |
0 10 |
||
Abbreviations: SARC-F: Strength, Assistance with walking, Rise from a chair, Climb stairs and Falls; score ≥4 is predictive of sarcopenia.
MSRA: Mini Sarcopenia Risk Assessment; 5-item ≤45 and 7-item ≤30 are indicative of risk of sarcopenia.
Table 4.
Summary of key findings
| Non-alcoholic fatty liver disease increases the risk of developing low muscle mass and low muscle strength, commonly manifesting as sarcopenic obesity. |
| Overlaps in pathophysiology between NAFLD and sarcopenia are related to insulin resistance, chronic systemic inflammation, decreased vitamin D levels, and sex hormones. |
| Screening for sarcopenia in NAFLD patients using the SARC-F Scoring and 7-item MSRA are crucial to prevent the progression of sarcopenia. |
| Weight reduction through an individualized hypocaloric Mediterranean Diet targeting 1200-1500 kcal/d or a reduction of 500-1000 kcal/d from baseline and a target protein intake of 1.2-1.5 g/kg/day is recommended. |
| In NAFLD alone, vigorous-intensity aerobic exercise is recommended. However, in NAFLD-associated sarcopenia, supervised resistance and hypertrophic training are recommended to revert muscular mass loss. |
| Emergent treatment options includes Beta-hydroxy-beta-methyl butyrate, branched-chain amino acid supplements, and testosterone therapy. |
Abbreviations: NAFLD = Non-alcoholic fatty liver disease; SARC-F = Strength, Assistance with walking, Rise from a chair, Climb stairs and Falls; MSRA = Mini Sarcopenia Risk Assessment
In patients with chronic liver disease, a cross-sectional study with 661 patients reported the usefulness of arm and calf circumferences, which are correlated with skeletal muscle index and grip strength, as simple surrogate markers for detecting sarcopenia. The optimal cut-off values of arm and calf circumferences were respectively determined to be 25.0 and 32.6 cm for males, and 22.7 and 32.1 cm for females.45 Recently, the lowest quintile of serum 25(OH)D level (4.85–15.26 ng/mL) was associated with increased occurrence of sarcopenia for both males and females, NAFLD in males, and sarcopenia-associated NAFLD in males but not in females. Hence, Vitamin D levels can also be a useful marker for sarcopenia and NAFLD, especially in males.46
MANAGEMENT
Specifically, for use in primary health care and community health promotion, the Asian Working Group for Sarcopenia (AWGS) 2019 introduced the concept of “possible sarcopenia,” defined as low muscle strength regardless of reduced physical performance. This aims to increase awareness of sarcopenia prevention through timely lifestyle interventions and to encourage referral to the hospital for confirmatory diagnosis.13 In this narrative review, we will focus on non-pharmacologic management and prevention, which remains the cornerstone of sarcopenia-associated NAFLD treatment.
Non-pharmacological management
Weight Reduction
Sarcopenic obesity, defined as low muscle mass with high levels of adiposity, is associated with poor clinical outcomes (i.e., increased mortality). There is strong evidence that weight reduction has a dose-dependent relationship with the improvement and resolution of NAFLD, remarkably with a weight loss of >10%.47-50 For every 1 kg of weight loss, a review of 43 studies reports a 0.83 U/L reduction in alanine aminotransferase (AST), a 0.56 U/L reduction in aspartate transaminase (AST), and a 0.77% reduction in steatosis assessed by radiology or histology.51 Normalweight NAFLD is defined as BMI ≤25 kg/m2 and ≤23 kg/m2 in non-Asian and Asian patients, respectively.
The American Gastroenterological Association (AGA) recommends a lower target weight-loss threshold for normal-weight NAFLD as it shows the following similar histologic benefits to NASH: fibrosis regression, steatosis improvement, decreased waist circumference, and decreased LDL levels. Non-alcoholic fatty liver disease resolution of 50% and 70% were observed with total body weight loss of 3%–5% and 7%–10%, respectively.47
Moreover, the concept of “hidden obesity” in sarcopenic patients is introduced as BMI ≤25 kg/m2 with the addition of body fat percentage >25% in males and >30% in females. Hence, monitoring is emphasized in normal-weight NAFLD patients. Although presenting with less severe disease, they are susceptible to low skeletal muscle index attributed to decreased IGF-1 production; whereas in females, poor bone mass density, vitamin D3 deficiency, and a decrease in estrogen production.52
Diet
Patients with advanced liver diseases, including cirrhosis (i.e., a complication of NAFLD), are in an accelerated state of starvation and catabolism owed to increased gluconeogenesis, fat oxidation, ketogenesis, and impaired protein turnover.53 To meet a patient’s caloric and nutritional requirements, a consultation with a specialized nutritionist is preferred. The AGA 2019 recommends: 1) an individualized hypocaloric diet targeting 1200–1500 kcal/d or a reduction of 500–1000 kcal/d from baseline; 2) a minimum protein intake of 1.2–1.5 g/kg, favorably chicken, fish, eggs, nuts, lentils, and/or soy; 3) small frequent meals, <4–6 hours in between meals; 4) bedtime snack containing protein and ≥50 g of complex carbohydrates.47 Similarly, the European Society for Clinical Nutrition and Metabolism (ESPEN) guideline recommends reducing periods of starvation by taking 3–5 meals/day and a late evening snack regardless of composition, to improve the total body protein status and nitrogen balance, reverse anabolic resistance, and manage sarcopenia of cirrhosis.53,54 A target protein intake of 1.2–1.5 g/kg/day is recommended for the upregulation of protein anabolism and improvement of total body protein in patients with cirrhosis and sarcopenia.53
In a Korean national survey with 4179 elderly participants with sarcopenia, the low protein intake group (<0.8 g/kg/day) had a significantly higher risk of sarcopenia (OR = 1.707). On the other hand, the high protein intake group (>1.2 g/kg/day) had lower prevalence of sarcopenia and sarcopenia-related factors such as insulin and total body fat.46 However, a nationally representative data-based study demonstrated that participants with a higher sodium intake, as assessed by urinary sodium excretion, had significantly higher risks of both NAFLD (OR, 1.46, 95% CI, 1.27 to 1.66; p<0.001) and sarcopenia (OR, 1.49; 95% CI, 1.28 to 1.73; p<0.001). In addition, the study reported an average daily sodium consumption of 3.3 g in the general Korean population, which is higher than the WHO-recommended 2 g/day. This emphasizes the importance of optimal sodium intake for the general population, especially those at risk for both NAFLD and sarcopenia.55
Alcohol and smoking cessation
Although there is contradicting data on the effect of various amounts of alcohol consumption on NAFLD, the AGA 2019 guidelines recommend the best practice of complete alcohol restriction on adult patients with NAFLD.47 A national prospective study reports a 22–40% reduction of incident cardiovascular disease with alcohol intake up to 49 g/day, but only in patients who were never smokers. Even low (0-9 g/day) alcohol intake produced elevated HRs for incident liver disease and cancer. Significantly elevated risks were shown for alcohol intakes of 10-19 g/day and >30 g/day.56 The American Heart Association's Life's Simple 7 (LS7) metrics as surrogates of healthy living were investigated on subjects with NAFLD. Ideal glycemic control of hemoglobin A1c <5.7%, smoking status corresponding to a current nonsmoker and having smoked <100 cigarettes total, and BP level<120/80 mmHg offered significant protection against premature all-cause deaths. Moreover, smoking was the only ideal LS7 metric that had a higher prevalence among the group with sarcopenia compared to non-sarcopenia.57
Physical activity
The Expert Review of AGA 2019 recommends 1) 150–300 minutes of moderate-intensity or 75–150 minutes of vigorous-intensity aerobic exercise (i.e., walking, stationary biking) per week and 2) resistance training (i.e., weight lifting) as complementary, but not an alternative for adults with NAFLD.47 In a systematic review by Hashida and colleagues, the median effective protocol for aerobic exercise was 4.8 metabolic equivalents (METs) for 40 min/session, 3 times/week for 12 weeks; whereas for resistance exercise, the median effective protocol was 3.5 METs for 45min/session, 3 times/week for 12 weeks.58
Specifically, activity management in sarcopenia-related NAFLD must be individualized, planned, and supervised.59 For patients with poor cardiovascular fitness or any mobility-limiting comorbidity that may cause intolerance to aerobic training, resistance training is performed.60 A systematic review of aerobic versus resistance training of equal duration, frequency, and period both showed improved hepatic steatosis, with no significant differences in BMI, ALT levels, and intrahepatic lipids. Total energy consumption (kcal/total period), percentage of maximum oxygen consumption, and metabolic equivalents were significantly lower in the resistance training group. On the other hand, energy consumption was lower in the aerobic exercise group (kcal/exercise).58 Nonetheless, both types of training downregulate circulating inflammatory markers such as IL-6, CRP, and TNF-α.61
In a meta-analysis on the impact of endurance and combined training (i.e., endurance and resistance) on the sarcopenia criteria in NAFLD, both improved physical performance but not lean body mass. Considering most studies were endurance training protocols, this type of training may not positively impact muscle mass as compared to resistance training, which improves the power-producing capacity and sensitizes muscles to other anabolic stimuli needed in the setting of sarcopenia.
On the other hand, endurance exercise increases the oxidative capacity of skeletal muscle and supports faster protein turnover during resistance training.61 Although the effect on muscle strength of the mentioned training cannot be determined due to a lack of studies, lower muscle strength, ideally evaluated through handgrip strength, is associated with a higher hepatic steatosis index.59 To revert muscular mass loss, hypertrophic training is recommended: intensity of 40–80% of the individual in one maximum repetition, with loads >60% to increase maximal force and muscular mass.62
Meanwhile, an emphasis on lifestyle intervention in addition to MD was adopted for 6 months in an RCT, resulting in significant improvements in ALT levels and liver stiffness, whereas only liver stiffness improved with MD alone. The physical activity program included at least 30 min/d of moderate-vigorous activity (i.e., fast or very fast walking, slow or fast running, dancing, tennis), 10000 steps/d measured via pedometer given, and assessed via the validated Athens Physical Activity Questionnaire.63 A similar study on the combination of aerobic physical activity and MD reduced the severity of liver steatosis and improved gut microbiota in a cohort of patients with NAFLD.64
Pharmacologic management
In patients who are unable to adhere to lifestyle interventions, pharmacological therapy is necessary. However, it should be emphasized that pharmacotherapy is not first-line and that there are no currently approved drugs for the treatment of sarcopenia-associated NAFLD. Instead, we will discuss emerging treatment options for sarcopenia in chronic liver diseases.
β-Hydroxy-β-methylbutyrate
Beta-hydroxy-beta-methyl butyrate (HMB) has been evaluated to increase muscle mass and performance by promoting protein synthesis and counteracting muscle catabolism. In a single-blind RCT, 12 weeks of HMB supplementation (3 g/day) resulted in a statistically significant increase in muscle function assessed through a chair stand test and six-minute walk test, an increase in quadriceps muscle mass measured by ultrasound, and a decrease in liver function index compared to placebo (sorbitol powder).65
In malnourished cirrhotic patients, commercially available oral nutritional supplementations both provided by Abbott Laboratories (Madrid, Spain) were compared in a double-blind RCT: Ensure® Plus Advance (HMB group; 1.5 kcal/mL, 24.3% protein, 28.8% fat, and 1.5 g of calcium HMB per service) and 220 mL of Ensure® Plus High Protein (HP group; 1.25 kcal/mL, 25.3% protein, 23.8% fat). Improvement in liver function scores and BMI, with a reduction of LDL cholesterol, was observed in both groups. Although the HMB group had improved muscle strength and reduced minimal hepatic encephalopathy, larger trials are recommended before using HMB supplements.66 In both RCTs, HMB supplementation was well tolerated by patients albeit with minimal gastrointestinal effects causing dropouts.
Branched-chain amino acid supplements
As a result of liver dysfunction and impaired ureagenesis, hyperammonemia occurs. The response of the body is to remove the excess extrahepatic ammonia occurring in the skeletal muscles through the synthesis of glutamine, in exchange for branched-chain amino acids (BCAAs). Moreover, there is an accelerated state of starvation causing increased gluconeogenesis and catabolism. Hence, there is decreased plasma BCAAs in patients with cirrhosis due to increased utilization as an energy source. Hyperammonemia-induced upregulation of myostatin, a negative regulator of muscle growth, is the key contributor to sarcopenia in patients with chronic liver disease.53 There are ongoing clinical trials on the effect of BCAA supplementation on muscle mass, quality, and molecular markers of muscle regeneration in patients with chronic liver disease (NCT04246918) and liver cirrhosis (NCT03633279). The BCAA 10-gram-packet consists of 952 mg L-Isoleucine, 1904 mg L-Leucine, and 1144 mg L-Valine. In a single-center, prospective study of adult patients with cirrhosis, 24 weeks of oral BCAA supplement twice a day resulted in a significant increase in strength assessed via hand grip, but not in muscle mass.67 Nonetheless, long-term BCAA supplementation (0.20–0.25 g/kg/day) has shown beneficial effects on protein metabolism (i.e., improved muscle mass) in patients with cirrhosis.53
Testosterone therapy
Low serum testosterone levels are seen in patients with decreased liver function, and liver cirrhosis, and even lower levels are seen in patients with sarcopenia and cirrhosis.53 A clinical trial on the efficacy and safety of testosterone therapy in improving sarcopenia in men with cirrhosis (NCT03995251) is ongoing. In a 12-month, double-blinded, placebo-controlled trial in men with cirrhosis and total testosterone <12 nmol/L or free testosterone <230 pmol/L, serial administration of 1000 mg intramuscular testosterone undecanoate resulted in a significant increase in appendicular lean mass, a substantial increase in total lean mass, total bone mass, bone mineral density, hemoglobin, with reduced fat mass and HbA1c.68
KNOWLEDGE GAPS AND FUTURE DIRECTIONS
Non-pharmacologic management has been established as the first-line treatment for sarcopenia-associated NAFLD. Although uncertainties remain on the most effective diet and type, duration, and frequency of physical activity due to limited inter-trial comparisons, RCTs, and heterogeneity of the reviewed studies, optimal management is still individualized and supervised.69 Socioeconomic factors that restrict patient’s adherence to diet and exercise such as culture, younger age, lower income, and lower educational level should be investigated in community-based and large nationwide trials using consistent outcomes measures.70,71 Key recommendations for future studies include local cuisine-based MD modifications, quality of life and need for palliative care referrals, complementary and alternative medicine, and pharmacologic options, especially for patients unable to adhere to lifestyle changes. This information is hoped to improve care, intervention sustainability, and acceptability for patients with sarcopenia-associated NAFLD.
Limitations
There are limitations to this narrative review that could affect how far its conclusions can be generalized. Aside from the aforementioned limitations (i.e., limited intertrial comparisons, RCTs, and heterogeneity of the reviewed studies), there are no existing guidelines for sarcopenia and its interrelationship with non-alcoholic fatty liver disease. The relative variability and lack of uniformity (i.e., definition, diagnostic criteria, and monitoring) among the existing papers such as those seen in the European, International, and Asian Working groups may affect the generalizability of the study. Furthermore, possible confounders of the study are race and age distribution.
CONCLUSION
In this narrative review, we discussed the overlapping pathophysiologic mechanisms between NAFLD and sarcopenia, muscle-related factors influencing NAFLD progression, and liver dysfunction-related factors that promote sarcopenia. Nonpharmacologic management remains the mainstay of treatment for NAFLD-associated sarcopenia: 1) screening and early diagnosis; 2) weight reduction; 3) diet; 4) alcohol and smoking cessation; and 5) resistance and hypertrophic training. For patients unable to perform the mentioned lifestyle interventions, clinical trials on pharmacologic options are ongoing to explore their utility.
Funding Statement
Funding Source None.
Statement of Authorship
All authors certified fulfillment of ICMJE authorship criteria.
CRediT Author Statement
FR: Conceptualization, Methodology, Validation, Investigation, Resources, Writing – review and editing, Supervision, Project administration; AA: Formal analysis, Investigation, Data Curation, Writing – original draft preparation, Visualization, Project administration; DC: Formal analysis, Investigation, Data Curation, Writing – original draft preparation, Visualization; NRB: Formal analysis, Investigation, Data Curation, Writing – original draft preparation, Visualization; SC: Conceptualization, Validation, Writing – review and editing, Supervision, Project administration; GPC: Validation, Data Curation, Writing – review and editing, Visualization, Project administration; MGY: Conceptualization, Methodology, Validation, Resources, Writing – review and editing, Supervision; EL: Validation, Data Curation, Writing – review and editing, Visualization, Project administration; KV: Validation, Data Curation, Writing – review and editing, Visualization, Project administration.
Author Disclosure
The authors declared no conflict of interest.
References
- 1.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. PMID: 28930295. 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Gadiparthi C, Spatz M, Greenberg S, et al. NAFLD epidemiology, emerging pharmacotherapy, liver transplantation implications and the trends in the United States. J Clin Transl Hepatol. 2020;28;8(2):215–21. PMID: 32832402. PMCID: . 10.14218/JCTH.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the study of liver diseases. Hepatology. Hepatology. 2018;67(1):328–57. PMID: 28714183. 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 4.Bang KB, Cho YK. Comorbidities and metabolic derangement of NAFLD. J Lifestyle Med. 2015;5(1):7–13. PMID: 26528424. PMCID: . 10.15280/jlm.2015.5.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 20191; 48(1):16–31. PMID: 30312372. PMCID: . 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dent E, Woo J, Scott D, Hoogendijk EO. Sarcopenia measurement in research and clinical practice. Eur J Int Med. 2021;90:1–9. PMID: 34238636. 10.1016/j.ejim.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Verstraeten LMG, de Haan NJ, Verbeet E, van Wijngaarden JP, Meskers CGM, Maier AB. Handgrip strength rather than chair stand test should be used to diagnose sarcopenia in geriatric rehabilitation inpatients: REStORing health of acutely unwell adults (RESORT). Age Ageing. 2022;51(11):afac242. PMID: 36413590. PMCID: . 10.1093/ageing/afac242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phu S, Kirk B, Bani Hassan E, et al. The diagnostic value of the short physical performance battery for sarcopenia. BMC Geriatr. 2020;20(1):242. PMID: 32660438. PMCID: . 10.1186/s12877-020-01642-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez BP, Gomes IB, de Oliveira CS, et al. Accuracy of the Timed Up and Go test for predicting sarcopenia in elderly hospitalized patients. Clinics (Sao Paulo). 2015;70(5):369–72. PMID: 26039955. PMCID: . 10.6061/clinics/2015(05)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ecarnot F, Rogoli D, Maggi S. Epidemiology of sarcopenia. Practical Issues in Geriatrics; 2021. 10.1007/978-3-030-80038-3_1. [DOI] [Google Scholar]
- 11.Petermann-Rocha F, Balntzi V, Gray SR, et al. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86–99. PMID: 34816624. PMCID: . 10.1002/jcsm.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56. PMID: 21527165. PMCID: . 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LK, Woo J, Assantachai P, et al. Asian Working Group for sarcopenia: 2019 consensus update on Sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300-7. PMID: 32033882. 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and metaanalysis of general population studies. J Diabetes Metab Disord. 2017;16:21. PMID: 28523252. PMCID: . 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joo SK, Kim W. Interaction between sarcopenia and nonalcoholic fatty liver disease. Clin Mol Hepatol. 2023;29(Suppl):S68-78. PMID: 36472051. PMCID: . 10.3350/cmh.2022.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology. 2017; 66(6):2055–65. PMID: 28777879. 10.1002/hep.29420. [DOI] [PubMed] [Google Scholar]
- 17.Juanola O, Martínez-López S, Francés R, Gómez-Hurtado I. Non-alcoholic fatty liver disease: Metabolic, genetic, epigenetic and environmental risk factors. Int J Environ Res Public Health. 2021;18(10):5227. PMID: 34069012. PMCID: . 10.3390/ijerph18105227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishikawa H, Asai A, Fukunishi S, Nishiguchi S, Higuchi K. Metabolic syndrome and sarcopenia. Nutrients. 2021;13(10):3519. PMID: 34684520. PMCID: . 10.3390/nu13103519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong J, Shin WK, Lee JW, Lee SY, Kim Y. Associations of serum vitamin D level with sarcopenia, non-alcoholic fatty liver disease (NAFLD), and sarcopenia in NAFLD among people aged 50 years and older: The Korea National Health and Nutrition Examination Survey IV–V. Metabolic Syndrome and Related Disorders. 2022;20(4): 210–8. PMID: 35100057. 10.1089/met.2021.0106. [DOI] [PubMed] [Google Scholar]
- 20.Gan D, Wang L, Jia M, et al. Low muscle mass and low muscle strength associate with nonalcoholic fatty liver disease. Clin Nutr. 2020;39(4):1124–30. PMID: 31053512. 10.1016/j.clnu.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Cai C, Song X, Chen Y, Chen X, Yu C. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Hepatol Int. 2020;14(1):115–26. PMID: 31290072. PMCID: . 10.1007/s12072-019-09964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du K, Goates S, Arensberg M, Pereira S, Gaillard T. Prevalence of sarcopenia and sarcopenic obesity vary with race/ethnicity and advancing age. Divers Equal Health Care. 2018;15(4):175-83. https://www.primescholars.com/articles/prevalence-of-sarcopenia-and-sarcopenic-obesity-vary-with-raceethnicity-and-advancing-age-94980.html. [Google Scholar]
- 23.Bigman G, Ryan A. Implications of race and ethnicity in sarcopenia US national prevalence of sarcopenia by muscle mass, strength, and function indices. Gerontol Geriatr Res. 2021;4(1):126. PMID: 35368515. PMCID: . [PMC free article] [PubMed] [Google Scholar]
- 24.Roh E, Hwang SY, Yoo HJ, et al. Impact of non-alcoholic fatty liver disease on the risk of sarcopenia: A nationwide multicenter prospective study. Hepatol Int. 2022;16(3):545–54. PMID: 34780030. 10.1007/s12072-021-10258-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang YM, Zhu KF, Zhou WJ, et al. Sarcopenia is associated with the presence of nonalcoholic fatty liver disease in Zhejiang Province, China: A cross-sectional observational study. BMC Geriatr. 2021;21(1):55. PMID: 33446095. PMCID: . 10.1186/s12877-020-01910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wijarnpreecha K, Aby ES, Ahmed A, Kim D. Association between sarcopenic obesity and nonalcoholic fatty liver disease and fibrosis detected by fibroscan. J Gastrointestin Liver Dis. 2021;18;30(2): 227–32. PMID: 33951121. 10.15403/jgld-3323. [DOI] [PubMed] [Google Scholar]
- 27.Chung GE, Kim MJ, Yim JY, Kim JS, Yoon JW. Sarcopenia Is Significantly Associated with Presence and Severity of Nonalcoholic Fatty Liver Disease. J Obes Metab Syndr. 2019;28(2):129–38. PMID: 31294345. PMCID: . 10.7570/jomes.2019.28.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakravarthy MV, Siddiqui MS, Forsgren MF, Sanyal AJ. Harnessing Muscle-Liver Crosstalk to Treat Nonalcoholic Steatohepatitis. Front Endocrinol (Lausanne). 2020;11:592373. PMID: 33424768. PMCID: . 10.3389/fendo.2020.592373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández-Mincone T, Contreras-Briceño F, Espinosa-Ramírez M, et al. Nonalcoholic fatty liver disease and sarcopenia: Pathophysiological connections and therapeutic implications. Expert Rev Gastroenterol Hepatol. 2020;14(12):1141–57. PMID: 32811209. 10.1080/17474124.2020.1810563. [DOI] [PubMed] [Google Scholar]
- 30.Kuchay MS, Martínez-Montoro JI, Kaur P, Fernández-García JC, Ramos-Molina B. Non-alcoholic fatty liver disease-related fibrosis and sarcopenia: An altered liver-muscle crosstalk leading to increased mortality risk. Ageing Res Rev. 2022;80:101696. PMID: 35843589. 10.1016/j.arr.2022.101696. [DOI] [PubMed] [Google Scholar]
- 31.Mikolasevic I, Pavic T, Filipec Kanizaj T, Bender DV, Domislovic V, Krznaric Z. Nonalcoholic fatty liver disease and sarcopenia: Where do we stand? Canadian Journal of Gastroenterology and Hepatology. 2020;2020:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mody A, White D, Kanwal F, Garcia JM. Relevance of low testosterone to nonalcoholic fatty liver disease. Cardiovasc Endocrinol. 2015;4(3): 83–9. PMID: 26405614. PMCID: . 10.1097/XCE.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu J, Ke Y, Wu F, et al. Circulating irisin levels in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterol Res Pract. 2020;2020:8818191. PMID: 33224193. PMCID: . 10.1155/2020/8818191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arhire LI, Mihalache L, Covasa M. IrisIn: A hope in understanding and managing obesity and metabolic syndrome. Front Endocrinol (Lausanne). 2019;10:524. PMID: 31428053. PMCID: . 10.3389/fendo.2019.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dichtel LE, Subudhi S, Drescher H, et al. Expression of IGF-1, IGF-1 receptor and growth hormone receptor in hepatic tissue in adults across the spectrum of nonalcoholic fatty liver disease (NAFLD). J Endocr Soc. 2021;5(Suppl 1): A538-9. 10.1210/jendso/bvab048.1097. [DOI] [Google Scholar]
- 36.Szczepańska E, Gietka-Czernel M. FGF21: A novel regulator of glucose and lipid metabolism and whole-body energy balance. Hormone and Metabolic Research. 2022;54(04):203–11. PMID: 33381084. PMCID: . 10.3389/fendo.2020.601290. [DOI] [PubMed] [Google Scholar]
- 37.Tillman EJ, Rolph T. FGF21: An emerging therapeutic target for non-alcoholic steatohepatitis and related metabolic diseases. Front Endocrinol (Lausanne). 2020;11:601290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai X, Yuan Y, Liao Z, et al. Α-ketoglutarate prevents skeletal muscle protein degradation and muscle atrophy through PHD3/ADRB2 pathway. FASEB J. 2017;32(1):488–99. PMID: 28939592. PMCID: . 10.1096/fj.201700670R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woo J, Leung J, Morley JE. Validating the SARC-F: A suitable community screening tool for sarcopenia ? J Am Med Dir Assoc. 2014;15(9):630–4. PMID: 24947762. 10.1016/j.jamda.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Ida S, Kaneko R, Murata K. SARC-F for screening of sarcopenia among older adults: A meta-analysis of screening test accuracy. J Am Med Dir Assoc. 2018;19(8):685–9. PMID: 29778639. 10.1016/j.jamda.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Rossi AP, Caliari C, Urbani S,et al. Sarcopenia risk evaluation in a sample of hospitalized elderly men and women: Combined use of the Mini Sarcopenia Risk Assessment (MSRA) and the SARC-F. Nutrients. 2021;13(2):635. PMID: 33669277. PMCID: . 10.3390/nu13020635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi AP, Micciolo R, Rubele S, Fantin F, Caliari C, Zoico E, et al. Assessing the risk of sarcopenia in the elderly: The Mini Sarcopenia Risk Assessment (MSRA) questionnaire. J Nutr Health Aging. 2017;21(6):743–9. PMID: 28537342. 10.1007/s12603-017-0921-4. [DOI] [PubMed] [Google Scholar]
- 43.Yang M, Hu X, Xie L, et al. Comparing mini sarcopenia risk assessment with SARC-F for screening sarcopenia in community-dwelling older adults. J Am Med Dir Assoc. 2019;20(1):53–7. PMID: 29909052. 10.1016/j.jamda.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Chun HS, Lee M, Lee HA, et al. Risk stratification for sarcopenic obesity in subjects with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2023;21(9):2298-307.e18. PMID: 36462755. 10.1016/j.cgh.2022.11.031. [DOI] [PubMed] [Google Scholar]
- 45.Endo K, Sato T, Kakisaka K, Takikawa Y. Calf and arm circumference as simple markers for screening sarcopenia in patients with chronic liver disease. Hepatol Res. 2020;51(2):176–89. PMID: 33141991. 10.1111/hepr.13589. [DOI] [PubMed] [Google Scholar]
- 46.Hong J, Shin WK, Lee JW, Kim Y. Relationship between protein intake and sarcopenia in the elderly with nonalcoholic fatty liver disease based on the fourth and fifth Korea National Health and Nutrition Examination Survey. Metab Syndr Relat Disord. 2021; 19(8):452–9. PMID: 34255575. 10.1089/met.2021.0011. [DOI] [PubMed] [Google Scholar]
- 47.Younossi ZM, Corey KE, Lim JK. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: Expert review. Gastroenterology. 2021;160(3):912–8. PMID: 33307021. 10.1053/j.gastro.2020.11.051. [DOI] [PubMed] [Google Scholar]
- 48.Finer N. Weight loss interventions and nonalcoholic fatty liver disease: Optimizing liver outcomes. Diabetes Obes Metab. 2021;24 (Suppl 2):44–54. PMID: 34622555. 10.1111/dom.14569. [DOI] [PubMed] [Google Scholar]
- 49.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterol. 2015;149(2): 367-78.e5. PMID: 25865049. 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Wong VW, Chan RS, Wong GL, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2013;59(3):536-42. PMID: 23623998. 10.1016/j.jhep.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 51.Koutoukidis DA, Koshiaris C, Henry JA, et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism. 2021;115: 154455. PMID: 33259835. 10.1016/j.metabol.2020.154455. [DOI] [PubMed] [Google Scholar]
- 52.Himoto T, Miyatake K, Maeba T, Masaki T. Verification of the nutritional and dietary factors associated with skeletal muscle index in Japanese patients with nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol. 2020;2020:3576974. PMID: 32695733. PMCID: . 10.1155/2020/3576974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim Y. Emerging treatment options for sarcopenia in chronic liver disease. Life. 2021;11(3):250. PMID: 33803020. PMCID: . 10.3390/life11030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bischoff SC, Bernal W, Dasarathy S, et al. ESPEN practical guideline: Clinical nutrition in liver disease. Clin Nutr. 2020;39(12):3533–62. PMID: 33213977. 10.1016/j.clnu.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Han E, Kim MK, Im S-S, Kim HS, Kwon TK, Jang BK. High sodium intake, as assessed by urinary sodium excretion, is associated with nonalcoholic fatty liver disease or sarcopenia. Gut Liver. 202315;17(3):456-65. PMID: 36317511. PMCID: . 10.5009/gnl220133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Åberg F, Puukka P, Salomaa V, et al. Risks of light and moderate alcohol use in fatty liver disease: Follow-up of population cohorts. Hepatology. 2020;71(3):835-48. PMID: 31323122. 10.1002/hep.30864. [DOI] [PubMed] [Google Scholar]
- 57.Van Dongen C, Paik JM, Harring M, et al. Sarcopenia, healthy living, and mortality in patients with chronic liver diseases. Hepatol Commun. 2022;6(11):3140-53. PMID: 35950286. PMCID: . 10.1002/hep4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hashida R, Kawaguchi T, Bekki M, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J Hepatol. 2017;66(1):142–52. PMID: 27639843. 10.1016/j.jhep.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 59.Gonzalez A, Valero-Breton M, Huerta-Salgado C, Achiardi O, Simon F, Cabello-Verrugio C. Impact of exercise training on the sarcopenia criteria in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur J Transl Myol. 2021;31(1):9630. PMID: 33709647. PMCID: . 10.4081/ejtm.2021.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakatsuka T, Tateishi R, Koike K. Changing clinical management of NAFLD in Asia. Liver Int. 2021;42(9):1955–68. PMID: 34459096. 10.1111/liv.15046. [DOI] [PubMed] [Google Scholar]
- 61.Arrese M, Cabello-Verrugio C, Arab JP, Barrera F, Baudrand R, Guarda FJ, et al. Sarcopenia in the setting of nonalcoholic fatty liver. Metab Target Org Damage. 2022; 2:2. 10.20517/mtod.2021.16. [DOI] [Google Scholar]
- 62.Wackerhage H, Schoenfeld BJ, Hamilton DL, Lehti M, Hulmi JJ. Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J Appl Physiol (1985). 2019;126(1):30–43. PMID: 30335577. 10.1152/japplphysiol.00685.2018. [DOI] [PubMed] [Google Scholar]
- 63.Katsagoni CN, Papatheodoridis GV, Ioannidou P, Deutsch M, Alexopoulou A, Papadopoulos N, et al. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: A randomised controlled clinical trial. Br J Nutr. 2018;120(2):164–75. PMID: 35565740. PMCID: . 10.3390/nu14091773. [DOI] [PubMed] [Google Scholar]
- 64.Calabrese FM, Disciglio V, Franco I, Sorino P, Bonfiglio C, Bianco A, et al. A low glycemic index Mediterranean diet combined with aerobic physical activity rearranges the gut microbiota signature in NAFLD patients. Nutrients. 2022;14(9):1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lattanzi B, Bruni A, Di Cola S, et al. The effects of 12-week beta-hydroxy-beta-methylbutyrate supplementation in patients with liver cirrhosis: Results from a randomized controlled single-blind pilot study. Nutrients. 2021;13(7):2296. PMID: 34371806. PMCID: . 10.3390/nu13072296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Espina S, Sanz-Paris A, Gonzalez-Irazabal Y, et al. Randomized clinical trial: Effects of β-hydroxy-β-methylbutyrate (HMB)-enriched vs. HMB-free oral nutritional supplementation in malnourished cirrhotic patients. Nutrients. 2022;14(11):2344. PMID: 35684144. PMCID: . 10.3390/nu14112344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uojima H, Sakurai S, Hidaka H, Kinbara T, Sung JH, Ichita C, et al. Effect of branched-chain amino acid supplements on muscle strength and muscle mass in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29(12):1402–7. PMID: 28984678. 10.1097/MEG.0000000000000968. [DOI] [PubMed] [Google Scholar]
- 68.Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J Hepatol. 2016;65(5):906–13. PMID: 27312945. 10.1016/j.jhep.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 69.Haigh L, Kirk C, El Gendy K, et al. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Clin Nutr. 2022;41(9):1913–31. PMID: 35947894. 10.1016/j.clnu.2022.06.037. [DOI] [PubMed] [Google Scholar]
- 70.Mendonça N, Gregório MJ, Salvador C, Henriques AR, Canhão H, Rodrigues AM. Low adherence to the Mediterranean diet is associated with poor socioeconomic status and younger age: A cross-sectional analysis of the EPIDOC cohort. Nutrients. 2022;14(6):1239. PMID: 35334895. PMCID: . 10.3390/nu14061239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tong TY, Imamura F, Monsivais P, et al. Dietary cost associated with adherence to the Mediterranean diet, and its variation by socioeconomic factors in the UK Fenland study. Br J Nutr. 2018;119(6): 685–94. PMID: 29553031. PMCID: . 10.1017/S0007114517003993. [DOI] [PMC free article] [PubMed] [Google Scholar]