Abstract
Aim: There is limited data available regarding the comparison of Sacituzumab govitecan (SG) vs. chemotherapy in metastatic breast cancer patients.
Materials & methods: We performed a systematic review and meta-analysis aimed to assess the safety profile of SG vs. chemotherapy for metastatic breast cancer (mBC) clinical trials.
Results: The pooled odds ratio for outcomes such as grade 3–4 and all grade neutropenia, leukopenia, anemia and other non-hematological adverse events showed a higher risk for patients receiving SG. No statistically significant differences were reported in terms of grade 3–4 fatigue, all grade nausea, febrile neutropenia and treatment discontinuation due to adverse events.
Conclusion: Our data, coupled with a statistically and clinically meaningful survival benefit, support the use of SG for mBC.
Keywords: : antibody–drug conjugate, breast, breast cancer, sacituzumab govitecan, triple-negative breast cancer
Plain language summary
Article highlights.
Herein, we aimed to compare the safety profile of sacituzumab govitecan (SG) vs. chemotherapy for metastatic breast cancer (mBC) clinical trials.
The pooled odds ratios for outcomes such as grade 3–4 and all grade neutropenia, leukopenia, anemia and other non-hematological adverse events showed a higher risk for patients receiving SG.
Grade 3–4 fatigue, all grade nausea, febrile neutropenia and treatment discontinuation due to adverse events reported no statistically significant differences.
Despite SG reported a higher risk of some treatment-related events, several toxicities showed no statistically significant differences between mBC patients receiving SG vs. chemotherapy.
Further analysis is needed in the future and real-world evidence is required to better explore this issue in this setting.
1. Background
Sacituzumab govitecan (SG) is an antibody–drug conjugate (ADC) whose structure includes an antitrophoblast cell-surface antigen-2 (Trop-2) antibody coupled to the cytotoxic SN-38 payload via a hydrolysable linker, the CL2A, which is used to bind SN-38 to the anti-TROP-2 immunoglobulin [1]. ADCs are a new class of therapeutic agents which deliver chemotherapeutic compounds selectively into tumor cells, with less adverse events (AEs) and toxicities compared with standard cytotoxic chemotherapy [2]. Interestingly, ADCs share some characteristics with other anticancer agents and their mechanism of action is able to boost the antitumor effect of the cytotoxic compound; in fact, ADCs target receptors which are typically over-expressed in cancer cells, but are low in normal tissues including HER2 (as in the case of trastuzumab emtansine and trastuzumab deruxtecan), CD33, CD30, CD22 and Trop-2. Trop-2 represents an epithelial antigen that is expressed in approximately 85–90% of all subtypes of breast cancer (BC), including hormone-responsive (HR) disease and several clinical trials aimed to assess the role of this agent in BC patients have been recently presented and published [3]. Firstly, the multicenter, IMMU-132-01 clinical trial evaluated safety and tolerability of SG as monotherapy and SG was administered in 21-day treatment cycles in pretreated patients with advanced epithelial tumors in this phase 1/2 study [4]. In the phase 1 dose-escalation, SG showed a tolerable pharmacokinetic and safety profile at doses of 8 and 10 mg/kg; in the phase 2 cohort expansion, objective response rate was 33%, including complete responses in three patients (2.8%) and an overall clinical benefit rate (CBR) of 45.4%. Based on the results of this study and with a good therapeutic index at 10 mg/kg, this dose was selected for future developments [4].
More recently, the phase 3 ASCENT trial (NCT02574455) compared SG vs. single-agent chemotherapy-including vinorelbine, capecitabine, eribulin or gemcitabine, according to physician's choice – in metastatic triple-negative breast cancer (mTNBC) patients [5]. In this trial, 468 patients without brain involvement were assigned to receive SG or chemotherapy (235 and 233 patients, respectively). SG improved median progression-free survival (PFS) and overall survival over chemotherapy (5.6 vs. 1.7 months and 12.1 vs. 6.7 months, respectively); according to ASCENT, overall response rate (ORR) was 35% with SG vs. 5% with chemotherapy [5]. The tolerability of SG was good, with a safety profile which was consistent with previous reports: the rate of adverse events (AEs) leading to treatment discontinuation was 4.7%, no treatment-related deaths were observed and diarrhea and neutropenia were the most frequent grade 3–4 AEs [5]. In another trial, the EVER-132-001 phase 2b study, 80 Chinese mTNBC patients received SG monotherapy [6]; objective response rate and median PFS were 38.8% and 5.55 months, respectively, with median overall survival (OS) of 93.8, 82.5 and 68.0% at 3, 6 and 9 months, respectively [6].
The results of these studies led to the US FDA-approval of SG for patients with mTNBC with two or more prior systemic therapies (Initial US Approval: 2020) and at least one of them for metastatic disease and prompted clinicians and researchers to test this agent in other settings, including hormone-responsive (HR) positive (HR+)/HER2- negative (HER2-) metastatic BC [7]. In the TROPiCS-02 trial (NCT03901339), SG was compared with the treatment of physician's choice (including systemic chemotherapy with vinorelbine, capecitabine, gemcitabine or eribulin) in HR+/HER2-, metastatic or locally recurrent inoperable, previously treated HR+/HER2- BC [8]. In this patient population, SG demonstrated a statistically and clinically meaningful median PFS improvement, with a 34% reduction in the risk of disease progression or death (median 5.5 vs. 4.0 months in the experiment and the control arm, respectively) and OS (14.4 months and 11.2 months) [8].
Despite SG has reported a manageable safety profile across different clinical trials, this ADC has a specific set of treatment-related AEs, which may limit adherence to treatment, including hematologic events, diarrhea, febrile neutropenia and infusion-related reactions [9]. However, limited data are available regarding the comparison of SG vs. chemotherapy in BC patients. Since the number of indications and cancer patients receiving SG is supposed to increase further in the near future, a systematic review and meta-analysis was performed aimed to systematically compared the safety profile of SG vs. chemotherapy in phase II and III clinical trials including metastatic BC patients.
2. Materials & methods
2.1. Search strategy
Phase II and III clinical trials published from 15 June 2008 to 18 March 2023, comparing SG vs. chemotherapy for relapsed or refractory metastatic breast cancer (mBC) patients, were retrieved by three different authors. Keywords used for searching on Cochrane Library, EMBASE and PubMed/Medline were the following: ‘sacituzumab govitecan’ or ‘sacituzumab’ or ‘antibody–drug conjugate’ or ‘ADC’ and ‘chemotherapy’ or ‘eribulin’ or ‘vinorelbine’ or ‘capecitabine’ or ‘gemcitabine’ and ‘breast cancer’ or ‘metastatic breast cancer’ or ‘relapsed or refractory breast cancer’. Only articles published in peer-reviewed journals and written in English language were included. Proceedings of the main international oncological meetings (such as European Society of Medical Oncology [ESMO], American Society of Clinical Oncology [ASCO], American Association for Cancer Research [AACR], European CanCer Organization [ECCO]) were also searched from 2008 onward for relevant trials and/or abstracts. The meta-analysis was conducted according to Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (Supplementary Appendix).
2.2. Assessment of risk of bias in included studies
The Cochrane Collaboration tool and risk of bias was used to assess the methodological quality of the included trials; three authors performed the search and analysis.
2.3. Type of outcomes measures
We examined grade 3–4 and all grade neutropenia, grade 3–4 and all grade leukopenia, grade 3–4 and all grade anemia, grade 3–4 and all grade nausea, grade 3–4 and all-grade diarrhea, grade 3–4 and all-grade fatigue, alopecia, febrile neutropenia and treatment discontinuation rate. For each trial, three different authors extracted data from the safety analysis. Data were obtained from the safety analysis of each study or from Supplementary Materials.
2.4. Statistical design
R studio was used to perform all statistical analyses. Dichotomous variables were analyzed by using odds ratios (ORs); ORs were combined with the Mantel-Haenszel method. Heterogeneity among the included trials was measured by the Chi-square test and I2 statistic, with substantial heterogeneity that was considered to exist when the I2 value was greater than 50% or there was a p-value lower than 0.10 in the Chi-square test. A fixed-effect model eas used to analyze quantitative data when I2 was lower than 50%; conversely, a random-effect model was used in case of substantial heterogeneity [10,11].
3. Results
3.1. Selected studies
739 potentially relevant reports were identified, which were restricted to two following the independent evaluation of three authors [5,8]; 737 records were excluded as nonpertinent reports (editorials, ongoing studies/trials in progress, review articles, case reports, preclinical studies, retrospective studies, systematic reviews and meta-analyses, single-arm trials, nonrandomized trials, etc.). Eligible studies were identified and selected (Figure 1); Table 1 reports a summary of the included trials.
Figure 1.

Diagram of all the trials included and excluded in the present meta-analysis.
Table 1.
Summary of all the included studies in the present meta-analysis.
| Trial (NCT number) | Study Design | Experimental arm (n° pts) | Control arm (n° pts) | Primary endpoint | Patient population | Trial Status |
|---|---|---|---|---|---|---|
| TROPiCS-02 (NCT03901339) | Phase 3 | Sacituzumab govitecan (n = 268) | Treatment of physician's choice (capecitabine, vinorelbine, gemcitabine or eribulin) (n = 249) | PFS | Metastatic or locally recurrent inoperable HR+/HER2- breast cancer that progressed after – At least 1 endocrine therapy, taxane and CDK4/6 inhibitor in any setting – At least 2, but no more than 4, lines of chemotherapy for metastatic disease |
Completed |
| ASCENT (NCT02574455) | Phase 3 | Sacituzumab Govitecan (n = 258) | Treatment of physician's choice (capecitabine, vinorelbine, gemcitabine or eribulin) (n = 224) | PFS | Metastatic or locally recurrent inoperable TNBC that progressed after – At least two systemic chemotherapy regimens for unresectable, locally advanced or metastatic disease |
Completed |
HR: Hormone-responsive; PFS: Progression-free survival; TNBC: Triple-negative breast cancer.
3.2. Risk of bias assessment
Studies examined were graded as having a ‘low risk’, ‘high risk’ or ‘unclear risk’ of bias across the specified domains of selection, performance, attrition and reporting bias. The lists of outcomes reported in the published papers were compared to those from study protocols or trial registries. A risk of bias graph was used to summarize the results of the assessment (Figure 2).
Figure 2.

Risk of bias graph; authors' judgments about each risk of bias item is presented as percentages across all included studies.
3.3. Grade 3–4 & all grade neutropenia
The pooled ORs for grade 3–4 and all grade neutropenia were 1.89 (95% CI: 1.46–2.44; Figure 3A) and 2.15 (95% CI: 1.66–2.78; Figure 3B) in mBC patients receiving SG vs. chemotherapy. A fixed effect model was used since low heterogeneity was observed in the analyses (I2 of 0%).
Figure 3.
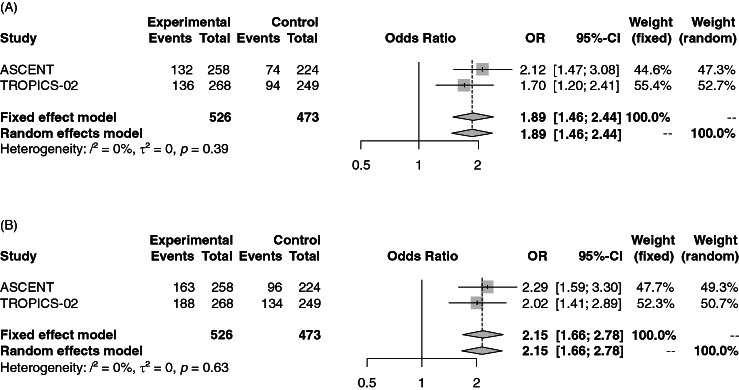
Forest plot of comparison between sacituzumab govitecan vs. chemotherapy for metastatic breast cancer. The outcome was the odds ratio of grade 3–4 (A) and all grade (B) neutropenia.
CI: Confidence interval; OR: Odds ratio.
3.4. Grade 3–4 & all grade leukopenia
The pooled ORs for grade 3–4 and all grade leukopenia were 1.84 (95% CI: 1.12–3.03) and 1.54 (95% CI: 1.05–2.26) (Supplementary Figures S1 & S2) for patients treated with SG vs. single-agent chemotherapy. Heterogeneity was 0% and thus, a fixed effect model was used for both analyses.
3.5. Grade 3–4 & all grade anemia
The pooled ORs for grade 3–4 and all grade anemia were 1.80 (95% CI: 1.02–3.18) and 1.60 (95% CI: 1.21–2.11) (Supplementary Figures S3 & S4) in mBC patients receiving SG vs. chemotherapy. Both analyses showed heterogeneity of 0% and a fixed effect model was used.
3.6. Grade 3–4 & all grade fatigue
The pooled OR for grade 3–4 fatigue was 1.16 (95% CI: 0.28–4.77; Supplementary Figure S5) in patients treated with SG vs. chemotherapy. The analysis was associated with substantial heterogeneity (I2 of 78%); thus, a random effect model was used. The analysis of all grade fatigue showed an OR of 1.62 (95% CI: 1.25–2.11; Supplementary Figure S6) and a fixed effect model was used due to low heterogeneity (I2 of 0%).
3.7. Grade 3–4 & all grade diarrhea
The pooled ORs for grade 3–4 and all grade diarrhea were 7.34 (95% CI: 2.57–21.01; Supplementary Figure S7) and 8.28 (95% CI: 5.23–13.12; Supplementary Figure S8) in mBC patients receiving SG vs. chemotherapy. The analysis of grade 3–4 diarrhea showed low heterogeneity and the fixed effect model was used, while those of all grade diarrhea presented substantial heterogeneity (I2 of 54%) and a random effect model was used.
3.8. Grade 3–4 & all grade nausea
The pooled ORs for grade 3–4 and all grade nausea were 0.45 (95% CI: 0.14–1.52) and 3.16 (95% CI: 2.43–4.12; Supplementary Figures S9 & S10) in mBC patients receiving SG vs. chemotherapy. Both analyses showed heterogeneity <50% and a fixed effect model was used.
3.9. Febrile neutropenia, alopecia & treatment discontinuation due to adverse events
The pooled ORs for febrile neutropenia and alopecia were 1.67 (95% CI: 0.9–3.12; Figure 4) and 4.45 (95% CI: 3.30–6.01; Supplementary Figure S11) in patients receiving SG vs. chemotherapy. Both analyses showed low heterogeneity and a fixed effect model was used. No statistically significant differences in terms of treatment discontinuation due to AEs were reported between SG and chemotherapy, with an OR of 1.12 (95% CI: 0.59–2.11; Figure 5). Heterogeneity was 33% and a fixed effect model was performed.
Figure 4.

Forest plot of comparison between sacituzumab govitecan vs. chemotherapy for metastatic breast cancer; the outcome was the odds ratio of febrile neutropenia.
CI: Confidence interval; OR: Odds ratio.
Figure 5.

Forest plot of comparison between sacituzumab govitecan vs. chemotherapy for metastatic breast cancer; the outcome was the odds ratio of treatment discontinuation due to adverse events.
CI: Confidence interval; OR: Odds ratio.
4. Discussion
Therapeutic agents with a novel mechanism of action, including ADCs, are being tested in mBC and are currently under evaluation, as single-agent or in combination with other anticancer drugs [12,13]. ADCs represent a novel class of agents that can deliver chemotherapeutic compounds selectively into cancer cells with a lower rate of adverse events compared with standard systemic chemotherapy and agents such as trastuzumab deruxtecan, SG, datopotamab deruxtecan have dramatically changed the treatment landscape of several solid tumors, reporting unprecedented responses [14]. This mechanism of action is based on the different parts of ADCs, which share some characteristics with monoclonal antibodies and small molecule drugs to deliver highly cytotoxic and effective payloads, something which in turn should permit to bypass toxicity on noncancer cells and increase the antitumoral cytotoxic effect [15]. Among ADCs, SG has reported notable efficacy in previously treated mBC patients, according to ASCENT and TROPiCS-02 trials and the results of SG monotherapy have prompted clinicians and researchers to consider the expansion and testing of this ADC in other settings, including the earlier stage of BC – such as neoadjuvant and adjuvant treatment [16]. SG consists of three parts: a humanized anti-Trop-2 antibody, the SN-38 payload and a linker for SN-38. As regards SN-38, this agent is more potent than the parent compound, the chemotherapeutic agent and topoisomerase I inhibitor irinotecan [17]. Of note, SN-38 was chosen for its moderate cytotoxicity, which permits delivery in high quantity to the tumor. Conversely, the linker for SN-38 is a hydrolysable, pH-sensitive linker which permits SN-38 release in targeted tumor cells and tumor microenvironment, allowing a bystander effect.
Metastatic BC remains an incurable disease, with a low percentage of patients surviving five years or more. There are substantial unmet needs for this patients population, not only in terms of additional treatment options and improved survival outcomes, but also to maintain quality of life, to reduce the incidence of AEs and optimize anticancer treatments. Ongoing trials are also testing combinatorial strategies including immunotherapy, chemotherapy and ADCs in this setting, in order to improve the anticancer activity of these agents.
Herein, we performed a comprehensive systematic review and meta-analysis with the aim of exploring grade 3–4 and all grade neutropenia, leukopenia, anemia, nausea, diarrhea and fatigue in phase II and III clinical trials comparing SG vs. chemotherapy for mBC patients. We also assessed the risk of alopecia, febrile neutropenia and treatment discontinuation rate in both groups. Two eligible trials were selected in this meta-analysis, encompassing a total of 999 patients (SG: 526; chemotherapy: 473). Despite our research revealing the presence of a higher risk of some AEs in mBC patients receiving SG than those treated with chemotherapy, no statistically significant differences were reported in terms of treatment discontinuation due to AEs and febrile neutropenia, which is commonly considered an important and often life-threatening event. Conversely, grade 3–4 diarrhea was more common in the SG group, which is in line with previous data from clinical trials; the number of events was low in this analysis and these results should be interpreted with caution. Based on the results of the current study, clinical monitoring of AEs should be key to optimize treatment and avoid discontinuation. At the same time, it is worth noting some data from clinical trials: in TROPiCS-02, while, for example, diarrhea worsened in the SG vs. the chemotherapy arms, other symptoms and functional scales were either improved or maintained with SG compared with the control arm [18–20]. In addition, in the same study including HR+/HER2- mBCs, SG showed the health-related quality of life benefit for previously treated patients: over time, a greater proportion of patients receiving chemotherapy experienced clinically meaningful worsening of quality of life vs. those who received SG [21,22]. In fact, median time to deterioration for global health status or quality of life (4.3 vs. 3.0 months; HR 0.75) and fatigue (2.2 vs. 1.4 months; HR 0.73) was significantly longer with SG vs. chemotherapy, while median time to deterioration for pain was similar between treatment groups. Of note, these data, coupled with a statistically significant and clinically meaningful OS benefit, support the use of SG as an important therapeutic option for this patient population with many unanswered questions.
The present meta-analysis has strengths and limitations to be acknowledged. The strengths of our analysis encompass the inclusion of randomized controlled, phase III clinical trials and the overall number of patients (SG: 526; chemotherapy: 473). However, our findings should be interpreted with caution and some limitations should be highlighted. First, although we used random-effect modelling to address heterogeneity, some analyses presented substantial heterogeneity, with I2 higher than 50%. Second, aggregate data were extracted from clinical trials results and used for the meta-analysis since individual patient data were not available. Third, the control arm included widely different chemotherapeutic agents (capecitabine, vinorelbine, gemcitabine or eribulin), which have different efficacy and non-superimposable safety profiles. In addition, it was not possible to include in the analysis some clinical trials, due to the study design (single arm).
5. Conclusion
Despite SG reported a higher risk of some treatment-related events, several toxicities showed no statistically significant differences between mBC patients receiving SG vs. chemotherapy. Our analysis confirms the overall tolerable safety profile of SG in these patients; in addition, we emphasized the presence of specific toxicities. Further analysis is needed in the future and real-world evidence is required to better explore this issue in this setting.
Supplementary Material
Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/14796694.2024.2354162
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Grinda T, Rassy E, Pistilli B. Antibody–drug conjugate revolution in breast cancer: the road ahead. Curr Treat Options Oncol. 2023;24(5):442–465. doi: 10.1007/s11864-023-01072-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontes MS, Vargas Pivato de Almeida D, Cavalin C, Tagawa ST. Targeted therapy for locally advanced or metastatic urothelial cancer (mUC): therapeutic potential of sacituzumab govitecan. Onco Targets Ther. 2022;15:1531–1542. doi: 10.2147/OTT.S339348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolò E, Repetto M, Boscolo Bielo L, Tarantino P, Curigliano G. Antibody–drug conjugates in breast cancer: what is beyond HER2? Cancer J. 2022;28(6):436–445. doi: 10.1097/PPO.0000000000000629 [DOI] [PubMed] [Google Scholar]; • A very interesting review discussing the role of antibody–drug conjugates in breast cancer patients.
- 4.Bardia A, Messersmith WA, Kio EA, et al. Sacituzumab govitecan, a Trop-2-directed antibody–drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021;32(6):746–756. doi: 10.1016/j.annonc.2021.03.005 [DOI] [PubMed] [Google Scholar]
- 5.Rugo HS, Tolaney SM, Loirat D, et al. Safety analyses from the phase 3 ASCENT trial of sacituzumab govitecan in metastatic triple-negative breast cancer. NPJ Breast Cancer. 2022;8(1):98. doi: 10.1038/s41523-022-00467-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The practice-changing phase III ASCENT trial exploring sacituzumab govitecan for triple-negative patients.
- 6.Xu B, Ma F, Wang T, et al. A Phase IIb, single arm, multicenter trial of sacituzumab govitecan in Chinese patients with metastatic triple-negative breast cancer who received at least two prior treatments. Int J Cancer. 2023;152(10):2134–2144. doi: 10.1002/ijc.34424 [DOI] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration www.accessdata.fda.gov/drugsatfda_docs/label/2020/761115s000lbl.pdf
- 8.Kalinsky K, Diamond JR, Vahdat LT, et al. Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol. 2020;31(12):1709–1718. doi: 10.1016/j.annonc.2020.09.004 [DOI] [PubMed] [Google Scholar]
- 9.Furlanetto J, Marmé F, Loibl S. Sacituzumab govitecan: past, present and future of a new antibody–drug conjugate and future horizon. Future Oncol. 2020;18(28):3199–3215. doi: 10.2217/fon-2022-0407 [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JPT, Thomas J, Chandler J, et al. Cochrane hand-book for systematic reviews of interventions. 2nd ed. Chichester (UK): John Wiley & Sons; 2019. doi: 10.1002/9781119536604 [DOI] [Google Scholar]
- 12.Nicolò E, Boscolo Bielo L, Curigliano G, Tarantino P. The HER2-low revolution in breast oncology: steps forward and emerging challenges. Ther Adv Med Oncol. 2023;15:17588359231152842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang Y, Chai Q, Tao W, Liao Y, Liu X, Wu B. Cost-effectiveness of sacituzumab govitecan vs. chemotherapy in advanced or metastatic triple-negative breast cancer. Breast. 2023;68:173–180. doi: 10.1016/j.breast.2023.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarantino P, Carmagnani Pestana R, Corti C, et al. Antibody–drug conjugates: Smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. 2022;72(2):165–182. doi: 10.3322/caac.21705 [DOI] [PubMed] [Google Scholar]
- 15.Thomas A, Teicher BA, Hassan R. Antibody–drug conjugates for cancer therapy. Lancet Oncol. 2016;17(6):e254–e262. doi: 10.1016/S1470-2045(16)30030-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey LA, Loirat D, Punie K, et al. Sacituzumab govitecan as second-line treatment for metastatic triple-negative breast cancer-phase 3 ASCENT study subanalysis. NPJ Breast Cancer. 2022;8(1):72. doi: 10.1038/s41523-022-00439-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veeraballi S, Khawar Z, Aslam HM, Muzaffar M. Role of sacituzumab govitecan in solid tumors. J Oncol Pharm Pract. 2022;28(7):1617–1623. doi: 10.1177/10781552221092359 [DOI] [PubMed] [Google Scholar]
- 18.Giugliano F, Curigliano G, Tarantino P. HER2-low expression in breast oncology: treatment implications in the smart chemotherapy era. Eur J Cancer Prev. 2023;32(2):149–154. doi: 10.1097/CEJ.0000000000000781 [DOI] [PubMed] [Google Scholar]
- 19.Najminejad Z, Dehghani F, Mirzaei Y, Mer AH, Saghi SA, Abdolvahab MH, Bagheri N, Meyfour A, Jafari A, Jahandideh S, Gharibi T, Amirkhani Z, Delam H, Mashatan N, Shahsavarani H, Abdollahpour-Alitappeh M. Clinical perspective: antibody–drug conjugates for the treatment of HER2-positive breast cancer. Mol Ther. 2023;31(7):1874–1903. doi: 10.1016/j.ymthe.2023.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YF, Xu YY, Shao ZM, Yu KD. Resistance to antibody–drug conjugates in breast cancer: mechanisms and solutions. Cancer Commun (Lond). 2023;43(3):297–337. doi: 10.1002/cac2.12387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rugo HS, Bardia A, Marmé F, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;402(10411):1423–1433. doi: 10.1016/S0140-6736(23)01245-X [DOI] [PubMed] [Google Scholar]; •• The practice-changing phase III TROPiCS-02 trial exploring Sacituzumab govitecan for hormone receptor-positive patients.
- 22.Rugo HS, Bardia A, Marmé F, et al. A plain language summary of the TROPiCS-02 study in patients with breast cancer (HR-positive/HER2-negative). Future Oncol. 2024;20(11):635–651. doi: 10.2217/fon-2023-0845 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


