Abstract
Aim: The present study investigated the antimicrobial effectiveness of a rhamnolipid complexed with arginine (RLMIX_Arg) against planktonic cells and biofilms of methicillin-resistant Staphylococcus aureus (MRSA). Methodology: Susceptibility testing was performed using the Clinical & Laboratory Standards Institute protocol: M07-A10, checkerboard test, biofilm in plates and catheters and flow cytometry were used. Result: RLMIX_Arg has bactericidal and synergistic activity with oxacillin. RLMIX_Arg inhibits the formation of MRSA biofilms on plates at sub-inhibitory concentrations and has antibiofilm action against MRSA in peripheral venous catheters. Catheters impregnated with RLMIX_Arg reduce the formation of MRSA biofilms. Conclusion: RLMIX_Arg exhibits potential for application in preventing infections related to methicillin-resistant S. aureus biofilms.
Keywords: : biofilms, medical catheter, MRSA, Staphylococcus aureus, surfactants
Plain language summary
Summary points.
80% of chronic resistant infections are associated with biofilms.
Methicillin-resistant Staphylococcus aureus (MRSA) is a pathogen associated with nosocomial infections, and particularly catheter-related infections.
The development of new therapeutic strategies is imperative to mitigate the impact of MRSA infections.
Amphiphilic structures, like rhamnolipids can modify the interaction between microorganisms and surfaces, disrupting cell adhesion and permeability.
The use of cationic rhamnolipids offers even greater versatility when compared with their anionic counterparts.
Minimum inhibitory concentrations (MICs) of RLMIX_Arg ranged from 4 to 16 μg/ml.
It was found that 37.5% (6) of the strains showed synergism, 56.25% (9) had an additive relationship and 6.25% (1) showed antagonism for the combination of RLMIX_Arg and oxacillin.
This research demonstrates the efficacy of RLMIX_Arg in inhibiting S. aureus biofilm formation and its potential for disassembling mature biofilms within peripheral venous catheters at a concentration of 4 mg/ml and 80 μg/ml.
After impregnating the catheter with 4 mg of RLMIX_Arg, the adhesion of S. aureus cells on the catheter surface was also reduced.
The rhamnolipid complexed with the amino acid arginine, has demonstrated antimicrobial potential against methicillin-resistant S. aureus.
Microbial resistance represents a significant contributor to the rising global mortality and morbidity rates, emerging as a major public health concern [1]. The management of resistant infections is linked to increased expenses for broad-spectrum drugs, as well as extended hospital stays [2]. Treating resistant infections in USA alone incurs an additional annual cost of approximately US$ 2.9 billion [3].
It is estimated that 80% of chronic resistant infections are associated with biofilms, which are defined as microbial communities adhering to biotic or abiotic surfaces immersed in an extracellular polymeric matrix [4]. The architecture of biofilms enables bacteria to survive adverse conditions, displaying antibiotic resistance 1000-fold greater than that of planktonic cells [5]. In the USA, these infections result in an annual cost of US$ 11.5 billion, encompassing surgical site infections, urinary tract infections and particularly those linked to catheter usage [6]. A study conducted in 57 hospitals in India demonstrated that the mortality rate increases by approximately 39% in patients with catheter-related infections [7]. The prevalence rate of biofilm-associated infections varied significantly between studies from different regions and countries [8].
Despite their crucial role, the use of catheters presents a significant risk of developing catheter-related bloodstream infections [9]. Catheters can become contaminated during handling, from the migration of normal skin flora, and through exposure to contaminated fluids, among other factors [10]. Approximately 250,000 bloodstream infections occur annually in Europe, with 36.5% of them being associated with catheter usage [11]. In Germany, Spain and Australia, the rates of catheter-related infections are 3.04, 1.17 and 0.39 per 1000 patients per day, respectively [12].
Staphylococcus aureus (MRSA) is a commensal pathogen associated with opportunistic infections, nosocomial infections, skin and soft tissue infections, and particularly catheter-related infections [13]. A study identified that out of 573 bacteremias caused by S. aureus, 189 originated from intravascular catheters (32.8%) [14]. Another significant concern is the emergence of methicillin-resistant variants of S. aureus, which currently account for 43% of S. aureus infections, resulting in the deaths of approximately 20,000 patients in the USA annually – comparable to the combined number of deaths from AIDS, tuberculosis and hepatitis in the country [15,16].
Considering this context, the development of new therapeutic strategies is imperative to mitigate the impact of MRSA infections. Biosurfactants, chemically defined as amphipathic molecules produced through microbial fermentation [17], have been extensively studied for their antimicrobial properties [18,19]. Several characteristics, including high biodegradability, low toxicity and tolerance to high temperatures and pH variations, render biosurfactants an intriguing option for the pharmaceutical industry [20]. Studies have indicated the efficacy of various rhamnolipids against S. aureus. Titanium disks coated with the rhamnolipid R89BS (4 mg/ml) inhibited the formation of S. aureus biofilms by up to 99% [21].
Amphiphilic structures can modify the interaction between microorganisms and surfaces, disrupting cell adhesion and permeability [22]. Consequently, the use of cationic rhamnolipids offers even greater versatility when compared with their anionic counterparts [23]. In that study, a rhamnolipid produced by Pseudomonas aeruginosa conjugated with the amino acid arginine (RLMIX_Arg) was used [24]. The study revealed that RLMIX_Arg exhibited activity against fungi of the Candida spp genus and demonstrated antiprotozoal efficacy [24]. However, its activity against S. aureus biofilms has yet to be investigated.
Materials & methods
Drugs & microorganisms
Fifteen methicillin-resistant S. aureus strains were utilized, including the MRSA strain ATCC 65398.Additionally, a strain of methicillin-sensitive S. aureus (ATCC 6538) was included. All strains used were sourced from the Laboratory for Bioprospecting of Antimicrobial Molecules (LABIMAN) at the Federal University of Ceará (UFC). A mixture of mono- and di-rhamnolipids conjugated with the amino acid arginine (RLMIX_Arg), which was synthesized and and manufactured following the method described by Da Silva et al. [24] and kindly provided by the Centro Superior de Investigación Científica of the University of Barcelona. The critical micellar concentration of the mixture measured at 0.016 g/l. Oxacillin and vancomycin were commercially obtained from Sigma-Aldrich (USA).
Antimicrobial sensitivity testing & synergism with oxacillin & vancomycin
The minimum inhibitory concentration (MIC) was determined according to protocol M07-A10 [25]. Oxacillin was tested at concentrations of 64–0.25 μg/ml, vancomycin at concentrations of 4–0.015 μg/ml, and RLMIX_Arg at concentrations of 64–0.25 μg/ml. To determine the minimum bactericidal concentration (MBC), a 10 μl aliquot of the suspension used in the sensitivity test was plated on Mueller-Hinton agar plates and incubated for 24 h at 37 °C. The MBC was defined as the lowest concentration capable of inhibiting bacterial growth by 99.9% in colonies on the plates [26]. The tolerance level was determined according to Das et al. [27]. To determine synergism, the checkerboard technique was used with subsequent determination of the fractional inhibitory concentration index (FICI) according to da Silva et al. [28].
Biofilms in 96-well plates
Assessment of the in vitro effect of Rhamnolipid on Biofilms in 96-well plates
The effect of RLMIX_Arg on mature biofilms was determined following the methodology to Costa et al. [29] with modifications. Bacterial biofilm testing was continued with only three MRSA (with similar MIC values) strains for logistical and laboratory material management reasons. To prepare the inoculum, Tryptic Soy Broth (TSB) + 1% glucose medium was used, achieving a concentration at the McFarland scale of 0.5 (1.5 × 108 cells). Subsequently, 200 μl of this suspension was added to 96-well plates. The plates were incubated for 48 h at 37°C. RLMIX_Arg was then introduced at varying concentrations: 4, 8, 16, 64 and 80 μg/ml. The plates were once again incubated for 24 h at 37 °C. For the assessment of cell viability, the 2 mg/ml MTT (3-4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide) reduction assay was employed. The readings were taken at 570 nm using a plate reader after a 24-h incubation period.
Assessing the in vitro sub-inhibitory effect of Rhamnolipid on biofilm formation in 96-well plates
To evaluate the interference of RL-MIX_Arg in the initial phase of biofilm adhesion, we used the method of Brambilla et al. [30], in which inoculum (1.5 × 108 cells) were added together with RL-MIX_Arg at concentrations: 0.5, 1, 2 and 4 μg/ml. The plates were incubated for 48 h at 37 °C. For the assessment of cell viability, the 2 mg/ml MTT (3-4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide) reduction assay was employed. The readings were taken at 570 nm using a plate reader after a 24-h incubation period.
Biofilms in peripheral venous catheters
Assessment of the in vitro effect of rhamnolipid on biofilms in peripheral venous catheters
The assessment of mature antibiofilm activity on catheters followed the protocol established by Weiss et al. [31] with modifications. Jelco 18G peripheral intravenous catheters (Descarpack) were employed, and they were cut into 5.0 mm section. These catheter segments were placed in 24-well plates along with a suspension of MRSA strains (1.5 × 108 cells) Tryptone Soy Broth (TSB) + 1% glucose medium and subsequently incubated for 48 h at 37 °C. After this incubation period, the catheter sections underwent three saline solution washes before being transferred to a new plate. RLMIX_Arg was added at concentrations of 4 μg/ml, 8 μg/ml, 16 μg/ml, 64 μg/ml, and 80 μg/ml. Following this period, the catheter sections were washed and transferred to tubes containing 1 ml of saline solution, then vortexed at 2500 rpm for 1.5 minutes. Subsequently, the samples were diluted, and a 10 μl aliquot was seeded onto Mueller-Hinton agar plates. The plates were then incubated for 12 h at 37 °C. The number of colony-forming units (CFU) was enumerated and multiplied by the dilution factor [32].
Effect of RLMIX_Arg -impregnated catheters on biofilm formation
RLMIX_Arg coating via physical adsorption was obtained acording to [22] by immersing silicone coupons in 1 ml of 4 μg/ml, 8 μg/ml, 16 μg/ml, 64 μg/ml, 80 μg/ml, 4 mg/ml and 8 mg/ml RLMIX_Arg solution for 24 h at 37°C. After impregnating catheter fragments with RLMIX_Arg, tests were performed to evaluate the effect on biofilm formation by three isolates of MRSA. Biofilm formation was induced, as previously described (in section X.). Chloroform impregnated catheters were used as biofilm growth control. A plate antibiogram [25] test with MRSA strain 2 was carried out to confirm the effect on inhibiting the growth of the strains after impregnation.
Assessment of cell membrane damage
The membrane integrity of MRSA cells was assessed using the propidium iodide exclusion test (2 mg/L). Cells were incubated with RLmixArg (4, 8 and 16 μg/ml) alone and combined with oxacillin for 20 h. A total of 10,000 events were evaluated [26].
TUNEL test
The deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was used for DNA fragmentation analysis. Cells were fixed in 7% paraformaldehyde and washed with 1% Triton X-100 for 10 min on ice. Subsequently, they were incubated with the TUNEL reaction mixture for 1 h at 37 °C [26].
Scanning electron microscopy analysis
The analysis was performed according to Do Amaral Valente Sá et al. [33]. First, methicillin-resistant S. aureus biofilms were formed on catheters (20 mm) and treated with 8 and 80 μg/ml. The biofilms were then fixed with 2.5% glutaraldehyde in 0.15M sodium cacodylate buffer and incubated overnight at 4 °C. Dehydration of the cells was performed using an increasing alcohol series followed by drying with hexamethyldisilazane (Polysciences Europe, Hirschberg an der Bergstraße, Germany). The samples were then coated with a 10 nm layer of gold (Emitech Q150T, Quorum Technologies Ltd, Laughton, UK) and analyzed with an FEI Inspect S50 microscope operating in high vacuum mode.
Statistical analysis
The sensitivity and checkerboard tests were performed in triplicate, and the average of the three experiments was used. The absorbances of the biofilms on polystyrene plates were evaluated using one-way and two-way analysis of variance (ANOVA) followed by the Tukey test (p < 0.05), and the damage was determined by cytometry. For biofilms formed on catheters, the mean of colony-forming units in triplicate was used followed by one-way and two-way analysis of variance (ANOVA) and the Tukey test (p < 0.05).
Results
RLMIX_Arg has bactericidal & synergistic activity with oxacillin
Minimum inhibitory concentrations (MICs) of RLMIX_Arg ranged from 4 to 16 μg/ml. The ATCC strains of methicillin-susceptible and methicillin-resistant S. aureus had MICs of 4 and 8 μg/ml, respectively, for RLMIX_Arg (Table 1). All clinical strains tested exhibited oxacillin resistance, with MIC values ranging from 16 to 256 μg/ml. MIC values equal to or greater than 4 for oxacillin are classified as resistant [34]. The oxacillin-sensitive S. aureus strain employed as a control demonstrated an MIC of 0.125. Regarding vancomycin, only one strain exhibited intermediate resistance with an MIC of 4 (Table 1). The remaining strains were considered vancomycin-sensitive, with MIC values less than or equal to 2 [34]. Regarding the minimum bactericidal concentration (MBC) the values ranged from 8–32 μg/ml. The CBM/MIC ratios ranged from 1 to 2, characterizing bactericidal activity (Table 1). It was found that 37.5% (6) of the strains showed synergism, 56.25% (9) had an additive relationship and 6.25% (1) showed antagonism for the combination of RLMIX_Arg and oxacillin (Table 2). For the combination of RLMIX_Arg and vancomycin, 50% (8) of the strains had additive effect and 50% (8) showed antagonistic affect. For this reason, the study continued using oxacillin as an alternative to a combination with the biosurfactant.
Table 1.
Antibacterial activity of RLMIX_Arg isolated against methicillin-resistant S. aureus (MRSA) strains.
| MIC† values μg/ml | |||||||
|---|---|---|---|---|---|---|---|
| Isolated | Tolerance level | ||||||
| Strains | Source | RL-MIX-ARG | Oxa | Vanco | MBC‡ | MBC/CIM | INTER§ |
| MRSA ATCC | ATCC | 8 | 8 | 2 | 8 | 1 | Bactericide |
| SA ATCC | ATCC | 4 | 0.125 | 0.5 | 8 | 2 | Bactericide |
| MRSA 1¶,# | Blood | 8 | 256 | 1 | 8 | 1 | Bactericide |
| MRSA 2¶ | Ocular secretion | 8 | 32 | 0.5 | 8 | 1 | Bactericide |
| MRSA 3 | Urine | 8 | 256 | 1 | 8 | 1 | Bactericide |
| MRSA 4¶ | Abscess secretion | 8 | 64 | 0.5 | 8 | 1 | Bactericide |
| MRSA 5 | Pleural fluid | 8 | 64 | 1 | 8 | 1 | Bactericide |
| MRSA 6 | Blood | 4 | 64 | 1 | 8 | 2 | Bactericide |
| MRSA 7 | Blood | 4 | 128 | 2 | 8 | 2 | Bactericide |
| MRSA 8 | Blood | 16 | 64 | 4 | 16 | 1 | Bactericide |
| MRSA 9 | Skin secretion | 8 | 32 | 1 | 16 | 2 | Bactericide |
| MRSA 10 | Blood | 16 | 64 | 2 | 32 | 2 | Bactericide |
| MRSA 11 | Skin secretion | 8 | 16 | 1 | 16 | 2 | Bactericide |
| MRSA 12 | Blood | 8 | 64 | 1 | 16 | 2 | Bactericide |
| MRSA 13 | Blood | 16 | 64 | 2 | 32 | 2 | Bactericide |
| MRSA 14 | Tracheal aspirated | 8 | 256 | 1 | 16 | 2 | Bactericide |
MIC (minimum inhibitory concentration) concentration capable of inhibiting 99.99% of visible growth.
MBC (minimum bactericidal concentration) less concentration capable of inhibiting 99.99% of bacterial.
MBC/MIC ≥16 bacteriostatic; MBC/MIC ≤4 bactericide.
Strains used in biofilm tests in plates, catheters and cytometry.
Strain used in scanning electron microscopy analysis.
Table 2.
Antibacterial activity of RLMIX_Arg in combination with oxacillin and vancomycin against methicillin-resistant S. aureus.
| MIC† values μg/ml | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Combined | |||||||||
| Strains | Source | RL-MIX-ARG | Oxa | ΣFICI‡ | INTER§ | Vanco | RL-MIX-ARG | ΣFICI‡ | INTER§ |
| MRSA ATCC | ATCC | 8 | 8 | 2 | ANT | 1 | 4 | 1,00 | ADD |
| SA ATCC | ATCC | 2 | 0,0625 | 1 | ADD | 0,5 | 4 | 2,00 | ANT |
| MRSA 1¶,# | Blood | 4 | 128 | 1 | ADD | 1 | 8 | 2,00 | ANT |
| MRSA 2¶ | Ocular secretion | 4 | 16 | 1 | ADD | 0,5 | 8 | 2,00 | ANT |
| MRSA 3 | Urine | 4 | 128 | 1 | ADD | 1 | 8 | 2,00 | ANT |
| MRSA 4¶ | Abscess secretion | 4 | 32 | 1 | ADD | 0,5 | 8 | 2,00 | ANT |
| MRSA 5 | Pleural fluid | 2 | 16 | 0,5 | SYN | 0,5 | 4 | 1,00 | ADD |
| MRSA 6 | Blood | 0,5 | 8 | 0,25 | SYN | 0,5 | 2 | 1,00 | ADD |
| MRSA 7 | Blood | 1 | 32 | 0,5 | SYN | 2 | 4 | 2,00 | ANT |
| MRSA 8 | Blood | 8 | 32 | 1 | ADD | 4 | 16 | 2,00 | ANT |
| MRSA 9 | Skin secretion | 1 | 4 | 0,25 | SYN | 0,5 | 4 | 1,00 | ADD |
| MRSA 10 | Blood | 4 | 16 | 0,5 | SYN | 1 | 8 | 1,00 | ADD |
| MRSA 11 | Skin secretion | 4 | 8 | 1 | ADD | 0,5 | 4 | 1,00 | ADD |
| MRSA 12 | Blood | 4 | 32 | 1 | ADD | 0,5 | 4 | 1,00 | ADD |
| MRSA 13 | Blood | 4 | 16 | 0,5 | SYN | 1 | 8 | 1,00 | ADD |
| MRSA 14 | Tracheal aspirated | 4 | 128 | 1 | ADT | 1 | 8 | 2,00 | ANT |
MIC (minimum inhibitory concentration) concentration capable of inhibiting 99.99% of visible growth.
ΣFICI: Fractional inhibitory concentration.
FIC ≤0,5 synergism (SYN); FIC >0.5 but <1 partial synergism (SYP); FIC = 1 additive (ADD); FIC >1 antagonismo (ANT).
Strains used in biofilm tests in plates, catheters and cytometry.
Strain used in scanning electron microscopy analysis.
INTER: Interpretation; OXA; Oxacilin; Vanco: Vancomicin.
RLMIX_Arg has an antibiofilm action against MRSA
For the pre-formed biofilms in the 96-well plates, a significant decrease in cell viability (p < 0.05) was observed for all three strains tested. None of the tested strains showed significant reduction (p < 0.05) when using MIC/2 (4 μg/ml), MIC (8 μg/ml) and 2xMIC (16 μg/ml) (Figure 1). MRSA 1 strain caused growth reductions of 91.7% and 95.1% at the concentrations of 64 and 80 μg/ml, respectively. MRSA 4 strain showed 77.6% and 80.9% reduction for 64 μg/ml and 80 μg/ml, respectively. MRSA 2 had the lowest levels of reduction, 52.5 and 69.4%, for the concentrations of 64 and 80 μg/ml. In all strains tested, the concentrations of 64 and 80 μg/ml were significant when compared with the other concentrations used. Regarding the reduction in cell biomass, the results found were: MRSA 1 a decrease of 31.67% at concentration of 80 μg/ml, MRSA 2 a decrease of 32.34% (80 μg/ml) and MRSA a decrease of 24.34% (80 μg/ml) (Supplementary Figure 1).
Figure 1.

Effect of RLMIX_Arg on the cell viability of preformed 48 h biofilms of methicillin-resistant S. aureus (MRSA) in 96-well plates after 24 h of incubation.
(A) * p < 0.05 compared with control / ** p < 0.01 compared with control/ *** p < 0.001 compared with control/ # compared with 16 μg/Ml. (B) Chemical structure of the new biosurfactants produced from the conjugation between mono and di-ramnolipids complex with the amino acid arginine, resulting in the mixture RLMIX_Arg. 1: di-ramnolipids complex; 2: mono-ramnolipids complex.
RLMIX_Arg inhibits the formation of MRSA biofilms on plates at sub-inhibitory concentrations
All the concentrations tested showed a reduction in biofilm formation. The greatest reduction was found in MRSA strain 1, showing a 52.2% reduction at a concentration of 2 μg/ml and 44.2% at 1 μg/ml. For MRSA strain 2, a 48.8% reduction was shown at 1 μg/ml and 47% at 2 μg/ml. The MRSA 4 strain showed lower levels of reduction in biofilm formation, 26% at 2 μg/ml. In all the strains tested, the concentrations of 1 μg/ml and 2 μg/ml were more effective in preventing biofilm formation in 96-well plates (Figure 2).
Figure 2.

Antibiofilm effect of RLMIX_Arg on the cell viability of methicillin-resistant S. aureus (MRSA) in 96-well plates after 48 h of incubation.
* p < 0.05 compared with control / ** p < 0.01 compared with control/ *** p < 0.001 compared with control.
RLMIX_Arg has antibiofilm action against MRSA in peripheral venous catheters
RLMIX_Arg significantly decreased the amount of viable cells/colony-forming units (CFU/ml) in the mature biofilms (48 h) at the concentration of 80 μg/ml after incubation for 24 h (p < 0.001) (Figure 3). At the concentrations of MIC (8 μg/ml) and 2xMIC (16 μg/ml), no significant difference was observed compared with the control. At the concentration of 80 μg/ml, inhibition of about 90% was observed in all strains.
Figure 3.
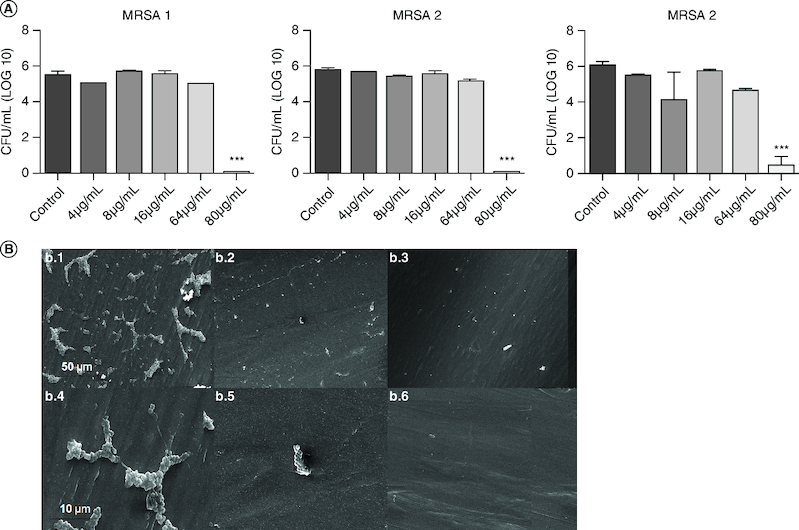
Effect of RLMIX_Arg on preformed biofilms against methicillin-resistant S. aureus in Jelco 18G intravenous peripheral catheters after 24 h of treatment and colony-forming units (CFU) at different concentrations.
(A) * p < 0.05 compared with control / ** p < 0.01 compared with control/ *** p < 0.001 compared with control/ # compared with 16 μg/ml. (B) (b.1) 2000×, scale 50 μm; (b.4) 10,000×, scale 10 μm) untreated catheter. (b.2) 2000×, scale 50 μm; (b.5) 10,000×, scale 10 μm) catheter treated with 8 μg/ml. (b.3) 2000×, scale 50 μm; (b.6) 10,000×, scale 10 μm) catheter treated with 80 μg/ml.
Catheters impregnated with RLMIX_Arg reduce the formation of MRSA biofilms
After impregnating the catheters at concentrations of 4, 8, 16, 64 and 80 μg/ml, no significant reductions in biofilm growth were found. The concentration of 4 mg/ml showed the greatest reduction in biofilm formation. For MRSA 1 there was a 28.1% reduction, for MRSA 2 the greatest reduction was obtained, 36.85% and MRSA 4 a 23.75% reduction. At a concentration of 8 mg/ml the rates of reduction in biofilm formation were lower than at 4 mg/ml. A halo of inhibition was observed only at the highest concentrations, 4 μg/ml and 8 μg/ml. No halo of inhibition was observed on the catheter used as a control (Figure 4).
Figure 4.
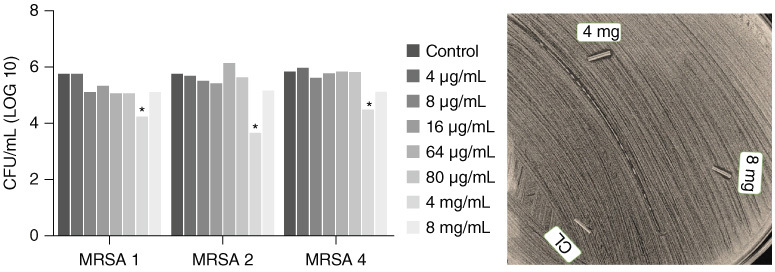
Effect of RLMIX_Arg impregnated catheter against methicillin-resistant S. aureus (MRSA).
(A) Colony-forming units at different concentrations. (B) Antibiogram test using strain MRSA 2.
CFU: Colony-forming units.
RLMIX_Arg causes damage to the MRSA cell membrane
In all three strains tested, there was a significant reduction in cell viability (p < 0.05). Oxacillin, which was used as a control, reduced cell viability by 60, 36.04 and 41.94% in MRSA 1, 2 and 4, respectively. RLMIX_Arg decreased cell viability in a concentration-dependent manner in all strains. The 2xMIC concentration (16 μg/ml) obtained reductions of 42.18% (MRSA 1), 46.15% (MRSA 4) and 43.09% (MRSA 2). The combination of RLMIX_Arg and oxacillin caused the highest levels of cell membrane damage, 81.40% (MRSA 1), 62.27% (MRSA 4) and 59.31% (MRSA 2) (Figure 5).
Figure 5.

Effect of RLMIX_Arg against methicillin-resistant S. aureus after 24 h of treatment with RLMIX_Arg, % of propidium iodide positive cells.
RLMIX_Arg causes damage to MRSA cell DNA
In all three strains tested, significant cell DNA damage was observed (p < 0.05). RLMIX_Arg increased DNA damage in a concentration-dependent manner, so that the combination RLMIX_Arg and oxacillin showed the highest levels of damage, 68.31% (MRSA 1), 57.36% (MRSA 4) and 63.39% (MRSA 2) (Figure 6).
Figure 6.
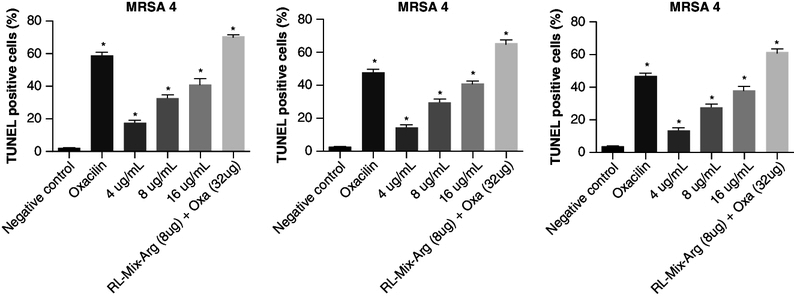
Effect of RLMIX_Arg against methicillin-resistant S. aureus after 24 h of treatment with RLMIX_Arg, % of TUNEL-positive cells.
RLMIX_Arg inhibits the formation of MRSA biofilms on peripheral venous catheters
Electron microscopy analysis has confirmed reduction in cell count following RLMIX_Arg treatments. At a concentration of 80 μg/ml, only cell remnants were discernible, exhibiting alterations to their original morphology (Figure 3B). In the untreated catheter, a complex network of the exopolymeric matrix, crucial for cell adhesion to surfaces, was readily observed.
Discussion
Our study has demonstrated that RLMIX_Arg, a rhamnolipid produced by Pseudomonas aeruginosa and complexed with the amino acid arginine, exhibits bactericidal properties against planktonic strains of methicillin-resistant S. aureus (MRSA) at concentrations ranging from 4 to 16 μg/ml. Our data corroborate the findings of Ramos da Silva et al., who found MICs of 8 μg/ml for S.aureus and 32 μg/ml for methicillin-resistant S. aureus (MRSA) [23]. On the other hand, investigations have found the antimicrobial activity of a mixture of ramnoliphids (M15RL, MDRL and DDRL) against S. aureus strains and the MIC ranged from 50 μg/ml to >100 μg/ml for MRSA [35].
Alternative approaches for investigating the antibacterial properties of rhamnolipids have also been documented in the literature. One study utilized zinc oxide nanoparticles coated with rhamnolipids but obtained higher MIC values (250 μg/ml) against S. aureus [36]. Therefore, the complexation with amino acids emerges as an intriguing strategy for reducing both the MIC values and the toxicity of these molecules, as suggested by [23].
Regarding synergism, study results demonstrated reduced MICs when RLMIX_Arg was concurrently administered with oxacillin and vancomycin. Our findings align with those of Samadi et al., who employed a combination of rhamnolipids and oxacillin, resulting in a 42.8% synergistic and a 57.2% partially synergistic relationship [37]. It is well-documented that surfactants intercalate into target cell membranes, disrupting cellular homeostasis and leading to increased membrane permeability. Consequently, the efficacy of oxacillin is enhanced, as it can more readily penetrate bacterial cells [38].
One study reported that concentrations of 30 and 40 μg/ml of a mixture of mono- and di-rhamnolipids, when added to 58 μg/ml of the aminoglycoside tobramycin, were capable of eradicating approximately 99.9% of planktonic S. aureus cells [39]. However, the same study did not observe a significant synergistic relationship when oxacillin and the same rhamnolipid mixture were used. The introduction of the amino acid arginine into the rhamnolipid mixture may account for this discrepancy in findings [23]. Arginine, as a basic compound with cationic properties, exhibits strong interactions with the negatively charged molecules found in bacterial membranes (lipoteichoic acid in Gram-positive bacteria), facilitating the release of cellular ions and subsequently enhancing the action of the biosurfactant [40–42].
The RLMIX_Arg exhibited effective dispersion activity on MRSA cells within mature biofilms (48 h) at concentrations of 64 μg/ml and 80 μg/ml. Another study that synthesized surfactants based on cationic amino acids with polar heads composed of amino acid pairs arginine-phenylalanine and arginine-tryptophan found that their compounds demonstrated dispersion capabilities on mature biofilms of Candida spp. at a concentration of 81.2 μg/ml, a concentration closely resembling that found in our study [42]. Furthermore, others have reported that 100 μg/ml of a rhamnolipid obtained from Pseudomonas BP03 strains inhibited S. aureus biofilm formation by 83.5% in plaques [43]. In this study, a lower concentration of 80 μg/ml was sufficient to inhibit biofilm formation by more than 90%. At subinhibitory concentrations, RlMIX_Arg was also able to inhibit biofilm formation on plates.
This research demonstrates the efficacy of RLMIX_Arg in inhibiting S. aureus biofilm formation and its potential for disassembling mature biofilms within peripheral venous catheters at a concentration of 4 mg/ml and 80 μg/ml. Others have shown that a commercially obtained rhamnolipid could eliminate 88.9% of the biofilm formed by S. aureus on plates using milk as a carbohydrate source and 35% when utilizing a conventional nutrient medium [44].
After impregnating the catheter with 4 mg of RLMIX_Arg, the adhesion of S. aureus cells on the catheter surface was also reduced, pointing to a possible alternative for reducing the risk of biofilm formation on medical materials. Additionally, others have reported an 85% reduction in the adhesion of S. aureus to silicone elastomeric disks when incubated concurrently with a mixture of mono- and di-rhamnolipids (R89BS) produced by P. aeruginosa after 72 h [45]. Interestingly, our study showed that higher concentrations (8 mg/ml) were less efficient at impregnating the catheter, reflecting a lower inhibitory activity. Probably, depending on the catheter material and the substance used, there is an ideal concentration for the impregnation process. In the abovementioned study, they used a concentration of 2 mg/ml, a lower concentration than that found in this study, of a mixture of rhamnolipids to impregnate catheters and obtained a 85% reduction in the adhesion of S. aureus cells [45]. There are two crucial strategies to potentially decrease the early infection risk of implantable devices: reducing the quantity of cells capable of adhering to the surface and delaying their adhesion. RLMX_Arg effectively tackles both requirements with its antimicrobial and anti-adhesive properties [22].
The antibiofilm activity of rhamnolipids against various microorganisms has been previously reported in numerous studies, although the underlying mechanism of action remains incompletely understood [17]. Rhamnolipids have been thought interact with cell surface structures, including lipopolysaccharides, proteins and phospholipids, leading to alterations in cellular hydrophobicity and a subsequent reduction in surface adhesion [46]. Consequently, biosurfactants possess the potential to impede the formation of S. aureus biofilms in catheters and other medical materials, ultimately diminishing the reliance on broad-spectrum antibiotics and curbing the development of microbial resistance [47].
RLMIX_Arg caused concentration-dependent membrane damage in S. aureus cells. Additionally, an increase in cellular DNA damage was observed. The presence of basic groups, such as arginine, enhances the interaction of the rhamnolipid molecule with DNA, owing to the presence of positive charges [42,48]. Therefore, the occurrence of simultaneous events (damage to membrane integrity and binding to DNA molecules) is possible, leading to the disruption of cellular homeostasis. However, one limitation of our study was the lack of molecular experiments that could shed more light on these events.
Conclusion
The rhamnolipid complexed with the amino acid arginine has demonstrated antimicrobial potential against methicillin-resistant S. aureus cells in both planktonic form and within plaque biofilms and biofilms formed on peripheral venous catheters. Catheters impregnated with RLMIX_Arg demonstrated reduced susceptibility to the development of S. aureus biofilms. It is critical to understand these dynamics and explore the potential of rhamnolipids to develop new applications for these molecules in the pharmaceutical industry. In this context, the development and application of formulations containing RLMIX_Arg emerge as a potential option for preventing biofilm formation on medical devices such as catheters, prostheses and implants.
Supplementary Material
Acknowledgments
The authors are grateful to the Central Analítica-UFC for support with the microscopy tests, CAPES for support and Grant PID2022-136354-NB100 funded by MCIN/AEI/10.13039/501100011033 and by “ERDF” A way of making Europe.
Supplementary data
To view the supplementary data that accompany this paper, please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fmb-2023-0271
Author contributions
F Daiana Dias Barroso: methodology, investigation, writing - original draft. L Juvêncio da Silva: methodology, investigation, writing - original draft. H Almeida Queiroz: methodology, investigation, writing - original draft. L Gurgel Valente do Amaral: methodology, investigation. A Ramos da Silva: methodology, investigation. C Rocha da Silva: conceptualization, validation, supervision. J Batista de Andrade Neto: methodology, investigation. B Coelho Cavalcante: methodology, investigation, formal analysis. H Vitoriano Nobre Júnior: conceptualization, validation, supervision. M. Odorico de Moraes: methodology, investigation, formal analysis. A Pinazo: visualization, formal analysis. L Pérez: visualization, formal analysis.
Financial disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interest disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Alarjani KM, Skalicky M. Antimicrobial resistance profile of Staphylococcus aureus and its in-vitro potential inhibition efficiency. J. Infect. Public Health. 14(12), 1796–1801 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Tsuzuki S, Yu J, Matsunaga N, Ohmagari N. Length of stay, hospitalisation costs and in-hospital mortality of methicillin-susceptible and methicillin-resistant Staphylococcus aureus bacteremia in Japan. Public Health 198, 292–296 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Shrestha P, Cooper BS, Coast Jet al. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob. Resist. Infect. Control. 7(1), 98 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Idrees M, Sawant S, Karodia N, Rahman A. Staphylococcus aureus biofilm: morphology, genetics, pathogenesis and treatment strategies. Int. J. Environ. Res. Public Health 18(14), 7602 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Breij A, Riool M, Cordfunke RAet al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. [Internet]. 10(eaan4044) (2018). Available from: https://www.science.org [DOI] [PubMed] [Google Scholar]
- 6.Singh A, Amod A, Pandey Pet al. Bacterial biofilm infections, their resistance to antibiotics therapy and current treatment strategies. Biomed. Mater. 17(2) (2022). [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal VD, Gupta D, Rajhans Pet al. Six-year multicenter study on short-term peripheral venous catheters-related bloodstream infection rates in 204 intensive care units of 57 hospitals in 19 cities of India: International Nosocomial Infection Control Consortium (INICC) findings. Am. J. Infect. Control 48(9), 1001–1008 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Cangui-Panchi SP, Ñacato-Toapanta AL, Enríquez-Martínez LJ, Reyes J, Garzon-Chavez D, Machado A. Biofilm-forming microorganisms causing hospital-acquired infections from intravenous catheter: a systematic review. Curr. Res. Microb. Sci. 3, 100175 (November) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrag HA, Hosny AEDMS, Hawas AM, Hagras SAA, Helmy OM. Potential efficacy of garlic lock therapy in combating biofilm and catheter-associated infections; experimental studies on an animal model with focus on toxicological aspects. Saudi Pharm. J. 27(6), 830–840 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa K, Furukawa K. Staphylococcus aureus bacteremia due to central venous catheter infection: a clinical comparison of infections caused by methicillin-resistant and methicillin-susceptible strains. Cureus 13(7), e16607 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salmanov A, Litus V, Vdovychenko Set al. Healthcare-associated infections in intensive care units. Wiad. Lek. 72(5 cz 2), 963–969 (2019). [PubMed] [Google Scholar]
- 12.Ray-Barruel G, Xu H, Marsh N, Cooke M, Rickard CM. Effectiveness of insertion and maintenance bundles in preventing peripheral intravenous catheter-related complications and bloodstream infection in hospital patients: a systematic review. Infect. Dis. Heal. 24(3), 152–168 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, She P, Xu Let al. Antimicrobial, antibiofilm, and anti-persister activities of penfluridol against Staphylococcus aureus. Front. Microbiol. 18(12), 727692 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnal C, Birgand G, Lolom Iet al. Staphylococcus aureus healthcare associated bacteraemia: an indicator of catheter related infections. Med. Mal. Infect. 45(3), 84–88 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Dadashi M, Nasiri MJ, Fallah Fet al. Methicillin-resistant Staphylococcus aureus (MRSA) in Iran: a systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 12(2010), 96–103 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Liu WT, Chen EZ, Yang Let al. Emerging resistance mechanisms for 4 types of common anti-MRSA antibiotics in Staphylococcus aureus: a comprehensive review. Microb. Pathog. 156, 104915 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Thakur P, Saini NK, Thakur VK, Gupta VK, Saini RV, Saini AK. Rhamnolipid the glycolipid biosurfactant: emerging trends and promising strategies in the field of biotechnology and biomedicine. Microb. Cell Fact. 20(1), 1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh P, Patil Y, Rale V. Biosurfactant production: emerging trends and promising strategies. J. Appl. Microbiol. 126(1), 2–13 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Ceresa C, Fracchia L, Sansotera AC, De Rienzo MAD, Banat IM. Harnessing the potential of biosurfactants for biomedical and pharmaceutical applications. Pharmaceutics 15(8), 2156 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Focuses on the main updates on biosurfactants and their applications in the biomedical field. It also points out their good compatibility with various known antimicrobial molecules and their compatibility with nanoparticles and liposomes.
- 20.Chong H, Li Q. Microbial production of rhamnolipids: opportunities, challenges and strategies. Microb. Cell Fact. 16(1), 137 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tambone E, Bonomi E, Ghensi Pet al. Rhamnolipid coating reduces microbial biofilm formation on titanium implants: an in vitro study. BMC Oral Health 21(1), 49 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ceresa C, Tessarolo F, Maniglio Det al. Medical-grade silicone coated with rhamnolipid R89 is effective against Staphylococcus spp. biofilms. Molecules 24(21),3843 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos da Silva A, Manresa MÁ, Pinazo A, García MT, Pérez L. Rhamnolipids functionalized with basic amino acids: synthesis, aggregation behavior, antibacterial activity and biodegradation studies. Coll. Surf. B Biointerf. 181(February), 234–243 (2019). [DOI] [PubMed] [Google Scholar]; • This study led to the synthesis of new cationic rhamnolipid derivatives conjugated to arginine and lysine.
- 24.da Silva A, Nobre H, Sampaio Let al. Antifungal and antiprotozoal green amino acid-based rhamnolipids: mode of action, antibiofilm efficiency and selective activity against resistant Candida spp. strains and Acanthamoeba castellanii. Coll. Surf. B Biointerf. 193(May), 111148 (2020). [DOI] [PubMed] [Google Scholar]
- 25.CLSI . M07-A10: methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically [Internet]. Available from: http://www.clsi.org
- 26.Batista de Andrade Neto J, Alexandre Josino MA, Rocha da Silva Cet al. A mechanistic approach to the in-vitro resistance modulating effects of fluoxetine against meticillin resistant Staphylococcus aureus strains. Microb. Pathog. 127(November 2018), 335–340 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Das B, Mandal D, Dash SKet al. Eugenol provokes ROS-mediated membrane damage-associated antibacterial activity against clinically isolated multidrug-resistant Staphylococcus aureus strains. Infect. Dis. Res. Treat. 9, IDRT.S31741 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Da Silva CR, De Andrade Neto JB, Costa Sidrim JJet al. Synergistic effects of amiodarone and fluconazole on Candida tropicalis resistant to fluconazole. Antimicrob. Agents Chemother. 57(4), 1691–1700 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa EM, Silva S, Madureira AR, Cardelle-Cobas A, Tavaria FK, Pintado MM. A comprehensive study into the impact of a chitosan mouthwash upon oral microorganism's biofilm formation in vitro. Carbohydr. Polym. 101(1), 1081–1086 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Brambilla LZS, Endo EH, Cortez DAG, Filho BPD. Anti-biofilm activity against Staphylococcus aureus MRSA and MSSA of neolignans and extract of Piper regnellii. Rev. Bras. Farmacogn. 27(1), 112–117 (2017). [Google Scholar]
- 31.Sidrim JJC, Amando BR, Gomes FIFet al. Chlorpromazine-impregnated catheters as a potential strategy to control biofilm-associated urinary tract infections. Fut. Microbiol. 14(12), 1023–1034 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Iwata Y, Sakai N, Yoneda Iet al. D-Serine inhibits the attachment and biofilm formation of methicillin-resistant Staphylococcus aureus. Biochem. Biophys. Res. Commun. 537, 50–56 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Do Amaral Valente Sá LG, Da Silva CR, De Andrade Neto JBet al. Antifungal activity of etomidate against growing biofilms of fluconazole-resistant Candida spp. strains, binding to mannoproteins and molecular docking with the ALS3 protein. J. Med. Microbiol. 69(10), 1221–1227 (2020). [DOI] [PubMed] [Google Scholar]
- 34.CLSI . CLSI.Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically;ApprovedStandard—Tenth Edition.CLSI documentM07-A10. Wayne, PA: Clinical and LaboratoryStandards Institute; 2015. 30th ed.2015
- 35.Buonocore C, Giugliano R, Della Sala Get al. Evaluation of antimicrobial properties and potential applications of Pseudomonas gessardii M15 rhamnolipids towards multiresistant Staphylococcus aureus. Pharmaceutics 15(2), 700 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malakar C, Patowary K, Deka S, Kalita MC. Synthesis, characterization, and evaluation of antibacterial efficacy of rhamnolipid-coated zinc oxide nanoparticles against Staphylococcus aureus. World J. Microbiol. Biotechnol. 37(11), 1–14 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Samadi N, Abadian N, Ahmadkhaniha Ret al. Structural characterization and surface activities of biogenic rhamnolipid surfactants from Pseudomonas aeruginosa isolate MN1 and synergistic effects against methicillin-resistant Staphylococcus aureus. Folia Microbiol. (Praha) 57(6), 501–508 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Otzen DE. Biosurfactants and surfactants interacting with membranes and proteins: same but different? Biochim. Biophys. Acta - Biomembr. 1859(4), 639–649 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Radlinski LC, Rowe SE, Brzozowski Ret al. Chemical induction of aminoglycoside uptake overcomes antibiotic tolerance and resistance in Staphylococcus aureus. Cell Chem. Biol. 26(10), 1355–1364.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinazo A, Manresa MA, Marques AM, Bustelo M, Espuny MJ, Pérez L. Amino acid-based surfactants: new antimicrobial agents. Adv. Colloid Interface Sci. 228, 17–39 (2016). [DOI] [PubMed] [Google Scholar]; •• This review article discusses the structural features that promote the antimicrobial activity of amino acid-based biosurfactants.
- 41.Patrone V, Campana R, Vittoria E, Baffone W. In vitro synergistic activities of essential oils and surfactants in combination with cosmetic preservatives against Pseudomonas aeruginosa and Staphylococcus aureus. Curr. Microbiol. 60(4), 237–241 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Serpa Sampaio Moreno L, Nobre Junior HV, Ramos da Silva Aet al. Arginine-phenylalanine and arginine-tryptophan-based surfactants as new biocompatible antifungal agents and their synergistic effect with Amphotericin B against fluconazole-resistant Candida strains. Coll. Surf. B Biointerf. 207, 112017 (2021). [DOI] [PubMed] [Google Scholar]; •• Study discusses the antifungal properties of a biosurfactant complexed with arginine and its synergistic potential with anfortecin B, as well as its action against biofilms.
- 43.Sabarinathan D, Vanaraj S, Sathiskumar Set al. Characterization and application of rhamnolipid from Pseudomonas plecoglossicida BP03. Lett. Appl. Microbiol. 72(3), 251–262 (2021). [DOI] [PubMed] [Google Scholar]
- 44.e Silva SS, Carvalho JWP, Aires CP, Nitschke M. Disruption of Staphylococcus aureus biofilms using rhamnolipid biosurfactants. J. Dairy Sci. 100(10), 7864–7873 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Allegrone G, Ceresa C, Rinaldi M, Fracchia L. Diverse effects of natural and synthetic surfactants on the inhibition of Staphylococcus aureus biofilm. Pharmaceutics 13(8),1172 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nickzad A, Déziel E. The involvement of rhamnolipids in microbial cell adhesion and biofilm development - an approach for control? Lett. Appl. Microbiol. 58(5), 447–453 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Sulaiman R, Trizna E, Kolesnikova Aet al. Antimicrobial and biofilm-preventing activity of l-borneol possessing 2(5H)-furanone derivative F131 against S. aureus—C.albicans mixed cultures. Pathogens 12(1), 26 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bera S, Zhanel GG, Schweizer F. Antibacterial activity of guanidinylated neomycin B- and kanamycin A-derived amphiphilic lipid conjugates. J. Antimicrob. Chemother. 65(6), 1224–1227 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


