Abstract
Objectives:
To investigate the effect of topical 0.05% cyclosporine A (CsA) eye drops as an adjunct to conventional therapy in maintaining post–femtosecond-assisted laser in situ keratomileusis (FS-LASIK) ocular surface stability.
Methods:
Sixty-six patients (eyes) undergoing FS-LASIK were randomized into 2 groups: 33 patients (eyes) in group I (conventional treatment group) and 33 patients (eyes) in group II (CsA group). Conventional treatments include topical levofloxacin, fluorometholone, and artificial tears. Group II received topical 0.05% CsA eye drops twice daily for three months in addition to conventional treatment. Ocular Surface Disease Index (OSDI), numerical rating scale (NRS), tear break-up time (TBUT), Schirmer I test (SIt), corneal fluorescein staining (CFS), conjunctival lissamine green (LG) staining, corneal sensitivity, and corneal nerve morphology were measured. In addition, tear inflammatory cytokine levels were measured using the Luminex assay. Follow-up was performed preoperatively and 1 and 3 months postoperatively.
Results:
In the CsA group, OSDI, TBUT, LG, corneal sensitivity, and corneal nerve fiber total branch density recovered better than in the conventional treatment group. As for tear inflammatory cytokines, interferon (INF) -γ, interleukin (IL)-10, and IL-6 levels were significantly higher in the conventional treatment group as compared with the CsA group. In addition, no significant differences in NRS, SIt, and CFS scores were observed between the two groups.
Conclusion:
In conclusion, 0.05% CsA eye drops is a useful adjunct to conventional treatment for restoring the ocular surface stability after corneal refractive surgery and is more potent in sustaining anti-inflammatory effects.
Key Words: Dry eye disease, Post-FS-LASIK, Ocular surface, Corneal nerves, Inflammatory cytokines, Treatment
Femtosecond-assisted laser in situ keratomileusis (FS-LASIK) is one of the most common refractive surgeries. It involves the creation of a corneal flap using a femtosecond laser, followed by excimer laser ablation of the stromal layer. The cutting of subbasal nerve fibers and the release of inflammatory mediators caused by laser application are both related to postoperative dry eye disease (DED).1 Postoperative DED affects approximately 94.8% of patients at 1 day, 85.4% at 1 week, and 59.4% at 1 month.2 While DED typically manifests transiently in the early postoperative period, it can progress and develop into a chronic condition. Approximately 8% to 20% of patients experience chronic DED for more than 6 months,3–5 leading to significant patient dissatisfaction and discomfort.
Chronic inflammation is increasingly recognized as an important factor in ocular surface instability, especially after refractive surgery.6 Topical corticosteroids are effective anti-inflammatory drugs, but their long-term use is limited by adverse ocular reactions, high intraocular pressure, cataracts, and other complications.7 Nonsteroidal anti-inflammatory drugs (NSAIDs) are a feasible option for controlling ocular inflammation; however, studies have shown that their long-term use may be related to corneal melting, which limits their long-term use.7,8 Ocular surface inflammation following corneal refractive surgery may persist for a long time. Clinically, safer anti-inflammatory drugs are needed to maintain ocular surface homeostasis in patients after corneal refractive surgery. However, the effects of conventional artificial tears are single, and the effects are limited.
Cyclosporine A (CsA), a nonsteroidal antiapoptotic agent and T-cell proliferation inhibitor, was approved by the Food and Drug Administration of the United States in 2002 for the treatment of dry eye.9 Previous studies showed that 0.05% cyclosporine A eye drops was widely used to improve the symptoms and signs of patients with DED.10 Corneal nerve disruption triggers a cascade of inflammatory events that exacerbate the underlying tear film instability, which has been suggested as a causative factor for postrefractive surgery DED.11 The aim of this study is to investigate the effect of topical 0.05% CsA eye drops as an adjunct to standard medical therapy for the recovery of post-FS-LASIK ocular surface stability.
MATERIALS AND METHODS
Participants
Participants eligible for inclusion were candidates for bilateral FS-LASIK, aged between 20 and 45 years, with a spherical range from −2.00 D to −9.00 D, cylinder less than −3.00 D, and stable refraction for 2 years. All participants had discontinued corneal contact lens wear for at least 2 weeks for soft lenses and 4 weeks for rigid gas-permeable lenses. Participants were excluded if they had active ocular diseases, such as corneal keratitis, keratoconus, or severe dry eye, or systemic diseases likely to cause potential secondary effects on the ocular surface, such as diabetes or connective tissue disease. Pregnant and nursing mothers were excluded from this study.
Sample size calculation for this study was conducted using PASS 15.0 software, with the primary outcome measure being the difference in OSDI scores between postrefractive surgery patients with and without cyclosporine A treatment, as documented in previous studies.12 Based on the mean OSDI scores and their standard deviations, assuming an alpha error of 0.05 and a power of 0.80, the calculated sample size required for statistical significance was 26 participants in each group. Assuming a 20% dropout, we increased the sample size accordingly, resulting in 33 participants needed per group. All tests were performed on both eyes, only the data from the right eye were used for analysis.
Methods
We performed a prospective randomized controlled trial in 66 patients who underwent FS-LASIK at a tertiary care ophthalmic center. This study was approved by the Ethics Committee of the Peking University Third Hospital and followed the principles of the Declaration of Helsinki (registration number: M2021398). Written informed consent was obtained from all the participants. This study is registered with the identifier NCT06043908 at ClinicalTrials. gov.
Sixty-six eyes from 66 patients were randomly assigned to 2 groups: 33 eyes in group I (Conventional treatment group) and 33 eyes in group II (CsA group), with an allocation ratio of 1:1 using a computer-generated randomizer. Group I received conventional treatment (routine postoperative regimen prescribed at our center): antibiotic eye drops (0.5% levofloxacin, Santen, Japan) four times daily for 2 weeks post-FS-LASIK. In addition, 0.1% fluorometholone eye drops (Santen, Japan) was administered four times daily for the first postoperative week, followed by weekly tapering, and discontinued four weeks post-FS-LASIK. Artificial tears (0.1% hyaluronic acid sodium eye drops) (HyloComod, Ursapharm, Saarbrucken, Germany) were prescribed at least four times per day for 1 month, and thereafter, their use was adjusted based on individual complaints and signs of dry eye. By contrast, group II received topical 0.05% cyclosporine A eye drops (Sinqi Pharmaceutical, Shenyang, China) twice daily for three months, in addition to conventional postoperative treatment.
Surgery Protocol
All surgeries were performed by the same surgeon (co-author, C.Y.G.) under topical anesthesia. Simultaneous bilateral procedures were performed on each subject, and only the right eye was included in this study. The flaps were created using a WaveLight FS200 femtosecond laser (Alcon Laboratories Inc, Fort Worth, TX) with an energy of 0.80 μJ, a thickness of 110 μm, and a diameter of 8.5 to 9 mm. The hinge position was superior. Laser ablation was performed using a WaveLight EX500 excimer laser (Alcon Laboratories Inc, Fort Worth, TX), with an optical zone of 6.0 to 6.5 mm. Emmetropia was the target postoperative refraction in all eyes. All the surgical procedures were uneventful. The participants were examined preoperatively and 1 and 3 months postoperatively.
Ocular Surface Evaluations
Ocular symptoms were assessed using the Ocular Surface Disease Index (OSDI) questionnaire.13 The numerical rating scale (NRS) is generally used to assess the severity of pain (score: 0–10).14 Tear break-up time (TBUT) was evaluated after applying a fluorescein strip previously moistened with an antiseptic saline solution to the inferior conjunctival fornix, and the participants were observed with a cobalt blue filter over the slitlamp biomicroscope. Corneal fluorescein staining (CFS) and conjunctival lissamine green (LG) staining were evaluated using the National Eye Institute Workshop guidelines (total score, 0–15)15 and Oxford grading panel (total score, 0–15),16 respectively. The Schirmer I test (SIt) was conducted using Schirmer paper strips (5×35 mm) without anesthesia.
Corneal Sensitivity and Corneal Nerve Morphology
Corneal sensitivity was assessed using a Cochet-Bonnet esthesiometer (Luneau Ophthalmologie, Chartres Cedex, France), comprising a 6.0-cm regolabile nylon monofilament. Starting at 6.0 cm, the monofilament length was gradually reduced in 5-mm increments until the first observable reaction occurred. A longer length indicates a greater corneal sensitivity.17 Evaluations were conducted both at the central cornea and in its peripheral quadrants, namely, the superior, inferior, nasal, and temporal areas (approximately 2 mm away from the tangent to the limbus).
Corneal nerve imaging was performed using an in vivo laser scanning confocal microscope (IVCM) (Heidelberg Retina Tomograph 3/Rostock Cornea Module; Heidelberg Engineering GmbH, Heidelberg, Germany). Five representative images of the subbasal plexus nerve of the central cornea were selected for analysis (resolution: 384×384 pixels; area: 400 μm×400 μm [0.16 mm2]). Automated analyses of corneal nerve fiber density (CNFD), corneal nerve branch density (CNBD), corneal nerve fiber length (CNFL), and corneal nerve fiber total branch density (CTBD) were acquired from the IVCM scans adopting ACCMetrics software (University of Manchester, United Kingdom).18–20
Analysis of Tear Cytokine Concentration
Approximately 5 μL of unstimulated basal tears from the right eye were carefully collected from the lower tear meniscus using a clean glass micropipette (Microcaps; Drummond Scientific Co, Broomall, PA) within a reasonable time frame (up to 5 min) without provoking reflex tear secretion. The samples were promptly stored at −80°C. The levels of inflammatory cytokines (interferon [IFN]-γ, interleukin [IL]-10, IL-17A, IL-1β, IL-23, IL-6, and tumor necrosis factor-α [TNF-α]) were detected using the MILLIPLEX Human High Sensitivity T Cell Magnetic Bead Panel (Millipore, Billerica, MA).
Statistical Analyses
Statistical analyses were performed using SPSS software (version 27.0; SPSS Inc, Chicago, IL). Figures were created using the GraphPad Prism 9.4 software package. The normality assumption was checked using the Shapiro–Wilk test. Linear mixed models were used to assess the changes in the studied variables over time between the two groups. The Bonferroni adjustment was used for multiple comparisons. Statistical significance was set at P<0.05.
RESULTS
Baseline Measurements
Four patients in group I (conventional treatment group) discontinued treatment at 3 months post-FS-LASIK for nonstudy-related reasons. No obvious demographic or other differences were found between the participants and those who completed all visits. Finally, we analyzed 29 eyes in group I and 33 eyes in group II (the CsA group). The demographic details and preoperative parameters are presented in Table 1. There were no statistically significant differences between the two groups in mean age, spherical equivalent, or ocular surface parameters, including OSDI, TBUT, CFS, LG, and SIt scores (P>0.05).
TABLE 1.
Preoperative Demographic Data of Participants
| Basic Information | Conventional Treatment Group | CsA Group | P |
| Participants/eyes, (n/n) | 29/29 | 33/33 | |
| Female/male, (n/n) | 25/4 | 29/4 | |
| Age (y), mean (SD) | 28.12±5.48 | 29.69±5.95 | 0.284 |
| Spherical equivalent (D), mean (SD) | −6.48±1.36 | −5.95±1.49 | 0.179 |
| OSDI (score), mean (SD) | 10.21±4.63 | 10.05±4.70 | 0.215 |
| TBUT (s), mean (SD) | 5.64±3.28 | 5.55±2.44 | 0.910 |
| CFS (score), mean (SD) | 0.39±0.90 | 0.34±0.67 | 0.810 |
| LG (score), mean (SD) | 0.45±1.09 | 0.24±0.69 | 0.370 |
| Schirmer I test (mm), mean (SD) | 16.82±9.71 | 15.86±8.25 | 0.680 |
n, number; y, year; D, diopter; OSDI, Ocular Surface Disease Index; TBUT, tear break-up time; CFS, corneal fluorescein staining; LG, lissamine green.
The two groups were compared using the independent samples t test.
Ocular Surface Parameters
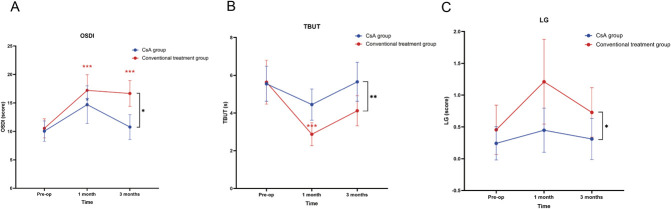
There was significantly better recovery in OSDI, TBUT, and LG scores in the CsA group compared with the conventional treatment group over time (P=0.013, P=0.003, and P=0.016, respectively) (Table 2). The mean OSDI scores significantly increased in the two groups at 1 month postoperatively compared with the preoperative values. Nevertheless, while the OSDI scores of the CsA group returned to preoperative levels at 3 months, the conventional treatment group still exhibited higher scores at this time compared with baseline (Fig. 1). The NRS scores increased at 1 month and 3 months in the conventional treatment group. However, no statistically significant increase was observed at any time point in the CsA group (Table 2). Tear break-up time scores decreased at 1 month and recovered to preoperative levels at 3 months in the conventional treatment group. In the CsA group, no significant differences were noted between the preoperative and postoperative TBUT levels (Table 2, Fig. 1). Lissamine green scores were significantly higher in the conventional treatment group than in the CsA group. No significant changes in the LG scores were observed in either group at 1 month or 3 months compared with baseline (Table 2, Fig. 1). The CFS scores for both groups increased at 1 month after surgery compared with baseline. However, no significant differences were observed in the CFS scores for both groups between 3 months and baseline (Table 2). In addition, no differences in the SIt and CFS scores were observed between the two groups (Table 2).
TABLE 2.
Ocular Surface Parameters of Participants Over Time
| Ocular Symptoms and Signs | Group | Pre-op | Post-op 1m | Post-op 3m | P |
| OSDI (score), mean (SD) | Conventional treatment group | 10.55±4.73 | 17.20±7.75a | 16.66±6.38b | 0.013 |
| CsA group | 10.05±4.70 | 14.71±8.68a | 10.77±5.72 | ||
| NRS (score), mean (SD) | Conventional treatment group | 0.36±0.55 | 0.79±0.86a | 0.67±0.60b | 0.101 |
| CsA group | 0.34±0.61 | 0.45±0.95 | 0.31±0.71 | ||
| TBUT (s), mean (SD) | Conventional treatment group | 5.64±3.29 | 2.88±1.73a | 4.12±2.25 | 0.003 |
| CsA group | 5.55±2.44 | 4.45±2.18 | 5.66±2.72 | ||
| CFS (score), mean (SD) | Conventional treatment group | 0.21±0.59 | 1.54±2.55a | 0.63±1.74 | 0.623 |
| CsA group | 0.34±0.67 | 1.21±1.67a | 0.38±0.72 | ||
| LG (score), mean (SD) | Conventional treatment group | 0.33±0.96 | 0.92±1.32 | 0.58±0.88 | 0.016 |
| CsA group | 0.24±0.69 | 0.45±0.91 | 0.31±0.85 | ||
| Schirmer I test (mm), mean (SD) | Conventional treatment group | 16.67±9.79 | 15.63±9.28 | 12.88±7.87 | 0.139 |
| CsA group | 15.86±8.26 | 17.31±8.69 | 21.62±9.14 |
In both groups, the changes in values 1 month postoperatively were significantly different compared with the preoperative values.
In both groups, the changes in values 3 months postoperatively were significantly different compared with the preoperative values.
OSDI, Ocular Surface Disease Index; NRS, numerical rating scale; TBUT, tear break-up time; CFS, corneal fluorescein staining; LG, lissamine green; pre-op, preoperatively; post-op, postoperatively.
P-value indicates a significant difference in values between the conventional treatment group and the CsA group over time with a mixed linear model.
FIG. 1.
Changes in OSDI (A), TBUT (B), and LG (C) scores over time for both groups. OSDI, Ocular Surface Disease Index; TBUT, tear break-up time; LG, lissamine green. Blue asterisks indicate significant within-group differences for the CsA group at postoperative time points compared with the baseline. Red asterisks indicate significant within-group changes for the conventional treatment group. Black asterisks between the two lines highlight significant differences between the CsA and conventional treatment groups over time. *P<0.05; **P<0.01; ***P<0.001; ns, no significance.
Corneal Sensitivity and Corneal Nerve Morphology
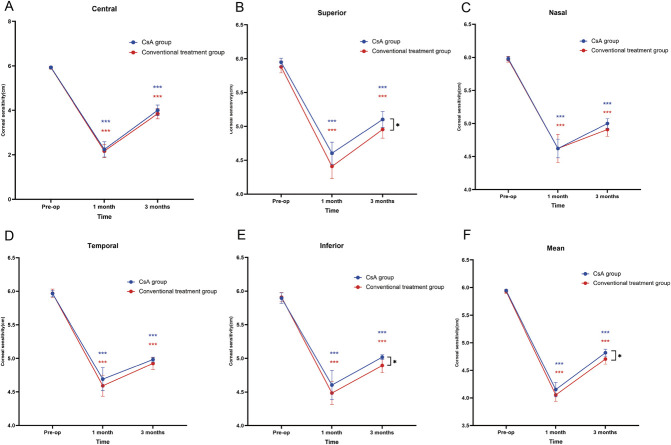
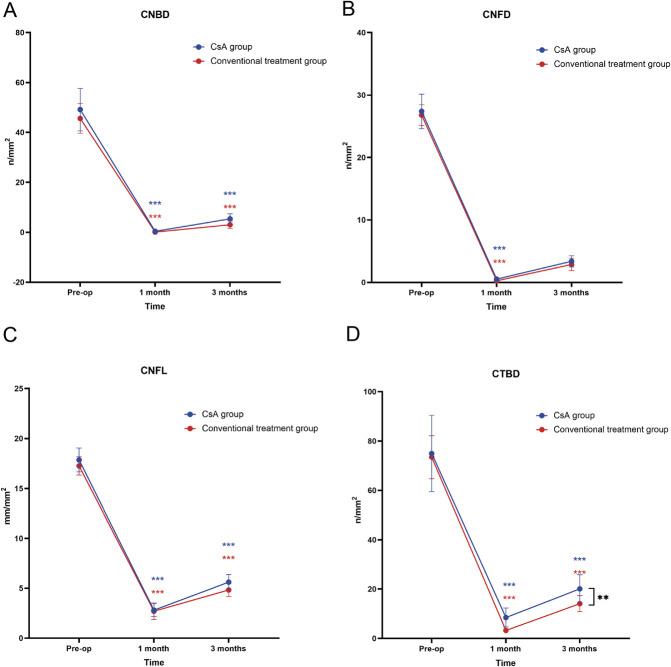
The postoperative corneal sensitivity and corneal nerve density decreased significantly in both groups (Table 3). The superior, inferior, and mean corneal sensitivities were significantly better recovery in the CsA group than in the conventional treatment group over time (P=0.044, P=0.031, and P=0.039, respectively) (Table 3, Fig. 2). The central, nasal, and temporal corneal sensitivities were comparable between the two groups (Table 3). Regarding corneal nerve morphological parameters, CTBD was significantly better recovery in the CsA group than in the conventional treatment group over time (P=0.003) (Table 3, Fig. 3). The CsA and conventional treatment groups exhibited comparable CNFD, CNBD, and CNFL levels (Table 3).
TABLE 3.
Ocular Surface Parameters of Participants Over Time
| Characteristics | Group | Pre-op | Post-op 1m | Post-op 3m | P |
| Corneal sensitivity (cm) | |||||
| Central, mean (SD) | Conventional treatment group | 5.92±0.18 | 2.17±0.84a | 3.85±0.66b | 0.373 |
| CsA group | 5.93±0.18 | 2.24±0.90a | 4.00±0.63b | ||
| Superior, mean (SD) | Conventional treatment group | 5.88±0.25 | 4.41±0.51a | 4.96±0.36b | 0.044 |
| CsA group | 5.95±0.16 | 4.60±0.43a | 5.10±0.31b | ||
| Inferior, mean (SD) | Conventional treatment group | 5.91±0.20 | 4.49±0.48a | 4.89±0.30b | 0.031 |
| CsA group | 5.89±0.21 | 4.60±0.57a | 5.01±0.09b | ||
| Nasal, mean (SD) | Conventional treatment group | 5.97±0.12 | 4.62±0.60a | 4.91±0.29b | 0.120 |
| CsA group | 5.98±0.09 | 4.62±0.37a | 5.00±0.19b | ||
| Temporal, mean (SD) | Conventional treatment group | 5.97±0.17 | 4.59±0.44a | 4.92±0.25b | 0.186 |
| CsA group | 5.97±0.13 | 4.69±0.45a | 4.98±0.09b | ||
| Mean, mean (SD) | Conventional treatment group | 5.93±0.11 | 4.06±0.33a | 4.71±0.26b | 0.039 |
| CsA group | 5.94±0.06 | 4.15±0.34a | 4.82±0.17b | ||
| Corneal nerve parameters | |||||
| CNFD (n/mm2), mean (SD) | Conventional treatment group | 25.83±4.47 | 0.22±0.59a | 2.72±3.14b | 0.219 |
| CsA group | 27.39±7.31 | 0.53±1.08a | 3.38±2.35b | ||
| CNBD (n/mm2), mean (SD) | Conventional treatment group | 44.22±16.05 | 0.07±0.32a | 2.60±4.58b | 0.081 |
| CsA group | 49.12±22.45 | 0.38±1.12a | 5.36±5.36b | ||
| CNFL (mm/mm2), mean (SD) | Conventional treatment group | 17.01±2.34 | 2.96±2.59a | 5.01±1.98b | 0.212 |
| CsA group | 17.86±3.13 | 2.80±1.70a | 5.61±1.98b | ||
| CTBD (n/mm2), mean (SD) | Conventional treatment group | 73.47±24.30 | 3.22±2.71a | 14.64±9.59b | 0.003 |
| CsA group | 74.96±40.61 | 8.47±10.04a | 20.05±15.01b |
In both groups, the changes in values 1 month postoperatively were significantly different compared with the preoperative values.
In both groups, the changes in values 3 months postoperatively were significantly different compared with the preoperative values.
CNFD, corneal nerve fiber density; CNBD, corneal nerve branch density; CNFL, corneal nerve fiber length; CTBD, corneal nerve fiber total branch density; pre-op: preoperatively; post-op: postoperatively.
P-value indicates a significant difference in values between the conventional treatment group and the CsA group over time with a mixed linear model.
FIG. 2.
Changes in central (A), superior (B), nasal (C), temporal (D), inferior (E), and mean corneal sensitivity (F) over time for both groups. Blue asterisks indicate significant within-group differences for the CsA group at postoperative time points compared with the baseline. Red asterisks indicate significant within-group changes for the conventional treatment group. Black asterisks between the two lines highlight significant differences between the CsA and conventional treatment groups over time. *P<0.05; **P<0.01; ***P<0.001; ns, no significance.
FIG. 3.
Changes in CNBD (A), CNFD (B), CNFL (C), and CTBD (D) values over time for both groups. CNFD, corneal nerve fiber density; CNBD, corneal nerve branch density; CNFL, corneal nerve fiber length; CTBD, corneal nerve fiber total branch density. Blue asterisks indicate significant within-group differences for the CsA group at postoperative time points compared with the baseline. Red asterisks indicate significant within-group changes for the conventional treatment group. Black asterisks between the two lines highlight significant differences between the CsA and conventional treatment groups over time. *P<0.05; **P<0.01; ***P<0.001; ns, no significance.
Tear Inflammatory Cytokines and Neuropeptide Concentrations
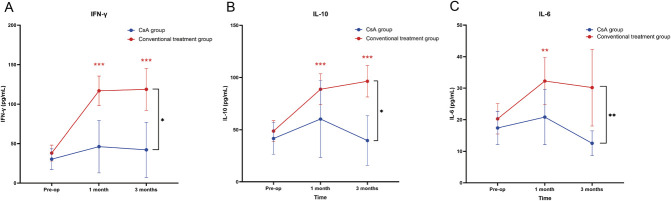
The increase in postoperative INF-γ, IL-10, and IL-6 levels from baseline was significantly greater in the conventional treatment group compared with the CsA group (P=0.025, P=0.013, P=0.008) (Table 4, Fig. 4). Tear IL-17A, IL-1β, IL-23, and TNF-α levels showed no statistically significant differences between the CsA group and the conventional treatment group (Table 4). In the conventional treatment group, there was a statistically significant increase in INF-γ, IL-10, IL-β, and TNF-α levels at 1 month and 3 months compared with baseline. However, no significant increases in the levels of these cytokines were observed in the CsA group (Table 4).
TABLE 4.
Concentrations of Tear Inflammatory Cytokines of Participants Over Time
| Inflammatory Cytokine Concentrations (pg/mL) | Group | Pre-op | Post-op 1m | Post-op 3m | P |
| IFN-γ, mean (SD) | Conventional treatment group | 38.04±28.37 | 116.88±52.57a | 118.78±75.00b | 0.025 |
| CsA group | 30.38±34.26 | 46.16±87.13 | 42.13±92.01 | ||
| IL-10, mean (SD) | Conventional treatment group | 48.71±28.01 | 88.78±41.64a | 96.41±42.37b | 0.013 |
| CsA group | 41.61±39.63 | 60.23±96.72 | 39.67±62.52 | ||
| IL-17A, mean (SD) | Conventional treatment group | 36.30±23.21 | 48.47±38.33 | 52.55±33.43b | 0.325 |
| CsA group | 34.18±27.13 | 48.95±63.24 | 34.80±36.36 | ||
| IL-1β, mean (SD) | Conventional treatment group | 3.02±2.99 | 7.87±6.57a | 9.46±7.53b | 0.344 |
| CsA group | 4.50±4.60 | 7.91±12.06 | 5.67±7.98 | ||
| IL-23, mean (SD) | Conventional treatment group | 1,598.81±963.36 | 2,115.24±4,303.46 | 2,379.20±5,194.68 | 0.994 |
| CsA group | 1,183.24±779.40 | 1,584.48±1937.48 | 1,045.79±1,064.67 | ||
| IL-6, mean (SD) | Conventional treatment group | 20.29±13.56 | 32.27±21.12a | 30.18±34.32 | 0.008 |
| CsA group | 17.39±13.74 | 20.83±22.89 | 12.53±10.34 | ||
| TNF-α, mean (SD) | Conventional treatment group | 17.17±8.80 | 30.20±23.61a | 32.98±18.67b | 0.735 |
| CsA group | 21.99±18.00 | 34.25±45.04 | 23.59±20.32 |
In both groups, the changes in values 1 month postoperatively were significantly different compared with the preoperative values.
In both groups, the changes in values 3 months postoperatively were significantly different compared with the preoperative values.
INF-γ, Interferon-γ; IL, interleukin; TNF-α, tumor necrosis factor-α; pre-op, preoperatively; post-op, postoperatively.
P-value indicates a significant difference in values between the conventional treatment group and the CsA group over time with a mixed linear model.
FIG. 4.
Changes in tear INF-γ (A), IL-10 (B), and IL-6 (C) levels over time for both groups. INF-γ, interferon-γ; IL, interleukin. Blue asterisks indicate significant within-group differences for the CsA group at postoperative time points compared with the baseline. Red asterisks indicate significant within-group changes for the conventional treatment group. Black asterisks between the two lines highlight significant differences between the CsA and conventional treatment groups over time. *P<0.05; **P<0.01; ***P<0.001; ns, no significance.
DISCUSSION
In this study, we observed that corneal refractive surgery altered tear film stability and changed the ocular surface state. The use of 0.05% CsA treatment was associated with a significant improvement in ocular dry eye symptoms and contributed to the rapid improvement of ocular parameters and ocular inflammation.
In recent years, significant attention has been paid to postoperative dry eye. Ocular surface inflammation contributes to dry eye disease and also has been observed in patients who have undergone corneal refractive surgery, resulting in a reduction in tear production. Topical steroids reduced ocular surface inflammation, which is an essential component of postoperative medication, in the entire study population. However, long-term use of topical glucocorticoids can lead to complications, such as steroid-induced glaucoma and cataracts.21 Furthermore, 0.05% cyclosporine emulsion drops (Restasis, Allergan Inc, CA) is an immunosuppressive agent widely used to treat various autoimmune diseases and has been approved by the United States Food and Drug Administration (FDA) for treating DED.9,22 This has been supported by human and animal studies showing that cyclosporine restores the body's ability to produce natural, healthy tears, thereby relieving the overall dry eye condition.22–26 However, Restasis uses oil-in-water emulsion to deliver cyclosporine to the ocular surface, but reports of ocular intolerance, ocular discomfort, or visual disturbances have also been noted.27 In our study, we utilized 0.05% cyclosporine A eye drops produced by Shenyang Sinqi Pharmaceutical Co, Ltd. This medication, a generic version of Restasis, employs Ailic-Tech nano-microemulsion technology. This results in a colorless, transparent microemulsion that significantly reduces the common adverse reactions associated with ocular intolerance. In addition, our study not only considered ocular symptoms and signs but also included assessments of corneal nerves, corneal sensitivity, and tear inflammatory cytokines. This approach allows for a more comprehensive analysis of ocular surface recovery following FS-LASIK with the combined use of 0.05% CsA eye drops.
In this study, patients treated with CsA showed faster improvement in dry eye-associated symptoms compared with conventional treatment based on OSDI scores. Our study showed that administration of CsA resulted in better stability of the tear film, as demonstrated by the TBUT results. The improvement in tear film following CsA treatment was accompanied by an improvement in conjunctival epithelial recovery, as indicated by conjunctive staining scores. These results confirm that additional CsA treatment is more effective than conventional treatment in addressing ocular surface dysfunction in patients with postcorneal refractive surgery. The patients in this study exhibited a preoperative TBUT of 5.5-6s. Given that mild dry eye is not a contraindication for FS-LASIK, these individuals were included in our analysis. Previous research has demonstrated that the presence of preoperative dry eye can lead to more severe and/or prolonged dry eye after refractive surgery.28 Therefore, careful attention should be given to the evaluation of preoperative dry eye in refractive surgery candidates.
Furthermore, in this study, the corneal flap was created superiorly. Research by Donnenfeld ED et al. indicates that dry eye signs and symptoms tend to be more severe in eyes with superior-hinge flaps and milder in those with nasal-hinge flaps.29 In addition, a meta-analysis covering eight randomized controlled trials, encompassing a total of 657 eyes, found that the TBUT was significantly longer and the percentage of patients with CFS was lower in the horizontal-hinge group compared with the vertical-hinge group at both 1 and 3 months postoperatively.30 Consequently, it is crucial for patients undergoing FS-LASIK with superior corneal flaps to focus on early and appropriate medication use to promote tear film stability.
This finding in this study also indicates that corneal refractive surgery causes direct damage to the corneal nerve fibers due to the preparation of the corneal flap. In this study, the corneal nerve plexus and corneal sensitivity in both groups showed significant reductions at 1 and 3 months postoperatively. This potentially explains the alterations in the tear film and the consequent postoperative discomfort. Corneal innervation is critical for the regulation of tear film secretion and corneal trophism because it has a trophic effect on the maintenance of corneal structure and function.31 We also observed a positive effect of 0.05% CsA eyedrops on the number and morphology of the nerve fibers. Corneal nerve recovery was faster with the additional CsA treatment than with the conventional treatment. This was accompanied by a similar improvement in corneal sensitivity.
The key finding of this study was a statistically significant increase in tear levels of INF-γ, IL-10, and IL-6 from the baseline in the conventional treatment group, whereas the increases observed after treatment with 0.05% CsA eye drops were not statistically significant. The corneal wound healing response following corneal refractive surgery is considered a complex inflammatory cascade, including the presence in the tear film of inflammatory cytokines, leukotrienes, oxygen-free radicals, proteolytic enzymes, and an increase in subbasal dendritic cells in all sectors of the central area of the cornea, which can be considered a sign of inflammation due to the release of inflammatory mediators.32,33 Previous studies have indicated a reduction in the expression of IL-6 in the conjunctival epithelium in patients with moderate to severe dry eye treated with 0.05% cyclosporine A eye drops for 6 months.25 In cases of chronic dry eye, topical cyclosporine is believed to function as a partial immunomodulator and an anti-inflammatory agent, mitigating the activation of T cells and T-cell–mediated cytokine release.24,34,35 Pflugfelder et al.'s study36 demonstrated significantly higher levels of IL-1α, IL-6, IL-8, TNF-α, and TNF-β1 RNA in the conjunctival epithelium of dry eye patients compared with control patients. However, this study is the first to demonstrate that additional topical 0.05% CsA eye drops treatment can significantly decrease several inflammatory markers for ocular surface recovery in postrefractive surgery patients. Artificial tears are useful for temporary palliative life; however, they lack the complex mixture of compositions found in healthy tear films. Therefore, the use of water supplements and tear retention alone is insufficient. Additional anti-inflammatory treatments were more effective than conventional treatments.
However, a previous study37 involving subjects without preoperative dry eye who underwent corneal refractive surgery revealed that additional topical cyclosporin did not provide a benefit for post-LASIK dry eye, as the additional use of cyclosporin did not alleviate symptoms or reduce inflammatory markers. This previous study did not include postoperative ocular surface signs, which is noteworthy because patients undergoing corneal refractive surgery may not report obvious ocular symptoms despite having positive ocular surface signs due to a significant decrease in corneal sensitivity. This highlights for clinicians the importance of paying attention to ocular surface signs. Furthermore, participants in our study had a preoperative TBUT of 5.5 to 6 s, with some patients presenting mild dry eye, which could lead to a more severe postoperative ocular surface inflammatory response and more noticeable changes in inflammatory markers. In comparison, patients without preoperative dry eye may show less variation in postoperative ocular surface inflammation. Moreover, differences in detection methods and the duration of corticosteroid application could influence the measured levels of inflammatory markers.
This study has several limitations. First, the follow-up period was short. In future studies, we plan to extend the follow-up period and determine the optimal medication duration. Second, we did not observe whether the patient's ocular surface condition could continue to improve after discontinuing the use of 0.05% CsA eye drops. This aspect is needed to further investigate in the future. In countries where CsA is not approved for dry eye, steroid eye drops may be used for a longer period of time, such as 0.1% fluorometholone eye drops. Future studies are needed to compare the levels of tear inflammatory cytokines and dry eye conditions when steroid eye drops are used for a longer period of time to guide the selection of more suitable and safer precise medication strategies for patients.
Overall, these results showed that corneal refractive surgery induces widespread and persistent inflammation of the corneal-conjunctival surface, leading to dry eye-associated signs and symptoms. The use of an additional 0.05% CsA eye drops could be considered a valuable component for postoperative therapeutic management following corneal refractive surgery.
ACKNOWLEDGMENT
The authors would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Supported by the National Natural Science Foundation of China under Grant No. 81974128 and No. 81570813.
Y. Chen and H. Qi authors contributed equally as co-corresponding authors.
Contributor Information
Lu Zhao, Email: Drzl1996@163.com.
Hongyu Duan, Email: hyduan167@163.com.
Baikai Ma, Email: 846259652@qq.com.
Tingting Yang, Email: ytt98620@163.com.
Yifan Zhou, Email: yankezhouyifan@126.com.
Yilin Liu, Email: 916352464@qq.com.
Jiawei Chen, Email: 2211210769@stu.pku.edu.cn.
Yueguo Chen, Email: chenyueguo@263.net.
REFERENCES
- 1.Battat L, Macri A, Dursun D, et al. Effects of laser in situ keratomileusis on tear production, clearance, and the ocular surface. Ophthalmology 2001;108:1230–1235. [DOI] [PubMed] [Google Scholar]
- 2.Yu EY, Leung A, Rao S, et al. Effect of laser in situ keratomileusis on tear stability. Ophthalmology 2000;107:2131–2135. [DOI] [PubMed] [Google Scholar]
- 3.Chao C, Stapleton F, Zhou X, et al. Structural and functional changes in corneal innervation after laser in situ keratomileusis and their relationship with dry eye. Graefes Arch Clin Exp Ophthalmol 2015;253:2029–2039. [DOI] [PubMed] [Google Scholar]
- 4.Salomão MQ, Ambrósio R, Wilson SE. Dry eye associated with laser in situ keratomileusis: Mechanical microkeratome versus femtosecond laser. J Cataract Refractive Surg 2009;35:1756–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denoyer A, Landman E, Trinh L, et al. Dry eye disease after refractive surgery: Comparative outcomes of small incision lenticule extraction versus LASIK. Ophthalmology 2015;122:669–676. [DOI] [PubMed] [Google Scholar]
- 6.Toda I. LASIK and the ocular surface. Cornea 2008;27:S70–S76. [DOI] [PubMed] [Google Scholar]
- 7.Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf 2017;15:575–628. [DOI] [PubMed] [Google Scholar]
- 8.Rigas B, Huang W, Honkanen R. NSAID-induced corneal melt: Clinical importance, pathogenesis, and risk mitigation. Surv Ophthalmol 2020;65:1–11. [DOI] [PubMed] [Google Scholar]
- 9.Perry HD, Donnenfeld ED, Kanellopoulos AJ, et al. Topical cyclosporin A in the management of postkeratoplasty glaucoma. Cornea 1997;16:284–288. [PubMed] [Google Scholar]
- 10.Rao SN. Topical cyclosporine 0.05% for the prevention of dry eye disease progression. J Ocul Pharmacol Ther 2010;26:157–164. [DOI] [PubMed] [Google Scholar]
- 11.Baudouin C, Aragona P, Messmer EM, et al. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the OCEAN group meeting. Ocul Surf 2013;11:246–258. [DOI] [PubMed] [Google Scholar]
- 12.Kanellopoulos AJ. Incidence and management of symptomatic dry eye related to LASIK for myopia, with topical cyclosporine A. Clin Ophthalmol 2019;13:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the ocular surface disease index. Arch Ophthalmol 2000;118:615–621. [DOI] [PubMed] [Google Scholar]
- 14.Satitpitakul V, Kheirkhah A, Crnej A, et al. Determinants of ocular pain severity in patients with dry eye disease. Am J Ophthalmol 2017;179:198–204. [DOI] [PubMed] [Google Scholar]
- 15.Lemp MA. Report of the national eye institute/industry workshop on clinical trials in dry eyes. CLAO J 1995;21:221–232. [PubMed] [Google Scholar]
- 16.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003;22:640–650. [DOI] [PubMed] [Google Scholar]
- 17.Sun CC, Chang CK, Ma DH, et al. Dry eye after LASIK with a femtosecond laser or a mechanical microkeratome. Optom Vis Sci 2013;90:1048–1056. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Graham J, Dabbah MA, et al. An automatic tool for quantification of nerve fibers in corneal confocal microscopy images. IEEE Trans Biomed Eng 2017;64:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petropoulos IN, Alam U, Fadavi H, et al. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Invest Ophthalmol Vis Sci 2014;55:2071–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang T, Ma B, Xie J, et al. Evaluation of ocular surface characteristics in dry eye disease with and without soft contact lens wear: A comparative study. Eye Contact Lens 2022;48:377–383. [DOI] [PubMed] [Google Scholar]
- 21.McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: Benefits and risks. Drug Saf 2002;25:33–55. [DOI] [PubMed] [Google Scholar]
- 22.Titiyal JS, Goswami A, Kaur M, et al. Impact of topical cyclosporine-A or topical chloroquine on post-LASIK ocular surface stability—A randomized controlled trial. Curr Eye Res 2023;48:557–563. [DOI] [PubMed] [Google Scholar]
- 23.Kaswan RL, Salisbury MA, Ward DA. Spontaneous canine keratoconjunctivitis sicca. A useful model for human keratoconjunctivitis sicca: Treatment with cyclosporine eye drops. Arch Ophthalmol 1989;107:1210–1216. [DOI] [PubMed] [Google Scholar]
- 24.Sall K, Stevenson OD, Mundorf TK, et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA phase 3 study group. Ophthalmology 2000;107:631–639. [DOI] [PubMed] [Google Scholar]
- 25.Turner K, Pflugfelder SC, Ji Z, et al. Interleukin-6 levels in the conjunctival epithelium of patients with dry eye disease treated with cyclosporine ophthalmic emulsion. Cornea 2000;19:492–496. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Zalisnak D, Nash D, Yeu E. Ocular surface diseases and corneal refractive surgery. Curr Opin Ophthalmol 2014;25:264–269. [DOI] [PubMed] [Google Scholar]
- 27.Sall K, Stevenson OD, Mundorf TK, et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology 2000;107:631–639. [DOI] [PubMed] [Google Scholar]
- 28.Maychuk DY. Prevalence and severity of dry eye in candidates for laser in situ keratomileusis for myopia in Russia. J Cataract Refractive Surg 2016;42:427–434. [DOI] [PubMed] [Google Scholar]
- 29.Donnenfeld ED, Solomon K, Perry HD, et al. The effect of hinge position on corneal sensation and dry eye after LASIK. Ophthalmology 2003;110:1023–1029. [DOI] [PubMed] [Google Scholar]
- 30.Feng YF, Yu JG, Wang DD, et al. The effect of hinge location on corneal sensation and dry eye after LASIK: A systematic review and meta-analysis. Graefes Archive Clin Exp Ophthalmol 2013;251:357–366. [DOI] [PubMed] [Google Scholar]
- 31.Lee BH, McLaren JW, Erie JC, et al. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci 2002;43:3660–3664. [PubMed] [Google Scholar]
- 32.De Cillà S, Fogagnolo P, Sacchi M, et al. Corneal involvement in uneventful cataract surgery: An in vivo confocal microscopy study. Ophthalmologica. 2014;231:103–110. [DOI] [PubMed] [Google Scholar]
- 33.Park Y, Hwang HB, Kim HS. Observation of influence of cataract surgery on the ocular surface. PLoS One 2016;11:e0152460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brignole F, Pisella PJ, Goldschild M, et al. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci 2000;41:1356–1363. [PubMed] [Google Scholar]
- 35.Power WJ, Mullaney P, Farrell M, et al. Effect of topical cyclosporin A on conjunctival T cells in patients with secondary Sjögren's syndrome. Cornea 1993;12:507–511. [DOI] [PubMed] [Google Scholar]
- 36.Pflugfelder SC, Jones D, Ji Z, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren's syndrome keratoconjunctivitis sicca. Curr Eye Res 1999;19:201–211. [DOI] [PubMed] [Google Scholar]
- 37.Hessert D, Tanzer D, Brunstetter T, et al. Topical cyclosporine A for postoperative photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refractive Surg 2013;39:539–547. [DOI] [PubMed] [Google Scholar]