Abstract
An essential prerequisite for evolution by natural selection is variation among individuals in traits that affect fitness1. The ability of a system to produce selectable variation, known as evolvability2, thus markedly affects the rate of evolution. Although the immune system is among the fastest-evolving components in mammals3, the sources of variation in immune traits remain largely unknown4,5. Here we show that an important determinant of the immune system’s evolvability is its organization into interacting modules represented by different immune cell types. By profiling immune cell variation in bone marrow of 54 genetically diverse mouse strains from the Collaborative Cross6, we found that variation in immune cell frequencies is polygenic and that many associated genes are involved in homeostatic balance through cell-intrinsic functions of proliferation, migration and cell death. However, we also found genes associated with the frequency of a particular cell type that are expressed in a different cell type, exerting their effect in what we term cyto-trans. The vertebrate evolutionary record shows that genes associated in cyto-trans have faced weaker negative selection, thus increasing the robustness and hence evolvability2,7,8 of the immune system. This phenomenon is similarly observable in human blood. Our findings suggest that interactions between different components of the immune system provide a phenotypic space in which mutations can produce variation with little detriment, underscoring the role of modularity in the evolution of complex systems9.
Subject terms: Immunogenetics, Evolutionary genetics, Gene expression, Evolutionary biology
By profiling immune cell variation in bone marrow of 54 genetically diverse mouse strains and analysing the evolutionary record, we found that genes associated with the frequency of a particular cell type but expressed in a different cell type increase the evolvability of the immune system.
Main
A key precondition for evolution by natural selection is the availability of suitable variation in natural populations. Early studies in evolutionary computation have shown that increased complexity also increases the probability that random mutations produce pleiotropic effects negatively affecting fitness10. In other words, complex systems have a greater potential for getting trapped in local fitness optima. Thus, for the Darwinian process of evolution through mutation and selection to work in complex biological systems, these systems need to have evolved evolvability—an architecture such that mutations are likely to result in more adaptive phenotypes2.
The immune system is a complex system intimately engaged in maintaining homeostasis and the struggle against pathogens, which makes it a prime target for the process of natural selection. This is evidenced by the fact that genes with immune function are among the fastest-evolving genes in mammalian and avian genomes3,11. In animals, immunity is achieved through an interplay between different types of immune cells12–15. The immune system of different species, or even that of individuals of the same species, can thus differ not only in individual cell types16,17 but also in the interaction between them18–20. The relative importance of these two contributions towards the evolvability of the immune system, however, is unclear.
The decisive factor for a system’s evolvability is how genotypic variation maps onto phenotypic variation. To address the question of evolvability of immune traits, we focused on the genetic determinants of the relative frequencies of individual immune cell types, also known as immune profiles. We chose this trait because immune profiles are highly variable between individuals5, are functionally important in both health and disease21–25, and are to a large extent determined by genetic factors4,26,27. At the same time, genetic determinants of immune profile variation remain elusive. Whereas a recent study that included more than half a million participants successfully identified the association of more than a 1,000 genes with the frequencies of five, low-resolution white blood cell types (neutrophils, monocytes, lymphocytes, basophils and eosinophils)28,29, studies looking at white blood cells with higher resolution, but conducted only on hundreds of individuals, identified only a small number of associated genes22–24,30–33. Approaches combining both higher cellular resolution and necessary sample size have only recently started to emerge34, illustrating the need for alternative approaches.
Here we performed a genetic association study of immune cell profiles in the Collaborative Cross (CC), a panel of highly genetically diverse yet inbred mouse strains6,35–37. The CC exhibits wide phenotypic variation in many functional immune traits38–41, as well as in immune homeostatic composition42,43, thereby allowing sufficient statistical power and reproducibility in a setting with minimal and equal environmental influences on immune cellular composition. We focused on immune cell profiles in the bone marrow, not readily accessible for association studies in humans. We associated and functionally analysed a large number of genes to immune cell abundance in a cell-specific manner. Finally we categorized exonic variants associated with immune cell frequency variation based on the respective genes being expressed or not in the respective immune cell type. Doing so enabled us to discover that those genes that are not expressed in the cell type whose variation they influence have accumulated variation under weak negative selection, supporting a role of intercellular interactions in immune system evolvability.
CC mice vary widely in their immune profiles
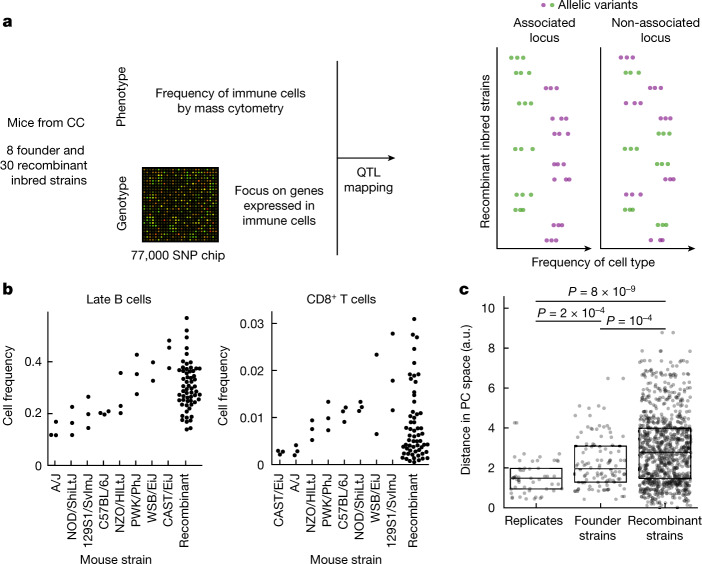
To quantify variation in immune profiles in well-defined genetic backgrounds we leveraged mice from the CC panel6. The CC panel was previously designed to maximize heterogeneity between strains while maintaining individual homozygosity—eight founder mouse strains, including five laboratory and three wild inbred strains, had been crossed and then inbred to achieve homozygosity, for a total of about 100 strains44. Of those we profiled the bone marrow of eight founder strains in triplicate and 30 recombinant inbred strains in duplicate (Fig. 1a and Supplementary Table 1). To enable simultaneous profiling of multiple immune cell subtypes we subjected the extracted cells to profiling with time-of-flight mass cytometry (CyTOF). Using a broad panel of antibodies, we quantified the abundance of nine immune cell populations—haematopoietic stem cells (HSCs), natural killer (NK) cells, CD8+ T cells, CD4+ T cells, total B cells, pro-B cells, late B cells, granulocytes and monocytes (for labelling and gating strategy see Methods, Supplementary Fig. 1 and Supplementary Tables 2 and 3). We observed a continuous range of immune cell frequencies among recombinant inbred strains, a typical phenotype of CC mice42, with a dynamic range comparable to or even larger than that observed between the most extreme founder strains (P = 10−4; Fig. 1b, Extended Data Fig. 1a and Supplementary Table 4). This is consistent with previously reported epistatic interactions36. Importantly for our study, variation between strains was much wider than that between mice of the same strain, allowing us to capture the genetic determinants of the variation with our study design (Fig. 1c and Extended Data Fig. 1b).
Fig. 1. Immune cell profiles are highly variable across CC mouse strains.
a, Schematic of the experimental approach. The genome of CC recombinant strains was reconstructed at each locus based on single-nucleotide polymorphism (SNP) chip data and the genomes of the eight founder strains; immune cell frequencies were quantified by mass cytometry; the association of variants with immune cell type frequency was then quantified. b, Swarm-plot of frequencies of selected immune cell types from both CC founder strains and CC recombinant inbred strains used for the association study. Cell frequency is shown as a fraction of total live bone marrow cells. Founder strains are ordered according to median frequency for each cell subset; see Extended Data Fig. 1a for plots of all immune cell types assayed. c, Strip-plot of pairwise distances in principal component analysis (PCA) space between immune profiles within the respective categories. PCA was computed using the immune profiles for all measured animals that had all target cell types detected (n = 42); the Euclidean distance in the space defined by the first two PC (Extended Data Fig. 1b) is shown for all pairs within the respective categories. Boxplots denote median, first and third quartiles. P values for two-sided t-test are shown. SNP chip created with Adobe Stock (https://stock.adobe.com).
Extended Data Fig. 1. Immune cell frequencies of CC parent and recombinant strains used in the exploratory part of the association study.
a. Swarm plots of bone marrow immune cell frequencies, profiled using mass cytometry. Parent strains are ordered according to the median value for each of the immune cell subsets. b. Principal component analysis using immune cell frequencies of all mice measured in the first cohort, for which all target cell types could be detected. The first two principal components are shown.
Variation in immune profiles is polygenic
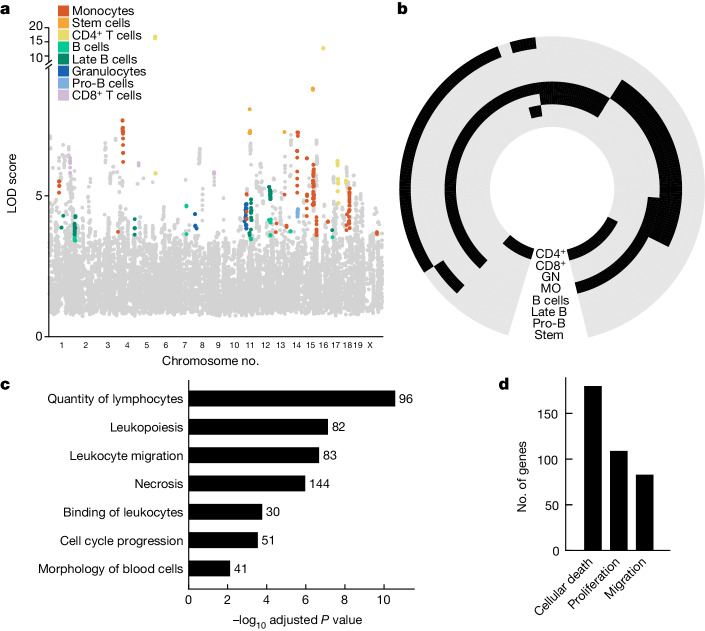
To link genotypic variation to phenotypic variation in immune profiles we associated the measured frequencies of different immune cell subpopulations with the known genetic profiles of the CC parental strains, which had all been previously sequenced45. To increase the interpretability of our association study we considered only genes with a potential function in the immune system as identified previously by the ImmGen consortium (a broad set of 7,965 genes)46,47 and such that at least one founder strain contains an exonic variant in this gene, for a total of 6,902 genes represented by 15,458 loci (Methods). The exclusion of intronic and intergenic variants was driven by our goal to associate variants to specific genes48. For each of the nine immune cell subsets we applied a previously developed pipeline for quantitative trait loci (QTL) mapping in outbred mice49, which determines the association of a given locus with the observed phenotype by taking into account the kinship between recombinant inbred strains—that is, the likelihood that a given strain contains the founder allele. In this way we identified genes associated with the frequency of one or more cell types with log odds ratio (LOD) greater than the 5% false discovery rate (FDR) threshold determined separately for each cell type by permutation analysis (Methods and Extended Data Fig. 2a). To ensure high stringency of our association study, we further measured immune cell profiles in a second, validation cohort of 48 mice stemming from 24 CC recombinant strains not included in the first cohort (Supplementary Tables 5 and 6). We performed analysis of variance to determine whether the variation in immune cell frequency in the validation cohort could be explained by allelic differences in each gene identified in the first cohort (Padj < 0.05), and retained genes only if the directionality of the effect was consistent across both cohorts. This stringent procedure resulted in 271 genes associated with high confidence with the frequency of one or more cell types in bone marrow (Fig. 2a). Due to the fact that we assayed functionally related traits, we leveraged signal propagation across traits to detect additional associations for this set of genes50–52 (Methods). Overall we report 543 gene–cell type frequency associations across all assayed cell types (Fig. 2b and Supplementary Table 7).
Extended Data Fig. 2. Genetic associations of immune cell frequencies.
a. False discovery rate (FDR) as a function of threshold for association as inferred from permutation analysis (Methods) for each cell type. Red dashed line indicates the FDR of 5% used to determine the cell type-specific LOD score threshold used to find associated genes in the exploratory cohort. b. Distribution of relative median LOD scores for gene clusters during signal propagation. Genes found significantly associated to at least immune cell type were clustered based on their pattern of association across all the assayed cell types. For each cluster, median LOD score for each cell type was calculated and then divided by the maximum value of this quantity across all cell types. The distribution of the resulting values is plotted, with two clear peaks – all gene cluster-cell type pairs with median LOD score above 0.4 were considered associated. c. Bar-plot of functionally enriched categories. Functional groups that were found enriched using Ingenuity Pathway Analysis; terms with p < 0.05 are shown. x-axis indicates -log10 of p-value from one-sided Fisher’s exact test.
Fig. 2. Regulation of the frequency of immune cell types in bone marrow is polygenic and related to quantitative homeostatic balance.
a, Manhattan plot for associated loci. Coloured dots denote cell type with which genes were found associated based on significance threshold set according to FDR for each population separately; grey dots denote genes with an association below this threshold. Associated loci that did not pass validation by the second cohort are not shown. b, Circular heatmap of associations between genes and immune cell types. Black denotes association, grey denotes no association determined. Genes are clustered according to their association profile across cell types. Cell types are indicated on each ring (CD4+, CD4+ T cells; CD8+, CD8+ T cells; GN, granulocytes; MO, monocytes); for NK cells no associations were determined. c, Functional enrichment of genes associated with the assayed immune traits as determined by ingenuity pathway analysis (IPA). Related functional terms were manually grouped and, for each group, the term with the lowest adjusted P value is shown; for details on grouping of terms see Extended Data Fig. 3b. One-sided Fisher’s exact test with Benjamini–Hochberg correction was applied. d, Number of genes associated with selected homeostatic functions.
To gain a biological understanding of the determinants of immune cell frequencies we performed functional enrichment analysis of trait-associated genes. The results suggested a role for genes involved in the cell-intrinsic functions of cellular movement, proliferation and death (Extended Data Fig. 2c). To expand the resolution of this functional insight we increased the number of genes analysed for functional enrichment by relaxing the stringency of the association analysis for a total of 785 associated genes (Methods). Functional enrichment of this larger set confirmed the role of cell-intrinsic functions (Fig. 2c and Supplementary Table 8), with 31% of genes annotated for at least one of the functions of proliferation, cell death and cellular movement (Fig. 2d)—that is, basic determinants of homeostatic balance. Taken together, akin to the control of messenger RNA and protein abundance, cell subset abundance is also subject to turnover rates.
Genes associated in cyto-trans
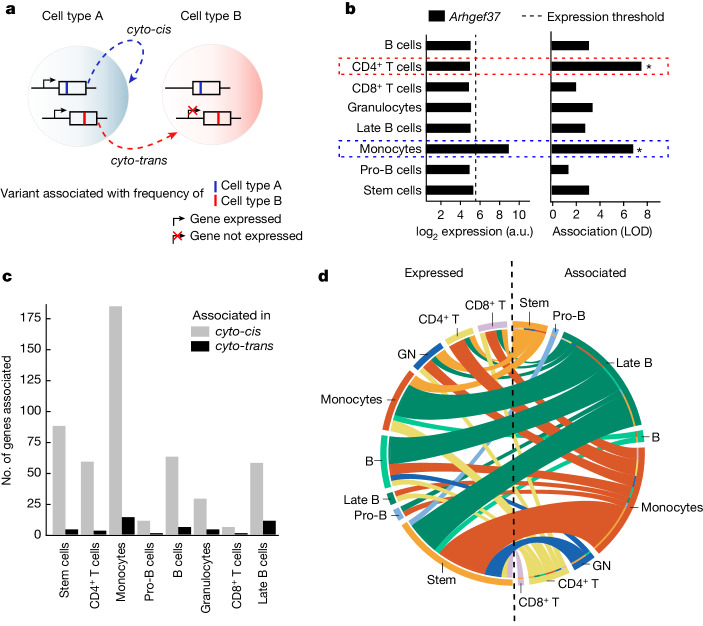
Because a considerable fraction of the genes that we discovered to be associated with immune cell frequencies are involved in cellular turnover, we wondered whether all variation in cell frequencies could be rationalized in terms of cell-intrinsic functions. In other words, we sought to identify genes that would influence the abundance of a cell type from outside of the given cell type, through direct or indirect interaction between the cell types. To explore this question we divided the associated genes into two groups (Fig. 3a)—those expressed in the cell type with whose abundance they are associated (henceforth cyto-cis genes) and those not expressed in the respective cell type (cyto-trans genes). To do this we took advantage of a comprehensive gene expression resource on sorted bone marrow cells from the Immunological Genome project46, and the negative threshold for microarray signal previously established by the consortium as corresponding to one per million reads in comparable RNA sequencing data53 (dashed vertical line in Fig. 3b). As an example, Arhgef37, a guanine nucleotide exchange factor, was found to be associated with both monocyte and CD4+ T cell frequency (Fig. 3b, right). However, Arhgef37 is expressed only in monocytes (Fig. 3b, left) and thus, with respect to monocyte frequency, Arhgef37 acts in cyto-cis whereas, with respect to the frequency of CD4+ T cells, Arhgef37 acts in cyto-trans.
Fig. 3. Genes associated with frequency of specific immune cell types are frequently not expressed in the associated cell type.
a, Schematic of the definition of a genetic association acting in cyto-cis or cyto-trans. A gene associated in cyto-cis is expressed in the cell type of the phenotype with which it is associated; conversely, a cyto-trans gene is not expressed in the associated cell type but rather in a different cell type. b, Example of data used to classify associations as cyto-cis or cyto-trans. Gene expression was determined by ImmGen consortium, and LOD scores for association with immune cell frequencies are shown for the selected gene, Arhgef37. Black dashed vertical line indicates gene expression threshold used for binarization of gene expression, and asterisks denote significant associations as determined by a permutation test (Methods). Coloured dashed rectangles highlight associations in cyto-cis and cyto-trans. c, Bar plot showing the number of genes associated in cyto-cis or cyto-trans for each respective cell type. Cell types are ordered by increasing proportion of cyto-trans associations. d, Split Circos plot depicting expression of genes associated with immune cell frequencies in cyto-trans. The colour of connecting lines is determined by the cell type with which the gene is associated; the colour of the rim on the left-hand side corresponds to the cell type in which the gene is expressed. For associated genes expressed in multiple cell types, multiple lines are shown. a.u., arbitrary units.
Classifying all associations between genes and immune cell frequencies as either cyto-cis or cyto-trans, we observed a cyto-cis relationship for the majority of associations (91.9%, 499 associations comprising 257 genes; Fig. 3c). However, a non-negligible fraction of the associations (8.1%, 44 associations comprising 28 genes) observed across all cell types (Fig. 3c) demonstrated cyto-trans regulation—that is, these genes have the ability to regulate the abundance of at least one immune cell type without being expressed in that cell type. However, our analysis was restricted to exonic variants, which generally require expression to manifest their phenotype54. Consequently, the relevant immune cell types must be influenced by these variants through some form of interaction with cell types that express the protein, possibly mediated by ligands, metabolites, yet other cell types or in a connection between a cell and its precursor. Although many cyto-trans genes were expressed in HSCs, suggesting the control of abundance of a specific cell type from upstream in the differentiation lineage, overall cyto-trans genes create a complex web of interactions between cell types (Fig. 3d). In addition, some cyto-trans associations were even shared across multiple cell types (Extended Data Fig. 3a). Compared with all associated genes, cyto-trans genes were enriched for a number of signalling pathways, congruent with their role in mediation of interactions between cell types (Extended Data Fig. 3b and Supplementary Table 9).
Extended Data Fig. 3. Analysis of cyto-trans associations.
a. Circular heatmap of associations between genes and immune cell types, classified according to expression in the respective cell type. Genes are clustered according to their association profile across cell types. Cell types are indicated on each ring (CD4+ – CD4+ T cells, CD8+ – CD8+ T cells, GN – Granulocytes, MO – monocytes). b. Bar-plot of functionally enriched categories in cyto-trans genes relative to all associated genes. Functional groups that were found enriched using Ingenuity Pathway Analysis (see Methods for details), terms with p < 0.05 are shown. x-axis indicates -log10 of p-value from one-sided Fisher’s exact test. c. Empirical cumulative distribution functions for amino acid conservation scores of genes found associated with a single immune cell type in this study, grouped by whether they were found associated in cyto-cis or in cyto-trans. P-value for one-sided Kolmogorov-Smirnov test between these two groups is stated.
Weaker negative selection in cyto-trans
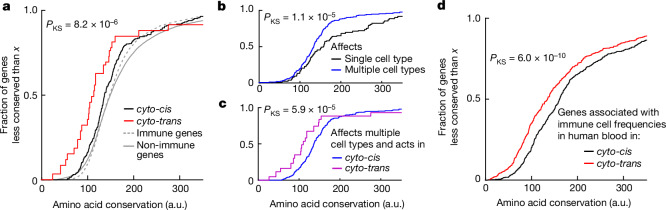
Having identified the phenomenon of genes associated with the frequency of immune cell types in which they are not expressed, we next asked whether these cyto-trans genes have a special role in the evolution of immune traits. More specifically, we hypothesized that genes determining the frequency of the immune cell type but that are not expressed in that cell type, and thus cannot have any additional function in that cell type, might face weaker negative selection. To test this hypothesis we compared the evolutionary sequence conservation for cyto-trans versus cyto-cis genes as determined for the mouse immune system. To do so we used scores calculated with PhastCons55, an established method used for quantification of evolutionary conservation based on a two-state hidden Markov model, across 60 vertebrate species including rodents, primates, carnivores and fish and spanning an evolutionary time of more than 500 million years56 (Methods). The PhastCons model classifies each site as belonging to a conserved element or not, as well as assigning a score to each site representing the probability of negative selection of that site across evolution of the ensemble of species55. For each gene associated with at least one immune cell type abundance in our dataset, we calculated its mean conservation score by averaging the scores of all conserved sites located in its coding region. We observed that genes for which we found a cyto-trans association in our study were under significantly lower negative selection than those for which we found no cyto-trans association (one-sided Kolmogorov–Smirnov test, PKS = 8.2 × 10−6; Fig. 4a).
Fig. 4. Genes with cyto-trans associations facilitate the evolution of immune traits.
a, Empirical cumulative distribution functions for amino acid conservation scores of genes grouped by whether they were found to be associated with immune cell frequencies in only cyto-cis or also in cyto-trans. P value for one-sided Kolmogorov–Smirnov test between these two groups is shown. Cumulative distributions of evolutionary conservation for immune genes considered in this study (Methods) and for non-immune genes in the mouse genome are shown for comparison in grey. b, As in a, except that genes are categorized according to whether they are associated with more than one immune cell type. c, As in a, except that only genes associated with frequencies of multiple immune cell types are considered. d, Empirical cumulative distribution functions for amino acid conservation scores of genes found to be associated with immune cell (neutrophil, monocyte, lymphocyte, basophil, eosinophil) frequencies in human blood28,29. Genes are grouped by whether they were found to be associated with immune cell frequencies only in cyto-cis or also in cyto-trans (Methods). P value for one-sided Kolmogorov–Smirnov test is shown.
Because cyto-trans regulation is, by definition, connected with interaction among different cell types, we wondered whether the recent evolution of a complex immune system in vertebrates had focused on coordinated changes in abundance across multiple immune cell types rather than just a single one. To test this hypothesis we classified genes to either (1) those associated with only a single cell type in our study (or with only closely related cell types within the same lineage; Methods) or (2) those associated with the frequency of multiple cell types, and quantified the evolutionary conservation as before. We indeed found that genes associated with the frequency of multiple cell types are evolutionarily less conserved than those affecting just one immune cell type (PKS = 1.1 × 10−5; Fig. 4b).
We wondered whether the phenomena of genes driving evolution by eliciting coordinated changes across multiple cell types and evading negative selection by being involved in interactions between cell types are overlapping. Cyto-trans genes were not enriched among genes associated with multiple cell types (Pχ2 = 0.61) and, when considering only genes associated with multiple cell types, we observed that those with at least one cyto-trans association are under weaker negative selection (PKS = 5.9 × 10−5; Fig. 4c). This suggests that genes involved in interactions between immune cell types have been an important source of variation, not merely because they would be more likely to affect multiple cell types but probably because they face fewer constraints related to their function.
The principle that cyto-trans genes promote evolvability cannot be specific to mice but, rather, should be true of any sufficiently advanced cell-based immune system. We therefore sought to validate our result using a larger experimental dataset from a different organism. To do so we analysed previously published genetic associations for the frequencies of immune cell types in human blood (1,046 genes in total)28,29 and juxtaposed them with gene expression data from the Human Protein Atlas57. Here too we found that genes associated with cell types in which they were not expressed (cyto-trans, 358 genes) were significantly less conserved than those expressed in the associated cell type (PKS = 6.0 × 10−10; Fig. 4d, Extended Data Fig. 4 and Supplementary Table 11).
Extended Data Fig. 4. Evolutionary conservation of genes associated with immune cell frequencies in the human blood.
a. As in Fig. 4d. Empirical cumulative distribution functions for amino acid conservation scores of genes found associated with immune cell frequencies in the human blood by previous studies. Genes are grouped by whether they were found associated with immune cell frequencies only in cyto-cis or also in cyto-trans (Methods). b. As in (a), except genes are categorised according to whether or not they are associated to more than one immune cell type or not. c. As in (a), except only genes associated with frequencies of a single immune cell type are considered d. As in (a), except only genes associated with frequencies of multiple immune cell types are considered. P-values for one-sided Kolmogorov-Smirnov test are stated.
Discussion
Natural selection as an evolutionary mechanism can act only on pre-existing population heterogeneity, which also needs to be heritable. The genetic contribution towards interindividual variation is thus a key variable determining evolvability. In the present study we leveraged heterogeneity in recombinant mice stemming from well-defined lineages to infer those genes influencing immune cell frequencies, an immune trait important in both health and disease5,21–25, and juxtaposed these genes with the vertebrate evolutionary record to infer the determinants of immune system evolvability.
We found 271 genes with variants that contribute towards variation in the frequencies of various immune cell types. Although many of these do so through the cell-intrinsic functions of cell differentiation, proliferation and death, we also found that about 10% of the associated genes affect the frequencies of those cell types in which they are not expressed. These genes must be doing so in what we term cyto-trans—that is, via expression in another cell type, through direct or indirect interaction with the cell type in question. Notably, we found that the coding sequences of cyto-trans genes have been under weaker negative selection during vertebrate evolution than those of the genes found acting in cyto-cis only. This implies that the genetic determinants of interactions between different immune cell types are, when mutated, more amenable to producing near-neutral variation in immune cell frequencies compared with genes involved in internal cell regulation. In addition, as a separate phenomenon, we found that the same is true for genes associated with, and potentially coordinating, frequencies of multiple cell types. This earmarks genes involved in interactions between immune cell types as an important source of near-neutral variation.
Variation with strong positive or negative fitness effects has an appreciated role in the evolution of biological species, but the importance of near-neutral variation has only lately emerged as an important consideration for the capacity of biological systems to evolve. Recent work on transcription factor and protein structure evolution2,7,58 has found that increased capacity for near-neutral variation—that is, increased mutational robustness—paradoxically facilitates evolution by supporting genetic diversity. This has solved the apparent conflict between evolvability and robustness8—the seemingly contradictory requirement that phenotypes be significantly altered by genetic changes to allow selection of fitter phenotypes but not be altered so much as to undergo frequent mutations without harm. Our results offer an appealing extension of this evolvability principle in the context of the immune system—an enhancement of near-neutral genetic diversity is achieved through modularity. Conceptually one can speculate that, whereas the genetic determinants of inner cell life might have been arranged early in evolution to ensure functioning of individual immune cell types, the interaction between different immune cell types provides a phenotypic space in which mutations can continue to produce variation with little detriment.
The cell, the atomic unit of life, is well suited to correspond to a module in the evolutionary process of the immune system, consistent with cyto-trans genes acting as an important source of evolutionary novelty. Nevertheless, our observation that genes associated with frequencies of multiple cell types, and thus possibly coordination among them, are also an important source of evolvability irrespective of the cyto-trans phenomenon suggests the existence of multiple layers of modularity. Such staged modular design, in which modules are iteratively combined into yet more complex modules, has previously been proposed as one of the unifying features of evolved complex systems, including those of human origin9. Although evolvability through modularity is not expected to be limited to the immune system, due to the high need for its rapid evolution, the immune system is poised to manifest modularity exceptionally strongly. Further research into immune system evolvability thus could not only enlighten the design principles behind immune responses but also contribute to biomimetic solutions—for example, in the system-of-systems approach to engineering, which is similarly based on interactions between functional units59. Our work thus acutely demonstrates the need to bring the study of evolvability through modularity, previously explored with success in the context of protein structure60, gene regulation61 and metabolism62, to complex cellular systems such as the immune system.
Methods
Mouse strains and sample collection
The CC founder strain males (n = 3 per strain) of laboratory strains A/J, C57BL/6 J, 129S1Sv/ImJ, NOD/ShiLtJ and NZO/H1LtJ, and of wild-derived strains CAST/EiJ, PWK/PhJ and WSB/EiJ, were purchased from The Jackson Laboratory, delivered to the Systems Genetics Core Facility at The University of North Carolina (UNC) and killed at 6–8 weeks. CC recombinant mice were purchased from, handled, bred and killed by the Systems Genetics Core Facility at UNC. All procedures involving animals were performed according to the Guide for the Care and Use of Laboratory Animals, with previous approval by the Institutional Animal Care and Use Committee within the Association for Assessment and Accreditation of Laboratory Animal Care-accredited programme at UNC at Chapel Hill (Animal Welfare Assurance no. A-3410-01). In total we profiled 129 mice aged 8–14 weeks from 54 different complete lines, at least two mice per strain (Supplementary Table 1). Bone marrow and blood tissues were collected from necropsy following killing by CO2. Bone marrow was flushed with a cold-cell staining medium (DPBS + 0.5% bovine serum albumin) from the femur and tibia using a 27.5-gauge needle and 10 ml syringe to achieve a single-cell suspension. Red blood cells from blood samples were eliminated by lysis buffer (BD Biosciences, catalogue no. 555899).
Cell staining
Primary conjugates of mass cytometry antibodies were prepared using the Maxpar antibody conjugation kit (Fluidigm Inc.) according to the manufacturer’s protocol (no. PRD002, Fluidigm), and optimal concentration was determined by titration according to the manufacturer’s protocol. Cells from each sample were washed twice with Cell Staining Medium (Maxpar) and a total of 3 million cells were used for extracellular staining. Cells were resuspended in 500 µl containing 1:2,000 rhodium DNA intercalator (Fluidigm) for 20 min of live/dead cell staining. Samples were washed with Cell Staining Medium and resuspended in a total of 100 µl of metal-tagged antibody mix for 1 h for cell surface marker staining. Cells were then fixed with 1.6% paraformaldehyde (Sigma-Aldrich) in a total volume of 200 µl and stored at 4 °C. Cells were centrifuged, paraformaldehyde removed and iridium DNA intercalator staining was performed for 20 min at 1:2,000 dilution in a 500 µl volume to differentiate cells from debris. Finally, fixed samples were washed three times with deionized water immediately before data acquisition.
Data acquisition and analysis
Samples were acquired using a CyTOF1 machine (DVS Sciences) at 500 events s−1 for a total of 100,000–200,000 events per sample. Internal metal isotope bead standards were added for sample normalization as described previously63. Acquired data were uploaded to a Cytobank web server (Cytobank) for data processing and gating out of dead cells and normalization beads. To account for intrarun declines in mean marker intensity over time, the acquisition records were truncated at the moment when any of the channels drifted by more than 5% from its mean since the start of acquisition. We noted that, for several strains (maximum of eight per cell type), some cell type markers were not observed, probably due to allelic variants being low affinity to the respective antibody, consistent with previous literature64, barring the direct use of unsupervised clustering approaches for cell subset characterization and quantification. We thus manually gated all samples (see Supplementary Fig. 1 for gating scheme) to estimate the frequency of nine major cell types across mouse strains. Mouse strains for which an antibody required to identify a cell subset was presumed low affinity were not included in the analysis of genetic associations for that cell subset (see Supplementary Tables 3 and 5 for exclusions). Resulting cell counts were then exported, adjusted to the total number of cells in the sample and are summarized in Supplementary Tables 4 and 6.
Initial mapping of associated genetic loci
Filtering of loci
The CC mice were genotyped using MegaMUGA SNPchip with about 78,000 markers36,65,66. To reduce the extent of multiple hypothesis testing we chose to focus on genomic loci containing immune-related genes with genetic variants. To do so we included only loci that passed both of the following criteria: (1) those located within a broad set of genes identified previously by the ImmGen consortium (7,965 genes) as having a potential function in the immune system46,47 and (2) those for which at least one of the CC founder strains harboured an exonic sequence variant. Using these two filtering criteria, we reduced the number of loci for testing from 77,725 to 15,458 located within 6,902 genes.
Genotype–phenotype association
We performed QTL mapping using the DOQTL R package49. In the first step, the haplotype of each recombinant mouse was reconstructed by per-locus estimation of the likelihood of it stemming from each of the founder strains. In the next step we searched for significant association between immune phenotype and each genomic locus, using an additive haplotype model that takes into account the kinship between recombinant CC mouse strains. To check for stability of observed associations we used a leave-one-out approach, always leaving one sample out and recalculating the strength of association; we retained only loci showing an association across all leave-one-out options according to the DOQTL procedure. Then, for each cell type we identified the significant association threshold as one that corresponds to 5% FDR (Extended Data Fig. 3a). We determined FDR by permutation analysis—repeatedly shuffling the labels of recombinant mouse strains (replicates were always assigned identical labels)—and calculated the strength of the cell type association.
Validation of gene–trait association
We used a second cohort of mice to validate our findings. We determined the frequencies of nine immune cell subsets as in the exploratory cohort. Each significant gene–phenotype association identified in the first cohort was tested for significance in the second. We used analysis of variance to determine whether the variation in immune cell frequencies could be explained by allelic differences in mice in the validation cohort. Only associations with Padj < 0.05, following correction for multiple hypothesis testing using the Benjamini–Hochberg approach, were retained. Next we required that the directionality of the phenotype–genotype association be consistent between the first and second cohorts. We calculated Spearman correlation between the first and the second cohorts for the mean of the given phenotype stratified by allele; only genes with correlation greater than 0 were retained for further analysis.
Signal propagation
Considering all genes that were validated to be associated with at least one assayed trait, we clustered them according to their association scores across all cell types using k-means clustering. The optimal number of clusters was determined using the silhouette method. For each cell type we determined associated clusters using their median association LOD scores. We considered a cluster as being associated with a given cell type frequency if the median LOD score of genes in the cluster for the given cell type was at least 40% of the highest median calculated for that cluster. This 40% threshold was chosen empirically based on the location of the minimum in the distribution of the relative LOD scores for gene cluster–cell type pairs, as shown in Extended Data Fig. 2b. The clusters were used solely for the purpose of signal propagation and not for any other analysis in this work.
Gene enrichment analysis
We performed gene enrichment analysis on the combined list of associated genes for all cell subtypes with IPA (Qiagen) software (build ‘ing_neptunite’ 2023-11-19), with the Core Analysis module for ‘Diseases & Functions’, using all genes considered in the association study as the background set. Biological filters were set such that only mouse genes were considered, and only immune-related terms: disease-related terms were manually filtered out and the remaining functional terms manually grouped into higher-level functions. We similarly performed gene enrichment analysis on a larger list of genes after relaxing the stringency of the association test so as to have no requirement for the directionality of effect to be conserved in the validation cohort (Padj < 0.1). Gene enrichment analysis of cyto-trans genes was performed similarly using IPA, with the Core Analysis module for ‘Pathways’ using all associated genes as the background set.
Cyto-cis and cyto-trans determination
We defined the expression of a particular gene in a particular cell type based on ImmGen Consortium expression data46 (GSE15907). We mapped between the populations defined by ImmGen and those profiled in our study (see Supplementary Table 10 for mapping). In the case where more than one ImmGen population was included in our cell subset definition, we required that at least one subpopulation express the gene of interest. To define each gene as expressed or not, we used an expression threshold defined by the ImmGen Consortium53. In brief, the consortium determined a threshold to consider a gene expressed by an empirical Gaussian mixture model, used to decompose the expression histograms into two Gaussian distributions. Low distribution was assumed to correspond to background noise, and the higher to the true signal. These yielded a conservative threshold for expression from the higher-expression Gaussian (above 120 a.u., 95% or greater probability of expression) and a threshold for the gene being silent from the lower-expression Gaussian (below 47 a.u., 95% or greater probability of the gene being silent). The range 47–120 a.u. was an intermediate range within which the gene might or might not be expressed. To be conservative towards calling cyto-trans based on the non-expression of the gene in the associated cell type, we considered the low ImmGen microarray threshold for gene expression of 47 a.u.
Evolutionary conservation
We obtained PhastCons55 conservation scores from the UCSC Genome Browser for 60 vertebrate species. Then, for each gene we calculated its conservation score by averaging the scores of all conserved elements located in its coding region. For the purposes of classification of genes as being associated with single or multiple cell types, all subtypes of B cells were considered a single cell type, as were granulocytes and monocytes.
Validation using human data
To validate in a different species that genes associated with immune traits in cyto-trans exhibit lower evolutionary conservation, we obtained published data from two human studies of immune cell frequencies in blood28,29. Both studies measured the abundance of five immune cell types: basophils, eosinophils, lymphocytes, monocytes and neutrophils. In total, 1,046 genes were found to be associated with abundance of at least one of these cell types.
Gene expression data for human immune cells were obtained from the Human Protein Atlas57 (http://www.proteinatlas.org). For lymphocytes, all genes expressed in both B and T cells were included. All genes associated with a cell type in which they were not expressed were considered as being associated in cyto-trans. We estimated conservation of the associated human genes in a similar manner as before for mouse genes (‘Evolutionary conservation’) using PhastCons55 conservation scores for human genes considering 100 vertebrate species, downloaded from the UCSC Genome Browser.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-024-07661-0.
Supplementary information
This file contains Supplementary Fig. 1 and legends for Tables 1–11.
Supplementary Tables 1–11.
Acknowledgements
We thank F. P. M. de Villena, D. Miller and G. Shaw for providing us with the CC mouse strains and their help with the mouse experiments; A. Rolls, T. Ben-Shannan, H. Azulay-Debby, B. Korin and M. Schiller for help with mouse work and manuscript revision; A. Ziv-Kennet for help with data preprocessing; and F. Iraqi for initial set-up of the system. We thank J. Tsang, M. Choder, A. Segre, D. Melamed, G. Atzmon, M. Lukačišinová, B. Perets and members of the Shen-Orr laboratory for fruitful discussions. This study was supported through generous support from the Israel Science Foundation (grant no. 1365/12 and 1626/20), Anonymous Foundation, MALAT and the Colleck Research Fund. M.L. was supported by the Rubenstein-Technion Integrated Cancer Center Fellowship and the Aly Kaufman Fellowship. We thank Y. Abraham for his contribution to the design and creation of the schematic in the original version of Fig 1a.
Extended data figures and tables
Author contributions
T.D., E.S. and S.S.S. carried out study design. T.D., M.L. and S.S.S. were responsible for conceptualization. Investigation was the responsibility of T.D., M.L., B.L., R.N., Y.A., A.A., Y.O. and M.G. Experimentation was carried out by T.D. and E.S. Data analysis was performed by T.D. and M.L. T.D., M.L. and S.S.S. wrote the manuscript draft. Writing, review and editing were carried out by T.D., M.L. and S.S.S. S.S.S. supervised and acquired funding.
Peer review
Peer review information
Nature thanks Pierre Pontarotti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The raw data files generated in CyTOF runs are available on community.cytobank.org (experiment nos. 116506 and 116507; first and second cohort, respectively). Mouse cell-specific gene expression was estimated using data generated by the ImmGen consortium, ImmGen Microarray Phase 1, GSE15907. Human cell-specific gene expression data were obtained from the Human Protein Atlas (https://www.proteinatlas.org/about/download).
Code availability
The analysis code is available on GitHub (https://github.com/shenorrLabTRDF/CCanalysis).
Competing interests
S.S.O. holds equity and is a consultant of CytoReason and holds an unpaid position with the Human Immunome Project. T.D., E.S. and Y.A., are employees and hold equity in CytoReason. R.N holds equity in CytoReason.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tania Dubovik, Martin Lukačišin
Extended data
is available for this paper at 10.1038/s41586-024-07661-0.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-024-07661-0.
References
- 1.Darwin, C. On the Origins of Species by Means of Natural Selection (Murray, 1859). [Google Scholar]
- 2.Payne, J. L. & Wagner, A. The causes of evolvability and their evolution. Nat. Rev. Genet.20, 24–38 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Shultz, A. J. & Sackton, T. B. Immune genes are hotspots of shared positive selection across birds and mammals. eLife8, e41815 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liston, A., Humblet-Baron, S., Duffy, D. & Goris, A. Human immune diversity: from evolution to modernity. Nat. Immunol.22, 1479–1489 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Brodin, P. & Davis, M. M. Human immune system variation. Nat. Rev. Immunol.17, 21–29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collaborative Cross Consortium. The genome architecture of the Collaborative Cross mouse genetic reference population. Genetics190, 389–401 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draghi, J. A., Parsons, T. L., Wagner, G. P. & Plotkin, J. B. Mutational robustness can facilitate adaptation. Nature463, 353–355 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner, A. The role of robustness in phenotypic adaptation and innovation. Proc. R. Soc. B Biol. Sci.279, 1249–1258 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon, H. A. The architecture of complexity. Proc. Am. Philos. Soc.106, 467–482 (1962). [Google Scholar]
- 10.Wagner, G. P. & Altenberg, L. Complex adaptations and the evolution of evolvability. Evol. Int. J. Org. Evol.50, 967–976 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Enard, D., Cai, L., Gwennap, C. & Petrov, D. A. Viruses are a dominant driver of protein adaptation in mammals. eLife5, e12469 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar, M. P. et al. Analysis of single-cell RNA-seq identifies cell-cell communication associated with tumor characteristics. Cell Rep.25, 1458–1468 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stubbington, M. J. T., Rozenblatt-Rosen, O., Regev, A. & Teichmann, S. A. Single cell transcriptomics to explore the immune system in health and disease. Science358, 58–63 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kveler, K. et al. Immune-centric network of cytokines and cells in disease context identified by computational mining of PubMed. Nat. Biotechnol.36, 651–659 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieckmann, J. C. et al. Social network architecture of human immune cells unveiled by quantitative proteomics. Nat. Immunol.18, 583–593 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Hagai, T. et al. Gene expression variability across cells and species shapes innate immunity. Nature563, 197–202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiao, A. et al. Single-cell sequencing reveals the evolution of immune molecules across multiple vertebrate species. J. Adv. Res.55, 73–87 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armingol, E., Officer, A., Harismendy, O. & Lewis, N. E. Deciphering cell–cell interactions and communication from gene expression. Nat. Rev. Genet.22, 71–88 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chua, R. L. et al. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat. Biotechnol.38, 970–979 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Thaiss, C. A., Levy, M., Itav, S. & Elinav, E. Integration of innate immune signaling. Trends Immunol.37, 84–101 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Tsang, J. S. et al. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell157, 499–513 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orrù, V. et al. Genetic variants regulating immune cell levels in health and disease. Cell155, 242–256 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira, M. A. R. et al. Quantitative trait loci for CD4:CD8 lymphocyte ratio are associated with risk of type 1 diabetes and HIV-1 immune control. Am. J. Hum. Genet.86, 88–92 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagou, V. et al. Genetic architecture of adaptive immune system identifies key immune regulators. Cell Rep.25, 798–810 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alpert, A. et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med.25, 487–495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall, M. A. et al. Genetic influence on peripheral blood T lymphocyte levels. Genes Immun.1, 423–427 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Evans, D. M., Frazer, I. H. & Martin, N. G. Genetic and environmental causes of variation in basal levels of blood cells. Twin Res.2, 250–257 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Vuckovic, D. et al. The polygenic and monogenic basis of blood traits and diseases. Cell182, 1214–1231 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen, M.-H. et al. Trans-ethnic and ancestry-specific blood-cell genetics in 746,667 individuals from 5 global populations. Cell182, 1198–1213 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patin, E. et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat. Immunol.19, 302–314 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Mangino, M., Roederer, M., Beddall, M. H., Nestle, F. O. & Spector, T. D. Innate and adaptive immune traits are differentially affected by genetic and environmental factors. Nat. Commun.8, 13850 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roederer, M. et al. The genetic architecture of the human immune system: a bioresource for autoimmunity and disease pathogenesis. Cell161, 387–403 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aguirre-Gamboa, R. et al. Differential effects of environmental and genetic factors on T and B cell immune traits. Cell Rep.17, 2474–2487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akbari, P. et al. A genome-wide association study of blood cell morphology identifies cellular proteins implicated in disease aetiology. Nat. Commun.14, 5023 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Churchill, G. A. et al. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat. Genet.36, 1133–1137 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Srivastava, A. et al. Genomes of the Mouse Collaborative Cross. Genetics206, 537–556 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chesler, E. J. et al. The Collaborative Cross at Oak Ridge National Laboratory: developing a powerful resource for systems genetics. Mamm. Genome19, 382–389 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snijders, A. M. et al. Influence of early life exposure, host genetics and diet on the mouse gut microbiome and metabolome. Nat. Microbiol.2, 16221 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Vered, K., Durrant, C., Mott, R. & Iraqi, F. A. Susceptibility to Klebsiella pneumonaie infection in collaborative cross mice is a complex trait controlled by at least three loci acting at different time points. BMC Genomics15, 865 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorè, N. I., Iraqi, F. A. & Bragonzi, A. Host genetic diversity influences the severity of Pseudomonas aeruginosa pneumonia in the Collaborative Cross mice. BMC Genet.16, 106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferris, M. T. et al. Modeling host genetic regulation of influenza pathogenesis in the Collaborative Cross. PLoS Pathog.9, e1003196 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelada, S. N. P. et al. Genetic analysis of hematological parameters in incipient lines of the collaborative cross. G3 (Bethesda)2, 157–165 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graham, J. B. et al. Extensive homeostatic T cell phenotypic variation within the Collaborative Cross. Cell Rep.21, 2313–2325 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noll, K. E., Ferris, M. T. & Heise, M. T. The Collaborative Cross: a systems genetics resource for studying host-pathogen interactions. Cell Host Microbe25, 484–498 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keane, T. M. et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature477, 289–294 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heng, T. S. P. et al. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol.9, 1091–1094 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Jojic, V. et al. Identification of transcriptional regulators in the mouse immune system. Nat. Immunol.14, 633–643 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borsari, B. et al. Enhancers with tissue-specific activity are enriched in intronic regions. Genome Res.31, 1325–1336 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gatti, D. M. et al. Quantitative trait locus mapping methods for diversity outbred mice. G3 (Bethesda)4, 1623–1633 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo, B. & Wu, B. Integrate multiple traits to detect novel trait–gene association using GWAS summary data with an adaptive test approach. Bioinformatics35, 2251–2257 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim, S. & Xing, E. P. Statistical estimation of correlated genome associations to a quantitative trait network. PLoS Genet.5, e1000587 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu, X. et al. Meta-analysis of correlated traits via summary statistics from GWASs with an application in hypertension. Am. J. Hum. Genet.96, 21–36 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ericson, J. et al. ImmGen microarray gene expression data: data generation and quality control pipeline. ImmGenhttps://www.immgen.org/Protocols/ImmGen%20QC%20Documentation_ALL-DataGeneration_0612.pdf (2012).
- 54.Castel, S. E. et al. Modified penetrance of coding variants by cis-regulatory variation contributes to disease risk. Nat. Genet.50, 1327–1334 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siepel, A. et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res.15, 1034–1050 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar, S. et al. TimeTree 5: an expanded resource for species divergence times. Mol. Biol. Evol.39, msac174 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uhlen, M. et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science366, eaax9198 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Zheng, J., Guo, N. & Wagner, A. Selection enhances protein evolvability by increasing mutational robustness and foldability. Science370, eabb5962 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Maier, M. W. Architecting principles for systems-of-systems. Syst. Eng.1, 267–284 (1998). [Google Scholar]
- 60.Rorick, M. M. & Wagner, G. P. Protein structural modularity and robustness are associated with evolvability. Genome Biol. Evol.3, 456–475 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kashtan, N. & Alon, U. Spontaneous evolution of modularity and network motifs. Proc. Natl Acad. Sci. USA102, 13773–13778 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ravasz, E., Somera, A. L., Mongru, D. A., Oltvai, Z. N. & Barabási, A. L. Hierarchical organization of modularity in metabolic networks. Science297, 1551–1555 (2002). [DOI] [PubMed] [Google Scholar]
- 63.Finck, R. et al. Normalization of mass cytometry data with bead standards. Cytometry A83, 483–494 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Philbrick, W. M., Maher, S. E., Bridgett, M. M. & Bothwell, A. L. A recombination event in the 5’ flanking region of the Ly-6C gene correlates with impaired expression in the NOD, NZB and ST strains of mice. EMBO J.9, 2485–2492 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Welsh, C. E. et al. Status and access to the Collaborative Cross population. Mamm. Genome23, 706–712 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morgan, A. P. et al. The mouse universal genotyping array: from substrains to subspecies. G3 (Bethesda)6, 263–279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Supplementary Fig. 1 and legends for Tables 1–11.
Supplementary Tables 1–11.
Data Availability Statement
The raw data files generated in CyTOF runs are available on community.cytobank.org (experiment nos. 116506 and 116507; first and second cohort, respectively). Mouse cell-specific gene expression was estimated using data generated by the ImmGen consortium, ImmGen Microarray Phase 1, GSE15907. Human cell-specific gene expression data were obtained from the Human Protein Atlas (https://www.proteinatlas.org/about/download).
The analysis code is available on GitHub (https://github.com/shenorrLabTRDF/CCanalysis).