Abstract
Spontaneously formed germinal centers (GCs) have been reported in most mouse models of human autoimmune disease and autoimmune patients, and have long been considered a source of somatically-mutated and thus high affinity autoantibodies, but their role in autoimmunity is becoming increasingly controversial, particularly in the context of systemic autoimmune diseases like lupus. On the one hand, there is good evidence that some pathogenic lupus antibodies have acquired somatic mutations that increase affinity for self-antigens. On the other hand, recent studies that have genetically prevented GC formation, suggest that GCs are dispensable for systemic autoimmunity, pointing instead to pathogenic extrafollicular (EF) B-cell responses. Furthermore, several lines of evidence suggest germinal centers may in fact be somewhat protective in the context of autoimmunity. Here we review how some of the conflicting evidence arose, and current views on the role of GC in autoimmunity, outlining mechanisms by which GC may eliminate self-reactivity. We also discuss recent advances in understanding extrafollicular B cell subsets that participate in autoimmunity.
1. Introduction
Germinal centers (GCs) are specific microstructures that develop in secondary lymphoid organs under antigen stimulation. B cells within GCs undergo clonal expansion, somatic hypermutation (SHM), selection, and eventually differentiate to affinity-matured plasma cells (PCs) or memory B cells. GC B cells are important source of high-affinity antibody required by long-term protective immunity.
GC is morphologically divided into two compartments: a dark zone (DZ) filled with dividing B cells (first called centroblasts) and a light zone (LZ) enriched with centrocytes (non-dividing progeny of centroblasts) (MacLennan, 1994;Victora and Nussenzweig, 2022). Activated B cells that have bound antigen and received cognate help form follicular helper T (TFH) cells at the T: B borders (Johnston et al., 2009;Nurieva et al., 2009;Yu et al., 2009). TFH cells are a specialized subset of CD4 T cells characterized by expression of transcription factor Bcl6 and surface markers CXC-chemokine receptor 5 (CXCR5), inducible costimulator (ICOS) and programmed cell death protein 1 (PD-1) (Johnston et al., 2009;Nurieva et al., 2009;Yu et al., 2009). Upon priming, activated B cells upregulate activation-induced cytidine deaminase (AID) that allows immunoglobulin isotype switching and commence cell division at the periphery of the follicle (Allen, Okada and Cyster, 2007;Roco et al., 2019). Those B cells that downregulate EBI-2 migrate towards the center of the follicle where they undergo rapid clonal expansion as B blasts before moving to the pole of the follicle closer to the T zone and differentiate into centroblasts, giving rise to the dark zone (Pereira et al., 2009). Proliferating centroblasts further upregulate the expression of, AID, which introduces mutations into the Ig variable region genes and thereby changes antibody affinity and specificity, a process termed somatic hypermutation (SHM) (Berek, Berger and Apel, 1991;Griffiths et al., 1984;Jacob et al., 1991).
Mutated dark zone B cells then exit cell cycle and move to LZ where they can bind antigen held in the form of immune complexes by a network of follicular dendritic cells (FDCs) that localizes in LZ (Kosco-Vilbois and Scheidegger, 1995). LZ B cells take up and process this antigen to present it to germinal center TFH cells. GC TFH cells govern B cell positive selection with GC and allow for optimal affinity-matured antibody responses. B cell clones with enhanced affinity tend to have an advantage in their competition for TFH cell help and exit GCs, differentiating into memory B cells and long-lived PCs (Figure 1). A proportion of cells recycles to the dark zone to undergo further rounds of SHM. GC B cells can thus shuttle back and forth between the two zones through CXCR4/CXCR5-mediated migration (Allen et al., 2004).
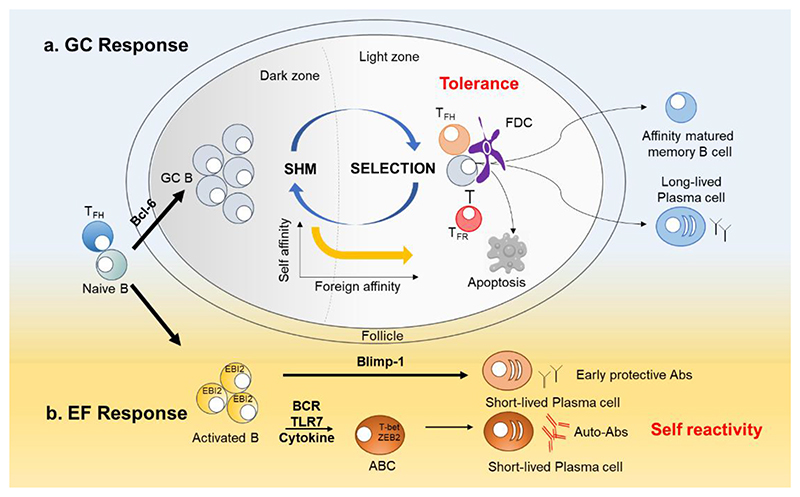
Figure 1. Germinal center and extrafollicular pathways in plasma cell differentiation.
a. Germinal center (GC) response. GC response incorporates B cell clonal expansion, somatic hypermutation (SHM) and selection, and is the source of affinity matured memory B cells and plasma cells. GC selection is regulated by the balance of two T cell subsets: T follicular helper (TFH) cells that promote GC responses and T follicular regulatory (TFR) cells that inhibit the response. Self-reactive cells accidentally generated in the GC are safely confined in GC and are either cleared by apoptosis, or undergo new rounds of SHM and selection, thus eliminating self-affinity while increasing affinity towards foreign antigens if present.
b. Extrafollicular (EF) response. Blimp-1 antagonizes the pro-GC signaling of Bcl-6 and promotes the development of short-lived PCs in EF sites (Shaffer et al., 2002). A subset of EF B cells known as age-associated B cells (ABCs or DN2 (IgD- CD27-) B cells are expanded in autoimmunity, the frequency of which correlates with autoantibodies and disease severity. The development of ABCs requires signals through the B cell receptor (BCR), TLR7, and cytokines such as IFN-γ and IL-21. ABCs differentiate readily into plasma cells and are considered a major source of autoantibodies.
This process is regulated by the balance of two T-cell subsets, TFH cells that promote GC responses and T follicular regulatory (TFR) cells that limit selection and differentiation of self-reactive B cells and terminate GC reactions (Chung et al., 2011;Gonzalez-Figueroa et al., 2021;Jacobsen et al., 2021;Linterman et al., 2011).
GCs are not the sole environment in which B cells participate in humoral immunity. Upon encountering antigens, proliferating B cells that maintain EBI-2 expression migrate to extrafollicular (EF) regions and initiate antibody responses (Hannedouche et al., 2011;Liu et al., 2011;Pereira et al., 2009). Blimp-1 promotes the development of short-lived PCs in EF sites and can counteract pro-GC signaling by Bcl-6 (Shaffer et al., 2002). The EF pathway enables rapid differentiation of B cells to short-lived PCs, that provides fast protection against pathogens. Distinction between GC and EF contributions to B cell self-reactivity is essential for understanding of autoimmune pathogenesis and subsequent targeted therapies.
Over the last decade, many pieces have been added to the puzzle of autoantibody responses, although we still do not have a complete picture. New evidence from improved understanding of B cell responses and availability of mice that cannot form germinal centers suggests that GCs are dispensable for the initiation and maintenance of systemic autoimmunity. An extrafollicular B cell response, which lacks the required tolerance check-points, is likely to be the key source of autoimmunity. In this review, we outline the current models of GC-independent autoimmunity, and the factors that maintain GC self-tolerance. We also discuss the likelihood that GCs serve to constrain self-reactive B cells while defending against foreign antigens and review the extrafollicular B cell subsets that are emerging as prime suspects of pathogenic autoantibody production.
2. Assumptions that led to a pathogenic interpretation of spontaneous GCs
For the last two decades, the field of autoimmunity tacitly accepted a pathogenic role of germinal centers in autoantibody generation based on assumptions and interpretation of facts in the context of an incomplete understanding of the events that occur inside or outside of germinal centers. Here we outline some of the observations that led to the conclusions that autoantibodies arose in germinal centers.
-
A)
Most lupus mouse models develop spontaneous GCs. Since a report in 2001 describing spontaneous GCs in young lupus-prone mice across a variety of genetic backgrounds (Luzina et al., 2001), multiple studies have reported similar findings across multiple models of human autoimmunity, contributing to the view that GCs promote autoimmunity. Most of these studies however have only provided indirect evidence of association between GCs and autoimmunity. However, studies have since been published and are described below in which either mice develop antinuclear antibodies in the absence of spontaneous germinal centers (Deng et al., 2017;Jang et al., 2019;O’Neill et al., 2011;Schwickert et al., 2019;Soni et al., 2020;Zhang et al., 2019), or lupus prone-mice that normally develop spontaneous GCs continue to produce autoantibodies despite genetic abrogation GC formation (Brown et al., 2022).
-
B)
Causal relationship between TFH overactivity and systemic autoimmunity. After the first association was made in Roquinsan mice between increased TFH cell numbers and lupus-like autoimmunity in 2005 (Vinuesa et al., 2005), followed by demonstration of a causal relationship (Linterman et al., 2009), a myriad of reports emerged collectively describing a near universal association between the magnitude of spontaneous GC reactions and baseline TFH activation and manifestations of autoimmunity (Domeier, Schell and Rahman, 2017). Whilst it is likely that controlling TFH cell numbers and reactivity via thymic selection is an important checkpoint against autoimmunity in GCs that may be breached in the presence of certain genetic susceptibility, it is also possible that the role of dysregulated TFH cells in promoting autoimmunity does not occur within GC reactions. When TFH cells were discovered to be an independent cell subset in 2009 (Johnston et al., 2009;Nurieva et al., 2009;Yu et al., 2009), their main function was considered to be their ability to drive and maintain GCs and this reinforced considering GCs pathogenic (Vinuesa, Sanz and Cook, 2009). It later became clear that TFH cells played a near equal important function in driving extrafollicular responses (Lee et al., 2011), including formation of age-associated B cells (ABCs) (Song et al., 2022), thought to be pathogenic in lupus. Additional extrafollicular peripheral helper T cell subsets have also been identified and shown to play important roles in driving autoreactive B cell responses as described below (Marks and Rao, 2022;Odegard et al., 2008;Rao et al., 2017).
-
C)
Pathogenic antibodies are nearly always isotype switched and isotype switching was until recently considered to occur predominantly in GCs. Except for a few exceptions including IgM antibodies in Guillain Barre Sd (Kaida et al., 2001), isotype switched antibodies are more pathogenic for their increased ability to fix complement and activate myeloid cells via their Fc gamma receptors (Takai, 2002). For decades, expression of AID – the enzyme required for both isotype switching and somatic hypermutation - had been considered to be a feature of GCs and this and other lines of evidence had led to the assumption that isotype switching mainly occurred in GCs. Indeed, it was not until 2019 that definitive evidence was provided demonstrating that isotype switching occurs prior to expression of Bcl-6 and the onset of somatic hypermutation (Roco et al., 2019), thus prior to B cells entering GCs. In terms of self/foreign discrimination, the GC pathway is likely to be more stringent and refined than the extrafollicular pathway, due to the requirement for TFH cell help prior to differentiation into plasma cells. In the case of an extrafollicular response, a second signal delivered by a TLR ligand is sufficient as a second signal for a B cell to become a plasma cell in the absence of T cell help (Eckl-Dorna and Batista, 2009).
-
D)
Multiple reports have described that pathogenic autoantibodies carry somatic mutations, and somatic hypermutation is generally associated with GC origin. These include early reports in the late 90s of somatic mutations that endow B cells with self-reactivity towards DNA (Ray, Putterman and Diamond, 1996), others directed against skin autoantigens (Di Zenzo et al., 2012), and more recent reports describing high affinity autoantibodies as a consequence of somatic mutations in 9G4 antibodies from SLE patients (Gomez-Banuelos et al., 2023;Ota et al., 2023). Given the stochastic nature of SHM, such self-reactive B-cell clones have long been assumed to be an inevitable by-product of GCs. However, somatic mutation, albeit limited, has been suggested to also occur extrafollicularly in the context of chronic bacterial infections such as Salmonella (Di Niro et al., 2015;Shlomchik et al., 1987) and in lupus-prone MRL.lpr mice in which self-reactive plasmablasts localize outside follicles (Sang et al., 2014;William et al., 2002). While these studies are compelling, definitive evidence of SHM requires genetic manipulation that prevents the development of GC responses in these models.
3. Evidence of GC-independent systemic autoimmunity
Certain models of autoimmune disease are accompanied by the presence of spontaneous GCs, whose precise role in autoimmunity remains unclear. In recent years, there has been increasing evidence that lupus may occur in a GC-independent manner in mice (Table 1) and humans.
Table 1. Roles of GCs in mouse models of autoimmunity.
| Mouse strain | Manifestations | GC formation | Autoantibody origin | Driven by TLR7? | Reference |
|---|---|---|---|---|---|
| Systemic autoimmunity without formation of spt-GCs | |||||
| cGVHD | Scleroderma, lymphocytic bronchitis, damage in the salivary and lacrimal glands, Anti-dsDNA-IgG and IgG deposition in the skin and thymus. | No or less GCs | T cell-dependent extrafollicular pathway | Not tested | (Deng et al., 2017) |
| C57BL/6 →BALB/c | |||||
| B cell-condition al Ikzf1 deficiency (Ikzf1B-) | Splenomegaly, systemic inflammation, ANA. | No GCs | Myd88-dependent extrafollicular pathway | Not tested | (Schwickert et al., 2019) |
| Bach2-/- | Lupus nephritis, elevated titers of IgG-switched autoantibodies | Defects in GC formation | T cell-dependent extrafollicular pathway | Not tested | (Jang et al., 2019) |
| B cell-intrinsic Ship-deficiency (ShipB-) | Excessive autoantibodies against ssDNA, dsDNA, dsRNA and chromatin. | Impaired GC B responses | Extrafollicular ABCs contributes to autoantibody production | Not tested | (O’Neill et al., 2011;Zhang et al., 2019) |
| Dnase1l3-/- | Glomerulonephritis, splenomegaly, rapid anti-dsDNA antibody responses. | Normal | IFN-I producing pDCs facilitate extrafollicular autoantibody responses. | TLR7 and TLR9 | (Soni et al., 2020) |
| Autoimmunity with spt-GCs | |||||
| TLR7Y264H, | Thrombocytopenia, proliferative glomerulonephritis, lymphoid infiltrates, splenomegaly, decreased survival and ANAs with nuclear. | Increased GCs | GC B cells are dispensable for the autoimmune phenotype and instead protect against it, suggesting that the pathogenic autoantibody response originates from the EF pathway. | Yes | (Brown et al., 2022) |
| TLR7Y264H Bcl6flox and TLR7Y264H Bcl6flox Cd23cre | |||||
| 564Igi and | Mesangioproliferative glomerulonephropathies, IgG autoantibodies | Increased GCs | GC block fail to mitigate autoimmunity, suggesting an extrafollicular origin of autoantibody responses. | Yes | (Berland et al., 2006;Voss et al., 2022) |
| 564Igi AicdaCre Bcl6flox | |||||
| R848 induced lupus | Splenomegaly, dsDNA IgG2c, immune complex deposition in glomeruli. | Increased GCs | EF response is sufficient to drive autoimmunity. | Yes | (Voss et al., 2022) |
| AicdaCre and AicdaCre Bclflox | |||||
| MRL.Fas lpr (MRL/lpr) | Lymphoproliferation, progressive renal failure, Lymphadenopathy and skin lesions, autoantibodies including ANA, anti-dsDNA, anti-Sm, anti-Ro and anti-La. | Increased in young mice but not in old mice | Autoantibodies are generated by somatic hypermutation at the T zone–red pulp border rather than in GCs. | Yes | (Cosgrove et al., 2023;William et al., 2002) |
| NZM2410-derived B6.Sle1.Sle2.Sle3 (TC) strain | Systemic autoimmunity with fatal glomeruloneph ritis, increased anti-chromatin and anti-dsDNA IgG2a. | Increased GCs | The EF foci rather than the GC are positively correlated with the production of autoantibodies | Partially | (Sang et al., 2014) |
| BAFF tg | Systemic autoimmunity with nephritis and salivary gland destruction | Numerous GCs | Shown to be T-independent, and thus likely extrafollicular | Driven by MyD88 and TLR7/9 | (Groom et al., 2007) |
Abbreviations: ANA, antinuclear antibodies; dsDNA, double-stranded DNA; Sm, Smith; ssDNA, single-stranded DNA; GC, germinal center; GVHD, Graft-versus-host disease; ABC, age-associated B cell; pDCs, plasmacytoid dendritic cells; IFN-I, Type I interferon.
3.1. Autoimmunity without spontaneous GC formation
cGVHD
The coexistence of autoantibodies and inadequate GC responses was firstly reported in chronic graft-versus-host disease (cGVHD) (Deng et al., 2017). After hematopoietic cell transplantation (HCT) using grafts containing a small number of C57BL/6 splenocytes, BALB/c recipients develop cGVHD, with elevated dsDNA-IgG and IgG deposition but less or no GC formation (Deng et al., 2017).
Ikaros deficiency
Ikaros, a transcription factor encoded by Ikaros family zinc finger 1 (IKZF1), functions as a regulator of differentiation, proliferation and self-tolerance in lymphocytes. IKZF1 is one of SLE risk genes identified by genome-wide association studies (GWAS) (Van Nieuwenhove et al., 2018), and conditional deficiency of IKZF1 in B cells leads to systemic autoimmunity in mice (Schwickert et al., 2019). Deletion of Ikaros stringently blocks GC formation, and it is proposed that a Myeloid differentiation primary-response protein-88 (MyD88)-dependent extrafollicular response is responsible for the autoimmune phenotype (Schwickert et al., 2019).
Bach2 deficiency
BACH2 is a transcription repressor required for class switch recombination (CSR) and SHM (Muto et al., 2004). Mice lacking Bach2 develop lupus nephritis but fail to form GCs. Bach2-deficient B cells tend to localize outside the follicle and induce lupus via a T cell-dependent extrafollicular pathway (Jang et al., 2019).
Ship deficiency
SHIP is an inositol 5’ -phosphatase that acts as a negative regulator of immune responses. B cell-intrinsic Ship-deficiency (ShipΔB) causes a lupus-like disease in mice, which exhibit a compromised GC response, with self-reactivity thought to originate primarily from extrafollicular CD11c+Tbet+ ABCs (O’Neill et al., 2011;Zhang et al., 2019).
Dnase1l3 deficiency
Deoxyribonuclease 1 like 3 (DNASE1L3) is a secreted DNA endonuclease responsible for degrading antigenic DNA. Loss of function (Lof) mutations in DNASE1L3 have been identified as causes of monogenic lupus (Al-Mayouf et al., 2011). Dnase1l3-/- mice do not show excessive expansion of GCs; instead, type I interferons secreted by plasmacytoid dendritic cells (pDCs) are thought to promote extrafollicular B cell differentiation and are required for the autoimmune phenotype (Soni et al., 2020). Combined deficiency of TLR7 and TLR9 has been shown to remove self-reactivity in this model (Soni et al., 2020).
3.2. Most lupus models require TLR7 signaling, but TLR7-dependent lupus does not require GC
Enhanced TLR7 signaling is a key mechanism in lupus pathogenesis and is becoming a hotspot for research and therapy. Increased TLR7 activation was reported in B cells from the majority of SLE patients (Jenks et al., 2018) and TLR7 gain-of-function was subsequently identified as a cause of human monogenic form of lupus (Brown et al., 2022). More recently, variants in UNC93B1 that disrupt degradative sorting of TLR7 (Mishra et al., 2024;Wolf et al., 2024) have also been shown to cause human lupus, underscoring the importance of this pathway in human SLE.
In mice, TLR7 overactivity appears to explain lupus disease in a large number of models. In some of these, lupus results from direct variation of TLR7 gene dose, or enhanced nucleic acid sensing function. These models include BXSB Yaa mice that harbour a chromosomal segment duplication from X onto Y that contains TLR7 (Pisitkun et al., 2006;Soni et al., 2014), Tlr7 transgenic (tg) mice (Walsh et al., 2012), TLR7-agonist (imiquimod) induced lupus (Hirobe et al., 2022;Voss et al., 2022), and kika mice that carry a TLR7 gain-of-function variant found in a girl with SLE (Brown et al., 2022). In another group of lupus models in which Tlr7 gene expression is intact, disease pathogenesis is also dependent on TLR7 signaling. Models in this group include 564 Igi tg mice (Berland et al., 2006), MRL.Faslpr (MRL/lpr) (Cosgrove et al., 2023;Shlomchik et al., 1987;William et al., 2002), Tnip1-deficient mice (Nanda et al., 2019), Unc93b1-deficient mice (Fukui et al., 2011), NZM2410-derived B6.Sle1.Sle2.Sle3 (TC) mice (Sang et al., 2014), and BAFF tg mice (Groom et al., 2007). Virtually all the mouse models in both groups show robust spontaneous GC formation, and deletion of TLR7 abrogates GC formation and autoimmune phenotypes (Christensen et al., 2006; Soni et al., 2014).
Toll-like receptor 7 (TLR7) signaling in B cells themselves has been shown to be required for spontaneous GC formation (Brown et al., 2022; Peterson et al., 2022; Soni et al., 2014) and fate-mapping of GC-derived B cells has revealed that TLR7-induced self-reactive B cells can be enriched in GCs (Castrillon et al., 2023;Degn et al., 2017). Besides inducing GCs, TLR7 overactivation is also associated with expansion of extrafollicular CXCR5-CD11c+Tbet+ B cells (known as IgD-CD27- DN2 B cells or age-associated B cells (ABCs)) as well as expansion of follicular and extrafollicular helper T cells (Brown et al., 2022; Jenks et al., 2018).
Whilst the evidence in mice described above has long suggested a pathogenic role of GCs in Tlr7-dependent lupus, definitive evidence on the role of such spontaneous GCs has only been obtained recently. By genetic manipulation of mice to prevent B cells from forming GCs (CD23Cre Bcl6flox mice), it has been found across three Tlr7-dependent lupus mouse models – kika, 564 Igi and imiquimod-induced lupus - that the absence of GC does not alleviate the autoimmune phenotype (Brown et al., 2022;Voss et al., 2022). Instead, defective GC formation leads instead to a more robust expansion of pathogenic ABCs and plasma cells and comparable or greater antinuclear antibody titres. These studies provide definitive evidence that TLR7-mediated autoimmunity can occur in a GC-independent manner and the extrafollicular response can be an important source of pathogenic B cells and autoantibodies.
4. GCs as a gatekeeper of B cell tolerance
The stochastic nature of SHM makes it possible to generate self-reactive B-cells, yet in healthy individuals, those cells can normally be effectively removed in GCs thanks to the existence of several tolerance checkpoints (Table 2).
Table 2. B cell self-tolerance checkpoints in GC.
| Factors | Mechanisms | Reference |
|---|---|---|
| B cell negative selection in germinal centers | BCR-mediated clonal deletion. | (Pulendran et al., 1995;Smith et al., 2000) |
| FAS-mediated cell apoptosis. | (Butt et al., 2015;Smith, Nossal and Tarlinton, 1995) | |
| TFR-mediated inhibition of self-reactive B cell differentiation into plasma cells. | (Chung et al., 2011;Jacobsen et al., 2021;Linterman et al., 2011;Wollenberg et al., 2011) | |
| Macrophage clearance of apoptotic cells. | (Grootveld et al., 2023;Hanayama et al., 2004). | |
| Control of positive GC B cell selection | Limiting TFH cell help for B cells presenting self-peptide. | (Merkenschlager et al., 2021;Vinuesa et al., 2005) |
| Self-reactive GC B cells are not selected into the memory pool. | (Cappione et al., 2005;Richardson et al., 2013) | |
| B cell confinement in germinal centers | GPCRs (e.g., S1PR2 and P2RY8) promote B cells to be confined within GC. | (He et al., 2022;Lu et al., 2019;Muppidi, Lu and Cyster, 2015) |
| Autoreactivity redemption by SHM | Self-reactive B cells mutate away from self-reactivity in GCs. | (Burnett et al., 2018;Sabouri et al., 2014) |
Abbreviations: BCR, B cell receptor; GC, germinal center; GPCR, G protein-coupled receptors; SHM, somatic hypermutation.
4.1. B cell negative selection in germinal centers
BCR-mediated clonal deletion
It was discovered more than 20 years ago that GC B cells undergo waves of apoptosis after encountering soluble self-antigens (Pulendran et al., 1995;Shokat and Goodnow, 1995). The pro-survival protein Bcl-2 is involved in regulating the negative selection of GC B cells: overexpression of Bcl-2 significantly reduces apoptosis of GC B cells (Pulendran et al., 1995;Smith et al., 2000).
FAS-mediated cell apoptosis
GC B cells exhibit high levels of death receptor FAS, and deletion of FAS in B cells results in fatal lymphoproliferation (Hao et al., 2008;Smith, Nossal and Tarlinton, 1995). FAS is dispensable for soluble antigen-induced clonal deletion of GC B cells (Smith, Nossal and Tarlinton, 1995), and instead is pivotal for the control of “rogue” GC B cells that escape affinity selection and readily differentiate into PCs (Butt et al., 2015).
TFR-mediated inhibition of self-reactive B cell differentiation into plasma cells
TFR cells are a population of Bcl6+Foxp3+ double-positive T cells that localize to the GC. They share some features with conventional TFH and TREG cells, but are distinct from both. TFR cells may counteract the selection signal provided by TFH cells, terminate the GC B response, and inhibit plasma cell differentiation (Chung et al., 2011;Jacobsen et al., 2021;Linterman et al., 2011;Merkenschlager et al., 2023;Wollenberg et al., 2011). Loss of TFR cell control results in expansion of non-antigen (Ag)-specific B cells in GC (Linterman et al., 2011), as well as autoimmunity and allergy characterized by self-reactive IgE and IgG1 (Clement et al., 2019;Fu et al., 2018;Gonzalez-Figueroa et al., 2021;Xie et al., 2019). TFR cells exert their tolerizing effects through their expression of cytotoxic T-lymphocyte-associated protein 4 (CTLA4) (Wing et al., 2014), PD-1 (Sage et al., 2013) and neuritin (Gonzalez-Figueroa et al., 2021). Neuritin can be taken up by B cells and inhibit their differentiation into PCs (Gonzalez-Figueroa et al., 2021), maintaining high BCL6 expression. Thus, TFR cells may prevent selection of self-reactive GC B cells into the periphery, maintain them in GC to undergo further rounds of mutation and TFH-mediated selection.
Macrophage clearance of apoptotic cells
GCs are sites of massive apoptosis. Tingible body macrophages (TBMs) are professional scavengers of apoptotic debris in GCs, preventing the activation of autoimmunity by self-nucleic acids decorating the apoptotic bodies. TBMs are derived from follicle-resident macrophage precursors and are triggered by apoptotic debris in situ (Grootveld et al., 2023). Milk fat globule epidermal growth factor (EGF) factor 8 (MFG-E8) is a soluble bridging protein that binds an apoptotic “eat me” signal phosphatidylserine, and promotes the phagocytosis of apoptotic debris by TBMs in GCs (Hanayama et al., 2004). Mice lacking the tyrosine kinases Tyro, Axel and Mer in macrophages cannot take up apoptotic GC B cells and develop lupus-like disease (Lu and Lemke, 2001).
4.2. Control of positive selection
Strict control of TFH mediated B cell positive selection is important to prevent excessive autoimmunity (Pratama and Vinuesa, 2014). Quality control of TFH cells starts in the thymus, where their precursors undergo negative selection resulting in elimination of T cells carrying self-reactive TCRs, preventing provision of help to B cells presenting self-peptides. Studies in human tonsils tracking the fate of autoreactive 9G4 B cells showed that in healthy individuals, despite some entering GCs, few matured into centroblasts, and they were not selected into the memory B cell pool (Cappione et al., 2005;Richardson et al., 2013). By contrast, autoreactive 9G4 B cells were found in the memory compartment of SLE patients, suggesting a breach in the control of TFH-mediated positive selection.
An example of a GC tolerance checkpoint that limits TFH cell expansion is posttranscriptional regulation of T cell mRNAs by Roquin. Roquin is an RNA-binding protein belonging to the family of E3 ubiquitin ligases. Mice homozygous for a loss-of-function mutation of Roquin (Roquinsan/san (sanroque)) exhibit substantial expansion of TFH cells and develop systemic autoimmunity. Roquin is known to bind to and degrade target mRNAs including Icos, inhibiting the number and function of TFH cells (Vinuesa et al., 2005). This regulatory mechanism adds to the finely tuned dynamic regulation of TFH cells within the GC, which is essential to maintain optimal high-affinity and avoid self-reactivity of GC responses (Merkenschlager et al., 2021;Shulman et al., 2013).
4.3. B cell confinement in germinal centers
Although there is no physical barrier surrounding GC, B cells are equipped with G protein-coupled receptors (GPCRs), such as P2RY8 (Gallman et al., 2021;Lu et al., 2019;Muppidi, Lu and Cyster, 2015) and S1PR2 (Green and Cyster, 2012), which confine the B cells to the GC region, thus allowing them to undergo cyclic rounds of SHM and selection. P2RY8 has been shown to be important to confine B cells to GCs (Muppidi, Lu and Cyster, 2015;Muppidi et al., 2014). Its ligand GGG is expressed in the periphery of the follicle (Lu et al., 2019). Recently, a P2RY8 L275F de novo variant was identified in a child with lupus nephritis (He et al., 2022). The L275F variant increases B cell migration and egress of GC B cells. P2RY8 was downregulated in some B cell subsets of lupus patients, and low expression of P2RY8 correlated with nephritis and extrafollicular ABCs (He et al., 2022). Notably, TLR7 ligands but not TLR9 ligand nor a large suite of cytokines were able to downregulate P2RY8. Therefore, P2RY8 is a gatekeeper that confines B cells in GC, and in doing this, may also limit pathogenic extrafollicular self-reactivity.
4.4. Autoreactivity redemption by SHM
Self-reactive B cells that are not immediately deleted by apoptosis and can present foreign antigen to T cells with cognate reactivity to such foreign antigen (e.g. in the case of immunization with self-foreign antigen conjugates), can undergo clonal redemption via rounds of SHM that eliminate autoreactivity whilst preserving reactivity to the foreign antigen. Single cell SHM analysis of SWHEL GC B cells (whose BCR binds hen egg lysozyme with high affinity) in mice expressing HEL as a neo-self antigen, revealed a rapid selection of BCR mutations that lead to a loss of autoreactivity to self (HEL) while presumably increasing affinity to a related or conjugated foreign antigen (Reed et al., 2016;Sabouri et al., 2014;Young, Lau and Burnett, 2022) . Studies of human antibody mutations by deep sequencing have also provided evidence that the antibody response to vaccinia virus can evolve from rhesus D alloantigen (RhD) responses, and IGHV4-34+ B cells can also mutate away from autoreactivity in GCs although they often also lose foreign reactivity in the process and are thus “unredeemable” (Reed et al., 2016) . Thus, the self-reactive cells accidentally produced in GCs, instead of being an obstacle to immunity, can serve as a pre-existing army against foreign antigens.
It should also be noted that SHM-mediated autoreactivity redemption relies on the in situ or proximal expression of high levels of self-antigens in GC. GC-derived B cells retain autoreactive potential to those antigens that are rare in GC, or tissue-specific antigens in distal organs (Chan et al., 2012). That is, although GCs have a series of measures to eliminate B-cell self-tolerance and greatly prevent systemic autoimmunity, GC-derived B cells still harbor pathogenic potential for organ-specific autoantigens and may thus still cause organ-specific autoimmunity.
5. Extrafollicular B cell responses in autoimmunity
In recent years, it has been increasingly recognized that the extrafollicular B cell response is an important source of pathogenic autoantibodies, and that, both in autoimmune-prone mouse models (see Table 1) and in patients with autoimmune diseases (Jenks et al., 2018;Ota et al., 2023), extrafollicular autoantibodies are a key causative factor in the pathogenesis of autoimmune diseases.
5.1. ABCs in autoimmunity
ABCs constitute an increasingly recognized extrafollicular subpopulation (Jenks et al., 2018;Song et al., 2022), initially named after observing their accumulation with age in humans and mice (Hao et al., 2011). Their accumulation is also evident in autoimmune diseases. ABCs are also referred to as atypical memory B cells and double negative 2 (DN2) in humans, due to lack of IgD and CD27 expression. ABCs are characterized by high expression of CD11c and/or CD11b, FcRL5, and CD19 and low expression of IgD, CD27, CD21, and CXCR5. Their differentiation is driven by the transcription factors T-bet and ZEB2 (Dai et al., 2024;Gao et al., 2022;Jenks et al., 2018;Nickerson et al., 2023;Song et al., 2022). ABC generation requires T cell help (Odegard et al., 2008;Song et al., 2022) and is mediated by signals through the B cell receptor (BCR), TLR7 expressed in B cells, and cytokines such as IFN-γ and IL-21 (Brown et al., 2022;Jenks et al., 2018;Nickerson et al., 2023;Rubtsov et al., 2011;Wang et al., 2018) (Figure 1).
ABCs have been implicated in autoimmune diseases including systemic lupus erythematosus (SLE) (Jenks et al., 2018;Nickerson et al., 2023;Rubtsov et al., 2011), rheumatoid arthritis (RA) (Qin et al., 2022) and Sjögren’s Syndrome (Saadoun et al., 2013). The frequency of circulating ABCs in human SLE correlates with autoantibody titres (e.g., anti-nuclear, anti-dsDNA, anti-RNA, and anti-chromatin), and disease severity measured by the SLE Disease Activity Index (SLEDAI) (Jenks et al., 2018;Wang et al., 2018;Wu et al., 2019). ABCs are particularly expanded in patients with lupus nephritis (LN), and negatively correlate with renal impairment (He et al., 2022;Sosa-Hernandez et al., 2022). It has been established that ABCs readily differentiate into PCs, and their absence results in reduced autoantibodies and histologic manifestation (Dai et al., 2024;Nickerson et al., 2023;Rubtsov et al., 2011). ABCs appear to contribute to both systemic inflammation and organ damage in autoimmunity. The precise mechanisms underlying ABCs’ function and their intricate interactions with other immune cells including conventional extrafollicular plasmablasts are still being elucidated, holding much promise for the development of targeted therapies for autoimmune diseases (Levack et al., 2022).
5.2. Extrafollicular T cell help
Depending on the absence or presence of T-cell help, extrafollicular B cell responses can be categorized into either the thymus-independent (TI) (Garcia de Vinuesa et al., 1999;Hsu et al., 2006;Martin, Oliver and Kearney, 2001) or thymus dependent (TD) pathways (Jang et al., 2019;Keller et al., 2021;Odegard et al., 2008;Rao et al., 2017), with the latter being prominent in autoimmune conditions.
Extrafollicular T helper (EF TH) cells
It is now recognized that Bcl6+ TFH cells first localize at the T-B border, where they prime B cells prior to their commitment to the GC or extrafollicular route of differentiation (Lee et al., 2011). Such extrafollicular or pre-GC TFH cells not only provide help for B cell to differentiate into to extrafollicular PCs (Kim et al., 2018;Lee et al., 2011), but are also necessary for the generation of pathogenic ABCs (Song et al., 2022). In addition, there is a group of ICOS+ TFH-like cells detected at extrafollicular sites that promote antibody responses via IL-21 and CD40L (Odegard et al., 2008;Poholek et al., 2010). Therefore, overactive TFH cells or TFH-like cells can also cause autoimmunity through an extrafollicular pathway.
Peripheral T helper (TPH) cells
In non-lymphoid tissues (e.g., blood and synovium), a subset of PD1hi CXCR5- CD4 T cells, known as TPH cells, are largely expanded in patients with autoimmune diseases such as RA (Rao et al., 2017) and SLE (Caielli et al., 2019;Ota et al., 2023). These cells guide the differentiation of extrafollicular B cells through IL-21 (Rao et al., 2017) or IL-10 (Caielli et al., 2019). To date, mechanisms underlying TPH development and pathogenesis have not been fully elucidated, and the relationship between TPH and conventional TFH cells remains largely unknown. Nevertheless, targeting extrafollicular TPH responses is being considered for treatment of autoimmune diseases.
6. Concluding remarks
It has been controversial for many years whether GCs in autoimmunity are pathogenic or protective. Evidence has continued to emerge in recent years suggesting that GCs are likely to be dispensable for the initiation of systemic autoimmune diseases, and in contrast, the extrafollicular pathway is sufficient to drive autoimmunity. Despite the risk of SHM in GCs generating self-reactive B cells, in healthy individuals these cells can remain confined to GCs and are either cleared by apoptosis, mutate away from autoreactivity, or are prevented from differentiation by the absence of self-reactive TFH cells and presence of TFR cells. Thus, at least for systemic autoimmunity, GC seems to be the gatekeeper of self-tolerance, rather than the kicker. An in-depth understanding of how to attain removal of self-reactivity in GCs while preventing Tlr7-driven self-reactive extrafollicular responses will be valuable in the fight against autoimmune diseases.
Acknowledgements
CV is funded by Shanghai Renji Hospital (grant 808.001.03), an LRA Global team award grant and Lupus Insight Prize, a Royal Society Wolfson Fellowship and the Francis Crick Institute (C2228), which receives its core funding from Cancer Research UK, the UK Medical Research Council, and the Wellcome Trust. YH is funded by the National Natural Science Foundation of China (32270946 & 32170903).
Footnotes
Competing interests
The authors declare no competing interests.
References
- Al-Mayouf SM, Sunker A, Abdwani R, Abrawi SA, Almurshedi F, Alhashmi N, Al Sonbul A, Sewairi W, Qari A, Abdallah E, Al-Owain M, et al. Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat Genet. 2011;43:1186. doi: 10.1038/ng.975. [DOI] [PubMed] [Google Scholar]
- Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- Berland R, Fernandez L, Kari E, Han JH, Lomakin I, Akira S, Wortis HH, Kearney JF, Ucci AA, Imanishi-Kari T. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 2006;25:429. doi: 10.1016/j.immuni.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Brown GJ, Canete PF, Wang H, Medhavy A, Bones J, Roco JA, He Y, Qin Y, Cappello J, Ellyard JI, Bassett K, et al. TLR7 gain-of-function genetic variation causes human lupus. Nature. 2022;605:349. doi: 10.1038/s41586-022-04642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett DL, Langley DB, Schofield P, Hermes JR, Chan TD, Jackson J, Bourne K, Reed JH, Patterson K, Porebski BT, Brink R, et al. Germinal center antibody mutation trajectories are determined by rapid self/foreign discrimination. Science. 2018;360:223. doi: 10.1126/science.aao3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt D, Chan TD, Bourne K, Hermes JR, Nguyen A, Statham A, O’Reilly LA, Strasser A, Price S, Schofield P, Christ D, et al. FAS Inactivation Releases Unconventional Germinal Center B Cells that Escape Antigen Control and Drive IgE and Autoantibody Production. Immunity. 2015;42:890. doi: 10.1016/j.immuni.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Caielli S, Veiga DT, Balasubramanian P, Athale S, Domic B, Murat E, Banchereau R, Xu Z, Chandra M, Chung CH, Walters L, et al. A CD4(+) T cell population expanded in lupus blood provides B cell help through interleukin-10 and succinate. Nat Med. 2019;25:75. doi: 10.1038/s41591-018-0254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappione A, 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115:3205. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon C, Simoni L, van den Broek T, van der Poel C, Akama-Garren EH, Ma M, Carroll MC. Complex subsets but redundant clonality after B cells egress from spontaneous germinal centers. Elife. 2023;12 doi: 10.7554/eLife.81012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TD, Wood K, Hermes JR, Butt D, Jolly CJ, Basten A, Brink R. Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity. 2012;37:893. doi: 10.1016/j.immuni.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, Fan HM, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement RL, Daccache J, Mohammed MT, Diallo A, Blazar BR, Kuchroo VK, Lovitch SB, Sharpe AH, Sage PT. Follicular regulatory T cells control humoral and allergic immunity by restraining early B cell responses. Nat Immunol. 2019;20:1360. doi: 10.1038/s41590-019-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove HA, Gingras S, Kim M, Bastacky S, Tilstra JS, Shlomchik MJ. B cell-intrinsic TLR7 expression drives severe lupus in TLR9-deficient mice. JCI Insight. 2023;8 doi: 10.1172/jci.insight.172219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D, Gu S, Han X, Ding H, Jiang Y, Zhang X, Yao C, Hong S, Zhang J, Shen Y, Hou G, et al. The transcription factor ZEB2 drives the formation of age-associated B cells. Science. 2024;383:413. doi: 10.1126/science.adf8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degn SE, van der Poel CE, Firl DJ, Ayoglu B, Al Qureshah FA, Bajic G, Mesin L, Reynaud CA, Weill JC, Utz PJ, Victora GD, et al. Clonal Evolution of Autoreactive Germinal Centers. Cell. 2017;170:913. doi: 10.1016/j.cell.2017.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng R, Hurtz C, Song Q, Yue C, Xiao G, Yu H, Wu X, Muschen M, Forman S, Martin PJ, Zeng D. Extrafollicular CD4(+) T-B interactions are sufficient for inducing autoimmune-like chronic graft-versus-host disease. Nat Commun. 2017;8:978. doi: 10.1038/s41467-017-00880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Niro R, Lee SJ, Vander Heiden JA, Elsner RA, Trivedi N, Bannock JM, Gupta NT, Kleinstein SH, Vigneault F, Gilbert TJ, Meffre E, et al. Salmonella Infection Drives Promiscuous B Cell Activation Followed by Extrafollicular Affinity Maturation. Immunity. 2015;43:120. doi: 10.1016/j.immuni.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Zenzo G, Di Lullo G, Corti D, Calabresi V, Sinistro A, Vanzetta F, Didona B, Cianchini G, Hertl M, Eming R, Amagai M, et al. Pemphigus autoantibodies generated through somatic mutations target the desmoglein-3 cis-interface. J Clin Invest. 2012;122:3781. doi: 10.1172/JCI64413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeier PP, Schell SL, Rahman ZS. Spontaneous germinal centers and autoimmunity. Autoimmunity. 2017;50:4. doi: 10.1080/08916934.2017.1280671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckl-Dorna J, Batista FD. BCR-mediated uptake of antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood. 2009;113:3969. doi: 10.1182/blood-2008-10-185421. [DOI] [PubMed] [Google Scholar]
- Fu W, Liu X, Lin X, Feng H, Sun L, Li S, Chen H, Tang H, Lu L, Jin W, Dong C. Deficiency in T follicular regulatory cells promotes autoimmunity. J Exp Med. 2018;215:815. doi: 10.1084/jem.20170901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui R, Saitoh S, Kanno A, Onji M, Shibata T, Ito A, Onji M, Matsumoto M, Akira S, Yoshida N, Miyake K. Unc93B1 restricts systemic lethal inflammation by orchestrating Toll-like receptor 7 and 9 trafficking. Immunity. 2011;35:69. doi: 10.1016/j.immuni.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Gallman AE, Wolfreys FD, Nguyen DN, Sandy M, Xu Y, An J, Li Z, Marson A, Lu E, Cyster JG. Abcc1 and Ggt5 support lymphocyte guidance through export and catabolism of S-geranylgeranyl-l-glutathione. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abg1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Shen Q, Roco JA, Frith K, Munier CML, Nekrasov M, Dalton B, He J-S, Jaeger R, Cook MC, Zaunders JJ, et al. ZEB2 regulates the development of CD11c+ atypical B cells. bioRxiv. 2022:2022.09.01.506173 [Google Scholar]
- Garcia de Vinuesa C, O’Leary P, Sze DM, Toellner KM, MacLennan IC. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur J Immunol. 1999;29:1314. doi: 10.1002/(SICI)1521-4141(199904)29:04<1314::AID-IMMU1314>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Gomez-Banuelos E, Yu Y, Li J, Cashman KS, Paz M, Trejo-Zambrano MI, Bugrovsky R, Wang Y, Chida AS, Sherman-Baust CA, Ferris DP, et al. Affinity maturation generates pathogenic antibodies with dual reactivity to DNase1L3 and dsDNA in systemic lupus erythematosus. Nat Commun. 2023;14:1388. doi: 10.1038/s41467-023-37083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Figueroa P, Roco JA, Papa I, Nunez Villacis L, Stanley M, Linterman MA, Dent A, Canete PF, Vinuesa CG. Follicular regulatory T cells produce neuritin to regulate B cells. Cell. 2021;184:1775. doi: 10.1016/j.cell.2021.02.027. [DOI] [PubMed] [Google Scholar]
- Green JA, Cyster JG. S1PR2 links germinal center confinement and growth regulation. Immunol Rev. 2012;247:36. doi: 10.1111/j.1600-065X.2012.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths GM, Berek C, Kaartinen M, Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984;312:271. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR, Mackay F. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootveld AK, Kyaw W, Panova V, Lau AWY, Ashwin E, Seuzaret G, Dhenni R, Bhattacharyya ND, Khoo WH, Biro M, Mitra T, et al. Apoptotic cell fragments locally activate tingible body macrophages in the germinal center. Cell. 2023;186:1144. doi: 10.1016/j.cell.2023.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, Pereira JP, Guerini D, Baumgarten BU, Roggo S, Wen B, Knochenmuss R, et al. Oxysterols direct immune cell migration via EBI2. Nature. 2011;475:524. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Duncan GS, Seagal J, Su YW, Hong C, Haight J, Chen NJ, Elia A, Wakeham A, Li WY, Liepa J, et al. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29:615. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Gallman AE, Xie C, Shen Q, Ma J, Wolfreys FD, Sandy M, Arsov T, Wu X, Qin Y, Zhang P, et al. P2RY8 variants in lupus patients uncover a role for the receptor in immunological tolerance. J Exp Med. 2022;219 doi: 10.1084/jem.20211004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirobe S, Yamasaki T, Ito S, Quan YS, Kamiyama F, Tachibana M, Okada N. Transcutaneous Administration of Imiquimod Promotes T and B Cell Differentiation into Effector Cells or Plasma Cells. Pharmaceutics. 2022;14 doi: 10.3390/pharmaceutics14020385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu MC, Toellner KM, Vinuesa CG, Maclennan IC. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc Natl Acad Sci U S A. 2006;103:5905. doi: 10.1073/pnas.0601502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Jacobsen JT, Hu W, TB RC, Solem S, Galante A, Lin Z, Allon SJ, Mesin L, Bilate AM, Schiepers A, Shalek AK, et al. Expression of Foxp3 by T follicular helper cells in end-stage germinal centers. Science. 2021;373 doi: 10.1126/science.abe5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang E, Kim UK, Jang K, Song YS, Cha JY, Yi H, Youn J. Bach2 deficiency leads autoreactive B cells to produce IgG autoantibodies and induce lupus through a T cell-dependent extrafollicular pathway. Exp Mol Med. 2019;51:1. doi: 10.1038/s12276-019-0352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, Tomar D, Woodruff MC, Simon Z, Bugrovsky R, Blalock EL, et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity. 2018;49:725. doi: 10.1016/j.immuni.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida K, Kusunoki S, Kamakura K, Motoyoshi K, Kanazawa I. Guillain-Barre syndrome with IgM antibody to the ganglioside GalNAc-GD1a. J Neuroimmunol. 2001;113:260. doi: 10.1016/s0165-5728(00)00451-3. [DOI] [PubMed] [Google Scholar]
- Keller B, Strohmeier V, Harder I, Unger S, Payne KJ, Andrieux G, Boerries M, Felixberger PT, Landry JJM, Nieters A, Rensing-Ehl A, et al. The expansion of human T-bet(high)CD21(low) B cells is T cell dependent. Sci Immunol. 2021;6:eabh0891. doi: 10.1126/sciimmunol.abh0891. [DOI] [PubMed] [Google Scholar]
- Kim ST, Choi JY, Lainez B, Schulz VP, Karas DE, Baum ED, Setlur J, Gallagher PG, Craft J. Human Extrafollicular CD4(+) Th Cells Help Memory B Cells Produce Igs. J Immunol. 2018;201:1359. doi: 10.4049/jimmunol.1701217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosco-Vilbois MH, Scheidegger D. Follicular dendritic cells: antigen retention, B cell activation, and cytokine production. Curr Top Microbiol Immunol. 1995;201:69. doi: 10.1007/978-3-642-79603-6_4. [DOI] [PubMed] [Google Scholar]
- Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, Ramiscal RR, Chan TD, Gatto D, Brink R, Yu D, et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med. 2011;208:1377. doi: 10.1084/jem.20102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levack RC, Newell KL, Cabrera-Martinez B, Cox J, Perl A, Bastacky SI, Winslow GM. Adenosine receptor 2a agonists target mouse CD11c(+)T-bet(+) B cells in infection and autoimmunity. Nat Commun. 2022;13:452. doi: 10.1038/s41467-022-28086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yang XV, Wu J, Kuei C, Mani NS, Zhang L, Yu J, Sutton SW, Qin N, Banie H, Karlsson L, et al. Oxysterols direct B-cell migration through EBI2. Nature. 2011;475:519. doi: 10.1038/nature10226. [DOI] [PubMed] [Google Scholar]
- Lu E, Wolfreys FD, Muppidi JR, Xu Y, Cyster JG. S-Geranylgeranyl-L-glutathione is a ligand for human B cell-confinement receptor P2RY8. Nature. 2019;567:244. doi: 10.1038/s41586-019-1003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- Luzina IG, Atamas SP, Storrer CE, daSilva LC, Kelsoe G, Papadimitriou JC, Handwerger BS. Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol. 2001;70:578. [PubMed] [Google Scholar]
- MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Marks KE, Rao DA. T peripheral helper cells in autoimmune diseases. Immunol Rev. 2022;307:191. doi: 10.1111/imr.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- Merkenschlager J, Berz RM, Ramos V, Uhlig M, MacLean AJ, Nowosad CR, Oliveira TY, Nussenzweig MC. Continually recruited naive T cells contribute to the follicular helper and regulatory T cell pools in germinal centers. Nat Commun. 2023;14:6944. doi: 10.1038/s41467-023-41880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager J, Finkin S, Ramos V, Kraft J, Cipolla M, Nowosad CR, Hartweger H, Zhang W, Olinares PDB, Gazumyan A, Oliveira TY, et al. Dynamic regulation of T(FH) selection during the germinal centre reaction. Nature. 2021;591:458. doi: 10.1038/s41586-021-03187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra H, Schlack-Leigers C, Lim EL, Thieck O, Magg T, Raedler J, Wolf C, Klein C, Ewers H, Lee-Kirsch MA, Meierhofer D, et al. Disrupted degradative sorting of TLR7 is associated with human lupus. Sci Immunol. 2024:eadi9575. doi: 10.1126/sciimmunol.adi9575. [DOI] [PubMed] [Google Scholar]
- Muppidi JR, Lu E, Cyster JG. The G protein-coupled receptor P2RY8 and follicular dendritic cells promote germinal center confinement of B cells, whereas S1PR3 can contribute to their dissemination. The Journal of experimental medicine. 2015;212:2213. doi: 10.1084/jem.20151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muppidi JR, Schmitz R, Green JA, Xiao W, Larsen AB, Braun SE, An J, Xu Y, Rosenwald A, Ott G, Gascoyne RD, et al. Loss of signalling via Gα13 in germinal centre B-cell-derived lymphoma. Nature. 2014;516:254. doi: 10.1038/nature13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Tashiro S, Nakajima O, Hoshino H, Takahashi S, Sakoda E, Ikebe D, Yamamoto M, Igarashi K. The transcriptional programme of antibody class switching involves the repressor Bach2. Nature. 2004;429:566. doi: 10.1038/nature02596. [DOI] [PubMed] [Google Scholar]
- Nanda SK, Petrova T, Marchesi F, Gierlinski M, Razsolkov M, Lee KL, Wright SW, Rao VR, Cohen P, Arthur JSC. Distinct signals and immune cells drive liver pathology and glomerulonephritis in ABIN1[D485N] mice. Life Sci Alliance. 2019;2 doi: 10.26508/lsa.201900533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson KM, Smita S, Hoehn KB, Marinov AD, Thomas KB, Kos JT, Yang Y, Bastacky SI, Watson CT, Kleinstein SH, Shlomchik MJ. Age-associated B cells are heterogeneous and dynamic drivers of autoimmunity in mice. J Exp Med. 2023;220 doi: 10.1084/jem.20221346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill SK, Getahun A, Gauld SB, Merrell KT, Tamir I, Smith MJ, Dal Porto JM, Li QZ, Cambier JC. Monophosphorylation of CD79a and CD79b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy. Immunity. 2011;35:746. doi: 10.1016/j.immuni.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M, Nakano M, Nagafuchi Y, Kobayashi S, Hatano H, Yoshida R, Akutsu Y, Itamiya T, Ban N, Tsuchida Y, Shoda H, et al. Multimodal repertoire analysis unveils B cell biology in immune-mediated diseases. Ann Rheum Dis. 2023;82:1455. doi: 10.1136/ard-2023-224421. [DOI] [PubMed] [Google Scholar]
- Pereira JP, Kelly LM, Xu Y, Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009;460:1122. doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JN, Boackle SA, Taitano SH, Sang A, Lang J, Kelly M, Rahkola JT, Miranda AM, Sheridan RM, Thurman JM, Rao VK, et al. Elevated Detection of Dual Antibody B Cells Identifies Lupus Patients With B Cell-Reactive VH4-34 Autoantibodies. Front Immunol. 2022;13:795209. doi: 10.3389/fimmu.2022.795209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, Crotty S, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratama A, Vinuesa CG. Control of TFH cell numbers: why and how? Immunol Cell Biol. 2014;92:40. doi: 10.1038/icb.2013.69. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Kannourakis G, Nouri S, Smith KG, Nossal GJ. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature. 1995;375:331. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- Qin Y, Cai ML, Jin HZ, Huang W, Zhu C, Bozec A, Huang J, Chen Z. Age-associated B cells contribute to the pathogenesis of rheumatoid arthritis by inducing activation of fibroblast-like synoviocytes via TNF-alpha-mediated ERK1/2 and JAK-STAT1 pathways. Ann Rheum Dis. 2022;81:1504. doi: 10.1136/ard-2022-222605. [DOI] [PubMed] [Google Scholar]
- Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, Donlin LT, Henderson LA, Wei K, Mizoguchi F, Teslovich NC, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542:110. doi: 10.1038/nature20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SK, Putterman C, Diamond B. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmune disease. Proc Natl Acad Sci U S A. 1996;93:2019. doi: 10.1073/pnas.93.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JH, Jackson J, Christ D, Goodnow CC. Clonal redemption of autoantibodies by somatic hypermutation away from self-reactivity during human immunization. J Exp Med. 2016;213:1255. doi: 10.1084/jem.20151978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C, Chida AS, Adlowitz D, Silver L, Fox E, Jenks SA, Palmer E, Wang Y, Heimburg-Molinaro J, Li QZ, Mohan C, et al. Molecular basis of 9G4 B cell autoreactivity in human systemic lupus erythematosus. J Immunol. 2013;191:4926. doi: 10.4049/jimmunol.1202263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roco JA, Mesin L, Binder SC, Nefzger C, Gonzalez-Figueroa P, Canete PF, Ellyard J, Shen Q, Robert PA, Cappello J, Vohra H, et al. Class-Switch Recombination Occurs Infrequently in Germinal Centers. Immunity. 2019;51:337. doi: 10.1016/j.immuni.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun D, Terrier B, Bannock J, Vazquez T, Massad C, Kang I, Joly F, Rosenzwajg M, Sene D, Benech P, Musset L, et al. Expansion of autoreactive unresponsive CD21-/low B cells in Sjogren’s syndrome-associated lymphoproliferation. Arthritis Rheum. 2013;65:1085. doi: 10.1002/art.37828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabouri Z, Schofield P, Horikawa K, Spierings E, Kipling D, Randall KL, Langley D, Roome B, Vazquez-Lombardi R, Rouet R, Hermes J, et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc Natl Acad Sci U S A. 2014;111:E2567. doi: 10.1073/pnas.1406974111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14:152. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang A, Niu H, Cullen J, Choi SC, Zheng YY, Wang H, Shlomchik MJ, Morel L. Activation of rheumatoid factor-specific B cells is antigen dependent and occurs preferentially outside of germinal centers in the lupus-prone NZM2410 mouse model. J Immunol. 2014;193:1609. doi: 10.4049/jimmunol.1303000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickert TA, Tagoh H, Schindler K, Fischer M, Jaritz M, Busslinger M. Ikaros prevents autoimmunity by controlling anergy and Toll-like receptor signaling in B cells. Nat Immunol. 2019;20:1517. doi: 10.1038/s41590-019-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, Staudt LM. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Shokat KM, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375:334. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, Victora GD. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KG, Light A, O’Reilly LA, Ang SM, Strasser A, Tarlinton D. bcl-2 transgene expression inhibits apoptosis in the germinal center and reveals differences in the selection of memory B cells and bone marrow antibody-forming cells. J Exp Med. 2000;191:475. doi: 10.1084/jem.191.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KG, Nossal GJ, Tarlinton DM. FAS is highly expressed in the germinal center but is not required for regulation of the B-cell response to antigen. Proc Natl Acad Sci U S A. 1995;92:11628. doi: 10.1073/pnas.92.25.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Antao OQ, Condiff E, Sanchez GM, Chernova I, Zembrzuski K, Steach H, Rubtsova K, Angeletti D, Lemenze A, Laidlaw BJ, et al. Development of Tbet- and CD11c-expressing B cells in a viral infection requires T follicular helper cells outside of germinal centers. Immunity. 2022;55:290. doi: 10.1016/j.immuni.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni C, Perez OA, Voss WN, Pucella JN, Serpas L, Mehl J, Ching KL, Goike J, Georgiou G, Ippolito GC, Sisirak V, et al. Plasmacytoid Dendritic Cells and Type I Interferon Promote Extrafollicular B Cell Responses to Extracellular Self-DNA. Immunity. 2020;52:1022. doi: 10.1016/j.immuni.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni C, Wong EB, Domeier PP, Khan TN, Satoh T, Akira S, Rahman ZS. B cell-intrinsic TLR7 signaling is essential for the development of spontaneous germinal centers. J Immunol. 2014;193:4400. doi: 10.4049/jimmunol.1401720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa-Hernandez VA, Romero-Ramirez S, Cervantes-Diaz R, Carrillo-Vazquez DA, Navarro-Hernandez IC, Whittall-Garcia LP, Absalon-Aguilar A, Vargas-Castro AS, Reyes-Huerta RF, Juarez-Vega G, Meza-Sanchez DE, et al. CD11c(+) T-bet(+) CD21(hi) B Cells Are Negatively Associated With Renal Impairment in Systemic Lupus Erythematosus and Act as a Marker for Nephritis Remission. Front Immunol. 2022;13:892241. doi: 10.3389/fimmu.2022.892241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002;2:580. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- Van Nieuwenhove E, Garcia-Perez JE, Helsen C, Rodriguez PD, van Schouwenburg PA, Dooley J, Schlenner S, van der Burg M, Verhoeyen E, Gijsbers R, Frietze S, et al. A kindred with mutant IKAROS and autoimmunity. J Allergy Clin Immunol. 2018;142:699. doi: 10.1016/j.jaci.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Nussenzweig MC. Germinal Centers. Annu Rev Immunol. 2022;40:413. doi: 10.1146/annurev-immunol-120419-022408. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, Roberts IS, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- Voss LF, Howarth AJ, Wittenborn TR, Hummelgaard S, Juul-Madsen K, Kastberg KS, Pedersen MK, Jensen L, Papanastasiou AD, Vorup-Jensen T, Weyer K, et al. The extrafollicular response is sufficient to drive initiation of autoimmunity and early disease hallmarks of lupus. Front Immunol. 2022;13:1021370. doi: 10.3389/fimmu.2022.1021370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh ER, Pisitkun P, Voynova E, Deane JA, Scott BL, Caspi RR, Bolland S. Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proc Natl Acad Sci U S A. 2012;109:16276. doi: 10.1073/pnas.1209372109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, Rahman S, Zerrouki K, Hanna R, Morehouse C, Holoweckyj N, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun. 2018;9:1758. doi: 10.1038/s41467-018-03750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41:1013. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Wolf C, Lim EL, Mokhtari M, Kind B, Odainic A, Lara-Villacanas E, Koss S, Mages S, Menzel K, Engel K, Duckers G, et al. UNC93B1 variants underlie TLR7-dependent autoimmunity. Sci Immunol. 2024:eadi9769. doi: 10.1126/sciimmunol.adi9769. [DOI] [PubMed] [Google Scholar]
- Wollenberg I, Agua-Doce A, Hernandez A, Almeida C, Oliveira VG, Faro J, Graca L. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187:4553. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- Wu C, Fu Q, Guo Q, Chen S, Goswami S, Sun S, Li T, Cao X, Chu F, Chen Z, Liu M, et al. Lupus-associated atypical memory B cells are mTORC1-hyperactivated and functionally dysregulated. Ann Rheum Dis. 2019;78:1090. doi: 10.1136/annrheumdis-2019-215039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie MM, Liu H, Corn C, Koh BH, Kaplan MH, Turner MJ, Dent AL. Roles of T Follicular Helper Cells and T Follicular Regulatory Cells in Autoantibody Production in IL-2-Deficient Mice. Immunohorizons. 2019;3:306. doi: 10.4049/immunohorizons.1900034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Lau AWY, Burnett DL. B cells in the balance: Offsetting self-reactivity avoidance with protection against foreign. Front Immunol. 2022;13:951385. doi: 10.3389/fimmu.2022.951385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, Ellyard JI, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang H, Liu S, Xia F, Kang Z, Zhang Y, Liu Y, Xiao H, Chen L, Huang C, Shen N, et al. Excessive CD11c(+)Tbet(+) B cells promote aberrant T(FH) differentiation and affinity-based germinal center selection in lupus. Proc Natl Acad Sci U S A. 2019;116:18550. doi: 10.1073/pnas.1901340116. [DOI] [PMC free article] [PubMed] [Google Scholar]