Abstract
With the rapid development of clinical diagnosis and treatment, many traditional and conventional in vitro diagnosis technologies are unable to meet the demands of clinical medicine development. In this situation, nanomaterials are rapidly developing and widely used in the field of in vitro diagnosis. Nanomaterials have distinct size-dependent physical or chemical properties, and their optical, magnetic, electrical, thermal, and biological properties can be modulated at the nanoscale by changing their size, shape, chemical composition, and surface functional groups, particularly because they have a larger specific surface area than macromaterials. They provide an amount of space to modify different molecules on their surface, allowing them to detect small substances, nucleic acids, proteins, and microorganisms. Combining nanomaterials with in vitro diagnosis is expected to result in lower detection limits, higher sensitivity, and stronger selectivity. In this review, we will discuss the classfication and properties of some common nanomaterials, as well as their applications in protein, nucleic acids, and other aspect detection and analysis for in vitro diagnosis, especially on aging-related nanodiagnostics. Finally, it is summarized with guidelines for in vitro diagnosis.
Keywords: Nanomaterials, Nanoparticles, In vitro diagnosis, Geriatric, Protein, Nucleic acid
Graphical abstract
1. Introduction
With the rapid advancement of clinical diagnosis and treatment, many traditional and conventional in vitro diagnosis technologies are unable to meet the demands of clinical medicine development. In this situation, nanotechnology grew drastically as an emergent discipline in the late 1980s and early 1990s. Nanomaterials are materials in which at least one dimension of the three-dimensional spatial scale is in the nanometer scale (1–100 nm), and their surface effect, volume effect, and quantum size influence drastically modify the structure of materials, resulting in nanomaterials with several outstanding properties that differ from traditional materials at the macro level. People began to research nanoparticles in the 1960s, and in 1963, Uyeda created metal nanoparticles using gas evaporation and condensation, then examined them with electron microscopy and electron diffraction [1]. In 1984, Gleiter of Saarland University in Germany and Siegal of Argonne Laboratory in the United States succeeded in producing nano-fine powders of pure substances, and also pressurized iron particles with a diameter of 6 nm into shape in situ in a high-vacuum and sintered nano-microscopic crystals, ushering in a new era of nanomaterial research [2]. The development of nanomaterials can be broadly divided into three stages based on the connotation and characteristics concentrated on the development of nanocrystalline or nanophase materials and the differences between them and ordinary materials. From 1990 to 1994, researchers focused on using nanomaterials’ physical and chemical properties to develop nanocomposites. The synthesis of composites and discovery of physical characteristics became the key goals of nanomaterials research. After 1994, the nanoassembly systems became the dominant trend. Their fundamental idea is to use nanoparticles or nanowires as building blocks, then combine them to form nanostructured systems in one, two, or three dimensions.
Nanomaterials have distinct size-dependent physical or chemical properties, and their optical, magnetic, electrical, thermal, and biological properties can be modulated at the nanoscale by changing their size, shape, chemical composition, and surface functional groups, particularly because they have a larger specific surface area than macromaterials. They have adequate surface area to modify numerous chemicals, allowing them to detect small substances, nucleic acids, proteins, and microorganisms. With the support of numerous specific properties of nanomaterials, scientists have made remarkable advances in various research fields, which also stimulates the applications of nanomaterials more and more widely, such as energy transduction [3,4], electronic fabrication [5], nanomedicine fields [[6], [7], [8], [9]].
In vitro diagnosis technology is a service that obtains meaningful clinical diagnostic information by evaluating body samples from outside the human body, such as blood, body fluids, and tissues, in order to diagnose disease or body function. Combining nanomaterials with in vitro diagnosis is expected to result in lower detection limits, higher sensitivity, and stronger selectivity. Currently, there are just a few reviews on nanomaterials in vitro diagnosis available. From this perspective, we will discuss the classfication and properties of some common nanomaterials, as well as their applications in protein, nucleic acid, and other aspect detection and analysis for in vitro diagnosis, especially on aging-related nanodiagnostics. Finally, it is summarized with guidelines for in vitro diagnosis.
2. Classification and properties of nanomaterials
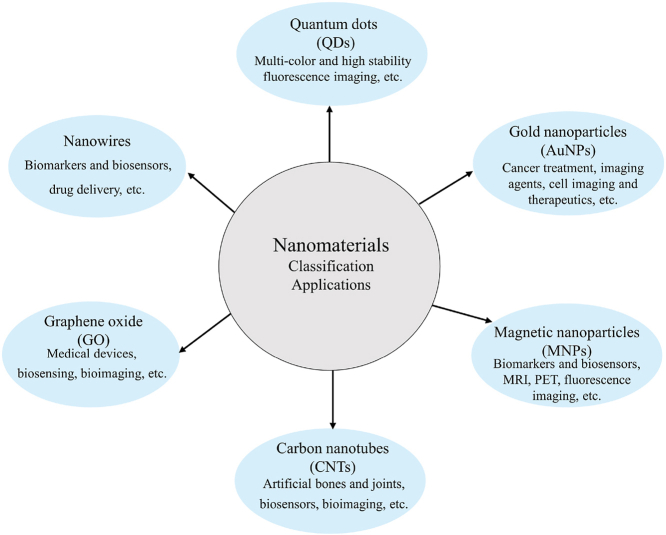
Nanomaterials are nanometer-scale materials (nm, 10−9 m) that can be classed as zero-dimensional (such as nanoparticles (NPs)), one-dimensional (such as nanofibers, nanowires, and nanotubes), two-dimensional (such as nanofilm), or three-dimensional (such as bulk material) [10,11]. In recent years, many nanomaterials have been extensively researched for biomedical applications due to their optical, magnetic, electrical, thermal, and biological properties. Quantum dots, gold nanoparticles, magnetic nanoparticles, graphene oxide, carbon nanotubes, and nanowires are among the most popular, having been widely used in a variety of in vitro diagnostic applications. Table 1 displays their diameters, properties, advantages and applications, limitations and disadvantages.
Table 1.
Comparison between different nanomaterials.
| Classification | Diameters | Properties/advantages | Applications | Limitations/disadvantages |
|---|---|---|---|---|
| Quantum dots (QDs) | 2–10 nm [12] | Broad absorption spectrum, narrow optical band, excellent photovoltaic stability; high penetration capacity in biological media, excellent optical properties. | Multi-color and high stability fluorescence imaging, drug delivery systems. | High light quenching rate, pH sensitivity, broad emission spectrum, difficulties dissolving in solution, toxicity, etc. |
| Gold nanoparticles (AuNPs) | 1–100 nm [20] | Surface plasmon resonance, Raman effect enhancement, near-infrared spectral absorption; suitable for photodynamic therapy, large surface area. | Cancer treatment, imaging agents, drug delivery vehicles and absorptive heating agents, cell imaging and therapeutics. | Low detection sensitivity; poor thermotherapy specificity, expensive for commercial production, non-biodegradability, etc. |
| Magnetic nanoparticles (MNPs) | 2–20 nm [42] | Magnetic responsiveness and superparamagnetism; easy elimination from the body, mechanical and chemical stability, size uniformity. | Biomarkers and biosensors, MRI, PET, fluorescence imaging, tissue repair, drug carriers, disease diagnosis and therapy. | High cost, low magnetic thermal efficiency, toxicity, low biocompatibility and elimination, challenge to direct MNPs to desired tissue in the absence of magnetic field, etc. |
| Carbon nanotubes (CNTs) | 0.2–100 nm [55] | High electrical and thermal conductivity; high strength, stiffness, and elasticity. | Artificial bones and joints, biosensors, bioimaging, and drug delivery systems. | High cost, particle aggregation, unstable under extreme conditions, potential toxicity, poor biocompatibility and elimination, etc. |
| Graphene oxide (GO) | Tens of nanometers to tens of micrometers [60] | High electrical and thermal conductivity; high specific surface area and excellent biocompatibility. | Medical devices, biosensing, bioimaging, and drug delivery systems. | High cost, particle aggregation, potential toxicity, processing difficulty, instability under harsh temperatures, low biocompatibility and elimination, etc. |
| Nanowires (NWs) | 1–100 nm [65] | High specific surface area and excellent electrochemical properties. | Biosensors and biomarkers, drug delivery systems. | High cost, potential toxicity, low stability, processing difficulties, etc. |
2.1. Quantum dots (QDs)
Quantum dots (QDs) are nanomaterials with exceptional optoelectronic characteristics with sizes ranging from 2 to 10 nm [12]. QDs electrons are restricted to three spatial dimensions due to their dimensions being far smaller than the Bragg lattice constants of conventional materials, resulting in the quantum size effect. This activity alters the energy band structure and optoelectronic properties of QDs, resulting in superior spectrum features and photoelectric conversion efficiency. Their size, shape, and composition can be slightly altered by the “quantum confinement effect” [13], which states that when a semiconductor particle's radius is smaller than its exciton Bohr radius, the particle's energy spectrum becomes discrete. This process produces a discrete band gap that varies with particle size. QDs can thus be made to emit a specific fluorescence spectrum spanning ultraviolet (UV) to near-infrared (NIR) wavelengths by selecting the appropriate size and composition. They outperform commonly used fluorochromes, such as organic dyes and fluorescent probes, due to their broad absorption spectrum, narrow optical band, and high photovoltaic stability. QDs have a narrow and symmetrical emission spectrum, making them more suited for detecting numerous fluorescence spectra at the same time, whereas ODs have a broader absorption spectrum, allowing for simultaneous emission of all fluorescent colors of various QDs from a single excitation source. Furthermore, QDs offer various benifits over ordinary organic fluorescent dyes, such as low photobleaching, large molar extinction coefficients, high quantum yields, and long fluorescence lifetimes, making them excellent as optical probes. As a result, QDs are recognized as a feasible substitute for organic fluorescent dyes and can be used in a wide range of bioanalysis, including biosensing, optical imaging, cell separation, and diagnosis [14,15]. However, ODs have some limitations and drawbacks, including a high light quenching rate, pH sensitivity, a broad emission spectrum, difficulties dissolving in solution, and toxicity, etc.
Biosynthetic quantum dots (BQDs), a form of fluorescent nanomaterial, were recently reported to be synthesized using Staphylococcus aureus [16]. Aside from BQDs, protein A binds to the Fc region of monoclonal antibodies, allowing nanomaterials to be selectively attached to them. BQDs have enabled the development of novel electrochemical biosensors. Ultra-low levels of prostate-specific antigen (PSA) can be detected using differential pulse voltammetry (DPV), electrochemical impedance spectroscopy (EIS), and electrochemiluminescence (ECL) as a transduction method. This idea is easily extendable to the ultrasensitive detection of vital illness signs such as proteins, viruses and pathogens [17,18].
2.2. Gold nanoparticles (AuNPs)
Gold nanoparticles (AuNPs) are metal particles with diameters ranging from 1 to 100 nm that have the advantages of low toxicity, easy surface modification, biocompatibility, and distinctive optical features, as well as the ability to fine-tune particle size, shape, and surface properties [19,20]. AuNPs can be used as contrast agents in imaging tests including computed tomography (CT), magnetic resonance imaging (MRI), and optical imaging for their good optical qualities, which allow clinicians to see patients’ lesions more clearly. AuNPs surpass traditional contrast agents in terms of biocompatibility and imaging resolution, potentially increasing clinical diagnosis accuracy and reliability [21,22]. Among of these, the surface plasma resonance (SPR) effect is a distinguishing optical property of AuNPs [23], induced by the resonance phenomenon that occurs when the incident light interacts with the free electrons on the surface layer of AuNPs. SPR occurs when the incident light frequency and the vibration frequency of free electrons on the surface layer of AuNPs coincide, allowing metal nanoparticles to significantly boost their light absorption and scattering capacities. According to Gans theory [24,25], two SPR bands (longitudinal band and the transverse band) will convert AuNPs from spheres to rods. When the aspect ratio of AuNPs increases, the longitudinal band redshifts dramatically, causing a color shift from blue to red [26]. Furthermore, plasmon resonance is controlled by modulating the shape, size, and composition of metal nanoparticles, as well as the wavelength and polarization state of incident light, allowing for precise regulation of the optical properties of metal nanoparticles, opening up new avenues for their applications in sensing, imaging, catalysis, and other fields. However, there are certain limitations and disadvantages of AuNPs, such as low detection sensitivity, poor thermotherapy specificity, expensive for commercial production, and non-biodegradability, etc.
In recent years, AuNPs have been widely used in the diagnosis of cardiac diseases such as myocardial infarction due to their capacity to detect target biomarkers with surface changes directly from serum samples, making them many times more sensitive than conventional biosensors [[27], [28], [29], [30], [31], [32]]. AuNPs have also been used successfully in a range of biological applications, including biomarkers, DNA sensors, molecular recognition systems, drug delivery systems, and optical imaging, because of their unique, advanced, and enhanced physicochemical features [[33], [34], [35], [36], [37], [38]]. For example, during the SARS-CoV-2 global pandemic in the previous two years, when the demand for rapid, simple, and cost-effective point-of-care (POC) diagnostic tests was unprecedentedly high, the combination of AuNPs with specific antibodies for detecting the presence of SARS-CoV-2 antigens by lateral flow immunochromatography assay (LFIA) greatly improved the sensitivity and specificity of traditional immunochromatographic techniques and provided effective support [39]. Prostate cancer, one of the most frequent malignant tumors in men, can be detected early with a biomarker called PSA. Alnaimi and his colleagues’ electrochemical biosensor detects PSA throughout a linear range of 1–100 ng/mL, with a minimum detection limit of 1 pg/mL. The detection binding time is 5 min, and the PSA is high sensitivity, so the sensor has the characteristics of high sensitivity, a wide linear range, a low detection limit, and a low cost, which is extremely important for the early diagnosis of prostate [40].
2.3. Magnetic nanoparticles (MNPs)
Magnetic nanoparticles (MNPs) are a significant form of nanoparticle with diameters ranging from 2 to 20 nm. They are typically made of pure metals (Fe, Co, Ni, and other rare earth metals) or metal-polymer mixtures [41,42]. Iron oxide nanoparticles have become widely used in biomedicine due to their biocompatibility, biodegradability, and superparamagnetism. The capacity of MNPs to magnetically modify an external magnetic field provides a considerable advantage [43]. MNPs not only exhibit unique nanomaterial properties such as small particle size, large specific surface area, and strong connectivity, but also show significant magnetic responsiveness and superparamagnetism [44]. MNPs are classified as paramagnetic, ferromagnetic, or superparamagnetic particles in medical and biological applications. Superpara- and ferro-magnetic particles have a magnetic core and a surface coating, whereas paramagnetic particles are predominantly chelates of paramagnetic ions with no discernible core or surface layer. MNPs' magnetic properties are primarily determined by their core material, as well as the size and form of the ferromagnetic particles. In general, a material's magnetic properties are highly dependent on temperature. When the temperature approaches the material's Curie temperature, its ferromagnetism and superparamagnetism disappear, and it shows paramagnetic activity [45]. MNPs have been used in a range of applications, including biomarkers and biosensors, MRI, positron emission tomography (PET), fluorescence imaging, tissue healing, drug carriers, disease detection and treatment [[46], [47], [48], [49], [50]]. Yu et al. discovered that oligomalic acid-modified MNPs can be used as bioprobes to specifically recognize and capture complementary nucleotide strands, as well as isolate and purify them in the presence of a magnetic field [51]. In cellular phagocytosis assays, it was observed that the aminoglycolic acid-modified particles resisted phagocytosis by phagocytes while being readily phagocytosed by cancer cells. This shows great promise in tumor diagnosis and treatment [44,[52], [53], [54]]. However, there are certain limitations and disadvantages of MNPs, such as their expensive cost, low magnetic thermal efficiency, potential toxicity, low biocompatibility and elimination, difficulties in directing MNPs to desired tissue in the absence of magnetic field, etc.
2.4. Carbon nanotubes (CNTs)
Carbon nanotubes (CNTs) are tubular structures made of a single layer of carbon atoms. They are divided into two types: single-walled CNTs, which have internal diameters between 0.2 and 2 nm and are composed of a single layer of carbon atoms, and multi-walled CNTs, which have internal diameters between 2 and 100 nm and are made of many concentric layers of carbon atoms. This structure confers a number of unique physical and chemical features to CNTs [55]. These distinctive characteristics include: First, because CNTs and graphite have comparable electronic structures, they have strong electrical and thermal conductivity. Second, because of its solid structure, CNTs have extraordinarily high strength and stiffness, with values approaching hundreds of megapascals, or many times the strength of steel. The elasticity is also high, with the capacity to bear severe bending and tensile deformation. Third, CNTs have a highly flat surface that facilitates extensive chemical interactions with other substances, as well as excellent physical and chemical properties. Because of these qualities, CNTs are ideal biological materials for application in prosthetic bones, joints, biosensors, and drug delivery systems. However, CNTs have some limitations and disadvantages, such as high cost, particle aggregation, instability under extreme conditions, potential toxicity, poor biocompatibility and elimination, etc.
CNTs’ unique optical properties can be used for bioimaging, and Huai et al. have shown that this imaging technique can aid in the clinical diagnosis of disease by detecting and characterizing tumor cells, as well as in the early detection of lesions, hence increasing treatment success rates [56]. Because of their strong electrical conductivity and chemical durability, CNTs can be used to create extremely sensitive biosensors. These sensors can detect numerous biomolecules, including lipids, proteins, DNA, making them suitable for disease detection and treatment. For examples, Ashkan et al. demonstrated higher lipid content in tumor tissue secretions than in normal tissues using the results of lipid content detection of more than 100 human breast biopsy tissue secretions by biosensors synthesized from CNTs, which complements clinical biopsy-based diagnostic rapid detection methods [57]. Aseel Alnaimi et al. developed biosensors built of multi-walled CNTs to add clinical value in the early detection of prostate cancer [40]. They achieved the goals of high efficiency, low cost, a low lower limit of detection, a broad linear range, and improved specificity for PSA detection in blood. ECL is a cutting-edge analytical method used in clinical monitoring and disease detection. CNTs can be used to improve ECL signals and gain new insights into the mechanisms underlying ECL onset, as well as for microsphere-based immunoassays and ECL microscopy-based bioimaging [58].
2.5. Graphene oxide (GO)
Graphene, which was first successfully isolated by two British scientists (Geim and Novoselov) in 2004 [59], has revolutionized a variety of fields, including electronics and energy. Scientists have made tremendous efforts over the last decade to explore graphene and learn more about its physical and chemical properties. Graphene and its derivatives have attracted a lot of attention because of its versatility and modifiability. Graphene's large specific surface area, thermal and electrical conductivity, and ease of modification or functionalization make it an attractive candidate for biological applications; however, its poor dispersibility in water and many organic solvents limits its use in biomedical applications.
Graphene oxide (GO) is formed by oxidizing graphene with diameters ranging from tens of nanometers to tens of micrometers [60], keeping the graphene's enormous planar structure and specific surface area while introducing hydrophilic groups such as epoxy, hydroxyl, and carboxylic acid groups. These hydrophilic groups can interact with other substances through hydrogen bonding, electrostatic interactions, and covalent bonding, making them an effective carrier platform for bioactive chemicals. As a result, GO has been proposed as a viable alternative to graphene in a number of biomedical applications, including drug delivery, cancer therapy, and therapeutic diagnostics. GO has high electrical conductivity, a large specific surface area, and excellent biocompatibility. It may interact selectively with biomolecules to detect biomarkers and disease-related chemicals [61]. Combining GO with biomolecular recognition elements allows for the development of highly sensitive and selective biosensors for rapid and accurate disease diagnosis [62]. Furthermore, because of its outstanding optical properties and biocompatibility, GO has been used in bioimaging and drug administration systems, resulting in novel approaches and technologies for clinical diagnosis and treatment [[62], [63], [64]]. Yang et al. showed that gadolinium-functionalized GO nanoparticles can be used as a contrast agent for magnetic resonance imaging to detect the location and extent of drug delivery across the blood-brain barrier to tumor tissues [63]. Han et al. found that titanium dioxide nanoparticles encapsulated in GO nanoparticles can detect the cardiac marker cTnI with a linear dynamic range of 1.0 × 10−13-1.0 × 10−10 mol/L and detection limit of 4.0 × 10−14 mol/L using potentiometric ECL. This approach offers a wide linear range and a low detection limit, making it a precise, sensitive, and fast tool for identifying myocardial injury [62]. However, there are certain limitations and disadvantages of GO, such as high cost, particle aggregation, potential toxicity, processing difficulty, instability under harsh temperatures, low biocompatibility and elimination, etc.
2.6. Nanowires (NWs)
Nanowires (NWs) are wire-like structures with microscopic scales and widths ranging from 1 to 100 nm [65]. Alternatively, they might be defined as structures with a lateral size of a few tens of nanometers or less but an unlimited longitudinal extent. Over the past two decades, several metallic, compound semiconducting, and dielectric materials have been developed for NWs. NWs, with their large specific surface area and excellent electrochemical characteristics, can be utilized as carriers for biosensors that detect biomarkers and disease-related chemicals [66]. Furthermore, researchers have employed functionalized NWs to creat very sensitive biosensors capable of rapidly detecting tumor markers and disease-related molecules such as viruses, thereby providing a new technique of clinical diagnosis [67,68]. Viruses are the leading cause of human disease, and there is growing worry about their propensity to be used as a biological weapon. To date, NWs have successfully detected a variety of viruses, including influenza A H3N2, Dengue, HIV, and H1N1 [69]. Nanowire-modified sensors detect IFN-γ levels below 150 T cells in individuals with tuberculosis and HIV infection, which routine tests cannot detect [70]. Du et al. demonstrated that silicon nanowire array biosensors have a considerable advantage in ctDNA detection, with an ultra-low detection limit of 10 aM and good linearity across the ctDNA concentration range of 0.1 fM to 100 pM [71]. However, NWs have some limitations and disadvantages, such as high cost, potential toxicity, low stability, and processing difficulties, etc.
3. Applications of nanomaterials in protein detection and analysis
3.1. Applications of nanomaterials in protein sequencing
The proteome may provide a more accurate description of a cell's or organism's physiology than DNA does. The sequence and spatial structure of a protein are both part of its information. Protein sequencing provides valuable information for disease diagnosis and treatment [72]. In recent years, electrochemical nanopore technologies have emerged as a viable alternative approach to proteomic analysis. Nanopore structures, which may be modified in size, shape, and surface properties to selectively detect and conduct protein molecules, are an important application of nanomaterials in protein sequencing. The combination of nanomaterials with nanopore technology can also enable high-throughput protein sequencing, increasing the speed and efficiency of the process.
The basic idea behind nanopores is that an insulating barrier layer the size of a nanopore separates an electrolyte-filled chamber into two microscopic compartments. When the electrolytes are charged, a single molecule can pass through the pore and partially prevent ions from entering the nanopore. During translocation, the ionic current level drops and then returns to its previous value. Nanopore size, surface properties, voltage, and solution can all influence ionic current. Residence duration, current amplitude, and capture rate are all important factors in molecular structure and dynamic motion. When a single molecule makes contact with the nanopore sensing surface, a conventional ionic current modulation occurs, revealing the transporter molecule's composition, charge distribution, structure, and sequence. As a result, nanopores' ability to read the sequences of individual protein residues is determined by well-defined fluctuations in ionic currents. Nanopore arrays, in particular, allow high-throughput parallel detection of low-abundance proteins, protein rescue in the absence of in vitro amplification methods, and the interpretation of long-read proteins without labeling and/or enrichment [73,74].
Several bio-nanopores are routinely employed, including α-hemolysin (α-HL), Mycobacterium smegmatis porin A (MspA), aerolysin (AeL), and Fragaceatoxin C (FraC) [[75], [76], [77], [78]]. α-HL nanopores were utilized to study the aggregation of Aβ associated with Alzheimer's disease (AD). Results showed that the amyloidosis process of Aβ is influenced by its initial structural properties and can be produced by β-CD [77]. FraC nanopores, both wild-type and mutant, were used to detect peptide/protein biomarkers [79]. The modified W116S FraC nanopore recognizes angiotensin I (Ang I), II (Ang II), III (Ang III), and IV (Ang IV) peptides in the mixture simultaneously, which is critical for regulating blood pressure and fluid balance [72,80].
3.2. Applications of protein-nanomaterials (PNs) hybrids in real-time and filed detection
With the assistance of rapidly advancing nanotechnology, the combination of protein and nanomaterials (PNs) hybrids, which integrates various physicochemical properties of nanomaterials while also synergizing the functionality and performance of proteins, provides an excellent opportunity to demonstrate significant advantages and potential in a variety of biological applications [[81], [82], [83]]. The protein and nanomaterial components of PNs hybrid biosensors work together in a more functionally efficient (synergistic) manner to complete the biosensing process, from analyte recognition to signal generation. Proteins have strong affinity and specific recognition targets, and nanopaticles’ high surface area and/or porous nature allow for a large number of immobilization sites for protein loading or analyte separation/concentration. The excellent biocompatibility of nanomaterials improves protein function; nanomaterial components around proteins can protect them from denaturation caused by external influences, boosting stability and repeatability. Meanwhile, nanomaterials with diverse physical and chemical properties can serve as direct markers or indirect fixed substrates/catalysts for signal generation, greatly extending and diversifying signal types. For example, the novel Ab2-Au@Ag–Cu2O hybrid displays outstanding electrocatalytic activity for H2O2 reduction with a low LOD of 0.003 pg/mL for PSA detection because to the synergy of Au, Ag, and Cu2O [84]. Furthermore, because proteins and nanomaterials have varied surface chemistry, they can be coupled in a variety of ways under basic conditions, yielding protein-nanomaterial hybrids with highly integrated characteristics and various functionalities in a compact space. This greatly simplifies the integration/assembly of several components to create performance-enhancing and compact biosensors. They can be easily combined with portable readers and/or integrated into miniature substrates, exposing distinct advantages and possibilities in field inspections when paired with portable and small equipment such as pH/glucose/temperature/gas analyzers, test strips, and microfluidic sheets [85].
3.3. Applications of nanomaterials in immune detection and analysis
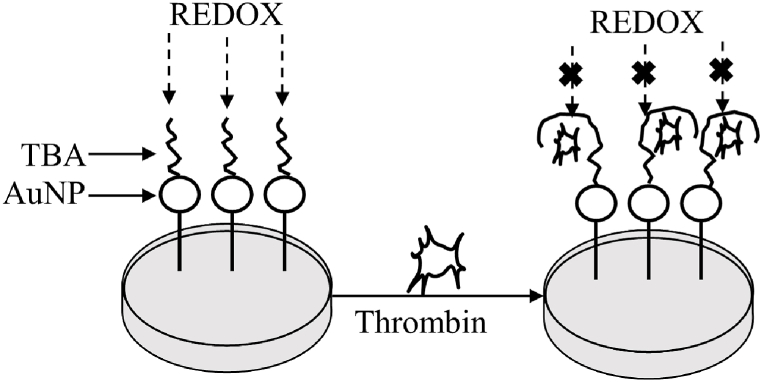
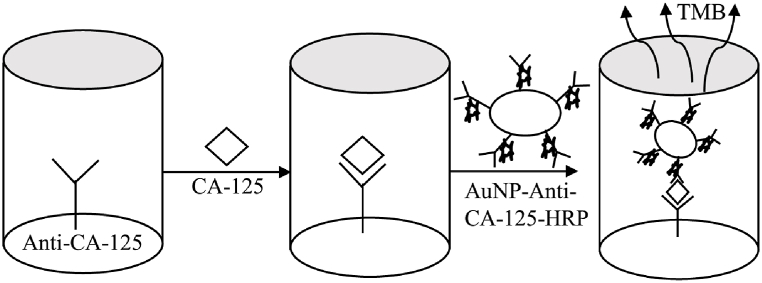
Noble metal nanoparticles have several advantages, including electrical conductivity, immobilization, and biosensor stability. AuNPs are the most widely used precious metal nanoparticles due to their biocompatibility and electrical properties. The primary use of AuNPs is to fix thiophene bridged aldehydes (TBAs), in which TBAs attach to thrombin, causing conformational changes that alter charge transfer resistance in the presence of REDOX (reduction-oxidation) couplings, which can then be measured electrochemically (Fig. 1). Meanwhile, silver and other noble metal nanoparticles have been used as electrochemical aptamers to thrombin detection [86,87]. For their local surface plasmon resonance (LSPR) features, AuNPs have a high extinction coefficient, making them ideal substrate materials for traditional substrate materials such as TMB in ELISA experiments (Fig. 2). The presence of analytes in ELISA may cause AuNPs to change size or form, allowing them to cluster together in colloidal solutions. The color of the colloidal solution allows the naked eye to perceive the AuNPs aggregation, which can then be measured using UV–visible spectrophotometry. Plasma ELISA is an ELISA technique that uses AuNPs as a substrate. The fundamental advantage of plasma ELISA over traditional ELISA is that the detection limit is substantially lower, yet he number of atomic analytes detectable with the naked eye is the greatest. AuNPs can be applied as multifunctional nanomaterials in ELISA immunoassays in the following ways: AuNPs can be used as three-dimensional antibody carriers, increasing detection sensitivity. They can also replace traditional enzymes in the preparation of ultra-sensitive and extremely stable immunoassay reagents, allowing for rapid and visual point-of-care testing systems in clinical applications [88].
Fig. 1.
Schematic diagram of AUNP-based detection methods. AuNP was linked to TBAs and fixed to the reaction plate. TBAs bound to thrombin, conformational modifications occurred, charge transfer resistance was changed in the presence of REDOX, and further determination was carried out using an electrochemical method.
Fig. 2.
AuNPs is an excellent substrate for typical substrate materials, such as TMB in ELISA tests. The plates were coated with Anti-CA-125 primary antibody, treated with serum containing CA-125, and then supplemented with AuNP-Anti-CA-125-HRP secondary antibody, producing a cascade amplification effect.
Nanomaterials can be employed as protein capture carriers due to their distint interactions with proteins. AuNPs and MNPs, for example, can change the surfaces of proteins to selectively trap them. Also, structural alterations like nanopores can be used to capture proteins in nanomaterials. Raman scattering happens when nanomaterials are exposed to excitation light, producing distinct Raman spectra. Because nanomaterials’ size and morphology have a substantial influence on their Raman spectra, the Raman effect can be used to investigate their structure and properties. At the same time, nanomaterials have a large specific surface area and a broad surface functional group repertoire, which can be selectively combined with biomarkers to provide highly sensitive detection of chemicals and cells in organisms. For example, p24, a viral capsid protein in serum, is an early virological biomarker of HIV-1 infection. The chemically amplified nanopore assay detects p24 in serum extreme sensitivity, with a detection limit as low as 0.5 pg/mL, which is 20–100 times more sensitive than the ELISA method currently used in clinical practice [89]. Magnetic nanoparticles are used in nanopore blocking sensors to reduce PSA reaction time and atypical binding, allowing for extremely sensitive, specific, and rapid detection of PSA concentrations in patient blood while also lowering the PSA minimum detection limit [90]. Metallic nanoparticles can considerably increase capacitive sensor sensitivity at the fM-pM level. The morphological complexity of nanoparticles does not always result in enhanced sensitivity, but rather broadens the linear response range. The simplest aptamer sensor, which only uses AuNPs, has one of the lowest LODs at roughly 0.14 fM, but the Ag Nanowire (Ag-NW)-based sensor has a linear range of almost five orders of magnitude. Meanwhile, due to their biocompatibility and anti-biological contamination features, aptamer sensors made of polymer materials may detect thrombin in whole blood analysis [91].
Cancer biomarkers are biological molecules that can be found in blood, tissues, and body fluids (including saliva, urine), including proteins (released or cell surface proteins), carbohydrates, and nucleic acids. Measuring cancer biomarker levels can help with early cancer detection. Protein markers such as CEA, AFP, PSA, and CA-125 are approved for cancer diagnosis, and their sensitivity can be increased through particular interactions with antibodies, antibody fragments, or aptamers. The interaction event is then turned into a quantitative signal. Because of their distinct properties, nanomaterials have been used to detect tumor markers with improved specificity and sensitivity. QDs stand out for their high quantum yield, high molar extinction coefficient, wide absorption range, strong photobleaching resistance, and outstanding degradation performance. Recent studies have used QD-based biosensors to detect cancer biomarkers, such as the detection of ovarian cancer CA-125 biomarker using QDs junction fitting [92], and the detection of PSA antigen by side-flow technology using QDs embedded with silica nanoparticles [93], as well as the detection of CEA and AFP antigens [[93], [94], [95]]. Meanwhile, AuNPs can detect cancer-related markers such as tumor DNA or cells and exosomes. Introducing many binding ligands to cells can have a multidimensional effect, boosting the specificity and sensitivity of the diagnosis [96].
4. Applications of nanomaterials in nucleic acid detection and analysis
Karry Mullis developed the polymerase chain reaction (PCR) in 1983. Multiple heat cycles are used to replicate specific DNA sequences from little amounts of DNA or RNA. PCR amplification of DNA templates involves several heat cycles in the presence of DNA polymerases and primers. During a heat event, the DNA template undergoes three major processes: denaturation, annealing, and extension, with the number of DNA strands increasing exponentially as the PCR cycle varies. Despite the fact that PCR is a sensitive technology capable of identifying small amounts of template DNA in the reaction (10–100 copies), it has several drawbacks [88]. The most common issue with conventional PCR is the production of non-specific amplification products that fail to amplify GC-rich areas of genomic DNA. As nanotechnology expanded and research advanced, nanoparticles were thoroughly investigated in assisted PCR technique [97]. A wide range of nanoparticles are utilized to increase PCR detection performance, including liposomes, carbon nanoparticles, metal nanoparticles, and QDs. In general, using nanoparticles in PCR detection can increase PCR yields, improve DNA replication fidelity, increase template DNA binding efficiency with primers and DNA polymerase, and facilitate heat cycling. Liposome-based PCR distinguishes itself from inorganic nanoparticle-based PCR by incorporating a distinct form of nano-PCR and providing cellular or nucleus-like hardware for PCR reactions [88].
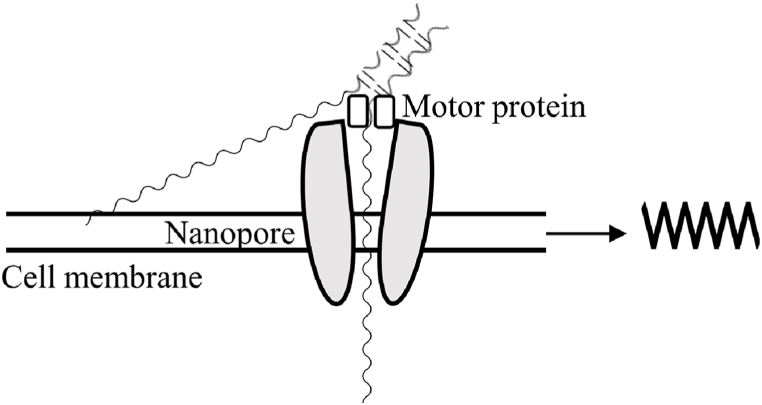
Many nanomaterials have been effectively employed to improve the specificity and efficiency of PCR, including QDs, AuNPs [98], CNTs [97], GO [97], reduced graphene oxide (rGO) [97], various metal oxide materials (e.g., zinc oxide, titanium dioxide), and other composites (such as polymers doped with AuNPs). RNA polyadenylation has an impact on RNA location, function, stability, and translation in the cell. Nanopore 3’ end capture sequencing (Nano 3P-seq) eliminates the requirement for RNA polyadenylation, PCR, or RNA junction ligation, allowing sequencing of RNA molecules from the 3′ end of the RNA molecule, with the ability to capture non-A bases in varied lengths of poly A tails (Fig. 3) [99]. Zhang et al. demonstrated that nanopore sequencing can detect the presence of Neisseria gonorrhoeae in clinical samples as well as the 13 most frequently used resistance genes, with a minimum detection limit of 31 copies/reaction for this method [100]. Circulating tumor DNA (ctDNA) in blood is a critical biomarker for non-invasive cancer diagnosis, evaluation, prediction, and treatment. The combination of hybridisation chain reaction (HCR) and nanopore sensors makes it possible to detect ctDNA in physiological fluids quickly, efficiently, and sensitively. KRAS G12DM is a small ctDNA biomarker used to detect colon cancer. The HCR nanopore sensing technology enables the rapid, specific, and sensitive detection of KRAS G12DM [101]. MicroRNAs are a type of non-coding RNAs that is short-stranded (18–22 nucleotides) and regulates the cell cycle, apoptosis, and signaling pathways. MicroRNAs are abnormally expressed in all tumor cells, and the types of microRNAs expressed vary by tumor cell type. Protein nanopore sensors must enhance membrane stability and high throughput capacity [102].
Fig. 3.
Nanopore sequencing model diagram. To break a double strand into a single strand, a single molecule of DNA passes through a pore protein, the pore protein has a protein molecule that acts as a converter, the single molecule of DNA stays in the pore, some ions cause current changes, and the current changes are different for different bases, and a computer calculation method is used to obtain the base sequence.
With the emergence of coronavirus disease 2019 (COVID-19), several researchers are proposing rapid and simple nucleic acids tests to help prevent the virus from spreading [103,104]. Lee et al., for example, developed two new point-of-care assays to rapidly detect infections [105]. One example is the nanoPCR, which uses core-shell magneto-plasmonic nanoparticles (MPNs). The gold shell significantly accelerates the thermal cycle by converting volume, plasma, and photothermal energy, whereas the magnetic core's magnetic gap allows for sensitive in situ fluorescence detection. When utilized to detect COVID-19, the SARS-CoV-2 RNA found in 17 min was as high as 3.2 copies/L, accurately identifying COVID-19 cases in 150 clinical samples, confirming its clinical utility [97].
5. Applications of nanomaterials on aging-related nanodiagnostics and other aspects
Alzheimer's disease (AD) is a neurodegenerative disease that is a leading cause of dementia and a significant contributor to mortality and disability of the elderly. As the aging of the population, insightful research and accurate diagnosis of AD becomes more important. miR-34a and amyloid beta (Aβ) are the two mains biomarkers for AD. A study conducted by Pereira et al. demonstrated that a novel electrochemical miRNA biosensor, developed using nanotechnology, exhibited high targeting affinity and specific detection capabilities for miR-34a. This provides a new, economic, and less invasive diagnostic tool for early detection of AD [106]. Chiu utilized immunomagnetic nanoparticle techniques to measure Aβ and tau proteins in plasma. The results indicated that Aβ1-42 × tau (642.58) can effectively differentiate patients with dementia from those in the preclinical stage of AD. The positive agreement percentage (PPA) is 84.9 %, the negative agreement percentage (NPA) is 78.8 %, and the overall agreement rate (ORA) is 83 % [107]. Furthermore, a review published by Somayeh and colleagues outlined the use of nanotechnology-manufactured biosensors for detecting Aβ specifically, offering a more precise approach for early detection of AD [108]. Parkinson's disease is the second most common neurodegenerative disease in the elderly population. The pathogenic mechanism is diverse, and there are no specific and reliable laboratory diagnostic markers. Clinical diagnosis is mainly based on clinical symptoms and laboratory analysis of certain indicators, such as neurotransmitters, alpha-synuclein nucleotides, and dopamine. Traditional diagnostic methods are limited by low sensitivity and specificity, as well as poor equipment performance. Biosensors manufactured using nanomaterials such as graphene oxide, carbon nanotubes, and gold nanoparticles can improve the specificity and sensitivity of detecting Parkinson-related biological markers. Additionally, these biosensors offer a detection method with low-cost, easy-to-operate, and easy-to-functionalize [109]. Diabetic nephropathy (DN) is a chronic disease caused by proteinuria and progressive reduction of glomerular filtration rate (GFR) resulted from long-term hyperglycemia. It is a common complication of diabetes and one of the common diseases in the elderly population. With the aging of the population, its incidence is increasing year by year. Microalbuminuria has been widely regarded as the main clinical indicator for the diagnosis of DN, but its specificity is relatively low. With the continuous progress in life science, many microRNAs (such as miR-34a) have been speculated as new biomarkers of DN. However, traditional clinical detection methods for these biomarkers have limitations in terms of sensitivity and specificity. AuNPs have been successfully applied in the development of various diagnostic analyses due to their optical properties [110]. Yuting Liu used biosensors integrated with AuNPs to detect biomarkers associated with DN. The method has a wide range of linear detection capabilities, ranging from 1 pg/mL to 10 ng/mL, and has a very low detection limit as low as 0.3 pg/mL, indicating it's high sensitivity and broad clinical application potential for DN-related biomarkers detection [111]. Nossier et al. conducted AuNPs-based analysis and achieved high (78 %, 72 %, 81 %, and 69 %) sensitivity and specificity of qualitative detection of urinary miR-210 and 34a. These data are consistent with the results from real-time fluorescent quantitative PCR. Therefore, AuNPs analysis is considered to be an effective method for non-invasive diagnosis of DN [112]. Diabetes is a chronic metabolic disease characterized by elevated blood glucose levels that can lead to severe damage to the organs and systems of the heart, blood vessels, eyes, kidneys, and nerves over time. In clinical practice, timely and accurate diagnosis and effective monitoring and management of diabetes are crucial to reducing the mortality of diabetes patients. In a review article, Yang suggested that, by combining nanomaterials such as carbon nanotubes, quantum dots, silicon nanowires, and other techniques such as electrochemical detection, optical detection, and qualitative spectrum detections, the newly formed analytical technology platforms can effectively improve metabolic analysis. This technological strategy has a huge potential to be used in the precise diagnosis of diabetic diseases in the early stages [113].
Bismuth sulfide nanoprobes have an in vivo half-life of up to 2 h and can be successfully boosted at the tumor site to enable CT imaging of the tumor. Additionally, bismuth sulfide has a low bandgap energy, allowing it to efficiently convert absorbed light energy into heat energy. CT-guided cancer treatment is now accessible, with tumor elimination is at 100 % [114]. Based on antigen-antibody specific recognition, AuNPs for coupling oligonucleotides rapidly capture Mycobacterium tuberculosis CFP-10, and the detection level of conjugated CFP-10 antibody by real-time immunofluorescence quantitative polymerase chain reaction (RT–IF–PCR), which reflects the proliferation of Mycobacterium tuberculosis and the progression of the disease, and the sensitivity of the RT–IF–PCR technique developed on the basis of a colorimetric sensor technology for glutathione detection was developed, based on the biological activity and stability of MnO2 nano-enzymes, providing low cost, simple, quick, extremely sensitive, highly selective, visual, and quantitative assays [115].
6. Summary and outlook
Nanotechnology, as an emerging technology, has received a lot of attention since its inception and has now become one of the most important technologies of the 21st century, driving research in various fields and the development of diverse industries, with visible progress in medicine. Nanomaterials can solve many problems that standard treatment cannot. By using nanomaterials into traditional medicine, we can increase sensitivity, specificity, and reliability in disease diagnosis. For example, in the context of stroke, nanomaterials can be programmed to release payloads in response to specific extracellular processes that occur around the clot and in the ischemic penumbral zone, allowing pathogenic characteristics to be detected at different stages of stroke progression [116]. Nanomaterial-based biosensors can detect more Leishmania protozoa in biological samples, hence boosting leishmaniasis diagnosis and detection [117]. Nanomaterials certainly have great potential in a varie of in vitro diagnostic applications, notably rapid detection. However, some obstacles must be addressed before the clinical transformation of in vitro diagnosis may be realized. To begin, nanomaterials for in vitro detection applications go through several phases, including design, synthesis, surface modification, and biocoupling, all of which are crucial to the overall performance of the nanoprobes. Because of the challenges of nanomaterial surface chemistry, improved preparation and modification procedures are necessary to offer nanoprobes with high reproducibility of performance, resilient surface coatings, and flexible functionalization and biocoupling processes. Second, the majority of current nanotechnology in vitro diagnosis entail many operational phases, such as sample extraction, purification, and detection, all of which require professional and qualified personnel to execute. As a result, automated testing and reduced human labor via the creation of integrated portable devices are both viable options. Third, many nanoassay kits are still in the pre-clinical stage, and it is critical to evaluate and extensively examine the efficacy of these technologies in a clinical setting using a large number of clinical samples. Early clinical evaluation of nanomaterial-based in vitro diagnostic technologies can help to accelerate their clinical acceptance by accounting for the diversity of patient samples during technology development. Nanomaterials have the potential to disrupt the he cell's autophagy process and change its fate. To advance nanomedicine, we must be more rigorous, concentrating only on nanotechnology and nanomaterials for safety research and evaluation, much as we do with cloning technology. Nanotechnology, like cloning technology, requires time for debate; scientific research is ongoing, and new science and technology will be better suited to serve humanity.
Funding statement
This work was supported by Beijing Natural Science Foundation (5222034) and Quanzhou City Science & Technology Program of China (2022C034R).
Additional information
No additional information is available for this article.
Data availability statement
There is no additional data available for this review.
CRediT authorship contribution statement
Huiying Lai: Writing – original draft, Supervision, Conceptualization. Rongfu Huang: Writing – original draft, Conceptualization. Xin Weng: Writing – original draft. Baoshan Huang: Writing – original draft. Jianfeng Yao: Writing – review & editing, Funding acquisition. Yaya Pian: Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Nishida I.A. An electron microscope and electron diffraction study of fine manganese particles prepared by evaporation in argon at low pressures. J. Phys. Soc. Jpn. 1969;26:1225–1239. doi: 10.1143/JPSJ.26.1225. [DOI] [Google Scholar]
- 2.Birringer R., Gleiter H., Klein H.P., et al. Nanocrystalline materials an approach to a novel solid structure with gas-like disorder? Phys. Lett. 1984;102:365–369. doi: 10.1016/0375-9601(84)90300-1. [DOI] [Google Scholar]
- 3.Xu D., Li Z., Li L., et al. Insights into the photothermal conversion of 2D MXene nanomaterials: synthesis, mechanism, and applications. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.202000712. [DOI] [Google Scholar]
- 4.Ding M., Cui S., Fang L., et al. NIR-I-Responsive single-band upconversion emission through energy migration in core-shell-shell nanostructures. Angew Chem. Int. Ed. Engl. 2022;61 doi: 10.1002/anie.202203631. [DOI] [PubMed] [Google Scholar]
- 5.Zhou P., Yu H., Zhong Y., et al. Fabrication of waterproof artificial compound eyes with variable field of view based on the bioinspiration from natural hierarchical micro-nanostructures. Nano-Micro Lett. 2020;12:166. doi: 10.1007/s40820-020-00499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah N., Zaman T., Rehan T., et al. Preparation and characterization of agar based magnetic nanocomposite for potential biomedical applications. Curr. Pharmaceut. Des. 2019;25:3672–3680. doi: 10.2174/1381612825666191011113109. [DOI] [PubMed] [Google Scholar]
- 7.Ha Y., Ko S., Kim I., et al. Recent advances incorporating superparamagnetic nanoparticles into immunoassays. ACS Appl. Nano Mater. 2018;1:512–521. doi: 10.1021/acsanm.7b00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang X., Li L., Chen Z., et al. Nanomedicine for the detection and treatment of ocular bacterial infections. Adv Mater. 2023 doi: 10.1002/adma.202302431. [DOI] [PubMed] [Google Scholar]
- 9.Zhu M., Kuang Z., Xu F., et al. Employing a redox reporter-modified self-assembly monolayer in electrochemical aptamer-based sensors to enable calibration-free measurements. ACS Appl. Bio Mater. 2023;6:1586–1593. doi: 10.1021/acsabm.3c00001. [DOI] [PubMed] [Google Scholar]
- 10.Chen J., Zhang X., Millican R., et al. Recent advances in nanomaterials for therapy and diagnosis for atherosclerosis. Adv. Drug Deliv. Rev. 2021;170:142–199. doi: 10.1016/j.addr.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan C.K.W., Zhang L., Cheng C.K., et al. Recent advances in managing atherosclerosis via nanomedicine. Small. 2018;14 doi: 10.1002/smll.201702793. [DOI] [PubMed] [Google Scholar]
- 12.Ajith S., Almomani F., Elhissi A., et al. Nanoparticle-based materials in anticancer drug delivery: current and future prospects. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobhanan J., Rival J.V., Anas A., et al. Luminescent quantum dots: synthesis, optical properties, bioimaging and toxicity. Adv. Drug Deliv. Rev. 2023;197 doi: 10.1016/j.addr.2023.114830. [DOI] [PubMed] [Google Scholar]
- 14.Pham X.H., Park S.M., Ham K.M., et al. Synthesis and application of silica-coated quantum dots in biomedicine. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221810116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yemets A., Plokhovska S., Pushkarova N., et al. Quantum dot-antibody conjugates for immunofluorescence studies of biomolecules and subcellular structures. J. Fluoresc. 2022;32:1713–1723. doi: 10.1007/s10895-022-02968-5. [DOI] [PubMed] [Google Scholar]
- 16.Chao C., Kang L., Dai W., et al. Microfluidic-based modulation of triplet exciton decay in organic phosphorescent nanoparticles for size-assisted photodynamic antibacterial therapy. J. Mater. Chem. B. 2023;11:3106–3112. doi: 10.1039/d2tb02662g. [DOI] [PubMed] [Google Scholar]
- 17.Wang W., Liu Y., Shi T., et al. Biosynthesized quantum dot for facile and ultrasensitive electrochemical and electrochemiluminescence immunoassay. Anal. Chem. 2020;92:1598–1604. doi: 10.1021/acs.analchem.9b04919. [DOI] [PubMed] [Google Scholar]
- 18.Yao X., Yan P., Tang Q., et al. Quantum dots based electrochemiluminescent immunosensor by coupling enzymatic amplification for ultrasensitive detection of clenbuterol. Anal. Chim. Acta. 2013;798:82–88. doi: 10.1016/j.aca.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Tsai D.H., Lu Y.F., DelRio F.W., et al. Orthogonal analysis of functional gold nanoparticles for biomedical applications. Anal. Bioanal. Chem. 2015;407:8411–8422. doi: 10.1007/s00216-015-9011-9. [DOI] [PubMed] [Google Scholar]
- 20.Yeh Y.C., Creran B., Rotello V.M. Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale. 2012;4:1871–1880. doi: 10.1039/c1nr11188d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., Li L., Li Y., et al. PSMA1-mediated ultrasmall gold nanoparticles facilitate tumor targeting and MR/CT/NIRF multimodal detection of early-stage prostate cancer. Nanomedicine. 2023;47 doi: 10.1016/j.nano.2022.102617. [DOI] [PubMed] [Google Scholar]
- 22.Hara D., Tao W., Totiger T.M., et al. Prostate cancer targeted X-ray fluorescence imaging via gold nanoparticles functionalized with prostate-specific membrane antigen (PSMA) Int. J. Radiat. Oncol. Biol. Phys. 2021;111:220–232. doi: 10.1016/j.ijrobp.2021.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Patra A., Ding T., Engudar G., et al. Component-specific analysis of plasma protein corona formation on gold nanoparticles using multiplexed surface plasmon resonance. Small. 2016;12:1174–1182. doi: 10.1002/smll.201501603. [DOI] [PubMed] [Google Scholar]
- 24.Indhu A.R., Keerthana L., Dharmalingam G. Plasmonic nanotechnology for photothermal applications-an evaluation. Beilstein J. Nanotechnol. 2023;14:380–419. doi: 10.3762/bjnano.14.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farokhnezhad M., Esmaeilzadeh M. Graphene coated gold nanoparticles: an emerging class of nanoagents for photothermal therapy applications. Phys. Chem. Chem. Phys. 2019;21:18352–18362. doi: 10.1039/c9cp03126j. [DOI] [PubMed] [Google Scholar]
- 26.Liu X.Y., Wang J.Q., Ashby C.R., Jr., et al. Gold nanoparticles: synthesis, physiochemical properties and therapeutic applications in cancer. Drug Discov. Today. 2021;26:1284–1292. doi: 10.1016/j.drudis.2021.01.030. [DOI] [PubMed] [Google Scholar]
- 27.Khan S., Hasan A., Attar F., et al. Gold nanoparticle-based platforms for diagnosis and treatment of myocardial infarction. ACS Biomater. Sci. Eng. 2020;6:6460–6477. doi: 10.1021/acsbiomaterials.0c00955. [DOI] [PubMed] [Google Scholar]
- 28.Roshanbinfar K., Kolesnik-Gray M., Angeloni M., et al. Collagen hydrogel containing polyethylenimine-gold nanoparticles for drug release and enhanced beating properties of engineered cardiac tissues. Adv Healthc Mater. 2023 doi: 10.1002/adhm.202202408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shevach M., Fleischer S., Shapira A., et al. Gold nanoparticle-decellularized matrix hybrids for cardiac tissue engineering. Nano Lett. 2014;14:5792–5796. doi: 10.1021/nl502673m. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q., Wang T., Lio C., et al. Surface hydrolysis-designed AuNPs-zwitterionic-glucose as a novel tool for targeting macrophage visualization and delivery into infarcted hearts. J. Contr. Release. 2023;356:678–690. doi: 10.1016/j.jconrel.2023.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Qiu J., Jiang P., Wang C., et al. Lys-AuNPs@MoS(2) nanocomposite self-assembled microfluidic immunoassay biochip for ultrasensitive detection of multiplex biomarkers for cardiovascular diseases. Anal. Chem. 2022;94:4720–4728. doi: 10.1021/acs.analchem.1c05061. [DOI] [PubMed] [Google Scholar]
- 32.Han G.R., Kim M.G. Highly sensitive chemiluminescence-based lateral flow immunoassay for cardiac troponin I detection in human serum. Sensors. 2020;20 doi: 10.3390/s20092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarfraz N., Khan I. Plasmonic gold nanoparticles (AuNPs): properties, synthesis and their advanced energy, environmental and biomedical applications. Chem. Asian J. 2021;16:720–742. doi: 10.1002/asia.202001202. [DOI] [PubMed] [Google Scholar]
- 34.Jin Y., Huang Z., Xu B., et al. Localization of multiple DNAzymes as a branchedzyme-powered nanodevice for the immunoassay of tumor biomarkers. Anal. Chim. Acta. 2023;1274 doi: 10.1016/j.aca.2023.341580. [DOI] [PubMed] [Google Scholar]
- 35.Tan Y., Zhou J., Xing X., et al. DNA assembly of plasmonic nanostructures enables in vivo SERS-based MicroRNA detection and tumor photoacoustic imaging. Anal. Chem. 2023 doi: 10.1021/acs.analchem.3c00775. [DOI] [PubMed] [Google Scholar]
- 36.Santhosh P.B., Tenev T., Šturm L., et al. Effects of hydrophobic gold nanoparticles on structure and fluidity of SOPC lipid membranes. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241210226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mutalik C., Saukani M., Khafid M., et al. Gold-based nanostructures for antibacterial application. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241210006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdellatif A.A.H., Ahmed F., Mohammed A.M., et al. Recent advances in the pharmaceutical and biomedical applications of cyclodextrin-capped gold nanoparticles. Int. J. Nanomed. 2023;18:3247–3281. doi: 10.2147/ijn.S405964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y., Zhan L., Shen J.W., et al. fM-aM detection of the SARS-CoV-2 antigen by advanced lateral flow immunoassay based on gold nanospheres. ACS Appl. Nano Mater. 2021;4:13826–13837. doi: 10.1021/acsanm.1c03217. [DOI] [PubMed] [Google Scholar]
- 40.Alnaimi A., Al-Hamry A., Makableh Y., et al. Gold nanoparticles-MWCNT based aptasensor for early diagnosis of prostate cancer. Biosensors. 2022;12 doi: 10.3390/bios12121130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu R., Ouyang L., Chen H., et al. Recent advances in biomolecular detection based on aptamers and nanoparticles. Biosensors. 2023;13 doi: 10.3390/bios13040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y., Li Z., Huang Y., et al. Amplifying cancer treatment: advances in tumor immunotherapy and nanoparticle-based hyperthermia. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1258786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farzin A., Etesami S.A., Quint J., et al. Magnetic nanoparticles in cancer therapy and diagnosis. Adv Healthc Mater. 2020;9 doi: 10.1002/adhm.201901058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivera-Rodriguez A., Rinaldi-Ramos C.M. Emerging biomedical applications based on the response of magnetic nanoparticles to time-varying magnetic fields. Annu. Rev. Chem. Biomol. Eng. 2021;12:163–185. doi: 10.1146/annurev-chembioeng-102720-015630. [DOI] [PubMed] [Google Scholar]
- 45.Rümenapp C., Gleich B., Haase A. Magnetic nanoparticles in magnetic resonance imaging and diagnostics. Pharm. Res. (N. Y.) 2012;29:1165–1179. doi: 10.1007/s11095-012-0711-y. [DOI] [PubMed] [Google Scholar]
- 46.Gutiérrez-Capitán M., Sanchís A., Carvalho E.O., et al. Engineering a point-of-care paper-microfluidic electrochemical device applied to the multiplexed quantitative detection of biomarkers in sputum. ACS Sens. 2023 doi: 10.1021/acssensors.3c00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L., Gao Y., Zhang Y., et al. A biomimetic nanogel system restores macrophage phagocytosis for magnetic resonance imaging-guided synergistic chemoimmunotherapy of breast cancer. Adv Healthc Mater. 2023 doi: 10.1002/adhm.202300967. [DOI] [PubMed] [Google Scholar]
- 48.Zară-Dănceanu C.M., Minuti A.E., Stavilă C., et al. Magnetic nanoparticle coating decreases the senescence and increases the targeting potential of fibroblasts and adipose-derived mesenchymal stem cells. ACS Omega. 2023;8:23953–23963. doi: 10.1021/acsomega.3c02449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boltman T., Meyer M., Ekpo O. Diagnostic and therapeutic approaches for glioblastoma and neuroblastoma cancers using chlorotoxin nanoparticles. Cancers. 2023;15 doi: 10.3390/cancers15133388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mamani J.B., Borges J.P., Rossi A.M., et al. Magnetic nanoparticles for therapy and diagnosis in nanomedicine. Pharmaceutics. 2023;15 doi: 10.3390/pharmaceutics15061663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu C., Zhao J., Guo Y., et al. A novel method to prepare water-dispersible magnetic nanoparticles and their biomedical applications: magnetic capture probe and specific cellular uptake. J. Biomed. Mater. Res. 2008;87:364–372. doi: 10.1002/jbm.a.31786. [DOI] [PubMed] [Google Scholar]
- 52.Wu M., Huang S. Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment. Mol Clin Oncol. 2017;7:738–746. doi: 10.3892/mco.2017.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fathi Karkan S., Mohammadhosseini M., Panahi Y., et al. Magnetic nanoparticles in cancer diagnosis and treatment: a review. Artif. Cells, Nanomed. Biotechnol. 2017;45:1–5. doi: 10.3109/21691401.2016.1153483. [DOI] [PubMed] [Google Scholar]
- 54.Gallo J., Long N.J., Aboagye E.O. Magnetic nanoparticles as contrast agents in the diagnosis and treatment of cancer. Chem. Soc. Rev. 2013;42:7816–7833. doi: 10.1039/c3cs60149h. [DOI] [PubMed] [Google Scholar]
- 55.Komane P., Kumar P., Choonara Y. Functionalised carbon nanotubes: promising drug delivery vehicles for neurovascular disorder intervention. AAPS PharmSciTech. 2023;24 doi: 10.1208/s12249-023-02651-3. [DOI] [PubMed] [Google Scholar]
- 56.Zhang H., Liu Y., Yao M., et al. Cathodic electrochemiluminesence microscopy for imaging of single carbon nanotube and nucleolin at single tumor cell. Anal. Chem. 2023;95:570–574. doi: 10.1021/acs.analchem.2c04425. [DOI] [PubMed] [Google Scholar]
- 57.Zandi A., Gilani A., Abbasvandi F., et al. Carbon nanotube based dielectric spectroscopy of tumor secretion; electrochemical lipidomics for cancer diagnosis. Biosens. Bioelectron. 2019;142 doi: 10.1016/j.bios.2019.111566. [DOI] [PubMed] [Google Scholar]
- 58.Rebeccani S., Wetzl C., Zamolo V.A., et al. Electrochemiluminescent immunoassay enhancement driven by carbon nanotubes. Chem. Commun. 2021;57:9672–9675. doi: 10.1039/d1cc03457j. [DOI] [PubMed] [Google Scholar]
- 59.Novoselov K.S., Geim A.K., Morozov S.V., et al. Electric field effect in atomically thin carbon films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 60.Bulakhe R.N., Nguyen A.P., Ryu C., et al. Facile synthesis of mesoporous nanohybrid two-dimensional layered Ni-Cr-S and reduced graphene oxide for high-performance hybrid supercapacitors. Materials. 2023;16 doi: 10.3390/ma16196598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng L., Wu L., Qu X. New horizons for diagnostics and therapeutic applications of graphene and graphene oxide. Adv Mater. 2013;25:168–186. doi: 10.1002/adma.201203229. [DOI] [PubMed] [Google Scholar]
- 62.Han Z., Shu J., Liang X., et al. Label-free ratiometric electrochemiluminescence aptasensor based on nanographene oxide wrapped titanium dioxide nanoparticles with potential-resolved electrochemiluminescence. Anal. Chem. 2019;91:12260–12267. doi: 10.1021/acs.analchem.9b02318. [DOI] [PubMed] [Google Scholar]
- 63.Yang H.W., Huang C.Y., Lin C.W., et al. Gadolinium-functionalized nanographene oxide for combined drug and microRNA delivery and magnetic resonance imaging. Biomaterials. 2014;35:6534–6542. doi: 10.1016/j.biomaterials.2014.04.057. [DOI] [PubMed] [Google Scholar]
- 64.Li S., Yang Y., Wang S., et al. Advances in metal graphitic nanocapsules for biomedicine. Exploration (Beijing) 2022;2 doi: 10.1002/exp.20210223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsu C.-Y., Rheima A.M., Abbas Z.s., et al. Nanowires properties and applications: a review study. South African Journal of Chemical Engineering. 2023;46:286–311. doi: 10.1016/j.sajce.2023.08.006. [DOI] [Google Scholar]
- 66.García-Carmona L., González M.C., Escarpa A. Vertically-oriented and shape-tailored electrocatalytic metal nanowire arrays for enzyme-free galactosemia rapid diagnosis. Chemistry. 2017;23:9048–9053. doi: 10.1002/chem.201701213. [DOI] [PubMed] [Google Scholar]
- 67.Shen F., Wang J., Xu Z., et al. Rapid flu diagnosis using silicon nanowire sensor. Nano Lett. 2012;12:3722–3730. doi: 10.1021/nl301516z. [DOI] [PubMed] [Google Scholar]
- 68.Ivanov Y.D., Romanova T.S., Malsagova K.A., et al. Use of silicon nanowire sensors for early cancer diagnosis. Molecules. 2021;26 doi: 10.3390/molecules26123734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azam Z., Singh A. Innovative Applications Of Nanowires For Circuit Design; Advances in Computer and Electrical Engineering. 2021. Various applications of nanowires; pp. 17–53. [DOI] [Google Scholar]
- 70.Liu Y., Rahimian A., Krylyuk S., et al. Nanowire aptasensors for electrochemical detection of cell-secreted cytokines. ACS Sens. 2017;2:1644–1652. doi: 10.1021/acssensors.7b00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li D., Chen H., Fan K., et al. A supersensitive silicon nanowire array biosensor for quantitating tumor marker ctDNA. Biosens. Bioelectron. 2021;181 doi: 10.1016/j.bios.2021.113147. [DOI] [PubMed] [Google Scholar]
- 72.Luo Y., Wu L., Tu J., et al. Application of solid-state nanopore in protein detection. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21082808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y., Ai L., Gong Y., et al. Preparation and usage of nanomaterials in biomedicine. Biotechnol. Bioeng. 2023 doi: 10.1002/bit.28472. [DOI] [PubMed] [Google Scholar]
- 74.Kieninger A.K., Forchhammer K., Maldener I. A nanopore array in the septal peptidoglycan hosts gated septal junctions for cell-cell communication in multicellular cyanobacteria. Int J Med Microbiol. 2019;309 doi: 10.1016/j.ijmm.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 75.Sheng Y., You Y., Cao Z., et al. Rapid and selective DNA-based detection of melamine using α-hemolysin nanopores. Analyst. 2018;143:2411–2415. doi: 10.1039/c8an00580j. [DOI] [PubMed] [Google Scholar]
- 76.Bhatti H., Jawed R., Ali I., et al. Recent advances in biological nanopores for nanopore sequencing, sensing and comparison of functional variations in MspA mutants. RSC Adv. 2021;11:28996–29014. doi: 10.1039/d1ra02364k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asandei A., Iftemi S., Mereuta L., et al. Probing of various physiologically relevant metals-amyloid-β peptide interactions with a lipid membrane-immobilized protein nanopore [corrected] J. Membr. Biol. 2014;247:523–530. doi: 10.1007/s00232-014-9662-z. [DOI] [PubMed] [Google Scholar]
- 78.Wang X., Stevens K.C., Ting J.M., et al. Translocation behaviors of synthetic polyelectrolytes through alpha-hemolysin (α-HL) and Mycobacterium smegmatis porin A (MspA) nanopores. J. Electrochem. Soc. 2022;169 doi: 10.1149/1945-7111/ac6c55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang G., Willems K., Soskine M., et al. Electro-osmotic capture and ionic discrimination of peptide and protein biomarkers with FraC nanopores. Nat. Commun. 2017;8:935. doi: 10.1038/s41467-017-01006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu Z.L., Huo M.Z., Ying Y.L., et al. Biological nanopore approach for single-molecule protein sequencing. Angew Chem. Int. Ed. Engl. 2021;60:14738–14749. doi: 10.1002/anie.202013462. [DOI] [PubMed] [Google Scholar]
- 81.Kim N.H., Chae S., Yi S.A., et al. Peptide-assembled single-chain atomic crystal enhances pluripotent stem cell differentiation to neurons. Nano Lett. 2023 doi: 10.1021/acs.nanolett.3c00966. [DOI] [PubMed] [Google Scholar]
- 82.Olorundare F.O.G., Sipuka D.S., Sebokolodi T.I., et al. An electrochemical immunosensor for an alpha-fetoprotein cancer biomarker on a carbon black/palladium hybrid nanoparticles platform. Anal. Methods. 2023 doi: 10.1039/d3ay00702b. [DOI] [PubMed] [Google Scholar]
- 83.Basso C.R., Crulhas B.P., Castro G.R., et al. Recent advances in functional nanomaterials for diagnostic and sensing using self-assembled monolayers. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241310819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang Y., Yan Q., Liu Q., et al. An ultrasensitive sandwich-type electrochemical immunosensor based on the signal amplification strategy of echinoidea-shaped Au@Ag-Cu(2)O nanoparticles for prostate specific antigen detection. Biosens. Bioelectron. 2018;99:450–457. doi: 10.1016/j.bios.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 85.Zhang L., Ying Y., Li Y., et al. Integration and synergy in protein-nanomaterial hybrids for biosensing: strategies and in-field detection applications. Biosens. Bioelectron. 2020;154 doi: 10.1016/j.bios.2020.112036. [DOI] [PubMed] [Google Scholar]
- 86.Xu H., Zhang T., Gu Y., et al. An electrochemical thrombin aptasensor based on the use of graphite-like C(3)N(4) modified with silver nanoparticles. Mikrochim. Acta. 2020;187:163. doi: 10.1007/s00604-020-4111-4. [DOI] [PubMed] [Google Scholar]
- 87.Qin B., Yang K. Voltammetric aptasensor for thrombin by using a gold microelectrode modified with graphene oxide decorated with silver nanoparticles. Mikrochim. Acta. 2018;185:407. doi: 10.1007/s00604-018-2924-1. [DOI] [PubMed] [Google Scholar]
- 88.Tabatabaei M.S., Islam R., Ahmed M. Applications of gold nanoparticles in ELISA, PCR, and immuno-PCR assays: a review. Anal. Chim. Acta. 2021;1143:250–266. doi: 10.1016/j.aca.2020.08.030. [DOI] [PubMed] [Google Scholar]
- 89.Wei X., Wang X., Zhang Z., et al. A click chemistry amplified nanopore assay for ultrasensitive quantification of HIV-1 p24 antigen in clinical samples. Nat. Commun. 2022;13:6852. doi: 10.1038/s41467-022-34273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu Y., Yao Y., Cheong S., et al. Selectively detecting attomolar concentrations of proteins using gold lined nanopores in a nanopore blockade sensor. Chem. Sci. 2020;11:12570–12579. doi: 10.1039/d0sc04552g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yousef H., Liu Y., Zheng L. Nanomaterial-based label-free electrochemical aptasensors for the detection of thrombin. Biosensors. 2022;12 doi: 10.3390/bios12040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Al-Ogaidi I., Gou H., Aguilar Z.P., et al. Detection of the ovarian cancer biomarker CA-125 using chemiluminescence resonance energy transfer to graphene quantum dots. Chem. Commun. 2014;50:1344–1346. doi: 10.1039/c3cc47701k. [DOI] [PubMed] [Google Scholar]
- 93.Bock S., Kim H.M., Kim J., et al. Lateral flow immunoassay with quantum-dot-embedded silica nanoparticles for prostate-specific antigen detection. Nanomaterials. 2021;12 doi: 10.3390/nano12010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shojaeian S., Allameh A., Jeddi-Tehrani M., et al. Quantum dot-labeled tags improve minimal detection limit of CA125 in ovarian cancer cells and tissues. Iran. J. Allergy, Asthma Immunol. 2018;17:326–335. doi: 10.18502/ijaai.v17i4.92. [DOI] [PubMed] [Google Scholar]
- 95.Mo G., He X., Zhou C., et al. A novel ECL sensor based on a boronate affinity molecular imprinting technique and functionalized SiO(2)@CQDs/AuNPs/MPBA nanocomposites for sensitive determination of alpha-fetoprotein. Biosens. Bioelectron. 2019;126:558–564. doi: 10.1016/j.bios.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 96.Dessale M., Mengistu G., Mengist H.M. Nanotechnology: a promising approach for cancer diagnosis, therapeutics and theragnosis. Int. J. Nanomed. 2022;17:3735–3749. doi: 10.2147/ijn.S378074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang Z., Shen B., Yue L., et al. Application of nanomaterials to enhance polymerase chain reaction. Molecules. 2022;27 doi: 10.3390/molecules27248854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dahiya B., Prasad T., Singh V., et al. Diagnosis of tuberculosis by nanoparticle-based immuno-PCR assay based on mycobacterial MPT64 and CFP-10 detection. Nanomedicine (Lond) 2020;15:2609–2624. doi: 10.2217/nnm-2020-0258. [DOI] [PubMed] [Google Scholar]
- 99.Begik O., Diensthuber G., Liu H., et al. Nano3P-seq: transcriptome-wide analysis of gene expression and tail dynamics using end-capture nanopore cDNA sequencing. Nat. Methods. 2023;20:75–85. doi: 10.1038/s41592-022-01714-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang C., Xiu L., Li Y., et al. Multiplex PCR and nanopore sequencing of genes associated with antimicrobial resistance in Neisseria gonorrhoeae directly from clinical samples. Clin. Chem. 2021;67:610–620. doi: 10.1093/clinchem/hvaa306. [DOI] [PubMed] [Google Scholar]
- 101.Sun H., Yao F., Su Z., et al. Hybridization chain reaction (HCR) for amplifying nanopore signals. Biosens. Bioelectron. 2020;150 doi: 10.1016/j.bios.2019.111906. [DOI] [PubMed] [Google Scholar]
- 102.Wang Y., Zheng D., Tan Q., et al. Nanopore-based detection of circulating microRNAs in lung cancer patients. Nat. Nanotechnol. 2011;6:668–674. doi: 10.1038/nnano.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma X. Isothermal amplification technology for diagnosis of COVID-19: current status and future prospects. Zoonoses. 2022;2 doi: 10.15212/zoonoses-2021-0022. [DOI] [Google Scholar]
- 104.Li X., To K.K.-W., Yuen K.-Y. Variants of SARS coronavirus-2 and their potential impact on the future of the COVID-19 pandemic. Zoonoses. 2021;1 doi: 10.15212/zoonoses-2021-1003. [DOI] [Google Scholar]
- 105.Lee C.Y., Degani I., Cheong J., et al. Development of integrated systems for on-site infection detection. Acc. Chem. Res. 2021;54:3991–4000. doi: 10.1021/acs.accounts.1c00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pereira R.L., Oliveira D., Pêgo A.P., et al. Electrochemical miRNA-34a-based biosensor for the diagnosis of Alzheimer's disease. Bioelectrochemistry. 2023;154 doi: 10.1016/j.bioelechem.2023.108553. [DOI] [PubMed] [Google Scholar]
- 107.Chiu M.J., Chen T.F., Hu C.J., et al. Nanoparticle-based immunomagnetic assay of plasma biomarkers for differentiating dementia and prodromal states of Alzheimer's disease - a cross-validation study. Nanomedicine. 2020;28 doi: 10.1016/j.nano.2020.102182. [DOI] [PubMed] [Google Scholar]
- 108.Hamd-Ghadareh S., Salimi A., Parsa S., et al. Development of three-dimensional semi-solid hydrogel matrices for ratiometric fluorescence sensing of Amyloid β peptide and imaging in SH-SY5 cells: improvement of point of care diagnosis of Alzheimer's disease biomarker. Biosens. Bioelectron. 2022;199 doi: 10.1016/j.bios.2021.113895. [DOI] [PubMed] [Google Scholar]
- 109.Mobed A., Razavi S., Ahmadalipour A., et al. Biosensors in Parkinson's disease. Clin. Chim. Acta. 2021;518:51–58. doi: 10.1016/j.cca.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 110.Masud M.K., Na J., Younus M., et al. Superparamagnetic nanoarchitectures for disease-specific biomarker detection. Chem. Soc. Rev. 2019;48:5717–5751. doi: 10.1039/c9cs00174c. [DOI] [PubMed] [Google Scholar]
- 111.Liu Y., Wang H., Xiong C., et al. A sensitive electrochemiluminescence immunosensor based on luminophore capped Pd@Au core-shell nanoparticles as signal tracers and ferrocenyl compounds as signal enhancers. Biosens. Bioelectron. 2016;81:334–340. doi: 10.1016/j.bios.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 112.Nossier A.I., Shehata N.I., Morsy S.M., et al. Determination of certain urinary microRNAs as promising biomarkers in diabetic nephropathy patients using gold nanoparticles. Anal. Biochem. 2020;609 doi: 10.1016/j.ab.2020.113967. [DOI] [PubMed] [Google Scholar]
- 113.Yang J., Huang L., Qian K. Nanomaterials-assisted metabolic analysis toward in vitro diagnostics. Exploration (Beijing) 2022;2 doi: 10.1002/exp.20210222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang Z., Wang L., Zhang J., et al. Application of bismuth sulfide based nanomaterials in cancer diagnosis and treatment. Nano Today. 2023;49 doi: 10.1016/j.nantod.2023.101799. [DOI] [Google Scholar]
- 115.Tang M., Zhang Z., Sun T., et al. Manganese-based nanozymes: preparation, catalytic mechanisms, and biomedical applications. Adv Healthc Mater. 2022;11 doi: 10.1002/adhm.202201733. [DOI] [PubMed] [Google Scholar]
- 116.Landowski L.M., Niego B., Sutherland B.A., et al. Applications of nanotechnology in the diagnosis and therapy of stroke. Semin. Thromb. Hemost. 2020;46:592–605. doi: 10.1055/s-0039-3399568. [DOI] [PubMed] [Google Scholar]
- 117.Kammona O., Tsanaktsidou E. Nanotechnology-aided diagnosis, treatment and prevention of leishmaniasis. Int J Pharm. 2021;605 doi: 10.1016/j.ijpharm.2021.120761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no additional data available for this review.