Abstract
Background
Intracranial dissecting aneurysms present a challenging subset linked to considerable morbidity and mortality, necessitating effective therapeutic strategies to prevent complications. Traditional treatments face technical limitations, while emerging endovascular modalities like stent-assisted coiling, multiple stenting, and flow-diverting devices (FDDs) offer promise in reducing periprocedural risks and enhancing patient outcomes. The aim of this study is to compare the safety and efficacy outcomes of endovascular treatment for intracranial dissecting aneurysms using FDDs versus stents (with or without coiling).
Methods
A systematic review and meta-analysis were conducted following established guidelines. The search included PubMed, Scopus, Web of Science, and Embase databases up to July 30, 2023. Eligible studies reporting outcomes of interest in both FDD and stent-treated groups were included, and the data was extracted and analyzed using STATA software.
Results
Six studies were analyzed, involving 131 patients in the FDD group and 199 patients in the stent group. The pooled rates for favorable functional outcomes (86.8% vs. 86%), mortality (3.9% vs. 6%), adequate occlusion (79.7% vs. 86.3%), aneurysm recurrence (1.3% vs. 13.3%), in-stent stenosis/thrombosis (7% vs. 6.9%), ischemic events/infarctions (6.7% vs. 7.8%), retreatment (7% vs. 8.6%), and technical success (100% vs. 98.7%) were comparable in individuals treated with FDDs and stents (p > 0.05 in all cases). Additionally, complete occlusion rates were not significantly different between FDD (62.7%) and stent-treated patients (75.2%) (p = 0.06). However, after excluding one study in a leave-one-out analysis of the random effects meta-analysis, a significant difference in the pooled rates of this outcome was observed between the FDD (59.2%) and stent (75.2%) groups (p = 0.034).
Conclusion
FDDs present a promising approach for the treatment of intracranial dissecting aneurysms, yielding outcomes that are roughly comparable to stent-based methods. However, the absence of randomized trials and data limitations highlight the need for further research to enhance treatment strategies.
Keywords: Flow diverters, stents, aneurysm, dissection, safety, efficacy
Introduction
Intracranial dissecting aneurysms constitute a distinct and challenging subset of intracranial aneurysms associated with considerable morbidity and mortality, particularly when rupture occurs.1,2 These aneurysms manifest as a disruption of the arterial wall, leading to the formation of an intramural hematoma and variable luminal changes, which can result in devastating consequences such as subarachnoid hemorrhage, ischemic stroke, and other neurological deficits. It is imperative to note the differentiation between subintimal and subadventitial dissections. Subintimal dissections, characterized by the separation of the intimal layer from the media, often lead to intramural hematoma formation, potentially causing luminal compromise through stenosis or thrombosis. The risk of rupture is heightened due to aneurysmal dilatation resulting from arterial wall weakening. On the other hand, subadventitial dissections, occurring between the adventitial layer and surrounding tissue, may not directly affect the vessel lumen but can induce external compression, leading to luminal compromise and pseudoaneurysm formation. This increases the risk of rupture by disrupting vessel wall integrity. Understanding these differences is crucial for tailoring treatment approaches and improving outcomes in the management of intracranial dissecting aneurysms.3–5
Furthermore, understanding the differentiation between anterior and posterior circulation is pivotal in grasping the diverse manifestations of intracranial dissecting aneurysms. For instance, the V4 segment of the vertebral artery, located in the posterior circulation, exhibits distinct characteristics in terms of wall architecture. This segment is particularly noteworthy for having the weakest wall architecture among cerebral arteries, characterized by thinner mural layers compared to other intracranial arteries such as the middle cerebral artery, anterior cerebral artery, posterior cerebral artery, and basilar artery. This structural variation predisposes the V4 segment to increased vulnerability to dissection and rupture. The differences in wall architecture have significant implications for the management of intracranial dissecting aneurysms.6–9
Traditionally, the treatment of intracranial dissecting aneurysms has been technically challenging. Clipping is often unfeasible due to the broad and often shallow anatomical characteristics of these aneurysms, and endovascular interventions carry the risk of increased morbidity and mortality.8,9 Consequently, in the process of selecting the most appropriate endovascular intervention, factors such as the dissecting aneurysm's location, its influence on critical branches, and the overall anatomical intricacy take precedence.7–9
Various endovascular treatment modalities have emerged in recent years as potential solutions to reduce periprocedural complications associated with intracranial dissecting aneurysms, aiming to enhance patient outcomes. These modalities include stent-assisted coil embolization, multiple stenting, and flow-diverting devices (FDDs).1,2 In this context, braided stents offer support to the arterial wall and prevent further dissection progression by acting as scaffolding. Additionally, laser-cut stents, characterized by precise patterns, provide effective coverage and flow diversion, contributing to dissecting aneurysm isolation and reconstruction.10–12 On the other hand, FDDs have shown promise in treating complex intracranial dissecting aneurysms by redirecting blood flow away from the aneurysm sac, inducing thrombosis, and promoting natural healing, ultimately isolating and sealing off the dissecting aneurysm.6,7 Understanding these differences is crucial for selecting the most appropriate intervention tailored to the specific characteristics of the dissecting aneurysm, thereby optimizing treatment outcomes.
By synthesizing and evaluating the existing body of evidence, the primary objective of this systematic review and meta-analysis is to offer a comparison of safety and efficacy outcomes pertaining to the endovascular treatment of intracranial dissecting aneurysms using flow diverters as opposed to stents.
Methods
This systematic review has been reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement guidelines. 13 A thorough literature review was conducted on July 30, 2023, encompassing four major databases: PubMed, Scopus, Web of Science, and Embase. For each database, specific search terms were crafted, comprising (“intracranial aneurysm*” OR “cerebral aneurysm*” OR “brain aneurysm*” OR “anterior circulation aneurysm*” OR “posterior circulation aneurysm*”) AND (“dissect*”) AND (“flow diverter” OR “flow diversion” OR “Pipeline” OR “FRED” OR “Tubridge” OR “SILK” OR “Surpass”). Additionally, a manual examination of references cited within the included studies was performed to ensure that no relevant papers were inadvertently overlooked. The assessment process involved a meticulous review of each article's title, abstract, and, the full text. Two co-authors carried out this review independently, with any uncertainties or ambiguities resolved through consultation with a senior co-author. The AutoLit platform, developed by Nested Knowledge in St Paul, Minnesota, USA, was utilized for deduplication, screening, and data extraction.
The inclusion criteria encompassed studies that examined treatment outcomes for intracranial dissecting aneurysms using FDDs and compared these outcomes with treatments involving stent placement (with or without coiling). These criteria were applied without restrictions on publication date, country of origin, study design, location of intracranial aneurysms, or their rupture status. The exclusion criteria included non-English literature, case reports, case series with fewer than five eligible patients, conference abstracts, editorial comments, and review articles.
Pertinent data were extracted from eligible studies, including the first author's name, publication year, study design, patients’ demographic and clinical characteristics, type of FDD and stent employed, adjunctive endovascular therapy, and safety and efficacy treatment outcomes.
Within our analysis, a modified Rankin Scale score of 2 or lower was considered indicative of a favorable functional outcome at the last clinical follow up, while adequate occlusion was defined as complete aneurysm occlusion along with a neck remnant, which was determined based on either O'Kelly-Marotta grades C and D or Raymond-Roy Occlusion Classification grades I and II,14,15 as assessed during the most recent follow-up angiography for each individual aneurysm.
To assess the methodological quality of the studies included in the analysis, the Joanna Briggs Institute's (JBI) tool for the critical appraisal of quasi-experimental studies was employed. 16 This structured tool played a pivotal role in systematically evaluating various dimensions of each study, including study design, group selection, comparability of groups, management of potential confounders, and the robustness of data collection and analysis methods. Incorporating the JBI tool into our meta-analysis ensured a comprehensive and consistent evaluation of internal validity and methodological soundness, thus enhancing the overall rigor and reliability of the evidence synthesis.
The certainty of the meta-analysis results for each specific outcome was rigorously evaluated using the GRADE methodology. 17 To facilitate this assessment, a summary of findings table was generated via GRADEpro GDT web software. 18 This evaluation focused on key factors such as methodological quality, precision, and potential publication bias to determine the certainty of each finding.
Statistical analysis
Data on the incidence of outcomes within both the FDD and stent groups were collected. Given the methodological heterogeneity among the included studies, a random-effects model for all the meta-analyses was adopted. An odds ratio (OR) meta-analysis was conducted to assess differences in outcomes between the FDD and stent groups. This analysis utilized the random-effects restricted maximum likelihood model, and the effect sizes were represented as ORs on forest plots. The level of heterogeneity was assessed using both the I-square statistic and Cochran's Q chi-squared test. A low level of heterogeneity was defined as an I-square value of 25% or lower, a moderate level as values near 50%, and a high level as I-squared values of 75% or higher. 19
A covariate of interest in this meta-analysis was the duration of follow up, which, as per a priori hypothesis, was presumed to significantly influence the reported outcomes. However, the limited number of studies included prevented a methodological evaluation of this variable's impact on the reported outcomes using meta-regression techniques. Nevertheless, data on the approximate mean follow-up time, expressed in months, was provided in each forest plot. Additionally, the study-specific effect sizes in these plots were organized based on follow-up time. This organization facilitated a subjective visual assessment of the trend in how follow-up duration may influence the observed outcomes.
Moreover, a leave-one-out analysis was carried out to investigate the potential influence of individual studies on the overall results. To assess publication bias, funnel plots were employed to identify any indications of asymmetry. Egger's regression-based test was not employed to assess asymmetry in the funnel plots because it is not recommended for meta-analyses with fewer than ten included studies for the outcomes. 20
All statistical analyses were conducted using STATA software (Version 17.0, Stata Corp, College Station, TX), and the built-in “meta” command in STATA was utilized for these analyses.
Results
Screening and selection of articles
During the systematic literature search, a predefined search strategy identified an initial pool of 934 articles. After eliminating duplicate records, 409 papers were subjected to screening based on their title and abstract, leading to the exclusion of 384 articles. Subsequently, the full text of the remaining 25 papers was obtained and reviewed. Following this evaluation, 19 articles that did not align with the study's objectives were excluded. In the end, six articles that met the predefined inclusion criteria were identified and included in the analysis. The screening process and the application of eligibility criteria are visually summarized in Figure 1, following PRISMA guidelines.
Figure 1.
PRISMA flow diagram showing the review process. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study characteristics
In this review, 6 studies were included involving 131 patients in the FDD group and 199 patients in the stent group, all diagnosed with intracranial dissecting aneurysms. These studies, published between 2019 and 2023, employed a retrospective cohort design. Geographically, half of these studies originated from China, with the others conducted in the United States, Germany, and South Korea. Supplemental Table 1 provides a comprehensive summary of these studies.
The baseline characteristics of the included studies vary considerably, reflecting a heterogeneous patient demographic and clinical settings. Catapano et al. 1 reported an average patient age of 53 years, with a balanced gender distribution (47% female). The study predominantly involved patients treated with devices like the Pipeline and Tubridge for dissecting aneurysms located mainly in the vertebral artery segments. Similarly, Cho et al. 2 described a cohort with a mean age of approximately 52.6 years, where 43% were female, highlighting prevalent comorbidities such as hypertension (44%) and diabetes (12%). Their subjects predominantly had aneurysms in the intracranial segment of the vertebral artery, treated with devices like LVIS Blue and Enterprise. Additionally, Kaschner et al. 6 provided data on a smaller cohort with ages averaging between 46.6 years for the FDD group and 50.3 years for the stent group, with a focus on aneurysms located in the V4 segment of the vertebral artery. Li et al. 7 noted a similar age range and also highlighted comorbid conditions like hypertension, which was present in 29% of the stent group and 36% of the FDD group. The majority of their patients had aneurysms exclusively in the intracranial vertebral artery. Further, Wu et al. 8 reported on the oldest cohort with mean ages of 58.3 years for the stent group and 55.1 years for the FDD group, reporting a significant presence of lifestyle risk factors such as alcohol abuse and smoking. The aneurysms were primarily located in the intracranial vertebral and basilar arteries. Lastly, Zhang et al. 9 examined a relatively younger FDD group (mean age 47.1 years) compared to the stent group (mean age 55.8 years), with a substantial proportion having aneurysms larger than 10 mm.
Among the included studies, the FDD group primarily utilized devices such as the Pipeline (Medtronic, USA) and Tubridge (MicroPort, China) flow diverters, while the stent group employed various stents, including LVIS Blue (Microvention, Tustin, CA, USA), Enterprise (Codman & Shurtleff, Raynham, MA, USA), Neuroform (Stryker, Kalamazoo, Michigan, USA), and Solitaire (Covidien, Irvine, CA, USA). Coiling was predominantly used in the stent group across these studies. None of the studies employed a randomization method for patient allocation between the stent and FDD groups. The decision to use FDD or stents was influenced by several factors, including patient preferences and healthcare provider opinions. Most studies reported dual antiplatelet therapy as their primary regimen.6–9 However, two studies did not specify their antiplatelet therapy regimens.1,2 Notably, two studies reported longer antiplatelet therapy durations in the FDD group, which could potentially confound and affect follow-up bleeding and ischemic rates in this subgroup.8,9
The intracranial vertebral artery, particularly the V4 segment, was the most frequently studied site for dissecting aneurysms, with two studies specifically focusing on intracranial vertebral arteries.2,7 The basilar artery and vertebrobasilar junction were other common sites for ruptured aneurysms in the studies. Importantly, the study by Catapano et al. 1 reported that all included aneurysms were in dominant vertebral artery whereas in the study by Cho et al., 2 only 26% of included aneurysms were in the dominant vertebral artery in the overall study cohort. The information regarding the dominancy of affected vertebral artery was not specifically reported in the rest of the included studies.
Outcomes in the studies were predominantly based on follow-up data, hence the duration of follow up was extracted separately for imaging and clinical outcomes. Retreatment rates, favorable functional outcomes, and mortality rates were derived from clinical follow up, while complete and adequate occlusion rates, recurrence rates, and ischemic event rates were based on imaging outcomes. The mean clinical follow-up times were notably long, generally exceeding 24 months in all studies, except for Kaschner et al., 6 which reported a mean follow up of 17.1 months in the stent group and 12 months in the FDD group. Conversely, imaging follow-up times were shorter in most studies, with mean or median times less than or around one year in three studies.6,8,9 Notably, the study by Catapano et al. did not specify the duration of imaging follow up. 1
Quality assessment
Supplemental Table 2 provides a summary of the risk of bias assessment results using the JBI tool. It is noteworthy that concerns about bias related to confounding factors were prevalent across all studies. This is primarily attributed to the retrospective nature of the studies and the absence of a randomization process, leading to a lack of control over potential confounding variables. Allocation decisions in the studies were influenced by various factors, including patient and medical team preferences, as well as considerations such as the type and location of the dissecting aneurysm, its severity, and baseline risk factors, all of which acted as potential confounders. Additionally, in two studies,8,9 there were differences in antiplatelet regimen protocols between the FDD and stent groups, representing another significant potential confounder.
The outcomes of the GRADE assessment are presented in the Supplemental Table 3. Notably, the certainty grade assigned to each assessed outcome was “very low” according to the GRADE methodology. This classification primarily arises from the low methodological quality of the included nonrandomized studies, coupled with small study populations. The small study population contributed to wide confidence intervals for the reported outcomes, indicative of low statistical power.
Quantitative synthesis
-
Favorable functional outcome
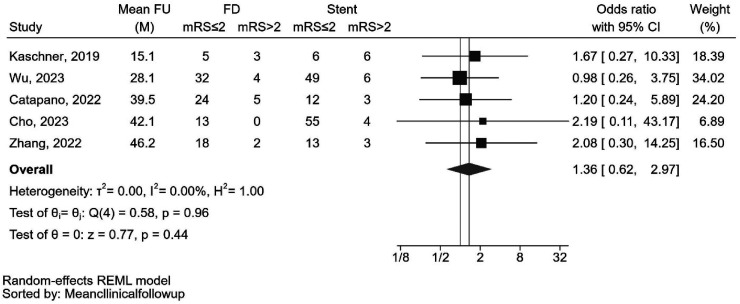
The pooled rate of favorable functional outcomes was 86.8% (92 out of 106 cases) for individuals treated with FDDs and 86.0% (135 out of 157 cases) for those treated with stents. The analysis showed that the reported rates of this outcome did not significantly differ between patients treated with FDDs or stents (OR: 1.36 [0.62, 2.97], p = 0.44). Minimal heterogeneity was observed for this outcome (I2 < 0.01%, Cochran's Q test: p = 0.96) (Figure 2). As illustrated in the forest plot, the follow-up durations exhibited significant variation among the included studies. One study reported a relatively short clinical follow-up duration of only 15 months, while others had longer mean durations ranging between 28 and 46 months. A leave-one-out analysis was conducted to evaluate the influence of excluding individual studies, but the results remained nonsignificant. A funnel plot assessing publication bias can be found in Supplemental Figure 1.
-
Mortality
The pooled mortality rate was 3.9% (4 out of 102 cases) for individuals who underwent FDD treatment, and 6.0% (11 out of 184 cases) for those treated with stents. The mortality rate did not show a significant difference between patients treated with FDDs or stents (OR: 0.79 [0.26, 2.39], p = 0.68). Minimal heterogeneity was observed for this outcome (I2 < 0.01%, Cochran's Q test: p = 0.90) (Figure 3). While the follow-up periods varied considerably among the included studies, including one study with a notably shorter follow-up time, a visual assessment of the forest plot does not indicate any discernible trend of effect concerning clinical follow-up duration. An analysis assessing the impact of excluding individual studies was conducted through a leave-one-out analysis, but the results remained nonsignificant. A funnel plot assessing publication bias is available in Supplemental Figure 2.
-
Complete occlusion
In cases treated with FDDs, complete occlusion was achieved in 62.7% (37 out of 59 cases), while cases treated with stents demonstrated a pooled complete occlusion rate of 75.2% (94 out of 125 cases). Nevertheless, the analysis indicated that the complete occlusion rate did not significantly differ between patients treated with FDDs or stents (OR: 0.50 [0.24, 1.04], p = 0.06). Heterogeneity for this outcome was low (I2 = 2.73%, Cochran's Q test: p = 0.26) (Figure 4). To evaluate the impact of excluding individual studies, a leave-one-out analysis was conducted. The forest plot in Figure 5 revealed that, after excluding Kaschner et al.'s study, 6 a significant difference in reported complete occlusion rates was observed between the two groups (OR: 0.44 [0.21, 0.94], p = 0.034). Significantly, the study conducted by Kaschner et al. had a notably low and potentially insufficient mean follow-up time of 6.7 months. A funnel plot assessing publication bias can be found in Supplemental Figure 3.
-
Adequate occlusion
Adequate occlusion was attained in 79.7% of cases (55 out of 69) treated with FDDs and 86.3% of cases (113 out of 131) treated with stents. However, the pooled rate of achieving adequate occlusion did not significantly differ between patients treated with FDDs or stents (OR: 0.61 [0.27, 1.36], p = 0.23). Heterogeneity for this outcome was minimal (I2 < 0.01%, Cochran's Q test: p = 0.23) (Supplemental Figure 4). To assess the impact of excluding individual studies, a leave-one-out analysis was conducted, but the results remained inconclusive. A funnel plot examining publication bias can be found in Supplemental Figure 5. The visual assessment of follow-up periods in the included forest plots did not reveal any potential trend for the effect of follow-up duration.
-
Aneurysm recurrence
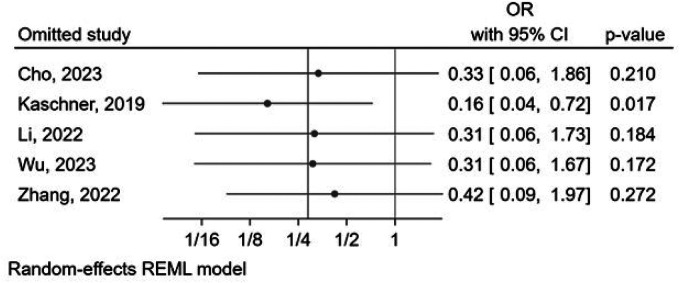
Aneurysm recurrence occurred in 1.3% (1 out of 75 cases) of patients treated with FDDs and in 13.3% (19 out of 143 cases) of those treated with stents. Although not reaching statistical significance (p = 0.07), there was a trend lower rate of aneurysm recurrence in those treated by FDDs compared to those treated by stents (OR: 0.29 [0.07, 1.13], p = 0.07). Heterogeneity for this outcome was minimal (I2 < 0.01%, Cochran's Q test: p = 0.41) (Figure 6). Importantly, the leave-one-out analysis revealed that after excluding Kaschner et al.'s study, 6 a significant difference in reported aneurysm recurrence rates could be observed between the two groups (OR: 0.16 [0.04, 0.72], p = 0.017), as presented in Figure 7. A funnel plot assessing publication bias is available in Supplemental Figure 6. Upon visual assessment, there was no discernible trend in the effect of follow-up duration based on the included forest plots.
-
In-stent stenosis/thrombosis
Among cases treated with FDDs, in-stent stenosis or thrombosis was observed in 7.0% (6 out of 86 cases), while cases treated with stents exhibited a pooled rate of 6.9% (7 out of 102 cases). The analysis revealed no significant difference in the pooled rate of this outcome between patients treated with FDDs or stents (OR: 1.25 [0.42, 3.77], p = 0.69). Heterogeneity for this outcome was minimal (I2 < 0.01%, Cochran's Q test: p = 0.78) (Supplemental Figure 7). The leave-one-out analysis yielded nonsignificant results. Supplemental Figure 8 shows a funnel plot assessing publication bias. The visual assessment of follow-up periods in the provided forest plots did not indicate any apparent trend for the effect of follow-up duration.
-
Ischemic event/infarction
Ischemic events or infarction were observed in 6.7% (7 out of 104 cases) of patients treated with FDDs and in 7.8% (9 out of 115 cases) of those treated with stents. The pooled rate of ischemic events/infarction did not significantly differ between patients treated with FDDs or stents (OR: 0.81 [0.24, 2.79], p = 0.74). Heterogeneity for this outcome was low (I2 = 14.76%, Cochran's Q test: p = 0.35) (Supplemental Figure 9). The results of a leave-one-out analysis remained nonsignificant. A funnel plot assessing publication bias can be found in Supplemental Figure 10. The visual assessment of follow-up periods in the included forest plots did not reveal any potential trend for the effect of follow-up duration.
-
Retreatment
The pooled rate of retreatment was 7.0% (6 out of 86 cases) for patients treated with FDDs and 8.6% (10 out of 116 cases) for those treated with stents. However, the retreatment rate did not exhibit a significant difference between patients treated with FDDs or stents (OR: 0.61 [0.15, 2.41], p = 0.48). Heterogeneity for this outcome was low (I2 = 25.64%, Cochran's Q test: p = 0.28) (Supplemental Figure 11). A leave-one-out analysis was conducted but the results remained nonsignificant. A funnel plot assessing publication bias is available in Supplemental Figure 12. The visual assessment of follow-up periods in the included forest plots did not reveal any discernible trend for the effect of follow-up duration.
-
Technical success
The pooled rate of technical success was 100% (55 out of 55 cases) for patients treated with FDDs and 98.7% (75 out of 76 cases) for those treated with stents. There was no significant difference in technical success rates between patients treated with FDDs or stents (OR: 1.30 [0.15, 11.05], p = 0.81). Heterogeneity for this outcome was low (I2 < 0.01%, Cochran's Q test: p = 0.85) (Supplemental Figure 13). The results of a leave-one-out analysis remained nonsignificant. Supplemental Figure 14 presents a funnel plot assessing publication bias in.
Figure 2.
Forest plot of the random-effects meta-analysis for reported favorable functional outcome rates in patients with dissecting aneurysms undergoing endovascular treatment with flow diverter devices compared to stents. CI: confidence interval; FD: flow diverter; FU: follow up; M: months; mRS: modified Rankin score; OR: odds ratio.
Figure 3.
Forest plot of the random-effects meta-analysis for reported mortality rates in patients with dissecting aneurysms undergoing endovascular treatment with flow diverter devices compared to stents. CI: confidence interval; FD: flow diverter; FU: follow up; M: months; mRS: modified Rankin score; OR: odds ratio.
Figure 4.
Forest plot of the random-effects meta-analysis for reported complete occlusion rates in patients with dissecting aneurysms undergoing endovascular treatment with flow diverter devices compared to stents. CI: confidence interval; FD: flow diverter; FU: follow up; M: months; mRS: modified Rankin score; OR: odds ratio.
Figure 5.
Forest plot of the leave-one-out analysis of the random-effects meta-analysis for reported complete occlusion rates in patients with dissecting aneurysms undergoing endovascular treatment with flow diverter devices compared to stents. CI: confidence interval; FD: flow diverter; FU: follow up; M: months; mRS: modified Rankin score; OR: odds ratio.
Figure 6.
Forest plot of the random-effects meta-analysis for reported recurrence rates in patients with dissecting aneurysms undergoing endovascular treatment with flow diverter devices compared to stents. CI: confidence interval; FD: flow diverter; FU: follow up; M: months; mRS: modified Rankin score; OR: odds ratio.
Figure 7.
Forest plot of the leave-one-out analysis of the random-effects meta-analysis for reported recurrence rates in patients with dissecting aneurysms undergoing endovascular treatment with flow diverter devices compared to stents. CI: confidence interval; FD: flow diverter; FU: follow up; M: months; mRS: modified Rankin score; OR: odds ratio.
Discussion
This systematic review and meta-analysis demonstrated that employing FDDs for treating intracranial dissecting aneurysms yielded similar rates for most safety and efficacy outcomes compared to stent-assisted coiling and standalone stenting.
Intracranial dissecting aneurysms can be treated through surgical or endovascular methods, with a growing preference for the latter due to reduced surgical risks.20,21 Endovascular treatment, less invasive in nature, involves reconstructing and blocking affected arteries. When considering endovascular approaches, the optimal strategy for preventing re-rupture particularly in the context of dissecting vertebral artery and posterior inferior cerebellar artery (PICA) aneurysms is endovascular trapping or sacrifice of the diseased segment. This approach has a strong rationale but carries the highest risk of ischemic stroke related to perforators in the treated segment or in the PICA territory.1,2 Stent deployment alone can partially seal the aneurysm's intimal tear, aiding healing but potentially falling short in preventing recurrence or rebleeding. 22 Stent-assisted coiling offers added benefits by reconstructing the parent artery, maintaining patency, and embolizing the aneurysm, lowering rebleeding risk. However, aneurysm recurrence remains a challenge. The use of multiple stents during coiling potentially enhances flow diversion and hemodynamics, reducing rupture and recurrence risk, but it raises the risk of periprocedural ischemic events and poses technical challenges for dissecting aneurysms involving perforating arteries.23–25
Flow diverters have enhanced the treatment of complex dissecting intracranial aneurysms through endovascular reconstruction and vascular remodeling. These devices have significantly improved treatment outcomes by reducing aneurysm porosity, diverting blood flow from the aneurysm sac, promoting thrombosis formation, and providing a physical scaffold at the neck to stimulate neointimal tissue growth.26–29
The present study initially showed no significant difference in the complete occlusion rate between patients treated with FDDs and those treated with stents. However, upon excluding Kaschner et al.'s study 6 from the analysis, a significant difference was observed between the two groups. The Kaschner et al.'s study was the sole study in our analysis with a majority of patients having ruptured aneurysms, which appeared to have a notable impact on the overall observed effect based on a leave-one-out analysis. It is important to highlight that the degree of complete occlusion tends to increase with the duration of follow-up in the context of flow diversion treatment for cerebral aneurysms. Furthermore, several factors can influence the occlusion rate. Firstly, the specific shape of dissecting aneurysms and the size of the aneurysm neck may impact the endothelialization process of the FDD. 30 Secondly, using overlapping FDDs can affect the extent of metal coverage, potentially enhancing thrombosis within the aneurysm cavity and achieving complete aneurysm occlusion.31,32 Additionally, the decision to employ adjunctive coiling following the deployment of a FDD without achieving the desired occlusion can also influence aneurysm thrombosis and complete occlusion. 7 These factors can account for variations in the degree of complete occlusion observed in different studies.
Rebleeding following the placement of an FDD may be associated with the use of antiplatelet medications both before and after the procedure, aimed at minimizing the risk of thrombotic ischemic events.33–35 However, recent advancements in intravascular FDDs incorporating Shield Technology have enhanced the treatment approach for wide-necked intracranial aneurysms. This technology builds on the established efficacy of the Pipeline embolization device and introduces an innovative implant surface modification. The design aims to improve flow diversion therapy, resulting in reduced thrombogenicity and the requirement for only a single antiplatelet agent. This innovative development holds promise for significantly decreasing rebleeding rates in cases involving such aneurysms.36,37
Our study results suggest that patients treated with FDDs are less prone to aneurysm recurrence compared to those treated with traditional stents, highlighting the promising outcomes associated with this approach. However, it is important to interpret these findings considering the study's limitations. Further well-designed prospective studies with larger sample sizes and longer follow-up periods are necessary to validate these results and uncover the underlying mechanisms behind the observed differences in recurrence rates between FDDs and traditional stents.
The majority of the included aneurysms in our study were situated in the posterior circulation. To date, there exists only one meta-analysis that compares the treatment outcomes of posterior circulation aneurysms with FDDs versus stent-assisted coiling. 38 The results suggest comparable efficacy in terms of occlusion rate and favorable clinical outcomes between FDDs and stent-assisted coiling. The study identified stroke as the most prevalent complication, regardless of the treatment modality used, with stent-assisted coiling exhibiting a lower periprocedural complication rate. However, this study did not specifically focus on dissecting aneurysms; instead, it included all nonsaccular aneurysms, including fusiform, dissecting, fusiform dissecting, dolichoectatic, and transitional types.
Currently, no randomized clinical trials establish the superiority of FDDs over other endovascular techniques for treating intracranial dissecting aneurysms. Nevertheless, the findings from this meta-analysis consistently affirm that FDDs represent a safe and effective treatment choice for these aneurysms, demonstrating comparable outcomes to stents across most variables examined in our study. However, it is important to consider that tailoring different techniques to specific circumstances may be the most practical approach for the future management of both unruptured and ruptured intracranial dissecting aneurysms.
Acknowledging the restricted data provided by the included studies, conducting subgroup analyses, particularly regarding the aneurysm rupture status, specific intracranial locations, and the individual types of FDDs and stents, proved unfeasible which constitutes a significant limitation of the current study. Additionally, the retrospective, single-center nature of these studies constrained the generalizability of the findings and potentially introduced confounding or selection bias. Further studies evaluating dissecting aneurysm treatment with common FDDs featuring antithrombogenic coatings, as well as with newer devices like the Pipeline Flex Shield, the p64MH HPC, and the Derivo Heal, and comparing the outcomes with other endovascular treatments are warranted to provide additional evidence for selecting the optimal treatment in this context.
Conclusion
FDDs present a promising treatment option for intracranial dissecting aneurysms, offering safety and efficacy outcomes comparable to those of stent-assisted coiling and standalone stenting. However, the absence of randomized clinical trials and limitations in available data emphasize the imperative for additional research to address methodological constraints and enhance the management of these intricate aneurysms.
Supplemental Material
Supplemental material, sj-docx-1-ine-10.1177_15910199241262070 for Comparative meta-analysis of endovascular strategies for intracranial dissecting aneurysms: Flow diverters versus stents with or without coiling by Melika Amoukhteh, Amir Hassankhani, Payam Jannatdoust, Parya Valizadeh, Sherief Ghozy, Cem Bilgin and David F Kallmes in Interventional Neuroradiology
Acknowledgements
We express our gratitude to the developers of Nested Knowledge, namely Karl Holub, Stephen Mead, Jeff Johnson, and Darian Lehmann-Plantenberg, for their invaluable contributions that facilitated our study. Their work on the AutoLit and Synthesis platforms for systematic review enhanced the efficiency of our research. Additionally, we would like to acknowledge the support provided by ChatGPT, an OpenAI language model based on the GPT-3.5 architecture. The model contributed to refining the language and enhancing the manuscript's readability during the editing process. It is important to emphasize that the authors bear full responsibility for the content of this publication, as they thoroughly reviewed and edited the material after utilizing the tool.
Footnotes
Contributors: The inception of the project was a collaborative effort by all authors. During the subsequent revision stages, DFK, SG and CB made significant scientific contributions, offering valuable insights. MA and AH were instrumental in conducting database searches, extracting data, drafting, and critically revising the content to ensure the incorporation of essential intellectual contributions. PV and PJ played pivotal roles in data analysis and interpretation. The final manuscript was thoroughly reviewed and approved by all authors.
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DFK holds equity in Nested Knowledge, Superior Medical Editors, Conway Medical, Marblehead Medical, and Piraeus Medical. He receives grant support from MicroVention, Medtronic, Balt, and Insera Therapeutics. Additionally, he has served on the Data Safety Monitoring Board for Vesalio and has received royalties from Medtronic.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Amir Hassankhani https://orcid.org/0000-0002-0529-5341
Sherief Ghozy https://orcid.org/0000-0001-5629-3023
Cem Bilgin https://orcid.org/0000-0002-6243-9495
David F Kallmes https://orcid.org/0000-0002-8495-0040
Supplemental material: Supplemental material for this article is available online.
References
- 1.Catapano JS, Ducruet AF, Cadigan MS, et al. Endovascular treatment of vertebral artery dissecting aneurysms: a 20-year institutional experience. J Neurointerv Surg 2022; 14: 257–261. [DOI] [PubMed] [Google Scholar]
- 2.Cho WC, Lee HJ, Choi JH, et al. Clinical and radiological outcomes of vertebral artery dissecting aneurysms treated with endovascular treatments: a 12-year single-center experience. World Neurosurg 2023; 175: e904–e913. [DOI] [PubMed] [Google Scholar]
- 3.Krings T, Choi I-S. The many faces of intracranial arterial dissections. Interv Neuroradiol 2010; 16: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander MD, Yuan C, Rutman A, et al. High-resolution intracranial vessel wall imaging: imaging beyond the lumen. J Neurol Neurosurg Psychiatry 2016; 87: 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehdi E, Aralasmak A, Toprak H, et al. Craniocervical dissections: radiologic findings, pitfalls, mimicking diseases: a pictorial review. Curr Med Imaging Rev 2018; 14: 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaschner MG, Kraus B, Petridis A, et al. Endovascular treatment of intracranial ‘blister’ and dissecting aneurysms. Neuroradiol J 2019; 32: 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Xu GQ, Gao HL, et al. Endovascular treatment of intracranial vertebral artery unruptured dissecting aneurysms: comparison of flow diversion and stent-assisted coiling or stenting alone. Front Neurol 2022; 13: 919866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Q, Wang C, Xu S, et al. Low-profile visualized intraluminal support-within-enterprise overlapping-stent technique versus flow diversion in the treatment of intracranial vertebrobasilar trunk dissecting aneurysms. Quant Imaging Med Surg 2023; 13: 3536–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Peng Q, Zhou Y, et al. Outcomes of reconstructive endovascular treatment of vertebrobasilar dissecting aneurysms with intramural hematoma. Front Neurol 2022; 13: 914878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iosif C, Biondi A. Braided stents and their impact in intracranial aneurysm treatment for distal locations: from flow diverters to low profile stents. Expert Rev Med Devices 2019; 16: 237–251. [DOI] [PubMed] [Google Scholar]
- 11.Borota L, Mahmoud E, Nyberg C. Neuroform Atlas stent in treatment of iatrogenic dissections of extracranial internal carotid and vertebral arteries: a single-centre experience. Interv Neuroradiol 2019; 25: 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latacz P, Simka M, Brzegowy P, et al. Endovascular management of carotid and vertebral artery dissections with new generation double-mesh stent and protection systems—single-center early and midterm results. Postepy Kardiol Interwencyjnej 2019; 15: 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’kelly CJ, Krings T, Fiorella D, et al. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol 2010; 16: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mascitelli JR, Moyle H, Oermann EK, et al. An update to the Raymond-Roy occlusion classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg 2015; 7: 496–502. [DOI] [PubMed] [Google Scholar]
- 16.Tufanaru C, Munn Z, Aromataris E, et al. Chapter 3: systematic reviews of effectiveness. In: Aromataris E, Munn Z (eds). JBI Manual for Evidence Synthesis. 2020. Available at: https://synthesismanual.jbi.global [Google Scholar]
- 17.Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available at: https://guidelinedevelopment.org/handbook. [Google Scholar]
- 18.GRADEpro GDT: GRADEpro Guideline development tool [Software]. McMaster University and Evidence Prime, 2024. Available at: https://gradepro.org.
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page MJ, Higgins JPT, Sterne JAC. Chapter 13: assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds.). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane 2023. Available from: www.training.cochrane.org/handbook [Google Scholar]
- 21.Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg 1997; 87: 141–162. [DOI] [PubMed] [Google Scholar]
- 22.Zenteno MA, Santos-Franco JA, Freitas-Modenesi JM, et al. Use of the sole stenting technique for the management of aneurysms in the posterior circulation in a prospective series of 20 patients. J Neurosurg 2008; 108: 1104–1118. [DOI] [PubMed] [Google Scholar]
- 23.Kim M, Levy EI, Meng H, et al. Quantification of hemodynamic changes induced by virtual placement of multiple stents across a wide-necked basilar trunk aneurysm. Neurosurgery 2007; 61: 1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng H, Wang Z, Kim M, et al. Saccular aneurysms on straight and curved vessels are subject to different hemodynamics: implications of intravascular stenting. AJNR Am J Neuroradiol 2006; 27: 1861–1865. [PMC free article] [PubMed] [Google Scholar]
- 25.Chung Y, Lee SH, Choi SK, et al. Triple stent therapy for the treatment of vertebral dissecting aneurysms: efficacy and safety. World Neurosurg 2017; 99: 79–88. [DOI] [PubMed] [Google Scholar]
- 26.Lee I, Kao Y-S, Lai Y-J, et al. Flow diverter retreatment for intracranial aneurysms: a meta-analysis of efficacy and feasibility. Interv Neuroradiol 2024; 30: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieber BB, Gounis MJ. The physics of endoluminal stenting in the treatment of cerebrovascular aneurysms. Neurol Res 2002; 24: S33–S42. [DOI] [PubMed] [Google Scholar]
- 28.Hanel RA, Cortez GM, Benalia VHC, et al. Patient outcomes after treatment of brain aneurysm in small diameter vessels with the silk vista baby flow diverter: a systematic review. Interv Neuroradiol 2024; 30: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuda Y, Chung J, Lopes DK. Analysis of neointima development in flow diverters using optical coherence tomography imaging. J Neurointerv Surg 2018; 10: 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravindran K, Casabella AM, Cebral J, et al. Mechanism of action and biology of flow diverters in the treatment of intracranial aneurysms. Neurosurgery 2020; 86: S13–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim YC, Shin YS, Chung J. Flow diversion via LVIS blue stent within enterprise stent in patients with vertebral artery dissecting aneurysm. World Neurosurg 2018; 117: 203–207. [DOI] [PubMed] [Google Scholar]
- 32.Suh SH, Kim BM, Park SI, et al. Stent-assisted coil embolization followed by a stent-within-a-stent technique for ruptured dissecting aneurysms of the intracranial vertebrobasilar artery. Clinical article. J Neurosurg 2009; 111: 48–52. [DOI] [PubMed] [Google Scholar]
- 33.Heller RS, Dandamudi V, Lanfranchi M, et al. Effect of antiplatelet therapy on thromboembolism after flow diversion with the pipeline embolization device. J Neurosurg 2013; 119: 1603–1610. [DOI] [PubMed] [Google Scholar]
- 34.Patel PD, Chalouhi N, Atallah E, et al. Off-label uses of the pipeline embolization device: a review of the literature. Neurosurg Focus 2017; 42: E4. [DOI] [PubMed] [Google Scholar]
- 35.White TG, Santhumayor BA, Turpin J, et al. Flow diverter surface modifications for aneurysm treatment: a review of the mechanisms and data behind existing technologies. Interv Neuroradiol 2023. doi: 10.1177/15910199231207550 [DOI] [PubMed] [Google Scholar]
- 36.Manning NW, Cheung A, Phillips TJ, et al. Pipeline shield with single antiplatelet therapy in aneurysmal subarachnoid haemorrhage: multicentre experience. J Neurointerv Surg 2019; 11: 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Girdhar G, Andersen A, Pangerl E, et al. Thrombogenicity assessment of pipeline flex, pipeline shield, and FRED flow diverters in an in vitro human blood physiological flow loop model. J Biomed Mater Res A 2018; 106: 3195–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domingo RA, Tripathi S, Perez-Vega C, et al. Treatment of posterior circulation non-saccular aneurysms with flow diversion versus stent-assisted coiling: a systematic review and meta-analysis. J Neurointerv Surg 2021; 13: 159–163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ine-10.1177_15910199241262070 for Comparative meta-analysis of endovascular strategies for intracranial dissecting aneurysms: Flow diverters versus stents with or without coiling by Melika Amoukhteh, Amir Hassankhani, Payam Jannatdoust, Parya Valizadeh, Sherief Ghozy, Cem Bilgin and David F Kallmes in Interventional Neuroradiology