Abstract
Background
The association between sleeve gastrectomy and halitosis remains relatively unknown. Therefore, this study aimed to evaluate the effect of sleeve gastrectomy on halitosis and the oral bacterial species associated with halitosis in patients with obesity.
Methods
This was a prospective longitudinal cohort study that examined patients before and after sleeve gastrectomy and followed the patients at three time intervals (1, 3, and 6 months) after sleeve gastrectomy. Clinical periodontal measurements (plaque index [PI], gingival index [GI], and probing depth [PD]) were obtained. In addition, plaque samples were collected for quantification of the periodontopathogenic bacteria: Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, and Fusobacterium nucleatum using real-time quantitative polymerase chain reaction (qPCR). In addition, breath samples were collected to analyze the concentration of volatile sulfur compounds (VSCs), namely hydrogen sulfide (H2S), methyl mercaptan (CH3SH), and dimethyl sulfide (CH3SCH3), via portable gas chromatography (Oral Chroma™).
Results
Of the 43 patients initially included, 39 completed the study, with a mean age of 32.2 ± 10.4 years. For PI and GI repeated measurements one way analysis of variance showed a significant increase (p-value < 0.001 for both) one month after surgery, with mean values of 1.3 and 1.59, respectively, compared to the baseline. During the same period, the number of P. gingivalis increased, with a p-value = 0.04. Similarly, the levels of hydrogen sulfide (H2S) and methyl mercaptan (CH3SH) increased significantly in the first month after surgery (p-value = 0.02 and 0.01, respectively).
Conclusion
This study demonstrated that sleeve gastrectomy may lead to increased halitosis one month post-surgery, attributed to elevated and P. gingivalis counts, contributing to the development of gingivitis in obese patients who underwent sleeve gastrectomy. This emphasizes the importance of including oral health professionals in the multidisciplinary team for the management of patients undergoing bariatric surgery
Keywords: Obesity, Bariatric surgery, Halitosis, Periodontal pathogens, Periodontal disease, Breath tests
1. Introduction
Obesity has become a worldwide epidemic and is characterized by low-grade systemic inflammation, which may be linked to several associated comorbidities, such as cardiovascular diseases and cancer (James, 2013). Obesity is defined as having a body mass index (BMI) greater than 30.0 kg/m2 (World Health Organization, 2019). In 2019, over 340 million children and adolescents aged 5–19 years were either overweight or obese (World Health Organization, 2019). Currently, bariatric surgical interventions are recognized as the most effective treatment for severe obesity (Colquitt et al., 2014). Surgical management with bariatric surgery (i.e., gastric banding, sleeve gastrectomy, and Roux-en-Y gastric bypass) can improve health and reduce mortality in patients with severe obesity (Wolfe et al., 2016). Sleeve gastrectomy is the most frequently performed bariatric and metabolic procedure in Saudi Arabia. The popularity of this procedure is due to the low incidence of short- and long-term postoperative complications (Nguyen et al., 2011, Cummings and Pratt, 2015). A growing body of literature recognizes the impact of bariatric surgery in the prevalence of oral changes, such as dental erosion, increased dental caries, hypersensitivity, increased plaque accumulation, and periodontal disease (Cummings and Pratt, 2015, Fontanille et al., 2018, Castilho et al., 2019). Recent reports have revealed that the most frequent complaints from patients after bariatric surgery include dry mouth, unpleasant taste, and halitosis (Oliveira, 2007, Dupim Souza et al., 2013). It has been proposed that unpleasant oral symptoms are facilitated by changes in diet, decreased salivary flow, and increased acidity due to frequent episodes of vomiting in the postoperative phase. These factors induce changes in the oral flora, leading to alterations in the qualitative and quantitative microbial composition of the dental biofilm (Sales-Peres et al., 2015). Such changes can influence the development of halitosis because the presence of some types of oral bacteria such as P. gingivalis influences the production of VSCs (Awano et al., 2002). Halitosis is defined as bad breath that can be caused by malodor due to oral causes, including those originating from the oral cavity, such as tongue coating or periodontal disease (Alzoman, 2021). In contrast, non-oral halitosis is caused by disturbances in the upper and lower respiratory tracts (Yaegaki and Coil, 2000) or metabolic changes (Alzahrani et al., 2021). Three major odoriferous components lead to oral malodors. VSCs include hydrogen sulfide (H2S), methyl mercaptan (CH3SH), and dimethyl sulfide (CH3SCH3) (Alzoman, 2008). Gas chromatography is considered a reliable method for detecting oral gases. It is specific and sensitive to all three VSCs. One cross-sectional study evaluated the occurrence of halitosis among patients before and after a specific Roux-en-Y gastric bypass surgery; however, did not find a notable difference. To the best of our knowledge, evidence on the effects of bariatric surgery on halitosis is lacking. Therefore, this study aimed to evaluate the effect of laparoscopic sleeve gastrectomy on intra-oral halitosis by assessing the surgery effect on the levels of VSCs in the breath and halitosis-related bacteria.
2. Materials and methods
2.1. Ethical consideration
Ethical approval was obtained from the International Review Board (IRB) no. E-18-3071, and College of Dentistry Research Center (CDRC) No: PR 0079, King Saud University, Riyadh. This study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its subsequent amendments. An informed consent form containing details regarding the nature of the study was provided to all the participants.
2.2. Study population
In this observational, prospective, longitudinal cohort study, volunteers were recruited from the list of patients scheduled to undergo Sleeve Gastrectomy at the Bariatric Surgery Unit of King Khaled University Hospital. Initially, 50 participants were recruited according to the following criteria:
2.3. Inclusion criteria
-
1.
Patients with obesity with a body mass index of 35–50.
-
2.
Age ranging from 18 to 50 years.
-
3.
Scheduled for laparoscopic sleeve gastrectomy surgery.
2.4. Exclusion criteria
Participants were excluded from the study if they met one of the following conditions:
-
1.
Evidence of diabetes.
-
2.
Presence of tonsillitis or sinusitis.
-
3.
Presence of periodontitis.
-
4.
Presence of dental abscesses.
-
5.
Presence of partially erupted 3rd molar.
-
6.
With removable partial denture.
-
7.
Smoking.
-
8.
Underwent periodontal therapy in the past three months.
-
9.
History of antibiotic use in the past three months.
Of the 50 patients recruited for the screening visits, 43 were included in the study.
2.5. Study protocol
-
•
Screening visit
During screening visits, patients were asked to participate in the study by signing an informed consent form explaining the objectives of the study as well as the potential risks and benefits. Moreover, a detailed questionnaire on general health was given to all subjects. This was followed by an intra-oral clinical examination to determine the subject’s eligibility for inclusion in the study. All subjects received scaling and oral hygiene instructions, as well as caries control therapy, one month prior to the scheduled day of operation.
-
•
Baseline visit
Baseline measurements were taken 1 day (d) before the scheduled operation room (OR) day. Clinical oral measurements (plaque index [PI], gingival index [GI], and probing depth [PD]) were performed at the baseline visit. Plaque samples were collected for microbiological analyses. Breath samples were collected to analyze the concentration of VSC. Intra-examiner reliability done on five patients not included in the study and Cronbach's alpha was 0.964c.
-
•
Bariatric surgery procedure
All bariatric procedures were performed laparoscopically at the Accredited Bariatric Surgery Center of King Khaled University Hospital. Sleeve gastrectomy procedures were performed in accordance with previously published studies from the Metabolic and Bariatric Surgery Center at King Khaled University Hospital, and the post-surgical diet in the first month started with clear liquid, colored liquid, and semiliquid (Al-Naami et al., 2013, Althuwaini et al., 2018a, Althuwaini et al., 2018b, Bamehriz et al., 2018, Alsaif et al., 2020).
-
•
Follow-up visit
Similar measurements were obtained on the 1st, 3rd, and 6th month post-surgery.
2.6. Periodontal examination
Clinical periodontal measurements, including PI (Silness and Loe, 1964), GI (Loe and Silness, 1963), and PD were performed on the Ramfjord teeth using a periodontal probe (William's probe; Hu-Friedy, Chicago, Ill., USA). All measurements were performed by a single examiner (HA).
PI: concerns the thickness of dental plaque biofilm along the gingival margin of Ramfjord teeth score it from 0 no plaque, 1 light film, 2 moderate film and 3 abundance of soft plaque biofilm to 4.
GI: scores gingival inflammation from 0 to 3 on the buccal, lingual and proximal surfaces of Ramfjord teeth.
PD: Periodontal pocket depths measured the distance from the gingival margin to the bottom of the gingival sulcus with gentle pressure around the cemento-enamel junctions (CEJ) at three sits buccal and three lingual having the probe placed parallel to the long axis of the tooth and 10 degrees tilted inward at the proximal points.
2.7. Breath analysis
VSC concentration in breath were analyzed via portable gas chromatography (Oral Chroma™; Abilit Corporation, Japan). The subjects were asked to refrain from eating, brushing their teeth, and rinsing their mouths for 3 h (h) prior to breath sample assessment. To measure oral malodor with the Oral Chroma™, the air within the oral cavity was sampled using a 1 mL syringe after closing the mouth for 60 second (s). The collected oral breath samples were immediately injected into the Oral Chroma device. The concentrations of hydrogen sulfide (H2S), methyl mercaptan (CH3SH), and dimethyl sulfide (CH3SCH3) were recorded in parts per billion (ppb).
2.8. Microbiological assessment
Subgingival plaque samples were collected via sterile paper point size 35 (Sure-endo, Korea) selected teeth in each quadrant received minimum of 3 paper points. After drying the area from saliva using an air spray and cotton, the supragingival plaque was carefully removed. Then, paper points were inserted to the deepest pocket depth and left for 30 s after removing paper points from the pockets, and the pooled samples were transferred into 2 mL Eppendorf tubes (SPL, Korea) containing 1.5 mL of phosphate-buffered saline (PBS) solution (Fig. 1). Tubes were collected in an ice box prior to storage at −80 °C until analysis. DNA was extracted from frozen bacterial sample using BioFACT™ Genomic DNA Prep Kit For Gram (−) Bactria (Biofact,Daejeon,Korea). To quantify the levels of P. gingivalis, Fusobacterium nucleatum, Tannerella forsythia, and Treponema denticola, a 16s ribosomal RNA gene-targeted qPCR was performed using EvaGreen Supermix (Solos Biodyne, Tartu, Estonia). The reactions were run on an ABI 7500 real-time PCR instrument (Applied Biosystems, Foster City, CA, USA) with a total reaction volume of 20 µL. P. gingivalis, F. nucleatum, T. forsythia, and T. denticola primers were used as previously described (Table 1) (Kato et al., 2005). Thirty-nine samples were analyzed per period (pre-operative, 1M, 3M, and 6M), for a total of 156 samples. DNAs was amplified and marked with the EvaGreen Supermix reagent (Applied Biosystems, Foster City, CA, USA) for qPCR. Specific primers were used for each targeted bacterium in this study, and a universal primer (16S) was used for the standard bacteria to verify the presence of bacterial DNA and allow for relative quantification (RQ). The primer sequences are listed in Table 1. The primers (Macrogen, Inc., Seoul, Korea) were diluted to a 10 pmol/µL concentration using RNase-free water. To assemble the qPCR plate (MicroAmp Optical 96-well reaction plates), 5 µL of DNA was added to the PCR master mix, which contained 4 µL of EvaGreen qPCR Supermix and 0.4 µL of each primer (forward and reverse) and is supplemented with 10.20 µL of RNase-free water in. This step was performed in triplicate for each sample. Then, the plate was sealed and centrifuged at 2000 rpm for 30 s at 4 °C in a Centurion Scientific K24R Benchtop Centrifuge (Wast Sussex, UK) to obtain a spinning action in order for the liquid to settle at the bottom of the wells, avoiding any trace in the walls of the wells. The plates were then transferred to a 7500 Real-Time PCR System thermocycler (Applied Biosystems). The thermocycling process was as follows: 95 °C for 12 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 20 s, and 72 °C for 20 s. Readings were obtained using the 7500 Real-Time PCR System software (Applied Biosystems). RQ of bacteria was conducted. The higher the RQ, the greater the number of bacteria. The RQ was calculated using the formula RQ = 2^ΔCt = 2^(Ct target bacteria-Ct universal primer) (Schmittgen and Livak, 2008).
Fig. 1.
(A) Subgingival plaque sample collection using sterile paper point size 35. (B) Plaque samples placed in an Eppendorf tube containing 1.5 mL of PBS.
Table 1.
Primers used for real-time PCR.
| Designation | Sequence | Target gene |
|---|---|---|
| Pg(F) | 5′-TACCCATCGTCGCCTTGGT-3′ | 16s rRNA |
| Pg(R) | 5′-CGGACTAAAACCGCATACACTTG-3′ | 16s rRNA |
| Fn(F) | 5′-CGCAGAAGGTGAAAGTCCTGTAT-3′ | 16s rRNA |
| Fn(R) | 5′-TGGTCCTCACTGATTCACACAGA-3′ | 16s rRNA |
| Tf(F) | 5′-ATCCTGGCTCAGGATGAACG-3′ | 16s rRNA |
| Tf(R) | 5′-TACGCATACCCATCCGCAA-3′ | 16s rRNA |
| Td(F) | 5′-AGAGCAAGCTCTCCCTTACCGT-3′ | 16s rRNA |
| Td(R) | 5′-TAAGGGCGGCTTGAAATAATGA-3′ | 16s rRNA |
| Uni(F) | 5′-CGCTAGTAATCGTGGATCAGAATG-3′ | 16s rRNA |
| Uni(R) | 5′-TGTGACGGGCGGTGTGTA-3′ | 16s rRNA |
Pg, Porphyromonas gingivalis; Fn, Fusobacterium; Tf, Tannerella forsythia; Td, Treponema denticola; Uni, universal primer; rRNA, ribosomal ribonucleic acid.
2.9. Sample size calculation
The sample size was determined using Cohen (1988) at α = 0.05, with an estimated standard deviation of 10 and effect size (F) of 0.8 with power 0.9 (90 %). To establish a statistical difference, the minimum number of subjects was 30. Due to the anticipated discontinuity of patients, the sample size was increased to 43.
3. Statistical test
All statistical analyses were performed using the SPSS statistical package (version 22.0; SPSS Inc., Chicago, IL, USA) for Windows. Descriptive statistics (means, standard deviations, frequencies, interquartile range, and percentages) are presented. For inferential statistics, a one-way analysis of variance was used if normality was satisfied to compare the independent variables with two or more levels of numerical response variables. If normality was not satisfied, the Friedman nonparametric test was used. Pearson’s and Spearman’s correlations were used to determine the relationship between two numerical variables. The significance level was set at p < 0.05.
4. Results
Initially, 43 patients were included in the study, and four patients dropped out due to personal circumstances. Therefore, 39 subjects had completed the study. The study subjects were 12 male and 27 females with a mean age of 32.2 ± 10.4 and ages ranging between 18–50 years (Table 2).
Table 2.
Demographic data of the study participants.
| Baseline measurements | |
|---|---|
| Variables | Mean (SD) |
| Age | 32.2 (10.4) |
| Male/female | 12/27 |
| BMI | 45.2 (4.6) |
| PI | 0.3 (0.2) |
| GI | 0.5 (0.3) |
| PD | 2.1 (0.3) |
4.1. Periodontal examination
At baseline, the PI was 0.3, and repeated measurements one-way (ANOVA) showed a significant increase one month after surgery, with a mean value of 1.4 (P < 0.05). Later, PI significantly decreased in the 3rd month and further decreased in the 6th month, with mean values of 0.7 and 0.6, respectively (P < 0.05).
The mean GI was 0.5 at baseline and significantly increased in the 1st month after surgery, with a mean value of 1.6 (P < 0.05). Afterward, GI significantly decreased in the 3rd month and further decreased in the 6th month, with mean values of 1.3 and 0.7, respectively (P < 0.05). The results showed that PD did not significantly change in any of the three time periods (Table 3).
Table 3.
Mean values of PI, GI, and PD at baseline and at 1, 3, and 6 months.
| Time | PI |
GI |
PD |
|||
|---|---|---|---|---|---|---|
| Mean | P | Mean | P | Mean | P | |
| Baseline | 0.3 | <0.05* | 0.5 | <0.05* |
2.0 | NS |
| 1 month | 1.4 | 1.6 | 2.1 | |||
| 3 months | 0.7 | 1.3 | 2.0 | |||
| 6 months | 0.6 | 0.7 | 2.0 | |||
Repeated measurements One Way ANOVA (P-Value)*, Plaque index (PI), gingival index (GI), and probing depth (PD).
4.2. VSC measurement results
At baseline, the median values of H2S and CH3SH were 125 ppb and 63 ppb, respectively. The Friedman nonparametric test showed a significant increase after one month for both gases, with median values of 291 ppb and 164 ppb, respectively (P < 0.02 and P < 0.01, respectively). Further, in the 3rd and 6th months, the levels of H2S decreased to 132 pbb and 140 ppb, respectively. Similarly, the levels of CH3SH decreased to 86 ppb and 46 ppb, respectively (Table 4). In contrast, CH3SCH3 showed no significant change during any of the three time periods.
Table 4.
VSC levels at baseline and at 1, 3, and 6 months.
| Time | H2S |
CH3SH |
CH3SCH3 |
|||
|---|---|---|---|---|---|---|
| Median (IQR) | P | Median (IQR) | P | Median (IQR) | p | |
| Baseline | 125 (300) | 0.02* | 63 (169) | 0.01* |
20 (34) | NS |
| 1 month | 291 (754) | 164 (715) | 21 (43) | |||
| 3 months | 132 (369) | 86 (165) | 22 (34) | |||
| 6 months | 140 (495) | 46 (199) | 16 (30) | |||
Friedman nonparametric test.
Hydrogen sulphide (H2S), Methyl mercaptan (CH3SH), and Dimethyl sulphide (CH3SCH3).
In addition, the results showed that the PI was significantly positively correlated with the levels of H2S and CH3SH (27.9 % and 25 %, respectively; p < 0.05).
4.3. qPCR quantification
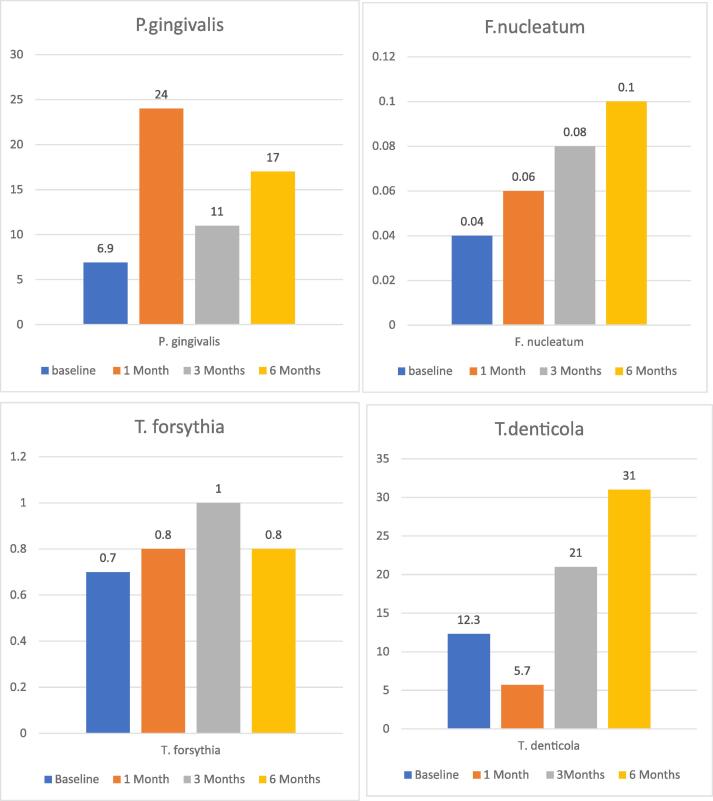
Regarding the bacterial RQ values, Friedman nonparametric test indicated a sudden increase in the median RQ of P. gingivalis at 1 month, followed by lower populations at three and six months (P = 0.004). The RQ values of F. nucleatum increased significantly from baseline during the study period (P = 0.003). No significant changes were found in the RQs of T. forsythia and T. denticola. Finally, P.gingivalis showed a positive correlation with GI of 17.3 % (p = 0.03) (Fig. 2).
Fig. 2.
Median bacterial RQ values at baseline and at 1, 3, and 6 months. Friedman nonparametric test.
5. Discussion
This study aimed to verify a possible association between sleeve gastrectomy and the occurrence of halitosis and its associated oral bacterial species and is the first longitudinal study to report oral changes as early as one month after sleeve gastrectomy as other studies have reported changes at 3, 6, and 12 months. Our results showed a sharp increase in VSC concentration, P. gingivalis counts, and periodontal measurements (GI and PI) one month after surgery, which decreased after three and six months. Halitosis is defined as an unpleasant odor that can be noticed by the patient themselves and was one of the most commonly reported oral side effects in patients after bariatric surgery in a cohort study in 2022 (Bastos et al., 2022). In the present study, the concentration of VSCs was measured using a portable oral chroma device at three time points: 1, 3, and 6 months post-surgery. The levels of H2S and CH3SH significantly increased 1 month post surgery, and then started to decrease 3 and 6 months postoperatively. H2S and CH3SH are the major contributors to oral malodor (Tangerman and Winkel, 2007). In this study, we performed DNA quantification using qPCR to detect the most common bacteria that are associated with subgingival plaque and have been reported to be responsible for VSC production, namely P. gingivalis, T. forsythia, T. denticola, and F. nucleatum (Persson et al., 1989, Persson et al., 1990). P. gingivalis is considered one of the most virulent periodontopathogens that induces chronic periodontal disease in humans (Hajishengallis et al., 2012). The RQ values reported in this study were distinctively high after one month and sustained a high value at three and six months afterward compared to baseline. This is consistent with the results of Sales-Peres et al. (2015), that observed an increased availability of P. gingivalis six months after gastric bypass surgery (Sales-Peres et al., 2015, Adawi et al., 2022). In contrast, Dupim Souza et al. (2013) conducted a cross-sectional study to evaluate the effect of Rouxen-Y gastric bypass on halitosis and found no difference in the frequency of halitosis between the control and case groups that could be due to the type of surgery (Cummings and Pratt, 2015)and/ or the type of study (Stiles, 2013). Furthermore, P. gingivalis is characterized by inflammation and bleeding due to the release of anticoagulation enzymes, such as gingipain (How et al., 2016). Gingipain was found to degrade fibrinogen and host heme proteins, which contribute to inhibition of blood coagulation and increased bleeding, thereby enhancing the availability of hemin for bacterial growth (Sroka et al., 2001). This may explain the positive correlation between the GI values and the levels of P. gingivalis in the current study. Nutritional deficiencies observed after bariatric surgery lead to vitamin C, D, and B12 deficiencies, which have been reported in the short and long term after bariatric surgery (Moizé et al., 2013). Studies have shown that vitamin deficiency can affect gingival health and oral microbial flora (Leggott et al., 1991, Abreu et al., 2016, Zong et al., 2016, Sulijaya et al., 2019). F. nucleatum counts increased significantly from baseline during the study period (P = 0.003). The Gram-negative anaerobe F. nucleatum is an oral bacterium that is present in the normal oral microbiome (Bolstad et al., 1996). However, it also has pathogenic potential and has been implicated in periodontal diseases and halitosis (Bolstad et al., 1996). Additionally, F. nucleatum is assumed to act as a “microbial bridge” as it can co-aggregate with early and late colonizers of dental plaque. The major outer membrane protein of F. nucleatum, FomA, functions as a nonspecific porin in lipid bilayer membranes. FomA has been recognized as a major immunogen of F. nucleatum (Wu et al., 2020). FomA has been reported to be involved in the binding of Fusobacteria to Streptococcus sanguis on the tooth surface and to P. gingivalis in the periodontal pockets. In addition, in this study, we found that PI was positively correlated with H2S and CH3SH. The plaque index was also high 1 month post-surgery and showed the same pattern as H2S and CH3SH at three and six months. Oral malodor is known to be partly due to bacterial plaque accumulation (Porter and Scully, 2006). Gingival disease is defined as the inflammation of gingival tissues caused by the accumulation of dental plaque and is clinically characterized by redness, swelling, and bleeding of the tissues.
In this study, we found a significant increase in the GI and PI one month post-surgery, with mean values of 1.6 and 1.4, respectively, and p-value < 0.001 for both. Meanwhile, no difference in PD was found before and after surgery. Consistent with our results, a systematic review and meta-analysis reported a statistically significant increase in periodontal inflammation (mean percentage of sites with bleeding on probing) after bariatric surgery compared with baseline data six months after surgery and no difference in probing depth before and after bariatric surgery (de Moura-Grec et al., 2014, Sales-Peres et al., 2015, Sales-Peres et al., 2017, Fontanille et al., 2018, Čolak et al., 2021). In contrast, at 12 months post-surgery, previous studies did not find any effect of the surgery compared to the baseline (Sales-Peres et al., 2015, Sales-Peres et al., 2017), which could be explained by the delay of examination time (12 months post-surgery) and controlling for confounding factors, such as the presence of subjects with diabetes and use of medication, which will have a direct effect on salivation (Scully, 2003, Farias et al., 2019).
Possible explanations for the changes in GI and PI after bariatric surgery are the presence of tongue coating (Dupim Souza et al., 2013), dry mouth, and stick plaque accumulation, which could be due to the type of diet in the first month, which started with clear liquid, colored liquid, and semi-liquid that led to a decrease in chewing function and stimulated salivation (Nakagawa et al., 2017). According to multiple studies, the tongue coating is primarily responsible for halitosis of oral origin (Lee et al., 2003, Pedrazzi et al., 2004, Tsai et al., 2008). Furthermore, it is possible to notice a change in diet with a higher feeding frequency of small meals and soft foods (Schneider and Knieknecht, 1986) that adhere to the tooth surface throughout the day, together with a reduction in salivary flow, which could explain the increase in PI and GI in these individuals (Hague and Baechle, 2008, Marsicano et al., 2011, Marsicano et al., 2012, Maria de Souza et al., 2018).
To the best of our knowledge, this is the first longitudinal clinical study that focused on the effect of sleeve gastrectomy on oral halitosis and followed up patients at 1, 3, and 6 months post-surgery. However, gastric reflux has been reported in patients undergoing sleeve gastrectomy, which may increase oral exposure to gastric acid (Sharma et al., 2014).
6. Conclusion
This study showed that sleeve gastrectomy increased the PI and GI in the first month after surgery, as well as the concentration of VSC and the presence of P. gingivalis, which is associated with intraoral halitosis. This change improved significantly in the 3rd and 6th months after the surgery. Our recommendations include that patients, physicians, and dentists should be aware of this effect of sleeve gastrectomy and that dentists should be one of the medical teams to overcome this effect through regular follow-up of the patient before and after surgery, focusing on performing good oral hygiene after the surgery, use of saliva substitutes, and prescription of multivitamin supplements, especially in the first month. Further studies with a larger sample size, tongue coating evaluation, saliva flow evaluation, oral hygiene evaluation, PH, and acidity tests are required to confirm this effect. In addition, this study highlights the effect of food type on plaque accumulation.
Funding
This study was funded by King Abdulaziz City for Science and Technology grant no (1-18-03-001-0050).
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Hanadi Alzahrani reports financial support was provided by King Abdulaziz City for Science and Technology grant no (1-18-03-001-0050). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Ms. Sarah Salman for her help in patients’ communication and recruitment.
Contributor Information
Hanadi G. Alzahrani, Email: dr.hanadialzahrani@gmail.com.
Mohammed A. AlSarhan, Email: malsarhan@ksu.edu.sa.
Abdullah Aldohayan, Email: adohayanssls@gmail.com.
Fahad Bamehriz, Email: fbamehriz@ksu.edu.sa.
Hamad A. Alzoman, Email: halzoman@ksu.edu.sa.
References
- Abreu O.J., Tatakis D.N., Elias-Boneta A.R., et al. Low vitamin D status strongly associated with periodontitis in Puerto Rican adults. BMC Oral Health. 2016;16:89. doi: 10.1186/s12903-016-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adawi H., Aggarwal A., Jain S., et al. Influence of bariatric surgery on oral microbiota: a systematic review. Eur. J. Dent. 2022 doi: 10.1055/s-0042-1753471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Naami M., Anjum M.N., Aldohayan A., et al. Robotic general surgery experience: a gradual progress from simple to more complex procedures. Int. J. Med. Robot. Comput. Assist. Surg. 2013;9:486–491. doi: 10.1002/rcs.1521. [DOI] [PubMed] [Google Scholar]
- Alsaif F.A., Alabdullatif F.S., Aldegaither M.K., et al. Incidence of symptomatic cholelithiasis after laparoscopic sleeve gastrectomy and its association with rapid weight loss. Saudi J. Gastroenterol. 2020 doi: 10.4103/sjg.SJG_472_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althuwaini S., Bamehriz F., Aldohayan A., et al. Prevalence and predictors of gastroesophageal reflux disease after laparoscopic sleeve gastrectomy. Obes. Surg. 2018;28:916–922. doi: 10.1007/s11695-017-2971-4. [DOI] [PubMed] [Google Scholar]
- Althuwaini S., Bamehriz F., Alobaid O., et al. Identification of bacterial and fungal pathogens in patients with post-laparoscopic sleeve gastrectomy leakage. Obes. Surg. 2018;28:3965–3968. doi: 10.1007/s11695-018-3442-2. [DOI] [PubMed] [Google Scholar]
- Alzahrani H.G., Alsarhan M.A., Aldohayan A., et al. Extraoral halitosis due to exhaled acetone in patients undergoing sleeve gastrectomy. J. Clin. Diagn. Res. 2021;15 [Google Scholar]
- Alzoman H. Periodontal disease and halitosis. Saudi Dent. J. 2008;20:3–9. doi: 10.1016/j.sdentj.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzoman H. The association between periodontal diseases and halitosis among Saudi patients. Saudi Dent. J. 2021;33:34–38. doi: 10.1016/j.sdentj.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awano S., Gohara K., Kurihara E., et al. The relationship between the presence of periodontopathogenic bacteria in saliva and halitosis. Int. Dent. J. 2002;52:212–216. doi: 10.1002/j.1875-595X.2002.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Bamehriz F., Althuwaini S., Alobaid O., et al. Is body mass index ≥50 kg/m(2) a predictor of higher morbidity for patients who have undergone laparoscopic sleeve gastrectomy? Saudi J. Anaesth. 2018;12:555–558. doi: 10.4103/sja.SJA_140_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos I.H.A., Alves E.S., Sousa C.D., et al. Bariatric surgery and oral health: a cohort study. Bariatric Surg. Pract. Patient Care. 2022;17:241–245. doi: 10.1089/bari.2021.0136. [DOI] [Google Scholar]
- Bolstad A.I., Jensen H.B., Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin. Microbiol. Rev. 1996;9:55–71. doi: 10.1128/CMR.9.1.55-71.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho A.V.S.S., Foratori-Junior G.A., Sales-Peres S.H.C. ABCD. Arquivos Brasileiros de Cirurgia Digestiva; São Paulo: 2019. Bariatric Surgery Impact on Gastroesophageal Reflux and Dental Wear: A Systematic Review; p. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. Perceived stress in a probability sample of the United States. The social psychology of health. Sage Publications, Inc; Thousand Oaks, CA, US: 1988. pp. 31–67. [Google Scholar]
- Čolak D., Gašperšič R., Kučič A.C., et al. The effect of bariatric surgery on periodontal health: systematic review and meta-analyses. Arch. Med. Sci. 2021;17:1118–1127. doi: 10.5114/aoms/135880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquitt J.L., Pickett K., Loveman E., et al. Surgery for weight loss in adults. Cochrane Database Syst. Rev. 2014 doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings, S., Pratt, J., 2015. Metabolic and bariatric surgery: nutrition and dental considerations. J. Am. Dent. Assoc. (1939). 146, 767–772. doi: 10.1016/j.adaj.2015.06.004. [DOI] [PubMed]
- de Moura-Grec P.G., Yamashita J.M., Marsicano J.A., et al. Impact of bariatric surgery on oral health conditions: 6-months cohort study. Int. Dent. J. 2014;64:144–149. doi: 10.1111/idj.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupim Souza A.C., Franco C.F., Pataro A.L., et al. Halitosis in obese patients and those undergoing bariatric surgery. Surg. Obes. Relat. Dis. 2013;9:315–321. doi: 10.1016/j.soard.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Farias T., Vasconcelos B., SoutoMaior J.R., et al. Influence of bariatric surgery on salivary flow: a systematic review and meta-analysis. Obes. Surg. 2019;29:1675–1680. doi: 10.1007/s11695-019-03784-w. [DOI] [PubMed] [Google Scholar]
- Fontanille I., Boillot A., Range H., et al. Bariatric surgery and periodontal status: a systematic review with meta-analysis. Surg. Obes. Relat. Dis. 2018;14:1618–1631. doi: 10.1016/j.soard.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Hague A.L., Baechle M. Advanced caries in a patient with a history of bariatric surgery. Journal of Dental Hygiene: JDH. 2008;82:22. [PubMed] [Google Scholar]
- Hajishengallis G., Darveau R.P., Curtis M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- How K.Y., Song K.P., Chan K.G. Porphyromonas gingivalis: an overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W.P. Obesity—a modern pandemic: the burden of disease. Endocrinologia y Nutricion: Organo De La Sociedad Espanola De Endocrinologia y Nutricion. 2013;60(Suppl. 1):3–6. doi: 10.1016/s1575-0922(13)70015-9. [DOI] [PubMed] [Google Scholar]
- Kato H., Yoshida A., Awano S., et al. Quantitative detection of volatile sulfur compound- producing microorganisms in oral specimens using real-time PCR. Oral diseases. 2005;11(Suppl 1):67–71. doi: 10.1111/j.1601-0825.2005.01096.x. [DOI] [PubMed] [Google Scholar]
- Lee C.-H., Kho H.-S., Chung S.-C., et al. The relationship between volatile sulfur compounds and major halitosis-inducing factors. J. Periodontol. 2003;74:32–37. doi: 10.1902/jop.2003.74.1.32. [DOI] [PubMed] [Google Scholar]
- Leggott P.J., Robertson P.B., Jacob R.A., et al. Effects of ascorbic acid depletion and supplementation on periodontal health and subgingival microflora in humans. J. Dent. Res. 1991;70:1531–1536. doi: 10.1177/00220345910700121101. [DOI] [PubMed] [Google Scholar]
- Loe H., Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta odontologica Scandinavica. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Maria de Souza G., Willya Douglas de Oliveira D., Santos Lages F., et al. Relationship between bariatric surgery and periodontal status: a systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2018;14:1205–1216. doi: 10.1016/j.soard.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Marsicano J.A., Grec P.G.M., Belarmino L.B., et al. Interfaces between bariatric surgery and oral health: a longitudinal survey. Acta Cirurgica Brasileira. 2011;26:79–83. doi: 10.1590/s0102-86502011000800015. [DOI] [PubMed] [Google Scholar]
- Marsicano J.A., Sales-Peres A., Ceneviva R., et al. Evaluation of oral health status and salivary flow rate in obese patients after bariatric surgery. Eur. J. Dent. 2012;6:191–197. [PMC free article] [PubMed] [Google Scholar]
- Moizé V., Andreu A., Flores L., et al. Long-term dietary intake and nutritional deficiencies following sleeve gastrectomy or Roux-En-Y gastric bypass in a mediterranean population. J. Acad. Nutr. Diet. 2013;113:400–410. doi: 10.1016/j.jand.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Nakagawa K., Matsuo K., Takagi D., et al. Effects of gum chewing exercises on saliva secretion and occlusal force in community-dwelling elderly individuals: a pilot study. Geriatr. Gerontol. Int. 2017;17:48–53. doi: 10.1111/ggi.12670. [DOI] [PubMed] [Google Scholar]
- Nguyen N.T., Masoomi H., Magno C.P., et al. Trends in use of bariatric surgery, 2003–2008. J. Am. Coll. Surg. 2011;213:261–266. doi: 10.1016/j.jamcollsurg.2011.04.030. [DOI] [PubMed] [Google Scholar]
- Oliveira, 2007. Impacto da saúde bucal na qualidade de vida de usuários do sistema de saúde da Polícia Militar de Minas Gerais submetidos à cirurgia bariátrica: um ponto de partida para a abordagem integral [Dissertação de Mestrado]. Fundação Comunitária Tricordiana de Educação: Universidade Vale do Rio Verde de Três Corações, Betim.
- Pedrazzi V., Sato S., de Mattos M.G.C., et al. Tongue-cleaning methods: a comparative clinical trial employing a toothbrush and a tongue scraper. J. Periodontol. 2004;75:1009–1012. doi: 10.1902/jop.2004.75.7.1009. [DOI] [PubMed] [Google Scholar]
- Persson S., Claesson R., Carlsson J. The capacity of subgingival microbiotas to produce volatile sulfur compounds in human serum. Oral Microbiol. Immunol. 1989;4:169–172. doi: 10.1111/j.1399-302X.1989.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Persson S., Edlund M.-B., Claesson R., et al. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol. Immunol. 1990;5:195–201. doi: 10.1111/j.1399-302X.1990.tb00645.x. [DOI] [PubMed] [Google Scholar]
- Porter S.R., Scully C. Oral malodour (halitosis) BMJ (Clin. Res. Ed.) 2006;333:632–635. doi: 10.1136/bmj.38954.631968.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales-Peres S.H., de Moura-Grec P.G., Yamashita J.M., et al. Periodontal status and pathogenic bacteria after gastric bypass: a cohort study. J. Clin. Periodontol. 2015;42:530–536. doi: 10.1111/jcpe.12410. [DOI] [PubMed] [Google Scholar]
- Sales-Peres S.H.C., Sales-Peres M.C., Ceneviva R., et al. Weight loss after bariatric surgery and periodontal changes: a 12-month prospective study. Surg. Obes. Relat. Dis. 2017;13:637–642. doi: 10.1016/j.soard.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Schneider H.G., Knieknecht G. The formation of dental plaque in relation to the ingestion of food. Nahrung. 1986;30:31–37. doi: 10.1002/food.19860300109. [DOI] [PubMed] [Google Scholar]
- Scully C. Drug effects on salivary glands: dry mouth. Oral Dis. 2003;9:165–176. doi: 10.1034/j.1601-0825.2003.03967.x. [DOI] [PubMed] [Google Scholar]
- Sharma A., Aggarwal S., Ahuja V., et al. Evaluation of gastroesophageal reflux before and after sleeve gastrectomy using symptom scoring, scintigraphy, and endoscopy. Surg. Obes. Relat. Dis. 2014;10:600–605. doi: 10.1016/j.soard.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Silness J., Loe H. Periodontal Disease In Pregnancy. II. Correlation Between Oral Hygiene And Periodontal Condtion. Acta odontologica Scandinavica. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- Sroka A., Sztukowska M., Potempa J., et al. Degradation of host heme proteins by lysine- and arginine-specific cysteine proteinases (gingipains) of Porphyromonas gingivalis. J. Bacteriol. 2001;183:5609–5616. doi: 10.1128/JB.183.19.5609-5616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles L.E. Comment on: Halitosis in obese patients and those undergoing bariatric surgery. Surg. Obes. Relat. Dis. 2013;9:321–322. doi: 10.1016/j.soard.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Sulijaya B., Takahashi N., Yamazaki K., et al. Nutrition as adjunct therapy in periodontal disease management. Curr. Oral Health Rep. 2019;6:61–69. doi: 10.1007/s40496-019-0216-4. [DOI] [Google Scholar]
- Tangerman A., Winkel E.G. Intra- and extra-oral halitosis: finding of a new form of extra-oral blood-borne halitosis caused by dimethyl sulphide. J. Clin. Periodontol. 2007;34:748–755. doi: 10.1111/j.1600-051X.2007.01116.x. [DOI] [PubMed] [Google Scholar]
- Tsai C.-C., Chou H.-H., Wu T.-L., et al. The levels of volatile sulfur compounds in mouth air from patients with chronic periodontitis. J. Periodontal Res. 2008;43:186–193. doi: 10.1111/j.1600-0765.2007.01011.x. [DOI] [PubMed] [Google Scholar]
- Wolfe B.M., Kvach E., Eckel R.H. Treatment of obesity. Circ. Res. 2016;118:1844–1855. doi: 10.1161/CIRCRESAHA.116.307591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2019. Obesity, health topic. Retrieved sptember, 2019, from https://www.who.int/topics/obesity/en/.
- Wu J., Cannon R.D., Ji P., et al. Halitosis: prevalence, risk factors, sources, measurement and treatment - a review of the literature. Aust. Dent. J. 2020;65:4–11. doi: 10.1111/adj.12725. [DOI] [PubMed] [Google Scholar]
- Yaegaki K., Coil J.M. Examination, classification, and treatment of halitosis; clinical perspectives. J. Can. Dent. Assoc. 2000;66:257–261. [PubMed] [Google Scholar]
- Zong G., Holtfreter B., Scott A.E., et al. Serum vitamin B12 is inversely associated with periodontal progression and risk of tooth loss: a prospective cohort study. J. Clin. Periodontol. 2016;43:2–9. doi: 10.1111/jcpe.12483. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]