Abstract
Globally, oral infections and inflammatory lesions persist as substantial public health concerns, necessitating the introduction of novel oral treatment protocols. Oral diseases are linked to various causative factors, with dental plaque/biofilm resulting from inadequate hygiene practices playing a predominant role. The strategic implementation of novel topical therapies holds promise for effectively controlling the biofilms, addressing oral infections and promoting enhanced oral wound healing. This review aims to providing a comprehensive overview of the available evidence pertaining to the potential efficacy of topical oxygen and lactoferrin-releasing biomaterials, exemplified by the blue®m formula, as novel oral care interventions within the scope of contemporary implantology, oral surgery and periodontology.
Keywords: Oral care, Oxygen therapy, Oral biofilm, Biomaterials, Periodontitis, Peri-implantitis
1. Introduction
The overall health of the population is profoundly influenced by the state of oral health (Allen, 2003). Consequently, individuals should diligently adhere to daily oral hygiene practices and seek adequate professional oral care to ensure the ability to eat, speak comfortably, and maintain self-esteem (Petersen and Yamamoto, 2005). As of 2020, the World Health Organization reported that oral lesions and diseases remain significant global public health concerns (World Health Organization, 2022). An alarming statistic reveals that over 3.5 billion individuals are afflicted by oral diseases, exerting substantial health, social, and economic impacts. Furthermore, oral cancers present a serious worldwide health issue, resulting in nearly 200,000 deaths annually (World Health Organization, 2022). In recent times, peri-implantitis has emerged as the most prevalent oral disease leading to dental implant failures (Hasan et al., 2022).
In many countries, oral healthcare systems predominantly rely on a dentist-centred model incorporating advanced technology (Petersen et al., 2020). However, these systems often fall short in adequately promoting oral disease prevention. Furthermore, millions of patients lack access to early treatment for oral diseases (Glick et al., 2102). Consequently, the introduction of preventive tools and the promotion of oral care in the public domain are deemed essential for fostering healthy lifestyles among both children and adults.
The aetiology of oral lesions is multifaceted, primarily attributed to poor hygiene. Nevertheless, dental plaque/biofilm stands out as the principal etiological factor. A comprehensive understanding of the causative factors behind oral diseases serves as the foundation for integrated strategies aimed at prevention and control (Sheiham and Watt, 2000, Genco and Genco, 2014).
2. Oral health and biofilm
The oral cavity harbours approximately 700 species of microorganisms, including bacteria, fungi, viruses, and protozoa (Willis and Gabaldón, 2020). The microbiome plays a crucial role in maintaining a healthy balance in both the oral and gut systems. However, pathogenic biofilms can form as microorganisms adhere to each other and dental surfaces (Samaranayake and Matsubara, 2017).
Biofilm development progresses through several stages. Initially, free-swimming bacteria (planktonic) reversibly attach to oral surfaces, with primary colonizers like Streptococcus, Veillonella, Actinomyces, and Neisseria being common on dental surfaces. Salivary proteins bind to dental surfaces, initiating a synergistic relationship with primary colonizers, leading to more bacterial attachments and biofilm formation. As bacteria consume polysaccharides, they form an initial layer of biofilm matrix, followed by the development of a multi-layer microcolony. Corynebacterium species anchor to primary colonizers, creating a 3D biofilm structure. Oxidative species, such as Haemophilus, Aggregatibacter, and Neisseriaceae, occupy the oxygen-rich periphery, while anoxic conditions at the biofilm centre allow the growth of pathogenic bacteria like Capnocytophaga, Leptotrichia, and Fusobacterium (Filoche et al., 2010). The mature biofilm is sustained by multiple pathogenic bacteria.
In anoxic periodontal pockets, Gram-negative bacteria (e.g., Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola) dominate the subgingival biofilm, facilitated by protein–protein and lectin-carbohydrate interactions. Bacterial cell signalling and communication play a crucial role in biofilm formation (Scapoli et al., 2012).
Recent studies indicate that oxygen therapy can inhibit biofilm formation. For example, Ahn and Burne found that oxygen impairs dental biofilms by S. mutans, reducing biofilm infection due to anaerobic metabolism (Ahn and Burne, 2007).
A 2017 global periodontal workshop addressed unresolved issues related to periodontal diseases, progression, and pathogenesis (Caton et al., 2018, Chapple et al., 2018). Over the past century, the understanding of periodontal pathogenesis and etiological factors has evolved through various stages, as depicted in Fig. 1.
Fig. 1.
History of > 100 years to understand the ‘etiologic factors’ of periodontal diseases.
It is now evident that supra- and subgingival biofilms associated with gingivitis and periodontitis originate from a collection of commensal organisms. Dysbiosis within the biofilm may occur, leading to the overgrowth of more virulent bacteria, exacerbating periodontal inflammation, infection, and tissue destruction. Therefore, maintaining and redirecting the oral biofilm composition toward a symbiotic state is crucial for gingival/periodontal health and good healing.
Developing new therapies to control biofilm formation in the oral cavity remains a challenge, and ongoing studies are investigating the potential utility of active oxygen to disrupt bacterial biofilms (Ammons, 2010, Wang et al., 2015).
3. Conventional strategies for oral care
In recent decades, various chemical agents have been employed to manage dental biofilms (Baehni and Takeuchi, 2003). Typically, these conventional antiseptic agents consist of different active ingredients recommended for specific clinical indications. Approximately 90 % of commonly used antiseptic products feature potent antibacterial chemicals such as chlorhexidine (CHX), cetylpyridinium chloride, alcohol, essential oils, iodine, peroxide, and others (McDonnell and Russell, 1999). For instance, CHX stands out as one of the most widely utilized antiseptics in oral care. Nevertheless, it is associated with a range of extensively documented adverse effects, such as pigmentation of the oral soft tissues and teeth, taste alteration, parotid gland swelling, burning sensation, oral mucosa ulceration or erosions, transient anesthetic sensation and paresthesia (Jones, 1997, Zanatta et al., 2010, Pałka et al., 2022).
Similar to antibiotics, these compounds exhibit broad-spectrum effects and do not specifically target pathogenic biofilms, potentially fostering bacterial resistance and diminishing the therapeutic efficacy of conventional antiseptics (Cieplik et al., 2019). Additionally, a protective matrix of extracellular polymeric materials, including exopolysaccharides, envelops harmful germs within the biofilm. This matrix enhances bacterial tolerance while restricting agent penetration (Bonez, 2013). Thus, for anti-biofilm therapy to be efficacious, it must target both the biofilm matrix and the specific microbial cells within it. Illustrated in Fig. 2, CHX (C22H30Cl2N102C6H12O7) is a cationic large molecule with a mass of 898 Da.
Fig. 2.
Image of large CHX molecule structure compare to small oxygen (O2) molecule.
Chlorhexidine can adhere to the negatively charged surface of bacterial cells, causing cell damage (Bonez, 2013). A recent clinical study showed that CHX mouthwash has a significant impact on the complex oral microbiome and increases oral acidic conditions, resulting in lower diversity of oral bacteria and greater risk of oral diseases. Moreover, a negative systemic effect on patients' blood pressure was observed (Bescos et al., 2020). Limited data exist on the efficacy of intra-wound irrigation with conventional antiseptics. A plethora of literature suggests that CHX, even in low doses, may be cytotoxic and impede the wound healing process, affecting and reducing the viability and cell migration of fibroblasts, osteoblasts, gingival cells, keratinocytes, PRF membranes and lymphocytes (Pucher and Daniel, 1992, Babich et al., 1995, Cabral and Fernandes, 2007, Liu et al., 2018, Gavrailov et al., 2022, Ortega-Llamas et al., 2022). Moreover, clinical reports have documented allergic reaction and serious anaphylactic shock related to CHX (Nakonechna et al., 2014, Abdallah, 2015, Fernandes et al., 2019, Rose et al., 2019, Chiewchalermsri et al., 2020). Finally, the potential detrimental impact of CHX irrigation in treating peri-implantitis has been shown, as it can alter the surface topography of dental implants, potentially influencing re-osseointegration (Krishnamoorthy et al., 2022).
As a result, therapeutic strategies have emerged to manage dental biofilms (Taubman et al., 2007). One approach involves inhibiting bacterial adhesion to dental and implant surfaces, and suture materials. The primary colonizer bacteria physically create an interface that facilitates the adhesion of more microbes to these surfaces and subsequent biofilm formation (Veerachamy et al., 2014). By influencing the sequence of bacteria (primary colonizer) cell adhesion, this strategy can lead to variations in overall biofilm development (Yoshinari et al., 2000). A second strategy involves the development of agents with controlled-release anti-biofilm properties, exerting antimicrobial activity over time in situ to prevent biofilm formation (Lu et al., 2019).
4. Topical oxygen therapy
The utilization of oxygen therapy in medicine has been commonplace for nearly a century (Jindal, 2008). Its application as a treatment for hypoxemia was discovered by Joseph Priestley in 1774 (Tibbles and Edelsberg, 1996).
Oxygen therapy can be broadly categorized as systemic (hyperbaric) or topical. There is substantial evidence supporting the use of systemic oxygen supplementation to enhance wound healing in various anatomical regions. Wound healing requires a variety of cells to increase their metabolic activity, resulting in a high oxygen demand. Oxygen at the wound site has been shown to promote wound healing by stimulating several processes, including neovascularization, collagen production, re-epithelialization, degradation of necrotic tissue, and also creates an unfavorable environment for anaerobic bacteria (Tibbles and Edelsberg, 1996, Rodriguez et al., 2008, Schreml et al., 2010, Eisenbud, 2012, Yip, 2015, de Smet et al., 2017).
The topical application of oxygen over injured tissue, while not extensively explored, remains a subject of controversy (Linden et al., 2014, Orsted and Poulson, 2012). It is hypothesized that it may rapidly reverse tissue hypoxia in situ. Additionally, it may eradicate anaerobic bacteria and enhance the functionality of immune cells to combat various pathogens. Elevating local oxygen levels in wounded tissues can promote more effective healing (Dissemond at al. 2015).
Recently, Dr Peter Blijdorp and his research group developed a novel topical oxygenating formula (Deliberador et al., 2020, Mattei et al., 2021, Ngeow et al., 2022). Clinically, the blue®m formula (blue®m, Wapenveld, Netherlands) is administered intra-orally to prevent the growth of biofilms causing oral infections (Shibli et al., 2021). Chemically, it primarily consists of sodium peroxoborate, glycerol, and cellulose. In an aqueous solution, the formula generates a low concentration of hydrogen peroxide, known for its antiseptic properties (Grootveld et al., 2020).
The technology behind blue®m is centred on the controlled release of topical oxygen. The low, stable and safe concentration of hydrogen peroxide released also exhibits bactericidal action (Ray et al., 2012). Importantly, the blue®m formula can gradually release active oxygen without generating hydroxyl radicals. Another significant component of the blue®m formula is Lactoferrin, a multifunctional glycoprotein, which supports multiple biological processes involved in wound healing. The use of lactoferrin, both systemically and locally, promotes the initial inflammatory phases of tissue healing, exhibits anti-inflammatory activity that neutralizes overabundant immune response, improves the formation of granulation tissue, enhances the synthesis of extracellular matrix components, such as collagen and hyaluronan, and stimulates the proliferation and migration of fibroblasts and keratinocytes, resulting in enhanced re-epithelialization (Kim et al., 2006, Takayama and Aoki, 2012, Icriverzi et al., 2020, Shi et al., 2020, Trybek et al., 2020, Chang et al., 2023, Tian and Tian, 2023). Gao et al., showed that local application of lactoferrin increased in a significant manner bone regeneration in a rat critical-sized calvarial defect model (Gao et al., 2018), while Görmez et al., demonstrated that combining bovine lactoferrin and anorganic bovine bone promotes bone regeneration in defects around implants (Görmez et al., 2015). The above data suggest that the profound effect of lactoferrin on soft and hard tissue regeneration has therapeutic potential to improve clinical treatments in dentistry.
As a result, the release of both oxygen and lactoferrin from blue®m agents may align with the five pillars of wound therapy: 1) increases cellular metabolism, 2) enhances collagen and epithelium synthesis, 3) facilitates the release of growth factors, 4) stimulates angiogenesis, and 5) impedes the growth of pathogenic bacteria and exerts bactericidal action, supported by the results of recent in animal and in vitro studies. Santos et al., showed the antimicrobial effectiveness of blue®m solution against S. mutans, its ability to modulate the expression of the gbpA gene and its low cytotoxicity on fibroblast cells (Santos et al., 2023). Another in vitro study compared the effect of blue®m gel and 0.12 % CHX on Porphyromonas gingivalis, demonstrating that blue®m at a dose of 100 % and 75 % is similar to CHX (Deliberador et al., 2020). Deliberador et al., investigated the effect of blue®m gel on the healing of standardised skin wounds caused in rats. The histological and immunohistochemical findings showed accelerated healing, with increased angiogenesis and better collagen fibre formation when blue®m gel was applied compared to distilled water that served as control (Deliberador et al., 2023).
5. Clinical applications of topical oxygen therapy
Dr. Peter Blijdorp pioneered the TOOTh (Topical Oral Oxygen Therapy) protocol for oral healthcare, as illustrated in Fig. 3. This clinical protocol aims to provide a significant strategy for controlling biofilms associated with oral infections. In parallel, the in situ release of oxygen and lactoferrin is expected to actively promote the healing of oral tissues (see Fig. 4).
Fig. 3.
Scheme of the TOOTh directive for oral healthcare.
Fig. 4.
Illustration of microbial homeostasis in oral biofilm (Symbiosis vs Dysbiosis).
5.1. Periodontal healthcare
Local oxygen-based therapy by blue®m is suggested to restore and maintain a healthy microbial homeostasis. Studies have demonstrated a significant shift in microbial composition toward a homeostasis state of biofilm after applying oxygen therapy (Fernandez, 2014, Filoche et al., 2010, Fernandez y Mostajo, M., Exterkate, R.A., Buijs, M.J., Crielaard, W., Zaura, E., , 2017). Niveda and Kaarthikeyan reported a significant reduction in probing pocket depth in patients with chronic periodontitis treated with blue®m oxygen therapy, confirming its clinical advantage on periodontitis (Niveda, and Kaarthikeyan, 2020). Toothpastes containing active oxygen and lactoferrin have been proven to have comparable antiplaque and antigingivitis efficacies compared to triclosan-containing toothpastes (Cunha et al., 2019). In a randomized controlled clinical trial, the efficacy of blue®m gel as an adjunct therapy, with and without antimicrobial photodynamic therapy, was assessed in the management of residual pockets in periodontal patients with type 2 diabetes mellitus. The findings of the study revealed that the adjunctive application of blue®m gel directly within the periodontal pockets, generating a controlled release of active oxygen, led to a statistically significant decrease in bleeding on probing and in the percentage of sites with pocket depth ≥ 4 mm at 90 and 180 days. Additionally, a significant reduction in tumour necrosis factor α levels was observed at the 90-day mark (Cláudio et al., 2023). Furthermore, recent clinical reports have highlighted that adjunctive topical oxygen-based therapy formulations of toothpaste, mouthwash and oral gel yielded better clinical outcomes after treatment of patients with gingivitis and periodontitis as effective adjunct to mechanical debridement, scaling and root planning (Basudan et al., 2024).
In Fig. 5, Fig. 6, Fig. 7, Fig. 8, a representative clinical case conducted by Dr. Marcelo Imano (Curitiba/PR, Brazil) is presented.
Fig. 5.
Aggressive periodontitis in a 36-year-old female patient. In the initial panoramic x-ray, notable bone loss is evident, particularly around the areas of teeth 26, 27, and 28.
Fig. 6.
In the provided clinical images: (A) illustrates the initial clinical situation, revealing bleeding on probing, probing depths exceeding 5 mm, and clinical attachment loss surpassing 40 %. A diagnosis of generalized aggressive periodontitis was made. The treatment protocol consisted of extraction of 26, 27, and 28 due to the severity of the bone loss, and non-surgical periodontal therapy combined with blue®m oxygen therapy for the rest of the dentition. (B) depicts supra and subgingival scaling and root planing, followed by the application of blue®m gel. The gel was meticulously introduced into the periodontal pockets and around the teeth. (C) shows the immediate effect of the gel application, with observable bubbles indicating the topical release of oxygen. (D) provides a closer view of the bubbles, confirming the oxygen release. Subsequent images include (E) one day post-therapy using blue®m gel and (F) the second application of blue®m gel.
Fig. 7.
In the provided clinical images: (A) illustrates the status two days post-therapy using blue®m gel. (B) illustrates the third application of blue®m gel. (C) displays the condition three days post-therapy. Subsequent follow-up assessments are represented by (D) after fifteen days, (E) after thirty days, and (F) after sixty days. The periodontal tissues exhibited a healthy status throughout these follow-up periods, characterized by the absence of bleeding on probing, reduced probing depths ranging from 1 to 4 mm, and excellent plaque control.
Fig. 8.
Five-year recall. Clinical views (A, B) and panoramic x-ray (C) reveal significant progress. The patient had undergone orthodontic treatment and lower arch rehabilitation with two implants. Notably, periodontal tissues exhibited a healthy status, characterized by the absence of bleeding on probing, reduced probing depths, and excellent plaque control.
5.2. Peri-implant healthcare
Clinical findings in patients with peri-implant mucositis and peri-implantitis, managed using blue®m therapy as an adjunct to non-surgical therapy revealed that local oxygen-based adjunct therapy was equally effective as conventionally used local adjuncts such as antibiotics, antibacterials, and probiotics (Shaheen et al., 2024). As similar pathogens are related to the aetiology of periodontitis and peri-implantitis, a low and stable concentration of O2 applied to subgingival biofilms containing peri-implant pathogens may demonstrate a selective effect compared to CHX, with a significant loss of the ability of periodontal bacteria to form biofilms.
In Fig. 9, a representative clinical case conducted by Dr. Alex Oliveira de Moura (Fortaleza/CE, Brazil) under the supervision of Professor Tatiana Deliberador (Curitiba/PR, Brazil) is presented.
Fig. 9.
Peri-implant mucositis. In the provided clinical images: (A) illustrates the initial clinical appearance of peri-implant tissues following the removal of the prosthesis, revealing signs of gingival inflammation and bleeding on probing. Probing depths measured between 2 to 4 mm, prompting a diagnosis of peri-implant mucositis. (B) showcases the application of blue®m gel, carefully spread across the inflamed region. Notably, the images capture the release of oxygen bubbles upon gel application. (C) presents the follow-up evaluation conducted after 48 h, tracking the progress of treatment. (D) exhibits the follow-up assessment after 7 days, revealing a notable improvement with a healthy appearance of the peri-implant tissues, indicating successful management of peri-implant mucositis.
Clinical investigations have emphasized the potential of the implant-abutment interface as a reservoir for microbial colonization, presenting a possible threat to peri-implant health and potentially compromising both short and long-term outcomes (Canullo et al., 2015, Fathi et al., 2023). The absence of standardized clinical protocols has necessitated the reliance on empirically derived methods to mitigate bacterial presence in the implant connection, predominantly employing CHX gels applied to the threads and subgingival regions of healing abutments and provisional implant restorations (Ghannad et al., 2015). Furthermore, the detachment and subsequent reattachment of healing abutments and prosthetic components not only facilitate oral bacteria ingress into the implant platform but also pose a risk of traumatizing peri-implant soft tissues, potentially disrupting the mucosal barrier and leading to compromised healing and marginal bone loss (Abrahamsson et al., 1997). Additionally, the local application of CHX on subgingival surfaces of healing abutments and prosthetic components may exert cytotoxic effects on soft tissue cells. An in vitro investigation utilizing human epidermal keratinocytes revealed that CHX impeded epithelial cell attachment onto the surface of implant provisional restorative materials, potentially impeding the integrity of the peri-implant soft tissue seal (Luchinskaya et al., 2017). As an alternative approach, clinicians have turned to oxygen and lactoferrin-releasing gel for treating and decontaminating the implant compartments and abutment surfaces, and at the same time aiming to actively promote the healing of the peri-implant soft tissues surrounding healing abutments and provisional restorations (Fig. 10).
Fig. 10.
Application of blue®m gel whenever fitting healing abutments (stock or customised) and implant restorations.
In Fig. 11, a representative clinical case conducted by Dr. Minas Leventis (London, UK) is presented.
Fig. 11.
Early implant placement in a 43-year-old healthy female patient. Clinical view four weeks after the “atraumatic” extraction of the lower right second premolar (A). A customized anatomical healing abutment (Cervico, VP Innovato Holdings Ltd, Cyprus) was fabricated. Blue®m gel was placed on the prosthetic screw and the subgingival part of the abutment (B). The customized abutment was immediately fitted onto the implant at implant placement appointment, and the flap was sutured tension-free around the healing abutment using 5–0 monofilament sutures (C). Application of blue®m gel immediately post-op and twice a day during the healing period (D). Clinical picture 3 months post-op (E). The unique anatomy of the anatomical healing abutment facilitated proper oral hygiene measures by the patient during the healing phase. This, in synergy with the daily local application of the oxygen and lactoferrin-releasing blue®m gel, allowed the patient to maintain the abutment free of plaque, and enhanced the healing process of the peri-implant tissues (F, G). Application of blue®m gel onto the implant platform and the peri-implant soft-tissues prior fitting the final restoration (H). Clinical view immediately after the instalment of the implant crown (I).
Another clinical scenario where oxygen topical therapy might be effective is the cleaning of contaminated titanium surfaces during the surgical treatment of peri-implantitis, as a safer alternative to CHX and citric acid. Currently, CHX and citric acid are the most commonly used chemotherapeutic agents, exhibiting high potential for biofilm removal from the contaminated implant surfaces, but the evidence is scarce. It is of clinical importance that these chemicals might be cytotoxic, restraining cell proliferation and collagen synthesis, and suppressing the re-attachment and spreading of fibroblasts. Moreover, citric acid may lead to corrosion of implant surfaces (Patil et al., 2022).
In Fig. 12, Fig. 13, Fig. 14, a representative clinical case conducted by Dr. Minas Leventis (London, UK) is presented.
Fig. 12.
Peri-implantitis in a 58-year-old male patient with non-contributory medical history. In the provided clinical images: (A, B) illustrate the initial clinical situation, revealing pus discharge on palpation, bleeding on probing, probing depths exceeding 8 mm, and bone loss affecting the implant #12. A diagnosis of localized peri-implantitis was made. The treatment protocol consisted of initial conservative therapy combined with application of blue®m gel with an interdental brush twice a day for six months (C), followed by surgical therapy.
Fig. 13.
Surgical approach six months after the conservative treatment. A site-specific full-thickness flap was raised and all inflammatory soft-tissues were thoroughly removed using sharp curettes and degranulation burrs (A). The exposed implant surfaces were cleaned with titanium brushes (B) and then polished (C). Subsequently, blue®m gel was topically applied for 5 mins to hydrate the bone and release oxygen and lactoferrin in the area, in an attempt to decontaminate the implant surfaces and the surrounding tissues, and to enhance the healing (D). After removing the gel and rinsing with sterile saline, guided bone regeneration was performed using a synthetic bone substitute (E) and the flap was repositioned and sutured using 5–0 PTFE sutures (F). Blue®m gel was applied immediately post-operatively and provided to the patient to use twice a day during the healing period (G).
Fig. 14.
Follow-up assessment after 3 months, revealing significant improvement with healthy peri-implant soft tissues without swelling, nor bleeding on probing (A), and reduced probing depths ranging from 3 to 4 mm (B). Periapical x-ray showing notable bone gain at the crestal level of the implant #12 (C).
5.3. Care of oral surgical wounds and oral lesions
Oral lesions, including traumatic lesions, aphthous ulcers, lichen planus, angular cheilitis, herpes infection, mucocele, and xerostomia, often require palliative and supportive treatment. Blue®m oxygen therapy has shown promise in managing such conditions, reducing postoperative pain, and promoting the healing of intraosseous cystic lesions. These elements when applied locally, can promoting angiogenesis and crucial cellular functions, favouring tissue oxygenation, regeneration, and controlling complications and pain after oral surgeries (Yip, 2015, Ngeow et al., 2022).
Topical application of oxygen in the forms of gel, solution or foam can help the decontamination of fresh extraction sockets (Fig. 15, Fig. 16), while oxygen and lactoferrin might play an essential role in healing of oral wounded tissues, promoting neovascularization, collagen synthesis and subsequent secondary intention re-epithelialization of open surgical wounds (Fig. 17, Fig. 18). The use of these agents topically on sutured flaps may promote and accelerate the healing by primary intention of incision lines and at the same time control the biofilms accumulated on the sutures (Fig. 19). In oral surgery the ability of pathogenic bacteria to adhere and colonize the threads of sutures can cause post-operative inflammation, infection and wound dehiscence, thus the application of antiseptic agents might limit complications and improve healing rates (Otten et al., 2005, Asher et al., 2019, Rodríguez Zorrilla et al., 2020). In Fig. 20, Fig. 21, Fig. 22, a representative clinical case conducted by Dr. Minas Leventis (London, UK) is presented.
Fig. 15.
Clinical pictures illustrating a fresh extraction socket (A). The removal of all soft tissues (B) is essential for successful healing. (C) Topical application for 5 mins of blue®m gel, followed by rising with sterile saline, can assist the decontamination of the site. (D) Clinical view 2 weeks post-operatively revealing the healthy granulation tissue inside the socket and newly-formed connective tissue and epithelium at the periphery of the wound.
Fig. 16.

Oxygen and lactoferrin can be also used (A) in the forms of foam (B) or solution (C, D) for the treatment of fresh extraction sockets.
Fig. 17.
Clinical images showing the uneventful open healing of grafted sockets (A). Six days post-operatively the graft is embedded in a fibrin matrix (B) and the topical application of oxygen and lactoferrin (C) twice a day during the healing period may enhance the production of new collagen and epithelium to promote the healing by secondary intention, and at the same time to control the bacteria, minimizing the risk for post-operative infection. (D) Three months post-operatively the alveolar ridge preservation procedure preserved the volume of the ridge and the grafted area is covered by newly-formed keratinized soft tissues. (E) The bone grafting resulted in the regeneration of adequate amount of hard tissues to facilitate the correct placement of dental implants.
Fig. 18.
(A) Wound dehiscence one week after implant placement of two implants and simultaneous guided bone regeneration in the upper anterior area. Incomplete closure of the wound with no signs of infection. The surgical site was treated with blue®m gel to address the wound dehiscence and promote the secondary intention healing of the area (B). To facilitate the application of the blue®m gel, the patient was instructed to apply it twice a day on the inner surface of the provisional partial denture, ensuring that the gel will stay for adequate time in contact with the healing tissues. The denture was inspected to ensure that no pressure was applied on the alveolar ridge (C, D). Three months later, excellent healing of the area is observed. The ridge is completely covered by healthy keratinised soft tissues (E).
Fig. 19.
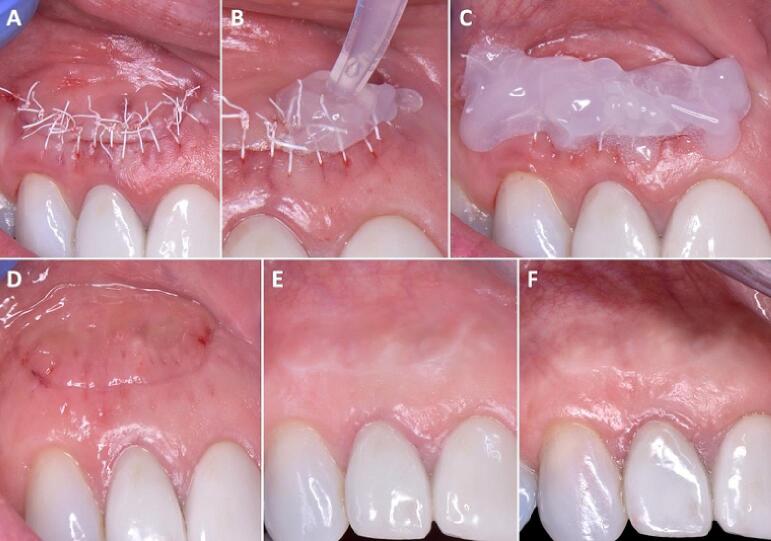
Controlling the bacteria that accumulate on the sutures and promoting the healing of the incision lines is essential for uneventful healing and for limiting the risk for infection, wound dehiscence and scar formation. A semilunar flap was sutured using 5–0 PTFE sutures (A). Application of blue®m gel immediately post-op and twice a day during the healing period (B, C). Clinical result after six days at suture removal (D), seven months post-operatively (E) and four years post-operatively (F).
Fig. 20.
Management of an impacted mandibular third molar with associated cystic lesion in a healthy 27-year-old male patient. Initial clinical view (A). The CBCT is showing the #48 in direct contact to the distal aspect of the distal root of the adjacent second molar, and the cystic lesion buccally to the roots of the impacted third molar (B, C). A full-thickness flap was raised (D) and subsequently bone was removed to expose the impacted #48, which was sectioned and removed in pieces. The cystic lesion (E) was enucleated in toto (F). The histopathological findings were consistent with a dentigerous cyst.
Fig. 21.
The site was thoroughly debrided of all soft tissues (A), and blue®m gel was applied for 5 mins and then rinsed with sterile saline (B). Subsequently, the bone defect was grafted using an injectable fully-resorbable synthetic bone substitute (C) and the flap was repositioned and sutured using 5–0 monofilament sutures (D). Blue®m gel was applied immediately post-operatively and provided to the patient to use twice a day during the healing period (E). (F, G) illustrate the clinical result eight months post-op. Adequate reconstruction of the soft and hard tissue topography and no residual periodontal pockets distally to the second molar.
Fig. 22.
Comparative CBCT images of the patient before (A, B) and eight months post-operatively (C, D). The treatment resulted in the regeneration of new bone and the reconstruction of the anatomy and architecture of the ridge.
6. Conclusions
In conclusion, the topical oxygen therapy, exemplified by the blue®m formula, emerges as a promising and novel approach in oral care therapy within clinical practice, based on both clinical observations and scientific findings. Oxygen and lactoferrin-releasing biomaterials seem to be safe and effective in controlling the pathogenic bacteria and in parallel promoting the healing in oral surgery, implantology and periodontology procedures, having applications in all phases of treatments. However, it is imperative to underscore the need for further clinical investigations to thoroughly validate and refine these therapeutic approaches. The clinical recommendations outlined in this review aim to guide dentists in maintaining their patients' oral health. While many suggestions and treatment protocols align with current evidence-based guidelines and relevant research findings, it is essential to acknowledge the existence of areas where sufficient published research is lacking. Recommendations in such instances are provided based on the consensus of experts in the field and traditional practices. Clinicians are advised to adhere to the clinical guidelines outlined in this review, taking into careful consideration the individual oral health status of each patient, the overarching treatment objectives, available resources, institutional policies, and alternative treatment solutions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdallah C. Perioperative chlorhexidine allergy: Is it serious? J. Anaesthesiol. Clin. Pharmacol. 2015;31(2):152–154. doi: 10.4103/0970-9185.155140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsson I., Berglundh T., Lindhe J. The mucosal barrier following abutment dis/reconnection. An experimental study in dogs. J. Clin. Periodontol. 1997;24(8):568–572. doi: 10.1111/j.1600-051x.1997.tb00230.x. PMID: 9266344. [DOI] [PubMed] [Google Scholar]
- Ahn, S.J., Burne, R.A., 2007. Effects of oxygen on biofilm formation and the AtlA autolysin of Streptococcus mutans. J. Bacteriol. 189 (17), 6293-6302. doi: 10.1128/JB.00546-07. Epub 2007 Jul 6. PMID: 17616606; PMCID: PMC1951938. [DOI] [PMC free article] [PubMed]
- Allen P.F. Assessment of oral health related quality of life. Health Qual. Life Outcomes. 2003;1:40. doi: 10.1186/1477-7525-1-40. PMID: 14514355; PMCID: PMC201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammons M.C. Anti-biofilm strategies and the need for innovations in wound care. Recent Pat. Antiinfect. Drug Discov. 2010;5(1):10–17. doi: 10.2174/157489110790112581. PMID: 19807676; PMCID: PMC7008005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher R., Chacartchi T., Tandlich M., Shapira L., Polak D. Microbial accumulation on different suture materials following oral surgery: a randomized controlled study. Clin Oral Investig. 2019;23(2):559–565. doi: 10.1007/s00784-018-2476-0. Epub 2018 May 2 PMID: 29717362. [DOI] [PubMed] [Google Scholar]
- Babich H., Wurzburger B.J., Rubin Y.L., Sinensky M.C., Blau L. An in vitro study on the cytotoxicity of chlorhexidine digluconate to human gingival cells. Cell Biol. Toxicol. 1995;11(2):79–88. doi: 10.1007/BF00767493. PMID: 7583874. [DOI] [PubMed] [Google Scholar]
- Baehni P.C., Takeuchi Y. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis. 2003;9(Suppl 1):23–29. doi: 10.1034/j.1601-0825.9.s1.5.x. PMID: 12974527. [DOI] [PubMed] [Google Scholar]
- Basudan A.M., Abas I., Shaheen M.Y., Alghamdi H.S. Effectiveness of Topical Oxygen Therapy in Gingivitis and Periodontitis: Clinical Case Reports and Review of the Literature. J. Clin. Med. 2024;13(5):1451. doi: 10.3390/jcm13051451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bescos R., Ashworth A., Cutler C., Brookes Z.L., Belfield L., Rodiles A., Casas-Agustench P., Farnham G., Liddle L., Burleigh M., White D. Effects of Chlorhexidine mouthwash on the oral microbiome. Sci. Rep. 2020;10(1):5254. doi: 10.1038/s41598-020-61912-4. PMID: 32210245; PMCID: PMC7093448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonez P.C., dos Santos Alves C.F., Dalmolin T.V., Agertt V.A., Mizdal C.R., da Costa Flores V., Marques J.B., Santos R.C.V., de Campos M.M.A. Chlorhexidine activity against bacterial biofilms. Am. J. Infect. Control. 2013;41(12):e119–e122. doi: 10.1016/j.ajic.2013.05.002. Epub 2013 Aug 1 PMID: 23910527. [DOI] [PubMed] [Google Scholar]
- Cabral C.T., Fernandes M.H. In vitro comparison of chlorhexidine and povidone–iodine on the long-term proliferation and functional activity of human alveolar bone cells. Clin. Oral Investig. 2007;11(2):155–164. doi: 10.1007/s00784-006-0094-8. Epub 2007 Jan 11 PMID: 17216529. [DOI] [PubMed] [Google Scholar]
- Canullo L., Peñarrocha-Oltra D., Covani U., Rossetti P.H.O. Microbiologic and Clinical Findings of Implants in Healthy Condition and with Peri-lmplantitis. Int. J. Oral Maxillofac. Implants. 2015;30(4):834–842. doi: 10.11607/jomi.3947. PMID: 26252036. [DOI] [PubMed] [Google Scholar]
- Caton J.G., Armitage G., Berglundh T., Chapple I.L., Jepsen S., Kornman K.S., Mealey B.L., Papapanou P.N., Sanz M., Tonetti M.S. A new classification scheme for periodontal and peri-implant diseases and conditions–Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018;45(Suppl 20):S1–S8. doi: 10.1111/jcpe.12935. PMID: 29926489. [DOI] [PubMed] [Google Scholar]
- Chang Y., Ping A., Chang C., Betz V.M., Cai L., Ren B. Lactoferrin mediates enhanced osteogenesis of adipose-derived stem cells: innovative molecular and cellular therapy for bone repair. Int. J. Mol. Sci. 2023;24(2):1749. doi: 10.3390/ijms24021749. PMID: 36675267; PMCID: PMC9864243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple I.L., Mealey B.L., Van Dyke T.E., Bartold P.M., Dommisch H., Eickholz P., Geisinger M.L., Genco R.J., Glogauer M., Goldstein M., Griffin T.J. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018;89(Suppl 1):S74–S84. doi: 10.1002/JPER.17-0719. PMID: 29926944. [DOI] [PubMed] [Google Scholar]
- Chiewchalermsri C., Sompornrattanaphan M., Wongsa C., Thongngarm T. Chlorhexidine allergy: current challenges and future prospects. J. Asthma Allergy. 2020;13:127–133. doi: 10.2147/JAA.S207980. PMID: 32210588; PMCID: PMC7069565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieplik F., Jakubovics N.S., Buchalla W., Maisch T., Al-Ahmad A. Resistance toward chlorhexidine in oral bacteria–is there cause for concern? Front. Microbiol. 2019;10:587. doi: 10.3389/fmicb.2019.00587. PMID: 30967854; PMCID: PMC6439480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cláudio, M.M., Garcia, V.G., Freitas, R.M., Rodrigues, J.V.S., Wainwright, M., Casarin, R.C., Duque, C., Theodoro, L.H., 2023. Association of active oxygen‐releasing gel and photodynamic therapy in the treatment of residual periodontal pockets in type 2 diabetic patients: A randomized controlled clinical study. J. Periodontol. doi: 10.1002/JPER.23-0125. Epub ahead of print. PMID: 38112075. [DOI] [PubMed]
- Cunha E.J., Auersvald C.M., Deliberador T.M., Gonzaga C.C., Esteban Florez F.L., Correr G.M., Storrer C.L.M. Effects of active oxygen toothpaste in supragingival biofilm reduction: a randomized controlled clinical trial. Int. J. Dent. 2019;2019:3938214. doi: 10.1155/2019/3938214. PMID: 31354823; PMCID: PMC6636571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smet G.H., Kroese L.F., Menon A.G., Jeekel J., van Pelt A.W., Kleinrensink G.J., Lange J.F. Oxygen therapies and their effects on wound healing. Wound Repair Regen. 2017;25(4):591–608. doi: 10.1111/wrr.12561. Epub 2017 Aug 7 PMID: 28783878. [DOI] [PubMed] [Google Scholar]
- Deliberador T.M., Weiss S.G., Rychuv F., Cordeiro G., Ten Cate M.C.L., Leonardi L., Brancher J.A., Scariot R. Comparative analysis in vitro of the application of blue® m oral gel versus chlorhexidine on Porphyromonas gingivalis: A pilot study. Advances in Microbiology. 2020;10(04):194–201. doi: 10.4236/aim.2020.104015. [DOI] [Google Scholar]
- Deliberador T.M., Macalossi J.M.S., Tenorio C., Dalĺ Agnol, G.D.S., Boia, M.F., Zielak, J.C. An oxygen-releasing agent promotes healing of skin wounds in rats. J. Wound Care. 2023;32(11):738–747. doi: 10.12968/jowc.2023.32.11.738. PMID: 37907358. [DOI] [PubMed] [Google Scholar]
- Dissemond J., Kröger K., Storck M., Risse A., Engels P. Topical oxygen wound therapies for chronic wounds: a review. J. Wound Care. 2015;24(2) doi: 10.12968/jowc.2015.24.2.53. PMID: 25647433, 53–54, 56–60, 62–63. [DOI] [PubMed] [Google Scholar]
- Eisenbud D.E. Oxygen in wound healing: nutrient, antibiotic, signaling molecule, and therapeutic agent. Clin. Plast. Surg. 2012;39(3):293–310. doi: 10.1016/j.cps.2012.05.001. Epub 2012 Jun 2 PMID: 22732377. [DOI] [PubMed] [Google Scholar]
- Fathi, A., Rismanchian, M., Dezaki, S.N., 2023. Effectiveness of different antimicrobial agents on malodor prevention in two-stage dental implants: a double-blinded randomized clinical trial. Eur. J. Dent. 17 (2), 524-529. doi: 10.1055/s-0042-1747954. Epub 2022 Jul 12. PMID: 35820442; PMCID: PMC10329548. [DOI] [PMC free article] [PubMed]
- Fernandes M., Lourenço T., Lopes A., Santos A.S., Santos M.C.P., Barbosa M.P. Chlorhexidine: a hidden life-threatening allergen. Asia Pac. Allergy. 2019;9(4):e29. doi: 10.5415/apallergy.2019.9.e29. PMID: 31720240; PMCID: PMC6826114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez y Mostajo, M., Exterkate, R.A., Buijs, M.J., Crielaard, W., Zaura, E., 2017. Effect of mouthwashes on the composition and metabolic activity of oral biofilms grown in vitro. Clin. Oral. Investig. 21 (4), 1221-1230. doi: 10.1007/s00784-016-1876-2. Epub 2016 Jun 23. PMID: 27337976; PMCID: PMC5399055. [DOI] [PMC free article] [PubMed]
- Fernandez y Mostajo, M., van der Reijden, W.A., Buijs, M.J., Beertsen, W., Van der Weijden, F., Crielaard, W., Zaura, E., 2014. Effect of an oxygenating agent on oral bacteria in vitro and on dental plaque composition in healthy young adults. Front. Cell Infect. Microbiol. 4, 95. doi: 10.3389/fcimb.2014.00095. PMID: 25101249; PMCID: PMC4107829. [DOI] [PMC free article] [PubMed]
- Filoche S., Wong L., Sissons C.H. Oral biofilms: emerging concepts in microbial ecology. J. Dent. Res. 2010;89(1):8–18. doi: 10.1177/0022034509351812. PMID: 19918089. [DOI] [PubMed] [Google Scholar]
- Gao, R., Watson, M., Callon, K.E., Tuari, D., Dray, M., Naot, D., Amirapu, S., Munro, J.T., Cornish, J., Musson, D.S., 2018. Local application of lactoferrin promotes bone regeneration in a rat critical‐sized calvarial defect model as demonstrated by micro‐CT and histological analysis. J. Tissue Eng. Regen. Med. 12 (1), e620-e626. doi: 10.1002/term.2348. Epub 2017 Apr 9. PMID: 27860377; PMCID: PMC5811776. [DOI] [PMC free article] [PubMed]
- Gavrailov T., Chenchev I., Gevezova M., Draganova M., Sarafian V. Effect on Cellular Vitality In Vitro of Novel APRF-Chlorhexidine Treated Membranes. J. Funct. Biomater. 2022;13(4):226. doi: 10.3390/jfb13040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco R.J., Genco F.D. Common risk factors in the management of periodontal and associated systemic diseases: the dental setting and interprofessional collaboration. J. Evid. Based Dent. Pract. 2014;14(Suppl):4–16. doi: 10.1016/j.jebdp.2014.03.003. Epub 2014 Mar 27 PMID: 24929584. [DOI] [PubMed] [Google Scholar]
- Ghannad F., Alkadi L.T., Wiebe C.B., Shen Y., Haapasalo M., Larjava H.S. Intra-operative application of chlorhexidine gel reduces bacterial counts in internal implant cavity. Eur. J. Oral Sci. 2015;123(6):425–431. doi: 10.1111/eos.12213. Epub 2015 Sep 28 PMID: 26411835. [DOI] [PubMed] [Google Scholar]
- Glick M., da Silva O.M., Seeberger G.K., Xu T., Pucca G., Williams D.M., Kess S., Eiselé J.L., Séverin T. FDI Vision 2020: shaping the future of oral health. Int. Dent. J. 2012;62(6):278–291. doi: 10.1111/idj.12009. PMID: 23252585; PMCID: PMC9374976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görmez U., Kürkcü M., Benlidayi M.E., Ulubayram K., Sertdemir Y., Dağlioğlu K. Effects of bovine lactoferrin in surgically created bone defects on bone regeneration around implants. J. Oral Sci. 2015;57(1):7–15. doi: 10.2334/josnusd.57.7. PMID: 25807903. [DOI] [PubMed] [Google Scholar]
- Grootveld M., Lynch E., Page G., Chan W., Percival B., Anagnostaki E., Mylona V., Bordin-Aykroyd S., Grootveld K.L. Potential advantages of peroxoborates and their ester adducts over hydrogen peroxide as therapeutic agents in oral healthcare products: chemical/biochemical reactivity considerations in vitro, ex vivo and in vivo. Dent. J. (basel) 2020;8(3):89. doi: 10.3390/dj8030089. PMID: 32784634; PMCID: PMC7559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan J., Bright R., Hayles A., Palms D., Zilm P., Barker D., Vasilev K. Preventing peri-implantitis: the quest for a next generation of titanium dental implant. ACS Biomater. Sci Eng. 2022;8(11):4697–4737. doi: 10.1021/acsbiomaterials.2c00540. Epub 2022 Oct 14 PMID: 36240391. [DOI] [PubMed] [Google Scholar]
- Icriverzi M., Dinca V., Moisei M., Evans R.W., Trif M., Roseanu A. Lactoferrin in bone tissue regeneration. Curr. Med. Chem. 2020;27(6):838–853. doi: 10.2174/0929867326666190503121546. PMID: 31258057. [DOI] [PubMed] [Google Scholar]
- Jindal S.K. Oxygen therapy: important considerations. Indian J. Chest Dis. Allied Sci. 2008;50(1):97–107. PMID: 18610694. [PubMed] [Google Scholar]
- Jones C.G. Chlorhexidine: is it still the gold standard? Periodontol. 1997;2000(15):55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. PMID: 9643233. [DOI] [PubMed] [Google Scholar]
- Kim C.W., Son K.N., Choi S.Y., Kim J. Human lactoferrin upregulates expression of KDR/Flk-1 and stimulates VEGF-A-mediated endothelial cell proliferation and migration. FEBS Lett. 2006;580(18):4332–4336. doi: 10.1016/j.febslet.2006.06.091. Epub 2006 Jul 7 PMID: 16842782. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy G., Narayana A., Balkrishanan D. Chlorhexidine for the treatment of peri-implantitis: Is it a benison? J. Long Term Eff. Med. Implants. 2022;32(1):19–23. doi: 10.1615/JLongTermEffMedImplants.2021039510. PMID: 35377990. [DOI] [PubMed] [Google Scholar]
- Linden, R., LeDez, K., Hopf, H., Fife, C., 2014. Topical oxygen therapy is not hyperbaric therapy and the two treatments should not be confused. Int. Wound J. 11 (2), 230-231. doi: 10.1111/j.1742-481X.2012.01069.x. Epub 2012 Aug 22. PMID: 22913614; PMCID: PMC7950685. [DOI] [PMC free article] [PubMed]
- Liu J.X., Werner J., Kirsch T., Zuckerman J.D., Virk M.S. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. J. Bone Jt. Infect. 2018;3(4):165–172. doi: 10.7150/jbji.26355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Hu W., Tian Z., Yuan D., Yi G., Zhou Y., Cheng Q., Zhu J., Li M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019;14:11. doi: 10.1186/s13020-019-0232-2. PMID: 30936939; PMCID: PMC6425673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchinskaya D., Du R., Owens D.M., Tarnow D., Bittner N. Various Surface Treatments to Implant Provisional Restorations and Their Effect on Epithelial Cell Adhesion: A Comparative. In Vitro Study. Implant Dent. 2017;26(1):12–23. doi: 10.1097/ID.0000000000000538. PMID: 28114240; PMCID: PMC7709421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei B.M., Imanishi S.A., de Oliveira Ramos G., de Campos P.S., Weiss S.G., Deliberador T.M. Mouthwash with active oxygen (blue® m) reduces postoperative inflammation and pain. Case Rep. Dent. 2021;2021:5535807. doi: 10.1155/2021/5535807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999;12(1):147–179. doi: 10.1128/CMR.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakonechna A., Dore P., Dixon T., Khan S., Deacock S., Holding S., Abuzakouk M. Immediate hypersensitivity to chlorhexidine is increasingly recognised in the United Kingdom. Allergol. Immunopathol. (madr) 2014;42(1):44–49. doi: 10.1016/j.aller.2012.08.001. Epub 2012 Dec 20 PMID: 23265264. [DOI] [PubMed] [Google Scholar]
- Ngeow W.C., Tan C.C., Goh Y.C., Deliberador T.M., Cheah C.W. A narrative review on means to promote oxygenation and angiogenesis in oral wound healing. Bioengineering (basel). 2022;9(11):636. doi: 10.3390/bioengineering9110636. PMID: 36354548; PMCID: PMC9688034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niveda R., Kaarthikeyan G. Effect of oxygen releasing oral gel compared to chlorhexidine gel in the treatment of periodontitis. J.P.R.I. 2020;32(19):75–82. doi: 10.9734/jpri/2020/v32i1930711. [DOI] [Google Scholar]
- Orsted, H.L., Poulson, R., Advisory Group, Baum, J., Christensen, D., Despatis, M., Goettl, K., Haligowski, D., Ho, C., Louis, K., O'Sullivan‐Drombolis, D., 2012. Evidence‐based practice standards for the use of topical pressurised oxygen therapy. Int. Wound J. 9 (3), 271-284. doi: 10.1111/j.1742-481X.2012.00956.x. Epub 2012 Apr 11. PMID: 22494402; PMCID: PMC7950340. [DOI] [PMC free article] [PubMed]
- Ortega-Llamas L., Quiñones-Vico M.I., García-Valdivia M., Fernández-González A., Ubago-Rodríguez A., Sanabria-de la Torre R., Arias-Santiago S. Cytotoxicity and wound closure evaluation in skin cell lines after treatment with common antiseptics for clinical use. Cells. 2022;11(9):1395. doi: 10.3390/cells11091395. PMID: 35563705; PMCID: PMC9099882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten J.E., Wiedmann-Al-Ahmad M., Jahnke H., Pelz K. Bacterial colonization on different suture materials—a potential risk for intraoral dentoalveolar surgery. J Biomed. Mater. Res. B Appl. Biomater. 2005;74(1):627–635. doi: 10.1002/jbm.b.30250. PMID: 15889436. [DOI] [PubMed] [Google Scholar]
- Pałka, Ł., Nowakowska-Toporowska, A., Dalewski, B., 2022, April. Is chlorhexidine in dentistry an ally or a foe? A narrative review. Healthcare (Basel). 10 (5), 764. doi: 10.3390/healthcare10050764. PMID: 35627901; PMCID: PMC9141996. [DOI] [PMC free article] [PubMed]
- Patil C., Agrawal A., Abullais S.S., Arora S., Khateeb S.U., Fadul A., Elagib M. Effectiveness of different chemotherapeutic agents for decontamination of infected dental implant surface: a systematic review. Antibiotics (basel). 2022;11(5):593. doi: 10.3390/antibiotics11050593. PMID: 35625237; PMCID: PMC9137954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, P.E., Baez, R.J., Ogawa, H., 2020. Global application of oral disease prevention and health promotion as measured 10 years after the 2007 World Health Assembly statement on oral health. Community Dent. Oral Epidemiol. 48 (4), 338-348. doi: 10.1111/cdoe.12538. Epub 2020 May 8. PMID: 32383537; PMCID: PMC7496398. [DOI] [PMC free article] [PubMed]
- Petersen P.E., Yamamoto T. Improving the oral health of older people: the approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2005;33(2):81–92. doi: 10.1111/j.1600-0528.2004.00219.x. PMID: 15725170. [DOI] [PubMed] [Google Scholar]
- Pucher J.J., Daniel C. The effects of chlorhexidine digluconate on human fibroblasts in vitro. J. Periodontol. 1992;63(6):526–532. doi: 10.1902/jop.1992.63.6.526. PMID: 1625152. [DOI] [PubMed] [Google Scholar]
- Ray, P.D., Huang, B.W., Tsuji, Y., 2012. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24 (5), 981-990. doi: 10.1016/j.cellsig.2012.01.008. Epub 2012 Jan 20. PMID: 22286106; PMCID: PMC3454471. [DOI] [PMC free article] [PubMed]
- Rodriguez P.G., Felix F.N., Woodley D.T., Shim E.K. The role of oxygen in wound healing: a review of the literature. Dermatol. Surg. 2008;34(9):1159–1169. doi: 10.1111/j.1524-4725.2008.34254.x. Epub 2008 May 29 PMID: 18513296. [DOI] [PubMed] [Google Scholar]
- Rodríguez Zorrilla S., Blanco Carrión A., García García A., Galindo Moreno P., Marichalar Mendía X., Seoane Prado R., Pérez Estévez A.J., Pérez-Sayáns M. Effect of antiseptic gels in the microbiologic colonization of the suture threads after oral surgery. Sci Rep. 2020;10(1):8360. doi: 10.1038/s41598-020-65007-y. PMID: 32433572; PMCID: PMC7239853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M.A., Garcez T., Savic S., Garvey L.H. Chlorhexidine allergy in the perioperative setting: a narrative review. Br. J. Anaesth. 2019;123(1):e95–e103. doi: 10.1016/j.bja.2019.01.033. Epub 2019 Apr 5 PMID: 30955832. [DOI] [PubMed] [Google Scholar]
- Samaranayake L., Matsubara V.H. Normal oral flora and the oral ecosystem. Dent Clin North Am. 2017;61(2):199–215. doi: 10.1016/j.cden.2016.11.002. PMID: 28317562. [DOI] [PubMed] [Google Scholar]
- Santos V.C.E.D., Maquera-Huacho P.M., Imbriani M.J.M., Minhaco V.M., Spolidorio D.M.P. Effects of BlueM® against Streptococcus mutans biofilm and its virulence gene expression. Braz. Dent. J. 2023;34(1):19–28. doi: 10.1590/0103-6440202305133. PMID: 36888841; PMCID: PMC10027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapoli L., Girardi A., Palmieri A., Testori T., Zuffetti F., Monguzzi R., Lauritano D., Carinci F. Microflora and periodontal disease. Dent. Res. J. (isfahan) 2012;9(Suppl 2):S202–S206. doi: 10.4103/1735-3327.109755. PMID: 23814584; PMCID: PMC3692174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreml S., Szeimies R.M., Prantl L., Karrer S., Landthaler M., Babilas P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010;163(2):257–268. doi: 10.1111/j.1365-2133.2010.09804.x. Epub 2010 Apr 15 PMID: 20394633. [DOI] [PubMed] [Google Scholar]
- Shaheen M.Y., Abas I., Basudan A.M., Alghamdi H.S. Local Oxygen-Based Therapy (blue® m) for Treatment of Peri-Implant Disease: Clinical Case Presentation and Review of Literature about Conventional Local Adjunct Therapies. Medicina. 2024;60(3):447. doi: 10.3390/medicina60030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiham A., Watt R.G. The common risk factor approach: a rational basis for promoting oral health. Community Dent. Oral Epidemiol. 2000;28(6):399–406. doi: 10.1034/j.1600-0528.2000.028006399.x. PMID: 11106011. [DOI] [PubMed] [Google Scholar]
- Shi P., Fan F., Chen H., Xu Z., Cheng S., Lu W., Du M. A bovine lactoferrin–derived peptide induced osteogenesis via regulation of osteoblast proliferation and differentiation. J. Dairy Sci. 2020;103(5):3950–3960. doi: 10.3168/jds.2019-17425. Epub 2020 Mar 18 PMID: 32197844. [DOI] [PubMed] [Google Scholar]
- Shibli, J.A., Rocha, T.F., Coelho, F., de Oliveira Capote, T.S., Saska, S., Melo, M.A., Pingueiro, J.M.S., de Faveri, M., Bueno-Silva, B., 2021. Metabolic activity of hydro-carbon-oxo-borate on a multispecies subgingival periodontal biofilm: a short communication. Clin. Oral Investig. 25 (10), 5945-5953. doi: 10.1007/s00784-021-03900-0. Epub 2021 Mar 28. PMID: 33774716; PMCID: PMC8443475. [DOI] [PMC free article] [PubMed]
- Takayama Y., Aoki R. Roles of lactoferrin on skin wound healing. Biochem. Cell Biol. 2012;90(3):497–503. doi: 10.1139/o11-054. Epub 2012 Feb 14 PMID: 22332789. [DOI] [PubMed] [Google Scholar]
- Taubman M.A., Kawai T., Han X. The new concept of periodontal disease pathogenesis requires new and novel therapeutic strategies. J. Clin. Periodontol. 2007;34(5):367–369. doi: 10.1111/j.1600-051X.2007.01065.x. PMID: 17448041. [DOI] [PubMed] [Google Scholar]
- Tian, M., Tian, D., 2023. The role of lactoferrin in bone remodeling: evaluation of its potential in targeted delivery and treatment of metabolic bone diseases and orthopedic conditions. Front. Endocrinol. (Lausanne). 14:1218148. doi: 10.3389/fendo.2023.1218148. PMID: 37680888; PMCID: PMC10482240. [DOI] [PMC free article] [PubMed]
- Tibbles P.M., Edelsberg J.S. Hyperbaric-oxygen therapy. N. Engl. J. Med. 1996;334(25):1642–1648. doi: 10.1056/NEJM199606203342506. PMID: 8628361. [DOI] [PubMed] [Google Scholar]
- Trybek G., Jedliński M., Jaroń A., Preuss O., Mazur M., Grzywacz A. Impact of lactoferrin on bone regenerative processes and its possible implementation in oral surgery–a systematic review of novel studies with metanalysis and metaregression. BMC Oral Health. 2020;20(1):232. doi: 10.1186/s12903-020-01211-6. PMID: 32843024; PMCID: PMC7448436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerachamy S., Yarlagadda T., Manivasagam G., Yarlagadda P.K. Bacterial adherence and biofilm formation on medical implants: a review. Proc. Inst. Mech. Eng. h. 2014;228(10):1083–1099. doi: 10.1177/0954411914556137. PMID: 25406229. [DOI] [PubMed] [Google Scholar]
- Wang Z., De La Fuente-Núñez C., Shen Y., Haapasalo M., Hancock R.E. Treatment of oral multispecies biofilms by an anti-biofilm peptide. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132512. PMID: 26168273; PMCID: PMC4500547 e0132512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis J.R., Gabaldón T. The human oral microbiome in health and disease: from sequences to ecosystems. Microorganisms. 2020;8(2):308. doi: 10.3390/microorganisms8020308. PMID: 32102216; PMCID: PMC7074908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2022. Global oral health status report: towards universal health coverage for oral health by 2030. Geneva: World Health Organization https://www.who.int/publications/i/item/9789240061484.
- Yip, W.L., 2015. Influence of oxygen on wound healing. Int Wound J. 12 (6), 620-624. doi: 10.1111/iwj.12324. Epub 2014 Jun 26. PMID: 24974913; PMCID: PMC7950466. [DOI] [PMC free article] [PubMed]
- Yoshinari M., Oda Y., Kato T., Okuda K., Hirayama A. Influence of surface modifications to titanium on oral bacterial adhesion in vitro. J. Biomed. Mater. Res. 2000;52(2):388–394. doi: 10.1002/1097-4636(200011)52:2<388::aid-jbm20>3.0.co;2-e. PMID: 10951380. [DOI] [PubMed] [Google Scholar]
- Zanatta, F.B., Antoniazzi, R.P., Rösing, C.K., 2010. Staining and calculus formation after 0.12% chlorhexidine rinses in plaque-free and plaque covered surfaces: a randomized trial. J. Appl. Oral Sci. 18 (5), 515-521. doi: 10.1590/s1678-77572010000500015. PMID: 21085810; PMCID: PMC4246385. [DOI] [PMC free article] [PubMed]