Abstract
Different spatial positions lead to inconsistent fermentation effects and flavors, however, the spatial heterogeneity of Qingxiangxing (QXX) Baijiu remains unknown. We investigated the microbes, flavors, and physicochemical properties of different layers in fermented grains of QXX Baijiu using Illumina HiSeq sequencing, two-dimensional gas chromatography–mass spectrometry (GC × GC–MS) and ultra-high performance liquid chromatography-mass (UHPLC-MS). A total of 79 volatiles, 1596 metabolites, 50 bacterial genera, and 52 fungal genera were identified. The contents distribution followed the order: upper layer > bottom layer > middle layer. Organic acids and derivatives were the main differential metabolites across the three layers. Starch, pH, and reducing sugar levels increased from the upper to bottom layer. Saccharomyces and Lactobacillus were dominant microbes. Pediococcus, the biomarker of upper layer, showed positive correlations with formic acid, ethyl lactate, acetic acid, ethyl linoleate, and ethyl oleate. These findings deepen our understanding of the fermentation and flavor formation mechanisms of QXX Baijiu.
Keywords: Qingxiangxing baijiu, Different layers of fermented grains, Microbial composition, Volatile compounds, Metabolites, Physicochemical properties
Highlights
-
•
The spatial heterogeneity of Qingxiangxing Baijiu fermentation was firstly explored.
-
•
The microbiota, flavor and physicochemical properties were comprehensively studied.
-
•
Upper-layer fermented grains played an important role in the flavor formation.
-
•
The Pediococcus promoted the production of flavor compounds in upper fermented grain.
-
•
Differential flavor compounds were closely correlated with microbial communities.
1. Introduction
Baijiu is a traditional distilled liquor in China with a long history. The production of Baijiu encompasses four key processes: traditional solid-state fermentation, distillation, aging, and blending. Qingxiangxing (QXX) is one of the four major aroma types of Baijiu in China, which is fermented primarily with sorghum as the raw material (Huang et al., 2020). It is characterized by a clear and mellow aroma, sweetness, softness, and a long aftertaste. Ethyl acetate and ethyl lactate are the principal aroma compounds of QXX Baijiu (Li et al., 2023).
As the carriers of microbes, fermented grains (FG) play an important role in the formation of Baijiu flavor. As a relatively independent ecosystem, the micro-ecology in the pit is different in different space environments (Qian et al., 2021). During microbial growth, reproduction, and metabolism, the physicochemical properties undergo changes during fermentation, and the fermentation effects in different layers of FG within pit vary (Qian et al., 2021). Most studies (Ao et al., 2022; Ding, Wu, Huang, & Zhou, 2016; Guan et al., 2023; Qian et al., 2021) suggest that the nutrients gradually sink with the water under gravity during the fermentation process, and the fermentation happening in the upper layer (UL) of FG is not as effective as that of the the bottom layer (BL), and consequently impacting the formation of flavor substances (Ding et al., 2016). Guan et al. (2023) demonstrated that concentrations of ethyl hexadecanoate, ethyl acetate, and ethyl butyrate progressively increased in the UL, middle layer (ML), and BL of Nongxiangxing (NXX) Baijiu (Guan et al., 2023). Temperature, oxygen, and acidity are closely associated with the composition of the microbial community (Guan et al., 2023). However, some studies (Ao et al., 2022; Ding et al., 2016) have found that the highest content of flavor substances occurs in the UL. Ao et al. (2022) indicated that at the end of Lu-flavor liquor fermentation, the content of major volatile flavor compounds (VFCs) in FG was highest in the UL, followed by the BL, and were lowest in the ML (Ao et al., 2022). Xiaolong Hu et al. (2020) employed 16S rRNA gene sequencing to analyze the bacterial communities in the FG of Luzhou-flavor liquor across various spatial locations during the fermentation. Their findings revealed that the bacterial community diversity in the ML's FG exceeded that in both in UL and BL at the same time in fermentation (Hu et al., 2020). However, existing studies have primarily focused on the spatial heterogeneity of NXX Baijiu and Jiangxiangxing (JXX) Baijiu (Ao et al., 2022; Guan et al., 2023; Hu et al., 2020). For QXX Baijiu, research has mainly been conducted on the entire FG microbial community, physicochemical characteristics, and flavor or changes during the fermentation process (Huang et al., 2020). Research on the distinct layers of FG in QXX Baijiu is limited, and the non-volatile flavor compounds (non-VFCs) are infrequently detected by metabonomics, hindering comprehensive analysis. Consequently, the limited understanding of the brewing mechanism of QXX Baijiu impedes the development and optimization of traditional brewing technologies.
In this study, we comprehensively investigated the physicochemical properties, microbial community composition, flavor substances, and metabolic pathways of the FG from UL, ML, and BL of QXX Baijiu using Illumina HiSeq sequencing, ultra-high performance liquid chromatography-mass (UHPLC-MS) for non-targeted metabolomics, and two-dimensional gas chromatography–mass spectrometry (GC × GC–MS). The relationship between key microorganisms and other factors was also explored. Differences between the layers of FG and their potential formation mechanism were analyzed. The results of this study will enhance our understanding of the spatial heterogeneity of FG and the brewing mechanism of QXX Baijiu. This study provides a theoretical basis for subsequent production optimization.
2. Materials and methods
2.1. Samples collection
QXX Baijiu was fermented in a stainless steel tank for 15 days at 25 °C using red glutinous sorghum as the raw material and Xiaoqu as the starter. The sorghum, Xiaoqu, and fermented grain sample used in our study were sourced from Sichuan Tujiu Liquor Co., Ltd.. Three tanks were selected for replicates.
The mixed FG samples were collected at the beginning of fermentation (day 0, before fermentation) as the BF group. Samples collected from UL, ML, and BL (each layer was approximately 50 cm high) were collected at the end of fermentation (day 15, after fermentation) as AF group. Subsequently, the samples from each layer were blended. One portion of the FG samples was stored at 4 °C for the analyses of physicochemical properties and VFCs analysis, while the other portion was stored at −80 °C for microbial and non-VFCs extraction and further analyses.
2.2. The physicochemical properties analysis
The physicochemical properties of FG, including acidity, pH, starch content, and reducing sugar levels were assessed using standard analytical methods (Guan et al., 2023). The moisture content in the various FG samples was determined by assessing the dry/wet weight at 105 °C.
2.3. DNA extraction, PCR, and Illumina HiSeq sequencing
The total DNA of FG samples was extracted using the Wizard® DNA kit (Promega, USA), following the manufacturer's protocol. The concentration and purity of the extracted DNA were measured using a Nanodrop 2000 ultra-micro spectrophotometer (Thermo Scientific, Illkirch, France) and agarose gel electrophoresis (Beijing Liuyi biotechnology Co.,Ltd.). The V3-V4 hypervariable regions of the 16S rRNA genes were amplified using the primer set 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) for bacteria. The ITS3-ITS4 region of the ITS rRNA genes were amplified using the ITS3R (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4F (5′-TCCTCCGCTTATTGATATGC-3′) primers for fungi. Library construction was carried out according to the instructions of the NextFlex Rapid DNA-SEQ Kit (BIOO Scientific, USA). High-throughput sequencing was conducted by the Illumina HiSeq X Ten (Illumina, San Diego, CA, USA) platform.
2.4. Extraction of volatile compounds
Volatile compounds from samples were extracted using the head space-solid phase microextraction (HS-SPME) method (Guan et al., 2023). Briefly, 2 g of the sample was placed into a 15 mL headspace vial with 5 mL sterile distilled water and 1.5 g NaCl, followed by performed ultrasonic treatment for 30 min. Subsequently, 20 μL of 0.822 mg/L 2-Octanol was added as internal standard. The vial was then sealed and equilibrated at 60 °C for 20 min. The 50/30 μm DVB/CAR/PDMS fibers (Supelco, Bellefonte, PA, USA) were inserted into the headspace of the vial to absorb the volatiles at 60 °C for 45 min. Afterward, the fiber was inserted into the GC injection port for desorption at 250 °C for 5 min.
2.5. GC × GC–MS detection and analysis of VFCs
VFCs in FG were analyzed using GC × GC–MS instrument (GCMS-QP2020 NX, Shimadzu, Japan) following previously reported methods (Fan et al., 2018). The one-dimensional (1D) column used was a DB wax column (30 m × 0.25 mm × 0.25 μm). The two-dimensional (2D) column comprised a DB-17MS column (1.2 m × 0.18 mm × 0.18 μm) and was equipped a solid-state thermal modulator HV (c720–21,005). Helium gas (purity >99.9999%) was used as a carrier gas for splitless injections at a flow rate of 1.0 mL/min. Additionally, 2D analysis time was synchronized with the 1D column, featuring a 4 s modulation period. The GC–MS test parameters were as previously described (Gao, Zhang, Regenstein, Yin, & Zhou, 2018).
VFCs were identified in the samples by matching their retention indices and mass spectra against the National Institute of Standards and Technology (NIST 20) spectral database. Relative quantification was performed by normalizing peak areas. The odor activity values (OAVs) were calculated based on the quantitative results and corresponding odor threshold values. The OAV of volatile substances was calculated by dividing concentration by their reported threshold value (Table S4).
2.6. Untargeted UHPLC-MS Metabolomic analysis
A UHPLC-Q Exactive HF-X system (Thermo Fisher Scientific, USA) was employed to separate and analyze the metabolites. A UHPLC system equipped with an ACQUITY HSS T3 column (100 mm × 2.1 mm i.d. × 1.8 μm; Waters, Milford, USA) facilitated the chromatographic separation of metabolites. Mobile phase A consisted of 0.1% formic acid in water: acetonitrile (95:5, v/v). Mobile phase B was composed of acetonitrile, isopropanol, and water in a ratio of 47.5:47.5:5 (v/v). All reagents were purchased from Fisher Chemical Co., Ltd. (USA). The flow rate was 0.40 mL/min, the injection volume was 2 μL, and the temperature of the chromatographic column was 40 °C. LC-MS was performed as previously described (Zeng et al., 2022). The mass spectrometric data were collected with an electrospray ionization (ESI) source operating in both positive and negative ion mode. Fermented grains (50 mg) were accurately weighed, and the metabolites were extracted using a 400 μL methanol:water (4:1, v/v) solution containing 0.02 mg/mL L-2-chlorophenylalanin as internal standard. The sample solution was placed in a high-throughput tissue crusher (Wonbio-96c, Shanghai Wanbo Biotechnology Co., LTD) and ground for 6 min at −10 °C, followed by ultrasonication at 40 kHz for 30 min at 5 °C. The samples were incubated at −20 °C for 30 min to precipitate proteins. Following centrifugation at 13000 g at 4 °C for 15 min, the supernatant was transferred to sample vials for LC-MS/MS analysis. All sample-derived supernatants were mixed in equivalent volumes to prepare the quality control sample (QC). The metabolites were identified and annotated by referencing the Human Metabolome (HMDB) database and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, pathway enrichment analysis was conducted by MetaboAnalyst 4.0.
2.7. Statistical analysis
SIMCA-P software (Version 14.1, Umetrics, Sweden) was used for principal component analysis (PCA) of the relative content of VFCs, and partial least squares discriminant analysis (PLS-DA). Histogram, venn diagram, and cluster analysis of the relative content of VFCs were conducted using Origin software (Version 7.00, OriginLab Corporation, Northampton, MA, USA). GraphPad Prism software (Version 8.2.1, Inc. San Diego, CA, USA) was utilized for significant difference analysis by Tukey test (P < 0.05 was considered significant). Shannon, Simpson, Chao, and Sobs indices were calculated with Mothur v1.30.1(version v.1.30.2). The Wilcoxon rank-sum test was used to analyze the differences between groups of Alpha diversity. Metabolites showing significant differences were selected based on variable importance in projection (VIP) scores (VIP > 1) from orthogonal partial least-squares discriminant analysis (OPLS-DA) and P-value (P < 0.05) from t-tests. R software (version1.6.2) was used to draw the heatmap. All measurements were carried out in triplicate, and the results were expressed as the means ± standard deviation.
3. Results
3.1. Microbial community composition of FG
To understand the characteristics of microbial communities in different layers of FG, the diversity and community composition were analyzed. The PCA plots (Fig. 1A, B) demonstrated that samples from the beginning and end of fermentation were distinct. While, FG samples from different layers were clustered together, but the distribution of microbial composition varied across layers. In bacterial and fungal PCA, samples from the BL and ML were closely aligned (Fig. 1A, B), indicating similar community compositions between these two layers.
Fig. 1.
Microbial community characteristics analysis of different FG. Beta diversity of the (A) bacterial and (B) fungal communities assessed by PCA analysis. The circos plots of the (C) bacterial and (D) fungal communities at the phylum level. The bar plots of the (E) bacterial and (F) fungal communities at the genus level.
The Shannon, Simpson, Chao, and Sobs indices of the microbial community were analyzed. The results showed that the bacterial community diversity and richness in FG significantly decreased after fermentation (Fig. S1A, S1B, S1C, S1D), indicating that the fermentation process optimized the bacterial structure. This phenomenon is the same as what has been reported (Huang et al., 2020). Disadvantaged microorganisms in the competition and the environment gradually disappeared after fermentation, while dominant microorganisms became more predominant. The Chao and Sobs indices were higher in the FG's ML compared to the other two layers (Fig. S1B, S1D), indicating greater community richness in the ML. After fermentation, the Chao, Simpson, and Sobs indices of the fungi in the FG gradually decreased in the UL, ML, and BL (Fig. S1E, S1F, S1G, S1H), although the diversity index remained significantly higher in the UL than before fermentation. The fungal community diversity and richness of fungi from the BL of FG were lower than pre-fermentation samples. This suggests that the high acidity and high water content of the BL may unsuitable for the survival of many fungi, whereas the UL provides an optimal environment for the growth and reproduction of aerobic fungi.
Venn diagram analysis revealed 18 unique bacterial genera in the ML (Fig. S1I, Table S3). Almost all of them were reported to be aerobic or facultative anaerobes (Rainey et al., 2015). Among them (Table S3), Chryseobacterium, as a cellulose-degrading bacterium, has high glucanase and xylanase activity (Tan et al., 2018). Sphingomonas has a special cell structure, and its cell membrane replaces lipopolysaccharide with sphingomonas (Aso et al., 2006), which has a metabolic mechanism to tolerate poor nutrition (Li et al., 2022). The characteristics of these genera may play important roles in the fermentation. Thus they survive in the ML with higher alcohol and acid content and lower oxygen content. Six genera (Fibrobacter, Cloacibacterium, Corynebacterium, Bifidobacterium, Propionibacterium, Cetobacterium) and two genera (Lactococcus, Roseburia) were unique to the UL and BL (Table S3), respectively. There were more unique genera in the ML than in the UL and BL (Fig. S1I). This could be attributed to the state of complete anaerobic fermentation in the middle and late stages of the fermentation, which inhibited the growth of aerobic microorganisms. The increase in alcohol and acid contents inhibited the growth of intolerant microorganisms in FG, especially in the BL. Thus, the environment in the ML may be more suitable for the survival of most facultative anaerobic bacteria, as well as acid-resistant microorganisms.
A total of 10 phyla and genera with relative abundance >0.01% were identified by composition analysis of bacterial community in FG. Firmicutes and Proteobacteria were the main phyla of FG before fermentation (BF), with relative abundance of 44.7% and 53.5%, respectively (Fig. 1C). At the end of fermentation, Firmicutes became the main phylum in all layers, with relative abundance of >99% and increasing from top to bottom. At the genus level, the bacterial community structure of FG tends to be simplified (Fig. 1E), which was consistent with our diversity analysis and also found in previous studies (Xue et al., 2023). Gluconobacter and Acetobacter were the dominant genera in the FG at the beginning of fermentation (Fig. 1E), with relative abundances of 37.36% and 20.39%, respectively. At the end of fermentation, Lactobacillus became the dominant genus in the FG (Fig. 1E), consistent with previous study (Luo et al., 2023; Xue et al., 2022). The relative abundances of Lactobacillus in the UL, ML, and BL were 91.58%, 95.69%, and 98.46% (Fig. 1E), respectively. The deeper the depth, the simpler the microbial community structure, which may be related to the high acidity of the BL (Fig. 2B). On the one hand, it may be due to the metabolism of acid-producing bacteria (Luo et al., 2023). On the other hand, the high-acid environment further promoted the replacement of microorganisms, and intolerant microorganisms were gradually eliminated (Xue et al., 2022). The 27 genera showed significant differences among the 4 groups (Fig. S2A). The first few genera with high abundance were Gluconobacter, Acetobacter, Achromobacter, and Bacillus. Except for the genera Lactobacillus and Bacillus, which showed a significant increase in abundance after fermentation, all other genera decreased significantly or even disappeared. More and more studies have shown that Bacillus plays an important role in the brewing process of Baijiu (Li, Lian, Ding, Nie & Zhang, 2014; Yang et al., 2020). The metabolites of Bacillus can promote the formation of Baijiu flavor (Li, Lian, Ding, Nie, & Zhang, 2014). Lefse analysis of FG in different layers (Fig. S1K) at the end of fermentation revealed that Pediococcus and unclassified__f__Lactobacillaceae were the biomarkers in the UL, while Weeksellaceae and Flavobacteriales were the biomarkers in the ML (Fig. S1K). The results showed that the relative abundance of Pediococcus increased at the beginning of fermentation, remained stable in the middle stage, and decreased at the later stage (Xue et al., 2023). Pediococcus is a type of lactic acid bacteria (LAB) with low pH resistance. It has been found to be the dominant bacteria in Baijiu and the main functional contributor in Baijiu fermentation (Hu et al., 2021), which can promote the synthesis of flavor compounds.
Fig. 2.
Physicochemical factors in different FG. (A) Moisture content; (B) Acidity; (C) pH value; (D) Starch content; (E) Reducing sugar content.
The results revealed that the diversity and richness of fungi in FG samples after fermentation (AF) (i.e., UL, ML, BL) were significantly different from those before fermentation (BF), and the diversity of the UL of FG was significantly higher than that of the other layers (Fig. S1E, S1F, S1G, S1H). Fourteen genera of fungi were unique to the UL of FG (Fig. S1J, Table S3), among which the genus Kloeckera was found to have a significant effect on the flavor and sensory qualities of wines (Tang, Zhao, Cui, Lai, & Zhang, 2023). The genus Thermoascus was reported to produce amylase, pectinases, cellulase, and xylanase (Wang et al., 2023), making it a potential functional genus for QXX Baijiu brewing. There were 9 unique genera (Cystobasidium, Neopestalotiopsis, Papiliotrema, Rhodotorula, Rhodosporidiobolus, Cryptococcus, Kregervanrija, Trichosporon, Neocucurbitaria) in the ML and 1 genus (Dekkera) in the BL (Table S3). Dekkera has been reported to have a higher ethanol yield than Saccharomyces cerevisiae under oxygen limitation (De Barros Pita et al., 2019). The diversity and abundance of BL in FG were lower than those before fermentation (Fig. S1E, S1F, S1G, S1H). This indicates that the high acidity and high moisture content of FG in the BL might not be suitable for the survival of most fungi. While the ML environment was more suitable.
Ascomycota was the sole fungal phylum before and after fermentation of FG (Fig. 1D), and the structure was relatively simple. Previous studies have shown that Ascomycota was the predominant fungus in the FG of Maotai-flavor Baijiu, NXX Baijiu, and QXX Baijiu, indicating that Ascomycetes are the key fungi in Baijiu brewing (Xue et al., 2023). The dominant genus of fungi in FG was Saccharomyces (68.70%) both before and after fermentation (Fig. 1F). At the end of fermentation, Saccharomyces decreased in the UL (40.56%), but increased in the ML (83.43%) and BL (83.06%), followed by the genera Cyberlindnera and unclassified_f_Metschnikowiaceae (Fig. 1F). The abundance of fungi exceeded that of bacteria at the end of fermentation, and there was no significant reduction. The composition of fungi in the FG of QXX Baijiu varied across multiple studies (Xue et al., 2023). Xue et al. (2023) showed that Issatchenkia and Saccharomycopsis were the dominant fungi in the fermentation process. Li, Fan, Huang, and Han (2022) found that Pichia was the dominant genus in the fermentation process. The analysis of differences between groups revealed that eight genera including Saccharomyces, Clavispora, and unclassified__f__Metschnikowiaceae exhibited significantly differences among the four groups (Fig. S2B). The abundance of Saccharomyces in the UL of FG was significantly lower than that in the other two groups, while the abundance of Clavispora, unclassified__f__Metschnikowiaceae was significantly higher than that in the other three groups. Lefse analysis revealed that Cutaneotrichosporon, Trichosporonaceae, and Trichosporonaless were biomarkers of the ML of FG at the end of fermentation (Fig. S1L).
3.2. The physicochemical properties of FG
The physicochemical properties of FG represent the growth environment of the microbial community, which is crucial to the fermentation of Baijiu brewing. The moisture content of FG at the end of fermentation was higher than that before fermentation (66.02 g/100 g) and increased gradually from top to bottom (67.39 g/100 g, 71.39 g/100 g, and 78.78 g/100 g, respectively) (Fig. 2A). These moisture contents may result from various physiological and biochemical reactions during fermentation and microbial metabolism. For instance, certain lactic acid bacteria naturally produce 2-butanol through anaerobic fermentation, utilizing acetyllactic acid as a substrate (Speranza et al., 1997). The presence of moisture lays the foundation for the smooth progress of saccharification and fermentation. During fermentation, the moisture content of FG affects the metabolism and community structure of microorganisms, and the growth metabolism of microorganisms also regulates the moisture content, which affects each other inseparably. Therefore, the difference in moisture content among different layers may be attributed to variations in microbial metabolic activity across spatial positions. Additionally, the moisture of FG gradually descended under gravity in the late stage of fermentation, with the order eventually becoming BL > ML > UL.
At the end of fermentation, the acidity of FG in the UL, ML, and BL increased gradually to 0.725, 0.75, and 0.835 mmol/10 g, respectively (Fig. 2B). Additionally, the acidity of post-fermentation in the BL was higher than that of the pre-fermentation samples. This is consistent with the existing studies (De Vuyst & Leroy, 2020). The pH decreased from top to bottom (4.115, 4.005, and 3.970 mmol/10 g, respectively) (Fig. 2C), all of which were lower than that of the pre-fermentation samples. As the oxygen content of the BL was lower than that of the UL and ML (Liu et al., 2023; Yang et al., 2023), facilitating the growth of anaerobic or facultative anaerobic bacteria in FG, the acidity of the FG in BL was the highest. Variations in microbial composition, acidity changes, and esterification effects may contribute to differences among FG layers.
The starch content of FG in the UL, ML, and BL showed an increasing trend, with values of 11.2, 11.6, and 14.9 g/100 g, respectively, which were lower than that of pre-fermentation samples (15.6 g/100 g) (Fig. 2D). The reducing sugar contents of FG in the UL, ML, and BL were 0.267, 0.5, and 0.53 g/100 g (Fig. 2E), respectively, and they also increased from the top to bottom. Reducing sugar content in FG was closely related to starch content. Starch is the material base of alcohol fermentation. After saccharification, starch and other polysaccharides were enzymatically hydrolyzed to glucose and other fermentable sugars. During the fermentation stage, glucose was converted to alcohol and trace flavor compounds. These substances provide the material basis and energy source for the growth and metabolism of microorganisms and make the fermentation proceed normally. The lowest content of starch and reduced sugar was found in the UL at the end of fermentation. This may be attributed to the decomposition of starch into reducing sugar in the UL of FG, with most of it converting into alcohol and esterifying into flavor substances. Therefore, its reduced sugar content is significantly lower than the other two layers.
3.3. The correlation of physicochemical properties and microbial community
To investigate the effects of various physicochemical environmental factors on microbial community distribution during Baijiu fermentation, we analyzed the correlation between environmental factors and dominant genera using canonical correspondence analysis (CCA). The result showed that CCA1 and CCA2 explained 98.93% of the bacterial species distribution (Fig. 3A), with CCA1 explaining 95.56% and CCA2 explaining 3.37%. This indicated that bacterial community is correlated with physicochemical properties. The CCA1 is corelated with most of the bacterial genus except Bacillus. Acidity and moisture exhibited a positive correlation with Lactobacillus (Fig. 3A). Acidity and moisture had a strong effect on Lactobacillus associated with CCA1. Lactobacillus metabolizes carbohydrates to produce acetic acid, lactic acid, ethanol, and other important flavor substances during of fermentation (Luo et al., 2023; Xue et al., 2022). The rapid accumulation of these substances can lead to the change of acidity and ethanol concentration in the fermentation environment. Due to the lower oxygen content of FG in the ML and BL compared to the UL (Liu et al., 2023; Yang et al., 2023), which promotes the growth of anaerobic or facultative anaerobic bacteria (Jiao et al., 2022), the acidity of FG in the ML and BL was higher. A negative correlation was observed between Bacillus and starch content (Fig. 3A). Bacillus has been reported to produce amylase and protease, which breaks down macromolecules such as starch and protein (Yang et al., 2020). The anaerobic environment and higher acidity in the ML and BL of FG led to a gradual decrease in amylase activity, potentially affecting the decomposition of macromolecules. Consequently, the BL exhibited the highest strach content.
Fig. 3.
The correlation network analysis between the microbial communities and physicochemical properties as well as flavor compounds of the FG. The CCA analysis of the correlation between the microbial communities (bacteria A, fungi B) and physicochemical properties at the genus level (P < 0.05). (C) The correlation between the microbial communities and VFCs by network correlation analysis. The line thickness indicates the correlation strength, with a yellow line denoting a positive correlation and a gray line signifying a negative correlation. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Bacteria such as Staphylococcus, Leuconostoc, Komagatacibacter, Weissella, and Acetobacter were positively correlated with pH (Fig. 3A). Most of these bacteria were LAB and acetic acid bacteria (AAB), known for acid production (De Vuyst & Leroy, 2020), leading to a reduction in pH in the brewing environment. Weissella and Komagatacibacter have been reported to be useful for the production of bacterial cellulose (Brugnoli et al., 2021; Ji et al., 2021). These genera adapt to high-acid environment by improving the intracellular microenvironment through increased energy accumulation, higher relative ethanol invertase activity, and alterations in cell membrane fatty acid composition (Todorov et al., 2023). Therefore they are more common in acidic environments.
Pediococcus was positively correlated with reducing sugars and negatively correlated with starch starch (Fig. 3A). Our microbial community analysis revealed that the abundance of Pediococcus in the UL of FG was significantly higher than that in the other two layers (Fig. S3A). The starch content in UL of FG was the lowest at the end of fermentation (Fig. 2D). Pediococcus may play an important role in starch hydrolysis. Pediococcus is effective in producing antimicrobial peptides and fermenting a variety of carbohydrates (Wang, Huang, & Huang, 2021). Most Pediococcus utilize the lactic acid oxidation system, with cells deriving energy from the oxidation of lactate to acetate (Wang, Huang, & Huang, 2021). In addition, Pediococcus is also known to produce lactic acid and plays crucial regulatory functions during fermentation (Zhang et al., 2021). Collectively, the composition of the bacterial community is primarily determined by acidity, pH, and starch content.
The interpretation rates of fungi on CCA1 and CCA2 were 84.78% and 11.36%, respectively (Fig. 3B). Analysis indicated that Pichia and Clavispora exhibited positive correlations with reducing sugar and negative correlations with acidity (Fig. 3B). The genus Pichia, which mainly comes from Daqu and fermentation environment, is an important ester-producing yeast during fermentation. It promotes the esterification reaction (Wang, Du, Zhang, & Xu, 2018). Issatchenkia, Cyberlindnera, and Diutina demonstrated positive correlations with pH and negative correlations with moisture (Fig. 3B). Issatchenkia exhibits resistance to acidity and heat, along with a proficient ability to produce alcohol (Wu et al., 2017). Diutina were recognized as traditional ester-producing yeast strains in Baijiu fermentation and produced various lipases (Wang et al., 2021b). Cyberlinlinnera was found to have the ability to produce aroma and ester (Bellut et al., 2019). Collectively, the fungal community was primarily affected by pH and reducing sugar levels. In conclusion, variations in physicochemical properties can result in alterations in the microbial community of different layers, subsequently impacting the fermentation process of Baijiu.
3.4. Analysis of volatile flavor compounds
A total of 79 VFCs were identified in FG using GC × GC–MS analysis (Table S1), comprising 26 alcohols, 18 esters, 7 acids, 5 ketones, 4 phenols, 1 aldehydes, and 19 other compounds (Fig. 4A). Alcohols and esters were the main flavor components in QXX Baijiu. The total amounts of VFCs in the UL, ML, and BL of FG were 424.82 mg/L, 32.31 mg/L, and 152.08 mg/L, respectively (Table S1). It showed that the total amount of VFCs in the UL was much more than that in the other two layers. The contents of VFCs varied across different layers of FG. (Fig. 4D).
Fig. 4.
VFCs analysis of FG in the different layers. (A) VFC types and quantities; (B) VFC relative content in different layers; (C) Venn diagram of types of VFC; (D) The heatmap of the VFCs; (E) VFCs displaying distinct differences between the groups are identified via multivariate VIP analysis; (F) Analysis of the main characteristic VFC of QXX Baijiu.
Alcohols were the most abundant VFCs (Fig. 4B), accounting for 67.46%, 52.24%, and 89.23% of the VFCs in the UL, ML, and BL of FG, respectively (Fig. 4B). The total amounts of alcohols in the UL, ML, and BL of FG were 424.82 mg/L, 32.31 mg/L, and 152.08 mg/L, respectively (Table S1). Among the alcohols in the UL, 6-Methyl-2-heptanol had the highest content of about 221.66 mg/L, which was much higher than 2-tridecanol (23.46 mg/L), the second highest content one in all three layers (Table S1). Additionally, pentanol, phenylethyl alcohol, 1-dodecanol, isoamylol, isobutanol, ethanol, and various other major alcohols were present in high content, primarily high-level alcohols, with concentrations ranging from 2 to 12 mg/L in decreasing order (Table S1). The ML and BL of FG exhibited the highest concentrations of 6-methyl-2-heptanol (10.45 mg/L) and 3,5-dimethyl-2-octanol (129.36 mg/L), respectively (Table S1). The formation of higher alcohols such as n-propanol and isobutanol endows the Baijiu with its main aroma and a sense of fullness and roundness. Isobutanol emits a sweet and musty aroma, while n-propanol has a sharp musty aroma (Gong, Ma, Li, Cheng, & Huang, 2023). Changes in fermentation environment impact the microbial composition of FG across different spatial locations. The functional and metabolic characteristics of different microorganisms vary, thus affecting the concentration of alcohols and other flavor compounds in FG (Hu et al., 2020; Zhang et al., 2021).
The number of major esters in the UL, ML, and BL of the FG was lower than that of alcohols (Fig. 4A), accounting for relative contents of 7.37%, 3.58%, and 0.78%, respectively (Fig. 4B). This indicates that the content of esters in the UL of FG was much higher than that in the other two layers, making a significant contribution to the flavor of QXX Baijiu. Among them, ethyl acetate, the main flavor substance of QXX Baijiu, had the highest content of 15.52 mg/L in the UL, followed by isoamyl acetate, diethyl succinate, formic acid, ethyl lactate, and ethyl hexanoate in decreasing order (Table S1). The study revealed that ethyl acetate had the highest concentration in QXX Baijiu, followed by ethyl lactate. The absolute content of these compounds, as well as their quantitative ratio relationship, significantly influenced the quality and style of QXX Baijiu. Ethyl acetate is the primary contributor to the delightful fruity aroma in QXX Baijiu, while ethyl caproate imparts a pineapple-like aroma, characteristic of fruity notes. These esters enhance the richness and fullness of Baijiu's taste (Qiao, Wang, Wang, Zhang, & Zheng, 2023).
The concentrations of acids in the UL, ML, and BL of FG were 11.15 mg/L, 5.99 mg/L, and 4.91 mg/L, respectively (Table S1). The relative contents were 2.62%, 18.55%, and 3.23%, respectively (Fig. 4B). The proportion of acids in the UL and BL of the FG was much lower than that in the ML. However, the VFC content in the UL was higher than that in the ML (Fig. 4D), indicating that the UL of FG exhibited greater richness in both the main types of VFCs and total contents.
The shared VFCs among different layers of FG were analyzed using a venn diagram. There were 34 common VFCs in the UL, ML, and BL (Fig. 4C), containing 12 alcohols, 8 esters and 1 acid, 2 phenols, 3 ketones, and 8 other compounds, with alcohols being the most abundant, followed by esters (Table S1). However, the VFC with the highest concentration varied across layers, indicating that the fermentation process in each layer was distinct and had its characteristics. The number of unique VFCs is highest in the BL (16), followed by the UL (13), and lowest in the ML (4). It is apparent that the UL and BL of FG contain a higher number of unique flavor substances compared to the ML. Additionally, a greater variety of distinctive flavor compounds contribute to the formation of the overall flavor profile of these two layers.
OPLS-DA was employed to analyze the difference in VFCs content among the FG layers and to investigate the impact of varying depths (Fig. 4E, Table S2). Thirty-five discriminated VFCs were identified using VIP scores (Table S2), with alcohols and esters being the most abundant, each comprising 11 compounds. Nine VFCs (VIP > 1) were identified, consisting of 1 alcohol, 6 esters, and 2 acids (Fig. 4E). Glycolic acid had the highest VIP value (2.1855), followed by formic acid (2.0385), ethyl lactate (1.9667), and ethyl butyrate (1.8672) (Table S2). Additionally, numerous esters exhibited significant differences among the three layers (Table S2, Fig. 4F). Ethyl butanoate, ethyl linoleate, phenethyl acetate, ethyl acetate, diethyl succinate, ethyl oleate and ethyl palmitate exhibited significant upregulation in the upper layer (Table S2, Fig. 4F). It's evident that the FG from UL contributed the most to the esters in the entire fermentation system. Phenylethyl acetate is recognized as the principal compound contributing to the aroma of Xiaoqu Baijiu, likely formed through esterification between acetic acid and phenylethanol, imparting a floral and fruity aroma (Dong et al., 2023). Glycolic acid contributes to the acidity of Baijiu and is produced during the maturation of Baijiu. When glycolic acid exceeds a certain level, it will affect the taste of Baijiu and make it sour and astringent (Toe et al., 2019). The content of glycolic acid in the UL was the lowest among the three layers (Table S1), indicating a potentially lesser impact on the bitterness of the produced Baijiu.
Interestingly, ethyl acetate, ethyl lactate, acetic acid, isobutanol, and isoamylol exhibited significantly higher levels in the UL compared to the other layers (Fig. 4F). All of these compounds are key flavor compounds of QXX Baijiu. Ethyl acetate exhibited a decreasing trend from the UL to the BL. The upper FG contributed predominantly to the flavor formation of QXX Baijiu. This flavor characteristic aligns with the findings of Ding et al., who identified the highest level of flavor components in the UL (Ding et al., 2016).
Some components with elevated concentrations and high thresholds may contribute relatively minimally to the overall aroma. In our study, ten aroma active substances with OAV > 1 were identified (Table S4), comprising 3 esters, 3 alcohols, 1 aldehydes, 2 ketones, and 1 phenols. In the UL, ML, and BL, there were 9, 5, and 6 aroma-active substances with OAV > 1, respectively. Ethyl hexanoate, 2-octanone, guaiacol, 2-nonanol, and 3-nonanol, all with OAV > 1, were present in all three samples (Table S4). Five kinds of aroma active substances with OAV > 1 were found to coexist in all three samples, namely ethyl hexanoate, 2-octanone, guaiacol, 2-nonanol, and 3-nonanol (Table S4). Guaiacol, with smoky and aromatic flavor, exhibited the highest OAV among all the aroma active substances (33–99), followed by 2-nonanol (4–28) with its fatty flavor, 2-octanone (2−21) with apple peel flavor, and ethyl hexanoate contributing a fruity and sweet flavor (Table S4). OAVs in the UL exceeded those in the other two layers. This indicated that UL had an important contribution to the flavor of fermented grains. Guaiacol made a substantial contribution to QXX Baijiu aromas of fermented grains. Guaiacol not only enhances the aroma and taste of liquor but also functions as a natural free radical scavenger, promoting microcirculation, enhancing human immunity, and mitigating the risk of cardiovascular and various other diseases (Wang et al., 2021). Studies have demonstrated that the addition of 4-methylguaiacol to Baijiu effectively reduce alcoholic liver injury as well as alleviate the liver function disorders and dysfunctions caused by alcohol (Niu et al., 2015). It is an important flavorful and healthy components in Chinese Baijiu.
In the existing literature, some researchers found that the flavor substances content in the UL was the highest (Ao et al., 2022; Ding et al., 2016). However, some researchers think that the flavor in the UL needs to be improved because the fermented nutrients in FG gradually sink under gravity (Guan et al., 2023; Qian et al., 2021). Nevertheless, these studies primarily focused on NXX Baijiu, whereas our study is the first to investigate QXX Baijiu fermentation and has revealed the UL's significant contribution to the overall flavor profile of FG. This may be related to the absence of pit mud in the fermentation process of QXX Baijiu, which eliminates the interference of pit mud microbes. The starter used in QXX Baijiu differs significantly from that employed in NXX and JXX Baijiu. The microbial composition of the Xiaoqu could also contribute to the different fermentation and flavor characteristics. This discovery sheds new light on QXX Baijiu fermentation, providing a theoretical foundation for subsequent layer-based distillation processes of QXX Baijiu.
3.5. The correlation between the microorganisms and VFCs
The association between microbes and VFCs was investigated by correlation network analysis. A total of 12 genera were found to be significantly associated with 33 VFCs (|r| ≥ 0.67, P < 0.05) (Fig. 3C). Furthermore, 162 correlation pairs were screened, comprising 157 positive correlations and 5 negative correlations (Fig. 3C). Therefore, it indicates that most microorganisms promote VFCs production. Notably, bacteria represented by Achromobacter and the fungi represented by Pichia were associated with a variety of VFCs with up to 22 pairs, indicating their significant influence on VFCs formation. Intriguingly, Pediococcus, identified as a biomarker in the UL, exhibited significant positive correlations with formic acid and ethyl lactate, the distinctive VFCs in the UL. Moreover, Pediococcus was positively correlated with acetic acid, ethyl linoleate, ethyl oleate, and ethyl palmitate. It is concluded that Pediococcus plays an important role in the ester and aroma production of QXX Baijiu, and has an important influence on the formation of the unique VFCs in the UL. Acetobacter was positively correlated with the major VFCs of QXX Baijiu, such as ethyl lactate, ethyl acetate, ethyl caproate, acetic acid, formic acid, pentanol, ethyl butyrate, and isobutyl alcohol. In conclusion, microbes play a crucial role in shaping the distinctive flavor profile of FG across layers.
3.6. Analysis of non-VFCs and metabolic pathways
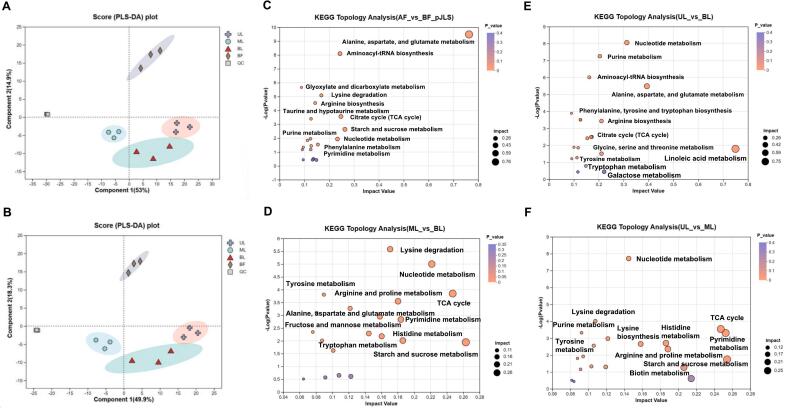
In total, 10,396 chromatographic peaks were extracted, resulting in the identification of 1596 metabolites. PLS-DA analysis indicated that the QC samples were closely clustered together (Fig. 5A, B), demonstrating the stable operation of the equipment and the reliability of the methods and results. Clear clustering separation was observed between the four groups of samples before and after fermentation (Fig. 5A, B). Post-fermentation samples from the three layers clustered together, while pre-fermentation samples were distributed separately (Fig. 5A, B). Significant differences were observed in the metabolites between AF and BF samples, as well as across different layers.
Fig. 5.
Multivariate statistical analysis of non-volatile metabolites in the fermentation grains of different layers. The PCA analysis of (A) negative and (B) positive ions. Topological analysis of KEGG pathway between (C) AF and BF, (D) ML and BL, (E) UL and BL, (F) UL and ML.
The heatmap (Fig. S4) depicted the top 50 significantly different metabolites among four groups, identified as potential biomarkers between groups using supervised OPLS-DA analysis and ANOVA, with VIP > 1.0 and P < 0.05. Table 1 presented the results of the differential analysis of samples at the beginning and end of fermentation, as well as across different layers. The highest number of differential metabolites (490) was observed between the ML and UL samples at the end of fermentation. This number was substantially higher than the differential metabolites identified between the AF and BF samples (380). This finding suggests significant differences in metabolism between the ML and UL samples. Variations in fermentation environments may result in distinct microbial compositions, thereby influencing microbial metabolism during fermentation.
Table 1.
The change and quantity statistical of differential metabolites in different groups.
| Group | Total sig metabolites | Down-regulated | Up-regulated |
|---|---|---|---|
| AF vs BF | 380 | 128 | 252 |
| BL vs ML | 237 | 182 | 55 |
| BL vs UL | 269 | 56 | 213 |
| ML vs UL | 490 | 42 | 448 |
AF vs BF: Comparison between AF group and BF group; BL vs ML: Comparison between BL group and ML group; BL vs UL: Comparison between BL group and UL group; ML vs UL: Comparison between ML group and UL group. Total sig metabolites: number of differential significant metabolites; up-regulated: number of up-regulated metabolites; and down-regulated: number of down-regulated metabolites.
Among different layers, organic acids and derivatives constituted the primary differential metabolites in the superclass, comprising approximately 40.61–44.53% (Fig. S5A, S5B, S5C, S5D). Lipids and lipid-like molecules followed, constituting approximately 13.11–15.64% (Fig. S5A, S5B, S5C, S5D). Organic oxygen compounds accounted for approximately 10.94–13.45% (Fig. S5A, S5B, S5C, S5D). Amino acids, peptides, and analogs were the predominant metabolites in the subclass, comprising approximately 34.75–38.11% (Fig. S5E, S5F, S5G, S5H), followed by carbohydrates and carbohydrate conjugates, accounting for about 5.66–10.49% (Fig. S5E, S5F, S5G, S5H). In conclusion, organic acids and their derivatives, as well as amino acids, peptides, and analogs, constituted the primary metabolites in FG, and they were significantly influenced by the fermentation environment and process. Previous studies have also found that organic acids account for a larger proportion of non-VFCs (Huang et al., 2020).
Based on the different metabolites, KEGG enrichment analysis and pathway topology analysis showed that 38 metabolic pathways were enriched in pre-fermentation and post-fermentation samples (Fig. 5C). Among them, alanine, aspartate, and glutamate metabolism pathways were the most important pathways noted. The levels of L-alanine, L-asparagine, and L-glutamate in FG significantly increased after fermentation compared to their levels before fermentation (Table S5). This increase may be attributed to microbial decomposition of macromolecules in FG, leading to the synthesis of amino acids.
Differential metabolites in the ML compared to the UL and BL were enriched in metabolic pathways dominated by alanine, aspartate, and glutamate metabolism, starch and sucrose metabolism, and the TCA cycle (Fig. 5D, F). Interestingly, the UL and BL showed significant enrichment in all 10 amino acid metabolic pathways (Fig. 5E), with the amino acid content notably higher in the UL compared to the BL. The amino acids involved in these pathways, including lysine, arginine, threonine, L-isoleucine, amino acid, and proline, are closely related to the metabolism of LAB, the primary microorganisms in the late fermentation stage of FG. This enhances the growth and reproduction of LAB, thereby enhancing its activity and biological potency (Wei et al., 2023).
Research has indicated that amino acids can notably influence the production of flavor compounds (Fleige, Kroll, & Steinbüchel, 2011). Allysine, Threonylleucine, and Isoleucylproline were involved in the metabolism of isoamyl alcohol, isobutanol, phenylethanol, and related ethyl esters (Wei et al., 2023, Wei et al., 2023), and then cause the flavor difference among groups. Yeast can also synthesize isoamyl alcohol through the amino acid synthesis pathway and the amino acid catabolism pathway (Fleige et al., 2011). Strong metabolism of amino acids in the UL may significantly impact flavor formation in this layer. Sucrose and trehalose exhibited significant down-regulation in the starch and sucrose metabolism pathway in the UL and BL compared to the ML. A large amount of macromolecules such as starch and sucrose was degraded during long-term fermentation, and these substances decreased significantly after fermentation. This could be attributed to microbial hydrolysis of macromolecules like starch and sucrose into smaller sugars in sorghum during fermentation. This suggests that starch and sucrose metabolism in the upper FG samples was more active, leading to increased decomposition of macromolecules. This is consistent with our previous physicochemical properties analysis.
In addition, notable down-regulation of cytidine, uracil, uridine, cytosine, and cytosine deoxyribonucleoside within the nucleotide metabolism pathway was observed in the UL and BL compared to the ML. This down-regulation may be attributed to the synthesis of significant amounts of microbial genetic material during fermentation. This suggests that microbial growth and reproduction were more active in the UL and BL compared to the ML.
Furthermore, the concentrations of citric acid, oxoglutaric acid, aconitic acid, and malic acid in the UL and BL of FG exhibited significant decreases in comparison to those in the ML. The intermediates of the TCA cycle can serve as carbon source to meet the growth needs of microorganisms. Studies have reported that Candida utilis can utilize malic acid and other intermediate products of TCA cycle as a sole carbon source or energy source. Candida utilis and Saccharomyces cerevisiae have been found to utilize the intermediate products of the TCA cycle to regulate their physiological metabolism (Lasserre et al., 2010).
Metabolites that were significantly different in the UL and BL were enriched in 30 metabolic pathways, primarily associated with linoleic acid metabolism (Fig. 5E). Linoleate may play a role in the formation of non-VFCs during the fermentation process as a precursor of Baijiu flavor. Linoleic acid is an essential long-chain fatty acid. Studies have shown that a high concentration of linoleic acid can enhance yeast survival in stressful environment by improving cellular resistance (Yang & Cadwallader, 2023). The dominant fungi in FG were all yeasts, including Issatchenkia, Saccharomyces, Cyberlindnera, Clavispora, and Pichia, with the highest fungal diversity observed in the UL. Linoleic acid may play a pivotal role in this process. Additionally, we observed significant up-regulation of ethyl linoleate in the UL, but notably not in the ML. Excessive levels of linoleic acid ethyl ester may lead to cloudiness and an oily odor in the liquor, hence requiring careful monitoring of its concentration. As the human body cannot synthesize high-level fatty acids, ethyl linoleate can be metabolized into linoleic acid upon ingestion, which is known to have cholesterol-lowering properties (Santiago, MacGilvray, Faustoferri, & Quivey Jr, 2012).
In previous studies, little attention was given to the contribution of non-VFCs to Baijiu flavor. Most studies primarily focused on organic acids, often employing proton nuclear magnetic resonance (1H NMR) and High Performance Liquid Chromatography (HPLC) techniques. However, in our study, LC-MS non-targeted metabolomics technology was utilized to comprehensively elucidate the non-VFCs profiles across various layers of FG in QXX Baijiu, deepening the understanding of the QXX Baijiu brewing process.
3.7. Association analysis of non-VFCs and microorganisms
Pearson correlation analysis was conducted to explore the relationship between microbial genus and non-volatile metabolites in FG. Strong correlation pairs (correlation coefficient > 0.9) were identified (Fig. S6, S7), with the majority exhibiting negative correlations.
Several bacterial genera significantly influence norleucine levels (Fig. S6). Norleucine exhibited a positive correlation with Lactobacillus and negative correlations with Acetobacter, Komagataeibacter, Leuconostoc, Gluconobacter, Enterobacter, Staphylococcus, Rhodococcus, and Oenococcus. Metabolome analysis revealed significantly higher levels of norleucine in post-fermentation compared to pre-fermentation samples (Fig. S3D), with BL showing higher levels than UL (Fig. S3C), consistent with Lactobacillus abundance trends (Fig. S3B). Lactobacillus may contribute to the production of norleucine. Norleucine, as a branched-chain amino acid, plays a central role in metabolism and participates in numerous signaling pathways. Its synthesis competes with pyruvate consumption, reducing the production of formic acid and other substances, thus alleviating stress (Peng et al., 2021). This contributes to the acid stress resistance of LAB. Currently, the potential relationship between norleucine and microorganisms in fermented grains remains unexplored, warranting further investigation.
Furthermore, Gluconobacter, Acetobacter, and Komagataeibacter classified as AAB, are capable of producing acetic acid. Acetobacter and Komagataeibacter bacteria demonstrate remarkable ability in oxidizing ethanol to acetic acid (Peng et al., 2023). Studies have demonstrated that Komagataeibacter europaeus JNP1 accelerates acetic acid production and reducing reducing sugar consumption, enhancing raw material utilization and fermented food quality (Nakano & Ebisuya, 2016). The presence of these microorganisms may contribute to the enrichment of non-VFCs in Baijiu to some extent.
The correlation between fungi and non-VFCs showed that the fungi closely related to metabolites belonged predominantly to the order Saccharomycetales. A variety of yeasts, such as unclassified_f__Metschnikowiaceae, Clavispora, and Hanseniaspora were closely related to various non-VFCs. Oxohexanoic acid was positively correlated with these three genera, whereas 5′-guanylic acid and 3′-adenylic acid were negatively correlated with them. Additionally, a notable negative correlation was observed between norleucine and Apiotrichum. Research has demonstrated that low norleucine concentrations are significant in inhibiting the growth of wild-type yeast strain WT (Liu, Fels, Dragone, & Mussatto, 2021). Moreover, 2-hydroxyisohexanoic acid, a by-product of norleucine metabolism, can impede the formation of Candida hypha (Liu et al., 2021). In this study, this genus was detected only in the pre-fermentation samples, and the species were absent in the samples at the end of fermentation. Thus, as Lactobacillus became the dominant species during fermentation, the norleucine content also increased significantly, norleucine may inhibit the growth of Apiotrichum to some extent. In subsequent studies, we will conduct further investigations to verify the relationship between non-VFCs and microorganisms, aiming to establish a foundation for the targeted regulation of the Baijiu fermentation process.
4. Conclusions
Our study firstly revealed that spatially varying environments led to the microbiome, physicochemical characteristics, flavor and metabolome heterogeneity of QXX Baijiu FG across different layers. The acidity, reducing sugar, and starch contents of FG varied across different depths, exhibiting an increasing trend from the top to the bottom layers at the end of fermentation. These changes influenced the growth and metabolism of microorganisms, consequently reshaping the microbial community structure within each layer. Metabolic variances of dominant microorgramisms (i.e. Lactobacillus and Saccharomyces) along with characteristic microorganisms (i.e. Pediococcus) in the pathway (e.g. amino acid metabolism, starch and sucrose metabolism,etc.) contribute to the difference of flavor compounds (i.e. organic acids and their derivatives, esters, etc.) across the layers of FG. Specifically, the VFC contents showed a trend of upper layer > lower layer > middle layer. This distribution pattern differs from other types of Baijiu, revealing the important role of the UL of FG in QXX Baijiu fermentation and flavor formation. Overall, this study provides a theoretical foundation for optimizing the fermentation process and distillation by layers, deepening our understanding of QXX Baijiu.
CRediT authorship contribution statement
Lei Tian: Writing – review & editing, Writing – original draft, Visualization, Supervision, Project administration, Funding acquisition, Data curation, Conceptualization. Pei Xu: Writing – review & editing, Visualization, Data curation. Junyu Chen: Investigation. Hang Chen: Visualization, Data curation. Ji Qin: Writing – review & editing, Supervision. Xiaotian Wu: Writing – review & editing. Chengzhe Liu: Resources. Zongjun He: Resources. Ying Liu: Supervision. Tongwei Guan: Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationship that could have appeared to influence the work reported in this paper.
The following are the supplementary data related to this article.
The richness, diversity, Lefse analysis of microbial communities in different FG layers. Shannon (A), Chao (B), Simpson (C), and Sobs (D) indices of bacteria. Shannon (E), Chao (F), Simpson (G), and Sobs (H) indices of fungi. Venn diagram of bacteria (I) and fungi (J) communities at genus level. Lefse analysis of bacteria (K) and fungi (L).
Microbial differences analysis among groups. (A) bacteria and (B) fungi (* P < 0.05) evaluated by Kruskal–Wallis H test.
Analysis of group differences in some characteristic microorganisms and metabolites. (A) The differences of genus Pediococcus among three layers. (B) The differences of Lactobacillus among four groups. (C) The differences of norleucine between UL and BL groups. (D) The differences of norleucine between AF and BF groups.
The top 50 significantly different metabolites with VIP > 1.0 and P < 0.05 were shown in the heatmap.
Functional annotation and classification of differential metabolites identified in the comparison between different layers. Classification of differential metabolites between (A) AF and BF, (B) UL and BL, (C) UL and ML, (D) BL and ML in the superclass. Classification of differential metabolites between (E) AF and BF, (F) UL and BL, (G) UL and ML, (H) BL and ML in the subclass.
The correlation between the bacterial genus and non-VFCs by heatmap analysis.
The correlation between the fungal genus and non-VFCs by heatmap analysis.
Supplementary material 1
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101508.
Acknowledgments
This research was funded by the Talent Introduction Project of Xihua University (Grant No. Z222061), the project of Nanchong science and technology (Grant No.23ZDYF0009) and the Sichuan Tujiu Liquor Co., Ltd. (Grant No. 222305).
Data availability
Data will be made available on request.
References
- Ao L., Mei J., Lang Z., Zeng S., Shen X., Lu Z., Zhang X., Chai L., Wang S., Shen C., SHI J., & XU Z. Changes of volatile compounds in different layers of fermented grains during Lu-flavor baijiu brewing. China Brewing. 2022;41(5):28–33. 10.11882/i.issn.0254-5071.2022.05.006 (In Chinese) [Google Scholar]
- Aso Y., Miyamoto Y., Mine Harada K., Momma K., Kawai S., Hashimoto W., Mikami B., Murata K. Engineered membrane superchannel improves bioremediation potential of dioxin-degrading bacteria. Nature Biotechnology. 2006;24(2):188–189. doi: 10.1038/nbt1181. [DOI] [PubMed] [Google Scholar]
- Bellut, K., Michel, M., Zarnkow, M., Hutzler, M., Jacob, F., Atzler, J. J.,Hoehnel,A., Lynch, K. M., & Arendt, E. K. (2019). Screening and application of Cyberlindnera yeasts to produce a fruity, non-alcoholic beer. Fermentation, 5(4), 103. doi: 10.3390/fermentation5040103. [DOI]
- Brugnoli M., Robotti F., La China S., Anguluri K., Haghighi H., Bottan S., Ferrari A., Gullo M. Assessing effectiveness of Komagataeibacter strains for producing surface-microstructured cellulose via guided assembly-based biolithography. Scientific Reports. 2021;11(1):19311. doi: 10.1038/s41598-021-98705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Barros Pita W., Teles G.H., Peña-Moreno I.C., Da Silva J.M., Ribeiro K.C., De Morais Junior M.A. The biotechnological potential of the yeast Dekkera bruxellensis. World Journal of Microbiology and Biotechnology. 2019;35:1–9. doi: 10.1007/s11274-019-2678-x. [DOI] [PubMed] [Google Scholar]
- De Vuyst L., Leroy F. Functional role of yeasts, lactic acid bacteria and acetic acid bacteria in cocoa fermentation processes. FEMS Microbiology Reviews. 2020;44(4):432–453. doi: 10.1093/femsre/fuaa014. [DOI] [PubMed] [Google Scholar]
- Ding X., Wu C., Huang J., Zhou R. Characterization of interphase volatile compounds in Chinese Luzhou-flavor liquor fermentation cellar analyzed by head space-solid phase micro extraction coupled with gas chromatography mass spectrometry (HS-SPME/GC/MS) LWT-Food Science and Technology. 2016;66:124–133. doi: 10.1016/j.lwt.2015.10.024. [DOI] [Google Scholar]
- Dong W., Dai X., Jia Y., Ye S., Shen C., Liu M., Lin F., Sun X., Xiong Y., Deng B. Association between baijiu chemistry and taste change: Constituents, sensory properties, and analytical approaches. Food Chemistry. 2023;137826 doi: 10.1016/j.foodchem.2023.137826. [DOI] [PubMed] [Google Scholar]
- Fan G., Sun B., Fu Z., Xia Y., Huang M., Xu C., Li X. Analysis of physicochemical indices, volatile flavor components, and microbial community of a light-flavor Daqu. Journal of the American Society of Brewing Chemists. 2018;76(3):209–218. doi: 10.1007/s11274-023-03865-0. [DOI] [Google Scholar]
- Fleige C., Kroll J., Steinbüchel A. Establishment of an alternative phosphoketolase-dependent pathway for fructose catabolism in Ralstonia eutropha H16. Applied Microbiology and Biotechnology. 2011;91:769–776. doi: 10.1007/s00253-011-3284-5. [DOI] [PubMed] [Google Scholar]
- Gao X., Zhang J., Regenstein J.M., Yin Y., Zhou C. Characterization of taste and aroma compounds in Tianyou, a traditional fermented wheat flour condiment. Food Research International. 2018;106:156–163. doi: 10.1016/j.foodres.2017.12.063. [DOI] [PubMed] [Google Scholar]
- Gong J., Ma Y., Li L., Cheng Y., Huang Y. Comparative characterization and contribution of key aroma compounds in the typical base liquor of Jiang-flavor baijiu from different distributions in the Chinese Chishui River basin. Food Chemistry: X. 2023;20 doi: 10.1016/j.fochx.2023.100932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan T., Wu X., Hou R., Tian L., Huang Q., Zhao F., Liu Y., Jiao S., Xiang S., Zhang J., Li D., Luo J., Jin Z., He Z. Application of clostridium butyricum, Rummeliibacillus suwonensis, and Issatchenkia orientalis for Nongxiangxing baijiu fermentation: Improves the microbial communities and flavor of upper fermented grain. Food Research International. 2023;169 doi: 10.1016/j.foodres.2023.112885. [DOI] [PubMed] [Google Scholar]
- Hu X., Wang K., Yu M., Tian R., Zhang Z., Wang Y., Wang Y., Zhao D., He P., Wei T. Microbial community succession pattern and spatial heterogeneity in fermented grains of strong-flavor baijiu. Food and Fermentation Industries. 2020;46(10):66–73. doi: 10.13995/j.cnki.11-1802/ts.023321. [DOI] [Google Scholar]
- Hu Y., Lei X., Zhang X., Guan T., Wang L., Zhang Z., Yu X., Tu J., Peng N., Liang Y., Zhao S. Characteristics of the microbial community in the production of Chinese rice-flavor baijiu and comparisons with the microflora of other flavors of baijiu. Frontiers in Microbiology. 2021;12 doi: 10.3389/fmicb.2021.673670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Fan Y., Lu T., Kang J., Pang X., Han B., Chen J. Composition and metabolic functions of the microbiome in fermented grain during light-flavor baijiu fermentation. Microorganisms. 2020;8(9):1281. doi: 10.3390/microorganisms8091281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X., Wang S., Luo Y., Yuan X., Wei Y., Zhang Q., Qin K., Tu Y. Green synthesis of weissella-derived fluorescence carbon dots for microbial staining, cell imaging and dual sensing of vitamin B12 and hexavalent chromium. Dyes and Pigments. 2021;184 doi: 10.1016/j.dyepig.2020.108818. [DOI] [Google Scholar]
- Jiao W., Xie F., Gao L., Du L., Wei Y., Zhou J., He G. Identification of core microbiota in the fermented grains of a Chinese strong-flavor liquor from Sichuan. LWT. 2022;158 doi: 10.1016/j.lwt.2022.113140. [DOI] [Google Scholar]
- Lasserre J.P., Nicaud J.M., Pagot Y., Joubert-Caron R., Caron M., Hardouin J. First complexomic study of alkane-binding protein complexes in the yeast Yarrowia lipolytica. Talanta. 2010;80(4):1576–1585. doi: 10.1016/j.talanta.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Li H., Lian B., Ding Y., Nie C., Zhang Q. Bacterial diversity in the central black component of Maotai Daqu and its flavor analysis. Annals of Microbiology. 2014;64(4):1659–1669. doi: 10.1007/s13213-014-0809-z. [DOI] [Google Scholar]
- Li H., Zhang X., Gao X., Shi X., Chen S., Xu Y., Tang K. Comparison of the aroma-active compounds and sensory characteristics of different grades of light-flavor baijiu. Foods. 2023;12(6):1238. doi: 10.3390/foods12061238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.-D., Zhu Z.-R., Zhang Y., Xu J., Wang H., Wang Z., Li H. The phyllosphere microbiome shifts toward combating melanose pathogen. Microbiome. 2022;10(1):1–17. doi: 10.1186/s40168-022-01234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Fan Y., Huang X., Han B. Microbial diversity and metabolites dynamic of light-flavor baijiu with stacking process. Fermentation. 2022;8(2):67. doi: 10.3390/fermentation8020067. [DOI] [Google Scholar]
- Liu S., Ren D., Qin H., Yin Q., Yang Y., Liu T., Zhang S., Mao J. Exploring major variable factors influencing flavor and microbial characteristics of upper jiupei. Food Research International. 2023;172 doi: 10.1016/j.foodres.2023.113057. [DOI] [PubMed] [Google Scholar]
- Liu Z., Fels M., Dragone G., Mussatto S.I. Effects of inhibitory compounds derived from lignocellulosic biomass on the growth of the wild-type and evolved oleaginous yeast Rhodosporidium toruloides. Industrial Crops and Products. 2021;170 [Google Scholar]
- Luo A., Yang N., Yang J., Hao J., Zhao J., Shi S., Hu B. Effects of microbial interspecies relationships and physicochemical parameters on volatile flavors in sorghum-based fermented grains during the fermentation of Shanxi light-flavored liquor. Food Science & Nutrition. 2023;11(3):1452–1462. doi: 10.1002/fsn3.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S., Ebisuya H. Physiology of Acetobacter and Komagataeibacter spp.: Acetic acid resistance mechanism in acetic acid fermentation. Acetic acid bacteria: ecology and physiology. 2016:223–234. doi: 10.1007/978-4-431-55933-7_10. [DOI] [Google Scholar]
- Niu Y., Yu D., Xiao Z., Zhu J., Song S., Zhu G. Use of stir bar sorptive extraction and thermal desorption for gas chromatography-mass spectrometry characterization of selected volatile compounds in Chinese liquors. Food Analytical Methods. 2015;8:1771–1784. doi: 10.1007/s12161-014-0060-z. [DOI] [Google Scholar]
- Peng, M. Y., Huang, T., Zhang, X. J., Chai, L. J., Lu, Z. M., Shi, J. S., & Xu, Z. H. (2023). Comparative genomics reveals the functional differences between Acetobacter pasteurianus and Komagataeibacter europaeus in vinegar pei of Zhenjiang aromatic vinegar, 638-655 (in Chinese). Doi: 10.13343/j.cnki.wsxb.20220414. [DOI]
- Peng M.Y., Zhang X.-J., Huang T., Zhong X.Z., Chai L.J., Lu Z.M.…Xu Z.H. Komagataeibacter europaeus improves community stability and function in solid-state cereal vinegar fermentation ecosystem: Non-abundant species plays important role. Food Research International. 2021;150 doi: 10.1016/j.foodres.2021.110815. [DOI] [PubMed] [Google Scholar]
- Qian W., Lu Z.M., Chai L.J., Zhang X.J., Li Q., Wang S.T.…Xu Z.H. Cooperation within the microbial consortia of fermented grains and pit mud drives organic acid synthesis in strong-flavor baijiu production. Food Research International. 2021;147 doi: 10.1016/j.foodres.2021.110449. [DOI] [PubMed] [Google Scholar]
- Qiao L., Wang J., Wang R., Zhang N., Zheng F. A review on flavor of baijiu and other world-renowned distilled liquors. Food Chemistry: X. 2023;100870 doi: 10.1016/j.fochx.2023.100870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey F., Kämpfer P., Trujillo M., Chun J., DeVos P., Hedlund B., Dedysh S. In: Bergey’s manual of systematics of archaea and bacteria. Whitman W.B., editor. Vol. 410. Wiley; Hoboken, NJ: 2015. [DOI] [Google Scholar]
- Santiago B., MacGilvray M., Faustoferri R.C., Quivey R.G., Jr. The branched-chain amino acid aminotransferase encoded by ilvE is involved in acid tolerance in Streptococcus mutans. Journal of Bacteriology. 2012;194(8):2010–2019. doi: 10.1128/JB.06737-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza G., Corti S., Fontana G., Manitto P., Galli A., Scarpellini M., Chialva F. Conversion of meso-2, 3-butanediol into 2-butanol by lactobacilli. Stereochemical and enzymatic aspects. Journal of Agricultural and Food Chemistry. 1997;45(9):3476–3480. doi: 10.1021/jf970103+. [DOI] [Google Scholar]
- Tan H., Miao R., Liu T., Yang L., Yang Y., Chen C., Lei J., Li Y., He J., Sun Q., Peng W., Gan B., Huang Z. A bifunctional cellulase–xylanase of a new Chryseobacterium strain isolated from the dung of a straw-fed cattle. Microbial Biotechnology. 2018;11(2):381–398. doi: 10.1111/1751-7915.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Zhao W., Cui M., Lai H., Zhang X. Identification and functional prediction of the key fungus of Hongxinqu for lighte–flavor baijiu brewing. LWT. 2023;115605 doi: 10.1016/j.lwt.2023.115605. [DOI] [Google Scholar]
- Todorov S.D., Dioso C.M., Liong M.T., Nero L.A., Khosravi-Darani K., Ivanova I.V. Beneficial features of pediococcus: From starter cultures and inhibitory activities to probiotic benefits. World Journal of Microbiology and Biotechnology. 2023;39(1):4. doi: 10.1007/s11274-022-03419-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toe C.J., Foo H.L., Loh T.C., Mohamad R., Abdul Rahim R., Idrus Z. Extracellular proteolytic activity and amino acid production by lactic acid bacteria isolated from Malaysian foods. International Journal of Molecular Sciences. 2019;20(7):1777. doi: 10.3390/ijms20071777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Huang Y., Huang Y. Microbiome diversity and evolution in stacking fermentation during different rounds of Jiang-flavoured baijiu brewing. LWT. 2021;143 doi: 10.1016/j.lwt.2021.111119. [DOI] [Google Scholar]
- Wang H., Sun C., Yang S., Ruan Y., Lyu L., Guo X., Wu X., Chen Y. Exploring the impact of initial moisture content on microbial community and flavor generation in Xiaoqu baijiu fermentation. Food Chemistry: X. 2023;20 doi: 10.1016/j.fochx.2023.100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Ma B., Feng T., Chen D., Yao L., Lu J., Sun M. Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities. Open Chemistry. 2021;19(1):518–529. doi: 10.1515/chem-2021-0039. [DOI] [Google Scholar]
- Wang X., Du H., Zhang Y., Xu Y. Environmental microbiota drives microbial succession and metabolic profiles during Chinese liquor fermentation. Applied and Environmental Microbiology. 2018;84(4) doi: 10.1128/AEM.02369-17. e02369–02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Lu J., Nie Y., Li C., Du H., Xu Y. Amino acids drive the deterministic assembly process of fungal community and affect the flavor metabolites in baijiu fermentation. Microbiology Spectrum. 2023;11(2) doi: 10.1128/spectrum.02640-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Zhang L.Q., Ran M.F., Wang S.T., Yang J.G., Shen C.H. Investigation on fresh bamboo replacing rice husks for brewing light-aroma baijiu. Journal of Food Science. 2023;88(4):1224–1236. doi: 10.1111/1750-3841.16481. [DOI] [PubMed] [Google Scholar]
- Wu Y.Y., Xing K., Zhang X.X., Wang H., Wang Y., Wang F., Li J.M. Influence of freeze concentration technique on aromatic and phenolic compounds, color attributes, and sensory properties of cabernet sauvignon wine. Molecules. 2017;22(6):899. doi: 10.3390/molecules22060899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue T.D., Zhang J.H., Wang T.R., Bai B.Q., Hou Z.X., Cheng J.F.…Fan S.H. Reveal the microbial communities and functional prediction during the fermentation of fen-flavor baijiu via metagenome combining amplicon sequencing. Annals of Microbiology. 2023;73(1):16. doi: 10.1186/s13213-023-01719-6. [DOI] [Google Scholar]
- Xue Y., Tang F., Cai W., Zhao X., Song W., Zhong J., Liu Z., Guo Z., Shan C. Bacterial diversity, organic acid, and flavor analysis of dacha and Ercha fermented grains of fen flavor baijiu. Frontiers in Microbiology. 2022;12 doi: 10.3389/fmicb.2021.769290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Liu Y., Chen L., Li J., Wang L., Du G. Genome sequencing and flavor compound biosynthesis pathway analyses of Bacillus licheniformis isolated from Chinese Maotai-flavor liquor-brewing microbiome. Food Biotechnology. 2020;34(3):193–211. doi: 10.1080/08905436.2020.1789474. [DOI] [Google Scholar]
- Yang L., Xian C., Li P., Wang X., Song D., Zhao L., Zhang C. The spatio-temporal diversity and succession of microbial community and its environment driving factors during stacking fermentation of Maotai-flavor baijiu. Food Research International. 2023;169 doi: 10.1016/j.foodres.2023.112892. [DOI] [PubMed] [Google Scholar]
- Yang W., Cadwallader K. Microbiology, Chemistry and Process Technology; 2023. Chemical components of Chinese baijiu. Science and engineering of Chinese liquor (baijiu) pp. 225–265. [DOI] [Google Scholar]
- Zeng X., Li J., Lyu X., Chen J., Chen X., Guo S. Untargeted metabolomics reveals multiple phytometabolites in the agricultural waste materials and medicinal materials of Codonopsis pilosula. Frontiers in Plant Science. 2022;12 doi: 10.3389/fpls.2021.814011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wang L., Tan Y., Wang H., Yang F., Chen L., Hao F., Lv X., Du H., Xu Y. Effect of Pichia on shaping the fermentation microbial community of sauce-flavor baijiu. International Journal of Food Microbiology. 2021;336 doi: 10.1016/j.ijfoodmicro.2020.108898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The richness, diversity, Lefse analysis of microbial communities in different FG layers. Shannon (A), Chao (B), Simpson (C), and Sobs (D) indices of bacteria. Shannon (E), Chao (F), Simpson (G), and Sobs (H) indices of fungi. Venn diagram of bacteria (I) and fungi (J) communities at genus level. Lefse analysis of bacteria (K) and fungi (L).
Microbial differences analysis among groups. (A) bacteria and (B) fungi (* P < 0.05) evaluated by Kruskal–Wallis H test.
Analysis of group differences in some characteristic microorganisms and metabolites. (A) The differences of genus Pediococcus among three layers. (B) The differences of Lactobacillus among four groups. (C) The differences of norleucine between UL and BL groups. (D) The differences of norleucine between AF and BF groups.
The top 50 significantly different metabolites with VIP > 1.0 and P < 0.05 were shown in the heatmap.
Functional annotation and classification of differential metabolites identified in the comparison between different layers. Classification of differential metabolites between (A) AF and BF, (B) UL and BL, (C) UL and ML, (D) BL and ML in the superclass. Classification of differential metabolites between (E) AF and BF, (F) UL and BL, (G) UL and ML, (H) BL and ML in the subclass.
The correlation between the bacterial genus and non-VFCs by heatmap analysis.
The correlation between the fungal genus and non-VFCs by heatmap analysis.
Supplementary material 1
Data Availability Statement
Data will be made available on request.