Abstract
The sensory quality of black tea (BT) influenced by various factors, among which tree age is particularly significant. People prefer BT produced by fresh leaves from old tea trees, yet the correlation between tree age and tea quality has not been thoroughly investigated. In this study, we analyzed the quality of BT from young trees (H-JYH) and old trees (H-OJYH) using e-tongue technology and sensory evaluation. Our findings revealed that H-OJYH had stronger sweetness and sourness, richer flavor, and diminished bitter-astringency compared to H-JYH. 1231 non-volatile metabolites and 504 volatile metabolites were discovered by ultra-performance liquid chromatography (UPLC) and gas chromatography–mass spectrometry (GC–MS). L-tartaric acid and trans-citridic acid were found to contribute to increase acidity, and 7,8-dihydroxy-6-methoxycoumarin and d-fructose 6-phosphate were associated with enhanced sweetness in H-OJYH. Additionally, lower levels of octyl gallate and vanillic acid in H-OJYH contributed to the diminished bitter-astringency. β-ionone, 2-phenylethanol and phenylacetaldehyde merged as characteristic compounds of older tree BT with stronger floral and sweet aroma. Our study serves as a guideline to explore the relationship between tree age and tea quality.
Keywords: Age; Black tea; Flavor; Aroma, characteristics
Highlights
-
•
In total, 1231 non-volatile metabolites and 504 volatile metabolites were detected.
-
•
3 non-volatile and 372 volatile different metabolites were screened.
-
•
OJYH had stronger sweetness, sourness, richness and weaker bitterness, astringency.
-
•
OJYH had stronger floral, fruit, sweet aroma and weaker spicy, wood aroma.
-
•
β-ionone, 2-phenylethanol, phenylacetaldehyde were floral-sweet identity substances.
1. Introduction
Tea ranks among the top three non-alcoholic beverages globally, known for its distinct taste, aroma, and associated health benefits (Shang, Li, Zhou, Gan, & Li, 2021). Tea can be classified into six main types based on the level of fermentation, including black tea, yellow tea, green tea, dark tea, white tea, and oolong tea (Cai et al., 2022). The flavor and aroma attributes of tea depend on the variety and quantity of its constituents (Zhang, Cao, Granato, Xu, & Ho, 2020). Umami, characterized by amino acids, especially L-theanine, accounting for over 50% from the total amino acid content in tea (Yu, Yeo, Low, & Zhou, 2014). The sweetness of tea infusion is associated with carbohydrates such as d-fructose 6-phosphate and D-galactose (Kim & Yaylayan, 2020). Additionally, the astringent quality of tea infusion is attributed to flavonol glycosides, which are more astringent than caffeoylquinic acid derivatives (Scharbert & Hofmann, 2005). The aroma profile of tea is determined by its key aroma components. For example, dihydro-β-ionone, 3,4-dehydro-β-ionone, and linalool oxide II has floral and fruity properties, while α-terpineol contributes woody and stale characteristics (Xie et al., 2023). Sensory evaluation of aroma compounds can be conducted using analytical methods such as gas chromatography-ion mobility spectrometry (GC-IMS) and olfactometry analysis (Liu et al., 2021), as well as gas chromatography–mass spectrometry−olfactometry (GC–MS/O) (Song & Liu, 2018).
Tea quality is predominantly assessed based on sensory characteristics, such as aroma, flavor, and appearance. The flavor and aroma qualities of tea are impacted by various factors including tea variety, cultivation and management practices, manufacturing processes (Feng et al., 2019), as well as the maturity and harvesting season of fresh tea leaves (Liu et al., 2021), growth environment (including climate, soil, and altitude), and genetic background (Jahan et al., 2022). The prolonged use of organic fertilizer is beneficial for enhancing both the yield and quality of tea plants (Jahan et al., 2022). Spring tea is renowned for its superior flavor quality, attributed to elevated levels of desirable flavor substances such as free amino acids, caffeine, gallocatechin, and non-gallocatechin (Ye, Guo, Li, Chen, & Qiao, 2022).
In addition to the aforementioned influencing factors, the age of the tea tree represents another significant but often overlooked factor. With the increasing age of tea trees, the active ingredients and quality-related components will undergo transformation and accumulation, resulting in differences in the health benefits and flavor quality of the tea. This phenomenon partly explains why teas sourced from older trees are often more popular in many tea-producing regions. However, the influence of tree age on taste and aroma-related substances in tea remains poorly understood. In this study, one bud and two leaves from BT obtained from both old and young trees were used as materials to analyze the discrepancies in the quality between BT from new and old trees by both electronic tongue (e-tongue) technology and traditional sensory evaluation. Furthermore, we adopted UPLC-ESI-MS/MS and GC–MS to determine the relative contents of substances and conducted differential analysis. Our objective was to elucidate the characteristic components and underlying reasons for the discrepancies in taste and aroma qualities between BT sourced from new and old trees.
2. Material and methods
2.1. Samples
We selected 60-year-old tea tree (referred to as H-OJYH) and 5-year-old tea tree (referred to as H-JYH) as materials, obtained from the HongYan Company (Yingde Country, Guangdong province, China). Both trees belonged to the Yinghong No. 9 tea tree. The tea gardens were cultivated and managed in the same condition, and harvested during the same season and adhered to the same picking standard, using one bud and two leaves. All tea samples underwent uniform processing procedures and parameters, which included withering for 16 h, rolling for 1.5 h, fermentation for 6 h, and drying at 120 °C for 30 min. Additionally, all tea samples were harvested and processed in April (spring).
2.2. Evaluation of sensory qualities
10 scholars specializing in tea science, who had received training in sensory evaluation, assessed the sensory qualities of BT according to the national standard method for sensory review of tea (GB/T 23776–2018). Prior to commencing the sensory review evaluation, informed consent was obtained from all participating tea reviewers. The assessors objectively and comprehensively evaluated the sensory qualities of BT, with particular emphasis on taste and aroma quality.
2.3. Electronic tongue analysis
Each of the H-JYH and H-OJYH samples, weighing 3 g each, was placed into a review cup, filled with 150 mL of boiling water was added to each cup, followed by covering them with a lid, and then brewed for 5 min. The resulting brews were then filtered through gauze to remove any residues. A volume of 100 mL from the filtrate was utilized for sensory testing at 25 °C, employing an electronic tongue system (TS-5000Z, INSENT, Japan). The unit of taste was measured using Weber's Fechner law. It is important to note that each tea sample was brewed twice, and measurements were taken three times for each brew.
2.4. Extraction and preparation of samples
H-JYH and H-OJYH samples were pricked and rapidly frozen using liquid nitrogen, then stored in a − 80 °C refrigerator. Subsequently, the tea samples were ground into powdered form by liquid nitrogen. Biological samples were freeze-dried using a vacuum freeze-dryer (Scientz-100F). The resulting freeze-dried samples were pulverized with a zirconia bead mixer pulverizer (MM 400, Retsch) at a frequency of 30 Hz for 1.5 min. 50 mg of lyophilized powder was dissolved in 1.2 mL of a 70% methanol solution and vortexed intermittently for 30 s per 30 min, repeating this process a total of 6 times. After centrifugation at 12000 rpm for 3 min, the extracts were filtrated (SCAA-104, 0.22 μm pore size; ANPEL, Shanghai, China) and subjected to analysis via UPLC-MS/MS.
2.5. UPLC conditions
The extracts from the aforementioned tea samples were analyzed by a UPLC-ESI-MS/MS system (UPLC, SHIMADZU Nexera X2; MS, Applied Biosystems 4500 Q TRAP). The UPLC setup included an Agilent SB-C18 column (1.8 μm, 2.1 mm × 100 mm), with mobile phases consisting of solvent A (pure water with 0.1% formic acid), and solvent B (acetonitrile with 0.1% formic acid). The sample determination employed a gradient program, commencing with 95% A and 5% B. The gradient linearly increased to 5% A and 95% B within 9 min and maintained this composition for 1 min. Subsequently, the composition was then adjusted to 95% A and 5.0% B within 1.1 min and held for 2.9 min. The flow rate was set at 0.35 mL per minute, the column temperature at 40 °C, and the injection volume at 4 μL. The stream was connected alternatively order to an ESI-triple quadrupole-linear ion trap (QTRAP)-MS.
Linear ion trap (LIT) and triple quadrupole (QQQ) scanning was obtained on a triple quadrupole linear ion trap mass spectrometer, API 4500 Q TRAP UPLC-MS/MS System, equipped with an ESI Turbo IonSpray interface operating in positive and negative ion modes and controlled by Analyst 1.6.3 software (AB Sciex). The ESI source operation parameters were referenced from previously published literature (Sun et al., 2023a). All MS raw data were processed using Analyst (v1.6.3) and MultiqQuant.
2.6. Sample extraction and preparation of volatile metabolites
H-JYH and H-OJYH samples were pricked, rapidly frozen in liquid nitrogen, and subsequently stored in a − 80 °C refrigerator. The samples were then ground into a fine powder using liquid nitrogen. Approximately 0.5 g of the powdered sample was immediately transferred into a 20 mL headspace vial (Agilent, Palo Alto, CA, USA) containing a NaCl saturated solution of sodium chloride. The vial was sealed with a gland equipped with a Tetrafluoroethylene-siloxane headspace septum (Agilent). Solid-phase microextraction (SPME) treatments were performed as follows: the extraction temperature was set to 60 °C, with each vial maintained at 60 °C for 5 min, and then a 120 μm DVB/CWR/PDMS fiber (Agilent) was brought into contact with the sample headspace at 60 °C for 15 min and desorbed at 250 °C for 5 min.
2.7. GC–MS conditions
Volatile organic compounds (VOCs) adsorbed onto fiber coatings were desorbed at 250 °C for 5 min in splitless mode at the inlet of a gas chromatograph (model 8890; Agilent). VOCs were identified and quantified using an Agilent Model 8890 GC coupled with a 7000D mass spectrometer (Agilent), equipped with a 30 m × 0.25 mm × 0.25 μm DB-5MS (5% phenyl-polymethylsiloxane) capillary column. The gas chromatographic conditions were adopted from previously published literature (Wen et al., 2023). MS analysis was performed in selected ion monitoring (SIM) mode with precise scanning of qualitative and quantitative ions (GB/T 23200.8–2016).
2.8. Metabolomics analysis
Unsupervised principal component analysis (PCA) was carried out using the statistics function prcomp within R (base package) version 3.5.1. Both Hierarchical cluster analysis (HCA) and Pearson correlation coefficients (PCC) were performed using the R package ComplexHeatmap, and their outcomes were visualized as heatmaps. Differential metabolites were identified based on the filter criteria of variable importance in projection (VIP) ≥ 1, absolute log2 fold change (FC) ≥ 1, P value ≥0.05. Orthogonal partial least squares discriminant analysis (OPLS-DA) result was performed using the R package MetaboAnalystR version 1.0.1. The volcano maps and heatmaps were plotted by R version 2.8.0 (ComplexHeatmap).
3. Results and discussion
3.1. Evaluation of sensory quality of BT from old and young tree by traditional sensory evaluation and e-tongue technique
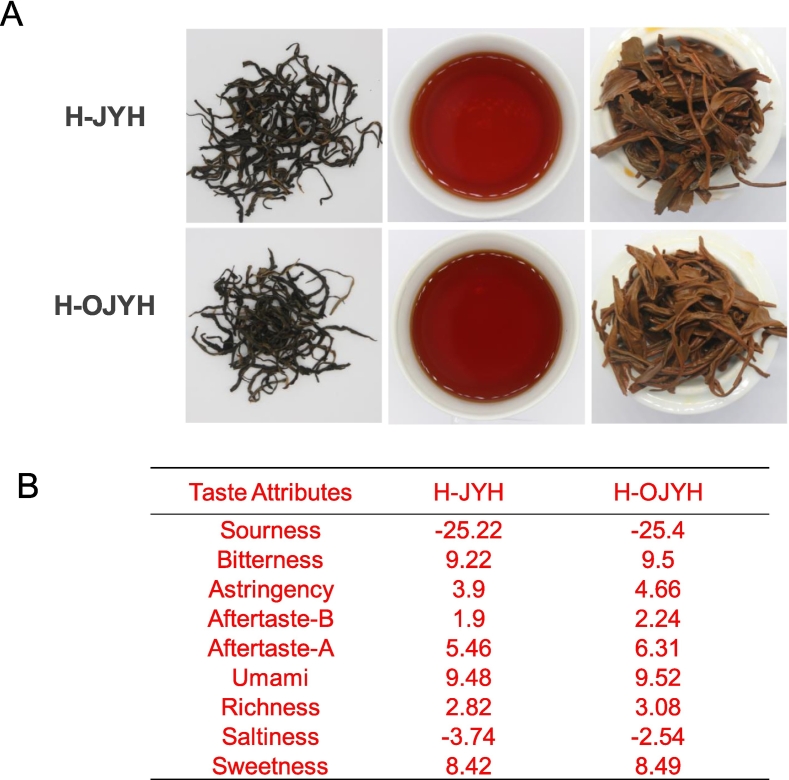
The quality of H-JYH and H-OJYH was analyzed using both the electronic tongue technique and traditional sensory evaluation. H-JYH presented a fruity and floral aroma, with a thick and slightly bitter taste, accompanied by a brilliant-red tea soup color (Table 1, Fig. 1A). In contrast, H-OJYH displayed a remarkably strong fruity and floral aroma, along with a sweet, mellow, and thick taste, and a similar brilliant-red tea soup color (Table 1, Fig. 1A). In short, compared to H-JYH, H-OJYH showed stronger fruity and floral aroma, as well as a sweeter and thicker taste. The e-tongue results indicated that H-OJYH had lower levels of bitter-astringency, and higher sweetness, and freshness compared to H-JYH (Fig. 1B). The results obtained from the e-tongue were consistent with those from traditional sensory evaluation regarding flavor attributes.
Table 1.
The sensory evaluation of young tree black tea (H-JYH) and old tree black tea (H-OJYH).
| appearance (25%) | aroma (30%) | soup color (10%) | taste (30%) | leaf base (10%) | Score | |
|---|---|---|---|---|---|---|
| H-JYH | tight and visible, dark brown and oily, well-proportioned, homogeneous |
fruity and floral | red and bright | thick and slightly bitter | some tender and soft, red | 92 |
| H-OJYH | tight and visible, some dark brown and oily, well-proportioned, homogeneous |
strong fruity and floral | red and bright | sweet, mellow and thick | tender and soft, red | 93 |
Fig. 1.
Quality analysis of black teas from old tree and new tree. (A) Traditional sensory evaluation results; (B) Electronic tongue results.
3.2. Overall analysis of all metabolites of BT from new and old trees
3.2.1. Overview of non-volatile and volatile metabolites
The Pearson correlation coefficients (R) for each sample within the groups of both the non-volatile and volatile metabolism categories were nearly 1, indicating a high level of biological reproducibility among the samples (Fig. 2C and 3C). Additionally, the Pearson's correlation coefficients of most volatile metabolites between H-OJYH and H-JYH were negative, indicating significant differences in volatile metabolites between H-OJYH and H-JYH (Fig. 3C). The three-dimensional results of PCA for nonvolatile metabolites showed that PC1 accounted for 36.04% of the variability, PC2 for 19%, and PC3 for 15.54%, with a cumulative interpretation of 100% for the first five principal components (Fig. 2A and B). Similarly, the PCA results for volatile metabolites showed that PC1 had a variable degree of explanation of 83.49%, PC2 had a variable degree of explanation of 7.4%, and PC3 had a variable degree of explanation of 3.64%, with a cumulative explanation of over 95% for the first five principal components (Fig. 3A and B). The results of the PCA demonstrated significant differences in all metabolites (both non-volatile and volatile) between H-OJYH and H-JYH, particularly in volatile metabolites (Fig. 3A). Samples within the respective groups of H-OJYH and H-JYH clustered first, followed by distinct clustering between H-OJYH and H-JYH, which indicated significant distinctions in non-volatile and volatile metabolites between old and young trees (Fig. 3D).
Fig. 2.
Overview of all non-volatile metabolites of black teas from old tree and new tree. (A) Principal component analysis (PCA) plot; (B) The plot of variance explained by each principal component; (C) Heatmap for correlation analysis; (D) Clustering heat map; (E) Classification chart for all non-volatile metabolites; (F) Trends of the all non-volatile metabolites change in different tree ages.
Note: PC1 denotes the first principal component, PC2 denotes the second principal component, PC3 denotes the third principal component, and the percentage denotes the explanation rate of that principal component for the dataset. Each point in the plot denotes a sample, and samples from the same group are represented using the same color.
Fig. 3.
Overview of all volatile metabolites of black teas from old tree and new tree. (A) Principal component analysis (PCA) plot; (B) The plot of variance explained by each principal component; (C) Heatmap for correlation analysis; (D) Clustering heat map; (E) Classification chart for all volatile metabolites; (F) Trends of the all volatile metabolites change in different tree ages.
Note: PC1 denotes the first principal component, PC2 denotes the second principal component, PC3 denotes the third principal component, and the percentage denotes the explanation rate of that principal component for the dataset. Each point in the plot denotes a sample, and samples from the same group are represented using the same color.
3.2.2. Analysis of categories and trends of non-volatile metabolites
There were 1231 non-volatile metabolites detected in both H-OJYH and H-JYH, which were classified into 12 categories (Fig. 2E), including 290 flavonoids (23.56%), 209 phenolic acids (16.98%), 154 lipids (12.51%), 133 others (10.80%), 101 amino acids and derivatives (8.20%), 93 organic acids (7.55%), 86 alkaloids (6.99%), 69 nucleotides and derivatives (5.61%), 36 lignans and coumarins (2.92%), 31 tannins (2.52%), 24 terpenoids (1.95%), and 5 quinones (0.41%). Flavonoids comprised the largest proportion of all non-volatile metabolites (23.56%), followed by phenolic acids (16.98%), with lipids ranking third (12.51%).
The total amount of non-volatile metabolites was slightly higher in H-OJYH compared to H-JYH (Fig. 2F). The relative amounts of amino acids and derivatives, flavonoids, alkaloids, and other substances in H-OJYH were similar to those in H-JYH. However, the levels of phenolic acids, tannins, and terpenoids were lower in H-OJYH than in H-JYH. The relative amounts of organic acids and lipids were higher in H-OJYH than in H-JYH. Moreover, H-JYH had lower levels of nucleotides and derivatives, lignans and coumarin compared to H-OJYH.
3.2.3. Analysis of categories and trends of volatile substances
Altogether, 504 volatiles were determined in both H-OJYH and H-JYH, categorized into 14 classes (Fig. 3E), including 118 terpenoids (23.41%), 83 esters (16.47%), 54 heterocyclic compounds (10.71%), 42 alcohols (8.33%), 41 ketones (8.13%), 41 aldehydes (8.13%), 36 aromatics (7.14%), 34 hydrocarbons (6.75%), 16 amines (3.17%), 15 acids (2.98%), 11 sulfur compounds (2.18%), 10 phenols (1.98%), 2 halogenated hydrocarbons (0.40%), and 1 others (0.20%). Terpenoids comprised the largest proportion of all volatile metabolites, followed by esters, with heterocyclic compounds ranking third.
The total relative content of volatile metabolite in H-JYH was significantly higher than H-OJYH (Fig. 3F). The relative amounts of phenolic acids, amines, nitrogen compounds, sulfur compounds, hydrocarbons, ketones, and others substances in H-OJYH were greater than those in H-JYH. Conversely, the relative amounts of alcohols, aromatics, halogenated hydrocarbons, aldehydes, terpenoids, heterocyclic compounds, and esters were lower in H-OJYH compared to H-JYH.
3.3. Differential analysis of non-volatile and volatile metabolites between old and young trees
3.3.1. Non-volatile differentially expressed metabolites and taste qualities between old and young trees
Three non-volatile differential metabolites were identified between H-OJYH and H-JYH using our screening criteria: VIP > 1, Log2FC (fold change) ≥ 2, and P value >0.05. Luteolin-6,8-di-C-glucoside and 1-α-linolenoyl-glycerol-2,3-di-O-glucoside significantly increased during storage, while 5,7-dihydroxy-3′,4′,5′-trimethoxyflavone decreased significantly (Fig. 4A). Although these substances were characteristic components for identifying between H-OJYH and H-JYH, they were not responsible for the discrepancies in taste quality because they were not flavor substances. Therefore, we adjusted the screening criteria to VIP > 1 and Log2FC (fold change) ≥ 1, and P value >0.05, resulting in the identification of 533 non-volatile differential metabolites, including 252 up-regulated and 281 down-regulated non-volatile differential metabolites (Fig. 4B).
Fig. 4.
Differential analysis of non-volatile metabolites between old tree black tea and new tree black tea, including (A) Volcano maps and (B) K-Means plot.
Among the 24 non-volatile differential metabolites with taste characteristics that were higher in H-OJYH (Table S1). 8 substances such as L-tyramine, exhibited bitterness, catechin with bitter and astringent taste, 3,4-dihydroxybenzoic acid (protocatechuic acid) with astringent taste (Chen et al., 2022). However, due to their high thresholds, these substances contributed less to the bitterness and astringency of the tea broth. Moreover, the decrease in bitter-astringent substances dominated in H-OJYH compared to H-JYH, resulting in weaker bitterness and astringency in H-OJYH than in H-JYH. Most of the differential metabolites with acidic and sweet tastes were significantly higher in H-OJYH than in H-JYH, resulting in improved acidity and sweetness in H-OJYH. These metabolites included trans-citridic acid, L-tartaric acid, and 2-methylsuccinic acid with sour taste (Hufnagel & Hofmann, 2008), salicylic acid with sweet and sour taste (Ahmed Ezzat, Zoltán, József, & Imre, 2016), as well as ribitol, fraxetin (7,8-dihydroxy-6-methoxycoumarin), d-fructose 6-phosphate, methyl 2-furoate, betaine, dulcitol, and D-galactose with sweet taste (Kim & Yaylayan, 2020; Yin et al., 2022). L-tartaric acid and trans-citridic acid enhanced the sourness of the tea soup, with their acidity thresholds of 41 and 50 mg/m3, respectively (Hufnagel & Hofmann, 2008). Fraxetin (7,8-dihydroxy-6-methoxycoumarin) and d-fructose 6-phosphate contributed to the sweetness of the tea broth in H-OJYH, with their sweetness thresholds of 571.5 and 570 mg/m3, respectively (Yin et al., 2022). In addition, H-OJYH had more special flavor substances, enhancing its richness and overall taste profile, including L-methionine with salty and sweet taste and antioxidant capacity (Zhang et al., 2022), and 3-methyl-1-pentanol affecting the green, apple, and subtle alcohol flavors of bighead carp (Xiao et al., 2021).
Forty-seven non-volatile differential metabolites with taste characteristics were found to be lower in H-OJYH. Among these, 22 substances, such as salicin, octyl gallate, and benzamide (Sun et al., 2023b) had bitterness characteristics, and 3 substances including theaflavin, epigallocatechin-3-gallate, and procyanidin B2 displayed both bitter and astringent characteristics (Chen et al., 2022), and 4 substances including theaflavic acid, vanillic acid, theaflavin-3-gallate, and quercetin-3-O-rutinoside (Rutin) exhibited astringency characteristics (Chen et al., 2022; Li et al., 2023) (Table S1). Rutin negatively impacted the sweet-mellow texture of BT (Li et al., 2019), and affected the bitter-astringent profile of green tea infusion (Chen et al., 2022). The reduction of bitter-astringent metabolites in H-OJYH, led to a weaker bitter-astringent flavor compared to H-JYH. Octyl gallate, chlorogenic acid (3-O-caffeoylquinic acid), neochlorogenic acid (5-O-caffeoylquinic acid), salicin, and octyl gallate greatly contributed to bitterness, with bitter thresholds being 49, 50, 50, 83, and 109 mg/m3. They played an essential role in attenuating the bitterness of H-OJYH. Additionally, vanillic acid and epigallocatechin-3-gallate contributed significantly to the astringency of the tea broth, with astringency thresholds of 53 and 87 mg/m3, respectively, thereby reducing the astringency in H-OJYH. Furthermore, several taste substances were higher in H-JYH compared to H-OJYH, contributing to the special flavor of H-JYH. For example, vanillin had a silky mouth coating sensation, while phenoxyacetic acid and citric acid contributed to a sweet and sour taste (Zhang et al., 2023; Zhang et al., 2023). Additionally, 9 metabolites had a sweet taste, such as 2-phenylethanol, and 2,4-dihydroxybenzoic acid. Compared to H-OJYH, H-JYH had significantly increased substances with better taste qualities, including cyclic 3′,5′-adenylic acid, L-theanine, hydroxypyruvic acid, and l-glutamine. Cyclic 3′,5′-adenylic acid and L-theanine contributed to an umami taste (Narukawa, Morita, & Hayashi, 2008), while hydroxypyruvic acid acted as a masking agent for bitterness, imparting a salty taste (Ley, Blings, Paetz, Krammer, & Bertram, 2006). Furthermore, l-glutamine exhibited salty, sweet, and umami taste (Bachmanov et al., 2016).
3.3.2. Volatile differentially expressed metabolites and aroma qualities between BT from old and young trees
In total, 372 volatile differential metabolites were detected in both H-OJYH and H-JYH, classified into 15 categories (Fig. 5A), including 89 terpenoids (23.99%), 64 esters (17.25%), 36 heterocyclic compounds (9.7%), 32 alcohols (8.63%), 31 ketones (8.36%), 30 aldehydes (8.09%), 28 hydrocarbons (7.55%), 22 aromatics (5.93%), 14 acids (3.77%), 10 amines (2.7%), 6 phenols (1.67%), 5 sulfur compounds (1.35%), 2 nitrogen compounds (0.54%), 2 halogenated hydrocarbons (0.27%), and 1 others (0.27%). The category with the highest proportion of volatile differential metabolites was terpenoids, followed by esters, with heterocyclic compounds being the third largest category.
Fig. 5.
Differential analysis of volatile metabolites between old tree black tea and new tree black tea. (A) Pie chart of the categories of volatile different metabolites; (B) Volcano maps; (C) Fold Change of volatile differential metabolites within top 10; (D) VIP value map of volatile differential metabolites within top 10.
Altogether, 371 differentially expressed metabolites were screened by differentially analyzing all volatiles of H-OJYH and H-JYH (Fig. 5B). Of these, 173 differential metabolites had a higher relative content in H-OJYH than in H-JYH, including 56 aroma substances and 32 substances with aroma thresholds, and 192 differential metabolites had a lower relative content in H-OJYH compared to H-JYH, including 93 substances with aroma attributes and 67 substances with aroma thresholds (Fig. 5B, Table S2). The relative amounts of volatile substances in H-OJYH were significantly higher than in H-JYH, mainly presenting sweet, floral and fruity odors, with some showing spicy, woody, nutty, and earthy odors. Among them, 27 volatile metabolites presented floral, fruity, and combinations of other aromas (floral and sweet, floral and fruity, fruity and nutty, etc.). Of these, 12 substances were fruity types, dominated by ketones and esters, including 6-propyloxan-2-one with a coconut-like odor (Bergamaschi et al., 2015), 1-(4-hydroxyphenyl)ethanone with a slight berry to sweet balsam aroma, 3-methylbutyl hexanoate, trans-2-undecen-1-ol, triacetin, and (3E,6E)-3,7,11-trimethyldodeca-1,3,6,10-tetraene with fruity odors, and heptan-2-yl acetate with fenugreek fruity aroma, (2E)-3,7-dimethylocta-2,6-dienal with strong lemon odor, [(Z)-hex-3-enyl] hexanoate and [(Z)-hex-3-enyl] pentanoate with tropical aroma, prop-2-enyl 3-cyclohexylpropanoate with a pineapple aroma with sweet notes of green apple, and (2E,6E)-2,6-dimethyl-10-methylidenedodeca-2,6,11-trienal with citrus-like aroma. 2-(4-Methylcyclohex-3-en-1-yl)propan-1-ol had a fruity and herbal aroma. Hexyl hexanoate had a fruity odor reminiscent of peach and apple, with a special odor of vegetable, herbal, and cut grass (Jordan, Margaria, Shaw, & Goodner, 2002). There were also 13 substances presenting sweet and floral aromas, including 2-methylnaphthalene, 4-(4-hydroxyphenyl)butan-2-one, methyl 2-phenylacetate, 4-acetylanisole, (2Z)-2-benzylideneheptan-1-ol, methyl tetradecanoate, (E)-7-methyloct-3-en-2-one, [(2E)-3,7-dimethylocta-2,6-dienyl] 2-methylbutanoate, 2,6-dimethyloct-7-en-2-ol, 1-(4-ethylphenyl)ethanone, 3-phenylpropyl acetate, (2Z,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol, and (3R)-3,7-dimethyloct-6-en-1-ol. The main contributors to floral aromas were 2-methylnaphthalene, 4-(4-hydroxyphenyl)butan-2-one, methyl 2-phenylacetate, 3-phenylpropyl acetate, (3R)-3,7-dimethyloct-6-en-1-ol, and 4-acetylanisole. 4-Acetylanisole emitted pleasant floral aromas reminiscent of hawthorn and heliotrope (D'Auria, Racioppi, Rana, & Laurita, 2014).
The relative amounts of volatile metabolites in H-OJYH were markedly reduced compared to those of H-JYH, mainly presenting spicy, wood, and earthy odors, with a portion of floral and fruity odors. There were 23 substances presenting spicy aroma and combinations of spicy with other odors such as floral, fruity, and nutty. The main contributing substances included (−)-carvone with a spearmint-like odor, 2-methoxy-4-vinylphenol with a powerfully spicy, clove-like roasted peanut aroma, 7-methyl-3-methylideneocta-1,6-diene with a peppery, spicy, and fruity aroma, (E,E)-2,4-undecadienal with complex flavors of oily, spicy, citrus, buttery, and baked odor (Hiromi Ikeura, 2010), 3,6-dimethyl-4,5,6,7-tetrahydro-1-benzofuran with an odor similar to that of menthol, methyl salicylate with a mint and wintergreen aroma (Ledo et al., 2018a), and 4-ethylbenzaldehyde with an almond and anise aroma. Volatile metabolites contributing to the woody aroma that were significantly higher in H-JYH than in H-OJYH including heptyl 2-methylpropanoate, (E)-4-(2,6,6-trimethylcyclohexen-1-yl)but-3-en-2-one with orange, jam, orris, raspberry, and cedar wood odor, isoborneol with a piney and camphor odor (Hong & Kyoung, 2016), (1S,2R,4S)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ol with a piney and camphoraceous aroma, citral with lemon peel, citrus, woody, and herbal odor (Hong & Kyoung, 2016). (E)-5-methyl-2-propan-2-ylhex-2-enal with herbal, woody, minty, musty, tomato, and lavender floral notes, L-fenchone with a camphor and woody aroma (Kang et al., 2019), (1R,2R,5S)-2-methyl-5-propan-2-ylbicyclo[3.1.0]hexan-2-ol with woody, balsam, and mint aroma, and endo-borneol with a sharp camphor-like odor. Volatile metabolites contributing to the earthy aroma were significantly higher in H-JYH than in H-OJYH, mainly including p-cresol, 4-methyl-2-nitrophenol, geranyl acetate, and 2-methylisoborneol.
The volatile differentially expressed metabolites with both Log2FC and VIP values meeting the top ten rankings were the most significant differential metabolites for the differences between H-OJYH and H-JYH. Combined with the aroma attributes of the differential metabolites and the aroma thresholds, we aimed to identify the characteristic components that affect the differences in the aroma qualities of H-JYH and H-OJYH (Fig. 5C and D). The relative amounts of seven volatiles were markedly more abundant in H-OJYH than in H-JYH, including 4-methyloctan-4-ylbenzene, (E)-4-(2,6,6-trimethylcyclohexen-1-yl)but-3-en-2-one, β-ionone, methyl salicylate, 2-phenylethanol, butylbenzene, and 2-phenylacetaldehyde. Among them, five volatile metabolites had aroma attributes. (E)-4-(2,6,6-Trimethylcyclohexen-1-yl)but-3-en-2-one had flowery, orange, seaweed, and cedar wood odors (Zhang, Li, et al., 2023; Zhang, Zhang, et al., 2023). β-Ionone had a violet floral aroma (Gong et al., 2023). Methyl salicylate had a mint and wintergreen aroma (Ledo et al., 2018b). 2-Phenylethanol had a rose-like aroma characteristic (Yan et al., 2020). 2-Phenylacetaldehyde had sweet, floral, chocolate, and cocoa aroma characteristics (Zhu et al., 2011). Volatile metabolites with aroma characteristics in tea primarily come from secondary metabolites, oxidation of carotenoids, and fatty acids, microbial metabolites formed after fermentation, and the products of the Maillard reaction developed by heating (Sales, Paulino, Pastore, & Bicas, 2018). The relative amounts of five volatiles in H-OJYH were significantly lower than those of H-JYH, including butanoic acid, 3-hydroxy-3-methyl-, (2E,4S,7E)-4-isopropyl-1,7-dimethylcyclodeca-2,7-dienol, (5E)-2,6-dimethylocta-5,7-dien-4-one, N-phenylnaphthalen-2-amine, and dehydromevalonic lactone, but they did not have aroma properties.
4. Conclusion
We found that H-OJYH had a more favorable flavor and a more pleasant aroma compared to H-JYH. H-OJYH showed stronger sweetness, sourness, and thickness, and diminished bitter-astringent taste compared to H-JYH, as determined by e-tongue technique and sensory evaluation. Metabolomics results confirmed the detection of 1231 non-volatiles and 504 volatiles. 3 components served as distinguishing markers for H-JYH and H-OJYH including Luteolin-6,8-di-C-glucoside (up-regulated), 1-α-linolenoyl-glycerol-2,3-di-O-glucoside (up-regulated), and 5,7-dihydroxy-3′,4′,5′-trimethoxyflavone (down-regulated). L-tartaric acid and trans-citridic acid increased the acidic flavor of H-OJYH, while 7,8-dihydroxy-6-methoxycoumarin and d-fructose 6-phosphate increased the sweetness of H-OJYH. Bitter metabolites such as octyl gallate, chlorogenic acid, neochlorogenic acid, and salicin, and astringent metabolites such as vanillic acid and epigallocatechin-3-gallate were significantly lower in H-OJYH than in H-JYH, resulting in diminished bitter-astringent taste in H-OJYH. Additionally, 372 volatile differential metabolites were screened, among which β-ionone (up-regulated), 2-phenylethanol (up-regulated), and phenylacetaldehyde (up-regulated) were characteristic components of H-OJYH with stronger floral and sweet aroma. This study investigated the characteristic components and internal reasons for the discrepancies in taste and aroma qualities between BT from new and old trees, providing insights into the correlation between different tree ages and tea quality, and laying a theoretical foundation for future tea quality evaluations. Subsequent studies would explore the effect of tree age on tea bioactivity.
CRediT authorship contribution statement
Ruohong Chen: Writing – original draft, Visualization, Methodology, Formal analysis, Data curation. Lingli Sun: Writing – original draft, Methodology, Formal analysis, Data curation. Suwan Zhang: Resources, Project administration, Funding acquisition. Shuai Wen: Resources, Project administration, Funding acquisition. Xingfei Lai: Resources, Project administration, Funding acquisition. Qian Li: Resources, Project administration, Funding acquisition. Junxi Cao: Resources, Project administration, Funding acquisition. Shili Sun: Writing – review & editing, Supervision, Project administration, Conceptualization.
Declaration of competing interest
All authors declare that there is no conflict of interest.
Acknowledgements
The research is supported by Food nutrition and health Collaborative Innovation Center of GDAAS (Project No.: XT202229); Agricultural competitive industry discipline team building project of Guangdong Academy of Agricultural Sciences (Project No.: 202126TD); Qingyuan Science and Technology Plan Projects (Project No.: 2021ZDZX002); Guangdong Basic and Applied Basic Research Foundation (Project No.: 2022A1515110190, 2023A1515010264, 2024A1515010696); Guangzhou Science and Technology Plan Projects (Project No.: 20212100005, 202201011455, 2023E04J0781); Special fund project for introduction of scientific and technological talents of Guangdong Academy of Agricultural Sciences (Project No.: R2021YJ-YB3014); Innovation Fund projects of Guangdong Academy of Agricultural Sciences (Project No.: 202115, 202135); Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science (Project No.: R2022PY-QY010); the Innovation Fund projects of Guangdong Key Laboratory of Tea Plant Resources Innovation and Utilization (Project No.: 2021CX02); The project of guangdong province rural science and technology commissioner stationed in the village and town (Project No.: KTP20210090). Funders did not have any role in study design, data collection, and data analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101470.
Contributor Information
Ruohong Chen, Email: chenruohong@tea.gdaas.cn.
Lingli Sun, Email: sunlingli@tea.gdaas.cn.
Qiuhua Li, Email: liqiuhua@tea.gdaas.cn.
Shuai Wen, Email: wenshuai@tea.gdaas.cn.
Xingfei Lai, Email: laixingfei@tea.gdaas.cn.
Qian Li, Email: liq@gdaas.cn.
Junxi Cao, Email: caojunxi@tea.gdaas.cn.
Shili Sun, Email: sunshili@zju.edu.cn.
Appendix A. Supplementary data
Supplementary Tables S1-2
Data availability
No data was used for the research described in the article.
References
- Ahmed Ezzat A.A., Zoltán S., József N., Imre J.H. Postharvest treatments with methyl jasmonate and salicylic acid. Effect of Methyl Jasmonate and Salicylic Acid on Apricot Fruit Quality. 2016;2(67):159–166. [Google Scholar]
- Bachmanov A.A., Bosak N.P., Glendinning J.I., Inoue M., Li X., Manita S.…Beauchamp G.K. Genetics of amino acid taste and appetite. Advances in Nutrition. 2016;7(4):806S–822S. doi: 10.3945/an.115.011270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi M., Aprea E., Betta E., Biasioli F., Cipolat-Gotet C., Cecchinato A.…Gasperi F. Effects of dairy system, herd within dairy system, and individual cow characteristics on the volatile organic compound profile of ripened model cheeses. Journal of Dairy Science. 2015;98(4):2183–2196. doi: 10.3168/jds.2014-8807. [DOI] [PubMed] [Google Scholar]
- Cai H., Zhong Z., Li Z., Zhang X., Fu H., Yang B., Zhang L. Metabolomics in quality formation and characterisation of tea products: A review. International Journal of Food Science and Technology. 2022;57(7):4001–4014. [Google Scholar]
- Chen Y.H., Zhang Y.H., Chen G.S., Yin J.F., Chen J.X., Wang F., Xu Y.Q. Effects of phenolic acids and quercetin-3-O-rutinoside on the bitterness and astringency of green tea infusion. npj Science of Food. 2022;6(1):8. doi: 10.1038/s41538-022-00124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Auria M., Racioppi R., Rana G.L., Laurita A. Studies on volatile organic compounds of some truffles and false truffles. Natural Product Research. 2014;28(20):1709–1717. doi: 10.1080/14786419.2014.940942. [DOI] [PubMed] [Google Scholar]
- Feng Z., Li Y., Li M., Wang Y., Zhang L., Wan X., Yang X. Tea aroma formation from six model manufacturing processes. Food Chemistry. 2019;285:347–354. doi: 10.1016/j.foodchem.2019.01.174. [DOI] [PubMed] [Google Scholar]
- Gong C., He N., Zhu H., Anees M., Lu X., Liu W. Multi-omics integration to explore the molecular insight into the volatile organic compounds in watermelon. Food Research International. 2023;166 doi: 10.1016/j.foodres.2023.112603. [DOI] [PubMed] [Google Scholar]
- Hiromi Ikeura K.K.X.L. Identification of (E,E)-2,4-undecadienal from coriander (Coriandrum sativum L.) as a highly effective deodorant compound against the offensive odor of porcine large intestine. Journal of Agricultural and Food Chemistry. 2010;58(20):11014–11017. doi: 10.1021/jf102297q. [DOI] [PubMed] [Google Scholar]
- Hong Y.S., Kyoung S.K. Identification of volatile flavor compounds in Jeju citrus fruits. Korean Journal of Food Preservation. 2016;7(23):977–988. [Google Scholar]
- Hufnagel J.C., Hofmann T. Quantitative reconstruction of the nonvolatile sensometabolome of a red wine. Journal of Agricultural and Food Chemistry. 2008;56(19):9190–9199. doi: 10.1021/jf801742w. [DOI] [PubMed] [Google Scholar]
- Jahan I., Shopan J., Rahman M.M., Sarkar A., Baset M.A., Zhang Z.…Hasan M.K. Long-term traditional fertilization alters tea garden soil properties and tea leaf quality in Bangladesh. Agronomy (Basel) 2022;12(9):2128. [Google Scholar]
- Jordan M.J., Margaria C.A., Shaw P.E., Goodner K.L. Aroma active components in aqueous kiwi fruit essence and kiwi fruit puree by GC-MS and multidimensional GC/GC-O. Journal of Agricultural and Food Chemistry. 2002;50(19):5386–5390. doi: 10.1021/jf020297f. [DOI] [PubMed] [Google Scholar]
- Kang G., Mishyna M., Appiah K.S., Yamada M., Takano A., Prokhorov V., Fujii Y. Screening for plant volatile emissions with allelopathic activity and the identification of L-Fenchone and 1,8-cineole from star Anise (Illicium verum) leaves. Plants (Basel) 2019;8(11) doi: 10.3390/plants8110457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.S., Yaylayan V. Profiling of glucose degradation products through complexation with divalent metal ions coupled with ESI/qTOF/MS/MS analysis. Current Research in Food Science. 2020;3:268–274. doi: 10.1016/j.crfs.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledo J.M., Flores H., Solano-Altamirano J.M., Ramos F., Hernández-Pérez J.M., Camarillo E.A.…Amador M.P. Experimental and theoretical study of methyl n-hydroxybenzoates. The Journal of Chemical Thermodynamics. 2018;124:1–9. [Google Scholar]
- Ledo J.M., Flores H., Solano-Altamirano J.M., Ramos F., Hernández-Pérez J.M., Camarillo E.A.…Amador M.P. Experimental and theoretical study of methyl n-hydroxybenzoates. The Journal of Chemical Thermodynamics. 2018;124:1–9. [Google Scholar]
- Ley J.P., Blings M., Paetz S., Krammer G.E., Bertram H.J. New bitter-masking compounds: Hydroxylated benzoic acid amides of aromatic amines as structural analogues of homoeriodictyol. Journal of Agricultural and Food Chemistry. 2006;54(22):8574–8579. doi: 10.1021/jf0617061. [DOI] [PubMed] [Google Scholar]
- Li J., Yao Y., Wang J., Hua J., Wang J., Yang Y.…Yuan H. Rutin, gamma-aminobutyric acid, gallic acid, and caffeine negatively affect the sweet-mellow taste of congou black tea infusions. Molecules. 2019;24(23) doi: 10.3390/molecules24234221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zhang Y., Chen C., Zhong S., Li M., Xu K.…Jin S. Chemical and quality analysis of beauty tea processed from fresh leaves of Tieguanyin variety with different puncturing degrees. Foods. 2023;12(9) doi: 10.3390/foods12091737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Xu Y., Wu J., Wen J., Yu Y., An K., Zou B. GC-IMS and olfactometry analysis on the tea aroma of Yingde black teas harvested in different seasons. Food Research International. 2021;150 doi: 10.1016/j.foodres.2021.110784. [DOI] [PubMed] [Google Scholar]
- Narukawa M., Morita K., Hayashi Y. L-theanine elicits an umami taste with inosine 5′-monophosphate. Bioscience, Biotechnology, and Biochemistry. 2008;72(11):3015–3017. doi: 10.1271/bbb.80328. [DOI] [PubMed] [Google Scholar]
- Sales A., Paulino B.N., Pastore G.M., Bicas J.L. Biogeneration of aroma compounds. Current Opinion in Food Science. 2018;19:77–84. [Google Scholar]
- Scharbert S., Hofmann T. Molecular definition of black tea taste by means of quantitative studies, taste reconstitution, and omission experiments. Journal of Agricultural and Food Chemistry. 2005;53(13):5377–5384. doi: 10.1021/jf050294d. [DOI] [PubMed] [Google Scholar]
- Shang A., Li J., Zhou D., Gan R., Li H. Molecular mechanisms underlying health benefits of tea compounds. Free Radical Biology and Medicine. 2021;172:181–200. doi: 10.1016/j.freeradbiomed.2021.06.006. [DOI] [PubMed] [Google Scholar]
- Song H., Liu J. GC-O-MS technique and its applications in food flavor analysis. Food Research International. 2018;114:187–198. doi: 10.1016/j.foodres.2018.07.037. [DOI] [PubMed] [Google Scholar]
- Sun L., Zhang S., Li Q., Yuan E., Chen R., Yan F.…Sun S. Metabolomics and electronic tongue reveal the effects of different storage years on metabolites and taste quality of oolong tea. Food Control. 2023;152 [Google Scholar]
- Sun L., Zhang S., Li Q., Yuan E., Chen R., Yan F.…Sun S. Metabolomics and electronic tongue reveal the effects of different storage years on metabolites and taste quality of Oolong Tea. Food Control. 2023;152 [Google Scholar]
- Wen S., Sun L., Zhang S., Chen Z., Chen R., Li Z.…Li Q. The formation mechanism of aroma quality of green and yellow teas based on GC-MS/MS metabolomics. Food Research International. 2023;172 doi: 10.1016/j.foodres.2023.113137. [DOI] [PubMed] [Google Scholar]
- Xiao N., Huang H., Liu J., Jiang X., Chen Q., Chen Q., Shi W. Comparison of different edible parts of bighead carp (Aristichthys nobilis) flavor. Journal of Food Biochemistry. 2021;45(11) doi: 10.1111/jfbc.13946. [DOI] [PubMed] [Google Scholar]
- Xie J., Wang L., Deng Y., Yuan H., Zhu J., Jiang Y., Yang Y. Characterization of the key odorants in floral aroma green tea based on GC-E-nose, GC-IMS, GC-MS and aroma recombination and investigation of the dynamic changes and aroma formation during processing. Food Chemistry. 2023;427 doi: 10.1016/j.foodchem.2023.136641. [DOI] [PubMed] [Google Scholar]
- Yan W., Zhang X., Qian X., Zhou J., Dong W., Ma J.…Jiang M. Comprehensive investigations of 2-phenylethanol production by high 2-phenylethanol tolerating Meyerozyma sp. strain YLG18. Enzyme and Microbial Technology. 2020;140 doi: 10.1016/j.enzmictec.2020.109629. [DOI] [PubMed] [Google Scholar]
- Ye F., Guo X., Li B., Chen H., Qiao X. Characterization of effects of different tea harvesting seasons on quality components, color and sensory quality of “Yinghong 9” and “Huangyu” large-leaf-variety black tea. Molecules. 2022;27(24) doi: 10.3390/molecules27248720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Q., Ji J., Zhang R., Duan Z., Xie H., Chen Z., Hu F., Deng H. Identification and verification of key taste components in wampee using widely targeted metabolomics. Food Chemistry: X. 2022;13 doi: 10.1016/j.fochx.2022.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Yeo A.S., Low M.Y., Zhou W. Identifying key non-volatile compounds in ready-to-drink green tea and their impact on taste profile. Food Chemistry. 2014;155:9–16. doi: 10.1016/j.foodchem.2014.01.046. [DOI] [PubMed] [Google Scholar]
- Zhang L., Cao Q., Granato D., Xu Y., Ho C. Association between chemistry and taste of tea: A review. Trends in Food Science & Technology. 2020;101:139–149. [Google Scholar]
- Zhang S., Li Q., Wen S., Sun L., Chen R., Zhang Z.…Chen Z. Metabolomics reveals the effects of different storage times on the acidity quality and metabolites of large-leaf black tea. Food Chemistry. 2023;426 doi: 10.1016/j.foodchem.2023.136601. [DOI] [PubMed] [Google Scholar]
- Zhang W., Han Y., Shi K., Wang J., Yang C., Xu X. Effect of different sulfur-containing compounds on the structure, sensory properties and antioxidant activities of Maillard reaction products obtained from Pleurotus citrinopileatus hydrolysates. LWT. 2022;171 [Google Scholar]
- Zhang Y.Y., Zhang P., Le M.M., Qi Y., Yang Z., Hu F.L.…Bao G.H. Improving flavor of summer Keemun black tea by solid-state fermentation using Cordyceps militaris revealed by LC/MS-based metabolomics and GC/MS analysis. Food Chemistry. 2023;407 doi: 10.1016/j.foodchem.2022.135172. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Zhou H., Hu Y., Tang J., Su M., Guo Y.…Liu B. Antityrosinase and antimicrobial activities of 2-phenylethanol, 2-phenylacetaldehyde and 2-phenylacetic acid. Food Chemistry. 2011;124(1):298–302. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables S1-2
Data Availability Statement
No data was used for the research described in the article.