Abstract
Amongst the various water pollutants, heavy metal ions require special attention because of their toxic nature and effects on humans and the environment. Preserving natural resources will have positive impacts on living conditions by reducing diseases and water treatment by nanotechnology is effective in solving this problem owing to the properties of nanomaterials. In this study, a goethite nanoparticle was prepared by hydrothermal method, while ZnO/goethite nanocomposite by co-precipitation was developed. The nanoparticles were characterized using Scanning Electron Microscopy (SEM), X-ray Diffraction (XRD), Fourier Transform Infrared Spectroscopy (FTIR), Transform Electron Microscopy (TEM), Thermogravimetric Differential Thermal Analysis (TGA-DTA), Dynamic Light Scattering (DLS), and Breunner-Emmet-Teller (BET) surface area analysis. The adsorption of Cd(II)-Pb(II) and Cd(II)-Pb(II)-Ni(II) ions systems on ZnO/goethite nanocomposite was investigated in a batch mode. The findings of the study showed that nanoparticles ZnO/goethite composite were mixed of spherical and rod-like shapes. The BET results revealed average particle sizes of 41.11 nm for nanoparticles for ZnO/goethite while TGA/DTA confirmed the stability of the adsorbents. The optimum adsorption capacities of the nanocomposite for Pb(II), Cd(II), and Ni(II) ions from the Pb-Cd-Ni ternary system were 415.5, 195.3, and 87.13 mg g−1, respectively. The adsorption isotherm data fitted well with the Langmuir isotherm model. The study concluded that the nanoparticle adsorbents are efficient for the remediation of toxic pollutants and are, therefore, recommended for wastewater treatment.
Keywords: Nanosorbent (ZnO/goethite), Competitive and selective sorption, Modeling studies, Cd-Pb-Ni ions
Introduction
The difficulties in estimating and describing the adsorption of pollutants from wastewater result from its multicomponent nature. Limited information on the use of inorganic nanomaterials as adsorbents coupled with fewer adsorbents performing satisfactorily in the removal of pollutants from wastewater is a huge challenge. In order to solve this problem, the field of nanotechnology has been explored in fabrication of nanomaterials and is presently receiving attention from many researchers around the world. Nanotechnology is used to create structural, chemical, and/or physical modifications of nanomaterials to enhance their properties above their bulk materials. Properties of materials such as particle size, shape, and surface chemistry are enhanced when they are brought to the nanoscale [1–6]. Furthermore, compositing materials can also improve their physical, structural and chemical properties resulting in increased efficiencies for different applications.
Various synthesis methods have been applied to synthesize composite nanomaterials of different lattice structures including co-precipitation [7], forced hydrolysis [8], surfactant-assisted methods and so on [9]. Many of these nanomaterials have been applied in the single and competitive adsorption of inorganic and organic pollutants, but few were explored for selective adsorption experiments. Selective adsorption is seldomly studied in adsorption research of pollutants from aqueous phases due to the complex nature and dimension of such multicomponent systems[10–14]. Adsorption works on systems involving two or more pollutants have been carried out with limited progress made. Hema and Arivoli [15] in their comparative study on adsorption of an organic pollutant on activated low-cost carbon, investigated carbon adsorption capacity from multiple systems. Janaki et al. [16] used polyaniline/bacterial extracellular polysaccharides composite in competitive adsorption of some pollutants from an aqueous solution. In the binary system, anionic pollutants competed resulting in antagonistic adsorption process with one having lower adsorption capacity. The binary adsorption study data were interpreted well by the Sheindorf–Rehbun–Sheintuch equation as compared to the extended Langmuir model with a constant interaction factor. Khalfa et al. [17] carried out a study on modeling competitive adsorption of some heavy metals onto natural and activated clay. The competitive adsorption study showed that the uptake of Pb ions were inhibited in the presence of zinc, and an antagonistic binary adsorption mechanism was observed. Rathore and Mondal [18] reported a study on adsorptive removal of arsenic and fluoride in a single as well as a bi-component system using aluminum oxide/hydroxide nanoparticles. The modified competitive Langmuir isotherm was most suitable for the bi-component system among different isotherms. Similarly, competitive adsorption of zinc and cadmium onto aluminum oxide nanoparticles was carried out by Stietiya and Wang [19], the results of the adsorption isotherms showed that Zn ions had a higher affinity for the Al2O3 nanoparticle surface than Cd ions in the binary-metal ions system. Chen et al. [20] performed a competitive adsorption experiment on adsorption of aqueous Cd2+, Pb2+, and Cu2+ ions by nano-hydroxyapatite in single- and multi-metal ions solution. The study showed that adsorption affinity of the adsorbent for Pb(II) ions were always higher than that of Cu(II) and Cd(II) ions; the adsorption maxima for the Cd, Pb and Cu ions followed the order Cd2+ < Cu2+ < Pb2+. They assumed that the trend could be inversely proportional to the hydrated ionic radii as Pb2+ (4.01 Å) > Cu2+ (4.19 Å) > Cd2+ (4.26 Å). The measured selectivity coefficients in the multi-metal ions (Cd–Pb–Cu) reaction system showed that Pb2+ has the highest adsorption selectivity on nano-hydroxyapatite among the metals investigated [16–20]. They concluded that the selectivity coincided well with the adsorption affinity order in mono-metal reaction systems. In another competitive adsorption study, Al-Jlil and Latif [21] evaluated the equilibrium isotherm models for the adsorption of Cu and Ni ions from wastewater on bentonite clay. They concluded that the adsorption studies were affected by a change in the pH and temperature of the process.
Zinc oxide (ZnO) is a semi-conductor and has been applied in its pure form and composited with other materials in various ways. It properties can be enhanced for better performance since it is relatively stable to the thermal, chemical, and physical treatment [21–26]. It has been applied in various applications as biosensor, solar cell, photodetector, photocatalyst and adsorbent in water treatments [27–32]. Structural and surface modifications of ZnO have been devised and explored with other materials in other to further improve its efficiency, selectivity and sensitivity [33–38]. One of the suggested ways is by compositing it with iron-based oxides and hydroxides. Iron oxyhydroxides containing ferric irons (FeOOH) have found applications in various ways due to their exceptional physical, chemical, structural and biological properties. These have made iron oxides materials of great interest and value in nanotechnology, science, and medicine [39]. Some of the areas of applications of iron-based nanomaterials include nano-disc formation [40], electrochemical sensing [41], photocatalysis [42], adsorption and so on [40, 41]. Goethite (α-FeOOH) has been applied in various fields including the fabrication of composite materials [42–44] and has shown excellent properties. ZnO is a semiconductor material with high resistance to corrosion and low toxicity. Goethite (α-FeOOH), on the other hand, is rich in hydroxyl group (OH) which can bind to metal ions pollutants using its lone pairs of electrons and it can form hydrogen bonds with halogenated pollutants. Therefore, combining the properties of ZnO and goethite to form ZnO/goethite nanocomposite can yield a more excellent material with high adsorption efficiency and capacity compared with their separate materials [45]. However, literatures have shown that ZnO/goethite nanohybrid has not been explored in adsorption of pollutants from aqueous mediums in any work so far. The ZnO/goethite synthesis route feasibility, application and optimum performance, especially in the area of adsorption of pollutants from aqueous phases has tremendously brought an improvement in the field of adsorption science in terms of environmental remediation. This is because ZnO/goethite nanocomposite, a novel adsorbent, has the capacity to remove various metal ions simultaneously from multiple systems without affecting its efficiency.
In this research work, a simple and facile co-precipitation method was designed and used to prepare nanohybrid of ZnO/goethite for competitive and selective adsorption study from Cd(II)-Pb(II) and Cd(II)-Pb(II)-Ni(II) sorption systems because of its exceptional performance in single component adsorption from aqueous phases.
Materials and methods
Chemicals
All chemical reagents used in this work were of analytical grade. Zinc nitrate hexahydrate, Zn(NO3)2·6H2O (96.0%) and cadmium nitrate, Cd(NO3)2 (99.0%) were purchased from Merck. Iron nitrate, Fe(NO3)2·9H2O (98.0%), sodium hydroxide, NaOH (97.0%), hydrochloric acid (96.0%), and potassium hydroxide, KOH (85.0%) were purchased from FINAR. Urea, CO(NH2)2 (99.0%) was purchased from NICE, while lead nitrate, Pb(NO3)2 (99.0%), was purchased from Fisher Scientific. All chemicals were used without further purification while double distilled water prepared in the laboratory was used throughout the entire experiment.
Synthesis of goethite nanoparticles
Goethite nanoparticles were prepared according to a method reported by John et al., 2022 [46]. A 5.0 M KOH solution (10 mL) was added to a 1.0 M iron nitrate salt solution (10 mL) under constant stirring (500 rpm) on a magnetic stirrer for 5 min. The resultant mixture was transferred into a 100 mL capacity Teflon autoclave Fig. 1. Double distilled water was added up to 70 – 80% volume of the autoclave and kept in an oven at 100 °C for 16 h for a hydrothermal reaction to occur. Upon reaction completion, the autoclave was allowed to cool to ambient temperature without interference. The orange-colored solid product was collected by decantation and washed with doubled distilled water and ethanol several times to completely remove impurities and any adsorbed ions. Finally, the precipitate was filtered and dried in an oven at 100 °C for 16 h
Fig. 1.
Synthesis of ZnO/goethite (ZGNPs) and its application in sorption of metal ions systems
Preparation of ZnO/goethite nanosorbent
Synthesis of ZnO/goethite was performed according to a method reported by John et al., 2020 [47] by dispersing 0.1 g of the freshly prepared α-FeOOH NPs in 75 mL of a solution containing 4.462 g Zn(NO3)2·6H2O in a beaker under stirring on a magnetic stirrer at 700 rpm as represented in Fig. 1 [48].
A 30 mL 0.4 M solution of NaOH was added dropwise to the solution and stirred for 1 h after the complete addition of the alkaline solution. The resulting precipitate was separated by filtration and washed with double distilled water until the pH of the filtrate was constant. The solid was finally dried at 120 °C for 10 h.
Characterization of nanosorbent
The XRD analysis of ZnO/goethite was done with an Empyrean series 2 diffractometer and the peaks were assigned using the PANanalytial (BBHD10-90.xrdmp) measurement software. The XRD analysis was done using CuKα radiation of a wavelength of 1.54060 Å (40 kV and 45 Ma) at a scan step of 24.765 s at 25 °C in the range of 2Ө = 20 to 90°.
The SEM analysis was performed with a Zeiss Evolution electronic microscope. A carbon tape was used to coat about 0.1 g of the sample and placed in the SEM machine. The SEM analysis was conducted at a speed scan of 7 with LaB6 filament at 40 µA.
The TEM analysis was done with a Tecnai G2 20 Twin electronic microscope. The experiment was performed on the sample by ultrasonically dispersing 0.1 g of it in 5 mL ethanol for 15 min. After complete dispersion, a drop of about 0.5 µL of the sample solution was placed on a carbon film and dried with infrared light for 2 min. The dried sample was loaded in a single tilt holder and inserted into the machine vacuum pipe for TEM analysis.
The FTIR analysis was carried out with a NICOLET 6700 digital spectrometer using the KBr pellet technique with 0.1 g of the nanomaterial sample using 0.1 mg KBr and the spectra were recorded from 4000 cm−1 to 500 cm−1.
The particle size and distribution of ZnO/goethite were measured using an Anton paar LENOVO leitesizier 500 equipped with a 660 nm laser and dynamic light-scattering (PCS) at 25˚C.
The Brunauer-Emmette-Teller (BET) method was used to determine the surface areas of the ZnO/goethite prepared. The N2 physisorption experiment was performed on a Quantachrone instrument by taking 0.02 g of the dry sample of the adsorbent and heating it under a vacuum at 105 °C for 10 h after the dispersion of the samples on a quantachrone cell.
The effect of mass loss as a result of temperature change was performed with a TGA/DTA Setaram, kep-tech SETSYS evolution instrument at a heating rate of 10 °C min−1. in an inert N2 atmosphere. The TGA/DTA was used to determine the mass loss steps, degradation process, and thermal stability of ZnO/goethite prepared.
Determination of ZnO/goethite point of zero charge (pHpzc)
The salt addition method was used to determine the pHpzc of ZnO/goethite by using a 0.1 M KNO3 solution [46]. Salt solutions of different initial pH (pHinitial) values in the 2.0—12.0 range were prepared and 0.1 g of the nanosorbent was added to each 50 mL of the salt solution in a conical flask, sealed, and placed in an orbital shaker with the mode: Smitta Scientific SS 30. The solution was stirred for 2 h at 120 rpm and was allowed to equilibrate for 2 h, and the final pH (pHfinal) was taken and plotted against the initial pH (pHinitial) from which the pHpzc curve was obtained and the pHpzc of ZnO/goethite was determined.
Competitive adsorption studies
To study the effect of multiple pollutants in adsorption processes, experiments were performed at optimum conditions for the single system [48]. A 0.1 g of ZnO/goethite was contacted with a binary solution of varying concentrations of Cd(II) and Pb(II) ions ranging from 200 to 1000 mg L−1 at pH 6 and agitated in a mechanical shaker for 2 h at 700 rpm. To investigate the performance of ZnO/goethite in adsorbing from multi-metal ions system, a ternary solution was prepared by introducing Ni(II) as the third metal ion, and tested in similar conditions as the binary system. The quantities (mg g−1) of each metal ion adsorbed and percentage removal (R, %) at equilibrium were determined in a similar way to the single adsorption using Eqs. (1) and (2), respectively.
| 1 |
| 2 |
where Co and Ce are the initial and equilibrium concentrations in mg L−1 respectively, while m and V are the mass (g) of adsorbent and volume (L) of solution respectively.
Selective adsorption experiment
The adsorption of Pb(II) ions by the synthesized ZnO/goethite nanosorbent in this adsorption study makes it necessary to further investigate its selectiveness for the metal ions. The selective adsorption experiment was performed in the same conditions as described in “Competitive adsorption studies” section, but with varying concentrations of the interfering ions of Cd(II) and Ni (II) from 100 to 500 mg L−1, while keeping the concentration of the targeted Pb(II) ions constant at 100 mg L−1. In similar conditions, the concentration of the targeted metal ions was increased to 200 mg L−1, while still varying the concentration of the interfering ions from 100 to 500 mg L−1.
The distribution coefficient, Kd, has been used extensively in many studies as a mobility index of a metal ion in a multi-ion adsorption system to investigate the interaction between two or more adsorbing species in aqueous solutions [45, 46, 49]. The Kd is a term used to describe the affinity of an adsorbate for a specific adsorbent amongst several competing ions and a dimensionless constant called the selectivity coefficient, α, for a specified metal ion among another competing ion can be estimated by Eqs. (3) and (4), respectively.
| 3 |
| 4 |
where; Co is the concentration of the metal ions in the aqueous phase before adsorption, Ce is the equilibrium concentration (mg g−1), V is the volume of solution (L), m is the mass of adsorbent used for the adsorption, T in Eq. (3) indicates the ternary (three metal ions) adsorption system, and are the distribution coefficients of the targeted and the interfering metal ions in solution designated as i and j respectively, while and are the targeted and the interfering metal ions respectively, with z indicating the metal ions valences. The adsorption data obtained from the optimized parameters were tested and analysed using extended and modified Langmuir, and extended Freundlich isotherms.
Determination of metal ions concentration using Atomic Absorption Spectroscopy (AAS)
The AAS technique requires standards with known content to establish the relation between the measured absorbance and the analyte concentration and relies on the Beer-Lambert law. According to the law, when a beam of monochromatic light of intensity, I0, passes through a medium that contains an absorbing substance, the intensity of transmitted radiation, I, depends on the length, l, of the absorbing medium and the concentration, c, of the solution[17, 50, 51]. Mathematically it can be represented in Eq. (5).
| 5 |
where;
- ε
molar absorption coefficient or molar extinction coefficient.
For a particular wavelength (λ), the absorbance, A, of a solution in a container of fixed path length is directly proportional to c of a solution (Eq. 6).
| 6 |
A plot of A and c is expected to be a linear plot, passing through the origin, and showing that Beer-Lambert’s law is obeyed. This plot, known as calibration curve, can also be employed in finding the c of a given solution.
Multiple adsorption isotherms
As a result of the multicomponent nature of wastewater, interferences and competitions occur for adsorption sites of adsorbents which requires the formulation of complex mathematical expressions for equilibrium adsorption of these pollutants from aqueous phases [12, 38, 46]. Different isotherms have been proposed and reported by various works for estimating and describing this equilibrium adsorption in multicomponent systems where only individual isotherm parameters are used (predictive models), and where individual isotherm parameters and correction factors are used (correlative models) [11–13, 52].
Extended Langmuir model (predictive), modified Langmuir model (correlative) and extended Freundlich model (correlative) adsorption isotherms were applied to the binary adsorption of the Cd-Pb system onto ZnO/goethite nanosorbent. The equilibrium adsorption data obtained in this work for binary solution of metal ions systems can also be explained in terms of adsorption capacity ratio (qe,binary/qe,single) as expressed in Eq. (7).
| 7 |
where qe,multi and qe, single are the maximum capacities of each metal ion adsorbed in multi-ion and single-ion systems by each adsorbent, and R is the adsorption capacity ratio. For R > 1, adsorption is enhanced by the presence of other metal ions (synergistic effect); for R = 1, no interaction occurred, and for R < 1, adsorption of one is suppressed by another (antagonistic effect) in the process.
Extended Langmuir Model (ELM)
The ELM is based on the same basic assumptions as those for the single component Langmuir isotherms. It follows that the energy of adsorption between the specific adsorbing; species and the adsorption site is constant and equal for each sit; there is no interaction between components and; there is equal competition between species for adsorption sites. The ELM [48, 53] is a predictive model that assumes a homogeneous surface with respect to the energy of adsorption [54]. The single-component parameter sets for the two heavy metal ions were substituted into Eq. (8) to enable the prediction of the binary component isotherms. The data obtained for equilibrium adsorption of Cd(II) and Pb(II) from Cd(II)-Pb(II) ions system onto ZnO/goethite nanosorbent in binary operation were estimated using nonlinear fitting of curves from ELM [46, 48, 55, 56] in Eqs. (8) - (10).
| 8 |
| 9 |
| 10 |
where qe,1 and qe,2 are the amount of energy of solutes adsorbed per unit mass of ZnO/goethite at equilibrium, Ce,1 and Ce,2 are equilibrium concentrations, qm,1 and qm,2 are their maximum amount adsorbed, and KL,1 and KL,2 are constants obtained from single-component adsorption isotherm analysis.
Modified Langmuir Model (MLM)
Adsorption systems involving more than one component are generally interactive and competitive. The adsorbent affinity changes due to equilibrium readjustment in the multiple component adsorption systems [57]. In order to account for these effects and incorporate them into a correlative model, an interaction factor,
 , has been introduced into the Eq. (11). The theoretical curves generated by this model are based on using the EL isotherm in Eq. (8) [57].
, has been introduced into the Eq. (11). The theoretical curves generated by this model are based on using the EL isotherm in Eq. (8) [57].
| 11 |
Extended Freundlich Model (EFM)
The principle of Freundlich isotherms assumes that the stronger adsorption binding sites are occupied first during the process of adsorption. Using EFM, the amounts of competing components in binary adsorption systems at equilibrium can be estimated using Eqs. (12) and (13), respectively [58].
| 12 |
| 13 |
where qe,1, and qe,2 are the equilibrium adsorption capacity or amount adsorbed (mg g−1) and Ce,1 and Ce,2 are the equilibrium concentrations (mg L−1) for unadsorbed components 1 and 2 in the binary adsorption of the Cd-Pb system. The values of Freundlich isotherm constants, KF1 and KF2, and adsorption intensity, n1 and n2, were obtained from the experimental data of individual Freundlich isotherms, while a set of the experimental values of qe,1 vs Ce,1 and qe,2 vs Ce,2 were used to obtain the values of the binary coefficients, X1, Y1, Z1 and X2, Y2, Z2 respectively, by minimizing the error in non-linear regression analysis [11, 48, 59].
The isotherm parameters in this adsorption study were estimated by minimizing the error function using Microsoft Excel Solver. The root means square of errors (RMSE) function was used in this work to measure the goodness of fit between the measured data and the theoretical models as expressed in Eq. (14).
| 14 |
where the qe,exp and qe,cal are the experimental and calculated amount adsorbed and N is the number of measurements taken from the adsorption experiment. A lower RMSE value indicated a better fitting curve of the isotherm models that described the binary adsorption system.
Results and discussions
Synthesis of goethite nanoparticles
During the formation of goethite in the reaction process, Fe2+ is oxidized to Fe3+ to form α- FeOOH according to Eqs. (15) - (17) [46] with the values of n ranging from 1 to 4.
| 15 |
| 16 |
| 17 |
Characterization of nanosorbent
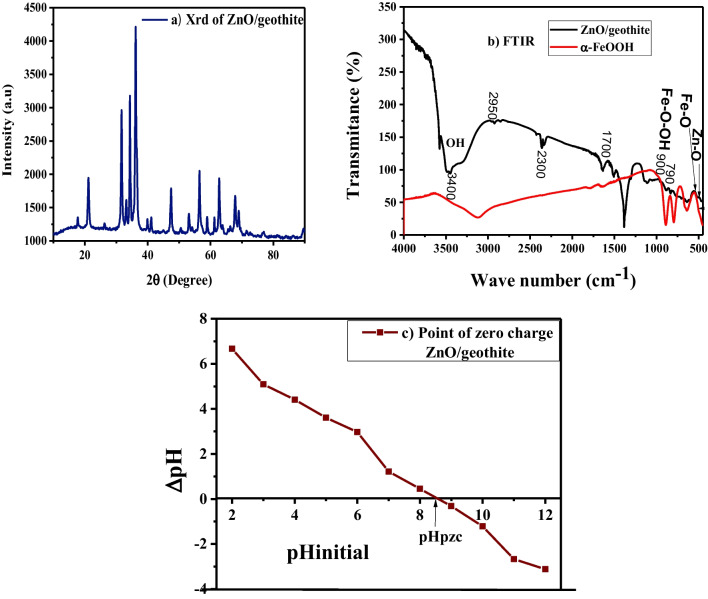
The XRD pattern for synthesized ZnO/goethite in Fig. 2a represents the formation of nanosorbent based on coprecipitation of ZnO in goethite nanoparticles. It can be observed that all the XRD peaks related to ZnO and α-FeOOH appeared in the XRD peaks as indicated in Fig. 2a. The peaks that appeared at 2Ө = 17.7, 21.1, 31.7, 33.2, 34.4, 36.1, 36. 6, 39.9, 41.1, 47.5, 53.1, 56.5, 58.9, 61.2, 62.7, 67.8, 68.9, and 76.8 are in agreement with XRD peaks for ZnO and goethite [39, 60]. The strongest peaks at 2θ = 31.705, 34.359 and 36.164 from the crystallographic plane (100), (002), and (101) corresponds to the hexagonal wurtzite structure of ZnO while, the peak positions at 2θ = 17.85, 21.27, 26.33, and 33.27 correspond to the crystal structure of pure orthorhombic goethite [1, 34–36]. The peak list and phase identifier plot in Fig. 2a showed a good proportion and growth of crystals of ZnO and α- FeOOH in ZnO/goethite. The broad nature of the peaks is an indication that the particles are within nano-range since peak broadening is inversely proportional to the size of the crystal [46, 48]. The average crystalline sizes of ZnO/goethite were estimated using the Debye-Scherer formula of diffraction peak in Eq. (18) [28, 55]:
| 18 |
where K is the average crystal size (Å), λ is the wavelength of radiation (CuKα = 1.54060 Å or 0.15406 nm), β is the full width at half maxima (FWHM) of the angle corresponding to the intense diffraction peak for a single peak in radian measured in Gaussian curve, while Ɵ is the diffraction angle known as Bragg’s angle obtained from 2Ɵ corresponding to the plane of the chosen diffraction peaks. According to Eq. (16), the mean crystalline size of ZnO/goethite was estimated as 35.60 nm.
Fig. 2.
XRD (a) FTIR (b) and point of zero charge (c) of Zn/goethite
The FTIR analysis of α- FeOOH and ZnO/goethite were performed in the range of 500 to 4000 cm−1 as shown in Fig. 2b. Different modes of bending and stretching vibrations were observed at different regions of the FTIR spectrum of the nanomaterial. The peak observed for ZnO/goethite between 4000 to 500 cm−1 indicates the characteristics vibration of n peaks of Zn-O and authenticates the formation of ZnO in the composite [13, 28]. The IR peak for goethite at about 3400 cm−1 can be assigned to the OH group bonding mode. It can be observed that goethite showed a peak at 556 cm−1 which is attributed to the bond vibration in Fe-O. The characteristic bands at 790 and 900 cm−1 can be assigned to Fe-O-OH bending vibration. The strong bands at 2300 cm−1 may be due to contamination by CO2 [8]. It can be observed that the IR bands of ZnO/goethite became broader. This implies that the nanocrystals have been distorted resulting in rearrangement of the crystal structure which occurs during the growth of the material resulting in some changes in structure of the composite adsorbent material leading to high adsorption capacity.
The point of zero-charge of ZnO/goethite represents the pH of the solution for which its overall surface charge is zero. The determination of this parameter is of great importance for the study of the adsorption of heavy metal ions. The point of zero charge (pHpzc) of the nanosorbent was determined by plotting the graph of the difference between pHi and the pHf (ΔpH) as shown in Fig. 2c and was found as 7.56. For pH below pHpzc 7.56, the ZnO/goethite material carries a positive overall charge while for pH above 7.56, its charge is negative [33–38].
The SEM images of goethite showed rod-like structures, while ZnO/goethite showed spherical-shaped clusters with nano-flake shapes embedded as represented in Fig. 3a, b. The images obtained are randomly oriented aggregation of nanoparticles with different particles size distributions. The aggregation of the nanoparticles as seen in the SEM images may be due to the high surface energy of the synthesized nanoparticles during the heating process [9].
Fig. 3.
SEM (a, b) and TEM (c, d) images for α-FeOOH and ZnO/goethite
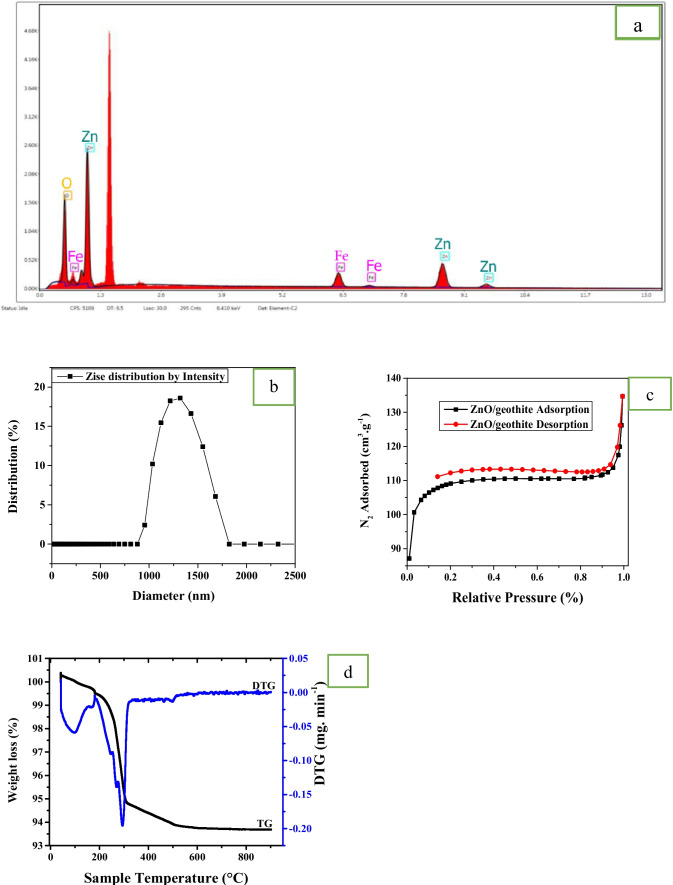
The TEM micrograph images obtained at different magnifications for α- FeOOH and ZnO/goethite are shown in Fig. 3c, d. The TEM images showed rod-like α-FeOOH and nano-flakes in spherical shapes for ZnO/goethite [48]. The purity of the sample can be seen from the EDAX spectrum presented in Fig. 4a, which revealed that the synthesized adsorbent material contains the respective elements without any other elements except carbon from the carbon tape used for sample preparation.
Fig. 4.
EDX (a), Zise distribution (b), N2 Nitrogen adsorption-desorption (c) and TG/DTg (d) for ZnO/goethite
The DLS analysis of ZnO/goethite in Fig. 4b showed that the average size of synthesized nanosorbent is 41.11 nm.
The N2 physisorption isotherm of ZnO/goethite is displayed in Fig. 4c, and the BET surface areas for the synthesized nanomaterials are presented in Table 1. It can be observed that ZnO/goethite has a higher surface area of 62.0 m2g−1 compared to nanoparticles of α- FeOO.
Table 1.
BET surface area analysis for synthesized nanosorbents
| Nanomaterial | BET (m2g−1) | Pore volume (cm3g−1) | Pore size (m2g−1) | Crystal size (nm) from X-ray analysis | Particle size (nm) distribution from DLS analysis |
|---|---|---|---|---|---|
| α- FeO2H | 58.35 | 1.20 | 24.72 | 35.89 | 58.10 |
| ZnO/goethite | 62.20 | 0.15 | 12.26 | 17.56 | 41.11 |
H with 58.35 m2g−1. The N2 adsorption-desorption isotherms showed that ZnO/goethite and α-FeOOH NPs are of type IV (H4 Hysteresis loop) according to the IUPAC classification of sorption isotherms [61] which are associated with narrow slit pores [62]. The mean pore sizes and volume of ZnO/goethite determined from BET surface area analysis are shown in Table 2 indicating mesoporous nanopowders. Heavy metals i.e. ionic radii of Ni2+ (0.072 nm), Pb2+ (0.118 nm) and Cd2+ (0.097 nm) can easily diffuse into the mesopores of ZnO/goethite [37, 51, 57, 58] and therefore ZnO/goethite contains pores which would favor the adsorption of Ni2+, Cd2+ and Pb2+ ions.
Table 2.
TGA-DTA studies for nanodsorbents between 10—900 °C under N2 flow at the rate of 10 °C min−1
| Nanomaterial | Initial mass (mg) | % Mass loss | Mass loss temperature range (oC) | Heating time (min) |
|---|---|---|---|---|
| α- FeO2H | 27.60 | 11.66 | 42.12 – 305.5 | 85.70 |
| ZnO/goethite | 22.96 | 6.398 | 184.6 – 361.8 | 32.80 |
The TGA/DTA graph for the synthesized ZnO/goethite is shown in Fig. 4d. The sample mass loss and associated thermal effects obtained by this method are summarized in Table 2. The TG/DTA curve indicated mass loss up to 361.8 °C for ZnO/goethite. The first mass loss steps for the nanomaterial occurred between 0 °C to 105 °C, indicating the removal of water molecules adsorbed on the surface of the sample. The mass loss continued to 362 °C which may be due to the loss of internally absorbed water molecules of the nanocomposite. The second step of mass loss may also be attributed to the decomposition and elimination of the volatile components that were still present on the surface of the synthesized nanomaterials. Anirudhan and Suchithra [63] reported that removal of physisorbed and interlayer water occurs between 90 to 200 °C. while dehydroxylation (-OH group loss) takes place between 300 to 380 °C. The adsorbent weight loss during TGA/DTA process is presented in Table 2. The total weight loss under thermal decomposition of the ZnO/goethite nanosorbent was 6.40%. This indicated that the adsorbent has about 93.60% component of ZnO and α- FeOOH nanoparticles that were stable under thermal treatment. It also implies that about 6.40% volatile components of the precursor materials for the synthesis of the nanomaterials were eliminated from the surface of the material in thermal process. Therefore, the newly synthesized nanomaterial showed high thermal stability.
Competitive adsorption studies
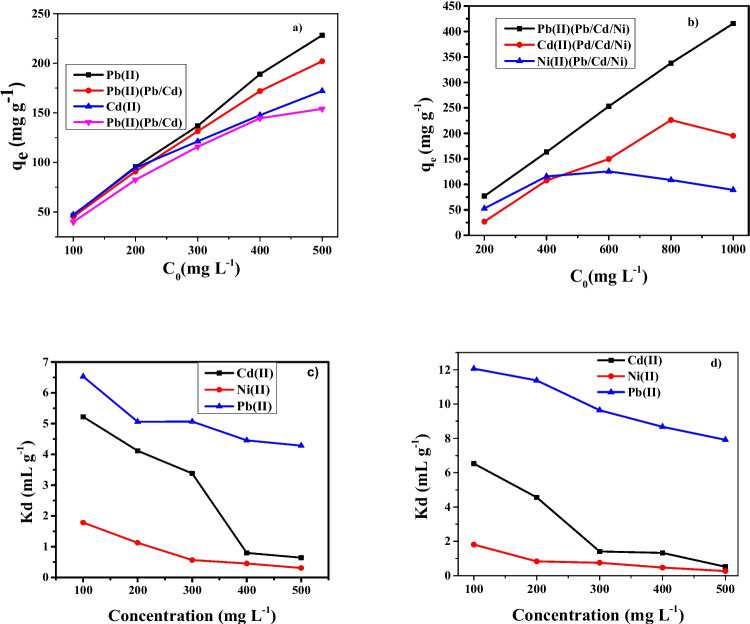
The quantities of each metal ions adsorbed at equilibrium from binary adsorption experiments were determined in a similar way to the single adsorption experiments and the combined results for both single and binary adsorption of Cd and Pb ions onto ZnO/goethite nanosorbent are shown in Fig. 5a. It can be observed that the quantities of the metal ions adsorbed on nanosorbent for the binary sorption experiment were relatively lower than for single adsorption with no significant difference in adsorption capacity.
Fig. 5.
Sorption systems: single and binary Pb(II) and Cd(II) (a), ternary Pb(II), Cd(II) and Ni(II) (b) (Conditions: distribution coefficients plot of 100 mg L−1 (c) and 200 mg L−1 of Pb(II) by varying concentration of Cd(II) and Ni(II) onto ZnO/goethite
Considering the initial metal ion concentrations from 200 to 1000 mg L−1, it can be observed in Table 3 and Fig. 5a, d that the adsorption capacities and efficiencies of ZnO/goethite nanosorbent in removal of Pb(II), Cd(II) and Ni(II) ions have reduced from 125.7 mg g−1 (92.96%) to 76.8 mg g−1 (76.8%), 94.56 mg g−1 (95.43%) to 26.85 (26.85%) and 81.32 (70.15%) to 52.75 mg g−1 (52.75%), respectively at the 200 mg L−1. Generally, the adsorption capacities trend for removal of the three metal ions from the aqueous phase followed the pattern: Ni(II) < Cd(II) < Pb(II). This could be attributed to the fact that Pb ions have faster rate of diffusion and higher adsorption affinity towards ZnO/goethite, which may be used to explain the adsorption mechanism [13, 15, 37]. Therefore, it can be assumed that one of the reasons for the higher adsorption of Pb(II) ions onto the nanosorbent is its higher electronegativity value and ionic radius compared with Cd and Ni [57, 62, 64]. Reports have shown that, in addition to vacant sites, the adsorption of metal ions from the multi-ion systems is influenced by ionic radius or electronegativity difference, while for single metal ion systems, vacant active sites of nanosorbent plays the most important role [65].
Table 3.
Adsorption capacities of Pb(II), Cd(II) and Ni(II) onto ZnO/goethite at different concentrations
| Concentration (mg L−1) | Pb(II) (mg g−1) | Cd(II) (mg g−1) | Ni(II) (mg g−1) | |||
|---|---|---|---|---|---|---|
| Single | Ternary | Single | Ternary | Single | Ternary | |
| 200 | 125.7 | 76.00 | 94.56 | 26.85 | 81.32 | 52.75 |
| 400 | 189.0 | 163.4 | 147.7 | 107.7 | 139.2 | 115.6 |
| 600 | 294.6 | 253.1 | 203.6 | 149.6 | 133.5 | 125.4 |
| 800 | 367.4 | 337.9 | 231.5 | 226.3 | 120.1 | 108.5 |
| 1000 | 477.8 | 415.5 | 209.2 | 195.3 | 110.2 | 89.13 |
Since the adsorption of Cd(II) and Pb(II) ions onto ZnO/goethite from the single [48] and binary systems in this adsorption study showed a very high adsorption capacity, Ni(II), a divalent ion with lower atomic and ionic radius (Table 4), was introduced to form a ternary ions system. The experimental results as presented in Fig. 5b and Table 5 showed that the presence of each of the metal ions in the multi-ion system influenced the uptake of the other in the adsorption process as reported in several research works [10–15, 49, 52]. Nevertheless, the antagonistic effect has a less pronouncement on the adsorption capacity of ZnO/goethite nanosorbent when compared with its sorption capacities in the single-ion system as can be observed in Table 3.
Table 4.
Some properties of the metal ions for the adsorption study
| Property | Cadmium (Cd) | Lead (Pb) | Nickel (Ni) |
|---|---|---|---|
| Atomic number | 48.0 | 82.0 | 28.0 |
| Atomic mass (a.m.u) | 112.41 | 207.20 | 58.69 |
| Atomic radius (Å) | 1.71 | 1.81 | 1.62 |
| Covalent radius (Å) | 1.48 | 1.47 | 1.17 |
| Ionic radius (Å) | 0.97 | 1.19 | 0.69 |
| Atomic volume (cm3 mol−1) | 13.10 | 18.17 | 6.59 |
| Coordination number | 4, 5 and 6 | 5, 6 and 8 | 4, 5 and 6 |
| Electronic configuration | [Kr]4d105S2 | [Xe]4F145d6S2 | [Ar]3d84S2 |
| Electronegativity (Paulings) | 1.69 | 2.33 | 1.91 |
| Magnetic moment | −1.0304 | + 1.009 | −0.9683 |
Table 5.
Adsorption capacities of competing (Cd and Ni) ions with ZnO/goethite nanosorbent from 100 mg L−1 of targeted (Pb) and varying concentrations
| Co (mg g−1) of competing ions, Cd(II) and Ni(II) | Cd(II) | Ni(II) | Pb(II) | |||
|---|---|---|---|---|---|---|
| qe (mg L−1) | Removal efficiency (%) | qe (mg L−1) | Removal efficiency (%) | qe (mg L−1) | Removal efficiency (%) | |
| 100 | 45.63 | 92.89 | 46.44 | 78.35 | 39.05 | 96.02 |
| 200 | 89.17 | 90.12 | 45.51 | 62.45 | 69.24 | 95.79 |
| 300 | 130.68 | 73.81 | 45.51 | 60.03 | 79.62 | 95.07 |
| 400 | 123.01 | 72.64 | 44.955 | 48.57 | 95.09 | 94.55 |
| 500 | 140.55 | 50.94 | 44.78 | 34.91 | 95.29 | 94.06 |
This study shows that irrespective of the presence of interfering metal ions, the removal efficiencies of ZnO/goethite nanocomposite remained close to 90% showing exceptionally high adsorption property in the multi-ion systems. At lower metal ion concentrations of 200 to 400 mg g−1 (Table 3), the adsorption capacities of the metal ions onto ZnO/goethite nanosorbent in single and multi-ion systems were not in close range for all metal ions studied. This could result from the fact that at initial lower concentrations, more of the adsorbent sites or pores were available so that the rate of competition amongst metal ions was insignificant. It can also be inferred that lower concentration canceled the effect of difference in electronegativity. Also, at lower concentration, selectivity of metal ions does not occur during adsorption of the ions since there is no pronounced competition among the available adsorption sites [10, 17, 66, 67].
Selective adsorption experiment
The plot of Kd values versus concentrations of the metal ions as represented in Fig. 5c, d showed that Ni Kd values were lower throughout the adsorption process. The significant reduction for Kd values as the concentrations were increased is an indication that ZnO/goethite nanosorbent has less adsorption affinity for Ni ions compared to Cd and Pb ions. At lower concentrations, each metal ion was adsorbed considerably onto the nanosorbent vacant sites, but as concentration was increased, the number of vacant sites decreased resulting in competitive adsorption for the few vacant sites available.
The Kd values for Pb ions slowly decreased as the concentration was increased. The significant drop in the Kd values for Ni and Cd ions suggested that there was massive displacement of the metal ions from their adsorbed sites in the presence of Pb ions in the process. Therefore, the scenario suggests that Pb ions, with the highest diffusion and adsorption rate were adsorbed to a greater extent by displacing Cd and Ni ions from most of their binding sites, especially the weakly bonded ones. This is clearly observed in the removal efficiencies of ZnO/goethite in Table 6 where Cd and Ni ions removal dropped from 92.89% to 56.22% and 78.35% to 38.12% respectively, while that of Pb ions remained constant between 92.89 to 89.55%.
Table 6.
The adsorption capacity of Pb(II) concentration was kept constant, 200 mg L−1 onto ZnO/goethite
| Co (mg L−1) of competing ions | Cd(II) | Ni(II) | Pb(II) | |||
|---|---|---|---|---|---|---|
| qe (mg g−1) | Removal efficiency (%) | qe (mg g−1) | Removal efficiency (%) | qe (mg g−1) | Removal efficiency (%) | |
| 100 | 46.445 | 92.89 | 39.175 | 78.35 | 48.01 | 92.89 |
| 200 | 90.12 | 89.17 | 62.45 | 69.24 | 47.10 | 91.01 |
| 300 | 110.72 | 87.12 | 90.05 | 53.08 | 47.54 | 91.02 |
| 400 | 145.29 | 61.51 | 97.15 | 47.55 | 47.28 | 89.91 |
| 500 | 127.34 | 56.22 | 87.28 | 38.12 | 47.03 | 89.55 |
This trend is similar to the results shown in Table 6 where the concentrations of the Pb(II) (targeted ions) were increased to 200 mg L−1, while still varying the concentrations of the interfering Cd and Ni ions (interfering ions).
However, it can be observed in Fig. 5a, b that, to some extent, there were regions (100—400 mg L−1) of competition between Ni(II) and Cd(II) ions for the available vacant sites. Research work on adsorption of some aqueous metal ions by nano-hydroxyapatite reported that competitive adsorption took place on the adsorbent vacant sites that can be occupied by all species, while non-competitive adsorption occurs on the sites only available to targeted ions with high adsorption affinity [12, 13, 15, 68]. The selectivity coefficients, α, estimated for Cd(II) and Ni(II) from the ternary system are presented in Tables 7 and 8.
Table 7.
Distribution coefficients (Kd) and selectivity coefficients (α) of ZnO/goethite sorption of 100 mg L−1 Pb(II) and varying concentrations of Cd(II) and Ni(II) ions
| Co (mg L−1) of interfering ions | Pb(II) | Cd(II) | Ni(II) | ||
|---|---|---|---|---|---|
| Kd | Kd | α | Kd | α | |
| 100 | 6.531 | 5.221 | 1.251 | 1.783 | 3.663 |
| 200 | 5.062 | 4.117 | 1.230 | 1.125 | 4.497 |
| 300 | 5.068 | 3.381 | 1.410 | 0.566 | 8.960 |
| 400 | 4.455 | 0.799 | 5.577 | 0.453 | 9.830 |
| 500 | 4.285 | 0.642 | 6.673 | 0.308 | 13.912 |
Table 8.
Distribution coefficients (Kd) and selectivity coefficients (α) of ZnO/goethite sorption of 200 mg L−1 Pb(II) and varying concentrations of Cd(II) and Ni(II) ions
| Co (mg L−1) of interfering ions | Pb(II) | Cd(II) | Ni(II) | ||
|---|---|---|---|---|---|
| Kd | Kd | α | Kd | α | |
| 100 | 12.063 | 6.532 | 1.847 | 1.809 | 6.666 |
| 200 | 11.376 | 4.561 | 2.494 | 0.832 | 13.681 |
| 300 | 9.642 | 1.409 | 6.841 | 0.751 | 12.838 |
| 400 | 8.674 | 1.328 | 6.534 | 0.472 | 18.368 |
| 500 | 7.918 | 0.519 | 15.253 | 0.268 | 29.525 |
It was observed that α values for Ni ions were higher than those for Cd ions, while the distribution coefficients, Kd, values of Cd ions were higher than those for Ni ions in the ternary system. This signifies that ZnO/goethite nanosorbent is selective for Ni ions in a medium where Cd ions have a higher distribution coefficient. Nevertheless, it was observed that Cd ions with higher distribution coefficients, were adsorbed more than Ni ions from the ternary solution as represented in Tables 5 and 6, while most of the Pb ions were adsorbed compared to both Cd and Ni ions in the selective adsorption studies. The general order of affinity for the metal ions onto the surface of ZnO/goethite, in terms of Kd values, in the ternary adsorption experiment, is in the order: Pb(II) > Cd(II) > Ni(II). The ZnO/goethite performed appreciably well for all the metal ions depending on the conditions of the adsorption process.
The present study showed that the nanohybrid material formed from goethite and ZnO (ZnO/goethite) yielded a more excellent material with enhanced properties. The ZnO is a semi-conductor material with high resistance to corrosion and low toxicity, while α-FeOOH, rich in hydroxyl group (OH), is a thermodynamically stable oxo-hydroxide of iron at ambient temperature and is often the end member of transformation of other iron oxides making it abundantly available. The OH of α-FeOOH group can bind to metal ions pollutants using its lone pairs of electrons and can form hydrogen bond with halogenated pollutants [39, 45, 69]. The optimum adsorption capacities of Pb(II), Cd(II) and Ni(II) ions at 1000 mg L−1 were 415.5 mg g−1, 195.3 mg g−1 and 87.13 mg g−1 in the ternary system respectively. Comparison of adsorption capacities of metal ions in multi-systems with various adsorbents as represented in Table 9 showed exceptionally higher adsorption capacities of ZnO/goethite nanocomposite for metal ions in the multiple adsorption systems.
Table 9.
Comparison of adsorption capacities of metal ions in multi-systems with various adsorbents and ZnO/goethite
| Adsorbent | System | Targeted pollutant | qe (mg g−1) | Reference |
|---|---|---|---|---|
| Natural clay | Pb/Cu | Pb(II) | 18.78 | [17] |
| Activated clay | Pb/Cu | Pb(II) | 39.02 | [17] |
| Nano-hydroxyapatite | Cd/Pb | Cd(II) | 66.70 | [20] |
| Pb(II) | 80.60 | [20] | ||
| Modified hydrotalcite | Cd/Cu | Cd(II) | 74.90 | [63] |
| Algae cystoseria indica | Ni/Cd | Ni(II) | 18.17 | [70] |
| Cd(II) | 55.34 | [70] | ||
| Bentonite clay | Cu/Ni | Ni(II) | 6.78 | [21] |
| Aluminum oxide | Zn/Cd | Cd(II) | 73.00 | [19] |
| Activated carbon | Cd/Cu | Cd(II) | 46.88 | [71] |
| Modified AAc graft PERT film | Ni/Cu/Co | Ni(II) | 85.00 | [13] |
| ZnO/goethite | Cd/Pb/Ni | Cd(II) | 195.3 | This work |
| Pb(II) | 415.5 | This work | ||
| Ni(II) | 87.13 | This work |
Isotherms adsorption studies
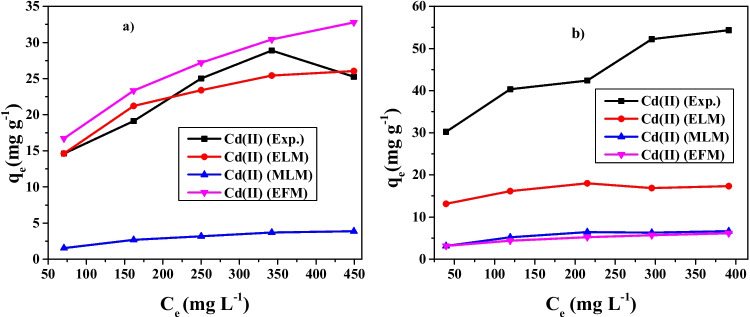
All the binary adsorption isotherm model parameters in this study for adsorption of Cd and Pb ions from the Cd-Pb system onto ZnO/goethite were obtained by using a non-linear regression method and compared with the experimental data. The results are represented in Fig. 6. The best amongst many models to experimental data is determined by how closer the adsorption data points are to the bisecting line at 45° [72]. This resulted in competition between adsorbing species in the binary adsorption system, and more or one of Cd and Pb ions are adsorbed more compared to others in the adsorption process as can be observed from Table 10.
Fig. 6.
Isotherm model plots for adsorption of Cd(II) (a), and Pb(II) from Cd(II)-Pb(II) binary systems (b) onto ZnO/goethite
Table 10.
Extended Langmuir (ELM), Modified Langmuir (MLM), and Extended Freundlich (EFM) models of Cd(II) and Pb(II) from Cd–Pb binary systems with ZnO/goethite
| Models | Parameters | Cd(II) | Pb(II) |
|---|---|---|---|
| Cd-Pb binary | qe,single(mg g−1) | 172.08 | 228.25 |
| qe,binary(mg g−1) | 154.04 | 202.05 | |
| R (qe,binary/qe,single) | 0.8952 | 0.8852 | |
| ELM | KL (L mg−1) | 0.0042 | 0.0082 |
| R2 | 0.8386 | 0.7302 | |
| SSE | 21.76 | 76.44 | |
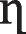 1 1
|
0.9699 | 0.3931 | |
| MLM |
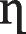 2 2
|
1.2858 | 0.4810 |
| R2 | 0.7778 | 0.8988 | |
| SSE | 31.92 | 36.16 | |
| EFM | X | 0.0077 | 0.0237 |
| Y | 0.0229 | 0.0385 | |
| Z | −0.1029 | −0.1751 | |
| R2 | 0.9716 | 0.8021 | |
| SSE | 61.48 | 96.89 |
The competition and interactions occurring in binary solutions of these metal ions may be significant and may affect each component’s adsorption to a different extent. Nevertheless, the ELM will not be suitable for representation with the occurrence of interaction and competition in these binary systems. This necessitated the incorporation of correction factor η to adapt the model to multicomponent systems and the coefficient in the ELM. By adding this factor, the isotherm model fitted well with the experimental data and the accuracy of the results can be improved.
The constant, η (specific to each adsorbate) of various components were calculated for a range of experimental values of qe,1 and qe,2. Figure 6a, b showed the comparison between curves of the experimental and theoretical data based on the interaction factor for the binary system, and Table 10 shows the values for the
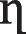 obtained for Cd and Pb ions in different systems. The corresponding values were substituted into Eq. (14) for the number of components in the binary adsorption system. Keith et al. [57] reported that the interaction factor was proposed and used with limited success for the sorption of some pollutants on carbon and peat. Consequently, comparing the present work with Keith’s prediction, it is not a surprise to see that the application of the interaction factor to the Cd-Pb ions system under study does not provide a satisfactory correlation of experimental data in the binary adsorption system except for adsorption of Cd from Cd-Pb ions system where it gave a relatively good fit to the experimental data. The reasons for the unsatisfactory correlation of the theoretical data to the experimental ones include, but are not limited to, the difference in molecular sizes of adsorbate; aggregation of materials in solution; electrolyte nature of the solutes which results in non-ideal system; and difference in saturation capacities of each adsorbate onto adsorbent in one-component systems [57].
obtained for Cd and Pb ions in different systems. The corresponding values were substituted into Eq. (14) for the number of components in the binary adsorption system. Keith et al. [57] reported that the interaction factor was proposed and used with limited success for the sorption of some pollutants on carbon and peat. Consequently, comparing the present work with Keith’s prediction, it is not a surprise to see that the application of the interaction factor to the Cd-Pb ions system under study does not provide a satisfactory correlation of experimental data in the binary adsorption system except for adsorption of Cd from Cd-Pb ions system where it gave a relatively good fit to the experimental data. The reasons for the unsatisfactory correlation of the theoretical data to the experimental ones include, but are not limited to, the difference in molecular sizes of adsorbate; aggregation of materials in solution; electrolyte nature of the solutes which results in non-ideal system; and difference in saturation capacities of each adsorbate onto adsorbent in one-component systems [57].
The RMSE values with the least error values determine the best model that explains the adsorption process well. The RMSE values obtained for the binary adsorption of Cd and Pb ions from the Cd-Pb system are represented in Table 10. Based on the RMSE data, the ELM showed a good fit for adsorption of Cd ions, while the MLM gave a good fit for Pb ions. By implication, the energy of adsorption between the Cd and Pb ions and the adsorption site is constant and equal for each site. It also implies that there is no interaction between components, and there is equal competition between Cd and Pb ions for adsorption sites. The ELM is a predictive model that assumes a homogeneous surface with respect to the energy of adsorption. The estimation of the values for adsorption capacity ratio, R, in Table 10 showed that all R values were less than 1 for the metal ions and this is an indication of antagonistic effect in the system. This was observed in the decreased adsorption capacities of each of the metal ions in the binary adsorption system than in a single system obtained in this adsorption study.
Conclusions
Nanosorbent (ZnO/goethite) was synthesized by co-precipitation method from zinc nitrate hexahydrate and hydrothermally synthesized α-FeOOH nanoparticles. The study showed that the nanohybrid material formed from goethite and ZnO (ZnO/goethite) yielded a more excellent material with enhanced properties. The nanosorbent was used in batch mode for competitive and selective adsorption from Cd(II)-Pb(II) and Cd(II)-Pb(II)-Ni(II) systems. The extended Langmuir model described the sorption process for Cd(II) ions in the binary system, indicating that adsorption between the ZnO/goethite nanosorbent and the metal ions was constant and equal for each site. However, it did not accurately predict the sorption of the Pb (II) ions, which implies that the adsorbent has a high uptake capacity for metals ions in the multicomponent systems.
Acknowledgements
Dr. John Godwin thanks the World Academy of Science (TWAS), and CSIR-Institute of Minerals and Materials Technology (CSIR-IMMT), India, for the Doctoral Fellowship award.
Dr. Jacques R. Njimou thanks the Indian National Science Academy for the ‘INSA–JRD TATA Fellowship No: DO/CCSTDS/113/2019. He is also grateful for the SEM MRI award Number:1827176and Fulbright grant PS00301505 that offered data sharing between the University of Ngaoundere and Indiana University of Pennsylvania.
We thank Prof. S. Basu, the Director of CSIR-IMMT, and Prof. Bankim C. Tripathy for hosting, supervising, and for the opportunity of data sharing between IMMT, University of Ilorin, Nigeria, and the University of Ngaoundere, Cameron.
Authors contribution
John Godwin, Jacques R. Njimou: Conceptualization, Methodology, Investigation, Formal analysis, Visualization, Writing original draft, Review & editing.
Nasalam Abdus-Salam: Conceptualization, Supervision, Investigation, Formal analysis.
Prasanna Kumar Panda: Data curation, Review & editing.
Haleemat Iyabode Adegoke: Formal analysis, Methodology, Data curation.
Bankim C. Tripathy: Investigation, Project administration, Supervision.
Sanda Andrada Maicaneanu: Supervision Methodology, Review & editing.
Declarations
Competing interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Enlei Z, Guosheng W, Xiaozhu L, Zhumin W. Synthesis and influence of alkaline concentration on α-FeOOH nanorods shapes. Indian Acad Sci. 2014;37:761–765. [Google Scholar]
- 2.Gottschalk F, Sun T, Nowack B. Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ Pollut. 2013;181:287–300. doi: 10.1016/j.envpol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Ararem A, Bouras O, Bouzidi A. Batch and continuous fixed-bed column adsorption of Cs+ and Sr2+ onto montmorillonite–iron oxide composite: comparative and competitive study. J Radioanal Nucl Chem. 2013;298:537–545. doi: 10.1007/s10967-013-2433-y. [DOI] [Google Scholar]
- 4.Liu L, Chen W-T, Kihara S, Kilmartin PA. Green synthesis of akaganéite (β-FeOOH) nanocomposites as peroxidase-mimics and application for discoloration of methylene blue. J Environ Manage. 2021;296:113163. doi: 10.1016/j.jenvman.2021.113163. [DOI] [PubMed] [Google Scholar]
- 5.Ighalo JO, Sagboye PA, Umenweke G, Ajala OJ, Omoarukhe FO, Adeyanju CA, et al. CuO nanoparticles (CuO NPs) for water treatment: a review of recent advances. Environ Nanotechnol Monit Manag. 2021;15:100443. [Google Scholar]
- 6.Njimou JR, Măicăneanu A, Indolean C, Nanseu-Njiki CP, Ngameni E. Removal of Cd (II) from synthetic wastewater by alginate-ayous wood sawdust (Triplochiton scleroxylon) composite material. Environ Technol. 2016;37:1369–1381. doi: 10.1080/09593330.2015.1116609. [DOI] [PubMed] [Google Scholar]
- 7.Farahmandjou M, Soflaee F. Synthesis and characterization of α-Fe2O3 nanoparticles by simple co-precipitation method. Phys Chem Res. 2015;3:191–196. [Google Scholar]
- 8.Fu D, Keech PG, Sun X, Wren JC. Iron oxyhydroxide nanoparticles formed by forced hydrolysis: dependence of phase composition on solution concentration. Phys Chem Chem Phys. 2011;13:18523–18529. doi: 10.1039/c1cp20188c. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Zhu X, Yu K. Surfactant-assisted nanorod synthesis of α-FeOOH and its adsorption characteristics for methylene blue. J Mater Res. 2014;29(4):509–518. doi: 10.1557/jmr.2013.387. [DOI] [Google Scholar]
- 10.Njimou JR, Godwin J, Pahimi H, Maicaneanu SA, Kouatchie-Njeutcha F, Tripathy BC, et al. Biocomposite spheres based on aluminum oxide dispersed with orange-peel powder for adsorption of phenol from batch membrane fraction of olive mill wastewater. Colloid Interface Sci Commun. 2021;42:100402. doi: 10.1016/j.colcom.2021.100402. [DOI] [Google Scholar]
- 11.Yangui A, Njimou JR, Cicci A, Bravi M, Abderrabba M, Chianese A. Competitive adsorption, selectivity and separation of valuable hydroxytyrosol and toxic phenol from olive mill wastewater. J Environ Chem Eng. 2017;5:3581–3589. doi: 10.1016/j.jece.2017.06.037. [DOI] [Google Scholar]
- 12.Li M, Li X, Wang L, Pei Y, An M, Liu J, et al. Highly efficient and selective removal of anionic dyes from water using a cellulose nanofibril/chitosan sponge prepared by dehydrothermal treatment. J Environ Chem Eng. 2021;9:105745. doi: 10.1016/j.jece.2021.105745. [DOI] [Google Scholar]
- 13.Rahman N, Sato N, Yoshioka S, Sugiyama M, Okabe H, Hara K. Selective Cu (II) adsorption from aqueous solutions including Cu (II), Co (II), and Ni (II) by modified acrylic acid grafted PET film. Int Sch Res Notices. 2013;2013. 10.1155/2013/536314.
- 14.Hadi P, Barford J, McKay G. Selective toxic metal uptake using an e-waste-based novel sorbent–single, binary and ternary systems. J Environ Chem Eng. 2014;2:332–339. doi: 10.1016/j.jece.2014.01.004. [DOI] [Google Scholar]
- 15.Hema M, Arivoli S. Comparative study on the adsorption kinetics and thermodynamics of dyes onto acid activated low-cost carbon. Int J Phys Sci. 2007;2:010–17. [Google Scholar]
- 16.Janaki V, Vijayaraghavan K, Ramasamy AK, Lee K-J, Oh B-T, Kamala-Kannan S. Competitive adsorption of reactive Orange 16 and reactive brilliant blue R on polyaniline/bacterial extracellular polysaccharides composite—A novel eco-friendly polymer. J Hazard Mater. 2012;241–242:110–117. doi: 10.1016/j.jhazmat.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Khalfa L, Bagane M, Cervera ML, Najjar S. Competitive adsorption of heavy metals onto natural and activated clay: equilibrium, kinetics and modeling. 2016 [cited 2022 Feb 14]; Available from: https://zenodo.org/record/1124515.
- 18.Rathore VK, Mondal P. Competitive adsorption of arsenic and fluoride onto economically prepared aluminum oxide/hydroxide nanoparticles: multicomponent isotherms and spent adsorbent management. Ind Eng Chem Res. 2017;56:8081–8094. doi: 10.1021/acs.iecr.7b01139. [DOI] [Google Scholar]
- 19.Stietiya MH, Wang JJ. Zinc and cadmium adsorption to aluminum oxide nanoparticles affected by naturally occurring ligands. J Environ Qual. 2014;43:498–506. doi: 10.2134/jeq2013.07.0263. [DOI] [PubMed] [Google Scholar]
- 20.Chen SB, Ma YB, Chen L, Xian K. Adsorption of aqueous Cd2+, Pb2+, Cu2+ ions by nano-hydroxyapatite: single-and multi-metal competitive adsorption study. Geochem J. 2010;44:233–239. doi: 10.2343/geochemj.1.0065. [DOI] [Google Scholar]
- 21.Al-Jlil SA, Latif MS. Evaluation of equilibrium isotherm models for the adsorption of Cu and Ni from wastewater on bentonite clay. Mater Technol. 2013;47:481–486. [Google Scholar]
- 22.Hassanein TF, Masoud AM, Mohamed WS, Taha MH, Guibal E. Synthesis of polyamide 6/nano-hydroxyapatite hybrid (PA6/n-HAp) for the sorption of rare earth elements and uranium. J Environ Chem Eng. 2021;9:104731. doi: 10.1016/j.jece.2020.104731. [DOI] [Google Scholar]
- 23.Saha S, Basu H, Rout S, Pimple MV, Singhal RK. Nano-hydroxyapatite coated activated carbon impregnated alginate: a new hybrid sorbent for uranium removal from potable water. J Environ Chem Eng. 2020;8:103999. doi: 10.1016/j.jece.2020.103999. [DOI] [Google Scholar]
- 24.Pandi K, Viswanathan N. Synthesis of alginate bioencapsulated nano-hydroxyapatite composite for selective fluoride sorption. Carbohydr Polym. 2014;112:662–667. doi: 10.1016/j.carbpol.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 25.Elkady MF, Mahmoud MM, Abd-El-Rahman HM. Kinetic approach for cadmium sorption using microwave synthesized nano-hydroxyapatite. J Non-Cryst Solids. 2011;357:1118–1129. doi: 10.1016/j.jnoncrysol.2010.10.021. [DOI] [Google Scholar]
- 26.Kuchi R, Dongquoc V, Van PC, Kim D, Jeong J-R. Synthesis of porous Fe3O4-SnO2 core-void-shell nanocomposites as high-performance microwave absorbers. J Environ Chem Eng. 2021;9:106585. doi: 10.1016/j.jece.2021.106585. [DOI] [Google Scholar]
- 27.Godwin J, Njimou JR, Nasalam A-S, Panda PK, Tripathy BC, Ghosh MK, et al. Nanoscale ZnO-adsorbents carefully designed for the kinetic and thermodynamic studies of Rhodamine B. Inorg Chem Commun. 2022;138:109287. doi: 10.1016/j.inoche.2022.109287. [DOI] [Google Scholar]
- 28.Njimou JR, Kouatchie FN, Njungab E, Talla A, Elambo Nkeng G. Treatment of agro-food wastewaters and valuable compounds recovery by column sorption runs. Sorption in 2020s, George Kyzas and Nikolaos Lazaridis, IntechOpen, 10.5772/intechopen.90087
- 29.Sieugaing Tamwa M, Njimou JR, Nguelo BB, Nanseu-Njiki CP, Vunain E, Tripathy BC, Ngameni E. Electrochemical sensor based on green-synthesized iron oxide nanomaterial modified carbon paste electrode for Congo red electroanalysis and capacitance performance. Mater Res Innov. 2022;27(4):243–252. doi: 10.1080/14328917.2022.2125694. [DOI] [Google Scholar]
- 30.John G, Abdus-Salam N, Adegoke IH, Njimou JR, Akor EJ, Etong ID, et al. Two step fabrication of nano-ZnO-α-FeOOH composite for experimental and modeling studies of removal of indigo carmine and alizarin red S in batch process. Chem Afr. 2022;1–15.
- 31.Nalwa K, Thakur A, Sharma N. Synthesis of ZnO nanoparticles and its application in adsorption. Adv Mater Proc. 2021;2:697–703. doi: 10.5185/amp/2017/696. [DOI] [Google Scholar]
- 32.Godwin J, Njimou JR, Nasalam A-S, Panda PK, Tripathy BC, Ghosh MK, et al. Nanoscale ZnO-adsorbents carefully designed for the kinetic and thermodynamic studies of Rhodamine B. Inorg Chem Commun. 2022;138:109287. doi: 10.1016/j.inoche.2022.109287. [DOI] [Google Scholar]
- 33.Prasad K, Jha AK. ZnO nanoparticles: synthesis and adsorption study. Nat Sci. 2009;1:129. [Google Scholar]
- 34.Azizi S, Mahdavi Shahri M, Mohamad R. Green synthesis of zinc oxide nanoparticles for enhanced adsorption of Lead ions from aqueous solutions: equilibrium, kinetic and thermodynamic studies. Mol Basel Switz. 2017;22:831. doi: 10.3390/molecules22060831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuvaraja G, Prasad C, Vijaya Y, Subbaiah MV. Application of ZnO nanorods as an adsorbent material for the removal of As(III) from aqueous solution: kinetics, isotherms and thermodynamic studies. Int J Ind Chem. 2018;9:17–25. doi: 10.1007/s40090-018-0136-5. [DOI] [Google Scholar]
- 36.Zhang F, Lan J, Yang Y, Wei T, Tan R, Song W. Adsorption behavior and mechanism of methyl blue on zinc oxide nanoparticles. J Nanoparticle Res. 2013;15:1–10. doi: 10.1007/s11051-013-2034-2. [DOI] [Google Scholar]
- 37.Hasanpour A, Niyaifar M, Asan M, Amighian J. Synthesis and characterization of Fe3O4 and ZnO nanocomposites by the sol–gelmethod. J Magn Magn Mater. 2013;334:41–44. doi: 10.1016/j.jmmm.2013.01.016. [DOI] [Google Scholar]
- 38.Pandey N, Shukla SK, Singh NB. Zinc oxide-urea formaldehyde nanocomposite film as low-cost adsorbent for removal of Cu (II) from aqueous solution. Adv Mater Lett. 2015;6:172–178. doi: 10.5185/amlett.2014.5604. [DOI] [Google Scholar]
- 39.Ghanbariasad A, Taghizadeh S-M, Show PL, Nomanbhay S, Berenjian A, Ghasemi Y, et al. Controlled synthesis of iron oxyhydroxide (FeOOH) nanoparticles using secretory compounds from Chlorella vulgaris microalgae. Bioengineered. 2019;10:390–396. doi: 10.1080/21655979.2019.1661692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jurkin T, Štefanić G, Dražić G, Gotić M. Synthesis route to δ-FeOOH nanodiscs. Mater Lett. 2016;173:55–59. doi: 10.1016/j.matlet.2016.03.009. [DOI] [Google Scholar]
- 41.Marinho JZ, Montes RHO, De Moura AP, Longo E, Varela JA, Munoz RAA, et al. Rapid preparation of α-FeOOH and α-Fe2O3 nanostructures by microwave heating and their application in electrochemical sensors. Mater Res Bull. 2014;49:572–576. doi: 10.1016/j.materresbull.2013.09.052. [DOI] [Google Scholar]
- 42.Sun M, Senthil RA, Pan J, Osman S, Khan A. A facile synthesis of visible-light driven rod-on-rod like α-FeOOH/α-AgVO3 nanocomposite as greatly enhanced photocatalyst for degradation of rhodamine B. Catalysts. 2018;8:392. doi: 10.3390/catal8090392. [DOI] [Google Scholar]
- 43.Khayat Sarkar Z, Khayat SF. Selective removal of lead (II) ion from wastewater using superparamagnetic monodispersed iron oxide (Fe3O4) nanoparticles as a effective adsorbent. Int J Nanosci Nanotechnol. 2013;9:109–114. [Google Scholar]
- 44.Nangah C, Merlain T, Nsami N, Tubwoh C, Mbadcam K, Dodoo-Arhin D. Synthesis and Characterization of Goethite Nanostructured powder: Application in the Simultaneous Removal of Co(II) and Ni(II) Ions from Aqueous Solution. MRSAdv. 2018;3(42–43):2675–2687. doi: 10.1557/adv.2018.527. [DOI] [Google Scholar]
- 45.Jinadasa KBPN, Dissanayake CB, Weerasooriya SVR. Sorption of toxic metals on goethite: study of cadmium, lead and chromium. Int J Environ Stud. 1995;48(1):7–16. doi: 10.1080/00207239508710972. [DOI] [Google Scholar]
- 46.Godwin J, Abdus-Salam N, Haleemat AI, Panda PK, Panda J, Tripathy BC. Facile synthesis of rod-like α-FeOOH nanoparticles adsorbent and its mechanism of sorption of Pb (II) and indigo carmine in batch operation. Inorg Chem Commun. 2022;140:109346. doi: 10.1016/j.inoche.2022.109346. [DOI] [Google Scholar]
- 47.Ge Y, Cui X, Liao C, Li Z. Facile fabrication of green geopolymer/alginate hybrid spheres for efficient removal of Cu(II) in water: batch and column studies. Chem Eng J. 2017;311:126–134. doi: 10.1016/j.cej.2016.11.079. [DOI] [Google Scholar]
- 48.Godwin J, Abdus-Salam N, Haleemat AI, Bello MO, Inyang ED, Alkali MI, et al. High performance nanohybrid ZnO-α-FeOOH nanocomposite prepared for toxic metal ions removal from wastewater: combined sorption and desorption studies. Inorg Chem Commun. 2022;145:109900. doi: 10.1016/j.inoche.2022.109900. [DOI] [Google Scholar]
- 49.Kaneti YV, Zakaria QMD, Zhang Z, Chen C, Yue J, Liu M, et al. Solvothermal synthesis of ZnO-decorated α-Fe2O3 nanorods with highly enhanced gas-sensing performance toward n-butanol. J Mater Chem A. 2014;2:13283–92. doi: 10.1039/C4TA01837K. [DOI] [Google Scholar]
- 50.Ghaedi M, Ahmadi F, Shokrollahi A. Simultaneous preconcentration and determination of copper, nickel, cobalt and lead ions content by flame atomic absorption spectrometry. J Hazard Mater. 2007;142:272–278. doi: 10.1016/j.jhazmat.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Massoudinejad M, Mohammadi A, Sadeghi S, Ghaderpoori M, Sahebi S, Alinejad A. Arsenic adsorption over dodecahedra ZIF-8 from solution aqueous: modelling, isotherms, kinetics and thermodynamics. Int J Environ Anal Chem. 2022;102:855–871. doi: 10.1080/03067319.2020.1727458. [DOI] [Google Scholar]
- 52.Zhou C, Wang X, Wang Y, Song X, Fang D, Ge S. The sorption of single- and multi-heavy metals in aqueous solution using enhanced nano-hydroxyapatite assisted with ultrasonic. J Environ Chem Eng. 2021;9:105240. doi: 10.1016/j.jece.2021.105240. [DOI] [Google Scholar]
- 53.Li L, Liu F, Jing X, Ling P, Li A. Displacement mechanism of binary competitive adsorption for aqueous divalent metal ions onto a novel IDA-chelating resin: isotherm and kinetic modeling. Water Res. 2011;45:1177–1188. doi: 10.1016/j.watres.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Girish CR. Various isotherm models for multi-components adsorption: a review. Int J Civ Eng Technol. 2017;8(10):80–86. [Google Scholar]
- 55.Zhou Y, Liu F, Yu S. Preparation and photo-catalytic activities of FeOOH/ZnO/MMT composite. Appl Surf Sci. 2015;355:861–867. doi: 10.1016/j.apsusc.2015.07.030. [DOI] [Google Scholar]
- 56.Lu P-J, Chang C-S, Chern J-M. Binary adsorption breakthrough curves in fixed bed: experiment and prediction. J Taiwan Inst Chem Eng. 2014;45:1608–1617. doi: 10.1016/j.jtice.2013.10.018. [DOI] [Google Scholar]
- 57.Choy KKH, Porter JF, McKay G. Langmuir isotherm models applied to the multicomponent sorption of acid dyes from effluent onto activated carbon. J Chem Eng Data. 2000;45:575–584. doi: 10.1021/je9902894. [DOI] [Google Scholar]
- 58.Khajavian M, Wood DA, Hallajsani A, Majidian N. Simultaneous biosorption of nickel and cadmium by the brown algae cystoseria indica characterized by isotherm and kinetic models. Appl Biol Chem. 2019;62:69. doi: 10.1186/s13765-019-0477-6. [DOI] [Google Scholar]
- 59.Adenekan AE, Patzek TW, Pruess K. Modeling of multiphase transport of multicomponent organic contaminants and heat in the subsurface: Numerical model formulation. Water Resour Res. 1993;29(11):3727–3740. doi: 10.1029/93WR01957. [DOI] [Google Scholar]
- 60.Njimou JR, Pengou M, Kouamo HT, Tamwa MS, Tizaoui C, Fannang U, et al. Removal of lead ions from aqueous solution by phosphate‐based geopolymer cement composite. J Chem Technol Biotechnol. 96:1358–1369. wileyonlinelibrary.com 10.1002/jctb.6657
- 61.Ulya HN, Taufiq A. Comparative structural properties of nanosized ZnO/Fe3O4 composites prepared by sonochemical and Sol-Gel methods. IOP Conf Ser: Earth Environ Sci. 2019;276(1):012059. 10.1088/1755-1315/276/1/012059.
- 62.ALOthma ZA. A review: fundamental aspects of silicate mesoporous materials. Materials. 2012; 5:2874–902.
- 63.Anirudhan TS, Suchithra PS. Equilibrium, kinetics and thermodynamic modelling for the adfosrption of heavy metals onto chemically modified hydrotalcite. Indian J Chem Technol. 2012;17:247–259. [Google Scholar]
- 64.Atun G, Acar ET. Competitive adsorption of basic dyes onto calcite in single and binary component systems. Sep Sci Technol. 2010;45:1471–1481. doi: 10.1080/01496395.2010.485603. [DOI] [Google Scholar]
- 65.Ayawei N, Ebelegi AN, Wankasi D. Modelling and interpretation of adsorption isotherms. J Chem. 2017;2017:11. doi: 10.1155/2017/3039817. [DOI] [Google Scholar]
- 66.Sotelo JL, Ovejero G, Rodríguez A, Álvarez S, Galán J, García J. Competitive adsorption studies of caffeine and diclofenac aqueous solutions by activated carbon. Chem Eng J. 2014;240:443–453. doi: 10.1016/j.cej.2013.11.094. [DOI] [Google Scholar]
- 67.Carreon-Alvarez A, Herrera-Gonzalez A, Casillas N, Prado-Ramirez R, Estarron-Espinosa M, Soto V, et al. Cu (II) removal from tequila using an ion-exchange resin. Food Chem. 2011;127:1503–1509. doi: 10.1016/j.foodchem.2011.02.004. [DOI] [Google Scholar]
- 68.Mobasherpour I, Salahi E, Pazouki M. Comparative of the removal of Pb2+, Cd2+ and Ni2+ by nano crystallite hydroxyapatite from aqueous solutions: adsorption isotherm study. Arab J Chem. 2012;5:439–446. doi: 10.1016/j.arabjc.2010.12.022. [DOI] [Google Scholar]
- 69.Mohan D, Pittman CU., Jr Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater. 2007;142:1–53. doi: 10.1016/j.jhazmat.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 70.Khajavian M, Wood DA, Hallajsani A, Majidian N. Simultaneous biosorption of nickel and cadmium by the brown algae cystoseria indica characterized by isotherm and kinetic models. Appl Biol Chem. 2019;62:1–12. doi: 10.1186/s13765-019-0477-6. [DOI] [Google Scholar]
- 71.Abdullah LC, Muhammad SSJ, Choong TS. Modelling of single and binary adsorptions of heavy metals onto activated carbon-equilibrium studies. Pertanika J Sci TechnolUniversiti Putra Malays Press. 2010;18:83–93. [Google Scholar]
- 72.Hilbrandt I, Lehmann V, Zietzschmann F, Ruhl AS, Jekel M. Quantification and isotherm modelling of competitive phosphate and silicate adsorption onto micro-sized granular ferric hydroxide. RSC Adv. 2019;9:23642–23651. doi: 10.1039/C9RA04865K. [DOI] [PMC free article] [PubMed] [Google Scholar]