Abstract

The 5-hydroxytryptamine-3 receptor (5-HT3R), a subtype of serotonin receptor, is a ligand-gated ion channel crucial in mediating fast synaptic transmission in the central and peripheral nervous systems. This receptor significantly influences various neurological activities, encompassing neurotransmission, mood regulation, and cognitive processing; hence, it may serve as an innovative target for neurological disorders. Multiple studies have revealed promising results regarding the beneficial effects of these phytoconstituents and extracts on conditions such as nausea, vomiting, neuropathic pain depression, anxiety, Alzheimer’s disease, cognition, epilepsy, sleep, and dyskinesia via modulation of 5-HT3R in the pathophysiology of neurological disorder. The review delves into a detailed exploration of in silico, in vitro, and in vivo studies and clinical studies that discussed phytoconstituents acting on 5-HT3R and attenuates difficulties in neurological diseases. The diverse mechanisms by which plant-derived phytoconstituents influence 5-HT3R activity offer exciting avenues for developing innovative therapeutic interventions. Besides producing an agonistic or antagonistic effect, some phytoconstituents exert modulatory effects on 5-HT3R activity through multifaceted mechanisms. These include γ-aminobutyric acid and cholinergic neuronal pathways, interactions with neurokinin (NK)-1, NK2, serotonergic, and γ-aminobutyric acid(GABA)ergic systems, dopaminergic influences, and mediation of calcium ions release and inflammatory cascades. Notably, the phytoconstituent’s capacity to reduce oxidative stress has also emerged as a significant factor contributing to their modulatory role. Despite the promising implications, there is currently a dearth of exploration needed to understand the effect of phytochemicals on the 5-HT3R. Comprehensive preclinical and clinical research is of the utmost importance to broaden our knowledge of the potential therapeutic benefits associated with these substances.
Keywords: 5-HT3 receptor, serotonin, neurological disorder, extract, phytoconstituents
Serotonin, 5-hydroxytryptophan (5-HT), is a neurotransmitter derived from tryptophan and is involved in regulating mood, appetite, and sleep. It exerts its action through seven receptor subtypes (5-HT1–5-HT7 receptors).1 Numerous preclinical and clinical investigations indicated the widespread distribution of 5-HT receptors in various brain areas like the hippocampus, amygdala, hypothalamus, olfactory bulb, choroid plexus, and islands of Calleja.2−9 They are involved in a variety of neuropsychological and neurodegenerative illnesses, including anxiety, depression, Alzheimer’s disease (AD), Parkinson’s disease (PD), and neuropathic pain, due to their widespread distribution in the brain.10,11 Additionally, 5-HT plays an important role in gut health, and its stimulation can cause nausea and vomiting, especially in conditions such as cancer, inflammatory bowel disease, and colitis.12−14
5-HT and its receptors play a crucial role in developing gastric and central nervous system (CNS) illnesses and may be used as a therapeutic target. The agonists and antagonists of the receptor have gained significant market share for their therapeutic effects.15,16 Except for the 5-hydroxytryptamine-3 receptor (5-HT3R), all other 5-HT receptors are G-protein-coupled. The 5-HT3R belongs to a ligand-gated cysteine-loop transmitter superfamily of ion channels, including the acetylcholine nicotinic receptor, the γ-aminobutyric acid A (GABAA) receptor, and the glycine receptor. It operates as a selective cation ion channel (Figure 1).17 5-HT3Rs are located in both the CNS and the peripheral nervous system (PNS). A study conducted by Kilpatrick et al. reported the distribution of 5-HT3R in the entorhinal cortex, retrosplenial cortex, hippocampus, and amygdala of the brain. Numerous autoradiographic labeling studies on rat brains showed the presence of 5-HT3Rs in the subthalamic nucleus.5,18In vitro autoradiographic assays and positron emission tomography imaging of mouse and rat brain slices suggested the highest 5-HT3R in the hippocampus.19 The subtypes of 5-HT3R are 5-HT3A and 5-HT3B. The immunostaining studies demonstrated a low occurrence of 5-HT3AR in the limbic system, particularly in the hippocampus, amygdala, and entorhinal cortex, whereas the highest occurrence was observed in the hippocampus (CA1-CA3) regions of the forebrain region and brain stem, such as the nucleus tractus solitarius and trigeminal nerve.20 The activation of 5-HT3AR causes a rapid influx of Ca2+ ions at the nerve endings, essential in controlling the release of neurotransmitters such as 5-HT, GABA, acetylcholine (Ach), glutamate, and dopamine (DA). It can regulate the release and inhibition of these neurotransmitters, which can result in a variety of neurological disorders like nausea, vomiting, neuropathic pain, depression, anxiety, cognition, sleep AD, dyskinesia, and epilepsy (Figure 2).21
Figure 1.
Structure of 5-HT3R (Created in Biorender.com)
Figure 2.
Mechanism of actions of phytochemicals on 5-HT3R (Created in Biorender.com).
Herbs, plant extracts, phytochemicals, and other natural substances have been utilized extensively since time immemorial (Table 1). Traditional medicine has become widespread and increasingly popular globally due to its diverse pharmacological properties. Alkaloids, flavonoids, tannins, glycosides, and terpenoids are phytoconstituents with tremendous potential for treating various chronic disorders by modulating 5-HT3R. Over the past 20 years, extensive research has been conducted on kampo medicine, a traditional Japanese remedy. Hoffman et al. conducted a study to screen the kampo remedies for their pharmacological effects. The effect of diluted ethanol tinctures of 121 kampo remedies on human 5-HT3AR was studied through a voltage clamp method. Ligusticum striatum (rhizoma) activated 5-HT3R, most strongly compared to other extracts, accounting for almost 30% of the 5-HT induced current. Further, the tinctures of different plant extracts such as Panax ginseng (radix), Syzygium aromaticum (flos), and Zingiber officinalis (rhizoma) were screened and found to have a potent 5-HT3R antagonistic effect. In addition, the phytoconstituents such as boldine and leonurine present in Lindera aggregate (radix) and Leonurus japonicus (herba), respectively, were found to be potent 5-HT3AR antagonists with IC50 values of 0.53 ± 0.15 and 2.17 ± 0.15 μM, respectively, on the Xenopus laevis oocytes. In contrast, the other constituents of kampo medicine, such as tannic acid and schizandrin, also exhibited lower antagonistic effects with IC50 137 ± 22 and 48.2 ± 4.1 μM, respectively (Table 2).22 Extracts from Bolivian plants Xanthium spinosum and Senecio mathewsii demonstrated promising results obtained from the electrophysiology patch clamp technique. These extracts blocked 5-HT3R and GABAA receptors. However, their investigations on in vivo preclinical therapeutics remain untouched on neurological disorders.23
Table 1. In Vivo Studies: Effects of Various Phytochemicals on 5-HT3R.
| S. No. | Plant Name | Chemical Constituent | Class | Doses | Disease | Animal Model | Effects | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | Ganoderma lucidum (Ganodermatace) | Ganoderic acid A, Ganoderic acid C2, Ganodermanotriol | Triterpenoid | Varying doses (1,3,10 and 30 mg/kg, i.p.) | Nausea and vomiting | Cisplatin (3 mg/kg, i.p.) in rats | Improved food intake and Antiemetic effects | (39) |
| 2. | Thymus persicus (Lamiaceae) | Carvacrol | Monoterpenoid | (50, 100, and 150 mg/kg, p.o.) | Neuropathic pain | Formalin induced paw licking in male mice | Antinociceptive and anti-inflammatory | (50) |
| 3. | Cannabis Sativa (Cannabaceae) | CBD, THC | Alkaloids | CBD (2.5 mg/kg, i.p.) with THC (1 mg/kg, i.p.) | Vomiting and abdominal retching | Lithium-chloride-induced vomiting and abdominal retching | Antiemetic effects | (42) |
| 4. | Salvia sclarea L. (Lamiaceae) | Sclareol | Diterpene | (15 mg/kg, p.o.) | Nausea and Vomiting | Copper sulfate-induced emesis in chicks | Decreased the number of retches and increased delay time | (43) |
| 5. | Zingiber officinale (Zingiberaceae) | Gingerol, Shogaol | Polyphenol | Z. officinale aq. extracts (100, 300, and 500 mg/kg, p.o.) | Neuropathic pain | Oxaliplatin induced (6 mg/kg, i.p.) | Antiallodynic and antihyperalgesia effects | (51) |
| 6. | Zingiber zerumbet (Zingiberaceae) | Zerumbone | Sesquiterpene | (10 mg/kg, i.p.) | Neuropathic pain | CCI-induced neuropathic pain in mice | Antiallodynic and antihyperalgesia effects | (52) |
| 7. | Veratrum grandiflorum (Melanthiaceae) | Resveratrol | Stilbene | (2 mg/kg, i.v.) | Neuropathic pain | Electrical stimulation (jaw opening reflex) wistar rats | Antinociceptive effects | (55) |
| 8. | Viola odorata (Violaceae) | Hydroxy flavoxone | Flavonoid | (1, 10, 30 mg/kg, i.p.) | Depression | FST and TST in mice | Reduce duration of immobility | (80) |
| 9. | Spinacia oleracea (Amaranthaceae) and Brassica oleracea (Brassicaceae) | Alpha-lipoic acid | Organo sulfur | (50, 100, 200 mg/kg, p.o.) | Depression | FST and chronic stress induced depression in rats | Antidepressant and reduced anhedonia | (87) |
| 10. | Terminalia catappa Linn (Combretaceae), Crocus sativus (Iridaceae), and Phyllanthus emblica L (Phyllanthaceae) | Gallic acid | Polyphenol | (30 and 60 mg/kg, p.o.) | Depression | FST and TST in mice | Antidepressant | (82) |
| 11. | Hypericum perforatum (Clusiaceae) | Hyperforin | Terpene | (10 or 25 mg/kg, p.o.) | Depression | FST in rats | Antidepressant and increase % entries in open arm maze | (84) |
| 12. | Gingko biloba (Ginkgoaceae) | (15, 30, and 60 mg/kg) | Cognition and memory | Scopolamine (3 mg/kg, i.p.) in male Swiss mice | Improve cognitive function through AchE Inhibition | (135) | ||
| 13. | Zingiber officinale (Zingiberaceae) | (25, 50, and 100 mg/kg, i.p.) | Epilepsy | PTZ (30 mg/kg, i.v.) in mice | Increase seizures threshold | (136) | ||
| 14. | Glycine max (Fabaceae) | Genistein | Flavonoid | (10 mg/kg, i.v.) | Epilepsy | PTZ (30 mg/kg, i.v.) in ovariectomized mice | Increase seizure threshold | (126) |
| 15. | Matricaria chamomilla (Asteraceae) | Apigenin | Flavonoid | (50 mg/kg, p.o.) | Depression | FST, TST | Reduce duration of immobility | (90) |
| 16. | Carpobrotus edulis (Aizoaceae) | Rutin | Flavonoid | (3 mg/kg, p.o.) | Depression | FST, TST | Reduce duration of immobility | (86, 137) |
Table 2. In Vitro Studies: Effect of Various Phytochemicals on 5-HT3R.
| S. No. | Plant Name | Chemical Constituents/Extracts | Class | In Vitro Model | Concentration (IC50) | Binding Site | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Panax ginseng (radix) (Araliaceae) | Ginsenoside | Steroid glycoside | Xenopus laevis oocytes | 27.6 ± 4.3 μM | Antagonism of 5-HT3AR | (22, 138) |
| 2. | Syzygium aromaticum (flos) (Myrtaceae) | Tannic acid | Polyphenol | Xenopus laevis oocytes | 48.2 ± 4.1 μM | Antagonism of 5-HT3AR | (22) |
| 3. | Lindera aggregate (radix) (Lauraceae) and Peumus boldus (Monimiaceae) | Boldine | Aporphine alkaloid | Xenopus laevis oocytes | 0.53 ± 0.15 μM | Antagonism of 5-HT3AR | (22, 139, 140) |
| 4. | Leonurus japonicas (herba) (Lamiaceae) | Leonurine | Alkaloid | Xenopus laevis oocytes | 2.17 ± 0.15 μM | Antagonism of 5-HT3AR | (22) |
| 5. | Schisandra chinesis (fructus) (Schisandraceae) | Schizandrin | Dibenzocyclooctadiene | Xenopus laevis oocytes | 48.2 ± 4.1 μM | Antagonism of 5-HT3AR | (22) |
| 6. | Zingiber officinale Roscoe (Zingiberaceae) | Galanolactone | Diterpene | Guinea pig ileum | 4.93 μM | Contractile inhibition of ileum via 5-HT3R | (27) |
| 7. | Rosa damascene (Rosaceae) | Citronellol | Monoterpenes | Xenopus laevis oocyte | 64 μM | Competitively inhibits 5-HT3AR | (40) |
| 8. | Cannabis sativa (Cannabaceae) | Cannabinoid cannabidiol | Alkaloids | Xenopus laevis oocyte | 121 nM to 29 μM | Noncompetitively inhibits 5-HT3AR | (41, 42) |
| 9. | Syzygium aromaticum (Myrtaceae) | Eugenol vanillin | Benzaldehyde | Xenopus laevis oocytes | 1159 μM eugenol and 4744 μM vanillin | Noncompetitive antagonist at 5-HT3AR | (40) |
| 10. | Zingiber officinale (Zingiberaceae) | 6-Shogaol | Polyphenol | Xenopus laevis oocytes | 30.6 ± 0.54 μM | Noncompetitive antagonist at 5-HT3AR | (40) |
| 11. | Zingiber officinale (Zingiberaceae) | 6-Gingerol | Polyphenol | Xenopus laevis oocytes | 46.6 ± 0.64 μM | Noncompetitive antagonist at 5-HT3AR | (40) |
| 12. | Hypericum perforatum (Hypericaceae) | Hyperforin | Terpene | Peritoneal cells | 3.4 μg/mL | Inhibiting serotonin uptake via 5-HT3R antagonism | (84) |
| 13. | Uncaria hook (Rubiaceae) | Hirsutine | Indole alkaloid | Xenopus laevis oocytes | 22.12 μM | Antagonism of 5-HT3R and 5-HT3ABR | (141) |
| 14. | Eucalyptus globulus (Myrtaceae) | Eucalyptol | Monoterpene | Xenopus laevis oocytes | 258 μM | Antagonism of 5-HT3R | (142) |
| 15. | Lippia alba (Varbenaceae) | Citral | Monoterpene | Xenopus laevis oocytes | 120 μM | Antagonism of 5-HT3R | (142) |
| 16. | Gingko biloba (Ginkgoaceae) | Bilobalide, ginkgolides B | Sesquiterpenoid | Xenopus laevis oocytes | Bilobalide 470 μM, Ginkgolide B730 μM | Antagonism of 5-HT3R | (110) |
| 17. | Cudrania tricuspidata (Moraceae) | Kaempferol | Flavonoid | Xenopus laevis oocytes | 12.8 ± 2.0 μM | Antagonism of 5-HT3R | (103) |
| 18. | Chili peppers (Solanaceae) | Capsaicin | Alkaloid | Xenopus laevis oocytes | 62 μM | Antagonism of 5-HT3R | (143) |
| 19. | Chili peppers (Solanaceae) | Capsaicin | Alkaloid | HEK-293 cells | 54 μM | Antagonism of 5-HT3R | (143) |
| 20. | Schisandra chinensis (Schisandraceae) | Schisandrin C | Lignan | Xenopus laevis oocytes | 27.0 μM | Competitively antagonized 5-HT3R | (144) |
| 21. | Xanthium spinosum (Asteraceae) | HEK-293 cells | 4.6 ± 0.8 μg/mL | Antagonism of 5-HT3R | (23) | ||
| 22. | Senecio mathewsii (Asteraceae) | HEK-293 cells | 5.0 ± 1.0 μg/mL | Antagonism of 5-HT3R | (23) |
Numerous chronic neurological diseases have shown resistance to available therapeutic options, leading to relapse and limited effectiveness. In light of these challenges, the potential benefits of phytoconstituents that target specific receptors and offer additional pharmacological effects have become a compelling area of exploration. The current review aims to explore and evaluate the potential of natural approaches in managing neurological disorders, focusing on modulation of 5-HT3R by phytochemicals. The goal of the review is to present a thorough understanding of how phytoconstituents interact with the 5-HT3R to mediate neurobehavioral activities, impacting neurological processes and perhaps having therapeutic advantages. Furthermore, to determine these natural chemicals’ potential as cutting-edge treatments for neurological disorders, the current review explores their modes of action, preclinical and clinical evidence, and safety profiles.
Nausea and Vomiting
5-HT3R is involved in the physiology of nausea and vomiting.21 CNS and PNS are activated during a vomiting response, stimulating the enterochromaffin cells in the intestinal mucosa and causing the gastrointestinal tract to produce 5-HT. It activates the peripheral 5-HT3R in the myenteric plexus, triggering synaptic and ganglionic transmission. This local 5-HT release induces vomiting by stimulating 5-HT3R, inhibiting gastric motility.24
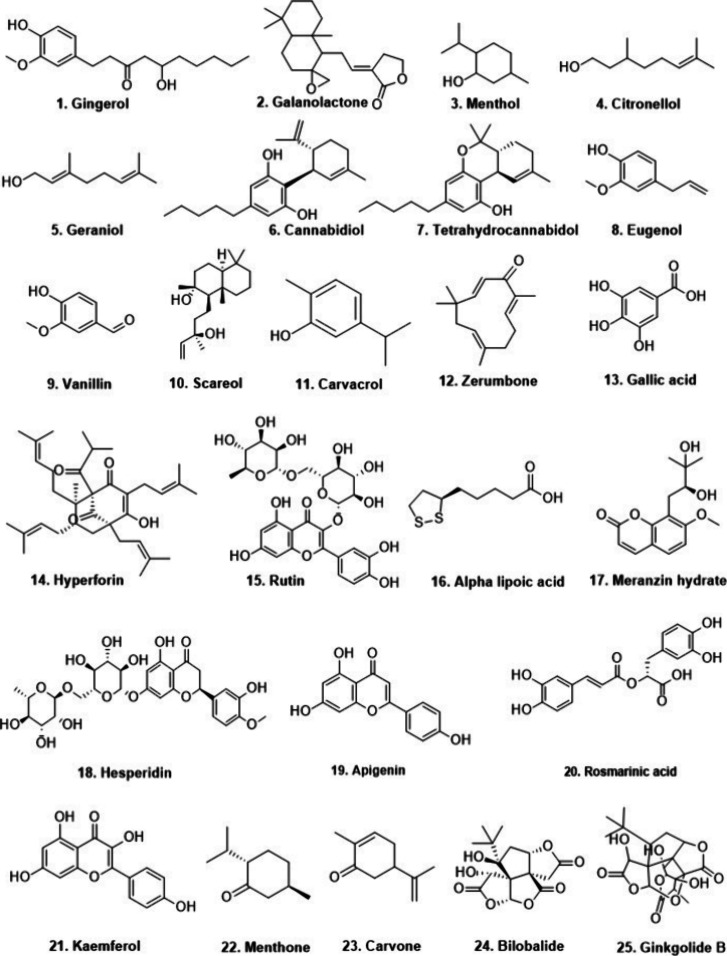
The use of the Zingiber officinale Roscoe (Zingiberaceae) plant extract in ameliorating vomiting reflexes was widely studied. The administration of gingerol (1) in guinea pigs effectively inhibited upper gastric motility through its antiserotonergic actions and was proposed to be effective in emesis (Figure 3).25 Investigations proved that gingerol strongly antagonizes 5-HT3AR.22,26 The other compounds of ginger, such as galanolactone (2), were also capable of suppressing contractile responses through antagonism of 5-HT3AR with an IC50 value of 4.93 μM against the effect of 2-methyl-5-HT, a 5-HT3R agonist.27 A clinical study regarding the effect of ginger root extract was conducted at St Bartholomew’s Hospital, London, on 60 women with major gynecological surgery. They were administered 0.5 g of ginger root extract (Zingiber officinale), which alleviated postoperative nausea and vomiting.28
Figure 3.
Various natural products reported as 5-HT3R antagonists.
In another randomized, double-blind, placebo-controlled, and crossover study, the patients with breast cancer were administered 500 mg of ginger-containing capsules; however, this was incapable of reducing chemotherapy-induced nausea and vomiting (CINV) in patients while the conventional medications such as ondansetron and domperidone reversed CINV.29 Six previously published randomized controlled studies have assessed the effectiveness of oral ginger in preventing CINV in cancer patients. However, each study focused on a different cancer population, utilized varying chemotherapy regimens, administered different dosages of ginger, and employed different outcome assessment methods. Consequently, the findings from these studies are contradictory: four studies reported a positive impact of ginger, whereas two studies indicated no significant benefits.30−35
Most anticancer medications have nausea and vomiting as side effects, affecting the effectiveness of the therapy. The reduction of the CINV is a challenging task. Palonosetron (Aloxi), a potent 5-HT3R antagonist, is effective and exhibits an antiemetic effect, but it induces headaches and constipation; therefore, it is less preferred.36 Various phytoconstituents have been studied to alleviate these problems and enhance efficacy of anticancer drugs by lowering CINV. Menthol (3), a monoterpenoid obtained from Mentha piperita (Lamiaceae), antagonized human 5-HT3R expressed in Xenopus laevis oocytes with an IC50 of 160 μM.37 Peppermint oil and menthol were evaluated for their binding affinity on 5-HT3R using different in vitro paradigms where they have used [14C] Guanidinium influx into N1E-115 cells for 5-HT3R expressions, isotonic contractions of the isolated rat ileum, and radioligand binding. Peppermint oil and menthol block 5-HT3R in a concentration-dependent manner and also reduce the 5-HT-induced contraction of rat ileum.38 The Ganoderma lucidum extract (GLE), a Chinese herb, was administered to rats at doses 1, 3, 10, and 30 mg/kg (i.p.) to evaluate its effect on cisplatin (3 mg/kg, i.p.) induced nausea. Results showed that GLE successfully increased food intake and minimized emesis.39
The essential oil of Rosa damascene, a flowering plant, contains monoterpenes, viz., citronellol (4) and geraniol (5). These compounds competitively inhibit 5-HT3AR with an IC50 value of 175 μM and 64.3 μM, respectively, and are effective against nausea and vomiting.40 Cannabidiol (6) (CBD), a nonpsychoactive substance, has shown inhibition in mice and human 5-HT3AR expressed on Xenopus laevis oocytes. CBD (2.5 mg/kg i.p.) and Δ9-tetrahydrocannabinol (THC, 7) (1.0 mg/kg i.p.) diminished the effects of lithium-chloride-induced vomiting and abdominal retching in shrews.41,42 Based on these findings, combining small doses of CBD with THC could enhance the antiemetic effect and mitigate the intoxicating effects associated with THC. Furthermore, research on eugenol (8) and vanillin (9) concluded that these have nonspecific inhibition of 5-HT3R expressed on Xenopus laevis oocytes with IC50 values of 1159 μM for eugenol and 4744 μM for vanillin.40 The study on chicks was also demonstrated for the antiemetic effects of sclareol (10). Its 15 mg/kg oral dose attenuated the vomiting refluxes induced by copper sulfate, and sclareol decreased the number of retches and increased delay time. These results were supported through in silico findings suggesting that sclareol binds to 5-HT3R and D1 receptors more strongly than ondansetron and domperidone.43
The FDA-approved drugs for CINV, such as granisetron, ondansetron, and palonosetron, are the best-selling medications in the market for several disorders associated with nausea and vomiting. Palonosetron has demonstrated its effects by selectively binding to 5-HT3AR for CINV, as was previously mentioned. However, the lack of selectivity for 5-HT3R subunits in the others may restrict their application in CINV.44 On the other hand, the selectivity of most of the previously investigated natural 5-HT3R antagonists supports their use in CINV, nausea, and vomiting.
Among these compounds, gingerol from Zingiber officinale, menthol from Mentha piperita, citronellol and geraniol from Rosa damascene, CBD, and eugenol have demonstrated effective inhibition of 5-HT3R activity and reduction of nausea and vomiting symptoms. These natural compounds offer promising alternatives for managing nausea and vomiting, including CINV, with potentially fewer side effects compared to conventional medications like palonosetron.
Neuropathic Pain
Neuropathic pain is a chronic and debilitating condition marked by severe pain, heightened sensitivity to pain (hyperalgesia), and abnormal processing of sensory signals (nociception). There is a disruption in somatosensory signals between the brain and spinal cord. 5-HT3R plays a significant role in transmitting pain in the central and peripheral nociceptive systems. The neurokinin (NK)-2 tachykinin receptor, substance P (SP), calcium calmodulin signaling, and protein kinase are also essential in transmitting central pain. SP is an 11-amino acid neuropeptide with a particular affinity for the neurokinin-2 receptor (NKR). Various types of non-neuronal cells, such as microglia and immune cells, express SP, which is essential to transmit nociceptive signals to second-order neurons in the brainstem and spinal cord via primary afferent fibers.
Additionally, the release of SP from primary afferent fibers increases the expression of the NK-2 receptor in dorsal horn neurons during neuropathic pain and inflammation.45 The proper functioning of NK-2 tachykinin receptors relies on the ability of 5-HT3R to facilitate the entry of Ca2+ ions. NK-2 receptors also play a role in the 5-HT3R-mediated release of SP. Substantial evidence supports the interdependence of 5-HT3R and the NK-2 tachykinin receptors, both crucial in transmitting pain signals within the CNS. Activation of NK-2 tachykinin receptors increases 5-HT release in the spinal cord, triggering 5-HT3R. This activation may contribute to the development of pain hypersensitivity. Research indicates that the coactivation of NK-2 tachykinin receptor and 5-HT3R enhances pain transmission in the spinal cord. When the 5-HT3Rs are activated, there is an increase in the level of calcium release, forming a calcium-calmodulin complex. This complex upregulates the nitric oxide synthase (NOS) enzyme, producing nitric oxide (NO). NO presence can then modulate NK-2 tachykinin receptor activity. Overexpression of neurokinin or SP on the NK-2 tachykinin receptor intensifies pain sensitivity. The study in isolated nodose neurons from adult guinea pigs displayed that 5-HT, NO, and meta-chlorophenyl-biguanide (CPBG), a 5-HT3R agonist, increased the response of SP (amplitude) in these neurons, which was absent with SP treatment alone. However, treatment with ICS205-930, a 5-HT3R antagonist, completely abolished these effects, indicating the involvement of 5-HT3R in pain stimuli.46 Moore et al.47 also showed that 5-HT promotes proinflammatory effects via 5-HT3R.
Furthermore, this activation may increase the afferent vagal nerve sensitivity to SP on the tachykinin receptors. The administration of the 5-HT3R antagonists, tropisetron and Y-25130 (at a concentration of 1 μM), did not result in noticeable alterations in the electrical characteristics of nodose neurons exposed to ovalbumin. These findings imply that 5-HT3R antagonists might have pain-relieving abilities.47 Several 5-HT3 agonists have been used to investigate the involvement of 5-HT3R in pain sensitivity. The intrathecal infusion of SR 57227, a 5-HT3R agonist, stimulates glial cells and neuronal hyperexcitability, raising hypersensitivity to pain. It offered a rudimentary understanding of the spinal 5-HT3R agonist as a pain mediator.48
A polyherbal formulation of 11 extracts from plants, viz., as Commiphora mukul, Terminalia bellirica, Terminalia chebula, Emblica officinalis, Tinospora cordifolia, Piper longum, Withania somnifera, Alpinia galanga, Glycyrrhiza glabra, Curcuma longa, and Asparagus racemosus, was evaluated against acetic acid triggered writhing in mice with ondansetron (0.5 mg/kg, i.p.) as standard. The polyherbal formulation at doses of 390 and 585 mg/kg p.o. displayed significant antinociceptive effects in the writhing and tail immersion tests, possibly due to modulation of 5-HT3R.49 Iranian Avishan is the common name for the perennial herb Thymus persicus belonging to the Lamiaceae family. It has historically been used to treat back pain and toothaches. Previous studies have proved its antinociceptive effects in neuropathic pain against formalin-induced paw licking in male mice.50 The essential oil Thymus persicus (TPEO) of aerial parts at doses of 50, 100, and 150 mg/kg p.o. significantly reduced allodynic effects and hyperalgesic stimuli, resulting in antinociceptive effects in mice. The study further elaborated on the impact of carvacrol (11) as a key compound in TPEO, which mediates the effect via the l-arginine/NO/cGMP/KATP signaling pathway and modulation of 5-HT3R. Moreover, it was postulated that the analgesic effect against neuropathic pain was due to a reduction in proinflammatory mediators.50
Further, certain studies explained the significance of Zingiber officinale Roscoe in analgesic effects at doses of 100, 300, and 500 mg/kg administered orally. One effect is against oxaliplatin-induced (2 mg/mL) cold and mechanical allodynia in mice.51 Zerumbone (12), a bioactive sesquiterpene derived from Zingiber zerumbet, has demonstrated antiallodynic and antihyperalgesic effects in a mouse model of neuropathic pain induced by chronic constriction injury. Another study revealed that zerumbone (10 mg/kg, i.p.) modulates the serotonergic system through 5-HT3R, producing antiallodynic and antihyperalgesic effects.52
For an extended period, tropisetron and ondansetron have been used to attenuate emesis. Still, their use in pain is also established by preclinical and clinical practices, which authenticate the crucial role of 5-HT3R antagonism in pain relief. The activation of spinal 5-HT3R regulates pain nociception, giving rise to allodynia. Further, 5-HT3R antagonism contributes to comfort from mechanical allodynia in spinal cord injury following neuropathic pain in rats.53 A double-blinded, placebo-controlled crossover study supported the finding from preclinical observations that 5-HT3R antagonism alleviates symptoms related to neuropathic pain.54
Resveratrol (2 mg/kg, i.v.) has been demonstrated to suppress the nociceptive, jaw-opening reflex in Wistar rats via 5-HT3R-mediated GABAergic inhibition measured by digastric muscle electromyograms (dEMGs). Resveratrol activates 5-HT3R, i.e., agonism, thereby inhibiting GABA receptor signaling and thus leading to inhibition of the Ca2+ channel, resulting in reduced pain as assessed by reduced amplification of dEMG.55 Other investigations are underway to differentiate the role of the 5-HT receptor in neuropathic pain evaluated by paclitaxel (2 mg/kg i.p.)-induced neuropathic pain in male Sprague–Dawley rats. These rats were treated with the standard drug, 60 μg of WAY100635 (a 5-HT1A receptor antagonist) or 30 μg of ketanserin (a 5-TH2A/2C receptor antagonist) or 30 μg of ondansetron (a 5-HT3R antagonist) or test drug Yokukansan, a Japanese medicine containing seven traditional mixture. Yokukansan attenuated PTX side effects by acting on the 5-HT1 and 5HT2 receptor and less via 5-HT3R. It improved allodynia and increased the withdrawal threshold, which supports its therapeutic use in neuropathic pain via 5HT1, 5-HT2, and 5-HT3R.56 Grape seed extract (GSE) was evaluated for action by 5-HT3 and GABA receptors in temporomandibular disorders characterized by dysfunctional jaw joints and muscles that control jaw movement. Animals were supplemented with 0.5% GSE dissolved in their water for 1 week, and nociception was induced by five injections of Freund adjuvant (each 100 μL per side) into the upper trapezius muscles. To investigate the mechanism of GSE, some animals were injected intracisternally with antagonists of 5-HT3, 5-HT7, GABAA, or GABAB receptors before jaw opening. The inhibitory effect of GSE is mediated via the activation of 5-HT3/7 receptors and GABAB to enhance central descending inhibitory pain pathways and suppress ongoing trigeminal nociception.57
The existing studies investigating the use of phytochemicals to regulate 5-HT3R for pain management, especially in neuropathic conditions, exhibit conflicting findings compared to similar research. This disparity complicates the comprehension of the impacts of 5-HT3R agonists and antagonists on pain. Faerber et al. conducted a study that clarified the effects of 5-HT3R agonists and antagonists. In their view, activating the spinal 5-HT3R increases the GABAergic activity, a crucial mediator of analgesic effects. On the other hand, 5-HT3R antagonists alleviate pain via NK1 and NK2 receptors by stopping the secretion of neurokinin, SP, and calcitonin gene-related peptides.21,58
Among these, carvacrol from Thymus persicus, zerumbone from Zingiber zerumbet, and resveratrol have demonstrated significant antinociceptive effects through 5-HT3R modulation. Carvacrol from Thymus persicus and zerumbone from Zingiber zerumbet exert their antinociceptive effects by modulating the l-arginine/NO/cGMP/KATP signaling pathway and through direct modulation of 5-HT3R activity. Resveratrol suppresses nociception by activating 5-HT3R-mediated GABAergic inhibition, leading to reduced pain sensitivity, and has demonstrated significant pain-relieving abilities by blocking 5-HT3R-mediated pain transmission pathways.
Currently, there is a lack of research that supports the use of phytochemicals as 5-HT3R agonists and antiagonists to treat central neuropathic pain pathways. Phytochemicals targeting 5-HT3R inevitably hold promise as treatments for neuropathic pain. More research is necessary to identify specific phytoconstituents and assess their impact on experimental models of neuropathic pain.
Anxiety
Anxiety is a neuropsychiatric illness characterized by restlessness, fear, apprehension, difficulty concentrating, irritability, muscle tension, and sleep disturbances. In recent times, considerable evidence has substantiated the pivotal involvement of the serotonergic system in anxiety disorders. Preclinical research has been instrumental in formulating and supporting this hypothesis.59,60
Antagonism of 5-HT3R demonstrates encouraging anxiolytic effects observed across different behavioral paradigms, including the elevated X-maze, elevated T-maze, light/dark test, and potentiated acoustic startle.61,62 Ondansetron, a 5-HT3R antagonist, effectively treated anxiety in the early investigations, as evidenced by the elevated plus maze results in mice.63Hallea ciliate and Datura stramonium were studied for their anxiolytic activity using a hole board test, increased head dips, sectional crossings, squares crossed, rearing in OFT, and decreased immobility time in force swim test (FST) in mice. This behavior pointed out that Datura has substantial anxiolytic and antidepressant-like activities through modulation of serotoninergic (5-HT3R) and adrenergic (α1) neurotransmissions.64 In contrast, Hallea ciliate mediates its anxiolytic effects via GABA and 5-HT3R antagonism.65 The anxiolytic effect of petroleum ether extract of Zingiber officinale (5–300 mg/kg p.o.) in mice has demonstrated a decreased preference for a closed arm of an elevated plus maze.66
Similarly, a combination of Ginkgo biloba and Zingiber officinale (0.5, 1, 10, and 100 mg/kg) via intragastric administration to male rats exhibited an anxiolytic effect without any amnesiac symptoms, as observed with benzodiazepines. Moreover, the combination displayed nootropic activity. Gingerol and galanolactone are the constituents of Zingiber officinale, both having antagonistic activity at the 5-HT3R and are considered to have the potential to treat fear and anxiety-related disorders.67
There has been little research on the anxiolytic effects of herbs, extracts, and phytoconstituents; molecular and clinical evidence is lacking, and behavioral assessments are the primary focus. Although some combinatorial effects like those of stramonium have been explored, little is known about particular phytoconstituents targeting anxiolytic effects via 5-HT and adrenergic receptors. More thorough research is necessary to investigate possible phytoconstituents acting on 5-HT3R and their molecular pathways for the anxiolytic effect.
Depression
The involvement of 5-HT3R in the progression of depression-like behavior is widely accepted; however, the role of 5-HT3R agonists and antagonists in the treatment of depression is still unclear and under investigation due to safety concerns.68 The treatment of depression has traditionally emphasized the role of 5-HT1 and 5-HT2 receptors. Their agonists and antagonists have an impressive market presence and are in high demand as therapeutics among physicians; FDA-approved medications for treating depression and anxiety include buspirone, a 5-HT1A agonist, methyl-5-HT, a 5-HT2AB agonist, and mirtazapine, a 5-HT2C receptor antagonist,69 in addition to selective serotonin reuptake inhibitors like fluoxetine, duloxetine, and escitalopram.70 The multifactorial pathophysiology of depression makes it challenging for researchers to identify compounds with multiple therapeutic effects with safety and efficacy. Buspirone, vortioxetine, mirtazapine, and quetiapine are some of the drugs utilized to alleviate neuropsychiatric symptoms that act through 5-HT3R modulation.71−75
Moreover, preclinical studies on phytochemicals and extracts have produced promising results in alleviating depressive symptoms through the regulation of 5-HT3R. In rodents, 5-HT3R agonism causes depressive symptoms; on the other hand, its antagonistic effects restore depressive-like behavior.75,76HTR3a knock out mice displayed antidepressant effects evaluated by FST and elevated plus maze in a chronic social defeat stress model.77 5-HT3R antagonism suppresses 5-HT binding to postsynaptic 5-HT3R, thereby enhancing its accessibility to other receptors such as 5-HT1A, 5-HT1B, and 5-HT1D, along with 5-HT2 receptors. This mechanism elicits antidepressant-like effects.78 In rodents, antidepressant effects were investigated using behavioral paradigms such as FST and the tail suspension test (TST). In FST, the antidepressant effects were reflected by an increase in serotonergic neurotransmission, which led to longer swimming durations (mobility), whereas those that were found to increase catecholaminergic neurotransmission led to longer struggling durations.79 The Sweet violet (Viola odorata L) extract consisting of compound 1, 5,7-dihydroxy-3,6-dimethoxyflavone, compound 2, 5,7,4′-trihydroxy-3′,5′dimethoxyflavone, and compound 3, 5,7,4′-trihydroxy-3′-methoxyflavone, was critically investigated for their antidepressant activity via 5-HT1, 5-HT2, and 5-HT3 receptors using WAY100635 (5-HT1A antagonist; 0.1 mg/kg i.p.), ketanserin (5-HT2A antagonist; 1 mg/kg, i.p.), or ondansetron (a 5-HT3R antagonist; 1 mg/kg i.p.) in mice. The extract (1, 10, and 30 mg/kg, i.p.) effectively reduced immobility duration in mice comparable to ketanserin and ondansetron in TST and FST as well as raised 5-HT levels in the brain. Molecular docking studies demonstrated that these compounds showed a strong interaction with 5-HT1 and 5-HT2 receptors with greater affinity and higher binding energy for 5-HT3R resulting in an antidepressant effect.80 Other in silico studies are also presented in Table 3. Another study along these lines evaluated gallic acid (GA, 13), which was derived from Phyllanthus emblica, Terminalia catappa Linn, and Crocus sativus, among other plant sources.81 It was discovered that GA was useful in controlling dopaminergic, serotonin, and adrenergic receptors. The mechanistic effects of GA (30 and 60 mg/kg, p.o.) in mice were examined using several pharmacological contexts involving certain 5-HT1A, 5-HT2A/2C, and 5-HT3R antagonists, such as NAN 190, ketanserin, and ondansetron, respectively. The higher dose 60 mg/kg GA was found to be effective to reduce the immobility time in TST and FST against above modulators which suggested that GA increased the 5-HT release in the synaptic cleft by interaction with 5-HT2R and 5HT3R.82
Table 3. In Silico Studies of Phytochemical Binding on 5-HT3R.
| S. No. | Constituents | Class | PDB ID | Binding Energy(kcal/mol) | Reference |
|---|---|---|---|---|---|
| 1. | Gingerols | Polyphenol | 4PIR | −8.7 | (145) |
| 2. | Shogaols | Polyphenol | 4PIR | − 8.31 | (145) |
| 3. | α-Lipoic acid | Organo sulfur | 6Y1Z | −5.4 | (87) |
| 4. | 5,7-Dihydroxy-3,6-dimethoxyflavone | Flavonoid | 4PIR | −8.15 | (80) |
| 5. | 5,7,4′-Trihydroxy-3′,5′dimethoxyflavone | Flavonoid | 4PIR | −7.90 | (80) |
| 6. | 5,7,4′-Trihydroxy-3′-methoxyflavone | Flavonoid | 4PIR | −7.39 | (80) |
| 7. | Kaempferol | Flavonoid | 6Y1Z | (103) | |
| 8. | Capsaicin | Alkaloid | −7.8 | (143) | |
| 9. | Psychotriasine | Alkaloid | 5AIN | −5.811 | (146) |
| 10. | Schisandrin C | Lignan | VHH15 | −5.62 | (144) |
| 11. | Meranzin hydrate | 6Y1Z | −8.3 | (88) | |
| 12. | Hesperidin | Flavonoid | 6Y1Z | −9.4 | (88) |
5-HT3R is widely distributed in the vagus nerve, nucleus tractus solitarius, guinea pig ileum, and peritoneal cells, among other places. Exposure to 5-HT activates these receptors.83 The antidepressant activity of Hypericum perforatum extract and its principal constituent, Hyperforin (14), was also explored using in vivo and in vitro models. In a research study employing a hypericum extract (CO2 extract) obtained through supercritical carbon dioxide extraction and hyperforin, notable spasmolytic effects were observed on the guinea pig ileum. The CO2 extract hindered 5-HT uptake in rat peritoneal cells. Specifically, hyperforin, the CO2 extract, and the ethanolic extract in peritoneal cells exhibited IC50 values of 3.4, 4.5, and 47.5 μg/mL, respectively, for inhibiting 5-HT uptake. Further, hyperforin exhibited superior inhibitory activity on guinea pig ileum contraction at concentrations of 0.1, 0.3, and 1 μg/mL, with inhibition constants of 10%, 80%, and 97%, respectively. Bezold-Jarisch (B-J) reflex is characterized as 5-HT-induced transient bradycardia resulting from the stimulation of 5-HT3R on the vagus nerve. Continuing the exploration of the B-J reflex, it was revealed that rats receiving only 5-HT bolus injections experienced a dose-dependent decrease in heart rates. Conversely, rats treated with hyperforin displayed less reduction in heart rate, possibly due to hyperforin’s 5-HT3R antagonist activity. The above in vitro investigation suggested that hyperforin can inhibit 5-HT reuptake with an IC50 value of 3.4 μg/mL. It also possesses 97% inhibitory activity on guinea pig ileum contraction at 1 μg/mL. It also reduced the 5-HT-induced bradycardia via 5-HT3R antagonism. These noteworthy findings regarding the in vitro 5-HT3R antagonism have set a promising direction for further research on hyperforin. Hyperforin (10 mg/kg) in rats significantly reduced immobility and increased % entries in the open arm in the behavioral despair test and elevated plus maze, which reflected its antidepressant and anxiolytic effects.84 The aforementioned research on antidepressant action has been thoroughly investigated and demonstrates that medicinal plants are useful not only for providing complicated or unique compounds but also for uncovering novel and therapeutically significant pharmacological pathways. The pathophysiological implications of 5-HT3R in depression have not been extensively investigated compared to those of 5-HT1A and 5-HT2 receptors. Nevertheless, literature data indicate that various types of antidepressants act as functional antagonists on 5-HT3R, suggesting that reducing 5-HT3R activity might play a role in the antidepressant mechanism of action.85 An extract of Schinus molle L. (Anacardiaceae) and its constituent rutin (15) (3 mg/kg p.o.) reduced immobility in FST and TST as well as the number of crossings in OFT. The suggested mechanism of action was due to the modulation of serotonergic, adrenergic, and dopaminergic supply. They further postulated that the effects of 5-HT1, 5HT2, and 5-HT3R primarily contribute to antidepressant action. They emphasized that 5-HT3R antagonism may be a crucial mediator for mitigating depression-like behavior in mice.86 α-Lipoic acid (14, ALA), a potent antioxidant commonly found in vegetables like spinach and cabbage, was found to increase % sucrose preference, decrease immobility duration, and restore levels of monoamines in the hippocampus. The docking study also revealed that ALA has a potent antagonistic effect on 5-HT3R, perhaps mitigating symptoms of depression. Furthermore, the fact that ALA’s nutraceutical qualities prevent stress-induced depression-like behavior is also highlighted.87Fructus Aurantii (FA) is the dried immature fruit of Citrus aurantium L. (Rutaceae). It contains multiple phytoconstituents that have neuroprotective effects. Meranzin hydrate (17, MH) and hesperidin (18) of FA demonstrated antidepressant action in behavioral and neurochemical paradigms and improvement in various biochemical indexes, including immobility time, gastric emptying, intestinal transit, corticotropin-releasing hormone CRH, ghrelin, ACTH, DA, noradrenaline NA, 5-HT, cortisol, and 5-HT3 receptor. These effects were achieved through regulation of the 5-HT3/growth hormone secretagogue receptor (GHSR) pathway. The study also validated the above-mentioned results using receptor antagonists and molecular docking, confirming the potential therapeutic mechanisms of MH in regulating the plasma biochemical indexes in rats with acute stress. The study also involved functional magnetic resonance imaging (fMRI) in providing anatomical and functional information about the active neural circuit regulating mood and gastroenteric motility. The study revealed the activation of excessive blood oxygen level-dependent (BOLD) signals in specific brain regions, including the left accumbens nucleus, left corpus callosum, and hypothalamus preoptic region, following acute stress stimuli. Additionally, it was found that FA and MH treatment reversed these BOLD signal changes to varying degrees.88Bacopa monnieri was tested for its effects on 5-HT receptors using antagonists of 5-HT1, 5-HT2, and 5-HT3 receptors. Furthermore, Bacopa monnieri (80 mg/kg p.o.) decreases immobility in FST and TST, which is indicative of antidepressant-like effects in albino mice. They proposed that 5-HT1 and 5-HT2, rather than 5-HT3R, receptors were responsible for Bacopa monnieri effects.89 Recently, using antagonists, apigenin (19, APG) was assessed for its effects on catecholaminergic and serotonergic systems. APG in mice at the dose of 50 mg/kg p.o significantly reduced immobility time in FST and TST as compared to PCPA and ondansetron. APG reduced immobility and increased swimming and climbing time better than fluoxetine (30 mg/kg).90
The phytoconstituents reported for antidepressant activity indicated that 5-HT3R antagonism is effective in treating depression like behavior. GA has demonstrated antidepressant effects by interacting with multiple 5-HT and dopamine receptors, including 5-HT3R, leading to increased 5-HT and dopamine release in the synaptic cleft. Hyperforin, derived, acts as a 5-HT3R antagonist, contributing to its antidepressant and anxiolytic effects. Similarly, ALA exerts its antidepressant activity by acting as a strong antagonist on 5-HT3R, thereby alleviating depression-like symptoms. Additionally, Meranzin hydrate + hesperidin (MH) demonstrated antidepressant effects by regulating the 5-HT3/growth hormone secretagogue receptor (GHSR) pathway. Among these molecules, GA and MH have been particularly well-investigated, showing promising results in preclinical studies.
Cognition
Cognitive decline encompasses a range of mental disorders involving impairments in learning, memory, attention, and challenges in language and motor skills. Evidence suggests the involvement of the cholinergic system is necessary for cognition and may be under the inhibitory control of 5-HT3R.91,92 Since 1990, it has been firmly established that 5-HT3R is implicated in cognitive processes. Studies have also been conducted on animal models of scopolamine-induced cognitive deficit in mice, rats, and marmosets, suggesting that 5-HT3R antagonism may reverse cognitive dysfunctions.93 Age-related decline in mental ability is also associated with decreased cholinergic neurotransmission in the CNS. Evidence suggests that inhibitory tone on the 5-HT receptor increases Ach release.94In vitro experiments have shown that 5-HT agonists decrease Ach release, whereas blocking 5-HT3R may increase Ach release and turnover in some brain regions. Ondansetron causing 5-HT3R blockade improves novel object recognition (NOR), reversal learning tasks, and cholinergic release. The action of 5-HT3R antagonists at such sites is suggested to facilitate cholinergic function and enhance the performance in cognition tests. The data strongly indicate that inhibiting the 5-HT3R pathway in the hippocampus, which affects cholinergic function, holds promise as a viable approach for enhancing cognitive function through various strategies.95
One more study conducted on rhesus monkeys using behavioral paradigms revealed that a low dose of ondansetron (0.00001–0.5 mg/kg, p.o.) improved the acquisition of visual object discrimination performance. This suggests that 5-HT3R antagonists might be beneficial in cognitive illnesses. In rats, another research study showed that azasetron injected bilaterally at doses of 0.32 and 1.0 μg/side significantly reduced working memory errors caused by intrahippocampal scopolamine administration at a 3.2 μg/side dose. Furthermore, cognitive dysfunction associated with depression was investigated in rats using autoradiography and NORT. Vortioxetine effectively binds to 5-HT3R with 95% occupancy and improved NORT. It was elucidated that these cognitive-enhancing effects of their 5-HT3R antagonist are likely mediated through cholinergic mechanisms. However, despite consistent findings in preclinical literature regarding the positive impact of 5-HT3R antagonists on memory function, it is crucial to note that ondansetron is considered to have limited clinical efficacy for improving cognitive function.96−98
Chronic administration of Zingiber officinale (Zingiberaceae) extract (50, 100, and 200 mg/kg, p.o.) improved memory function altered by morphine (5 mg/kg, i.p.) in male Wistar rats. The study suggested that these beneficial effects are attributed to the active constituents found in ginger, such as 6-Gingerol and Zingerone, which act on the 5-HT3R as an antagonist and inhibit cholinesterase activity.99 It was reported previously that scopolamine negatively affects cognitive functions in rats via modulation of 5-HT3R associated with α7 nAChR, leading to cholinergic manipulation. Behavioral and gene expression investigations were conducted to assess the impact of the rosmarinic acid (20)-rich extract of Ocimum basilicum L (Lamiaceae) on 5-HT3R.100 Scopolamine (0.5 mg/kg, i.v.) was injected into male Wistar rats and showed memory deficits in novel object discrimination in support of these elevated gene expressions of 5-HT3A R, NA7, M1, and nNOS leading to cognitive decline in the hippocampus of rats. At the same time, treatment with Ocimum basilicum L extract (200 and 400 mg/kg p.o.) for 14 days improved functions in the novel object recognition (NOR) test and restored cognitive functions by reducing expressions of 5-HT3AR, NA7, M1, and NOS levels. These results further corroborated the previously reported studies and supported the involvement of 5-HT3R in memory and cognitive processes. 5-HT3R has been observed to coexpress and mutually regulate with NA7 (Neuronal acetylcholine receptor subunit alpha-7, also known as nAChRα7). Notably, 5-HT3R shows significant expression in the hippocampus, prompting investigations into its role in learning and memory.101
Kaemferol (21), extracted from Cudriania tricuspidata leaves, exhibits promising cognitive enhancement effects in various experimental models-galactose/AlCl3-induced cognitive dysfunctions and chlorpyrifos-induced dementia. In mice, kaemferol (60 mg/kg i.p) demonstrated good blood–brain barrier permeability and improved cognitive function, escape latency, and distance of target quadrants in MWM via increasing monoamine levels (5-HT, DA, NE) and antioxidant effects, and reduced neuroinflammation in in vivo settings.102 Further studies on kaempferol using in vitro electrophysiological investigations were performed with Xenopus oocytes expressing the human 5-HT3AR to investigate the molecular relationship with IC50 12.8 ± 2.0 μM. The study confirmed a molecular-level interaction between kaempferol and 5-HT3AR. Notably, kaemferol exhibited concentration-dependent inhibition of 5-HT3AR, independent of voltage variations.103
Peppermint often contains high amounts of the monoterpenes menthone (22) and menthol. Essential oils and menthol independently affect GABAA, glycine, and 5-HT3R receptors in the in vitro study. They also showed cholinesterase inhibitory properties. These components of monoterpenes may have a variety of impacts that are directly related to brain function. Among these are the effects of essential oils on cholinesterase inhibition;104 menthol also affects GABAA and 5-HT3R receptors and negative allosteric modulation of GABAA receptors by carvone (23). Research has indicated that they can improve cognitive function and mitigate the increase in mental exhaustion linked to extended cognitive assessments.105
Ginkgo biloba has proven to be effective in various neurological illnesses, and it is a well-identified herb for interaction with the serotonergic system in memory-enhancing effects in the passive avoidance test.106 Bilobalide, a sesquiterpenoid lactone of Ginkgo biloba, is effective in C. elegans for 5-HT-mediated cholinergic modulation.107Ginkgo biloba was investigated in bisphenol A-induced cognitive deficit, where it demonstrated its effects via increasing hippocampal levels of DA, NE, and 5-HT and improved antioxidant potential, further protecting CA3 neurons.108 Its constituents, such as bilobalide (24) and Ginkgolide B (25), block 5-HT3AR expressed on Xenopus oocytes with an IC50 value for bilobalide of 470 μM and for Ginkgolide B of 730 μM. However, the binding sites of these molecules on 5-HT3R differ from that of granisetron. They were found to block the receptor pores.109,110 Radioligand binding studies have revealed the presence of 5-HT3R in the cholinergic-rich entorhinal cortex of the hippocampus. In vitro investigations have demonstrated that the activation of these 5-HT3Rs leads to a decrease in Ach release in the cerebral cortex, while using a 5-HT3R antagonist enhances the release of Ach.111 This explanation correlates with the fact that 5-HT3AR antagonism by Ginkgo biloba supports their correlation with cognition improvement and cholinergic enhancement. 5-HT3R blockade plays a pivotal role in cognitive function through cholinergic mediation. Experimental evidence suggests that inhibiting 5-HT3R enhances Ach release, highlighting the potential therapeutic significance of targeting this receptor in the modulation of cognitive processes and potential treatment avenues for cognitive disorders.
Various molecules have shown potential as cognitive enhancers through their interactions with 5-HT3R and modulation of cholinergic function. Notable candidates include Zingiber officinale extracts (containing 6-Gingerol and Zingerone), rosmarinic acid-rich extract of Ocimum basilicum L, kaemferol from Cudriania tricuspidata leaves, and compounds found in peppermint, such as menthol and menthone. Ginkgo biloba constituents like bilobalide and Ginkgolide B have also demonstrated 5-HT3R blockade activity. Among these, kaempferol and Ginkgo biloba constituents bilobalide and Ginkgolide B stand out as promising cognitive enhancers due to their ability to block 5-HT3R and their potential to enhance cholinergic function, which is crucial for cognitive processes. More studies are required in this direction.
Sleep
CNS is crucial in regulating sleep and is associated with neurotransmitters, including 5-HT and melatonin. An appropriate serotonergic supply ensures the adequate formation of melatonin, which is responsible for sound sleep. Sleep is regulated by two process rapid eye movement (REM) and non rapid eye movement (NREM). REM is define as rapid movement of the eyes, vivid dreams, increased brain activity and NREM is a sleep phase that consists of three stages, ranging from light to deep sleep. It is important for physical restoration, energy conservation, and memory consolidation. NREM sleep accounts for about 75−80% of total sleep time.112 Previously conducted studies in relation to 5-HT3R and sleep were discussed. It was proven that activation of 5-HT3R by the selective agonist m-CPBG has been shown to decrease the REM in rats. This proposed effect is related to the activation of glutamatergic interneurons expressing 5-HT3R, leading to an increase in 5-HT release at postsynaptic sites involved in the induction of REM.113 Another study explained the effect of zacopride, a 5-HT3R antagonist, effective in treating sleep apnea.114 Moreover, antidepressants such as vortioxetine and paroxetine increase NREM;115 a randomized clinical trial was conducted on Rosmarinus officinalis (RO) leaves for patients with withdrawal syndrome from opium abuse and sleep disturbances. The study demonstrated that patients administered RO capsules (16 mg) improved sleep and reduced insomnia via serotonergic interaction.116,117 Suanzaorentang, a traditional Chinese herbal remedy, consists of Anemarrhenae rhizoma, Poria cocos, Zizyphi spinosi semen, Ligusticum wallichii, and glycyrrhizae in a ratio of 4:2:2:2:1. This herbal remedy is believed to regulate sleep by exerting an antagonistic effect on 5-HT3R, which is involved in sleep regulation.118
Numerous studies have demonstrated the efficacy of synthetic drugs on 5-HT3R antagonism in addressing sleep-related difficulties. A couple of studies were conducted using phytoconstituents; however, there remains a paucity of research investigating the role of phytoconstituents in treating sleep disorders through modulation of 5-HT3R. Considering the side effects and habituation involved with synthetic drugs, the need for further exploration and attention toward phytochemicals in the context of sleep regulation via 5-HT3R is highly desired.
Alzheimer’s Disease (AD)
It has been found that 5-HT interacts with 5-HT3R in the hippocampus, a brain region involved in memory and learning. Activation of 5-HT3R receptors can decrease the brain’s cholinergic tone, exacerbating cognitive deficits in AD. The recent development of a novel molecule (R3487/MEM3454) with dual properties of 5-HT3R blocking and cholinesterase inhibitory activity holds promise as a potential therapeutic approach for AD. Acetylcholinesterase inhibitors (AChEIs) are currently the mainstay of treatment for AD, as they help increase the levels of Ach in the brain by blocking its degradation. They have shown improved attentional performance in rats with AD-like conditions.119
Moreover, there is evidence that ondansetron, a known 5-HT3R antagonist, can potentiate the effects of donepezil, an AChEI.120 Furthermore, RG3487, an α7-nAChR partial agonist/5-HT3 antagonist, demonstrated a noteworthy increase in cortical and hippocampal DA and ACh effluxes upon acute administration.121 This finding suggests that combining 5-HT3R antagonism with AChEIs might synergistically improve cognitive function in AD patients. Elevated HTR3A positive interneurons levels were observed in the brains of transgenic AD (Tg AD) mice and post-mortem AD patients, preceding Aβ plaque appearance. 5-HT3R is a Ca2+ permeable ionotropic receptor with heightened presynaptic activity in the hippocampus, leading to increased basal Ca2+ levels in the hippocampus and cortex. Chronic tropisetron administration mitigated cognitive deficits and reduced Aβ plaque levels in Tg AD mice, likely via an HTR3-dependent mechanism and by attenuating Ca2+ and calcineurin signaling.122
The presence of the β-amyloid protein significantly influences the pathogenesis of AD, which exacerbates several neuronal malfunctions. The in vitro study has shown β-amyloid 25–35 protein (10 μM) induced glutamate excitotoxicity and elevated calcium release, ROS, and caspase-3 leading to neuronal death. Such effects were observed to be reduced by 5-HT3R antagonists tropanyl-3,5-dichlorobenzoate (MDL72222, 0.1–10 μM). The study also suggested that 100 μM m-CPBG, an agonist of 5-HT3R, resists the neuroprotective effects of MDL72222.123 This study notifies the crucial role of 5-HT3R antagonists in AD, attenuating neurotoxic effects, and provides neuroprotection.
Many synthetic substances have been examined in rodents and in patients for their potential to alleviate AD by interacting with 5-HT3R. These substances have helped to reduce AD symptoms induced by scopolamine and amyloid beta. Although the advantages of 5-HT3R antagonist therapy for AD are widely known, no studies on phytoconstituents and 5-HT3R interactions have yet been documented. Consequently, future studies should investigate whether or not phytoconstituents can reduce AD by interacting with 5-HT3R in animal models.
Epilepsy
Epilepsy involves sudden abnormal neuronal discharges and is characterized by neuroinflammation and oxidative stress. An imbalance between glutamate and GABA plays a role in its pathogenesis. Past research data also pointed to the serotonergic supply, specifically 5-HT3R, which could be correlated with epilepsy.124 A study described the potential neuroprotective and antiepileptic benefits of hydroethanolic extract of Zingiber officinale (Zingiberaceae) (25, 50, 100 mg/kg, i.p.) in PTZ-induced tonic-clonic seizures in mice. The study discussed the antagonism of 5-HT3R involved in releasing various neurotransmitters, including GABA. It also inhibited calcium release, contributing to fast excitatory synaptic transmission in the CNS.
Ginger extract has a potency to diminish the PTZ-induced seizures via modulation of 5-HT3R.125 Some other natural 5-HT3R antagonists are effective in PTZ-induced seizures in ovariectomized mice. Genistein (10 mg/kg, i.p.), a flavonoid obtained from soya or soya products, provides phytoestrogen and exhibits estrogenic activity. Administering estrogen to ovariectomized female rats demonstrates neuroprotective effects against hippocampal damage induced by status epilepticus and was found to increase the seizure threshold, thereby reducing the chances of epileptic attacks. Similarly, administration of m-CPBG, a 5-HT3R agonist, supports the anticonvulsant effects of genistein by increasing the seizure threshold. In contrast, the tropisetron (1 mg/kg, i.p.) 5-HT3R antagonist reversed the anticonvulsant effects of genistein. Such results indicated that genistein provided potential antiepileptic effects in the PTZ-induced ovariectomized mice model via serotonergic pathways and 5-HT3R modulation.126 Traditional Chinese medicine reports pearl powder treatment for convulsion and tranquilization effects. Zhang et al. reported prevention of PTZ-induced generalized myoclonic seizures, reduced locomotor activity, and increased convulsion latency observed in mice in the pearl treatment group. These effects were further corroborated by receptor expression, measured by immunohistochemistry. It showed decreased 5-HT3R and enhanced GABA release, supporting the anticonvulsant properties of conventional pearl powder.127
Molecules targeting 5-HT3R have shown potential in treating epilepsy by modulating neurotransmitter release and inhibiting calcium influx, thus reducing seizure activity.
Dyskinesia
Levodopa is the standard treatment for PD. However, its chronic use leads to dyskinesia.128 The strategy to treat levodopa-induced dyskinesia (LID) involves modulation of NMDA and 5-HT receptors. AV101 (L-4-Chlorokinurenine) is an NMDA antagonist that reduced dyskinesia in primates, and proof of concept for the phase II trial in 20 PD patients continues.128,129 The growing evidence suggests that 5-HT3R might be involved in the development of dyskinesia.130 5-HT3R is present in the basal ganglia, and its stimulation shows DA release.131,132 Many studies have demonstrated that selective blockade of 5-HT3R with ondansetron and granisetron reduces the LID without affecting the antiparkinsonian activity of levodopa. Blockade of 5-HT3R reduces the DA release within the basal ganglia, suggesting that it could mitigate the aberrant DA release that characterizes the dyskinetic state.133,134 Selective blockade of 5-HT3R with medications like ondansetron and granisetron has been shown to reduce LID without affecting the antiparkinsonian activity of levodopa. This suggests that targeting 5-HT3R could potentially mitigate the aberrant DA release associated with dyskinesia, making it a molecule with significant potential for managing LID in PD patients.
It is noteworthy that several pharmacological agents that target 5-HT3R have demonstrated potential in reducing LID in PD. There is a dearth of research examining the potential effects of phytochemicals on dyskinesia through 5-HT3R modulation.
Conclusion and Future Perspectives
Exploring natural approaches for treating neurological disorders, particularly by targeting 5-HT3R modulation through phytochemicals, holds significant promise for advancing our understanding of and managing these complex conditions. Phytochemicals, owing to their safety and cost-effectiveness and, additionally, higher lipophilic balance, readily traverse the BBB, influencing 5-HT3R and impacting vital neurotransmitter systems such as GABA, α7-nAChR, NK-R, 5-HT1A, and D2. This broad influence implicates them in alleviating a spectrum of symptoms ranging from nausea, vomiting, and cognitive impairment to conditions such as AD, sleep disorders, depression, anxiety, and epilepsy. The antagonistic action of phytochemicals on 5-HT3R plays a pivotal role in regulating calcium release, inhibiting SP action, and mitigating inflammatory responses and oxidative stress. Consequently, these mechanisms contribute to a reduction in mitochondrial damage and caspase-3 activity, offering potential avenues for therapeutic intervention. Many constituents, such as gingerol, galanolactone, citronellol, geraniol, CBD, THC, eugenol, and vanillin, are reported to inhibit nausea and vomiting due to their 5-HT3R antagonism. Similarly, zerumbone, resveratrol, carvacrol, gallic acid, genistein, resveratrol, and alpha-lipoic acid are found to be neuroprotective.
However, despite the promising implications, the current body of evidence exploring the effects of phytochemicals on 5-HT3R is limited. Urgent attention is warranted for comprehensive preclinical and clinical studies to deepen our understanding of the therapeutic benefits these compounds may offer. The challenges of identifying optimal dosages, potential side effects associated with these molecules, and the development of standardized extracts may hamper the translation of these molecules into effective therapeutic strategies. Also, safety pharmacology studies and toxicity testing of these phytoconstituents are needed. Such investigations can shed light on their potential efficacy in treating not only common symptoms like nausea and cognitive impairment but also addressing more complex disorders like AD, dyskinesia, and epilepsy. Future perspectives should elucidate the specific molecular pathways and signaling cascades involved in the interaction between phytochemicals and 5-HT3R.
Glossary
Abbreviations
- 5-HT3AR
5-Hydroxytryptamine receptor 3A
- 5-HT
5-Hydroxytryptophan
- ALA
Alpha-lipoic acid
- AChEIs
Cholinesterase inhibitors
- α7-nAChR
α7-nicotinic acetylcholine receptor
- AD
Alzheimer’s disease
- B-J
Bezold-Jarisch
- CBD
Cannabidiol
- CNS
Central nervous system
- CINV
Chemotherapy-induced nausea vomiting
- CA1
Cornu Ammonis 1
- CA2
Cornu Ammonis 2
- dEMG
Digastric muscle electromyograms
- cGMP
Cyclic guanosine 3′,5′-monophosphate
- FDA
The Food and Drug Administration
- GABA
γ-Aminobutyric acid
- GA
Gallic acid
- GLE
Ganoderma lucidum extract
- GIT
Gastrointestinal tract
- FST
Force swim test
- KATP
ATP-sensitive potassium channels
- LID
Levodopa induced dyskinesia
- m-CPBG
Meta-chlorophenyl-biguanide
- NKR
Neurokinin-2 receptor
- NOS
Nitric oxide synthase
- NO
Nitric oxide
- NMDA
N-Methyl-d-aspartate
- NA7
Neuronal acetylcholine receptor subunit alpha 7
- OFT
Open field test
- PD
Parkinson disease
- PET
Positron emission tomography
- PNS
Peripheral nervous system
- PTZ
Pentylenetetrazole
- SP
Substance P
- RO
Rosmarinus officinalis
- TPEO
Thymus persicus aerial parts essential oil
- THC
Tetrahydrocannabinol
- TST
Tail suspension test
Author Contributions
Likhit Akotkar: Data curation, formal analysis, investigation, writing-original draft; Urmila Aswar: Conceptualization, validation, writing-review and editing and supervision; Ankit Ganeshpurkar: Data curation, formal analysis, reviewing, editing, drawing, and drafting of MS; Kundlik Rathod: Compilation of data, writing-original draft; Pradnya Bagad: Data curation, formal analysis, editing, drawing, and drafting of manuscript; Shailendra Gurav: Conceptualization and design of the study, validation, writing-review and editing.
The authors declare no competing financial interest.
References
- Hoyer D.; Hannon J. P.; Martin G. R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol., Biochem. Behav. 2002, 71, 533–554. 10.1016/S0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Pazos A.; Palacios J. M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985, 346, 205–230. 10.1016/0006-8993(85)90856-X. [DOI] [PubMed] [Google Scholar]
- Hoyer D.; Pazos A.; Probst A.; Palacios J. M. Serotonin receptors in the human brain. II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Res. 1986, 376, 97–107. 10.1016/0006-8993(86)90903-0. [DOI] [PubMed] [Google Scholar]
- Xu T.; Pandey S. C. Cellular localization of serotonin(2A) (5HT(2A)) receptors in the rat brain. Brain Res. Bull. 2000, 51, 499–505. 10.1016/S0361-9230(99)00278-6. [DOI] [PubMed] [Google Scholar]
- Kilpatrick G. J.; Jones B. J.; Tyers M. B. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature 1987, 330, 746–748. 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- Waeber C.; Sebben M.; Nieoullon A.; Bockaert J.; Dumuis A. Regional distribution and ontogeny of 5-HT4 binding sites in rodent brain. Neuropharmacology 1994, 33, 527–541. 10.1016/0028-3908(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Plassat J.-L.; Boschert U.; Amlaiky N.; Hen R. The mouse 5HT5 receptor reveals a remarkable heterogeneity within the 5HT1D receptor family. EMBO journal 1992, 11, 4779–4786. 10.1002/j.1460-2075.1992.tb05583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. C.; Reavill C.; East S. Z.; Harrison P. J.; Patel S.; Routledge C.; Leslie R. A. The distribution of 5-HT6 receptors in rat brain: an autoradiographic binding study using the radiolabelled 5-HT6 receptor antagonist [125I] SB-258585. Brain research 2002, 934, 49–57. 10.1016/S0006-8993(02)02360-0. [DOI] [PubMed] [Google Scholar]
- Gustafson E. L.; Durkin M. M.; Bard J. A.; Zgombick J.; Branchek T. A. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br. J. Pharmacol. 1996, 117, 657–666. 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdère P.; Di Giovanni G. Serotonin in health and disease. Int. J. Mol. Sci. 2020, 21, 3500. 10.3390/ijms21103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagheddu C.; Aroni S.; De Felice M.; Lecca S.; Luchicchi A.; Melis M.; Muntoni A. L.; Romano R.; Palazzo E.; Guida F.; Maione S.; Pistis M. Enhanced serotonin and mesolimbic dopamine transmissions in a rat model of neuropathic pain. Neuropharmacology 2015, 97, 383–393. 10.1016/j.neuropharm.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Botrel T. E.; Clark O. A.; Clark L.; Paladini L.; Faleiros E.; Pegoretti B. Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support Care Cancer 2011, 19, 823–832. 10.1007/s00520-010-0908-8. [DOI] [PubMed] [Google Scholar]
- Coates M. D.; Tekin I.; Vrana K. E.; Mawe G. M. Review article: the many potential roles of intestinal serotonin (5-hydroxytryptamine, 5-HT) signalling in inflammatory bowel disease. Aliment Pharmacol Ther 2017, 46, 569–580. 10.1111/apt.14226. [DOI] [PubMed] [Google Scholar]
- Alvarado D. M.; Ciorba M. A. Serotonin Receptors Regulate Inflammatory Response in Experimental Colitis. J. Nutr. 2020, 150, 1678–1679. 10.1093/jn/nxaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z.-C.; Qi W.-J.; Wang J.-Y.; Luo F. Chronic administration of 5-HT1A receptor agonist relieves depression and depression-induced hypoalgesia. Scientific World Journal 2014, 2014, 1. 10.1155/2014/405736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bétry C.; Pehrson A. L.; Etiévant A.; Ebert B.; Sánchez C.; Haddjeri N. The rapid recovery of 5-HT cell firing induced by the antidepressant vortioxetine involves 5-HT3 receptor antagonism. International Journal of Neuropsychopharmacology 2013, 16, 1115–1127. 10.1017/S1461145712001058. [DOI] [PubMed] [Google Scholar]
- Nichols D. E.; Nichols C. D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- Kwan C.; Lévesque C.; Bédard D.; Frouni I.; Yesuf J. M.; Hamadjida A.; Lévesque D.; Clarke P. B.; Huot P. Autoradiographic labelling of 5-HT3 receptors in the hemi-parkinsonian rat brain. Neuroscience Research 2022, 177, 135–144. 10.1016/j.neures.2021.12.004. [DOI] [PubMed] [Google Scholar]
- Mu L.; Müller Herde A.; Ruefli P. M.; Sladojevich F.; Milicevic Sephton S.; Kramer S. D.; Thompson A. J.; Schibli R.; Ametamey S. M.; Lochner M. Synthesis and Pharmacological Evaluation of [11C] granisetron and [18F] fluoropalonosetron as PET Probes for 5-HT3 Receptor Imaging. ACS chemical neuroscience 2016, 7, 1552–1564. 10.1021/acschemneuro.6b00192. [DOI] [PubMed] [Google Scholar]
- Miquel M. C.; Emerit M. B.; Nosjean A.; Simon A.; Rumajogee P.; Brisorgueil M. J.; Doucet E.; Hamon M.; Verge D. Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. Eur. J. Neurosci 2002, 15, 449–457. 10.1046/j.0953-816x.2001.01872.x. [DOI] [PubMed] [Google Scholar]
- Faerber L.; Drechsler S.; Ladenburger S.; Gschaidmeier H.; Fischer W. The neuronal 5-HT3 receptor network after 20 years of research—evolving concepts in management of pain and inflammation. European journal of pharmacology 2007, 560, 1–8. 10.1016/j.ejphar.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. M.; Herbrechter R.; Ziemba P. M.; Lepke P.; Beltran L.; Hatt H.; Werner M.; Gisselmann G. Kampo Medicine: Evaluation of the Pharmacological Activity of 121 Herbal Drugs on GABAA and 5-HT3A Receptors. Front Pharmacol 2016, 7, 219. 10.3389/fphar.2016.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedtke R.; Bell-Horner C.; Volk M.; Reinecke M.; Dillon G. Pharmacological survey of medicinal plants for activity at ligand-gated ion channels: Selective interaction with 5-HT3 receptors. Pharmaceutical biology 2004, 42, 73–82. 10.1080/13880200490505654. [DOI] [Google Scholar]
- Sheikhi M. A.; Ebadi A.; Talaeizadeh A.; Rahmani H. Alternative methods to treat nausea and vomiting from cancer chemotherapy. Chemotherapy research and practice 2015, 2015, 1. 10.1155/2015/818759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahara J.; Rong H. Q.; Iwamoto M.; Kobayashi G.; Matsuda H.; Fujimura H. Active components of ginger exhibiting anti-serotonergic action. Phytotherapy Research 1989, 3, 70–71. 10.1002/ptr.2650030208. [DOI] [Google Scholar]
- Lumb A. B. Mechanism of antiemetic effect of ginger. Anaesthesia 1993, 48, 1118. 10.1111/j.1365-2044.1993.tb07572.x. [DOI] [PubMed] [Google Scholar]
- Huang Q. R.; Iwamoto M.; Aoki S.; Tanaka N.; Tajima K.; Yamahara J.; Takaishi Y.; Yoshida M.; Tomimatsu T.; Tamai Y. Anti-5-hydroxytryptamine3 effect of galanolactone, diterpenoid isolated from ginger. Chem. Pharm. Bull. (Tokyo) 1991, 39, 397–399. 10.1248/cpb.39.397. [DOI] [PubMed] [Google Scholar]
- Bone M. E.; Wilkinson D. J.; Young J. R.; McNeil J.; Charlton S. Ginger root--a new antiemetic. The effect of ginger root on postoperative nausea and vomiting after major gynaecological surgery. Anaesthesia 1990, 45, 669–671. 10.1111/j.1365-2044.1990.tb14395.x. [DOI] [PubMed] [Google Scholar]
- Thamlikitkul L.; Srimuninnimit V.; Akewanlop C.; Ithimakin S.; Techawathanawanna S.; Korphaisarn K.; Chantharasamee J.; Danchaivijitr P.; Soparattanapaisarn N. Efficacy of ginger for prophylaxis of chemotherapy-induced nausea and vomiting in breast cancer patients receiving adriamycin–cyclophosphamide regimen: a randomized, double-blind, placebo-controlled, crossover study. Supportive Care in Cancer 2017, 25, 459–464. 10.1007/s00520-016-3423-8. [DOI] [PubMed] [Google Scholar]
- Ryan J. L.; Heckler C. E.; Roscoe J. A.; Dakhil S. R.; Kirshner J.; Flynn P. J.; Hickok J. T.; Morrow G. R. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Supportive care in cancer 2012, 20, 1479–1489. 10.1007/s00520-011-1236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manusirivithaya S.; Sripramote M.; Tangjitgamol S.; Sheanakul C.; Leelahakorn S.; Thavaramara T.; Tangcharoenpanich K. Antiemetic effect of ginger in gynecologic oncology patients receiving cisplatin. International Journal of Gynecologic Cancer 2004, 14, 1063. 10.1111/j.1048-891X.2004.14603.x. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Saadat A.; Sahebkar A.; Hashemian F.; Taghikhani M.; Abolhasani E. Effect of ginger on acute and delayed chemotherapy-induced nausea and vomiting: a pilot, randomized, open-label clinical trial. Integrative cancer therapies 2012, 11, 204–211. 10.1177/1534735411433201. [DOI] [PubMed] [Google Scholar]
- Pillai A. K.; Sharma K. K.; Gupta Y. K.; Bakhshi S. Anti-emetic effect of ginger powder versus placebo as an add-on therapy in children and young adults receiving high emetogenic chemotherapy. Pediatric blood & cancer 2011, 56, 234–238. 10.1002/pbc.22778. [DOI] [PubMed] [Google Scholar]
- Sontakke S.; Thawani V.; Naik M. Ginger as an antiemetic in nausea and vomiting induced by chemotherapy: A randomized, cross-over, double blind study. Indian journal of pharmacology 2003, 35, 32–36. [Google Scholar]
- Zick S. M.; Ruffin M. T.; Lee J.; Normolle D. P.; Siden R.; Alrawi S.; Brenner D. E. Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Supportive care in cancer 2009, 17, 563–572. 10.1007/s00520-008-0528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon A. D. Palonosetron (Aloxi): a second-generation 5-HT3 receptor antagonist for chemotherapy-induced nausea and vomiting. Baylor University Medical Center Proceedings 2006, 19, 413–416. 10.1080/08998280.2006.11928210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashoor A.; Nordman J. C.; Veltri D.; Yang K. H.; Shuba Y.; Al Kury L.; Sadek B.; Howarth F. C.; Shehu A.; Kabbani N.; Oz M. Menthol inhibits 5-HT3 receptor-mediated currents. J. Pharmacol Exp Ther 2013, 347, 398–409. 10.1124/jpet.113.203976. [DOI] [PubMed] [Google Scholar]
- Heimes K.; Hauk F.; Verspohl E. J. Mode of action of peppermint oil and (−)-menthol with respect to 5-HT3 receptor subtypes: binding studies, cation uptake by receptor channels and contraction of isolated rat ileum. Phytotherapy Research 2011, 25, 702–708. 10.1002/ptr.3316. [DOI] [PubMed] [Google Scholar]
- Wang C.-Z.; Basila D.; Aung H. H.; Mehendale S. R.; Chang W.-T.; McEntee E.; Guan X.; Yuan C.-S. Effects of Ganoderma lucidum extract on chemotherapy-induced nausea and vomiting in a rat model. American Journal of Chinese Medicine 2005, 33, 807–815. 10.1142/S0192415X05003429. [DOI] [PubMed] [Google Scholar]
- Ziemba P. M.; Schreiner B. S.; Flegel C.; Herbrechter R.; Stark T. D.; Hofmann T.; Hatt H.; Werner M.; Gisselmann G. Activation and modulation of recombinantly expressed serotonin receptor type 3A by terpenes and pungent substances. Biochem. Biophys. Res. Commun. 2015, 467, 1090–1096. 10.1016/j.bbrc.2015.09.074. [DOI] [PubMed] [Google Scholar]
- Yang K. H.; Galadari S.; Isaev D.; Petroianu G.; Shippenberg T. S.; Oz M. The nonpsychoactive cannabinoid cannabidiol inhibits 5-hydroxytryptamine3A receptor-mediated currents in Xenopus laevis oocytes. J. Pharmacol Exp Ther 2010, 333, 547–554. 10.1124/jpet.109.162594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock E. M.; Parker L. A. Synergy between cannabidiol, cannabidiolic acid, and Δ9-tetrahydrocannabinol in the regulation of emesis in the Suncus murinus (house musk shrew). Behavioral neuroscience 2015, 129, 368. 10.1037/bne0000057. [DOI] [PubMed] [Google Scholar]
- Bappi M. H.; Prottay A. A. S.; Al-Khafaji K.; Akbor M. S.; Hossain M. K.; Islam M. S.; Asha A. I.; Medeiros C. R.; Tahim C. M.; Lucetti E. C. P. Antiemetic effects of sclareol, possibly through 5-HT3 and D2 receptor interaction pathways: In-vivo and in-silico studies. Food Chem. Toxicol. 2023, 181, 114068 10.1016/j.fct.2023.114068. [DOI] [PubMed] [Google Scholar]
- Smith H. S.; Cox L. R.; Smith E. J. 5-HT3 receptor antagonists for the treatment of nausea/vomiting. Ann. Palliat Med. 2012, 1, 115–120. 10.3978/j.issn.2224-5820.2012.07.07. [DOI] [PubMed] [Google Scholar]
- Zieglgansberger W. Substance P and pain chronicity. Cell Tissue Res. 2019, 375, 227–241. 10.1007/s00441-018-2922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. A.; Taylor G. E.; Weinreich D. Serotonin unmasks functional NK-2 receptors in vagal sensory neurones of the guinea-pig. Journal of Physiology 1999, 514, 111–124. 10.1111/j.1469-7793.1999.111af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. A.; Oh E. J.; Weinreich D. 5-HT3 receptors mediate inflammation-induced unmasking of functional tachykinin responses in vitro. J. Appl. Physiol. 2002, 92, 2529–2534. 10.1152/japplphysiol.00974.2001. [DOI] [PubMed] [Google Scholar]
- Gu M.; Miyoshi K.; Dubner R.; Guo W.; Zou S.; Ren K.; Noguchi K.; Wei F. Spinal 5-HT3 receptor activation induces behavioral hypersensitivity via a neuronal-glial-neuronal signaling cascade. J. Neurosci. 2011, 31, 12823–12836. 10.1523/JNEUROSCI.1564-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chandrasekar S.; Divakar K.; Pawar A.; Goli D.; Harisha R.; Bhanumathy M.; Sushma G.; Shivaprasad H. Involvement of Opioid, Adenosine and 5-HT3 Receptors in Antinociceptive Effects of an Ayurvedic Polyherbal Formulation. Medical Principles and Practice 2010, 20, 66–70. 10.1159/000319763. [DOI] [PubMed] [Google Scholar]
- Abed D. Z.; Sadeghian R.; Mohammadi S.; Akram M. Thymus persicus (Ronniger ex Rech. f.) Jalas alleviates nociceptive and neuropathic pain behavior in mice: Multiple mechanisms of action. J. Ethnopharmacol 2022, 283, 114695 10.1016/j.jep.2021.114695. [DOI] [PubMed] [Google Scholar]
- Lee J. H.; Min D.; Lee D.; Kim W. Zingiber officinale Roscoe Rhizomes Attenuate Oxaliplatin-Induced Neuropathic Pain in Mice. Molecules 2021, 26, 548. 10.3390/molecules26030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia J. S. M.; Farouk A. A. O.; Mohamad A. S.; Sulaiman M. R.; Perimal E. K. Zerumbone alleviates chronic constriction injury-induced allodynia and hyperalgesia through serotonin 5-HT receptors. Biomedicine & Pharmacotherapy 2016, 83, 1303–1310. 10.1016/j.biopha.2016.08.052. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Oatway M. A.; Weaver L. C. Blockade of the 5-HT3 receptor for days causes sustained relief from mechanical allodynia following spinal cord injury. J. Neurosci Res. 2009, 87, 418–424. 10.1002/jnr.21860. [DOI] [PubMed] [Google Scholar]
- McCleane G. J.; Suzuki R.; Dickenson A. H. Does a single intravenous injection of the 5HT3 receptor antagonist ondansetron have an analgesic effect in neuropathic pain? A double-blinded, placebo-controlled cross-over study. Anesthesia & Analgesia 2003, 97, 1474–1478. 10.1213/01.ANE.0000085640.69855.51. [DOI] [PubMed] [Google Scholar]
- Hirata K.; Nishiki Y.; Goto R.; Inagaki M.; Oshima K.; Shimazu Y.; Takeda M. Resveratrol suppresses nociceptive jaw-opening reflex via 5HT(3) receptor-mediated GABAergic inhibition. Neurosci Res. 2020, 160, 25–31. 10.1016/j.neures.2019.10.012. [DOI] [PubMed] [Google Scholar]
- Yokomi H.; Kato T.; Narasaki S.; Kamiya S.; Taguchi S.; Horikawa Y. T.; Tsutsumi Y. M. The Serotonin-Mediated Anti-Allodynic Effect of Yokukansan on Paclitaxel-Induced Neuropathic Pain. Medicina (Kaunas) 2024, 60, 359. 10.3390/medicina60030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison L. E.; Woodman S. E.; Durham P. L. 5-HT3/7 and GABA(B) receptors mediate inhibition of trigeminal nociception by dietary supplementation of grape seed extract. Nutr Neurosci 2022, 25, 1565–1576. 10.1080/1028415X.2021.1880211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saria A.; Javorsky F.; Humpel C.; Gamset R. 5-HT3 receptor antagonists inhibit sensory neuropeptide release from the rat spinal cord. Neuroreport 1990, 1, 104–106. 10.1097/00001756-199010000-00005. [DOI] [PubMed] [Google Scholar]
- Olivier B.; van Wijngaarden I.; Soudijn W. 5-HT3 receptor antagonists and anxiety; a preclinical and clinical review. European Neuropsychopharmacology 2000, 10, 77–95. 10.1016/S0924-977X(99)00065-6. [DOI] [PubMed] [Google Scholar]
- Lopes L. T.; Canto-de-Souza L.; Baptista-de-Souza D.; de Souza R. R.; Nunes-de-Souza R. L.; Canto-de-Souza A. The interplay between 5-HT2C and 5-HT3A receptors in the dorsal periaqueductal gray mediates anxiety-like behavior in mice. Behavioural Brain Research 2022, 417, 113588 10.1016/j.bbr.2021.113588. [DOI] [PubMed] [Google Scholar]
- Costall B.; Domeney A. M.; Kelly M. E.; Tomkins D. M.; Naylor R. J.; Wong E. H.; Smith W. L.; Whiting R. L.; Eglen R. M. The effect of the 5-HT3 receptor antagonist, RS-42358–197, in animal models of anxiety. Eur. J. Pharmacol. 1993, 234, 91–99. 10.1016/0014-2999(93)90710-Y. [DOI] [PubMed] [Google Scholar]
- Gargiulo P. A.; Viana M. B.; Graeff F. G.; Silva M. A.; Tomaz C. Effects of anxiety and memory of systemic and intra-amygdala injection of 5-HT3 receptor antagonist BRL 46470A. Neuropsychobiology 1996, 33, 189–195. 10.1159/000119276. [DOI] [PubMed] [Google Scholar]
- Rodgers R.; Cole J.; Tredwell J. M. Profile of action of 5-HT 3 receptor antagonists, ondansetron and WAY 100289, in the elevated plus-maze test of anxiety of mice. Psychopharmacology 1995, 117, 306–312. 10.1007/BF02246105. [DOI] [PubMed] [Google Scholar]
- Akanji M. A.; Elijah O. O.; Oyedolapo A. A.; Aderonke A. A.; Opeoluwa O. L.; Omoloye A. A.; Oladoja F. A.; Olatundun S. O.; Edatomolaosi O. L. Datura stramonium abrogates depression- and anxiety-like disorders in mice: possible involvement of monoaminergic pathways in its antidepressant activity. Drug Metab Pers Ther 2022, 37, 305–314. 10.1515/dmpt-2021-0166. [DOI] [PubMed] [Google Scholar]
- Stephanie N. K. J.; Gisele N. N.; Clarisse M. O. F.; Germain T. S.; Neteydji S.; Simon P.; Rigobert A. M. E.; Elisabeth N. B. ANXIOLYTIC - LIKE PROPERTIES OF Hallea ciliata IN MICE. Afr J. Tradit Complement Altern Med. 2016, 13, 1–7. 10.21010/ajtcam.v13i4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwakarma S.; Pal S.; Kasture V. S.; Kasture S. Anxiolytic and antiemetic activity of Zingiber officinale. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 2002, 16, 621–626. 10.1002/ptr.948. [DOI] [PubMed] [Google Scholar]
- Topic B.; Hasenohrl R. U.; Hacker R.; Huston J. P. Enhanced conditioned inhibitory avoidance by a combined extract of Zingiber officinale and Ginkgo biloba. Phytother Res. 2002, 16, 312–315. 10.1002/ptr.870. [DOI] [PubMed] [Google Scholar]
- Bétry C.; Etiévant A.; Oosterhof C.; Ebert B.; Sanchez C.; Haddjeri N. Role of 5-HT3 receptors in the antidepressant response. Pharmaceuticals 2011, 4, 603–629. 10.3390/ph4040603. [DOI] [Google Scholar]
- Fawcett J.; Barkin R. L. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J. Affect Disord 1998, 51, 267–285. 10.1016/S0165-0327(98)00224-9. [DOI] [PubMed] [Google Scholar]
- Schweizer E.; Rickels K.; Hassman H.; Garcia-Espana F. Buspirone and imipramine for the treatment of major depression in the elderly. J. Clin Psychiatry 1998, 59, 175–183. 10.4088/JCP.v59n0406. [DOI] [PubMed] [Google Scholar]
- Kohler S.; Cierpinsky K.; Kronenberg G.; Adli M. The serotonergic system in the neurobiology of depression: Relevance for novel antidepressants. J. Psychopharmacol 2016, 30, 13–22. 10.1177/0269881115609072. [DOI] [PubMed] [Google Scholar]
- Park Y. S.; Oh H.; Sung K. W. Atypical antidepressant mirtazapine inhibits 5-hydroxytryptamine3 receptor currents in NCB-20 cells. J. Pharmacol Sci. 2023, 151, 63–71. 10.1016/j.jphs.2022.12.002. [DOI] [PubMed] [Google Scholar]
- Gonda X.; Sharma S. R.; Tarazi F. I. Vortioxetine: a novel antidepressant for the treatment of major depressive disorder. Expert Opin Drug Discov 2019, 14, 81–89. 10.1080/17460441.2019.1546691. [DOI] [PubMed] [Google Scholar]
- Aswar U.; Shende H.; Aswar M. Buspirone, a 5-HT1A agonist attenuates social isolation-induced behavior deficits in rats: a comparative study with fluoxetine. Behav Pharmacol 2022, 33, 309–321. 10.1097/FBP.0000000000000679. [DOI] [PubMed] [Google Scholar]
- Dale E.; Grunnet M.; Pehrson A. L.; Frederiksen K.; Larsen P. H.; Nielsen J.; Stensbøl T. B.; Ebert B.; Yin H.; Lu D. The multimodal antidepressant vortioxetine may facilitate pyramidal cell firing by inhibition of 5-HT3 receptor expressing interneurons: An in vitro study in rat hippocampus slices. Brain research 2018, 1689, 1–11. 10.1016/j.brainres.2017.12.025. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y.; Ishima T.; Takashima T. The 5-HT3 receptor agonist attenuates the action of antidepressants in the forced swim test in rats. Brain Res. 1998, 786, 189–193. 10.1016/S0006-8993(97)01459-5. [DOI] [PubMed] [Google Scholar]
- Martin V.; Riffaud A.; Marday T.; Brouillard C.; Franc B.; Tassin J. P.; Sevoz-Couche C.; Mongeau R.; Lanfumey L. Response of Htr3a knockout mice to antidepressant treatment and chronic stress. Br. J. Pharmacol. 2017, 174, 2471–2483. 10.1111/bph.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S.; Devadoss T.; Manjula S. N.; Rajangam J. 5-HT3 receptor antagonism: a potential therapeutic approach for the treatment of depression and other disorders. Current neuropharmacology 2021, 19, 1545. 10.2174/1570159X18666201015155816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankelevitch-Yahav R.; Franko M.; Huly A.; Doron R. The forced swim test as a model of depressive-like behavior. JoVE (Journal of Visualized Experiments) 2015, 97, e52587 10.3791/52587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim N.; Khan I.; Abdelhalim A.; Khan A.; Halim S. A. Antidepressant potential of novel flavonoids derivatives from sweet violet (Viola odorata L): Pharmacological, biochemical and computational evidences for possible involvement of serotonergic mechanism. Fitoterapia 2018, 128, 148–161. 10.1016/j.fitote.2018.05.016. [DOI] [PubMed] [Google Scholar]
- Shabani S.; Rabiei Z.; Amini-Khoei H. Exploring the multifaceted neuroprotective actions of gallic acid: A review. International Journal of Food Properties 2020, 23, 736–752. 10.1080/10942912.2020.1753769. [DOI] [Google Scholar]
- Can O. D.; Turan N.; Demir Ozkay U.; Ozturk Y. Antidepressant-like effect of gallic acid in mice: Dual involvement of serotonergic and catecholaminergic systems. Life Sci. 2017, 190, 110–117. 10.1016/j.lfs.2017.09.023. [DOI] [PubMed] [Google Scholar]
- Sevoz C.; Nosjean A.; Callera J. C.; Machado B.; Hamon M.; Laguzzi R. Stimulation of 5-HT3 receptors in the NTS inhibits the cardiac Bezold-Jarisch reflex response. Am. J. Physiol. 1996, 271, H80–H87. 10.1152/ajpheart.1996.271.1.H80. [DOI] [PubMed] [Google Scholar]
- Chatterjee S. S.; Noldner M.; Koch E.; Erdelmeier C. Antidepressant activity of hypericum perforatum and hyperforin: the neglected possibility. Pharmacopsychiatry 1998, 31 (Suppl 1), 7–15. 10.1055/s-2007-979340. [DOI] [PubMed] [Google Scholar]
- Eisensamer B.; Rammes G.; Gimpl G.; Shapa M.; Ferrari U.; Hapfelmeier G.; Bondy B.; Parsons C.; Gilling K.; Zieglgänsberger W. Antidepressants are functional antagonists at the serotonin type 3 (5-HT3) receptor. Molecular psychiatry 2003, 8, 994–1007. 10.1038/sj.mp.4001314. [DOI] [PubMed] [Google Scholar]
- Machado D. G.; Kaster M. P.; Binfare R. W.; Dias M.; Santos A. R.; Pizzolatti M. G.; Brighente I. M.; Rodrigues A. L. Antidepressant-like effect of the extract from leaves of Schinus molle L. in mice: evidence for the involvement of the monoaminergic system. Prog. Neuropsychopharmacol Biol. Psychiatry 2007, 31, 421–428. 10.1016/j.pnpbp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Akotkar L.; Aswar U.; Patil R.; Kumar D.; Aswar M.; Pandey J.; Gurav S. Antidepressant Effect of Alpha Lipoic Acid in Rats Exposed to Chronic Unpredictable Mild Stress: Putative Role of Neurotransmitters and 5HT3 Receptor. Future Pharmacology 2023, 3, 407–425. 10.3390/futurepharmacol3020025. [DOI] [Google Scholar]
- Zhao Q.; Liu J.; Chen L.; Gao Z.; Lin M.; Wang Y.; Xiao Z.; Chen Y.; Huang X. Phytomedicine Fructus Aurantii-derived two absorbed compounds unlock antidepressant and prokinetic multi-functions via modulating 5-HT3/GHSR. Journal of Ethnopharmacology 2024, 323, 117703 10.1016/j.jep.2024.117703. [DOI] [PubMed] [Google Scholar]
- Girish C.; Oommen S.; Vishnu R. Evidence for the involvement of the monoaminergic system in the antidepressant-like activity of methanolic extract of Bacopa monnieri in albino mice. International Journal of Basic and Clinical Pharmacology 2016, 5, 914–922. 10.18203/2319-2003.ijbcp20161545. [DOI] [Google Scholar]
- Al-Yamani M. J.; Mohammed Basheeruddin Asdaq S.; Alamri A. S.; Alsanie W. F.; Alhomrani M.; Alsalman A. J.; Al Mohaini M.; Al Hawaj M. A.; Alanazi A. A.; Alanzi K. D.; Imran M. The role of serotonergic and catecholaminergic systems for possible antidepressant activity of apigenin. Saudi J. Biol. Sci. 2022, 29, 11–17. 10.1016/j.sjbs.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Ariza M.; García-Alloza M.; Lasheras B.; Del Río J. n.; Ramírez M. a.-J. GABAA receptor antagonists enhance cortical acetylcholine release induced by 5-HT3 receptor blockade in freely moving rats. Brain research 2002, 956, 81–85. 10.1016/S0006-8993(02)03483-2. [DOI] [PubMed] [Google Scholar]
- Chutia P.; Srivastava S. Vortioxetine and Cognition in Older Adults: A Mini Review. Indian Journal of Clinical Psychiatry 2022, 2, 29–35. [Google Scholar]
- Lochner M.; Thompson A. J. The muscarinic antagonists scopolamine and atropine are competitive antagonists at 5-HT3 receptors. Neuropharmacology 2016, 108, 220–228. 10.1016/j.neuropharm.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves D. C.; Lummis S. C. The molecular basis of the structure and function of the 5-HT 3 receptor: a model ligand-gated ion channel. Molecular membrane biology 2002, 19, 11–26. 10.1080/09687680110110048. [DOI] [PubMed] [Google Scholar]
- Barnes J. M.; Costall B.; Coughlan J.; Domeney A. M.; Gerrard P. A.; Kelly M. E.; Naylor R. J.; Onaivi E. S.; Tomkins D. M.; Tyers M. B. The effects of ondansetron, a 5-HT3 receptor antagonist, on cognition in rodents and primates. Pharmacol., Biochem. Behav. 1990, 35, 955–962. 10.1016/0091-3057(90)90385-U. [DOI] [PubMed] [Google Scholar]
- Ohno M.; Watanabe S. Differential effects of 5-HT3 receptor antagonism on working memory failure due to deficiency of hippocampal cholinergic and glutamatergic transmission in rats. Brain research 1997, 762, 211–215. 10.1016/S0006-8993(97)00448-4. [DOI] [PubMed] [Google Scholar]
- du Jardin K. G.; Jensen J. B.; Sanchez C.; Pehrson A. L. Vortioxetine dose-dependently reverses 5-HT depletion-induced deficits in spatial working and object recognition memory: a potential role for 5-HT1A receptor agonism and 5-HT3 receptor antagonism. European Neuropsychopharmacology 2014, 24, 160–171. 10.1016/j.euroneuro.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Arnsten A. F.; Lin C. H.; Van Dyck C. H.; Stanhope K. J. The effects of 5-HT3 receptor antagonists on cognitive performance in aged monkeys. Neurobiol Aging 1997, 18, 21–28. 10.1016/S0197-4580(96)00162-5. [DOI] [PubMed] [Google Scholar]
- Gomar A.; Hosseini A.; Mirazi N. Memory enhancement by administration of ginger (Zingiber officinale) extract on morphine-induced memory impairment in male rats. Journal of Acute Disease 2014, 3, 212–217. 10.1016/S2221-6189(14)60047-0. [DOI] [Google Scholar]
- Ohno M.; Watanabe S. Differential effects of 5-HT3 receptor antagonism on working memory failure due to deficiency of hippocampal cholinergic and glutamatergic transmission in rats. Brain Res. 1997, 762, 211–215. 10.1016/S0006-8993(97)00448-4. [DOI] [PubMed] [Google Scholar]
- Yaakob N. S.; Seyed M. A.; Mohd-Zahid M. H.; Tee H.-H.; Yap S.-Y.; Ho S.-W.; Kamisah Y.; Azmi N. Neuroprotective Effects of Ocimum basilicum L. var. thyrsiflora on Scopolamine-Induced Non-Spatial Memory Deficits in Rats. Sains Malaysiana 2023, 52, 837–850. 10.17576/jsm-2023-5203-12. [DOI] [Google Scholar]
- Lin H.; Wang X.; Zhao J.; Lin Z. Protective effect of kaempferol against cognitive and neurological disturbances induced by d-galactose and aluminum chloride in mice. Journal of Functional Foods 2023, 100, 105385 10.1016/j.jff.2022.105385. [DOI] [Google Scholar]
- Lee S.; Seol H. S.; Eom S.; Lee J.; Kim C.; Park J. H.; Kim T. H.; Lee J. H. Hydroxy Pentacyclic Triterpene Acid, Kaempferol, Inhibits the Human 5-Hydroxytryptamine Type 3A Receptor Activity. Int. J. Mol. Sci. 2022, 23, 544. 10.3390/ijms23010544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D. O. Phytochemicals for improving aspects of cognitive function and psychological state potentially relevant to sports performance. Sports medicine 2019, 49, 39–58. 10.1007/s40279-018-1007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.; Okello E.; Chazot P.; Howes M.-J.; Ohiomokhare S.; Jackson P.; Haskell-Ramsay C.; Khan J.; Forster J.; Wightman E. Volatile terpenes and brain function: investigation of the cognitive and mood effects of Mentha× Piperita L. essential oil with in vitro properties relevant to central nervous system function. Nutrients 2018, 10, 1029. 10.3390/nu10081029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov V.; Belcheva S.; Konstantinova E.; Kehayov R.; Petkov V.; Hadjiivanova C. Participation of the serotonergic system in the memory effects of Ginkgo biloba L. and Panax ginseng CA Mey. Phytotherapy Research 1994, 8, 470–477. 10.1002/ptr.2650080807. [DOI] [Google Scholar]
- Brown M. K.; Luo Y. Bilobalide modulates serotonin-controlled behaviors in the nematode Caenorhabditis elegans. BMC Neurosci 2009, 10, 62. 10.1186/1471-2202-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Tabaa M. M.; Sokkar S. S.; Ramadan E. S.; Abd El Salam I. Z.; Zaid A. Neuroprotective role of Ginkgo biloba against cognitive deficits associated with Bisphenol A exposure: An animal model study. Neurochem. Int. 2017, 108, 199–212. 10.1016/j.neuint.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Zhang C.; Ogle W. O.. The Serotonergic System and Neuroplasticity are Implicated in the Effect of Phytochemicals on Mood and Cognitive Disorders. In Recent Advances in Theories and Practice of Chinese Medicine; 2012; 10.5772/26579. [DOI] [Google Scholar]
- Thompson A. J.; Jarvis G. E.; Duke R. K.; Johnston G. A.; Lummis S. C. Ginkgolide B and bilobalide block the pore of the 5-HT3 receptor at a location that overlaps the picrotoxin binding site. Neuropharmacology 2011, 60, 488–495. 10.1016/j.neuropharm.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J. M.; Barnes N. M.; Costall B.; Naylor R. J.; Tyers M. B. 5-HT3 receptors mediate inhibition of acetylcholine release in cortical tissue. Nature 1989, 338, 762–763. 10.1038/338762a0. [DOI] [PubMed] [Google Scholar]
- Bravo R.; Matito S.; Cubero J.; Paredes S. D.; Franco L.; Rivero M.; Rodriguez A. B.; Barriga C. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age (Dordr) 2013, 35, 1277–1285. 10.1007/s11357-012-9419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti J. M.; Jantos H.; Catenaccio V.; Xavier S. Blockade of the NMDA and AMPA/kainate receptors in the dorsal raphe nucleus prevents the 5-HT(3) receptor agonist m-chlorophenylbiguanide-induced suppression of REM sleep in the rat. Prog. Neuropsychopharmacol Biol. Psychiatry 2011, 35, 1341–1348. 10.1016/j.pnpbp.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Carley D. W.; Depoortere H.; Radulovacki M. R-zacopride, a 5-HT3 antagonist/5-HT4 agonist, reduces sleep apneas in rats. Pharmacol., Biochem. Behav. 2001, 69, 283–289. 10.1016/S0091-3057(01)00535-4. [DOI] [PubMed] [Google Scholar]
- Leiser S. C.; Iglesias-Bregna D.; Westrich L.; Pehrson A. L.; Sanchez C. Differentiated effects of the multimodal antidepressant vortioxetine on sleep architecture: Part 2, pharmacological interactions in rodents suggest a role of serotonin-3 receptor antagonism. J. Psychopharmacol 2015, 29, 1092–1105. 10.1177/0269881115592347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbardar M. G.; Hosseinzadeh H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iranian journal of basic medical sciences 2020, 23, 1100. 10.22038/ijbms.2020.45269.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solhi H.; Salehi B.; Alimoradian A.; Pazouki S.; Taghizadeh M.; Saleh A. M.; Kazemifar A. M. Beneficial Effects of Rosmarinus Officinalis for Treatment of Opium Withdrawal Syndrome during Addiction Treatment Programs: A Clinical Trial. Addict Health 2013, 5, 90–94. [PMC free article] [PubMed] [Google Scholar]
- Yi P. L.; Lin C. P.; Tsai C. H.; Lin J. G.; Chang F. C. The involvement of serotonin receptors in suanzaorentang-induced sleep alteration. J. Biomed Sci. 2007, 14, 829–840. 10.1007/s11373-007-9197-8. [DOI] [PubMed] [Google Scholar]
- Cappelli A.; Gallelli A.; Manini M.; Anzini M.; Mennuni L.; Makovec F.; Menziani M. C.; Alcaro S.; Ortuso F.; Vomero S. Further studies on the interaction of the 5-hydroxytryptamine3 (5-HT3) receptor with arylpiperazine ligands. Development of a new 5-HT3 receptor ligand showing potent acetylcholinesterase inhibitory properties. Journal of medicinal chemistry 2005, 48, 3564–3575. 10.1021/jm0493461. [DOI] [PubMed] [Google Scholar]
- Skovgård K.; Agerskov C.; Kohlmeier K. A.; Herrik K. F. The 5-HT3 receptor antagonist ondansetron potentiates the effects of the acetylcholinesterase inhibitor donepezil on neuronal network oscillations in the rat dorsal hippocampus. Neuropharmacology 2018, 143, 130–142. 10.1016/j.neuropharm.2018.09.017. [DOI] [PubMed] [Google Scholar]
- Huang M.; Felix A. R.; Kwon S.; Lowe D.; Wallace T.; Santarelli L.; Meltzer H. Y. The alpha-7 nicotinic receptor partial agonist/5-HT 3 antagonist RG3487 enhances cortical and hippocampal dopamine and acetylcholine release. Psychopharmacology 2014, 231, 2199–2210. 10.1007/s00213-013-3373-5. [DOI] [PubMed] [Google Scholar]
- Liu L. F.; Liu Y. T.; Wu D. D.; Cheng J.; Li N. N.; Zheng Y. N.; Huang L.; Yuan Q. L. Inhibiting 5-hydroxytryptamine receptor 3 alleviates pathological changes of a mouse model of Alzheimer’s disease. Neural Regen Res. 2023, 18, 2019–2028. 10.4103/1673-5374.366492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban J. Y.; Seong Y. H. Blockade of 5-HT3 receptor with MDL72222 and Y25130 reduces β-amyloid protein (25–35)-induced neurotoxicity in cultured rat cortical neurons. European journal of pharmacology 2005, 520, 12–21. 10.1016/j.ejphar.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Lin Y.; Chen S.; Li X.; Huo H. 5-HT3 Receptors: A Potential Therapeutic Target for Epilepsy. Curr. Neuropharmacol 2018, 16, 29–36. 10.2174/1570159X15666170508170412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini A.; Mirazi N. Acute administration of ginger (Zingiber officinale rhizomes) extract on timed intravenous pentylenetetrazol infusion seizure model in mice. Epilepsy Res. 2014, 108, 411–419. 10.1016/j.eplepsyres.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Amiri Gheshlaghi S.; Mohammad Jafari R.; Algazo M.; Rahimi N.; Alshaib H.; Dehpour A. R. Genistein modulation of seizure: involvement of estrogen and serotonin receptors. Journal of natural medicines 2017, 71, 537–544. 10.1007/s11418-017-1088-3. [DOI] [PubMed] [Google Scholar]
- Zhang J. X.; Li S. R.; Yao S.; Bi Q. R.; Hou J. J.; Cai L. Y.; Han S. M.; Wu W. Y.; Guo D. A. Anticonvulsant and sedative-hypnotic activity screening of pearl and nacre (mother of pearl). J. Ethnopharmacol 2016, 181, 229–235. 10.1016/j.jep.2016.01.039. [DOI] [PubMed] [Google Scholar]
- Zheng C.-Q.; Fan H.-X.; Li X.-X.; Li J.-J.; Sheng S.; Zhang F. Resveratrol alleviates levodopa-induced dyskinesia in rats. Frontiers in Immunology 2021, 12, 683577 10.3389/fimmu.2021.683577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot P.; Kang W.; Kim E.; Bédard D.; Belliveau S.; Frouni I.; Kwan C. Levodopa-induced dyskinesia: A brief review of the ongoing clinical trials. Neurodegenerative Disease Management 2022, 12, 51–55. 10.2217/nmt-2021-0051. [DOI] [PubMed] [Google Scholar]
- Aboulghasemi N.; Jahromy M. H.; Ghasemi A. Anti-dyskinetic efficacy of 5-HT3 receptor antagonist in the hemi-parkinsonian rat model. IBRO reports 2019, 6, 40–44. 10.1016/j.ibror.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu P. S.; Kulkarni S. K. Reversal of neuroleptic-induced orofacial dyskinesia by 5-HT3 receptor antagonists. European journal of pharmacology 2001, 420, 113–117. 10.1016/S0014-2999(01)00986-4. [DOI] [PubMed] [Google Scholar]
- Benuck M.; Reith M. E. Dopamine releasing effect of phenylbiguanide in rat striatal slices. Naunyn Schmiedebergs Arch Pharmacol 1992, 345, 666–672. 10.1007/BF00164581. [DOI] [PubMed] [Google Scholar]
- Kwan C.; Nuara S. G.; Bedard D.; Gaudette F.; Gourdon J. C.; Beaudry F.; Huot P. Selective blockade of the 5-HT3 receptor acutely alleviates dyskinesia and psychosis in the parkinsonian marmoset. Neuropharmacology 2021, 182, 108386 10.1016/j.neuropharm.2020.108386. [DOI] [PubMed] [Google Scholar]
- Kwan C.; Frouni I.; Bédard D.; Hamadjida A.; Huot P. Ondansetron, a highly selective 5-HT3 receptor antagonist, reduces L-DOPA-induced dyskinesia in the 6-OHDA-lesioned rat model of Parkinson’s disease. Eur. J. Pharmacol. 2020, 871, 172914 10.1016/j.ejphar.2020.172914. [DOI] [PubMed] [Google Scholar]
- Das A.; Shanker G.; Nath C.; Pal R.; Singh S.; Singh H. K. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: anticholinesterase and cognitive enhancing activities. Pharmacol., Biochem. Behav. 2002, 73, 893–900. 10.1016/S0091-3057(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Hosseini A.; Mirazi N. Acute administration of ginger (Zingiber officinale rhizomes) extract on timed intravenous pentylenetetrazol infusion seizure model in mice. Epilepsy research 2014, 108, 411–419. 10.1016/j.eplepsyres.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Machado D. G.; Bettio L. E.; Cunha M. P.; Santos A. R.; Pizzolatti M. G.; Brighente I. M.; Rodrigues A. L. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: evidence for the involvement of the serotonergic and noradrenergic systems. Eur. J. Pharmacol. 2008, 587, 163–168. 10.1016/j.ejphar.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Lee B. H.; Lee J. H.; Lee S. M.; Jeong S. M.; Yoon I. S.; Lee J. H.; Choi S. H.; Pyo M. K.; Rhim H.; Kim H. C.; Jang C. G.; Lee B. C.; Park C. S.; Nah S. Y. Identification of ginsenoside interaction sites in 5-HT3A receptors. Neuropharmacology 2007, 52, 1139–1150. 10.1016/j.neuropharm.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Walstab J.; Wohlfarth C.; Hovius R.; Schmitteckert S.; Röth R.; Lasitschka F.; Wink M.; Bönisch H.; Niesler B. Natural compounds boldine and menthol are antagonists of human 5-HT 3 receptors: implications for treating gastrointestinal disorders. Neurogastroenterology & Motility 2014, 26, 810–820. 10.1111/nmo.12334. [DOI] [PubMed] [Google Scholar]
- Akotkar L.; Aswar U.; Ganeshpurkar A.; Raj R.; Pawar A. An Overview of Chemistry, Kinetics, Toxicity and Therapeutic Potential of Boldine in Neurological Disorders. Neurochem. Res. 2023, 48, 3283–3295. 10.1007/s11064-023-03992-y. [DOI] [PubMed] [Google Scholar]
- Nakamura Y.; Ishida Y.; Kondo M.; Shimada S. Yokukansan contains compounds that antagonize the 5-HT3 receptor. Phytomedicine 2018, 43, 120–125. 10.1016/j.phymed.2018.04.034. [DOI] [PubMed] [Google Scholar]
- Jarvis G. E.; Barbosa R.; Thompson A. J. Noncompetitive Inhibition of 5-HT3 Receptors by Citral, Linalool, and Eucalyptol Revealed by Nonlinear Mixed-Effects Modeling. J. Pharmacol Exp Ther 2016, 356, 549–562. 10.1124/jpet.115.230011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebrisi E. E.; Prytkova T.; Lorke D. E.; Howarth L.; Alzaabi A. H.; Yang K.-H. S.; Howarth F. C.; Oz M. Capsaicin is a negative allosteric modulator of the 5-HT3 receptor. Frontiers in Pharmacology 2020, 11, 1274. 10.3389/fphar.2020.01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom S.; Lee J.; Baek Y.-B.; Yeom H. D.; Lee S.; Kim C.; Park Y.; Park S.-I.; Lee C.-M.; Lee J. H. Identification and molecular study on the interaction of Schisandrin C with human 5-HT3A receptor. Eur. J. Pharmacol. 2021, 906, 174220 10.1016/j.ejphar.2021.174220. [DOI] [PubMed] [Google Scholar]
- Lohning A. E.; Marx W.; Isenring L. In silico investigation into the interactions between murine 5-HT3 receptor and the principle active compounds of ginger (Zingiber officinale). Journal of Molecular Graphics and Modelling 2016, 70, 315–327. 10.1016/j.jmgm.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Bristy T. A.; Barua N.; Montakim Tareq A.; Sakib S. A.; Etu S. T.; Chowdhury K. H.; Jyoti M. A.; Aziz M. A.; Reza A.; Caiazzo E.; Romano B.; Tareq S. M.; Emran T. B.; Capasso R. Deciphering the Pharmacological Properties of Methanol Extract of Psychotria calocarpa Leaves by In Vivo, In Vitro and In Silico Approaches. Pharmaceuticals (Basel) 2020, 13, 183. 10.3390/ph13080183. [DOI] [PMC free article] [PubMed] [Google Scholar]