Abstract
Scabies, caused by the Sarcoptes scabiei var hominis mite burrowing into the skin, is a highly contagious disease characterized by intense nocturnal itching. Its global impact is considerable, affecting more than 200 million individuals annually and posing significant challenges to healthcare systems worldwide. Transmission occurs primarily through direct skin‐to‐skin contact, contributing to its widespread prevalence and emergence as a substantial public health concern affecting large populations. This review presents consensus‐based clinical practice guidelines for diagnosing and managing scabies, developed through the fuzzy Delphi method by dermatology, parasitology, pediatrics, pharmacology, and public health experts. The presence of burrows containing adult female mites, their eggs, and excreta is the diagnostic hallmark of scabies. Definitive diagnosis typically involves direct microscopic examination of skin scrapings obtained from these burrows, although dermoscopy has become a diagnostic tool in clinical practice. Treatment modalities encompass topical agents, such as permethrin, balsam of Peru, precipitated sulfur, and benzyl benzoate. In cases where topical therapy proves inadequate or in instances of crusted scabies, oral ivermectin is recommended as a systemic treatment option. This comprehensive approach addresses the diagnostic and therapeutic challenges associated with scabies, optimizing patient care, and management outcomes.
Keywords: scabies, neglected tropical diseases, diagnostics, treatment, infestations, elimination, prevention
Introduction
Scabies is an infectious skin disease caused by the mite Sarcoptes scabiei var hominis, which burrows into the skin and causes severe itching. Its global prevalence exceeds 200 million cases annually, burdening affected individuals and healthcare systems, especially in developing countries. 1 , 2 The quantifiable burden determined by the “disability‐adjusted life‐years” (DALY) metric is significant. Scabies can also have significant psychosocial and economic impacts due to stigmatization and loss of workforce. It causes severe sleep disturbances due to itching and secondary infections resulting from damage to the skin barrier. It is a widespread issue globally, particularly in communities with inadequate access to healthcare and limited resources. In 2017, the World Health Organization (WHO) included scabies in the list of neglected tropical diseases, acknowledging its significant impact on public health. 3
These guidelines aim to provide effective and practical approaches for diagnosing and treating the disease, particularly on a national basis. Renowned experts in dermatology, parasitology, pediatrics, pharmacology, and public health have developed this consensus text using the fuzzy Delphi method. Clear and concise algorithms present clinical practices and approaches to diagnosing and treating scabies.
Biology and immunology of the pathogen, infectiousness, and transmission
The causative agent of scabies in humans and animals is the S. scabiei var hominis mite, a highly specialized and adaptable species capable of thriving in a wide range of host animals. The males of this species measure 210–290 μm, while the females measure 300–500 μm in length. The eggs, which are oval‐shaped, measure 150–200 μm.
The scabies mites have the capacity to modulate the host's immune response and can survive outside the host for 2–5 days. 4 , 5 Although the host develops both innate and acquired immune responses against the mites, it is not protected from re‐infestations.
Transmission typically occurs through prolonged skin‐to‐skin contact between an infested individual and one not infested. However, it is also possible for transmission to occur through fomites such as bedding. Transmission through brief contact, such as handshakes and medical examinations, is rare since it usually requires 15–20 minutes. The risk of transmission is significantly higher in individuals with a higher parasitic load, particularly in crusted scabies, where millions of mites are present on the skin; transmission can occur even through short‐term contact with these patients. Additionally, transmission can also occur through shed scales from these patients. 6 , 7
Scabies are commonly found in other family members of infested individuals, especially spouses. 8 It is crucial to recognize that the absence of itching in a family member does not necessarily signify the absence of the disease.
Sexual contact is also a significant route of transmission, particularly among young and middle‐aged individuals. Scabies can be spread along with other sexually transmitted diseases during sexual intercourse. 9 Studies have shown that scabies is a common comorbidity in HIV patients, with an incidence of 18.6%. 10
Contact with bedding previously used by a known scabies patient showed that less than 1% of those exposed acquired the disease. In a study of healthy volunteers, scabies transmission was only detected when volunteers wore underwear previously used by scabies patients while still at body temperature, and even then, only two out of 32 volunteers were infested with scabies. It is important to note that good hygiene practices, such as regular and frequent body washing, do not significantly reduce the risk of transmission. 11 , 12
Scabies is a highly prevalent infectious disease among healthcare workers in refugee/asylum‐seeker centers, as well as among inmates in prisons and residents of communal living spaces, such as nursing homes for the elderly. 11 , 13 Similarly, the risk of transmission through close contact between children attending daycare centers and schools is exceptionally high.
Transmission of other variants of S. scabiei from animals to humans is rare, with the most common transmission occurring from dogs. Lesions resulting from mite penetration usually appear on skin areas that have been in contact with the animal. Discontinuing contact usually resolves the clinical presentation without requiring treatment. 14
Occurrence
Scabies is a parasitic infestation that affects individuals of all ages, races, genders, and socioeconomic groups. However, it is a highly prevalent disease in low‐ and middle‐income tropical countries. 15 Age‐specific data indicate that scabies is more common among children, young adults, and the elderly. Scabies is more prevalent during the fall and winter months. 16 There is a significant negative correlation with temperature and a positive correlation with humidity. Scabies outbreaks are known to occur in situations such as wars, disasters, famine, overcrowding, malnutrition, migration, homelessness, and poor hygiene. 17 , 18 , 19 , 20 In fact, scabies has been reported as the third most common infectious disease in refugee or asylum‐seeker populations. The prevalence of the disease varies geographically, ranging from 0.3 to 46% in different regions. 17 In tropical areas, the prevalence among children can vary between 5 and 50%. 1
The incidence of scabies in Turkey has been on the rise. A retrospective cross‐sectional study conducted between 2013 and 2018 revealed a gradual increase in the incidence from 0.4 to 1%. 21 In a multicenter study that compiled data on scabies patients in 12 tertiary dermatology clinics across various geographical regions of the country, 17,803 patients (14,574 adults and 3,229 children) were diagnosed with scabies between 2014 and 2019. 22 Notably, the number of cases increased approximately 7‐fold between 2017 and 2018 and 30‐fold between 2017 and 2019. 22 A scabies outbreak occurred in Turkey between 2018 and 2019, as noted in a retrospective study conducted in two tertiary hospitals in Istanbul. 23 A cross‐sectional study in Northeastern Turkey in 2021 reported an incidence of scabies of 10.9%. 24 It is believed that the number of cases is significantly higher than reported due to unrecorded cases.
The increase in the incidence of scabies in Turkey may have additional contributions from irregular migration movements that facilitate transmission and spread as well as the recent earthquake disaster that affected millions of people.
Clinical features
Scabies present with varying clinical signs and symptoms depending on the host's age and immunologic status, as well as the presence of comorbidities such as diabetes mellitus. 25 , 26 These factors can lead to diagnostic difficulties and delays of several months.
Common scabies
A spectrum of clinically specific and nonspecific lesions can be observed in common scabies in immunocompetent individuals. 27 However, the wide range of clinical symptoms of varying severity makes diagnosis challenging. 26
Common scabies is characterized by pruritus as the primary symptom, which results from a hypersensitivity reaction to components in the excretions and secretions of the mite. Clinical symptoms usually start about 4 weeks (2–6 weeks) after the initial infestation, with an average of 6–15 burrows present. 25 It is important to note that individuals who were previously infested and become sensitized may experience a more rapid immunologic reaction, in which case clinical symptoms may develop 1–3 days (or even hours) after reinfection. 11 , 18
Itching affects the entire body except for the head and neck, although the elderly and infants may also experience symptoms in these areas. Itching is typically worse at night and can cause insomnia. 28
Scabies can present with both specific and nonspecific lesions in immunocompetent individuals.
Specific lesions
The presence of the female mite's burrow is pathognomonic for clinical diagnosis of scabies infestation. 18 , 27 The clinical manifestations of scabies result from the body's immune response to the mites in the skin. However, during epidemics, the incidence of nonspecific lesions and atypical forms increases while the likelihood of specific findings, such as the presence of burrows, decreases. Burrows are not always visible, especially in individuals who bathe frequently. 18 , 27 They can be identified by their thread‐like formation, which resembles a broken line or the letter S. Typically, burrows are whitish, grayish, or brownish in color and measure about 0.5 mm wide and 10 mm long. They are slightly raised from the skin, and at the end of them, a pearl‐like vesicle known as a “vesicle Perle” may be observed (Figure 1).
Figure 1.
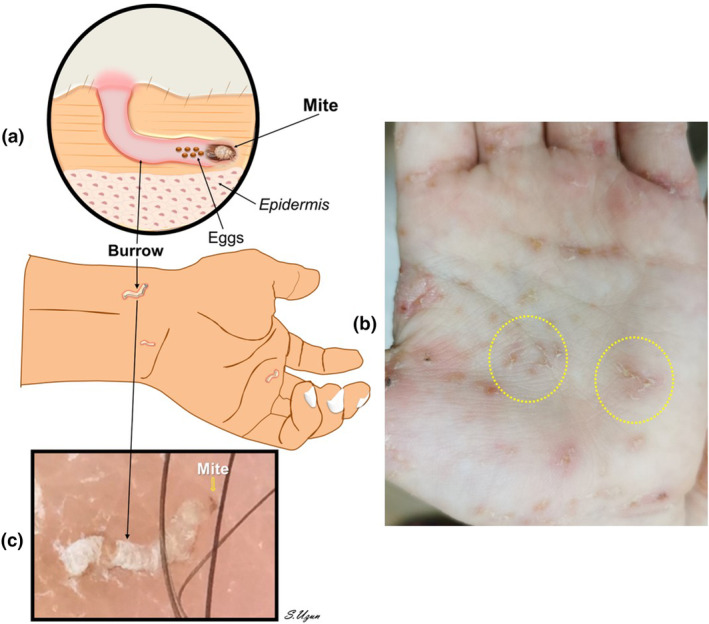
Schematic (a), clinical (b), and dermoscopic appearance (c) of a scabies burrow
Scabies lesions commonly appear on various parts of the body, such as the wrists, hands, fingers, umbilicus, waist, axillae, intergluteal cleft, and feet (Figure 2). 8
Figure 2.
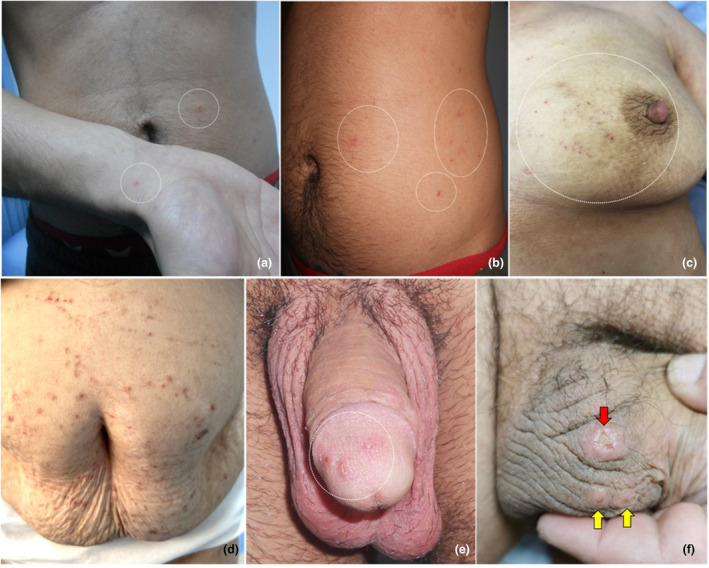
The most common symptoms of scabies on the skin are erythematous papules with excoriated tops due to scratching (prurigo papules). The most frequently affected areas are the wrists (a), belt area (b), around the breasts and areola in women (c), axillary regions and around the navel in both sexes (d), and the genital area in men, especially the glans penis (e) and scrotum (yellow arrows) (f). In some cases, ecthymatoid ulcerations may occur in the genital area (red arrow) (F)
Lesions associated with scabies typically present as prurigo papules or nodules that are often excoriated due to scratching. The genital area (penis and scrotum) is frequently affected in males, while in females, the areola and surrounding areas are commonly involved. The presence of pruritic papules, particularly on the glans penis in adult males, is almost always pathognomonic for scabies. 8 Additionally, in some cases, ecthymatoid ulcerations, also known as “scabies chancre,” may be present in the genital area (Figure 2f, red arrow). 27
Mites tend to avoid areas with high‐density pilosebaceous follicles. Therefore, in healthy adults, burrows or other signs of scabies, and consequently itching, are not commonly observed on the skin above the neck, face, and scalp. However, infants, the elderly, those with crusted scabies, and individuals with immunodeficiency may experience involvement of the face and scalp. 18 , 28
Nonspecific lesions
A clinically distinct polymorphism occurs with the intermingling of excoriations and erythematous papules, occasional secondary eczematizations, pustular lesions, and pyodermic lesions such as impetigo and furuncles. 27
Crusted scabies
Crusted scabies are characterized by widespread hyperkeratotic crusted lesions and are caused by an unlimited multiplication of mites in the skin, resulting in thousands or even millions of specimens. 29 , 30 It is a highly contagious form of scabies frequently transmitted through objects like bed covers and sofas. Accordingly, patients with crusted scabies are involved in the majority of outbreaks. 30 , 31 The incubation period of this form may be much shorter than that of common scabies, with symptoms appearing 4–5 days after transmission due to the extraordinary mite burden. 32 Host factors play a more significant role in crusted scabies than pathogen‐specific characteristics such as the parasite's virulence. Patients with amputations, paralysis, Alzheimer's disease, finger deformities, nutritional disorders, Down syndrome, or those with weak cognitive abilities or physical impairments, may not feel itching due to sensory loss or decreased scratching ability. 33 , 34 Although it is commonly believed that pruritus is absent in crusted scabies, half of all patients with this condition experience some degree of pruritus, which gradually diminishes over time. 2 , 30
More than half of crusted scabies patients have identifiable immunosuppressive risk factors, possibly indicating an increased tendency to develop Th2 immune responses. 2 , 5 , 18 , 30 In a case series of crusted scabies, HIV infection was reported as the most common comorbidity in 45% of patients. 35
The cutaneous manifestations of crusted scabies are slow and insidious, characterized by localized keratotic plaques and a diffuse erythematous appearance. These plaques have a parakeratotic crust that can exceed 1 cm in thickness and closely resemble psoriasis or Darier's disease. 27 , 28 , 33 They exhibit a range of colors, including creamy, gray, yellow‐brown, or yellow‐green variations, and are firmly attached to the underlying skin areas. 27 , 33 Upon removal, the surface of the skin appears rough, like a “pumice stone,” while the underlying skin is smooth, erythematous, and moist. Nails are a significant source of relapses and have a discolored, thickened, and dystrophic appearance. 36 Generalized lymphadenopathy is frequently detected in crusted scabies, peripheral blood eosinophilia, and increased serum IgE levels. Additionally, secondary bacterial infections are common and can even result in mortality. 30 , 34
Nodular scabies
It presents with highly pruritic reddish‐brown nodules and papules that typically develop following adequate scabies treatment. Nodules are commonly found in the anogenital area, including the penis and scrotum in men and the vulva and surrounding areas in women as well as in the inguinal and gluteal regions, breasts, and axillary folds. 27 , 28 In pediatric patients, nodules are more commonly observed in the axillary region, around the abdomen, and in the genital area. Nodular scabies are often misdiagnosed as mastocytoma, cutaneous B‐cell lymphoma, and urticaria pigmentosa. 37
Itching is especially pronounced at night. Notably, these nodules do not necessarily indicate an active infestation and typically do not contain mites. Nodular scabies can persist for weeks or even months after effective scabies treatment. 27 , 28
Clinical features in specific age groups and special populations
Scabies in infants and young children
In this age group, scabies may present with a rash that is predominantly vesicular, papulovesicular, or papulopustular. 28 Lesions may be more widespread, affecting the neck, face, scalp, nails, palms, soles of the feet, and back. Infants may exhibit prominent symptoms, such as crying, irritability, and restlessness, whereas older children may experience insomnia, fatigue, and lethargy due to increased itching at night. 2
Scabies in the elderly
The clinical presentation of scabies may also differ in elderly individuals due to their decreased immune response. Crusted scabies may develop in elderly patients. 8 , 25 Facial and scalp involvement is frequently observed in the elderly, while the frequency of burrows on the soles of the feet is also increased. In bedridden patients, involvement of the back is more prominent. 31 , 38
Scabies in HIV/AIDS patients and other immunocompromised individuals
Scabies is a common cause of itching in HIV/AIDS patients, affecting 2–4% of individuals in this group. 27 Different forms of scabies, including crusted, atypical, papulonodular, and common forms, can develop, while crusted scabies is particularly common in HIV/AIDS patients. 39
Scabies in pregnancy
Scabies are frequently observed during pregnancy and can cause significant itching. It is important to avoid confusion with other pruritic skin diseases during pregnancy, as delayed diagnosis can lead to maternal and fetal complications.
Clinical features of itching in scabies
Scabies should be considered a differential diagnosis in anyone presenting with itching, especially those with a history of contact with nonfamily individuals. The severity of itching can be unbearable and is the most burdensome symptom caused by scabies in 90–99% of the patients. 40 Severe itching that awakens a patient from sleep primarily suggests scabies. It is important to note that itching in scabies typically intensifies at night (nocturnal crescendo) and can cause sleep disturbances in approximately 80% of patients. 2 , 41
Diagnosis of scabies
The recommended diagnostic algorithm for scabies is illustrated in Figure 3.
Figure 3.
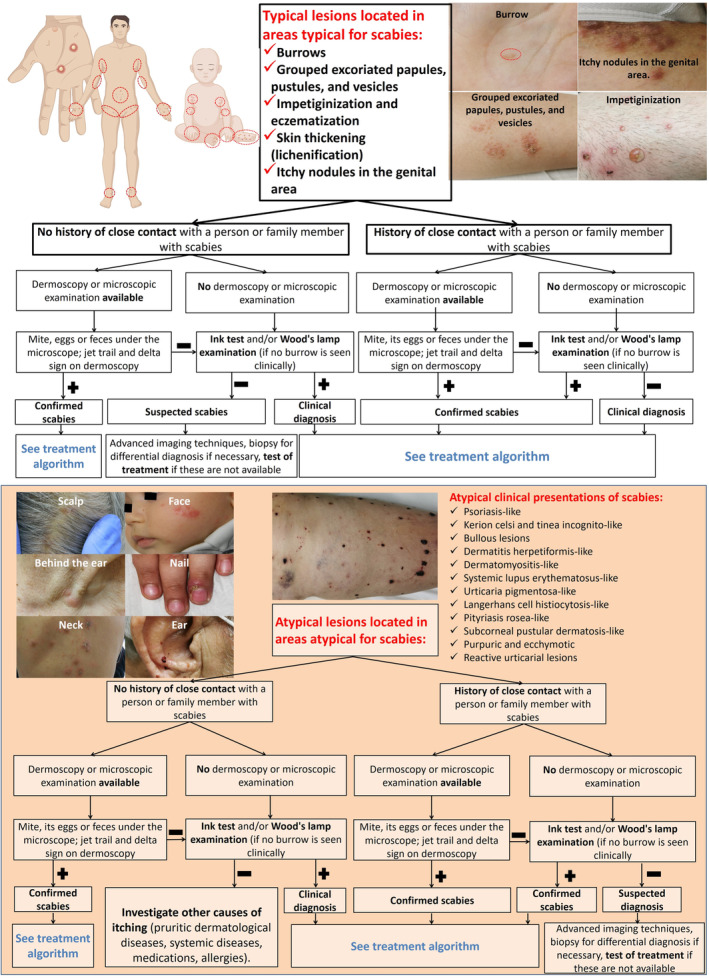
Recommended algorithm for scabies diagnosis
Clinical diagnosis
A comprehensive clinical examination can provide valuable clues for diagnosing scabies and differential diagnosis. Patients should undergo a full‐body examination in a well‐lit environment, with special attention to the head and face in infants and elderly individuals. If there is itching, the scalp should be examined in situations where a complete examination is not possible, such as in field surveys; at least a four‐extremity examination should be performed.
Scabies can be diagnosed if the patient has a history of contact with a scabies‐infested individual, itching that commenced subsequent to the contact, and exhibits typical distribution and lesions. If any patient meets even one of these criteria, the condition is considered “suspected scabies.” In cases where itching and lesions are distributed atypically, suspicion of scabies arises if the patient reports both itching and a history of contact with a scabies‐infested individual. 26
Visualizing burrows, a specific clinical sign of scabies facilitates the diagnosis. If dermoscopy is unavailable, burrows can be revealed with an ink test by applying ink to the suspicious area and then wiping it with an alcohol‐soaked tissue. Linear ink residues on the skin indicate the presence of burrows. 42 Another effective method is to use Wood's lamp in a dark room, which can detect burrows that reflect a bright yellow fluorescence. 43
Microscopy
The most effective method for diagnosing scabies involves gently scraping the skin with a blunt‐edged scalpel blade (size 10 or 15) while placing 1–2 drops of mineral oil onto the sampling area to help the scraping material adhere to the blade. In cases where children are afraid of the blade, a curette can be used instead. 44 To visualize mites, eggs, and fecal material, 10% potassium hydroxide (KOH) solution should be added to the collected samples and left for half an hour. This will partially dissolve keratinized tissues and make observing mites, eggs, and/or fecal pellets easier. It is important to note that the movements of mites cannot be observed when KOH is applied. However, using mineral oil may hinder the identification of mites, eggs, and fecal material. Therefore, mineral oil is recommended over KOH examination for patients with crusted scabies, while for common scabies patients, KOH examination is the preferred method. A positive microscopic examination confirms the clinical diagnosis of scabies, whereas a negative result does not exclude it. A meta‐analysis study of 1,544 patients found that skin scraping microscopic examination had a sensitivity of 56.3% and a specificity of 100%. 45
Superficial skin biopsy is a less invasive and highly effective technique for obtaining microscopic samples. 46 This method is superior to skin scraping in detecting mites and eggs. To perform this technique, a drop of cyanoacrylate adhesive is added to a slide, the slide is pressed to the sampling area for 30 seconds, and then lifted. After immersing the sample in oil, it can be examined under a microscope. 47
Transparent adhesive tape can also be used to obtain microscopic samples. After applying the tape to the sampling area and pressing it onto the skin to adhere, it can be pulled off after a few seconds. The adhesive side of the tape can then be placed on a slide for examination under a microscope. The tape method is the most effective way to detect mites between the fingers or toes. 48 A meta‐analysis has shown that the tape method has a sensitivity of 68.4%, compared to 56.3% for the standard scraping method. 45
Dermoscopy
Dermoscopy, with a specificity of approximately 85%, is an effective method for diagnosing scabies and monitoring treatment. 49 , 50 During the examination, the observer should identify the brown‐black triangle, which represents the mite itself (known as the delta sign), followed by a white smoke sign, which represents the burrow (known as the jet trail) (Figure 1b). 51 For a more suitable dermoscopic examination of the genital area, a videodermoscope or handheld dermoscope connected to a cell phone can be used. 26 It is important to remember that handheld dermoscopes cannot detect eggs and feces, however, mite bodies and eggs can be detected using polarized dermoscopy after dropping blue ink. 52 In crusted scabies, a noodle pattern is created due to numerous burrows. 53 Additionally, transillumination with dermoscopy light can be used to observe burrows more easily on the skin between the fingers of patients. 54
By adding ultraviolet (UV) light, a modified dermoscopy method known as “UV‐dermoscopy” allows the mite's entire body to be visible with a bright reflection. This facilitates the observation of the “ball sign,” which is more noticeable than the delta sign. 55 , 56
Treatment of scabies
Topical treatment
The characteristics of topical medications for the treatment of scabies are summarized in Table 1.
Table 1.
The characteristics of topical medications for the treatment of scabies
| Permethrin | Sulfur | Benzyl benzoate | Crotamiton | |
|---|---|---|---|---|
| Formulation | 5% cream or lotion | 2–12.5% cream or ointment | 10–25% lotion | 10% cream or lotion |
| Application | Leave on the skin for 8–12 h, then wash off. Repeat after 1 week | Apply for 3 consecutive days and repeat after 1 week | Apply for 2–3 consecutive days and repeat after 1 week | Apply for two consecutive nights |
| Side effects | Itching, burning, stinging, eczema | Bad odor and xerotic eczema | Itching, burning, stinging, pustules, skin irritation, eczema, neurological complications | Itching, skin irritation, redness, eczema, anaphylaxis |
| Children | >2 months a | Safe, at 6% concentration in infants | Safe for children over 2 years old | Not recommended |
| Pregnancy | Category B | Category C (limited use, probably safe, at 6% concentration) | Not recommended | Category C |
| Lactation | Safe | It is unknown whether it passes into breast milk, but it can be used at 6% concentration | Not recommended, safety for breastfeeding unknown | It is unknown whether it passes into breast milk |
Though not officially approved, permethrin is the most commonly recommended medication by pediatric dermatologists also in infants younger than 2 months.
Permethrin
Permethrin (as a 5% concentration cream or lotion) has been considered the gold standard treatment for scabies, but recent studies have shown a high resistance of scabies mites to this medication. 57 However, it is important to note that some studies suggest that this resistance may not be accurate and that the ineffectiveness may be due to application errors. 58 A meta‐analysis comparing scabies treatments has shown that permethrin is significantly more effective than oral ivermectin, lindane, and crotamiton. 59
For optimal efficacy, it is recommended to apply permethrin twice at a 1‐week interval, leaving it on the body for 8–12 hours after each application and then washing it off. Topical application of permethrin is safe during pregnancy and lactation, as less than 2% of the substance is absorbed, and its inactive metabolites are rapidly metabolized. 60 To ensure safety, it is recommended to clean the permethrin applied to the breast and surrounding areas before breastfeeding and reapply after breastfeeding. To ensure the safety of children when applying permethrin to their facial areas, it is essential to apply the product at a distance that is out of licking range. Permethrin is safe for use in infants younger than 2 months old, despite the FDA recommendation for children over 2 months old. 61 Although systemic absorption through the skin can cause metabolic acidosis and neurotoxicity, permethrin remains a safe and effective treatment option for neonatal scabies. 62 To mitigate these side effects, washing the body after 4–5 hours of treatment in infants is recommended. 63 Permethrin should be applied to the entire body, avoiding the eyes and mouth, especially to the hairy skin in infants and the elderly. 64
Permethrin may cause irritant contact dermatitis in some individuals, which can lead to discontinuation of treatment in approximately 5% of patients. 65
Sulfur
Compounded preparations containing 3–10% sulfur in petroleum jelly and ready‐to‐use preparations containing 12.5% sulfur, such as Wilkinson's ointment, are commonly used for topical treatment of scabies in Turkey. They are applied to the whole body after a bath, then reapplied without bathing on the second and third days, then a bath is taken, and clean clothes are worn on the fourth day. 66 This process should be repeated a week later. Xerotic eczema has been observed in about one‐third of patients following high‐concentration sulfur mixtures. This relatively inexpensive and safe treatment is recommended at a concentration of 6%, especially for pregnant women, breastfeeding mothers, and infants, especially when other topical treatments, such as permethrin, are ineffective. 67 , 68 A recent study reported that 10% precipitated sulfur was more effective than permethrin and was considered a promising treatment alternative for cases that did not respond to permethrin. 57
Balsam of Peru
Balsam of Peru is used in the same way as sulfur‐containing formulations. It should be noted that balsam of Peru may cause contact dermatitis. 69 The antiparasitic effect is attributed to its content of thymol and benzyl benzoate. 70
Benzyl benzoate
Benzyl benzoate is used in a 25% formulation for adults and 10–12.5% formulations for children. 71 The primary factor constraining its utilization is the potential irritation it may induce. 72 After bathing, the medication is applied to the whole body once a day for 2–3 consecutive days, while contact with the eyes should be avoided. 73
Crotamiton
The 10% cream or lotion of crotamiton is applied to the whole body once or twice a day for five consecutive days in adults and children. Taking a bath before the first application and on the sixth day is recommended. Another recommended method is to reapply the medication within 24 hours after the first application without bathing and then to bathe 2 days after the last application. 74 Crotamiton facilitates the healing of lesions in secondary impetigo due to its antibacterial effect, while its antipruritic effect is an important benefit for patients. 75 , 76 In comparative studies, crotamiton is less effective than both topical lindane permethrin and oral ivermectin. 77 , 78 , 79 Crotamiton is generally preferred in infants and young children because of its low toxicity and rare irritation.
Systemic treatment
Systemic treatment is considered a second‐line option in cases where topical treatment is ineffective for common scabies, while it is the first‐line treatment for crusted scabies, often in combination with topical therapy.
Oral ivermectin
Ivermectin, a semisynthetic macrocyclic oral antibiotic with potent activity against nematodes and arthropods, has not yet received FDA approval for treating scabies. 80 In Turkey, oral ivermectin (Ziver® 3 mg, Humanis, Armec® 3 mg, Ali Raif) has been approved for scabies treatment since 2022.
A limited number of studies and our clinical experience suggest that an oral dose of 200 μg/kg of ivermectin taken twice at a 1‐week interval is an effective treatment for scabies (Tables 2 and 3). The instructions for using the product in Turkey state that the tablets should be taken on an empty stomach, without eating 2 hours before and after treatment. It has been reported that taking the drug after a fatty meal may increases its concentration in the blood. 81 , 82 The results reported suggest that this situation either increases bioavailability and efficacy or, conversely, increases the risk of toxicity without a significant increase in efficacy. 81 , 82
Table 2.
Oral ivermectin doses for scabies treatment 87
| Common scabies | Single dose of 200 μg/kg taken on an empty stomach, repeated 1 week later |
| Crusted scabies | Depending on clinical severity a : 3 doses: 200 μg/kg on days 1, 2, and 8; 5 doses: 200 μg/kg on Days 1, 2, 8, 9, and 15; 7 doses: 200 μg/kg on Days 1, 2, 8, 9, 15, 22, and 29 |
It is recommended to use 5% permethrin or benzyl benzoate daily for seven consecutive days (and then twice a week until clinical improvement) along with oral ivermectin treatment and, if necessary, by using topical keratolytics.
Table 3.
Dosing of oral ivermectin based on body weight 88
| Body weight (kg) | Single oral dose (number of 3 mg tablets a ) |
|---|---|
| 15–24 | 1 tablet |
| 25–35 | 2 tablets |
| 36–50 | 3 tablets |
| 51–65 | 4 tablets |
| 66–79 | 5 tablets |
| ≥80 | Calculated as 200 μg/kg |
In Turkey, oral ivermectin is available in packages containing 4, 10, 12, and 20 tablets of 3 mg each.
The serum half‐life of ivermectin is 18 hours, and elimination of the drug occurs through renal excretion of inactive metabolites after metabolism in the liver. 81 While dose adjustment is not required in patients with renal insufficiency, the safety of multiple doses of ivermectin in individuals with severe liver disease has not been studied. 80
It is recommended to administer three to seven doses of ivermectin for crusted scabies, depending on the severity of the disease (Table 2). 80 , 83 In those patients, oral ivermectin treatment should be given along with medication for topical scabies (e.g., permethrin or benzyl benzoate).
Oral ivermectin may also be used for prophylaxis in close contact with scabies‐infected individuals (especially in crusted scabies cases). A single oral dose of 200 μg/kg of oral ivermectin effectively prevents scabies in a large population. 84 , 85
Side effects of oral ivermectin and its use in different patient groups
To date, the use of ivermectin for the treatment of scabies has not been associated with any serious adverse events. 81 However, ivermectin is not recommended for use in children younger than 5 years of age or weighing less than 15 kg due to a lack of safety data and theoretical concerns about potential neurotoxicity. Reports supporting the safety and efficacy of ivermectin in children are increasing. 83 , 86 , 87 A recent literature review of 19 studies of oral ivermectin treatment for various parasitoses, including 1,088 children weighing <15 kg, found a low adverse event rate (1.4%) and reported that adverse events were mild and self‐limiting. 88 , 89 A recent large retrospective study in France of 170 scabies‐infected children weighing <15 kg who received oral ivermectin reported that the treatment was effective in 85% of the children, with only mild side effects such as eczema and gastrointestinal discomfort reported in nine patients. 87
While the use of oral ivermectin for the treatment of scabies in the senior age group is considered safe, it is classified as category C in pregnancy. 31 However, its use in pregnant women may be warranted if the potential benefits outweigh the potential risks. 90 The European guideline does not recommend the use of oral ivermectin for the treatment of scabies in pregnant women. 91 Nevertheless, reports documenting accidental administration of the drug to pregnant women have not reported adverse outcomes regarding the course of pregnancy and fetal health. 80 , 92 Oral ivermectin is used in France for the treatment of scabies during pregnancy as a first‐ or second‐line therapy. 93
In Turkey, the instructions for the use of ivermectin‐containing products state that less than 2% of the ivermectin applied is found in breast milk and that there are no data on its safety in newborns, recommending its use only if the expected benefit outweighs the potential risk in nursing mothers. 90 The same document suggests postponing treatment of nursing mothers until 1 week after delivery, although there are also reports that oral ivermectin can be safely used during breastfeeding. 15 , 93
The Centers for Disease Control and Prevention states that the potential risk of ivermectin in pregnant women is low and does not pose a risk to breastfeeding. However, due to limited data on the use of ivermectin in pregnant and breastfeeding women, treatment with permethrin should be preferred. 94
Consensus recommendations for scabies treatment in Turkey
According to the consensus recommendations for scabies treatment developed by the authors using the fuzzy Delphi method, the first‐line treatment for classic scabies is topical therapy. Accordingly, permethrin, balsam of Peru, sulfur mixtures, or benzyl benzoate should be used first. If treatment is unsuccessful, oral ivermectin is recommended. A combination of 5% permethrin or benzyl benzoate with oral ivermectin treatment is suggested for crusted scabies. The recommended treatment algorithm for scabies in Turkey and the details of the treatment are shown in Figure 4.
Figure 4.
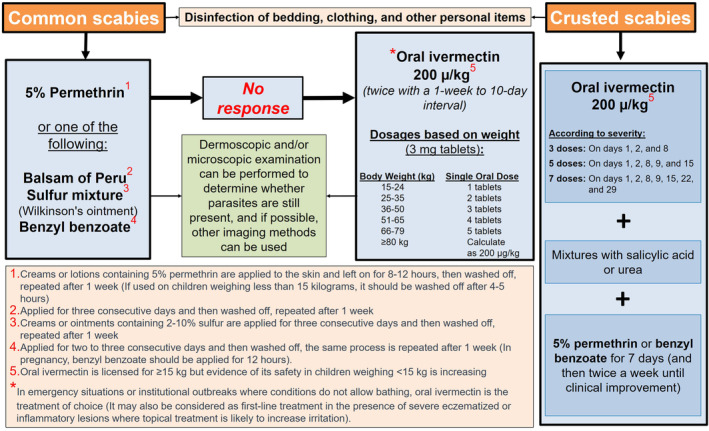
Recommended treatment algorithm for scabies in Turkey
Recommendations for effective treatment, prevention, and care
After effective treatment, pruritus usually resolves gradually within 7–10 days, but complete resolution may take up to 4 weeks. 28 In those receiving oral ivermectin treatment, pruritus may increase rather than decrease after the first dose. Therefore, patients should be warned not to skip the second dose of medication under the impression that their symptoms have worsened. In addition, eczema, which can develop due to irritation from scabies or the medicines used, may also cause pruritus, in which case second‐generation antihistamines and topical corticosteroids are recommended.
Because of the high potential for scabies to spread rapidly, especially in institutional settings, such as nursing homes, shelters, prisons, hospitals, military barracks, refugee camps, and disaster relief camps, close contacts should also be treated, and measures should be taken to minimize transmission.
Family members and those in close contact with the scabies patient should also be treated, even if there is no clinical evidence of scabies. 28 , 31
Although studies have shown that the risk of transmission by fomites is low (<3%), traditionally, washing all items that may carry mites at high temperatures (>50 °C) or storing items, such as clothing, linens, bedding, children's fabrics, and stuffed animals in sealed plastic bags, may reduce the spread of scabies. These recommendations should be strictly followed in crusted scabies patients. 83 , 95
Table 4 summarizes effective scabies treatment and prevention recommendations and important information about scabies infestations.
Table 4.
Recommendations for effective scabies treatment and prevention
|
Treatment and management of institutional scabies outbreaks
Scabies poses a high risk for outbreaks in institutions such as nursing homes, hospitals, prisons, and daycare centers. Because the incidence of scabies in these settings should be minimal, a patient with scabies symptoms should be treated for scabies even if no parasites are found during examinations after ruling out other diseases that may cause itching. 96
To declare a scabies outbreak in a long‐term or residential care facility, there must be at least one positive case or at least two clinically suspected cases. 97 Only one case of crusted scabies is considered an outbreak.
Table 5 summarizes the options for managing scabies outbreaks in institutional settings.
Table 5.
Steps in managing institutional scabies outbreaks
|
Conclusion
Scabies is a global health concern, particularly impacting low‐ and middle‐income countries. Precise diagnostics, like microscopic examination and dermoscopy, aid in tailored treatment. A holistic approach addressing environmental and socioeconomic factors is crucial. Implementing these strategies can alleviate symptoms and reduce transmission. These guidelines aim to promote a comprehensive understanding and effective management of scabies in Turkey and other countries where clear guidelines for controlling this disease do not exist and reflect a collaborative effort to mitigate the impact of this ancient disease.
Conflict of interest: None.
Funding source: None.
References
- 1. World Health Organization . Scabies. Available from: https://www.who.int/news‐room/fact‐sheets/detail/scabies (last accessed 09 December 2023).
- 2. Ständer S, Ständer S. Itch in scabies‐what do we know? Front Med (Lausanne). 2021;8:628392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chosidow O, Hay RJ. Control of scabies and secondary impetigo: optimising treatment effectiveness in endemic settings. Lancet Infect Dis. 2019;19:454–456. [DOI] [PubMed] [Google Scholar]
- 4. Mellanby K. Scabies. 2nd ed. UK: Hampton: E. W. Classey; 1972. [Google Scholar]
- 5. Mumcuoğlu KY, Taylan‐Özkan A, Gazi U. Uyuz etkeninin entomolojik ve immünolojik özellikleri. In: Uzun S, Durdu M, editors. Her Yönüyle Uyuz. Antalya: Kongre Kitabevi; 2023. p. 21–34. [Google Scholar]
- 6. Arlian LG, Estes SA, Vyszenski‐Moher DL. Prevalence of Sarcoptes scabiei in the homes and nursing homes of scabietic patients. J Am Acad Dermatol. 1988;19:806–811. [DOI] [PubMed] [Google Scholar]
- 7. Arlian LG, Runyan RA, Sorlie LB, Estes SA. Host‐seeking behavior of Sarcoptes scabiei . J Am Acad Dermatol. 1984;11:594–598. [DOI] [PubMed] [Google Scholar]
- 8. Uzun S. Uyuzun klinik özellikleri ve ayırıcı tanısı. In: Uzun S, Durdu M, editors. Her Yönüyle Uyuz. Antalya: Kongre Kitabevi; 2023. p. 49–68. [Google Scholar]
- 9. Cohen PR. Two for one: concurrent acquisition of molluscum contagiosum infection and scabies infestation after a single sexual encounter. Cureus. 2021;13:e20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montaño‐Castellón I, Marconi CSC, Saffe C, Brites C. Clinical and laboratory outcomes in HIV‐1 and HTLV‐1/2 coinfection: a systematic review. Front Public Health. 2022;10:820727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mellanby K. Transmission of scabies. Br Med J. 1941;2:405–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson CG, Mellanby K. The parasitology of human scabies. Parasitology. 1942;34:285–290. [Google Scholar]
- 13. Kouotou EA, Nansseu JRN, Sangare A, Bogne LLM, Sieleunou I, Adegbidi H, et al. Burden of human scabies in sub‐Saharan African prisons: evidence from the west region of Cameroon. Australas J Dermatol. 2018;59:e6–e10. [DOI] [PubMed] [Google Scholar]
- 14. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767–1774. [DOI] [PubMed] [Google Scholar]
- 15. Thomas C, Coates SJ, Engelman D, Chosidow O, Chang AY. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533–548. [DOI] [PubMed] [Google Scholar]
- 16. Korycinska J, Dzika E, Kloch M. Epidemiology of scabies in relation to socio‐economic and selected climatic factors in north‐east Poland. Ann Agric Environ Med. 2020;27:374–378. [DOI] [PubMed] [Google Scholar]
- 17. Tefera S, Teferi M, Ayalew A, Belete T, Hadush H. Prevalence of scabies and associated factors among primary school children in Raya Alamata District, Tigray, Ethiopia, 2017/2018. J Infect Dis Epidemiol. 2020;6:154. [Google Scholar]
- 18. Leung AKC, Lam JM, Leong KF. Scabies: a neglected global disease. Curr Pediatr Rev. 2020;16:33–42. [DOI] [PubMed] [Google Scholar]
- 19. Lassa S, Campbell MJ, Bennett CE. Epidemiology of scabies prevalence in the U.K. from general practice records. Br J Dermatol. 2011;164:1329–1334. [DOI] [PubMed] [Google Scholar]
- 20. Worku ED, Asemahagn MA, Endalifer ML. Determinants of scabies outbreak in Takusa district of Amhara Region, Northwest Ethiopia. J Public Health Afr. 2020;11:1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aktas H, Cebecik A. Changes in incidence and age distribution of scabies: a retrospective cohort study in a tertiary hospital. Arch Clin Exp Med. 2019;4:21–24. [Google Scholar]
- 22. Özden MG, Ertürk K, Kartal SP, Yayli S, Göktay F, Doğramacı CA, et al. An extraordinary outbreak of scabies in Turkey. J Eur Acad Dermatol Venereol. 2020;34:e818–e820. [DOI] [PubMed] [Google Scholar]
- 23. Baykal C, Atci T, Kutlay A, Baykut B, Türkoğlu Z. Scabies outbreak in Turkey in 2018‐2019. J Eur Acad Dermatol Venereol. 2021;35:e384–e385. [DOI] [PubMed] [Google Scholar]
- 24. Karaca Ural Z, Çatak B, Ağaoğlu E. Prevalence of scabies in the Covid‐19 pandemic period and determination of risk factors for scabies: a hospital‐based cross‐sectional study in Northeast Turkey. Acta Parasitol. 2022;67:802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Orion E, Matz H, Wolf R. Ectoparasitic sexually transmitted diseases: scabies and pediculosis. Clin Dermatol. 2004;22:513–519. [DOI] [PubMed] [Google Scholar]
- 26. Engelman D, Yoshizumi J, Hay RJ, Osti M, Micali G, Norton S, et al. The 2020 International alliance for the control of scabies consensus criteria for the diagnosis of scabies. Br J Dermatol. 2020;183:808–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tüzün Y, Parlak AH. Uyuz, bitlenme ve diğer paraziter hastalıklar. In: Tüzün Y, Gürer MA, Serdaroğlu S, Oğuz O, Aksungur VL, editors. Dermatoloji. 3rd ed. İstanbul: Nobel Tıp Kitapevleri; 2008. p. 683–728. [Google Scholar]
- 28. Uzun S, Durdu M. Derimiz için öneriler. 1st ed. İstanbul: İstanbul Tıp Kitapevleri; 2021. p. 668–673. [Google Scholar]
- 29. Walton SF. The immunology of susceptibility and resistance to scabies. Parasite Immunol. 2010;32:532–540. [DOI] [PubMed] [Google Scholar]
- 30. Roberts LJ, Huffam SE, Walton SF, Currie BJ. Crusted scabies: clinical and immunological findings in seventy‐eight patients and a review of the literature. J Infect. 2005;50:375–381. [DOI] [PubMed] [Google Scholar]
- 31. Raffi J, Suresh R, Butler DC. Review of scabies in the elderly. Dermatol Ther (Heidelb). 2019;9:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Executive committee of guideline for the diagnosis and treatment of scabies . Guideline for the diagnosis and treatment of scabies in Japan (third edition): executive committee of guideline for the diagnosis and treatment of scabies. J Dermatol. 2017;44:991–1014. [DOI] [PubMed] [Google Scholar]
- 33. Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340–347. [DOI] [PubMed] [Google Scholar]
- 34. Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79–90. [DOI] [PubMed] [Google Scholar]
- 35. Dia D, Dieng MT, Ndiaye AM, Ndiaye B, Develoux M. La gale croûteuse à Dakar a propos de 11 cas observés en une année [Crusted scabies in Dakar apropos of 11 cases seen in a year]. Dakar Med. 1999;44:243–245. [PubMed] [Google Scholar]
- 36. Goldstein BG, Goldstein AO. Scabies: epidemiology, clinical features, and diagnosis. UpToDate. Available from: https://medilib.ir/uptodate/show/4038 (last accessed 09 December 2023). [Google Scholar]
- 37. Yanes DA, Faith EF. Nodular scabies: a persistent nodular eruption. Dermatol Online J. 2018;24:13030. [PubMed] [Google Scholar]
- 38. Suresh R, Raffi J, Murase J, Butler D. Scabies in the elderly. Int J Women's Dermatol. 2019;5:282–283. [Google Scholar]
- 39. Orkin M. Scabies in AIDS. Semin Dermatol. 1993;12:9–14. [PubMed] [Google Scholar]
- 40. Jannic A, Bernigaud C, Brenaut E, Chosidow O. Scabies itch. Dermatol Clin. 2018;36:301–308. [DOI] [PubMed] [Google Scholar]
- 41. Nair PA, Vora RV, Jivani NB, Gandhi SS. A study of clinical profile and quality of life in patients with scabies at a rural tertiary care centre. J Clin Diagn Res. 2016;10:WC01–WC05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Durdu M, Yürekli A. Uyuzun tanısı. In: Uzun S, Durdu M, editors. Her Yönüyle Uyuz. Antalya: Kongre Kitabevi; 2023. p. 81–100. [Google Scholar]
- 43. Yürekli A, Can İ, Oğuz M. Using ultraviolet light in diagnosing scabies: scabies' sign via Wood's lamp. J Am Acad Dermatol. 2023;89:e195–e196. [DOI] [PubMed] [Google Scholar]
- 44. Engelman D, Cantey PT, Marks M, Solomon AW, Chang AY, Chosidow O, et al. The public health control of scabies: priorities for research and action. Lancet. 2019;394:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shoukat Q, Rizvi A, Wahood W, Coetzee S, Wrench A. Sight the mite: a meta‐analysis on the diagnosis of scabies. Cureus. 2023;15:e34390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zorbozan O, Türk BG, Acar A, Oraloğlu G, Ünver A, Töz S, et al. Comparison of skin scraping and standard superficial skin biopsy in the laboratory diagnosis of scabies. Turkiye Parazitol Derg. 2020;44:164–167. [DOI] [PubMed] [Google Scholar]
- 47. Neynaber S, Muehlstaedt M, Flaig MJ, Herzinger T. Use of superficial cyanoacrylate biopsy (SCAB) as an alternative for mite identification in scabies. Arch Dermatol. 2008;144:114–115. [DOI] [PubMed] [Google Scholar]
- 48. Katsumata K, Katsumata K. Simple method of detecting Sarcoptes scabiei var hominis mites among bedridden elderly patients suffering from severe scabies infestation using an adhesive‐tape. Intern Med. 2006;45:857–859. [DOI] [PubMed] [Google Scholar]
- 49. Errichetti E. Dermoscopy in monitoring and predicting therapeutic response in general dermatology (Non‐tumoral dermatoses): an up‐to‐date overview. Dermatol Ther (Heidelb). 2020;10:1199–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dupuy A, Dehen L, Bourrat E, Lacroix C, Benderdouche M, Dubertret L, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007;56:53–62. [DOI] [PubMed] [Google Scholar]
- 51. Wolfe C, Rudd E, Ng J, Weir J, Fuller LC. An unusual dermoscopic appearance of scabies. Br J Dermatol. 2021;185:e69. [DOI] [PubMed] [Google Scholar]
- 52. Trane L, Scarfì F, Silvestri F, Venturi F, Pisano L, De Giorgi V. Fluorescence advanced videodermatoscopy for the rapid and safe diagnosis of misdiagnosed scabies. Photodermatol Photoimmunol Photomed. 2021;37:230–232. [DOI] [PubMed] [Google Scholar]
- 53. Chavez‐Alvarez S, Villarreal‐Martinez A, Argenziano G, Ancer‐Arellano J, Ocampo‐Candiani J. Noodle pattern: a new dermoscopic pattern for crusted scabies (Norwegian scabies). J Eur Acad Dermatol Venereol. 2018;32:e46–e47. [DOI] [PubMed] [Google Scholar]
- 54. Kaur I, Jakhar D. Transillumination dermoscopy of finger web spaces for scabies. J Am Acad Dermatol. 2020;82:e87–e88. [DOI] [PubMed] [Google Scholar]
- 55. Nie J, Gou T, Xu L, Wang W, Zhang L, Lu Y. Misdiagnosed scabies correctly diagnosed by dermoscopy using ultraviolet light mode. Clin Exp Dermatol. 2021;46:1601–1603. [DOI] [PubMed] [Google Scholar]
- 56. Yürekli A. A new sign with UV dermoscope in the diagnosis of scabies: ball sign. Skin Res Technol. 2023;29:e13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ertugrul G, Aktas H. Comparison of sulfur ointment and permethrin treatments in scabies. Dermatol Ther. 2022;35:e15897. [DOI] [PubMed] [Google Scholar]
- 58. Yürekli A. Is there a really resistance to scabies treatment with permethrin? In vitro killing activity of permethrin on Sarcoptes scabiei from patients with resistant scabies. Dermatol Ther. 2022;35:e15260. [DOI] [PubMed] [Google Scholar]
- 59. Thadanipon K, Anothaisintawee T, Rattanasiri S, Thakkinstian A, Attia J. Efficacy and safety of antiscabietic agents: a systematic review and network meta‐analysis of randomized controlled trials. J Am Acad Dermatol. 2019;80:1435–1444. [DOI] [PubMed] [Google Scholar]
- 60. Porto I. Antiparasitic drugs and lactation: focus on anthelmintics, scabicides, and pediculicides. J Hum Lact. 2003;19:421–425. [DOI] [PubMed] [Google Scholar]
- 61. Thomas C, Rehmus W, Chang AY. Treatment practices in the management of scabies in infants younger than two months. Pediatr Dermatol. 2021;38:431–435. [DOI] [PubMed] [Google Scholar]
- 62. Goksugur SB, Karatas Z, Goksugur N, Bekdas M, Demircioglu F. Metabolic acidosis in an infant associated with permethrin toxicity. Pediatr Dermatol. 2015;32:e15–e17. [DOI] [PubMed] [Google Scholar]
- 63. Hoffmann JC, Mößner R, Schön MP, Lippert U. Topical scabies therapy with permethrin is effective and well tolerated in infants younger than two months. J Dtsch Dermatol Ges. 2019;17:597–600. [DOI] [PubMed] [Google Scholar]
- 64. Modi K, Patel D, Shwayder T. Scalp‐to‐toes application of permethrin for patients with scabies. Dermatol Online J. 2018;24:13030. [PubMed] [Google Scholar]
- 65. Veraldi S, De Micheli P, Schianchi R, Pontini P. A new treatment regimen with permethrin in scabies. G Ital Dermatol Venereol. 2018;153:491–493. [DOI] [PubMed] [Google Scholar]
- 66. Durdu M. Uyuzun topikal tedavisi. In: Uzun S, Durdu M, editors. Her Yönüyle Uyuz. Antalya: Kongre Kitabevi; 2023. p. 135–145. [Google Scholar]
- 67. Elgart ML. A risk‐benefit assessment of agents used in the treatment of scabies. Drug Saf. 1996;14:386–393. [DOI] [PubMed] [Google Scholar]
- 68. Roos TC, Alam M, Roos S, Merk HF, Bickers DR. Pharmacotherapy of ectoparasitic infections. Drugs. 2001;61:1067–1088. [DOI] [PubMed] [Google Scholar]
- 69. Balsam of Peru in the treatment of scabies. Hospital (Lond 1886). 1907;41:426–427. [PMC free article] [PubMed] [Google Scholar]
- 70. Seo SM, Park HM, Park IK. Larvicidal activity of ajowan (Trachyspermum ammi) and Peru balsam (Myroxylon pereira) oils and blends of their constituents against mosquito, Aedes aegypti, acute toxicity on water flea, Daphnia magna, and aqueous residue. J Agric Food Chem. 2012;60:5909–5914. [DOI] [PubMed] [Google Scholar]
- 71. King RE. The benzyl benzoate treatment of scabies. Br Med J. 1940;2:626–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ly F, Caumes E, Ndaw CA, Ndiaye B, Mahé A. Ivermectin versus benzyl benzoate applied once or twice to treat human scabies in Dakar, Senegal: a randomized controlled trial. Bull World Health Organ. 2009;87:424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mytton OT, McGready R, Lee SJ, Roberts CH, Ashley EA, Carrara VI, et al. Safety of benzyl benzoate lotion and permethrin in pregnancy: a retrospective matched cohort study. BJOG. 2007;114:582–587. [DOI] [PubMed] [Google Scholar]
- 74. Crotamiton . [WWW document]. https://reference.medscape.com/drug/eurax-crotamiton-topical-343500 Accessed 9 Dec 2023
- 75. Cubela V, Yawalkar SJ. Clinical experience with crotamiton cream and lotion in treatment of infants with scabies. Br J Clin Pract. 1978;32:229–231. [PubMed] [Google Scholar]
- 76. Konstantinov D, Stanoeva L, Yawalkar SJ. Crotamiton cream and lotion in the treatment of infants and young children with scabies. J Int Med Res. 1979;7:443–448. [DOI] [PubMed] [Google Scholar]
- 77. Rao MA, Raza N, Faheem M, Saleem MA. Comparison of efficacy of permethrin 5% cream with crotamiton 10% cream in patients with scabies. J Ayub Med Coll Abbottabad. 2019;31:230–232. [PubMed] [Google Scholar]
- 78. Walker GJ, Johnstone PW. Interventions for treating scabies. Cochrane Database Syst Rev. 2000;2:CD000320. [DOI] [PubMed] [Google Scholar]
- 79. Goldust M, Rezaee E, Raghifar R. Comparison of oral ivermectin versus crotamiton 10% cream in the treatment of scabies. Cutan Ocul Toxicol. 2014;33:333–336. [DOI] [PubMed] [Google Scholar]
- 80. Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717–725. [DOI] [PubMed] [Google Scholar]
- 81. Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol. 2002;42:1122–1133. [DOI] [PubMed] [Google Scholar]
- 82. Miyajima A, Hirota T, Sugioka A, Fukuzawa M, Sekine M, Yamamoto Y, et al. Effect of high‐fat meal intake on the pharmacokinetic profile of ivermectin in Japanese patients with scabies. J Dermatol. 2016;43:1030–1036. [DOI] [PubMed] [Google Scholar]
- 83. Morgado‐Carrasco D, Piquero‐Casals J, Podlipnik S. Tratamiento de la escabiosis [Treatment of scabies]. Aten Primaria. 2022;54:102231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kentley J, Fuller LC. Ivermectin. In: Wakelin SH, Maibach HI, Archer CB, editors. Handbook of systemic drug treatment in dermatology. 3rd ed. Oxon: CRC Press; 2023. p. 159–163. [Google Scholar]
- 85. Romani L, Whitfield MJ, Koroivueta J, Kama M, Wand H, Tikoduadua L, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305–2313. [DOI] [PubMed] [Google Scholar]
- 86. Del Mar Saez‐De‐Ocariz M, McKinster CD, Orozco‐Covarrubias L, Tamayo‐Sánchez L, Ruiz‐Maldonado R. Treatment of 18 children with scabies or cutaneous larva migrans using ivermectin. Clin Exp Dermatol. 2002;27:264–267. [DOI] [PubMed] [Google Scholar]
- 87. Levy M, Martin L, Bursztejn AC, Chiaverini C, Miquel J, Mahé E, et al. Ivermectin safety in infants and children under 15 kg treated for scabies: a multicentric observational study. Br J Dermatol. 2020;182:1003–1006. [DOI] [PubMed] [Google Scholar]
- 88. Jittamala P, Monteiro W, Smit MR, Pedrique B, Specht S, Chaccour CJ, et al. A systematic review and an individual patient data meta‐analysis of ivermectin use in children weighing less than fifteen kilograms: is it time to reconsider the current contraindication? PLoS Negl Trop Dis. 2021;15:e0009144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wilkins AL, Steer AC, Cranswick N, Gwee A. Question 1: is it safe to use ivermectin in children less than five years of age and weighing less than 15 kg? Arch Dis Child. 2018;103:514–519. [DOI] [PubMed] [Google Scholar]
- 90. Ziver tablet kısa ürün bilgisi dökümanı. Available from: https://titck.gov.tr/storage/Archive/2022/kubKtAttachments/UYGUNKB22.11.22_ca4cabaa‐9373‐4854‐b350‐f691a95076d0.pdf (last accessed 11 December 2023).
- 91. Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248–1253. [DOI] [PubMed] [Google Scholar]
- 92. Nicolas P, Maia MF, Bassat Q, Kobylinski KC, Monteiro W, Rabinovich NR, et al. Safety of oral ivermectin during pregnancy: a systematic review and meta‐analysis. Lancet Glob Health. 2020;8:e92–e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weill A, Bernigaud C, Mokni M, Gil S, Elefant E, Chosidow O. Scabies‐infested pregnant women: a critical therapeutic challenge. PLoS Negl Trop Dis. 2021;15:e0008929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. CDC, STI Tx quick guide, scabies. Available from: https://www.cdc.gov/stiapp/scabies.html (last accessed 11 December 2023)
- 95. Sunderkötter C, Feldmeier H, Fölster‐Holst R, Geisel B, Klinke‐Rehbein S, Nast A, et al. S1 guidelines on the diagnosis and treatment of scabies ‐ short version. J Dtsch Dermatol Ges. 2016;14:1155–1167. [DOI] [PubMed] [Google Scholar]
- 96. Mounsey KE, Murray HC, King M, Oprescu F. Retrospective analysis of institutional scabies outbreaks from 1984 to 2013: lessons learned and moving forward. Epidemiol Infect. 2016;144:2462–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mueller SM, Gysin S, Schweitzer M, Schwegler S, Haeusermann P, Itin P, et al. Implementation and evaluation of an algorithm for the management of scabies outbreaks. BMC Infect Dis. 2019;19:200. [DOI] [PMC free article] [PubMed] [Google Scholar]


