Abstract
This case report presents a 9-year-old child without underlying pathology, with a severe life-threatening non-diabetic metabolic ketoacidosis occurring less than 48 h after the onset of fasting and vomiting. The patient was admitted to the pediatric intensive care unit. He received volume expansion and maintenance fluid therapy which allowed a favorable evolution. Because of the unusual rapid onset of intense ketonemia and acidosis, a hereditary metabolic disease was investigated. The association between short fasting period and severe metabolic ketoacidosis has never been described in children outside of the neonatal period. This clinical case emphasizes urgent recognition, rigorous diagnostic and appropriate management in clinical practice.
Keywords: Ketoacidosis, Non-diabetic, Pediatric, Intensive care
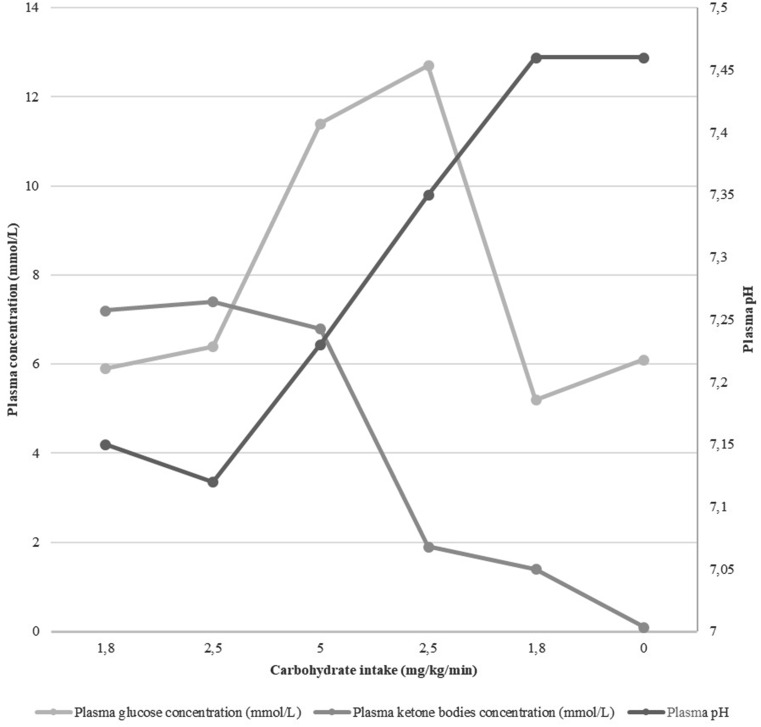
A 9-year-old child is brought to the pediatric emergency unit for vomiting. Abdominal pain and complete intake refusal began less than 48 h ago, followed by 10 episodes of vomiting in the last 12 h. There was no infectious context, no drug or toxic intake and no polyuro-polydipsic syndrome reported by the parents. This child had a factor VII deficiency (32%) without other medical, surgical, allergy or family past medical history. On physical examination, he was asthenic and pale, without fever. A Kussmaul’s dyspnea was noted, without abnormal oxygen saturation (99%). Hemodynamic examination revealed tachycardia at 138 bpm, skin recoloration time of 3 s, cold extremities and normal blood pressure (114/89mmHg). He had a 10% loss of his healthy weight (33 kg) with moderate dehydration signs (dark circles and dry mucous membrane). He had isolated ideomotor slowdown. Abdominal examination was normal. He received volume expansion with 10 mL/kg of 0.9% normal saline to restore peripheral circulation. He then had maintenance fluid therapy with isotonic and unbalanced 5% glucose solution at 1.5 L/m² per day. The first blood gas had a pH of 7.15, a pCO2 of 25.4 mmHg and a bicarbonate of 8.5 mmol/L. Plasma glucose concentration was 5.9 mmol/L and plasma ketone bodies concentration was 7.2 mmol/L. Euglycemia excludes the diabetic origin of the metabolic keto-acidosis. Biological assessment to calculate the patient’s anion gap (AG) were extend in order to exclude other metabolic acidosis etiologies. Laboratory findings were sodium 141 mmol/L; potassium 5 mmol/L; chloride 103 mmol/L; bicarbonate 7 mmol/L; blood urea nitrogen 8.8 mmol/L; creatinine 55 µmol/L; albumin 53 g/L; measured osmolality 334 mosmol/L and calculated osmolality 307 mosmol/L; lactate 1.8 mmol/L. Corrected AG was 27.75 (normal range 8–16). There was no aspirin intake. The glycol and methanol molecule’s dosages were negative. Three hours after the beginning of the fluid replacement blood tests were performed: pH of 7.12, a pCO2 of 24.8 mmHg and a bicarbonate of 8 mmol/L. Sodium was 145 mmol/L, chloride 117 mmol/L, lactate 1.7 mmol/L. Plasma glucose concentration was 6.4 mmol/L and plasma ketone bodies concentration was 7.4 mmol/L. The patient was admitted to the pediatric intensive care unit. Fluid therapy was increased to 3 L/m² per day with a third balanced fluid (Isofundin©) and two-thirds isotonic first 5%, then 10% glucose solution. Carbohydrate intake was increased to 5 mg/kg per minute (Fig. 1). The evolution was favorable with a pH normalization after 12 h and ketonemia reversing after 24 h. The patient’s cardio-pulmonary and neurologic examination was normal. He resumed an oral diet without any vomiting. Because of the onset of intense ketonemia with acidosis in a school-aged child with no medical history and with an unusual fasting time of less than 48 h, the work-up was completed in search of an underlying hereditary metabolic disease. The patient’s urinary and plasma organic acid chromatography reflected a profile compatible with significant ketosis of prolonged fasting and did not point to a particular pathology; it was considered as normal, as well as the acylcarnitine profiling studies and the redox points performed remotely, thus ruling out an inborn error of metabolism. Parent reported the usual consumption of 3 L of soda per day.
Fig. 1.
pH, Plasma glucose and ketone bodies concentration according to carbohydrate intake
Metabolic acidosis is a frequently encountered pathology in pediatric emergencies. It is defined by a drop in plasma pH below 7.38 associated with a drop in plasma bicarbonate concentration below 20mmol/L. Severe acidosis is defined by a pH less than 7.2 and can be life-threatening [1, 2]. We distinguish the acute forms (occurring in a few days) from the chronic forms (evolving for several weeks to years). Metabolic acidosis results from 3 possible mechanisms: (1) a decrease in bicarbonates may be caused by digestive or renal losses; (2) an increase in acid due to excessive endogenous production or exogenous ingestion of acid; (3) an increase in acid due to a decrease in acid excretion. To distinguish between these mechanisms, the AG must be calculated in any metabolic acidosis according to the formula AG = (Na+ + K+)– (HCO3− + Cl−). The presence of a non-measured anion will result in an increase in AG while a loss of bicarbonate will be compensated by an increase in chloremia and therefore will not modify the balance between anions and cations. Hypoalbuminemia leads to a decrease in the AG value, so AG must be corrected according to the formula AGc = AG + (40– albuminemia (g/L) x 0.25). A normal AG should be 12 ± 4 mmol/L. In the event of an increased AG, it is necessary to verify the absence of an increase in lactic acid and ketone bodies. Acute renal failure with increased urea must be ruled out as well as the intake of acetylsalicylic acid. When these four elements do not explain the increase in the AG, exogenous intoxication by methanol or glycol molecules must be also ruled out. The calculation of the osmotic gap (measured osmolality– calculated osmolality) helps support the diagnosis. An algorithm was proposed by the “Société de Réanimation de Langue Française” to investigate clinical pictures of acidosis [1].
In acidosis due to increased ketone bodies, the most common etiology in school-aged children is diabetes. Hyperglycemia greater than 11 mmol/L will complete the diagnosis. In case of euglycemia, organic aciduria should be investigated by performing chromatography of urinary organic acids. Ketoacidosis must suggest a deficit in ketolysis in the event of a rapid onset of symptoms or their persistence despite a well-conducted treatment. Redox points with determination of plasma glucose, lactate, pyruvate, free fatty acids, and ketone bodies can support this diagnosis. In case of the investigations ruled out all other diagnosis, the isolated elevation of ketonemia in a situation of fasting ketoacidosis should be retained to explain this acidosis. However, this situation appeared unusual given the age of the child and the onset speed of symptoms.
Severe fasting ketoacidosis cases have been reported in healthy adults after a prolonged sugar-free diet of several weeks [3–4]. Short fasting, i.e. less than 48 h, most often leads to isolated ketosis. It can be more severe and associated with acidosis under stress or insulin resistance. Thus, several cases of severe ketoacidosis post-short-fasting-period have been described in perioperative period and in pregnant or breastfeeding women [5–8]. A case has been described in a woman with a history of acute recurrent pancreatitis probably related to an insufficient pancreatic insulin response [9].
In children, although fasting ketosis is a reason for frequent visits to pediatric emergencies department, fasting ketoacidosis, a fortiori severe, is rare [10]. A few cases are described in infants, in a context of perioperative stress or underlying metabolic disease [11]. To our knowledge, there is no clinical case reported in the literature of severe ketoacidosis post-short-fasting-period in school-aged children without any associated pathology.
Insulin plays a major role in controlling energy production during fasting periods. The drop in insulinemia secondary to fasting leads to the initiation of neoglucogenesis and lipopysis in order to maintain cellular energy supply via the formation of ketone bodies. The elevation of ketone levels induces a retroactive control of the catabolic pathway with the participation of insulin. Thus, insulin insufficiency as in type 1 diabetes or insulin resistance impairs the modulation of ketone body production. In such cases, during fasting, lipolysis and gluconeogenesis are not slowed down and quickly lead to significant hyperketonemia [12].
In children, the risk of developing obesity or metabolic syndrome is increased by 60% for each additional serving of sugar-sweetened drinks consumed daily [13]. Also, its directly associated with an increase in insulin resistance [14]. In this young patient, with a high daily consumption of sugar-sweetened beverages, the hypothesis of a rapid runaway production of ketone bodies in response to fasting by insulin resistance is possible.
The therapeutic management of metabolic acidosis is the subject of experts’ clinical recommendations in France [1]. Management of the underlying pathology will allow for metabolic acidosis correction in most cases.
In the case of hyperketonemia in a fasting context, the first objective is to stimulate carbohydrate energy metabolism by providing a significant carbohydrate intake. Normal hepatic glucose production in adolescents is estimated to be around 2 to 4 mg/kg per minute [15]. It is necessary to provide a parenteral glucose dose at least equivalent to normal hepatic production [15]. The second objective is to correct the hydro-electrolyte disturbances. Isotonic crystalloid solutions are widely used to ensure volume expansion and maintenance. A 0.9% normal saline bolus is the most readily available and most often used solution in this context. However, its responsible for major chlorine inputs and can induce hyperchloremic metabolic acidosis. Balanced solutions such Isofundin© or PlasmaLyte© have compositions that are closer to the composition of plasmatic serum. These solutions have been developed in order to reduce the risk of hyperchloremic acidosis by limiting chlorine intake and replacing chloride with buffers. In adult critical care, the use of balanced fluids has demonstrated a significant decrease on the occurrence of hyperchloremia associated with a decrease in the rate of acute renal failure and mortality [16].
In children, several studies have shown benefits of balanced solutions [17]. The 2018 diabetic ketoacidosis consensus statement addresses this discussion and leave the choice to the practitioners regarding balanced solution use [18]. The 2021 European Resuscitation Council’s recommendations have been updated in favor of balanced crystalloid solutions to be used as first choice for bolus volume expansion [19].
In conclusion, several important points are raised by the description of this unique clinical case of severe fasting metabolic ketoacidosis. Short fasting periods can induce severe life-threatening metabolic ketoacidosis even in healthy children. Practitioners may encounter this more often due to the increase in children’s daily carbohydrate intake. The diagnostic approach in view of an acidosis picture must be systematic and can be supported by algorithms in order not to ignore all the possible etiologies. Hereditary metabolic diseases should be investigated for non-typical metabolic acidosis pictures. While managing metabolic acidosis, balanced crystalloid solutions are recommended for hydroelectrolytic disorders correction, in order not to worsen acidosis with hyperchloremia.
Abbreviations
- AG
Anion Gap
Author contributors
Dr C.R conceptualized and designed the study, drafted the initial manuscript, and critically reviewed and revised the manuscript. Drs C.F, C.A, C.L, A.A and I.G collected data, carried out the initial analyses, and critically reviewed and revised the manuscript. Dr D.B conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
No funding was secured for this study.
Data availability
Data will be made available on reasonable request.
Declarations
Conflict of interest
All authors have no conflicts of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jung B, Martinez M, Claessens Y-E, et al, et al. Diagnostic et prise en Charge de l’Acidose Métabolique recommandations formalisées d’experts communes Société de réanimation de langue française (SRLF)– Société française de médecine d’urgence (SFMU) Méd Intensive Réa. 2019;28(6):481–502. [Google Scholar]
- 2.Kraut JA, Madias NE. Metabolic acidosis: pathophysiology, diagnosis and management. Nat Rev Nephrol. 2010;6(5):274–85. [DOI] [PubMed]
- 3.Shah P, Isley WL. Ketoacidosis during a low-carbohydrate diet. N Engl J Med. 2006;354(1):97–8. doi: 10.1056/NEJMc052709. [DOI] [PubMed] [Google Scholar]
- 4.Mubarik A, Jupalli A, Iqbal AM, Muddassir S, Eddib A. Isolated starvation ketoacidosis: a Rare cause of severe metabolic acidosis presenting with a pH less than 7. Cureus. 2019;11(2):e4086. doi: 10.7759/cureus.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mostert M, Bonavia A. Starvation ketoacidosis as a cause of unexplained metabolic acidosis in the Perioperative Period. Am J Case Rep. 2016;17:755–8. doi: 10.12659/AJCR.900002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou W, Luo L. Preoperative prolonged fasting causes severe metabolic acidosis: a case report. Med (Baltim) 2019;98(41):e17434. doi: 10.1097/MD.0000000000017434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha N, Venkatram S, Diaz-Fuentes G. Starvation ketoacidosis: a cause of severe anion gap metabolic acidosis in pregnancy. Case Rep Crit Care. 2014;2014:906283. doi: 10.1155/2014/906283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monnier D, Goulenok T, Allary J, Zarrouk V, Fantin B. Cétose de jeûne chez une femme allaitante [Starvation ketosis in a breastfeeding woman] Rev Med Interne. 2015;36(12):854–8. doi: 10.1016/j.revmed.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Chan KH, Ramahi A. A rare case of severe starvation-induced ketoacidosis in a patient with recurrent pancreatitis. Cureus. 2020;12(3):e7368. doi: 10.7759/cureus.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Millar R, Chiappazzo A, Palmer B. Starvation ketosis in the Pediatric Emergency Department: a prospective Estimate of Prevalence. Pediatr Emerg Care. 2022;38(1):e147–50. doi: 10.1097/PEC.0000000000002188. [DOI] [PubMed] [Google Scholar]
- 11.Dennhardt N, Beck C, Huber D, et al. Impact of preoperative fasting times on blood glucose concentration, ketone bodies and acid-base balance in children younger than 36 months: a prospective observational study. Eur J Anaesthesiol. 2015;32(12):857–61. doi: 10.1097/EJA.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 12.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357(9255):505–8. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 14.Bremer AA, Auinger P, Byrd RS. Relationship between insulin resistance-associated metabolic parameters and anthropometric measurements with sugar-sweetened beverage intake and physical activity levels in US adolescents: findings from the 1999–2004 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2009;163(4):328–35. doi: 10.1001/archpediatrics.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labarthe F, Willot S, Roullet-Renoleau N, et al. Nutrition des maladies métaboliques rares en pédiatrie. Réanimation. 2010;19(5):441–7. doi: 10.1016/j.reaurg.2010.04.004. [DOI] [Google Scholar]
- 16.Semler MW, Self WH, Rice TW. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(20):1951. doi: 10.1056/NEJMc1804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehr AR, Rached-d’Astous S, Barrowman N, et al. Balanced Versus Unbalanced Fluid in critically Ill children: systematic review and Meta-analysis. Pediatr Crit Care Med. 2022;23(3):181–91. doi: 10.1097/PCC.0000000000002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfsdorf JI, Glaser N, Agus M et al. (2018). Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State: A Consensus Statement from the International Society for Pediatric and Adolescent Diabetes. Pediatric Diabetes. 2018;19(27):155–177. [DOI] [PubMed]
- 19.Van de Voorde P, Turner NM, Djakow J, et al. European Resuscitation Council guidelines 2021: paediatric life support. Resuscitation. 2021;161:327–87. doi: 10.1016/j.resuscitation.2021.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on reasonable request.