Abstract
Background
This study was designed to compare the effects of four meal patterns on liver steatosis, fibrosis, and biochemical factors in patients with Nonalcoholic fatty liver disease (NAFLD).
Methods
The 12-week intervention was performed on 123 patients with NAFLD who were randomly allocated into four groups: “3-meals”, “skipping breakfast”, “skipping dinner”, and " 3 meals and 3 snacks per day.” group. The assessment of liver steatosis, fibrosis, biochemical factors, and anthropometrical evaluation were performed at baseline and at end of the study.
Results
A significant improvement was found in the liver steatosis and fibrosis among the patients who consumed 3 meals, 3 snacks compared to the other groups (P < 0.001). In addition, a higher reduction was observed in serum levels of alanine amino transferase (ALT) (20.93 ± 23.37 mg/dl, P < 0.001), aspartate aminotransferase (AST) (17.15 ± 16.48 mg/dl, P < 0.001), gamma-glutamyl transferase(GGT) (13.43 ± 13.41 mg/dl; P < 0.001), and alkaline phosphatase (ALK) (47.19 ± 60.51 mg/dl; P = 0.004) in patients who consumed 3 meals, 3 snacks, while the concentration of liver enzymes in patients who consumed 3 meals increased significantly. At the end of the study, there was a significant increase in the fasting blood sugar (FBS) concentration in the “skipping breakfast” group (17.51 ± 38.85 mg/dl; P = 0.011) and “3-meals” group (17.51 ± 38.85 mg/dl, P = 0.03).
Conclusion
Consuming 3 meals, 3 snack per day significantly improves disease severity and biochemical factors in NAFLD patients. Further studies are warranted.
Trial registration number
: IRCT20201010048982N2. Name of the registry: Urmia University of Medical Sciences. Date of registration: 2021-08-22, 1400/05/31. URL of trial registry record: https://www.irct.ir/search/result?query=IRCT20201010048982N2.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-023-01375-2.
Keywords: Meal pattern, Non-alcoholic fatty Liver Disease, Liver enzymes, Clinical trial, Steatosis
Introduction
Non-alcoholic fatty liver disease (NAFLD) is an emerging public health issue, not only being a major cause of liver-related morbidity and mortality worldwide, but also an independent risk factor for other chronic disorders [1, 2]. This disease is asymptomatic in the early stages, however, if untreated, it progresses to steatosis and liver fibrosis. In fact, the disease includes a wide range of liver damage, from steatosis to non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and hepatocellular carcinoma [3]. The prevalence of this disease varies in different countries for reasons such as differences in lifestyle, dietary pattern and other environmental factors. Based on the recent evaluation, NAFLD affects more than one billion individuals worldwide [4].
Various risk factors are involved in the etiology of NAFLD, such as type 2 diabetes (T2DM), obesity, inactive lifestyle and unhealthy eating patterns [5]. A systematic review study that evaluated data of 222,816 patients with T2DM from 25 countries found that more than 60% of diabetic patients had NAFLD [6]. On the other hand, obesity is a major risk factor for NAFLD through various mechanisms such as increased insulin resistance and inflammation [7].
There is no approved pharmacological therapy for the treatment of NAFLD. Nutrition is one of the environmental factors in the development and progression of NAFLD. Numerous studies have reported that adherence to diets contained higher amounts of sugars, fructose, saturated fatty acids (SFAs) and some calorie dense foods increases the risk of NAFLD [8, 9].
The circadian system temporally organizes numerous physiological and behavioral variables such as sleep–wake cycle, metabolism, body temperature, blood pressure, or endocrine secretions [10, 11]. Therefore, a link has been established between circadian rhythms and components of metabolic syndrome, such as glucose metabolism and obesity, and and meal frequency cause a considerable change in output of the peripheral clocks [12]. It is possible that in communities that experience more night eating and less sleep at night, the circadian rhythm is more prominent. Night light, nutritional intake, and weather are vital contributors to circadian rhythm [13]. So, the timing of food consumption, the amount of meals consumed during the day, and the regularity of these meals are associated with an increased risk of obesity, metabolic syndrome, and some other chronic disorders such as NAFLD [14–16]. Some studies have reported an inverse relationship between the number of meals and snacks and the risk of obesity and metabolic syndrome. In addition, it has been reported that increasing eating frequency can help energy metabolism, glucose and insulin management, and reduce body weight and body fat [17]. However, other researchers believe that increasing the number of meals and snacks increases the risk of obesity and abdominal and liver fat due to higher calorie intake, food stimuli, hunger, and the desire to eat [18]. Ha et al. in a cross-sectional study among the Korean population showed that there was an inverse correlation between meal timing and frequency with metabolic syndrome prevalence [19]. Furthermore, a study by Stote et al. that restricted meals to one meal per day for eight weeks in healthy adults resulted in a reduction in their weight, but no apparent difference in the levels of serum lipids, glucose or insulin between them and those who consumed three meals per day [20].
Insulin resistance (IR) is a main risk factor for NAFLD. It has been reported that some dietary behaviors such as eating frequency and timing influence insulin resistance and systemic inflammation [21]. Therefore, it is possible that higher eating frequency can suppress a sharp increase in the insulin concentration in serum and reduce the risk of NAFLD [22]. However, some studies showed beneficial effects for various fasting diets in the prevention and treatment of fatty liver disease. A RCT study reported that intermittent fasting for 4 weeks among the patients with NAFLD led to a significant reduction in weight, triglyceride, and liver enzymes [23]. Similar findings were shown in some others studies [24, 25], which increases the discrepancy about the existence of a clear relationship between the meal frequency and intervals with NAFLD.
Due to the importance of a healthy diet and behavior for the prevention and treatment of NAFLD and equivocal results regarding skipping meals and fasting diets, the aim of the present study was to compare the effect of four meal patterns on liver fibrosis, steatosis and liver enzymes, lipid profile, and anthropometric indices in patients with NAFLD.
Materials and methods
Recruitment and eligibility screening
This parallel-arm RCT was conducted from June 2021 to September 2021 at the Imam Khomeini Teaching Hospital in Urmia, Iran. This study was an open-label clinical trial on patients with Nonalcoholic fatty liver disease (NAFLD) referred to the Hepatology clinic. The diagnosis of NAFLD was made based on the presence of steatosis on Fibroscan examination. Men and women, 18–70 years old, without any of the following exclusion criteria were eligible to participate: (1) adherence to specific diets such as weight loss diets, (2) pregnancy or lactation, (3) BMI > 40, (4) viral hepatitis and ascites, (5) evidence of excessive alcohol use (> 10 g/day), (6) taking medications to lower blood sugar or lipids, (7) mental disorders, (8) therapy with approved medicine that may have potential benefit in the treatment of NAFLD (i.e. vitamin E, betaine, pioglitazone, rosiglitazone, pentoxifylline and gemfibrozil), (9) chronic diseases such as cardiovascular disease, diabetes, cancer, and chronic kidney failure.
Study design
This study was designed as a randomized open labeled controlled trial with four parallel groups. The sample size required for this study was calculated using the following formula based on the frequency of NAFLD treatment percentage (64% in the dietitian-led lifestyle modification program group and 20% in the usual care group) in previous study [26]. With 95% confidence interval and 80% test power, the sample size was calculated 16 patients in each group. However, considering 20% loss for the groups, the final sample size for each group was calculated to be 28 people.
 |
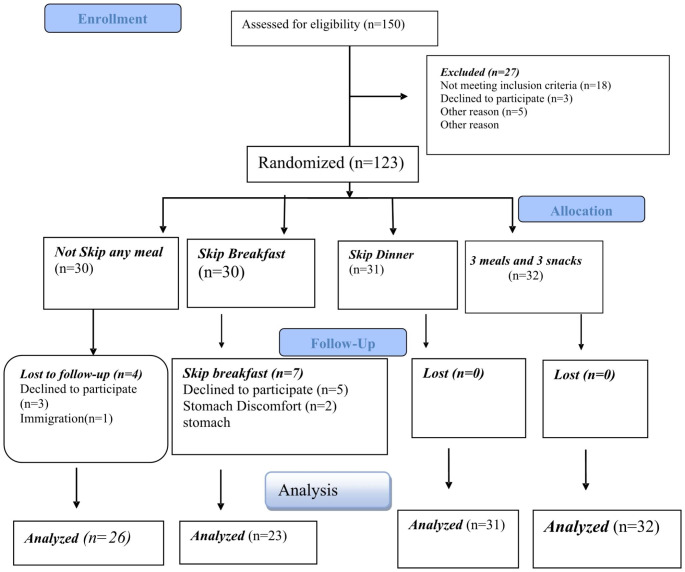
A total of 150 patients with NAFLD were consecutively screened from June 2021 to September 2021 and among them 123 subjects had necessary criteria (Fig. 1). Informed and written consent was received from all patients who met the necessary criteria to enter the study. All study procedures were approved by Ethics Committee at Urmia University of Medical Sciences (IR.UMSU.REC.1399.350) and registered in the Iranian Registry of Clinical Trials (IRCT20201010048982N2).
Fig. 1.
Flowchart of the study protocol
Patients were randomly allocated into four groups: (1) the first group (skipping breakfast): who was recommended to eliminate their breakfast and during the day, in addition to lunch and dinner, eat two snacks in the evening and at the end of the night, (2) the second group (skipping dinner): who was recommended to eliminate their dinner and in addition to breakfast and lunch, eat two snacks during the day and their last daily meal should be in the evening, (3) the third group (3 meals): who was recommended to eat only three main meals (breakfast, lunch and dinner) during the day, and (4) the fourth group (3 meals, 3 snacks): who was recommended to eat three main meals (breakfast, lunch and dinner) and three snacks. In order to homogenize groups regarding consumption of food groups, all of participants were advised to follow the recommendations of the Food and Agriculture Organization (FAO) for Iranians. All participants asked not to change their physical activity and medications during the study.
Clinical, para clinical and dietary intake assessments
At the first visit (week 0), in each group, necessary explanations regarding the types of meal patterns were given to participants and baseline data was collected. To evaluate the food consumption and physical activity, three days 24-hour food recall (including at least one weekday and at least one weekend) and a classified physical activity questionnaire based on Metabolic Equivalent of Task (MET) at baseline and each month during study was used, respectively. The information obtained from 24-h food recall questionnaire was analyzed by Nutritionist IV software to determine the mean caloric intake and consumed food groups [27].
To evaluate biochemical variables, at the beginning and end of the study, 10 cc of blood was taken from all participants after 12 h of fasting. All biochemical tests were measured in the same laboratory by an expert team. Serum levels of G-glutamyltransferase (GGT) was assessed by enzymatic colorimetric assay using special kit (Parsazmoun, Tehran, Iran). However, we used the photometric assay and special kits (Parsazmoun, Tehran, Iran) for measuring serum levels of ALT, AST and ALP. Total cholesterol, low density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were measured by enzymatic photometric method, using Parsazmoon test kits (Parsazmoun, Tehran, Iran). At the beginning and end of the study, liver steatosis and fibrosis were measured by Transient Elastography using a fibroscan (EchoSens, Paris, France) by a gastroenterologist. Fibrosis score was classified into three categories based on its severity: F0 to F1 (2 to 7 kPa) means there is little or no scarring on the liver. A fibrosis score of F2 (7.5 to 10 kPa) indicates moderate scarring has spread outside the liver. A fibrosis score of F3 (10 to 14 kPa) indicates severe spread scarring which disrupts normal blood flow [28].
The weight of all participants was measured using standard scale (Seca, Germany) with the least clothing and 0.1 kg accuracy. However, a standard height meter with an accuracy of 0.5 cm was used to measure the height.
Follow-up
To follow up, patients were asked to come to the University Nutrition Clinic each month during the study to check their diet. Also, patients were called every week and the necessary reminders were made. The counseling program for patients included an in-person counseling sessions, presentation of training booklets, and related text messages. If the rate of adherence to meal patterns was less than 85%, they excluded from the study. The final patient follow-up was in December 2021 included a transient elastography assay in addition to all of the follow-up assessments.
Primary and secondary outcomes
The primary outcome of this study was the significant changes in the liver steatosis and fibrosis. Secondary outcome measures were significant changes in serum concentrations of liver enzymes, lipid profile, FBS, and anthropometric measures.
Statistical analysis
To minimize attrition bias, we used the both intention-to-treat principle (ITT) and per-protocol (PP) analysis and in the ITT analysis, participants were evaluated according to the baseline sample size. Missing data were inserted using the last-observation-carried-forward procedure. Normality of data was examined by Shapiro–Wilk test and abnormal data were log-transformed for analysis. General and demographic information related to the participants in the study was collected from all participants at the beginning of the study using specific questionnaire. Also, for demographical data assessment, we used the one-way analysis of variance. Pre- and post-intervention items in each of the meal pattern groups were assessed by paired t test. The repeated measure ANOVA was used to compare the change in dietary intake and physical activity in different groups and time frames (baseline, 1st, 2nd and 3rd months). Also, we used the ANCOVA test for adjustment of covariates in two models: model 1 was crude and model 2 adjusted for disease duration, family income, supplements used, energy intake, and food groups consumption. Moreover, we used the Pearson Chi-square test for evaluation of liver steatosis and fibrosis between groups and also Generalized Linear Model (GLM) for adjusting models. SPSS version 26 for Windows (SPSS Inc., Chicago, IL, USA) was used for data evaluation. P < 0.05 was considered as significant level.
Results
At the beginning of the study, 123 patients with NAFLD were included in the study, and 112 of them completed the three-month intervention period. Of the 11 people who were excluded from the study, eight people were excluded from the study due to unwillingness to continue participating in the study, two patients due the stomach discomfort, and one patient due to the migration. In ITT analysis, however, all 123 participants were included. Information on the demographic characteristics of the study participants is reported in Table 1. The mean age of all participants was 43.70 ± 11.45 years, and we didn’t find any significant difference between four groups in term of age (P = 0.611). Disease duration in all of the patients was 2.74 ± 1.10 years, that was significantly different between groups (P < 0.001). Also, we found a significant different between groups in term of nutritional supplements usage (P = 0.03). However, there wasn’t significant differences between groups in term of family size (P = 0.188), education (P = 0.562), and height (P = 0.911).
Table 1.
General characteristics of the non-alcoholic fatty liver disease participants
| Variable | Total (n = 123) | G1 (n = 30) | G2 (n = 30) | G3(n = 31) | G4 (n = 32) | P-value1 |
|---|---|---|---|---|---|---|
| Age (years) | 43.70 (11.45) | 45.46 (11.65) | 41.9 (7.90) | 44.64 (13.80) | 42.84 (11.78) | 0.611 |
| Height(cm) | 171.60 (9.90) | 170.93(8.79) | 170.93(9.57) | 172.29 (9.78) | 172.21 (11.55) | 0.911 |
| Education (years) | 9.11 (3.10) | 8.80(3.01) | 9.63(3.21) | 9.38(3.05) | 8.65(3.19) | 0.562 |
| Family size (numbers) | 4.43(1.36) | 4.63(1.49) | 4.00 (1.17) | 4.38(1.30) | 4.68 (1.42) | 0.188 |
| Monthly income (Million Tomans) | 1.67(2.48) | 2.56(2.81) | 1.13(1.83) | 1.90(1.35) | 2.95(2.88) | < 0.001 |
| Nutritional supplements usage (NO/YES %) | 91.9/8.1 | 96.7/3.3 | 100/0.00 | 100/0.00 | 71.9/28.1 | 0.03 |
| Disease duration (years) | 2.74(1.10) | 2.03(1.18) | 2.93(0.9) | 3.22(0.92) | 2.78(1.06) | < 0.001 |
Data are presented as mean (SD) for quantitative variables, 1. The difference between groups calculated using ANOVA test, G1, group without any meal skipping; G2, group with breakfast skipping; G3, group with dinner skipping; G4, 3 meals and 3 snacks group
Comparison of dietary intake and physical activity between groups
The results for dietary intake and physical activity are shown in Table 2. There was no significant difference in the amount of fruit consumption among all the participants in the study over time (P = 0.834) and also in terms of the interaction of time and groups (P = 0.194). However, a significant difference was observed between individuals by group (P < 0.001). Cereal consumption was also significant in the participants in terms of time intervals, group differences (P < 0.001), and group and time interaction (P < 0.01). In term of meat and poultry, vegetables, and dairies intake, only the group difference was significant (p < 0.05). We also found a significant difference in group, time, and time and group interaction for total energy intake (P < 0.001) and fat intake (P < 0.05), while only difference of group and interaction of group and time were significant in sugar consumption (p < 0.001). In term of physical activity, we didn’t find any significant difference between groups during the study (P = 0.707).
Table 2.
Comparison of dietary food intakes and physical activity among 4 groups including daily 3 meals, skipping breakfast, skipping dinner, and 3 meals and 3 snacks at the baseline and following intervals1
| Variable2 | Time | G1 (n = 30) | G2 (n = 30) | G3(n = 31) | G4(n = 32) | Pgroup3 | Ptime4 | Pgroup*time5 |
|---|---|---|---|---|---|---|---|---|
| Total fruits (servings/day) | Baseline | 2.65 (0.96) | 2.15 (0.64) | 2.48 (0.81) | 1.87 (0.51) | < 0.001 | 0.834 | 0.194 |
| 1st month | 2.80 (0.61) | 2.81 (0.73) | 1.83 (0.59) | 2.59 (0.44) | ||||
| 2nd month | 2.58 (0.6) | 2.71(0.65) | 1.79 (0.77) | 2.83 (0.44) | ||||
| 3rd month | 2.74 (0.62) | 2.67(0.67) | 1.9 (0.7) | 2.78 (0.53) | ||||
| Cereals (servings/day) | Baseline | 8.10 (0.85) | 8.20 (0.84) | 8.24 (0.54) | 7.88 (0.68) | < 0.001 | < 0.001 | < 0.001 |
| 1st month | 8.25 (0.72) | 6.84 (0.75) | 7.16 (0.62) | 8.00 (0.57) | ||||
| 2nd month | 8.65 (0.89) | 6.44 (0.83) | 6.83 (0.95) | 8.12 (0.51) | ||||
| 3rd month | 8.60 (0.95) | 5.97 (1.97) | 6.48(1.15) | 7.97 (0.56) | ||||
| Meats and poultry (servings/day) | Baseline | 2.51 (0.74) | 2.38 (0.35) | 2.41 (0.44) | 2.60 (0.83) | 0.001 | 0.232 | 0.124 |
| 1st month | 2.50 (0.35) | 2.41 (0.5) | 2.12 (0.67) | 2.82 (0.88) | ||||
| 2nd month | 2.43 (0.59) | 2.41 (0.51) | 2.25 (0.6) | 2.89 (0.87) | ||||
| 3rd month | 2.58 (0.43) | 2.40 (0.63) | 2.41 (0.46) | 2.80 (0.71) | ||||
| Dairies (servings/day) | Baseline | 1.71(0.59) | 1.98 (0.7) | 1.64 (0.39) | 1.42 (0.4) | < 0.001 | 0.526 | 0.054 |
| 1st month | 1.50 (0.41) | 1.19 (0.45) | 1.27 (0.36) | 1.63 (0.5) | ||||
| 2nd month | 1.51 (0.49) | 1.03 (0.34) | 1.25 (0.28) | 1.68 (0.43) | ||||
| 3rd month | 1.59 (0.47) | 0.91 (0.37) | 1.51 (0.39) | 1.63 (0.43) | ||||
| Vegetables (servings/day) | Baseline | 2.81 (0.53) | 2.85 (0.54) | 3.35 (0.76) | 2.33 (0.47) | <0.001 | 0.635 | 0.164 |
| 1st month | 2.36 (0.54) | 2.71 (0.67) | 3.16 (0.41) | 2.76(0.47) | ||||
| 2nd month | 2.36(0.75) | 2.56(0.65) | 3.12(0.4) | 2.85(0.57) | ||||
| 3rd month | 2.38 (0.62) | 2.60 (0.63) | 3 (0.54) | 2.98 (0.51) | ||||
| Sugars (servings/day) | Baseline | 4.08 (0.5) | 4.03 (0.7) | 4.98 (0.52) | 3.23 (0.9) | <0.001 | 0.911 | < 0.001 |
| 1st month | 4.26 (1.05) | 4.23(0.98) | 5.09 (0.65) | (1.22) 2.96 | ||||
| 2nd month | 4.51 (1.20) | 5.40 (0.86) | 4.62 (0.56) | 2.87 (3.31) | ||||
| 3rd month | 4.61 (1.34) | 5.64 (1.88) | 4.82 (0.63) | 2.82 (1.5) | ||||
| Fats and oils (servings/day) | Baseline | 5.71 (0.86) | 4.71 (0.61) | 5.64 (0.7) | 5.41 (0.57) | < 0.001 | 0.001 | < 0.001 |
| 1st month | 5.72(0.94) | 5.70 (0.56) | 4.93 (0.77) | 5.21 (0.71) | ||||
| 2nd month | 5.97 (1.07) | 6.06 (0.68) | 4.80 (0.92) | 5 (0.76) | ||||
| 3rd month | 6.10 (1.22) | 6.31 (0.84) | 4.79 (0.9) | 4.95 (0.65) | ||||
| Energy intake (kcal/day) | Baseline | 1690 (200) | 1642(115) | 1734 (148) | 1518 (104) | < 0.001 | 0.001 | < 0.001 |
| 1st month | 1683 (158) | 1631 (127) | 1482 (102) | 1579(117) | ||||
| 2nd month | 1752 (196) | 1646 (102) | 1465 (122) | 1600 (120) | ||||
| 3rd month | 1765 (231) | 1633 (111) | 1502 (127) | 1588 (120) | ||||
| Physical activity (METs.hr/day) | Baseline | 32.84 (0.85) | 32.81(1.18) | 32.56 (1.04) | 32.93 (0.96) | 0.08 | 0.228 | 0.707 |
| 1st month | 32.90 (1.08) | 32.62(1.28) | 32.56 (0.99) | 32.70 (1.07) | ||||
| 2nd month | 33 (0.91) | 32.50(1.29) | 32.40 (1.00) | 32.58 (0.98) | ||||
| 3rd month | 33.17 (0.95) | 32.90(1.20) | 32.71 (0.94) | 32.49(0.92) |
1.Based on the intention to treat model; 2. Data are presented as mean (SD); 3. Calculated using repeated measure ANOVA to compare intakes during three months. G1, daily 3 meals; G2, skipping breakfast; G3, skipping dinner and G4, 3 meals and 3 snacks group. P3, P for group; P4, P for time differences; P5, P for time and groups interactions
Comparison of anthropometrical and biochemical variables in different meal groups
Table 3 presents comparison of the mean (SD) of the liver enzymes, anthropometric measurements, FBS, and lipid profile between four groups at the baseline and end of the study. In the per-protocol analysis “3 meals − 3 snacks” group (-5.32 ± 1.81 kg) and “skipping dinner” group (-6.08 ± 2.08 kg) had significantly higher weight reduction compared to other groups both in crude and adjusted models (P < 0.001). In term of waist circumference, 3 meals − 3 snacks group (-4.87 ± 3.67 cm) had significantly higher reduction compared to other 3 groups and “skipping dinner” group (-1.59 ± 1.97 kg) had significantly higher reduction compared to other 2 groups both in crude (P < 0.001) and adjusted (P = 0.001) models.
Table 3.
Metabolic biomarkers and hepatic characteristic changes before and after 12 weeks interventions among 4 groups including daily 3 meals, skipping breakfast, skipping dinner, and 3 meals and 3 snacks
| Variable | G1 (n = 26) | G2(n = 23) | G3 (n = 31) | G4 (n = 32) | P2 | P3 | |
|---|---|---|---|---|---|---|---|
| Weight (Kg) | Baseline | 81.09 (12.46) | 79.47 (9.65) | 83.17 (12.46) | 85.92 (17.47) | 0.275 | 0.151 |
| End of the study | 80.25 (12.46) | 79.39 (9.95) | 77.09 (9.58) | 80.59 (16.78) | 0.678 | 0.158 | |
| Changes | -0.84 (3.64) cd | 0.08 (2.40) cd | -6.08 (2.08) ab | -5.32 (1.81) ab | < 0.001 | < 0.001 | |
| p-value1 | 0.248 | 0.86 | < 0.001 | < 0.001 | - | ||
| WC (cm) | Baseline | 99.57 (7.44) | 98.82 (4.67) c | 104.90 (4.66) b | 104.75 (10.57) | 0.006 | 0.004 |
| End of the study | 100.25 (6.38) | 99.54 (5.15) | 103.30 (7.23) | 99.87 (9.36) | 0.188 | 0.042 | |
| Changes | 0.67 (3.73) cd | 0.71 (1.61) cd | -1.59 (1.97) abd | -4.87 (3.67) abc | < 0.001 | 0.001 | |
| p-value1 | 0.362 | 0.045 | < 0.001 | < 0.001 | |||
| FBS (mg/dl) | Baseline | 97.42 (11.30) d | 96.86 (17.92) cd | 104.77 (12.48) ab | 105.84 (14.15) ab | 0.028 | 0.031 |
| End of the study | 114.94 (39.18) cd | 119.95 (40.79) cd | 93.11 (9.43) ab | 93.46 (15.07) ab | < 0.001 | 0.029 | |
| Changes | 17.51(38.85) cd | 23.08 (40.06) cd | -11.66 (7.95) ab | -12.37 (15.07) ab | < 0.001 | 0.003 | |
| p-value1 | 0.03 | 0.011 | < 0.001 | < 0.001 | |||
| TG (mg/dl) | Baseline | 167.46 (100.79) | 183.60 (114.33) | 190.93 (89.15) | 194.09 (86.47) | 0.74 | 0.548 |
| End of the study | 239.11 (99.39) cd | 224.43 (116.47) cd | 139.54 (46.70) ab | 144.14 (55.45) ab | < 0.001 | 0.115 | |
| Changes | 71.65 (83.68) cd | 40.81 (73.89) cd | -51.38 (62.59) ab | -49.95 (55.93) ab | < 0.001 | < 0.001 | |
| p-value1 | < 0.001 | 0.015 | < 0.001 | < 0.001 | |||
| Cholesterol (mg/dl) | Baseline | 171.26 (35.08) | 180.08 (52.35) | 186.61 (86.91) | 181.65 (35.08) | 0.806 | 0.882 |
| End of the study | 214.03 (44.85) cd | 195.04 (45.10) cd | 149.93 (59.87) ab | 146.09 (34.60) ab | < 0.001 | < 0.001 | |
| Changes | 42.76 (50.07) cd | 14.95 (48.86) cd | -36.67 (46.67) ab | -35.56 (32.19) ab | < 0.001 | < 0.001 | |
| p-value1 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||
| LDL (mg/dl) | Baseline | 106.03 (37.03) | 105.24 (21.69) | 115.25 (36.73) | 116.89 (30.99) | 0.418 | 0.686 |
| End of the study | 124.92 (37.78) | 132.76 (23.61) d | 128.65 (51.22) | 102.51 (35.92) b | 0.017 | 0.660 | |
| Changes | 18.88 (31.97) d | 27.51 (19.85) d | 13.39 (49.99) d | -14.37 (20.58) abc | < 0.001 | 0.174 | |
| p-value1 | 0.002 | < 0.001 | 0.029 | < 0.001 | |||
| HDL (mg/dl) | Baseline | 53.30 (15.35) cd | 47.08 (8.61) c | 37.52 (11.70) ab | 42.16 (35.08) a | < 0.001 | 0.001 |
| End of the study | 44.36 (11.57) | 41.41 (13.15) | 45.24 (9.22) | 46.07 (9.10) | 0.43 | 0.288 | |
| Changes | -8.94 (18.27) cd | -5.67 (10.76) cd | 7.71 (9.94) ab | 3.89 (10.81) ab | < 0.001 | 0.008 | |
| p-value1 | 0.02 | 0.019 | 0.035 | 0.48 | |||
| ALT (mg/dl) | Baseline | 45.76 (41.37) | 42.17 (21.82) | 45.88 (19.47) | 53.12 (28.06) | 0.53 | 0.482 |
| End of the study | 73.65 (81.67) cd | 54.31 (29.87) | 31.65 (16.34) a | 32.18 (14.47) a | 0.001 | 0.062 | |
| Changes | 27.89 (47.54) cd | 12.14 (28.06) cd | -14.22 (19.36) ab | -20.93 (23.37) ab | < 0.001 | 0.001 | |
| p-value1 | < 0.001 | 0.05 | 0.017 | < 0.001 | |||
| AST (mg/dl) | Baseline | 31.49 (16.62) d | 33.34 (12.55) d | 44.27 (16.49) | 46.90 (23.84) ab | 0.003 | 0.011 |
| End of the study | 51 (32.33) cd | 38.53 (12.34) | 31.08 (14.80) a | 29.75 (18.20) a | 0.001 | 0.179 | |
| Changes | 19.50 (29.50) cd | 5.18 (15.34) cd | -13.19 (16.40) ab | -17.15 (16.48) ab | < 0.001 | 0.001 | |
| p-value1 | < 0.001 | 0.119 | 0.01 | < 0.001 | |||
| ALP (mg/dl) | Baseline | 195.09 (98.01) | 181.30 (69.14) | 184.96 (52.67) | 207.25 (84.42) | 0.28 | 0.726 |
| End of the study | 234.47 (97.43) cd | 211.06 (85.78) c | 158.72 (41.69) ab | 170.06 (47.68) a | < 0.001 | 0.001 | |
| Changes | 39.38 (109.29) cd | 29.76 (58.33) cd | -26.24 (42.05) ab | -47.19 (60.51) ab | 0.019 | 0.019 | |
| p-value1 | 0.58 | 0.023 | 0.002 | < 0.001 | |||
| GGT (mg/dl) | Baseline | 45.21 (36.83) | 30.21 (14.33) d | 29.74 (10.49) d | 61.48 (36.55) bc | < 0.001 | 0.012 |
| End of the study | 55.13 (37.61) c | 45.86 (14.52) c | 22.88 (6.62) abd | 48.04 (35.62) c | < 0.001 | 0.028 | |
| Changes | 9.91 (11.09) cd | 15.65 (7.22) cd | -6.85 (8.04) ab | -13.43 (13.41) ab | < 0.001 | < 0.001 | |
| p-value1 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
P1, Within group difference calculated by paired sample t test; P2, The difference between groups calculated by ANOVA (crude model); P3, The difference between groups calculated by ANOVA adjusted for disease duration, Family income, Supplements used, Energy intake, and food groups. abcd Bonferroni Post Hoc test was used to show pairwise difference. The superscript “a” indicates a significant difference with G1, “b” indicates a significant difference with G2, “c” indicates a significant difference with G3, and “d” indicates a significant difference with G4. G1, daily 3 meals; G2, skipping breakfast; G3, skipping dinner and G4, 3 meals and 3 snacks group
The results of ITT analysis in term of anthropometric variables were similar to per-protocol analysis.
We significantly observed reduction in FBS in “3 meals – 3 snacks” (-12.37 ± 15.07 mg/dl) and “skipping dinner” (-11.66 ± 7.95 mg/dl) groups and increase in FBS in daily “3 meals” (17.51 ± 38.85 mg/dl) and “skipping breakfast” (23.08 ± 40.06 mg/dl) groups both in crude (P < 0.001) and adjusted (P = 0.003) models.
We observed significantly reduction in TG and cholesterol in “3 meals – 3 snacks” and “skipping dinner” groups and increase in TG and cholesterol in daily “3 meals” and “skipping breakfast” groups both in crude and adjusted models (P < 0.001) (Table 3). Only in crude model, in “3 meals – 3 snacks” group LDL-C significantly reduced, however in other 3 groups increased (P < 0.001) (Table 3). We observed significantly increase in HDL-C in “3 meals – 3 snacks” and “skipping dinner” groups and decrease in HDL-C in daily “3 meals” and “skipping breakfast” groups both in crude (P < 0.001) and adjusted models (P = 0.001) (Table 3). In liver enzymes we showed significantly reduction in ALT, AST, ALP, and GGT in “3 meals – 3 snacks” and “skipping dinner” groups and increase in them in daily “3 meals” and “skipping breakfast” groups both in crude and adjusted models (P < 0.05) (Table 3).
In the ITT analysis, similar results were observed in term of lipid profile and liver enzymes.
Comparison of liver steatosis and fibrosis in different meal groups
The classification of patients in terms of liver fibrosis score and severity of steatosis at the beginning and end of the study is shown in Table 4. At baseline, there wasn’t a significant difference between groups in term of liver steatosis (p = 0.363). At the end of the three months of the intervention, both in crude and adjusted model, the percentage of patients improved in the “3 meals − 3 snacks” group was higher than the other groups (21.4%, P < 0.001), while we showed higher percentage of aggravation in the “skipping breakfast” group (8%, P < 0.001).
Table 4.
Liver fibrosis score and steatosis before and after 12 weeks interventions among 4 groups including daily 3 meals, skipping breakfast, skipping dinner, and 3 meals and 3 snacks
| Variable* | PP | |||||||
|---|---|---|---|---|---|---|---|---|
| G1 (n = 26) | G2 (n = 23) | G3(n = 31) | G4(n = 32) | P1 | P2 | |||
| Steatosis | Baseline | Mild | 3(2.6) | 1(0.89) | 2(1.78) | 2(1.78) | 0.87 | 0.363 |
| Moderate | 14(12.5) | 10(8.9) | 14(12.5) | 14(8.9) | ||||
| Severe | 9(8) | 12(10.7) | 15(13.4) | 16(%) | ||||
| End of the study | Mild | 9(8) | 0(0.0) | 7(6.3) | 21(18.8) | < 0.001 | < 0.001 | |
| Moderate | 8(7.1) | 3(2.7) | 8(7.1) | 5(4.5) | ||||
| Severe | 9(8) | 20(17.9) | 16(14.3) | 6(5.4) | ||||
| Changes | Improved | 7(6.3) | 0(0) | 8(7.1) | 24(21.4) | < 0.001 | < 0.001 | |
| No Change | 17(15.2) | 14(12.5) | 18(16.1) | 5(4.5) | ||||
| Aggravated | 2(1.8) | 9(8) | 5(4.5) | 3(2.7) | ||||
| Fibrosis | Baseline | Mild (n) | 12(10.7) | 13(11.6) | 17(15.2) | 21(18.8) | 0.402 | 0.697 |
| Moderate | 7 (6.3) | 4(3.6) | 7(6.3) | 7(6.3) | ||||
| Severe | 7(6.3) | 6(5.4) | 7(6.3) | 4(3.6) | ||||
| End of the study | Mild (n) | 11(9.8) | 3(2.7) | 7(15.2) | 21(18.8) | 0.019 | 0.001 | |
| Moderate | 8(7.1) | 10(8.9) | 3(2.7) | 9(8) | ||||
| Severe | 7(6.3) | 10(8.9) | 11(9.8) | 2(1.8) | ||||
| Changes | Improved | 3(2.7) | 1(0.9) | 2(1.8) | 9(8) | 0.128 | 0.28 | |
| No Change | 20(17.9) | 10(8.9) | 22(19.6) | 13(11.6) | ||||
| Aggravated | 3(2.7) | 12(10.7) | 7(6.3) | 10(8.9) | ||||
Data are presented as frequency (percentage); P1 the difference between groups calculated by Pearson Chi-square test; P2 the difference between groups calculated by Generalized Linear Model (GLM) and adjusted for Disease duration, Family income, Supplements used, Energy intake, and food groups; PP, Per-protocol analysis; the results not changed in the ITT test compared to PP test; G1, daily 3 meals; G2, skipping breakfast; G3, skipping dinner and G4, 3 meals and 3 snacks group
In term of liver fibrosis, there was not a significant difference between groups at the baseline (P = 0.697). At the end of the study, we found a significant difference between groups in term of liver fibrosis in the crude (P = 0.019) and adjusted model (P = 0.001) and the percentage of mild fibrosis was higher in “3-meals – 3 snacks” (18.8%) and “skipping dinner” groups (15.2%). However, analysis of changes in fibrosis showed that there were no significant differences in both crude and adjusted models following four studied interventions (P > 0.05) (Table 4).
Discussion
The present study was designed to evaluate the effects of meal patterns on liver steatosis and fibrosis, liver enzymes, lipid and glycemic profile, and anthropometric variables. The results of our study showed that consuming “3 meals and 3 snakes” during the day had beneficial effects on NAFLD compared to “3- meals”, “skipping breakfast”, and “skipping dinner” meal patterns.
A healthy diet is beneficial for NAFLD patients, and unhealthy dietary patterns are associated with the development and progression of NAFLD. The frequency, size, timing, and eating speed of meals are important factors related to dietary habits [29]. In this study, we found that 3 meals, 3 snacks per day and dinner skipping meal patterns led to a significant reduction in weight and waist circumference compared to the eating 3 meals or skipping breakfast. Obesity is one of the main risk factors for NAFLD and stimulates insulin resistance and inflammation in hepatocellular [30]. In line with our findings, Schoenfeld et al. in a meta-analysis study showed that increasing feeding frequency is positively related to decreasing fat mass and body fat percentage, as well as increasing FFM [31]. It’s common to see claims that eating more frequently is a good way to reduce body fat. This assertion is typically supported by the idea that frequent feedings promote postprandial thermogenesis, which is the rise in heat generation that lasts for up to 8 h following a meal [32, 33]. Le Blanc et al. in animal study, showed that compared to ingesting the same amount of total calories as a large, single meal, feeding dogs with four short meals increased significantly the thermogenic effect [34]. Similar results were seen in human studies, and the researchers attributed these effects to repeated stimulation of the sympathetic nervous system following an increase in the number of meals [35].
The result of the present study showed that participants in the “3 meals, 3 snacks” group had significantly improvement in the glycemic and lipid profile. In line with our findings, Tąpolska et al. in a population study among the Polish population found that higher meal frequency was associated with lower fasting TG and higher HDL-C [36]. Also, in a cross-sectional study, which was conducted in the Norfolk population of the European prospective investigation into cancer, it has been reported that there was an inverse association between meal frequency with TC and LDL-C levels [37]. Shahraki et al. in animal study showed that higher meal frequency in thirty female Wistar rats led to a significant reduction in the serum levels of total cholesterol, triglyceride, and LDL-C [38]. Moreover, Stote et al. in a RCT study examined the effects of 8 weeks meal frequency on blood variables and found that meal frequency led to a significant reduction in fat mass, total cholesterol, LDL-C, and increase in HDL-C concentrations [20]. In contrast with these findings, Salehi et al. in a RCT study evaluated the effects of 3 months 6 isocaloric meal diet compared to the control diet on lipid profile and glycemic profile among the patients with diabetes and found that HbA1c and BMI values decreased significantly in the 6 isocaloric meal diet, however they didn’t find any significant differences in the total cholesterol, LDL-C, HDL-C, triglyceride, MDA, and FBS levels [39].
Some studies have suggested that the beneficial effects of increasing the number of meals on reducing blood cholesterol are due to the effect on insulin secretion. By increasing the number of meals, insulin secretion does not create a peak and insulin concentration decreases [40]. Another favorable effect of 3 meals, 3 snacks in patients with NAFLD is due to the favorable effect on insulin sensitivity. Insulin resistance is one of the main risk factors in the NAFLD pathogenesis. A randomized, crossover study conducted for 24 weeks among the patients with polycystic ovary syndrome revealed that six meal consumption compared to the three-meal led to a significant reduction in the insulin resistance [41, 42]. Similar findings were reported in the study by Grangeiro et al. [43]. Insulin plays an important role in the hepatic synthesis of cholesterol through its effect on the HMG-CoA reductase enzyme, which is the rate-limiting enzyme in cholesterol biosynthesis and the target for cholesterol-lowering therapy [44]. Moreover, it has been reported that frequent meals could increase return of cholesterol from peripheral tissues to the liver through reverse cholesterol transport process in the postprandial phase after a fat-containing meal, reduce free fatty acids in the blood, and have an inhibitory effect on the accumulation of fatty acids in liver cells [45].
We also found a significant reduction in the liver enzymes and liver steatosis and fibrosis in subjects who consumed 3 meals- 3 snakes. Yokoyama et al. in a population-based study among patients with fatty liver reported that patients who consumed “three times/day” compared subjects who intake “two times/day” and “once/day or rare”, had lower risk for fatty liver [46]. Also, Trovato et al. in a cross sectional study among the 708 non-diabetic youngsters, 458 women, and 250 men reported that the number of meals/day are smaller in NAFLD subjects compared to the healthy group [47]. However, some studies have reached contradictory results. In an analytical- cross-sectional study among 630 Iranian clients, researchers found that there was not a significant association between consuming breakfast and snack during the day and NAFLD [48]. The reason for the conflicting result of this study can be related to the lack of assessment of fasting times, the number of main meals consumed and the number of snacks consumed in patients compared to non-NAFLD individuals.
In the present study, we found that breakfast skipping led to a significant increase in the FBS, TG, cholesterol, and significant reduction in HDL-C. In line with our findings, several studies were reported that breakfast skipping significantly was associated with increase in TG [49], total, and LDL-C [49, 50]. Also, Monzani et al. in a systematic review study showed that skipping breakfast associated with obesity and metabolic disorders among adolescents [51]. Zeballos et al. found that people who skipped one meal had higher energy intake in their subsequent meal. For example, skipping breakfast led to a greater energy intake at lunch. Skipping meals especially breakfast skipping also led to a decrease in diet quality [52]. These points can explain negative effect of breakfast skipping on the lipid profile and FBS in our study. However, they found that among all skipping patterns, skipping dinner had higher and lowest negative association with energy intake and diet quality, respectively [52]. Therefore, if patients with NAFLD want to use meal skipping as an approach to weight loss, skipping dinner can be the best strategy compared to other skipping patterns.
Based on our knowledge, this study was the first clinical trial which evaluated the effects of four meal patterns on several biomarkers among the patients with NAFLD. One of the strengths of this study was the use of fibroscan method to evaluate liver fibrosis and steatosis. Since the liver biopsy is an invasive and expensive procedure, alternative methods such as ultrasonography, computed tomography (CT), and magnetic resonance (MRI) for assessing liver tissue are used in all medical centers and in most research projects. The sensitivity and specificity of fibroscan is higher than other methods and is a reliable method for examining liver fibrosis and steatosis [53]. However, this study also had some limitation that should be considered in interpretation of the results. One of the main limitations of this study was the lack of an accurate tool to follow-up patients’ adherence to the meal patterns. Although patients were reminded by phone call and text message, there may have been over-or under-reporting in meal timing and meal frequency. Also, the over-or under-reporting may reappear in the patient’s dietary intake report. Finally, based on the results of previous studies, to observe significant changes in liver fibrosis following a nutritional intervention requires a period of at least six months, and the duration of the intervention of three months to observe changes in liver fibrosis may be short.
Conclusion
This study showed promising results for the effects of 3 meals − 3 snacks pattern on liver steatosis, lipid profile, FBS, liver enzymes, and anthropometrical variables. However, more studies are needed to confirm the findings of the present study, and further evaluations are needed, especially to determine the mechanisms involved in the observed findings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to express their gratitude towards the Urmia University of Medical Sciences, for the facilities and financial support. The authors would like to thank the patients who participated in the present study.
Author contributions
The authors’ responsibilities were as follows MA and FA: conceived and designed the study and collected of blood sample and analyzed the data; MH: provided material and technical support, SB: wrote the manuscript; MA: critically revised the manuscript for important intellectual content; all authors: read and approved the final manuscript.
Data availability
Datasets used and/or analyzed during the current study are available from the corresponding author on reasonable requests.
Declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The present study was conducted following the deceleration of Helsinki and was approved by Ethics committee at the Urmia University of Medical Sciences, Urmia, Iran (Ethic number: IR.UMSU.REC.1399.350 Date: 2021.01.23).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Farkhondeh Alami, Email: Far.alami28@gmail.com.
Somayyeh Bararnia Adabi, Email: s.adabi1992@gmail.com.
References
- 1.Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic fatty liver: epidemiology and natural history. Rev Recen Clin Trial. 2014;9(3):126–33. doi: 10.2174/1574887109666141216111143. [DOI] [PubMed] [Google Scholar]
- 2.Bellentani S. The epidemiology of non-alcoholic fatty Liver Disease. Liver Int. 2017;37(Suppl 1):81–4. doi: 10.1111/liv.13299. [DOI] [PubMed] [Google Scholar]
- 3.Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Reviews Gastroenterol Hepatol. 2016;13(4):196–205. doi: 10.1038/nrgastro.2016.3. [DOI] [PubMed] [Google Scholar]
- 4.Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and Disease burden of nonalcoholic fatty Liver Disease. World J Gastroenterol. 2017;23(47):8263–76. doi: 10.3748/wjg.v23.i47.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadiparthi C, Spatz M, Greenberg S, Iqbal U, Kanna S, Satapathy SK, et al. NAFLD epidemiology, emerging pharmacotherapy, liver transplantation implications and the trends in the United States. J Clin Translational Hepatol. 2020;8(2):215. doi: 10.14218/JCTH.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 Diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Ezquerro S, Mocha F, Frühbeck G, Guzmán-Ruiz R, Valentí V, Mugueta C, et al. Ghrelin reduces TNF-α–induced human hepatocyte apoptosis, autophagy, and pyroptosis: role in obesity-associated NAFLD. J Clin Endocrinol Metabolism. 2019;104(1):21–37. doi: 10.1210/jc.2018-01171. [DOI] [PubMed] [Google Scholar]
- 8.Chung GE, Youn J, Kim YS, Lee JE, Yang SY, Lim JH, et al. Dietary patterns are associated with the prevalence of nonalcoholic fatty Liver Disease in Korean adults. Nutrition. 2019;62:32–8. doi: 10.1016/j.nut.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Hydes TJ, Ravi S, Loomba R, Gray ME. Evidence-based clinical advice for nutrition and dietary weight loss strategies for the management of NAFLD and NASH. Clin Mol Hepatol. 2020;26(4):383. doi: 10.3350/cmh.2020.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis P, Oster H, Korf HW, Foster RG, Erren TC. Food as a circadian time cue—evidence from human studies. Nat Reviews Endocrinol. 2020;16(4):213–23. doi: 10.1038/s41574-020-0318-z. [DOI] [PubMed] [Google Scholar]
- 11.Moreno JP, Crowley SJ, Alfano CA, Hannay KM, Thompson D, Baranowski T. Potential circadian and circannual rhythm contributions to the obesity epidemic in elementary school age children. Int J Behav Nutr Phys Activity. 2019;16(1):1–10. doi: 10.1186/s12966-019-0784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Challet E. The circadian regulation of food intake. Nat Reviews Endocrinol. 2019;15(7):393–405. doi: 10.1038/s41574-019-0210-x. [DOI] [PubMed] [Google Scholar]
- 13.Orozco-Solis R, Sassone-Corsi P. Epigenetic control and the circadian clock: linking metabolism to neuronal responses. Neuroscience. 2014;264:76–87. doi: 10.1016/j.neuroscience.2014.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pot GK, Hardy R, Stephen AM. Irregular consumption of energy intake in meals is associated with a higher cardiometabolic risk in adults of a British birth cohort. Int J Obes. 2014;38(12):1518–24. doi: 10.1038/ijo.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang P, Turek FW. Timing of meals: when is as critical as what and how much. Am J Physiology-Endocrinology Metabolism. 2017;312(5):E369–E80. doi: 10.1152/ajpendo.00295.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkey CS, Rockett HR, Gillman MW, Field AE, Colditz GA. Longitudinal study of skipping breakfast and weight change in adolescents. Int J Obes. 2003;27(10):1258–66. doi: 10.1038/sj.ijo.0802402. [DOI] [PubMed] [Google Scholar]
- 17.Keast DR, Nicklas TA, O’Neil CE. Snacking is associated with reduced risk of overweight and reduced abdominal obesity in adolescents: National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Clin Nutr. 2010;92(2):428–35. doi: 10.3945/ajcn.2009.28421. [DOI] [PubMed] [Google Scholar]
- 18.Duval K, Strychar I, Cyr M-J, Prud’homme D, Rabasa-Lhoret R, Doucet E. Physical activity is a confounding factor of the relation between eating frequency and body composition. Am J Clin Nutr. 2008;88(5):1200–5. doi: 10.3945/ajcn.2008.26220. [DOI] [PubMed] [Google Scholar]
- 19.Ha K, Song Y. Associations of meal timing and frequency with obesity and metabolic syndrome among Korean adults. Nutrients. 2019;11(10):2437. doi: 10.3390/nu11102437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981–8. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marinac CR, Sears DD, Natarajan L, Gallo LC, Breen CI, Patterson RE. Frequency and circadian timing of eating may influence biomarkers of inflammation and insulin resistance associated with Breast cancer risk. PLoS ONE. 2015;10(8):e0136240. doi: 10.1371/journal.pone.0136240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leidy HJ, Campbell WW. The effect of eating frequency on appetite control and food intake: brief synopsis of controlled feeding studies. J Nutr. 2011;141(1):154–7. doi: 10.3945/jn.109.114389. [DOI] [PubMed] [Google Scholar]
- 23.Cai H, Qin Y-L, Shi Z-Y, Chen J-H, Zeng M-J, Zhou W, et al. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty Liver Disease: a randomised controlled trial. BMC Gastroenterol. 2019;19(1):1–8. doi: 10.1186/s12876-019-1132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasineni K, Jordan CW, Thomes PG, Kubik JL, Staab EM, Sweeney SA, et al. Contrasting effects of fasting on liver-adipose axis in alcohol-associated and non-alcoholic fatty liver. Front Physiol. 2021;12:188. doi: 10.3389/fphys.2021.625352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Memel ZN, Wang J, Corey KE. Intermittent fasting as a treatment for nonalcoholic fatty Liver Disease: what is the evidence? Clin Liver Disease. 2022;19(3):101. doi: 10.1002/cld.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong VW-S, Chan RS-M, Wong GL-H, Cheung BH-K, Chu WC-W, Yeung DK-W, et al. Community-based lifestyle modification programme for non-alcoholic fatty Liver Disease: a randomized controlled trial. J Hepatol. 2013;59(3):536–42. doi: 10.1016/j.jhep.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 28.Lai M, Afdhal NH. Liver fibrosis determination. Gastroenterol Clin. 2019;48(2):281–9. doi: 10.1016/j.gtc.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Oddy WH, Herbison CE, Jacoby P, Ambrosini GL, O’sullivan TA, Ayonrinde OT, et al. The western dietary pattern is prospectively associated with nonalcoholic fatty Liver Disease in adolescence. Official J Am Coll Gastroenterology| ACG. 2013;108(5):778–85. doi: 10.1038/ajg.2013.95. [DOI] [PubMed] [Google Scholar]
- 30.Duseja A, Chawla YK. Obesity and NAFLD: the role of bacteria and microbiota. Clin Liver Dis. 2014;18(1):59–71. doi: 10.1016/j.cld.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Jon Schoenfeld B, Albert Aragon A, Krieger JW. Effects of meal frequency on weight loss and body composition: a meta-analysis. Nutr Rev. 2015;73(2):69–82. doi: 10.1093/nutrit/nuu017. [DOI] [PubMed] [Google Scholar]
- 32.Louis-Sylvestre J, Lluch A, Neant F, Blundell JE, editors. Highlighting the positive impact of increasing feeding frequency on metabolism and weight management. Forum of nutrition; 2003. [PubMed]
- 33.Tai MM, Castillo P, Pi-Sunyer FX. Meal size and frequency: effect on the thermic effect of food. Am J Clin Nutr. 1991;54(5):783–7. doi: 10.1093/ajcn/54.5.783. [DOI] [PubMed] [Google Scholar]
- 34.LeBlanc J, Diamond P. Effect of meal size and frequency on postprandial thermogenesis in dogs. Am J Physiology-Endocrinology Metabolism. 1986;250(2):E144–E7. doi: 10.1152/ajpendo.1986.250.2.E144. [DOI] [PubMed] [Google Scholar]
- 35.LeBlanc J, Mercier I, Nadeau A. Components of postprandial thermogenesis in relation to meal frequency in humans. Can J Physiol Pharmacol. 1993;71(12):879–83. doi: 10.1139/y93-133. [DOI] [PubMed] [Google Scholar]
- 36.Tąpolska M, Spałek M, Skrypnik D, Bogdański P, Owecki M. The influence of meal frequency on lipid profile in the Polish population. Neuro Endocrinol Lett. 2019;40(7–8):325–8. [PubMed] [Google Scholar]
- 37.Titan SM, Bingham S, Welch A, Luben R, Oakes S, Day N, et al. Frequency of eating and concentrations of serum cholesterol in the Norfolk population of the European prospective investigation into cancer (EPIC-Norfolk): cross sectional study. BMJ. 2001;323(7324):1286. doi: 10.1136/bmj.323.7324.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahboob S, RASHIDI M, Mesgari M. The Effect of Meal frequency on serum lipid and lipoproteins in rats. Med J Tabriz Univ Med Sci. 2013;26(3):33–7. [Google Scholar]
- 39.Salehi M, Kazemi A, Zadeh JH. The effects of 6 isocaloric meals pattern on blood lipid profile, glucose, hemoglobin a1c, insulin and malondialdehyde in type 2 diabetic patients: a randomized clinical trial. Iran J Med Sci. 2014;39(5):433. [PMC free article] [PubMed] [Google Scholar]
- 40.Farshchi H, Taylor M, Macdonald I. Regular meal frequency creates more appropriate insulin sensitivity and lipid profiles compared with irregular meal frequency in healthy lean women. Eur J Clin Nutr. 2004;58(7):1071–7. doi: 10.1038/sj.ejcn.1601935. [DOI] [PubMed] [Google Scholar]
- 41.Papakonstantinou E, Kontogianni M, Mitrou P, Magriplis E, Vassiliadi D, Nomikos T, et al. Effects of 6 vs 3 eucaloric meal patterns on glycaemic control and satiety in people with impaired glucose tolerance or overt type 2 Diabetes: a randomized trial. Diabetes Metab. 2018;44(3):226–34. doi: 10.1016/j.diabet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Paoli A, Bosco G, Camporesi EM, Mangar D. Ketosis, ketogenic diet and food intake control: a complex relationship. Front Psychol. 2015;6:27. doi: 10.3389/fpsyg.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grangeiro ÉD, Trigueiro MS, de Oliveira Siais L, Paiva HM, Sola-Penna M, Rosado EL. Effect of Meal frequency on the loss weight, Glycemia, lipid Profile, plasma ghrelin and energy expenditure of women with obesity. A Clinical Trial; 2020.
- 44.Zhong S, Li L, Liang N, Zhang L, Xu X, Chen S, et al. Acetaldehyde dehydrogenase 2 regulates HMG-CoA reductase stability and cholesterol synthesis in the liver. Redox Biol. 2021;41:101919. doi: 10.1016/j.redox.2021.101919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutherland WH, de Jong SA, Walker RJ. Effect of dietary cholesterol and fat on cell cholesterol transfer to postprandial plasma in hyperlipidemic men. Lipids. 2007;42(10):901–11. doi: 10.1007/s11745-007-3101-1. [DOI] [PubMed] [Google Scholar]
- 46.Yokoyama A, Taniki N, Nakamoto N, Tomita K, Hara S, Mizukami T, et al. Associations among Liver Disease, serum lipid profile, body mass index, ketonuria, meal skipping, and the alcohol dehydrogenase-1B and aldehyde dehydrogenase‐2 genotypes in Japanese men with alcohol dependence. Hepatol Res. 2020;50(5):565–77. doi: 10.1111/hepr.13475. [DOI] [PubMed] [Google Scholar]
- 47.Trovato FM, Martines GF, Brischetto D, Catalano D, Musumeci G, Trovato GM. Fatty Liver Disease and lifestyle in youngsters: diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int. 2016;36(3):427–33. doi: 10.1111/liv.12957. [DOI] [PubMed] [Google Scholar]
- 48.Mansour-Ghanaei R, Mansour-Ghanaei F, Naghipour M, Joukar F. The lifestyle characteristics in non-alcoholic fatty Liver Disease in the PERSIAN Guilan Cohort Study. Open Access Maced J Med Sci. 2019;7(19):3313–8. doi: 10.3889/oamjms.2019.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shafiee G, Kelishadi R, Qorbani M, Motlagh ME, Taheri M, Ardalan G, et al. Association of breakfast intake with cardiometabolic risk factors. Jornal De Pediatria. 2013;89(6):575–82. doi: 10.1016/j.jped.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 50.Smith KJ, Gall SL, McNaughton SA, Blizzard L, Dwyer T, Venn AJ. Skipping breakfast: longitudinal associations with cardiometabolic risk factors in the Childhood determinants of Adult Health Study. Am J Clin Nutr. 2010;92(6):1316–25. doi: 10.3945/ajcn.2010.30101. [DOI] [PubMed] [Google Scholar]
- 51.Monzani A, Ricotti R, Caputo M, Solito A, Archero F, Bellone S, et al. A systematic review of the association of skipping breakfast with weight and cardiometabolic risk factors in children and adolescents. What should we Better Investigate in the Future? Nutrients. 2019;11(2):387. doi: 10.3390/nu11020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeballos E, Todd JE. The effects of skipping a meal on daily energy intake and diet quality. Public Health Nutr. 2020;23(18):3346–55. doi: 10.1017/S1368980020000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan®) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease-where do we stand? World J Gastroenterol. 2016;22(32):7236. doi: 10.3748/wjg.v22.i32.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets used and/or analyzed during the current study are available from the corresponding author on reasonable requests.