Abstract
Redox biocatalysis plays an increasingly important role in modern organic synthesis. The recent integration of novel media such as deep eutectic solvents (DESs) has significantly impacted this field of chemical biology. Alcohol dehydrogenases (ADHs) are important biocatalysts where their unique specificity is used for enantioselective synthesis.
This review explores aspects of redox biocatalysis in the presence of DES both with whole cells and with isolated ADHs. In both cases, the presence of DES has a significant influence on the outcome of reactions albeit via different mechanisms. For whole cells, DES was shown to be a useful tool to direct product formation or configuration - a process of solvent engineering. Whole cells can tolerate DES as media components for the solubilization of hydrophobic substrates. In some cases, DES in the growth medium altered the enantioselectivity of whole cell transformations by solvent control. For isolated enzymes, on the other hand, the presence of DES promotes substrate solubility as well as enhancing enzyme stability and activity. DES can be employed as a smart solvent or smart cosubstrate particularly for cofactor regeneration purposes.
From the literatures examined, it is suggested that DES based on choline chloride (ChCl) such as ChCl:Glycerol (Gly), ChCl:Glucose (Glu), and ChCl:1,4-butanediol (1,4-BD) are useful starting points for ADH-based redox biocatalysis. However, each specific reaction will require optimisation due to the influence of several factors on biocatalysis in DES. These include solvent composition, enzyme source, temperature, pH and ionic strength as well as the substrates and products under investigation.
Keywords: Deep eutectic solvents, Alcohol dehydrogenases, Solvent engineering, Biocatalysis, Enantioselectivity
Graphical abstract
Highlights
-
•
Deep eutectic solvents (DES) can dramatically alter biocatalytic processes.
-
•
DES can influence the enantioselectivity of chiral synthesis in whole cells.
-
•
DES can enable enhanced reaction yields by enhancing enzyme performance (stability, activity, specificity).
-
•
DES components can be a useful cosubstrate in cofactor regeneration.
1. Introduction
The paradigm of sustainable and green chemistry introduces a new perspective to the fields of chemistry and chemical engineering. Sustainability principles have evolved over time, guiding the design, development, and implementation of chemical products and processes [1]. These principles empower scientists and engineers to actively contribute to the well-being of the economy, humanity, and the planet [2]. A key focus is the development of innovative approaches to minimise waste, conserve energy, and identify substitutes for hazardous substances [3]. The utilisation of inorganic chemicals, organic reagents/solvents, the subsequent downstream processes and the use of complex multi-step synthesis procedures contribute to waste generation in chemical manufacture. A potential solution to this challenge is the integration of biocatalysts (mostly enzymes) into chemical processes [4,5]. Biocatalysis can enable the production of products that are not readily achievable through conventional chemical synthesis. They may also use alternative, and cheaper, raw materials and thus reduce operational costs. Moreover, they often require minimal fixed infrastructure [6]. Some biocatalysts have demonstrated operational stability in organic solvents and this opens up scope for biocatalysts in organic synthesis [7]. However, using organic solvents for certain reactions reduces the environmental desirability of this type of biocatalysis. Considering this shortcoming, the use of modern solvents, such as ionic liquids (IL) [8,9] and more recently DES [10], in biocatalysis is one of the major breakthroughs in improving the sustainability of organic synthesis (see Supplementary Information Fig. S1). Additionally, DESs, when used as a solvent or cosolvent, can enhance the solubility of hydrophobic substrates. For instance, the introduction of DESs has improved the solubility of halogenated hydrocarbons, epoxides, and several drugs, while also reducing unwanted reactions. DES can help to enhance the yield, stability, and, in many cases, the activity of enzymes [[10], [11], [12], [13], [14], [15], [16]]. In some cases, DES can influence the enantioselectivity of reactions, adding to their growing importance [[17], [18], [19], [20]]. This review largely considers the reduction of carbonyl compounds to yield chiral alcohols through ADH mediated reactions, an example of one of the key enantioselective reactions that play a central role in organic synthesis [21]. This enantioselective ability, makes ADH a powerful, and industrially significant biocatalyst [22].
1.1. Alcohol dehydrogenase: a powerful redox biocatalyst
ADH, is a fully reversible alcohol oxidising enzyme that is capable of alcohol oxidation and ketone/aldehyde reduction as well as aldehyde oxidation/dismutation (see Table 1).
Table 1.
The variety of reactions catalysed by ADHs.
| No. | Reaction | Scheme | Ref. |
|---|---|---|---|
| 1 |
Alcohol oxidation |
 |
[23] |
| 2 |
Carbonyl reduction |
 |
[[24], [25], [26]] |
| 3 |
Aldehyde oxidation |
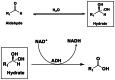 |
[27] |
| 4 | Aldehyde dismutation | 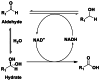 |
[[28], [29], [30], [31]] |
Note: direct aldehyde oxidation to the carboxylic acid is normally only observed under NADH recycling conditions when NADH concentrations are low. Prior hydration of the aldehyde is shown (reaction 3) for aldehyde oxidation. The hydration produces a gem-diol which is structurally analogues to a secondary alcohol. The oxidation of the hydrated aldehyde to form a carboxylic acid is not reversible.
ADH has a wide range of applications [32,33], and has been utilised in diverse multi-step cascades to transform simple starting materials into complex products [22,34,35]. For instance, beginning with a cyclic ketone and diol, a redox-neutral ADH-monooxygenase cascade for producing lactones has been demonstrated [36].
Redox biocatalysis requires a cofactor to complete the redox cycle. The cofactor is reduced, or oxidised, according to the reaction type [22,37]. There are two types of cofactors for ADH, nicotinamide adenine dinucleotide non-phosphorylated (NADH/NAD+) and its phosphorylated form (NADPH/NADP+; see Fig. 1), with both forms presenting similar physiochemical properties [22].
Fig. 1.
Nicotinamide adenine dinucleotide non-phosphorylated (NADH/NAD+) and its phosphorylated ribose (NADPH/NADP+) form. The adenine dinucleotide portion of the molecule is indicated as R. The site of phosphorylation, on the ribose moiety, is highlighted in red. The reversible transfer of a hydride ion is indicated. Note that the nicotinamide portion of the NAD(P) is the recipient or donor of a hydride ion during redox catalysis.
A major challenge in the synthetic application of ADHs is cofactor regeneration; cofactors are expensive and consequently, regeneration is necessary [38]. This limitation has been addressed by using whole cells, as opposed to an isolated (purified) enzyme, as the biocatalyst source. Early studies with purified enzymes concentrated on ADHs from horse liver [32] and yeast [39] but in recent years a number of other ADH have been employed. A selection of purified ADH enzymes used in biocatalysis is shown in Table 2. In addition, a variety of ADHs used in whole cells have been described [10].
Table 2.
Some ADHs used in biocatalysis and their structural features.
| Enzyme | Structural Features | Ref. |
|---|---|---|
| Yeast ADH | Medium chain, tetramer, 347 amino acid residues per polypeptide chain and contains a catalytic zinc ion | [39,40] |
| Thermoanaerobacter brockii ADH | Medium chain, tetramer, 352 amino acids residues per polypeptide and contains a catalytic zinc ion | [40] |
| Horse liver ADH | Medium chain, dimer, 374 amino acid residues per polypeptide chain and contains a catalytic zinc ion | [39,40] |
| Candida parapsilosis ADH | Medium chain, dimer, 279 amino acids per polypeptide and contains a catalytic zinc ion | [41] |
| Drosophila ADH | Short chain, dimer, 250 amino acid residues per polypeptide chain, no metal ion | [40] |
| Clostridium beijerinckii ADH | Medium chain, tetramer, ∼351 amino acid residues per polypeptide and contains a catalytic zinc ion | [40] |
| Pyrococcus furiosus AdhD | Short chain, monomer with 278 amino acids, no metal ion | [42] |
| Entamoeba histolytica ADH2 | Medium chain, tetramer, 453 amino acids residues per chain and contains iron | [43,44] |
| Geobacillus thermodenitrificans ADH2 | Long chain, homodimer with 387 amino acids, contains iron | [45,46] |
| Geobacillus thermodenitrificans ADH1 | Long chain, homooctamer with 395 amino acids, contains iron | [45,46] |
| Sphingobium yanoikuyae ADH | Short chain, 262 amino acids | [47] |
| Ralstonia ADH | Short chain, trimer in solution, 249 amino acids | [48] |
Whole cell biocatalyst sources are often favoured over the use of isolated enzymes due to their affordability since using whole cells directly reduces the production and purification cost of isolating an enzyme [20]. Despite these advantages, whole cell approaches come with drawbacks, such as, the formation of side products, demanding additional downstream steps in product isolation/purification, and potential cell toxicity induced by substrates/products [49]. To fully appreciate the use of ADH in redox biocatalysis, it is important to understand these factors and the mechanism of ADH-catalysed reactions.
1.2. The mechanism of ADH catalysed reactions
ADHs tend to be broad specificity enzymes capable of binding a range of aliphatic and aromatic alcohols/carbonyls. The reaction is fully reversible, and the enzyme can reduce aldehydes or ketones to the corresponding alcohol (see Table 1). A detailed mechanism of a catalytic zinc-containing ADH is shown in Fig. 2. The catalytic activity of the ADH is initiated by the binding of cofactor [NAD(P)+/NAD(P)H] to the enzyme surface followed by the binding of substrate (alcohol or carbonyl compound). Hydride ion transfer occurs between cofactor and substrate [22].
Fig. 2.
Mechanism of ADH catalysed reduction and oxidation involving an active site metal (zinc). The nicotinamide cofactor is shown coordinated to the active site zinc. The binding of the alcohol compound leads to hydride transfer to the nicotinamide moiety of NAD+ and the binding of the carbonyl compound (reverse reaction) leads to the abstraction of hydride ion from the nicotinamide moiety of NADH. Note: The R group attached to the nicotinamide represents the remainder of the cofactor (see Fig. 1). R1 and R2 represent alkyl or aryl substituents. The hydride ion transferred during the redox reaction is highlighted in red. Adapted from Ref. [22].
Many ADHs exist as metalloenzymes (See Fig. 3a and b), and in these cases a metal ion (e.g. zinc or iron ) is present in their active sites [[50], [51], [52]]. The metal ion assists in the coordination of both the cofactor and the substrate keeping them in close proximity such that the hydride ion transfer becomes more favourable [22].
Fig. 3.
a) Classification of ADH based on the metal ion in the active site [39,40,53] from different species. b) 3D structure of horse liver ADH containing zinc ion, inset shows the zinc ion complexed to NADH and cyclohexyl formamide (PDB code-1LDY) [54]. Created with BioRender.com.
There are four possibilities to transfer the hydride ion from the cofactor to the substrate in the reduction of a prochiral ketone. Two re (back) face transfer and two si (front) face transfer of the hydride ion [22]. ADH can be Prelog-categorised as Prelog and anti-Prelog ADH, based on the transfer of the hydride ion [55]. If an ADH adds a hydride ion to the si face of the prochiral ketone to produce an (R)-alcohol, or if the ADH abstracts protons from (R)-alcohols, it is referred to as an anti-Prelog ADH. Whereas, if the addition of the hydride ion occurs through the re face to form (S)-alcohol, or the ADH abstracts the hydride ion from (S)-alcohols, then the ADH is termed as Prelog ADH (see Fig. 4a) [22,55]. Alternatively, Keinan and colleagues explained the stereoselectivity of TbADH for the reduction of ketones, as being modulated by large and small alkyl binding sites on the enzyme surface. Above a certain bulk, the smaller alkyl site was unoccupied causing enantiomeric inversion. When the small alkyl groups were methyl, ethyl, isopropyl or cyclopropyl, the hydride transfer occurred through the si face of the substrate giving an (R)-alcohol product. This was not the case with n-propyl and bulkier groups where inversion of configuration occurs (see Fig. 4b) [56].
Fig. 4.
Stereochemical recognition in asymmetric reduction reactions catalysed by ADHs. a) Prelog model, which was based on the addition/transfer of hydride ion (H−) to/from the cofactor to the substrate giving corresponding (R) alcohol (anti-Prelog ADH)/(S) alcohol (Prelog ADH) or the carbonyl compound [55]. b) Keinan model to explain enantiomeric outcomes in ADH-catalysed reactions which was based on the bulkiness of groups in the space surrounding the carbonyl moiety: above a certain bulk, some alkyl groups cannot bind to a smaller site (occupied by R1 above) and will enter the larger site leading to the observed transition from R to S configuration [56]. Created with BioRender.com.
1.3. Modulating ADH catalysed reactions for enantioselectivity
With the different hydride transfer possibilities, ADH catalysis is enantioselective [57]. Recent studies have shown that the reaction can be judiciously manipulated by altering the reaction conditions, such as varying the solvent to yield excess R or S alcohol products from corresponding ketones [58,59]. Along with solvent, temperature and pH, play a significant role in achieving the desired enantiomeric conversion [22,37,[60], [61], [62], [63], [64]].
In chemical kinetics, the rate of a chemical reaction doubles with every 10 °C rise in temperature [65]. However, this is not the case with biocatalysis where there is a trade-off between activity and enzyme stability at higher temperatures [66,67]. The optimal temperature for an enzyme is the intersection point of the activity (increasing) curve and the stability (decreasing) curve [22,61].
The effect of pH on organic synthesis by ADHs often depends on whether oxidation or reduction is considered. The reduction of carbonyl compounds is favoured in slightly acidic conditions while the oxidation of alcohols is favoured in alkaline conditions [68]. However, changing the pH of the solution significantly may affect the enzyme's conformation and activity by changing the state of ionisation of catalytic amino acids [67]. Isolated ADH is generally highly active at neutral pH; however, the reduced form of the cofactor (NAD(P)H) is most stable in an alkaline medium and its oxidised form is most stable in an acidic medium [62].
A prominent consideration in biocatalysis is the nature of the solvent(s) used for substrate solubilization and their effect on the enzyme(s). Recent advances in conventional organic, and neoteric solvents require careful consideration of the variation in ADH activity in the chosen solvent system [63]. Thus, enantiomers formed in certain reactions may depend on the composition of the solvent and it has been reported that DES can dramatically alter enantioselectivity [25]. This approach of enhancing enantioselectivity is an example of solvent engineering in biocatalysis [10,[18], [19], [20]].
Given that this is a significant area of rapid development, this review focuses specifically on the influence of DESs on ADH reactions either, (i) as catalysed via whole cell-based ADH, or (ii) catalysed by isolated cell-free enzymes. Much of the focus is on enantioselective reduction reactions, the main application area for ADH. This review also considers the strategies for cofactor regeneration in DES.
2. Deep eutectic solvents and whole cell biocatalysis
DESs are a special category of eutectic mixture, which are typically created between hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs) or mixtures of Lewis acids and bases. Widely used HBDs are urea, glycerol, ethylene glycol, glucose and widely used HBAs are ammonium or phosphonium-based salts. An essential criterion is that the acidity difference between the components of DES should be minimal [69]. They are prepared either by mixing the components with gentle heating and stirring or by mixing components in a common solvent followed by solvent removal. This ease of preparation is an advantage of DES in comparison to ionic liquids [69]. Perhaps surprisingly a number of cell types (e.g. cultured plant cells, microbial cells) showed improved cell permeability, stability and reduced cytotoxicity in the presence of DES [64,[70], [71], [72], [73]]. For example, whole Cyberlindnera saturnus cells have been used for the synthesis of (S)-1-[3,5 bis(trifluoromethyl)phenyl]ethanol [(S)- BTPE] from 3,5-bis(trifluoromethyl)acetophenone (BTAP) in DES, l-carnitine:lysine (C:Lys). The introduction of DES (C:Lys) increased cell permeability to substrate and reduced the substrate (BTAP) toxicity [73]. Thus, the reduction of carbonyl compounds by whole cells in the presence of DES offered significant advantages over aqueous media. The examples given below are for ketone reduction to chiral alcohols where there is evidence that the main enzyme involved in the whole cell transformation is an ADH.
2.1. Whole cell carbonyl reductions in deep eutectic solvents
Numerous industrially relevant processes require alcohol functionalities. Reductions, particularly the reduction of prochiral ketones into their corresponding chiral alcohols, are an important reaction in organic chemistry [22,35]. Such reactions are efficiently performed through biocatalysis. By the end of the 20th century, the use of traditional biocatalytic reactions had transitioned from the use of organic solvents to ionic liquids (IL). More recently, the move to the use of DES, and the combination of DES with IL, has gained ground. For example, a biphasic medium of DES [ChCl:ethylene glycol (EG)] and IL [1-butyl-3-methylimidazolium hexafluorophosphate (C4MIM·PF6)] was used to reduce 2-octanone to its corresponding (R)-alcohol with the help of Acetobacter pasteurianus Gim1.158 cells (see Supplementary Information Table S1). The addition of IL to this DES system increased the solubility of both product and substrate as well as the operational stability of the whole cell. The DES (ChCl:EG) showed good biocompatibility and served to increase cell permeability [11]. In general, the reaction yield is contingent on the chosen DES and, therefore, careful selection of DES is pivotal [10,18]. For example, different DESs were tested for the reduction of 2-hydroxyacetophenone (HAP) to (R)-1-phenyl-1,2-ethanediol (PED) by using K. gibsonii SC0312 cells [70]. Here, the use of ChCl:1,4-BD provided good yield (80 %) in conjunction with good cell biocompatibility in comparison to other DESs tested [ChCl:urea (U), ChCl:triethylene glycol (TEG), ChCl:EG, ChCl:Gly (details in Supplementary Information Table S2)]. This system, 80 mM HAP in ChCl:1,4-BD yielded 80 % (R)-PED with an optical purity >99 % [70].
DESs can also be prepared using a variety of oligopeptides and amino acids (Supplementary Information Tables S3, S4, S5). For instance, comparing amino acid and oligopeptide-based DESs [ChCl with glutathione (GSH)], an oligopeptide-based DESs (ChCl:GSH) demonstrated an enhanced yield (from 70.4 % to 87.6 %) for the reduction of BTAP to the corresponding (R)-BTPE using T. asperellum ZJPH0810 cells (see Supplementary Information Table S3) [71]. However, by using the same substrate (BTAP) with the yeast isolate Cyberlindnera saturnus ZJPH1807 in C: Lys in the presence of Tween-80, a different enantioselectivity was favored producing (S)-BTPE (see Supplementary Information Table S6) [73]. It should be emphasised that Tween 80, a surfactant, is utilised in the reaction in conjunction with the DES. Tween 80 is also used as a surfactant in the synthesis of (S)-4-chloro-3-hydroxybutanoate [(S)-CHBE]. The conversion of ethyl 4-chloro-3-oxobutanoate (COBE) to (S)-CHBE was conducted with recombinant E. coli CCZU-T15 cells in the presence of Tween 80 and a ChCl:Gly-water mixture. Here an enantiomeric excess (ee) of >99 % was obtained (see Supplementary Information Table S7) [74]. In short, the use of a surfactant can increase the dispersion of water-insoluble substrates through the formation of a micellar system in DES, increasing substrate mass transfer and, therefore, product yield [73,74]. The same strategy was employed in the reduction of 2-chloro-1-(2,4-dichlorophenyl) ethanone (CPE) to (R)-2-chloro-1-(2,4-dichlorophenyl)ethanol [(R)-CPEO] using C. saturnus ZJPH1807 cells in C:trehalose (Tre), supplemented with cyclodextrin (CD) (see Supplementary Information Table S8) [75]. Similarly, for the transformation of 2ʹ-(trifluoromethyl) acetophenone by G. silvicola in choline acetate (ChAc):Cys, the addition of methylated-β-cyclodextrin (MCD) serves a comparable role (see Scheme 1) [76].
Scheme 1.
Asymmetric reduction of 1-(2-(trifluoromethyl)phenyl)ethan-1-one with G. silvicola ZJPH1811. ee is enantiomeric excess.
CD and its derivatives, akin to Tween 80, enhance substrate solubility and can boost yield [75,76]. For instance, in the conversion of 2ʹ-(trifluoromethyl) acetophenone, the yield in ChAc:Cys increased from 72.3 % to 83.2 % with the addition of MCD [76]. The addition of organic solvent to DES can also increase the substrate and product solubility. For example in the biotransformation of COBE into (R)-CHBE with E. coli CgCR, a high (R)-CHBE yield of 97.6 % was obtained in the bioreaction system containing 7 % (v/v) betaine:lactic acid (B:LA) and 50 % (v/v) ethyl acetate [77].
However, there are certain challenges when using DESs, primarily due to their inherent viscosity and cytotoxicity [78]. To overcome this, the inclusion of a cosolvent (buffer/water) is often necessary. For example, maximum enantioselectivity was achieved in ChCl:Gly with a 30 % (v/v) water system during asymmetric reduction of 1-(3,4- dimethylphenyl) ethenone using S. cerevisiae [Baker's Yeast (BY)], details provided in Supplementary Information Table S9 [79]. Moreover, the reaction yield was also dependent on the HBA chosen. In the initial studies on DES in biocatalysis, ChCl was commonly employed as the HBA [[80], [81], [82]]. However, each whole cell system exhibits distinct behavior with different solvents. It was found that at high concentrations of ChCl, HLADH activity diminishes [25]. Thus, it is important to assess a variety of DESs to identify the optimal solvent system for a given enzymatic reaction. In comparing DESs based on ChAc and ChCl as HBAs, enzyme activity was notably more efficient in ChAc when amino acids were used as HBDs in the bioconversion of 2-chloro-1-(3,4-difluorophenyl) ethanone (CFPO) into (S)-2-chloro-1-(3,4-difluorophenyl) ethanol [(S)-CFPL] using recombinant E. coli resting cells (see Supplementary Information Table S10) [78]. The most favourable results were achieved using ChAc:Lys, with an ee >99.99 %. Additionally, the inclusion of ChAc:Lys significantly improved cofactor regeneration, outperforming ChCl-based DESs. Here, optimal conditions were established at a temperature of 30 °C and a pH below 7.6, with DES concentrations less than 1 % (w/v). Above 1 % (w/v) DES concentration, the yield decreases, possibly due to increased cytotoxicity [78]. ChAc demonstrated greater efficiency than ChCl which has been attributed to the cytotoxicity of ChCl, induced by the chloride ion binding to the cell plasma membrane [78]. In many acid-based DESs, enzyme activity typically decreases e.g.; ChCl:oxalic acid (OA), ChCl:malic acid (MA) [83]. Recently, it has been discovered that acidic DESs can enhance bioreduction. In the conversion of CFPO to (S)-CFPL using recombinant E. coli cells containing the NADH-dependent reductase (CmCR) in 7.5 %, v/v ChCl:LA DES [84] instead of ChAc:Lys [78], substantially higher yield (90.8 %) was obtained, marking 1.4-fold increase over a purely aqueous culture medium. The reason suggested for the improved yield is the enhanced cell membrane permeability facilitated by the low viscosity of ChCl:LA (7.5 % v/v) (Supplementary Information Table S11) [84].
The stability of DESs is significantly influenced by the preparation parameters and the application under consideration. For example, during the preparation of ChCl:LA, 2 mol% choline was found to be converted to a lactic acid choline ester at 60 °C (see Scheme 2). This increases to 4 mol% at 80 °C and 7 mol% at 100 °C. Long-term storage can increase the ester formation [85]. This stability issue is not limited to ChCl:LA, but it was also observed with other DES components such as malonic acid, glycolic acid, and levulinic acid [85]. For instance, malonic acid can decompose into acetic acid and carbon dioxide in DES [85]. In glucose-based DES, caramelization of glucose can occur at temperatures between 100 °C and 120 °C [86]. These degradation aspects are significant since most DESs preparation procedures involve heating at above 60 °C to achieve a homogeneous solution [85]. The impact of these degradation products on enzyme stability and activity remains unknown and requires further study.
Scheme 2.
Ester formation in carboxylic acid-based DES during preparation and long-term storage.
Another important aspect to consider is the pH associated with the DES, which can vary with temperature. For example, the pH of ChCl:F (d-fructose) solution appears to decrease as the temperature increases. At 25 °C, the pH of ChCl:F (1:1) solution was 6.1 [87]. It is well known for ADHs that the reaction rates for alcohol oxidation increases with increasing pH up to pH 9–10, beyond which they decline [88]. In most cases, the fluctuating pH of reactions is controlled using buffer as component of the reaction mixture [25].
DESs are often mixed with water or an aqueous buffer in various ratios during their application. It is essential to understand DES can retain its properties after mixing with water up to a certain concentration [12,89]. For example, ChCl:U can maintain its nanostructural properties in up to 50 % w/w water [90]. ChCl:Gly (1:2) maintains its properties up to 35 % w/w water; at higher water levels, DES solutions become an aqueous electrolyte mixture [91]. The same principles apply to other DESs under consideration. Another critical factor is the water activity in DESs. A certain amount of free water is necessary for proper enzyme function. For instance, HLADH remains active in systems containing free water, while its activity decreases in neat DESs [25].
Other than choline derivatives, betaine (B), l-proline (P), l-carnitine (C), cetyltrimethylammonium bromide, and FeCl3 can be used as HBAs in DES formulations (see Table 3) [73,[92], [93], [94]].
Table 3.
List of (NA)DES having a HBA other than ChCl [92] where B is Betaine, P is Proline, C is carnitine, Gly is Glycerol, Glu is Glucose, Lys is Lysine and G is Glycine.
| No. | DES | Molar ratio |
|---|---|---|
| 1 | B:Glu:Gly | 1:1:2 |
| 2 | P:Glu:Gly | 1:1:2 |
| 3 | C:Glu:Gly | 1:1:2 |
| 4 | B:Lys | 1:2 |
| 5 | P:Lys | 1:2 |
| 6 | C:Lys | 1:2 |
| 8 | B:G | 1:1 |
l-carnitine based DES (C:Lys) was found to be efficient for the reduction of 2,6-dichloro-3-fluoroacetophenone (see Supplementary Information Table S12) [92]. A betaine-based DES (B:Lys) was found to be efficient in several biocatalytic reductions as in the case of the reduction of 4-(trifluoromethyl) acetophenone by G. geotrichum ZJPH1810 cells and also with recombinant E. coli (see Supplementary Information Tables S13 and S14) [92]. Betaine based DES as an efficient solvent in the synthesis of 2,5-furandimethanol (FDM) from 5-hydroxymethylfurfural (see Scheme 3) has been reported [[95], [96], [97]].
Scheme 3.
Synthesis of FDM from 5-hydroxymethylfurfural by P. putida S12 cells in betaine based DES.
FDM is widely used in resin industries, in the production of synthetic fibres and fine chemicals [98]. ChCl:Gly (7.5% v/v) was found to be efficient in the synthesis of furfuryl alcohol from furfural [99] (see Scheme 4).
Scheme 4.
Synthesis of furfuryl alcohol from furfural with recombinant E. coli CF containing a reductase and cofactor recycling by formate dehydrogenase (FDH).
The use of plant cells, such as carrot and sugar beet, in DES is gaining attention in ADH-based reductive biocatalysis [64,79]. For example, cultured carrot roots were employed in the biocatalytic reduction of 1-(3,4-dimethylphenyl) ethenone (see Supplementary Information Table S15) [64]. Although superior conversion was achieved in pure aqueous solution, ChCl with glucose (Glu) as HBD demonstrated the highest conversion among the different DESs investigated. ChCl with EG showed the lowest conversion [64].
The activity of ADH sourced from different species may be different in the same DES. In the case of Thermoanaerobacter ethanolicus ADH (TeSADH), horse liver ADH (HLADH), and Ralstonia sp. ADH (RasADH) which were overexpressed in E.coli; each showed different activity in a ChCl:Gly (1:2) DES system. Here, the activity of TeSADH and HLADH was influenced by the concentration of DES, as the activity for both enzymes declined with increasing DES concentration above 90 % (v/v). However, RasADH was active at high DES levels: at a DES concentration of 95 % (v/v) (ChCl:Gly), half of the initial activity persists (see Supplementary Information Table S16) [100].
While the capabilities of whole cells in suspension is acknowledged, the potential application of immobilised whole cells is an emerging field with significant potential. This is because immobilised cells offer several advantages over free cells. These include simplified separation from the reaction medium, enhanced substrate conversion, reduced inhibition by products, shorter reaction times, and greater control over cell replication [101]. For instance, immobilised Acetobacter sp. CCTCC M209061 cells were used for the bioreduction of 3-chloropropiophenone to (S)-3-chloro-1-phenylpropanol in ChCl:U. DES showed biocompatibility with the cells and increased cell permeability and stability (see Scheme 5) [102].
Scheme 5.
Asymmetric reduction of 3-chloropropiophenone with immobilised Acetobacter sp. CCTCC M209061 cells.
As these reductions of this prochiral ketone can give a route to the corresponding enantiomers, careful adjustment of DES can selectively produce a desired enantiomer [83,100].
2.2. Deep eutectic solvent engineering to enhance the enantioselectivity of carbonyl reduction
An enantioselective reaction is a chemical reaction that produces predominantly one enantiomer of a chiral compound over the other [103]. The distinctive stereochemistry of each enantiomer underscores the significance of enantioselective reactions [57]. For example, selection of appropriate enantiomers can reduce the side effects and enhance the therapeutic efficacy of (bio)pharmaceutical compounds [104]. Beyond (bio)pharmaceuticals, enantioselective reactions are crucial across a spectrum of industries, encompassing, agrochemicals, flavour, fragrance, materials science and beyond [10,22,105]. Stereoselectivity can be tuned by judiciously adjusting the (NA)DES composition. NADES are modified DES which incorporate natural components as HBA and HBD (e.g. B:Lys) and these provide a cell-like environment to the whole cell (see Supplementary Information Fig. S1) [92]. For example, utilisation of BY in the bio-reduction of ethyl acetoacetate (EAA) in the presence of DES showed such stereoselectivity. Here, the reactions in pure water yielded the (S)-alcohol, [(S)-ethyl 3-hydroxybutyrate (EHB); ee > 95 %] whereas, using ChCl:Gly (1:2) resulted in (R)-alcohol, [(R)-ethyl 3-hydroxybutyrate; ee > 95 %]. A clear stereo-inversion occurred in the product while using pure water versus DES >80 % v/v and a racemic mixture was obtained in DES:water (80:20) (see Supplementary Information Table S17) [58]. For the bio-reduction of several arylpropanones with BY in DES, water, and DES-water mixtures, showed a similar stereo-inversion. When water was used as the solvent, it resulted in synthesis of excess (S)-alcohol (96 % ee); however, employing DES (ChCl:Gly) resulted in excess (R)-alcohol (96 % ee; see Scheme 6) [59].
Scheme 6.
Enantioselective reduction of different ketones with Baker's yeast. The enantioselectivity varies with the reaction conditions [59].
In the two examples mentioned above, BY in ChCl:Gly primarily yielded (R)-alcohols. It is important to note that BY in ChCl:Gly does not consistently exhibit (R) selectivity, as the formation of (S)-alcohol was observed during the reduction of aryl ketones with BY cells in water and ChCl:Gly mixtures (20 w% water; see Supplementary Information Table S18) [106]. Thus, the DES, the whole cells used, and the nature of substrate are the important factors which influence the enantioselectivity of biocatalytic reaction [56].
Enantioselective enrichment has also been observed in reductions involving whole cells derived from plants. In the reduction of the prochiral ketone, 1-(3,4-dimethylphenyl) ethanone to its chiral, (1R)-1-(3,4-dimethylphenyl) ethanol using sugar beet (Beta vulgaris L. subsp. vulgaris), the highest enantioselectivity for the (S)-alcohol was achieved in pure water (see Supplementary Information Table S19) [72]. Conversely, in DESs, particularly ChCl-based DESs, the predominant product was the (R)-alcohol, ranging from 18.1 % ee to 88.7 % ee. This selectivity increased with decreasing water content. The highest selectivity was observed in ChCl:Glu (88.7 % ee). Similarly, the reduction of 1-(3,4-dimethylphenyl) ethenone using carrot root in water resulted in high enantioselectivity for the (S)-alcohol (95.6 % ee). However, in the presence of DESs (ChCl:Glu), the (R)-enantiomer becomes dominant (see Supplementary Information Table S15) [64]. Additionally, in the pH range from 3.9 to 6.0 of the medium, (R)-alcohol was prominent, whereas at other pH values the (S)-alcohol was favoured. Based on these studies it was argued that, the preferential formation of (R)-enantiomer was either due to the inhibitory action of the DES on cell ADHs (towards Prelog ADH; see Section 1.1) or due to the altered pH of the medium [64].
Utilising the same DES and whole cells, it is possible to synthesise different enantiomers with different substrates. For example, with the same ADH source strain, G. geotrichum ZJPH1810, different enantiomers were obtained by maintaining the DES, B: Lys constant. For the substrate 2,6-dichloro-3-fluoroacetophenone, the (S)-alcohol was obtained predominantly (>99.9 % ee) in B: Lys while for 4-(trifluoromethyl) acetophenone, the (R)-alcohol was the major product (63.7 % ee) (see Supplementary Information Tables S20 and S21) [92]. This indicates the influence of substrate as suggested by the Keinan model (see Fig. 4b above) [56].
The stereochemistry of different ketone derivatives were also observed to be tunable with RasADH (overexpressed in E.coli) with different DES compositions, ChCl:Gly (1:1.5), ChCl:U (1:2) and ChCl:EG (1:2). The highest yield was obtained in ChCl:Gly [(S)-alcohol)] and the ee ratio increased with the use of DES even at 90 % (v/v; >95 % ee) (see Supplementary Information Table S16), but a low ee was obtained without DES [100]. Extending this work, different ADH activities from yeast, three strains of Yarrowia lipolytica (AM71, AM72, P26A), Candida viswanathi AM120, Hansenula anomala C2, Saccharomyces cerevisiae K1, and Saccharomyces pombe C1 were investigated for the reduction of α-butyrolactone (see Scheme 7) [63]. Among these, the Yarrowia strains reduced the α-butyrolactone most efficiently, but yielded the anti-diastereomer; Candida viswanathi AM120 reduced the substrate with less stereoselectivity, and no substrate transformation was observed with Saccharomyces. Yarrowia lipolytica AM71 converted the α-butyrolactone into its corresponding (3R,1ˈR) product and Candida viswanathi AM120 converted the substrate into mixture of (3S, 1ˈS) and (3R,1ˈR) with a trace amount of (3R,1ˈS).
Scheme 7.
Asymmetric reduction of 3-acetyldihydrofuran-2(3H)-one with Y.lipolytica AM7 and C.viswanathi AM120. Kpi is potassium phosphate.
This suggests that enantioselectivity depends on DES, the whole cell involved and the nature of the substrate.
The concept of solvent control can be applied in this context, where altering the DES composition can induce stereo inversion. ChCl:Gly can be considered as the first option DES for ADH-based reduction of carbonyl compounds and enantioselective reactions [58,59,63,64,72,74,79,83,100,106]. The mechanism behind stereo inversion may involve the inhibition of (R) or (S) selective ADH enzymes present in the whole cells, although the mechanistic details are yet to be proven. Conversely, acidic DESs have demonstrated lower efficiency in biocatalysis [83]. Hence, the pivotal element in biocatalysis is the meticulous choice of DESs and the water/buffer:DES composition [83]. These two factors stand out as the primary optimisation parameters in the solvent system. Following these, additional molecules such as surfactants, which enhance substrate solubility, come into play [75,76]. Subsequently, NADES have proven to be more efficient for whole cell-based reduction reactions since they can provide a more suitable environment for the chosen whole cell [92].
Despite several advantages, the use of whole cells pose various operational challenges such as byproduct formation, difficulty in product isolation, and substrate/product toxicity to the cells [49]. In many cases, using isolated enzymes may be a more convenient arrangement, both in terms of handling, operation and product separation.
3. Isolated ADH-based reductions of carbonyl compounds in deep eutectic solvents
The use of isolated enzymes in DES-buffer systems for bioreduction offers a solution to some of the barriers presented by whole cell enzyme sources. Isolated ADH was used in the reduction of different aryl prochiral ketones into their corresponding alcohols [24]. Interestingly, in the conversion of propiophenone to 1-phenyl-1-propanol, with the Codex® ADH screening kit, the isolated enzyme was inactive in ChCl:U and ChCl:LA DESs systems; whereas it showed activity in ChCl:Gly and ChCl:sorbitol (Sor). Certain ADHs retained activity with increasing DES concentration from 50 % to 80 % (w/w), suggesting this solvent system is a suitable environment for biocatalysis. Significantly in this example, the use of DESs led to a notable improvement in ee of the final product, indicating the influential role of DESs in enantioselectivity (see Supplementary Information Table S22) [24]. Furthermore, the water content and the components of DESs, especially HBDs, can play a significant role in determining the reaction yield for isolated ADH-based reductions. For example, the most efficient bioreduction of cinnamaldehyde to cinnamyl alcohol was achieved at a higher HBA:HBD ratio of 1:9 in ChCl:Gly using HLADH (see Scheme 8) [25].
Scheme 8.
Asymmetric reduction of cinnamaldehyde with HLADH in ChCl:Gly.
An interesting approach to simplify product recovery involves a hydrophobic DES with aqueous buffer as a two-phase system. The strategy employs a thermomorphic multiphase system (TMS). In this arrangement, the reaction takes place when the buffer, containing the enzyme, and the DES [lidocaine:oleic acid (L:Ole); 1:1)], containing the substrate, are brought into a single-phase at lower temperature (<lower critical solution temperature). This step is followed by increasing the temperature (>lower critical solution temperature) to re-form the two-phase regime such that the enzyme associates with the aqueous phase and the substrate/products self-isolate into the hydrophobic phase (see Fig. 5). The major advantage of using this system is the separation of product and ease of enzyme recovery (see Supplementary Information Table S23) [26].
Fig. 5.
Schematic illustraiton of thermomorphic multiphase system (TMS). T is the system temperature, TLCST is the lower critical solution temperature. Created with BioRender.com.
In another application, a series of halogenated ketones can be reduced by lyophilised E. coli ADH [(R)-selective ADH from Lactobacillus brevis (LbADH) and (S)-selective ADHs from Rhodococcus ruber (ADH-A); Supplementary Information Table S24] [107].
The utilisation of isolated enzymes is constrained by the requirement for expensive cofactors. Therefore, it is essential to explore and devise strategies for efficient cofactor regeneration (see section 4 below).
4. Cofactor regeneration in deep eutectic solvents
Cofactors play a crucial role in redox biocatalysis in general, and ADH-based reactions specifically. As they can be expensive (See Supplementary Information Table S25), auto regeneration within the reaction system is a key requirement for a sustainable bioprocess. This can be achieved by carefully designing a co-reaction to regenerate the cofactor. The regeneration of a cofactor requires the addition of a cosubstrate. The cosubstrate will get oxidised (e.g. iso-propanol to acetone) and the cofactor will get reduced (e.g. NAD+ to NADH) accomplishing in-situ regeneration of cofactor. In some instances, the cosubstrate can also influence the conversion of substrate negatively. In the case of the reduction of propiophenone to (S)-1-phenylpropan-1-ol by using isolated RasADH in ChCl:Gly (1:2), the addition of propan-1-ol and propan-2-ol as cosubstrates enhanced the reactivity, whereas ethanol hampered the conversion [100]. One of the advancements in this area is the introduction of a smart cosubstrate. 1,4-BD was added as the cosubstrate in the reduction of various ketones in presence of Tris-HCl buffer (pH 7.0) with ADH. The 1,4-BD was oxidised to γ-butyrolactone (GBL) which is a thermodynamically irreversible and kinetically inert coproduct [108]. An advancement in this concept is the use of HLADH for the conversion of cyclohexanone to cyclohexanol; 1,4-BD in the DES, ChCl:1,4-BD acts as the cosubstrate, resulting in a more sustainable bioprocess. Furthermore, 1,4-BD acts as a smart cosubstrate, as it becomes irreversibly oxidised to a coproduct GBL (See Fig. 6) [109].
Fig. 6.
Overview of cofactor regeneration strategies over time with the introduction of DES in ADH catalysed reactions. a) Coupled enzyme approach in which the oxidation of phosphite by PTDH facilitates cofactor regeneration [110]. b) Smart cosubstrate, in which the 1,4-BD is getting oxidised to thermodynamically irreversible and kinetically inert coproduct facilitating the cofactor regeneration [108]. c) Extended coupled enzyme, in which PTDH, GDH, FDH are used for cofactor regeneration by oxidising phosphite, glucose, formate respectively [111]. d) smart solvent, in this case, glucose acts as HBD in DES and as a cosubstrate in the enzyme reaction for cofactor regeneration [112]. e) smart solvent and smart cosubstrate, 1,4-BD acts as HBD in the DES as well as being oxidised to a corresponding thermodynamically irreversible and kinetically inert coproduct [109]. The central yellow arrow denotes time. Abbreviations: BVMO is Baeyer-Villiger monooxygenases, HLADH is horse liver alcohol dehydrogenase, PTDH is phosphite dehydrogenase, GDH is glucose dehydrogenase and FDH is formate dehydrogenase. Created with BioRender.com.
A coupled enzyme reaction concept was developed as an efficient approach for the regeneration of a cofactor. For example, the Baeyer-Villiger monooxygenases (BVMO), coupled with NADPH-regenerating phosphite dehydrogenase (PTDH), was employed for a sustainable reduction reaction (see Fig. 6) [110]. This approach was further extended by coupling BVMO and ADH with regenerating enzymes like PTDH, glucose dehydrogenase (GDH) and formate dehydrogenase (FDH). The best result was obtained from the PTDH coupled enzyme as the oxidation of phosphite is irreversible [111]. These reactions were conducted in an aqueous buffer solution (Tris-HCl, pH 7.5) and, in this arrangement, the cosubstrate (glucose, formate, phosphite) had to be externally added. When DES is used as a smart solvent, it avoids this need for external additions to the reaction mixture. In this arrangement, the DES can act as a solvent and the HBD component of the DES can act as a cosubstrate, thereby eliminating the need for external addition of cosubstrate. For example, when ChCl:Glu was used for the reduction of acetophenone using LbADH, there was no need to add glucose as cosubstrate for cofactor regeneration [112]. Instead, glucose present as the HBD in the DES is oxidised to D-glucono-1,5-lactone by GDH and, concomitantly, NADP+ is converted to NADPH, resulting in cofactor regeneration. This dual-purpose utilisation of the solvent and its components marked a significant advancement in cofactor regeneration techniques (see Fig. 6). This concept was initially investigated in batch reactions [112] and later translated to a continuous flow reactor (see Fig. 7) [113]. This novel use of DES, as an approach to remove the need for addition of external cosubstrate to achieve cofactor regeneration, underscores the efficiency and promiscuity of DESs as a redox reaction enhancing solvent.
Fig. 7.
Advancement of the smart solvent concept from batch reaction to continuous flow reaction in ADH catalysed reactions. (a) Batch reaction in which glucose acts as both HBD of DES and as a cosubstrate for cofactor regeneration [112]. (b) Continuous flow system with the concept of smart solvent for the reduction of 3-oxo-3-(thiophen-2-yl)propanenitrile [113]. Imm. R. rubra is immobilised whole cells of Rhodotorula rubra MIM14. Created with BioRender.com.
5. Conclusion
Biocatalysis is evolving as a sustainable approach for generating products currently unattainable, or causing environmental damage, through conventional chemical synthesis. The transition from organic solvents to DES marked a breakthrough in this field. This review explored the benefits of incorporating DES in redox biocatalysis using whole cells or isolated enzymes. The use of DES in biocatalysis, in redox or other reactions, benefits from lower toxicity, biodegradability, and ease of preparation. Beyond these, DES-based biocatalysis can significantly enhance the stability, activity, and enantioselectivity of enzymes; making them powerful solvents in redox biocatalysis. DESs can be tailored, through judicious solvent engineering, to induce changes in reaction output. For instance, altering the DES composition in some reactions can change the product's configuration as well as enhancing enantioselectivity, enzyme activity and stability. Additionally, adjusting the molar ratio of HBD components can enhance reaction yield.
This review of the literature suggests ChCl:Gly as a useful starting point for ADH-based enantioselective carbonyl reduction reactions. ChCl:Gly is stable, as neither ChCl or Gly participate in the ADH reaction and which supports the hydrogen bonding between the components. The enantioselectivity observed for certain whole-cell reactions in the presence of DES, especially in ChCl:Gly, is understood to be as a result of the inhibitory action of (R) or (S) selective ADH, although this concept lacks mechanistic confirmation and requires further investigation.
Looking to the future of redox biocatalysis in DES, a major challenge is the involvement of expensive cofactors. Current research focusses on extending the concepts of smart solvent and smart cosubstrate as potential solutions and these illuminate the path for future sustainable development. In certain reactions, the HBD of DES (e.g., Glu in ChCl:Glu, 1,4-BD in ChCl:1,4-BD) can serve both as a component in DES and as a cosubstrate for cofactor regeneration. This eliminates the need for external addition of cosubstrate for cofactor regeneration which is the concept of smart solvent. The smart cosubstrate concept involves the irreversible oxidation of the cosubstrate to a kinetically inert coproduct, leading to cofactor regeneration (eg: oxidation of 1,4-BD to GBL). Both concepts have been successfully applied in ChCl:1,4-BD and the concept of smart solvent has been adopted to continuous flow reactions, marking a milestone in biocatalysis. Extending, and scaling up, these fundamental concepts will provide the next steps for researchers working towards developing sustainable redox biocatalytic systems.
Abbreviations
| ADH | Alcohol dehydrogenase |
| 1,4-BD | 1,4-butanediol |
| B | Betaine |
| BTAP | 1-(3,5-dimethylphenyl)ethan-1-one |
| BTPE | 1-(3,5-dimethylphenyl)ethan-1-ol |
| BVMO | Baeyer-Villiger monooxygenases |
| BY | Baker's Yeast |
| C | l-Carnitine |
| CD | Cyclodextrin |
| C4MIM·PF6 | 1-butyl-3-methylimidazolium hexafluorophosphate |
| ChCl | Choline chloride |
| ChAc | Choline acetate |
| Cys | Cysteine |
| CFPL | 2-chloro-1-(3,4-difluorophenyl)ethanol |
| CFPO | 2-chloro-1-(3,4-difluorophenyl)ethenone |
| CHBE | 4-chloro-3-hydroxybutanoate |
| COBE | Ethyl 4-chloro-3-oxobutanoate |
| CPE | 2-chloro-1-(2,4-dichlorophenyl)ethanone |
| CPEO | 2-chloro-1-(2,4-dichlorophenyl)ethanol |
| DES | Deep eutectic solvents |
| ee | Enantiomeric excess |
| EHB | Ethyl 3-hydroxybutyrate |
| EG | Ethylene glycol |
| EAA | Ethyl acetoacetate |
| F | Fructose |
| FDH | Formate dehydrogenase |
| FDM | Furandimethanol |
| G | Glycine |
| GBL | γ-butyrolactone |
| GDH | Glucose dehydrogenase |
| Gly | Glycerol |
| Glu | Glucose |
| GSH | Glutathione |
| HAP | Hydroxyacetophenone |
| HLADH | Horse liver alcohol dehydrogenase |
| HBA | Hydrogen bond acceptors |
| HBD | Hydrogen bond donors |
| IL | Ionic liquids |
| KRED | Ketoreductases |
| L | Lidocaine |
| LA | Lactic acid |
| LBADH | Lactobacillus brevis alcohol dehydrogenase |
| Lys | Lysine |
| MA | Malic acid |
| MCD | Methylated-β-cyclodextrin |
| MOAP | 4′-methoxyacetophenone |
| MOPE | 1-(4-methoxyphenyl)ethanol |
| NADES | Natural deep eutectic solvents |
| NADPH | Nicotinamide adenine dinucleotide phosphate hydrogen |
| OA | Oxalic acid |
| P | l-Proline |
| PED | 1-phenyl-1,2-ethanediol |
| PBS | Phosphate buffered saline |
| PTDH | Phosphite dehydrogenase |
| ADH-A | Rhodococcus ruberADH |
| RasADH | Ralstonia sp. ADH |
| Sor | Sorbitol |
| TMS | Thermomorphic multiphasic system |
| TesADH | Thermoanaerobacter ethanolicus ADH |
| Tre | Trehalose |
| TEG | Triethylene glycol |
| U | Urea |
Data availability
All the relevant data are included in the manuscript and the supplementary document. No separate repository is attached.
CRediT authorship contribution statement
Ebin K. Baby: Writing – original draft. Rangasamy Savitha: Writing – review & editing. Gemma K. Kinsella: Writing – review & editing, Funding acquisition. Kieran Nolan: Writing – review & editing, Funding acquisition. Barry J. Ryan: Writing – review & editing, Funding acquisition. Gary T.M. Henehan: Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Prof. Gary Henehan reports financial support was provided by Science Foundation Ireland. Prof. Gary Henehan reports a relationship with Technological University Dublin that includes: employment. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research was funded under Science Foundation Ireland (SFI) Programme, Frontiers for the Future: grant number 21/FFP-A/9898.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e32550.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Doble M., Kruthiventi A.K. Green Chemistry and Engineering. Elsevier; 2007. Introduction; pp. 1–26. [DOI] [Google Scholar]
- 2.Asif M., Imran Mohd. Handbook of Greener Synthesis of Nanomaterials and Compounds. Elsevier; 2021. A chapter on synthesis of various heterocyclic compounds by environmentally friendly green chemistry technologies; pp. 69–108. [DOI] [Google Scholar]
- 3.Lewandowski T.A. Encyclopedia of Toxicology. Elsevier; 2014. Green chemistry; pp. 798–799. [DOI] [Google Scholar]
- 4.Sheldon R.A., Woodley J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018;118:801–838. doi: 10.1021/acs.chemrev.7b00203. [DOI] [PubMed] [Google Scholar]
- 5.Alcántara A.R., Domínguez De María P., Littlechild J.A., Schürmann M., Sheldon R.A., Wohlgemuth R. Biocatalysis as key to sustainable industrial chemistry. ChemSusChem. 2022;15 doi: 10.1002/cssc.202102709. [DOI] [PubMed] [Google Scholar]
- 6.On advances and challenges in biocatalysis. Nat. Catal. 2018;1:635–636. doi: 10.1038/s41929-018-0157-7. [DOI] [Google Scholar]
- 7.Zaks A., Klibanov A.M. Enzymatic catalysis in organic media at 100°C. Science. 1984;224:1249–1251. doi: 10.1126/science.6729453. [DOI] [PubMed] [Google Scholar]
- 8.Schindl A., Hagen M.L., Muzammal S., Gunasekera H.A.D., Croft A.K. Proteins in ionic liquids: reactions, applications, and futures. Front. Chem. 2019;7:347. doi: 10.3389/fchem.2019.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorke J., Srienc F., Kazlauskas R. Toward advanced ionic liquids. Polar, enzyme-friendly solvents for biocatalysis. Biotechnol Bioproc E. 2010;15:40–53. doi: 10.1007/s12257-009-3079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotor-Fernández V., Paul C.E. Deep eutectic solvents for redox biocatalysis. J. Biotechnol. 2019;293:24–35. doi: 10.1016/j.jbiotec.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Xu P., Du P.-X., Zong M.-H., Li N., Lou W.-Y. Combination of deep eutectic solvent and ionic liquid to improve biocatalytic reduction of 2-octanone with Acetobacter pasteurianus GIM1.158 cell. Sci. Rep. 2016;6 doi: 10.1038/srep26158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhoraningoga A., Kinsella G.K., Henehan G.T., Ryan B.J. β-glucosidase from Streptomyces griseus: ester hydrolysis and alkyl glucoside synthesis in the presence of Deep Eutectic Solvents. Current Research in Green and Sustainable Chemistry. 2021;4 doi: 10.1016/j.crgsc.2021.100129. [DOI] [Google Scholar]
- 13.Pätzold M., Siebenhaller S., Kara S., Liese A., Syldatk C., Holtmann D. Deep eutectic solvents as efficient solvents in biocatalysis. Trends Biotechnol. 2019;37:943–959. doi: 10.1016/j.tibtech.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Stepankova V., Vanacek P., Damborsky J., Chaloupkova R. Comparison of catalysis by haloalkane dehalogenases in aqueous solutions of deep eutectic and organic solvents. Green Chem. 2014;16:2754–2761. doi: 10.1039/C4GC00117F. [DOI] [Google Scholar]
- 15.Morrison H.G., Sun C.C., Neervannan S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int. J. Pharm. 2009;378:136–139. doi: 10.1016/j.ijpharm.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 16.Lindberg D., De La Fuente Revenga M., Widersten M. Deep eutectic solvents (DESs) are viable cosolvents for enzyme-catalyzed epoxide hydrolysis. J. Biotechnol. 2010;147:169–171. doi: 10.1016/j.jbiotec.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Smith E.L., Abbott A.P., Ryder K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014;114:11060–11082. doi: 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- 18.Aranda C., De Gonzalo G. Biocatalyzed redox processes employing green reaction media. Molecules. 2020;25:3016. doi: 10.3390/molecules25133016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibn Majdoub Hassani F.Z., Amzazi S., Lavandera I. The versatile applications of DES and their influence on oxidoreductase-mediated transformations. Molecules. 2019;24:2190. doi: 10.3390/molecules24112190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnodo D., Maffeis E., Marra F., Nejrotti S., Prandi C. Combination of enzymes and deep eutectic solvents as powerful toolbox for organic synthesis. Molecules. 2023;28:516. doi: 10.3390/molecules28020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magano J., Dunetz J.R. Large-scale carbonyl reductions in the pharmaceutical industry. Org. Process Res. Dev. 2012;16:1156–1184. doi: 10.1021/op2003826. [DOI] [Google Scholar]
- 22.De Miranda A.S., Milagre C.D.F., Hollmann F. Alcohol dehydrogenases as catalysts in organic synthesis. Front. Catal. 2022;2 doi: 10.3389/fctls.2022.900554. [DOI] [Google Scholar]
- 23.Stone C.L., Li T.K., Bosron W.F. Stereospecific oxidation of secondary alcohols by human alcohol dehydrogenases. J. Biol. Chem. 1989;264:11112–11116. doi: 10.1016/S0021-9258(18)60436-3. [DOI] [PubMed] [Google Scholar]
- 24.Cicco L., Ríos-Lombardía N., Rodríguez-Álvarez M.J., Morís F., Perna F.M., Capriati V., García-Álvarez J., González-Sabín J. Programming cascade reactions interfacing biocatalysis with transition-metal catalysis in Deep Eutectic Solvents as biorenewable reaction media. Green Chem. 2018;20:3468–3475. doi: 10.1039/C8GC00861B. [DOI] [Google Scholar]
- 25.Bittner J.P., Zhang N., Huang L., Domínguez De María P., Jakobtorweihen S., Kara S. Impact of deep eutectic solvents (DESs) and individual DES components on alcohol dehydrogenase catalysis: connecting experimental data and molecular dynamics simulations. Green Chem. 2022;24:1120–1131. doi: 10.1039/D1GC04059F. [DOI] [Google Scholar]
- 26.Meyer L., Andersen M.B., Kara S. Ein thermomorphes stark eutektisches Lösungsmittelmehrphasensystem für biokatalytische Anwendungen. Angew. Chem. 2022;134 doi: 10.1002/ange.202203823. [DOI] [Google Scholar]
- 27.Oppenheimer N.J., Henehan G.T.M. In: Enzymology and Molecular Biology of Carbonyl Metabolism 5. Weiner H., Holmes R.S., Wermuth B., editors. Springer US; Boston, MA: 1995. Horse liver alcohol dehydrogenase-catalyzed aldehyde oxidation; pp. 407–415. [DOI] [PubMed] [Google Scholar]
- 28.Winberg J.-O., McKINLEY-McKEE J.S. Drosophila melanogaster alcohol dehydrogenase: mechanism of aldehyde oxidation and dismutation. Biochem. J. 1998;329:561–570. doi: 10.1042/bj3290561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henehan G.T.M., Oppenheimer N.J. Horse liver alcohol dehydrogenase-catalyzed oxidation of aldehydes: dismutation precedes net production of reduced nicotinamide adenine dinucleotide. Biochemistry. 1993;32:735–738. doi: 10.1021/bi00054a001. [DOI] [PubMed] [Google Scholar]
- 30.Mee B., Kelleher D., Frias J., Malone R., Tipton K.F., Henehan G.T.M., Windle H.J. Characterization of cinnamyl alcohol dehydrogenase of Helicobacter pylori: an aldehyde dismutating enzyme. FEBS J. 2005;272:1255–1264. doi: 10.1111/j.1742-4658.2005.04561.x. [DOI] [PubMed] [Google Scholar]
- 31.Velonia K., Smonou I. Dismutation of aldehydes catalyzed by alcohol dehydrogenases. J. Chem. Soc., Perkin Trans. 2000;1:2283–2287. doi: 10.1039/b001039l. [DOI] [Google Scholar]
- 32.Davies J., Jones J.B. Enzymes in organic synthesis. 16. Heterocyclic ketones as substrates of horse liver alcohol dehydrogenase. Stereospecific reductions of 2-substituted tetrahydrothiopyran-4-ones. J. Am. Chem. Soc. 1979;101:5405–5410. doi: 10.1021/ja00512a048. [DOI] [Google Scholar]
- 33.Irwin A.J., Lok K.P., Huang K.W.-C., Jones J.B. Enzymes in organic synthesis. Influence of substrate structure on rates of horse liver alcohol dehydrogenase-catalysed oxidoreductions. J. Chem. Soc., Perkin Trans. 1978;1:1636. doi: 10.1039/p19780001636. [DOI] [PubMed] [Google Scholar]
- 34.Wu S., Snajdrova R., Moore J.C., Baldenius K., Bornscheuer U.T. Biocatalysis: enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. 2021;60:88–119. doi: 10.1002/anie.202006648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hauer B. Embracing nature's catalysts: a viewpoint on the future of biocatalysis. ACS Catal. 2020;10:8418–8427. doi: 10.1021/acscatal.0c01708. [DOI] [Google Scholar]
- 36.Scherkus C., Schmidt S., Bornscheuer U.T., Gröger H., Kara S., Liese A. Kinetic insights into ϵ‐caprolactone synthesis: improvement of an enzymatic cascade reaction. Biotech & Bioengineering. 2017;114:1215–1221. doi: 10.1002/bit.26258. [DOI] [PubMed] [Google Scholar]
- 37.Chadha A., Padhi S.K., Stella S., Venkataraman S., Saravanan T. Microbial alcohol dehydrogenases: recent developments and applications in asymmetric synthesis. Org. Biomol. Chem. 2024;10:1039. doi: 10.1039/D3OB01447A. D3OB01447A. [DOI] [PubMed] [Google Scholar]
- 38.Jia Q., Zheng Y.-C., Li H.-P., Qian X.-L., Zhang Z.-J., Xu J.-H. Engineering isopropanol dehydrogenase for efficient regeneration of nicotinamide cofactors. Appl. Environ. Microbiol. 2022;88 doi: 10.1128/aem.00341-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raj S.B., Ramaswamy S., Plapp B.V. Yeast alcohol dehydrogenase structure and catalysis. Biochemistry. 2014;53:5791–5803. doi: 10.1021/bi5006442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korkhin Y., Kalb Gilboa A.J., Peretz M., Bogin O., Burstein Y., Frolow F. NADP-dependent bacterial alcohol dehydrogenases: crystal structure, cofactor-binding and cofactor specificity of the ADHs of Clostridium beijerinckii and Thermoanaerobacter brockii 1 1Edited by R. Huber. J. Mol. Biol. 1998;278:967–981. doi: 10.1006/jmbi.1998.1750. [DOI] [PubMed] [Google Scholar]
- 41.Tian K., Li Z. A simple biosystem for the high‐yielding cascade conversion of racemic alcohols to enantiopure amines. Angew. Chem. Int. Ed. 2020;59:21745–21751. doi: 10.1002/anie.202009733. [DOI] [PubMed] [Google Scholar]
- 42.Solanki K., Abdallah W., Banta S. Extreme makeover: engineering the activity of a thermostable alcohol dehydrogenase (AdhD) from Pyrococcus furiosus. Biotechnol. J. 2016;11:1483–1497. doi: 10.1002/biot.201600152. [DOI] [PubMed] [Google Scholar]
- 43.Espinosa A., Yan L., Zhang Z., Foster L., Clark D., Li E., Stanley S.L. The bifunctional entamoeba histolytica alcohol dehydrogenase 2 (EhADH2) protein is necessary for amebic growth and survival and requires an intact C-terminal domain for both alcohol dehydrogenase and acetaldehyde dehydrogenase activity. J. Biol. Chem. 2001;276:20136–20143. doi: 10.1074/jbc.M101349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espinosa A., Perdrizet G., Paz-y-Mino C G., Lanfranchi R., Phay M. Effects of iron depletion on Entamoeba histolytica alcohol dehydrogenase 2 (EhADH2) and trophozoite growth: implications for antiamoebic therapy. J. Antimicrob. Chemother. 2009;63:675–678. doi: 10.1093/jac/dkp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji Y., Mao G., Wang Y., Bartlam M. Crystallization and preliminary X-ray characterization of an NAD(P)-dependent butanol dehydrogenase A from Geobacillus thermodenitrificans NG80-2. Acta Crystallogr F Struct Biol Cryst Commun. 2013;69:184–187. doi: 10.1107/S1744309113000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X., Dong Y., Zhang J., Zhang A., Wang L., Feng L. Two novel metal-independent long-chain alkyl alcohol dehydrogenases from Geobacillus thermodenitrificans NG80-2. Microbiology. 2009;155:2078–2085. doi: 10.1099/mic.0.027201-0. [DOI] [PubMed] [Google Scholar]
- 47.Lavandera I., Kern A., Resch V., Ferreira-Silva B., Glieder A., Fabian W.M.F., De Wildeman S., Kroutil W. One-way biohydrogen transfer for oxidation of sec -alcohols. Org. Lett. 2008;10:2155–2158. doi: 10.1021/ol800549f. [DOI] [PubMed] [Google Scholar]
- 48.Man H., Kędziora K., Kulig J., Frank A., Lavandera I., Gotor-Fernández V., Rother D., Hart S., Turkenburg J.P., Grogan G. Structures of alcohol dehydrogenases from Ralstonia and sphingobium spp. reveal the molecular basis for their recognition of ‘bulky–bulky’ ketones. Top. Catal. 2014;57:356–365. doi: 10.1007/s11244-013-0191-2. [DOI] [Google Scholar]
- 49.Betancor L., Berne C., Luckarift H.R., Spain J.C. Coimmobilization of a redox enzyme and a cofactor regeneration system. Chem. Commun. 2006:3640. doi: 10.1039/b604689d. [DOI] [PubMed] [Google Scholar]
- 50.Conway T., Sewell G.W., Osman Y.A., Ingram L.O. Cloning and sequencing of the alcohol dehydrogenase II gene from Zymomonas mobilis. J. Bacteriol. 1987;169:2591–2597. doi: 10.1128/jb.169.6.2591-2597.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leskovac V., TriviÄ S., PeriÄin D. The three zinc-containing alcohol dehydrogenases from baker's yeast, Saccharomyces cerevisiae. FEMS Yeast Res. 2002;2:481–494. doi: 10.1111/j.1567-1364.2002.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 52.Jörnvall H., Persson B., Krook M., Atrian S., Gonzalez-Duarte R., Jeffery J., Ghosh D. Short-chain dehydrogenases/reductases (SDR) Biochemistry. 1995;34:6003–6013. doi: 10.1021/bi00018a001. [DOI] [PubMed] [Google Scholar]
- 53.Larson S.B., Jones J.A., McPherson A. The structure of an iron-containing alcohol dehydrogenase from a hyperthermophilic archaeon in two chemical states. Acta Crystallogr F Struct Biol Commun. 2019;75:217–226. doi: 10.1107/S2053230X19001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramaswamy S., Scholze M., Plapp B.V. Binding of formamides to liver alcohol dehydrogenase. Biochemistry. 1997;36:3522–3527. doi: 10.1021/bi962491z. [DOI] [PubMed] [Google Scholar]
- 55.Prelog V. Specification of the stereospecificity of some oxido-reductases by diamond lattice sections. Pure Appl. Chem. 1964;9:119–130. doi: 10.1351/pac196409010119. [DOI] [Google Scholar]
- 56.Keinan Ehud, Hafeli E.K., Seth K.K. Raphael. Lamed, Thermostable enzymes in organic synthesis. 2. Asymmetric reduction of ketones with alcohol dehydrogenase from Thermoanaerobium brockii. J. Am. Chem. Soc. 1986;108:162–169. doi: 10.1021/ja00261a026. [DOI] [Google Scholar]
- 57.McConathy J., Owens M.J. Stereochemistry in drug action. Prim. Care Companion CNS Disord. 2003;5 doi: 10.4088/PCC.v05n0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maugeri Z., Domínguez de María P. Whole‐cell biocatalysis in deep‐eutectic‐solvents/aqueous mixtures. ChemCatChem. 2014;6:1535–1537. doi: 10.1002/cctc.201400077. [DOI] [Google Scholar]
- 59.Vitale P., Abbinante V.M., Perna F.M., Salomone A., Cardellicchio C., Capriati V. Unveiling the hidden performance of whole cells in the asymmetric bioreduction of aryl‐containing ketones in aqueous deep eutectic solvents. Adv. Synth. Catal. 2017;359:1049–1057. doi: 10.1002/adsc.201601064. [DOI] [Google Scholar]
- 60.Gorke J.T., Srienc F., Kazlauskas R.J. Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem. Commun. 2008:1235. doi: 10.1039/b716317g. [DOI] [PubMed] [Google Scholar]
- 61.Almeida V.M., Marana S.R. Optimum temperature may be a misleading parameter in enzyme characterization and application. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chenault H.K., Whitesides G.M. Regeneration of nicotinamide cofactors for use in organic synthesis. Appl. Biochem. Biotechnol. 1987;14:147–197. doi: 10.1007/BF02798431. [DOI] [PubMed] [Google Scholar]
- 63.Mączka W., Wińska K., Grabarczyk M., Żarowska B. Yeast-mediated stereoselective reduction of α-acetylbutyrolactone. Appl. Sci. 2018;8:1334. doi: 10.3390/app8081334. [DOI] [Google Scholar]
- 64.Panić M., Elenkov M.M., Roje M., Bubalo M.C., Redovniković I.R. Plant-mediated stereoselective biotransformations in natural deep eutectic solvents. Process Biochem. 2018;66:133–139. doi: 10.1016/j.procbio.2017.12.010. [DOI] [Google Scholar]
- 65.Connors K.A. Wiley-VCH; New York, NJ: 1990. Chemical Kinetics: the Study of Reaction Rates in Solution, Nachdr. [Google Scholar]
- 66.Daniel R.M., Peterson M.E., Danson M.J., Price N.C., Kelly S.M., Monk C.R., Weinberg C.S., Oudshoorn M.L., Lee C.K. The molecular basis of the effect of temperature on enzyme activity. Biochem. J. 2010;425:353–360. doi: 10.1042/BJ20091254. [DOI] [PubMed] [Google Scholar]
- 67.Kabir M.F., Ju L.-K. On optimization of enzymatic processes: temperature effects on activity and long-term deactivation kinetics. Process Biochem. 2023;130:734–746. doi: 10.1016/j.procbio.2023.05.031. [DOI] [Google Scholar]
- 68.Chang A., Scheer M., Grote A., Schomburg I., Schomburg D. BRENDA, AMENDA and FRENDA the enzyme information system: new content and tools in 2009. Nucleic Acids Res. 2009;37:D588–D592. doi: 10.1093/nar/gkn820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abranches D.O., Coutinho J.A.P. Everything you wanted to know about deep eutectic solvents but were afraid to Be told. Annu. Rev. Chem. Biomol. Eng. 2023;14:141–163. doi: 10.1146/annurev-chembioeng-101121-085323. [DOI] [PubMed] [Google Scholar]
- 70.Peng F., Chen Q.-S., Li F.-Z., Ou X.-Y., Zong M.-H., Lou W.-Y. Using deep eutectic solvents to improve the biocatalytic reduction of 2-hydroxyacetophenone to (R)-1-phenyl-1,2-ethanediol by Kurthia gibsonii SC0312. Mol. Catal. 2020;484 doi: 10.1016/j.mcat.2020.110773. [DOI] [Google Scholar]
- 71.Li J., Wang P., He Y.-S., Zhu Z.-R., Huang J. Toward designing a novel oligopeptide-based deep eutectic solvent: applied in biocatalytic reduction. ACS Sustainable Chem. Eng. 2019;7:1318–1326. doi: 10.1021/acssuschemeng.8b04989. [DOI] [Google Scholar]
- 72.Pavoković D., Košpić K., Panić M., Radojčić Redovniković I., Cvjetko Bubalo M. Natural deep eutectic solvents are viable solvents for plant cell culture-assisted stereoselective biocatalysis. Process Biochem. 2020;93:69–76. doi: 10.1016/j.procbio.2020.03.020. [DOI] [Google Scholar]
- 73.Bi S., Liu H., Lin H., Wang P. Integration of natural deep-eutectic solvent and surfactant for efficient synthesis of chiral aromatic alcohol mediated by Cyberlindnera saturnus whole cells. Biochem. Eng. J. 2021;172 doi: 10.1016/j.bej.2021.108053. [DOI] [Google Scholar]
- 74.Dai Y., Huan B., Zhang H.-S., He Y.-C. Effective biotransformation of ethyl 4-chloro-3-oxobutanoate into ethyl (S)-4-Chloro-3-Hydroxybutanoate by recombinant E. coli CCZU-T15 whole cells in [ChCl][Gly]–Water media. Appl. Biochem. Biotechnol. 2017;181:1347–1359. doi: 10.1007/s12010-016-2288-0. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y., Duan Z.-W., Liu H.-Y., Qian F., Wang P. Synergistic promotion for microbial asymmetric preparation of (R)-2-chloro-1-(2,4-dichlorophenyl)ethanol by NADES and cyclodextrin. Mol. Catal. 2022;526 doi: 10.1016/j.mcat.2022.112376. [DOI] [Google Scholar]
- 76.Xiong L., Kong X., Liu H., Wang P. Efficient biosynthesis of (S)-1-[2-(trifluoromethyl)phenyl]ethanol by a novel isolate Geotrichum silvicola ZJPH1811 in deep eutectic solvent/cyclodextrin-containing system. Bioresour. Technol. 2021;329 doi: 10.1016/j.biortech.2021.124832. [DOI] [PubMed] [Google Scholar]
- 77.Yang L., Xu D., Jiang L., He Y. Asymmetric bioreduction of ethyl 4-chloroacetoacetate into ethyl 4-Chloro-3-hydroxybutyrate by recombinant Escherichia coli CgCR in ethyl acetate-betaine:lactic acid-water. Processes. 2023;11:3144. doi: 10.3390/pr11113144. [DOI] [Google Scholar]
- 78.He Y., Huang Q., Wang P. Design and evaluation of novel bio‐based deep eutectic solvents for highly efficient bioproduction of chiral aryl alcohol. J. Appl. Chem. Biotechnol. 2020;95:1980–1988. doi: 10.1002/jctb.6386. [DOI] [Google Scholar]
- 79.Panić M., Delač D., Roje M., Radojčić Redovniković I., Cvjetko Bubalo M. Green asymmetric reduction of acetophenone derivatives: Saccharomyces cerevisiae and aqueous natural deep eutectic solvent. Biotechnol. Lett. 2019;41:253–262. doi: 10.1007/s10529-018-2631-3. [DOI] [PubMed] [Google Scholar]
- 80.Ullah R., Atilhan M., Anaya B., Khraisheh M., García G., ElKhattat A., Tariq M., Aparicio S. A detailed study of cholinium chloride and levulinic acid deep eutectic solvent system for CO 2 capture via experimental and molecular simulation approaches. Phys. Chem. Chem. Phys. 2015;17:20941–20960. doi: 10.1039/C5CP03364K. [DOI] [PubMed] [Google Scholar]
- 81.Abbott A.P., Boothby D., Capper G., Davies D.L., Rasheed R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004;126:9142–9147. doi: 10.1021/ja048266j. [DOI] [PubMed] [Google Scholar]
- 82.Abbott A.P., Capper G., Davies D.L., Rasheed R.K., Tambyrajah V. Novel solvent properties of choline chloride/urea mixturesElectronic supplementary information (ESI) available: spectroscopic data. 2003. http://www.rsc.org/suppdata/cc/b2/b210714g/,Chem.Commun See. [DOI]
- 83.Cvjetko Bubalo M., Mazur M., Radošević K., Radojčić Redovniković I. Baker's yeast-mediated asymmetric reduction of ethyl 3-oxobutanoate in deep eutectic solvents. Process Biochem. 2015;50:1788–1792. doi: 10.1016/j.procbio.2015.07.015. [DOI] [Google Scholar]
- 84.Kong L., Fan B., He Y.-C. Efficient whole-cell biosynthesis of (S)-2-chloro-1-(3,4-difluorophenyl)-ethanol from 2-chloro-1-(3,4-difluorophenyl) ethanone in a sustainable reaction system. Mol. Catal. 2023;550 doi: 10.1016/j.mcat.2023.113570. [DOI] [Google Scholar]
- 85.Rodriguez Rodriguez N., Van Den Bruinhorst A., Kollau L.J.B.M., Kroon M.C., Binnemans K. Degradation of deep-eutectic solvents based on choline chloride and carboxylic acids. ACS Sustainable Chem. Eng. 2019;7:11521–11528. doi: 10.1021/acssuschemeng.9b01378. [DOI] [Google Scholar]
- 86.Marchel M., Cieśliński H., Boczkaj G. Thermal instability of choline chloride-based deep eutectic solvents and its influence on their Toxicity─Important limitations of DESs as sustainable materials. Ind. Eng. Chem. Res. 2022;61:11288–11300. doi: 10.1021/acs.iecr.2c01898. [DOI] [Google Scholar]
- 87.Hayyan A., Mjalli F.S., AlNashef I.M., Al-Wahaibi T., Al-Wahaibi Y.M., Hashim M.A. Fruit sugar-based deep eutectic solvents and their physical properties. Thermochim. Acta. 2012;541:70–75. doi: 10.1016/j.tca.2012.04.030. [DOI] [Google Scholar]
- 88.Zhao Q., Hou Y., Gong G.-H., Yu M.-A., Jiang L., Liao F. Characterization of alcohol dehydrogenase from permeabilized brewer's yeast cells immobilized on the derived attapulgite nanofibers. Appl. Biochem. Biotechnol. 2010;160:2287–2299. doi: 10.1007/s12010-009-8692-y. [DOI] [PubMed] [Google Scholar]
- 89.Monteiro H., Paiva A., Duarte A.R.C., Galamba N. Structure and dynamic properties of a glycerol–betaine deep eutectic solvent: when does a DES become an aqueous solution? ACS Sustainable Chem. Eng. 2022;10:3501–3512. doi: 10.1021/acssuschemeng.1c07461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sapir L., Harries D. Restructuring a deep eutectic solvent by water: the nanostructure of hydrated choline chloride/urea. J. Chem. Theor. Comput. 2020;16:3335–3342. doi: 10.1021/acs.jctc.0c00120. [DOI] [PubMed] [Google Scholar]
- 91.Ferreira A.S.D., Craveiro R., Duarte A.R., Barreiros S., Cabrita E.J., Paiva A. Effect of water on the structure and dynamics of choline chloride/glycerol eutectic systems. J. Mol. Liq. 2021;342 doi: 10.1016/j.molliq.2021.117463. [DOI] [Google Scholar]
- 92.Xia N., Xiong L., Bi S., Qian F., Wang P. Development of biocompatible DES/NADES as co-solvents for efficient biosynthesis of chiral alcohols. Bioprocess Biosyst Eng. 2020;43:1987–1997. doi: 10.1007/s00449-020-02387-5. [DOI] [PubMed] [Google Scholar]
- 93.Tang W., Fan B., Wang X., Huang C., Tang Z., He Y. Facilitating enzymatic hydrolysis efficiency of rape straw by pretreatment with a novel glycerol-based deep eutectic solvent. Ind. Crop. Prod. 2023;206 doi: 10.1016/j.indcrop.2023.117587. [DOI] [Google Scholar]
- 94.Zhang D., Liu J., Xu H., Liu H., He Y.-C. Improving saccharification efficiency of corn stover through ferric chloride-deep eutectic solvent pretreatment. Bioresour. Technol. 2024;399 doi: 10.1016/j.biortech.2024.130579. [DOI] [PubMed] [Google Scholar]
- 95.Zhang S., Wu C., Ma C., Li L., He Y.-C. Transformation of bread waste into 2,5-furandimethanol via an efficient chemoenzymatic approach in a benign reaction system. Bioresour. Technol. 2023;371 doi: 10.1016/j.biortech.2023.128579. [DOI] [PubMed] [Google Scholar]
- 96.Peng Y., Jiang L., Di J., Ma C., Li Q., He Y. A hybrid process for valorization of d -fructose to 2,5-Bis(hydroxymethyl)furan by bridging chemocatalysis and biocatalysis in a betaine:benzenesulfonic acid system. ACS Sustainable Chem. Eng. 2022;10:12165–12176. doi: 10.1021/acssuschemeng.2c02702. [DOI] [Google Scholar]
- 97.Zhang S., Ma C., Li Q., Li Q., He Y.-C. Efficient chemoenzymatic valorization of biobased D-fructose into 2,5-bis(hydroxymethyl)furan with deep eutectic solvent Lactic acid:Betaine and Pseudomonas putida S12 whole cells. Bioresour. Technol. 2022;344 doi: 10.1016/j.biortech.2021.126299. [DOI] [PubMed] [Google Scholar]
- 98.Halilu A., Ali T.H., Atta A.Y., Sudarsanam P., Bhargava S.K., Abd Hamid S.B. Highly selective hydrogenation of biomass-derived furfural into furfuryl alcohol using a novel magnetic nanoparticles catalyst. Energy Fuels. 2016;30:2216–2226. doi: 10.1021/acs.energyfuels.5b02826. [DOI] [Google Scholar]
- 99.Li Q., Ren J.-Q., Li Q., Di J.-H., Ma C., He Y. Sustainable conversion of biomass-derived d -xylose to furfuryl alcohol in a deep eutectic solvent–water system. ACS Sustainable Chem. Eng. 2021;9:10299–10308. doi: 10.1021/acssuschemeng.1c02965. [DOI] [Google Scholar]
- 100.Müller C.R., Lavandera I., Gotor‐Fernández V., Domínguez de María P. Performance of recombinant‐whole‐cell‐catalyzed reductions in deep‐eutectic‐solvent–aqueous‐media mixtures. ChemCatChem. 2015;7:2654–2659. doi: 10.1002/cctc.201500428. [DOI] [Google Scholar]
- 101.Duarte J.C., Rodrigues J.A.R., Moran P.J.S., Valença G.P., Nunhez J.R. Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. AMB Expr. 2013;3:31. doi: 10.1186/2191-0855-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu P., Xu Y., Li X.-F., Zhao B.-Y., Zong M.-H., Lou W.-Y. Enhancing asymmetric reduction of 3-chloropropiophenone with immobilized acetobacter sp. CCTCC M209061 cells by using deep eutectic solvents as cosolvents. ACS Sustainable Chem. Eng. 2015;3:718–724. doi: 10.1021/acssuschemeng.5b00025. [DOI] [Google Scholar]
- 103.Gold V., editor. The IUPAC Compendium of Chemical Terminology: the Gold Book. fourth ed. International Union of Pure and Applied Chemistry (IUPAC); Research Triangle Park, NC: 2019. [DOI] [Google Scholar]
- 104.Coelho M.M., Fernandes C., Remião F., Tiritan M.E. Enantioselectivity in drug pharmacokinetics and toxicity: pharmacological relevance and analytical methods. Molecules. 2021;26:3113. doi: 10.3390/molecules26113113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ni Y., Xu J.-H. Biocatalytic ketone reduction: a green and efficient access to enantiopure alcohols. Biotechnol. Adv. 2012;30:1279–1288. doi: 10.1016/j.biotechadv.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 106.Vitale P., Perna F., Agrimi G., Pisano I., Mirizzi F., Capobianco R., Capriati V. Whole-cell biocatalyst for chemoenzymatic total synthesis of rivastigmine. Catalysts. 2018;8:55. doi: 10.3390/catal8020055. [DOI] [Google Scholar]
- 107.Ibn Majdoub Hassani F.Z., Amzazi S., Kreit J., Lavandera I. Deep eutectic solvents as media in alcohol dehydrogenase‐catalyzed reductions of halogenated ketones. ChemCatChem. 2020;12:832–836. doi: 10.1002/cctc.201901582. [DOI] [Google Scholar]
- 108.Kara S., Spickermann D., Schrittwieser J.H., Leggewie C., Van Berkel W.J.H., Arends I.W.C.E., Hollmann F. More efficient redox biocatalysis by utilising 1,4-butanediol as a ‘smart cosubstrate. Green Chem. 2013;15:330. doi: 10.1039/c2gc36797a. [DOI] [Google Scholar]
- 109.Chanquia S.N., Huang L., García Liñares G., Domínguez De María P., Kara S. Deep eutectic solvents as smart cosubstrate in alcohol dehydrogenase-catalyzed reductions. Catalysts. 2020;10:1013. doi: 10.3390/catal10091013. [DOI] [Google Scholar]
- 110.Torres Pazmiño D.E., Snajdrova R., Baas B., Ghobrial M., Mihovilovic M.D., Fraaije M.W. Self‐sufficient baeyer–villiger monooxygenases: effective coenzyme regeneration for biooxygenation by fusion engineering. Angew. Chem. Int. Ed. 2008;47:2275–2278. doi: 10.1002/anie.200704630. [DOI] [PubMed] [Google Scholar]
- 111.Woodyer R., Van Der Donk W.A., Zhao H. Relaxing the nicotinamide cofactor specificity of phosphite dehydrogenase by rational design. Biochemistry. 2003;42:11604–11614. doi: 10.1021/bi035018b. [DOI] [PubMed] [Google Scholar]
- 112.Mourelle-Insua Á., Lavandera I., Gotor-Fernández V. A designer natural deep eutectic solvent to recycle the cofactor in alcohol dehydrogenase-catalysed processes. Green Chem. 2019;21:2946–2951. doi: 10.1039/C9GC00318E. [DOI] [Google Scholar]
- 113.Annunziata F., Guaglio A., Conti P., Tamborini L., Gandolfi R. Continuous-flow stereoselective reduction of prochiral ketones in a whole cell bioreactor with natural deep eutectic solvents. Green Chem. 2022;24:950–956. doi: 10.1039/D1GC03786B. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the relevant data are included in the manuscript and the supplementary document. No separate repository is attached.


















