Abstract
Focal segmental glomerulosclerosis (FSGS), a common cause of primary glomerulonephritis, has a poor prognosis and is pathologically featured by tubulointerstitial injury. Thrombospondin-1 (TSP-1) is an extracellular matrix protein that acts in combination with different receptors in the kidney. Here, we analyzed the tubular expression of TSP-1 and its receptor integrin β3 (ITGB3) in FSGS. Previously the renal interstitial chip analysis of FSGS patients with tubular interstitial injury showed that the expression of TSP-1 and ITGB3 were upregulated. We found that the expression of TSP-1 and ITGB3 increased in the tubular cells of FSGS patients. The plasma level of TSP-1 increased and was correlated to the degree of tubulointerstitial lesions in FSGS patients. TSP-1/ITGB3 signaling induced renal tubular injury in HK-2 cells exposure to bovine serum albumin and the adriamycin (ADR)-induced nephropathy model. THBS1 KO ameliorated tubular injury and renal fibrosis in ADR-treated mice. THBS1 knockdown decreased the expression of KIM-1 and caspase 3 in the HK-2 cells treated with bovine serum albumin, while THBS1 overexpression could induce tubular injury. In vivo, we identified cyclo-RGDfK as an agent to block the binding of TSP-1 to ITGB3. Cyclo-RGDfK treatment could alleviate ADR-induced renal tubular injury and interstitial fibrosis in mice. Moreover, TSP-1 and ITGB3 were colocalized in tubular cells of FSGS patients and ADR-treated mice. Taken together, our data showed that TSP-1/ITGB3 signaling contributed to the development of renal tubulointerstitial injury in FSGS, potentially identifying a new therapeutic target for FSGS.
Keywords: FSGS, TSP-1, renal tubulointerstitial injury, ITGB3
Focal segmental glomerulosclerosis (FSGS), one common cause of primary glomerulonephritis, has a high incidence and a poor prognosis (1). Pathologically characterized by podocyte injury, FSGS is often accompanied by renal tubulointerstitial lesions of varying degrees (2). FSGS accounts for 40% of adults with nephrotic syndromes (3). Podocyte injury is the first event that may result in podocyte loss, glomerular matrix accumulation, and glomerular filtration membrane dysfunction, followed by tubular injury, interstitial inflammation, fibroblast accumulation, and interstitial fibrosis (4, 5). It has long been believed the tubules are the victim of injury. More studies have shown that renal tubular injury plays a critical part in renal tubulointerstitial fibrosis and acts as a driving force in the progression of renal diseases (6). In response to injury, tubular epithelial cells turn inflammatory and fibrogenic, thus producing various bioactive molecules that drive interstitial inflammation and fibrosis (6, 7, 8). Chronic tubulointerstitial fibrosis has been proven as an independent prognostic factor of FSGS (9). The tubular injury involves interstitial inflammatory infiltration, fibroblast accumulation, and matrix deposition, contributing to interstitial fibrosis and progression into kidney failure (10). Therefore, new therapeutic strategies are in urgent need to prevent or delay renal tubular injury in FSGS patients.
Thrombospondin1 (TSP-1) belongs to the family of five glycoproteins encoded by a body of genes. It is released from activated platelets and many other cell types, such as endothelial cells, smooth muscle cells, kidney mesangial cells, and tubular cells (11, 12). TSP-1, a 420 to 450 kDa homotrimer of subunits of approximately 145 kDa, contains several domains that can interact with specific cell surface receptors/molecules to modulate cell adhesion, growth, motility, differentiation, and survival (13, 14). TSP-1 is only expressed in about 1% of the renal tubular and interstitial areas (tissues) in the normal human kidneys (11) and it has been shown to interact with various receptors to drive the development of ischemia-reperfusion injury (IRI), unilateral ureteral obstruction (UUO), and diabetic nephropathy (DN) (15, 16, 17, 18). However, the role of TSP1 in nondiabetic proteinuric kidney disease has not been explored.
In the transcriptome analysis, we have found that the expression of TSP-1 increases in the tubulointerstitial tissues of FSGS patients. Once binding to surface receptors, including integrins, CD36 and CD47, TSP-1 transduces signals from extracellular to intracellular (19). TSP-1 also employs its cognate receptor CD47 and integrin-β3 (ITGB3) to regulate various physiological processes, including immune response, angiogenesis, cell migration, adhesion, and apoptosis (20). Blocking the TSP-1/CD47 signaling decreases inflammatory response (21) and regenerates epithelial cells in several animal models of acute kidney injury (22). The CD47-blocking antibody can ameliorate fibrotic histological changes in mice (23, 24). Li et al. have identified that ITGB3 expression increases in tubular cells and is positively correlated with fibrosis degree in chronic kidney disease patients (25). Furthermore, a high level of plasma TSP-1 is associated with a decline in estimated glomerular filtration in chronic kidney disease patients (23). However, the underlying mechanism of TSP-1 in mediating FSGS-induced renal injury remains unknown.
In this study, therefore, we investigated the role of the TSP-1/ITGB3 signaling pathway in glomerulosclerosis, renal inflammation, and fibrosis based on the mouse model of primary FSGS. Our results suggested that plasma TSP-1 could serve as a potential biomarker in FSGS and the TSP-1/ITGB3 signaling pathway may be pointed to a new potential therapeutic target in FSGS with tubular interstitial injury, even if the primary glomerular pathology is not attenuated.
Results
TSP-1 expression in renal tubulointerstitial tissues of FSGS patients
Renal tubulointerstitial tissues from FSGS patients and controls were microdissected under a stereomicroscope and an Affymetrix HTA 2.0 microarray was used to profile the gene expression patterns in the tissues. The CEL files were accessed through the Gene Expression Omnibus, GSE121211 (26). Among the differentially expressed genes, the mRNA level of THBS1 was significantly higher than that in the control group (Fig. S1). We performed immunohistochemical (IHC) staining for TSP1 in normal renal tissues adjacent to cancer undergoing nephrectomy for renal cancer (n = 15) (controls) and on tissues from FSGS patients that presented with tubulointerstitial injury (n = 56). The FSGS patients were grouped according to the degree of acute tubulointerstitial injury. Patients’ demographics are described in Table 1. IHC staining showed that TSP-1 was negative in normal renal tissues, and the expression of TSP-1 was upregulated in renal tubular epithelial cells in FSGS patients (Fig. 1, A and B). There was no significant difference in the positive expression of TSP-1 between the subgroups with different degrees of tubulointerstitial injury, although the positive rate of TSP-1 expression was negatively correlated with the levels of urine RBP, urine protein, and serum creatinine (Table S1).
Table 1.
Baseline characteristics of FSGS population
| Characteristic | Overall cohort (N = 56) | Mild group (N = 22) | Moderate group (N = 18) | Severe group (N = 16) |
|---|---|---|---|---|
| Male sex, n | 40 | 17 | 14 | 9 |
| Age, years | 24.5 (19–40.75) | 22.5 (19–26.75) | 28 (20.5–41.25) | 44 (18.25–61.75)a |
| Duration of AKI (d) | 7 (4–10) | 7 (4–9.25) | 7.5 (2.75–10.25)b | 8 (5–15.75)a |
| Urine protein g/24 h | 9.47 (6.47–14.86) | 8.53 (6.17–14.12) | 10.9 (7.55–15.52) | 10.85 (5.88–15.19) |
| Serum albumin, g/L | 19.65 ± 3.12 | 20.28 ± 2.73c | 18.16 ± 2.75b | 20.46 ± 3.57 |
| Serum creatinine, umol/L | 143.21 (99.01–202.44) | 99.01 (83.98–119.34) | 156.47 (136.14–205.09)b | 217.46 (167.08–325.31)a |
| Urine NAG, U/(g.Cr) | 68.88 ± 31.24 | 61.76 ± 35.54 | 64.27 ± 22.27b | 85.51 ± 34.89a |
| Urine RBP, mg/L | 15.84 ± 12.71 | 10.63 ± 9.93 | 17.94 ± 10.62 | 20.96 ± 16.39 |
| Serum cholesterol, mmol/L | 11.34 (8.79–13.3) | 11.61 (9.6–13.36) | 10.99 (8.73–15.42) | 11.39 (8.42–12.94) |
| TSP-1 positive rate,% | 12.82 + 7.18 | 14.41 ± 6.18 | 10.03 ± 6.61 | 13.78 ± 8.48 |
AKI, acute kidney injury; FSGS, focal segmental glomerulosclerosis; NAG, N-acetyl-β- D-glucosaminidase; RBP, retinol-binding protein; TSP-1, thrombospondin 1.
Mild group versus severe group, p < 0.05.
Moderate group versus severe group, p < 0.05.
Mild group versus moderate group, p < 0.05.
Figure 1.
Expression of TSP-1 in renal tubulointerstitial tissues of FSGS patients.A and B, immunohistochemical analysis of TSP-1 in FSGS patients (n = 56) and normal controls (n =15). TSP-1 was expressed in the renal tubular cells of patients with FSGS. C, platelet-poor plasma was collected and TSP1 levels were measured by ELISA. Results are graphed by different degrees of proteinuria of podocytopathies (minimal change disease and FSGS). D, correlation between the level of plasma TSP-1 level and the degree of proteinuria in podocytopathies. E, plasma TSP1 levels are graphed by tubulointerstitial lesion score. F, correlation between the level of plasma TSP-1 level and the tubulointerstitial lesion score in podocytopathies. For statistical analysis, a two-tailed Student’s t test was used for B, one-way ANOVA was used for C and E. Pearson’s correlation was used for D and F. ∗p < 0.05 compared among different groups. The scale bars represent 50 μm (A). FSGS, focal segmental glomerulosclerosis; TSP-1, thrombospondin 1.
Plasma TSP-1 elevates in FSGS patients
The plasma TSP-1 level increased in patients with CKD, suggesting a significantly negative relationship with estimated glomerular filtration (23). To explore the role of TSP-1 in FSGS with tubulointerstitial injury, we enrolled FSGS patients (n = 21) and minimal change disease patients (n = 10), and have measured the TSP-1 concentrations in their platelet-poor plasma. We observed a significant increase in plasma TSP-1 level with the increase inurinary protein (Fig. 1C) and with the severity of tubulointerstitial injury (Fig. 1E). The linear regression analysis demonstrated that TSP-1 expression was correlated with urinary protein level (Fig. 1D) (r2 = 0.651, p < 0.05) and tubulointerstitial lesion score (Fig. 1F) (r2 = 0.612, p < 0.05).
TSP-1 expression correlates with progressive tubulointerstitial injury in adriamycin-induced nephropathy model
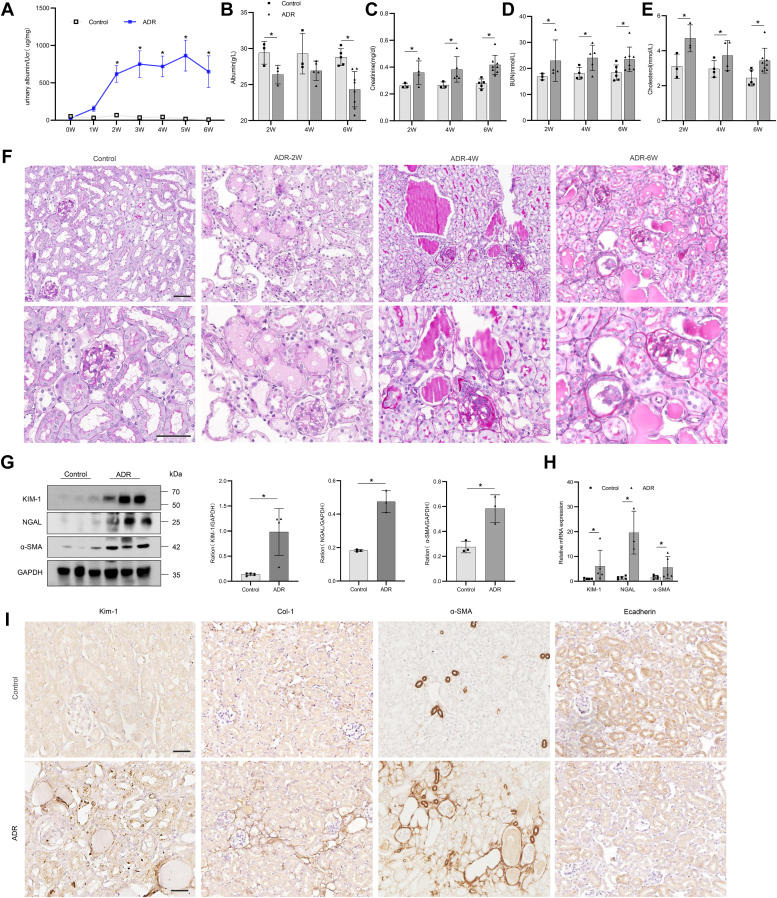
We analyzed TSP-1 expression in glomeruli of BALB/c WT mice at several time points after adriamycin (ADR)-induced nephropathy. Renal TSP-1 mRNA level increased as the disease progressed. Urinary albumin levels (Fig. 2A) and serum albumin, creatinine, urea, and cholesterol levels were significantly higher in the ADR group than in the control group (Fig. 2, B–E). Glomerular pathology was accompanied by prominent tubulointerstitial changes, including tubular cell with brush border loss, tubular dilation, intraluminal cast formation, and expansion of the interstitial volume due to deposition of extracellular matrix (Fig. 2F). Western blot analysis showed that the expression of renal tubular injury markers KIM-1 and NGAL and fibrosis marker α-SMA increased significantly in the renal tissue of ADR nephropathy (Fig. 2G). Quantitative polymerase chain reaction results showed that the mRNA levels of kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, and fibrosis marker α-smooth muscle actin increased significantly in the renal tissue of ADR nephropathy (Fig. 2H). IHC staining showed that the expression of KIM-1, collagen I (Col-I), and α-SMA in the interstitium increased significantly, but that of E-cadherin decreased (Fig. 2I). Thus, similar to that in human FSGS, TSP-1 was also overexpressed in ADR-induced glomerulosclerosis in mice (Fig. 3, C–F).
Figure 2.
Renal functional impairment and pathology in mice with adriamycin nephropathy.A, albuminuria expressed as ACR (mg/mg) was evaluated in spot urine samples at baseline and weekly intervals thereafter until week 6 of ADR nephropathy. B–E, plasma levels of albumin (B), creatinine (C), urea (D), and cholesterol (E) were determined at week 2, 4, and 6 in ADR-treated mice. F, ADR-treated mice developed severe glomerular lesions with segmental to global sclerosis, tubular injury, and interstitial fibrosis indicated by Periodic acid–Schiff-positive glomerular deposits. G, Western blot analysis of KIM-1, NGAL, and α-SMA in tubulointerstitial tissues of mice treated with ADR. H, RT-PCR analysis of KIM-1, NGAL, and α-SMA in tubulointerstitial tissues of mice treated with ADR. I, immunohistochemical staining of KIM-1, COL-1, α-SMA, and E-cadherin in renal tissues of mice treated with ADR. For statistical analysis, one-way ANOVA was used for A, and a 2-tailed Student’s t test was used for B–E, G, and H. ∗p < 0.05 compared with control mice. Scale bars, 50 μm (F and I). Col-1, collagen I.
Figure 3.
TSP-1/ITGB3 Signaling is upregulated in tubular cells of ADR-treated mice.A, ITGB3 mainly expressed on renal tubule (from public database: Tissue expression of ITGB3 - Staining in kidney-The Human Protein Atlas). The scale bars represent 200 μm. B, immunohistochemical staining of ITGB3 in renal tissues of FSGS patients. C, immunofluorescence staining of TSP-1 and ITGB3 in renal tissues of FSGS patients. D, Western blot analysis of TSP-1 and ITGB3 in tubulointerstitial tissues of mice treated with ADR. E, RT-PCR analysis of TSP-1 and ITGB3 in tubulointerstitial tissues of mice treated with ADR. F and G, immunohistochemical and immunofluorescence staining of TSP-1 and ITGB3 in renal tissues of mice treated with ADR. H, double immunofluorescence for TSP-1 (red) with ITGB3 (green) shows that both proteins are expressed by renal tubulointerstitial injury in FSGS patients. I, double immunofluorescence for TSP-1 (green) with ITGB3 (red) shows that both proteins are expressed by renal tubulointerstitial injury in renal tissues of mice treated with ADR. For statistical analysis, two-tailed Student’s t test was used for D and E. ∗p < 0.05 compared with control mice. The scale bars represent 50 μm (B, C, F–H). The scale bars represent 20 μm (I). ADR, adriamycin; FSGS, focal segmental glomerulosclerosis; ITGB3, integrin β3; TSP-1, thrombospondin 1.
TSP-1/ITGB3 signaling regulates tubulointerstitial injury in humans with FSGS and ADR-induced nephropathy model
Our transcriptomic analysis has shown that ITGB3 is significantly upregulated in FSGS patients. ITGB3, also known as CD61 or GPIIIA, can respond to the stromal and immune microenvironment and promote cellular senescence in many types of tumors. The single-cell RNA-seq and IHC staining of human renal tissues from a public database (Human Protein Atlas) showed that ITGB3 was mainly expressed in proximal tubular cells (Fig. 3A). ITGB3 expression also increased in renal tubules in the ischemia-reperfusion injury model (Fig. S2). Thus, we first determined the levels of TSP-1 and its receptor ITGB3 in renal tubulointerstitial tissues from FSGS patients. IHC and immunofluorescence (IF) staining confirmed that the levels of ITGB3 and TSP-1 increased in the tubulointerstitial tissues of FSGS patients (Figs. 1A, and 3, B and C). Similar to that in human FSGS, ITGB3 was upregulated in renal tubular epithelial cells of mice with ADR-induced glomerulosclerosis by WB (Fig. 3D) and quantitative polymerase chain reaction (Fig. 3E), as well as by IHC and IF staining (Fig. 3, F and G). The IF results supported the colocalization of ITGB3 and TSP-1 (Fig. 3H).
We therefore induced ADR nephropathy in WT and TSP-1 −/− mice to compare their phenotypes (Fig. S3). TSP-1 −/− mice developed significantly less nephrotic-range albuminuria accompanied by increased plasma albumin levels (Fig. 4B) and hyper-cholesterolemia compared with WT controls (Fig. 4, A–E). Low plasma urea and creatinine levels indicated less renal functional impairment in TSP-1 −/− mice (Fig. 4, C and D). Moreover, Periodic acid–Schiff staining revealed milder tubulointerstitial damage in the kidney tissues in TSP-1 −/− mice, which was paralleled by reduced renal expression of tubular injury and interstitial fibrosis markers (Fig. 4, F and G). Together, TSP-1 deficiency ameliorated functional and structural tubulointerstitial injury in ADR models.
Figure 4.
THBS1 deficiency reduces albuminuria and renal functional impairment in mice with ADR nephropathy.A, level of Albuminuria (ug/mg) in WT and THBS1-KO mice treated with ADR. B–E, plasma levels of albumin, creatinine, urea, and cholesterol were determined at week 6 in WT and THBS1-KO mice treated with ADR. F, periodic acid–Schiff staining of renal sections in WT and THBS1-KO mice treated with ADR. G, Western blot analysis of TSP-1, KIM-1, and α-SMA in tubulointerstitial tissues of WT and THBS1-KO mice treated with ADR. For statistical analysis, one-way ANOVA was used for A and B. ∗p <0.05 compared with control WT mice; # p < 0.05 compared with ADR-treated mice. Original magnifications: ×200, the scale bars represent 50 μm (F). ADR, adriamycin; TSP-1, thrombospondin 1.
TSP-1/ITGB3 signaling induced renal tubular injury in HK-2 cells
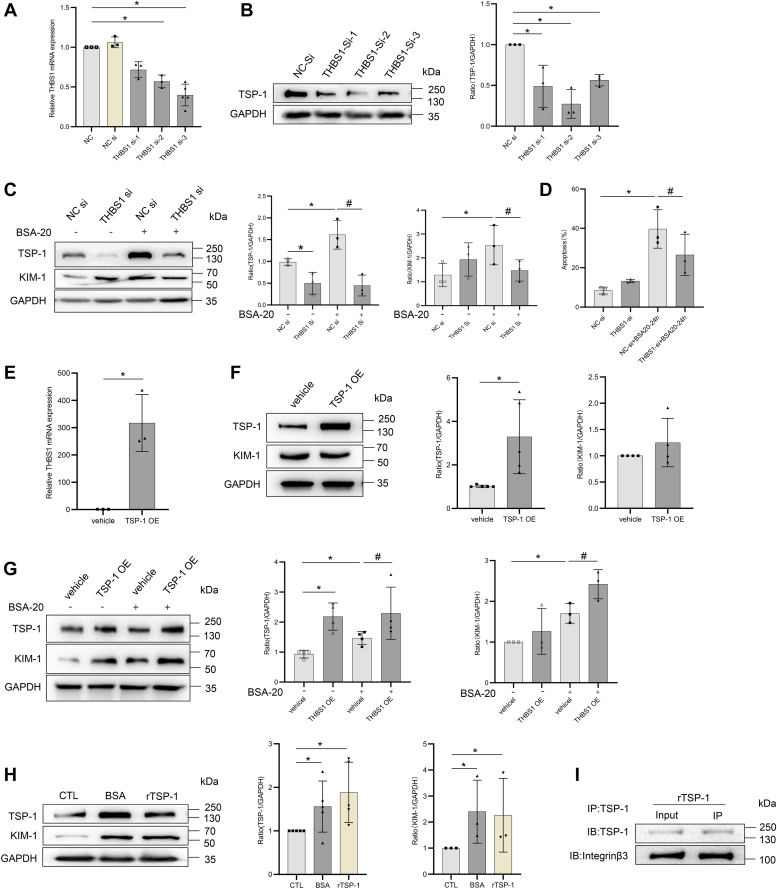
Human renal tubular (HK-2) cells were cultured and treated in vitro. Bovine serum albumin (BSA) can be used to induce in vitro damage on renal tubular epithelial cells ((27, 28)). WB revealed that the expression of KIM-1 and cleaved caspase 3 increased following BSA stimulation (Fig. 5, A and B), which was further supported by the IF of KIM-1 and the apoptosis assay (Fig. 5, C and D). The high expression of TSP-1 and ITGB3 was confirmed by WB, quantitative polymerase chain reaction, and IF (Fig. 5, E–G). Therefore, we investigated whether THBS1 is involved in the progression of renal tubular injury. We silenced THBS1 expression in HK-2 cells transfected with siRNA (Fig. 6, A and B). WB revealed that the expression of KIM-1 and caspase-3 was significantly decreased in the HK-2 cells treated with BSA (Fig. 6C) and the apoptotic rate decreased (Fig. 6D). In addition, we transfected HK-2 cells with a THBS1-expressing plasmid. The expression of TSP-1 and KIM-1 was significantly upregulated in TSP-1–overexpressed cells treated with BSA (Fig. 6, E–G). As an extracellular matrix protein, TSP-1 can be secreted to bind to receptors or ligands on the cell membrane, thus activating downstream pathways. Therefore, we directly stimulated HK-2 cells with recombinant human TSP-1 protein (rTSP-1). Consistent with the results in BSA-stimulated HK-2 cells, the expression of KIM-1 was significantly increased in HK-2 cells stimulated by rTSP-1 (Fig. 6H). The co-immunoprecipitation (CO-IP) results supported the colocalization of ITGB3 and THBS1 (Fig. 6I). These results suggested that TSP-1/ITGB3 signaling might induce renal tubular injury.
Figure 5.
Induction of the damage of renal tubular epithelial cells in BSA-treated HK-2 cells.A and B, Western blot analysis of caspase 3, cleaved caspase 3, and KIM-1 in HK-2 cells exposed to BSA (10, 20, 50 mg/ml) for 24 h. C, immunofluorescence staining of KIM-1 in HK-2 cells exposed to BSA (20 mg/ml) for 24 h. D, incubation with BSA (10, 20 mg/ml) for 24 h induced apoptosis of HK-2 cells by flow cytometry. E, RT-PCR analysis of TSP-1 and ITGB3 in HK-2 cells exposed to BSA (10, 20 mg/ml) for 12 and 24 h. F, Western blot analysis of TSP-1 and integrinβ3 (ITGB3) in HK-2 cells exposed to BSA (10, 20, 50 mg/ml) for 24 h. G, double immunofluorescence for TSP-1 (green) with ITGB3 (red) shows that both proteins are expressed in HK-2 cells exposed to BSA (20 mg/ml) for 24 h. For statistical analysis, one-way ANOVA was used for B, E, and F. ∗p < 0.05 compared with control cells. Original magnifications: ×400, the scale bars represent 50 μm (C and G). BSA, bovine serum albumin; TSP-1, thrombospondin 1.
Figure 6.
TSP-1 aggravated HK-2 cells injury by combined with ITGB3.A and B, verification of THBS1 knockdown in HK-2 cells. RT-PCR analysis of THBS1 (A) and Western blot analysis of TSP-1 (B) in HK-2 cells treated with THBS1-siRNA. C, Western blot analysis of TSP-1 and KIM-1 in HK-2 cells treated with THBS1-siRNA exposed to BSA (20 mg/ml) for 24 h. D, THBS1 knockdown effect the apoptosis of HK-2 cells exposed to BSA (20 mg/ml) for 24 h by flow cytometry. E, RT-PCR analysis of THBS1in HK-2 cells treated with THBS1 plasmid. F, Western blot analysis of TSP-1 and KIM-1 in HK-2 cells treated with THBS1 plasmid. G, Western blot analysis of TSP-1 and KIM-1 in HK-2 cells treated with THBS1 plasmid exposed to BSA (20 mg/ml) for 24 h. H, Western blot analysis of TSP-1 and KIM-1 in HK-2 cells exposed to rTSP-1 (1 ug/ml) and BSA (20 mg/ml) for 24 h. I, IP analysis of the binding between TSP-1 and ITGB3 in HK-2 cells treated with rTSP-1 (1 ug/ml). For statistical analysis, one-way ANOVA was used for A–G. ∗p < 0.05 compared with control cells. # p < 0.05 compared with cells exposed to BSA. BSA, bovine serum albumin; Col-1, collagen I; ITGB3, integrin β3; IP, immunoprecipitation; rTSP-1, recombinant human TSP-1 protein; TSP-1, thrombospondin 1.
Inhibiting ITGB3 by cyclo-RGDfK attenuates tubulointerstitial injury in the mouse model of ADR-induced nephropathy
To further confirm the effect of ITGB3 inhibition (cyclo-RGDfK), another modified ADR-induced nephropathy model was established through treatment with the cyclo-RGDfK (Fig. S4). We found that the urinary albumin level decreased significantly in the cyclo-RGDfK group (Fig. 7A). The serum albumin level significantly increased, and the serum albumin, creatinine, urea, and cholesterol levels decreased significantly compared with those in the control group (Fig. 7, B–E). Periodic acid–Schiff and Masson staining showed reduction of renal tubular injury and fibrotic lesions in the cyclo-RGDfK group (Fig. 7F ). IHC and WB analyses revealed that the inhibition of the TSP-1/ITGB3 signaling pathway attenuated the tubulointerstitial injury in the ADR model, and that the expression levels of ITGB3, KIM-1, Col-1, α-SMA, and F4/80 were also significantly decreased in the cyclo-RGDfK group (Fig. 7, G and H). Quantitative reverse transcriptase PCR showed significantly decreased mRNA expression of renal injury and inflammation markers (KIM-1, NGAL, Col-I, α-SMA, fibronectin, transforming growth factor β, IL-6, and IL-1β) (Fig. 7I). These findings indicated that cyclo-RGDfK may represent a targeted approach to alleviate tubular cell injury and kidney fibrosis.
Figure 7.
Effect of ITGB3 inhibition by cyclo-RGDfK on the tubulointerstitial injury of ADR-treated mice.A, level of albuminuria (ug/mg) in ADR- and cyclo-RGDfK–treated mice. B–E, serum levels of albumin, creatinine, urea, and cholesterol were determined at week 6 in mice treated with ADR and cyclo-RGDfK. F, PAS and Masson staining of renal sections in mice treated with ADR and cyclo-RGDfK. G, immunohistochemical staining of TSP-1, ITGB3, KIM-1, E-cadherin, COL-1, α-SMA, and F4/80 in tubulointerstitial tissues of mice treated with ADR and cyclo-RGDfK. H, Western blot analysis of TSP-1, ITGB3, KIM-1, NGAL, E-cadherin, α-SMA, and COL-1 in tubulointerstitial tissues of mice treated with ADR and cyclo-RGDfK. I, RT-PCR analysis of THBS1, ITGB3, NGAL, KIM-1, α-SMA, IL-6, TGF-β, IL-1β, COL-1, and fibronectin in tubulointerstitial tissues of mice treated with ADR and cyclo-RGDfK. For statistical analysis, one-way ANOVA was used for A, B, H, and I. ∗p < 0.05 compared with control mice. # p <0.05 compared with ADR-treated mice. Original magnifications: ×200, the scale bars represent 50 μm (F and G). ADR, adriamycin; BSA, bovine serum albumin; Col-1, collagen I; ITGB3, integrin β3; TGF-β, transforming growth factor-β; TSP-1, thrombospondin 1.
Inhibiting ITGB3 by cyclo-RGDfK attenuates renal tubular injury in HK-2 cells
Based on the result of in vivo models, we found that the TSP-1/ITGB3 signaling pathway may be involved in the development and progression of renal tubulointerstitial injury in FSGS. We further examined the role of the TSP-1/ITGB3 signaling pathway in renal tubular injury in vitro. We silenced ITGB3 expression in HK-2 cells transfected with siRNA (Fig. 8, A and B). Then WB revealed that when ITGB3 expression was silenced, the expression levels of TSP-1 and KIM-1 were significantly decreased in the HK-2 cells treated with rTSP-1 (Fig. 8C) and BSA (Fig. 8D) after transfection with ITGB3 siRNA. Furthermore, the ITGB3 inhibitor cyclo-RGDfK significantly prevented the upregulation of KIM-1 and α-SMA in HK-2 cells treated with rTSP-1 (Fig. 8E). These results suggested that silencing ITGB3 and using cyclo-RGDfK to prevent the binding between TSP-1 and ITGB3 can attenuate injury in renal tubular epithelial cells.
Figure 8.
Effect of ITGB3 inhibition by cyclo-RGDfK on HK-2 cells injury.A, verification of ITGB3 knockdown in HK-2 cells. RT-PCR analysis of ITGB3 (A) and Western blot analysis of integrinβ3 (B) in HK-2 cells treated with ITGB3-siRNA. C, Western blot analysis of integrinβ3, TSP-1, and KIM-1 in HK-2 cells treated with ITGB3-siRNA exposed to rTSP-1 (1 ug/ml) for 24 h. D, Western blot analysis of integrinβ3, TSP-1, and KIM-1 in HK-2 cells treated with ITGB3-siRNA exposed to BSA (20 mg/ml) for 24 h. E, Western blot analysis of integrinβ3, TSP-1, KIM-1, and α-SMA in HK-2 cells treated with cyclo-RGDfK (0.5 uM) and rTSP-1 (1 ug/ml) for 24 h. For statistical analysis, one-way ANOVA was used for A–C. ∗p < 0.05 compared with control cells. # p < 0.05 compared with cells exposed to BSA (20 mg/ml) or rTSP-1 (1 ug/ml). BSA, bovine serum albumin; Col-1, collagen I; rTSP-1, recombinant human TSP-1 protein; TSP-1, thrombospondin 1; ITGB3, integrin β3.
Discussion
The annual incidence of FSGS has continued to rise globally (29). FSGS is often characterized by renal tubulointerstitial injury, which may co-occur with podocyte injury. FSGS progresses into kidney failure within 5 to 20 years in about 30 to 50% of patients (30). The tubulointerstitial injury could lead to more serious renal dysfunction than glomerular injury. At present, most treatments for FSGS continue to focus on podocytes, but with poor effects. Therefore, new therapeutic targets for renal tubulointerstitial injury in FSGS remain to be explored.
Several studies have shown that TSP-1 is mainly expressed in the tubulointerstitial area of the kidney, and it is also found that TSP-1 is highly expressed in the tubulointerstitial area of the kidney through ischemia-reperfusion model (15), UUO model (17), and diabetic nephropathy model (31). Moreover, TSP-1 has been observed to activate podocytes in the FSGS group, compared with the control group (32). TSP-1 expression is also prominent in the sclerotic lesions in the remnant kidney affected by glomerular and tubulointerstitial fibrosis (33). In this study, we observed the expression of TSP-1 in the renal tubulointerstitial of the renal tissue with FSGS patients and ADR nephropathy, which is activated in the renal tubulointerstitial and appears to be involved in the development of FSGS.
In the study, the expression of TSP-1 in the renal tissue was significantly higher in FSGS patients with tubulointerstitial injury than in the control group, but the expression level of TSP-1 was negatively correlated with the degree of urinary proteinuria. This suggests that the expression of TSP-1 increases in the early stage of renal tubular injury in FSGS. It is speculated that the increased expression of TSP-1 in the early stage causes renal tubular injury and further activates the downstream pathway to aggravate the damage. With the aggravation of fibrosis, the expression of TSP-1 does not increase markedly. We also explored the role of plasma TSP-1 in FSGS with tubulointerstitial injury. We observed that plasma TSP-1 level rose significantly with urinary protein and positively correlated with tubulointerstitial lesion score. Our result suggested that plasma TSP-1 elevates in FSGS result from tubulointerstitial injury or secondary vascular damage and inflammation. These results are also consistent with previous findings (15, 23). The underlying mechanisms remain to be investigated in the future. Moreover, we also found that the expression levels of TSP-1 and its receptor ITGB3 were increased in FSGS patients with tubulointerstitial injury and the ADR model. On the other hand, THBS1 knockout developed less nephrotic-range albuminuria accompanied by increased plasma albumin levels and low plasma creatinine levels compared with WT controls. It is shown that TSP-1 deficiency ameliorated functional and structural tubulointerstitial injury in ADR models.
FSGS can be treated with efforts to reduce proteinuria, improve the remission rate, and delay the progression of the disease. Previous studies on FSGS focus on podocyte injury, confirming that long-term serious albuminuria can damage renal tubular epithelial cells, induce renal tubular epithelial cells to produce various chemokines and inflammatory factors, and mediate interstitial inflammation and fibrosis (34, 35). Urinary protein can be controlled to reduce the reabsorption of protein in renal tubules, and further alleviate renal tubulointerstitial injury. Therefore, cultured tubular cells exposed to BSA were used as surrogates for in vivo exposure of tubular cells to albumin in FSGS. In the model of BSA-induced renal tubular epithelial cell injury, we found that the levels of TSP-1 and ITGB3 increased in HK-2 cells. IP assay further showed that the binding between TSP-1 and ITGB3 was enhanced in HK-2 cells after BSA-induced renal tubular epithelial cell injury. These suggest that TSP-1 and its receptor may play an important role in exerting its pathogenic role, and selective TSP-1–related therapy may represent a potential direction in the treatment of renal tubulointerstitial injury in FSGS. Therefore, an intervention targeting the binding of TSP-1 to ITGB3 may be a new therapeutic approach.
At present, studies have focused on the role and mechanism of ITGB3 in podocytes, rarely on renal tubular injury. However, it has been found in some public transcription databases that the expression of ITGB3 increases in the tubular tissue of DN, FSGS, and lupus nephritis patients (36). Li et al. have found that ITGB3 is mainly located in the renal tubules by using UUO mouse models. It has been found that ITGB3 overexpression could induce p53 pathway activation and secretion of transforming growth factor β, resulting in senescent and profibrotic phenotype change in tubular cells. ITGB3 antagonist (ISL) could inhibit ITGB3, which, in turn, attenuated cellular senescence and renal fibrosis (25). However, how ITGB3 induces renal tubulointerstitial injury in FSGS is still unclear. Here, for the first time, we found that ITGB3 and TSP-1 may be involved in the pathogenesis of FSGS renal tubulointerstitial lesions.
TSP-1 combines with its receptor ITGB3 to regulate an array of pathological processes, such as inflammatory response, apoptosis, and tumor angiogenesis ((37, 38, 39)). The binding of TSP-1 with its receptor ITGB3 can activate the downstream transforming growth factor β signaling pathway to initiate the development of breast tumors (40). The expression levels of TSP-1 and its receptor ITGB3 increase under hypoxia, and TSP-1–neutralizing antibodies or ITGB3 inhibitors can inhibit cell migration and subsequent progression of atherosclerotic disease (41). The TSP-1/ITGB3 complex may also be involved in vascular endothelial lesions in patients with kidney failure (42). In kidney diseases, the ITGB3 inhibitor cyclo-RGDfk can alleviate lipopolysaccharide-induced kidney injury and proteinuria in DN (43, 44). In the beginning, cyclo RGDfk was used to treat the classic ADR model, and it was found that there was a significant decrease in urinary protein in the third week (Fig. S5) and mild renal tubular injury, which may be due to the inhibition on podocyte ITGB3 expression and the alleviation on podocyte injury. Therefore, we established the modified ADR-induced nephropathy model according to the reference (45, 46), which could maintain a high degree of proteinuria and damage to tubulointerstitial tissues. In the present study, the level of urinary protein did not increase until week 5, which may be related to the fact that reabsorption increases as tubular damage subsides. Our results showed that cyclo-RGDfk could improve renal function and reduce renal tubular injury and renal interstitial fibrosis in ADR mice. The mRNA and protein levels of KIM-1, NGAL, Col-1, and α-SMA decreased, and the number of F4/80 positive cells decreased in the renal interstitium. In the in vitro model, our result showed that silencing ITGB3 or treating with ITGB3 inhibitor cyclo-RGDfK prevented the binding between TSP-1 and ITGB3, thus attenuating renal tubular epithelial cell injury. Furthermore, we confirmed the interaction between TSP-1 and ITGB3 by co-IP experiments, indicating that blocking the binding of TSP-1 to ITGB3 may attenuate renal tubulointerstitial injury.
In conclusion, TSP-1 is suggested to mediate renal tubulointerstitial injury in FSGS by binding to its receptor ITGB3. Blocking the binding of TSP-1 to ITGB3 can alleviate renal tubulointerstitial injury and interstitial fibrosis, which may serve as a new therapeutic target for FSGS. Further studies will explore whether the application of this treatment leads to improved patient outcomes.
Experimental procedures
Human samples
Eight FSGS patients who underwent renal biopsies at Jingling Hospital were recruited for tubulointerstitial transcriptome analysis. The other 56 FSGS patients were enrolled for the validation study. Control tissues were obtained from the unaffected portion of surgical nephrectomies and were confirmed to be normal through light microscopic analysis.
Renal specimens were kept in the Renal Biobank of the National Clinical Research Center of Kidney Diseases at Jinling Hospital. Informed consent was obtained from each participant.
Plasma TSP1 measurement
Samples were collected from the Renal Biobank of the National Clinical Research Center of Kidney Diseases, Renal Biobank of Jiangsu Provincial Science and Technology Resources Coordination Service Platform. All subjects provided written consent. Patients (n = 31) diagnosed with podocytopathy were recruited and did not have an intercurrent illness or acute kidney injury. Blood was collected into EDTA tubes without a tourniquet using a 23-gauge needle and placed immediately on ice. Platelet-poor plasma was generated by centrifugation at 2500 rpm for 15 min at 4 °C without brake and then stored a −80 °C until analysis. Plasma TSP1 concentration was determined by ELISA (DTSP10, R&D systems).
Reagents and materials
The following antibodies were used in this study: antibodies against TSP-1 (MA5-13398), from Invitrogen; antibodies against TSP-1 (ab85762), TIM 1 (ab47634), and NGAL (ab63929) from Abcam; and antibodies against F4/80 (sc-377009) from Santa Cruz Biotechnology; antibodies against TIM 1(ab47635)from R&D Systems; antibodies against integrin-β3 (13166), collagen alpha-1 (I) chain (72026), cleaved caspase-3 (9661), and E-cadherin (24E10) from Cell Signaling Technology; antibodies against integrin-b3 AP5 (EBW107) from Kerafast, Inc; antibodies against TIM-1 from NOVUS Biologicals; antibodies against F(α-SMA) (GTX100034) from Gene Tex; and antibodies against GAPDH (60004-1-Ig) from Proteintech.
The following reagents were used in this study: rTSP-1 (3074-TH, R&D systems); cyclo-RGDfK (S7834, Selleck); human doxorubicin hydrochloride (D1515, Millipore Sigma); BSA (A8806, Sigma).
Cell culture, transfection, and treatments
HK-2 cells were purchased from the American Type Culture Collection. Cells in passages 2 to 6 were used for experiments. Immortalized tubular epithelial cells (HK-2) were cultured in Dulbecco's modified Eagle's medium/F12 medium supplemented with 10% fetal bovine serum and incubated at 37 °C with 5% CO2. After synchronization, cells were treated with varying quantities of BSA, 1 ug/ml rTSP-1 (R&D Systems), or cyclo-RGDfK (S7834, Selleck). HK2 cells in 6-well plates were ready for transfection. For transfection of THBS1 plasmids or shRNA, ITGB3 shRNA, Lipofectamine 3000 (Thermo Fisher Scientific), and jetPRIME in vitro DNA & shRNA transfection reagent (pt-114-15, PolyPlus Transfection) were used according to the manufacturer’s instructions. Cells were harvested at 48 h after transfection.
Animals
We performed all animal care and experiments according to the guidelines of the Institutional Animal Care and Use Committee at Jinling Hospital of Nanjing University.
Male inbred BALB/c mice weighing 22 to 25 g and aged 8 weeks and THBS1 KO transgenic mice were purchased and validated from Nanjing GemPharmatech Company and given free access to water and food and provided a 12/12 h light/dark cycle. All experimental procedures were approved and conducted in accordance with the institutional guidelines for animal care.
Male BALB/c mice were injected with 11 mg/kg ADR (Millipore Sigma) in the tail vein at days 0 and 14 (45) and sacrificed 6 weeks after injection (26, 47). To investigate the role of ITGB3 in the TSP-1 signaling pathway, we intraperitoneally injected the mice with cyclo-RGDfK (Selleck) (2 mg/kg body weight, dissolved in PBS) or PBS once daily starting from day 14 after the last ADR injection to day 1 before the analyses (week 6) (Fig. S4) (43, 44). THBS1 KO and C57BL/6 control mice were assigned to receive a single intravenous injection of ADR (11 mg/kg, Millipore Sigma) and sacrificed at 6 weeks after injection. The mice were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally), and kidney tissue and blood samples were collected for further experiments. Mouse tubulointerstitial fractions were obtained from the kidney cortex using established methods (48).
Urinary albumin and creatinine levels in the mice were measured using Albuwell M (Exocell) and QuantiChrom Creatinine Assay Kit (Bioassay systems) according to the manufacturer’s instructions. The results were presented as albumin/creatinine ratio (ACR, μg/mg). Plasma values for albumin, cholesterol, creatinine, and urea were measured with an enzymatic method and expressed in millimoles per liter.
Cell apoptosis
The apoptosis of HK-2 cells induced by BSA was examined using the Annexin V-FITC/PI Apoptosis Detection Kit by flow cytometry according to the manufacturer’s protocol (A211-01,Vazyme). The cells were immediately analyzed with the flow cytometer mentioned above.
Western blotting analysis
Tissues or cells were extracted in a commercial radio-immunoprecipitation assay lysis buffer. The bicinchoninic acid assay Protein Kit (Beyotime) was used to quantify protein concentration. After loading samples on 4 to 12% SDS-PAGE gels, the resolved proteins were transferred onto nitrocellulose membranes (Millipore). The membranes were incubated overnight at 4 °C using antibodies against TSP-1 (1:200), ITGB3 (1:1000), KIM-1 (1:500), Col-1 (1:1000), caspase 3 (1:2000), α-SMA (1:2000), E-cadherin (1:1000), GAPDH (1:10,000), and β-actin (1:5000) and then incubated with secondary antibodies (1:5000, Proteintech) for 2 h at 37 °C. After washing with Tween, the protein bands were visualized by enhanced chemiluminescence plus the chemiluminescence substrate method. ImageJ software v1.8.0 (National Institutes of Health, Bethesda, MD) was used for relative protein quantification.
Co-IP assay
For the Co-IP assay, HK-2 cells were lysed with IP buffer containing 10 mmol/L N-ethylmaleimide and a mixture of mammalian protease inhibitors and phosphatase inhibitors (Sigma-Aldrich) on ice. Protein G Sepharose beads (MedChemExpress) were incubated with 10 μl of anti-TSP-1 (1 mg/ml) for 4 h at 4 °C with constant shaking. After washing, the beads were incubated with the prepared proteins at 4 °C overnight. Immunoprecipitated proteins were subjected to gel electrophoresis and analyzed using Western blot.
RNA extraction and real-time PCR
Total RNA was extracted from renal sections and HK-2 cells using a FastPure Cell/Tissue Total RNA Isolation Kit (Vazyme). Reverse transcription was carried out with an RT 2 First Strand Kit (the FastPure Cell/Tissue Total RNA Isolation Kit (Vazyme)). QuantiTect SYBR Green PCR Master Mix (Applied Biosystems) was used for gene expression level measurement. The primers for PCR analysis are listed in Table S2.
IHC staining
IHC was carried out as described previously in paraffin-embedded tissue sections 4 μm thick. Paraffin-embedded sections were deparaffinized and rehydrated, and then repaired at high temperature. The sections were incubated for 1 h at room temperature with primary antibody diluted in 1% BSA in PBS. Primary antibodies were anti-TSP-1 (1:50, Abcam), anti-ITGB3 (1:100, Cell Signaling Technology), anti-F4/80 (1:50, Santa Cruz), anti-KIM-1 (1:100, Abcam), and anti-α-SMA (1:200, Gene Tex). The staining was visualized with the polyvalent horseradish peroxidase-diaminobenzidine detection (1:50, MXB Biotechnologies). The area of versican expression was quantified by the Image Pro Plus software (National Institutes of Health, Bethesda, MD).
IF staining
Renal sections or cultured cells were fixed with 4% paraformaldehyde. Then the renal sections or cells were blocked with 1% BSA, incubated in primary antibodies, and incubated overnight at 4 °C. The sections were then incubated with secondary antibodies (1:100, DAKO) diluted in a blocker for 45 min.
Statistics
All data were expressed as the means ± SD or medians (interquartile range). The data from multiple groups were analyzed with one-way ANOVA followed by Tukey’s post hoc test. Data from two groups were compared by a two-tailed Student’s t test. Pearson correlation analysis was used to determine the relationship between plasma TSP-1 and clinic pathological indicators. Statistical significance was accepted at values of p <0.05.
Data availability
The data that support this study are available from the corresponding authors upon request.
Study approval
The study was carried out by the principles of the Declaration of Helsinki and was approved by the ethics committees of Jinling Hospital (2022DZGZR-143). All participants provided informed consent. Animals used in this study were approved by the Institutional Animal Care and Use Committee of Jinling Hospital (2022DZGKJDWLS-00133).
Supporting information
This article contains supporting information (26).
Conflict of interest
The authors declare that they have no conflict of interests with the contents of this article.
Acknowledgments
We thank the physicians, patients, and volunteers who contributed to this study.
Author contributions
Y. F., S. D., Y. X., X. Y., Q. L., S. L., M. Z., F. Y., and L. L. data curation; Y. F., S. D., Y. X., X. Y., and C. Z. formal analysis; Y. F., S. D., Y. X., X. Y., Q. L., F. X., D. L., S. L., M. Z., F. Y., Y. J., L. L., X. Z., H. B., Z. C., and C. Z. writing–original draft; F. X., D. L., S. L., M. Z., and X. Z. investigation; Y. X., F. X., D. L., S. L., M. Z., Y. J., H. B., Z. C., and C. Z. supervision; Y. J. and C. Z. conceptualization; Y. J. and C. Z. methodology; Y. F., S. D., Y. X., X. Y., Q. L., F. X., D. L., S. L., M. Z., F. Y., Y. J., L. L., X. Z., H. B., Z. C., and C. Z. writing–reviewing and editing.
Funding and additional information
This work is supported by grants from the National Natural Science Foundation of China (82070793), the Medical Scientific Research Project of Jiangsu Provincial Health Commission (ZD2021018) and Jiangsu Provincial Science and Technology Resources Coordination Service Platform, No.BM2015004-1.
Reviewed by members of the JBC Editorial Board. Edited by Qi-Qun Tang
Supporting information
References
- 1.Korbet S.M. Treatment of primary FSGS in adults. J. Am. Soc. Nephrol. 2012;23:1769–1776. doi: 10.1681/ASN.2012040389. [DOI] [PubMed] [Google Scholar]
- 2.Tang X., Xu F., Chen D.M., Zeng C.H., Liu Z.H. The clinical course and long-term outcome of primary focal segmental glomerulosclerosis in Chinese adults. Clin. Nephrol. 2013;80:130–139. doi: 10.5414/CN107607. [DOI] [PubMed] [Google Scholar]
- 3.D'Agati V.D., Kaskel F.J., Falk R.J. Focal segmental glomerulosclerosis. N. Engl. J. Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 4.Sethi S., Glassock R.J., Fervenza F.C. Focal segmental glomerulosclerosis: towards a better understanding for the practicing nephrologist. Nephrol. Dial. Transpl. 2015;30:375–384. doi: 10.1093/ndt/gfu035. [DOI] [PubMed] [Google Scholar]
- 5.De Vriese A.S., Sethi S., Nath K.A., Glassock R.J., Fervenza F.C. Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J. Am. Soc. Nephrol. 2018;29:759–774. doi: 10.1681/ASN.2017090958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu B.C., Tang T.T., Lv L.L., Lan H.Y. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018;93:568–579. doi: 10.1016/j.kint.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Qi R., Yang C. Renal tubular epithelial cells: the neglected mediator of tubulointerstitial fibrosis after injury. Cell Death Dis. 2018;9:1126. doi: 10.1038/s41419-018-1157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takaori K., Nakamura J., Yamamoto S., Nakata H., Sato Y., Takase M., et al. Severity and frequency of proximal tubule injury determines renal prognosis. J. Am. Soc. Nephrol. 2016;27:2393–2406. doi: 10.1681/ASN.2015060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexopoulos E., Stangou M., Papagianni A., Pantzaki A., Papadimitriou M. Factors influencing the course and the response to treatment in primary focal segmental glomerulosclerosis. Nephrol. Dial. Transpl. 2000;15:1348–1356. doi: 10.1093/ndt/15.9.1348. [DOI] [PubMed] [Google Scholar]
- 10.Diamond J.R., Ricardo S.D., Klahr S. Mechanisms of interstitial fibrosis in obstructive nephropathy. Semin. Nephrol. 1998;18:594–602. [PubMed] [Google Scholar]
- 11.Xie X.S., Li F.Y., Liu H.C., Deng Y., Li Z., Fan J.M. LSKL, a peptide antagonist of thrombospondin-1, attenuates renal interstitial fibrosis in rats with unilateral ureteral obstruction. Arch. Pharm. Res. 2010;33:275–284. doi: 10.1007/s12272-010-0213-6. [DOI] [PubMed] [Google Scholar]
- 12.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J. Cell Mol. Med. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Resovi A., Pinessi D., Chiorino G., Taraboletti G. Current understanding of the thrombospondin-1 interactome. Matrix Biol. 2014;37:83–91. doi: 10.1016/j.matbio.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Goicoechea S., Orr A.W., Pallero M.A., Eggleton P., Murphy-Ullrich J.E. Thrombospondin mediates focal adhesion disassembly through interactions with cell surface calreticulin. J. Biol. Chem. 2000;275:36358–36368. doi: 10.1074/jbc.M005951200. [DOI] [PubMed] [Google Scholar]
- 15.Thakar C.V., Zahedi K., Revelo M.P., Wang Z., Burnham C.E., Barone S., et al. Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J. Clin. Invest. 2005;115:3451–3459. doi: 10.1172/JCI25461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou Y., Xin Y., Liu S., Li Y., Meng X., Wang J., et al. A biocompatible nanoparticle-based approach to inhibiting renal ischemia reperfusion injury in mice by blocking thrombospondin-1 activity. Am. J. Transpl. 2022;22:2246–2253. doi: 10.1111/ajt.17052. [DOI] [PubMed] [Google Scholar]
- 17.Bige N., Shweke N., Benhassine S., Jouanneau C., Vandermeersch S., Dussaule J.C., et al. Thrombospondin-1 plays a profibrotic and pro-inflammatory role during ureteric obstruction. Kidney Int. 2012;81:1226–1238. doi: 10.1038/ki.2012.21. [DOI] [PubMed] [Google Scholar]
- 18.Hohenstein B., Daniel C., Hausknecht B., Boehmer K., Riess R., Amann K.U., et al. Correlation of enhanced thrombospondin-1 expression, TGF-beta signalling and proteinuria in human type-2 diabetic nephropathy. Nephrol. Dial. Transpl. 2008;23:3880–3887. doi: 10.1093/ndt/gfn399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H., Herndon M.E., Lawler J. The cell biology of thrombospondin-1. Matrix Biol. 2000;19:597–614. doi: 10.1016/s0945-053x(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez L.S., Gutierrez J. Thrombospondin 1 in metabolic diseases. Front. Endocrinol. (Lausanne) 2021;12 doi: 10.3389/fendo.2021.638536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X., Xu M., Jia J., Zhang Z., Gaut J.P., Upadhya G.A., et al. CD47 blockade reduces ischemia/reperfusion injury in donation after cardiac death rat kidney transplantation. Am. J. Transpl. 2018;18:843–854. doi: 10.1111/ajt.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers N.M., Zhang Z.J., Wang J.J., Thomson A.W., Isenberg J.S. CD47 regulates renal tubular epithelial cell self-renewal and proliferation following renal ischemia reperfusion. Kidney Int. 2016;90:334–347. doi: 10.1016/j.kint.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 23.Julovi S.M., Sanganeria B., Minhas N., Ghimire K., Nankivell B., Rogers N.M. Blocking thrombospondin-1 signaling via CD47 mitigates renal interstitial fibrosis. Lab. Invest. 2020;100:1184–1196. doi: 10.1038/s41374-020-0434-3. [DOI] [PubMed] [Google Scholar]
- 24.El-Rashid M., Ghimire K., Sanganeria B., Lu B., Rogers N.M. CD47 limits autophagy to promote acute kidney injury. FASEB J. 2019;33:12735–12749. doi: 10.1096/fj.201900120RR. [DOI] [PubMed] [Google Scholar]
- 25.Li S., Jiang S., Zhang Q., Jin B., Lv D., Li W., et al. Integrin beta3 induction promotes tubular cell senescence and kidney fibrosis. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.733831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han R., Hu S., Qin W., Shi J., Hou Q., Wang X., et al. C3a and suPAR drive versican V1 expression in tubular cells of focal segmental glomerulosclerosis. JCI Insight. 2019;4 doi: 10.1172/jci.insight.122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morigi M., Macconi D., Zoja C., Donadelli R., Buelli S., Zanchi C., et al. Protein overload-induced NF-kappaB activation in proximal tubular cells requires H(2)O(2) through a PKC-dependent pathway. J. Am. Soc. Nephrol. 2002;13:1179–1189. [PubMed] [Google Scholar]
- 28.Nakajima H., Takenaka M., Kaimori J.Y., Hamano T., Iwatani H., Sugaya T., et al. Activation of the signal transducer and activator of transcription signaling pathway in renal proximal tubular cells by albumin. J. Am. Soc. Nephrol. 2004;15:276–285. doi: 10.1097/01.asn.0000109672.83594.02. [DOI] [PubMed] [Google Scholar]
- 29.McGrogan A., Franssen C.F., de Vries C.S. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol. Dial. Transpl. 2011;26:414–430. doi: 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
- 30.Thomas D.B., Franceschini N., Hogan S.L., Ten Holder S., Jennette C.E., Falk R.J., et al. Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int. 2006;69:920–926. doi: 10.1038/sj.ki.5000160. [DOI] [PubMed] [Google Scholar]
- 31.Daniel C., Schaub K., Amann K., Lawler J., Hugo C. Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes. 2007;56:2982–2989. doi: 10.2337/db07-0551. [DOI] [PubMed] [Google Scholar]
- 32.Kim J.H., Kim B.K., Moon K.C., Hong H.K., Lee H.S. Activation of the TGF-beta/Smad signaling pathway in focal segmental glomerulosclerosis. Kidney Int. 2003;64:1715–1721. doi: 10.1046/j.1523-1755.2003.00288.x. [DOI] [PubMed] [Google Scholar]
- 33.Hugo C., Kang D.H., Johnson R.J. Sustained expression of thrombospondin-1 is associated with the development of glomerular and tubulointerstitial fibrosis in the remnant kidney model. Nephron. 2002;90:460–470. doi: 10.1159/000054735. [DOI] [PubMed] [Google Scholar]
- 34.Wu C.C., Chen J.S., Lu K.C., Chen C.C., Lin S.H., Chu P., et al. Aberrant cytokines/chemokines production correlate with proteinuria in patients with overt diabetic nephropathy. Clin. Chim. Acta. 2010;411:700–704. doi: 10.1016/j.cca.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 35.Liu W.J., Xu B.H., Ye L., Liang D., Wu H.L., Zheng Y.Y., et al. Urinary proteins induce lysosomal membrane permeabilization and lysosomal dysfunction in renal tubular epithelial cells. Am. J. Physiol. Ren. Physiol. 2015;308:F639–F649. doi: 10.1152/ajprenal.00383.2014. [DOI] [PubMed] [Google Scholar]
- 36.Na J., Sweetwyne M.T., Park A.S., Susztak K., Cagan R.L. Diet-induced podocyte dysfunction in Drosophila and mammals. Cell Rep. 2015;12:636–647. doi: 10.1016/j.celrep.2015.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hotchkiss K.A., Matthias L.J., Hogg P.J. Exposure of the cryptic Arg-Gly-Asp sequence in thrombospondin-1 by protein disulfide isomerase. Biochim. Biophys. Acta. 1998;1388:478–488. doi: 10.1016/s0167-4838(98)00211-8. [DOI] [PubMed] [Google Scholar]
- 38.Isenberg J.S., Romeo M.J., Yu C., Yu C.K., Nghiem K., Monsale J., et al. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood. 2008;111:613–623. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashton A.W., Cheng Y., Helisch A., Ware J.A. Thromboxane A2 receptor agonists antagonize the proangiogenic effects of fibroblast growth factor-2: role of receptor internalization, thrombospondin-1, and alpha(v)beta3. Circ. Res. 2004;94:735–742. doi: 10.1161/01.RES.0000122043.11286.57. [DOI] [PubMed] [Google Scholar]
- 40.Harpel J.G., Schultz-Cherry S., Murphy-Ullrich J.E., Rifkin D.B. Tamoxifen and estrogen effects on TGF-beta formation: role of thrombospondin-1, alphavbeta3, and integrin-associated protein. Biochem. Biophys. Res. Commun. 2001;284:11–14. doi: 10.1006/bbrc.2001.4922. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi M., Oka M., Ikeda T., Akiba S., Sato T. Role of thrombospondin-1 in hypoxia-induced migration of human vascular smooth muscle cells. Yakugaku Zasshi. 2008;128:377–383. doi: 10.1248/yakushi.128.377. [DOI] [PubMed] [Google Scholar]
- 42.Pandolfi A., Di Pietro N., Sirolli V., Giardinelli A., Di Silvestre S., Amoroso L., et al. Mechanisms of uremic erythrocyte-induced adhesion of human monocytes to cultured endothelial cells. J. Cell Physiol. 2007;213:699–709. doi: 10.1002/jcp.21138. [DOI] [PubMed] [Google Scholar]
- 43.Lang Y., Zhao Y., Zheng C., Lu Y., Wu J., Zhu X., et al. MiR-30 family prevents uPAR-ITGB3 signaling activation through calcineurin-NFATC pathway to protect podocytes. Cell Death Dis. 2019;10:401. doi: 10.1038/s41419-019-1625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madhusudhan T., Ghosh S., Wang H., Dong W., Gupta D., Elwakiel A., et al. Podocyte integrin-beta 3 and activated protein C coordinately restrict RhoA signaling and ameliorate diabetic nephropathy. J. Am. Soc. Nephrol. 2020;31:1762–1780. doi: 10.1681/ASN.2019111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vielhauer V., Berning E., Eis V., Kretzler M., Segerer S., Strutz F., et al. CCR1 blockade reduces interstitial inflammation and fibrosis in mice with glomerulosclerosis and nephrotic syndrome. Kidney Int. 2004;66:2264–2278. doi: 10.1111/j.1523-1755.2004.66038.x. [DOI] [PubMed] [Google Scholar]
- 46.Wilkening A., Krappe J., Muhe A.M., Lindenmeyer M.T., Eltrich N., Luckow B., et al. C-C chemokine receptor type 2 mediates glomerular injury and interstitial fibrosis in focal segmental glomerulosclerosis. Nephrol. Dial. Transpl. 2020;35:227–239. doi: 10.1093/ndt/gfy380. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y., Wang Y.P., Tay Y.C., Harris D.C. Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int. 2000;58:1797–1804. doi: 10.1046/j.1523-1755.2000.00342.x. [DOI] [PubMed] [Google Scholar]
- 48.Yang L., Besschetnova T.Y., Brooks C.R., Shah J.V., Bonventre J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support this study are available from the corresponding authors upon request.