Keywords: arterial baroreflex, menopause, muscle sympathetic nerve activity, sympathetic transduction
Abstract
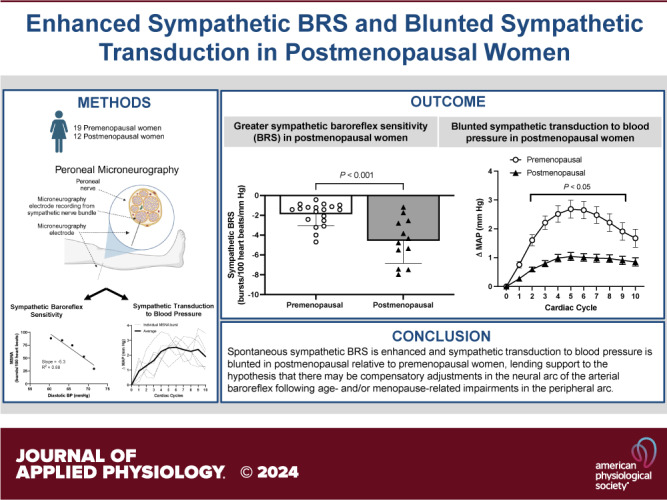
The sympathetic nervous system is critical for regulating blood pressure (BP) via the arterial baroreflex and sympathetic transduction in the peripheral vasculature. These mechanisms interact, and both may be altered with aging and impacted by menopause. Although age-related decreases in sympathetic transduction have been demonstrated in women, it remains unclear whether sympathetic baroreflex sensitivity (BRS) is impaired in postmenopausal women (POST). We tested the hypothesis that sympathetic BRS would be enhanced in POST compared with premenopausal women (PRE). We examined beat-by-beat BP and muscle sympathetic nerve activity (MSNA) in 19 PRE (22 ± 2 yr, 22 ± 3 kg/m2) and 12 POST (57 ± 5 yr, 24 ± 2 kg/m2) during 10 min of rest. Spontaneous sympathetic BRS was quantified as the slope of a linear regression between MSNA burst incidence and diastolic BP. Sympathetic transduction to mean arterial pressure (MAP) for the 10 cardiac cycles following spontaneous MSNA bursts was assessed via signal averaging method. Resting MAP was similar (PRE: 82 ± 8 vs. POST: 85 ± 8 mmHg, P = 0.43), whereas resting MSNA was elevated in POST (PRE: 10 ± 6 vs. POST: 45 ± 16 bursts/100 heart beats, P < 0.0001). Spontaneous sympathetic BRS was enhanced in POST (PRE: −2.0 ± 1.2 vs. POST: −5.2 ± 1.9 bursts/beat/mmHg, P < 0.0005). Sympathetic transduction to MAP was attenuated in POST (time: P < 0.001, group: P < 0.001, interaction: P < 0.01). These data suggest that sympathetic BRS may be enhanced in POST. Consistent with recent hypotheses, enhanced sensitivity of the arterial baroreflex’s neural arc may signify a compensatory response to reduced efficiency of the peripheral arterial baroreflex arc (i.e., sympathetic transduction) to preserve BP buffering capacity.
NEW & NOTEWORTHY Studies examining sympathetic baroreflex function with aging remain equivocal, with some studies showing an increase, decrease, or no change in sympathetic baroreflex sensitivity (BRS) in older adults compared with younger adults. With aging, women experience unique physiological changes due to menopause that influence autonomic function. For the first time, we show that postmenopausal women exhibit a greater sympathetic BRS compared with young premenopausal women.
INTRODUCTION
The arterial baroreflex serves as the primary regulatory mechanism for maintaining blood pressure (BP) at its homeostatic setpoint via modulations in heart rate and sympathetic nerve activity. Baroreflex control of sympathetic nerve activity, or sympathetic baroreflex sensitivity (BRS), is commonly assessed in humans as the slope or gain of changes in muscle sympathetic nerve activity (MSNA) in response to changes in diastolic BP. However, the influence of aging on sympathetic BRS remains incompletely understood. Although a majority of studies have found no significant change in sympathetic BRS with advancing age (1–5), there is also evidence for both attenuated (6) and augmented (7) sympathetic baroreflex function in older adults. Moreover, there is a dearth of studies that examine the sex-specific impact of aging on sympathetic BRS. This is an important question to directly assess as women face unique physiological changes during the aging process (i.e., menopause), which differentially influence the autonomic function among other systems (8). Although there have been comparisons of sympathetic BRS between young and older women (9), as well as pre- and postmenopausal women (10, 11), the inclusion of men in these studies limits the ability to directly determine the influence of menopause.
The arterial baroreflex is a closed-loop, negative feedback reflex that regulates arterial pressure via adjustments in autonomic nervous system activity in concert with other afferent inputs, autonomic reflexes, and projections from other neural networks or brain regions to the cardiovascular control centers in the medulla oblongata. Under conditions of adequate arterial pressure, the stretch of barosensitive primary afferent fibers in the carotid sinus and aortic arch ensure the tonic activation of secondary interneurons in the nucleus tractus solitarius (NTS) which, in turn, ensure the tonic inhibition of sympathetic premotor neurons. When arterial pressure is low, the firing rate of these barosensitive primary afferent fibers decreases, thereby removing the tonic activation of the secondary interneurons in the NTS, and thus promoting the generation of sympathetic outflow to raise arterial pressure (12). The arterial baroreflex can be divided into two “arcs”: the neural arc, which describes afferent information to the medulla from the baroreceptors, and the peripheral arc, which describes the efferent signals produced in the medulla targeted at the periphery (i.e., sympathetic outflow). Sympathetic BRS can be used as an index of the neural arc, whereas sympathetic transduction can be used as an index of the peripheral arc (13).
Recently, some have proposed that age-related alterations in one arc of the arterial baroreflex may induce a compensatory response in the other arc to support BP homeostasis (9, 14). D'Souza et al. (9) found reduced sympathetic transduction to BP (i.e., impaired peripheral arc) alongside elevated sympathetic baroreflex gain (i.e., enhanced neural arc) in older compared with younger adults. Importantly, two of the seven older women in this study were in perimenopause, which induces large fluctuations in sex hormones and other physiological perturbations (15). In addition, young women were studied at various menstrual or oral contraceptive pill (OCP) phases, which may alter sympathetic BRS (16, 17) and its relation with sympathetic transduction to BP.
Therefore, we examined the sympathetic BRS and sympathetic transduction to BP in young, premenopausal young women (PRE) and older, postmenopausal women (POST). We also examined whether sympathetic BRS (neural arc) was related to sympathetic transduction to BP (peripheral arc) across age/menopausal groups. Accordingly, we tested the hypothesis that sympathetic transduction to BP would be reduced, whereas sympathetic BRS would be enhanced, in older, postmenopausal women relative to young, premenopausal women.
METHODS
Participants
The study procedures conformed to the Declaration of Helsinki and were approved by the University of Delaware’s Institutional Review Board. To test the novel hypothesis for sympathetic BRS, we analyzed data from previous (18–20) and ongoing studies at the University of Delaware. Resting MSNA has been previously published in these women (18–20). POST (n = 12) were at least 1 year since their last menses and not taking hormone therapy. PRE (n = 19) were tested during the early follicular phase (days 1–7) of the menstrual cycle (n = 8) or placebo phase of oral contraceptives (n = 11). After providing written informed consent, all women completed a standard medical screening at the University of Delaware Nurse Managed Primary Care Center and were found to be normotensive and free from known tobacco use or neural, cardiovascular, respiratory, or metabolic diseases. Height, weight, and resting brachial artery BP and resting HR were measured.
Experimental Measurements
Participants reported to the laboratory after fasting for at least 4 h, refraining from alcohol and caffeine for 12 h, and avoiding strenuous physical activity for 24 h. Beat-by-beat arterial BP was measured noninvasively via finger photoplethysmography (Finometer; Finapres Medical Systems, Amsterdam, the Netherlands) of the middle finger of the nondominant hand. Automated BPs were taken throughout the visit to verify Finometer-derived BP measurements. Heart rate was measured using a lead II electrocardiogram (Dinamap Dash 2000; GE Medical Systems, Milwaukee, WI). Respiration was measured using a strain-gauge transducer belt (ADInstruments, Colorado Springs, CO).
Multiunit postganglionic MSNA was recorded via peroneal microneurography performed according to the best practices as previously described in detail (19–21). In brief, a tungsten microelectrode was inserted through unanesthetized skin just below the fibular head into muscle nerve fascicles of the peroneal nerve. The insertion point was determined by external electrical stimulation to elicit involuntary muscle twitches of the lower leg. A reference microelectrode was also inserted into the skin ∼2–3 cm away. Neural signals were amplified (70,000-fold), band-pass filtered (700–2,000 Hz), rectified, and integrated (0.1-s time constant) to obtain mean voltage neurograms (Nerve Traffic Analyzer, model 662c-3; University of Iowa Bioengineering, Iowa City, IA). Neural recordings were verified to be MSNA recordings when spontaneously occurring bursts were pulse synchronous, responsive to an end-expiratory breath hold or Valsalva maneuver, and unresponsive to arousal stimuli or skin stimulation. Cardiovascular and sympathetic variables for the present analysis were measured during a stable supine baseline period of 10 min.
Data Analysis
The sympathetic neurogram was analyzed to detect spontaneous MSNA bursts on a beat-to-beat basis using custom LabVIEW software (18, 22). MSNA data processing and analyses were conducted by laboratory members trained in the visual inspection of MSNA bursts. The following criteria were used to detect MSNA bursts: 1) >3:1 signal-to-noise ratio, 2) characteristic MSNA burst morphology, 3) pulse synchronicity, and 4) lack of response to arousal stimuli. MSNA burst frequency (bursts/min), burst incidence (bursts/100 cardiac cycles), and total activity (AU/min) were quantified during the resting baseline period.
Spontaneous sympathetic BRS was assessed using methodology detailed in the recent Cores of Reproducibility in Physiology article on best practices for assessing spontaneous sympathetic BRS (23). We examined the linear regression between spontaneous fluctuations in diastolic BP and MSNA burst incidence during the 10-min resting baseline period. MSNA was averaged over diastolic BP ranges (3-mmHg bins) to minimize the influence of MSNA bursts arising from nonbaroreflex mechanisms. To conform with the best practice guidelines and in an effort to improve the rigor of the study, only subjects with a weighted linear regression correlation coefficient above −0.7 were included (23). We excluded 4 subjects (2 PRE and 2 POST) from our group comparison of sympathetic BRS, leaving 17 PRE and 10 POST included in the analysis for this measure. The slope of the linear regression between diastolic BP and MSNA burst incidence was used as an index of sympathetic BRS or gain in line with previous reports from our laboratory (24) and others (14, 25–27).
Sympathetic transduction to MAP was assessed using a spike-triggered signal averaging methodology that has been previously described (22, 28, 29). In brief, each cardiac cycle throughout the recording was identified as either being associated with or without a burst of MSNA. For cardiac cycles associated with a burst, mean arterial pressure (MAP) was measured for the 10 subsequent cardiac cycles following the cardiac cycle with which the burst occurred (i.e., cardiac cycle 0). This measurement was consistent across all cardiac cycles associated with a burst of MSNA, regardless of burst sequence (i.e., singlet, doublet, etc.). Average MAP responses following bursts were determined for each subject and averaged to provide group means. These methods reliably quantify beat-to-beat changes in BP induced by bursts of MSNA (22, 28–31). We also assessed the changes in MAP following cardiac cycle that were not associated with a burst of MSNA to measure declines in MAP when sympathetic support is removed.
As it is well known that cardiovagal BRS declines with aging, we assessed spontaneous cardiovagal BRS using the sequence method in PRE and POST. In brief, HemoLab software (Harald Stauss Scientific, Iowa City, IA) was used to detect sequences in which systolic BP and R-R interval changed in the same direction for four or more consecutive cardiac cycles. Linear regression analyses were then performed between R-R interval and systolic BP during these sequences. We included only those linear regressions with a correlation coefficient of ≥0.8. The regression slopes of different sequences, with either positive or negative slopes, were then averaged and used to characterize cardiovagal BRS.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism v. 9.1. Independent t tests were used to assess group differences in baseline characteristics, cardiovagal BRS, sympathetic BRS, peak transduction to MAP following MSNA bursts, and nadir transduction to MAP following cardiac cycle not associated with an MSNA burst between PRE and POST. Beat-to-beat changes in MAP were compared between groups using two-way (group × time) repeated-measures ANOVAs with Bonferroni’s post hoc tests. The relation between spontaneous sympathetic BRS and peak transduction to MAP was assessed using a Pearson correlation. The level of significance was set a priori at P < 0.05. Data are presented as means ± SD.
RESULTS
Subject characteristics are listed in Table 1. By design, POST were older compared with PRE. All women were normotensive and nonobese. Resting blood pressures and body mass index (BMI) were not different between groups. Resting heart rate was significantly lower in POST relative to PRE. Resting MSNA burst frequency, burst incidence, burst amplitude, and total activity were elevated in POST relative to PRE.
Table 1.
Participant characteristics
| Premenopausal (n = 19) | Postmenopausal (n = 12) | |
|---|---|---|
| Age, yr | 22 ± 2 | 57 ± 5* |
| BMI, kg/m2 | 22 ± 3 | 23 ± 1 |
| SBP, mmHg | 110 ± 10 | 114 ± 10 |
| DBP, mmHg | 69 ± 8 | 70 ± 9 |
| MAP, mmHg | 82 ± 8 | 85 ± 8 |
| HR, beats/min | 69 ± 10 | 56 ± 6* |
| MSNA, bursts/min | 7 ± 4 | 26 ± 10* |
| MSNA, bursts/100 hb | 10 ± 6 | 45 ± 16* |
| MSNA, AU/beat | 6 ± 3 | 19 ± 8* |
| MSNA, AU/min | 353 ± 162 | 1076 ± 510* |
| Postmenopause, yr | 9 ± 6 | |
| OCPs, n | 11 | |
| Race | 14 White, 2 Hispanic, 2 Asian, 2 Not Reported** | 10 White, 2 Not Reported** |
Values are presented as means ± SD. Unpaired t tests were used to determine differences between groups. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; MSNA, muscle sympathetic nerve activity; hb, heart beat; OCPs, oral contraceptive pills.
*P < 0.001 vs. Premenopausal;
**subject did not report race.
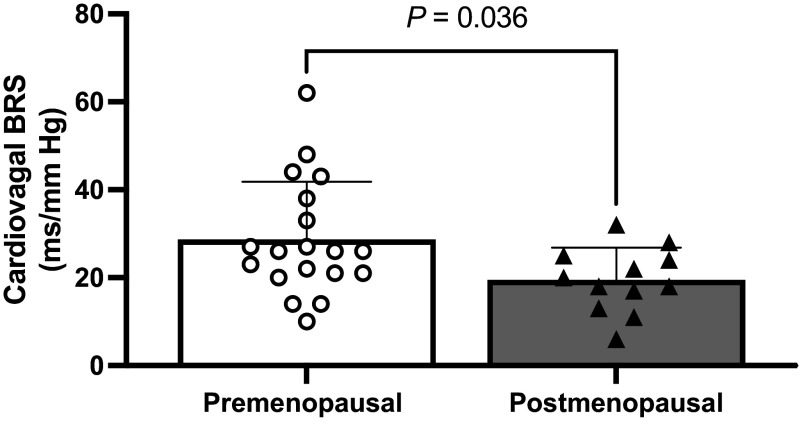
As expected, spontaneous cardiovagal BRS was significantly reduced in POST compared with PRE (Fig. 1; PRE: 28 ± 13.2 vs. POST: 19.4 ± 7.4 ms/mmHg; P = 0.036).
Figure 1.
Spontaneous cardiovagal baroreflex sensitivity in premenopausal and postmenopausal women. White circles denote premenopausal women (n = 19). Black triangles denote postmenopausal women (n = 12). An unpaired t test was used to evaluate a group difference. Values are presented as means ± SD. BRS, baroreflex sensitivity.
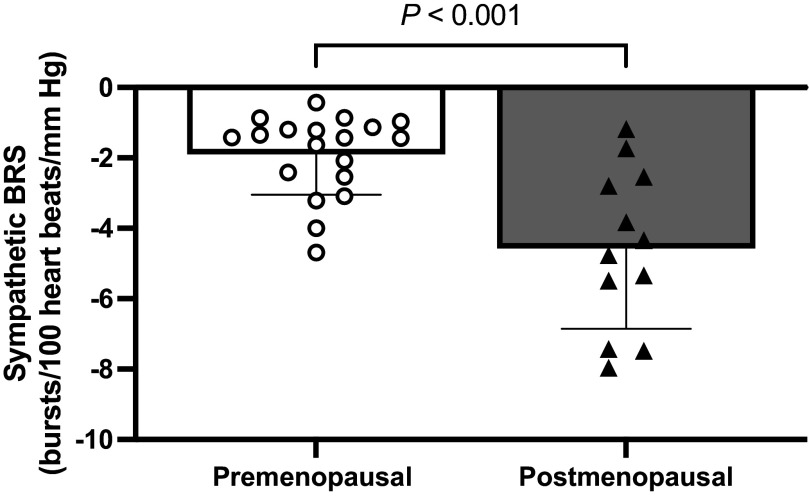
Spontaneous sympathetic BRS was significantly greater in POST compared with PRE (Fig. 2; PRE: −1.9 ± 1.1 vs. POST: −4.6 ± 2.1 bursts/100 heart beats/mmHg; P < 0.005). Mean diastolic BP during the recording did not differ between groups (PRE: 62 ± 15 vs. POST: 57 ± 8 mmHg; P = 0.305). The average correlation coefficient of the linear regression between diastolic BP and MSNA burst incidence was −0.83 ± 0.09 for PRE and −0.91 ± 0.08 for POST.
Figure 2.
Spontaneous sympathetic baroreflex sensitivity in premenopausal and postmenopausal women. White circles denote premenopausal women (n = 17). Black triangles denote postmenopausal women (n = 10). Four women (2 premenopausal and 2 postmenopausal) were excluded from the larger cohort for this comparison as they did not have a weighted linear regression correlation coefficient above −0.7 in accordance with best practice guidelines (23). An unpaired t test was used to evaluate a group difference. Values are presented as means ± SD. BP, blood pressure; BRS, baroreflex sensitivity; MSNA, muscle sympathetic nerve activity.
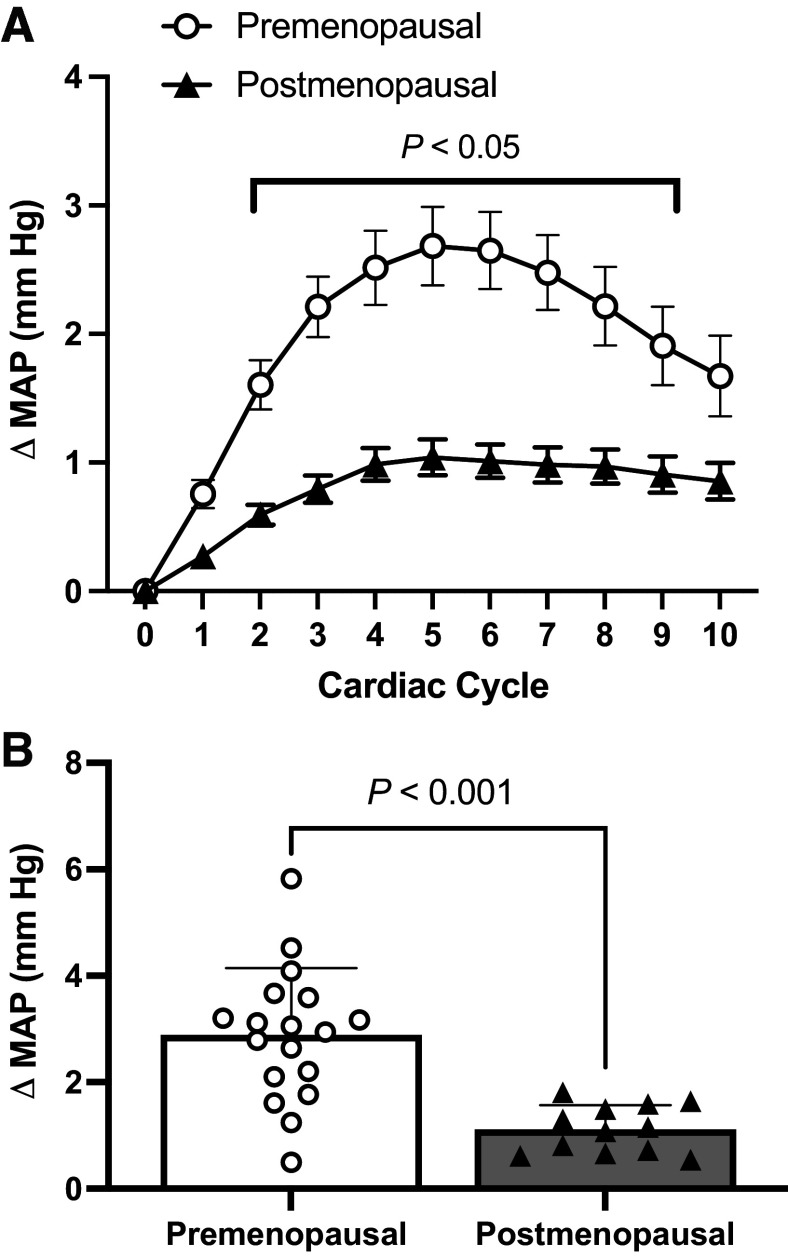
Increases in MAP over the 10 cardiac cycles following an MSNA burst were significantly lower in POST relative to PRE (Fig. 3A; time: P < 0.0001; group: P < 0.0001; interaction: P = 0.008). Bonferroni post hoc tests revealed that group differences arose at cardiac cycle 2 (P = 0.029), cardiac cycle 3 (P < 0.001), cardiac cycle 4 (P < 0.0001), cardiac cycle 5 (P < 0.0001), cardiac cycle 6 (P < 0.0001), cardiac cycle 7 (P = 0.0001), cardiac cycle 8 (P = 0.002), and cardiac cycle 9 (P = 0.032). The peak increase in MAP within the 10 cardiac cycles following an MSNA burst was significantly lower in POST compared with PRE (Fig. 3B; PRE: 2.7 ± 1.4 vs. POST: 1.1 ± 0.4 mmHg; P < 0.001).
Figure 3.
A: signal-averaged sympathetic transduction of mean arterial pressure over the 10 cardiac cycles following a spontaneous MSNA burst in premenopausal and postmenopausal women. Circles denote premenopausal women (n = 18). Triangles denote postmenopausal women (n = 12). A repeated-measures analysis of variance (ANOVA) was used to evaluate the main effects of time and menopausal group. Bonferroni-corrected post hoc analyses were performed when a significant ANOVA interaction was observed. P < 0.05, significant difference pre- and postmenopausal women. B: peak increase in mean arterial pressure over the 10 cardiac cycles following a spontaneous MSNA burst in premenopausal and postmenopausal women. White circles denote premenopausal women (n = 18). Black triangles denote postmenopausal women (n = 12). An unpaired t test was used to evaluate a group difference. Values are presented as means ± SD. MAP, mean arterial pressure; MSNA, muscle sympathetic nerve activity.
There were significantly larger decreases in MAP over the 10 cardiac cycles following a cardiac cycle not associated with an MSNA burst (i.e., nonburst) in POST relative to PRE (time: P < 0.0001; group: P < 0.0001; interaction: P = 0.142). The nadir in MAP within the 10 cardiac cycles following a nonburst cardiac cycle was significantly larger in POST compared with PRE (PRE: −0.4 ± 0.4 vs. POST: −1.2 ± 1.1 mmHg; P = 0.012).
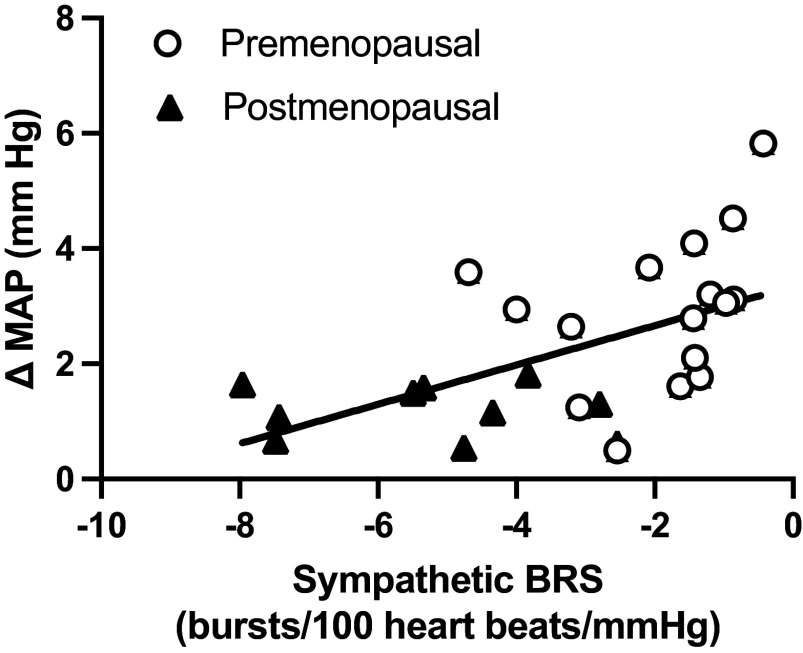
Finally, there was a significant inverse relation between spontaneous sympathetic BRS and peak transduction to MAP in all women (Fig. 4; r = 0.55, P = 0.004).
Figure 4.
Pearson correlation between spontaneous sympathetic baroreflex sensitivity and peak transduction to mean arterial pressure across both premenopausal and postmenopausal groups (r = 0.55, P = 0.004). Circles denote premenopausal women (n = 17). Triangles denote postmenopausal women (n = 10). BRS, baroreflex sensitivity; MAP, mean arterial pressure.
DISCUSSION
The primary novel finding of this study is an enhanced spontaneous sympathetic BRS in POST compared with PRE. In line with previous studies (22, 32), we also observed attenuated sympathetic transduction to blood pressure in POST compared with PRE. However, we have extended this finding by showing that sympathetic transduction to BP is inversely associated with sympathetic BRS in women. These data lend further support to the suggestion that chronic, age-related, and/or menopause-related increases in sympathetic baroreflex function may signify a compensatory adjustment to age-related reductions in sympathetic transduction.
Changes in BRS with Aging
Declines in cardiovagal BRS with advancing age are well established (33–36). Consistent with previous studies, we also observed a decline in cardiovagal BRS in POST relative to PRE. However, there are conflicting reports as to whether there is indeed a change in sympathetic baroreflex gain with aging. Studies using pharmacologically induced changes in BP have found no change in sympathetic baroreflex gain with aging (1–5). However, changes in sympathetic baroreflex function can be identified if falls and rises in pressure are examined separately (37). Older adults were found to have attenuated increases in MSNA during falls in BP induced by sodium nitroprusside (i.e., reduced sympathetic BRS), but augmented sympathoinhibition to rises in BP induced by phenylephrine (i.e., greater sympathetic BRS) (37). Lower MSNA responses during falls in BP were interpreted to reflect a reduction in mechanical baroreceptor unloading caused by stiffening of the barosensory vessels with age, whereas greater sympathoinhibition to pressure increases may be due to more sensitive central neural control with age (37).
Although the literature contains reports of changes in sympathetic BRS with aging, these reports often conflict with one another. Matsukawa et al. (6) found older subjects to have impaired baroreflex gain to falls in BP during phase II of the Valsalva maneuver compared with young participants. On the other hand, Davy et al. (7) found augmented sympathetic BRS in older subjects assessed during hypovolemia induced by lower body negative pressure. The heterogenous stimuli used in these studies that differentially unload the cardiopulmonary, aortic, and carotid baroreceptors may contribute to these incongruent findings (37). Still, the influence of aging on sympathetic BRS remains controversial (6, 9, 34, 37).
Relatively few of these studies examining the change in sympathetic BRS with age have included women (3–5, 9, 37). This is an important consideration, given the known sex differences in autonomic function (38), sympathetic nerve activity (39), adrenergic receptor responsiveness (40), and baroreflex function (34). Furthermore, direct comparisons of sympathetic baroreflex gain have yet to be made between young and older women. This is a question worthy of separate investigation given the distinct difference in the aging process between men and women (i.e., menopause), which can differentially influence autonomic function (8).
Barnes et al. (10) investigated the influence of sex and menopause status on cardiovagal and sympathetic baroreflex sensitivity during the modified Oxford technique. Attenuated cardiovagal baroreflex sensitivity was observed in older women compared with young men and women, but no differences in sympathetic baroreflex sensitivity were identified (10). The lack of difference in sympathetic baroreflex sensitivity was likely related to study power; exploratory comparison between the young premenopausal and older postmenopausal women presented in the study by Barnes et al. (10) identifies a trend for augmented sympathetic baroreflex gain with aging and/or menopause in women (P = 0.06).
In the present study, we found higher spontaneous sympathetic BRS in POST relative to PRE (Fig. 3A). These data suggest that there is enhanced sympathetic baroreflex function with aging and/or menopause in women. They also lend further support for D'Souza et al.’s (9) recent finding that older adults exhibit greater integrated MSNA baroreflex threshold gain and medium-sized MSNA action potential cluster baroreflex gain relative to young adults. The current work builds upon this previous study by D'Souza et al. by incorporating two important methodological controls when measuring autonomic function in women: menopause status and menstrual or OCP phase. First, our older POST group consisted of only women who were at least 1 year postmenopausal. Second, we controlled for menstrual cycle in our PRE, studying them only during the low-hormone phase of their menstrual or OCP cycle. Among other considerations, these are both direct determinants of circulating sex hormones that are known to alter sympathetic outflow (18, 41, 42) and may alter sympathetic baroreflex function in women (16, 17, 43).
Influence of Menopause and Sex Hormones on Baroreflex Function
Compared with young men, young women rely more so on cardiac changes to defend BP during a carotid hypertensive challenge (e.g., neck pressure) (44). Young adults of both sexes seem to respond with similar baroreflex-mediated pressor responses to a carotid hypotensive challenge (i.e., neck suction) (44). With advancing age, however, women begin displaying a greater reliance on vascular responses to defend BP during neck suction and blunted cardiac responses to defend BP during neck pressure and suction (45). There is a significant relation between resting MSNA and total peripheral resistance in older POST, which is absent in PRE due to β-adrenergic-mediated vasodilation (40). Importantly, the present data reflect baroreflex control of BP in a resting state that differs from the hypo- and hypertensive challenges employed by Credeur et al. (45). Still, our finding of augmented sympathetic baroreflex gain aligns with these previous observations of blunted cardiac and greater vascular reliance of baroreflex control of BP with aging in women.
Whether changes in baroreflex function between young PRE and older POST are attributable to menopause, chronological aging, or both is difficult to determine. Understanding the influence of sex hormones on baroreflex function may help to elucidate the predominant mechanism. Minson et al. compared sympathetic baroreflex gain, assessed using the modified Oxford technique, between young women during the early follicular (low estrogen and low progesterone) and mid-luteal (high estrogen, high progesterone) phases of the menstrual cycle. Sympathetic baroreflex gain was found to be greater during the mid-luteal phase (16). Surprisingly, the opposite is true for young women taking oral contraceptives; sympathetic baroreflex gain is augmented during the low-hormone phase (placebo pills) relative to the high-hormone phase of combined ethinyl estrogen-progestin pills (17). Unchanged sympathetic baroreflex gain during upright tilt and the Valsalva maneuver has also been observed across the menstrual cycle (46). Furthermore, estrogen supplementation has been shown to either enhance (47, 48) or unalter (49) sympathetic baroreflex gain in POST and ovariectomized rats. Taken together, the influence of sex hormones on baroreflex control of MSNA remains equivocal.
Possible Causes of Changes in Sympathetic BRS with Aging/Menopause
Another possible mechanism to explain a decline in sympathetic BRS with aging and/or menopause is the age-related decline in sympathetic transduction to BP. Age-related elevations in MSNA have been suggested to be a possible homeostatic mechanism that compensates for reduced sympathetic transduction (50). Similarly, Hissen et al. (14) purport that the neural and peripheral arcs of the arterial baroreflex may hold a dynamic compensatory relationship to maintain BP when one arc is reduced. This stems from their finding of a significant inverse relation between sympathetic BRS and sympathetic transduction, which was evident in young men but not in young women (14). They propose that because it is associated with greater sympathetic transduction, low sympathetic BRS in these young men may reflect a baroreflex buffering capacity similar to those with high sympathetic BRS and lower sympathetic transduction. Theoretically, the arterial baroreflex compensates for a smaller pressor response by becoming more sensitive to changes in BP and allowing for the generation of MSNA more liberally. Indeed, D'Souza et al. (9) demonstrated that in older adults compared with younger adults, there is an upward shift of the sympathetic BRS operating point whereby MSNA bursts occur with a greater probability at the same diastolic BP.
An increase in sympathetic baroreflex gain during postexercise ischemia in young healthy adults is coupled with a reduction in sympathetic transduction (13). This suggests that, acutely, the decrements in one arc of the arterial baroreflex can be offset by an activation of the other to maintain adequate baroreflex control of BP. D'Souza et al.’s recent findings support the idea that this compensatory relationship could also be observed in chronic adjustments to arterial baroreflex function with aging. Therefore, we chose to investigate whether measures of the peripheral arc of the arterial baroreflex (i.e., sympathetic transduction) might be related to measures of the neural arc (i.e., sympathetic BRS) across PRE and POST.
In agreement with Vianna et al. and D'Souza et al., we found significantly blunted sympathetic transduction to MAP in women with aging (Fig. 3A) (22, 32). These results differ from the report by Briant et al. (51) who observed augmented sympathetic transduction in POST compared with PRE. This discrepancy likely lies with the different analytical techniques used between these studies, with the former using the signal averaging method and the latter using a linear regression method. We also found significantly larger decreases in MAP following nonburst cardiac cycles in women with aging in agreement with previous work (22), suggesting greater reliance on sympathetic support of BP in POST relative to PRE.
D'Souza et al. (32) have recently shown that older adults display blunted sympathetic transduction to leg vascular conductance (LVC) and that nadir LVC responses to spontaneous bursts of MSNA are inversely associated with sympathetic BRS in young males and older males, whereas this association is not significant in young females or older females. We found that sympathetic transduction to MAP was inversely related to spontaneous sympathetic BRS across both groups of women (Fig. 4). This suggests, in agreement with D'Souza et al. (9), that age-related reductions in sympathetic transduction may be involved in mediating changes in sympathetic baroreflex function with aging and/or menopause in women.
Limitations
There are important methodological considerations to take into context when interpreting the present findings. First, we used the spontaneous method of sympathetic baroreflex gain analysis on data from subjects during quiet rest. Assessment of sympathetic baroreflex function in a resting state has important limitations as it is difficult to determine the causality of the association between MSNA and spontaneous changes in BP and because of the nonbaroreflex inputs that influence MSNA (52). However, spontaneous sympathetic baroreflex gain has been shown to be strongly associated with sympathetic baroreflex gain measured during the modified Oxford technique (52).
Second, this investigation characterizes the sympathetic transduction of a spontaneous burst of MSNA into changes in MAP, not vascular conductance (i.e., the end-organ response to the MSNA burst). Because MAP is determined by both neural and nonneural factors, nonneural factors could impact measures of sympathetic transduction to MAP. Still, measurements of sympathetic transduction to BP are well accepted because of the inherit contribution of vascular conductance to BP regulation (53) and the parallel nature of changes in LVC and MAP following a spontaneous burst of MSNA (29).
Finally, we used the signal averaging method for measurement of sympathetic transduction versus the linear regression method. The signal averaging method has been primarily used in studies examining young adults with relatively low MSNA burst frequency. The linear regression method was developed as an attempt to overcome potential limitations of the signal averaging method in populations with high resting MSNA burst frequency, although, this has yet to be verified in subsequent studies (53). Still, the MSNA burst frequency in PRE and POST groups is similar to data in other well-accepted investigations using the signal averaging method (22, 54). We recognize that the sample size in the current study is small. As this was a retrospective study, we did not conduct a prior power analysis. Additional studies with a larger sample size are warranted to confirm these findings.
Conclusions
In conclusion, spontaneous sympathetic BRS is enhanced in POST relative to PRE. In agreement with previous findings, POST also exhibit lower sympathetic transduction to BP relative to PRE. Still, lower sympathetic transduction to BP is associated with higher sympathetic BRS among PRE and POST, lending further support to the hypothesis that an enhanced neural arc with age and/or menopause may signify a compensatory adjustment to age-related impairments in the peripheral arc of the arterial baroreflex to preserve BP buffering capacity.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01 HL146558, P20 GM113125, and K99/R00 HL133414.
DISCLOSURES
J.L.G. and M.M.W. are paid consultants for Orchestra Biomed. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
E.L.M., J.L.G., and M.M.W. conceived and designed research; E.L.M., J.L.G., and M.M.W. performed experiments; S.J.M., J.L.G., and M.M.W. analyzed data; S.J.M., J.L.G., J.K.S., and M.M.W. interpreted results of experiments; S.J.M. and M.M.W. prepared figures; S.J.M. and M.M.W. drafted manuscript; S.J.M., E.L.M., J.L.G., J.K.S., and M.M.W. edited and revised manuscript; S.J.M., E.L.M., J.L.G., J.K.S., and M.M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the participants for volunteering for the study. The Graphical Abstract was created with BioRender.com.
REFERENCES
- 1. Davy KP, Tanaka H, Andros EA, Gerber JG, Seals DR. Influence of age on arterial baroreflex inhibition of sympathetic nerve activity in healthy adult humans. Am J Physiol Heart Circ Physiol 275: H1768–H1772, 1998. doi: 10.1152/ajpheart.1998.275.5.H1768. [DOI] [PubMed] [Google Scholar]
- 2. Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol Heart Circ Physiol 263: H798–H803, 1992. doi: 10.1152/ajpheart.1992.263.3.H798. [DOI] [PubMed] [Google Scholar]
- 3. Matsukawa T, Sugiyama Y, Mano T. Age-related changes in baroreflex control of heart rate and sympathetic nerve activity in healthy humans. J Auton Nerv Syst 60: 209–212, 1996. doi: 10.1016/0165-1838(96)00057-4. [DOI] [PubMed] [Google Scholar]
- 4. Matsukawa T, Sugiyama Y, Iwase S, Mano T. Effects of aging on the arterial baroreflex control of muscle sympathetic nerve activity in healthy subjects. Environ Med 38: 81–84, 1994. [PubMed] [Google Scholar]
- 5. Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol 276: H1691–H1698, 1999. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- 6. Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Baroreflex control of muscle sympathetic nerve activity is attenuated in the elderly. J Auton Nerv Syst 73: 182–185, 1998. doi: 10.1016/s0165-1838(98)00128-3. [DOI] [PubMed] [Google Scholar]
- 7. Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension 32: 298–304, 1998. doi: 10.1161/01.hyp.32.2.298. [DOI] [PubMed] [Google Scholar]
- 8. Vongpatanasin W. Autonomic regulation of blood pressure in menopause. Semin Reprod Med 27: 338–345, 2009. doi: 10.1055/s-0029-1225262. [DOI] [PubMed] [Google Scholar]
- 9. D'Souza AW, Klassen SA, Badrov MB, Lalande S, Shoemaker JK. Aging is associated with enhanced central, but impaired peripheral arms of the sympathetic baroreflex arc. J Appl Physiol (1985) 133: 349–360, 2022. doi: 10.1152/japplphysiol.00045.2022. [DOI] [PubMed] [Google Scholar]
- 10. Barnes JN, Matzek LJ, Charkoudian N, Joyner MJ, Curry TB, Hart EC. Association of cardiac baroreflex sensitivity with blood pressure transients: influence of sex and menopausal status. Front Physiol 3: 187, 2012. doi: 10.3389/fphys.2012.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hart EC, Wallin BG, Curry TB, Joyner MJ, Karlsson T, Charkoudian N. Hysteresis in the sympathetic baroreflex: role of baseline nerve activity. J Physiol 589: 3395–3404, 2011. doi: 10.1113/jphysiol.2011.208538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dampney RAL. Resetting of the baroreflex control of sympathetic vasomotor activity during natural behaviors: description and conceptual model of central mechanisms. Front Neurosci 11: 461, 2017. doi: 10.3389/fnins.2017.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ogoh S, Fisher JP, Young CN, Raven PB, Fadel PJ. Transfer function characteristics of the neural and peripheral arterial baroreflex arcs at rest and during postexercise muscle ischemia in humans. Am J Physiol Heart Circ Physiol 296: H1416–H1424, 2009. doi: 10.1152/ajpheart.01223.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hissen SL, Macefield VG, Brown R, Taylor CE. Sympathetic baroreflex sensitivity is inversely related to vascular transduction in men but not women. Am J Physiol Heart Circ Physiol 317: H1203–H1209, 2019. doi: 10.1152/ajpheart.00501.2019. [DOI] [PubMed] [Google Scholar]
- 15. Hale GE, Robertson DM, Burger HG. The perimenopausal woman: endocrinology and management. J Steroid Biochem Mol Biol 142: 121–131, 2014. doi: 10.1016/j.jsbmb.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 16. Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 17. Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation 102: 1473–1476, 2000. doi: 10.1161/01.cir.102.13.1473. [DOI] [PubMed] [Google Scholar]
- 18. Wenner MM, Greaney JL, Matthews EL, McGinty S, Kaur J, Vongpatanasin W, Fadel PJ. Influence of age and estradiol on sympathetic nerve activity responses to exercise in women. Med Sci Sports Exerc 54: 408–416, 2021. doi: 10.1249/MSS.0000000000002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matthews EL, Sebzda KN, Wenner MM. Altered baroreflex sensitivity in young women with a family history of hypertension. J Neurophysiol 121: 1011–1017, 2019. doi: 10.1152/jn.00471.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greaney JL, Matthews EL, Wenner MM. Sympathetic reactivity in young women with a family history of hypertension. Am J Physiol Heart Circ Physiol 308: H816–H822, 2015. doi: 10.1152/ajpheart.00867.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hart EC, Head GA, Carter JR, Wallin BG, May CN, Hamza SM, Hall JE, Charkoudian N, Osborn JW. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Heart Circ Physiol 312: H1031–H1051, 2017. doi: 10.1152/ajpheart.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 302: H2419–H2427, 2012. doi: 10.1152/ajpheart.01105.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holwerda SW, Carter JR, Yang H, Wang J, Pierce GL, Fadel PJ. CORP: standardizing methodology for assessing spontaneous baroreflex control of muscle sympathetic nerve activity in humans. research-article. Am J Physiol Heart Circ Physiol 320: H762–H771, 2021. doi: 10.1152/ajpheart.00704.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wenner MM, Rose WC, Delaney EP, Stillabower ME, Farquhar WB. Influence of plasma osmolality on baroreflex control of sympathetic activity. Am J Physiol Heart Circ Physiol 293: H2313–H2319, 2007. doi: 10.1152/ajpheart.01383.2006. [DOI] [PubMed] [Google Scholar]
- 25. Vianna LC, Fernandes IA, Martinez DG, Teixeira AL, Silva BM, Fadel PJ, Nóbrega ACL. Water drinking enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in healthy young humans. Exp Physiol 103: 1318–1325, 2018. doi: 10.1113/EP087095. [DOI] [PubMed] [Google Scholar]
- 26. Taylor CE, Witter T, El Sayed K, Hissen SL, Johnson AW, Macefield VG. Relationship between spontaneous sympathetic baroreflex sensitivity and cardiac baroreflex sensitivity in healthy young individuals. Physiol Rep 3: e12536, 2015. doi: 10.14814/phy2.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Durocher JJ, Klein JC, Carter JR. Attenuation of sympathetic baroreflex sensitivity during the onset of acute mental stress in humans. Am J Physiol Heart Circ Physiol 300: H1788–H1793, 2011. doi: 10.1152/ajpheart.00942.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wallin BG, Nerhed C. Relationship between spontaneous variations of muscle sympathetic activity and succeeding changes of blood pressure in man. J Auton Nerv Syst 6: 293–302, 1982. doi: 10.1016/0165-1838(82)90002-9. [DOI] [PubMed] [Google Scholar]
- 29. Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol 304: H759–H766, 2013. doi: 10.1152/ajpheart.00842.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fairfax ST, Holwerda SW, Credeur DP, Zuidema MY, Medley JH, Dyke PC, Wray DW, Davis MJ, Fadel PJ. The role of α-adrenergic receptors in mediating beat-by-beat sympathetic vascular transduction in the forearm of resting man. J Physiol 591: 3637–3649, 2013. doi: 10.1113/jphysiol.2013.250894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fairfax ST, Padilla J, Vianna LC, Holwerda SH, Davis MJ, Fadel PJ. Influence of spontaneously occurring bursts of muscle sympathetic nerve activity on conduit artery diameter. Am J Physiol Heart Circ Physiol 305: H867–H874, 2013. doi: 10.1152/ajpheart.00372.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. D'Souza AW, Hissen SL, Manabe K, Takeda R, Washio T, Coombs GB, Sanchez B, Fu Q, Shoemaker JK. Age- and sex-related differences in sympathetic vascular transduction and neurohemodynamic balance in humans. Am J Physiol Heart Circ Physiol 325: H917–H932, 2023. doi: 10.1152/ajpheart.00301.2023. [DOI] [PubMed] [Google Scholar]
- 33. Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol 293: R3–R12, 2007. doi: 10.1152/ajpregu.00031.2007. [DOI] [PubMed] [Google Scholar]
- 34. Fu Q, Ogoh S. Sex differences in baroreflex function in health and disease. J Physiol Sci 69: 851–859, 2019. doi: 10.1007/s12576-019-00727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res 29: 424–431, 1971. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- 36. Bristow JD, Gribbin B, Honour AJ, Pickering TG, Sleight P. Diminished baroreflex sensitivity in high blood pressure and ageing man. J Physiol 202: 45P–46P, 1969. [PubMed] [Google Scholar]
- 37. Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol 587: 2049–2057, 2009. doi: 10.1113/jphysiol.2009.170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation 111: 494–498, 2005. doi: 10.1161/01.cir.0000153864.24034.a6. [DOI] [PubMed] [Google Scholar]
- 39. Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol Regul Integr Comp Physiol 275: R1600–R1604, 1998. doi: 10.1152/ajpregu.1998.275.5.R1600. [DOI] [PubMed] [Google Scholar]
- 40. Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the beta-adrenergic receptors. J Physiol 589: 5285–5297, 2011. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weitz G, Elam M, Born J, Fehm HL, Dodt C. Postmenopausal estrogen administration suppresses muscle sympathetic nerve activity. J Clin Endocrinol Metab 86: 344–348, 2001. doi: 10.1210/jcem.86.1.7138. [DOI] [PubMed] [Google Scholar]
- 42. Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation 103: 2903–2908, 2001. doi: 10.1161/01.cir.103.24.2903. [DOI] [PubMed] [Google Scholar]
- 43. Fu Q, Levine BD. Autonomic circulatory control during pregnancy in humans. Semin Reprod Med 27: 330–337, 2009. doi: 10.1055/s-0029-1225261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim A, Deo SH, Vianna LC, Balanos GM, Hartwich D, Fisher JP, Fadel PJ. Sex differences in carotid baroreflex control of arterial blood pressure in humans: relative contribution of cardiac output and total vascular conductance. Am J Physiol Heart Circ Physiol 301: H2454–H2465, 2011. doi: 10.1152/ajpheart.00772.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Credeur DP, Holwerda SW, Boyle LJ, Vianna LC, Jensen AK, Fadel PJ. Effect of aging on carotid baroreflex control of blood pressure and leg vascular conductance in women. Am J Physiol Heart Circ Physiol 306: H1417–H1425, 2014. doi: 10.1152/ajpheart.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 587: 2019–2031, 2009. doi: 10.1113/jphysiol.2008.168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hunt BE, Taylor JA, Hamner JW, Gagnon M, Lipsitz LA. Estrogen replacement therapy improves baroreflex regulation of vascular sympathetic outflow in postmenopausal women. Circulation 103: 2909–2914, 2001. doi: 10.1161/01.cir.103.24.2909. [DOI] [PubMed] [Google Scholar]
- 48. He XR, Wang W, Crofton JT, Share L. Effects of 17beta-estradiol on sympathetic activity and pressor response to phenylephrine in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 275: R1202–R1208, 1998. doi: 10.1152/ajpregu.1998.275.4.R1202. [DOI] [PubMed] [Google Scholar]
- 49. He XR, Wang W, Crofton JT, Share L. Effects of 17beta-estradiol on the baroreflex control of sympathetic activity in conscious ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 277: R493–R498, 1999. doi: 10.1152/ajpregu.1999.277.2.R493. [DOI] [PubMed] [Google Scholar]
- 50. Taylor JA, Tan CO. BP regulation VI: elevated sympathetic outflow with human aging: hypertensive or homeostatic? Eur J Appl Physiol 114: 511–519, 2014. doi: 10.1007/s00421-013-2731-7. [DOI] [PubMed] [Google Scholar]
- 51. Briant LJB, Burchell AE, Ratcliffe LEK, Charkoudian N, Nightingale AK, Paton JFR, Joyner MJ, Hart EC. Quantifying sympathetic neuro-haemodynamic transduction at rest in humans: insights into sex, ageing and blood pressure control. J Physiol 594: 4753–4768, 2016. doi: 10.1113/jp272167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: a nonpharmacological measure of baroreflex sensitivity. Am J Physiol Heart Circ Physiol 298: H816–H822, 2010. doi: 10.1152/ajpheart.00924.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Young BE, Greaney JL, Keller DM, Fadel PJ. Sympathetic transduction in humans: recent advances and methodological considerations. Am J Physiol Heart Circ Physiol 320: H942–H953, 2021. doi: 10.1152/ajpheart.00926.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robinson AT, Babcock MC, Watso JC, Brian MS, Migdal KU, Wenner MM, Farquhar WB. Relation between resting sympathetic outflow and vasoconstrictor responses to sympathetic nerve bursts: sex differences in healthy young adults. Am J Physiol Regul Integr Comp Physiol 316: R463–R471, 2019. doi: 10.1152/ajpregu.00305.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.