Abstract
Background
Although previous studies have reported a bidirectional relationship between ischemic stroke (IS) and epilepsy, the existence of a causal nexus and its directionality remains a topic of controversy.
Methods
The single nucleotide polymorphisms (SNPs) associated with IS were extracted from the Genome-Wide Association Study (GWAS) database. Pooled genetic data encompassing all epilepsy cases, as well as generalized and focal epilepsy subtypes, were acquired from the International League Against Epilepsy's GWAS study. In this study, the primary analysis approach utilized the inverse variance weighting (IVW) method as the main analytical technique. To enhance the robustness of the findings against potential pleiotropy, additional sensitivity analyses were conducted.
Results
In the forward analysis, the IVW method demonstrated that IS was associated with an increased risk of all epilepsy (odds ratio (OR) = 1.127, 95 % confidence interval (CI) = 1.038–1.224, P = 0.004) and generalized epilepsy (IVW: OR = 1.340, 95 % CI = 1.162–1.546, P = 5.70 × 10–5). There was no substantial causal relationship observed between IS and focal epilepsy (P > 0.05). Furthermore, generalized epilepsy, focal epilepsy, and all epilepsy did not show a causal relationship with IS.
Conclusion
This Mendelian randomization (MR) analysis demonstrates that IS increases the risk of developing epilepsy, especially generalized epilepsy. Conversely, no clear causal association was found between epilepsy and the onset of stroke. Therefore, the possible mechanisms of the effect of epilepsy on the pathogenesis of IS still need to be further investigated.
Keywords: Ischemic stroke, Generalized epilepsy, Focal epilepsy, Mendelian randomization, Casual analysis
1. Introduction
Epilepsy is an intricate neurological condition that impacts more than 70 million individuals globally, with nearly 80 % of those affected residing in low-income and middle-income nations [1]. A significant treatment disparity is evident, as over 75 % of these individuals do not receive systematic medical care [2,3]. Premature mortality in people with epilepsy poses a great public health problem as some deaths are preventable [4]. Comorbidities are the most important cause of death. More than 50 % of patients have one or more comorbidities, including anxiety, depression, dementia, and autoimmune disorders.
Stroke is an abrupt onset disorder characterized by disruptions in cerebral blood circulation, comprising hemorrhagic stroke and ischemic stroke (IS). IS constitutes roughly 71 % of all stroke cases, making it the most prevalent type [5]. In the United States, IS stands as the primary cause of both fatalities and disabilities, impacting approximately 700,000 individuals annually [6]. Studies have indicated that IS recurrence stands at around 8.5 %, with a disability rate of 3.6 %, and a prevalence of dysphagia ranging from 37 % to 78 % [7,8].
Accumulating evidence indicates a strong link between IS and epilepsy. Both basic laboratory investigations and clinical studies have indicated a detrimental cycle between IS and seizures. To elaborate, in the initial stages following a stroke, factors like hypoxia, metabolic irregularities, ion channel dysfunction, and acute-phase disruptions of the blood-brain barrier can emerge [9]. These can manifest epileptogenic potential in the course of the disease process. At the same time, within 1 year of stroke onset, approximately 20 % of patients with post-stroke epilepsy exhibit drug resistance. This is one of its most unique and dangerous features [10,11]. Post-stroke epilepsy has received extensive research attention, and stroke is widely acknowledged as a major causative factor for epilepsy. However, only a few studies have conclusively determined the genetic factors that lead to the development of epilepsy in stroke patients, and the full extent to which genetic factors impact the occurrence of epilepsy remains not entirely clear [12,13].
In parallel, epidemiological investigations have indicated a significant correlation between epileptic seizures and subsequent increased risk of stroke, leading to the proposition that epilepsy may be one of the etiological factors for stroke. However, this viewpoint lacks support from studies on the pathophysiological mechanisms, and further research is needed to explore the causal relationship between epilepsy and stroke [14]. A recent cohort study found that the risk of IS was increased by 2.89 times in patients with epilepsy compared with normal subjects [15]. In another study, Selassie found similar results [16]. Even in numerous cross-sectional studies, such as the research conducted by Gaitatzis and Chang, an elevated risk of stroke has been observed in individuals with epilepsy. However, this does not imply a bilateral causal relationship. These studies, which investigate the incidence of stroke in epilepsy patients, still adhere to traditional observational methods [14,17]. Given the limitations of traditional observational studies in addressing reverse causality and potential confounding variables, researchers were also unable to determine the precise temporal relationship between epilepsy and subsequent stroke occurrences, leading to unjustified delays in stroke diagnosis. Consequently, the drawn conclusions remain rather tenuous. Even if these studies exhibit a robust statistical connection, they do not definitively establish a causal link between epilepsy and IS. Therefore, it is debatable whether there is a causal association between epilepsy and IS.
Mendelian randomization (MR) employs genetic variants as instrumental variables (IVs) based on the principles of Mendelian genetics to explore the causal association between an exposure and an outcome [18,19]. Utilizing this approach makes it feasible to mitigate the impact of confounding factors in the postnatal environment. This addresses the concern of potential false negative or false positive outcomes that can arise from confounding variables and reverse causation in medical observational studies [20].
We collected data on IS and epilepsy from extensive genetic studies. The primary aim is to investigate the potential reciprocal cause-and-effect link between these two conditions through bidirectional Two-sample MR (TSMR) analysis. The results of this study not only aimed to enhance our overall understanding of both disorders but also aimed to furnish substantial evidence to support the development of practical strategies for preventing both epilepsy and IS in clinical settings.
2. Materials and methods
2.1. Study design
Fig. 1 illustrates the comprehensive framework of our two-sample Mendelian randomization (MR) study. To enhance the credibility of our MR approach, our investigation aimed to fulfill the following three underlying hypotheses commonly employed in MR studies [1]: The single nucleotide polymorphisms (SNPs) used as IVs should exhibit a high degree of correlation with the exposure under investigation [2]; The SNPs employed as IVs should not be associated with any confounding factors that could influence the association between the exposure and the outcome [3]; The SNPs should not exert a direct effect on the outcome unless this effect is mediated through their association with the exposure of interest.
Fig. 1.
An overall design of our bidirectional two-sample mendelian randomization analysis. GWAS, genome-wide association study; IS, ischemic stroke; MR, Mendelian randomization; SNP, single nucleotide polymorphism.
2.2. Data sources
To perform our MR analysis, we utilized publicly accessible summary statistics derived from the largest meta-analyses of genome-wide association studies (GWAS) for each specific trait. The summary statistics for each disease were obtained from the IEU Open GWAS project. For comprehensive details on recruitment procedures and diagnostic criteria, we refer to the original publications.
2.3. Ischemic stroke
The genetic data of IS was derived from a GWAS conducted by Malik R et al., in 2018, encompassing a cohort of 440,328 individuals of European descent, with 34,217 individuals in the case group and 406,111 individuals in the control group. The research encompasses an analysis of 7,537,579 SNPs.
2.4. All epilepsy
All epilepsy genetic variants were obtained from the International League Against Epilepsy Complex Consortium (ILAE), comprising 15212 patients and 29677 controls with epilepsy. The study included 4,880,492 SNPs. The summary statistics for all epilepsy datasets can be found on the following online platform: http://gwas.mrcieu.ac.uk/datasets/ieu-b-8/.
2.5. Generalized epilepsy
Genetic variants associated with generalized epilepsy were extracted from a large GWAS conducted by ILAE. The study encompassed 3,769 epilepsy cases and 29,677 controls. A total of 4,867,068 SNPs were included in the analysis. Summary statistics for the datasets related to generalized epilepsy can be accessed through the following online portal: http://gwas.mrcieu.ac.uk/datasets/ieu-b-9/.
2.6. Focal epilepsy
The genetic association analysis for focal epilepsy involved 39,348 individuals from the ILAE, including 9,671 epilepsy cases and 29,677 controls. A total of 4,862,782 SNPs were included in this study. Summary statistics for the datasets related to focal epilepsy can be accessed through the following online portal: http://gwas.mrcieu.ac.uk/datasets/ieu-b-10/.
Data sources for IS and epilepsy have been assembled and made publicly accessible online (Table 1). As this study was based on published data, it did not require ethical approval or informed consent.
Table 1.
GWAS dataset used in the mendelian randomization.
2.7. Primary MR analysis
2.7.1. Selection
At the beginning of the study, we identified SNPs that showed a strong association with IS based on published data, using a primary screening criterion of P < 5 × 10−8. To ensure the independence of the instrumental variables used for exposure, we excluded SNPs that exhibited linkage disequilibrium (LD) (r2<0.001, clumping window = 10,000 kb). Next, we retrieved the information on IVs from the epilepsy GWAS, excluding palindromic SNPs. Subsequently, we aligned the exposure data with the outcome data, ensuring that the impact of the SNP on both exposure and outcome corresponded to the same allele. In our study, we performed F-statistic calculations to address potential bias stemming from weak instrumental variables. The F statistic was computed using the formula F R2 (n-k-1)/[k (1-R2)], with R2 indicating the extent to which the instrumental variable accounts for the variability in the exposure. N represents the sample size of the exposure GWAS study, while K denotes the number of IVs. A robustness threshold was set at an F statistic exceeding 10, indicating that our results remain unaffected by bias arising from weak instrumental variables.
2.8. MR analysis
In our current study, we employed MR analytical approaches to investigate the potential bidirectional causal relationship between IS and epilepsy risk. We utilized three commonly used MR methods, namely inverse-variance weighted (IVW), MR Egger, and Weighted median. IVW was employed as the main analytical approach in MR studies. IVW is distinguished by regression models that do not include an intercept term and are fitted accordingly. Additionally, we employed the MR-Egger method as an alternative approach to evaluate the validity of the IVW results. The weighted median method exhibits a notable capability in detecting causality and can serve as a valuable complementary approach to assess the robustness of its findings to a certain extent. Even in situations where a substantial proportion of the SNPs are invalid IVs, the weighted median method demonstrates a tendency to provide accurate estimates consistently.
2.9. Sensitivity analysis
We evaluated the potential heterogeneity among instrumental variables in each analysis using Cochran's Q test. A p-value >0.05 indicates the absence of significant heterogeneity. To assess horizontal pleiotropy, we employed the MR-Egger intercept test. A p-value >0.05 suggests no evidence of horizontal pleiotropy. Furthermore, we utilized the Mendelian Randomization Pleiotropy Residual Sum and Outlier (MR-PRESSO) test to identify and address pleiotropy in the horizontal direction by removing outliers. Despite employing diverse methods to enhance the accuracy of IVs during their assessment, it is unavoidable that a subset of the identified IVs may introduce potential risks to the outcomes. Consequently, we employed the leave-one-out method for sensitivity analysis to further verify the reliability of our findings.
2.10. Reverse MR analysis
The identical procedure was employed for the reverse MR analysis, and IVW was utilized as the principal analytical technique.
2.11. Statistical analysis
The TSMR analyses in this study were performed using R software version 4.0.3, in conjunction with the “Two Sample MR” package (version 0.5.6) and the “MRPRESSO” package. The R package, developed by Hemani et al. can be downloaded from GitHub (http://mrcieu.github.io/TwoSampleMR/). The entire study employed R software for data mapping.
3. Results
3.1. Causal analysis of ischemic stroke on epilepsy
To investigate the causal impact of IS as an exposure factor on the prevalence of outcomes, we employed the IV screening process described earlier to eliminate ineligible SNPs, while considering the independence and cascade imbalance of variants as IVs. From the IS GWAS dataset, we identified a total of 7 SNPs as instrumental variables. Fig. 2 provides detailed information on these IVs with the exposure factors. For the IVs associated with IS, all F-statistics exceeded 10, ranging from 30.39 to 60.47. The median F-statistic was 34.60, indicating the exclusion of bias caused by weak IVs and providing further validation for the robustness of the results (Additional file 1: Table S1).
Fig. 2.
Correlation analysis results of ischemic stroke and epilepsy.
The IVW analysis revealed a causal association between IS and epilepsy, with an odds ratio (OR) of 1.127 (95 % confidence interval [CI] = 1.038–1.224, P = 0.004). The weighted median analysis [OR = 1.128, 95 % CI = 1.025–1.242, P = 0.013] also supported the causal association, further demonstrating the reliability of the findings. These findings highlight the robustness of the results. In addition, a genetic predisposition to IS was found to be significantly associated with an increased risk of generalized epilepsy (IVW: OR = 1.340, 95 % CI = 1.162–1.546, P = 5.70 × 10–5). However, no causal association was observed between IS and focal epilepsy (IVW: OR = 1.073, 95 % CI = 0.975–1.181, P = 0.144) (Additional file 2: Fig. S1A, Fig. S2A, Fig. S3A, Fig. 2).
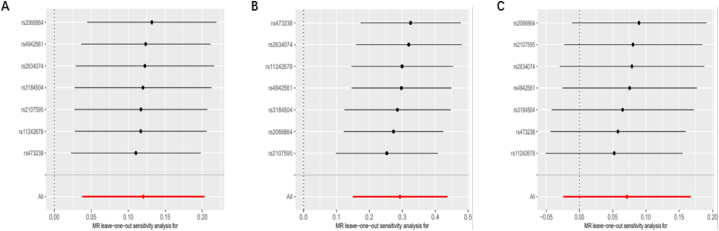
The heterogeneity tests for IS concerning all epilepsy, generalized epilepsy, and focal epilepsy yielded P-values >0.05, suggesting no substantial heterogeneity. Furthermore, the MR Egger test for horizontal pleiotropy indicated P-values >0.05 for all three conditions (IS with all epilepsy, generalized epilepsy, and focal epilepsy), indicating the absence of horizontal pleiotropy (Table 2). Funnel plots (Additional file 2: Fig. S1D, Fig. S2D, and Fig. S3D) and scatter plots (Additional file 2: Fig. S1C, Fig. S2C, and Fig. S3C) consistently support the same findings. In the “leave-one-out” analysis, each SNP was separately omitted from the analysis, and no single SNP was found to significantly impact the robustness of the results. Therefore, the TSMR correlation analysis results are deemed consistent and trustworthy (Fig. 3A–C).
Table 2.
Heterogeneity and pleiotropy test result ischemic stroke and epilepsy.
| Outcome | Methods | Horizontal pleiotropy |
Heterogeneity |
|||
|---|---|---|---|---|---|---|
| MR-Egger regression |
MR-presso |
Q | Q_Value | |||
| Egger intercept | P-intercept | Global-P | ||||
| All epilepsy | IVW | −0.013 | 0.591 | 0.986 | 1.142 | 0.979 |
| MR Egger | – | – | – | 0.814 | 0.976 | |
| Focal epilepsy | IVW | −0.008 | 0.773 | 0.847 | 2.918 | 0.818 |
| MR Egger | – | – | – | 2.826 | 0.726 | |
| Generalized epilepsy | IVW | 0.018 | 0.675 | 0.721 | 4.060 | 0.668 |
| MR Egger | – | – | – | 3.862 | 0.569 | |
Fig. 3.
Sensitivity analysis of the causal effect of ischemic stroke on epilepsy. (A) Leave-one-out plot of the causal relationship between ischemic stroke and all epilepsy. (B) Leave-one-out plot of the causal relationship between ischemic stroke and generalized epilepsy. (C) Leave-one-out plot of the causal relationship between ischemic stroke and focal epilepsy.
3.2. Causal relationship analysis between epilepsy and ischemic stroke
When investigating the causal effects of overall epilepsy, generalized epilepsy, and focal epilepsy as exposure variables on the outcome, we identified 3 SNPs, 11 SNPs, and 1 SNP, respectively, using the same method as described above. In this study, all F-statistics exceeded 10, ranging from 29.84 to 54.31. The median F-statistic was 33.25 for all epilepsy and 33.39 for generalized epilepsy (Additional file 1: Table S2, Table S3, and Table S4).
We employed IVW, weighted median, and MR Egger methods to examine the causal effects between all epilepsy, generalized epilepsy, focal epilepsy, and IS. However, no significant causal association was observed (P > 0.05) (Additional file 2: Fig. S4A, Fig. S5A, Table 3).
Table 3.
MR analysis for the causality of epilepsy with ths risk of ischemic stroke.
| Method | nSNP | SE | OR (95%CI) | P-val | Horizontal pleiotropy |
Heterogeneity |
|||
|---|---|---|---|---|---|---|---|---|---|
| MR-Egger regression |
MR-presso |
Q | Q_value | ||||||
| Egger intercept | P_value | Global_p | |||||||
| All epilepsy and ischemic stroke | |||||||||
| MR Egger | 3 | 0.484 | 1.380 (0.534–3.567) | 0.626 | – | – | – | 0.249 | 0.617 |
| Weightedmedian | 3 | 0.151 | 1.044 (0.775–1.406) | 0.774 | – | – | – | ||
| IVW | 3 | 0.126 | 1.096 (0.854–1.405) | 0.469 | −0.013 | 0.708 | – | 0.492 | 0.781 |
| Generalized epilepsy and ischemic stroke | |||||||||
| MR Egger | 11 | 0.381 | 1.170 (0.554–2.470) | 0.689 | – | – | – | 8.586 | 0.476 |
| Weightedmedian | 11 | 0.058 | 1.080 (0.964–1.210) | 0.183 | – | – | – | ||
| IVW | 11 | 0.041 | 1.066 (0.983–1.157) | 0.118 | −0.007 | 0.812 | 0.591 | 8.646 | 0.565 |
| Focal epilepsy and ischemic stroke | |||||||||
| Wald ratio | 1 | 0.190 | 0.998 (0.686–1.450) | 0.992 | – | – | – | – | – |
The heterogeneity test results for all epilepsy, generalized epilepsy, and focal epilepsy with IS were P-values >0.05, indicating no significant heterogeneity and suggesting unbiased results (Additional file 2: Fig. S4D and Fig. S5D). Similarly, the MR Egger test for horizontal pleiotropy revealed no clear evidence of horizontal pleiotropy between all epilepsy, generalized epilepsy, focal epilepsy, and IS, with P-values >0.05 (Additional file 2: Fig. S4C and Fig. S5C, Table 3). Sensitivity analysis using the leave-one-out approach demonstrated that no individual SNP significantly influenced the study results. Therefore, the TSMR correlation analysis results are deemed stable and reliable (Additional file 2: Fig. S4B and Fig. S5B).
4. Discussion
This bidirectional MR study investigated the potential causal connection between IS and epilepsy. In terms of genetics, IS increases the risk of generalized epilepsy and all epileptic potentials. No clear causal relationship was found between IS and focal epilepsy. When conducting reverse TSMR analysis, it does not establish a causal relationship between all types of epilepsy, including generalized and focal epilepsy and IS.
To date, Considerable research has been conducted on the underlying mechanisms of post-stroke epilepsy. An ischemic event triggers a myriad of processes within brain tissue, including excitotoxicity, blood-brain barrier compromise, ion channel dysfunction, and inflammatory response, all of which can contribute to the onset of epilepsy [21].
Recently the role of inflammatory response in promoting epileptic seizures has been increasingly emphasized and confirmed [22]. Also, the inflammatory response is one of the non-negligible pathologic processes after cerebral infarction, starting in the acute phase and continuing throughout the course of the disease [23]. During the acute phase of cerebral infarction, microglia and astrocytes are activated and gradually produce TNF-α, IL-1β, IL-6, and other related inflammatory factors. Inflammatory factors and related mediators gradually accumulate, and an inflammatory waterfall effect occurs in the subacute or even recovery phase of cerebral infarction, which induces seizures [24]. The neurological inflammatory response after stroke alters the permeability of the blood-brain barrier, decreases neuronal response thresholds, and increases neuronal depolarization and sensitivity [25].
In addition, the blood-brain barrier is disrupted and albumin enters the brain parenchyma and binds to transforming growth factor receptors on astrocytes, thereby reducing the uptake of potassium ions and glutamate in the synaptic gap [26]. The ensuing rise in extracellular potassium and glutamate triggered neuronal hyperexcitability, culminating in epilepsy.
Another is the excitotoxicity theory, in which large amounts of glutamate and other neurotransmitters are released from injured cells into the extracellular space after an IS [27]. Glutamate functions as an excitotoxic neurotransmitter, and excessive extracellular glutamate concentrations lead to substantial neuronal loss, concurrently inducing epileptiform firing in viable neurons, resulting in seizures. At the same time, glutamate is also thought to produce epileptoid firing in living neurons, which then causes seizures [28].
Neuronal ion channel deficits are also involved in post-stroke epilepsy. Injury from IS causes an increase in intracellular calcium and sodium, which decreases the depolarized seizure threshold [29]. In experimental studies, increased extracellular potassium levels cause neuronal depolarization and promote seizures [30]. In addition, the occurrence of stroke leads to altered gene expression, which is involved in the mechanism of post-stroke epilepsy. Lu found in a rat model of permanent middle cerebral artery occlusion that ischemia upregulated genes that mediate inflammation, cell death, cytoskeletal function, and metabolism [31]. Altered gene expression may be associated with an immune response and impairment of neuronal plasticity, leading to seizures [32].
This study leveraged information on SNPs derived from genetic variations associated with IS as instrumental variables. The causal association of IS on the development of epilepsy was explored genetically, with results indicating a potential causal association between IS and both epilepsy and generalized epilepsy.
This MR analysis effectively mitigated the influences of reverse causation and confounding variables. From the perspective of genetic variation, whether epilepsy has a clear causal association with the onset of IS was analyzed. Contrary to earlier observational studies, the findings revealed that there was no evident causal connection between all epilepsy, generalized epilepsy, and focal epilepsy and the development of IS. A highly plausible explanation is that most studies on post-epileptic stroke, especially in the process of prospective studies, collected information about the state of epilepsy after stroke, which was affected by reverse cause and effect. Previous studies have found that seizures may be caused by potential premonitory risk factors of stroke, such as hemosiderin deposition and microvascular embolism [33]. For example, patients with cerebrovascular disease may have no obvious clinical symptoms other than epilepsy and are difficult to detect by conventional magnetic resonance imaging. Because the clinical symptoms and imaging examination of these microvascular strokes are easy to ignore, epilepsy caused may be regarded as primary epilepsy and may progress to stroke with obvious clinical symptoms and obvious imaging results in the follow-up. As a result, the false positive results were obtained [34,35].
Another possible reason is that the clinical treatment of patients with epilepsy affects the occurrence of IS, not caused by epilepsy itself. A national cohort study in 2011 showed that patients who received antiepileptic drugs had an increased risk of IS compared to patients who did not receive antiepileptic drugs [36]. The Olesen study found that patients treated with carbamazepine, sodium valproate, phenytoin, and clonazepam had an increased risk of IS [37]. In previous studies, it has been found that antiepileptic drugs can cause metabolic side effects such as lipid abnormality, obesity, hyperhomocysteinemia, and subclinical hypothyroidism [[38], [39], [40]].
All of these side effects have been identified as risk factors for stroke and are involved in the onset and progression of IS. As a result, this study posited that the connection between epilepsy and IS, as observed in conventional epidemiological investigations, cannot be straightforwardly attributed to epilepsy having a direct causal impact on the occurrence of IS.
Our study uses MR analysis as the overall research framework. The genetic variation data identified by GWAS were used as tool variables to simulate exposure factors to control the confounding factor bias in traditional observational studies. Moreover, the genotype dictated the phenotype without interfering with subsequent disease progression, significantly diminishing the likelihood of reverse causation. In addition, the exposure and outcome data come from the largest alliance study at present, which ensures the test efficiency and reliability of the statistical results.
There are several limitations in this study that warrant further improvement. Firstly, the sample sizes for epilepsy subtypes and IS were relatively small. Replication of the current findings requires studies with larger sample sizes. Secondly, the participants in this study were predominantly of European ancestry. Future investigations should include GWAS data from diverse ethnic populations to assess the generalizability of the results across different groups. Thirdly, MR analysis should be limited to risk factors that have suitable genetic variants. Genetic variants often have a limited impact on most risk factors, which can lead to false-negative results. Fourthly, it is important to note that publicly available data resources for post-stroke epilepsy in the specific field are currently limited. Future research should focus on validating our findings in larger and more diverse cohorts. Additionally, we utilized both GWAS-specific data and publicly available summary data, which may introduce confounding bias due to potential sample overlaps. Future studies incorporating larger GWAS datasets are expected to enhance statistical power and provide a more precise assessment of the genetic influences on epilepsy and stroke. Prospective studies are also being conducted to clarify underlying mechanisms.
5. Conclusion
In conclusion, this MR study suggests that the onset of IS potentially increases the likelihood of developing epilepsy. Therefore, early intervention in patients with IS may reduce the risk of epilepsy. The importance of early prevention and management of epilepsy in patients with IS was emphasized. In reverse MR analysis, no clear causal association was shown between epilepsy (including all epilepsy, generalized epilepsy, and focal epilepsy) and IS. Given the constraints of this study, larger GWAS datasets and prospective studies are needed to discover potential mechanisms of causal influence between epilepsy and IS.
Funding statement
This study was supported by the Special Project for Health Research Talents of Jilin Province [2023SCZ34] and the Medical Science and Technology Innovation and Health Management Program Project of Jilin Province [202307-A01].
Consent for publication
Not applicable.
Ethics statement
All data used in this study were obtained from publicly available databases; further ethical approval was not required.
Data availability statement
The data generated or analyzed during this study are available in this published article and its supplementary information files, further inquiries can be directed to the corresponding authors.
CRediT authorship contribution statement
Zongzhi Jiang: Writing – original draft. Yining Sun: Methodology, Formal analysis. Ziyi Wang: Software. Songyan Liu: Writing – review & editing.
Declaration of competing interest
Songyan Liu reports financial support was provided by the Health Research Talent Special project of Jilin Province. Songyan Liu reports financial support was provided by the Medical Science and Technology Innovation and Health Management Plan project of Jilin Province. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e32532.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Thijs R.D., Surges R., O'Brien T.J., Sander J.W. Epilepsy in adults. Lancet (London, England) 2019;393(10172):689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 2.Löscher W., Potschka H., Sisodiya S.M., Vezzani A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol. Rev. 2020;72(3):606–638. doi: 10.1124/pr.120.019539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiest K.M., Sauro K.M., Wiebe S., Patten S.B., Kwon C.S., Dykeman J., et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296–303. doi: 10.1212/WNL.0000000000003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanner A.M., Bicchi M.M. Antiseizure medications for adults with epilepsy: a review. JAMA. 2022;327(13):1269–1281. doi: 10.1001/jama.2022.3880. [DOI] [PubMed] [Google Scholar]
- 5.Campbell B.C.V., De Silva D.A., Macleod M.R., Coutts S.B., Schwamm L.H., Davis S.M., Donnan G.A. Ischaemic stroke. Nat. Rev. Dis. Prim. 2019;5(1):70. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- 6.Walter K. What is acute ischemic stroke? JAMA. 2022;327(9):885. doi: 10.1001/jama.2022.1420. [DOI] [PubMed] [Google Scholar]
- 7.Vyas M.V., Fang J., Austin P.C., Kapral M.K. Proportion of life spent in Canada and stroke incidence and outcomes in immigrants. Ann. Epidemiol. 2022;74:58–65. doi: 10.1016/j.annepidem.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Yang C., Pan Y. Risk factors of dysphagia in patients with ischemic stroke: a meta-analysis and systematic review. PLoS One. 2022;17(6) doi: 10.1371/journal.pone.0270096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doria J.W., Forgacs P.B. Incidence, implications, and management of seizures following ischemic and hemorrhagic stroke. Curr. Neurol. Neurosci. Rep. 2019;19(7):37. doi: 10.1007/s11910-019-0957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lattanzi S., Meletti S., Trinka E., Brigo F., Turcato G., Rinaldi C., et al. Individualized prediction of drug resistance in people with post-stroke epilepsy: a retrospective study. J. Clin. Med. 2023;12(11) doi: 10.3390/jcm12113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lattanzi S., Rinaldi C., Cagnetti C., Foschi N., Norata D., Broggi S., et al. Predictors of pharmaco-resistance in patients with post-stroke epilepsy. Brain Sci. 2021;11(4) doi: 10.3390/brainsci11040418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H., Song Z., Yang G.P., Zhang B.K., Chen M., Wu T., Guo R. The ALDH2 rs671 polymorphism affects post-stroke epilepsy susceptibility and plasma 4-HNE levels. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang B., Chen M., Yang H., Wu T., Song C., Guo R. Evidence for involvement of the CD40/CD40L system in post-stroke epilepsy. Neurosci. Lett. 2014;567:6–10. doi: 10.1016/j.neulet.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Chang C.S., Liao C.H., Lin C.C., Lane H.Y., Sung F.C., Kao C.H. Patients with epilepsy are at an increased risk of subsequent stroke: a population-based cohort study. Seizure. 2014;23(5):377–381. doi: 10.1016/j.seizure.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Cleary P., Shorvon S., Tallis R. Late-onset seizures as a predictor of subsequent stroke. Lancet (London, England) 2004;363(9416):1184–1186. doi: 10.1016/S0140-6736(04)15946-1. [DOI] [PubMed] [Google Scholar]
- 16.Selassie A.W., Wilson D.A., Martz G.U., Smith G.G., Wagner J.L., Wannamaker B.B. Epilepsy beyond seizure: a population-based study of comorbidities. Epilepsy Res. 2014;108(2):305–315. doi: 10.1016/j.eplepsyres.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Gaitatzis A., Carroll K., Majeed A., J W.S. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45(12):1613–1622. doi: 10.1111/j.0013-9580.2004.17504.x. [DOI] [PubMed] [Google Scholar]
- 18.Zheng J., Baird D., Borges M.C., Bowden J., Hemani G., Haycock P., et al. Recent developments in mendelian randomization studies. Current epidemiology reports. 2017;4(4):330–345. doi: 10.1007/s40471-017-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birney E. Mendelian randomization. Cold Spring Harbor perspectives in medicine. 2022;12(4) doi: 10.1101/cshperspect.a041302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekula P., Del Greco M.F., Pattaro C., Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J. Am. Soc. Nephrol. 2016;27(11):3253–3265. doi: 10.1681/ASN.2016010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wimmer I., Zrzavy T., Lassmann H. Neuroinflammatory responses in experimental and human stroke lesions. J. Neuroimmunol. 2018;323:10–18. doi: 10.1016/j.jneuroim.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Jayaraj R.L., Azimullah S., Beiram R., Jalal F.Y., Rosenberg G.A. Neuroinflammation: friend and foe for ischemic stroke. J. Neuroinflammation. 2019;16(1):142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alyu F., Dikmen M. Inflammatory aspects of epileptogenesis: contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr. 2017;29(1):1–16. doi: 10.1017/neu.2016.47. [DOI] [PubMed] [Google Scholar]
- 24.Endres M., Moro M.A., Nolte C.H., Dames C., Buckwalter M.S., Meisel A. Immune pathways in etiology, acute phase, and chronic sequelae of ischemic stroke. Circ. Res. 2022;130(8):1167–1186. doi: 10.1161/CIRCRESAHA.121.319994. [DOI] [PubMed] [Google Scholar]
- 25.Preininger M.K., Zaytseva D., Lin J.M., Kaufer D. Blood-brain barrier dysfunction promotes astrocyte senescence through albumin-induced TGFβ signaling activation. Aging Cell. 2023;22(2) doi: 10.1111/acel.13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cacheaux L.P., Ivens S., David Y., Lakhter A.J., Bar-Klein G., Shapira M., et al. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J. Neurosci. : the official journal of the Society for Neuroscience. 2009;29(28):8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neves D., Salazar I.L., Almeida R.D., Silva R.M. Molecular mechanisms of ischemia and glutamate excitotoxicity. Life Sci. 2023;328 doi: 10.1016/j.lfs.2023.121814. [DOI] [PubMed] [Google Scholar]
- 28.Szydlowska K., Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47(2):122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Kamp M.A., Dibué M., Schneider T., Steiger H.J., Hänggi D. Calcium and potassium channels in experimental subarachnoid hemorrhage and transient global ischemia. Stroke Res. Treat. 2012;2012 doi: 10.1155/2012/382146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weilinger N.L., Maslieieva V., Bialecki J., Sridharan S.S., Tang P.L., Thompson R.J. Ionotropic receptors and ion channels in ischemic neuronal death and dysfunction. Acta Pharmacol. Sin. 2013;34(1):39–48. doi: 10.1038/aps.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu X.C., Williams A.J., Yao C., Berti R., Hartings J.A., Whipple R., et al. Microarray analysis of acute and delayed gene expression profile in rats after focal ischemic brain injury and reperfusion. J. Neurosci. Res. 2004;77(6):843–857. doi: 10.1002/jnr.20218. [DOI] [PubMed] [Google Scholar]
- 32.Ramos-Cejudo J., Gutiérrez-Fernández M., Rodríguez-Frutos B., Expósito Alcaide M., Sánchez-Cabo F., Dopazo A., Díez-Tejedor E. Spatial and temporal gene expression differences in core and periinfarct areas in experimental stroke: a microarray analysis. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira-Atuesta C., Döhler N., Erdélyi-Canavese B., Felbecker A., Siebel P., Scherrer N., et al. Seizures after ischemic stroke: a matched multicenter study. Ann. Neurol. 2021;90(5):808–820. doi: 10.1002/ana.26212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferlazzo E., Gasparini S., Beghi E., Sueri C., Russo E., Leo A., et al. Epilepsy in cerebrovascular diseases: review of experimental and clinical data with meta-analysis of risk factors. Epilepsia. 2016;57(8):1205–1214. doi: 10.1111/epi.13448. [DOI] [PubMed] [Google Scholar]
- 35.Pitkänen A., Roivainen R., Lukasiuk K. Development of epilepsy after ischaemic stroke. Lancet Neurol. 2016;15(2):185–197. doi: 10.1016/S1474-4422(15)00248-3. [DOI] [PubMed] [Google Scholar]
- 36.Olesen J.B., Abildstrøm S.Z., Erdal J., Gislason G.H., Weeke P., Andersson C., et al. Effects of epilepsy and selected antiepileptic drugs on risk of myocardial infarction, stroke, and death in patients with or without previous stroke: a nationwide cohort study. Pharmacoepidemiol. Drug Saf. 2011;20(9):964–971. doi: 10.1002/pds.2186. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh C.Y., Lai E.C., Yang Y.H., Lin S.J. Comparative stroke risk of antiepileptic drugs in patients with epilepsy. Epilepsia. 2013;54(1):172–180. doi: 10.1111/j.1528-1167.2012.03693.x. [DOI] [PubMed] [Google Scholar]
- 38.Phabphal K., Limapichat K., Sathirapanya P., Setthawatcharawanich S., Geater A. Characterization of glucose homeostasis and lipid profile in adult, seizure-free, epileptic patients in Asian population. Eur. J. Neurol. 2012;19(9):1228–1234. doi: 10.1111/j.1468-1331.2012.03708.x. [DOI] [PubMed] [Google Scholar]
- 39.Katsiki N., Mikhailidis D.P., Nair D.R. The effects of antiepileptic drugs on vascular risk factors: a narrative review. Seizure. 2014;23(9):677–684. doi: 10.1016/j.seizure.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Ashjazadeh N., Fathi M., Shariat A. Evaluation of homocysteine level as a risk factor among patients with ischemic stroke and its subtypes. Iran. J. Med. Sci. 2013;38(3):233–239. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated or analyzed during this study are available in this published article and its supplementary information files, further inquiries can be directed to the corresponding authors.