Abstract
Dedifferentiated liposarcoma is a rare cancer with a poor prognosis. A 52-year-old man presented with a chief complaint of a mass in his left scrotum. He came with suspected testicular tumor, but all the measured tumor markers were negative. Imaging test showed approximately 2 cm diameter mass accompanied by calcification with some substantial components between the testis and epididymis. Left high testicular resection was performed. The tumor had no continuity between the testis and epididymis, and the spermatic cord transection was negative. Pathological findings showed well differentiated fatty component and a dedifferentiated component around the trabecular bone-like tissue. We observed dedifferentiated dysmorphic cells mixed with fatty droplets of unequal size. Immunostaining led to the diagnosis of dedifferentiated liposarcoma. No additional postoperative therapy was performed. The possibility of dedifferentiated liposarcoma should be kept in mind even if mass is confined to the scrotum and consisted of calcification. In the case of an intrascrotal calcified mass with malignant perspective, radical surgery is highly recommended.
Keywords: Dedifferentiated liposarcoma, Scrotum, Calcification
Introduction
Dedifferentiated liposarcoma (DDLPS) is a rare cancer with a poor prognosis. It is the one of the five major classifications of malignant adipocytic tumors in World Health Organization (WHO). Well-differentiated liposarcoma/atypical fatty tumor, dedifferentiated liposarcoma, myxoid liposarcoma, pleomorphic liposarcoma, and myxoid pleomorphic liposarcoma. The peak incidence of DDLPS occurs in the sixth and seventh decades. It is discovered as a painless swelling, most commonly occurring in the retroperitoneum, but also in the extremities, spermatic cord, or trunk [1, 2]. We report a case of DDLPS diagnosed after the excision of a calcified mass in the scrotum, along with a review of the literature.
Case presentation
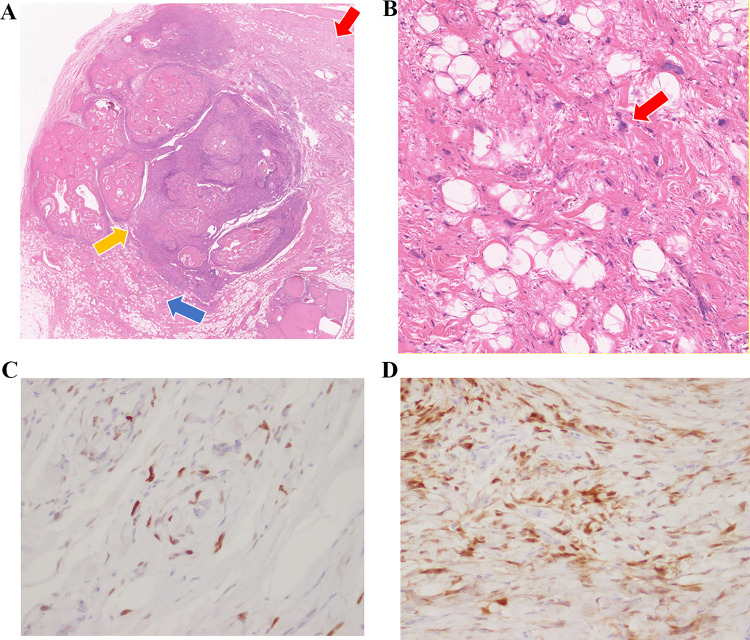
The patient first came with a chief complaint of a mass in the left scrotum that had been present for 2 months. Tumor markers elevated in testicular tumor, such as LDH, HCG-beta and AFP were negative. Echography revealed a 20 mm × 22 mm × 25 mm calcified mass between the testis and epididymis. The primary cause was unclear, and inflammatory disease was considered as a possible differential diagnosis. However, since testicular malignancy could not be ruled out, we decided to perform a left high testicular resection on a wait-and-see basis. Preoperative imaging findings: Computed tomography (CT) showed a calcified mass between the testis and epididymis, without metastasis; and the magnetic resonance imaging (MRI) scan showed the tumor consisting of few solid components without fat (Fig. 1). Left high testicular resection was performed under for suspected left testicular tumor with stage of cT1N0M0 (Fig. 2). The patient was discharged on the third postoperative day. 18-FDG positron emission tomography (PET) -CT taken on postoperative day 106, MRI every 3 months after postoperative day and all showed no evidence of metastasis or recurrence. Histologically, lipo-fibrous component surrounds calcifying core which contains trabecular-bone like ossifications and atypical giant cells (Fig. 3a, b). In the fibrous lesion, short spindle to polygonal cells with bizarre nucleus proliferate, and some mature adipocytes are mixed. Immunohistochemically, atypical cells are positive for p16, and MDM2 (Fig. 3c, d). These findings lead to the diagnosis of dedifferentiated liposarcoma.
Fig. 1.
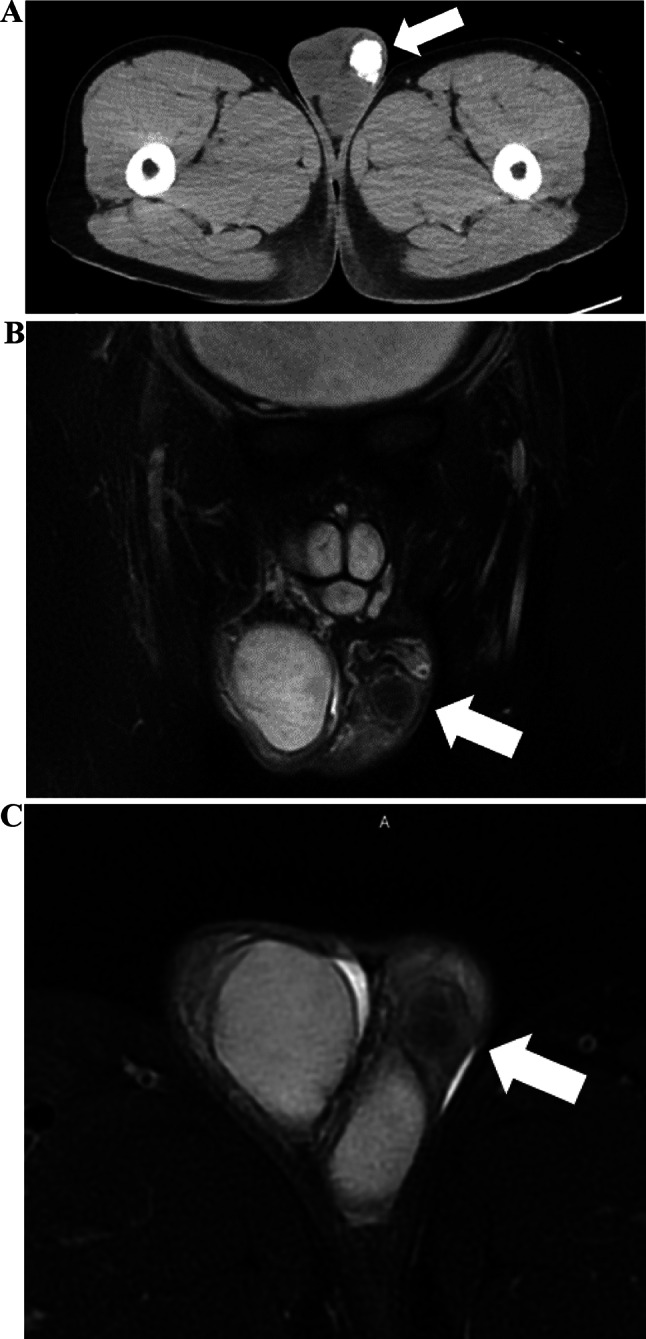
(A) CT (axial) It reveals an inguinal mass (white arrow), which mainly consisted of calcification. There is no metastasis. (B) MRI (T2-weighted coronal image with fat suppression). It reveals the tumor consisting of few solid ingredients. (C) MRI (T2-weighted horizontal image with fat suppression). It shows no fat signal area on the tumor
Fig. 2.
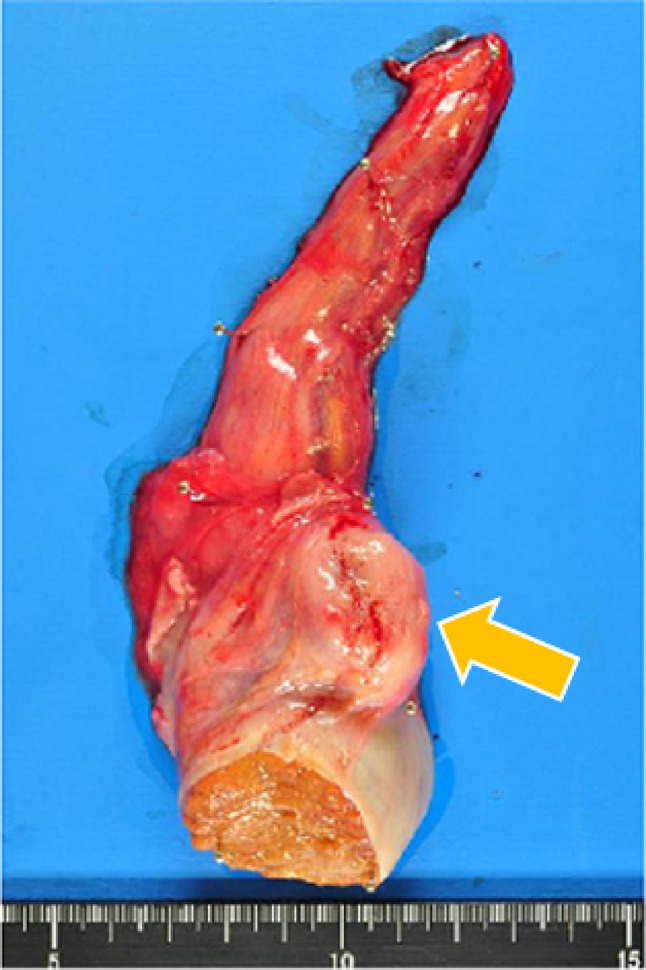
Extracted specimen, before formalin fixation. Tumor (yellow arrow) reveals between testis and epididymis
Fig. 3.
(A) HE stains × 4: Well differentiated fat components (blue arrow) and dedifferentiated components (red arrow) are observed around the spongy bone-like tissue (yellow arrow). (B) HE stains × 100: Atypical cells mixed in adipose tissue (red arrow). (C) MDM2 stains × 400: The tumor cells are partially positive for MDM2. (D) p16 stains × 400: The tumor cells are diffusely positive for p16
Discussion
DDLPS was first described by Evans et al. in 1979 as a malignant fibrous histiocytoma image in parts of a well-differentiated liposarcoma. Molecular genetics is characterized by amplifications of the q13–15 region of chromosome 12, and the MDM2, p53, and CDK4 genes [12]. It consists of a biphasic structure consisting of a fatty mass and a solid soft tissue mass on CT and MRI. There are plenty fibrous tissue and partial calcification with internal necrosis [13]. The histological features are described as fibrosarcoma like and malignant fibrous histiocytoma like lesions consisting of atypical spindle-shaped cell proliferation without fatty cells, with multifocal vacuoles characteristic of DDLPS. In the present case, both well-differentiated lipomatosis components and dedifferentiated components were observed around the trabecular bone-like tissue. Also observed were dedifferentiated spindle-shaped atypical cells mixed with fatty droplets. Ossifying lesions were predominant, but there are few reports of ossification within this tumor [14]. However, immunostaining showed that the tumor cells were partially positive for CD34, MDM2 and p16 positive, resulting in a diagnosis of DDLPS. The treatment is based on definitive surgical resection. There are reports that the local recurrence rate is approximately 40%, the metastatic rate is 15–30%, and the overall mortality rate is 28%. Dedifferentiation from a favorable prognostic histology, such as the well-differentiated type suggests a low 5-year survival rate (30%), and poor prognosis [14]. In the case of postoperative recurrence, additional resection of the recurrent site is considered first. If additional resection is not feasible or if there is clear residual disease, drug therapy is an option. The standard for primary drug therapy is single agent doxorubicin, as demonstrated in a retrospective study by Italiano A. et al. [15]. Second-line therapy included eribulin, a non-taxane microtubule inhibitor, and trabectedin [16, 17]. In addition, MDM2 and CDK4 inhibitors may be an option after a relapse [18]. However, the combination of each agent has not led to a satisfactory clinical outcome. Most liposarcomas occur mainly in the retroperitoneum. The incidence of liposarcoma at inguinal to intrascrotal is 9.1% and DDLPS account for only 6–10% of liposarcoma [19], then DDLPS confined to the scrotum are extremely rare. There were only 19 cases of scrotal localized DDLPS reported in Japan during the 15-year period from 2008 to 2022 (Table 1). High testicular resection was performed in all cases. Eight (42.1%) had positive margins, and four of these had metastases and additional surgical resection. The median observation period since the last surgery was 18.9 months, and in all cases (excluding those with unknown outcome), there was no recurrence at the reporting point. In cases such as the present one, where the margins were negative and the tumor was considered to have been surgically cured, frequent postoperative imaging evaluation is recommended for the early detection. There is no established treatment strategy for postoperative therapy to prevent recurrence. Since the risk of side effects associated with chemotherapy is believed to be high, the early detection of recurrence and additional resection is more realistic. The possibility of DDLPS should be kept in mind even if mass is confined to the scrotum and consisted of calcification. In the case of an intrascrotal mass with malignant perspective, radical surgery is highly recommended.
Table 1.
Report cases of DDLPS which confined at the inguinal to the scrotum from Japan last 15 years. (High Testiclectomy: HT, Additional Dermatomy: AD, Radiation Therapy: RT)
| Case | Year | Author | Age | Left or Right | Treatment | Resection margin | Additional treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 2022 | Ishibashi [3] | 52 | R | HT | + | Additional resection 2 months later | No recurrence for 8 months |
| 2 | 2020 | Kumada [4] | 56 | R | HT | − | – | No recurrence for 9 months |
| 3 | 2019 | Takaoka [5] | 74 | R | HT | − | – | No recurrence for 12 months |
| 4 | 2019 | Tobiume [6] | 51 | L | HT | + | Additional resection of retroperitoneal area 1 month later | No recurrence for 12 months |
| 5 | 2019 | Shinohara [7] | 67 | R | HT + AD | − | – | Unknown |
| 6 | 2018 | Ando [8] | 62 | L | HT | + | Additional resection + RT60 Gy | Unknown |
| 7 | 2018 | Oshikawa [9] | 91 | R | HT | − | – | No recurrence for 18 months |
| 8 | 2016 | Hodotuka [6] | 63 | R | HT | − | – | No recurrence for 36 months |
| 9 | 2015 | Tajima [6] | 64 | R | HT | Unknown | Unknown | Unknown |
| 10 | 2016 | Yoshino [6] | 92 | R | HT | + | – | No recurrence for 8 months |
| 11 | 2013 | Hatananaka [6] | 77 | R | HT | − | – | No recurrence for 24 months |
| 12 | 2013 | Tanaka [10] | 77 | L | HT + AD | + | Additional resection 3 months later | No recurrence for 16 months |
| 13 | 2013 | Okano [6] | 70 | R | HT | − | Unknown | Unknown |
| 14 | 2012 | Ohata [6] | 50 s | L | HT | + | – | No recurrence for 36 months |
| 15 | 2009 | Yoshino [6] | 71 | L | HT | − | – | No recurrence for 4 months |
| 16 | 2009 | Itoh [6] | 61 | L | HT | − | – | No recurrence for 12 months |
| 17 | 2009 | Akaihata [11] | 76 | L | HT | − | – | No recurrence for 22 months |
| 18 | 2008 | Funahashi [6] | 79 | R | HT | + | – | No recurrence for 12 months |
| 19 | 2008 | Kakuta [6] | 69 | L | HT | + | – | No recurrence for 54 months |
Acknowledgements
We express our sincere thanks to Ehime Urological Association for the cost of publication.
Data availability
The data in this report are available on request from the corresponding author.
Declarations
Conflict of interest
The authors declare that have no conflict of interest.
Approval of the research protocol by an Institutional Reviewer Board
Not applicable.
Informed consent
Informed consent was obtained.
Registry and the Registration No. of the study/trial
Not applicable.
List of supporting information
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Haruna Arai, Email: k447001y@mails.cc.ehime-u.ac.jp.
Ryuta Watanabe, Email: watanabe.ryuta.cu@ehime-u.ac.jp.
References
- 1.Nishio J, et al. Contributions of cytogenetics and molecular cytogenetics to the diagnosis of adipocytic tumors. J Biomed Biotechnol. 2011;2011:524067. doi: 10.1155/2011/524067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waters R, Horvai A, Greipp P, et al. Atypical lipomatous tumour/well-differentiated liposarcoma and de-differentiated liposarcoma in patients aged ≤40 years: a study of 116 patients. Histopathology. 2019;75:833–842. doi: 10.1111/his.13957. [DOI] [PubMed] [Google Scholar]
- 3.Ishibashi Y, et al. A case of dedifferentiated liposarcoma of the spermatic cord. Acta Urol. 2022;68:17–21. doi: 10.14989/ActaUrolJap_68_1_17. [DOI] [PubMed] [Google Scholar]
- 4.Kumada N, et al. A case of liposarcoma of the spermatic cord. Toyota J Med. 2020;29:62–66. [Google Scholar]
- 5.Takaoka N, et al. A case of dedifferentiated liposarcoma of the spermatic cord. Acta Urol. 2019;65:529–532. doi: 10.14989/ActaUrolJap_65_12_529. [DOI] [PubMed] [Google Scholar]
- 6.Tobiume M, et al. A case of dedifferentiated liposarcoma of the spermatic cord. Acta Urol. 2019;65:429–434. doi: 10.14989/ActaUrolJap_65_10_429. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara M, et al. A case of dedifferentiated liposarcoma in the scrotum. J Urol Surg. 2019;32:307. [Google Scholar]
- 8.Ando T, et al. Dedifferentiated spermatic cord liposarcoma with macroscopic ossification. IJU. 2018;1:5–8. doi: 10.1002/iju5.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oshikawa Y, et al. A case of liposarcoma in the scrotum in the very elderly. Nishi Nihon Hinyokika. 2018;80:305. [Google Scholar]
- 10.Tanaka S, et al. A case of dedifferentiated liposarcoma of the spermatic cord. J Urol Surg. 2013;26:754. [Google Scholar]
- 11.Akaihata H, et al. A case of liposarcoma occurring in the scrotum. Jpn J Urol. 2009;63:811–815. [Google Scholar]
- 12.Evans HL, et al. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3:507–523. doi: 10.1097/00000478-197912000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Gondo T, et al. Retroperitoneal dedifferentiated liposarcoma: case report. Jpn J Urol. 2007;98:795–799. doi: 10.5980/jpnjurol1989.98.795. [DOI] [PubMed] [Google Scholar]
- 14.Nishio J, et al. Biology and management of dedifferentiated liposarcoma: state of the art and perspectives. J Clin Med. 2021;10(15):3230. doi: 10.3390/jcm10153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livingston JA, Bugano D, Barbo A, et al. Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: defining the benefit and challenges of the standard. Sci Rep. 2017;19:11836. doi: 10.1038/s41598-017-12132-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387:1629–1637. doi: 10.1016/S0140-6736(15)01283-0. [DOI] [PubMed] [Google Scholar]
- 17.Demetri GD, Von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34:786–793. doi: 10.1200/JCO.2015.62.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer TM, Gounder MM, Weise AM, et al. A phase 1 study of MDM2 inhibitor DS-3032b in patients with well/de-differentiated liposarcoma (WD/DD LPS), solid tumors (ST) and lymphomas (L) J Clin Oncol. 2018;36:11514. doi: 10.1200/JCO.2018.36.15_suppl.11514. [DOI] [Google Scholar]
- 19.Nagae I, et al. A case of differentiated liposarcoma of the spermatic cord with widely infiltrative local recurrence. Jpn J Gastroenterol Surg. 2007;40:349–354. doi: 10.5833/jjgs.40.349. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this report are available on request from the corresponding author.