Abstract
Paraneoplastic neurological syndromes (PNS) are neurological disorders that occur in close association with tumors without direct metastasis or invasion of the tumors and in which anti-neural antibodies may be present. Cerebellar ataxia is a common form of PNS in patients with breast cancer. However, reports of symptom improvement with breast cancer treatment are more common in patients with positive anti-neural antibodies and are rarely seen in those with negative anti-neural antibodies. In addition, there have been few quantitative evaluations of symptom improvement. We report a case in which neurological symptoms significantly improved after surgical treatment for breast cancer. The patient was a 78-years-old woman with subacute progressive cerebellar ataxia. A subsequent diagnosis of breast cancer led to the diagnosis of “PNS probable”. A comprehensive search for anti-neural antibodies was negative in all cases. The quantitative index of the Scale for the Assessment and Rating of Ataxia (SARA) score, a standard evaluation method for ataxia in spinocerebellar degeneration, improved after breast cancer surgery. This case may provide a rationale for treating breast cancer patients negative for anti-neural antibodies, with the possibility of improving neurological symptoms.
Keywords: Breast cancer, Paraneoplastic neurological syndrome, Subacute cerebellar dysfunction, Scale for the assessment and rating of ataxia score
Introduction
Paraneoplastic neurological syndromes (PNS) are neurological disorders that occur in close association with a tumor without direct metastasis or invasion of the tumor, and new diagnostic criteria were proposed in 2021 [1]. There are three risk categories depending on the neurological symptoms and associated autoantibodies, further divided into a final diagnosis of definite, probable, or possible, depending on the presence or absence of tumor and the presence or type of anti-neural antibodies. Although cancer treatment and immunotherapy are often provided, there are few comprehensive reports and treatment decisions are often difficult to make.
Subacute cerebellar ataxia is one of symptoms of PNS and that often occurs in breast cancer patients [2]. Although the expression of anti-Yo and anti-Ri antibodies is high in breast cancer [1], not all PNS patients have anti-neural antibodies and the diagnosis of "PNS probable" can be made without the presence of anti-neuronal antibodies [2]. Furthermore, neurological symptoms rarely improve after cancer treatment [2, 3], especially in antibody-negative patients. However, patients with PNS are at a high risk for cancer treatment owing to neurological symptoms, and it is important to determine whether cancer treatment improves neurologic symptoms.
Recently, autoimmune cerebellar ataxia (ACA) has attracted attention as one of the cerebellar ataxia [4], and Dalmau and Graus proposed the diagnostic criteria for ACA [5]. Cerebellar ataxia of the PNS is included in ACA, and ACA requires early treatment including immunotherapy and cancer treatment to maintain the cerebellar reserve [4, 6]. So, if a patient with PNS also has a diagnosis of ACA, early treatment may improve neurological symptoms.
Here, we report a case of PNS with ACA and breast cancer in which the Scale for the Assessment and Rating of Ataxia (SARA) score, a measure of cerebellar ataxia [7], significantly improved after immunotherapy and surgical treatment for breast cancer, although anti-neural antibodies were negative within the search range. There are few reports of breast cancer cases with concomitant anti-neural antibody-negative PNS, and this case provides a rationale for considering breast cancer treatment when selecting a treatment strategy for cases of anti-neural antibody-negative PNS.
Case report
A 78-years-old postmenopausal woman with a positive medical history of controlled diabetes mellitus and rheumatoid arthritis presented with a 9-month history of blurred vision, diplopia, and progressive gait disturbance. Two months earlier, a physical examination revealed dysarthria, numbness in the extremities, bilateral poor finger–nose finger test, bilateral poor knee–heel test, trunk ataxia, unstable stance, and open-legged gait. The patient was diagnosed with an SARA score of 22 points.
Physical therapy included joint range of motion training, muscle strengthening training, basic movement training, balance training, and standing gait practice; occupational therapy included fine motor training, upper limb function training, activities of daily living, and activities parallel to daily living training; and articulation training as speech and hearing therapy.
There was no unusual protein fraction in this patient's serum. Contrast-enhanced cerebral magnetic resonance imaging (MRI) and non-contrast-enhanced cervical spine MRI were performed without any specific findings. 123 I-IMP cerebral perfusion single-photon emission computerized tomography revealed cerebellar hypoperfusion and mild hypoperfusion in the left cerebral hemisphere and frontal lobe predominantly (Fig. 1). Subacute cerebellar degeneration owing to PNS was suspected based on subacutely progressive cerebellar ataxia symptoms predominantly in the left hemisphere. A whole-body contrast-enhanced computed tomography scan was performed, which suggested left breast cancer. Upper and lower gastrointestinal endoscopy and pelvic contrast MRI showed no other findings suggestive of primary cancer.
Fig. 1.
a–d 123 I-IMP cerebral perfusion single-photon emission computerized tomography imaging. Cerebellar hypoperfusion and mild hypoperfusion in the left cerebral hemisphere and frontal lobe predominantly are shown
After close examination, the patient was diagnosed with left breast cancer T1cN0M0, estrogen receptor (ER)-positive, progesterone receptor (PR)-positive, and human epidermal growth factor receptor 2 (HER2)-negative. The diagnosis of breast cancer led to a PNS diagnosis. Comprehensive testing for anti-neural antibodies was performed, all of which were negative (Yo, Tr, Ri, Hu, amphiphysin-IgG, CV2, PNMA2, recoverin, SOX1, titin, zic4, GAD65, VGCC, NMADR, LGI1, CASPR2, AMPAR, GABABR, DPPX, and Sez6l2). Based on these findings, the diagnosis of "PNS probable" was made, and according to the ACA diagnostic criteria, the diagnosis of "probable ACA" was made [5]. Shortly after the diagnosis of breast cancer, venoglobulin 20 g/day was administered for 5 days as intravenous immunoglobulin therapy, and the SARA score showed mild improvement, with a score of 19. The patient underwent a left mastectomy and sentinel lymph node biopsy. Postoperative pathology revealed that she had invasive ductal carcinoma, pT2 (40 mm), pN0 (0/1), histological grade 1, ER 100%, PR 90%, HER2 score 0, and Ki67 6.2%. Only letrozole for 5 years was started as postoperative treatment. The patient showed marked improvement in cerebellar ataxia symptoms even after surgery, with an SARA score of 15 at 1 month postoperatively and an SARA score of 7 at 6 months postoperatively (Table 1, Fig. 2). Six months after the surgery, the patient had no recurrence.
Table 1.
SARA score changes over time for each finding
| At diagnosis | Post IVIg | 1 POM | 6 POM | |
|---|---|---|---|---|
| Total | 22 | 19 | 15 | 7 |
| Gait | 6 | 6 | 5 | 2 |
| Stance | 6 | 5 | 4 | 1 |
| Sitting | 4 | 2 | 0 | 0 |
| Speech disturbance | 1 | 1 | 1 | 0 |
| Finger chase | 1 | 1 | 1 | 1 |
| Nose–finger test | 1 | 1 | 1 | 1 |
| Fast alternating hand movements | 1 | 1 | 1 | 1 |
| Heal–shin slide | 2 | 2 | 2 | 1 |
SARA, scale for the assessment and rating of ataxia; IVIg, intravenous immunoglobulin; POM, postoperative month
Fig. 2.
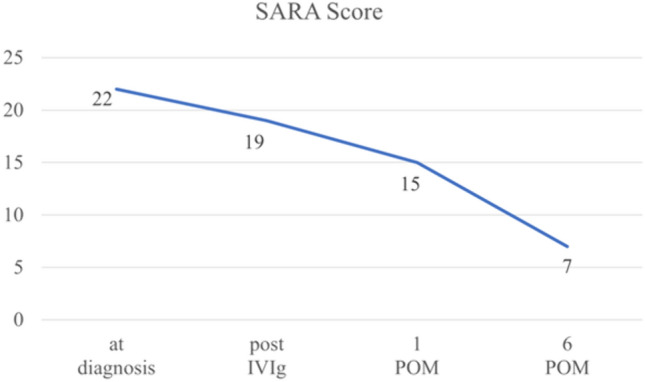
Change over time in total SARA score. Total SARA score gradually improved after IVIg and breast surgery. SARA, scale for the assessment and rating of ataxia; IVIg, intravenous immunoglobulin; POM, postoperative month
Discussion
This study had two important findings. First, there is a possibility that anti-neural antibodies are not identified in the PNS. Second, an appropriate diagnosis and curative treatment of the tumor may improve the neurological symptoms of PNS.
According to a comprehensive single-center analysis [8], PNS occurs in 0.3% of breast cancer cases, and in 57% of cases, as in the present case, neurological symptoms precede the diagnosis of breast cancer. Cerebellar ataxia was the most common symptom in 27% (15/56). Anti-neural antibodies were found in 54% of cases, including anti-Yo antibodies in 33%, anti-amphiphysin-IgG antibodies in 30%, anti-ANNA-2 (or anti-Ri) antibodies in 17%, and AChR, AMPA, VGCC-N, GAD65 in a few cases. Ninety-one percent (51/56) of the patients received some form of immunotherapy such as IVIg in this case. Since this report did not mention whether improvement of neurological symptoms was achieved after breast cancer treatment, we collected case report, resulting in 11 hits in 14 cases [9–19] (Table 2).
Table 2.
Literature review of breast cancer-related PNS
| Reference | Year | Age | Stage | Subtype | IVIg | Onconeural antibody | Operation | Chemotherapy | Improvement of PNS | Indicator of PNS evaluation |
|---|---|---|---|---|---|---|---|---|---|---|
| Sancho, et al. [9] | 2006 | 45 | 2B | ER + , HER2 + | Yes | Yo | Yes | Yes | Yes | None |
| Rupasinghe, et al. [10] | 2007 | 61 | 4 | HER2 + | Yes | Yo | Yes | None | None | None |
| Ogita, et al. [11] | 2008 | 32 | 3C | ER-, HER2 + | Yes | Yo | Yes | Yes | Temporary | None |
| Poudel, et al. [12] | 2013 | 66 | 1A | ER-, HER2- | Yes | Yo | Yes | Yes | Yes | None |
| Adama, et al. [13] | 2015 | 72 | 3C | ER + | Unknown | Yo | Yes | Yes | Yes | None |
| Ng, et al. [14] | 2015 | 58 | 2A | ER-, HER2- | Unknown | Yo negative | Yes | None | Yes | None |
| Ng, et al. [14] | 2015 | 69 | 3C | ER-, HER2 + | Unknown | Unknown | Yes | Yes | Yes | None |
| Kato, et al. [15] | 2017 | 42 | 1 (T1mi) | Unknown | Yes | Yo | Yes | None | Temporary | SARA score etc |
| Martin, et al. [16] | 2017 | 52 | 2A | ER-, HER2 + | Yes | Yo | Yes | Yes | Temporary | None |
| May, et al. [17] | 2018 | 53 | 2B | ER-, HER2 + | Yes | Yo | Yes | Yes | Minimal | None |
| Laguna, et al.[18] | 2018 | 48 | 2A (T0N1) | ER-, HER2 + | Yes | Yo | Yes | None | None | None |
| Laguna, et al.[18] | 2018 | 51 | 1A | ER-, HER2 + | Yes | Yo | Yes | None | Minimal | None |
| Laguna, et al.[18] | 2018 | 48 | 1 (T1a) | ER + , HER2- | Yes | None | Yes | None | Temporary | None |
| Sena, et al. [19] | 2020 | 72 | 2A | ER + , HER2- | Unknown | Ri | Yes | Yes | Minimal | None |
| Present case | 2023 | 78 | 2A | ER + , HER2- | Yes | None | Yes | None | Yes | SARA score |
14 cases of breast cancer related PNS have been found in the literature. Table is sorted by year of publication
PNS, paraneoplastic neurological syndromes; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; SARA, scale for the assessment and rating of ataxia
In 86% (12/14) of cases, neurological symptoms improved after treatment for breast cancer. However, 33% (4/12) relapsed. Among the patients in whom neurological symptoms improved, 83% (10/12) were positive for anti-Yo antibodies. Although there were no obvious positive findings for anti-neural antibodies in a previous study [14], it is unknown what anti-neural antibodies were tested for other than confirming anti-Yo antibody negativity in case 1. To date, only one such case has been reported [18] in which there were no obvious anti-neural antibodies and yet neurological symptoms improved, and even that case did not specify which anti-neural antibody test was performed. No other case has been reported in which all anti-neural antibodies were negative and where the patient's symptoms improved, even after an exhaustive search, as in this case. Some immunologic mechanism may be involved in the development of anti-neural antibodies. However, none of these 14 cases had an autoimmune disease. ER-negative and HER2-positive breast cancer tends to be more frequent. Different immune responses of different breast cancer subtypes [20] may affect PNS.
In this case, the SARA score provided a quantitative assessment of the improvement in cerebellar symptoms. The SARA score has been reported as an objective and quantitative clinical scale for measuring the severity of ataxia and has demonstrated reliability in patients with spinocerebellar degeneration [7]. It may also be useful for assessing the severity of PNS with cerebellar ataxia symptoms. Neurological symptoms in the PNS are often difficult to quantify, and most reports of improvement have been based only on qualitative assessment. The SARA score has been used in a study [15] and reported a score of 13 at diagnosis, worsening to 16 at surgery, and improving to 13 after surgery. This is in contrast with the present case, which improved by a total of 15 points. This interpretation was obtained by quantitative assessments, such as SARA score, suggesting the usefulness of using quantitative indices to evaluate cerebellar ataxia symptoms in PNS.
The first reason for the improvement in neurological symptoms after breast cancer surgery, even in the presence of negative anti-neural antibodies, is the possibility of anti-neural antibodies outside the search range such as AChR in the present case. However, it is difficult to perform a comprehensive search because of the limited number of laboratories available and the cost involved. Furthermore, less-sensitive antibodies, such as anti-Yo antibodies [21], may not have been detected. For other reasons, the possibility of the existence of unknown anti-neural antibodies cannot be completely ruled out. Although negative in this case, many novel anti-neural antibodies have been discovered, including Sez6l2 [22, 23], and the concept of PNS is changing, including the proposal of ASA [5]. Further research on unknown anti-neural antibodies in the PNS and adaptation to new disease concepts are needed.
In the present case, all PNS anti-neural antibodies were negative after a comprehensive search, and there was a clear improvement in neurological symptoms after breast cancer surgery based on the SARA score. The search for all anti-neural antibodies is difficult, and neurological symptoms in the early stage may improve after tumor treatment. Therefore, proper diagnosis and curative treatment of the tumor may improve neurological symptoms, which are very important in PNS, with or without anti-neural antibodies.
Funding
Jps kakenhi, keiko tanaka
Data availability statement
The data supporting the findings of this case report are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
Keiko Tanaka received research funding from JPS KAKENHI. Other authors declare that they have no known competing financial interests, personal relationships, or any other relevant disclosures that could have influenced the work reported in this study.
Informed consent
Witten informed consent was obtained from the patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cai MT, Qiao S, Lai QL, et al. Evaluation of the Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes in China. Front Immunol. 2022;13:790400. doi: 10.3389/fimmu.2022.790400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voltz R. Paraneoplastic neurological syndromes: an update on diagnosis, pathogenesis, and therapy. Lancet Neurol. 2002;1:294–305. doi: 10.1016/s1474-4422(02)00135-7. [DOI] [PubMed] [Google Scholar]
- 3.Gatti G, Simsek S, Kurne A, et al. Paraneoplastic neurological disorders in breast cancer. Breast. 2003;12:203–207. doi: 10.1016/s0960-9776(03)00011-0. [DOI] [PubMed] [Google Scholar]
- 4.Yaguchi H, Kudo A, Yabe I. Invited review article: Autoimmune cerebellar ataxia. Clinical and Experimental Neuroimmunology n/a. 2023 doi: 10.1111/cen3.12752. [DOI] [Google Scholar]
- 5.Dalmau J, Graus F. Autoimmune Encephalitis and Related Disorders of the Nervous System. Cambridge: Cambridge University Press; 2022. [Google Scholar]
- 6.Kudo A, Yaguchi H, Tanaka K, et al. A retrospective study of autoimmune cerebellar ataxia over a 20-year period in a single institution. J Neurol. 2023 doi: 10.1007/s00415-023-11946-1. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz-Hubsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66:1717–1720. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- 8.Murphy BL, Zalewski NL, Degnim AC, et al. Breast cancer-related paraneoplastic neurologic disease. Breast Cancer Res Treat. 2018;167:771–778. doi: 10.1007/s10549-017-4566-0. [DOI] [PubMed] [Google Scholar]
- 9.Sancho MI, Lopez MR, Martinez LC, et al. Subacute cerebellar degeneration as paraneoplastic syndrome: initial symptom of breast cancer with HER2 overexpression. Clin Breast Cancer. 2006;7:79–80. doi: 10.3816/cbc.2006.n.016. [DOI] [PubMed] [Google Scholar]
- 10.Rupasinghe J, Butler E. Progressive ataxic gait disorder. J Clin Neurosci. 2007;14:153–157. doi: 10.1016/j.jocn.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 11.Ogita S, Llaguna OH, Feldman SM, Blum R. Paraneoplastic cerebellar degeneration with anti-Yo antibody in a patient with HER2/neu overexpressing breast cancer: a case report with a current literature review. Breast J. 2008;14:382–384. doi: 10.1111/j.1524-4741.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 12.Poudel CK, Achar KN. Gross cerebellar paraneoplastic neurological disorder in a patient with an occult breast cancer. BMJ Case Rep. 2013 doi: 10.1136/bcr-2012-008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adama D, Moussa B, Emmanuel M, Dennis U. Breast cancer revealed by a paraneoplastic cerebellar syndrome: about one case and literature review. Pan Afr Med J. 2015;22:25. doi: 10.11604/pamj.2015.22.25.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng YR, Ho CD, Ng WL, Tan SM. Paraneoplastic cerebellar degeneration and dermatomyositis as first manifestations of underlying breast malignancy: a report of two cases and a brief review of the subject. Surg Case Rep. 2015;1:59. doi: 10.1186/s40792-015-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato N, Hashida G, Konaka K. Rehabilitation for a patient with anti-Yo antibody-positive paraneoplastic cerebellar degeneration caused by breast cancer: A case report and literature review. Medicine (Baltimore) 2017;96:e8468. doi: 10.1097/MD.0000000000008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin AN, Dillon PM, Jones DE, et al. Anti-Yo Mediated Paraneoplastic Cerebellar Degeneration Associated with Pseudobulbar Affect in a Patient with Breast Cancer. Case Rep Oncol Med. 2017;2017:8120689. doi: 10.1155/2017/8120689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le May M, Dent S. Anti-Yo antibody-mediated paraneoplastic cerebellar degeneration associated with cognitive affective syndrome in a patient with breast cancer: a case report and literature review. Curr Oncol. 2018;25:e585–e591. doi: 10.3747/co.25.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laguna B, Hayward JH, Lee AY, et al. Paraneoplastic neurologic syndrome as a presentation of underlying breast malignancy. Breast J. 2018;24:1038–1042. doi: 10.1111/tbj.13121. [DOI] [PubMed] [Google Scholar]
- 19.Sena G, Gallo G, Vescio G, et al. Anti-Ri-associated paraneoplastic ophthalmoplegia-ataxia syndrome in a woman with breast cancer: a case report and review of the literature. J Med Case Rep. 2020;14:67. doi: 10.1186/s13256-020-02410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onkar SS, Carleton NM, Lucas PC, et al. The Great Immune Escape: Understanding the Divergent Immune Response in Breast Cancer Subtypes. Cancer Discov. 2023;13:23–40. doi: 10.1158/2159-8290.CD-22-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krakenes T, Herdlevaer I, Raspotnig M, et al. CDR2L Is the Major Yo Antibody Target in Paraneoplastic Cerebellar Degeneration. Ann Neurol. 2019;86:316–321. doi: 10.1002/ana.25511. [DOI] [PubMed] [Google Scholar]
- 22.Yaguchi H, Yabe I, Takahashi H, et al. Identification of anti-Sez6l2 antibody in a patient with cerebellar ataxia and retinopathy. J Neurol. 2014;261:224–226. doi: 10.1007/s00415-013-7134-5. [DOI] [PubMed] [Google Scholar]
- 23.Abe M, Yaguchi H, Kudo A, et al. Sez6l2 autoimmunity in a large cohort study. J Neurol Neurosurg Psychiatry. 2023;94:667–668. doi: 10.1136/jnnp-2022-330194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this case report are available from the corresponding author upon reasonable request.



