Abstract
The microbial analysis of fish is critical for ensuring overall health. Uncooked fish can serve as a conduit for transmitting several types of microbes; the current investigation sought to assess the bacterial levels in various kinds of fish from Nasser Lake, Aswan, Egypt, considered the chief source of potable water in Egypt. Two hundred and fifty fish samples, including 50 of each Oreochromis niloticus, Sander lucioperca, Lates niloticus, Clarias gariepinus, and Mormyrus kannume, from Nasser Lake, Aswan, Egypt, were collected to detect the bacterial load, isolation, and identification of Aeromonas hydrophila, Pseudomonas aeruginosa, and Vibrio parahaemolyticus and their virulence genes. The findings revealed that Oreochromis niloticus and Clarias gariepinus exhibited higher bacterial loads than other fish species. Incidences of bacterial contamination among examined fishes were 28.8%, 20.4%, and 16% for Aeromonas hydrophila, Pseudomonas aeruginosa, and Vibrio parahaemolyticus, respectively. Additionally, PCR analysis detected the presence of aerA (60%) and Act (40%) genes in A. hydrophila, rpoB (70%) and LasB (30%) genes in P. aeruginosa, and ToxR (70%) and tdh (50%) genes in V. parahaemolyticus. The study suggested that the bacterial contamination levels in Oreochromis niloticus and Clarias gariepinus could be notably more significant than in other species that could potentially be harmful to the consumers, especially considering the identification of particular bacteria known to cause foodborne illnesses. Further recommendations emphasized that regular monitoring and assessments are required to preserve their quality.
1. Introduction
Fish is an excellent source of many essential nutrients for human health, including high-quality protein, a plentiful supply of omega-3 fatty acids, vitamins, necessary amino acids, phosphates, and calcium [1]. Egypt currently generates over 73.8% of all fish raised in Africa and ranks ninth globally, producing 1.54% [2]. Despite the advantages, contamination is still possible due to bacteria or other physical, chemical, or biological contaminants. Bacterial contamination is the primary cause of fish food contamination among the dangers [3]. Microbial growth and residence in food are the primary factors contributing to food spoilage, producing undesirable metabolites that impart unpleasant flavours and aromas. This environment produces undesirable metabolites, which impart unpleasant flavours and aromas to the food [4]. Foodborne diseases are recognized to occur regularly and are related to low-income countries, probably due to improper food handling and hygiene, lack of food safety laws and weak implementation systems, lack of economic assets to procure safety tools, and lack of education and training for different food handlers [5]. Foodborne pathogens are those microorganisms which cause human diseases via virulence machinery, even sometimes at their low infectious dose [6]. Fish is contaminated with pathogenic and spoilage bacteria at any production and supply chain stage [7].
A variety of pathogenic bacterial species, including E. coli, Salmonella spp., Staphylococcus aureus, Aeromonas spp., Pseudomonas spp., and Vibrio spp., are responsible for fish foodborne illnesses [8]. Salmonella spp. are a significant public health concern since they are the most commonly documented cause of occasional cases and outbreaks of gastroenteritis globally [9]. Within the same framework, E. coli is a paramount bacterial contaminant, frequently employed as a surveillant for fish deterioration and contamination [10]. Moreover, among food poisonings, the highlights are those caused by S. aureus, which is responsible for food poisoning outbreaks worldwide [11]. Psychrotolerant Pseudomonas aeruginosa is the most commonly isolated bacterium from spoilt seafood; it causes unpleasant smells and odours in addition to slime, which degrades the product's quality. Many virulence factors, some of which are related to the bacterial cell surface and include lipopolysaccharide, flagella, type IV pili, type III secretion system, exotoxin A, proteases, and alginate, are linked to P. aeruginosa's pathogenicity. These factors also aid in transforming active proteins and the adherence and colonisation of bacteria within a host cell [12].
Furthermore, Aeromonas hydrophila, widely distributed in aquatic environments, can not only cause diseases in fish and humans directly but also increase histamine levels, posing a chemical hazard to human health [13]. The pathogenicity of A. hydrophila has been related to numerous putative virulence agents, such as aerolysin (aerA) and cytotoxic enterotoxin (Act) [14]. In addition, Vibrio parahaemolyticus is the most researched Vibrio spp., and it is known to be harmful to people, causing gastroenteritis and diarrhoea in people worldwide [15]. Certain virulence factors, including tdh and toxR, are present in V. parahaemolyticus strains and are primarily associated with hemolysis and cytotoxicity in the host cell [16]. The microbiological contamination of fish and fishery products is associated with aquatic environments and the sanitary conditions from the farm to the table, including cultivation, harvest, processing, storage, and transportation. Thus, ensuring food safety is the responsibility of the authorities and those who produce and market the processing product [17].
Therefore, it was crucial to monitor the bacterial load and its pattern of occurrence in fish to offer relevant information on the risk profile of fish for public health. Furthermore, the traditional method could be more efficient and time-consuming, with only one test possible. Consequently, the conventional culture technique has proven inadequate in identifying the proliferating multitude of bacterial species. Polymerase chain reaction (PCR) is one of the most common molecular techniques, and it is widely used to detect fish pathogenic bacteria [5] rapidly. Nevertheless, despite numerous studies investigating various fish species sourced from aquacultures in Egypt, there needs to be more information concerning the safety and quality of freshwater fish from sources such as Nasser Lake, a critical source for Egypt's national fisheries. Oreochromis niloticus (Nile tilapia), Lates niloticus (Nile perch), Sander lucioperca (pike perch), Clarias gariepinus (African catfish), and Mormyrus kannume (elephant snout) are the most commonly captured in South Egypt's Nasser Lake [18] and one of the most extensively consumed fish species in Egypt [16, 19]. Therefore, this work assesses fish safety and bacterial quality at Nasser Lake, Egypt.
2. Material and Methods
2.1. The Research Site
The latitudes of Nasser Lake are 22°00′ to 23°58′N, while the longitudes are 31°19′ to 33°19′E [20]. It covers approximately 5248 km2 and has numerous side expansions (khors). The obtained fish were from two khors situated on the northern-western and southern-eastern shores of Nasser Lake (El-Ramla Khor and Khor Abu Simbel, respectively) during March and April 2023 (Figure 1).
Figure 1.
Map of Nasser Lake showing the position of Khor Ramla and Khor Abu Simbel.
2.2. Sample Collection and Preparation
Two hundred and fifty freshly caught fish samples (each weight ~200 g and ~30 cm length) include 50 of each Oreochromis niloticus (Nile Tilapia), Sander lucioperca (pike perch), Lates niloticus (Nile perch), Clarias gariepinus (African sharptooth catfish), and Mormyrus kannume (elephant snout) were collected from Nasser Lake in Aswan, Egypt, during March to April 2023. The samples were placed in sterile bags and refrigerated in an ice box before being sent to the laboratory. A sterile stainless-steel knife removes the scales, head, fins, tails, and bones after the fish has been eviscerated, and the two back fillets were kept in the refrigerator at 4°C for further examination. Aseptically, 25 g of flesh samples were put into stomacher bags with 225 ml of peptone water and then blended at 200 rpm for 2 min (Seward™ Stomacher™ Model 400 Circulator Lab Blender, 110 V). Homogenates were serially diluted in peptone water at 0.1% (nonselective preenrichment) at 37°C for 24 hr. One millimeter from each previously prepared serial dilution was inoculated separately into three appropriately marked triplicated Petri dishes for the following analysis:
Total aerobic count (TAC): ascertained by applying the pour plating technique with plate count agar (M091A, HiMedia) and incubating the plates for 48 hr at 35°C. The result is expressed as log cfu/g [21]
E. coli count: one millimeter of each serial dilution was spread-plated on Eosin Methylene Blue (EMB) agar (HiMedia MM022) using a conventional plate count method. Plates were incubated for 48 hr at 37°C. The number of metallic sheen colonies on plates ranging from 30 to 300 was determined utilising a digital colony counter (DC-8 OSK 100086, Kayagaki, Japan), and the result was expressed as log cfu/g [22]
Staphylococcus aureus count: one millimeter of each serial dilution was incubated at 35°C for 48 hr on Baird Parker Agar Base (Oxoid CM 0275) with egg yolk tellurite emulsion. After incubation, black, glossy colonies with translucent zones were identified and counted as S. aureus, and the finding was expressed as log cfu/g [23]
Salmonella count: 0.1 ml of nonselective preenrichment was placed into 10 ml of Rappaport-Vassiliadis broth (CM0866, Oxoid) and incubated at 42°C for 24 hr. A loop of selective enrichment broth was scattered onto Xylose Lysine Deoxycholate (XLD, CM0469, Oxoid) agar and incubated at 37°C for 24 hr using a digital colony counter. Suspected Salmonella colonies that showed up as red colour with black cores on XLD agar were counted and expressed as log cfu/g [24]
Aeromonas hydrophila isolation and identification: 1 ml of the homogenate was incubated for 24 hr at 28°C in 9 ml of brain heart infusion broth (BHI). Streaked Aeromonas isolation medium base agar was fortified with ampicillin (Biolife, CN0801) with one loop of enrichment broth, and the mixture was then aerobically incubated for 18 to 24 hr at 37°C. Green colonies with dark centers suggest A. hydrophila [25]. Preliminary screening and identification were conducted using gram staining, oxidase, and catalase tests. Gram-negative, oxidase, and catalase-positive isolates were preserved on a blood agar slant, and further biochemical characterizations such as hydrogen sulphide, indole test, urease test, Voges-Proskauer test, and sugars (glucose, inositol, and mannitol) were performed [26]
Pseudomonas aeruginosa isolation and identification: 1 ml of the previous dilution was dispersed with Pseudomonas agar base medium (M085, HiMedia) improved with glycerol, and the regionalized colonies (greenish-yellow colonies) were produced after 48 hr of incubation at 25°C [27]. The purified P. aeruginosa colonies were identified biochemically [28]
Vibrio parahaemolyticus isolation and identification:a loopful of nonselective preenrichment was streaked onto thiosulfate-citrate-bile salt-sucrose agar (GM189, HiMedia), and the mixture was incubated for 24 hr at 37°C. The presumed V. parahaemolyticus (smooth green colonies) was collected, purified, and biochemically identified [29]
Determination of some food poisoning virulence genes: 10 randomly obtained pure isolates of A. hydrophila, P. aeruginosa, and V. parahaemolyticus were incubated in nutrient broth for 24 hr at 37°C. Predefined act and aerA genes in A. hydrophila, rpoB and LasB genes in P. aeruginosa, and tdh and ToxR genes in V. parahaemolyticus were targeted using the sequences of the primers (Table 1) synthesised by Willowfort Company (United Kingdom) with the thermocycling program shown in Table 2, and according to its manufacture instructions, the extraction of genomic DNA from bacterial isolates using the GeneJET Genomic DNA Purification Kit (Catalog no. K0721, Thermo Scientific, USA) and COSMO PCRRED Master Mix (Code no. W1020300X, Willowfort, United Kingdom) was resolved on 1.5% agarose gel electrophoresis. Animal Health Research Institute, Dokki, Giza, Egypt, provided positive control for each target gene
Table 1.
The specific sequences of oligonucleotide primers for PCR.
| Bacteria | Gene | Primer sequence (5′-3′) | bp | References |
|---|---|---|---|---|
| P. aeruginosa | rpoB | CAGTTCATGGACCAGAACAACCCG | 759 | [30] |
| ACGCTGGTTGATGCAGGTGTTC | ||||
| LasB | GGAATGAACGAG GCGTTCTC | 300 | ||
| GGTCCAGTAGTAGCGGTTGG | ||||
|
| ||||
| V. parahaemolyticus | tdh | GTAAAGGTCTCTGACTTTTGGAC | 251 | [31] |
| TGGAATAGAACCTTCATCTTCACC | ||||
| ToxR | GTCTTCTGACGCAATCGTTG | 366 | ||
| ATACGAGTGGTTGCTGTCATG | ||||
|
| ||||
| A. hydrophila | aerA | AACCGAACTCTCCAT | 301 | [32] |
| TTGTCCGGGTTGTACTCGTC | ||||
| act | GAGAAGGTGACCACCAAGAAC | 232 | ||
| AACTGACATCGGCCTTGAACTC | ||||
Table 2.
Thermocycling program for gene primers.
| Bacteria | Target gene | Primary denaturation | Secondary denaturation | Annealing | Extension | No. of cycles | Final extension |
|---|---|---|---|---|---|---|---|
| P. aeruginosa | rpoB | 94°C 3 min |
94°C 60sec |
58°C 60sec. |
72°C 2 min |
30 | 72°C 2 min |
| LasB | 94°C 5 min |
94°C 60 sec |
60 °C 1 min |
72°C 1 min |
35 | 72°C 7 min |
|
|
| |||||||
| V. parahaemolyticus | Tdh | 94°C 5 min |
94°C 1 min |
55°C 1 min |
72°C 1 min |
30 | 72°C 7 min |
| ToxR | 94°C 10 min |
94°C 1 min |
63°C 1.5 min |
72°C 1.5 min |
20 | 72°C 10 min |
|
|
| |||||||
| A. hydrophila | aerA | 94°C 5 min |
94°C 30 sec |
54°C 30 sec |
72°C 1 min |
30 | 72°C 10 min |
| Act | |||||||
2.3. Statistical Analysis
Performed statistical analysis to ascertain the prevalence of bacterial contamination in various fish species. Descriptive statistics were primarily used, with percentages calculated to represent the proportion of fish samples contaminated by each species of bacteria. ANOVA was evaluated to analyze all the findings statistically, and a p ≤ 0.5 was considered significant.
3. Results
Concerning the bacterial load in the examined samples, Table 3 reveals that the mean of the total aerobic count (log cfu/g) of the analyzed fish was the highest in M. kannume (5.73 ± 0.24) and O. niloticus (5.62 ± 0.39) followed by C. gariepinus (5.08 ± 0.36), while L. niloticus (4.32 ± 0.3) and S. lucioperca (4.23 ± 1 × 0.3) recorded the lowest value. Furthermore, all samples were accepted according to the Egyptian National Food Safety Authority (NFSA) [33] as TAC < 106 cfu/g for fresh fish. Furthermore, the mean value of E. coli count (log cfu/g) was 4.48 ± 0.15, 4.65 ± 0.23, 5.85 ± 0.23, 6.11 ± 0.37, and 3.75 ± 0.14 for O. niloticus, L. niloticus, S. lucioperca, C. gariepinus, and M. kannume, respectively (Table 4). Furthermore, all samples did not match NFSA regulations, and there is a significant difference between the examined samples at p ≤ 0.05. On the other hand, Table 5 shows that the mean of the S. aureus count was 5.94 ± 0.42, 4.1 ± 0.23, 3.20 ± 0.32, 5.04 ± 0.39, and 3.03 ± 0.21 log cfu/g for O. niloticus, L. niloticus, S. lucioperca, C. gariepinus, and M. kannume. According to the NFSA, the accepted count of S. aureus was <104. As a result, all tested samples were approved for L. niloticus, S. lucioperca, and M. kannume. At the same time, only 54% of O. Niloticus and 60% of C. gariepinus were accepted, according to the NFSA. Additionally, the findings shown in Table 6 show that the mean count of Salmonella spp., C. gariepinus, and M. kannume recorded the highest value with a mean of 6.42 ± 0.36 and 5.81 ± 0.22 log cfu/g, followed by L. niloticus and O. niloticus with a mean of 5.48 ± 0.39 and 5.32 ± 0.48 log cfu/g, while S. lucioperca reported a mean value of 5.08 ± 0.25 log cfu/g. The samples contaminated with Salmonella did not match the regulations established by the NFSA. The variances between the specimens were significant at p ≤ 0.05.
Table 3.
Total aerobic count (log cfu/g) of analyzed fish (n = 50 each).
| Sample | Minimum | Maximum | Mean ± SE | Accepted samples | ||
|---|---|---|---|---|---|---|
| NFSA∗ | No. | % | ||||
| O. niloticus | 5.39 | 5.72 | 5.62 ± 0.39a | <106 cfu/g | 100 | 100 |
| L. niloticus | 4.83 | 5.61 | 4.32 ± 0.3c | 100 | 100 | |
| S. lucioperca | 3.62 | 5.70 | 4.23 ± 1 × 0.33c | 100 | 100 | |
| C. gariepinus | 3.67 | 5.26 | 5.08 ± 0.36b | 100 | 100 | |
| M. kannume | 4.92 | 6.08 | 5.73 ± 0.24a | 100 | 100 | |
∗Egyptian National Food Safety Authority [33] for fresh fish. p ≤ 0.04210 is considered a significant difference. The mean values with the same letters in each column do not show a significant difference.
Table 4.
E. coli count (log cfu/g) among the analyzed samples (n = 50 each).
| Sample | Minimum | Maximum | Mean ± SE | Accepted samples | ||
|---|---|---|---|---|---|---|
| NFSA∗ | No. | % | ||||
| O. niloticus | 3.30 | 5.58 | 4.48 ± 0.15c | <4 × 10 cfu/g | 0 | 0 |
| L. niloticus | 2.61 | 5.78 | 4.65 ± 0.23c | 0 | 0 | |
| S. lucioperca | 2.54 | 6.18 | 5.85 ± 0.23b | 0 | 0 | |
| C. gariepinus | 5.61 | 6.30 | 6.11 ± 0.37a | 0 | 0 | |
| M. kannume | 2.49 | 5.04 | 3.75 ± 0.14d | 0 | 0 | |
∗Egyptian National Food Safety Authority [33] for fresh fish. A difference at p ≤ 0.05 was regarded as significant. Same-letter mean values in each column do not significantly differ from one another.
Table 5.
S. aureus count (log cfu/g) among examined samples (n = 50 each).
| Sample | Minimum | Maximum | Mean ± SE | Accepted samples | ||
|---|---|---|---|---|---|---|
| NFSA∗ | No. | % | ||||
| O. niloticus | 2.68 | 6.46 | 5.94 ± 0.42a | <104 cfu/g | 27 | 54 |
| L. niloticus | 2. 48 | 4.56 | 4.1 ± 0.23b | 0 | 0 | |
| S. lucioperca | 2.11 | 3.76 | 3.20 ± 0.32c | 0 | 0 | |
| C. gariepinus | 2.23 | 5.52 | 5.04 ± 0.39a | 30 | 60 | |
| M. kannume | 2.34 | 3.45 | 3.03 ± 0.21c | 0 | 0 | |
∗Egyptian National Food Safety Authority [33] for fresh fish. The investigated samples differ significantly at p ≤ 0.05. No discernible difference exists between values with identical letters in any column.
Table 6.
Salmonella count (log cfu/g) among examined samples (n = 50 each).
| Sample | Minimum | Maximum | Mean ± SE | Accepted samples | ||
|---|---|---|---|---|---|---|
| NFSA∗ | No. | % | ||||
| O. niloticus | 4.98 | 5.48 | 5.32 ± 0.48b | Free | 0 | 0 |
| L. niloticus | 5.20 | 5.58 | 5.48 ± 0.39b | 0 | 0 | |
| S. lucioperca | 5.70 | 6.41 | 5.08 ± 0.25b | 0 | 0 | |
| C. gariepinus | 5.56 | 6.30 | 6.42 ± 0.36a | 0 | 0 | |
| M. kannume | 5.52 | 6.00 | 5.81 ± 0.22a | 0 | 0 | |
∗Egyptian National Food Safety Authority [33] for fresh fish. Defined a significant difference at p ≤ 0.05. No discernible difference exists between values with identical letters in any column.
Furthermore, Figure 2 shows the incidence of some bacterial contamination among the examined fish samples. 28.8% of the analyzed fish samples had A. hydrophila distributed as 46%, 24%, 20%, 34%, and 20% in O. niloticus, L. niloticus, S. lucioperca, C. gariepinus, and M. kannume, respectively. Moreover, 20.4% of the inspected fish had P. aeruginosa, with distribution rates of 38%, 14%, 10%, 24%, and 16% in O. niloticus, L. niloticus, S. lucioperca, C. gariepinus, and M. kannume, respectively. V. parahaemolyticus was recognized in 16% of examined fishes, with incidences of 28%, 14%, 10%, 16%, and 12% in O. niloticus, L. niloticus, S. lucioperca, C. gariepinus, and M. kannume, respectively. Differential biochemical tests confirmed that all positive isolates at the species level were A. hydrophila (Table 7). Moreover, the results of the occurrence of different virulence genes in the studied bacteria were confirmed by PCR. Figure 3 shows the PCR electrophoresis of A. hydrophila virulence genes (aerA and Act), identified at 60% and 40%, respectively. Figure 4 also displays the PCR electrophoresis of P. aeruginosa virulence genes, with incidence rates of the rpoB and lasB genes at 70% and 30%, respectively. Besides, 70% and 50% of the examined isolates had the toxR and tdh genes of V. parahaemolyticus (Figure 5).
Figure 2.
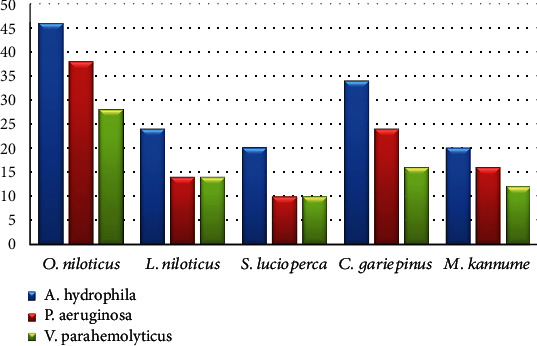
Occurrence of some bacterial contamination in the examined fish.
Table 7.
Biochemical properties of isolated bacteria.
| Characters | A. hydrophila | P. aeruginosa | V. parahaemolyticus |
|---|---|---|---|
| Gram stain | Negative | Negative | Negative |
| Shape | Rods | Rods | Rods |
| Motility | Motile | Motile | Motile |
| Catalase | + | + | + |
| Oxidase | + | + | + |
| H2S | + | − | − |
| Glucose fermentation | + | − | + |
| Growth in vibriostatic agent 0/129 at 10 μg | + | − | − |
| Indole | + | − | + |
| Urease | + | − | + |
| Voges-Proskauer | + | − | − |
Figure 3.
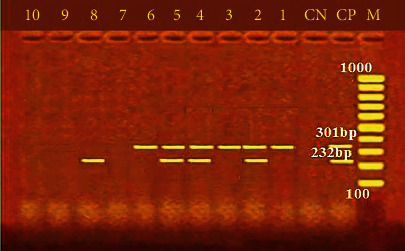
PCR electrophoresis of A. hydrophila virulence genes. M: 100 bp ladder; CP: positive control for aerA at 301 bp and Act at 232 bp; CN: negative control; lane (1, 2, 3, 4, 5, and 6): positive for aerA at 301 bp; lane (2, 4, 5, and 8): positive for Act at 232 bp.
Figure 4.
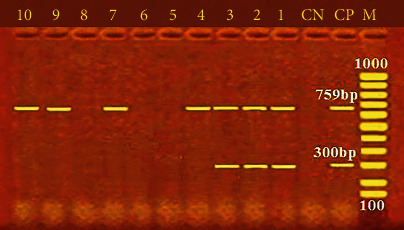
PCR electrophoresis of P. aeruginosa virulence genes. M: 100 bp ladder; CP: positive control for rpoB at 759 bp and LasB at 300 bp; CN: negative control; lane (1, 2, 3, 4, 7, 9, and 10): positive for the rpoB at 759 bp; lane (1, 2, and 3): positive for the LasB at 300 bp.
Figure 5.
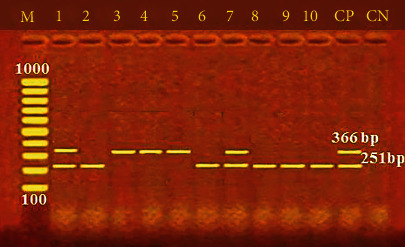
PCR electrophoresis of V. parahaemolyticus virulence genes. M: 100 bp ladder; CP: positive control for the ToxR at 366 bp and the tdh at 251 bp; CN: negative control; lane (1, 3, 4, 5, and 7): positive for the ToxR at 366 bp; lane (1, 2, 6, 7, 8, 9, and 10): positive for tdh at 251 bp.
4. Discussion
The microbiological status of fish meat is an essential marker of the sanitary conditions relevant to the environment for fish breeding, handling, and storing, as fish is a safe diet and healthy fish muscles are considered sterile [13]. The total bacterial count, which represents a significant guide for evaluating the level of meat cleanliness and hygienic value, plays an integral role in this process. Consequently, the information obtainable in Table 3 demonstrated the variations in the total aerobic count (log cfu/g) observed in the inspected fish. There were significant variations across the studied samples (p ≤ 0.05). TAC was the highest in M. kannume and O. niloticus, followed by C. gariepinus, while L. niloticus and S. lucioperca recorded the lowest value. According to NFSA [33], the allowable count of TBC was <106; therefore, the analysis of all samples was concluded to be acceptable. The microbial quality of fish significantly impacts food safety. Fish is an exceptionally perishable food item that must be preserved and handled carefully. Three broad categories can be used to categorise pathogenic bacteria and spoilage linked to fish: native bacteria found in fish's natural commensal microbiota, exogenous enteric bacteria from nearby contaminated water, and bacterial contamination during processing, storage, or food preparation.
Microbial deterioration is considered a primary factor in the change in fish muscle's acceptability as a raw material for the food industry. Pathogenic and spoilage microorganisms can be introduced into fish and fish products throughout the production and supply chain [13]. The variance in the initial bacterial load of the examined fish samples can be attributed to the microbial load of the water in which they reside and the proliferation of bacteria that thrive under storing conditions [17]. Fishes are affected by the bacterial load and the bacterial species that are present in the ecosystem; also, Ali et al. [34] showed that fishes collected from polluted sites (drainage) recorded higher bacterial loads (in different fish organs) than fishes collected from nonpolluted sites. The results supported Amuneke et al. [35] and Khairy et al. [36]. On the other hand, the current research findings were higher than those provided by Mamdouh et al. [37], who reported 4.83 ± 0.43 log cfu/g in O. niloticus and lower than the finding of Ali [38], who reported TAC of 6.36 to 7.89 log cfu/g for fishes in Abu Simbel Khor.
Among the range of pathogenic bacterial species that cause fish foodborne diseases is E. coli. The pathogenic strains of E. coli cause diarrhoea by producing and releasing toxins and can also cause food spoilage in fish [39]. The findings (Table 4) indicate the mean count of E. coli (log cfu/g); a statistically significant association was found (p ≤ 0.05) between the various sample types and the occurrence of E. coli. Detecting E. coli in some fishes might represent postharvest cross-contamination [40]. The permissible E. coli count, as specified by the NFSA [33], is < 4 log cfu/g, which has been given as the quality criteria for this organism. As a result, none of the fish samples evaluated in the present investigation met the required standard, suggesting that improper handling and improper hygiene might lead to the contamination of ready-to-eat foods, eventually affecting the consumers' health. The occurrence of this bacterium in food is directly related to faecal contamination. This bacterium is the most abundant facultative anaerobe of the human intestinal microflora. Furthermore, E. coli is broadly present in the intestinal tracts of warm-blooded animals, and E. coli in ready-to-eat foods is undesirable because it suggests poor hygienic conditions that lead to contamination [39]. The results obtained in the present study were consistent with the values indicated by Yohans et al. [39], which ranged from 4.45 to 5.89 log cfu/g in the investigated samples. The E. coli load in raw fish samples was higher than that observed by Dhanapal et al. [41], who found 4.67 log cfu/g, but lower than that identified by Wendwesen et al. [42], who found 4.63 log cfu/g in Nile tilapia fillet samples. This potential disagreement might arise from the difference in the sample size used, the ecosystem of the study area, or the sampling methods.
Staphylococcus aureus is an opportunistic common pathogen associated with colonising humans' skin and musical surfaces. S. aureus may grow in food using temperature and time, producing heat-stable intoxication and cooking-resistant enterotoxins [17]. S. aureus is not a normal microbiota of fish; therefore, its presence in fish indicates cross-contamination originating from the harvest area or improper handling by fish handlers [43]. The mean of S. aureus count ranges from 5.94 ± 0.42 log cfu/g in O. niloticus to 3.03 ± 0.21 log cfu/g in M. kannume (Table 5). The variances between the specimens were significant at p ≤ 0.05. According to the NFSA [33], the accepted count of S. aureus was <104. As a result, all tested samples were approved for L. niloticus, S. lucioperca, and M. kannume. At the same time, only 54% of O. niloticus and 60% of C. gariepinus were accepted, according to the NFSA [33]. This result shows that it is essential to implement effective regulations and good hygiene practices in the fish industry to ensure food hygiene. The outcomes matched those noted by Danba et al. [44] and Budiati et al. [45] but were lower than the findings of Mamdouh et al. [37]. S. aureus constitutes a substantial public health concern due to heat-stable staphylococcal enterotoxins (SEs) during its growth. Even if food is heated before consumption, the toxins can still cause illness even after the bacterial cells are destroyed by heat [46]. S. aureus is a product of contamination of the aquatic environment where they come from and poor production and handling conditions in hygiene [17]. The difference in S. aureus count and incidence rates found in this study among examined samples and other studies may result from differences in fish handlers' processing and hygienic practices.
Salmonella spp. is not part of the healthy fish microbiota, and its presence indicates faecal contamination either from polluted water or cross-contamination during the production chain (unhygienic fish handling, fish processing, or marketing) [47]. Salmonella spp. is the primary source of intestinal infections in animals and humans, and these bacteria are responsible for millions of illnesses globally. Nontyphoidal Salmonella spp. is mainly associated with foodborne diseases, making it a significant zoonotic agent [48]. Salmonella can be found in various sources, including soil, water, animal faeces, insects, equipment surfaces, and food processing plants [49]. The findings shown in Table 6 show the mean count of Salmonella spp. The observed distinctions among the samples were statistically significant at p ≤ 0.05. The samples contaminated with Salmonella did not comply with the regulatory thresholds established by the NFSA [33]. The recovery rate in the current study result was higher, and this could be due to contamination of a water source, the absence of hygienic practices, and strict follow-up of this sector by the concerned authorities. These findings are lower than those of Tanyag et al. [50]. Freshwater fish can act as passive carriers of Salmonella, excreting it without showing any clinical symptoms. The results agreed with Yohans et al. [39] and Kagambèga et al. [51]. The occurrence of Salmonella in this investigation is comparatively higher than that recorded in other studies [52–54]. The differences in prevalence rates can be attributed to sample size and sample types. Salmonella spp. are found in animal or human reservoirs, and their presence in raw fish in this study suggests poor hygienic practices during production, handling, processing, and marketing, which could be a result of direct or indirect faecal contamination, posing a risk to people consuming raw or undercooked contaminated fish.
Aeromonas hydrophila is regarded as one of the primary significant foodborne zoonotic pathogens. They are the natural inhabitants of different aquatic environments, such as freshwater, brackish, and marine water. It causes severe health effects in people, such as meningitis, septic arthritis, diarrhoea (traveller's diarrhoea), fulminating septicemia, and gastroenteritis [54]. In this study, 28.8% of the analyzed fish samples had A. hydrophila, O. niloticus reported the highest percentage (46%), while S. lucioperca and M. kannume had the lowest percentage (20%) (Figure 2). Differential biochemical tests confirmed that all isolates at the species level were A. hydrophila (Table 7). A statistically significant difference was found among fish samples and the occurrence of A. hydrophila (p < 0.05). Fish can be contaminated with pathogenic bacteria by polluted water or by handling, processing, and unhygienic storage conditions [55]. Almost identical to the outcome (43.8%) was recorded by Wamala et al. [56] and Kishk et al. [57]. Moreover, a high finding was reported by Yohans et al. [39], who recorded that 76.6% to 80% of examined samples had A. hydrophila. In addition, Morshdy et al. [54] and Dhanapala et al. [41] observed A. hydrophila with 14% and 9.3% from freshwater fish.
The pathogenicity of A. hydrophila has been related to numerous putative virulence agents, such as aerolysin (aerA) and cytotoxic enterotoxin (Act) [14]. On the other hand, A. hydrophila virulence-associated genes (aerA and Act) were found in 60% and 40% of the inspected A. hydrophila isolates (Figure 3). These results matched Emeish et al. [58] and Elbarbary et al. [59], who recorded 64.3% and 60% of the inspected A. hydrophila isolates. The results were lower than those of Ahangarzadeh et al. [60], who detected aerA and act genes in 51.61% and 74.19%, respectively. Morshdy et al. [54] noticed aerA in 75% of the examined A. hydrophila isolates. Previous research has demonstrated that hemolysin and aerolysin donate to A. hydrophila pathogenicity in fish and humans, which allows the bacteria to colonise, replicate, and damage the host tissues [61]. Determining virulence determinants is the key to identifying the pathogenic potentials due to these virulence determinants' multifunctional and multifactorial roles in A. hydrophila pathogenicity [62]. Previous data indicate that A. hydrophila has modest changes in incidence rates, and according to Hafez et al. [63], the different species, sample location and time, geographic region, postcapture contamination, fish species, types of water, handling, and manipulations during fish handling, storage, and transportation can all be factors in the changes of Aeromonas species incidence.
Pseudomonas aeruginosa is a rod-shaped, gram-negative bacterium with a high pathogenic potential. It is classified as a member of the γ-proteobacteria and is abundant in various environments, including water, plants, soil, and animals [38]. Psychotolerant Pseudomonas species are the most commonly isolated bacterium from spoilt seafood; they cause unpleasant smells, odours, and slime, which degrade the product's quality [64]. P. aeruginosa infection, encompassing digestive tract infections, is prevalent in underdeveloped countries at a rate of 17% and in Europe at 11.5%. Enterotoxigenic P. aeruginosa-contaminated fish induces diarrhoea, gastrointestinal disorders, and skin infections, particularly in immunocompromised patients [65]. In the current study, 51% of the inspected fish had P. aeruginosa, and its incidence ranged from 10% in S. lucioperca to 38% in O. niloticus (Figure 2). Differential biochemical assays verified the identity of each isolate as P. aeruginosa at the species level (Table 7). Additionally, a significant difference was found among fish samples and the incidence of P. aeruginosa (p < 0.05). These recordings corresponded with the data from Abd El-Maogoud et al. [66], who noted similar frequencies (47.3%). A previous study by Mamdouh et al. et al. [39] confirmed higher occurrences (65.0%) of P. aeruginosa in the examined samples, while Ali et al. [38], Elbarbary et al. [59], and Abd El-Maogoud et al. [66] recorded lower incidences of 13.8%, 5.0%, and 29%. Pseudomonas is a part of the usual fish microflora and can be opportunistic and developed into virulent and disseminated in distressed fish [67]. The variation in the results between different species may be due to the difference in hygiene measures applied during catching, handling, freezing, storage, and processing [68].
By producing a wide range of virulence factors, P. aeruginosa can adjust to the unfavourable environment of its hosts and enhance the likelihood of illness and infection [69]. Many virulence issues, some of which are related to the bacterial cell surface and comprise lipopolysaccharide, flagella, type IV pili, type III secretion system, exotoxin A, proteases, and alginate, are linked to P. aeruginosa's pathogenicity. These factors also aid in transforming active proteins and the adherence and colonisation of bacteria within a host cell [38]. The incidence of virulence genes of P. aeruginosa mainly identified in this study was 70% and 30% for rpoB and lasB, respectively (Figure 4). This finding validates the variability of P. aeruginosa reported by numerous publications [64, 65] and emphasises the identification method's great discriminating power based on the rpoB gene. Additionally, it was shown that P. aeruginosa had unique virulence genes, such as lasB, and this could be justified by the fact that P. aeruginosa secretes elastase (LasB), a metalloproteinase involved in host colonisation and tissue damage [30]. The findings of this study revealed lower incidence rates of rpoB and lasB genes in P. aeruginosa isolates compared to the analysis shown by Benie et al. [64], who detected rpoB and lasB I with incidences of 91.1% and 89.2%, respectively. Meanwhile, the current results were higher than those reported by Elbarbary et al. [59] and Shahrokhi et al. [70], who confirmed that the lasB gene (50%) had been the primary detected virulence gene in inspected P. aeruginosa isolates. These results indicate that some virulence factors assist bacterial establishment and colonisation on the surface of the host, while others expedite the invasion of numerous tissues. P. aeruginosa can invade tissue. P. aeruginosa also produces toxins and enzymes that disrupt carnal barriers by disrupting cell membranes despite the host's immune system [30].
Vibrio spp., prevalent in numerous types of seafood, are microbial foodborne pathogens of water sources that increase human vulnerability to hazards to public health [71]. V. parahaemolyticus stands out for its prevalence among pathogens carried by food of marine origin [72] and is recognized in 16% of inspected fishes, with incidences ranging from 28% in O. niloticus to 10% in S. lucioperca (Figure 2). Differential biochemical analyses identified all isolates as V. parahaemolyticus at the species level (Table 7). A statistically significant difference was found between fish samples and the incidence of V. parahaemolyticus (p < 0.05), suggesting the possibility of transmission of V. parahaemolyticus through the consumption of fish. Similar outcomes obtained by Ahmed et al. [73] recorded 15.1%. High outcomes were achieved by Asran et al. [31] and Morshdy et al. [74], who identified V. parahaemolyticus in 50% and 42.3% of inspected fish; meanwhile, Elbarbary et al. [59], Suresh et al. [75], and Yen et al. [76] reported low percentages of 2.3%, 3.89%, and 7.5%. A statistically significant difference was found between fish samples and the incidence of V. parahaemolyticus (p < 0.05).
Cross-contamination, improper handling, a lack of cleanliness, and temperature fluctuations during keeping may explain the low variation in Vibrio isolation incidences [77]. PCR-based assays targeting the highly conserved toxR and tdh genes in V. parahaemolyticus have become a prevalent molecular procedure for discovering and identifying V. parahaemolyticus in seafood samples [78]. The toxR gene, linked to the cytotoxic activity and hemolysis of V. parahaemolyticus in the host cell, confirms the pathogenicity of V. parahaemolyticus connected with seafood [73]. In the current study, 70% and 50% of the examined bacteria had toxR and tdh genes, respectively (Figure 5). A high rate (100%) of V. parahaemolyticus isolates harboured the toxR gene by Morshdy et al. [74], Yen et al. [76], and Shahrokhi et al. [70], while Almejhim et al. [52] found a lower percentage (21.7%) held the toxR gene. The restricted number of strains and different primer designs may be to blame for the variations in the prevalence of virulence gene identification. It could also be explained by the species, various aquatic salinities, the quantity of fish investigated, and ecological conditions. Food safety is a worldwide health priority, and foodborne infections have a substantial global impact. Detecting microbiological pathogens in food is thus the solution to preventing and recognizing health and safety hazards.
5. Conclusion
The current study focused on examining fish available on the market and popular among consumers in Aswan, Egypt, concerning consumer safety. Additionally, the present study revealed that most fish samples had higher bacterial burdens than recommended, and increased bacterial contamination observed in O. niloticus and C. gariepinus compared to other species may constitute health hazards, mainly due to the detection of some food-poisoning bacteria such as A. hydrophila, P. aeruginosa, and V. parahaemolyticus. More research was needed to assess fish bacterial quality nationwide and develop faster, cheaper, and more sensitive methods for isolating and identifying foodborne pathogens and their virulence genes. Continuous enforcement of hygienic conditions in food handling, food contact surfaces, personal sanitary practices, and consuming fully cooked fish should be recommended to help eliminate foodborne pathogens.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at Northern Border University, Arar, KSA, for funding this research “work through the project number” NBU-FPEJ-2024-3165-01.
Data Availability
The article contains the requisite data to substantiate the conclusions drawn in this study.
Ethical Approval
All techniques employed in this study were directed in compliance with applicable standards and regulations. The agreement was acquired from the New Valley Research Ethics Committee (number 02-3/11-2023-5).
Conflicts of Interest
The authors claim no conflicts of interest.
Authors' Contributions
Nady Elbarbary, Mohamed Dandrawy, and Reda A. Gomaa conducted conceptualization, data curation, and methodology. Ghada Hadad and Amna Osman conducted a formal analysis and investigation. Maha Abdelhaseib and Fagelnour Elnoamany managed the validation. Ghada A. Ibrahim conducted visualisation. Nady Kh. Elbarbary, Maha Abdelhaseib, and Neveen Abdelmotilib wrote the original draft. Rawaf Alenazy and Ibrahim Elbagory wrote, reviewed, and edited the paper. All authors contributed equally and approved the final manuscript.
References
- 1.Byrd K. A., Thilsted S. H., Fiorella K. J. Fish nutrient composition: a review of global data from poorly assessed inland and marine species. Public Health Nutrition . 2021;24(3):476–486. doi: 10.1017/s1368980020003857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food and Agriculture Organization (FAO) The State of World Fisheries and Aquaculture 2012. Sales and Marketing Group Publishing Policy and Support Branch Office of Knowledge Exchange . Vialedelle Terme di Caracalla 00153 Rome, Italy: Research and Extension FAO; 2012. [Google Scholar]
- 3.World Health Organization (WHO) Risk Assessment of Vibrio parahaemolyticus in Seafood: Interpretative Summary and Technical Report . World Health Organization; 2011. [Google Scholar]
- 4.Fikadu T., Bezuayehu A. Assessment on the microbial safety status of fish products at fish retail areas in Addis Ababa, Ethiopia. Journal of Entomology and Zoology Studies . 2021;9:41–46. [Google Scholar]
- 5.Mitiku B. A., Mitiku M. A., Ayalew G. G., Alemu H. Y., Geremew U. M., Wubayehu M. T. Microbiological quality assessment of fish origin food along the production chain in upper Blue Nile watershed, Ethiopia. Food Science & Nutrition . 2023;11(2):1096–1103. doi: 10.1002/fsn3.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yousef A. E., Abdelhamid A. G. Food Microbiology: Fundamentals and Frontiers . 5th. Washington, DC: ASM Press; 2019. Chapter 1: Behavior of microorganisms in food: growth, survival, and death; pp. 3–21. [DOI] [Google Scholar]
- 7.Food and Drug Administration's (FDA's) Fish and Fishery Products Hazards and Controls Guidance . 4th. 2021. pp. 75–345. http://fda.gov/media/80637 .
- 8.Lina S., Luxin W. The microbial safety of fish and fish products: recent advances in understanding its significance, contamination sources, and control strategies. Comprehensive Reviews in Food Science and Food Safety . 2021;20(1):738–786. doi: 10.1111/1541-4337.12671. [DOI] [PubMed] [Google Scholar]
- 9.Yang X., Huang J., Su Y., et al. Incidence and antimicrobial resistance of Salmonella serovars in fresh retail aquatic products from China. LWT . 2022;171, article 114123 doi: 10.1016/j.lwt.2022.114123. [DOI] [Google Scholar]
- 10.Kim J. S., Lee M. S., Kim J. H. Recent updates on outbreaks of Shiga toxin-producing Escherichia coli and its potential reservoirs. Front Cell Infect Microbiology . 2020;10:1–10. doi: 10.3389/fcimb.2020.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S., Huang J., Wu Q., et al. Prevalence and characterization of Staphylococcus aureus isolated from retail vegetables in China. Frontiers in Microbiology . 2018;9:p. 1263. doi: 10.3389/fmicb.2018.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussein M. A., El-tahlawy A. S., Abdelmoneim H. M., Abdallah K., Bayomi R. Pseudomonas aeruginosa in fish and fish products: a review on the incidence, public health significance, virulence factors, antimicrobial resistance, and biofilm formation. Journal of Advanced Veterinary Research . 2023;13:1464–1468. https://www.advetresearch.com/index.php/AVR/article/view/1403 . [Google Scholar]
- 13.Sheng L., Wang L. The microbial safety of fish and fish products: recent advances in understanding its significance, contamination sources, and control strategies. Comprehensive Reviews in Food Science and Food Safety . 2021;20(1):738–786. doi: 10.1111/1541-4337.12671. [DOI] [PubMed] [Google Scholar]
- 14.Castro-Escarpulli G., Figueras M. J., Aguilera-Arreola G., et al. Characterisation of Aeromonas spp. isolated from frozen fish intended for human consumption in Mexico. International Journal of Food Microbiology . 2003;84(1):41–49. doi: 10.1016/S0168-1605(02)00393-8. [DOI] [PubMed] [Google Scholar]
- 15.You K. G., Bong C. W., Lee C. W. Antibiotic resistance and plasmid profiling of Vibrio spp. in tropical waters of Peninsular Malaysia. Environmental Monitoring and Assessment . 2016;188(3):p. 171. doi: 10.1007/s10661-016-5163-0. [DOI] [PubMed] [Google Scholar]
- 16.Zaher H. A., Nofal M. I., Hendam B. M., Elshaer M. M., Alothaim A. S., Eraqi M. M. Prevalence and antibiogram of Vibrio parahaemolyticus and Aeromonas hydrophila in the flesh of Nile tilapia, with special reference to their virulence genes detected using multiplex PCR technique. Antibiotics . 2021;10(6):p. 654. doi: 10.3390/antibiotics10060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen P., Takahisa M., Thi T. Prevalence and antibiotic resistance of Staphylococcus aureus isolated from Pangasius fish and fish processing handlers in the Mekong Delta, Viet Nam. CTU Journal of Innovation and Sustainable Development . 2023;15(3):103–109. doi: 10.22144/ctujoisd.2023.055. [DOI] [Google Scholar]
- 18.Elhaweet A., Adam E., Sangq Y., El-Far A. Assessment of Lake Nasser fisheries. Egyptian Journal of Aquatic Research . 2008;34(2):285–298. [Google Scholar]
- 19.Mohamed W., Taha E., Osman A. An economic study of fish production and consumption in Egypt and its role in food security achieving. SVU-International Journal of Agricultural Sciences . 2022;4(1):223–235. doi: 10.21608/svuijas.2022.120401.1173. [DOI] [Google Scholar]
- 20.El-Shabrawy G. M. Lake Nasser-Nubia. In: Dumont H. J., editor. The Nile: Origin, Environments, Limnology and Human Use, vol. 89 . Springer Science & Business Media; 2009. pp. 125–131. [Google Scholar]
- 21.American Public Health Association (APHA) Revisions to Standard Methods for the Examination of Water and Wastewater . (Supplement). American Public Health; 2001. [Google Scholar]
- 22.Fawole M. O., Oso B. A. Laboratory Manual of Microbiology . Revised Edition. Ibadan, Nigeria: Spectrum Books Ltd; 2001. [Google Scholar]
- 23.Nady E. K., Abdelmotilib N. M., Gomaa R. A., et al. Impact of thawing techniques on the microstructure, microbiological analysis, and antioxidants activity of Lates niloticus and Mormyrus kannume fish fillets. Egyptian Journal of Aquatic Research . 2023;49(4):530–536. doi: 10.1016/j.ejar.2023.10.004. [DOI] [Google Scholar]
- 24.Elbarbary N. K., Abdelmotilib N. M., Salem-Bekhit M. M., Salem M. M., Singh S., Dandrawy M. K. Antibacterial efficiency of apple vinegar marination on beef-borne Salmonella. Open Veterinary Journal . 2024;14(1):274–283. doi: 10.5455/OVJ.2024.v14.i1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin B., Austin D. A. Bacterial Fish Pathogens. Disease of Farmed and Wild Fish . 6th. Springer Nature publishing; 2016. Aeromonadaceae representatives (Motile Aeromonads) pp. 161–214. [Google Scholar]
- 26.Cheesbrough M. District Laboratory Practice: In Tropical Countries . Part 2, 2nd. Cambridge: Cambridge University Press; 2006. [DOI] [Google Scholar]
- 27.Elbarbary N. K., Abdelmotilib N. M. Effect of natural antimicrobials on the reduction of Pseudomonas aeruginosa in frozen chicken products. Journal of Advanced Veterinary Research . 2023;13(3):501–507. https://www.advetresearch.com/index.php/AVR/article/view/1263 . [Google Scholar]
- 28.Quinn P. J., Markey B. K., Carter M. E., Donnelly W. J., Leonard F. C. Veterinary Microbiology and Microbial Disease . 36. Vol. 26. Iowa State Univ. Press, Blackwell Science Ltd; 2002. [Google Scholar]
- 29.International Organization for Standardization (ISO) Microbiology of food and animal feeding stuffs-horizontal method for the detection of potentially enteropathogenic. Vibrio spp. Part 1: Detection of Vibrio parahaemolyticus and Vibrio cholerae (2007) 2007. https://www.iso.org/standard/38278 .
- 30.Benie C. K., Dadié A., Guessennd N., et al. Characterization of virulence potential of Pseudomonas aeruginosa isolated from bovine meat, fresh fish, and smoked fish. European Journal of Microbiology and Immunology . 2017;7(1):55–64. doi: 10.1556/1886.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asran E. A., Khalil S. A., Awad Y. N., Hussein M. Molecular characterization of Vibrio species isolated from fish using PCR technique. Alexandria Journal for Veterinary Sciences . 2020;67(2):9–19. doi: 10.5455/ajvs.117363. [DOI] [Google Scholar]
- 32.Hu M., Wang N., Pan Z. H., Lu C. P., Liu Y. J. Identity and virulence properties of Aeromonas isolates from diseased fish, healthy controls and water environment in China. Letters in Applied Microbiology . 2012;55(3):224–233. doi: 10.1111/j.1472-765x.2012.03281.x. [DOI] [PubMed] [Google Scholar]
- 33.National Food Safety Authority (NFSA), Egypt (1/2021) Microbiological standards for foodstuffs. Al-Waqa'i' al-Misriyya . 2021;57(A):p. 31. [Google Scholar]
- 34.Ali S. M., Yones E. M., Kenawy A. M., Ibrahim T. B., Abbas W. T. Effect of El-Sail drain wastewater on Nile tilapia (Oreochromis niloticus) from River Nile at Aswan, Egypt. Journal of Aquaculture Research & Development . 2014;6:p. 294. doi: 10.4172/2155-9546.1000294. [DOI] [Google Scholar]
- 35.Amuneke E., Igbodiegwu G., Patrick O., Aloysius A. Bacteriological profile of selected fish species and water sample from Otuocha River Anambra state. International Research Journal of Agriculture and Rural Development . 2020;18(1):11–26. doi: 10.4314/jafs.v18i1.2. [DOI] [Google Scholar]
- 36.Elbarbary K. N., Maky H. H., Gomaa R. A., Hassan M. A. Evaluation of the quality of fish burger formulated with Moringa oleifera leaves during frozen storage. Egyptian Journal of Aquatic Biology & Fisheries . 2024;28(3):473–490. [Google Scholar]
- 37.Mamdouh D., Mohamed Hassan A., Elbahy Fawzy E. Bacterial evaluation of the quality of farmed fish of Kafr El- Sheikh City in Egypt. Benha Veterinary Medical Journal . 2022;41(2):16–21. doi: 10.21608/bvmj.2021.80430.1433. [DOI] [Google Scholar]
- 38.Ali S. M. Bacterial load of tilapia fish residing El-Ramla and Abu Simbel Khors, Lake Nasser, Egypt. Egyptian Journal of Aquatic Biology & Fisheries . 2022;26(5):149–160. doi: 10.21608/ejabf.2022.258921. [DOI] [Google Scholar]
- 39.Yohans H., Mitiku B. A., Tassew H. Levels of Escherichia coli as bio-indicator of contamination of fish food and antibiotic resistance pattern along the value chain in Northwest Ethiopia. Veterinary medicine . 2022;13(13):299–311. doi: 10.2147/VMRR.S373738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saad M. S., Hassan A., Hassnien F. S., Abdel-Aal M. M., Zakar A., Elshfey S. A. Prevalence of Escherichia coli in fish obtained from retail fish markets in Gharbia Governorate, Egypt. Benha Veterinary Medical Journal . 2018;34(1):254–260. doi: 10.21608/bvmj.2018.54248. [DOI] [Google Scholar]
- 41.Dhanapal K. G., Vidya S. L., Binay B. N., Venkateswarlu G., Reddy A. D., Basu S. Effect of cooking on physical, biochemical, bacteriological characteristics and fatty acid profile of tilapia (Oreochromis mossambicus) fish steaks. Archives of Applied Science Research . 2012;4:1142–1149. [Google Scholar]
- 42.Wendwesen T., Dagmar N., Yitbarek G., Matusala M. Microbiological quality of frozen raw and undercooked Nile tilapia (Oreochromis niloticus) fillets and food safety practices of fish handlers in Arba Minch town, SNNPR, Ethiopia. Journal of Veterinary Medicne and Animimaal Health . 2017;9(3):55–62. doi: 10.5897/JVMAH2015.0424. [DOI] [Google Scholar]
- 43.Kumar L. R., Kasim A. K., Lekshmi M., Nayak B. B., Kumar S. Incidence of methicillin-resistant staphylococci in fresh seafood. Advances in Microbiology . 2016;6(6):399–406. doi: 10.4236/aim.2016.66039. [DOI] [Google Scholar]
- 44.Danba E. P., Bichi A. H., Ishaku S., et al. Occurrence of pathogenic bacteria associated with Clarias gariepinus in selected fish farms of Kumbotso local government area of Kano state, Nigeria. Bayero Journal of Pure and Applied Sciences . 2015;7(2):145–149. doi: 10.4314/bajopas.v7i2.25. [DOI] [Google Scholar]
- 45.Budiati T., Rusul G., Wan N., Wan A., Rosma A., Yahya M. Microbiological quality of catfish (Clarias gariepinus) and tilapia (Tilapia mossambica) obtained from wet markets and ponds in Malaysia. Journal of Aquaculture Research and Development . 2014;6(1):291–295. doi: 10.4172/2155-9546.1000291. [DOI] [Google Scholar]
- 46.Soriano J. M., Mañes J., Soler C. Rapid whole protein quantitation of staphylococcal enterotoxins A and B by liquid chromatography/mass spectrometry. Journal of Chromatography . 2012;1238:54–59. doi: 10.1016/j.chroma.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Mumbo M. T., Nyaboga E. N., Kinyua J. K., et al. Antimicrobial resistance profiles of Salmonella spp. and Escherichia coli isolated from fresh Nile tilapia (Oreochromis niloticus) fish marketed for human consumption. BMC Microbiology . 2023;23(1):p. 306. doi: 10.1186/s12866-023-03049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van T. H., Nguyen H. K., Smooker P. M., Coloe P. J. The antibiotic resistance characteristics of non-typhoidal Salmonella enterica isolated from food-producing animals, retail meat and humans in South East Asia. International Journal of Food Microbialogy . 2012;154(3):98–106. doi: 10.1016/j.ijfoodmicro.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 49.Silva N., Junqueira V. A., Silveira N. A., Taniwaki M. H., Nascimento M., Gomes R. A. Manual of Methods for Microbiological Analysis of Food . 1st. London: CRC Press; 2017. [DOI] [Google Scholar]
- 50.Tanyag B., Quiambao J. J., Ko A. A., Singh A., Cambia F., Montojo U. Prevalence of invA gene of Salmonella spp. in fish and fishery resources from Manila Bay aquaculture farms using real-time PCR. Applieed Microbiology . 2021;1(3):510–519. doi: 10.3390/applmicrobiol1030033. [DOI] [Google Scholar]
- 51.Kagambèga A., Salifou B., Elizabeth A. M., et al. Genome analysis of Salmonella strains isolated from imported frozen fish in Burkina Faso. Annals of Microbiology . 2021;71(1):p. 32. doi: 10.1186/s13213-021-01642-8. [DOI] [Google Scholar]
- 52.Almejhim M., Aljeldah M., Elhadi N. Improved isolation and detection of toxigenic Vibrio parahaemolyticus from coastal water in Saudi Arabia using immunomagnetic enrichment. Peer Joournal . 2021;9, article e12402 doi: 10.7717/peerj.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhuiyan A. N., Rezvi M., Kaysar M. D., Hossain M., Uddin D., Haider M. D. N. Isolation and identification of Salmonella spp. and Escherichia coli from water used during live transportation of Pangasius catfish, Pangasianodon hypophthalmus. Journal Of Advanced Biotechnology And Experimental Therapeutics . 2022;5(3):676–686. doi: 10.5455/jabet.2022.d146. [DOI] [Google Scholar]
- 54.Morshdy A. M., Nehal S., Rasha M., Karima A. Prevalence of antibiotic-resistant Aeromonas and molecular identification of Aeromonas hydrophila isolated from some marketed fish in Egypt. Journal of Advanced Veterinary Research . 2022;12(6):717–721. https://www.advetresearch.com/index.php/AVR/article/view/1096 . [Google Scholar]
- 55.Sarkar A., Saha M., Roy P. Detection of 232bp virulent gene of pathogenic Aeromonas hydrophila through PCR based technique: (a rapid molecular diagnostic approach) Advanced Microbiology . 2013;3(1):83–87. doi: 10.4236/aim.2013.31013. [DOI] [Google Scholar]
- 56.Wamala K. S. P., Mugimba K., Mutoloki S., et al. Occurrence and antibiotic susceptibility of fish bacteria isolated from Oreochromis niloticus (Nile tilapia) and Clarias gariepinus (African catfish) in Uganda. Fisheries and Aquatic Sciences . 2018;21(1):1–10. doi: 10.1186/s41240-017-0080-x. [DOI] [Google Scholar]
- 57.Kishk D., Moustafa N. Y., Kirrella G. A. Prevalence and virulence characteristics of Aeromonas species isolated from fish farms in Egypt. Kafrelsheikh Veterinary Medical Journal . 2020;18(2):5–8. doi: 10.21608/kvmj.2020.115274. [DOI] [Google Scholar]
- 58.Emeish W. F., Mohamed H. M., Elkamel A. A. Aeromonas infections in African sharptooth catfish. Journal of Aquatic Research Development . 2018;9(9):p. 548. doi: 10.4172/2155-9546.1000548. [DOI] [Google Scholar]
- 59.Elbarbary N. K., Abd El-aziz D. S., Maky M. A. M., Khalifa M. I., Karmi M. Assessment of the bacteriological quality of some fish products. Egyptian Journal of Veterinary Sciences . 2024;55(1):183–196. doi: 10.21608/ejvs.2023.225910.1551. [DOI] [Google Scholar]
- 60.Ahangarzadeh M., Ghorbanpour M., Peyghan R., Houshmand H., Rohani M. S., Soltani M. Detection and distribution of virulence genes in Aeromonas hydrophila isolates causing infection in cultured carps. Veterinary Research Forum . 2022;13(1):55–60. doi: 10.30466/vrf.2020.115998.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aslani M. M., Hamzeh H. S. Characterization and distribution of virulence factors in Aeromonas hydrophila strains isolated from fecal samples of diarrheal and asymptomatic healthy persons in Ilam, Iran. Iran Biomedical Journal . 2004;8:199–203. [Google Scholar]
- 62.Nawaz M., Khan S. A., Khan A. A., et al. Detection and characterization of virulence genes and integrons in Aeromonas veronii isolated from catfish. Food Microbiology . 2010;27(3):327–331. doi: 10.1016/j.fm.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Hafez A. E., Darwish W. S., Elbayomi R. M., Hussein M. A., El Nahal S. M. Prevalence, antibiogram and molecular characterization of Aeromonas hydrophila isolated from frozen fish marketed in Egypt. Slovenian Veterinary Research . 2018;55:445–454. doi: 10.26873/SVR-671-2018. [DOI] [Google Scholar]
- 64.Benie C. K. D., Dadie A., Guessennd N. Molecular identification and virulence factors of Pseudomonas aeruginosa strains isolated from animal products. Journal of Bacteriology and Mycology . 2017;4(3):91–96. doi: 10.15406/jbmoa.2017.04.00094. [DOI] [Google Scholar]
- 65.Losito A. R., Raffaelli F., Del Giacomo P., Tumbarello M. New drugs for the treatment of Pseudomonas aeruginosa infections with limited treatment options: a narrative review. Antibiotics . 2022;11(5):p. 579. doi: 10.3390/antibiotics11050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abd El-Maogoud H., Abo Baker M. E., Ayman H. M., Mohamed A. M. Occurrence and characterization of Pseudomonas species isolated from fish marketed in Sohag Governorate, Egypt. South Valley University International Journal of Veterinary Science . 2021;4(2):76–84. doi: 10.21608/svu.2021.64991.1111. [DOI] [Google Scholar]
- 67.Zilberberg M. D., Shorr A. F. Epidemiology of healthcare-associated pneumonia (HCAP) Seminars in Respiratory and Critical Care Medicine . 2009;30(1):10–15. doi: 10.1055/s-0028-1119804. [DOI] [PubMed] [Google Scholar]
- 68.Salem A., Osman I., Shehata S. Assessment of psychrotrophic bacteria in frozen fish with special reference to Pseudomonas species. Benha Veterinary Medical Journal . 2018;34(2):140–148. doi: 10.21608/bvmj.2018.29423. [DOI] [Google Scholar]
- 69.Vidaillac C., Chotirmall S. H. Pseudomonas aeruginosa in bronchiectasis: infection, inflammation, and therapies. Expert Review of Respiratory Medicine . 2021;15(5):649–662. doi: 10.1080/17476348.2021.1906225. [DOI] [PubMed] [Google Scholar]
- 70.Shahrokhi G. R., Rahimi E., Shakerian A. The prevalence rate, pat-tern of antibiotic resistance, and frequency of virulence factors of Pseudomonas aeruginosa strains isolated from fish in Iran. Journal of Food Quality . 2022;2022:8. doi: 10.1155/2022/8990912.8990912 [DOI] [Google Scholar]
- 71.Semenza J. C., Paz S. Climate change and infectious disease in Europe: impact, projection and adaptation. The Lancet Regional Health-Europe . 2021;9, article 100230 doi: 10.1016/j.lanepe.2021.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Victória M., Janaína N., Flávia M., Hilana V. Vibriosis and its impact on microbiological food safety. Food Science and Technology . 2021;42(1) doi: 10.1590/fst.65321. [DOI] [Google Scholar]
- 73.Ahmed H. A., Mohamed M. E., Rezk M. M., Gharieb R. M., Abdel-Maksoud S. A. Aeromonas hydrophila in fish and humans; prevalence, virulotyping, and antimicrobial resistance. Slovenian Veterinary Research . 2024;55:113–124. doi: 10.26873/SVR-636-2018. [DOI] [Google Scholar]
- 74.Morshdy A. M., Ahmed R., Mohamed A., Rasha M. Prevalence of antibiotic-resistant Vibrio isolated from some marketed fish in Egypt with a decontamination trial by lemon juice. Journal of Advanced Veterinary Research . 2022;12(4):353–357. https://www.advetresearch.com/index.php/AVR/article/view/1013 . [Google Scholar]
- 75.Suresh Y., Subhashini N., Kiranmayi C. B., et al. Isolation, molecular characterization and antimicrobial resistance patterns of four different Vibrio species isolated from fresh water fishes. International Journal of Current Microbiology and Applied Sciences . 2018;7(7):3080–3088. doi: 10.20546/ijcmas.2018.707.359. [DOI] [Google Scholar]
- 76.Yen P. T., Linh N. Q., Tram N. D. The identification and determination of toxin genes of Vibrio strains causing hemorrhagic disease on red drum (Sciaenops ocellatus) using PCR. AMB Express . 2021;11 doi: 10.1186/s13568-020-01161-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Letchumanan V., Pusparajah P., Tan L. T., Yin W. F., Lee L. H., Chan K. G. Occurrence and antibiotic resistance of Vibrio parahaemolyticus from shellfish in Selangor, Malaysia. Frontier of Microbiology . 2015;6:p. 1417. doi: 10.3389/fmicb.2015.01417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zaafrane S., Maatouk K., Alibi S., Ben Mansour H. Occurrence and antibiotic resistance of Vibrio parahaemolyticus isolated from the Tunisian coastal seawater. Journal of Water and Health . 2022;20(2):369–384. doi: 10.2166/wh.2022.243. [DOI] [PubMed] [Google Scholar]
- 79.Narayanan S. V., Joseph T. C., Peeralil S., Mothadaka M. P., Lalitha K. V. Prevalence, virulence characterization, AMR pattern and genetic relatedness of Vibrio parahaemolyticus isolates from retail seafood of Kerala, India. Frontier of Microbiology . 2020;11:p. 592. doi: 10.3389/fmicb.2020.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The article contains the requisite data to substantiate the conclusions drawn in this study.


