Abstract
Methods
We systematically searched the PubMed, Web of Science, African Journals Online, Embase, Cochrane Library, and Google Scholar databases for studies conducted up to March 1, 2023, that estimated the prevalence of HBV in Tanzania based on HBV surface antigen measurements. The DerSimonian–Laird random effects model was used to estimate the overall prevalence of HBV with 95% confidence intervals (CIs). Potential sources of heterogeneity were also investigated.
Results
Thirty-one studies with a total sample size of 37,988 were included in the meta-analysis. The overall average HBV prevalence estimate in Tanzania was 6.91% (95% CI = 5.18–8.86%). Subgroup analysis revealed the highest prevalence in the northern zone (9.32%, 95% CI; 2.24–20.36%), among the blood donors (18.72%, 95% CI: 17.43–20.05%) and among the community volunteers (8.76%, 95% CI: 4.55–14.15%). The lowest prevalence was observed in the lake zone at 4.66% (95% CI: 3.49–5.99) and in pregnant women at 4.72% (95% CI: 3.42–6.21). The overall between-study variability showed significant heterogeneity (I2 = 97.41%, P < 0.001).
Conclusions
Our results showed that Tanzania is a country with moderately high HBV endemicity, with large interregional differences and significantly high numbers of HBV infections within the community. This underscores the need for immediate development of targeted prevention strategies and further epidemiological studies to better understand the pattern of the disease.
1. Introduction
Hepatitis B virus (HBV) infection poses a significant global public health burden, with approximately 296 million people living with chronic hepatitis B (CHB) worldwide [1]. HBV infection can lead to serious liver diseases, such as cirrhosis and liver cancer. In sub-Saharan Africa, including Tanzania, the prevalence of HBV infection is particularly high. Available subpopulation studies in different parts of Tanzania have shown the prevalence of HBV to be 1.2–11.2% [2].
Despite the significant global burden associated with HBV infection, recent estimates indicate that only approximately 10% of people living with this disease have been formally diagnosed, and only 13% of those diagnosed are receiving treatment [3]. This global estimate coincides with the local data from Tanzania, where less than 10% of the population was screened for HBV, with minimal detection and treatment rates [4]. This concerning gap in diagnosis and subsequent treatment exposes a substantial number of individuals to an increased lifetime risk of liver-related complications, such as cirrhosis, hepatocellular carcinoma (HCC), and events of end-stage liver disease and death. Indeed, more than 66% of cases in the country with HCC were correlated with HBV infection [5].
Globally, there have been several efforts to address this issue, with the goal of eliminating HBV as a public health threat by 2030 [6]. Prevention through vaccination and antiviral treatment is a key strategy for achieving this goal. However, according to the recent report from the World Health Organization (WHO) [7], this target is likely to be missed, particularly in low- and middle-income countries, mainly due to a lack of comprehensive viral hepatitis prevention and control programs and poor surveillance systems for the disease. According to this report, there is still a high number of new infections and deaths; 1.5 million people were newly infected with HBV in 2019, with 1.1 million deaths.
In response to the global HBV elimination goal, Tanzania launched a national strategic plan for viral hepatitis in 2018 [4]. However, the effective policy implementation is still limited, as vaccination rates for high-risk groups have remained below the desired level, 2% in pregnant women [8], 74% in healthcare workers (HCWs) [9], and 0% in people living with HIV (PLWHIV) [2]. The WHO [6] recommends the HBV vaccination for all individuals at high risk of HBV infection. Additionally, the number of eligible patients receiving antiviral treatment for HBV is limited in the country [10]. The inconsistency of the data, coupled with insufficient surveillance systems in the country, may be the major factor hindering effective policy implementation, subsequent preventive strategies, and resource allocation. To address this issue, a systematic review and meta-analysis of available studies in Tanzania were conducted to estimate the prevalence of CHB in the country, describing the disease pattern.
2. Materials and Methods
2.1. Search Strategy
This study was registered with the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42023472128 and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. A systematic literature search was conducted in the following electronic databases: PubMed, Web of Science, African Journals Online, Embase, Cochrane Library, and Google Scholar, for studies up to March 2023. For PubMed, the following MeSH terms and texts were used: “Hepatitis B” [MeSH] OR “HBV” [MeSH] OR “Hepatitis B prevalence” [MeSH] OR “Viral hepatitis” [MeSH] AND “Hepatitis B virus” [MeSH] AND “Hepatitis B Tanzania” [MeSH] OR “Hepatitis B Mwanza” [MeSH] OR “Hepatitis B Kilimanjaro” [MeSH] OR “Hepatitis B Dar es salaam” [MeSH] OR “Hepatitis B Mbeya” [MeSH] OR “Hepatitis B Morogoro” [MeSH] OR “Hepatitis B Iringa” [MeSH] OR “Hepatitis B Pwani” [MeSH] OR “Hepatitis B Zanzibar” [MeSH] OR “Hepatitis B Kagera” [MeSH] OR “Hepatitis B Arusha” [MeSH] OR “Hepatitis B Manyara” [MeSH] OR “Hepatitis B Njombe” [MeSH] OR “Hepatitis B Katavi” [MeSH] OR “Hepatitis B Tanga” [MeSH] OR “Hepatitis B Ruvuma” [MeSH] OR “Hepatitis B Singida” [MeSH] OR “Hepatitis B Shinyanga” [MeSH] OR “Hepatitis B Simiyu” [MeSH] OR “Hepatitis B Geita” [MeSH] OR “Hepatitis B Mara” [MeSH] OR “Hepatitis B Rukwa” [MeSH] OR “Hepatitis B Mtwara” [MeSH] OR “Hepatitis B Kigoma” [MeSH] OR “Hepatitis B Lindi” [MeSH] OR “Hepatitis B Tabora” [MeSH] OR “Hepatitis B Pemba” [MeSH] OR “Hepatitis B Dodoma” [MeSH]. The latter is a list of all administrative regions in Tanzania. Two independent authors (SBK and VDK) screened the titles and abstracts of the manuscripts to determine their relevance, and the full texts of the selected studies were subsequently reviewed. Similar search strategies were used for other databases that included terms such as hepatitis B virus, viral infections, prevalence, Tanzania, and prevalence of hepatitis in the various target groups.
2.2. Eligibility
2.2.1. Inclusion Criteria
The inclusion criteria were studies conducted in Tanzania, published in English before March 2023, used the hepatitis B surface antigen (HBsAg) test for diagnosing HBV infection, and included participants of any age.
2.2.2. Exclusion Criteria
Case reports, reviews, preprints, and studies with insufficient or inaccessible data were excluded.
2.3. Data Collection and Management
Mendeley software was used as the reference manager to remove duplicates of the studies that were identified from the electronic databases and to generate bibliographies. The titles and abstracts that were produced from electronic databases were independently screened by two authors as per eligibility criteria. The full texts of the qualified studies were then reviewed, and the data were extracted and entered into a Microsoft Excel-designed extraction form using the following variables: publication year, study design, study location (regional/zone), population (general, PLWHIV, pregnant women, HCW, people who inject drugs (PWID), and children), study year, sample size, prevalence of HBV, and diagnosis method. A third reviewer was involved in double-checking the correctness of the data entry and resolving disagreements.
2.4. Quality Assessment
Quality assessment was done using the Newcastle-Ottawa Quality Assessment Scale [12]. All the parameters of this scale (standardized methods for confirming diagnosis, large sample size, multicenter study, appropriate statistical methods that report results, accounting for confounders, clear methodology of selection of participants, and representativeness of the population) were considered in our meta-analysis. The quality assessment was carried out by two independent reviewers, and disagreements were resolved by a third reviewer.
2.5. Statistical Analysis
Quantitative data on the overall number of subjects with HBV were extracted, as were the data in the subgroups. The HBV prevalence across studies was calculated, and due to heterogeneity, the data are presented as ranges of values with 95% confidence interval (CI). Furthermore, weighted means were calculated considering the different study sizes.
For the meta-analysis, contingency tables were created in an Excel spreadsheet to compare the prevalence of HBV in different study populations. Given the inherent variability among observational studies, we used the random effects model of DerSimonian and Laird to estimate the HBV prevalence in the included studies, as used elsewhere [13]. Heterogeneity across studies was assessed using the heterogeneity index (I2); I2 > 70% suggested high heterogeneity, 50–69 indicated substantial heterogeneity, and <49 indicated low heterogeneity.
3. Results
3.1. Study Identification
The flowchart for the selection of studies is presented in Figure 1. A total of 120 studies were found in various electronic databases. After removing duplicates, 66 articles remained. After screening the titles and abstracts, 6 more studies were removed and 48 studies were chosen for full manuscript reading. From those, 17 studies were excluded due to missing prevalence (4), conducted outside Tanzania (5), and 8 being reviews leaving 31 studies for the final review. The risk of bias assessment according to the Newcastle-Ottawa Quality Assessment Scale is shown in Supplementary Table 1.
Figure 1.
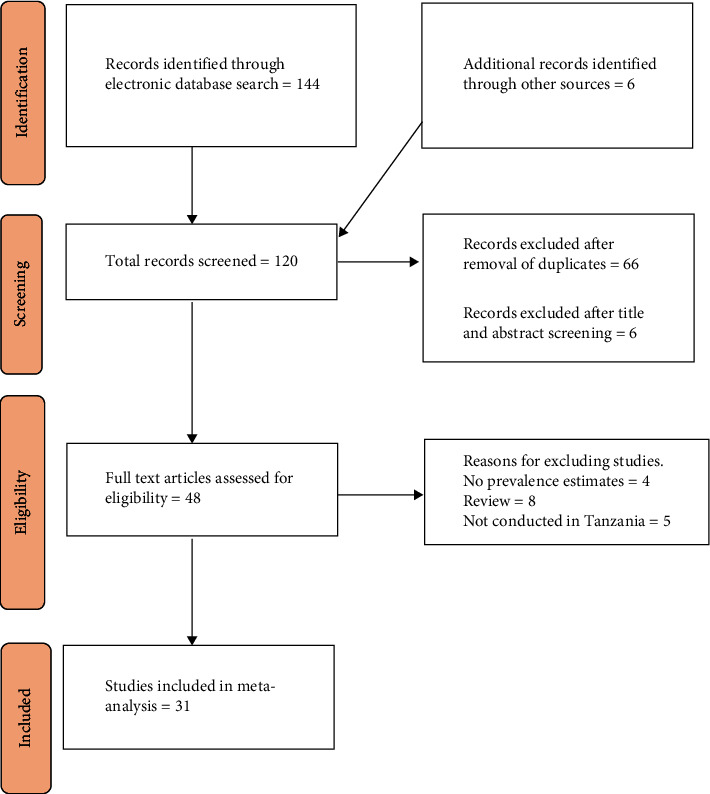
PRISMA flow diagram for identification and selection of articles for inclusion in the review.
3.2. Study Characteristics
We included all studies that met the eligibility criteria and were conducted before 01 March 2023 in our review. Table 1 shows the characteristics of the studies that were eligible for our meta-analysis. A total of 31 studies were analyzed, with a combined sample size of 37,988. The duration of the studies ranged from two months to nine years, with the majority being cross-sectional studies (26 out of 31; 83.9%). Approximately one-third of the studies (11 out of 31; 35.5%) were conducted in the eastern zone of the country, specifically in the Dar es Salaam and Morogoro regions. The Zanzibar, southern, and central zones had minimal representation, with only one study (3.2%) each. Among the reviewed studies, Hawkins et al. [31] had the largest sample size (17,539), while Machange et al. [23] had the smallest sample size (68). The majority of the studies (17 out of 31; 54.8%) were conducted between 2013 and 2023, while only 5 out of 31 (16.1%) were conducted between 1991 and 2001. Rapid diagnostic tests (RDTs) were the most commonly used method for diagnosing HBV in 17 (54.8%) studies.
Table 1.
Characteristics of the included studies in the systematic review and meta-analysis for the prevalence of hepatitis B in Tanzania from 1994 to 2023.
| Study author | Publication year | Study design | City | Population type | Study year | Sample size | Prevalence (%) | Method |
|---|---|---|---|---|---|---|---|---|
| Pellizzer et al. [14] | 1994 | Cross-sectional | Dodoma | Outpatients | 1991-1992 | 1004 | 4.4 | ELISA |
| Stark et al. [15] | 2000 | Cross-sectional | Kilimanjaro | Community | 1996 | 211 | 16.6 | ELISA |
| Matee et al. [16] | 2006 | Cross-sectional | Dar es salaam | Blood donors | 2004-2005 | 1599 | 8.8 | ELISA |
| Kilonzo et al. [17] | 2017 | Cross-sectional | Mwanza | PLWHIV | 2014-2015 | 743 | 6.6 | RDT |
| Miller et al. [18] | 1998 | Cross-sectional | Dar es salaam | Community | 1992 | 403 | 6 | ELISA |
| Msuya et al. [19] | 2006 | Cross-sectional | Kilimanjaro | Outpatients | 1999 | 382 | 4.2 | ELISA |
| Rashid et al. [20] | 2014 | Cross-sectional | Dar es salaam | Pregnant women | 2010 | 310 | 3.9 | ELISA |
| Mirambo et al. [21] | 2016 | Cross-sectional | Mwanza | Pregnant women | 2014 | 211 | 3.8 | RDT |
| Froeschl et al. [22] | 2021 | Cohort | Mbeya | Community | 2002–2010 | 2363 | 5 | RDT |
| Machange et al. [23] | 2017 | Cross-sectional | Kilimanjaro | HCW | 2014 | 68 | 13.2 | ELISA |
| Valerian et al. [24] | 2018 | Cross-sectional | Kilimanjaro | Blood donors | 2016 | 1829 | 29.6 | ELISA |
| Christian et al. [25] | 2019 | Cohort | Dar es salaam | PLWHIV | 2014-2015 | 494 | 33.4 | RDT |
| Meschi et al. [26] | 2010 | Cross-sectional | Zanzibar | Outpatients | 2007 | 120 | 1.7 | ELISA |
| Meschi et al. [26] | 2010 | Cross-sectional | Iringa | Outpatients | 2007 | 157 | 6.4 | ELISA |
| Ramírez-Mena et al. [27] | 2016 | Cohort | Morogoro | PLWHIV | 2005–2015 | 3097 | 7.3 | RDT |
| Kamenya et al. [28] | 2017 | Cross-sectional | Kilimanjaro | PLWHIV | 2015 | 300 | 2.3 | RDT |
| Kapinga and Aboud [29] | 2018 | Cross-sectional | Kagera | Pregnant women | 2017 | 600 | 7.2 | RDT |
| Muro et al. [30] | 2013 | Cross-sectional | Kilimanjaro | Children | 2006–2009 | 547 | 4.2 | ELISA |
| Hawkins et al. [31] | 2013 | Cohort | Dar es salaam | PLWHIV | 2004–2011 | 17539 | 6.2 | RDT |
| Mueller et al. [32] | 2015 | Cross-sectional | Mwanza | HCW | 2012 | 598 | 7.2 | ELISA |
| Shao et al. [33] | 2018 | Cross-sectional | Kilimanjaro | HCW | 2015-2016 | 442 | 5.7 | RDT |
| Geffert et al. [8] | 2020 | Cross-sectional | Mwanza | Pregnant women | 2014-2015 | 743 | 3 | RDT |
| Shayo et al. [34] | 2021 | Cross-sectional | Dar es salaam | SCD patients | 2018-2019 | 325 | 1.2 | RDT |
| Manyahi et al. [35] | 2017 | Cross-sectional | Dar es salaam | Pregnant women | 2014 | 249 | 8 | ELISA |
| Ng'wamkai et al. [36] | 2019 | Cross-sectional | Mwanza | Pregnant women | 2018 | 499 | 5.8 | RDT |
| Kawambwa et al. [37] | 2020 | Cross-sectional | Dar es salaam | PWID | 2017 | 219 | 7.8 | RDT |
| Mirambo et al. [38] | 2020 | Cross-sectional | Mwanza | Students | 2016 | 1211 | 3.1 | RDT |
| Shedura et al. [39] | 2023 | Cross-sectional | Mtwara | PLWHIV | 2022 | 220 | 10 | RDT |
| Franzeck et al. [40] | 2013 | Prospective | Morogoro | PLWHIV | 2011-2012 | 272 | 9.2 | RDT |
| Kilonzo et al. [41] | 2021 | Cross-sectional | Mwanza | PWID | 2019-2020 | 253 | 3.6 | RDT |
| Menendez et al. [42] | 1999 | Cross-sectional | Morogoro | Pregnant women | 1995 | 980 | 6.3 | ELISA |
ELISA: enzyme-linked immunosorbent assay; PLWHIV: people living with HIV; HCW: healthcare worker; RDT: rapid diagnostic test; PWID: people who inject drugs.
3.3. Prevalence of HBV Infection in Tanzania
The combined prevalence of HBV among the 37,988 participants was 6.91% (95% CI = 5.18–8.86%), with a high level of heterogeneity at 97.41% (P < 0.001). The prevalence varied significantly across the included studies, ranging from 1.23% (95% CI = 0.48–3.12%) in individuals with sickle cell disease to 33.40% (95% CI = 29.38–37.67%) in PLWHIV (Figure 2).
Figure 2.
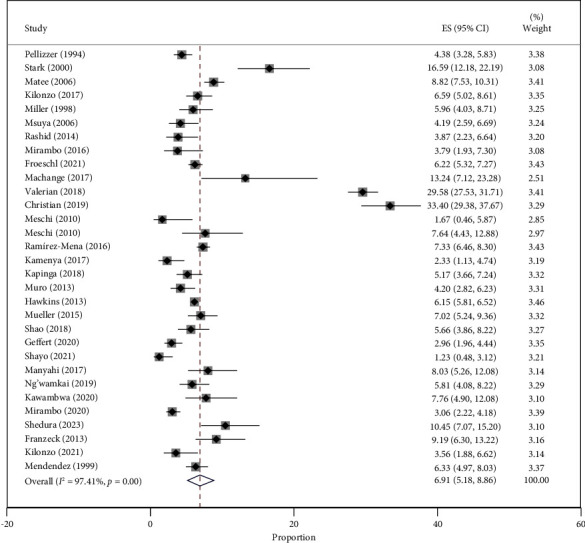
Forest plot of the studies on HBV prevalence in Tanzania published from 1994 to 2023.
3.4. Subgroup Analysis of HBV Prevalence
As shown in Table 2, our meta-analysis divided the studies into groups based on geographical location, year of study, studied population (PLWHIV, blood donors, HCW, pregnant women, and PWID), and HBV diagnosis method. When considering the geographical location of the country, the highest prevalence of HBV was found in the northern zone, with 9.32% (95% CI; 2.24–20.36%) and a total of 3779 participants. The eastern zone had the next highest prevalence of 7.93% (95% CI; 5.57–10.66%) with a sample size of 25,487. The lowest prevalence of 4.66% (3.49–5.99) was observed in the lake zone. The southern, central, and Zanzibar zones were each represented by one study (Figure 3).
Table 2.
Subgroup analysis of the estimated prevalence of HBV in Tanzania in the studies published from 1994 to 2023.
| Variable | Number of studies | Sample size | Prevalence % (95% CI) | Heterogeneity (%) | P value |
|---|---|---|---|---|---|
| Geographical zone | |||||
| Southern | 1 | 220 | 10.45 (7.07–15.20) | NA | NA |
| Northern | 7 | 3779 | 9.32 (2.24–20.36) | 98.74 | <0.001 |
| Eastern | 11 | 25487 | 7.93 (5.57–10.66) | 96.73 | <0.001 |
| Southern highlands | 2 | 2520 | 6.19 (5.27–7.18) | NA | NA |
| Lake | 8 | 4858 | 4.66 (3.49–5.99) | 77.19 | <0.001 |
| Central | 1 | 1004 | 4.38 (3.28–5.83) | NA | NA |
| Zanzibar | 1 | 120 | 1.67 (0.46–5.87) | NA | NA |
| Year of study | |||||
| 1991–2001 | 5 | 2980 | 6.27 (4.22–9.73) | 87.93 | <0.001 |
| 2002–2012 | 9 | 23505 | 5.97 (4.94–7.08) | 76.95 | <0.001 |
| 2013–2023 | 17 | 11503 | 7.48 (3.96–11.97) | 98.39 | <0.001 |
| Diagnosis method | |||||
| ELISA | 14 | 8457 | 7.79 (4.08–12.53) | 97.98 | <0.001 |
| RDT | 17 | 29531 | 6.24 (4.65–6.62) | 95.63 | <0.001 |
| Study group: general population | |||||
| Blood donors | 2 | 3428 | 18.72 (17.43–20.05) | 0 | <0.001 |
| Community | 3 | 2977 | 8.76 (4.55–14.15) | 91.17 | <0.001 |
| PLWHIV | 7 | 22665 | 7.31 (5.22–9.72) | 93.50 | <0.001 |
| Healthcare workers | 3 | 1108 | 7.06 (4.63–9.93) | 56.42 | 0.10 |
| PWID | 2 | 472 | 5.32 (3.44–7.57) | 0 | <0.001 |
| Pregnant women | 7 | 3592 | 4.72 (3.42–6.21) | 62.23 | 0.02 |
| Others | 7 | 3746 | 4.18 (2.81–5.79) | 0 | <0.001 |
NA: not applicable; CI: confidence interval; ELISA: enzyme-linked immunosorbent assay; RDT: rapid diagnostic test; PLWHIV: people living with HIV; PWID: people who inject drugs.
Figure 3.
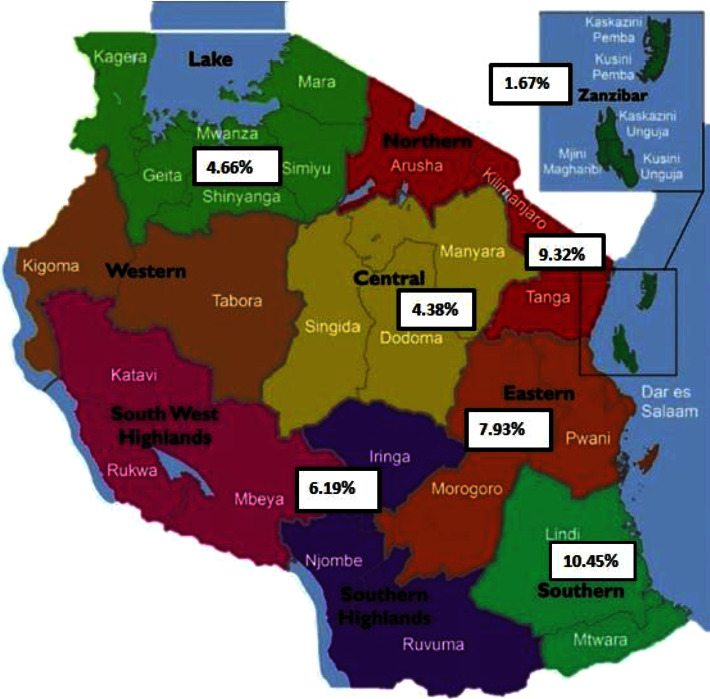
Map of Tanzania showing the prevalence of hepatitis B infection in different geographical zones of the country. Modified map adopted from Suleiman [43]. Tanzania regions are classified into 9 zones: (1) Eastern Zone (Morogoro, Pwani, and Dar es Salaam); (2) Northern Zone (Arusha, Kilimanjaro, and Tanga); (3) Lake Zone (Kagera, Mwanza, Simiyu, Mara, Shinyanga, and Geita); (4) Western Zone (Kigoma and Tabora); (5) Central Zone (Dodoma, Manyara, and Singida); (6) Southern Highlands (Katavi, Mbeya, Rukwa, Iringa, and Njombe); (7, 8) Southern Zone (Lindi, Ruvuma, and Mtwara); and (9) Zanzibar Zone.
Recent studies conducted from 2013–2023 (sample size of 11,503) reported the highest pooled HBV prevalence of 7.48% (95% CI = 3.96–11.97%) compared to older studies. Among the two HBV diagnostic methods used, studies that used enzyme-linked immunosorbent assay (ELISA) (sample size 8457) reported a higher HBV prevalence of 7.79% (95% CI; 4.08–12.53) compared to studies that used RDT (6.24%, 95% CI; 4.65 – 6.62%). Additionally, HBV prevalence was highest in the general population (blood donors and community volunteers) (18.72%, 95% CI: 17.43–20.05%) and (8.76%, 95% CI: 4.55–14.15%), respectively, compared to high-risk groups such as PLWHIV (7.31%, 95% CI: 5.22–9.72%), HCW (7.06%, 95% CI: 4.63–9.93%), and PWID (5.32%, 95% CI: 3.44–7.57). Pregnant women had the lowest prevalence of 4.72% (95% CI: 3.42–6.21%).
4. Discussion
Effective policy implementation requires data on the magnitude of disease. This meta-analysis review reports the prevalence of HBV in different populations in Tanzania from 1994 to 2023. Our findings showed a pooled prevalence of 6.91%, with variations observed among different geographical zones, study years, diagnostic methods, and population subgroups. This prevalence is similar to that reported in Ethiopia (6.0%) [13] and the average prevalence in four other East African countries (6.03%). Tanzania had a lower prevalence of 5.16% compared to Kenya and Uganda, which had higher prevalence of 8.54% and 8.45%, respectively [44]. Similarly, a neighboring country, Malawi, had a higher prevalence of 8.1% [45]. These results suggest that Tanzania has intermediate-high endemicity of HBV, while most neighboring countries have high endemicity according to the WHO classification [46]. The lower prevalence in Tanzania may be attributed to the government's efforts to improve preventive measures, such as early infant HBV vaccination. Tanzania has higher cumulative vaccination coverage rates (89.6%) compared to Uganda (77.6%) and Kenya (86.7%) [47]. Other factors, including HBV genotypes and high-risk behaviors, may also contribute to these differences. Nonetheless, Tanzania being intermediate-high HBV endemicity, more aggressive public health measures are needed in the country to control HBV and align with global targets for elimination by 2030.
Our research found significant differences in the occurrence of HBV in different areas of the country. Studies in the northern and eastern regions reported higher rates than the national average, while lower rates were reported in the lake and southern highlands regions. These findings suggest that there may be variations in the factors that contribute to HBV infection, such as prenatal screening, vaccination rates, community awareness, and the persistence of the infection. However, further investigation is necessary to understand the underlying reasons for this pattern. Similar disparities in HBV prevalence have been reported in other countries, including Iran [48], China [49], and Kenya [50], independent of factors like the studied population or diagnostic methods used. As for the southern, Zanzibar, and central regions, it is challenging to determine the overall prevalence of HBV due to the limited number of studies conducted in each area, with different populations and timeframes. In the southern region, Shedura et al. [39] analyzed the prevalence of HBV among pregnant women attending antenatal clinics in 2022. In Zanzibar [26], febrile outpatients were analyzed in 2007 while the analyzed population in the central region included all outpatients attending districts and regional hospitals from 1991 to 1992 [14].
Our study also assessed the prevalence of HBV infection in different populations, and one significant finding was the higher prevalence of HBV in the general population (blood donors and community volunteers) compared to high-risk groups such as PLWHIV, HCW, PWID, and pregnant women. This contrasts with findings from other studies in intermediate- and high-endemic areas. For example, in Bangladesh, the national average prevalence of HBV in the general population is 4%, lower than that in PWID (7.5%) and HCW (7.3%) [51]. Similarly, in China, a recent meta-analysis reported a national HBV prevalence of 3.8% in the general population, with higher rates in high-risk populations such as PLWHIV (10.7%) and PWID (15.0%) [52]. Another study in Sierra Leone found a higher prevalence of 15.9% among PLWHIV compared to the national average of 13.0%, while pregnant women and HCW had lower percentages of 9.7% and 11.9%, respectively [53]. One possible explanation for our findings is that the high-risk populations consistently have access to healthcare facilities, making them more likely to receive health education and vaccination against HBV infection compared to the general population. Previous studies in Tanzania have shown that more than two-thirds of HCW were vaccinated against HBV infection, and general knowledge about HBV was good [54, 55]. These findings were backed up by another study by Ndunguru et al. [56] that further revealed that knowledge and HBV vaccine uptake among the HCW were significantly higher in the urban areas compared to rural areas. However, even though the prevalence in high-risk populations is lower than the community, it is still higher than the national average as found in our study. Therefore, these findings highlight the importance of public health measures and call for simultaneous efforts to strengthen HBV elimination programs both in the community and healthcare settings.
In terms of the diagnosis methods, the prevalence rate of HBV detected using RDT was found to be lower than that of studies using ELISA. This may be due to the lower sensitivity of RDT compared to ELISA, with reported sensitivities of 70.0% and 78.0% in Togo and Gabon, respectively [57, 58]. The performance of RDT can also be influenced by factors such low levels of HBsAg and HBV deoxyribonucleic acid (DNA), HBV genotypes, specific brand of HBsAg tests used, and the HIV status of the individual [59]. Due to these shortcomings, the WHO recommends using RDT only in the settings where laboratory testing is limited and/or in populations where access to rapid testing would facilitate linkage to care and treatment [60]. In this meta-analysis, the majority (54.8%) of published studies, including those involving PLWHIV, used RDT as a diagnostic method, which may have led to an underestimation of the true prevalence of HBV in our study.
4.1. Strengths
This study employed an extensive search approach across significant data sources and included a large number of studies and various subgroup populations from most geographical zones of the country. Consequently, our findings provide an accurate representation of the current HBV situation in Tanzania.
4.2. Limitations
One significant drawback of this meta-analysis was the uneven representation of certain subgroups such as children and various geographical zones. Only one study [30] focused on the population of infants who are particularly susceptible to HBV infection through maternal transmission. Similarly, only one study was available for some geographical zones such as southern [39], central [14], and Zanzibar [26]. Therefore, it is important to be cautious when interpreting the overall findings of our study.
5. Conclusion
To the best of our understanding, this study is the first to provide a meta-analysis on the prevalence of HBV in Tanzania. The current literature suggests that Tanzania is a country with an intermediate-high endemicity of HBV, but with significant variations between regions. Additionally, this study confirms a considerable number of HBV infections within the community. These results highlight the immediate need for targeted prevention strategies, such as awareness programs, universal immunization, and relevant policies. Moreover, further research should be conducted to better understand the underlying factors influencing the observed infection patterns.
Acknowledgments
We wish to thank the Catholic University of Health and Allied Sciences (CUHAS) administration for the technical support.
Abbreviations
- CHB:
Chronic hepatitis B
- CI:
Confidence interval
- ELISA:
Enzyme-linked immunosorbent assay
- HBsAg:
Hepatitis B surface antigen
- HBV:
Hepatitis B virus
- DNA:
Deoxyribonucleic acid
- HCW:
Healthcare worker
- HCC:
Hepatocellular carcinoma
- MESH:
Medical subject heading
- PLWHIV:
People living with HIV
- PRISMA:
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO:
International Prospective Register of Systematic Reviews
- PWID:
People who inject drugs
- RDT:
Rapid diagnostic test.
Data Availability
The data supporting the findings of this study are available within the article and the supplemental files.
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Authors' Contributions
SBK, VDK, and IN were responsible for concept and design. VDK, SBK, and ERS were responsible for acquisition of data. HMJ, VDK, LR, SEM, and MMM were responsible for analysis and interpretation of data. HMJ, LR, SBK, and IN drafted the article. ERS, SEM, IN, and MMM were responsible for critical review of the manuscript. All authors have approved the final version of the manuscript.
Supplementary Materials
Supplementary Table 1: risk of bias assessment for selected studies.
References
- 1.Cdc. Fast facts on global hepatitis B, centers for disease control and prevention. 2017. https://www.cdc.gov/globalhealth/immunization/diseases/hepatitis-b/data/fast-facts.html%23print .
- 2.Kilonzo S. B., Gunda D. W., Mpondo B. C. T., Bakshi F. A., Jaka H. M. Hepatitis B virus infection in Tanzania: current status and challenges. The Journal of Tropical Medicine . 2018;2018:10. doi: 10.1155/2018/4239646.4239646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui F., Blach S., Manzengo Mingiedi C., et al. Global reporting of progress towards elimination of hepatitis B and hepatitis C. The Lancet Gastroenterology and Hepatology . 2023;8(4):332–342. doi: 10.1016/s2468-1253(22)00386-7. [DOI] [PubMed] [Google Scholar]
- 4.United Republic of Tanzania. National Strategic Plan for the Control of Viral Hepatitis 2018/19-2022/23 . Dar es Salaam, Tanzania: Ministry of Health; 2018. [Google Scholar]
- 5.Jaka H., Mshana S. E., Rambau P. F., Masalu N., Chalya P. L., Kalluvya S. E. Hepatocellular carcinoma: clinicopathological profile and challenges of management in a resource-limited setting. World Journal of Surgical Oncology . 2014;12(1):246–249. doi: 10.1186/1477-7819-12-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Who. Draft Global Health Sector Strategies. Viral Hepatitis, 2016–2021 . Geneva, Switzerland: The World Health Organization; 2015. [Google Scholar]
- 7.Who. Interim Guidance for Country Validation of Viral Hepatitis Elimination . Geneva, Switzerland: World Health Organization; 2021. [Google Scholar]
- 8.Geffert K., Maponga T. G., Henerico S., et al. Prevalence of chronic HBV infection in pregnant woman attending antenatal care in a tertiary hospital in Mwanza, Tanzania: a cross-sectional study. BMC Infectious Diseases . 2020;20(1):395–410. doi: 10.1186/s12879-020-05096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah S. M., Rodin H., Pogemiller H., et al. Hepatitis B awareness and vaccination patterns among healthcare workers in Africa. The American Journal of Tropical Medicine and Hygiene . 2020;103(6):2460–2468. doi: 10.4269/ajtmh.20-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilonzo S. B., Nkandala I., Jaka H. M., Marmo L. Antiviral therapy eligibility and low treatment coverage among hepatitis B virus infected patients in Tanzania. Open Journal of Gastroenterology . 2023;13(10):299–312. doi: 10.4236/ojgas.2023.1310027. [DOI] [Google Scholar]
- 11.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews . 2015;4(1) doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson J., Welch V., Losos M., Tugwell P. J. The Newcastle-Ottawa scale (NOS) for assessing the quality of non-randomized studies in meta-analyses. Ottawa Hospital Research Institute . 2011;2(1):1–12. [Google Scholar]
- 13.Yazie T. D., Tebeje M. G. An updated systematic review and meta-analysis of the prevalence of hepatitis B virus in Ethiopia. BMC Infectious Diseases . 2019;19:917–1013. doi: 10.1186/s12879-019-4486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pellizzer G., Blé C., Zarnperetti N., et al. Serological survey of hepatitis B infection in Tanzania. Public Health . 1994;108(6):427–431. doi: 10.1016/s0033-3506(94)80100-2. [DOI] [PubMed] [Google Scholar]
- 15.Stark K., Poggensee G., Höhne M., Bienzle U., Kiwelu I., Schreier E. Seroepidemiology of TT virus, GBC-C/HGV, and hepatitis viruses B, C, and E among women in a rural area of Tanzania. Journal of Medical Virology . 2000;62(4):524–530. doi: 10.1002/1096-9071(200012)62:4<524::aid-jmv19>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Matee M. I. N., Magesa P. M., Lyamuya E. F. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses and syphilis infections among blood donors at the Muhimbili National Hospital in Dar Es Salaam, Tanzania. BMC Public Health . 2006;6(1):21–26. doi: 10.1186/1471-2458-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilonzo S. B., Gunda D. W., Kashasha F., Mpondo B. C. Liver fibrosis and hepatitis B coinfection among ART naive HIV-infected patients at a tertiary level hospital in northwestern Tanzania: a cross-sectional study. The Journal of Tropical Medicine . 2017;2017:6. doi: 10.1155/2017/5629130.5629130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller W. C., Shao J. F., Weaver D. J., et al. Seroprevalence of viral hepatitis in Tanzanian adults. Tropical Medicine and International Health . 1998;3(9):757–763. doi: 10.1046/j.1365-3156.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- 19.Msuya S. E., Mbizvo E. M., Hussain A., Sam N., Stray-Pedersen B. Seroprevalence of hepatitis B and C viruses among women of childbearing age in Moshi urban, Tanzania. East African Medical Journal . 2006;83(2):91–94. doi: 10.4314/eamj.v83i2.9394. [DOI] [PubMed] [Google Scholar]
- 20.Rashid S., Kilewo C., Aboud S. Seroprevalence of hepatitis B virus infection among antenatal clinic attendees at a tertiary hospital in Dar es Salaam Tanzania. Tanzania Journal of Health Research . 2014;16(1):9–15. doi: 10.4314/thrb.v16i1.2. [DOI] [PubMed] [Google Scholar]
- 21.Mirambo M. M., Mbena P. B., Mushi M. F., et al. Prevalence of Hepatitis B surface antigen among pregnant women attending antenatal clinic at Nyamagana district hospital Mwanza Tanzania. Tanzania Journal of Health Research . 2015;18(1):8–10. doi: 10.4314/thrb.v18i1.10. [DOI] [Google Scholar]
- 22.Froeschl G., Hoelscher M., Maganga L. H., et al. Hepatitis B, C and D virus prevalence in children and adults in Mbeya Region, Tanzania: results from a cohort study 2002-2009. The Pan African medical journal . 2021;39(1) doi: 10.11604/pamj.2021.39.174.26553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machange R. E., Mosha D., Pyuza J. J., Nyombi B. B., Shao E. R. Seroprevalence and knowledge of hepatitis B virus infection among LaboratoryWorkers at Kilimanjaro christian medical centre in moshi, Tanzania. The East African Health Research Journal . 2017;1(2):80–85. doi: 10.24248/EAHRJ-D-16-00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valerian D. M., Mauka W. I., Kajeguka D. C., et al. Prevalence and causes of blood donor deferrals among clients presenting for blood donation in northern Tanzania. PLoS One . 2018;13(10) doi: 10.1371/journal.pone.0206487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christian B., Fabian E., Macha I., et al. Hepatitis B virus coinfection is associated with high early mortality in HIV-infected Tanzanians on antiretroviral therapy. AIDS . 2019;33(3):465–473. doi: 10.1097/qad.0000000000002073. [DOI] [PubMed] [Google Scholar]
- 26.Meschi S., Schepisi M. S., Nicastri E., et al. The prevalence of antibodies to human herpesvirus 8 and hepatitis B virus in patients in two hospitals in Tanzania. Journal of Medical Virology . 2010;82(9):1569–1575. doi: 10.1002/jmv.21852. [DOI] [PubMed] [Google Scholar]
- 27.Ramírez-Mena A., Glass T. R., Winter A., et al. Prevalence and outcomes of hepatitis B coinfection and associated liver disease among antiretroviral therapy- naive individuals in a rural Tanzanian human immunodeficiency virus cohort. Open Forum Infectious Diseases . 2016;3(3) doi: 10.1093/ofid/ofw162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamenya T., Damian D. J., Ngocho J. S., Philemon R. N., Mahande M. J., Msuya S. E. The prevalence of hepatitis B virus among HIV-positive patients at Kilimanjaro Christian Medical Centre referral hospital, northern Tanzania. The Pan African medical journal . 2017;28 doi: 10.11604/pamj.2017.28.275.11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapinga D., Aboud S. Seroprevalence and factors associated with hepatitis B virus infection in pregnant women attending antenatal clinic in Karagwe district council Tanzania. International Journal of Infectious Diseases . 2018;73:p. 369. doi: 10.1016/j.ijid.2018.04.4249. [DOI] [Google Scholar]
- 30.Muro F. J., Fiorillo S. P., Sakasaka P., et al. Seroprevalence of hepatitis B and C viruses among children in Kilimanjaro region Tanzania. Journal of the Pediatric Infectious Diseases Society . 2013;2(4):320–326. doi: 10.1093/jpids/pit018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawkins C., Christian B., Ye J., et al. Prevalence of hepatitis B coinfection and response to antiretroviral therapy among HIV-infected patients in Tanzania. AIDS . 2013;27(6):919–927. doi: 10.1097/qad.0b013e32835cb9c8. [DOI] [PubMed] [Google Scholar]
- 32.Mueller A., Stoetter L., Kalluvya S. E., et al. Prevalence of hepatitis B virus infection among health care workers in a tertiary hospital in Tanzania. BMC Infectious Diseases . 2015;15(1):386–389. doi: 10.1186/s12879-015-1129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao E. R., Mboya I. B., Gunda D. W., et al. Seroprevalence of hepatitis B virus infection and associated factors among healthcare workers in northern Tanzania. BMC Infectious Diseases . 2018;18:474–510. doi: 10.1186/s12879-018-3376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shayo G., Makundi I., Luzzatto L. The prevalence of human immunodeficiency and of hepatitis B viral infections is not increased in patients with sickle cell disease in Tanzania. BMC Infectious Diseases . 2021;21(1) doi: 10.1186/s12879-021-06726-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manyahi J., Msigwa Y., Mhimbira F., Majigo M. High sero-prevalence of hepatitis B virus and human immunodeficiency virus infections among pregnant women attending antenatal clinic at Temeke municipal health facilities, Dar es Salaam Tanzania: a cross sectional study. BMC Pregnancy and Childbirth . 2017;17(1) doi: 10.1186/s12884-017-1299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng’wamkai G., Msigwa K. V., Chengula D., et al. Treponema pallidum infection predicts sexually transmitted viral infections (hepatitis B virus, herpes simplex virus-2, and human immunodeficiency virus) among pregnant women from rural areas of Mwanza region, Tanzania. BMC Pregnancy and Childbirth . 2019;19(1):p. 392. doi: 10.1186/s12884-019-2567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawambwa R. H., Majigo M. V., Mohamed A. A., Matee M. I. High prevalence of human immunodeficiency virus, hepatitis B and C viral infections among people who inject drugs: a potential stumbling block in the control of HIV and viral hepatitis in Tanzania. BMC Public Health . 2020;20:177–7. doi: 10.1186/s12889-020-8294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirambo M. M., Mkumbo E., Selega H., et al. Hepatitis B virus infections among health professional students in Mwanza city, Tanzania in 2016. Archives of Public Health . 2020;78(1):76–85. doi: 10.1186/s13690-020-00459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shedura V. J., Mchau G. J., Kamori D. High seroprevalence and associated risk factors for hepatitis B virus infection among pregnant women living with HIV in Mtwara region, Tanzania. Bulletin of the National Research Centre . 2023;47(1):p. 43. doi: 10.1186/s42269-023-01019-8. [DOI] [Google Scholar]
- 40.Franzeck F. C., Ngwale R., Msongole B., et al. Viral hepatitis and rapid diagnostic test based screening for HBsAg in HIV-infected patients in rural Tanzania. PLoS One . 2013;8(3):p. e58468. doi: 10.1371/journal.pone.0058468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kilonzo S. B., Gunda D. W., Majinge D. C., et al. Seroprevalence of hepatitis B virus infection, anti-HCV antibodies and HIV and knowledge among people who use drugs attending methadone therapy clinic in Tanzania; a cross-sectional study. BMC Infectious Diseases . 2021;21:699–9. doi: 10.1186/s12879-021-06393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menendez C., Sanchez-Tapias J. M., Kahigwa E., et al. Prevalence and mother-to-infant transmission of hepatitis viruses B, C, and E in Southern Tanzania. Journal of Medical Virology . 1999;58(3):215–220. doi: 10.1002/(sici)1096-9071(199907)58:3<215::aid-jmv5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 43.Suleiman R. Agriculture for food security 2030 report. Local and regional variations in conditions for agriculture and food security in Tanzania: A Review . 2018 [Google Scholar]
- 44.Kafeero H. M., Ndagire D., Ocama P., Kudamba A., Walusansa A., Sendagire H. Prevalence and predictors of hepatitis B virus (HBV) infection in east Africa: evidence from a systematic review and meta-analysis of epidemiological studies published from 2005 to 2020. Archives of Public Health . 2021;79(1):167–219. doi: 10.1186/s13690-021-00686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stockdale A. J., Mitambo C., Everett D., Geretti A. M., Gordon M. A. Epidemiology of hepatitis B, C and D in Malawi: systematic review. BMC Infectious Diseases . 2018;18(1):516–610. doi: 10.1186/s12879-018-3428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Who. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. 2015. https://www.who.int/publications/i/item/9789241549059. [PubMed]
- 47.Who. Hepatitis B vaccination coverage. https://immunizationdata.who.int/pages/coverage/hepb.html.
- 48.Salehi-Vaziri M., Sadeghi F., Almasi Hashiani A., Gholami Fesharaki M., Alavian S. M. Hepatitis B virus infection in the general population of Iran: an updated systematic review and meta-analysis. Hepatitis Monthly . 2016;16(4):1–8. doi: 10.5812/hepatmon.35577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H., Men P., Xiao Y., et al. Hepatitis B infection in the general population of China: a systematic review and meta-analysis. BMC Infectious Diseases . 2019;19:811–910. doi: 10.1186/s12879-019-4428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Makokha G. N., Zhang P., Hayes C. N., Songok E., Chayama K. The burden of hepatitis B virus infection in Kenya: a systematic review and meta-analysis. Frontiers in Public Health . 2023;11 doi: 10.3389/fpubh.2023.986020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uz-Zaman M. H., Rahman A., Yasmin M. Epidemiology of hepatitis B virus infection in Bangladesh: prevalence among general population, risk groups and genotype distribution. Genes . 2018;9(11):p. 541. doi: 10.3390/genes9110541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z., Lin C., Mao X., et al. Changing prevalence of chronic hepatitis B virus infection in China between 1973 and 2021: a systematic literature review and meta-analysis of 3740 studies and 231 million people. Gut . 2023;72(12):2354–2363. doi: 10.1136/gutjnl-2023-330691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yendewa G. A., Wang G. M., James P. B., et al. Prevalence of chronic hepatitis B virus infection in Sierra Leone, 1997–2022: a systematic review and meta-analysis. The American Journal of Tropical Medicine and Hygiene . 2023;109(1):105–114. doi: 10.4269/ajtmh.22-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shao E. R., Mboya I. B., Lyamuya F., et al. Uptake of cost-free hepatitis B vaccination among healthcare workers in northern Tanzania. Tanzania Medical Journal . 2021;32(2):39–56. [Google Scholar]
- 55.Aaron D., Nagu T. J., Rwegasha J., Komba E. Hepatitis B vaccination coverage among healthcare workers at national hospital in Tanzania: how much, who and why? BMC Infectious Diseases . 2017;17:786–787. doi: 10.1186/s12879-017-2893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ndunguru B., Wilfred D., Kapesa A., et al. Low uptake of hepatitis B vaccination among healthcare workers in primary health facilities in Mwanza region, north-western Tanzania. Frontiers in Public Health . 2023;11 doi: 10.3389/fpubh.2023.1152193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salou M., Ehlan A., Dossim S., et al. Assessment of the performance of six in vitro diagnostic kits for qualitative detection of hepatitis B virus surface antigen (HBsAg) in human serum or plasma in Lomé, Togo. African Journal of Clinical and Experimental Microbiology . 2020;21(3):211–216. [Google Scholar]
- 58.Eko Mba J. M., Bisseye C., Mombo L. E., et al. Assessment of rapid diagnostic tests and fourth‐generation Enzyme‐Linked Immunosorbent Assays in the screening of Human Immunodeficiency and hepatitis B virus infections among first‐time blood donors in Libreville (Gabon) Journal of Clinical Laboratory Analysis . 2019;33(3) doi: 10.1002/jcla.22824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amini A., Varsaneux O., Kelly H., et al. Diagnostic accuracy of tests to detect hepatitis B surface antigen: a systematic review of the literature and meta-analysis. BMC Infectious Diseases . 2017;17(S1):698–737. doi: 10.1186/s12879-017-2772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Who. Gudelines in hepatitis B and C testing. 2017. https://iris.who.int/bitstream/handle/10665/254621/9789241549981-eng.pdf .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: risk of bias assessment for selected studies.
Data Availability Statement
The data supporting the findings of this study are available within the article and the supplemental files.


