Living well with chronic pain: A 12-month randomized controlled trial revealing impact from the digital pain self-management program EPIO: Findings from a 12-month randomized controlled trial revealed long-term positive psychological changes for patients with chronic pain receiving EPIO, a digital pain self-management intervention program.
Keywords: Chronic pain, eHealth, Digital pain self-management, Psychosocial self-management, Pain interference, Cognitive behavioral therapy, Acceptance and commitment therapy, Self-regulatory fatigue, Anxiety, Depression
Abstract
Introduction:
Chronic pain affects a wide range of physical and psychological aspects of life for those impacted. Psychosocial treatment approaches may be of support, but outreach is still limited.
Objectives:
To evaluate the efficacy of EPIO, an evidence-informed, user-centered digital self-management intervention for people with chronic pain, in a 12-month randomized controlled trial.
Methods:
People living with chronic pain (N = 266) were randomized to the EPIO intervention (n = 132) or a usual-care control group (n = 134). The intervention was delivered in a simple blended care model, and outcome measures collected at baseline, 6 months, and 12 months. Generalized linear models for repeated measures were fitted to compare groups over time.
Results:
Participants were primarily female (81%), median age 49 years (range 22–78), with heterogeneous pain conditions, and had lived with pain >5 years (77.6%). A mixed linear model with all timepoints included revealed no statistically significant group differences for the primary outcome of pain interference. Significant psychological benefits in favor of the intervention group were however detected for depression (P = 0.022), self-regulatory fatigue (P = 0.024), vitality (P = 0.016), and mental health (P = 0.047). Baseline to 12-month changes showed additional favorable effects for anxiety (between-group mean differences [MDs] = 0.79, P = 0.047), depression (MD = 1.08, P = 0.004), self-regulatory fatigue (MD = 2.42, P = 0.021), pain catastrophizing (MD = 2.62, P = 0.009), and health-related quality of life.
Conclusions:
The EPIO program aims to improve outreach of evidence-based pain self-management interventions. Findings demonstrate how using EPIO can lead to sustainable psychological change, enhancing mental health and health-related quality of life for people suffering from pain, providing a chance to live well with the pain.
1. Introduction
Chronic pain continues to be a serious personal and public health concern, impacting physical and psychological well-being, sleep, physical and social activities, private and professional roles, and relationships.44,52 Living with chronic pain naturally also impacts quality of life and ultimately ability to cope.30,31,74 The multitude of challenges presented by living with chronic pain may also impact ability to regulate thoughts, feelings, and behavior (ie, self-regulation).46–48,58
The significant impact and interference of chronic pain is accompanied by a recognition of chronic pain as a condition with contributing biological, psychological and social factors.15 Evidence-based biopsychosocial treatment methods have therefore been recommended, including psychosocial self-management approaches such as cognitive behavioral therapy (CBT)6,25,72 and more recently also acceptance and commitment therapy (ACT).20,25,27,32 Despite the established, growing evidence of such approaches, outreach remains limited, indicating the need for innovative delivery methods.20
Digital solutions in the form of applications (apps) may have the potential to expand outreach of pain self-management approaches, and research has shown how digital interventions may reduce pain intensity and improve physical and psychosocial functioning.45 Challenges with existing digital pain management interventions nevertheless include limited or lack of theoretical basis19,75; limited or lack of user (ie, people with chronic pain and health care providers) involvement during the design and development processes60; challenges with program attrition/adherence2,41; limited efficacy testing/evidence of effect,38,61 particularly from trials longer than 3 months45; and also limited planning for, or evidence of, implementation poststudy.67
Aiming to improve outreach of pain self-management interventions, considering current issues with digital interventions, this research team developed EPIO (ie, inspired by the Greek goddess for the soothing of pain; Epione), a digital psychosocial pain self-management program.10,11,36,37,66 EPIO was designed and developed using evidence-based, user-centered processes, with iterative user testing and evidence-informed content,36,37,66 aiming to be of support to anyone living with chronic pain (ie, not sex, age, or pain type/diagnosis-specific). In accordance with the Medical Research Council framework for complex intervention evaluation,16,57 the EPIO program was tested in a feasibility pilot study, with participants living with chronic pain rating the program as useful, with excellent system usability.10 Participants also described EPIO as facilitating motivation to learn, aiding in making peace with the presence of pain, and experienced EPIO as a friend, promoting communication and support.11
This study explored findings from a 12-month (ie, primary end point) randomized controlled trial (RCT) testing the use of EPIO in people living with chronic pain (ie, noncancer, nonmigraine pain lasting ≥3 months). Participants in the EPIO intervention group, compared with participants in a usual-care control group, were hypothesized to report significant improvements in pain interference (ie, primary outcome) and anxiety, depression, self-regulatory fatigue, health-related quality of life (HRQoL), pain catastrophizing, and pain acceptance (ie, all secondary outcomes). Short-term (ie, 3-month) explorations, published after 12-month RCT completion, showed decreased symptoms of depression and self-regulatory fatigue for participants having access to EPIO, with EPIO described as useful and easy to use.9
2. Methods
2.1. Study design
A 2-armed 12-month RCT with participants with chronic pain randomly assigned to (1) the digital pain self-management intervention program EPIO or (2) a usual-care control group.
2.2. Participants and recruitment
People living with chronic pain were recruited through a major medical institution, collaborating health care practices, social media, or patient organizations' web pages. Eligibility criteria (ie, self-reported) were as follows: (1) living with chronic pain (ie, not pain condition/diagnosis-specific), (2) having lived with pain ≥3 months, (3) being 18 years and older, (4) having access to a smartphone/tablet, (5) understanding oral/written Norwegian, and (6) being able to attend an introduction session either at a health care facility or through a secure video link. Exclusion criteria included self-reported cancer-related pain, migraine, or severe untreated psychological illness.
2.3. The EPIO intervention program
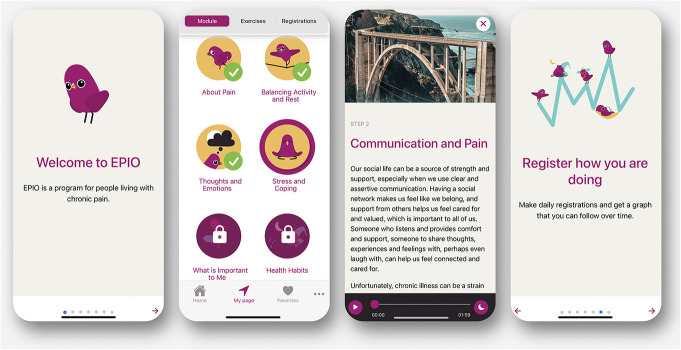
The EPIO program content is primarily CBT-based, with value aspects of ACT, and centers around well-known components for pain self-management,36 with 9 modules containing a combination of psychoeducational information (eg, about pain, importance of activity pacing, and use of coping strategies) and exercises (eg, thought challenges and diaphragmatic breathing).36 See Figure 1 for program content overview. The EPIO program can be individualized through options for reading and/or listening, choice of favorites, and graphs related to sleep, rest, activity, and mood.36 See Figure 2 for example screenshots.
Figure 1.
EPIO program content.
Figure 2.
EPIO program screenshots.
2.4. Study procedure
Study methods and results are reported following the CONSORT 2010 checklist for parallel group randomized trials,53 and the intervention described in accordance with the TIDieR checklist.28 The Regional Committee for Medical and Health Research Ethics (REK 2018/8911) and the Hospital Privacy Protection Committee (PVO 2017/6697) provided study approvals. The study was ClinicalTrials.gov (NCT 03705104) registered before enrollment, and all participants signed informed consent prior to preparticipation.
Study information was provided through a study website, related social media, or verbally/through flyers by collaborating health care practices. Interested participants could submit a contact form or call the study phone, receiving additional study information from a project team member.
Enrolled participants completed baseline outcome measures before computerized randomization (ie, using R-tool, a local software program), stratified by sex, to either the intervention or control group (ie, study arms 1:1, block size 20). Because patients were assigned to the EPIO intervention or not, true blinding was not possible. Outcome measures and system use data were collected electronically through a secure server (ie, Services for Sensitive Data, University of Oslo) using encrypted connections.
2.4.1. Intervention group
Participants randomized to the intervention group received (1) a face-to-face (ie, either in-person or by video during COVID-19) introduction session with study personnel, (2) access to the app-based EPIO program for 12 months, and (3) two brief follow-up phone calls at approximately 3 and 7 weeks to ask how participants were doing with the program and whether they had any program-related questions. The introduction session was conducted by 1 to 2 members of the project team using a structured manual, describing rationale for the EPIO program (ie, pain self-management), program downloading, and introducing content examples. Study personnel conducting the introduction sessions and follow-up phone calls were public health scientists or registered nurses, trained and supervised by the Principal Investigator; a licensed clinical health psychologist. Participants could contact the project team through a study phone during regular working hours for study-related assistance.
2.4.2. Usual-care control group
The control group received no follow-up apart from reminders to complete outcome measures but could call the project study phone in case of questions. No information was obtained to control for engagement in other types of self-management interventions during the study period.
2.5. Data collection and outcome measures
Outcome measures were collected at baseline (ie, including a study-specific sociodemographic/disease-specific measure), 3,9 6, and 12 months. Notes from follow-up phone calls were written down immediately after phone calls.
2.5.1. Psychosocial outcome measures
2.5.1.1. Pain interference
Pain interference (ie, primary outcome) and pain severity were measured with the short version of the Brief Pain Inventory.34 Brief Pain Inventory pain interference consists of 7 items measuring impact of pain on daily function, including activity/affective aspects of interference,3 and 4 items measuring pain severity. The Brief Pain Inventory has been validated in a Norwegian cancer pain population35 and has shown acceptable internal consistency and reliability in patients with noncancer pain.34 Score range is 0 to 10, with higher scores indicating higher interference/severity.
2.5.1.2. Symptoms of anxiety and depression
Symptoms of anxiety and depression were measured with the Hospital Anxiety and Depression Scale,76 a 14-item scale with 7 items gauging symptoms of anxiety and depression, respectively. Score range is 0 to 21 for each subscale, and higher scores indicate higher symptom presence. The Hospital Anxiety and Depression Scale has acceptable internal consistency and reliability and has been validated in a Norwegian sample.39
2.5.1.3. Self-regulatory fatigue
Self-regulatory fatigue was measured with the Self-Regulatory Fatigue scale,47 with 18 items gauging cognitive, emotional, and behavioral components of capacity to self-regulate. The Self-Regulatory Fatigue-18 has been validated in Norwegian cancer populations7,8 and has acceptable internal consistency and reliability.47 Score range is 18 to 90, with higher scores indicating higher self-regulatory fatigue.
2.5.1.4. Health-related quality of life
Health-related quality of life was measured using the noncommercial SF-36 Short Form Health Survey, RAND-36,70 with 36 items measuring physical, role, emotional, cognitive, and social function, as well as physical, general, and global health. The RAND-36 has acceptable internal consistency and reliability and has been validated in a Norwegian chronic pain sample.40 Score range is 0 to 100, with higher scores indicating higher HRQoL.
2.5.1.5. Pain catastrophizing
Pain catastrophizing was measured with the Pain Catastrophizing Scale,59 a 13-item scale measuring catastrophic thinking and maladaptive responses to pain, including subscales measuring helplessness, magnification, and rumination. The Pain Catastrophizing Scale has acceptable internal consistency and reliability and has been validated in a Norwegian chronic pain sample.26 Score range is 0 to 52, with higher scores referencing higher level of catastrophic thoughts/feelings about pain.
2.5.1.6. Pain acceptance
Pain acceptance was measured through the Chronic Pain Acceptance Questionnaire short form,42 an 8-item pain acceptance measure gauging pain willingness and activity engagement (4 items each). The Chronic Pain Acceptance Questionnaire has acceptable internal consistency and reliability and has been validated in a Norwegian chronic pain sample.23 Score range is 0 to 24, with higher scores indicating higher pain acceptance.
2.5.2. Program use
Data related to program use and progress were collected automatically through a secure research server. Program completers were defined as participants completing at least 6 of the 9 EPIO modules (67%) during the study period.8–10,71
2.6. Power analysis and sample estimates
Previous studies with digital health interventions have reported Cohen d effect sizes of 0.30 to 0.40 on pain interference (ie, primary outcome here) for comparable samples.13,62 To allow a detection of d = 0.4 for the primary outcome, with an alpha of 0.05% and 80% power (based on a 2-sided t test), a sample size of 200 participants was required. Considering probable attrition, and adequate power in potential secondary analyses, total study sample included 266 participants.
2.7. Statistical and thematic analyses
Baseline characteristics and user patterns were summarized with mean and SD for normally distributed variables, and median and ranges for variables with skewed distributions. Categorical data were presented as counts and percentages. For the analysis of between-group differences in outcome measurements, generalized linear models (GLMs) for repeated measures were fitted. To account for statistical dependencies as each individual was measured several times and time spans between completed measurements varied, an unstructured covariance matrix was used to model covariances. Models for each outcome consisted of 3 covariates: measurement (time), group, and interaction term (ie, time and group). All measured timepoints (ie, for outcome variables) were considered, and all overall between-group differences were, therefore, adjusted for baseline differences.
Because statistically significant differences were observed between the intervention and the usual-care control group for age, disability benefits at baseline, and years living with pain, these variables were included in the analysis as possible confounders. All analyses were conducted according to intention-to-treat principles, including all participants in each group independently of how much the intervention group used the intervention. Between-group differences are reported as the intervention group change from the baseline to 6 and 12 months, minus the usual-care control group change from baseline. Exploratory subgroup analyses for the intervention group only, using GLMs, were performed to detect potential differences in outcomes between intervention completers and noncompleters of the EPIO program. In addition, the effect of years living with pain (3 categories; <5, 5–10, >10 years), level of education (3 categories; Elementary/high school, University/college ≤4 years, University/college >4 years), and diagnosis group (4 International Classification of Diseases version 11 (ICD-11) categories; primary pain, nociceptive pain/secondary musculoskeletal pain, neuropathic pain, posttraumatic/postoperative) were explored. The effect of these variables on the outcome was assessed using a GLM model with an interaction term (the assessed variable × group) to explore whether such a variable might affect the groups differently (ie, intervention vs controls). P-values < 0.05 were considered statistically significant. The results are presented as marginal means evaluated at mean age and given timepoint, estimated mean differences (MDs) between groups, with 95% confidence intervals and effect sizes (ie, Standardized Coefficients β).54 Effect sizes were interpreted as small (0.10–0.29), medium (0.30–0.49), and large (≥0.50).50 Statistical analyses were performed using the Statistical Package for the Social Sciences (release 28; SPSS, Inc, Chicago, IL) and Stata (version 17).
Qualitative data (ie, derived from 267 follow-up phone call notes, equaling approximately 55 single-line pages) were uploaded by coauthor K.B. to the software program NVivo version 12 (QSR International, Victoria, Australia) and analyzed using a thematic analysis process (ie, coding reliability).12,14 Authors L.S.N., E.B., and E.B.S. then further analyzed findings and discussed until consensus was achieved.
3. Results
3.1. Enrollment and sample description
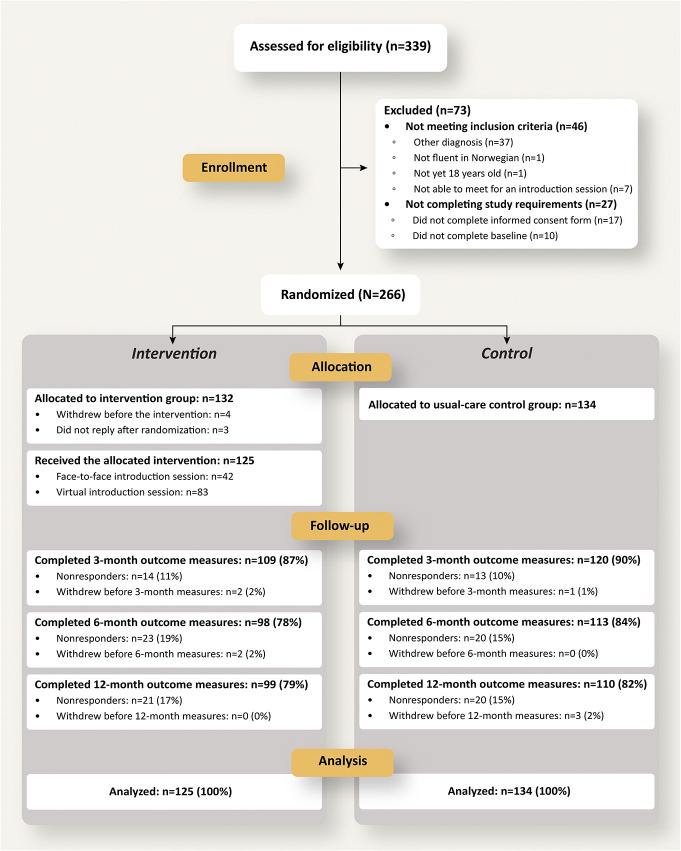
From November 2019 to February 2021, 339 adults living with chronic pain were screened and 266 enrolled. Of these, 7 participants randomized to the intervention group were excluded or withdrew from the study, resulting in a total study sample of 259 participants, allocated to the intervention (n = 125) or control (n = 134) groups. See Figure 3 CONSORT flow diagram for an overview of recruitment and retention details.
Figure 3.
CONSORT flow diagram describing enrollment, allocation, follow-up for the 12-month EPIO study. Participants not completing outcome measures are referred to as “Nonresponders.”
Two-thirds (66%) of the included participants were primarily recruited through social media, and the remainder (34%) through collaborating health care practices. The final 259 participants were primarily self-identified female (81%) and Anglo-American (97%). At inclusion, participants were median 49 years (range 22–78), most reported being on sick leave or disability benefits (186/259, 72%), suffered from fibromyalgia or unspecific musculoskeletal pain (166/259, 64%), and described having lived with pain ≥10 years (158/259, 61%; Table 1). Follow-up phone calls were conducted with n = 184 participants {(ie, at 3 [n = 102] and 7 [n = 82] weeks)}.
Table 1.
Sociodemographic-related and disease-related characteristics at baseline (N = 259).
| Characteristics | All participants (N = 259) | Intervention (n = 125) | Control (n = 134) | P | |||
|---|---|---|---|---|---|---|---|
| Age (y), median (range) | 49 | 22–78 | 50 | 26–74 | 48 | 22–78 | 0.020 |
| Gender, n (%) | 0.601 | ||||||
| Female | 210 | 81 | 103 | 82.4 | 107 | 79.9 | |
| Male | 49 | 19 | 22 | 17.6 | 27 | 20.1 | |
| Marital status, n (%) | 0.291 | ||||||
| Married/cohabitating | 172 | 66.4 | 79 | 63.2 | 93 | 69.4 | |
| Single/divorced | 87 | 33.6 | 46 | 36.8 | 41 | 30.6 | |
| Education, n (%) | 0.482 | ||||||
| Elementary/high school | 108 | 41.7 | 49 | 39.2 | 59 | 44.0 | |
| University/college ≤4 y | 104 | 40.2 | 55 | 44.0 | 49 | 36.6 | |
| University/college >4 y | 47 | 18.1 | 21 | 16.8 | 26 | 19.4 | |
| Employment, n (%) | 0.583 | ||||||
| Full-time/part-time work | 57 | 22.0 | 30 | 24.0 | 27 | 20.1 | |
| Sick leave/disability benefits | 186 | 71.8 | 86 | 68.8 | 100 | 74.6 | |
| Retired/others | 16 | 6.2 | 9 | 7.2 | 7 | 5.2 | |
| 100% disability benefits, n (%) | 78 | 30.1 | 45 | 36.6 | 33 | 24.6 | 0.042 |
| Income status, (EUR*), n (%) | 0.913 | ||||||
| <40.000 | 54 | 20.8 | 28 | 22.4 | 26 | 19.4 | |
| >40.000–60.000 | 41 | 15.8 | 19 | 15.2 | 22 | 16.4 | |
| >60.000–80.000 | 63 | 24.3 | 32 | 25.6 | 31 | 23.1 | |
| >80.000–100.000 | 55 | 21.2 | 24 | 19.2 | 31 | 23.1 | |
| >100.000 | 56 | 17.8 | 22 | 17.6 | 24 | 17.9 | |
| Self-reported pain conditions,† n (%) | |||||||
| Unspecific musculoskeletal pain‡ | 59 | 22.8 | 27 | 24.1 | 32 | 26.0 | 0.736 |
| Unspecified disk disorder§ | 31 | 12.0 | 15 | 13.4 | 16 | 13.0 | 0.931 |
| Osteoarthritis§ | 48 | 18.5 | 22 | 19.6 | 26 | 21.1 | 0.776 |
| Rheumatoid arthritis§ | 30 | 11.6 | 16 | 14.3 | 14 | 11.5 | 0.521 |
| Fibromyalgia‡ | 107 | 41.3 | 53 | 47.3 | 54 | 43.9 | 0.599 |
| Neuropathic pain‖ | 19 | 7.3 | 10 | 8.9 | 9 | 7.3 | 0.651 |
| Postinjury or postsurgery¶ | 24 | 9.3 | 13 | 11.6 | 11 | 8.9 | 0.501 |
| Other‡‖# | 43 | 16.6 | 25 | 22.5 | 18 | 14.6 | 0.120 |
| Years living with pain, n (%) | 0.029 | ||||||
| <3 years | 34 | 13.1 | 12 | 9.6 | 22 | 16.4 | 0.104 |
| 3–5 years | 24 | 9.3 | 7 | 5.6 | 17 | 12.7 | 0.049 |
| >5–10 years | 43 | 16.6 | 19 | 15.2 | 24 | 17.9 | 0.558 |
| >10 years | 158 | 61.0 | 87 | 69.6 | 71 | 53.0 | 0.006 |
EUR, 1 EURO is approximately 1.1 USD; approximately 10 Norwegian kroner (fall 2023).
Participants could report having several types of self-reported conditions.
Categorized for study purposes as primary pain (ICD-11).
Categorized for study purposes as nociceptive pain/secondary musculoskeletal pain (ICD-11).
Categorized for study purposes as neuropathic pain (ICD-11).
Categorized for study purposes as posttraumatic/postoperative pain (ICD-11).
Includes categories such as (chronic fatigue syndrome) CFS/ME, Complex Regional Pain Syndrome, and nonspecific “other.”
Participants in the intervention group were statistically significantly older compared with the control group (median 50 and 48 years, respectively), and more participants in the intervention group reported having lived with pain ≥10 years compared with the control group (87/125, 69.6% vs 71/134, 53%, respectively). In addition, a higher proportion of the intervention group reported being on 100% disability at baseline (24/125, 36.6% vs 33/134, 24.6%). Age, years living with pain, and disability status were therefore included in the linear mixed model analyses as potential confounders. No harm or unintended effects were reported or detected in either group during the study.
3.2. Between-group differences
Including all timepoints in the mixed model, no statistically significant between-group differences were observed over the 12 months for the primary outcome of pain interference (Table 2). There were however statistically significant differences in favor of the EPIO intervention group for the secondary outcomes of depression and self-regulatory fatigue, as well as for the HRQoL subscales vitality and mental health (Table 2).
Table 2.
Effects of EPIO at 6 and 12 months.*
| Intervention group n = 125 | Control group n = 134 | Between-group differences | Time-trend | |||
|---|---|---|---|---|---|---|
| M (95% CI) | M (95% CI) | MD (95% CI) | P | Effect size β† | P | |
| Pain interference (BPI)‡ | 0.795 | |||||
| Baseline | 5.1 (4.7–5.5) | 5.6 (5.2–6.0) | ||||
| 6 months | 4.6 (4.2–5.1) | 5.0 (4.6–5.4) | −0.16 (−0.67 to 0.34) | 0.515 | −0.04 | |
| 12 months | 4.6 (4.1–5.1) | 5.0 (4.5–5.4) | −0.14 (−0.67 to 0.40) | 0.612 | −0.03 | |
| Pain severity (BPI)§ | 0.450 | |||||
| Baseline | 5.1 (4.8–5.4) | 5.4 (5.1–5.6) | ||||
| 6 months | 4.9 (4.5–5.6) | 5.3 (5.0–5.6) | 0.16 (−0.18 to −51)) | 0.354 | 0.06 | |
| 12 months | 4.7 (4.3–5.1) | 5.2 (4.9–5.6) | 0.27 (−0.07 to 0.62) | 0.122 | 0.10 | |
| Anxiety (HADS-A‖) | 0.253 | |||||
| Baseline | 7.1 (6.2–7.9) | 7.7 (6.8–8.5) | ||||
| 6 months | 6.3 (5.4–7.2) | 7.2 (6.4–8.0) | 0.30 (−0.48 to 1.07) | 0.456 | 0.05 | |
| 12 months | 6.1 (5.1–7.0) | 7.4 (6.6–8.3) | 0.79 (0.01 to 1.57) | 0.047 | 0.12 | |
| Depression (HADS-D¶) | 0.022 | |||||
| Baseline | 7.0 (6.2–7.7) | 7.1 (6.3–7.8) | ||||
| 6 months | 5.8 (5.1–6.6) | 6.6 (4.9–7.2) | 0.62 (−0.12 to 1.35) | 0.100 | 0.10 | |
| 12 months | 5.7 (4.8–6.5) | 6.8 (6.0–7.6) | 1.08 (0.35 to 1.82) | 0.004 | 0.18 | |
| Self-regulatory fatigue (SRF-18#) | 0.024 | |||||
| Baseline | 54.8 (52.6–57.0) | 55.7 (53.6–57.8) | ||||
| 6 months | 53.3 (51.1–55.6) | 55.1 (53.0–57.2) | 0.80 (−1.25 to 2.84) | 0.446 | 0.05 | |
| 12 months | 51.9 (49.5–54.2) | 55.1 (52.8–57.3) | 2.42 (0.36 to 4.47) | 0.021 | 0.14 | |
| HRQoL (RAND-36**) | ||||||
| Physical functioning | 0.309 | |||||
| Baseline | 52.7 (48.1–57.2) | 49.6 (45.2–53.9) | ||||
| 6 months | 57.1 (52.3–62.0) | 55.0 (50.4–59.6) | 0.99 (−3.03 to 5.00) | 0.630 | ||
| 12 months | 56.1 (51.3–61.0) | 55.5 (51.0–60.1) | 2.59 (−1.43 to 6.62) | 0.207 | ||
| Role-physical | 0.918 | |||||
| Baseline | 16.4 (11.3–21.6) | 8.40 (3.5–13.3) | ||||
| 6 months | 22.5 (15.7–29.2) | 16.2 (10.0–22.4) | 1.33 (−7.55 to 10.22) | 0.768 | 0.02 | |
| 12 months | 23.1 (16.3–30.0) | 16.1 (9.7–22.5) | 1.01 (−7.89 to 9.91) | 0.824 | 0.01 | |
| Bodily pain | 0.918 | |||||
| Baseline | 26.4 (23.4–29.4) | 24.9 (22.1–27.8) | ||||
| 6 months | 31.6 (28.8–35.3) | 28.6 ([25.2–32.0 | −1.47 (−5.96 to 3.02) | 0.521 | −0.04 | |
| 12 months | 33.7 ([29.5–38.1 | 28.1 (24.1–32.1) | −4.17 (−8.67 to 0.32) | 0.069 | −0.11 | |
| General health | 0.234 | |||||
| Baseline | 35.4 (31.5–39.4) | 35.6 (31.9–39.3) | ||||
| 6 months | 39.3 (35.0–43.6) | 36.9 (32.8–40.9) | −2.41 (−6.22 to 1.39) | 0.214 | −0.08 | |
| 12 months | 39.8 (35.4–44.1) | 38.8 (31.9–40.1) | −3.94 (−7.76 to −0.12) | 0.043 | −0.13 | |
| Vitality | 0.016 | |||||
| Baseline | 26.0 (22.2–29.7) | 24.6 (21.0–28.2) | ||||
| 6 months | 28.8 (24.6–33.0) | 27.7 (23.8–31.7) | 0.26 (−3.96 to 4.48) | 0.905 | 0.01 | |
| 12 months | 32.0 (27.7–36.4) | 26.0 (21.6–29.8) | −4.97 (−9.20 to −0.74) | 0.021 | −0.14 | |
| Social functioning | 0.297 | |||||
| Baseline | 45.5 (40.7–50.2) | 44.3 (39.8–48.9) | ||||
| 6 months | 55.1 (49.8–60.4) | 49.9 (44.9–54.8) | −4.02 (−10.17 to 2.12) | 0.200 | −0.08 | |
| 12 months | 54.7 (49.4–59.9) | 47.9 (43.0–52.7) | −5.66 (−11.82 to 0.50) | 0.072 | −0.11 | |
| Role-emotional | 0.210 | |||||
| Baseline | 45.7 (37.1–54.4) | 51.8 (43.5–60.1) | ||||
| 6 months | 50.2 (41.0–59.4) | 48.9 (40.4–57.4) | −7.24 (−20.25 to 5.78) | 0.276 | −0.07 | |
| 12 months | 59.4 (50.2–68.7) | 50.6 (42.0–59.3) | −14.52 (−27.57 to −1.47) | 0.029 | −0.14 | |
| Mental health | 0.047 | |||||
| Baseline | 65.2 (61.5–68.8) | 64.7 (61.3–68.1) | ||||
| 6 months | 65.1 (61.0–69.1) | 64.4 (60.7–68.2) | −0.05 (−3.81 to 3.71) | 0.980 | −0.00 | |
| 12 months | 67.2 (63.1–71.3) | 62.8 (58.9–66.7) | −4.05 (−7.82 to −0.28) | 0.035 | −0.13 | |
| Pain catastrophizing (PCS††) | ||||||
| Rumination | 0.136 | |||||
| Baseline | 7.4 (6.7–8.2) | 8.2 (7.5–9.0) | ||||
| 6 months | 6.2 (5.4–7.0) | 7.8 (7.0–8.6) | 0.81 (−0.05 to 1.67) | 0.064 | 0.12 | |
| 12 months | 5.6 (4.7–6.4) | 7.4 (6.6–8.2) | 1.06 (0.20 to 1.92) | 0.016 | 0.15 | |
| Magnification | 0.327 | |||||
| Baseline | 3.5 (3.0–4.0) | 3.8 (3.3–4.3) | ||||
| 6 months | 3.0 (2.5–3.5) | 3.7 (3.2–4.2) | 0.28 (−0.25 to 0.81) | 0.303 | 0.06 | |
| 12 months | 2.7 (2.2–3.2) | 3.6 (3.1–4.1) | 0.57 (0.04 to 1.10) | 0.035 | 0.13 | |
| Helplessness | 0.407 | |||||
| Baseline | 8.5 (7.5–9.5) | 9.7 (8.8–10.7) | ||||
| 6 months | 7.6 (6.6–8.6) | 9.4 (8.4–10.3) | 0.49 (−0.54 to 1.52) | 0.347 | 0.06 | |
| 12 months | 7.0 (5.9–8.1) | 9.2 (8.2–10.2) | 1.01 (−0.03 to 2.04) | 0.056 | 0.12 | |
| PCS total | 0.185 | |||||
| Baseline | 19.5 (17.4–21.5) | 21.9 (19.3–23.8) | ||||
| 6 months | 16.9 (14.8–19.0) | 20.9 (19.0–22.9) | 1.59 (−0.36 to 3.55) | 0.111 | 0.10 | |
| 12 months | 15.3 (13.1–17.6) | 20.3 (18.1–22.4) | 2.62 (0.66 to 4.58) | 0.009 | 0.16 | |
| Chronic pain acceptance (CPAQ‡‡) | ||||||
| Willingness | 0.704 | |||||
| Baseline | 13.7 (13.1–14.2) | 13.8 (13.3–14.3) | ||||
| 6 months | 13.7 (13.1–14.2) | 13.8 (13.2–14.3) | −0.05 (−0.81 to 0.72) | 0.908 | 0.01 | |
| 12 months | 13.9 (13.3–14.5) | 13.6 (13.1–14.2) | −0.42 (−1.19 to 0.35) | 0.288 | 0.07 | |
| Activity engagement | 0.086 | |||||
| Baseline | 13.5 (12.8–14.2) | 13.8 (13.1–14.5) | ||||
| 6 months | 13.8 (13.1–14.5) | 14.1 (13.5–14.8) | 0.12 (−0.67 to 0.92) | 0.770 | 0.02 | |
| 12 months | 14.4 (13.7–15.1) | 14.3 (13.7–15.0) | −0.43 (−1.23 to 0.37) | 0.293 | −0.07 | |
| CPAQ total | 0.225 | |||||
| Baseline | 27.2 (26.1–28.3) | 27.6 (26.6–28.7) | ||||
| 6 months | 27.5 (26.4–28.6) | 27.9 (26.9–29.0) | 0.07 (−1.20 to 1.34) | 0.913 | 0.01 | |
| 12 months | 28.3 (27.3–29.4) | 28.0 (26.9–29.0) | −0.83 (−2.11 to 0.44) | 0.201 | 0.08 | |
Estimated means from generalized linear mixed models.
Three-months findings reported elsewhere.9
Effect size β study-specific interpretations: small = 0.10 to 0.29, medium = 0.30 to 0.49, and large ≥0.5.50
Subscale of the Brief Pain Inventory (score range 0–10; a higher score indicates higher interference in life).
Subscale of the Brief Pain Inventory (score range 0–10; a higher score indicates higher severity).
HADS-A: Hospital Anxiety and Depression Scale-Anxiety subscale (score range 0–21; a higher score indicates a higher degree of anxiety).
HADS-D: Hospital Anxiety and Depression Scale-Depression subscale (score range 0–21; a higher score indicates a higher degree of depression).
SRF-18: Self-regulatory Fatigue 18 scale (score range 18–90; a higher score indicates higher self-regulatory fatigue).
RAND-36: RAND 36-Item scale (score range 0–100; a higher score indicates higher emotional well-being).
PCS: Pain Catastrophizing Scale (score range 0–52; a higher score indicates higher catastrophizing).
CPAQ: Chronic Pain Acceptance Questionnaire (score range 0–52; a higher score indicates a higher acceptance of pain).
BPI: Brief Pain Inventory; CI, confidence interval; HRQoL, health-related quality of life; MD, mean difference; β, standardized coefficients beta.
The statistical significance for the bold entries is listed under P and Time-trend P.
The largest intervention effects in favor of the intervention group were observed at 12 months, and between-group changes from baseline to 12 months were statistically significant in favor of the intervention group for several measures, including symptoms of anxiety (between-group MD = 0.79, P = 0.047), symptoms of depression (MD = 1.08, P = 0.004), self-regulatory fatigue (MD = 2.42, P = 0.021), HRQoL subscales general health (MD = −3.94, P = 0.043), vitality (MD = −4.97, P = 0.021), role-emotional (MD = −14.52, P = 0.029), and mental health (MD = −4.5, P = 0.035), as well as pain catastrophizing rumination (MD = 1.06, P = 0.016), magnification (MD = 0.57, P = 0.035), and total (MD = 2.62, P = 0.009; Table 2).
3.3. Program use
Timespan from first to last use varied from 1 to 364 days (medium 182), with use 1 to 315 days (medium 30). Two-thirds (82/125, 66%) of the participants in the intervention group completed ≥6/9 modules within the 12-month study period (ie, completers). Of those, 55% completed all 9 modules. No statistically significant differences in outcome measures were observed between intervention completers and noncompleters.
3.4. Exploratory analyses
There were no statistically significant differences for the separate activity and affective components of pain interference, and no outcome differences in time trajectories between intervention and control groups when stratified by years living with pain, level of education, or diagnosis group.
3.5. Follow-up phone calls—qualitative analyses
Notes from follow-up phone calls were analyzed into 4 themes as follows: (1) raising awareness, (2) a useful toolbox, (3) use, and (4) barriers for use. Participants described EPIO as particularly raising awareness about the connection between thoughts and feelings, motivation for change, and need for activity pacing, planning, and self-care. EPIO was described as a useful toolbox, providing coping strategies and helping participants deal with the pain, even if the pain did not go away. Some described using EPIO with others (eg, partner/health care provider) as beneficial and suggested incorporating EPIO in, and after, pain rehabilitation care. Participants reported implementing simple exercises (eg, diaphragmatic breathing) into daily life, without the app, and described practicing breathing exercises as contributing to helpful distraction, relaxation, calmness, and pain reduction. Struggling to prioritize use was described as the most frequent barrier for use.
4. Discussion
4.1. Principal findings
Considering all timepoints over the course of 12 months in a linear mixed model revealed no statistically significant between-group differences for the primary outcome of pain interference on function. Participants having access to EPIO did however report significantly lower symptoms of depression and self-regulatory fatigue as well as improved HRQoL vitality and mental health, compared with the control group. The largest between-group changes were observed from baseline to 12 months, with statistically significant findings in favor of the intervention group for symptoms of anxiety, depression, self-regulatory fatigue, HRQoL (ie, general health, vitality, role-emotional, and mental health), and pain catastrophizing.
Taking all findings into account, the EPIO intervention was associated with significant changes for psychological variables, yet nonsignificant changes for pain-related variables including physical components (ie, pain intensity, pain interference, and physical HRQoL scales). This might partly be explained by the fact the EPIO program is built to increase agency, raise awareness about psychosocial components, provide knowledge, and foster engagement in helpful strategies. As such, EPIO does not encourage people to think life can be pain free. Rather, EPIO seeks to show how pain is just a part of life, but that living well with pain is possible, a fact also reflected upon by participants in the follow-up phone calls.
Effects of existing pain self-management interventions seem to fade after 3 months.72 This study, with significant changes in psychological domains after 12 months in favor of the intervention group, even stronger than 3-month findings,9 therefore provides a major contribution to the pain literature. The nature of the EPIO intervention, with participants having access anywhere and anytime for 12 months, may explain these long-term effects, perhaps together with the fact that learning takes time, particularly when aiming for change after having lived with pain for years.
4.2. Mental health, quality of life, and self-regulation
The statistically significant psychological findings in favor of the intervention group indicate that using EPIO can strengthen mental health and quality of life for people suffering from chronic pain. Seeking to meet current recommendations for digital health solutions,10,11,29,36,37,41,60,66 including evidence-based content and user involvement in design and development processes, likely contributed to these improvements. This is also consistent with research showing potential positive impact of CBT and ACT for people living with chronic pain.22, 64,72.
Psychological distress and unexplained bodily symptoms are part of the ICD-11 diagnostic criteria for “primary musculoskeletal pain,” which includes fibromyalgia,49,73 conditions reported by 64% of participants in this study. The positive psychological impact for people receiving EPIO could hence indicate contribution to improved health above and beyond pain-related outcomes.
Studies have shown capacity for self-regulation to be a limited source that can be fatigued,4,5,55 and the complexity of chronic pain likely negatively affects ability to self-regulate.46,48,58,69 The improvement in self-regulatory capacity seen in this study might therefore be explained through the primarily CBT-based EPIO content,36 targeting cognitive, emotional, and behavioral factors necessary for self-regulation.9,43 Self-regulatory capacity could also be associated with the current improvement seen in pain catastrophizing (ie, exaggerating, ruminating on, and feeling helpless about the pain).65
4.3. Pain and pain interference
The change of focus from pain intensity to also capturing interference of pain has supported a broader understanding of individual pain impact.21,63 This was also why this study chose interference on function34,35 as a primary outcome. Measuring pain interference is nevertheless complicated because it depends on how pain affects a person's willingness to experience pain, their acceptance of living with pain, and their values and activities. Pain's impact on daily life therefore varies depending on the individual, and the same pain intensity score can be associated with large individual differences in pain interference.
Living with pain for many years, as was the case for most participants in this study, likely also makes changes in pain and pain interference challenging, perhaps even unlikely. The heterogeneous sample and large data variability may also have contributed to the rather small study effect sizes (β < 0.2), although not uncommon for psychosocial interventions in chronic pain.72
4.4. System use
At the end of the 12-month study, 66% of participants in the intervention group were considered completers, an accomplishment given the substantial adherence/attrition challenges for digital interventions.33,56 This is also an increase from the 50% completion seen at 3 months,9 indicating EPIO fostered continued program engagement over the year, and more than 3 months might be needed for people with chronic pain to engage in pain self-management interventions.
4.5. Study limitations and strengths
This study has some limitations. First, participants volunteered for study participation, which indicates high motivation. Most participants were also female, Anglo-American, and with higher education, all potential limitations for generalizability. Second, high heterogeneity (eg, pain conditions) could mean limited statistical power to explore subgroup comparisons. Future studies may therefore increase study sample and/or homogeneity to enhance chance of detecting change, for whom and when. Third, performing multiple statistical tests may inflate significance level (ie, type I error). Analyses therefore focused on confidence interval's, effect sizes and their interpretations and are described in detail in section 2.7 for transparency.77 Fourth, because the study did not monitor whether the control group engaged in any types of self-management training during the study period, analyses could not control for this aspect. Finally, most participants had lived with pain for many years and were on sick leave/receiving disability benefits. Being approved for disability is an arduous process and describing improvement in pain-related outcomes could potentially interfere with identity for some.
This study also has several strengths. The EPIO intervention program is designed and developed with sound theoretical foundation, stakeholder involvement and testing, qualitative and quantitative feasibility explorations, implementation planning, and taking adherence/attrition challenges into account10,11,36,37,66 before RCT. The blended care delivery method used may also have contributed to program engagement and completion, and the RCT allowed for thorough examination of system use and self-reported outcome measures over an entire year.
4.6. Future directions
Digital intervention programs such as EPIO may improve outreach of evidence-based pain self-management treatment. The therapeutic relationship from face-to-face interventions likely contributes to engagement, however, and to ensure impact, digital interventions must incorporate ways to encourage engagement, for example through blended care delivery.1,11,51,60 Study participants recommended incorporating EPIO into pain rehabilitation programs and including EPIO in a multidisciplinary setting (eg, combined with physical therapy24), might enhance impact, particularly where fear of pain and movement are impediments for physical improvement.17,18,68
Pain is a subjective phenomenon that can only be assessed through self-report, and a combination of quantitative and qualitative measures might therefore enable more in-depth explorations, as seen through the EPIO feasibility pilot.10,11 Digital wearables could also complement self-report measures, for example to gauge factors such as physical activity and sleep. Digital pain self-management might not be for everyone though, and future research should explore who would benefit from purely in-person treatment, blended care hybrid models, or simply access to digital treatment.
5. Conclusion
The EPIO project seeks to improve outreach of evidence-informed pain self-management interventions. Pain is challenging, sometimes impossible, to treat. However, this 12-month RCT shows how EPIO can contribute to psychological well-being and quality of life, even if living with pain. As most existing pain self-management interventions struggle to show effect beyond 3 months, these 12-month findings represent a major contribution to pain research. Complicated concepts such as how to live well with pain might not easily be solved or made sense of without considering psychosocial factors, as seen impacted in this study.
Disclosures
L.S.N. is an unpaid board member of the company dHealth AS, aiming to market the EPIO program for commercialization, but has no financial interest in the company. The remaining authors have no conflicts of interest to declare.
This study was funded by the Norwegian Research Council (Grant #256574, Principal Investigator L.Solberg Nes).
Data sets from this study are, due to the nature of patient sensitive information, not available for public sharing through public archives or repositories. Deidentified data from this study will however be made available in accordance with institutional standards through contacting the corresponding author.
Trial Registration: ClinicalTrials.gov NCT03705104.
Acknowledgements
The authors thank the patients who participated in this study, the collaborating partners, and health care providers who contributed with study recruitment, and the project study support team at the Department of Digital Health Research.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Elin Børøsund, Email: elin.borosund@rr-research.no.
Cecilie Varsi, Email: cecilie.varis@usn.no.
Hilde Eide, Email: hilde.eide@usn.no.
Lori B. Waxenberg, Email: lwaxenbe@PHHP.UFL.EDU.
Karen E. Weiss, Email: Weiss.Karen2@mayo.edu.
Eleshia J. Morrison, Email: morrison.Elsehia@mayo.edu.
Hanne Stavenes Støle, Email: HanneStavenes@fhi.no.
Ólöf B. Kristjansdottir, Email: Olof.Birna.Kristjansdottir@heilsugaeslan.is.
Katrine Bostrøm, Email: katrine.bostrom@gmail.com.
Elin Bolle Strand, Email: elin.bolle.strand@rr-research.no.
Milada Cvancarova Småstuen Hagen, Email: milas@oslomet.no.
Audun Stubhaug, Email: audun.stubhaug@medisin.uio.no.
Karlein M.G. Schreurs, Email: k.m.g.schreurs@utwente.nl.
References
- [1].Aerts JEM, Dam AV. Blended e-Health in cognitive behavioural therapy: usage intensity, attitude and therapeutic alliance in clinical practice. Psychology 2018;09:2422–35. [Google Scholar]
- [2].Amagai S, Pila S, Kaat AJ, Nowinski CJ, Gershon RC. Challenges in participant engagement and retention using mobile health apps: literature review. J Med Internet Res 2022;24:e35120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Atkinson TM, Halabi S, Bennett AV, Rogak L, Sit L, Li Y, Kaplan E, Basch E; Cancer and Leukemia Group B. Measurement of affective and activity pain interference using the Brief Pain Inventory (BPI): Cancer and Leukemia Group B 70903. Pain Med 2012;13:1417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: is the active self a limited resource? J Pers Soc Psychol 1998;74:1252–65. [DOI] [PubMed] [Google Scholar]
- [5].Baumeister RF, Tice DM, Vohs KD. The strength model of self-regulation: conclusions from the second decade of willpower research. Perspect Psychol Sci 2018;13:141–5. [DOI] [PubMed] [Google Scholar]
- [6].Beck JS. Cognitive behavior therapy. London: The Guilford Press, 2020. [Google Scholar]
- [7].Børøsund E, Ehlers SL, Clark MM, Andrykowski MA, Cvancarova Smastuen M, Solberg Nes L. Digital stress management in cancer: testing StressProffen in a 12-month randomized controlled trial. Cancer 2022;128:1503–12. [DOI] [PubMed] [Google Scholar]
- [8].Børøsund E, Varsi C, Clark MM, Ehlers SL, Andrykowski MA, Sleveland HRS, Bergland A, Nes LS. Pilot testing an app-based stress management intervention for cancer survivors. Transl Behav Med 2020;10:770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bostrøm K, Børøsund E, Eide H, Varsi C, Kristjansdottir ÓB, Schreurs KMG, Waxenberg LB, Weiss KE, Morrison EJ, Stavenes Støle H, Cvancarova Småstuen M, Stubhaug A, Solberg Nes L. Short-term findings from testing EPIO, a digital self-management program for people living with chronic pain: randomized controlled trial. J Med Internet Res 2023;25:e47284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bostrøm K, Børøsund E, Varsi C, Eide H, Flakk Nordang E, Schreurs KM, Waxenberg LB, Weiss KE, Morrison EJ, Cvancarova Småstuen M, Stubhaug A, Solberg Nes L. Digital self-management in support of patients living with chronic pain: feasibility pilot study. JMIR Form Res 2020;4:e23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bostrøm K, Varsi C, Eide H, Børøsund E, Kristjansdottir ÓB, Schreurs KMG, Waxenberg LB, Weiss KE, Morrison EJ, Nordang EF, Stubhaug A, Nes LS. Engaging with EPIO, a digital pain self-management program: a qualitative study. BMC Health Serv Res 2022;22:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Braun V, Clarke V. One size fits all? What counts as quality practice in (reflexive) thematic analysis? Qual Res Psychol 2021;18:328–52. [Google Scholar]
- [13].Buhrman M, Fredriksson A, Edström G, Shafiei D, Tärnqvist C, Ljótsson B, Hursti T, Gordh T, Andersson G. Guided Internet-delivered cognitive behavioural therapy for chronic pain patients who have residual symptoms after rehabilitation treatment: randomized controlled trial. Eur J Pain 2013;17:753–65. [DOI] [PubMed] [Google Scholar]
- [14].Clarke V, Braun V. Successful qualitative research: a practical guide for beginners. London: Sage, 2013. [Google Scholar]
- [15].Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet 2021;397:2082–97. [DOI] [PubMed] [Google Scholar]
- [16].Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M; Medical Research Council Guidance. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].den Hollander M, Goossens M, de Jong J, Ruijgrok J, Oosterhof J, Onghena P, Smeets R, Vlaeyen JWS. Expose or protect? A randomized controlled trial of exposure in vivo vs pain-contingent treatment as usual in patients with complex regional pain syndrome type 1. PAIN 2016;157:2318–29. [DOI] [PubMed] [Google Scholar]
- [18].den Hollander M, Smeets R, van Meulenbroek T, van Laake-Geelen CCM, Baadjou VA, Timmers I. Exposure in vivo as a treatment approach to target pain-related fear: theory and new insights from research and clinical practice. Phys Ther 2022;102:pzab270. [DOI] [PubMed] [Google Scholar]
- [19].Devan H, Farmery D, Peebles L, Grainger R. Evaluation of self-management support functions in apps for people with persistent pain: systematic review. JMIR Mhealth and Uhealth 2019;7:e13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Driscoll MA, Edwards RR, Becker WC, Kaptchuk TJ, Kerns RD. Psychological interventions for the treatment of chronic pain in adults. Psychol Sci Public Interest 2021;22:52–95. [DOI] [PubMed] [Google Scholar]
- [21].Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J; IMMPACT. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. PAIN 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- [22].Eccleston C, Morley SJ, Williams AC. Psychological approaches to chronic pain management: evidence and challenges. Br J Anaesth 2013;111:59–63. [DOI] [PubMed] [Google Scholar]
- [23].Eide H, Leren L, Sørebø Ø. The Norwegian versions of the Chronic Pain Acceptance Questionnaire CPAQ-20 and CPAQ-8—validation and reliability studies. Disabil Rehabil 2017;39:1441–8. [DOI] [PubMed] [Google Scholar]
- [24].Eiken AG, Nordanger D, Solberg Nes L, Varsi C. Patients' experiences of using an eHealth pain management intervention combined with psychomotor physiotherapy: qualitative study. JMIR Form Res 2022;6:e34458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Feliu-Soler A, Montesinos F, Gutiérrez-Martínez O, Scott W, McCracken LM, Luciano JV. Current status of acceptance and commitment therapy for chronic pain: a narrative review. J Pain Res 2018;11:2145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fernandes L, Storheim K, Lochting I, Grotle M. Cross-cultural adaptation and validation of the Norwegian pain catastrophizing scale in patients with low back pain. BMC Musculoskelet Disord 2012;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hayes SC. Acceptance and commitment therapy, relational frame theory, and the third wave of behavioral and cognitive therapies—republished article. Behav Ther 2016;47:869–85. [DOI] [PubMed] [Google Scholar]
- [28].Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan AW, Michie S. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- [29].Huygens MW, Vermeulen J, Swinkels IC, Friele RD, van Schayck OC, de Witte LP. Expectations and needs of patients with a chronic disease toward self-management and eHealth for self-management purposes. BMC Health Serv Res 2016;16:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Institute of Medicine Committee on Advancing Pain Research, Care, and Education. The National Academies Collection: reports funded by National Institutes of Health. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington: National Academies Press (US), 2011. [PubMed] [Google Scholar]
- [31].Interagency Pain Research Coordination Committee. National pain strategy: a comprehensive population health-level strategy for pain, United States: National Institute of Health; 2016. [Google Scholar]
- [32].Joypaul S, Kelly F, McMillan SS, King MA. Multi-disciplinary interventions for chronic pain involving education: a systematic review. PLoS One 2019;14:e0223306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kelders SM, Kok RN, Ossebaard HC, Van Gemert-Pijnen JE. Persuasive system design does matter: a systematic review of adherence to web-based interventions. J Med Internet Res 2012;14:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain 2004;20:309–18. [DOI] [PubMed] [Google Scholar]
- [35].Klepstad P, Loge JH, Borchgrevink PC, Mendoza TR, Cleeland CS, Kaasa S. The Norwegian Brief Pain Inventory questionnaire: translation and validation in cancer pain patients. J Pain Symptom Manage 2002;24:517–25. [DOI] [PubMed] [Google Scholar]
- [36].Ledel Solem IK, Varsi C, Eide H, Kristjansdottir OB, Børøsund E, Schreurs KMG, Waxenberg LB, Weiss KE, Morrison EJ, Haaland-Øverby M, Bevan K, Zangi HA, Stubhaug A, Solberg Nes L. A user-centered approach to an evidence-based electronic health pain management intervention for people with chronic pain: design and development of EPIO. J Med Internet Res 2020;22:e15889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ledel Solem IK, Varsi C, Eide H, Kristjansdottir OB, Mirkovic J, Børøsund E, Haaland-Øverby M, Heldal K, Schreurs KM, Waxenberg LB, Weiss KE, Morrison EJ, Solberg Nes L. Patients' needs and requirements for eHealth pain management interventions: qualitative study. J Med Internet Res 2019;21:e13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lee JA, Choi M, Lee SA, Jiang N. Effective behavioral intervention strategies using mobile health applications for chronic disease management: a systematic review. BMC Med Inform Decis Mak 2018;18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Leiknes KA, Dalsbø TK, Siqveland J. Psychometric assessment of the Norwegian version of the Hospital Anxiety and Depression Scale (HADS). Report 2016. Oslo: Folkehelseinstituttet, 2016. [Google Scholar]
- [40].Loge JH, Kaasa S, Hjermstad MJ, Kvien TK. Translation and performance of the Norwegian SF-36 Health Survey in patients with rheumatoid arthritis. I. Data quality, scaling assumptions, reliability, and construct validity. J Clin Epidemiol 1998;51:1069–76. [DOI] [PubMed] [Google Scholar]
- [41].Ludden GD, van Rompay TJ, Kelders SM, van Gemert-Pijnen JE. How to increase reach and adherence of web-based interventions: a design research viewpoint. J Med Internet Res 2015;17:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McCracken LM, Vowles KE, Eccleston C. Acceptance of chronic pain: component analysis and a revised assessment method. PAIN 2004;107:159–66. [DOI] [PubMed] [Google Scholar]
- [43].McGonigal K. The willpower instinct: how self-control works, why it matters, and what you can do to get more of it. New York: Avery Publishing Group, 2013. [Google Scholar]
- [44].Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth 2019;123:e273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Moman RN, Dvorkin J, Pollard EM, Wanderman R, Murad MH, Warner DO, Hooten WM. A systematic review and meta-analysis of unguided electronic and mobile health technologies for chronic pain-is it time to start prescribing electronic health applications? Pain Med 2019;20:2238–55. [DOI] [PubMed] [Google Scholar]
- [46].Nes LS, Carlson CR, Crofford LJ, de Leeuw R, Segerstrom SC. Self-regulatory deficits in fibromyalgia and temporomandibular disorders. PAIN 2010;151:37–44. [DOI] [PubMed] [Google Scholar]
- [47].Nes LS, Ehlers SL, Whipple MO, Vincent A. Self-regulatory fatigue in chronic multisymptom illnesses: scale development, fatigue, and self-control. J Pain Res 2013;6:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nes LS, Ehlers SL, Whipple MO, Vincent A. Self-regulatory fatigue: a missing link in understanding fibromyalgia and other chronic MultiSymptom illnesses. Pain Pract 2017;17:460–9. [DOI] [PubMed] [Google Scholar]
- [49].Nicholas M, Vlaeyen JWS, Rief W, Barke A, Aziz Q, Benoliel R, Cohen M, Evers S, Giamberardino MA, Goebel A, Korwisi B, Perrot S, Svensson P, Wang SJ, Treede RD; IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: chronic primary pain. PAIN 2019;160:28–37. [DOI] [PubMed] [Google Scholar]
- [50].Nieminen P. Application of standardized regression coefficient in meta-analysis. BioMedInformatics 2022;2:434–58. [Google Scholar]
- [51].Obro LF, Heiselberg K, Krogh PG, Handberg C, Ammentorp J, Pihl GT, Osther PJS. Combining mHealth and health-coaching for improving self-management in chronic care: a scoping review. Patient Educ Couns 2021;104:680–8. [DOI] [PubMed] [Google Scholar]
- [52].Rice ASC, Smith BH, Blyth FM. Pain and the global burden of disease. PAIN 2016;157:791–6. [DOI] [PubMed] [Google Scholar]
- [53].Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schäfer T, Schwarz MA. The meaningfulness of effect sizes in psychological research: differences between sub-disciplines and the impact of potential biases. Front Psychol 2019;10:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychol Sci 2007;18:275–81. [DOI] [PubMed] [Google Scholar]
- [56].Sieverink F, Kelders SM, van Gemert-Pijnen JE. Clarifying the concept of adherence to eHealth technology: systematic review on when usage becomes adherence. J Med Internet Res 2017;19:e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, Boyd KA, Craig N, French DP, McIntosh E, Petticrew M, Rycroft-Malone J, White M, Moore L. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021;374:n2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Solberg Nes L, Roach AR, Segerstrom SC. Executive functions, self-regulation, and chronic pain: a review. Ann Behav Med 2009;37:173–83. [DOI] [PubMed] [Google Scholar]
- [59].Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- [60].Talboom-Kamp EPWA, Verdijk NA, Kasteleyn MJ, Numans ME, Chavannes NH. From chronic disease management to person-centered eHealth; a review on the necessity for blended care. Clin eHealth 2018;1:3–7. [Google Scholar]
- [61].Thurnheer SE, Gravestock I, Pichierri G, Steurer J, Burgstaller JM. Benefits of mobile apps in pain management: systematic review. JMIR Mhealth Uhealth 2018;6:e11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Trompetter HR, Bohlmeijer ET, Veehof MM, Schreurs KM. Internet-based guided self-help intervention for chronic pain based on Acceptance and Commitment Therapy: a randomized controlled trial. J Behav Med 2015;38:66–80. [DOI] [PubMed] [Google Scholar]
- [63].Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, Cleeland C, Dionne R, Farrar JT, Galer BS, Hewitt DJ, Jadad AR, Katz NP, Kramer LD, Manning DC, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robinson JP, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Witter J. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. PAIN 2003;106:337–45. [DOI] [PubMed] [Google Scholar]
- [64].Urits I, Hubble A, Peterson E, Orhurhu V, Ernst CA, Kaye AD, Viswanath O. An update on cognitive therapy for the management of chronic pain: a comprehensive review. Curr Pain Headache Rep 2019;23:57. [DOI] [PubMed] [Google Scholar]
- [65].Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. PAIN 2002;96:319–24. [DOI] [PubMed] [Google Scholar]
- [66].Varsi C, Ledel Solem IK, Eide H, Børøsund E, Kristjansdottir OB, Heldal K, Waxenberg LB, Weiss KE, Schreurs KMG, Morrison EJ, Stubhaug A, Solberg Nes L. Health care providers' experiences of pain management and attitudes towards digitally supported self-management interventions for chronic pain: a qualitative study. BMC Health Serv Res 2021;21:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Varsi C, Solberg Nes L, Kristjansdottir OB, Kelders SM, Stenberg U, Zangi HA, Børøsund E, Weiss KE, Stubhaug A, Asbjørnsen RA, Westeng M, Ødegaard M, Eide H. Implementation strategies to enhance the implementation of eHealth programs for patients with chronic illnesses: realist systematic review. J Med Internet Res 2019;21:e14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Vlaeyen JWS, Crombez G, Linton SJ. The fear-avoidance model of pain. PAIN 2016;157:1588–9. [DOI] [PubMed] [Google Scholar]
- [69].Vogel TA, Desjardins S, Chabot B, Otto AR, Falk CF, Roy M. Cognitive fatigue weakens the effects of distraction on pain. PAIN 2023;164:2845–51. [DOI] [PubMed] [Google Scholar]
- [70].Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- [71].Werneburg BL, Herman LL, Preston HR, Rausch SM, Warren BA, Olsen KD, Clark MM. Effectiveness of a multidisciplinary worksite stress reduction programme for women. Stress Health 2011;27:356–64. [Google Scholar]
- [72].Williams ACdC, Fisher E, Hearn L, Eccleston C. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2020;8:CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016;46:319–29. [DOI] [PubMed] [Google Scholar]
- [74].Woo AK. Depression and anxiety in pain. Rev Pain 2010;4:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhao P, Yoo I, Lancey R, Varghese E. Mobile applications for pain management: an app analysis for clinical usage. BMC Med Inform Decis Mak 2019;19:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- [77].Roberts A, Roche M, Sainani KL. Exploratory analyses: how to meaningfully interpret and report them. PMR 2023;15:800–4. [DOI] [PubMed] [Google Scholar]