Abstract
The clinical success of implanted biomaterials such as dental implants is largely determined by the molecular signaling that occurs at the tissue-implant interface. The modification of surface topography is a widely-employed strategy for optimizing tissue integration with dental implants. However, little is known regarding the direct, cellular-level effects of substratum topography on platelet signaling and adhesion, despite these cells being the first to encounter the implant surface during surgical placement. Here we compared platelet adhesion and secretion on four (4) different titanium surfaces, notably, the modifications applied to commercially available dental implants: smooth (S) titanium; acid-etched (AE), sandblasted (SB) and a combined acid-etching/sandblasting procedure (SLA). Platelets were isolated from human blood, washed, and seeded on to the 4 test surfaces; platelet adhesion was quantified by microscopy. In addition, the secretion of critical molecules stored in platelet granules (platelet factor 4, PF4; soluble P-selectin, sCD62P; transforming growth factor-beta1, TGF-β1; platelet-derived growth factor-AB, PDGF-AB) was measured by enzyme-linked immunosorbent assay (ELISA) analysis of the supernatants. There was greater platelet adhesion to the rougher AE and SB surfaces, however, the concentration of the secreted growth factors was comparable on all surfaces. We conclude that while surface topography can be engineered to modulate initial platelet adhesion, granule secretion is likely regulated as a separate and independent process.
Keywords: Platelets, Implant, Surface topography, Cell adhesion
Graphical abstract
1. Introduction
Titanium is a widely-used biomaterial for dental and orthopedic implants, due to its suitable mechanical properties, biocompatibility, and its ability to integrate with bone, termed osseointegration.1,2 A key approach to optimizing the success of dental and orthopedic implants is through modification of the implant surface to elicit cellular responses conducive for wound healing.3,4 In this regard, the properties of titanium biomaterials are determined by their surface characteristics, notably, surface topography,5 chemical composition,6 and electrical charge.7 The modification of surface topography is a particularly well-documented method to improve the titanium osseointegration.5,8 Topographic modifications applied to commercially available dental implants include sandblasting, acid-etching, and a combination of sandblasting and acid-etching (termed SLA).3
Considerable efforts have been dedicated to studying the effect of surface topography on the adhesion and differentiation of osteogenic cells on titanium.9 This is understandable given that bone cells are directly responsible for osseointegration.10 However, there has been less intensive study on the effects of surface topography on blood cells, despite blood being the first tissue to contact the implant surface during surgical placement.11, 12, 13 After insertion, the wound site of the implant is immediately covered by a blood clot,14 which precedes the migration of inflammatory and osteogenic cells to the implant surface.15 Consequently, blood cells are ideally positioned to serve as a critical bridge between the titanium surface and the healing peri-implant tissues.
Platelets are cellular fragments derived from megakaryocytes in the bone marrow.16 Platelets store >30 bioactive cytokines, chemokines, and growth factors in 3 distinct granular compartments, termed alpha (α)-granules, dense granules and lysosomes.17, 18, 19 In their resting form, platelets circulate in the blood as discoid cells that rapidly aggregate and change shape upon activation by soluble agonists or by encountering the wall of a damaged blood vessel.20, 21, 22 Platelet aggregation and shape change are accompanied by the secretion of their granular contents which include pro-hemostatic, pro-angiogenic, and pro-inflammatory molecules17, 18, 19; accordingly, platelet function is critical for wound healing.23 Consequently, the platelet response to the dental implant surface would represent a key event that would set the stage for osseointegration and the implant's clinical success.
In addition to secreting the contents of their granules, activated platelets contribute to wound healing in part by recruiting and stimulating the proliferation and migration of osteogenic cells.24, 25, 26 Previous studies have suggested that platelet activation can be modulated by titanium surface hydrophilicity27 as well as by surface topography.13,27,28 A common feature in the experimental designs of these studies is the use of platelets retained in plasma (PRP). As a result, the exact, cellular-level response of platelets at the interface with the titanium surface remains incompletely characterized. Here we provide the first report on the interactions between purified platelets and titanium surfaces with topographies found on commercially available dental implants. We report that surface roughness created by acid-etching and/or sandblasting promotes early adhesion of platelets to the implant surface. However, the tested surface topographies did not have a significant effect on the secretion of specific platelet-derived cytokines (soluble P-selectin, sCD62P; platelet factor 4, PF4; platelet-derived growth factor-AB; PDGF-AB; transforming growth factor-beta1; TGF-β1). We conclude that while initial platelet adhesion and granule secretion can be modulated by surface topography, the 2 processes are likely governed independently.
2. Materials and methods
2.1. Preparation of titanium substrata
Commercially pure grade II (CP2) titanium (Ti) plates were prepared for use in this study. Two different plate dimensions were used: 10 mm × 10 mm × 1 mm plates were used for the platelet adhesion assay, whereas 15 mm × 15 mm × 1 mm plates were used for measurement of platelet secretion by enzyme-linked immunosorbent assay (ELISA). Plate size selection was based on compatibility with the chosen application (e.g. adhesion or secretion assay). All plates were sequentially polished with silicon carbide papers (grades 220, 320, 550 and 1000) under running water using a polishing machine (Lunn Major, Struers, Denmark). The plates were then ultrasonically cleaned in acetone for 15 min for degreasing, air-dried at room temperature for 30 min and then randomly allocated for preparation of the following 4 test surface topographies.
-
•
Group 1 (Smooth control, S): Polished Ti plates without further treatment, which served as controls.
-
•
Group 2 (Acid-etched, AE): Ti plates were soaked in a mixture of H2SO4 and HCl (6:1) at 60°C for 6 min.
-
•
Group 3 (Sandblasted, S): Both sides of each specimen were evenly air-abraded with alumina powder (LEMAT NT4, Wassermann, Germany) with a mean particle size of 250 μm at a constant pressure of 3.4 bar from a distance of 15 cm, perpendicularly to the disc surface. Blasting was performed for 30 s per side for the smaller Ti plates (10 × 10 × 1 mm), and for 67–68 s per side for the larger plates (15 × 15 × 1 mm).
-
•
Group 4 (Sandblasted, large grit, acid-etched, SLA): Specimens were sandblasted with Al2O3 powder and then acid-etched, essentially combining the methods described for the preparation of Group 2 and Group 3 substrata above.
Each Ti plate was cleaned as previously described13: 10 min acetone rinse, 10 min sonication (Branson, model 1210) in ddH2O (Milli-Q, Millipore), followed by 1 h of sonication in 2% Decon-75 detergent (BDH) followed by 5 min of sonication in ddH2O. The Ti plates were washed 3 times with ddH2O for 5 min, then washed twice in 100% ethanol. After cleaning, 6 specimens were randomly chosen from each group (3 samples of each size) for characterization of the surface topography. The remaining samples were stored in absolute ethanol and sealed until use. Prior to conducting experiments, samples were removed from the ethanol, air dried, and used within 24 h.
2.2. Surface characterization of the Ti substrata
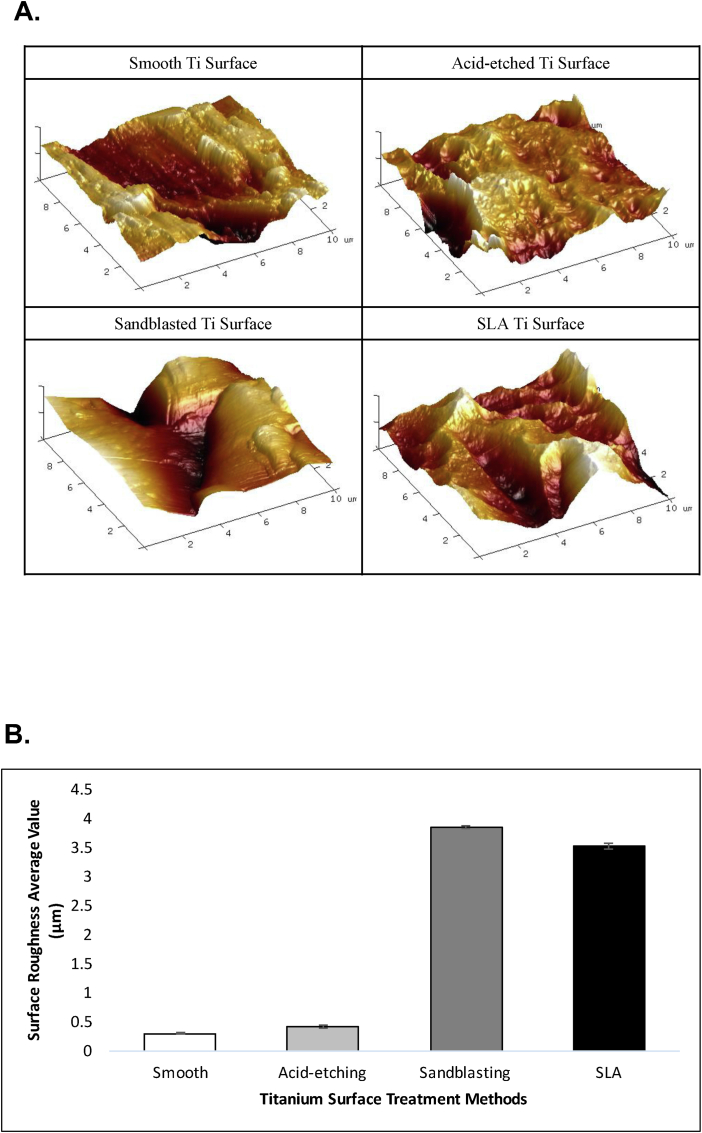
The. roughness average (Ra) is one of the most commonly used parameters in describing materials surface topography.5 The Ra values of the substrata used in this study were measured with a Surtronic 3+ device (Taylor Hobson Ltd., Leicester, UK). Each sample was measured 3 times in different directions with a track of 0.8 mm and the mean values of the measurements were calculated. The Ra values of the Ti substrata used in this study are depicted in Fig. 2.
Fig. 2.
Quantification of surface roughness by atomic force microscopy (AFM). A. The surface characteristics of the different test Ti surfaces were quantified by atomic force microscopy (AFM). B. Bar graph depicts the roughness average (Ra) of the 4 test surfaces, as determined by AFM. Data are mean ± SD.
2.3. Scanning electron microscopy (SEM) and atomic force microscopy (AFM)
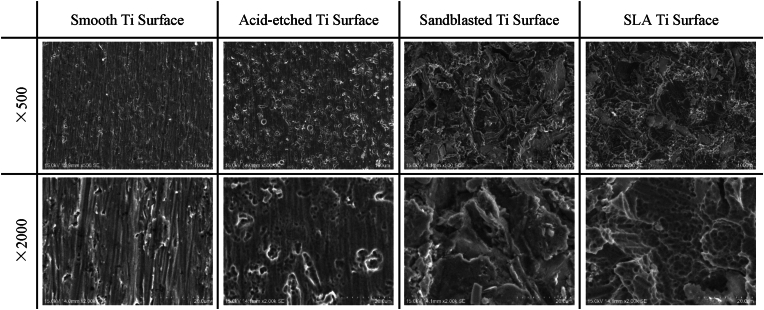
The Ti specimens in different groups were visualized by SEM (S–3400 N, Hitachi, Tokyo, Japan) which was performed at 20.0 kV operating voltage (Fig. 2). Further measurements of surface topography were performed with atomic force microscopy (AFM) using an Autoprobe CP microscope (Park Scientific Instruments, Santa Clara, CA). All measurements were performed in the non-contact mode using the Autoprobe CP's Nano World NCSTR tip at room temperature. A minimum of four samples were measured for each sample type. An area of 100 μm2 for each Ti plate surface was scanned and numerical surface roughness parameters were measured from each scan. The AFM images in Fig. 2 show the detail of the treated Ti surfaces.
2.4. Human platelet preparation
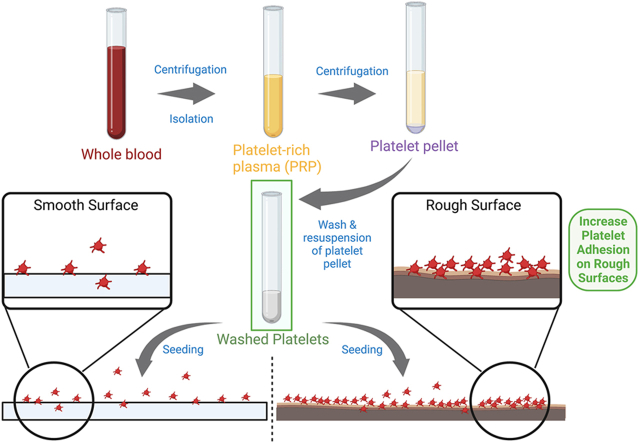
Whole blood was obtained by venipuncture from healthy human volunteers with informed consent in accordance with the Declaration of Helsinki, and with institutional ethical approval (protocol number H13-01805). Blood was collected in tubes containing a citrated anticoagulant buffer. Whole blood was centrifuged at 900 rpm for 20 min to obtain platelet-rich plasma (PRP); the PRP was then removed and centrifuged at 1000g for 5 min to separate the platelets. The platelet pellet was then washed twice by resuspending in a washing buffer (140 mM NaCl, 5 mM KCl, 12 mM trisodium citrate, 10 mM glucose, 12.5 mM sucrose, pH = 6.0) with prostaglandin E1 (PGE-1) and re-centrifugation. After washing, the platelets were resuspended in a resuspension buffer (10 mM HEPES, 140 mM NaCl, 3 mM KCl, 0.5 mM MgCl2, 5 mM NaHCO3, 10 mM glucose, pH = 7.4) and allowed to rest at room temperature for 30 min prior to use. Platelet concentration was determined using an ADVIA 120 hematological analyzer (Bayer Diagnostics, Tarrytown, NY).
2.5. Platelet adhesion assay
To quantify platelet adhesion to the different surface topographies, platelets (5 × 106 cells/mL) were seeded on to the different Ti surfaces (10 × 10 × 1 mm, placed in 24-well plates), and allowed to spread for up to 30 min. At specific time points (1, 2, 5, 15, or 30 min), and after washing to remove non-adherent platelets, cells were fixed with 4% paraformaldehyde, and permeabilized with 0.1% (v/v) Triton X-100. The platelets were then stained with fluorescein isothiocyanate (FITC)-conjugated phalloidin (Invitrogen, Eugene, OR) to label F-actin. Platelets were then visualized with a fluorescence microscope (Axio IMAGER M1m, Zeiss, Oberkochen, Germany). For each test surface, a minimum of 4 fields of view were captured for each time point. The number of platelets per field of view were counted using ImageJ software (Version 1.48, National Institutes of Health, Bethesda, MD).
2.6. Platelet secretion assay
To test the effect of surface topography on platelet granule release, platelets (1.4 × 108 cells/mL) were seeded on to the Ti plates (15 × 15 × 1 mm) placed in multi-well culture plates and allowed to spread for specific times (1, 2, 5, 15 or 30 min) at 37°C. At the chosen time points, platelet supernatants were harvested; the secretion reaction was stopped29 by centrifugation at 4°C and the supernatants were stored at −80°C. The quantity of specific mediators secreted by the platelets (platelet factor 4, PF4; platelet-derived growth factor AB, PDGF-AB; transforming growth factor-beta1, TGF-β1; soluble P-selectin, sCD62P) was measured by sandwich enzyme-linked immunosorbent assay (ELISA) kits (Quantikine, R&D Systems, Minneapolis, MN) in accordance with the manufacturer's instructions. All samples were measured in duplicate.
2.7. Statistical analysis
An analysis of variance (ANOVA) and Bonferroni post-hoc multiple comparison tests were used to determine the effect of surface topography (C, AE, S, SLA) on the chosen parameters (platelet adhesion and platelet secretion). Statistical significance was set at p < 0.05.
3. Results
To evaluate the role of surface topography in modulating the platelet response during initial wound healing, we compared the platelet response to 3 different titanium substrates (acid-etched, sandblasted, and SLA) relative to a smooth control surface. The efficacy of the surface treatments in generating the desired topographies was verified by both scanning electron microscopy (Fig. 1) and by atomic force microscopy (Fig. 2).
Fig. 1.
Topographical characterization of titanium (Ti) surface treatments. Scanning electron micrographs (SEM) taken at low magnification (500×, top panels) and high magnification (2000×, bottom panels) illustrate the microscopic features of the different surface treatments: acid-etching, sand-blasting and combined etching/blasting (SLA).
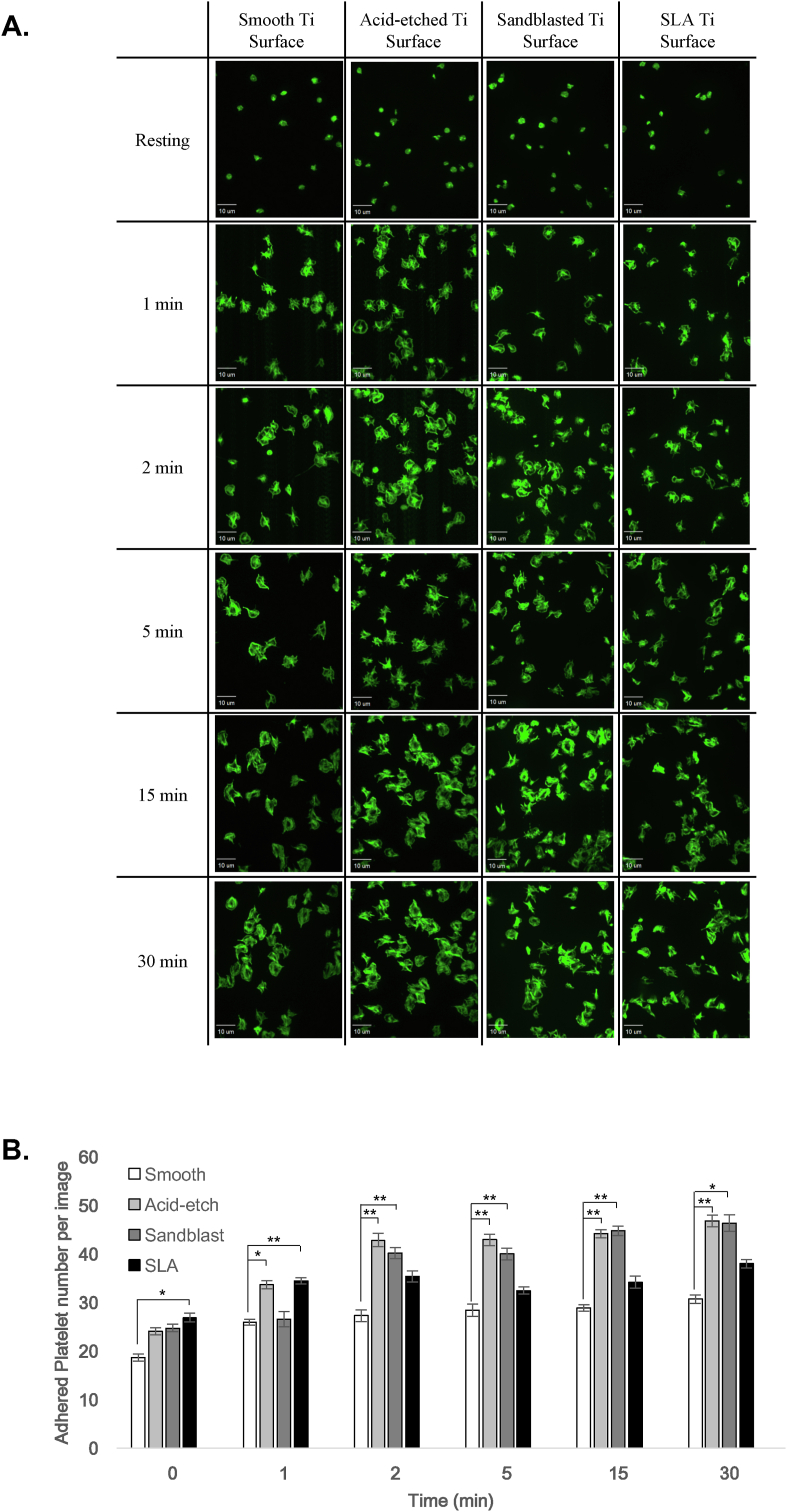
3.1. Surface roughness increases initial platelet adhesion
We first compared the numbers of adherent platelets seeded onto the 4 different test surfaces at specific times post-plating (Fig. 3A). Based on counts of adherent platelets (stained with phalloidin) the rougher acid-etched and sandblasted surfaces harbored significantly (p < 0.05) greater numbers of adherent platelets relative to the smooth control surface, at the 2, 5, 15 and 30 min time points (Fig. 3B). Interestingly, the SLA surface, treated with a combination of acid-etching and sandblasting, exhibited significantly (p < 0.05) greater platelet adhesion than the control surface at 1 min but not at the later time points (Fig. 3B). These data indicate that acid-etched and sandblasted surfaces favor the initial adhesion of platelets, however, the combined effects of etching and blasting (SLA) appears to have a more limited effect with regards to promoting platelet adhesion.
Fig. 3.
Platelet adhesion to Ti surfaces is modulated by surface topography. A. Confocal micrographs depict the F-actin staining (by FITC-phalloidin) of platelets adhering to smooth, acid-etched, sand-blasted or SLA titanium surfaces at the indicated time points. B. Bar graph depicts the mean number of platelets (per field of view) adhering to smooth Ti (white bars), acid-etched Ti (light grey bars), sand-blasted Ti (dark grey bars) and SLA-treated Ti (black bars). Data are mean ± SEM. *, p < 0.05; **, p < 0.001, based on ANOVA and Bonferroni post-hoc multiple comparison tests.
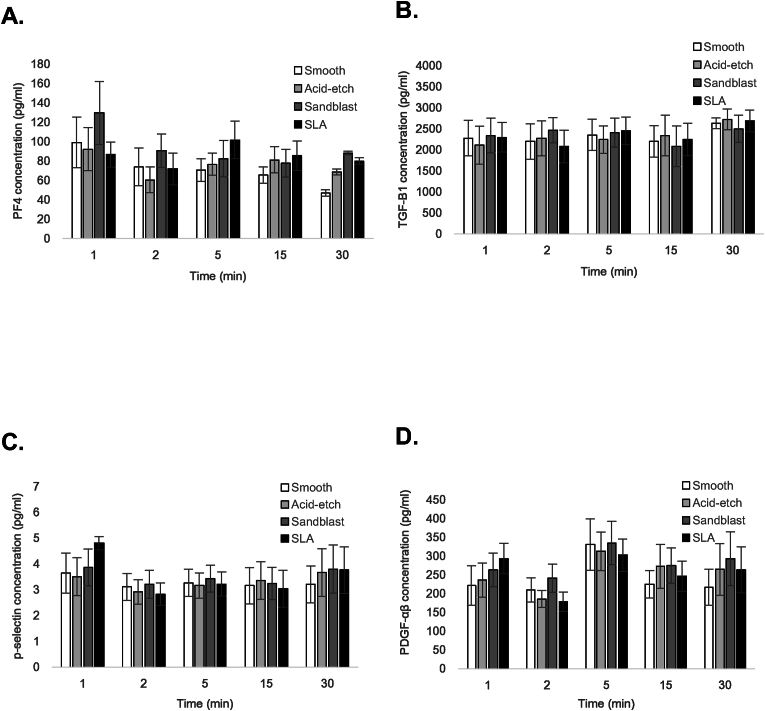
3.2. Platelet secretion is unaffected by surface topography
A critical aspect of platelet function, particularly in the context of wound healing, is the secretion of bioactive molecules from the intracellular granules.17, 18, 19 The majority of platelet cytokines and growth factors are stored in the alpha (α)-granules.17,19 We profiled the release of 4 different molecules: platelet factor 4 (PF4) and soluble P-selectin (sCD62P), which are major constituents of α-granules, as well as transforming growth factor-beta1 (TGF-β1) and platelet-derived growth factor-AB (PDGF-AB), which have well-documented roles in wound healing.30 Of the PDGF isoforms, we chose to examine PDGF-AB since this is the most abundant form of PDGF in platelets31 and the most abundant in platelet-rich plasma.32 For each of the 4 secreted molecules, enzyme-linked immunosorbent assay (ELISA) analysis of the supernatants did not reveal any significant differences in platelet-derived cytokine/growth factor release between the test surfaces (p > 0.05), at each of the time points examined (Fig. 4A-D). The time points for examination were selected based on evidence indicating that the bulk of granule secretion occurs within 30 min following platelet activation.29 These findings suggest that the increased adhesion of platelets to textured implant surfaces is not accompanied with a concomitant increase in cytokine release. Moreover, the data support the notion that platelet adhesion and secretion are regulated independently.
Fig. 4.
Platelet secretion on Ti surfaces is unaffected by surface topography. Bar graphs depict the concentration of secreted platelet factor 4 (PF4) (A), transforming growth factor-beta1 (TGF-β1) (B), soluble P-selectin (sCD62P) (C), and platelet-derived growth factor-AB (PDGF-AB) (D) from human platelets on smooth Ti (white bars), acid-etched Ti (light grey bars), sand-blasted Ti (dark grey bars) and SLA-treated Ti (black bars). Data are mean ± SEM and represent a minimum of 3 independent experiments using blood from different donors.
4. Discussion
Platelets are critical for mediating wound healing and tissue regeneration.23 In the context of dental implants, platelets are the first cells to contact the implant surface.12,13 Since activated platelets are abundant sources of growth factors that promote wound healing and osseointegration,17, 18, 19 it is therefore of considerable interest to identify and dissect the signaling mechanisms that trigger specific platelet responses, at the cellular level. Previous studies evaluating the response of platelets to implant surface topography studied platelets retained in plasma.13,27,28 To examine the pure cellular-level response to surface topography, we employed purified platelets in our study design. Our findings indicate that rougher surface topographies favor initial platelet adhesion although this increased adhesion is not accompanied by a commensurate increase in cytokine secretion.
Our finding of enhanced early platelet adhesion on the rougher implant surfaces is in agreement with data from Alfarsi et al., who also reported increased adhesion of platelets on SLA surfaces relative to smooth controls.27 The increased platelet adhesion could promote osseointegration since platelets recruit and/or bind directly to other inflammatory cells that participate in wound healing, including neutrophils and monocytes/macrophages.33
An interesting aspect of our data is the finding that the release of several cytokines (PF4, PDGF-AB, TGF-β1, sCD62P) remained unchanged despite the increased number of adherent platelets. Since there was no addition of exogenous platelet agonist (e.g. thrombin or collagen), it is expected that the platelet activation observed in our experimental system was primarily induced by contact with the Ti surface. Moreover, published data suggest that the release kinetics of specific platelet molecules can occur independently of the platelet's activation status.29 Based on the data, it is conceivable that the rougher surface topography may in fact constrain the initial activation and/or release of the platelets' granular contents. The clinical implications of this are unclear since platelet granules contain both pro-angiogenic/pro-inflammatory as well as anti-angiogenic/anti-inflammatory molecules34 and evidence suggests that the release of specific molecules may be targeted through specific platelet activation pathways.29,34 Further research is required to identify novel surface topographical modifications that could induce specific platelet responses with regards to adhesion and/or granule secretion. For example, the secretion of platelet-derived cytokines and/or growth factors deemed critical for osseointegration could potentially serve as an important test parameter for newly developed implant surface topographies. Similarly, for surface topographies under development, the ability to promote initial platelet adhesion could serve as another test criterion. Importantly, the data from the present study suggest that platelet adhesion and secretion are distinct functions that should be assessed independently when evaluating different implant surfaces.
Our secretion data stand in slight contrast with those of a previous study comparing the platelet response to different surface topographies.28 The authors of that study reported decreased platelet counts and increased release of vascular endothelial growth factor (VEGF) and PDGF on the acid-etched and SLA surfaces.28 The authors concluded that the rougher surface topographies promoted early platelet activation as determined by VEGF and PDGF levels. Notably, their study incubated platelet-rich plasma with the implant surfaces whereas we used purified washed platelets that were separated from plasma. This fundamental difference in the study designs may explain the disparate results since the presence of plasma proteins in PRP could affect the nature of the signaling between the implant surface and the platelet. Our study was therefore designed with the intent to examine direct cellular-level responses at the implant-platelet interface. Moreover, by excluding circulating cytokines (from the plasma), the use of purified platelets allows for the precise measurement of cytokines that are expressly released from the platelet granules upon contact with the titanium surface.
One aspect of the platelet response that has not been studied with regards to implant surface topography is the pro-hemostatic response. Specifically, the platelet dense granules, in contrast to the α-granules, house molecules (e.g. ADP, ATP) whose primary role is to promote hemostasis.17,35 This is relevant in light of published evidence that suggests that α-granule and dense granule release may be regulated via separate signaling pathways.35
In summary, our data suggest that the studied dental implant surface topographies have minimal direct effect on cytokine secretion from platelets. It is nonetheless conceivable that these surface topographies could indirectly accelerate wound healing by promoting initial platelet adhesion, and by extension, the recruitment of other cells to the implant. It would be of considerable interest to identify and develop novel surface topographies capable of modulating both platelet adhesion and granule secretion simultaneously. From a clinical perspective, such information would also be applicable to the design of barrier membranes and bone grafting materials for regenerative procedures.
Funding
This research was supported by a Canadian Institutes of Health Research Operating Grant (MOP-142450) and Clinician-Scientist Salary Award (to HK), a UBC Centre for Blood Research Summer Studentship (to RM) and by a UBC Faculty of Dentistry Postdoctoral Fellowship Award (to CG).
Declaration of competing interest
The authors report no conflicts of interest.
Acknowledgements
The authors thank George Fokas (Faculty of Dentistry, University of Hong Kong) for facilitating the preparation of the titanium substrata and Felix Hong for preparing the graphical abstract.
References
- 1.Guglielmotti M.B., Olmedo D.G., Cabrini R.L. Research on implants and osseointegration. Periodontol 2000. 2019;79:178–189. doi: 10.1111/prd.12254. [DOI] [PubMed] [Google Scholar]
- 2.Bosshardt D.D., Chappuis V., Buser D. Osseointegration of titanium, titanium alloy and zirconia dental implants: current knowledge and open questions. Periodontol 2000. 2017;73:22–40. doi: 10.1111/prd.12179. [DOI] [PubMed] [Google Scholar]
- 3.Sivaswamy V., Bahl V. Surface modifications of commercial dental implant systems: an overview. J Long Term Eff Med Implants. 2023;33:71–77. doi: 10.1615/JLongTermEffMedImplants.2022042612. [DOI] [PubMed] [Google Scholar]
- 4.Luke Yeo I.S. Dental implants: enhancing biological response through surface modifications. Dent Clin North Am. 2022;66:627–642. doi: 10.1016/j.cden.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Wennerberg A., Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009;20(Suppl 4):172–184. doi: 10.1111/j.1600-0501.2009.01775.x. [DOI] [PubMed] [Google Scholar]
- 6.Chouirfa H., Bouloussa H., Migonney V., Falentin-Daudre C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019;83:37–54. doi: 10.1016/j.actbio.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Kim K.H., Ramaswamy N. Electrochemical surface modification of titanium in dentistry. Dent Mater J. 2009;28:20–36. doi: 10.4012/dmj.28.20. [DOI] [PubMed] [Google Scholar]
- 8.Shirazi S., Ravindran S., Cooper L.F. Topography-mediated immunomodulation in osseointegration; ally or enemy. Biomaterials. 2022;291 doi: 10.1016/j.biomaterials.2022.121903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyan B.D., Lotz E.M., Schwartz Z. (*) roughness and hydrophilicity as osteogenic biomimetic surface properties. Tissue Eng Part A. 2017;23:1479–1489. doi: 10.1089/ten.tea.2017.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albrektsson T., Tengvall P., Amengual L., Coli P., Kotsakis G.A., Cochran D. Osteoimmune regulation underlies oral implant osseointegration and its perturbation. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1056914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies J.E. Understanding peri-implant endosseous healing. J Dent Educ. 2003;67:932–949. [PubMed] [Google Scholar]
- 12.Park J.Y., Davies J.E. Red blood cell and platelet interactions with titanium implant surfaces. Clin Oral Implants Res. 2000;11:530–539. doi: 10.1034/j.1600-0501.2000.011006530.x. [DOI] [PubMed] [Google Scholar]
- 13.Park J.Y., Gemmell C.H., Davies J.E. Platelet interactions with titanium: modulation of platelet activity by surface topography. Biomaterials. 2001;22:2671–2682. doi: 10.1016/s0142-9612(01)00009-6. [DOI] [PubMed] [Google Scholar]
- 14.Vanegas-Acosta J.C., Garzon-Alvarado D.A., Lancellotti V. Numerical investigation into blood clotting at the bone-dental implant interface in the presence of an electrical stimulus. Comput Biol Med. 2013;43:2079–2088. doi: 10.1016/j.compbiomed.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Khosravi N., DaCosta R.S., Davies J.E. New insights into spatio-temporal dynamics of mesenchymal progenitor cell ingress during peri-implant wound healing: provided by intravital imaging. Biomaterials. 2021;273 doi: 10.1016/j.biomaterials.2021.120837. [DOI] [PubMed] [Google Scholar]
- 16.Boscher J., Guinard I., Eckly A., Lanza F., Leon C. Blood platelet formation at a glance. J Cell Sci. 2020;133 doi: 10.1242/jcs.244731. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y., Yuan Y., Li W. Sorting machineries: how platelet-dense granules differ from alpha-granules. Biosci Rep. 2018;38 doi: 10.1042/BSR20180458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golebiewska E.M., Poole A.W. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29:153–162. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blair P., Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broos K., Feys H.B., De Meyer S.F., Vanhoorelbeke K., Deckmyn H. Platelets at work in primary hemostasis. Blood Rev. 2011;25:155–167. doi: 10.1016/j.blre.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Gremmel T., Frelinger A.L., 3rd, Michelson A.D. Platelet physiology. Semin Thromb Hemost. 2016;42:191–204. doi: 10.1055/s-0035-1564835. [DOI] [PubMed] [Google Scholar]
- 22.van der Meijden P.E.J., Heemskerk J.W.M. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16:166–179. doi: 10.1038/s41569-018-0110-0. [DOI] [PubMed] [Google Scholar]
- 23.Etulain J. Platelets in wound healing and regenerative medicine. Platelets. 2018;29:556–568. doi: 10.1080/09537104.2018.1430357. [DOI] [PubMed] [Google Scholar]
- 24.Gruber R., Varga F., Fischer M.B., Watzek G. Platelets stimulate proliferation of bone cells: involvement of platelet-derived growth factor, microparticles and membranes. Clin Oral Implants Res. 2002;13:529–535. doi: 10.1034/j.1600-0501.2002.130513.x. [DOI] [PubMed] [Google Scholar]
- 25.Oprea W.E., Karp J.M., Hosseini M.M., Davies J.E. Effect of platelet releasate on bone cell migration and recruitment in vitro. J Craniofac Surg. 2003;14:292–300. doi: 10.1097/00001665-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Gruber R., Karreth F., Kandler B., et al. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets. 2004;15:29–35. doi: 10.1080/09537100310001643999. [DOI] [PubMed] [Google Scholar]
- 27.Alfarsi M.A., Hamlet S.M., Ivanovski S. Titanium surface hydrophilicity enhances platelet activation. Dent Mater J. 2014;33:749–756. doi: 10.4012/dmj.2013-221. [DOI] [PubMed] [Google Scholar]
- 28.Kammerer P.W., Gabriel M., Al-Nawas B., Scholz T., Kirchmaier C.M., Klein M.O. Early implant healing: promotion of platelet activation and cytokine release by topographical, chemical and biomimetical titanium surface modifications in vitro. Clin Oral Implants Res. 2012;23:504–510. doi: 10.1111/j.1600-0501.2011.02153.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim H., Falet H., Hoffmeister K.M., Hartwig J.H. Wiskott-Aldrich syndrome protein (WASp) controls the delivery of platelet transforming growth factor-beta1. J Biol Chem. 2013;288:34352–34363. doi: 10.1074/jbc.M113.459750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrientos S., Stojadinovic O., Golinko M.S., Brem H., Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 31.Hart C.E., Bailey M., Curtis D.A., et al. Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all three PDGF dimers in human platelets. Biochemistry. 1990;29:166–172. doi: 10.1021/bi00453a022. [DOI] [PubMed] [Google Scholar]
- 32.Colciago A., Celotti F., Casati L., et al. In vitro effects of PDGF isoforms (AA, BB, AB and CC) on migration and proliferation of SaOS-2 osteoblasts and on migration of human osteoblasts. Int J Biomed Sci. 2009;5:380–389. [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang S.Z., To J.L., Hughes M.R., McNagny K.M., Kim H. Platelet signaling at the nexus of innate immunity and rheumatoid arthritis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.977828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Battinelli E.M., Markens B.A., Italiano J.E., Jr. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118:1359–1369. doi: 10.1182/blood-2011-02-334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul M., Golla K., Kim H. Gelsolin modulates platelet dense granule secretion and hemostasis via the actin cytoskeleton. Thromb Haemost. 2023;123:219–230. doi: 10.1055/s-0042-1758800. [DOI] [PubMed] [Google Scholar]